
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 31 March 2020
Sec. Evolutionary Ecology of Social Behaviour
Volume 8 - 2020 | https://doi.org/10.3389/fevo.2020.00076
This article is part of the Research Topic Microbial Drivers of Sociality – from Multicellularity to Animal Societies View all 18 articles
Reproduction is a very critical step in the life of an organism. Females must balance their investment in different life-history traits while reproducing. During the process of colony founding in social organisms, such as ants or bees, a trade-off between reproduction and immunity might be very stringent, because queens might be constrained to invest into immune protection of themselves and their developing offspring until the first workers emerge. Here we investigate how different levels of microbial pressure affect colony founding success of Lasius niger ant queens and whether investment into immune defense traits comes at a substantial cost to the queens. In a first experiment mated queens were exposed to four different environments: sterile housing, autoclaved soil, untreated soil and soil containing two opportunistic pathogens. In this experiment, we investigated an immediate cost, i.e., the success of producing the first brood, and a potential delayed cost, i.e., queen survival and colony founding success after hibernation. For the latter, we removed the first brood after hibernation to reveal hidden costs via the application of an additional stressor. We found that irrespective of the microbial environment all queens successfully managed to start a colony, with queens in the soil treatments showing a higher worker production than the queens in the sterile environment. This suggests that either soil components or soil microbes benefit colony growth. After hibernation queens in microbe soil showed significantly lower survival and could not replace a lost brood. In a second experiment, we investigated whether external immune defense in the form of formic acid use can explain part of the costs imposed on queens. We found that queens used formic acid to sanitize their new nest suggesting that queens founding a colony under high microbial pressure are forced to pay a substantial cost by investing in both reproduction and immunity simultaneously. Our results suggest that early, simultaneous investment in reproduction and immunity can allow colony growth under microbial pressure but may be costly in terms of resistance to later challenges. Ant queens may thus be trading off insurance against future challenges for increased pathogen immunity.
Reproduction is one of the most critical steps in the life of an organism (Harshman and Zera, 2007). Especially females have to balance their investment in different life-history traits while reproducing (Zuk and Stoehr, 2002). For example, depending on the degree of microbial pressure in the environment, in addition to reproduction, an individual has to invest in immunity (Schwenke et al., 2016). To ensure not only its own survival but also the one of its offspring it will have to invest in internal and external immune traits (Otti et al., 2014). In social organisms, such as ants, wasps from temperate regions or bumblebees, this trade-off might be very steep during the process of colony founding and might, thinking in terms of a superorganism, rather represent an investment into growth than reproduction per se (Hölldobler and Wilson, 2008). In ants, starting a colony comes with great challenges (Wheeler, 1910; Hölldobler and Wilson, 1990; Schmid-Hempel, 1998). Before founding a colony, virgin queens leave their nest in search for a mating partner. After successful mating, they bite off their wings and search for a suitable nest site to start laying the first eggs (Hölldobler and Wilson, 1990). Over 95% of queens are estimated to die during this process (Baer et al., 2006; Cole, 2009; Marti et al., 2015), i.e., colony founding events have a very high failure rate (Wilson, 1971). Microbes have been suggested as a major driver of this mortality. For example, in leaf cutter ants 74% of queen deaths during colony founding were associated with pathogens (Baer et al., 2006). Leaving the well-protected maternal colony increases the probability of encountering novel pathogens in the environment. In addition to facing a high microbial pressure herself (Rosengaus and Traniello, 1993; Schmid-Hempel, 2005; Cronin et al., 2013; Gálvez and Chapuisat, 2014; Cole et al., 2018), a recently mated queen founding a colony needs to protect also her offspring. Protection through immunity can occur via activation of internal immune defenses (Cremer et al., 2007; Otti et al., 2014), improving the chances of the queen’s survival, or by application of external immune defenses (Otti et al., 2014). The use of formic acid is an example to reduce the microbial pressure in the nest (Tragust, 2016; Baracchi and Tragust, 2017). Obviously, this will come at a cost adding to the common costs of producing the first brood, which depends exclusively on parental care (Andersson, 1984; Cole et al., 2018). As queens rely exclusively on their own energy reserves during colony foundation (Hölldobler and Wilson, 1990; Cronin et al., 2013; Norman et al., 2016) queens experience energetic stress (Camargo et al., 2011; Gálvez and Chapuisat, 2014). This stress might constrain the investment in immune defenses and result in a trade-off between colony growth (reproduction) and immunity (Calleri et al., 2007; Schwenke et al., 2016; Cole et al., 2018) as an increase in energy expenditure during the founding can strongly affect survival (Camargo et al., 2011). Two strategies might thus be pursued by colony founding queens to optimize the use of resources imposed by the constraints of this growth-immunity trade-off: (1) investment in immune defense of herself and/or her offspring or (2) investment in colony growth either producing high numbers of offspring (Schwenke et al., 2016) and/or speed up its development (Bordoni et al., 2017). With the latter strategy, queens might benefit from later protection against pathogens, because workers will not only forage for food, but they will also provide protection for the colony (Cremer et al., 2007; Otti et al., 2014; Tragust, 2016; Baracchi and Tragust, 2017). We assume both strategies are highly dependent on the microbial pressure in and around the nest site of a new colony and the queen’s current condition (Harshman and Zera, 2007; Schwenke et al., 2016). In founding queens, the production of a first brood is of paramount importance. Consequently, environmental stressors such as the presence of microbial pathogens might not only lead to short- but also long-term fitness effects. Costs induced by stressors might only show months later (Bordoni et al., 2017) or might be alleviated in the long run, as lost resources during colony founding might be compensated for by the colony as a whole later in life. To reveal potential hidden costs of long-term effects not only the direct effects of microbial pressure on the queen should be investigated. If the cost is context-dependent (Moret and Schmid-Hempel, 2000) an additional stressor, such as loss of brood, might uncover a potential hidden cost of microbial pressure.
Here we investigated how microbial pressure affects colony founding success of Lasius niger ant queens and to reveal potential hidden costs we exposed the queens to an additional stressor, i.e., the loss of the first brood, after founding. In a first experiment, we measured colony founding success in four different environments, ranging from a sterile housing to soil containing a bacterial and fungal pathogen. After collection in the field, we recorded queen survival and the production of workers. We assumed that queens exposed to microbe-enriched soil will have to invest more in their first brood than the queens in the other treatments. To reveal such a cost of investment we removed the complete offspring and transferred all queens to a sterile environment to minimize differences between treatments after hibernation (Bordoni et al., 2017). Then, we assessed their ability to start a second brood without food and any help by workers. We also assumed that investment into immune defense traits comes at a cost to the queens during colony founding. In a second experiment, we therefore investigated whether the application of formic acid to the environment as an external immune defense trait explains part of the costs imposed on queens during the first round of colony foundation.
