
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 05 November 2019
Sec. Behavioral and Evolutionary Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00419
This article is part of the Research TopicThe Next Step: Disentangling the Role of Plant-Soil Feedbacks in Plant Performance and Species Coexistence Under Natural ConditionsView all 15 articles
The plant-soil feedback framework allows researchers to target the interaction of plants and root-associated microbes and to determine its interplay on plant-plant interactions. Plant-soil feedbacks in terrestrial ecology are well-documented, but the strength and direction of feedbacks as influenced by abiotic environmental factors, such as temperature and soil moisture, has not been fully explored. In our study, we examined plant-soil feedback responses of both cool- and warm-season native and non-native grasses to elevated temperatures (ambient and +5°C) and soil moisture (100 and 75% field capacity). In a previous experiment, grasses were grown under temperature and soil moisture conditions similar to our current study. The resultant trained soil communities served as the inoculum sources for our current experiment. We found that consistent training and experimental temperatures resulted in negative PSF, where plants produced greater biomass in soils conditioned by heterospecifics. However, the direction of PSF was reversed when training and experimental conditions were mismatched. That is, when training and experimental temperatures mirrored one another, negative PSF occurred, suggesting coexistence between the two species is likely under these conditions. However, when only training or testing temperatures were elevated, positive PSF were detected, favoring the non-native species. These alterations in plant-soil feedbacks were relatively consistent across pairings of warm- and cool-season grasses. Overall, our results indicate inconsistent year-to-year environmental conditions, such as extreme temperatures, may undermine the stabilizing forces of negative PSF and favor of non-native grasses.
Interactions between plants and their associated soils play an important role in the formation of plant communities and maintenance of biodiversity (Mangan et al., 2010; Bauer et al., 2015; Bever et al., 2015). Plants affect the biotic and abiotic conditions of associated soils, with subsequent reciprocal interactions known as plant-soil feedbacks (PSFs) (Kulmatiski et al., 2008; Bever et al., 2010). These feedbacks range from positive to negative, and the direction of the feedback is largely driven by the presence and abundance of certain soil biota. Negative PSFs occur when a plant performs better in soils conditioned by heterospecifics, thus promoting community diversity, whereas positive PSFs are created when a plant's growth is increased in conspecific-conditioned soil, often resulting in monotypic stands (Bever et al., 1997; van der Putten et al., 2013). Generally, PSFs are negative, particularly between native plant species and species that are phylogenetically unrelated (Meiners et al., 2017; Crawford et al., 2019), suggesting that soil microbes are likely to contribute to coexistence of native and phylogenetically diverse plant species. In recent years, the role of PSFs in the success of non-native invasive plant species has been the focus of a growing body of research (Inderjit and van der Putten, 2010; van der Putten et al., 2013; Kulmatiski, 2018).
Previous research has shown the presence of invasive plant species can alter the density and composition of arbuscular mycorrhizal (AM) fungal communities, which may influence the feedback interactions that affect subsequent growth and establishment of both native and invasive species (Reinhart and Callaway, 2006; Vogelsang and Bever, 2009; Allen et al., 2018). Plant invasion can disrupt mutualistic interactions between native plants and soil microbial communities, further increasing ecosystem susceptibility to invasion. Past and current empirical studies suggest that invasive plants often create positive PSFs, thus suppressing native biodiversity and promoting growth of conspecifics (Reinhart and Callaway, 2006; Crawford and Knight, 2017; Crawford et al., 2019). This can occur through a number of mechanisms. Non-native plant species are often less dependent on native AM fungi, compared to native species, decreasing AM fungal densities with a concomitant decrease of native plant growth rates (Pringle et al., 2009; Vogelsang and Bever, 2009; Zubek et al., 2016; Grove et al., 2017). Alternatively, invasive species can be highly dependent on AM fungal associations, yet alter local soil microbial community composition, resulting in a loss of native plant growth and survival (Wilson et al., 2012; Zubek et al., 2016; Ba et al., 2018; Zhang et al., 2019).
