
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 28 April 2020
Sec. Behavioral and Evolutionary Ecology
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.00099
Oscine songbirds are an ideal system for investigating how early experience affects vocal behavior. Young songbirds face a challenging task: how to recognize and selectively learn only their own species’ song, often during a time-limited window. Because birds are capable of hearing birdsong very early in life, early exposure to song could plausibly affect recognition of appropriate models; however, this idea conflicts with the traditional view that song learning occurs only after a bird leaves the nest. Thus, it remains unknown whether natural variation in acoustic exposure prior to song learning affects the template for recognition. In a population where sister species, golden-crowned and white-crowned sparrows, breed syntopically, we found that nestlings discriminate between heterospecific and conspecific song playbacks prior to the onset of song memorization. We then asked whether natural exposure to more frequent or louder heterospecific song explained any variation in golden-crowned nestling response to heterospecific song playbacks. We characterized the amount of each species’ song audible in golden-crowned sparrow nests and showed that even in a relatively small area, the ratio of heterospecific to conspecific song exposure varies from 0 to 20%. However, although many songbirds hear and respond to acoustic signals before fledging, golden-crowned sparrow nestlings that heard different amounts of heterospecific song did not behave differently in response to heterospecific playbacks. This study provides the first evidence that song discrimination at the onset of song learning is robust to the presence of closely related heterospecifics in nature, which may be an important adaptation in sympatry between potentially interbreeding taxa.
Juvenile experience can set the stage for behavior later in life. The effects of early sensory experience have been studied in many taxa in the context of mate choice (Hebets, 2003; Verzijden and ten Cate, 2007; Balakrishnan et al., 2009; Delaney and Hoekstra, 2018) as well as in non-mating contexts (Colombelli-Négrel et al., 2012; König et al., 2015). How evolution shapes the timing and selectivity of learning has received considerable theoretical attention (Lachlan and Servedio, 2004; Olofsson et al., 2011; Verzijden et al., 2012) and, in a few cases, has been demonstrated empirically with adult mate choice (Grant and Grant, 1996; Magurran and Ramnarine, 2005). Learning is especially well studied in oscine songbirds, in which exposure to acoustic cues influences what songs a bird will later sing (Bateson, 1979; Nelson et al., 1997; Marler and Peters, 2010). Young birds that are not exposed to conspecific song tend to develop abnormal songs (Marler, 1970; Petrinovich, 1985). It is critical that songbird fledglings quickly identify conspecific models; failure to learn conspecific song can limit a male’s chances of successfully attracting conspecific mates (e.g., Grant and Grant, 1997; Qvarnström et al., 2006). Accordingly, songbirds exhibit selective song learning, e.g., by preferentially learning song from their own species, as demonstrated by decades of laboratory studies (Marler, 1970; Marler and Peters, 1977; Soha and Marler, 2000). Furthermore, learning is often limited to a sensitive period, beginning shortly after fledging, in which young birds are able to rapidly memorize song syllables they hear and will later sing (Bateson, 1979; Nelson et al., 1997). Songs heard after this developmental window are not sung later (Marler, 1970), nor are songs exclusively heard by nestlings (i.e., before the sensitive period) ever produced later (Slater, 1983b), suggesting that imitative male song learning does not occur in the nest (although see the discussion of embryonic learning below). Female learning is less well studied, but there is evidence that female songbirds also learn songs and song preferences (Konishi, 1965; Baptista and Morton, 1982; Lauay et al., 2004). How young birds of both sexes accomplish selective learning, avoiding mistakenly learning from heterospecifics, is a crucial adaptation that is likely important to evolutionary processes such as sexual selection and cultural evolution, as well as reproductive isolation between closely related taxa. This is an especially critical task in populations where closely related taxa coexist during the breeding season.
In order for selective song learning to take place, young birds must be able to discriminate between conspecific and heterospecific songs. This is hypothesized to be accomplished using an “acoustic template,” a neural representation of a key feature or features of appropriate conspecific song, which is in place at the onset of the learning period (Marler, 1970; Soha, 2017). Some experimental evidence, such as preferential learning or responsiveness to conspecific songs in young birds cross-fostered by heterospecifics (Marler and Peters, 1977; Wheatcroft and Qvarnström, 2017), suggests that acoustic templates at the onset of song learning are “innate”—i.e., genetically encoded and not influenced by early acoustic exposure (Wheatcroft and Qvarnström, 2015). However, recent work also shows that songbirds are capable of responding to and learning from acoustic experiences as nestlings (Davies et al., 2004; Madden and Davies, 2006; Haff and Magrath, 2012), or even as embryos inside eggs (Colombelli-Négrel et al., 2012, 2014; Mariette and Buchanan, 2016; Colombelli-Négrel and Kleindorfer, 2017; Katsis et al., 2018; Kleindorfer et al., 2018; Rivera et al., 2019). The body of work testing for early song discrimination has shown that species recognition is detectable at a relatively early age, including in wild nestling birds (Shizuka, 2014; McFarlane et al., 2016; Hudson and Shizuka, 2017; Wheatcroft and Qvarnström, 2017; Hudson et al., 2019b). Combining behavioral tests of species recognition in nestlings with measurements of natural variation in early song exposure allows us to investigate the extent to which young birds are sensitive to their acoustic environment while they are still in the nest. The critical question is whether early experience affects the ability of nestlings to recognize their own species—specifically, whether early exposure to heterospecific song may act in opposition to the well-documented tendency of young songbirds to preferentially respond to conspecific song. Alternatively, exposure to heterospecific song could improve a bird’s ability to discriminate against other species’ songs (Campbell and Hauber, 2010). Intuitively, the task of discriminating against heterospecific song to avoid costly learning mistakes should be most important when sister species breed at the same site (and thus may experience hybridization, which is frequently costly). Is the behavior of young birds in such populations affected by the acoustic presence of their sister species, or has selection acted to mitigate exposure to heterospecific song (e.g., by favoring nestlings that ignore heterospecific song)? The first step in addressing this question is establishing the amount of heterospecific song, if any, that is heard by nestling birds in a natural context. The responses of nestlings that are exposed to relatively more heterospecific song in the nest could then be compared with those nestlings that hear relatively less heterospecific song.
To address this longstanding question of the role of early experience on species recognition, we focused on the golden-crowned sparrow (Zonotrichia atricapilla), a large sparrow found in western North America. The golden-crowned sparrow’s sister species, the white-crowned sparrow (Zonotrichia leucophrys), breeds across a wide swath of the United States and Canada and is one of the model organisms for studying the timeline of avian vocal learning (reviewed in Soha, 2017). In white-crowned sparrows, as in many bird species, song is known to play a key role in mate selection (Becker, 1982), making the task of learning the correct song critical for males’ reproductive success (females of both species do not sing during the breeding season). Their close phylogenetic relationship with the well-studied white-crowned sparrow provides a solid basis for inferring some aspects of the golden-crowned sparrow song learning process; namely, that learning begins only after fledging, at ∼10 days after hatching. Moreover, in many parts of their breeding range, these two species breed simultaneously in the same treeline habitat, making them an ideal species pair in which to study how the presence of closely related species affects learning. It has been noted that hybridization often occurs across large genetic distances in birds, with viable offspring produced from hybridizing parent species separated by tens of millions of years (Price and Bouvier, 2002; McCarthy, 2006). Despite this potential for hybridization, the formation of hybrid pairs between golden- and white-crowned sparrows appears to be very rare (never seen at this site during fieldwork in June and July of 2013, 2015, and 2017, although two possible F1 hybrids have been described outside the breeding season in California; Miller, 1940; Morton and Mewaldt, 1960). Thus, despite the apparently ample opportunity to hybridize, a reproductive barrier clearly exists between these two closely related taxa, although whether this barrier is based on pre-mating behavioral barriers is not known.