In July 2015 we collected 200 Lasius niger wing-less queens after their mating flight on the campus of the University of Bayreuth and in Bindlach, a suburb of Bayreuth. These queens were randomly assigned to the following three soil treatments and a control treatment (N = 50 queens per treatment): untreated soil, autoclaved soil, autoclaved soil with added microbes (henceforth called microbe-enriched soil) and a sterile control without any soil (for details see “Preparation of Soil Treatments”). We used 15 ml Falcon tubes as nest for the queens. First, 5 ml autoclaved H2O was added to each tube and then a cotton ball placed into the tube to guarantee a certain degree of humidity. Then 1.5 g from one of the three soil treatments were added onto the cotton ball, whereas the sterile control remained without any soil. Finally, an ant queen was placed into the tube and the tube was closed with the lid.
The soil for the three treatments was collected in the same area on the campus of the University of Bayreuth as the ant queens. Then we split up the soil in two parts. One part was left as it was collected, i.e., served as the untreated soil treatment. Fifty 15 ml tubes were provided with untreated soil. The other part was autoclaved at 125°C for 20 min to kill the microbes present in the soil (Trevors, 1996). Once the soil had cooled down it was added to a hundred 15 ml tubes. Fifty tubes were left without further processing and to the other fifty tubes with autoclaved soil 1 ml of a mix of two opportunistic pathogens, one fungus (Metarhizium anisopliae, isolate KVL 03-143, obtained from the Faculty of Life Science, University of Copenhagen, Denmark) and one bacterium (Serratia marcescens, strain DSM12481, DSMZ Braunschweig, Germany), was added. Metarhizium fungi frequently occur in soil and are responsible for natural infections of ants (Hughes et al., 2004; Reber and Chapuisat, 2012). Serratia marcescens is pathogenic in a range of insects (Grimont and Grimont, 2006) and can cause immune system activation in the ant Camponotus floridanus (Ratzka et al., 2011). M. anisopliae spores were harvested from a malt-extract agar plate, their viability checked to be above 90% by scoring approximately 500 spores for germination after 18 h of incubation on an agar plate. Then spores were mixed to a concentration of ∼2 × 108 spores/ml. S. marcescens was produced by plating out from a glycerol stock on LB agar from which a single colony was picked to prepare an overnight culture in LB broth. 5 ml of the overnight culture (concentration ∼109 bacteria/ml) was mixed together with 5 ml of the M. anisopliae solution. To this we added 1,000 ml autoclaved tap water resulting in a bacteria concentration of 5 × 106 bacteria/ml and a fungal spore concentration of 1 × 106 spores/ml. This third soil treatment represents the microbe-enriched soil. The two other soil treatments received 1 ml of autoclaved H2O to account for the added liquid in the microbe-enriched soil treatment.
After housing the queens in the respective treatments (N = 50 queens per treatment), we started the founding experiment under constant darkness in a climate chamber at 22°C and 70% humidity. We checked for queen survival (checks done at 2 days and 1 month after the start of the experiment) and 4 months later (83 days) we put all tubes into hibernation by placing them into a refrigerator at 4°C. After 5 months of hibernation (day 221–225, for treatments autoclaved soil, untreated soil, microbe-enriched soil and sterile control without any soil respectively) all tubes were opened, the number of workers produced was counted and all offspring and dead queens were removed. Live queens (untreated soil: 26, autoclaved soil: 33, microbe-enriched soil: 19, sterile control: 27) were put into a fresh, sterile 15 ml Falcon tube provided with 5 ml autoclaved H2O blocked off with a cotton ball without any soil like the sterile control treatment before hibernation. They were then forced to produce a second brood to measure a potential hidden or delayed cost of colony founding. As mentioned above all brood was removed beforehand, and no food was provided. The tubes were again kept under constant darkness in climate chamber at 15°C and 70% humidity until the April 04, 2016 (day 257 of the experiment). For the queens surviving until the April 04, 2016 (untreated soil: 21, autoclaved soil: 21, microbe-enriched soil: 12, sterile control: 24), we raised the temperature to 22°C to initiate brood production and checked queen survival and brood production over the next 3 months.
In July 2016 we collected 200 Lasius niger wing-less queens after their mating flight on the campus of the University of Bayreuth to measure the use of formic acid under different environmental challenges. For this we collected soil from the premises of the University of Bayreuth and prepared three different soil supernatants as environmental challenges for the colony founding period. The soil was split in two parts of 500 ml and each part was filled into a one liter Duran glass bottle to which tap water was added up to the 1 l mark of the bottle. The mix was left on the bench top at room temperature for 48 h and occasionally shaken. After this it was left to settle for another 48 h. Then one mix was autoclaved at 125°C for 20 min to kill the microbes (Trevors, 1996). The other mix was left untreated. From both mixes we then took the supernatant, which yielded in 400 ml each. The supernatant of the autoclaved mix was split again into two parts. 200 ml were left as they were and we added the same mix at the same concentration of two opportunistic pathogens as in the founding experiment, i.e., M. anisopliae and S. marcescens, to the other 200 ml. We will refer to these three supernatants in the following as untreated soil, autoclaved soil and microbe-enriched soil.
To measure the use of formic acid during the colony founding stage we prepared 6-well microtiter plates (Cellstar 657185, Greiner Bio-One, Germany) as follows. First, we padded five wells of a plate with cotton wool on the side and placed a blue litmus paper (34 × 10 mm, 37135, Fluka, Germany) along the bottom center into each well. Blue litmus paper turns red under acidic conditions below pH = 4.5 (Supplementary Figure S1, and Supplementary Material). The cotton wool in the wells was then soaked with 3 ml of either of the four treatment solutions in a random fashion to four wells on a plate, i.e., sterile control, untreated soil, autoclaved soil, and microbe-enriched soil. For the sterile control we used autoclaved tap water. To the fifth well with cotton wool we added 3 ml autoclaved tap water as a control for humidity effects on the litmus paper. Into the sixth well we put a blue litmus paper without anything else as a reference. No discoloration of the litmus paper was seen in both of these control wells. To minimize the handling effect and to avoid excessive formic acid use while placing the queens into the wells, they were immobilized on ice for 30 min. Then we placed queens individually into a well (N = 200) and put them into a climate chamber at 22°C and 70% humidity and kept them under constant darkness.
We checked every week for queen survival, brood production and brood development until the first workers hatched. We also took a picture (Olympus Pen F) of the blue litmus paper every week until the end of the experiment. Once a week we also added 1 ml of autoclaved tap water to the cotton wool.