Current climate models predict warmer, drier conditions across much of North America (IPCC, 2014), and these conditions, coupled with continued pressure by invasive plant species, will likely exacerbate losses of native biodiversity. Alterations in climatic conditions will also likely have dramatic effects on the interactions of plants, their associated soil microbiota, and subsequent strength and direction of PSF, as soil microbes can mediate plant responses to drought (Kivlin et al., 2013; Xi et al., 2018). Warmer, drier conditions have been shown to reduce AM biomass (Duell et al., 2016; Yu et al., 2018), which likely alter PSFs. Drought has been shown to shift PSF in co-existing plants from negative or positive to neutral, depending on the species, suggesting that drought may neutralize PSF (Heinze et al., 2017; Fry et al., 2018), and these alterations in PSFs may be persistent (Kaisermann et al., 2017). While drought and invasive species individually alter the strength and direction of PSFs, there has been very little research linking the two and assessing the coupled effects on native plant growth.
Plant-microbe interactions can also mediate plant adaptation to perturbations in climate. Mycorrhizal fungi, for example, can increase drought tolerance of their host (Delavaux et al., 2017; Bowles et al., 2018). Even for plants that do not associate with mycorrhizal fungi such as mustards, changes in their microbiome can mediate tolerance to drought. For example, Lau and Lennon (2011) found that plants that were associated with more diverse microbial communities and subjected to drought exhibited greater growth and changes in phenological traits, compared to plants grown with less diverse microbial communities. Additional work by Lau and Lennon (2012) suggests that plant productivity and fitness is greatest when previous and contemporary environmental conditions were similar, as opposed to mismatched conditions. This suggests that plants may benefit from soil microbial communities trained under particular environmental conditions, and that variation in climate and weather patterns will affect certain species' abilities to persist.
To assess potential effects of climate change on PSF dynamics and the potential for microbes to mediate plant response to changing climate, a greenhouse experiment was conducted to: (1) assess the strength and direction of native and non-native grass PSFs under ambient conditions (well-watered and moderate temperatures) and (2) examine the strength and direction of native and non-native grass PSFs under projected climate scenarios (drought conditions and elevated temperatures). Based on results from Duell et al. (2016), we hypothesized 1a) soil microbial alterations resulting from non-native species will result in positive PSF under ambient conditions and 1b) soil microbial alterations resulting from native species will result in negative PSF under ambient conditions. Further, we hypothesized (2) PSFs of both non-native and native species will be exacerbated under elevated temperatures and drought conditions, relative to ambient conditions. Finally, we hypothesized (3) plants will perform best when grown in soils of matching environments as their current environment.
In our experiment, we used paired native and non-native warm- and cool-season perennial grasses to test the effects of soil moisture and temperature on PSF dynamics of these species. Schizachyrium scoparium (Michx.) Nash is a native, warm-season perennial bunchgrass found throughout the North America, especially in temperate, grass-dominated ecosystems. Bothriochloa ischaemum (L.) Keng is a non-native, warm-season perennial grass found throughout southern and central North American grasslands. Native to Europe, Asia, and northern Africa, B. ischaemum was introduced into North American grasslands in the early 1900's as a fast-growing livestock forage (Celarier and Harlan, 1955). Pascopyrum smithii (Rydb.) Á Löve is a native, cool-season perennial grass found throughout the Great Plains region of North America, and is a dominant component of northern grassland plant communities. Bromus inermis Leyss. is a non-native, cool-season perennial grass that can be found throughout North America. B. inermis was introduced into North America in the late 1800's from Eurasia as livestock forage and for its role in soil stabilization on degraded landscapes (Larson et al., 2001). Both B. ischaemum and B. inermis are widely-considered as invasive plants, often forming monocultures and decreasing biodiversity at many trophic levels (Hickman et al., 2006; Gabbard and Fowler, 2007; Dillemuth et al., 2009; Stotz et al., 2017).
Native tallgrass prairie soil was collected from the Konza Prairie Biological Station, Manhattan, KS, USA, where all four species used in this experiment can be commonly found. Soil was sieved through a 10 mm sieve to remove rocks and coarse plant material. Soil was steam-pasteurized at 80°C for 2 h and transported to Oklahoma State University greenhouse facilities.