In this study, we take advantage of natural variation in heterospecific (white-crowned sparrow) abundance at our field site to test the effect of exposure to heterospecific signals on the behavior of nestling golden-crowned sparrows. We investigate (1) whether nestling golden-crowned sparrows can distinguish heterospecific and conspecific songs prior to the sensitive period for vocal learning and (2) whether the acoustic environment in the nest affects this ability. If so, we might expect the response to vary depending on the level of heterospecific sound exposure. Another possibility is that nestling birds only attend to the loudest songs audible at the nest, or those above some threshold amplitude; in this case, we would expect nestlings to respond most to the song types that are loudest at their nest. If nestlings show no early effect of acoustic experience, this would suggest that species recognition is not overwritten by heterospecific exposure, even when this exposure occurs at a very early stage.
All recordings and playbacks were conducted at Hatcher Pass Management Area, Alaska in June and July 2017. During this period, sunrise time varied between 4:11 and 4:46 a.m. Therefore, to capture the dawn chorus with a conservative margin of time before and after sunrise, we continuously recorded between midnight and 7 a.m. local time using five Wildlife Acoustics Song Meters (SM4) (Song meter SM4 acoustic recorder, 2016). Nests were all constructed on the ground in this population, so recorders were placed on the ground at approximately 1 m from each golden-crowned sparrow nest, for one dawn chorus per nest, within 24 h of conducting playback experiments (N = 23 nests). Because of the relatively low density of singing birds at this location, and in particular, the fact that males almost never sing at their own nests, we consider the acoustic environment on the ground for a 1 m radius around the nest to be equivalent. Recordings were saved as consecutive 20 min WAV files, sampled at 16 or 24 kHz (16 bits per sample). Six researchers annotated recordings between 2 and 6 a.m., corresponding approximately to the 2 h before and after sunrise, noting golden-crowned and white-crowned sparrow songs if they were visible on the spectrogram in the program Syrinx (J. Burt, Seattle, WA, United States). Overlapping songs from different birds were counted as two separate songs if they overlapped for less than one-third of their duration. White-crowned sparrow exposure was then calculated for each nest by dividing the number of white-crowned songs detected by the total number of golden-crowned and white-crowned songs detected. We used ratio of songs because we reasoned that it may be a more consistent property of each nest over time; e.g., on a rainy morning when fewer songs are produced, both white-crowned number of songs and golden-crowned songs should both be decreased by a similar amount. Song data were only recorded at more than one date for a subset of five nests, and song ratios were moderately repeatable and significantly correlated across two days (Pearson’s product-moment correlation P = 0.017, R = 0.94; Figure 4 and Supplementary Table S1).
We also quantified the relative amplitude of both golden-crowned and white-crowned sparrow songs. Relative amplitudes were compared only between songs recorded on the same day with the same SM4 unit. We generated a Gaussian-windowed spectrogram of these songs in Matlab (as in Gardner and Magnasco, 2006), which results in a time-by-frequency matrix of signal intensity. For each annotated song, we calculated the mean and total amplitude of each song using the spectrogram matrix values within the time and frequency bounds identified in Syrinx. However, different nests had different levels of environmental background noise, which affects our amplitude calculations. To remove environmental background noise from our song amplitude measurements, we took the average amplitude from a period of time without any bird song (between 12 a.m. and 1:30 a.m.) in the same recording, and subtracted this average background value from the average amplitude of annotated songs for each nest (see Supplementary Figure S1 for an example). Because of the relatively low diversity and abundance of birds at this site, there is not a separate community of species that sings at night. The long silent stretches during hours after midnight thus provide an adequate measure of abiotic background noise (primarily streams). After subtracting this background amplitude from each song, we were able to estimate the relative amplitude of golden-crowned sparrow songs and white-crowned sparrow songs separately at each nest.
Previous studies showed that nestling golden-crowned sparrows that were played either conspecific song or that of the sympatric white-crowned sparrow chirp more in response to conspecific songs (Shizuka, 2014; Hudson and Shizuka, 2017; Hudson et al., 2019b). To test the role of early song exposure in modulating this behavior, we followed the same protocol as previous studies by conducting playbacks when nestlings were about 8–10 days old, as estimated based on hatch date or length of exposed primary feather (>6mm for the majority of nestlings). In total, 82 nestlings from 23 nests were included in the study; depending on the comparison being tested, not all nests were able to be used for all analyses below, in which case the smaller sample size will be given. All eggs in a nest typically hatch within 24 h (Norment et al., 1998), making the nestlings in each brood roughly the same age. All nestlings in a nest (2–6 per nest, mean 4.2, variance 0.79) were temporarily removed and randomly assigned to one of two playback treatments (golden-crowned or white-crowned sparrow song), each consisting of 6 stimulus files created from a unique recording of a different individual male to avoid pseudoreplication (recorded > 6 years prior at sites > 100 km away). These golden-crowned sparrow song recordings are of the same dialect type that males at this study site produce (Shizuka et al., 2016), and have been effective at eliciting strong responses from adult males and nestlings in this population previously (Shizuka, 2014; Hudson et al., 2019b). As in Hudson and Shizuka (2017) and Hudson et al. (2019b), each stimulus file consisted of 1 min of white noise, 2 min of song presentation (the same song recording repeated every 10 s), and an additional minute of white noise. For each trial, an individual nestling subject was placed alone in a collapsible cloth pet carrier (26 × 27 × 48 cm). Songs were broadcast from an iPod Nano mp3 player (Apple) through a speaker (iHome model IM60 or IM70, SDI Technologies, Inc., Rahway, NJ, United States) placed immediately outside the pet carrier. Playback volume was standardized to 60 dB at 1m from the speaker for consistency with previous studies in this population (Shizuka, 2014; Hudson and Shizuka, 2017; Hudson et al., 2019b). The response variable recorded was the number of times a nestling produced a chirp (“fledgling location chirp”; Marler, 1970), a behavior that has been used in the past to measure discrimination between song types by nestlings (Shizuka, 2014; Hudson and Shizuka, 2017; Hudson et al., 2019b) and fledglings (Soha and Marler, 2001). The observer recorded the number of chirps the nestling produced during the 1 min pre-playback period, 2 min of song playback, and 1 min post-playback period. A previous study using the same protocol found high inter-observer agreement in chirp numbers when trial videos were re-scored later by a different individual (Hudson et al., 2019b).
We collected nestling blood from the brachial vein immediately following playback trials and stored the blood on FTA filter paper cards. We determined the sex of individual nestlings using a standard DNA-based sexing protocol (Griffiths et al., 1998), which has been validated for this species (Chaine et al., 2011).
All statistical analyses were carried out in R (version 3.6.0, R Core Team 2019). After correcting for background noise as described above, we compared the amplitude of all annotated white-crowned sparrow songs with that of all annotated golden-crowned sparrow songs using a Wilcoxon rank-sum test.
We initially used the “lm” function in R to test the effect of proportion of white-crowned sparrow songs at a nest on average nest response to white-crowned song. We also performed this analysis with average amplitude of white-crowned sparrow songs at a nest replacing song exposure as a fixed effect. We next verified that nestlings were able to discriminate between conspecific (golden-crowned sparrow) and heterospecific (white-crowned sparrow) songs with a generalized linear mixed model with quasi-Poisson error distribution using the “glmmPQL” function in the “MASS” package (Venables and Ripley, 2002). We used penalized quasi-likelihood (PQL) because the data (number of chirps during the playback period) were overdispersed (c = 640, where c > 1 indicates overdispersion). The fixed effects were playback type, pre-track response, brood size, white-crowned sparrow song exposure, feather length, nestling sex, and the interaction of playback type and exposure; nestling nest of origin was included a random effect.