All statistical analyses were performed using R 3.6.1 (R Core Team, 2019). Survival data was analyzed with a Cox proportional hazard regression (COXPH) with environmental challenge (sterile control, autoclaved soil, untreated soil, or microbe-enriched soil) as predictor (package “survival”, Therneau and Grambsch, 2000). The proportion of queens producing workers and the number of workers produced in the founding experiment (before hibernation) was analyzed in two separate models (generalized linear model, GLM, with binomial errors for the proportional data – package lme4, Bates et al., 2015 – and zero-inflated generalized linear model for the count data – package glmmTMB, Brooks et al., 2017) with environmental challenge as a predictor. The proportion of queens producing brood after hibernation was similarly analyzed with environmental challenge as a predictor in a GLM with binomial errors. Only queens surviving until the initiation of brood production in the founding experiment after hibernation were used for this analysis and separate models were constructed for the different brood types (eggs, larvae, pupae, and workers). The proportion of queens externalizing formic acid during colony foundation was analyzed in a generalized liner mixed model (GLMER, package lme4, Bates et al., 2015) with binomial errors, environmental challenge, time in weeks and their interaction as fixed predictors and a random effect with random slopes for queen and random intercepts for time in weeks accounting for the repeated measure of formic acid use over time for each queen. Only data from the first 4 weeks was analyzed as upon week four workers started to eclose. These workers can externalize their own formic acid and thus bias the results of formic acid use of queens in the following weeks. To assess significance of predictors in all analyses models were compared to null (intercept only) or reduced models (for those with multiple predictors) using Likelihood Ratio (LRT) or Chi-square tests. Pairwise comparisons between factor levels of a significant predictor were performed using pairwise post-hoc tests adjusting the family-wise error rate according to the method of Westfall (package multcomp, Bretz et al., 2010). Model assumptions of all (zero-inflated) generalized linear and mixed models were checked using model diagnostic tests (overdispersion and zero-inflation) and plots (qq-plot and residual vs. predicted plot) (package DHARMa, Hartig, 2019).
Before hibernation until day 35 of the founding experiment, mortality among queens was under 20% for all environmental challenges (sterile control: 10%; autoclaved soil: 4%; untreated soil: 6%; microbe-enriched soil: 16%). Mortality however, rose to a maximum of 62% for founding queens in microbe-enriched soil until the end of hibernation on day 221–225 (sterile control: 46%; autoclaved soil: 34%; untreated soil: 46%) and thereafter. Overall the environmental challenge of microbe-enriched soil led to a significantly lower survival of founding queens in the founding experiment, while the other environmental challenges did not differ between each other (Figure 1 COXPH, overall LR-test, χ2 = 9.687, df = 3, P = 0.021, post-hoc Tukey comparisons: microbe-enriched soil vs. all other treatments: P < 0.048, all other comparisons: ns). This indicates a survival cost of queens founding a new colony in microbe-enriched soil.
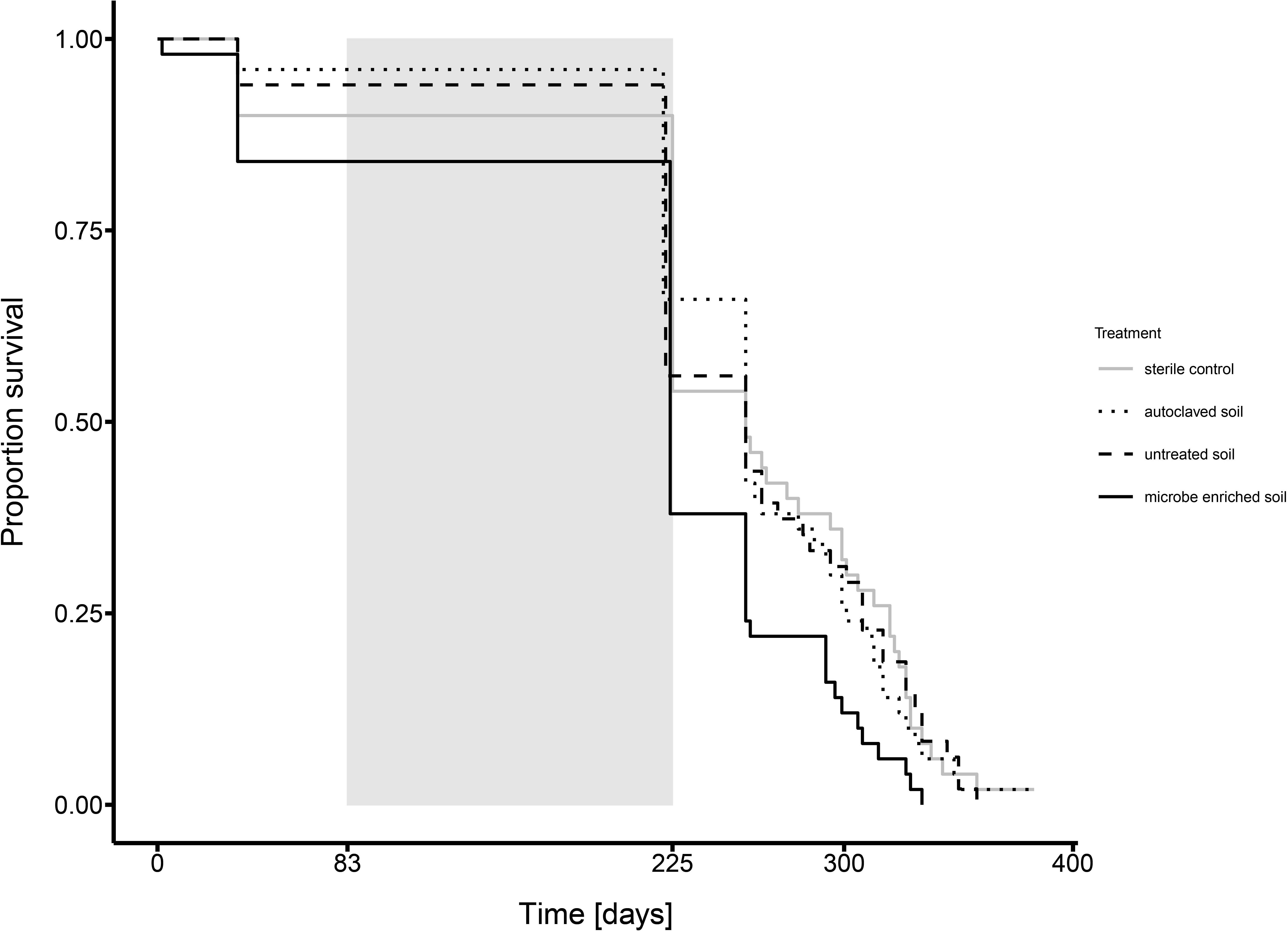
Figure 1. Survival of queens founding a colony under different environmental conditions over the complete experimental period (gray solid line: sterile control, dotted line: autoclaved soil, dashed line: untreated soil, solid black line: microbe-enriched soil), i.e., from the start of the founding experiment until the end of the experiment, with the time of hibernation (day 83 to 221–225 depending upon treatment, see materials and methods) indicated by the gray rectangle.
