- 1Department of Animal and Plant Sciences, University of Sheffield, Sheffield, United Kingdom
- 2Department of Zoology, University of Cambridge, Cambridge, United Kingdom
- 3School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
Interacting with relatives provides opportunities for fitness benefits via kin-selected cooperation, but also creates potential costs through kin competition and inbreeding. Therefore, a mechanism for the discrimination of kin from non-kin is likely to be critical for individuals of many social species to maximize their inclusive fitness. Evidence suggests that genetic cues to kinship are rare and that learned or environmental cues offer a more parsimonious explanation for kin recognition in most contexts. This is particularly true among cooperatively breeding birds, where recognition of familiar individuals is usually regarded as the most plausible mechanism for kin discrimination. In this article, we first review the evidence that familiarity provides an effective decision rule for discrimination of kin from non-kin in social birds. We then consider some of the complexities of familiarity as a cue to kinship, especially the problems of how individuals become familiar, and how familiar individuals are recognized. We conclude that while familiarity as a mechanism for kin recognition may be more parsimonious and widespread than genetic mechanisms, its apparent simplicity as a decision rule governing social interactions may be deceptive. Finally, we identify directions for future research on familiarity as a kin recognition mechanism in social birds and other taxa.
Introduction
Kin selection is often invoked to explain the evolution of cooperation among relatives in social animals (Rubenstein and Abbott, 2017). Here, we use ‘social' to describe species that exhibit cooperative breeding, following the widely used definition of cooperative breeding as a reproductive system in which more than a pair of individuals collectively raise young in a single brood or litter (Emlen and Vehrencamp, 1985; Koenig and Dickinson, 2016). Hamilton's rule predicts that cooperation confers indirect fitness benefits and will be selected for providing that the coefficient of relatedness between actor and recipient, multiplied by the benefits of cooperation to the recipient exceed the costs to the actor (Hamilton, 1964). Therefore, differential treatment of conspecifics that vary in genetic relatedness, i.e., kin discrimination (Sherman et al., 1997), is an important consideration in studies of social evolution. In addition to kin-selected fitness benefits, kin discrimination may also play an important role in inbreeding avoidance when passive processes, such as sex-biased dispersal, are insufficient to reduce inbreeding risk (Pusey and Wolf, 1996). These functional benefits of discriminating kin from non-kin are well-established, but the mechanisms through which this is realized are keenly debated.
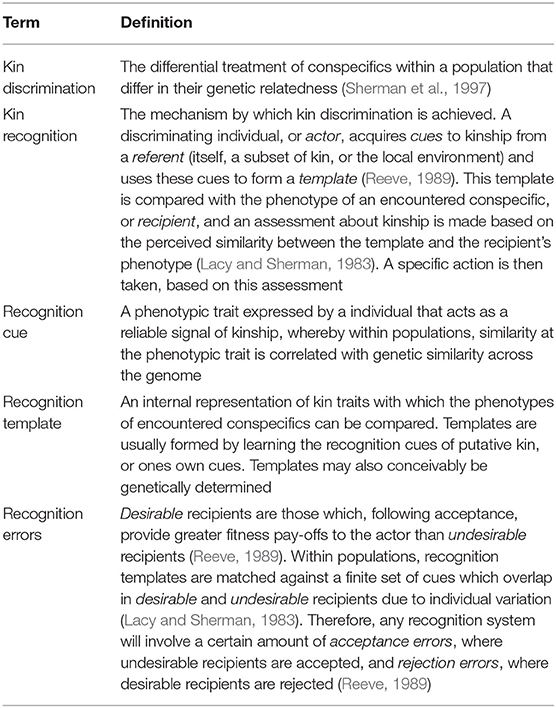
Our current framework for understanding kin recognition systems involves three components: the production of external cues; the perception of these cues and formation of recognition templates; and the action taken based on the perceived similarity between a template and an encountered phenotype (Beecher, 1982; Reeve, 1989; Gamboa et al., 1991; Table 1). Both the cue and the template may be either genetically determined or acquired from the biotic or abiotic environment (Sherman et al., 1997). Recognition systems will also be prone to errors; in the case of positive discrimination in favor of kin for helping behavior, these will be either rejection errors, in which kin are not recognized as such and rejected as social partners, or acceptance errors in which non-kin are erroneously recognized as kin and accepted as social partners (Reeve, 1989; Table 1). The extent to which cues and templates are determined genetically and/or environmentally, and the risk of making rejection/acceptance errors will vary greatly between and within species (Sherman et al., 1997; Komdeur et al., 2008).
This framework leads to three broad categories of kin recognition mechanism. Recognition may be based on familiarity, in which discriminating individuals learn the recognition cues of relatives (e.g., parents and/or siblings) at a sensitive phase during development (Komdeur and Hatchwell, 1999) and discriminate these familiar individuals from unfamiliar ones later in life. Second, recognition may be based on phenotype matching, whereby individuals use their own phenotype and/or those of their familiar kin to form a generalized template with which to compare the phenotypes of other individuals (Lacy and Sherman, 1983). Familiarity and phenotype-matching are considered alternative processes (Holmes and Sherman, 1983), but both involve matching phenotypes to learned templates; the two mechanisms differ only in the specificity of the template employed (Reeve, 1989). Thirdly, it is also possible that both cues and templates are genetically-determined rather than environmentally-acquired or learned, thereby satisfying Grafen's (1990) definition of kin recognition as requiring discrimination of true genetic relatives, although note that here we use the less restrictive definition of Sherman et al. (1997), as stated above.
The ecological and social circumstances in which a recognition system evolves is likely to have a profound effect on the probable mechanism of recognition (Komdeur et al., 2008). Likewise, a species' kin recognition mechanism will have consequences for the accuracy of discrimination and the degree of resolution between different categories of kin. For example, kin recognition that requires prior association for the learning of cues or templates allows individuals to recognize familiar kin only, whereas recognition that is based on phenotype matching may permit recognition of unfamiliar kin (Mateo, 2004). Among cooperatively breeding birds, recognition of familiar individuals is usually regarded as the most plausible mechanism for kin recognition (Komdeur and Hatchwell, 1999). However, the term familiarity is often ill-defined, the recognition cues are poorly understood, and very little is known about the conditions under which a previous association constitutes familiarity in the context of kin recognition. In this article, we first review the evidence for alternative kin recognition mechanisms in social birds, concluding that recognition based on familiarity is the best-supported decision rule for discrimination of kin from non-kin in most studies. We then consider some of the complexities of familiarity as a cue to kinship, suggesting that while such a mechanism for kin recognition may appear more parsimonious and widespread than phenotype matching, its apparent simplicity is deceptive. Finally, we discuss possible directions for future research on familiarity as a kin recognition mechanism in social birds and other taxa.
Mechanisms of Kin Recognition
Kin recognition may be achieved via a variety of mechanisms that range from simple to complex. In the simplest form of recognition, individuals encountered in a particular area are recognized as kin. As long as relatives are predictably distributed in space, location can correlate reliably with genetic relatedness (Komdeur and Hatchwell, 1999). Some researchers suggest this is not a true form of kin recognition, as individuals are responding to location, rather than phenotypic cues (Halpin, 1991; Tang-Martinez, 2001). However, in many natural populations, it is rare for unrelated individuals to be encountered in the nest for example, and a simple decision rule such as “treat anything in my nest as kin,” is an effective and widely used mechanism for offspring recognition in birds (Beecher, 1991), despite its potential for exploitation by intra- and inter-specific brood parasites (Davies, 2000). Other contextual cues may modify this simple rule; for example, polyandrous male dunnocks Prunella modularis are more likely to feed the young of females with which they mated during their fertile period (Burke et al., 1989; Davies et al., 1992), thereby maximizing their chance of directing their care toward offspring. Spatial cues to offspring recognition may be superseded by individual recognition when fledglings leave the nest (Beecher, 1988), but, in most cases, parent-offspring recognition does not persist beyond the period of offspring dependence.
