
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 23 January 2020
Sec. Urban Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00525
Increasing anthropogenic environmental impacts lead to rapid transitions of ecosystems and species. Species persisting in changing environments may respond to changes by altering phenotypic traits across space and/or time. Here we tested whether the frequencies of three color morphs in the ground beetle Harpalus affinis differed across spatial and temporal gradients. The gradients extended from urban to rural regions, and from the early twentieth century until today, in the Berlin-Brandenburg area, Germany. Specimens comprised beetles from the entomological collection of the Museum für Naturkunde, Berlin and recently collected material. As a result of differing environments, we expected to observe differences in color frequencies in beetles between habitats and across time, responding to different levels of urbanization. Our results revealed sexual dichromatism in H. affinis as well as some habitat dependent differences in trait frequency. Frequencies of color morphs remained generally constant in males across space and time. Females likewise showed no differences in color frequencies between habitats, urban and rural regions, and between different time periods in rural regions. In contrast color morph frequencies changed in urban regions over time in females: Bronze color decreased, whereas green color became more dominant over time. We assume that bronze color was selectively advantageous in times with high levels of soot pollution in the city, whereas green is more cryptic and thus advantageous in times with less polluted air. The color change of females thus could have been driven by natural selection. In contrast, the persistence of predominately green males through all times and habitats, more likely can be explained by sexual selection.
Increasing levels of urbanization and agricultural land use, mostly due to rapid population growth (Bairoch and Goertz, 1986; Antrop, 2004), cause rapid environmental transitions from natural to degraded or novel ecosystems and thus are of major ecological and socio-economic interest (Hobbs et al., 2013; Jeltsch et al., 2013). Novel ecosystems are defined as systems that differ in their (species) composition and/or function from present and past systems due to anthropogenic impacts such as land use and climate change (Harris et al., 2006; Root and Schneider, 2006; Ricciardi, 2007). Due to human induced environmental changes (Figure 1), the native fauna and flora will be heavily impacted by habitat degradation, fragmentation, and conversion, as well as the invasion of new species, and the creation of new habitats (Ribera et al., 2001; Haila, 2002). Consequently, many native species will be lost (Maas et al., 2002; Brunk and Wiegleb, 2006; Kenis et al., 2009; Ziegler, 2011). However, there are some native species that persist throughout these environmental transitions (Van't Hof et al., 2011; Doudna and Danielson, 2015). How these species deal with the changing environments, especially if rapid transitions triggered any changes in their phenotypes or if particular (pre-)adaptations enable these species to persist, whilst other species disappeared (Palkovacs et al., 2011), has been so far mostly neglected.
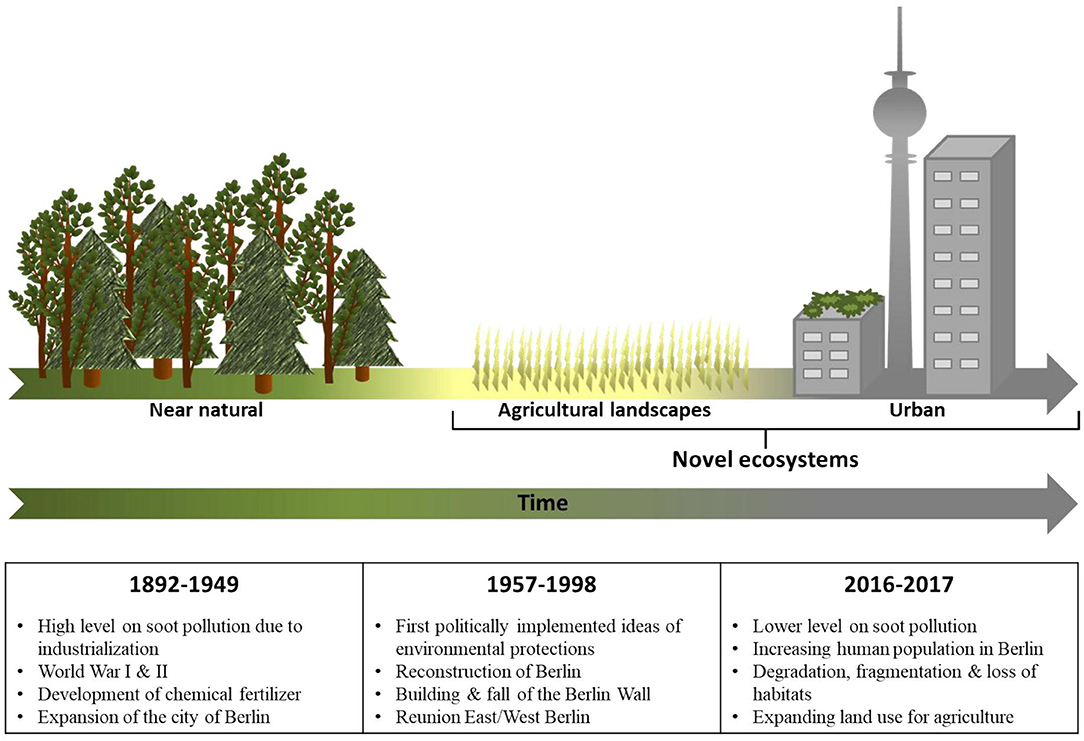
Figure 1. History and anthropogenic impacts on environments in space and time in the German Berlin-Brandenburg area. Different habitat types nowadays in space are reflected over time. Important events influencing environments are connected with the respective time period.
Whereas changes in behavior and physiology usually cannot be directly assessed over long time periods, certain morphological traits may be collected from time series of preserved specimens in historical collections (Rocha et al., 2014; Doudna and Danielson, 2015). Morphological traits that are known to be influenced by environmental conditions are body size (Sustek, 1992; McCabe and Patridge, 1997; Ribera et al., 2001), body proportions (Atkinson, 1994), including fluctuating asymmetry (Palmer and Strobeck, 1992), and coloration (Schultz and Rankin, 1985). These traits can be used as proxies for a species' ecological and physiological modification during its adaptation to a novel ecosystems. The historical datasets can finally be completed with recent samples to cover spatio-temporal gradients across environmental transitions (Van't Hof et al., 2011; Doudna and Danielson, 2015).