Our acoustic monitoring revealed variation in the amount of white-crowned sparrow song recorded at each golden-crowned sparrow nest. While some golden-crowned sparrow nests had no audible white-crowned sparrow songs (N = 8), the majority of golden-crowned sparrow nests were exposed to some white-crowned sparrow song (N = 15), at levels between 0.03 and 19% of the combined amount of golden-crowned and white-crowned-sparrow song (Figure 1 and Supplementary Table S2). In line with previous findings, nestling golden-crowned sparrows chirped more to conspecific song (median 32, range 0–124) than to white-crowned sparrow song (median = 1, range = 0–81; Figure 2, P < 0.001), supporting previous findings in this population (Shizuka, 2014; Hudson and Shizuka, 2017). We found that white-crowned sparrow songs recorded at golden-crowned sparrow nests were louder on average than golden-crowned sparrow songs (Wilcoxon rank-sum test, P < 0.001, N = 20, Figure 3A). We also found, from three nests at which 24 h recordings were annotated, that song production of both species was highest between 2 and 7 a.m. (Supplementary Figure S2), which supported our decision to focus on this timeframe.
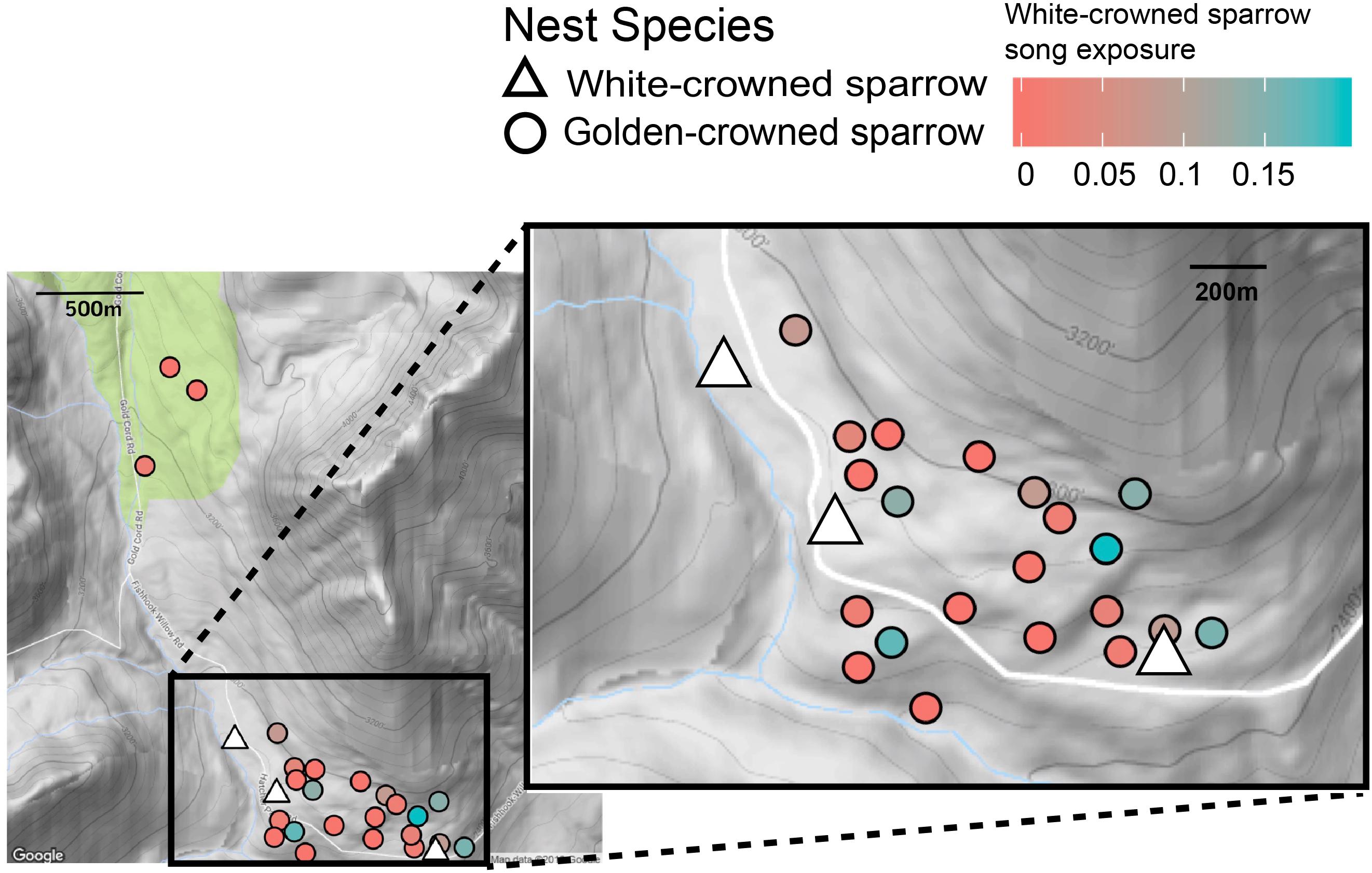
Figure 1. Positions of nests of playback subjects at Hatcher Pass Management area. White triangles represent active white-crowned sparrow nests; circles represent golden-crowned sparrow nests, with color representing the ratio of white-crowned sparrow song to total Zonotrichia songs recorded at that nest. At these golden-crowned sparrow nests, we exposed golden-crowned sparrows to playbacks of golden-crowned and white-crowned sparrow song and measured the response of nestlings in number of chirps during the playback period. Inset shows a detail of the densest area of nests. Interestingly, the proportion of heterospecific song at a golden-crowned sparrow nest does not seem to be predicted by its distance to the nearest white-crowned sparrow nest.
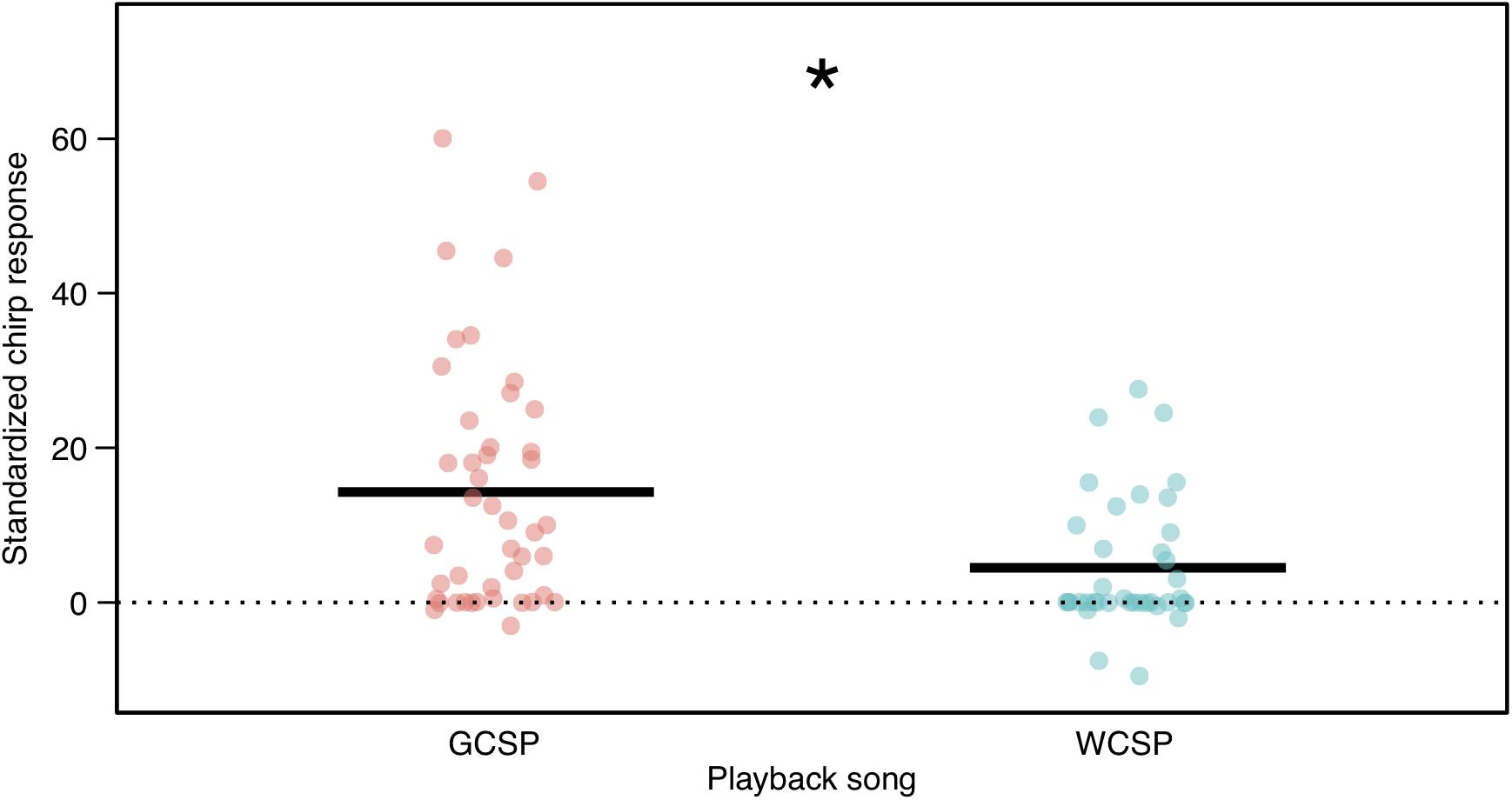
Figure 2. Response of golden-crowned sparrow nestlings to playbacks of conspecific (left) and white-crowned sparrow (right) songs (mean responses represented by horizontal bold lines). To better visualize responses, here the number of chirps in the 1-min pre-playback period was doubled and subtracted from the number of chirps during the 2-min playback period. Statistically, the effect of pre-trial chirping was accounted for by adding it as a fixed effect to the mixed-effects model (see section “Materials and Methods”). The nestling response to conspecific, golden-crowned sparrow songs was significantly higher than to white-crowned sparrow songs (P = 0.0007) as indicated by an asterisk.