Before hibernation until day 83 the proportion of queens producing workers was lowest in the sterile control treatment (66%), followed by the microbe-enriched soil (70%), untreated soil (76%) and autoclaved soil (84%) treatment with no significant differences between treatments (Figure 2A, GLM, overall χ2-test, Deviance = -4.958, df = 3, P = 0.175). A similar pattern was observed for the number of workers produced, but with queens in the sterile control treatment producing a significantly lower number of workers than queens in the untreated and the microbe-enriched soil treatment (Figure 2B, glmmTMB, overall χ2-test, χ2 = 8.164, P = 0.043, post-hoc Tukey comparisons: sterile control vs. untreated soil and microbe-enriched soil: P = 0.049, all other comparisons: P > 0.059). After hibernation, the proportion of surviving queens (untreated soil: 21, autoclaved soil: 21, microbe-enriched soil: 12, sterile control: 24) producing brood successively declined with advancing brood development (Figure 3) but was not significantly affected by environmental challenge (eggs: GLM, overall χ2-test, Deviance = -1.775, df = 3, P = 0.9811; larvae: GLM, overall χ2-test, Deviance = -0.076, df = 3, P = 0.995; pupae: GLM, overall χ2-test, Deviance = -7.484, df = 3, P = 0.058; worker: GLM, overall χ2-test, Deviance = -4.694, df = 3, P = 0.196).
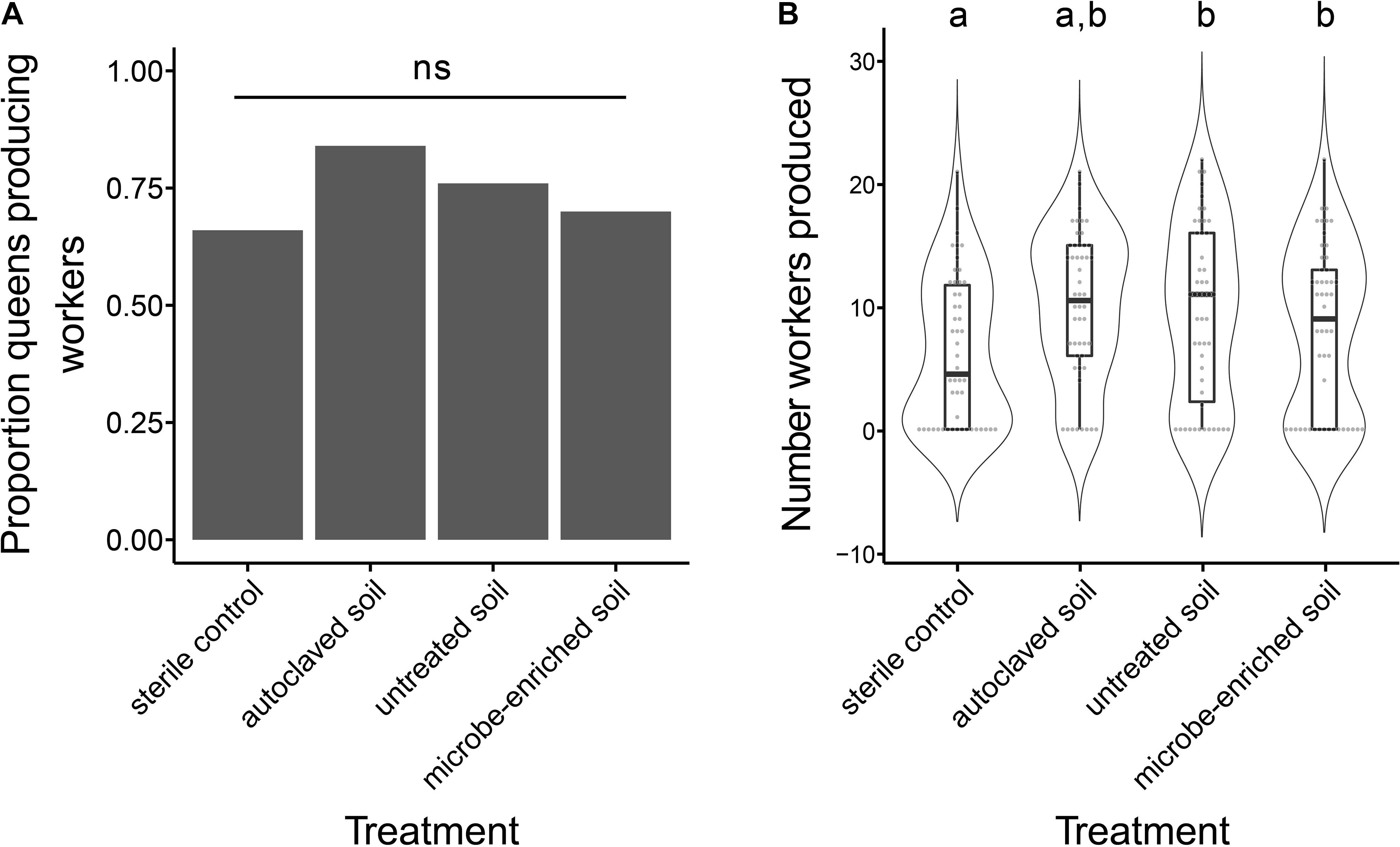
Figure 2. The proportion of queens producing workers (A) and the number of workers produced (B) before hibernation for the different environmental treatments (sterile control, autoclaved soil, untreated soil, and microbe-enriched soil) in the founding experiment. Bars represent the proportion of queens producing workers while boxplots show the median number of workers, as well as the upper and lower quartiles, with whiskers encompassing 1.5 times the interquartile range. Violins around the boxplots show the probability density of the data and gray points show the distribution into discrete bins. Small letters indicate statistically significant different groups at α = 0.05, while ns indicates non-significant groups.