Coloration is often related to background matching (Cott, 1940; Kettlewell, 1956; Endler, 1984, 1988; Storfer et al., 1999), and represents a trait that may change due to urbanization (Harrison and Garrett, 1926; Kettlewell, 1955, 1956; Clarke and Sheppard, 1966; Bishop, 1972). Most colors, based on pigments, fade after death (Doucet and Hill, 2009). By contrast structural colors like multilayer reflectors, as often found in Coleoptera (Parker, 1998; Noyes et al., 2007; Kinoshita et al., 2008), develop during metamorphosis by secretion of thin parallel layers of chitin by the epidermis and harden during sclerotization. One or more colors will be produced by constructive interference if the spacing of these layers approaches the wavelength of visible light (Land, 1972). Because these colors are depending on structure and not on pigments, they retain their properties under normal museum conditions (Parker and McKenzie, 2003). This makes them a useful trait for spatial and temporal comparisons (Hadley et al., 1988; Tyler, 2010).
One species that is characterized by multilayer reflectors and occurs in three distinctly different metallic color morphs (Seago et al., 2009) is the ground beetle Harpalus affinis. It is a well-known insect species persisting in natural and modified habitats such as agricultural fields (Sunderland et al., 1995), semi-natural landscapes (Anjum-Zubair et al., 2015), natural landscapes (Townsend, 1992), and urban green spaces (Deichsel, 2006).
The aim of this study was to investigate whether or not H. affinis exhibit changes in frequencies of color morphs across space (rural to urban and between different habitat types) and time (past to now) in the Berlin-Brandenburg area of Germany. This area is characterized by increasing levels of urbanization in which rapid transitions, from near natural to novel ecosystems occur(ed) (Cochrane and Jonas, 1999). The city of Berlin is a fast-growing metropolis, whose expansion and population increase started toward the end of the nineteenth century, including a phase of rapid reconstruction in the direct aftermath of World War II (Kraetke, 2000). This led to a high level of urbanized habitats (Antrop, 2000). Berlin is surrounded by the German federal state of Brandenburg, which, in contrast to Berlin, mostly consists of rural landscapes with habitats ranging from near natural to intensively managed agricultural monocultures (Cochrane and Jonas, 1999). For our study species, we predicted differences in color frequencies between urban, agricultural and near natural habitats as well as between urban and rural regions. We further predicted changes in color frequencies in rural and urban regions over time, spanning the end of the nineteenth century until nowadays. Color differences and changes should emerge depending on the respective level of land use and urbanization in the respective habitat, region and time, responding in higher frequencies of dark morphs in habitats and times with higher levels of urbanization and higher frequencies of green morphs in less urbanized habitats, regions and times.
The ground beetle Harpalus affinis (Schrank, 1781) (Coleoptera, Carabidae) is a Palearctic species (Wrase, 2004). The medium-sized (8.5–12 mm), diurnal, predominantly phytophagous species, is occasionally also preying on insect larvae (Townsend, 1992; Sunderland et al., 1995). Adults are winged and volant (Townsend, 1992), and may occur in either of three metallic color morphs: uniform metallic green, uniform bronze, or a mixture of green and bronze body parts (Wrase, 2004; Figure 2). Males differ from females by wider tarsal segments of the pro- and mesothoracic legs (Townsend, 1992; Loevei and McCambridge, 2002). Because this species persisted in our study area over time, occurs in different habitat types and different color morphs, and lastly is well presented in the collection of the Museum für Naturkunde, Berlin, it is a particularly well suited study species for our research question.

Figure 2. Photographs of the three Harpalus affinis color morphs “green,” “bronze,” and “mixed,” from the collections of the Museum für Naturkunde, Berlin. (A) (green ♀): Berlin, Prenzlauer Berg, Oderbruchkippe, 17, June 1972. (B) (bronze ♂): Brandenburg, nature reserve Mallnow near Seelow, 24, August 1975. (C) (mixed ♀): Berlin, Prenzlauer Berg, Oderbruchberg, 24, August 1969.
In total 546 vouchers, deposited in the Coleoptera collection of the Museum für Naturkunde, Berlin have been examined for this study. They originate from the Berlin-Brandenburg area, Germany, and span the years 1892–1998. Urban (Berlin) beetles with habitat information available have been collected at ruderal sites, dumpsites, roadsides, garden plots, and parks. Vouchers from rural Brandenburg with habitat information stem from meadows and forest edges (Appendix A in Supplementary Material). For some vouchers only rough information about their origin (Berlin or Brandenburg) were available. These beetles were only used for comparisons between regions and for temporal comparisons within regions and were excluded from comparisons between specific habitat types.
In total 114 recently collected specimens were used for this study. Specimens, collected in rural districts of Brandenburg were originated from agricultural winter wheat and soy fields and from fallow, grass strips in between these fields, located in: Nordwestuckermark (May to June 2016), Uckermark (June 2017), Maerkisch-Oderland (May to July 2017), and on dry grasslands in Blankenfelde-Mahlow of Teltow-Flaeming (June to July 2017). Specimens from urban sites were collected within the city of Berlin on 18 dry grassland sites (June to July 2017; Appendix A in Supplementary Material). These sites were comparable to sites from urban museum vouchers because dry grassland sites were located in the midst of the city. In general, due to classification into similar regions and habitats, we made sure that habitat types of specimens nowadays were comparable with those of vouchers from the collection. Specimens were collected with pitfall traps by ourselves and by cooperation partners.
The colors of thorax and abdomen were assessed for females and males separately. If the colors of both body parts were identical, we classified the respective individual as “green” or “bronze.” When thorax and abdomen were differently colored, we classified the specimen as “mixed.” Color categorization were always assessed under the same light conditions and by the same person, categories were unambiguous (Figure 2), and were made blind as to the origin of the specimen.
A total of 660 of H. affinis from the Berlin-Brandenburg area were examined for this study: 546 from the collection of the Museum für Naturkunde, Berlin, covering a 106 year time period (1892–1998), 77 specimens collected in 2016 and 2017 from agricultural winter wheat fields, soy fields and small green spaces in between these fields in Brandenburg, and 37 beetles originated from urban habitats in Berlin (Table 1).
Each H. affinis specimen was classified according to sex, color, and origin. Specimens originated from the city of Berlin were classified as “urban.” Beetles originated from its campestral surrounding, Brandenburg, were classified as “rural.” These classifications were used for observations between regions and within regions over time. If habitat information of the museum vouchers were available, specimens were used for observations between different habitat types. Specimens from the rural region, Brandenburg, were further classified as originated from “agricultural landscapes” or, when sampled in protected landscapes or nature conservation areas, as originated from “near natural” habitats. Specimens from the urban region, Berlin, were used for observations between habitats when further habitat information were given that individuals were sampled in ruderal sites, dumpsites, roadsides, garden plots or parks (Appendix A in Supplementary Material).