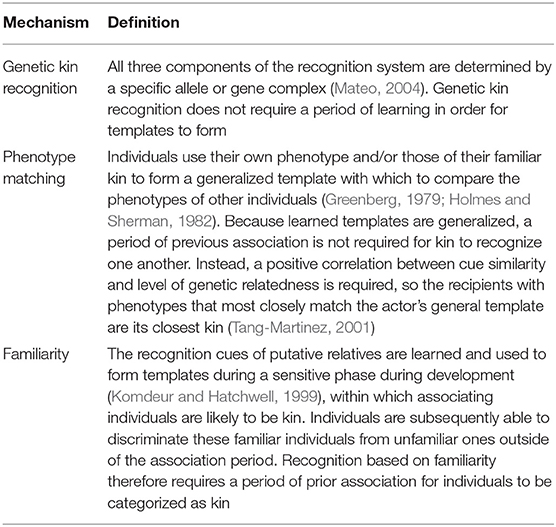
Such simple rules work well in non-social species, in which there is little or weak selective pressure to recognize kin beyond offspring independence. However, in social species there are often indirect fitness benefits to be gained from cooperating with close kin during adulthood or fitness costs of inbreeding, and, consequently, selection for mechanisms of kin recognition that persist beyond the period of parental care (Komdeur and Hatchwell, 1999; Cornwallis et al., 2009). In this review, we focus on mechanisms in social birds that might permit kin recognition over an individual's lifetime, or at least the period over which cooperative behavior or the risk of inbreeding exists. Such mechanisms may be based on genetic kin recognition, phenotype matching or familiarity (Table 2).
Genetic Recognition
Genetic kin recognition requires discrimination of kin from non-kin based entirely on genetically acquired cues without a period of associative learning. Here, recognition alleles, dubbed “greenbeard genes” by Dawkins (1976) or gene complexes encode the production of phenotypic cues, the templates and the perception of the cue and performance of a discriminatory action. Such a system relies on polymorphic recognition genes for reliable discrimination, yet paradoxically, kin-selected fitness benefits are predicted to reduce allelic diversity at these loci. This is because in cooperative contexts, individuals bearing common cues are more likely to encounter equivalent individuals and receive altruistic benefits than those with rare cues. These individuals will gain higher fitness, and eventually the common alleles become fixed and the recognition system breaks down (Crozier, 1986). Alternatively, mutation will interfere with genetic kin recognition, and mutant cheats who carry the phenotypic cues but not the associated relatedness, may evolve and spread through the population (Hamilton, 1964). Finally, in the case of a gene complex orchestrating recognition, recombination could disrupt kin recognition. In each of these theoretical scenarios, the required correlation between similarity in the inherited phenotypic cue and kinship among pairs of individuals would decrease over time, rendering such a cue useless for kin recognition (Gardner and West, 2007). There are no convincing cases of genetic kin recognition in cooperatively breeding birds (Table 3). Indeed, empirical evidence of genetic kin recognition across taxa is scarce, the clear exceptions being the slime mold, Dictyostelium discoideum (Queller et al., 2003) and fire ant Solenopsis invicta (Keller and Ross, 1998; Wang et al., 2013).
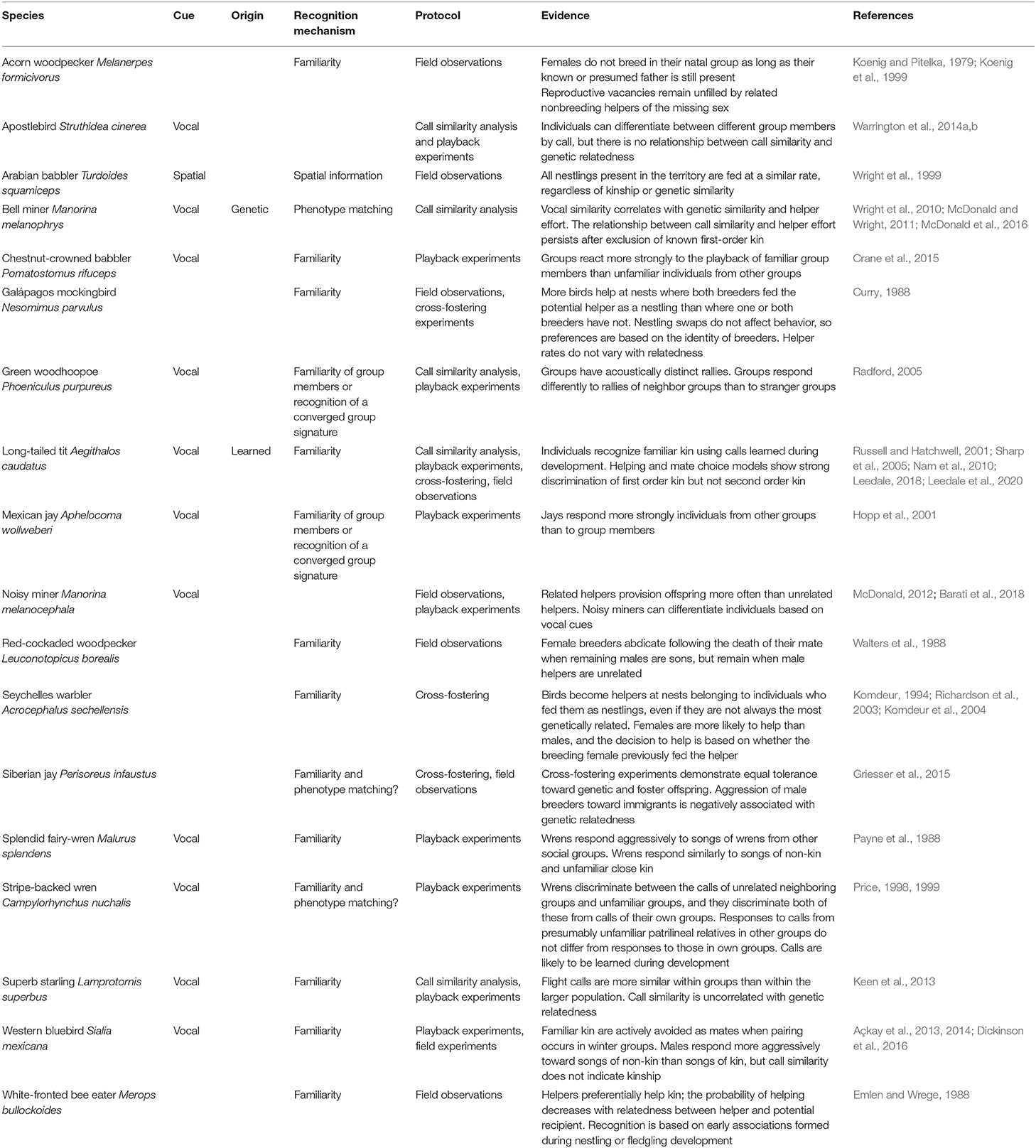
Table 3. Summary of empirical field studies of cooperatively breeding birds in which kin or group discrimination has been identified.
Phenotype Matching
The second candidate mechanism for kin recognition is phenotype matching. The distinction between phenotype matching and genetic kin recognition is that template formation requires the learning of phenotypic cues that reliably reflect genetic similarity. However, because individuals can use their own phenotype or the phenotypes of a subset of known kin to learn a generalized “kin” template, this does not require a period of prior association, or familiarity between matching individuals. Phenotype matching is an attractive potential mechanism for kin recognition, particularly in the context of inbreeding avoidance, because it allows individuals to recognize unfamiliar kin. Phenotype matching has been demonstrated in the decorated cricket Gryllodes sigillatus (Capodeanu-Nägler et al., 2014) and in several social mammals (e.g., Boyse et al., 1991; Pfefferle et al., 2013). Although in some species, such as the Belding's ground squirrel Spermophilus beldingi both phenotype-matching and familiarity seem to play a role (Holmes and Sherman, 1982). However, empirical support for phenotype matching in cooperatively breeding birds remains rare and inconclusive (Table 3).
One of the first studies to suggest phenotype matching as a plausible kin recognition mechanism in a cooperative bird was conducted by Price (1998, 1999) on stripe-backed wrens Campylorhynchus nuchalis. A series of playback experiments demonstrated that wrens were able to discriminate between vocalizations made by their own group, familiar neighboring groups and unfamiliar groups, consistent with a recognition system based on familiarity (Price, 1998). Subsequent experiments showed that the behavioral responses of wrens to calls from patrilineal relatives in the unfamiliar groups did not differ from their responses to calls from patrilineal relatives in their own group, which could indicate phenotype matching (Price, 1999). However, patrilineal relatives in unfamiliar groups are dominant males that have dispersed from their natal group, so a period of association between the dominant male in each group cannot be ruled out. As male helpers may follow the dominant male in their behavioral responses to intruders, this result could be achieved through recognition based on familiarity.