To assess the consequences of alterations in soil microbial communities, including AM fungal communities, soil inoculum was collected from a previous climate perturbation experiment (Duell et al., 2016) which investigated the effects of elevated temperatures and reduced soil moisture on both native and non-native grasses. In Duell et al. (2016), we conducted two experiments, one used two warm-season grass species [one native (S. scoparium) and one invasive (B. ischaemum)] and a second that used two cool-season grass species [one native (P. smithii) and one invasive (B. inermis)] grown under two climatic regimes to “train” microbial communities for inocula. Warm-season species were maintained at ambient (24°C) and elevated (29°C) temperatures and cool-season species were grown at ambient (17°C) and elevated temperatures (22°C). Temperature treatments were combined with two levels of soil moisture [field capacity and drought (35% less than field capacity)]. Temperature and soil moisture treatments were initiated following seedling establishment. Temperatures represent the mean daily high temperature for each respective treatment for the entirety of the experiment. Once plants were established, soil moisture was monitored twice per week using the gravimetric water content of each pot. The complete experimental design that produced our inoculum consisted of 16 treatment combinations: 4 plant species × 2 temperature treatments × 2 soil moisture treatments, arranged in a complete block design with 6 replications for a total of 96 pots (Duell et al., 2016). Training phase conditions will hereafter be referred to as source conditions (e.g., source temperature).
The experimental design of feedback test experiment was based on the feedback approach described in Bever (1994). Separate feedback test experiments were conducted for the warm-season grasses and the cool-season grasses (Zaiger, 2016). Warm- and cool-season grasses were germinated in vermiculite. After 14–21 days (second-leaf stage), individual seedlings were transplanted into pots (6 cm diameter × 25 cm deep: DeePots; stuewe.com, Tangent, Oregon), filled with 600 g (dry weight) of soil partitioned into three layers: 400 g of steam-pasteurized soil [80°C for 2 h and allowed to cool for 72 h to eliminate biotic communities but retain abiotic soil traits (Hetrick et al., 1990; Wilson and Hartnett, 1997, Johnson et al., 2010)], followed by 100 g of soil inoculum (inoculum described above), followed by 100 g of steam-pasteurized soil to protect cross-contamination during the growing period. One seedling was planted per pot and inoculated with soil trained by the non-native or native grass under all combinations of temperature and soil moisture treatments in Duell et al. (2016) (described above). Each of two feedback test experiments consisted of a full factorial design with three factors (plant species, temperature, and soil moisture) each consisting of the same temperature and soil moisture treatments used in the training phase (Figure 1). In total, both the cool-season and warm-season experimental studies consisted of 392 pots [8 inocula × 2 plants × 2 temperature treatment levels × 2 soil moisture treatment levels × 6 replications + 8 sterile controls (no inoculum)], for a total of 784 pots. Sterile controls consisted of 600 g of steam-pasteurized soil. Environmental conditions tested during this phase of the experiment will hereafter be referred to as experimental conditions (e.g., experimental temperature). After 16 weeks, prior to shoot senescence, plants were harvested, and root and shoot biomass was separated. Roots were washed free of soil, and all biomass was dried at 60°C for 48 h, and weighed.
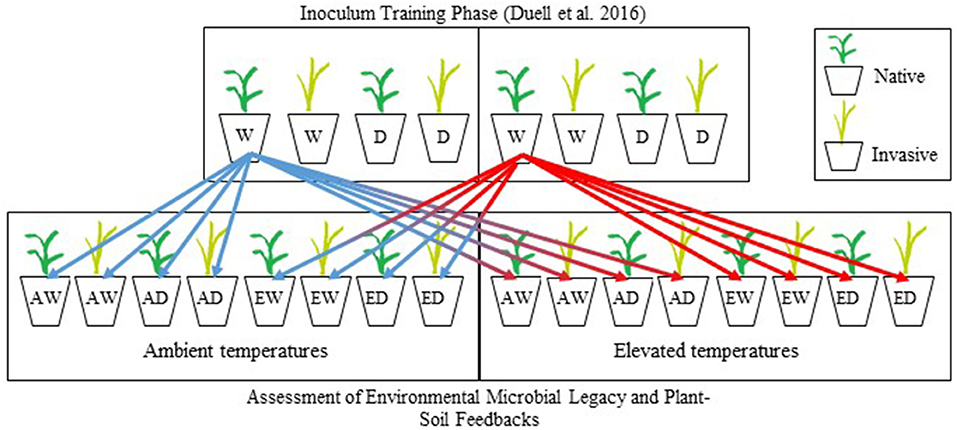
Figure 1. Schematic diagram for the experimental design of the training and experimental testing phases of this research. In the training phase, two warm-season grasses (Schizachyrium scoparium, Bothriochloa ischaemum) were grown under four combinations of soil moisture and temperature, replicated 6 times for a complete experimental design of 48 pots. Eight different soil communities conditioned in the training phase were used as inocula in the testing of PSF. The schematic shows only one paired native and invasive grass. As shown by our schematic, each soil treatment from the training phase was divided eight times and used as inoculum for native or invasive plant species of the same functional group, maintained at two soil moistures (well-watered, drought), and 2 temperatures (ambient, elevated). This experimental was replicated using cool-season grasses (Pascopyrum smithii, Bromus inermis). W and D, well-watered and drought, respectively; A and E, ambient and elevated temperatures, respectively.