For observations over time urban and rural, beetles were divided into three time periods from 1892 to 1949, 1957 to 1998, or 2016 to 2017. No sampling material was existing for times from 1999 to 2015, therefore this time period could not be included. Time period classification was based on a combination of practical reasons (availability of sufficient numbers of beetles) and biological relevance. During the first time period, the Berlin-Brandenburg area was mainly impacted by the effects of industrial revolution and the First and Second World War. Numerous newly established factories resulted in intense air pollution in Berlin (Wey, 1982; Ribbe et al., 2002a,b). Due to the development of the Haber-Bosch process for fixing nitrogen in the 1920's (Erisman et al., 2008), chemical fertilizers were for the first time applied in large quantities. These developments led to a high level of environmental pollution at that time without attempts of environmental protection (Pamme, 2003). Furthermore, Berlin was expanded to “Groß-Berlin” in the 1920's, population size exceeding the current state and leading to rapid construction of buildings and infrastructures (Buesch and Haus, 1987). During the period from 1957 to 1998, Berlin's reconstruction after World War II was finalized (Schildt and Sywottek, 1993). At the end of the 1950's, first measures concerning environmental protection were implemented (Pamme, 2003), resulting in decreasing emissions of air pollutants (UNEP/WHO, 1993; UBA, 1998), and reduced application of chemical fertilizers in the surroundings of the city (Tilman et al., 2002; Erisman et al., 2008). Therefore, this time period can be considered as an intermediate stage of environmental pollution due to urbanization between time period one and today. From 1999 to today, the human population within Berlin has been again steadily increasing (United Nations, 2002), but air pollution, mostly due to soot, decreased since industrialization because of environmental protection measures (UNEP/WHO, 1993; UBA, 1998; SenStadtWohn, 2018).
For statistical analyses, every beetle was tagged by region (urban, rural), habitat type (urban, agricultural, near natural), time period (1892–1949, 1957–1998, 2016–2017), and color (green, bronze, mixed). We used the R- Project, version 3.4.0 (R Core Team, 2017) with the package RVAideMemoire (Hervé, 2013). For the analysis of the differences between color composition between habitats, regions and regions over time, the G-test of independence was used (see Forsman and Shine, 1995; Cahan et al., 2002). To examine if there were differences in color frequencies between different habitats, regions and time periods of a region, and to investigate if there were frequency differences of colors within the same habitat, region and time period per region, the pairwise G-test with Bonferroni correction for multiple testing was used.
Across all vouchers, we observed significant differences in the frequencies of color morphs between sexes (G = 15.505, df = 2, p < 0.001). Females showed significantly more often “mixed” colors than males (p < 0.001). The frequencies of “green” and “bronze” colors did not differ between sexes. Significantly more females were “green” compared to “bronze” (p = 0.002) or “mixed” (p = 0.019). Males likewise were more often “green” than “bronze” (p = 0.014) or “mixed” (p < 0.001). “Bronze” males occurred more often than “mixed” (p < 0.001) ones (Figure 3). Both sexes also partly exhibited different frequencies of color morphs with regard to habitat type, region and region over time.
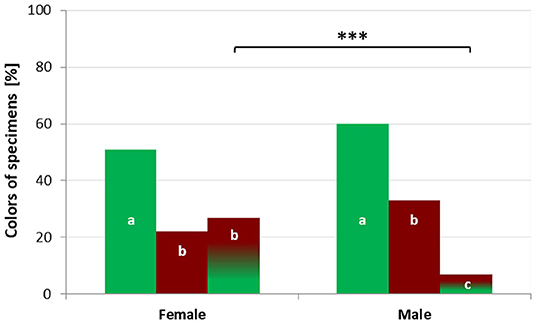
Figure 3. Percentage of different color morphs in female and male Harpalus affinis; significant color differences between sexes are indicated by stars (***p < 0.001); significant differences between color morphs within a sex are indicated by differing letters.
In females we observed no differences in coloration between urban, agricultural and near natural habitats (G = 4.8017; df = 4, p = 0.308). However, in urban habitats “green” dominated over “bronze” (p < 0.001) and “mixed” (p < 0.001) ones. In agricultural habitats frequencies of “green” were higher than “bronze” (p < 0.001) and “mixed” ones (p = 0.002). In near natural habitats “green” were more frequent than “bronze” (p = 0.004) ones (Figure 4A).
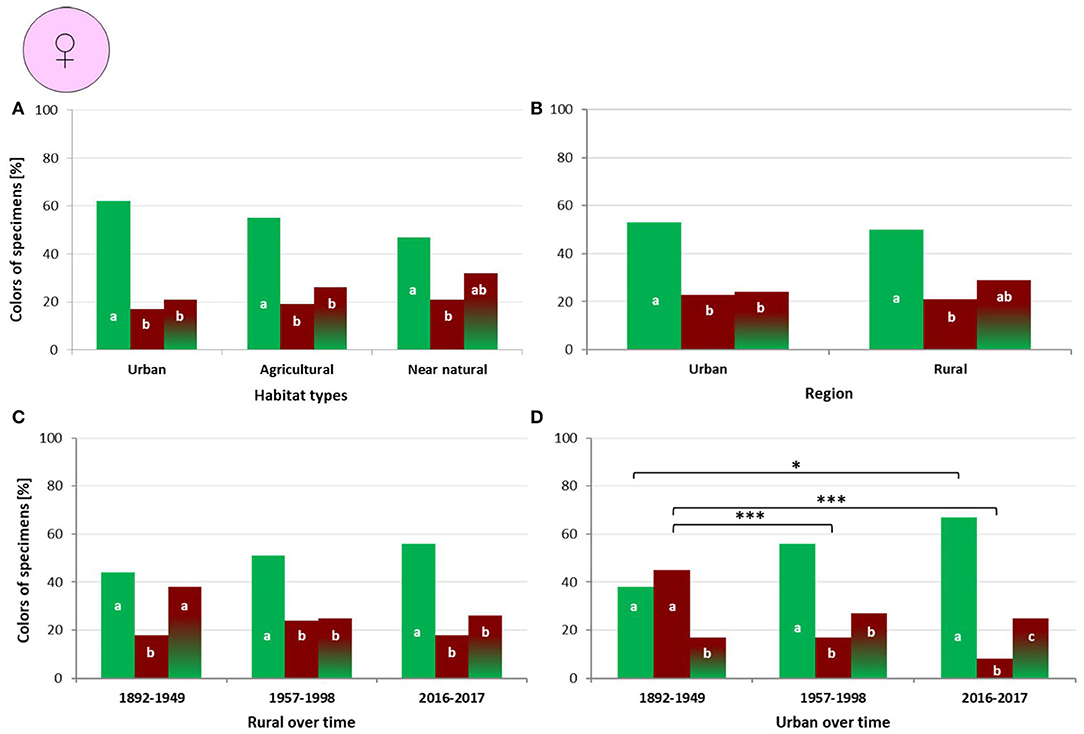
Figure 4. Percentage of female Harpalus affinis color morphs in different habitat types (A), regions with different urbanization level (B), and within a particular region during different time periods (C,D); different letters indicate significant differences in frequencies of the three color morphs; brackets with stars indicate significant difference between time periods (*p < 0.05; ***p < 0.01).