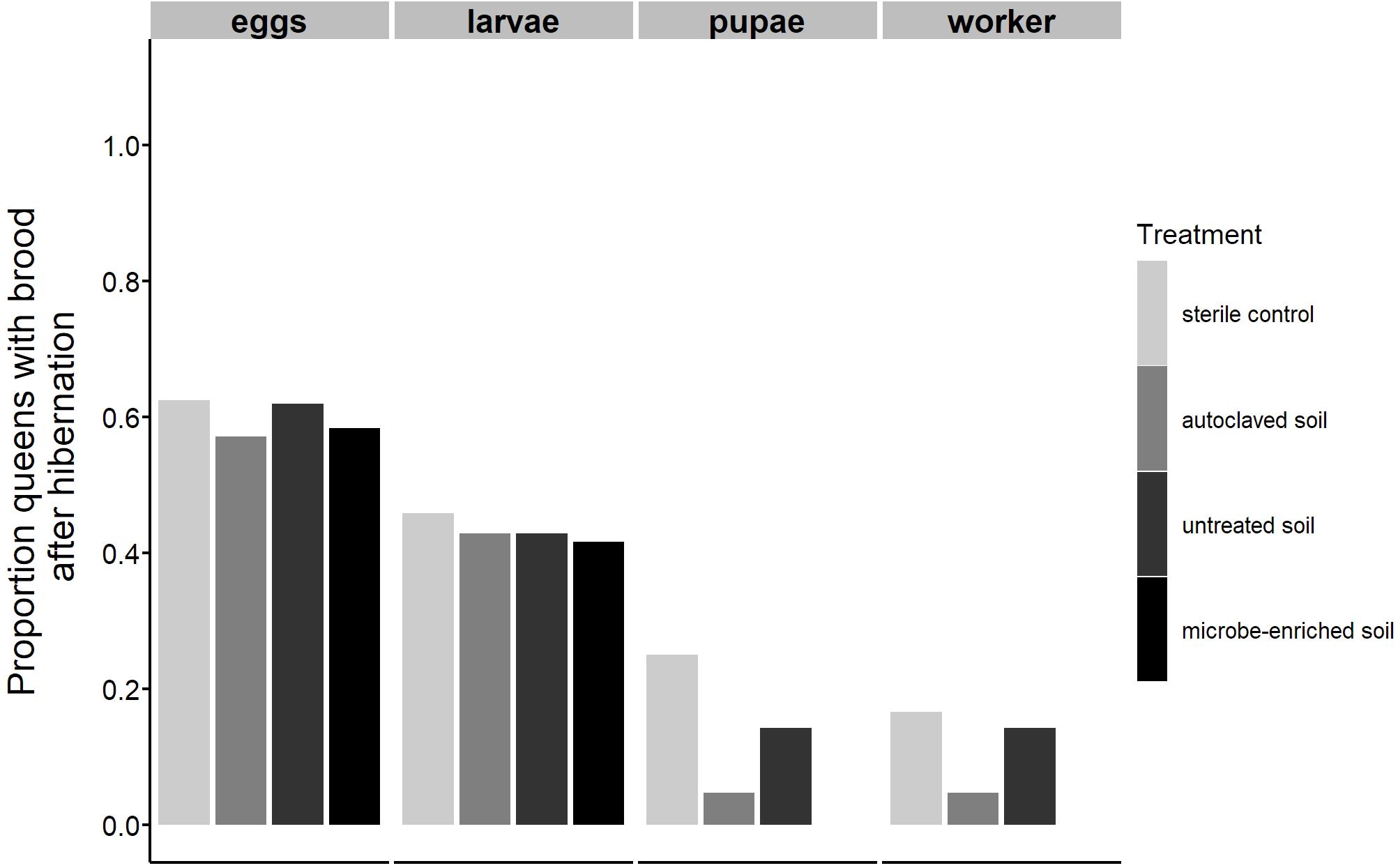
Figure 3. The proportion of queens producing brood after hibernation in the founding experiment. Bars represent the proportion of queens producing eggs, larvae, pupae, and workers under different environmental conditions (sterile control, autoclaved soil, untreated soil, and microbe-enriched soil). Only queens surviving hibernation are taken into account.
The use of formic acid during colony founding was not significantly influenced by environmental challenge (Figure 4; GLMER; interaction treatment × time: χ2 = 3.551, df = 3, P = 0.314; time: χ2 = 0.089, df = 1, P = 0.765; treatment χ2 = 2.598, df = 3, P = 0.458). However, the use of formic acid followed a conspicuous pattern over time. Formic acid use peaked, except for the sterile control, in week two (sterile control: 10%; autoclaved soil: 7%; untreated soil: 9%; microbe-enriched soil: 19%) and declined in the weeks thereafter. This pattern was most pronounced for queens founding in microbe-enriched soil and appeared to be generally linked to brood development, specifically the appearance of larvae.
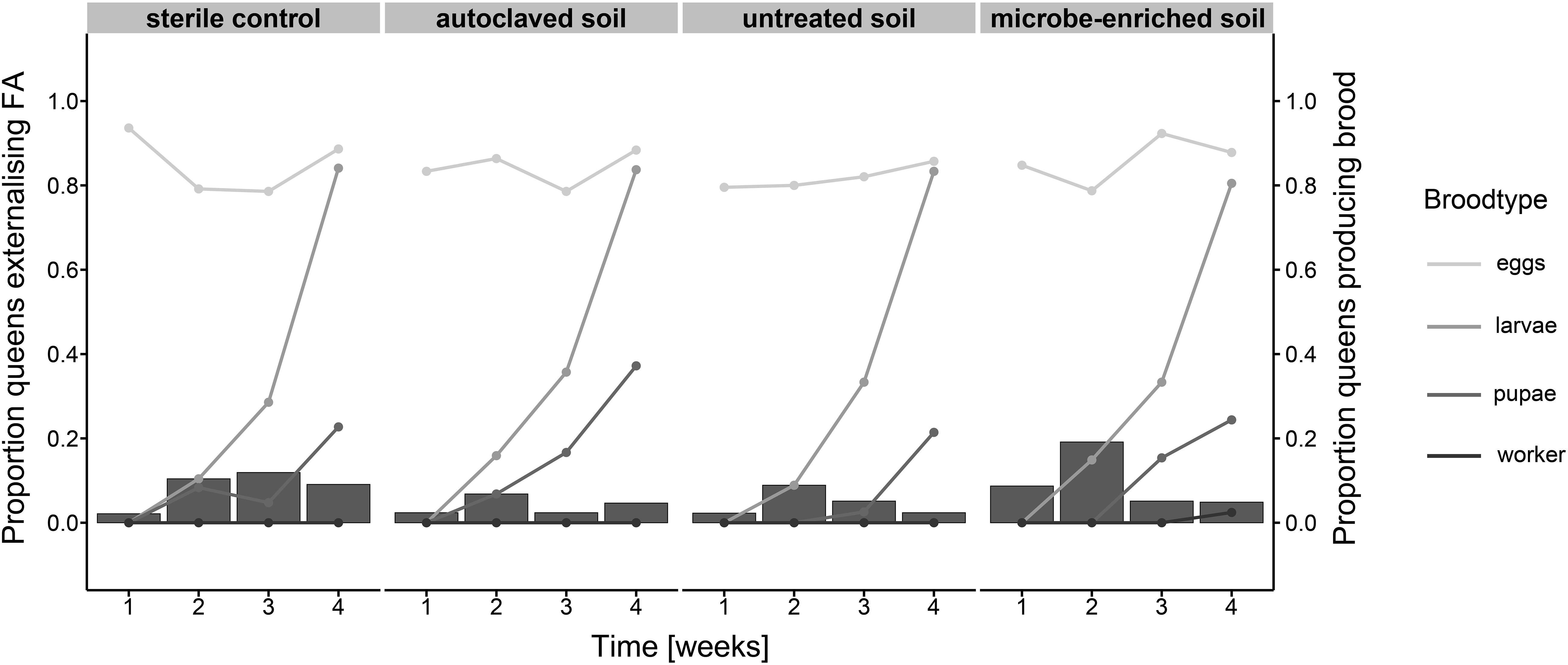
Figure 4. The proportion of queens externalizing formic acid (FA) in their environment (bars) and producing brood (points with connecting lines with gray shades from light gray to black for eggs, larvae, pupae, and workers respectively) over the course of 4 weeks under different environmental conditions (sterile control, autoclaved soil, untreated soil, and microbe-enriched soil).