Feedbacks were calculated for total biomass. Interaction coefficients were calculated to quantify PSF between native and non-native plants grown with inoculum trained by either conspecific or heterospecific plants. We used the following equation (Equation 1):Is = G(A)α − G(A)β − G(B)α + G(B)β, where Is is the feedback interaction coefficient, G(A)α is growth of plant species A inoculated with conspecific soil, G(A)β is growth of plant species A inoculated with heterospecific soil, G(B)α is growth of plant species B inoculated with heterospecific soil and, G(B)β is growth of plant species B inoculated with conspecific soil (Bever et al., 1997). When Is values are positive (Is > 0), a net positive feedback on plant growth is generated by the soil community, and coexistence between plant species does not occur. Conversely, when Isvalues are negative (Is< 0), a net negative feedback on plant growth is generated by the soil community, and coexistence between plant species does occur (Bever, 2003). Interaction coefficient values were calculated for each temperature and drought combination of both inoculum training and experimental conditions.
Using PROC-GLM in SAS, we constructed a general linear model using log-transformed (for normalization of biomass data due to extreme values caused by drought) biomass and percent colonization as the dependent variables. Species identity, drought, and temperature treatments from the inoculum source and from the feedback study (6 total) were used as factors with all possible interactions. Analyses of these experiments were split by drought treatments due to low survival of water-limited warm-season plants. In the experiment with warm-season grasses, mortality under drought prevented analysis of growth, but in the experiment with cool-season grasses, analyses of growth responses was possible under well-watered and drought conditions. Therefore, the model included total of 5 factors (experimental temperature, experimental plant identity, source temperature, source soil moisture (source water) treatment, and source plant identity) examined in the analysis. For each treatment combination, three of the six replicates were scored for mycorrhizal root colonization. Pairwise feedback was tested within the “plant_species*source_plant_species” interaction where source_plant_species represents the plant species that trained the soil (Bever, 1994). In fact, for full factorial experiments with two plant species training (source) and experimental as are ours, significance of pairwise feedback is tested directly as the “species* source species” interaction and the dependence of pairwise feedback on environmental conditions (either training or experimental conditions or their interaction) is tested directly as the interaction of “species*source species*environmental condition.” For example, a significant “species*source species*source temperature” interaction indicates that the strength of pairwise feedback depends upon the experimental temperature, while a significant “species*source species*source temperature*temperature” interaction indicates that pairwise feedback varies significantly with the interaction of source and experimental temperature. For all significant pairwise interactions between current plant 8 treatments and source treatments interaction coefficients were calculated using the formula (Equation 1, Bever et al., 1997). Differences in biomass under either soil moisture or temperature treatments with significant feedback interactions were assessed using a two-way analysis of variance (ANOVA) and Tukey's Honest Significant Difference (HSD) in R version 3.2.3 (R Core Team, 2015). Biomass and colonization were analyzed using the PROC GLM procedure in (SAS Institute, Cary, NC, U.S.A.), version 9.4 of the SAS System for Windows.
Regardless of species, plants subjected to reduced soil moisture exhibited high mortality, and therefore were removed from analyses. When subjected to well-watered experimental conditions, the main effects of plant species [F(1, 94) = 30.87, p ≤ 0.001], experimental temperature [F(1, 94) = 23.81, p ≤ 0.001], and plant species of the training phase (source species) [F(1, 94) = 9.62, p = 0.002] were significant for our warm-season grasses. The non-native species, B. ischaemum, produced greater total biomass in all environmental treatments, compared to the native species, S. scoparium (Figures 2A–D). In addition, plants grown under elevated temperatures produced greater biomass, compared to plants subjected to ambient temperatures.