We observed no difference in female coloration between urban and rural regions (G = 0.65073, df = 2, p = 0.722). In urban regions, “green” dominated over “bronze” (p = 0.002) and “mixed” (p = 0.003) ones. In rural regions frequencies of “green” were higher than “bronze” (p = 0.001) ones (Figure 4B).
We found no changes in female coloration in rural regions over time (G = 6.0192, df = 4, p = 0.198). From 1892 to 1949 “green” (p = 0.002) and “mixed” (p = 0.021) dominated over “bronze” ones. From 1957 to 1998 “green” dominated over “bronze” (p = 0.005) and “mixed” (p = 0.008) ones. From 2016 to 2017 “green” were more frequent than “bronze” (p < 0.001) and “mixed” (p = 0.002) ones (Figure 4C).
In contrast, we observed significant differences in female coloration in urban regions over time (G = 42.03, df = 4, p < 0.001). This concerned comparisons between time periods from 1892 to 1949 and from 1957 to 1998 (p < 0.001), and from 1892 to 1949 and from 2016 to 2017 (p < 0.001). “Green” were more frequent from 2016 to 2017 than from 1892 to 1949 (p < 0.013). “Mixed” color frequencies remained constant across the three time periods. “Bronze” colors were more frequent from 1892 to 1949 than from 1957 to 1998 (p < 0.001) and from 2016 to 2017 (p < 0.001), but did not differ between 1957–1998 and 2016–2017 (Figure 4D).
In males we observed no coloration differences between urban, agricultural and near natural habitats (G = 7.532, df = 4, p = 0.110). However, in urban habitats “green” were more abundant than “bronze” (p < 0.001) and “mixed” (p < 0.001), and “bronze” were more frequent than “mixed” (p < 0.001) ones. In agricultural habitats the frequencies of “green” and “bronze” did not differ, but “green” (p < 0.001) and “bronze” (p < 0.001) were more abundant than “mixed” ones. In near natural habitats “green” were more frequent than “bronze” (p < 0.001) and “mixed” (p < 0.001), and “bronze” were more frequent than “mixed” (p = 0.001) ones (Figure 5A).
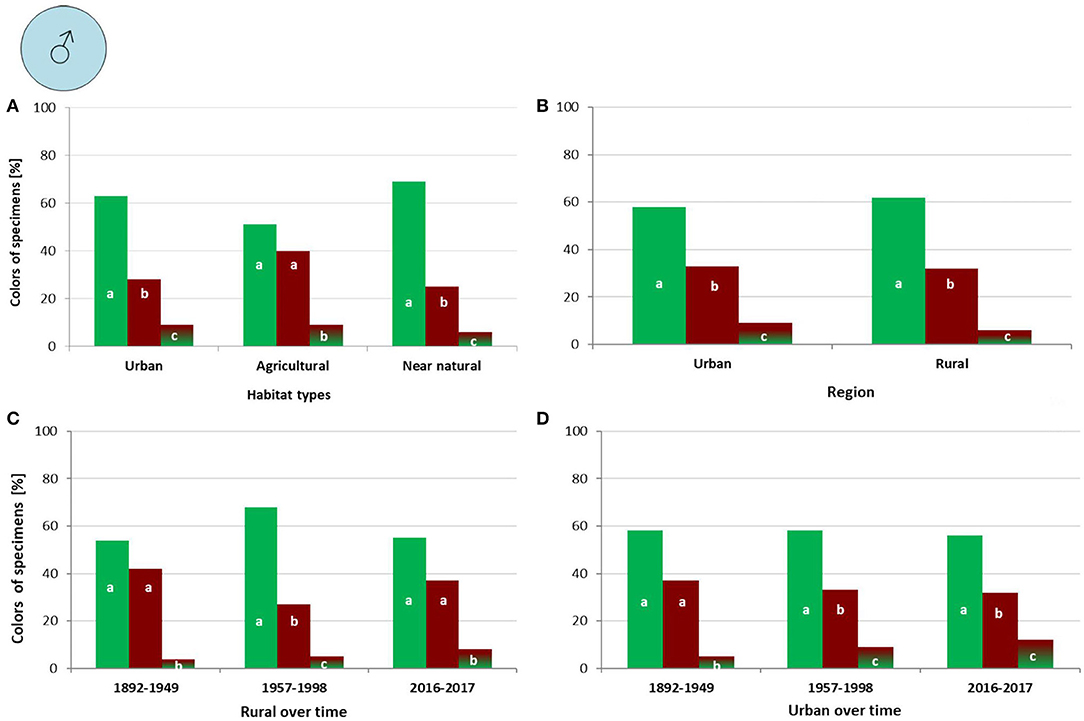
Figure 5. Percentage of male Harpalus affinis color morphs in different habitat types (A), regions with different urbanization level (B), and within a particular region during different time periods (C,D); different letters indicate significant differences in frequencies of the three color morphs.
We observed no differences in male color morph composition between urban and rural regions (G = 0.98696, df = 2, p = 0.912). In urban regions “green” males were more frequent than “bronze” (p = 0.025) and “mixed” (p < 0.001) ones and “bronze” were more frequent than “mixed” (p < 0.001) ones. In rural regions the frequencies of “green” was higher than “bronze” (p = 0.005) and “mixed” (p < 0.001); and “bronze” were more frequent than “mixed” (p < 0.001) ones (Figure 5B).
No changes were found in male coloration in rural regions over time (G = 6.9052, df = 4, p = 0.141). From 1892 to 1949 frequencies of “green” and “bronze” did not differ, but both morphs were more frequent than “mixed” (p < 0.001) ones. From 1957 to 1998 “green” were more frequent than “bronze” (p < 0.001) and “mixed” (p < 0.001), and “bronze” were more frequent than “mixed” (p < 0.001) ones. From 2016 to 2017 the frequencies of “green” and “bronze” did not differ, but both morphs were more frequent than “mixed” (p < 0.001) ones (Figure 5C).