A recent study on Siberian jays Perisoreus infaustus, a species that exhibits kin-based sociality although not cooperative breeding, has suggested that phenotype matching is used to recognize kin in some contexts. Within family groups, breeders are more aggressive toward immigrants than to their own offspring, but aggression of breeders toward immigrants was negatively associated with the immigrant's genetic relatedness to the breeding male (Griesser et al., 2015). In this study, individuals were considered unfamiliar if they had not interacted between fledging and dispersal, although the possibility that individuals had prior association could not be ruled out unequivocally.
Studies of bell miners Manorina melanophrys provide the best evidence for kin recognition via phenotype matching in cooperatively breeding birds (McDonald and Wright, 2011). Certain features of the bell miner's social system have important consequences for their recognition systems. They form large colonies, often comprising hundreds of individuals, within which individuals are organized into coteries of numerous breeding pairs assisted by non-breeding helpers of varying relatedness that provision multiple nests within their coterie. Like many cooperative breeders, kinship appears to be the most important factor in explaining the patterns of cooperation between breeders and helpers (Wright et al., 2010) and the shared provisioning efforts of helpers within social networks (McDonald et al., 2016). From an early age, however, young interact with both related and unrelated group members, making spatial or association-based recognition unreliable. Instead, the provisioning effort of helpers correlates with their vocal similarity to the breeding male, an apparently innate signal that also correlates with genetic relatedness (McDonald and Wright, 2011). However, whether vocal similarity permits kin recognition on a continuous scale or on a binary scale, whereby conspecifics are categorized as either kin or non-kin based on a threshold of template-phenotype similarity, remains unclear. Furthermore, although no evidence of call learning has been found, a putative association period during which kin may be learned has not been excluded empirically.
In the closely related noisy miner Manorina melanocephala, which has a similar social system, helpers direct their help toward genetic relatives (Barati et al., 2018), and discriminate between individuals based on acoustic cues (McDonald, 2012). Still, individuals may also rely on prior association to identify relatives, and whether kin recognition is based on phenotype matching or familiarity remains untested in this species.
The problem with recognition via phenotype matching of inherited cues is that, like genetic kin recognition, it is vulnerable to mutation and recombination, and requires sufficient polymorphism to permit precise discrimination. Another important consideration is that there may be selection for individuals to conceal kinship at certain life stages or in certain situations. For example, when paternity is uncertain, effective kin recognition by parents would be adaptive in order to direct care toward genetic offspring. However, from the offsprings' perspective, it would not be beneficial to display an obvious cue to genetic relatedness, as this could exclude cuckolded care-givers (Beecher, 1988; Davies et al., 1992). This conflict of interest between parent and offspring may make it difficult for phenotype matching of genetic cues to evolve as a recognition mechanism. Even if recognition cues are learned, the formation of a generalized template may still select for convergence, as individuals with a more common phenotype are more likely to be accepted as social partners than those with rarer cues. On the other hand, theory suggests that genetic diversity at recognition loci may be maintained if rare alleles confer an extrinsic selective advantage, such as resistance to certain parasites (Rousset and Roze, 2007). Indeed, the highly polymorphic major histocompatibility complex (MHC), has been implicated as a kinship marker during mate choice in vertebrates, detected through odor cues. MHC diversity affects parasite resistance (Kurtz et al., 2004), perhaps explaining how MHC polymorphism is maintained despite its putative role in kin recognition. However, the role of MHC in kin recognition is contested, as disassortative mate preference based on MHC haplotype may arise from the improved immunity associated with heterozygosity at MHC loci itself, rather than MHC haplotype acting as a reliable signal of genetic similarity across the genome (Green et al., 2015).
Familiarity
Familiarity is the most widely supported mechanism of kin recognition in cooperatively breeding birds (Komdeur and Hatchwell, 1999; Komdeur et al., 2008; Riehl and Stern, 2015; Table 3). Kin association during extended brood care provides a sensitive period during which reliable recognition templates can form. This period of association also offers an opportunity for learning of cues that are more similar within a family than in the general population, termed a family or kin “signature” (Beecher, 1982). Once recognition cues are fixed, individuals are potentially able to recognize familiar kin outside of the association context. When extra-pair paternity (EPP) and brood parasitism is rare, association during this period accurately reflects kinship, and a simple rule such as “assist anyone who was present in my natal nest” can be selected for (Komdeur and Hatchwell, 1999). For example, in cooperative contexts, Galápagos mockingbirds Nesomimus parvulus and white-fronted bee-eaters Merops bullockoides discriminate based on previous association, rather than kinship (Curry, 1988; Emlen and Wrege, 1988). In complex societies, a more precise rule, such as “assist anyone that fed me as a nestling” may be more reliable (Komdeur, 1994). In most cooperatively breeding birds, males are the predominant helping sex, but in the Seychelles warbler Acrocephalus sechellensis females are more likely to help than males, and choose to help at nests belonging to female breeders who fed them as nestlings, even if they are not the closest genetic relatives (Komdeur, 1994; Richardson et al., 2003). This makes evolutionary sense in species with high levels of extra-pair paternity, such as Seychelles warblers, because helpers are often unrelated to the male that fed them (Richardson et al., 2003). Cross-fostering experiments confirm that female subordinates base their helping decisions on associative learning and it is unlikely that young can discriminate between their mother and any other female helper (Komdeur et al., 2004).
Playback experiments show that cues enabling recognition of familiar individuals beyond the association period are encoded vocally (Table 3). An early study on the splendid fairy-wren Malurus splendens showed that while fairy-wrens responded aggressively to the songs of fairy-wrens from other social groups, they exhibited a similar response to the songs of both non-kin and unfamiliar close kin (Payne et al., 1988). More recent experiments have demonstrated that vocalizations signal group membership in Mexican jays Aphelocoma wollweberi (Hopp et al., 2001), green woodhoopoes Phoeniculus purpureus (Radford, 2005) and superb starlings Lamprotornis superbus (Keen et al., 2013). These studies suggest that vocalizations reflect social association rather than kinship per se, as would be expected if cues and templates are learned within groups.
In the context of inbreeding avoidance, good evidence for avoidance of kin as reproductive partners based on familiarity comes from studies of two species of social woodpecker: acorn woodpeckers Melanerpes formicivorus and red-cockaded woodpeckers Picoides borealis. Acorn woodpeckers exhibit high within-group relatedness, with most individuals being parents, siblings or offspring of everyone else within the group (Koenig and Haydock, 2004). Acorn woodpecker females do not breed in their natal group when the reproductive male in their natal group at the time of their birth (their assumed father) is still present (Koenig and Pitelka, 1979). Furthermore, when a dominant male or female dies, reproductive vacancies remain unfilled when non-breeding helpers of the missing sex are present, and breeding does not usually occur until the vacancy is filled by immigrants from outside the group (Koenig et al., 1999). Similarly, red-cockaded woodpecker females will abdicate a breeding position following the death of their mate when the remaining males are their sons, but will remain when they are unrelated to the male helpers (Walters et al., 1988). The mechanism behind these decisions has not been examined experimentally in either species.
The most compelling cases of kin recognition based on familiarity come from cooperative breeders in which helping occurs within kin neighborhoods (Dickinson and Hatchwell, 2004), where individuals routinely interact socially with both kin and non-kin so that selection for effective kin discrimination is likely to be strong (Cornwallis et al., 2009). In western bluebirds Sialia mexicana there is a strong kin preference in helping behavior (Dickinson et al., 1996) and active kin avoidance during mate choice (Dickinson et al., 2016). However, males do not reduce their provisioning effort in response to behavioral cues to paternity loss, such as extra-pair male intrusion or witnessing female acceptance of extra-pair copulations (Dickinson, 2003). This suggests, along with earlier studies (Leonard et al., 1995), that males do not recognize their own offspring, and that discrimination by both parents and offspring is based on social experience in the nest, rather than genetic relatedness (Dickinson, 2003). Playback experiments have shown that individuals discriminate kin based on vocal cues (Açkay et al., 2013) even though these vocalizations are poor indicators of genetic relatedness, because they are most similar among neighbors, regardless of kinship (Açkay et al., 2014). These findings collectively suggest that western bluebirds recognize familiar individuals, rather than kin, using vocal cues.