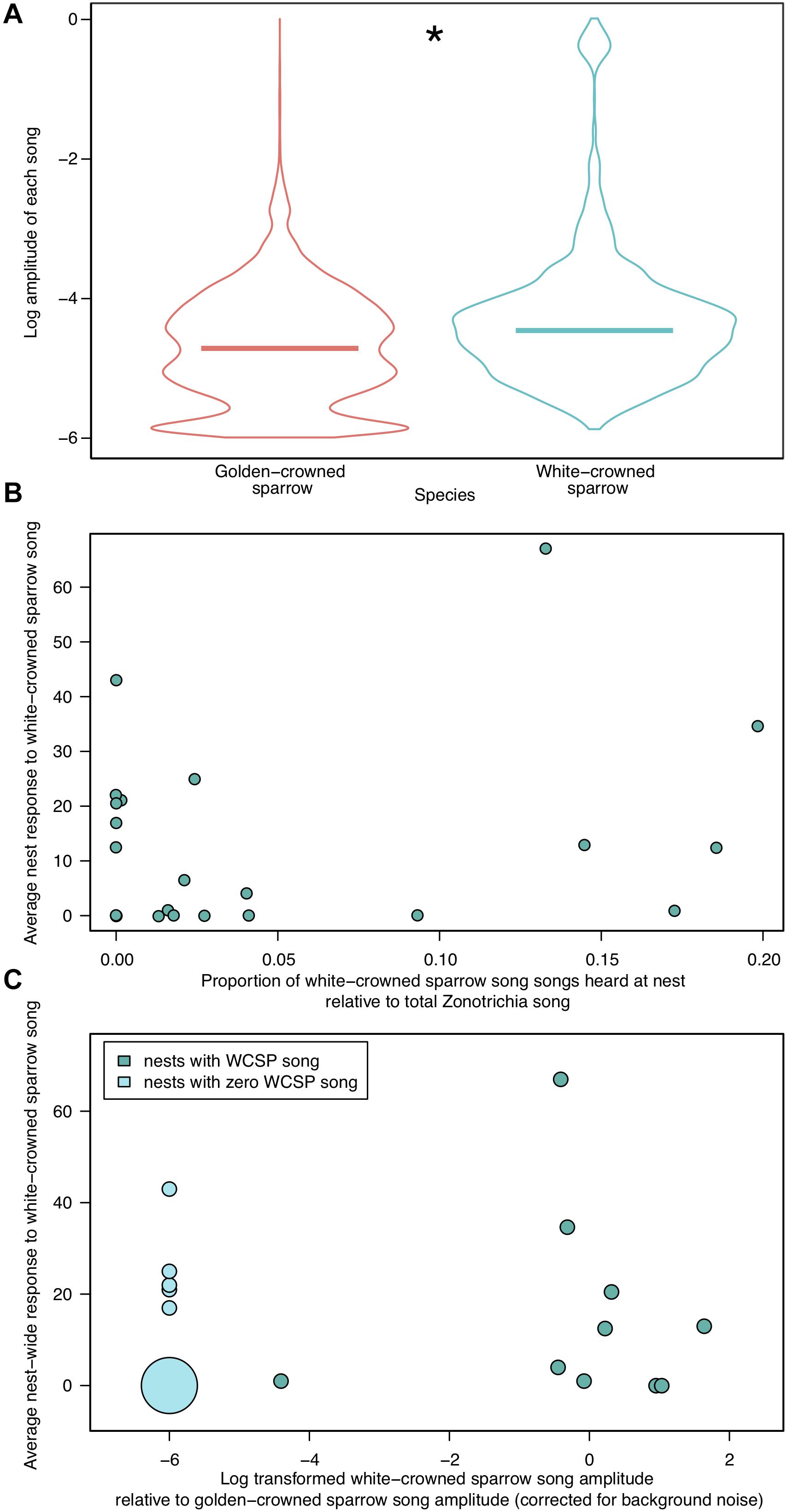
Figure 3. (A) The amplitudes of all recorded Zonotrichia songs at each golden-crowned sparrow nest (N = 20), log-transformed to aid visualization. White-crowned sparrow songs (right), although less frequently heard, were on average louder than golden-crowned sparrow songs (left) heard at golden-crowned sparrow nests (medians shown with horizontal bars; Wilcoxon rank-sum test, P < 0.001). (B) Average response of golden-crowned sparrow nestlings to white-crowned sparrow playback, as explained by proportion of white-crowned sparrow song to total Zonotrichia song heard at the nest during the dawn chorus. There was no effect of proportion of white-crowned sparrow song on average responsiveness (linear model; P = 0.252, N = 23). For results of the full generalized linear model, see Section “Results” and Table 1. (C) Average response of golden-crowned sparrow nestlings to white-crowned sparrow song playback, as explained by relative amplitude of white-crowned sparrow songs at each nest. Ratios of average white-crowned sparrow amplitude to average golden-crowned sparrow amplitude are transformed to a log scale to aid visualization; larger negative values represent nests with higher ratios of golden-crowned sparrow amplitude relative to white-crowned sparrow amplitude, and vice versa. Nests with no white-crowned sparrow songs (zero amplitude) are represented in lighter blue and assigned to an arbitrary value of -7 on this scale. The area of each circle represents the number of points at identical coordinates. The amplitude of white-crowned song did not predict the response to white-crowned song (linear model P = 0.65, N = 20).