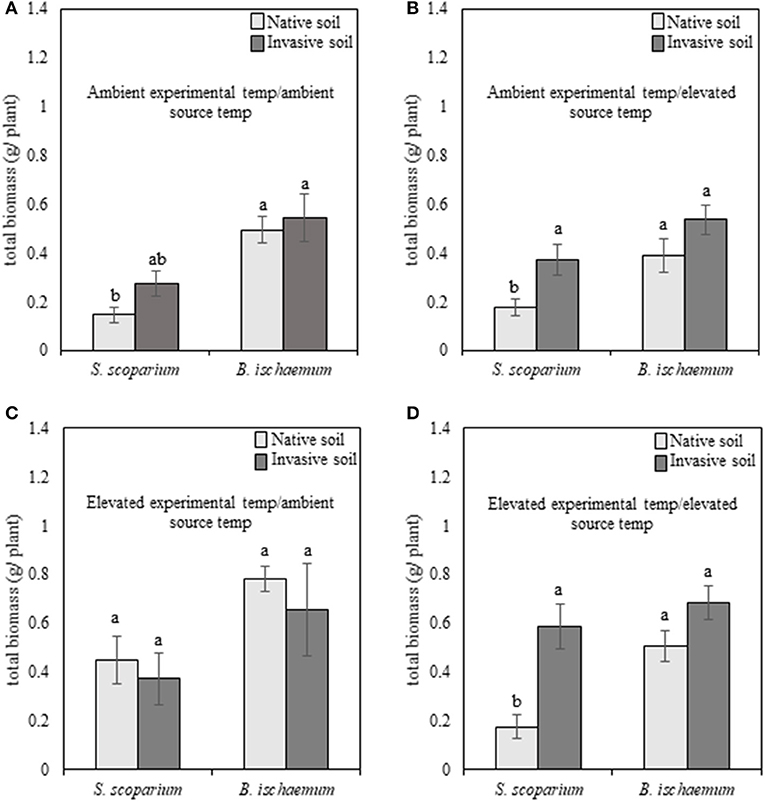
Figure 2. Total biomass production of native (Schizachyrium scoparium) and invasive (Bothriochloa ischaemum) warm-season grasses grown under well-watered conditions in response to source soil plant identity and source soil temperature. Light bars indicate inoculum derived from native Schizachyrium scoparium and dark gray bars represent inoculum derived from invasive Bothriochloa ischaemum. In each panel, pairings of bars on the left represent native S. scoparium, and bars on the right represent invasive B. ischaemum. Panels represent the following treatment combinations: (A) ambient experimental temperatures, ambient source temperatures, (B) ambient experimental temperatures, elevated source temperatures, (C) elevated experimental temperatures, ambient source temperatures, and (D) elevated experimental temperatures, elevated source temperatures. Different letters indicate significant differences within panel (p ≤ 0.05).
When subjected to well-watered experimental conditions, cool-season biomass production of native P. smithii was significantly greater as compared to invasive B. inermis [F(1, 149) = 4.89, p = 0.03]; however, no differences were found between native and non-native plant species when analyzed by combinations of experimental and source conditions (Figure 3). Overall, cool-season grasses subjected to elevated experimental temperatures produced greater total biomass, relative to individuals grown at ambient experimental temperatures [F(1, 149) = 5.31, p = 0.02]. Regardless of species, plants grown with inoculum trained under elevated temperatures produced consistently greater biomass than plants inoculated with microbes trained under ambient experimental temperatures [F(1, 149) = 5.26, p = 0.02]. Under well-watered experimental conditions none of the interactions and no feedback effects were significant (Supplementary Table 2).