Kin recognition has also been extensively studied in another species that helps within kin neighborhoods, the long-tailed tit Aegithalos caudatus. Long-tailed tits have a kin-selected cooperative breeding system in which failed breeders preferentially redirect their care to help relatives (Russell and Hatchwell, 2001; Hatchwell et al., 2014). Playback experiments show that long-tailed tits are able to discriminate between the calls of close kin and non-kin (Hatchwell et al., 2001; Sharp et al., 2005), and the calls thought to be used as recognition cues are individually distinctive, repeatable and more similar among close kin than among non-kin (Sharp and Hatchwell, 2005; Leedale et al., 2020). Cross-fostering experiments showed that nestlings and/or fledglings acquire their recognition templates from familiar kin during an associative learning period, when the cues themselves develop (Sharp et al., 2005), and that cross-fostered offspring subsequently help at the nest of foster siblings (Hatchwell et al., 2001). Moreover, there is strong evidence for effective discrimination of first-order kin, but not second-order kin, both in the context of helping behavior and mate choice (Leedale, 2018; Leedale et al., 2020). These results are all consistent with the idea that long-tailed tits categorize conspecifics as either kin or non-kin based on early association in the context of brood care (Sharp et al., 2005). On the other hand, Nam et al. (2010) and Leedale et al. (2020) both found that long-tailed tit helpers modified their effort according to their relatedness to the helped brood, suggesting that assessment of kinship is not based on a simple dichotomous rule of familiar (kin) vs. unfamiliar (non-kin) birds. Indeed, this suggests a mechanism of phenotype matching, with a gradation of similarity in vocalizations providing a fine-grained, continuous estimation of kinship. However, bioacoustic analysis did not support this suggestion (Leedale et al., 2020), so even in this relatively well-studied system, the mechanism underlying graded discrimination remains unknown.
This review focuses on kin recognition, but familiarity also provides a potential mechanism by which individual recognition may be achieved; for example, some cooperative bird species, such as the chestnut-crowned babbler Pomatostomus ruficeps have individually distinct vocalizations (Crane et al., 2015). However, although individual recognition has been identified in several social mammals, including chacma baboons Papio hamadryas (Bergman, 2003) and golden hamsters Mesocricetus auratus (Johnston and Bullock, 2001), there are no conclusive examples of individual recognition in cooperatively breeding birds (Table 3). The difference between individual and group recognition depends on the specificity of the templates acquired during the association period, which in turn depends on the nature of the interactions that occur between individuals during that time. In practice, this makes distinguishing individual from kin or group recognition difficult (Tibbetts and Dale, 2007). We discuss this in more detail in the following section.
Overall, there is substantial evidence that familiarity is a widespread kin recognition mechanism in cooperatively breeding birds. The limitation of familiarity is that non-kin will be considered kin if they are encountered during the putative associative learning stage, and kin not encountered during this period will not be recognized as such. However, in most cases, proximity at certain life stages is a reliable indicator of kinship. This is particularly true of birds, which have a prolonged period of parental care at the nest where encountered individuals are likely to be close kin. A second assumed limitation of recognition based on familiarity is that it may result in a binary recognition rule, in which individuals are categorized as either kin or non-kin. A more sophisticated mechanism that permits relatedness to be assessed on a continuous scale would be adaptive, in accordance with Hamilton's rule (Hamilton, 1964), although, as already discussed, such mechanisms may be evolutionarily unstable. Kin recognition through familiarity or prior association is also considered the most likely mechanism of kin recognition in social birds because it is simpler to evolve and arguably less cognitively demanding than an assessment of genetic relatedness based on phenotypic similarity. Yet, while a recognition system based on familiarity may be more parsimonious and widespread than phenotype matching and genetic mechanisms, we argue below that its apparent simplicity is deceptive.
The Complexities of Familiarity as a Cue to Kinship
Despite the general acceptance of familiarity as an important means of kin recognition and discrimination, much remains unknown about how associating individuals are categorized as kin and how familiar individuals are recognized after the associative learning period. Here, we suggest that progress will be made in understanding familiarity as a mechanism of kin recognition only when certain gaps in knowledge can be addressed: (i) the meaning of “familiarity,” (ii) the sensitive period for association; (iii) the cues used for recognition; and (iv) the distinction between familiarity and phenotype matching.
What Is “Familiarity”?
Familiarity in the context of kin recognition is difficult to define and to quantify. What is the specific series of events during which an individual learns who is familiar? In the kin recognition literature, familiarity generally refers to some previous social association among individuals, usually during early life stages (Hepper, 1986; Komdeur and Hatchwell, 1999), but the nature of this association is often vague. For instance, is spatial proximity sufficient, or do individuals need to interact in specific ways in order to become familiar? In studies of social birds, such as long-tailed tits, spatial proximity of nestlings may provide the basis for future helping among siblings, but helping also occurs across generations indicating that association when provisioning a brood or when being provisioned also provides the basis for future helping (Sharp et al., 2005; Nam et al., 2010). Precisely when the interactions took place, how many interactions there were, their duration, and the specific behavior and information transfer that took place during these interactions may influence how individuals are recognized and treated later in life. A critical issue here is that individuals often become familiar with and recognize many conspecifics through their lifetime, including mates (Blumenrath et al., 2007), territorial neighbors (Stoddard, 1996) or flock mates (Nowicki, 1983), so is it the timing, frequency or nature of the social interaction that results in some individuals being treated as kin and others not? A particularly nice example of such context-specificity in kin recognition is suggested by Komdeur et al.'s (2004) finding that Seychelles warbler helpers assist in the rearing of half-siblings that are the offspring of their mother but not those of their father, even though both parents would have provisioned the helper when it was young.
It may also be possible for individuals to acquire cues to kinship based on observations of the behavior of their familiar relatives toward other individuals. For example, unfamiliar individuals observed engaging in positive interactions with one's parents could be treated as kin. Indeed, such “indirect familiarity” could provide a kin recognition mechanism through which individuals recognize their younger siblings, despite not being reared together. Although we are not aware of any evidence for indirect familiarity among cooperative breeders, this idea parallels the social interaction expected under indirect reciprocity, in which help is directed toward an individual who has been observed providing help to others (Nowak and Sigmund, 2005). However, indirect cues to kinship are likely to be more error-prone than those learned through direct association because the link between kinship and familiarity will tend to be diluted. For example, in the case of direct association among parents, offspring and siblings during rearing, kinship of familiar individuals will usually be consistently high. But, if an offspring observes their parent interacting positively with an uncle, say, its relatedness to the “indirectly familiar” individual is lower than that between directly familiar individuals. If the offspring subsequently helps its uncle, and this is observed by their offspring, the relatedness between such “indirectly familiar” individuals is further reduced. As with direct familiarity, the frequency and nature of the interactions observed must also be considered, which, overall, may make the behavior of others a noisy and unstable cue to kinship.
Social network analysis is being used increasingly to quantify the strength of association between individuals and can be applied at different life history stages (Kurvers et al., 2013; McDonald et al., 2016). A social network inevitably reflects the nature of the behavior used to construct it (Madden et al., 2012), and they do not necessarily reflect genetic relatedness alone (Godfrey et al., 2014). For example, Napper and Hatchwell (2016) found that helping decisions in long-tailed tits reflected not only kinship, but also individuals' spatial distribution and their social associations during the previous winter. More work is needed to evaluate how prior association affects kin-directed behaviors using precisely quantified social networks in different contexts and life history stages.
When Is the Sensitive Period?
There is good evidence that kin recognition requires a period of learning, but when is this critical period? Many vocal learners have a sensory learning phase or window when they learn songs that they sing during adulthood (Kroodsma, 1978). Once this window closes, most songbirds are unable to learn new songs, although their repertoire may later be modified in some species (Mooney et al., 2008). Studies of songbirds show that that the window can be very short with a long delay between the sensory learning phase and the sensorimotor phase, during which the song is rehearsed and perfected, e.g., swamp sparrows Melospiza georgiana (Marler and Peters, 1982). Likewise, offspring that imprint on parents have a sensitive imprinting period (Bateson, 1964), and it has been suggested that learning of parental calls may even precede hatching, resulting in a parent-specific password, in superb fairy-wrens Malurus cyaneus (Colombelli-Négrel et al., 2012). This is interpreted as defense against inter-specific brood parasitism, but selection for early parent-offspring recognition would also be expected whenever there is a substantial risk of mis-directed parental care. For example, parents in colonial bank swallows Riparia riparia accept any offspring in their nest before 15 days, then recognize their own offspring at 15–17 days, i.e., just before fledging (Beecher, 1982, 1988, 1991).