In this study, we investigated how microbial pathogen pressure in the environment affects colony founding success of Lasius niger ant queens. We tested whether the need to simultaneously invest into immune defense and the production of the first cohort of offspring comes at a substantial cost to the queens and whether the use of formic acid as external immune defense trait explains part of the costs imposed on queens. Irrespective of the microbial pathogen pressure in the environment, queens showed a high success rate in founding a new colony, indicated by the high proportion of queens producing workers. While no significant differences were found in the proportion of queens producing workers, the number of workers produced was significantly lower in the sterile control treatment than the other treatments. In all experimental environments, 2–19% of queens (depending on week) invested in formic acid as an external immune defense to sanitize their nest. Together, we take this as evidence that no trade-off between colony growth (reproduction) and immunity (Calleri et al., 2007; Schwenke et al., 2016; Cole et al., 2018) is present in L. niger founding queens. Queens invested in both, brood production and nest sanitation. This “mixed” strategy comes at a significant cost to queens under microbial pressure. We found that queens in microbe-enriched soil experienced a significantly lower survival during and after hibernation when forced to produce a second batch of brood. Other long-term or potentially hidden costs induced by the loss of the first batch of brood after hibernation could not be detected in queens surviving hibernation, because the proportion of queens producing the second batch of brood did not differ between environmental treatments. However, as our experimental design did not include incipient colonies without a brood loss after hibernation, we are unable to disentangle the relative contributions of microbial challenge and brood loss on survival patterns, respectively. Thus, we cannot completely rule out the existence of hidden costs induced by the loss of the first batch of brood after hibernation.
The mixed strategy of investing in both colony production and immunity represents an alternative in addition to prioritize either colony growth or immunity (Morris, 1987; Schwenke et al., 2016; Duffield et al., 2017). Mixing the investment in reproduction and immunity was recently also found in termites (Cole et al., 2018). There, microbial stress reduced the survival of termite kings and queens, the likelihood of oviposition and total egg number. The onset of oviposition or egg quality did not change in the face of disease, indicating that termite queens choose to maintain offspring quality over quantity (Cole et al., 2018). Similar to termite kings and queens, we suggest that L. niger queens maintain a high reproductive output and a high external immune investment in the face of microbial pressure in the environment. They do this despite the potential costs of lower survival. The strategy of L. niger queens makes intuitive sense, because once a colony with the first cohort of workers is established, workers will forage for food alleviating the energetic costs of reproduction and immunity on queens (Hölldobler and Wilson, 1990). In addition, once a colony is established the cost of external immune defense via the use of formic acid is partitioned up among workers, alleviating the cost of external immune defense in microbe-rich environments.
It is likely that queens in microbe-enriched soil must invest heavily in their own protection during colony foundation. Thus, further studies might still uncover a trade-off between colony growth and immunity investigating the investment in internal immune system activation of the queen in a similar experiment. Another possibility is that this trade-off is not as bilateral as it seems. The evidence suggests that the investment in colony growth, immunity and in the insurance against unexpected challenges, i.e., the loss of the first brood, are traded off against each other. Queens invested equally in growth regardless of pathogen level. However, the queens surviving an immune challenge seem to have fewer resources left over as an insurance and are thus unprotected from an unexpected failure, while some unchallenged queens could produce a second brood. Ant queens may thus be trading off insurance against later challenges for increased pathogen immunity. But, as previously mentioned, future studies will have to disentangle the relative contribution of stressors, i.e., microbial challenge and loss of the first brood, to the costs imposed on founding queens.
Interestingly, our experiments revealed that queens in the sterile environment treatment showed the lowest probability of producing workers and also produced significantly fewer workers than queens in untreated and microbe-enriched soil. This might indicate a general positive effect of soil presence. It has been proposed that the spinning of a cocoon by ant larvae requires small particulate matter (Wheeler, 1910). Therefore, small particulate soil material might have been an underestimated beneficial factor in our experimental design. Alternatively or complementary to this, microbes naturally occurring in the soil might provide an as yet unknown and undescribed benefit to founding queens and the first cohort of brood. Ants entertain a variety of interactions with microbes spanning the continuum of symbiotic, mutualistic and parasitic interactions (Chomicki and Renner, 2017; Russell et al., 2017), which are embedded in a wider microbial community including the microbial community of an individual but also free-living microbial communities in the environment of an individual (Dittmer et al., 2016; Adair and Douglas, 2017; Brinker et al., 2019a). Therefore, the right microbial environment might be very important to ants. Indeed it has been found that ants often influence microbial communities surrounding them, causing a microbial shift between nest soil and soil adjacent to their nest (Brinker et al., 2019b and references therein). The importance of the microbial community in the environment might also explain the use of formic acid by queens in all our experimental environments, as it might not only function to sanitize the nest but might represent an external immune defense trait as originally defined, i.e., a trait acting outside an organism improving protection from pathogens or manipulating the composition of the microbial community in favor of the organism (Otti et al., 2014).
Over the first 4 weeks of colony founding, we also found that the changes in the use of external immune defense between the treatments showed a conspicuous pattern. The use of formic acid increased until week two (week three for the sterile control), followed by a decline over several weeks. Queens in the microbe-enriched soil treatment showed the largest increase and decrease in the use of formic acid, suggesting they were very limited in their use of external immune defense. Also, week two approximately coincides with the appearance of the first larvae. This pattern might indicate an adaptive use of formic acid as external immune defense according to brood developmental stage. It has recently been argued that pupae in cocoons might be less susceptible to the negative effects of formic acid (Pull et al., 2018). An adaptive use of formic acid as external immune defense according to brood developmental stage would therefore make sense. We would argue that the observed pattern indicates that the environment and the development status of the colony can both define the investment in external immune defense.
However, other mechanisms might also be at play here. Challenged by microbial pressure during colony founding, queens could benefit from immune priming their worker offspring (Moret and Schmid-Hempel, 2000; Sadd et al., 2005). Ant colonies normally stay in the same location over the years. Therefore, they are likely to repeatedly encounter the same or similar pathogens and trans-generational immune priming (Gálvez and Chapuisat, 2014; Roth et al., 2018) could be a beneficial strategy to assure the successful establishment of a strong and healthy colony. Indeed, several studies on colony founding, migration and nest building have shown a high preference of pathogen rich nesting sites compared to uninfected sites (founding: Brütsch et al., 2014, immigration: Pontieri et al., 2014, nest-structure: Leclerc et al., 2018), though the evidence for the existence of transgenerational immune priming in ants is currently mixed (Bordoni et al., 2018; Fuchs et al., 2018).