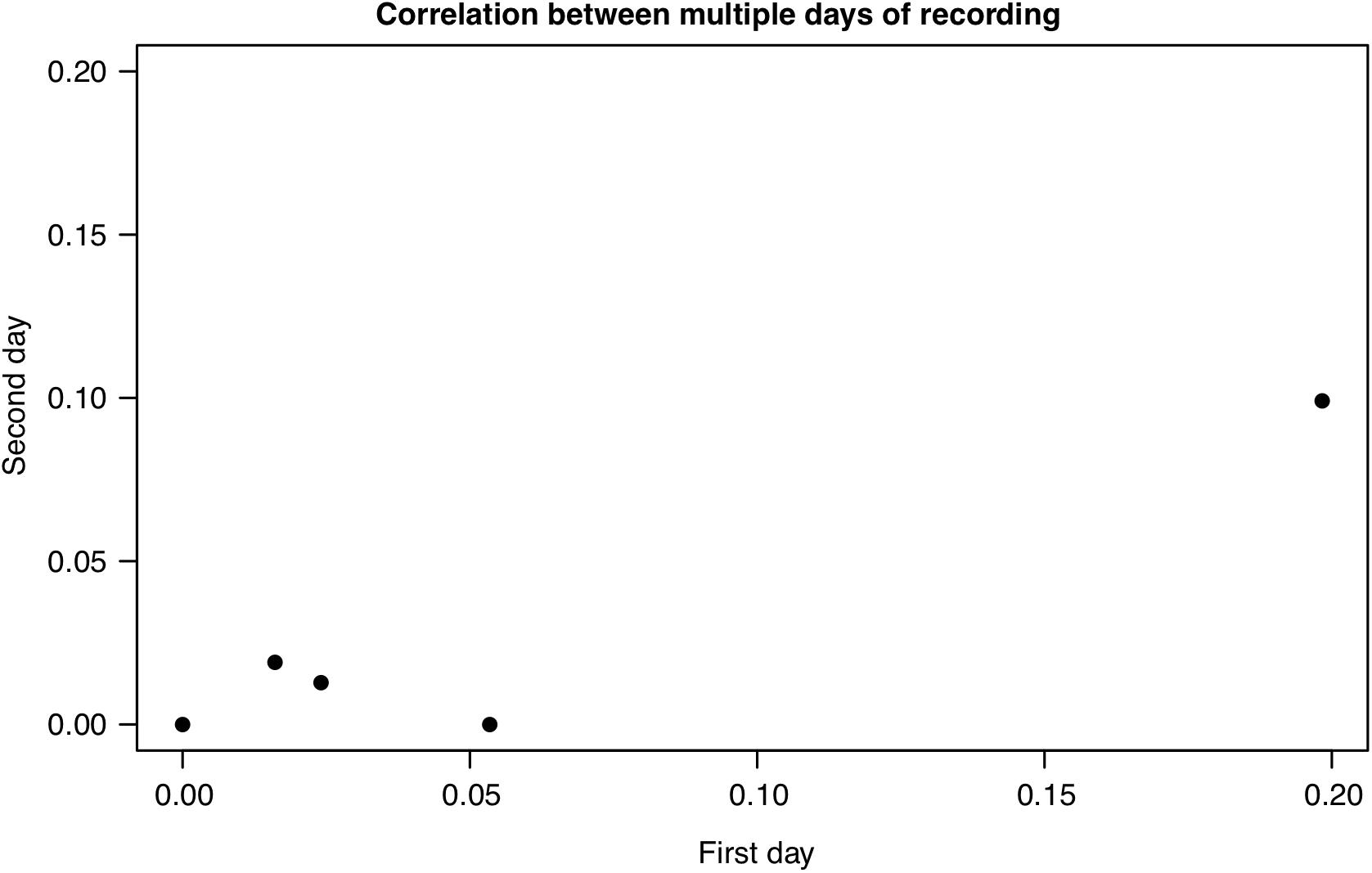
Figure 4. The correlation between the first and second day of recording for five nests at which 2 days of recordings were available (Pearson’s product-moment correlation P = 0.017, R = 0.94). All nests were recorded on consecutive dates, except the nest at (0,0) which was recorded six days apart (see Supplementary Table S1).
Exposure to white-crowned sparrow song did not increase average responsiveness to white-crowned song at a given nest (linear model; P = 0.252, N = 23, Figure 3B), nor did the amplitude of white-crowned song predict the response to white-crowned song (linear model P = 0.6, N = 20, Figure 3C). Using the difference between average nest-wide chirps to golden-crowned and white-crowned sparrow song as the response variable produced similar non-significant results (P = 0.112, n = 23); therefore, we only present total individual chirp values hereafter to maintain consistency with previous studies in this population (Shizuka, 2014; Hudson and Shizuka, 2017).
Overall, the number of chirps prior to playback, and the species of song playback (golden-crowned sparrow or white-crowned sparrow) had the greatest effect on golden-crowned nestling response (Table 1). In other words, chicks tested with golden-crowned sparrow song, and chicks that chirped more during the pre-stimulus period, both chirped significantly more during the playback period. Similarly to the nest-wide results reported above, white-crowned sparrow song exposure did not significantly affect individual nestlings’ chirp response (P = 0.24, Table 1). The pattern of results was the same whether white-crowned sparrow exposure was measured as the proportion of white-crowned sparrow to total Zonotrichia song or as the rate of white-crowned sparrow song (number of songs per hour, Supplementary Table S3). Notably, the interaction between playback type and white-crowned sparrow song proportion was not a significant predictor of the chirp response (Table 1). Together, these results do not support the hypothesis that nestling golden-crowned sparrows’ response to conspecific song varies depending on the level of heterospecific sound exposure and instead support the hypothesis that species recognition cannot be overwritten by early heterospecific exposure.
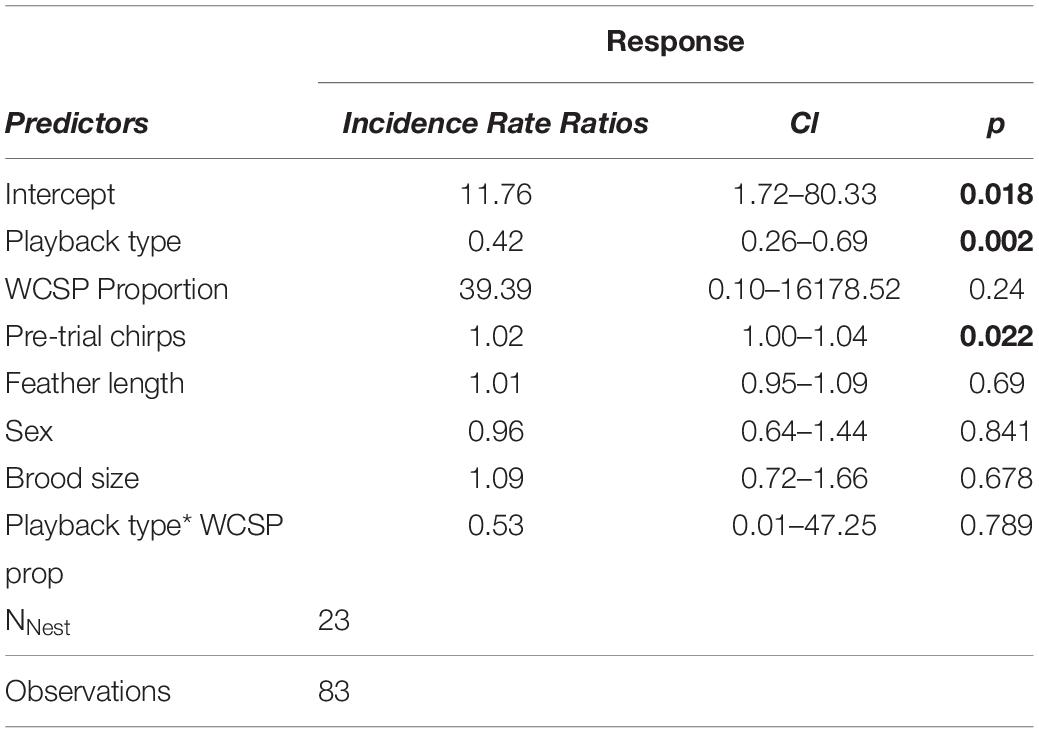
Table 1. Generalized linear mixed effects model with quasi-poisson error distribution and nest of origin as a random effect (N = 23).