We further observed no changes in male coloration in urban regions over time (G = 3.4427, df = 4, p = 0.487). From 1892 to 1949 the frequencies of “green” and “bronze” did not differ, but both were more frequent than “mixed” (p < 0.001) ones. From 1957 to 1998 “green” dominated over “bronze” (p = 0.025) and “mixed” (p < 0.001), and “bronze” were more frequent than “mixed” (p < 0.001). From 2016 to 2017 “green” dominated over “bronze” (p = 0.030) and “mixed” (p < 0.001), and “bronze” dominated over “mixed” (p < 0.001) ones (Figure 5D).
In summary frequencies of color morphs were different between sexes. The most frequent male color morph in males was “green,” followed by “bronze,” and “mixed.” This pattern of color morph frequency was similar in every habitat, region and regions over time. In females the most frequent color morph was “green” as well, followed by “mixed” and “bronze.” This applied to every habitat, region and in rural regions over time. However, in urban regions over time, frequencies of “bronze” morphs proportionally decreased, whereas the “green” morph increased over time.
Environmental alterations can lead to trait changes in species persisting in their altered habitats, and these changes can be observed across space and time (Van't Hof et al., 2011; Doudna and Danielson, 2015). Our study focused on changes in frequencies of color morphs in the diurnal ground beetle species Harpalus affinis in the Berlin-Brandenburg area, Germany, an area experiencing increasing urbanization and agriculture intensification during the last 125 years, with heavy air pollution in the early twentieth century due to industrialization, but with decreasing levels of air pollution after the Second World War (UBA, 1998). Generally, we observed that H. affinis displayed sexual color dimorphism. In females the frequencies of color morphs changed in urban regions over time, resulting in a decrease of “bronze,” and an increase of “green” color morphs whereas color frequencies remained nearly constant in rural areas over time as well as between regions and habitats. In contrast, males were displaying no differences or changes in their color frequencies, neither in urban and rural regions over time, nor between regions and habitats. In general “green” was the main color morph in H. affinis. It was the most frequent color morph in males in every observed habitat, region and regions over time as well as in females in habitats, regions, and rural regions over time. Generally, “green” is the most common multilayer color in beetles and has been hypothesized to have an important function in crypsis, e.g., to match substrates (Crowson, 1981; Parker, 1998). “Green” color seems to be the most advantageous color in a variety of habitats.
Although, “green” was shown to be the main color morph in H. affinis, in our first time period from 1892 to 1949, females in the city were more frequently “bronze” than “green.” In the later time periods, 1957–1998 and 2016–2017, the frequencies of “bronze” decreased, whereas frequencies of “green” increased, becoming the most frequent color morph, as observed in males and rural females. The most likely explanation of this early urban deviation from the predominating color frequencies is habitat alternation in that time. The time from 1892 to 1949 was influenced by the effects of industrial revolution and the city of Berlin was affected by a high level of air pollution, especially by soot (Wey, 1982). In addition, Berlin rapidly expanded its urban space during that time period, resulting in rapid construction of buildings and streets (Buesch and Haus, 1987).
These changes might have resulted in natural selection for “bronze” color morphs. The selective advantage for a species to be polychromatic lies in the ability to be cryptic in different habitats: “Green” morphs in green environments are less visible for predators than other color morphs, living in the same habitat (Thiele, 1977).
However, in less green habitats like heath lands (Thiele, 1977) or habitats with less vegetation like urban environments, “bronze” color morphs may be better camouflaged than “green” ones. Birds are the main predators of ground beetles (Larochelle, 1980) and due to their excellent visual capabilities (Goldsmith, 2006) able to distinguish between both of the color morphs. This may have led to a selection for the “bronze,” the least conspicuous, color morph in such soot polluted urban and vegetation-sparse habitats. Here the “bronze” beetles presumably were experiencing less predation pressure than “green” specimens (Thiele, 1977; Endler, 1988).
During the second time period, 1957–1998, first initiatives concerning environmental protection started (Pamme, 2003), and air pollution due to industrial soot commenced to decrease (UNEP/WHO, 1993; UBA, 1998), a process that continued until the most recent study period, 2016–2017 (SenStadtWohn, 2018). With decreasing pollution, “bronze” color morphs could have become again more conspicuous than the “green” morphs, the frequencies of both morphs consequently reversing over time. Such fast changes in color frequencies due to predation pressure are well known in insect populations. Prominent examples concern environmental melanism (Harris, 1988), in the Two-spot Ladybird Adila bipunctata (Lusis, 1962; Creed, 1966), or the Peppered Moth, Biston betularia, the latter being a classic case of industrial melanism with reversions to whitish gray morphs, following reduction of air pollution (Kettlewell, 1955, 1956; Clarke and Sheppard, 1966; Bishop, 1972). Industrial melanism in polluted urban areas was observed in the twenties in England (Harrison and Garrett, 1926; Mokyr, 2010), a time period similar to our first time period and thus backing our interpretation of the predominant “bronze” colors in female beetles during that time.
In contrast, “green” remained the most frequent color morph in our first time period in rural regions, presumably less affected by construction works and pollution (Wey, 1982) so that “bronze” color morphs stayed more conspicuous and therefore more prone to predation in these regions, predominantly consisting of agricultural and near natural environments. Similar observations of color change in heavily air polluted regions only, changes being absent in less polluted regions, have been reported for Biston betularia by Bishop (1972). These findings are underlining our assumption that deviation in color frequencies in the first time period could be resulted due to effects of industrialization. Unfortunately, we do not have vouchers of H. affinis, predating 1892 in order to test if females of the Berlin population had been predominately “green” prior to urbanization. The dominance of that color morph in all other time periods and habitats however, is a strong argument for that assumption.
In contrast to females, we did not observe any habitat or time related changes in color frequencies in males, “green” always being the dominant color morphs. Although ecological and behavioral observations in beetles are comparatively rarely published (Seago et al., 2009), intraspecific differences in coloration suggests their potential role in intraspecific communication, like sexual signaling. Osawa and Nishida (1991) show that male elytral color is an important factor in female mate selection in the ladybird beetle Harmonia axyridis. Likewise, Arrow (1951) and Vulinec (1997) suggested that the iridescent surface of particular dung beetle males is preferred by females. Sexual selection by female choice could also be the reason for male H. affinis to remain predominantly “green,” even in a polluted environment, if females more likely choose “green” males for mating. Then despite a higher predation risk males should remain “green” even in times with high levels of air pollution. Such tradeoff between mating success and survival is also described by Nokelainen et al. (2012) for the Wood Tiger Moth, Parasemia plantaginis: Here yellow color morphs provide better protection from predators due to aposematic coloration than the white morph. However, white males have higher mating success. In some birds, coloration has shown to be a signal of male quality (Hamilton and Zuk, 1982; Hill, 1991), and female mate choice is hypothesized to be an important selective driver toward brilliant colors in males (Darwin, 1874; Andersson, 1994; Hill, 2006). Similarly, other traits which should be eliminated by natural selection, are often favored by females' selection, like huge antlers or horns in various mammals or huge and elongated trails in peacocks (Darwin, 1874).