Based on these parallels between bird song learning and associative learning of kin, we postulate that the critical period for learning the template for recognition of kin through familiarity is similar to the sensory learning phase in many vocal learners. Thus, individuals could discriminate kin from non-kin even though they also associate with non-kin before they start vocalizing (Radford, 2005) or cooperating, and any associations that occur after the sensory learning phase (but before the sensorimotor phase) might result in non-kin being disregarded as social partners (i.e., associated but not “familiar”). Cross-fostering experiments provide strong empirical support for this putative learning period (Hatchwell et al., 2001; Komdeur et al., 2004; Sharp et al., 2005). However, although the time of call development is known in some species (e.g., Sharp et al., 2005), the precise timing of kin recognition template formation has not been identified in any cooperatively breeding species. Furthermore, while this mechanism may be effective as a rule for reliably directing care toward kin when mature offspring help their parents or siblings to raise subsequent broods, as is typical of many cooperatively breeding birds (Cockburn et al., 2017), there are species in which helpers care for the offspring of a younger generation of breeders (e.g., Richardson et al., 2007; Nam et al., 2010), suggesting that older birds can learn the identity of younger relatives, a process that must occur outside the putative critical learning period. A similar conclusion must be drawn when parents avoid breeding with younger relatives, as in acorn woodpeckers (Koenig et al., 1999).
Therefore, while the parallels with song-learning are intuitive and appealing, there are clearly situations in which a single sensitive period for learning kin identity do not apply. Cross-fostering experiments targeted at different life history stages and social network analysis across lifetimes provide invaluable tools with which to address this problem, but there remain formidable challenges to achieving a better understanding of the putative learning phase in natural populations.
What Are the Recognition Cues?
Another challenge when determining the role of familiarity is determining the cues used in kin recognition. Vocal cues are the most likely mechanism in birds (Table 3), but this has been the default sensory modality in all of the cited studies, so visual and olfactory cues cannot be ruled out. Kin recognition mechanisms in several non-cooperatively breeding birds, particularly in the context of inbreeding avoidance, have focused on odor cues (Coffin et al., 2011; Krause et al., 2012). Storm petrels Hydrobates pelagicus prefer non-kin odors when choosing mates (Bonadonna and Sanz-Aguilar, 2012) and odor has also been suggested as a recognition cue in zebra finches Taenopygia guttata (Caspers et al., 2013, but see Ihle and Forstmeier, 2013). These studies should encourage future work on olfactory kin recognition in cooperatively breeding birds for two reasons. First, most recent evidence of odor-based kin recognition comes from species with enclosed nests, which may retain odor more readily than open nests, thereby promoting the learning and familiarization of nest odors. Many cooperative breeders nest in domed nests or cavities (Price and Griffith, 2017), suggesting that olfactory cues to kinship are plausible. Second, most species in which odor-based kin recognition has been identified live in flocks or breed in colonies, even though they do not breed cooperatively, suggesting that there might be common selection pressures for odor-based kin recognition to evolve. Interestingly, the finding that preen gland secretion chemicals are positively correlated with MHC relatedness in black-legged kittiwakes Rissa tridactyla (Leclaire et al., 2014) suggests that phenotype-matching of odor cues is a feasible recognition mechanism, just as in mammals (Green et al., 2015). However, it should also be noted that even less is known about the timing of development, individuality and repeatability of odor profiles than is known about vocal cues.
For any kin recognition cue, whether vocal or odor, to be effective it must carry either an individual or family signature and be individually repeatable from its initial development to the time of discrimination; the same logic applies to a recognition template. Signal convergence therefore presents a significant problem for the stability of any recognition system. Frequent interactions may lead to an increase in phenotypic similarity among individuals. Vocal convergence can be adaptive for coordinated foraging (Bradbury and Balsby, 2016), particularly when birds forage in annual winter flocks that disband each spring. For example, black-capped chickadees Parus atricapillus, exhibit vocal plasticity throughout adulthood and vocal convergence can occur within a week of winter flock formation (Nowicki, 1989). However, such species do not breed cooperatively and individuals do not gain indirect fitness benefits from associating with kin. In kin-selected systems, kin recognition cues must be fixed during early development and cannot be updated during adulthood, even when interactions with non-kin are frequent (Radford, 2005; Keen et al., 2013). In long-tailed tits, vocalizations do not change significantly over an individual's lifetime once learned (Sharp and Hatchwell, 2005), but more studies that investigate the plasticity of putative recognition cues are needed. In addition, while the idea of a signature system, a specific profile of phenotypic components that vary in their combination from individual to individual, is well-established (Beecher, 1982), most studies continue to focus on a single recognition modality, rather than recognizing that familiarity is likely to be based on a combination of cues, which may minimize convergence and maintain recognition cue diversity and integrity.
Familiarity vs. Phenotype Matching
Although in principle the mechanisms of familiarity and phenotype matching are readily distinguished, in practice this may often not be the case. The two mechanisms differ in the predictions they make about whether the ability to discriminate requires prior association and about the resolution of discrimination. First, familiarity is explicitly dependent on social partners having prior knowledge of each other, whereas phenotype-matching allows recognition of unfamiliar kin. In practice, it is extremely difficult to rule out prior association in most field studies, even in cross-fostering experiments where there is often a period of association between parents and offspring prior to separation (e.g., Hatchwell et al., 2001). Kin recognition cues may even develop during gestation (e.g., Hepper, 1987) or incubation (e.g., Colombelli-Négrel et al., 2012; Dowling et al., 2016). Secondly, familiarity is generally assumed to result in dichotomous classification of conspecifics into familiar (kin) and unfamiliar (non-kin) individuals, while cue-template similarity under phenotype-matching is assumed to be continuous. However, if the recognition system involves a threshold for acceptance/rejection of social partners (Reeve, 1989), then discrimination based on phenotype-matching and familiarity may appear very similar in practice. Equally, it is possible that familiarity could be assessed as a continuous trait, with conspecifics discriminated according to their degree of familiarity.
Thus, the extent to which recognition cues permit kinship to be perceived on a continuous or binary scale is an important aspect of the kin recognition mechanism. When group membership is used to categorize relatives, as in Arabian babblers Turdoides squamiceps (Wright et al., 1999), kin discrimination is binary. When recognition is based on phenotype, e.g., white-fronted bee eaters Merops bullockoides (Emlen and Wrege, 1988), it may be binary or continuous, depending on the algorithm used to assess kinship. Binary or threshold kin discrimination will be effective in most cooperative breeders living on stable territories that, at least with regard to the helping sex, are mostly made up of first-order relatives, facilitating a decision rule based on prior association (Curry, 1988; Payne et al., 1988; Komdeur et al., 2004). In contrast, a recognition cue that permits individuals to discriminate kin varying in relatedness has been identified only in the bell miner (Wright et al., 2010), even though such fine-scale discrimination has been reported in at least one other species (Nam et al., 2010; Leedale et al., 2020).
The ability of helpers to assess the relatedness of conspecifics continuously may have been overlooked in some cases because of the way in which cooperative behavior is measured. For example, some studies focus on the probability of helping (Curry, 1988; Creel et al., 1991; Dickinson et al., 1996), whereas others measure the amount of help given (Dunn et al., 1995; Wright et al., 1999; Clutton-Brock et al., 2001), and both have been measured in just a few (Emlen and Wrege, 1988; Komdeur, 1994; Russell and Hatchwell, 2001; Nam et al., 2010). Moreover, consideration must also be given to how relatedness is assessed by helpers, especially the possibility of error and degree of resolution achievable (Leedale et al., 2020). These problems pose formidable challenges to empiricists, with more sophisticated observations and experiments required to determine how relatedness is perceived.
Conclusions
Familiarity is an intuitively plausible mechanism of kin recognition in social birds that, at first sight, appears more parsimonious than alternatives. However, we think that this apparent parsimony is deceptive, so that although most empirical studies support familiarity as the most likely mechanism, we argue that there is a great deal we do not understand about this process. In particular, we have identified four specific issues that would benefit from further investigation, although in making these recommendations, we acknowledge the difficulty of addressing them in natural populations.
Author Contributions
AL collated research and drafted the manuscript with BH. AL, BH, and JL contributed to the final version of the manuscript.