In our experimental setup, costs incurred by queens were discovered under microbial pressure and by enforcing an additional stressor, i.e., the removal of the first brood. This raises the rather interesting question whether in the absence of the additional stressor colonies could have fully recovered (Bordoni et al., 2017) or if the microbial pressure at the start of the colony cycle would have led to a shorter colony lifespan. It could well be that once foraging workers are present in the colony, resource costs paid early in life can be compensated by the work force. However, successful colony founding does also depend on the quality of workers. Under microbial stress queens might produce workers of low quality (Smith and Fretwell, 1974; Negroni et al., 2016), which would only delay the crash of a colony. More studies are needed, investigating the general quality of workers (e.g., body size, fat content or foraging efficiency) and in more detail the immune potential and internal immune system activation of the queen. Because our results suggest that early, simultaneous investment in reproduction and immunity can allow growth under a microbial challenge but may be costly in terms of resistance to later challenges, only long-term studies of colony development will be able to reveal the long-term/total costs of an early life investment in multiple life history traits.
Data underlying the study are included in the Supplementary Material.
ST, PB, and OO conceived the study, designed and performed the analysis, and wrote the manuscript. ST, PB, NR, and OO supervised and participated in data collection. NR contributed substantially to revisions. All authors read and approved of the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the two reviewers for their input which helped to improve our manuscript. Pina Brinker was supported by the Equal Opportunities Fund of the University of Bayreuth. Oliver Otti was supported by the Marvel Universe. He is especially grateful to Thor and Hulk for being such an entertaining duo and who gave everything to relieve the writing stress. We thank Simon Bräu and Gampertbräu for great support with their brews that helped spawn fresh and new ideas. Finally, we acknowledge the financial support within the funding program Open Access Publishing by the German Research Foundation (DFG).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00076/full#supplementary-material
Adair, K. L., and Douglas, A. E. (2017). Making a microbiome: the many determinants of host-associated microbial community composition. Curr. Opin. Microbiol. 35, 23–29. doi: 10.1016/j.mib.2016.11.002
Baer, B., Armitage, S. A. O., and Boomsma, J. J. (2006). Sperm storage induces an immunity cost in ants. Nature 441, 872–875. doi: 10.1038/nature04698
Baracchi, D., and Tragust, S. (2017). “Venom as a component of external immunedefense in hymenoptera,” in Evolution of Venomous Animals and Their Toxins, ed. A. Malhotra (Dordrecht: Springer), 213–233. doi: 10.1007/978-94-007-6458-3_3
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bordoni, A., Dapporto, L., Tatini, I., Celli, M., Bercigli, M., Barrufet, S. R., et al. (2018). Trans-generational immunization in the acrobat ant Crematogaster scutellaris. Biol. Lett. 14:20170761. doi: 10.1098/rsbl.2017.0761
Bordoni, A., Miroddi, M. A., Dapporto, L., and Turillazzi, S. (2017). Long-term assessment reveals the hidden and hiding effects of experimental stress on ant colonies. Behav. Ecol. Sociobiol. 71:144. doi: 10.1007/s00265-017-2373-6
Bretz, F., Hothorn, T., Westfal, P., and Westfall, P. H. (2010). Multiple Comparisons Using R. London: Chapman & Hall.
Brinker, P., Fontaine, M. C., Beukeboom, L. W., and Salles, J. F. (2019a). Host, symbionts, and the microbiome: the missing tripartiteinteraction. Trends Microbiol. 27, 480–488. doi: 10.1016/j.tim.2019.02.002
Brinker, P., Weig, A., Rambold, G., Feldhaar, H., and Tragust, S. (2019b). Microbial community composition of nest-carton and adjoining soil of the ant Lasius fuliginosus and the role of host secretions in structuring microbial communities. Fung. Ecol. 38, 44–53. doi: 10.1016/j.funeco.2018.08.007
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400.
Brütsch, T., Felden, A., Reber, A., and Chapuisat, M. (2014). Ant queens (Hymenoptera: Formicidae) are attracted to fungal pathogens during the initial stage of colony founding. Myrmecol. News 20, 71–76.
Calleri, D. V., Rosengaus, R. B., and Traniello, J. F. A. (2007). Immunity and reproduction during colony foundation in the dampwood termite, Zootermopsis angusticollis. Physiol. Entomol. 32, 136–142. doi: 10.1111/j.1365-3032.2007.00559.x
Camargo, R. S., Forti, L. C., Fujihara, R. T., and Roces, F. (2011). Digging effort in leaf-cutting ant queens (Atta sexdens rubropilosa) and its effects on survival and colony growth during the claustral phase. Insectes Soc. 58, 17–22. doi: 10.1007/s00040-010-0110-5
Chomicki, G., and Renner, S. S. (2017). The interactions of ants with their biotic environment. Proc. R. Soc. B 284:20170013. doi: 10.1098/rspb.2017.0013
Cole, B. J. (2009). “The ecological setting of social evolution: the demography of ant populations,” in Organization of Insect Societies?: From Genome to Sociocomplexity, eds J. Fewell and J. Gadau (Cambridge, MA: Harvard University Press), 74–104.
Cole, E. L., Ilieş, I., and Rosengaus, R. B. (2018). Competing physiological demands during incipient colony foundation in a social insect: consequences of pathogenic stress. Front. Ecol. Evol. 6:103. doi: 10.3389/fevo.2018.00103
Cremer, S., Armitage, S. A. O., and Schmid-Hempel, P. (2007). Social immunity. Curr. Biol. 17, 693–702. doi: 10.1016/j.cub.2007.06.008
Cronin, A. L., Molet, M., Doums, C., Monnin, T., and Peeters, C. (2013). Recurrent evolution of dependent colony foundation across eusocial insects. Annu. Rev. Entomol. 58, 37–55. doi: 10.1146/annurev-ento-120811-153643
Dittmer, J., van Opsta, E. J., Shropshire, J. D., Bordenstein, S. R., Hurst, G. D. D., and Brucker, R. M. (2016). Disentangling a holobiont – Recent advances and perspectives in Nasonia wasps. Front. Microbiol. 7:1478. doi: 10.3389/fmicb.2016.01478
Duffield, K. R., Bowers, E. K., Sakaluk, S. K., and Sadd, B. M. (2017). A dynamic threshold model for terminal investment. Behav. Ecol. Sociobiol. 71:185. doi: 10.1007/s00265-017-2416-z
Fuchs, S., Sundstroem, L., Bos, N., Stucki, D., and Freitak, D. (2018). Induced immune responses in Formica fusca (Hymeno ptera: Formicidae). Myrmecol. News 28, 53–66. doi: 10.25849/myrmecol.news
Gálvez, D., and Chapuisat, M. (2014). Immune priming and pathogen resistance in ant queens. Ecol. Evol. 4, 1761–1767. doi: 10.1002/ece3.1070
Grimont, F., and Grimont, P. A. D. (2006). “The genus enterobacter,” in The Prokaryotes: Volume 6: Proteobacteria: Gamma Subclass, eds M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (New York, NY: Springer), 219–244.