Due to limitation of the dataset and the sexual dichromatism found in H. affinis, specimens had to be divided into sexes before observing differences and changes in space and over time. This made sample sizes lower and influenced robustness of our findings. Alternative explanations for sex-specific differences in color adaptability in changing environments could be differences in behavior, different food preferences or living in different habitats. However, we have no hints that any of such differences exist and sexual dichromatism, found in H. affinis, is addition underlining our theory that sexual selection is the main driver of this effect.
In our study we could show a sexual dichromatism in H. affinis and we observed different, sex specific drivers for selection on color morphs. The appearance of “bronze” colored females during times of rapid urbanization and heavy environmental pollution is most likely due to natural selection. In contrast, males maintained their predominant “green” morph, presumably due to sexual selection. Further we could show that structural coloration is a useful trait for testing trait changes in species persisted in altered environments over space and time. Our results suggest that structural colorations may be able to change and reverse with changing environmental conditions within the relatively short time frame of 125 years or even less.
All datasets generated for this study are included in the article/Supplementary Material.
SK, M-OR, JF, JM, and FM contributed conception and design of the study. JF organized the database. SK performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was funded by the German Federal Ministry of Education and Research BMBF within the Collaborative Project Bridging in Biodiversity Science—BIBS (funding number: 01LC1501A-H).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank S. Buchholz (Technische Universität, Berlin); R. Platen (Leibniz-Centre for Agricultural Landscape Research, Müncheberg), and L. Schaub (University of Potsdam) for supplying voucher specimens. We further thank B. Jäger for support while working in the collection and B. Schurian (both Museum für Naturkunde, Berlin) for introducing to the Leica Z16 system, Leica Suite and Helicon Focus software. We further thank two reviewers for their constructive criticism. The permission for sampling invertebrates in Brandenburg was issued by Landesamt für Umwelt, Abteilung Naturschutz, city of Potsdam. The permission for sampling invertebrates in Berlin was issued by Senatsverwaltung für Umwelt, Verkehr und Klimaschutz, city of Berlin.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00525/full#supplementary-material
Anjum-Zubair, M., Entling, M. H., Bruckner, A., Drapela, T., and Thomas, F. (2015). Differences of spring carabid beetle assemblages between semi-natural and adjoining winter wheat. Agric. For. Entomol. 17, 355–365. doi: 10.1111/afe.12115
Antrop, M. (2000). Changing patterns in the urbanized countryside of Western Europe. Landsc. Ecol. 15, 257–270. doi: 10.10/A:1008151109252
Antrop, M. (2004). Why landscapes of the past are important for the future. Landsc. Urban Plan. 70, 21–34. doi: 10.1016/j.landurbplan.2003.10.002
Arrow, G. J. (1951). Horned beetles: a study of the fantastic in nature. The Hague: W. Junk, Publishers.
Atkinson, D. (1994). Temperature and organism size: a biological law for ectotherms. Adv. Ecol. Res. 25, 1–58. doi: 10.1016/S0065-2504(08)60212-3
Bairoch, P., and Goertz, G. (1986). Factors of urbanisation in the nineteenth century developed countries: a descriptive and econometric analysis. Urban Stud. 23, 285–305. doi: 10.1080/00420988620080351
Bishop, J. A. (1972). An experimental study of the cline of industrial melanism in Biston betularia (L.) (Lepidoptera) between urban Liverpool and rural North Wales. J. Anim. Ecol. 41, 209–243. doi: 10.2307/3513
Brunk, I., and Wiegleb, G. (2006). Laufkäfer gestörter Landschaften der Niederlausitz-Bergbaufolgelandschaften. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 15, 379–382.
Buesch, O., and Haus, W. (1987). Berlin als Hauptstadt der Weimarer Republik 1919–1933. New York, NY: Walter de Gruyter & Co. Berlin.
Cahan, S. H., Parker, D. J., Rissing, W. S., Johnson, A. R., Polony, S. T., Weiser, et al. (2002). Extreme genetic differences between queens and workers in hybridizing Pogonomyrmex harvester ants. Proc. R. Soc. Lond. 269, 1971–1877. doi: 10.1098/rspb.2002.2061
Clarke, C. A., and Sheppard, M. P. (1966). A local survey of the distribution of industrial melanic forms of the moths Biston betularia and estimates of the selective value of these in an industrial environment. Proc. R. Soc. B. 165, 424–439. doi: 10.1098/rspb.1966.0075
Cochrane, A., and Jonas, A. (1999). Reimagining Berlin: World city, national capital or ordinary place? Eur. Urban Reg. Stud. 6, 145–164. doi: 10.1177/096977649900600204
Creed, E. R. (1966). Geographic variation in the Two-spot Ladybird in England and Wales. Heredity 21, 57–72. doi: 10.1038/hdy.1966.4
Darwin, C. (1874). The Descent of Man and Selection in Relation to Sex. 2nd Edn. London: John Murray.