Funding
This work was funded by the Natural Environment Research Council (NERC, UK, 1517208) and Chinese Scholarship Council grant code (201706515025).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Açkay, Ç., Hambury, K. L., Arnold, J. A., Nevins, A. M., and Dickinson, J. L. (2014). Song sharing with neighbours and relatives in a cooperatively breeding songbird. Anim. Behav. 92, 55–62. doi: 10.1016/j.anbehav.2014.03.029
Açkay, Ç., Swift, R. J., Reed, V. A., and Dickinson, J. L. (2013). Vocal kin recognition in kin neighborhoods of western bluebirds. Behav. Ecol. 24, 898–905. doi: 10.1093/beheco/art018
Barati, A., Andrew, R. L., Gorrell, J. C., Etezadifar, F., and McDonald, P. G. (2018). Genetic relatedness and sex predict helper provisioning effort in the cooperatively breeding noisy miner. Behav. Ecol. 29, 1380–1389. doi: 10.1093/beheco/ary109
Bateson, P. P. G. (1964). Changes in chicks' responses to novel moving objects over the sensitive period for imprinting. Anim. Behav. 12, 479–489. doi: 10.1016/0003-3472(64)90068-5
Beecher, M. D. (1982). Signature systems and kin recognition. Am. Zool. 22, 477–490. doi: 10.1093/icb/22.3.477
Beecher, M. D. (1991). “Successes and failures of parent-offspring recognition,” in Kin Recognition, ed P. G. Hepper (Cambridge: Cambridge University Press), 94–124. doi: 10.1017/CBO9780511525414.006
Bergman, T. J. (2003). Hierarchical classification by rank and kinship in baboons. Science 302, 1234–1236. doi: 10.1126/science.1087513
Blumenrath, S. H., Dabelsteen, T., and Pedersen, S. B. (2007). Vocal neighbour–mate discrimination in female great tits despite high song similarity. Anim. Behav. 73, 789–796. doi: 10.1016/j.anbehav.2006.07.011
Bonadonna, F., and Sanz-Aguilar, A. (2012). Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition. Anim. Behav. 84, 509–513. doi: 10.1016/j.anbehav.2012.06.014
Boyse, E. A., Beauchamp, G. K., Yamazaki, K., and Bard, J. (1991). “Genetic components of kin recognition in mammals,” in Kin Recognition, ed P. G. Hepper (Cambridge: Cambridge University Press), 148–161. doi: 10.1017/CBO9780511525414.008
Bradbury, J. W., and Balsby, T. J. S. (2016). The functions of vocal learning in parrots. Behav. Ecol. Sociobiol. 70, 293–312. doi: 10.1007/s00265-016-2068-4
Burke, T., Davies, N. B., Bruford, M. W., and Hatchwell, B. J. (1989). Parental care and mating behavior of polyandrous dunnocks Prunella modularis related to paternity by DNA fingerprinting. Nature 338, 249–251. doi: 10.1038/338249a0
Capodeanu-Nägler, A., Rapkin, J., Sakaluk, S. K., Hunt, J., and Steiger, S. (2014). Self-recognition in crickets via on-line processing. Curr Biol, 24, R1117–18. doi: 10.1016/j.cub.2014.10.050
Caspers, B. A., Hoffman, J. I., Kohlmeier, P., Krüger, O., and Krause, E. T. (2013). Olfactory imprinting as a mechanism for nest odour recognition in zebra finches. Anim. Behav. 86, 85–90. doi: 10.1016/j.anbehav.2013.04.015
Clutton-Brock, T. H., Brotherton, P. N. M., Russell, A. F., O'Riain, M. J., Gaynor, D., Kansky, R., et al. (2001). Cooperation, control, and concession in meerkat groups. Science 291, 478–481. doi: 10.1126/science.291.5503.478
Cockburn, A., Hatchwell, B. J., and Koenig, W. D. (2017). “Sociality in birds,” in Comparative Social Evolution, eds D. R. Rubenstein and P. Abbott (Cambridge: Cambridge University Press), 320–353. doi: 10.1017/9781107338319.012
Coffin, H. R., Watters, J. V., and Mateo, J. M. (2011). Odour-based recognition of familiar and related conspecifics: a first test conducted on captive humboldt penguins (Spheniscus humboldti). PLoS ONE. 6:e25002. doi: 10.1371/journal.pone.0025002
Colombelli-Négrel, D., Hauber, M. E., Robertson, J., Sulloway, F. J., Hoi, H., Griggio, M., et al. (2012). Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr. Biol. 22, 2155–2160. doi: 10.1016/j.cub.2012.09.025
Cornwallis, C. K., West, S. A., and Griffin, A. S. (2009). Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. J. Evol. Biol. 22, 2445–2457. doi: 10.1111/j.1420-9101.2009.01853.x
Crane, J. M. S., Pick, J. L., Tribe, A. J., Vincze, E., Hatchwell, B. J., and Russell, A. F. (2015). Chestnut-crowned babblers show affinity for calls of removed group members: a dual playback without expectancy violation. Anim. Behav. 104, 51–57. doi: 10.1016/j.anbehav.2015.02.022
Creel, S. R., Monfort, S. L., Wildt, D. E., and Waser, P. M. (1991). Spontaneous lactation is an adaptive result of pseudopregnancy. Nature 351, 660–662. doi: 10.1038/351660a0
Crozier, R. H. (1986). Genetic clonal recognition abilities in marine invertebrates must be maintained by selection for something else. Evolution 40, 1100–1101. doi: 10.1111/j.1558-5646.1986.tb00578.x
Curry, R. L. (1988). Influence of kinship on helping behavior of Galápagos mockingbirds. Behav. Ecol. Sociobiol. 22, 141–152. doi: 10.1007/BF00303549
Davies, N. B., Hatchwell, B. J., Robson, T., and Burke, T. (1992). Paternity and parental effort in dunnocks Prunella modularis: how good are male chick-feeding rules? Anim. Behav. 43, 729–745. doi: 10.1016/S0003-3472(05)80197-6
Dickinson, J. L. (2003). Male share of provisioning is not influenced by actual or apparent loss of paternity in western bluebirds. Behav. Ecol. 14, 360–366. doi: 10.1093/beheco/14.3.360
Dickinson, J. L., Akçay, C., Ferree, E. D., and Stern, C. A. (2016). A hierarchical analysis of incest avoidance in a cooperative breeder. Behav. Ecol. 27, 1132–1140. doi: 10.1093/beheco/arw020
Dickinson, J. L., and Hatchwell, B. J. (2004). “Fitness consequences of helping,” in Ecology and Evolution of Cooperative Breeding in Birds, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 48–66. doi: 10.1017/CBO9780511606816.004
Dickinson, J. L., Koenig, W. D., and Pitelka, F. A. (1996). Fitness consequences of helping behavior in the western bluebird. Behav. Ecol. 7, 168–177. doi: 10.1093/beheco/7.2.168
Dowling, J. L., Colombelli-Négrel, D., and Webster, M. S. (2016). Kin signatures learned in the egg? Red-backed fairy-wren songs are similar to their mother's in-nest calls and songs. Front. Ecol. Evol. 4, 48. doi: 10.3389/fevo.2016.00048
Dunn, P. O., Cockburn, A., and Mulder, R. A. (1995). Fairy-wren helpers often care for young to which they are unrelated. Proc. Biol. Sci. 259, 339–343. doi: 10.1098/rspb.1995.0050
Emlen, S. T., and Vehrencamp, S. L. (1985). “Cooperative breeding strategies among birds,” in Experimental Behavioral Ecology, eds B. H..olldobler and M. Lindauer (Stuttgart: Gustav Fischer, 359–374.