In this study of golden-crowned sparrows, we quantified the amount of conspecific song, as well as the amount of song of a congeneric sister species, audible in the nest in the wild. We show that the amount and amplitude of white-crowned sparrow song heard by golden-crowned sparrow nestlings does not influence their response to playbacks of either species’ song. Documenting the opportunity for exposure to heterospecifics, especially closely related species, is important for understanding the evolution of recognition and its role in reproductive isolation. We found that even within a small area (∼0.5 km2), nests of golden-crowned sparrows varied moderately in the amount of relative white-crowned sparrow song exposure. This is likely due to the fact that white-crowned sparrows occupy larger territories than golden-crowned sparrows at this site, with each white-crowned male overlapping the territories of several golden-crowned males. Interestingly, however, the level of exposure did not seem to be purely explained by distance to the closest white-crowned sparrow nest (Figure 1). This surprising result may be partially explained by the extremely uneven terrain at the site; nests that were nearby in geographic distance were often separated by a ridge or other obstruction that interfered with sound transmission. Furthermore, for both white-crowned and golden-crowned sparrows, the location of nests within territories does not, from our observations, correlate in any way with the territory center, or most frequently occupied areas. In both species, we observed that males repeatedly use preferred singing perches that are within their territory, but not necessarily close to the nest. In species pairs that have overlapping territories, this can lead to the counterintuitive pattern that the heterospecific neighbors’ songs can be heard louder at a nest than the songs of the father or conspecific neighbors. Future work could confirm this explanation by documenting the location of all singing perches relative to focal nests. We are confident that the nests with high levels of white-crowned exposure in the core area of the study site are not explained by the presence of undetected white-crowned sparrow nests nearby; since all male birds on our study site were banded, an unbanded singing white-crowned sparrow with a nest in this area would almost certainly have been noted during our ∼8 h of daily observations. Furthermore, white-crowned sparrow song exposure was significantly correlated at the limited number of nests at which we were able to compare recordings between 2 days, supporting the idea that this aspect of the environment is consistent at each nest for at least some of the breeding season. Our findings suggest that even within the same population, individuals likely experience widely varying acoustic environments. This intuitive but rarely documented fact should be considered when studying learning, discrimination, and sympatry in the wild.
The present study is consistent with previous results suggesting that conspecific songs are more salient to nestling birds than heterospecific songs (Shizuka, 2014; Hudson and Shizuka, 2017). This has often been explained as an innate predisposition, consistent with results from young birds kept in isolation (Soha, 2017). An intuitive alternative explanation is that this predisposition is shaped by very early acoustic experience. As a first test of this alternative hypothesis, we sampled song exposure in a limited window of time at nests in a natural field context, and found no nest-wide effect of ambient heterospecific song exposure on golden-crowned sparrow nestling responses to heterospecific playbacks; on both an individual and nest-by-nest basis, white-crowned sparrow song exposure during the limited period of time we measured did not explain a significant amount of variation in nestling response. Only the playback type (golden-crowned sparrow or white-crowned sparrow song) and pre-playback activity level (chirps prior to the start of the stimulus) predicted response to the playback. Thus, our results support a commonly assumed (Nelson and Marler, 1993), but difficult to test, idea that preferential responses toward conspecific songs in fledglings are not learned via experience in the nest.
Many songbirds, including sparrows in the genus Zonotrichia, show a large degree of within-species geographic variation in their songs (Marler and Tamura, 1962; Nottebohm, 1969; Shizuka et al., 2016); our results raise new questions about how early experience with varied dialects affects behavior in young birds. A previous study showed that nestling golden-crowned sparrows in the present population discriminate against foreign dialects of conspecific song, responding to playbacks of these unfamiliar dialects as little as to white-crowned sparrow song (Hudson et al., 2019b). The results of the previous study suggest that nestlings either have an innately determined preference for local song, or, in contrast to our results with heterospecific song here, early nestling experience with song (in this case, with local variants of conspecific song) increases its salience. In either case, further study is required to explain the lack of response to conspecific, but unfamiliar, songs. In some taxa (Magurran and Ramnarine, 2004, 2005), selection is hypothesized to favor genetic assimilation of learned responses (e.g., innately encoding the ability to discriminate against heterospecifics) when learning errors are costly. It may be the case that in golden-crowned sparrows, learning from white-crowned sparrows is so costly that nestlings have evolved to filter out their songs as nestlings, setting the stage for accurate learning later in development. However, conspecific stimuli may still pass this early filter, and lead nestlings to respond preferentially to the local dialect they hear most often. This explanation fits our results and is consistent with the theory that young birds might initially accept only a narrow range of conspecific signals based on filial imprinting, which is later broadened via experience with other conspecifics (Irwin and Price, 1999). To better understand how recognition systems can evolve, this study focused on measuring naturally occurring variation in heterospecific song exposure in a population dominated by conspecifics. However, an informative extension of our results would be artificially manipulating the proportion of heterospecific song exposure to include more extreme values. Perhaps heterospecific exposure below some threshold does not influence juvenile behavior, but at high levels, recognition is affected. Manipulating nestlings’ experience by broadcasting high levels of heterospecific song (or foreign dialects of conspecific song) at the nesting site, similar to the approach used by Mennill et al. (2018), would be an important step in understanding learned recognition when abundances are unequal, such as at range limits or in hybrid zones.
Historically, selective song learning has been thought to begin only after fledging, as many species [white-crowned sparrows (Marler, 1970), swamp sparrows (Marler and Peters, 2010), zebra finches (Eales, 1985), and others (Slater, 1983b)] do not sing conspecific songs that were presented exclusively during the nestling period. However, it is possible for birds to demonstrate auditory learning earlier in development than the fledgling stage: in some crossbill species, nestlings have been shown to learn familial contact calls (Sewall, 2011) and brood parasites can adapt their begging calls to their host species (Davies et al., 2004; Madden and Davies, 2006; Liu et al., 2016). In addition, embryonic birds of multiple species have been shown to respond to Grier et al. (1967), Gottlieb (1988), and even learn from Colombelli-Négrel et al. (2012, 2014), Kleindorfer et al. (2018), species-specific sounds. These results show that, even if song learning per se has never been documented in the nest, learning related to vocalizations is possible in nestlings. Considered in this light, the variation in nestling chirp responses to heterospecific song (shown in Hudson and Shizuka, 2017; Hudson et al., 2019b) could plausibly be evidence of a behavioral effect of early heterospecific exposure. In this study, we set out to test whether, even if an innate template accurately guides young birds in this population to learn conspecific song, they might be affected in other ways by early experience, which could be manifested in different chirping responses.
Rigorously testing for early learning has been difficult in songbirds for two main reasons. First, a lack of song production does not necessarily mean a lack of learning: birds may retain songs in memory (Clayton, 1988), even if they do not produce them as part of their adult repertoire. Second, the most straightforward test of the role of very early learning requires raising birds in acoustic and social isolation from hatching (or even earlier, to avoid embryonic learning; Colombelli-Négrel et al., 2014), which is a logistical challenge in most songbirds. An alternative approach is cross-fostering, in which nestling birds are raised by a heterospecific foster parent, to see what effect early exposure to heterospecifics has on later behavior (Immelmann, 1972; Saether et al., 2007). These studies generally measure mate choice in adults, and so cannot directly test when in development heterospecific experience is most influential. We assayed behavioral discrimination at the nestling stage to test for early learning in real time. Our findings are consistent with the standard model of song learning, in which nestlings possess an innate, species-specific auditory template that is not dependent on early experience.
Early exposure to heterospecific sounds in the nest could potentially affect preference in two ways: either it could enable more effective species recognition, strengthening a predisposition to respond to conspecifics (Campbell and Hauber, 2010); or, it could interfere with species recognition and diminish the difference in response to heterospecifics versus conspecifics (Slagsvold et al., 2002; Eriksen et al., 2009). In contrast, our finding—that a significantly lower response to heterospecific song playback was maintained despite natural variation in heterospecific song exposure in the nest—provides a new line of support for the prediction that early-life experience with heterospecifics is not enough to override species-selective learning mechanisms. Disentangling the role of genetically inherited predispositions versus experience requires insight into the details of the learning process, natural history, and the ecological context in which learning takes place. Due to their history in behavioral ecology research and their unique biogeography, white- and golden-crowned sparrows are a promising system in which to study the interaction of these factors.