Deichsel, R. (2006). Species change in an urban setting - ground and rove beetles (Coleoptera: Carabidae and Staphylinidae) in Berlin. Urban Ecosyst. 9, 161–178. doi: 10.1007/s11252-006-8588-3
Doucet, S. M., and Hill, E. G. (2009). Do museum specimens accurately represent wild birds? A case study of carotenoid, melanin, and structural colours in long-tailed manakins Chiroxiphia linearis. J. Avian Biol. 40, 146–156. doi: 10.1111/j.1600-048X.2009.03763.x
Doudna, J. W., and Danielson, J. B. (2015). Rapid morphological change in the masticatory structures of an important ecosystem service provider. PLoS ONE 10:e0127218. doi: 10.1371/journal.pone.0127218
Endler, J. A. (1984). Progressive background matching in moths, a quantitative measure of crypsis. Biol. J. Linn. Soc. 22, 187–231. doi: 10.1111/j.1095-8312.1984.tb01677.x
Endler, J. A. (1988). Frequency-dependent predation, crypsis and aposematic coloration. Philos. Trans. R. Soc. Lond. B. 319, 505–523. doi: 10.1098/rstb.1988.0062
Erisman, J. W., Sutton, A. M., Galloway, J., Klimont, Z., and Winiwarter, W. (2008). How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639. doi: 10.1038/ngeo325
Forsman, A., and Shine, R. (1995). The adaptive significance of colour pattern polymorphism in the Australian scincid lizard Lampropholis delicate. Biol. J. Linn. Soc. 55, 273–291. doi: 10.1111/j.1095-8312.1995.tb01066.x
Goldsmith, T. H. (2006). What birds see. Sci. Am. 295, 68–75. doi: 10.1038/scientificamerican0706-68
Hadley, N. F., Schultz, D. T., and Savill, A. (1988). Spectral reflectances of three subspecies of the tiger beetle Neocicindela perhispida: correlations with their respective habitat substrates. N. Z. J. Zool. 15, 343–346. doi: 10.1080/03014223.1988.10422624
Haila, Y. (2002). A conceptual genealogy of fragmentation research: from island biogeography to landscape ecology. Ecol. Appl. 12, 321–334. doi: 10.1890/1051-0761(2002)012[0321:ACGOFR]2.0.CO;2
Hamilton, D. W., and Zuk, W. (1982). Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387. doi: 10.1126/science.7123238
Harris, A. C. (1988). Cryptic colouration and melanism in the sand-burrowing beetle Chaerodes trachyscelides (Coleoptera: Tenebrionodae). J. R. Soc. N. Z. 18, 333–339. doi: 10.1080/03036758.1988.10426474
Harris, J. A., Hobbs, R. J., Higgs, E., and Aronson, J. (2006). Ecological restoration and global climate change. Restor. Ecol. 14, 170–176. doi: 10.1111/j.1526-100X.2006.00136.x
Harrison, J. W. H., and Garrett, F. C. (1926). The induction of melanism in the Lepidoptera and its subsequent inheritance. Proc. R. Soc. Lond. 99, 241–263. doi: 10.1098/rspb.1926.0012
Hervé, M. (2013). Diverse Basic Statistical and Graphical Functions (RVAide Memoire). R Package. Available online at: https://CRAN.R-project.org/package=RVAideMemoire (accessed July 4, 2017).
Hill, G. E. (1991). Plumage coloration is a sexually selected indicator of male quality. Nature 350, 337–339. doi: 10.1038/350337a0
Hill, G. E. (2006). “Female choice for ornamental coloration,” in Bird Coloration, Function and Evolution, Vol. 2, eds G. E. Hill and K. J. McGraw. Cambridge, MA: Harvard University Press.
Hobbs, R. J., Higgs, S. E., and Hall, C. (2013). Novel Ecosystems: Intervening in the New Ecological World Order. New York, NY: Wiley-Blackwell. doi: 10.1002/9781118354186
Jeltsch, F., Blaum, N., Brose, U., Chipperfield, D. J., Clough, Y., Farwig, N., et al. (2013). How can we bring together empiricists and modellers in functional biodiversity research? Basic Appl. Ecol. 14, 93–101. doi: 10.1016/j.baae.2013.01.001
Kenis, M., Auger-Rozenberg, A. M., Roques, A., and Timms, L. L. (2009). Ecological effects of invasive alien insects. Biol. Invasions 11, 21–45. doi: 10.1007/s10530-008-9318-y
Kettlewell, H. B. D. (1955). Selection experiments on industrial melanism in the Lepidoptera. Heredity 9, 323–342. doi: 10.1038/hdy.1955.36
Kettlewell, H. B. D. (1956). Further selection experiments on industrial melanism in the Lepidoptera. Heredity 10, 287–301. doi: 10.1038/hdy.1956.28
Kinoshita, S., Yoshioka, S., and Miyazaki, J. (2008). Physics of structural colors. Rep. Prog. Phys. 71, 1–30. doi: 10.1088/0034-4885/71/7/076401
Kraetke, S. (2000). Berlin: the metropolis as a production space. Eur. Plan. Stud. 8, 7–27. doi: 10.1080/096543100110901
Land, M. F. (1972). The physics and biology of animal reflectors. Progr. Biophys. Mol. Biol. 24, 75–106. doi: 10.1016/0079-6107(72)90004-1
Larochelle, A. (1980). A list of birds of Europe and Asia as predators of carabid beetles including Cicindelini (Coleoptera: Carabidae). Cordulia 6, 1–19.
Loevei, G. L., and McCambridge, M. (2002). Adult mortality and minimum lifespan of the ground beetle Harpalus affinis (Coleoptera: Carabidae) in New Zealand. N. Z. J. Zool. 29, 1–4. doi: 10.1080/03014223.2002.9518283
Lusis, J. J. (1962). On the biological meaning of colour polymorphism of lady beetle Adalia bipunctata L. Izv. Byuro Genet. Leningrad 6, 89–163.
Maas, S., Detzel, P., and Staudt, A. (2002). Gefährdungsanalyse der Heuschrecken Deutschlands - Verbreitungsatlas, Gefährdungseinstufung und Schutzkonzepte. Münster-Hiltru: Bundesamt für Naturschutz; Landwirtschaftsverlag.
McCabe, J., and Patridge, L. (1997). An interaction between environmental temperature and genetic variation for body size for the fitness of adult female Drosophila melanogaster. Evolution 51, 1164–1174. doi: 10.1111/j.1558-5646.1997.tb03964.x
Mokyr, J. (2010). The Enlightened Economy an Economic History of Britain 1700–1850. New Haven, CT: Yale University Press.
Nokelainen, O., Hegna, H. R., Reudler, J. H., Lindstedt, C., and Mappes, J. (2012). Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc. R. Soc. B. 279, 257–265. doi: 10.1098/rspb.2011.0880
Noyes, J. A., Vukusic, P., and Hooper, I. R. (2007). Experimental method for reliably establishing the refractive index of buprestid beetle exocuticle. Opt. Express 15, 4351–4358. doi: 10.1364/OE.15.004351
Osawa, N., and Nishida, T. (1991). Seasonal variation in elytral colour polymorphism in Harmonia axyridis (the ladybird beetle): the role of non-random mating. Heredity 69, 297–307. doi: 10.1038/hdy.1992.129
Palkovacs, E. P., Kinnison, T. M., Correa, C., Dalton, M. C., and Hendry, P. A. (2011). Fates beyond traits: ecological consequences of human-induced trait change. Evol. Appl. 5, 183–191. doi: 10.1111/j.1752-4571.2011.00212.x
Palmer, R. A., and Strobeck, C. (1992). Fluctuating asymmetry as a measure of developmental stability: Implications of non-normal distributions and power of statistical traits. Acta Zool. Fenn. 191, 57–72.