Emlen, S. T., and Wrege, P. H. (1988). The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 23, 305–315. doi: 10.1007/BF00300577
Gamboa, G. J., Reeve, H. K., and Holmes, W. G. (1991). Conceptual issues and methodology in kin-recognition research - a critical discussion. Ethology 88, 109–127. doi: 10.1111/j.1439-0310.1991.tb00267.x
Gardner, A., and West, S. A. (2007). Social evolution: the decline and fall of genetic kin recognition. Curr. Biol. 17, R810–R812. doi: 10.1016/j.cub.2007.07.030
Godfrey, S. S., Ansari, T. H., Gardner, M. G., Farine, D. R., and Bull, C. M. (2014). A contact-based social network of lizards is defined by low genetic relatedness among strongly connected individuals. Anim. Behav. 97, 35–43. doi: 10.1016/j.anbehav.2014.08.019
Grafen, A. (1990). Do animals really recognize kin? Anim. Behav. 39, 42–54. doi: 10.1016/S0003-3472(05)80724-9
Green, J. P., Holmes, A. M., Davidson, A. J., Paterson, S., Stockley, P., Beynon, R. J., et al. (2015). The genetic basis of kin recognition in a cooperatively breeding mammal. Curr. Biol. 25, 2631–2641. doi: 10.1016/j.cub.2015.08.045
Greenberg, L. (1979). Genetic component of bee odour in kin recognition. Science 206, 1095–1097. doi: 10.1126/science.206.4422.1095
Griesser, M., Halvarsson, P., Drobniak, S. M., and Vilà, C. (2015). Fine-scale kin recognition in the absence of social familiarity in the Siberian jay, a monogamous bird species. Mol. Ecol. 24, 5726–5738. doi: 10.1111/mec.13420
Halpin, Z. T. (1991). “Kin recognition cues of vertebrates,” in Kin Recognition, ed P. G. Hepper (Cambridge: Cambridge University Press), 220–258. doi: 10.1017/CBO9780511525414.010
Hamilton, W. D. (1964). The genetical evolution of social behavior (I and II). J. Theor. Biol. 7, 1–52. doi: 10.1016/0022-5193(64)90038-4
Hatchwell, B. J., Gullett, P. R., and Adams, M. J. (2014). Helping in cooperatively breeding long-tailed tits: a test of Hamilton's rule. Philos. Trans. Biol. Sci. 369:20130565. doi: 10.1098/rstb.2013.0565
Hatchwell, B. J., Ross, D. J., Fowlie, M. K., and McGowan, A. (2001). Kin discrimination in cooperatively breeding long-tailed tits. Proc. Biol. Sci. 268, 885–890. doi: 10.1098/rspb.2001.1598
Hepper, P. G. (1986). Kin recognition: functions and mechanisms a review. Biol. Rev. 61, 63–93. doi: 10.1111/j.1469-185X.1986.tb00427.x
Hepper, P. G. (1987). The amniotic fluid: an important priming role in kin recognition. Anim. Behav. 35, 1343–1346. doi: 10.1016/S0003-3472(87)80006-4
Holmes, W. G., and Sherman, P. W. (1982). The ontogeny of kin recognition in two species of ground squirrels. Am. Zool. 22, 491–517. doi: 10.1093/icb/22.3.491
Hopp, S. L., Jablonski, P., and Brown, J. L. (2001). Recognition of group membership by voice in Mexican jays, Aphelocoma ultramarina. Anim. Behav. 62, 297–303. doi: 10.1006/anbe.2001.1745
Ihle, M., and Forstmeier, W. (2013). Revisiting the evidence for inbreeding avoidance in zebra finches. Behav. Ecol. 24, 1356–1362. doi: 10.1093/beheco/art074
Johnston, R. E., and Bullock, T. A. (2001). Individual recognition by use of odours in golden hamsters: the nature of individual representations. Anim. Behav. 61, 545–557. doi: 10.1006/anbe.2000.1637
Keen, S. C., Meliza, C. D., and Rubenstein, D. R. (2013). Flight calls signal group and individual identity but not kinship in a cooperatively breeding bird. Behav. Ecol. 24, 1279–1285. doi: 10.1093/beheco/art062
Keller, L., and Ross, K. G. (1998). Selfish genes: a green beard in the red fire ant. Nature 394, 573–575. doi: 10.1038/29064
Koenig, W. D., and Dickinson, J. L. (2016). Cooperative Breeding in Vertebrates. Cambridge: Cambridge University Press. doi: 10.1017/CBO9781107338357
Koenig, W. D., and Haydock, J. L. (2004). “Incest and incest avoidance,” in Ecology and Evolution of Cooperative Breeding in Birds, eds W. D. Koenig and J. L. Dickinson (Cambridge: Cambridge University Press), 142–156. doi: 10.1017/CBO9780511606816.010
Koenig, W. D., and Pitelka, F. A. (1979). Relatedness and inbreeding avoidance: counterploys in the communally nesting acorn woodpecker. Science 206, 1103–1105. doi: 10.1126/science.206.4422.1103
Koenig, W. D., Stanback, M. T., and Haydock, J. (1999). Demographic consequences of incest avoidance in the cooperatively breeding acorn woodpecker, Anim. Behav. 57, 1287–1293. doi: 10.1006/anbe.1999.1093
Komdeur, J. (1994). The effect of kinship on helping in the cooperative breeding Seychelles warbler (Acrocephalus sechellensis). Proc. Biol. Sci. 256, 47–52. doi: 10.1098/rspb.1994.0047
Komdeur, J., and Hatchwell, B. J. (1999). Kin recognition: function and mechanism in avian societies. Trends Ecol. Evol. 14, 237–241. doi: 10.1016/S0169-5347(98)01573-0
Komdeur, J., Richardson, D. S., and Burke, T. (2004). Experimental evidence that kin discrimination in the Seychelles warbler is based on association and not on genetic relatedness. Proc. Biol. Sci. 271, 963–969. doi: 10.1098/rspb.2003.2665
Komdeur, J., Richardson, D. S., and Hatchwell, B. J. (2008). “Kin-recognition mechanisms in cooperative breeding systems: ecological causes and behavioral consequences of variation,” in Ecology of Social Evolution, eds J. Korb and J. Heinze (Berlin; Heidelberg: Springer), 175–193. doi: 10.1007/978-3-540-75957-7_8
Krause, E. T., Kruger, O., Kohlmeier, P., and Caspers, B. A. (2012). Olfactory kin recognition in a songbird. Biol. Lett. 8, 327–329. doi: 10.1098/rsbl.2011.1093
Kroodsma, D. E. (1978). “Aspects of learning in the ontogeny of birdsong,” in The Development of Behavior: Comparative and Evolutionary Aspects, eds G. M. Burghardt and M. Bekoff (New York, NY: Garland, 215–230.
Kurtz, J., Kalbe, M., Aeschlimann, P. B., Häberli, M. A., Wegner, K. M., Reusch, T. B. H., et al. (2004). Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc. Biol. Sci. 271, 197–204. doi: 10.1098/rspb.2003.2567
Kurvers, R. H. J. M., Adamczyk, V. M. A. P., Kraus, R. H. S., Hoffman, J. I., van Wieren, S. E., van der Jeugd, H. P., et al. (2013). Contrasting context dependence of familiarity and kinship in animal social networks. Anim. Behav. 86, 993–1001. doi: 10.1016/j.anbehav.2013.09.001
Lacy, R. C., and Sherman, P. W. (1983). Kin recognition by phenotypic matching. Am. Nat. 121, 489–512. doi: 10.1086/284078
Leclaire, S., Van Dongen, W. F., Voccia, S., Merkling, T., Ducamp, C., Hatch, S. A., et al. (2014). Preen secretions encode information on MHC similarity in certain sex-dyads in a monogamous seabird. Sci. Rep. 4:6920. doi: 10.1038/srep06920
Leedale, A. E. (2018). Functions and mechanisms of kin recognition in long-tailed tits (Ph.D. thesis). University of Sheffield, Sheffield, United Kingdom.