The datasets generated for this study are available on request to the corresponding author or at https://zenodo.org/record/3745691. The code used to run the analyses can be found at https://github.com/CreanzaLab/CulturalMigrationAndConnectivity.
The animal study was reviewed and approved by the University of Nebraska-Lincoln IACUC.
DS and EH conceived of the study and wrote the manuscript with substantial contributions from NC. EH carried out fieldwork and analyzed the data. NC conceived of and wrote the code to implement the amplitude analyses.
This work was supported by the University of Nebraska, Vanderbilt University, a GAANN fellowship to EH and Layman Fund Grant to DS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Mia Azizah and Paden Derr for assistance with collecting this data in the field, and Elie-Benjamin Elkahwaji, Jackson Fischer, Sabina Medrano, and Carter Svec for annotating song files. This manuscript has been released as a Pre-Print at https://doi.org/10.1101/756445 (Hudson et al., 2019a).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00099/full#supplementary-material
Balakrishnan, C. N., Sefc, K. M., and Sorenson, M. D. (2009). Incomplete reproductive isolation following host shift in brood parasitic indigobirds. Proc. R. Soc. B Biol. Sci. 276, 219–228. doi: 10.1098/rspb.2008.0733
Baptista, L. F., and Morton, M. L. (1982). Song dialects and mate selection in montane White-crowned Sparrows. Auk 99, 537–547.
Bateson, P. (1979). How do sensitive periods arise and what are they for? Anim. Behav. 27, 470–486. doi: 10.1016/0003-3472(79)90184-2
Becker, P. H. (1982). “The coding of species-specific characteristics in bird sounds,” in Acoustic Communication in Birds, eds Kroodsma E. H. Miller and H. Ouellet (Amsterdam: Elsevier), 213–252. doi: 10.1016/b978-0-08-092416-8.50016-4
Campbell, D. L. M., and Hauber, M. E. (2010). Conspecific-only experience during development reduces the strength of heterospecific song discrimination in Zebra Finches (Taeniopygia guttata): a test of the optimal acceptance threshold hypothesis. Journal of Ornithology vol. 151, 379–389. doi: 10.1007/s10336-009-0466-3
Chaine, A. S., Tjernell, K. A., Shizuka, D., and Lyon, B. E. (2011). Sparrows use multiple status signals in winter social flocks. Anim. Behav. 81, 447–453. doi: 10.1016/j.anbehav.2010.11.016
Clayton, N. S. (1988). Song discrimination learning in zebra finches. Anim. Behav. 36, 1016–1024. doi: 10.1016/s0003-3472(88)80061-7
Colombelli-Négrel, D., Hauber, M. E., and Kleindorfer, S. (2014). Prenatal learning in an Australian songbird: habituation and individual discrimination superb fairy-wren embryos. Proc. Biol. Sci. 281:pii: 20141154.
Colombelli-Négrel, D., and Kleindorfer, S. (2017). Prenatal environment affects embryonic response to song. Biol. Lett. 13:pii: 20170302.
Colombelli-Négrel, D., Mark, E. H., Jeremy, R., Frank, J. S., Herbert, H., Matteo, G., et al. (2012). Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr. Biol. 22, 2155–2160. doi: 10.1016/j.cub.2012.09.025
Davies, N. B., Madden, J. R., and Butchart, S. H. M. (2004). Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. Biol. Sci. 271, 2297–2304. doi: 10.1098/rspb.2004.2835
Delaney, E. K., and Hoekstra, H. E. (2018). Sexual imprinting and speciation between two Peromyscus species. Evolution 72, 274–287. doi: 10.1111/evo.13409
Eales, L. A. (1985). Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim. Behav. 33, 1293–1300. doi: 10.1016/s0003-3472(85)80189-5
Eriksen, A., Lampe, H. M., and Slagsvold, T. (2009). Interspecific cross-fostering affects song acquisition but not mate choice in pied flycatchers, Ficedula hypoleuca. Anim. Behav. 78, 857–863. doi: 10.1016/j.anbehav.2009.07.005
Gardner, T. J., and Magnasco, M. O. (2006). Sparse time-frequency representations. Proc. Natl. Acad. Sci. U.S.A. 103, 6094–6099.
Gottlieb, G. (1988). Development of species identification in ducklings: XV. Individual auditory recognition. Dev. Psychobiol. 21, 509–522. doi: 10.1002/dev.420210602
Grant, B. R., and Grant, P. R. (1996). Cultural inheritance of song and its role in the evolution of Darwin’s finches. Evolution 50:2471. doi: 10.2307/2410714
Grant, P. R., and Grant, B. R. (1997). Hybridization, sexual imprinting, and mate choice. Am. Nat. 149, 1–28. doi: 10.1086/285976
Grier, J. B., Counter, S. A., and Shearer, W. M. (1967). Prenatal auditory imprinting in chickens. Science 155, 1692–1693. doi: 10.1126/science.155.3770.1692
Griffiths, R., Double, M. C., Orr, K., and Dawson, R. J. (1998). A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x
Haff, T. M., and Magrath, R. D. (2012). Learning to listen? Nestling response to heterospecific alarm calls. Anim. Behav. 84, 1401–1410. doi: 10.1016/j.anbehav.2012.09.005
Hebets, E. A. (2003). Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl. Acad. Sci. U.S.A. 100, 13390–13395. doi: 10.1073/pnas.2333262100
Hudson, E. J., Creanza, N., and Shizuka, D. (2019a). The role of early acoustic experience in song discrimination. Biorxiv [Preprint] doi: 10.1101/756445
Hudson, E. J., Hahn, M., and Shizuka, D. (2019b). Nestling and adult sparrows respond differently to conspecific dialects. Behav. Ecol. 30, 48–56. doi: 10.1093/beheco/ary148
Hudson, E. J., and Shizuka, D. (2017). Introductory whistle is sufficient for early song recognition by golden-crowned sparrow nestlings. Anim. Behav. 133, 83–88. doi: 10.1016/j.anbehav.2017.09.018
Immelmann, K. (1972). Sexual and other long-term aspects of imprinting in birds and other species. Adv. Study Behav. 4, 147–174. doi: 10.1016/s0065-3454(08)60009-1
Irwin, D. E., and Price, T. (1999). Sexual imprinting, learning and speciation. Heredity 82(Pt 4), 347–354. doi: 10.1038/sj.hdy.6885270
Katsis, A. C., Sonia, K., Buchanan, K. L., Hauber, M. E., Mylene, M. M., et al. (2018). Prenatal exposure to incubation calls affects song learning in the zebra finch. Sci. Rep. 8:15232.
Kleindorfer, S., Evans, C., Hauber, M. E., and Colombelli-Négrel, D. (2018). Could prenatal sound discrimination predict vocal complexity later in life? BMC Zool. 3, 1–9. doi: 10.1186/s40850-018-0038-1
König, K., Elena, K., Sören, B., Cornelia, G., Ines, B., Christian, K., et al. (2015). Does early learning drive ecological divergence during speciation processes in parasitoid wasps? Proc. Biol. Sci. 282:20141850. doi: 10.1098/rspb.2014.1850
Konishi, M. (1965). The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z. Tierpsychol. 22, 770–783.