Pamme, H. (2003). “Das Politikfeld Umweltpolitik,” in Verwaltungshandeln in Politikfeldern, ed D. Grunow (Opladen: Leske and Budrich), 185–224.
Parker, A. (1998). The diversity and implications of animal structural colours. J. Exp. Biol. 201, 2343–2347.
Parker, A. R., and McKenzie, D. (2003). The cause of 50 million year-old colour. Proc. R. Soc. B. 270, 151–153. doi: 10.1098/rsbl.2003.0055
R Core Team (2017). R: A Language and Environment Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org/ (accessed July 4, 2018).
Ribbe, W., Bohm, E., Schich, W., and Schulz, K. (2002a). Geschichte Berlins. Band 1: Von der Frühgeschichte bis zur Industrialisierung. München: Beck.
Ribbe, W., Bohm, E., Schich, W., and Schulz, K. (2002b). Geschichte Berlins. Band 2: Von der Märzrevolution bis zur Gegenwart. München: Beck.
Ribera, I., Dolédec, S., Dowmie, S. I., and Foster, N. G. (2001). Effects of land disturbance and stress on species traits of ground beetle assemblages. Ecology 82, 1112–1129. doi: 10.1890/0012-9658(2001)082[1112:EOLDAS]2.0.CO;2
Ricciardi, A. (2007). Are modern biological invasions an unprecedented form of global change? Conserv. Biol. 21, 329–336. doi: 10.1111/j.1523-1739.2006.00615.x
Rocha, L. A., Aleixo, A., Allen, G., Almeda, F., Baldwin, C. C., Barclay, L. V. M., et al. (2014). Specimen collection: an essential tool. Science 344, 814–815. doi: 10.1126/science.344.6186.814
Root, T. L., and Schneider, H. S. (2006). Conservation and climate change: the challenges ahead. Conserv. Biol. 20, 706–708. doi: 10.1111/j.1523-1739.2006.00465.x
Schildt, A., and Sywottek, A. (1993). Modernisierung im Wiederaufbau. - Die westdeutsche Gesellschafft der 50er Jahre. Bonn: Verlag J. H. W. Dietz.
Schultz, T. D., and Rankin, A. M. (1985). Developmental changes in the interference reflectors and colorations of tiger beetles (Cicindela). J. Exp. Biol. 117, 111–118.
Seago, A. E., Brady, P., Vigneron, J., and Schultz, D. (2009). Gold bugs and beyond: a review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Sock. Interface 6, 165–184. doi: 10.1098/rsif.2008.0354.focus
SenStadtWohn (Senatsverwaltung für Stadtentwicklung und Wohnen Berlin) (2018). Langjährige Entwicklung der Luftqualität. Available online at: https://www.stadtentwicklung.berlin.de/umwelt/umweltatlas/d312_03.htm (accessed September18, 2019).
Storfer, A., Cross, J., Rush, V., and Caruso, J. (1999). Adaptive coloration and gene flow as a constraint to local adaptation in the streamside salamander, Ambystoma barbouri. Evolution 53, 889–898. doi: 10.1111/j.1558-5646.1999.tb05383.x
Sunderland, K. D., Loevei, L. G., and Fenlon, J. (1995). Diets and reproductive phenologies of the introduced ground beetles Harpalus affinis and Cilvina australasiae (Coleoptera: Carabidae) in New Zealand. Aust. J. Zool. 43, 39–50. doi: 10.1071/ZO9950039
Sustek, Z. (1992). Changes in representation of carabid life forms along an urbanization gradient (Coleoptera, Carabidae). Biol. Brat. 47, 417–430.
Thiele, H.-U. (1977). Carabid Beetles in Their Environments. Berlin; Heidelberg; New York: Springer.
Tilman, D., Cassman, K. G., Matson, A. P., Naylor, R., and Polasky, S. (2002). Agricultural sustainability and intensive production practices. Nature 418, 671–677. doi: 10.1038/nature01014
Townsend, J. I. (1992). Harpalus affinis (Schrank) (Coleoptera: Carabidae) recently established in the North Island of New Zealand. N. Z. Entomol. 15, 25–29. doi: 10.1080/00779962.1992.9722624
Tyler, G. (2010). Variability in colour, metallic luster, and body size of Carabus arvensis Herbst, 1784 (Coleoptera: Carabidae) in relation to habitat properties. Entomol. Fenn. 21, 90–96. doi: 10.33338/ef.84514
United Nations (2002). World Population Prospects: The 2001 Revision. Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat.
Van't Hof, A., Edmonds, E., Dalikova, M., Marec, F., and Saccheri, J. I. (2011). Industrial melanism in British Peppered Moths has a singular and recent mutational origin. Science. 332, 958–960. doi: 10.1126/science.1203043
Vulinec, K. (1997). Iridescent dung beetles: a different angle. Fla. Entomol. 80, 132–141. doi: 10.2307/3495550
Wey, K.-G. (1982). Umweltpolitik in Deutschland. Kurze Geschichte des Umweltschutzes in Deutschland seit 1900. Opladen: Westdeutscher Verlag.
Wrase, D. W. (2004). “Harpalina,” in Die Käfer Mitteleuropas: Adephaga 1: Carabidae (Laufkäfer), eds H. Freude, K. W. Harde, G. A. Lohse, B. Klausberger, and G. Müller-Motzfeld (Heidelberg; Berlin: Spektrum-Verlag).
Keywords: color, urbanization, novel ecosystems, museum collection, ground beetle, Harpalus affinis
Citation: Keinath S, Frisch J, Müller J, Mayer F and Rödel M-O (2020) Spatio-Temporal Color Differences Between Urban and Rural Populations of a Ground Beetle During the Last 100 Years. Front. Ecol. Evol. 7:525. doi: 10.3389/fevo.2019.00525
Received: 04 October 2019; Accepted: 31 December 2019;
Published: 23 January 2020.
Edited by:
Stephanie Ann Yarwood, University of Maryland, College Park, United StatesReviewed by:
Melissa Lin Grunst, University of Antwerp, BelgiumCopyright © 2020 Keinath, Frisch, Müller, Mayer and Rödel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Keinath, c2lsdmlhLmtlaW5hdGhAbWZuLmJlcmxpbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.