Leedale, A. E., Lachlan, R. F., and Hatchwell, B. J. (2020). Helping decisions and kin recognition in long-tailed tits: is call similarity used to direct help towards kin? Philos Trans Biol Sci. doi: 10.5061/dryad.sbcc2fr2p
Leonard, M. L., Dickinson, J. L., Horn, A. G., and Koenig, W. (1995). An experimental test of offspring recognition in western bluebirds. Auk 112, 1062–1064. doi: 10.2307/4089043
Madden, J. R., Nielsen, J. F., and Clutton-Brock, T. H. (2012). Do networks of social interactions reflect patterns of kinship? Curr. Zool. 58, 319–328. doi: 10.1093/czoolo/58.2.319
Marler, P., and Peters, S. (1982). Long-term storage of learned birdsongs prior to production. Anim. Behav. 30, 479–482. doi: 10.1016/S0003-3472(82)80059-6
Mateo, J. M. (2004). Recognition systems and biological organization: the perception component of social recognition. Ann. Zool. Fenn. 41, 729–745. Available online at: https://www.jstor.org/stable/23736140
McDonald, P. G. (2012). Cooperative bird differentiates between the calls of different individuals, even when vocalizations were from completely unfamiliar individuals. Biol. Lett. 8, 365–368. doi: 10.1098/rsbl.2011.1118
McDonald, P. G., Rollins, L. A., and Godfrey, S. (2016). The relative importance of spatial proximity, kin selection and potential “greenbeard” signals on provisioning behavior among helpers in a cooperative bird. Behav. Ecol. Sociobiol. 70, 133–143. doi: 10.1007/s00265-015-2032-8
McDonald, P. G., and Wright, J. (2011). Bell miner provisioning calls are more similar among relatives and are used by helpers at the nest to bias their effort towards kin. Proc. Biol. Sci. 278, 3403–3411. doi: 10.1098/rspb.2011.0307
Mooney, R., Prather, J., and Roberts, T. (2008). “Neurophysiology of birdsong learning,” in Learning and Memory: A Comprehensive Reference, ed J. H. Byrne (Oxford: Elsevier), 441–474. doi: 10.1016/B978-012370509-9.00116-9
Nam, K.-B., Simeoni, M., Sharp, S. P., and Hatchwell, B. J. (2010). Kinship affects investment by helpers in a cooperatively breeding bird. Proc. Biol. Sci. 277, 3299–3306. doi: 10.1098/rspb.2010.0737
Napper, C. J., and Hatchwell, B. J. (2016). Social dynamics in nonbreeding flocks of a cooperatively breeding bird: causes and consequences of kin associations. Anim. Behav. 122, 23–35. doi: 10.1016/j.anbehav.2016.09.008
Nowak, M., and Sigmund, K. (2005). Evolution of indirect reciprocity. Nature 437, 1291–1298. doi: 10.1038/nature04131
Nowicki, S. (1983). Flock-specific recognition of chickadee calls. Behav. Ecol. Sociobiol. 12, 317–320. doi: 10.1007/BF00302899
Nowicki, S. (1989). Vocal plasticity in captive black-capped chickadees: the acoustic basis and rate of call convergence. Anim. Behav. 37, 64–73. doi: 10.1016/0003-3472(89)90007-9
Payne, R. B., Payne, L. L., and Rowley, I. (1988). Kin and social relationships in splendid fairy-wrens - recognition by song in a cooperative bird. Anim. Behav. 36, 1341–1351. doi: 10.1016/S0003-3472(88)80203-3
Pfefferle, D., Ruiz-Lambides, A. V., and Widdig, A. (2013). Female rhesus macaques discriminate unfamiliar paternal sisters in playback experiments: support for acoustic phenotype matching. Proc. Biol. Sci. 281:20131628. doi: 10.1098/rspb.2013.1628
Price, J. J. (1998). Family- and sex-specific vocal traditions in a cooperatively breeding songbird. Proc. Biol. Sci. 265, 497–502. doi: 10.1098/rspb.1998.0322
Price, J. J. (1999). Recognition of family-specific calls in stripe-backed wrens. Anim. Behav. 57, 483–492. doi: 10.1006/anbe.1998.1018
Price, J. J., and Griffith, S. C. (2017). Open cup nests evolved from roofed nests in the early passerines. Proc. Biol. Sci. 284:20162708. doi: 10.1098/rspb.2016.2708
Pusey, A. E., and Wolf, M. (1996). Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. doi: 10.1016/0169-5347(96)10028-8
Queller, D. C., Ponte, E., Bozzaro, S., and Strassmann, J. E. (2003). Single-gene greenbeard effects in the social amoeba Dictyostelium discoideum. Science 299, 105–106. doi: 10.1126/science.1077742
Radford, A. N. (2005). Group-specific vocal signatures and neighbour-stranger discrimination in the cooperatively breeding green woodhoopoe. Anim. Behav. 70, 1227–1234. doi: 10.1016/j.anbehav.2005.04.002
Reeve, H. K. (1989). The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435. doi: 10.1086/284926
Richardson, D. S., Burke, T., and Komdeur, J. (2003). Sex-specific associative learning cues and inclusive fitness benefits in the Seychelles warbler. J. Evolution. Biol. 16, 854–861. doi: 10.1046/j.1420-9101.2003.00592.x
Richardson, D. S., Burke, T., and Komdeur, J. (2007). Grandparent helpers: the adaptive significance of older, postdominant helpers in the Seychelles warbler. Evolution 61, 2790–2800. doi: 10.1111/j.1558-5646.2007.00222.x
Riehl, C., and Stern, C. A. (2015). How cooperatively breeding birds identify relatives and avoid incest: New insights into dispersal and kin recognition. BioEssays 37, 1303–1308. doi: 10.1002/bies.201500120
Rousset, F., and Roze, D. (2007). Constraints on the origin and maintenance of genetic kin recognition. Evolution 61, 2320–2330. doi: 10.1111/j.1558-5646.2007.00191.x
Rubenstein, D. R., and Abbott, P. (2017). Eds Comparative Social Evolution. New York, NY: Cambridge University Press. doi: 10.1017/9781107338319
Russell, A. F., and Hatchwell, B. J. (2001). Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. Biol. Sci. 268, 2169–2174. doi: 10.1098/rspb.2001.1790
Sharp, S. P., and Hatchwell, B. J. (2005). Individuality in the contact calls of cooperatively breeding long-tailed tits (Aegithalos caudatus). Behaviour. 142, 1559–1575. doi: 10.1163/156853905774831918
Sharp, S. P., McGowan, A., Wood, M. J., and Hatchwell, B. J. (2005). Learned kin recognition cues in a social bird. Nature 434, 1127–1130. doi: 10.1038/nature03522
Sherman, P. W., Reeve, H. K., and Pfennig, D. W. (1997). “Recognition systems,” in Behavioral Ecology: An Evolutionary Approach, eds J. R. Krebs, and N. B. Davies (Cambridge: Blackwell Science Ltd., 69–96.
Stoddard, P. K. (1996). “Vocal recognition of neighbours by territorial passerines,” in Ecology and Evolution of Acoustic Communication in Birds, eds D. E. Kroodsma and M. H. Miller (Ithaca, NY: Cornell University Press), 356–374. doi: 10.7591/9781501736957-028
Tang-Martinez, Z. (2001). The mechanisms of kin discrimination and the evolution of kin recognition in vertebrates: a critical re-evaluation. Behav. Processes 53, 21–40. doi: 10.1016/S0376-6357(00)00148-0
Tibbetts, E. A., and Dale, J. (2007). Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537. doi: 10.1016/j.tree.2007.09.001
Walters, J. R., Doerr, P. D., and Carter, J. H. III. (1988). The cooperative breeding system of the red-cockaded woodpecker. Ethology 78, 275–305. doi: 10.1111/j.1439-0310.1988.tb00239.x
Wang, J., Wurm, Y., Nipitwattanaphon, M., Riba-Grognuz, O., Huang, Y.-C., Shoemaker, D., et al. (2013). A Y-like social chromosome causes alternative colony organization in fire ants. Nature 493, 664–668. doi: 10.1038/nature11832
Warrington, M. H., McDonald, P. G., and Griffith, S. C. (2014a). Within-group vocal differentiation of individuals in the cooperatively breeding apostlebird. Behav. Ecol. 26, 493–501. doi: 10.1093/beheco/aru217
Warrington, M. H., McDonald, P. G., Rollins, L. A., and Griffith, S. C. (2014b). All signals are not equal: acoustic signalling of individuality, sex and breeding status in a cooperative breeder. Anim. Behav. 93, 249–260. doi: 10.1016/j.anbehav.2014.05.007
Wright, J., McDonald, P. G., te Marvelde, L., Kazem, A. J. N., and Bishop, C. M. (2010). Helping effort increases with relatedness in bell miners, but ‘unrelated' helpers of both sexes still provide substantial care. Proc. Biol. Sci. 227, 437–445. doi: 10.1098/rspb.2009.1360
Keywords: kin discrimination, kin recognition, cooperation, familiarity, social birds
Citation: Leedale AE, Li J and Hatchwell BJ (2020) Kith or Kin? Familiarity as a Cue to Kinship in Social Birds. Front. Ecol. Evol. 8:77. doi: 10.3389/fevo.2020.00077
Received: 11 July 2019; Accepted: 10 March 2020;
Published: 03 April 2020.
Edited by:
Mark A. Elgar, The University of Melbourne, AustraliaReviewed by:
Sjouke Anne Kingma, Wageningen University and Research, NetherlandsAndrew Young, University of Exeter, United Kingdom
Copyright © 2020 Leedale, Li and Hatchwell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy E. Leedale, YWVsNTdAY2FtLmFjLnVr
†Present address: Amy E. Leedale, Department of Zoology, University of Cambridge, Cambridge, United Kingdom
 Amy E. Leedale
Amy E. Leedale Jianqiang Li
Jianqiang Li Ben J. Hatchwell
Ben J. Hatchwell