Harshman, L. G., and Zera, A. J. (2007). The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86. doi: 10.1016/j.tree.2006.10.008
Hartig, F. (2019). DHARMa: Residual Diagnostics for Hierarchical (multi-level / mixed) Regression Models. R package Version 0.2.4. Available online at: https://cran.r-project.org/web/packages/DHARMa/index.html
Hölldobler, B., and Wilson, E. O. (2008). The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. New York, NY: W. W. Norton & Company.
Hughes, W. O. H., Thomsen, L., Eilenberg, J., and Boomsma, J. J. (2004). Diversity of entomopathogenic fungi near leaf-cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Inv. Pathol. 85, 46–53. doi: 10.1016/j.jip.2003.12.005
Leclerc, J. B., Pinto Silva, J., and Detrain, C. (2018). Impact of soil contamination on the growth and shape of ant nests. R. Soc. Open Sci. 5:180267. doi: 10.1098/rsos.180267
Marti, H. E., Carlson, A. L., Brown, B. V., and Mueller, U. G. (2015). Foundress queen mortality and early colony growth of the leafcutter ant, Atta texana (Formicidae, Hymenoptera). Insectes Soc. 62, 357–363. doi: 10.1007/s00040-015-0413-7
Moret, Y., and Schmid-Hempel, P. (2000). Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168. doi: 10.1126/science.290.5494.1166
Morris, D. M. (1987). Optimal allocation of parental investment. Oikos 49, 332–339. doi: 10.2307/3565769
Negroni, M. A., Jongepier, E., Feldmeyer, B., Kramer, B. H., and Foitzik, S. (2016). Life history evolution in social insects: a female perspective. Curr. Opin. Insect Sci. 16, 51–57. doi: 10.1016/j.cois.2016.05.008
Norman, V. C., Pamminger, T., and Hughes, W. O. H. (2016). Behavioural development, fat reserves and their association with productivity in lasius flavus founding queens. Sci. Nat. 103:23. doi: 10.1007/s00114-016-1350-7
Otti, O., Tragust, S., and Feldhaar, H. (2014). Unifying external and internal immune defences. Trends Ecol. Evol. 29, 625–634. doi: 10.1016/j.tree.2014.09.002
Pontieri, L., Vojvodic, S., Graham, R., Pedersen, J. S., and Linksvayer, T. A. (2014). Ant colonies prefer infected over uninfected nest sites. PLoS One 9:e111961. doi: 10.1371/journal.pone.0111961
Pull, C. D., Metzler, S., Naderlinger, E., and Cremer, S. (2018). Protection against the lethal side effects of social immunity in ants. Curr. Biol. 28, R1139–R1140. doi: 10.1016/j.cub.2018.08.063
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ratzka, C., Liang, C., Dandekar, T., Gross, R., and Feldhaar, H. (2011). Immune response of the ant Camponotus floridanus against pathogens and its obligate mutualistic endosymbiont. Insect Biochem. Mol. Biol. 41, 529–536. doi: 10.1016/j.ibmb.2011.03.002
Reber, A., and Chapuisat, M. (2012). Diversity, prevalence and virulence of fungal entomopathogens in colonies of the ant Formica selysi. Insect. Soc. 59, 231–239. doi: 10.1007/s00040-011-0209-3
Rosengaus, R. B., and Traniello, J. F. A. (1993). Disease risk as a cost of outbreeding in the termite Zootermopsis angusticollis. Proc. Natl. Acad. Sci. U.S.A. 90, 6641–6645. doi: 10.1073/pnas.90.14.6641
Roth, O., Beemelmanns, A., Barribeau, S. M., and Sadd, B. M. (2018). Recent advances in vertebrate and invertebrate transgenerational immunity in the light of ecology and evolution. Heredity (Edinb). 121, 225–238. doi: 10.1038/s41437-018-0101-2
Russell, J. A., Sanders, J. G., and Moreau, C. S. (2017). Hotspots for symbiosis: Function, evolution, and specificity of ant-microbe associations from trunk to tips of the ant phylogeny (Hymenoptera: Formicidae). Myrmecol. News 24, 43–69.
Sadd, B. M., Kleinlogel, Y., Schmid-Hempel, R., and Schmid-Hempel, P. (2005). Trans-generational immune priming in a social insect. Biol. Lett. 1, 386–388. doi: 10.1098/rsbl.2005.0369
Schmid-Hempel, P. (2005). Natural insect host-parasite systems show immune priming and specificity: Puzzles to be solved. BioEssays 27, 1026–1034. doi: 10.1002/bies.20282
Schwenke, R. A., Lazzaro, B. P., and Wolfner, M. F. (2016). Reproduction – immunity trade-offs in insects. Annu. Rev. Entomol. 61, 239–256. doi: 10.1146/annurev-ento-010715-023924.Reproduction
Smith, C. C., and Fretwell, S. D. (1974). The optimal balance between size and number of offspring. Am. Nat. 108, 499–506. doi: 10.1086/282929
Therneau, T. M., and Grambsch, P. M. (2000). Modeling Survival Data: Extending the Cox Model. New York, NY: Springer.
Tragust, S. (2016). External immune defence in ant societies (Hymenoptera: Formicidae): the role of antimicrobial venom and metapleural gland secretion. Myrmecol. News 23, 119–128.
Trevors, J. T. (1996). Sterilization and inhibition of microbial activity in soil. J. Microbiol. Methods 26, 53–59. doi: 10.1016/0167-7012(96)00843-3
Wheeler, W. M. (1910). Ants: Their Structure, Development and Behavior. New York, NY: Columbia University Press.
Keywords: external immune system, eusociality, colony founding costs, pathogen pressure, antimicrobial secretion, antimicrobial venom, life-history trade off
Citation: Tragust S, Brinker P, Rossel N and Otti O (2020) Balancing Life History Investment Decisions in Founding Ant Queens. Front. Ecol. Evol. 8:76. doi: 10.3389/fevo.2020.00076
Received: 01 September 2019; Accepted: 10 March 2020;
Published: 31 March 2020.
Edited by:
Dino McMahon, Freie Universität Berlin, GermanyReviewed by:
Tomer J. Czaczkes, University of Regensburg, GermanyCopyright © 2020 Tragust, Brinker, Rossel and Otti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon Tragust, c2ltb24udHJhZ3VzdEB6b29sb2dpZS51bmktaGFsbGUuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.