Lachlan, R. F., and Servedio, M. R. (2004). Song learning accelerates allopatric speciation. Evolution 58, 2049–2063. doi: 10.1111/j.0014-3820.2004.tb00489.x
Lauay, C., Gerlach, N. M., Adkins-Regan, E., and DeVoogd, T. J. (2004). Female zebra finches require early song exposure to prefer high-quality song as adults. Anim. Behav. 68, 1249–1255. doi: 10.1016/j.anbehav.2003.12.025
Liu, W. C., Rivers, J. W., and White, D. J. (2016). Vocal matching and intensity of begging calls are associated with a forebrain song circuit in a generalist brood parasite. Dev. Neurobiol. 76, 615–625. doi: 10.1002/dneu.22348
Madden, J. R., and Davies, N. B. (2006). A host-race difference in begging calls of nestling cuckoos Cuculus canorus develops through experience and increases host provisioning. Proc. Biol. Sci. 273, 2343–2351. doi: 10.1098/rspb.2006.3585
Magurran, A. E., and Ramnarine, I. W. (2004). Learned mate recognition and reproductive isolation in guppies. Anim. Behav. 67, 1077–1082. doi: 10.1016/j.anbehav.2003.10.010
Magurran, A. E., and Ramnarine, I. W. (2005). Evolution of mate discrimination in a fish. Curr. Biol. 15, R867–R868.
Mariette, M. M., and Buchanan, K. L. (2016). Prenatal acoustic communication programs offspring for high posthatching temperatures in a songbird. Science 353, 812–814. doi: 10.1126/science.aaf7049
Marler, P. (1970). A comparative approach to vocal learning: song development in white-crowned sparrows. J. Comp. Physiol. Psychol. 71, 1–25. doi: 10.1037/h0029144
Marler, P., and Peters, S. (1977). Selective vocal learning in a sparrow. Science 198, 519–521. doi: 10.1126/science.198.4316.519
Marler, P., and Peters, S. (2010). A sensitive period for song acquisition in the song sparrow, melospiza melodia: a case of age-limited learning. Ethology 76, 89–100. doi: 10.1111/j.1439-0310.1987.tb00675.x
Marler, P., and Tamura, M. (1962). Song ‘dialects’ in three populations of white-crowned sparrows. Condor 64, 368–377. doi: 10.2307/1365545
McFarlane, S. E., Söderberg, A., Wheatcroft, D., and Qvarnström, A. (2016). Song discrimination by nestling collared flycatchers during early development. Biol. Lett. 12: pii: 20160234.
Mennill, D. J., Ryan, N., Ian, P. T., Williams, H., Moran, I. G., Amy, N., et al. (2018). Wild birds learn songs from experimental vocal tutors. Curr. Biol. 28, 3273–3278.e4. doi: 10.1016/j.cub.2018.08.011
Miller, A. H. (1940). A hybrid between Zonotrichia coronata and Zonotrichia leucophrys. Condor 42, 45–48. doi: 10.2307/1364317
Morton, M. L., and Mewaldt, L. R. (1960). Further evidence of hybridization between Zonotrichia atricapilla and Zonotrichia leucophrys. Condor 62, 485–486.
Nelson, D. A., and Marler, P. (1993). Innate recognition of song in white-crowned sparrows: a role in selective vocal learning? Anim. Behav. 46, 806–808. doi: 10.1006/anbe.1993.1258
Nelson, D. A., Marler, P., Soha, J. A., and Fullerton, A. L. (1997). The timing of song memorization differs in males and females: a new assay for avian vocal learning. Anim. Behav. 54, 587–597. doi: 10.1006/anbe.1996.0456
Norment, C. J., Hendricks, P., and Santonocito, R. (1998). “Golden-crowned Sparrow (Zonotrichia atricapilla),” in The Birds of North America Online, eds A. F. Poole and F. B. Gill (Ithaca, NY: Cornell Lab of Ornithology), doi: 10.2173/bna.352
Nottebohm, F. (1969). The song of the chingolo, zonotrichia capensis, in argentina: description and evaluation of a system of dialects. Condor 71, 299–315. doi: 10.2307/1366306
Olofsson, H., Frame, A. M., and Servedio, M. R. (2011). Can reinforcement occur with a learned trait? Evolution 65, 1992–2003. doi: 10.1111/j.1558-5646.2011.01286.x
Petrinovich, L. (1985). Factors influencing song development in the white-crowned sparrow (Zonotrichia leucophrys). J. Comp. Psychol. 99, 15–29. doi: 10.1037/0735-7036.99.1.15
Price, T. D., and Bouvier, M. M. (2002). The evolution of F1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089. doi: 10.1111/j.0014-3820.2002.tb00133.x
Qvarnström, A., Haavie, J., Saether, S. A., Eriksson, D., and Pärt, T. (2006). Song similarity predicts hybridization in flycatchers. J. Evol. Biol. 19, 1202–1209. doi: 10.1111/j.1420-9101.2006.01140.x
Rivera, M., Cealie, M., Hauber, M. E., Kleindorfer, S., and Liu, W. C. (2019). Neural activation in response to conspecific songs in zebra finch (Taeniopygia guttata) embryos and nestlings. Neuroreport 30, 217–221. doi: 10.1097/wnr.0000000000001187
Saether, S. A., Thomas, B., Wiley, C., Nina, S., and Anna, Q. (2007). Sex chromosome-linked species recognition and evolution of reproductive isolation in flycatchers. Science 318, 95–97. doi: 10.1126/science.1141506
Sewall, K. B. (2011). Early learning of discrete call variants in red crossbills: implications for reliable signaling. Behav. Ecol. Sociobiol. 65, 157–166.
Shizuka, D. (2014). Early song discrimination by nestling sparrows in the wild. Anim. Behav. 92, 19–24.
Shizuka, D., Ross Lein, M., and Chilton, G. (2016). Range-wide patterns of geographic variation in songs of Golden-crowned Sparrows (Zonotrichia atricapilla). Auk 133, 520–529.
Slagsvold, T., Hansen, B. T., Johannessen, L. E., and Lifjeld, J. T. (2002). Mate choice and imprinting in birds studied by cross-fostering in the wild. Proc. Biol. Sci. 269, 1449–1455.
Slater, P. J. B. (1983b). “Bird song learning: theme and variations,” in Perspectives in ornithology, eds G. H. Brush and G. A. J. Clark (Cambridge: Cambridge University Press), 475–512.
Soha, J. (2017). The auditory template hypothesis: a review and comparative perspective. Anim. Behav. 124, 247–254.
Soha, J. A., and Marler, P. (2000). A species-specific acoustic cue for selective song learning in the white-crowned sparrow. Anim. Behav. 60, 297–306.
Soha, J. A., and Marler, P. (2001). Cues for early discrimination of conspecific song in the white-crowned sparrow (Zonotrichia leucophrys). Ethology 107, 813–826.
Song meter SM4 acoustic recorder (2016). Wildlife Acoustics. https://www.wildlifeacoustics.com/store.
Venables, W. N., and Ripley, B. D. (2002). “Modern Applied statistics with S,” in Statistics and Computing (New York, NY: Springer-Verlag), 496.
Verzijden, M. N., Carelten, C., Maria, R. S., Genevieve, M. K., Jenny, W. B., et al. (2012). The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 27, 511–519.
Verzijden, M. N., and ten Cate, C. (2007). Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 3, 134–136.
Wheatcroft, D., and Qvarnström, A. (2015). A blueprint for vocal learning: auditory predispositions from brains to genomes. Biol. Lett. 11:20150155.
Keywords: Song (or singing), avian, Zonotrichia atricapilla, recognition, learning
Citation: Hudson EJ, Creanza N and Shizuka D (2020) The Role of Nestling Acoustic Experience in Song Discrimination in a Sparrow. Front. Ecol. Evol. 8:99. doi: 10.3389/fevo.2020.00099
Received: 13 September 2019; Accepted: 27 March 2020;
Published: 28 April 2020.
Edited by:
Rita Covas, University of Porto, PortugalReviewed by:
Mylene M. Mariette, Deakin University, AustraliaCopyright © 2020 Hudson, Creanza and Shizuka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emily J. Hudson, ZW1pbHkuai5odWRzb25AdmFuZGVyYmlsdC5lZHU=; ZW1pbHlqYW5laHVkc29uQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.