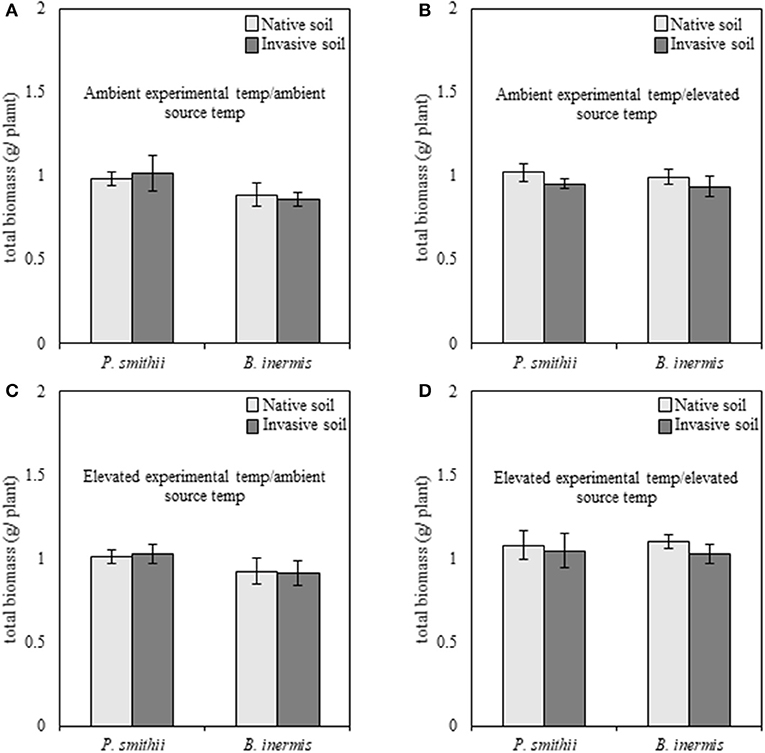
Figure 3. Total biomass production of native (Pascopyrum smithii) and invasive (Bromus inermis) cool-season grasses grown under well-watered conditions in response to source soil plant identity and source soil temperature. Light bars indicate inoculum derived from native Pascopyrum smithii and dark gray bars represent inoculum derived from invasive Bromus inermis. In each panel, pairings of bars on the left represent native P. smithii, and bars on the right represent invasive B. inermis. Panels represent the following treatment combinations: (A) ambient experimental temperatures, ambient source temperatures, (B) ambient experimental temperatures, elevated source temperatures, (C) elevated experimental temperatures, ambient source temperatures, and (D) elevated experimental temperatures, elevated source temperatures.
In the warm-season experiment, we observed a significant interaction [F(1, 94) = 3.93, p = 0.05] between native and non-native plant species, source plant species (i.e., plant species that trained the soil), experimental temperature, and source temperature (i.e., temperature at which the soil was trained) for our warm-season pairing, indicating that pairwise PSF varies with the interaction of source and experimental temperature. We illustrate this significant interaction in Figure 4A. When the source and experimental temperatures were consistent, PSF was negative, but when source and experimental temperatures were reversed, PSF was neutral or positive, and this reversal is significant (Supplementary Table 1). Specifically, total biomass of warm-season grasses grown under ambient temperatures was characterized by negative (ambient source soil temperature) and neutral (elevated source soil temperature) PSF (Figure 4A). When subjected to elevated experimental temperatures, the direction of PSF reversed, resulting in positive (ambient inoculum) or negative (elevated inoculum) PSF (Figure 4A). The negative PSF detected in warm-season total biomass under elevated temperatures with inoculum trained under elevated conditions was driven by significantly greater S. scoparium biomass production in B. ischaemum inoculum, compared to inoculum trained by the conspecific; whereas no difference was observed in B. ischaemum production with inoculum trained by either plant species.
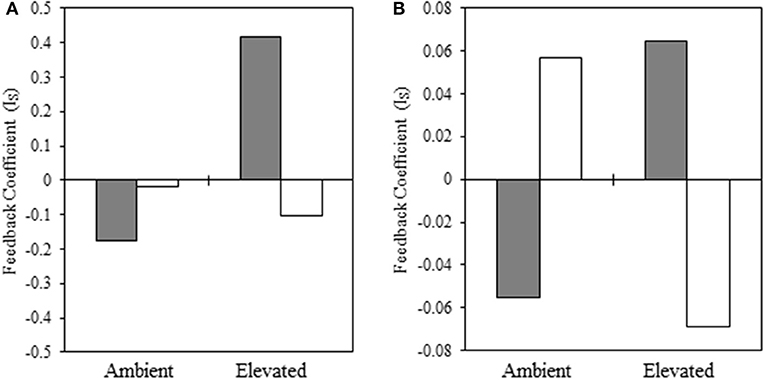
Figure 4. Interaction coefficient for PSF related to biomass production of native and non-native (A) warm-season and (B) cool-season grasses. Filled bars indicate inoculum from ambient temperatures and open bars represent inoculum conditioned in elevated temperatures. In each panel, the left pair of bars represent plants grown at ambient temperatures, while the right-hand pair of bars represent plants grown at elevated temperatures (Note: y-axes differ in scale).
In our cool-season experiment, the main effects were not significant under drought conditions (Supplementary Table 3). However, under drought conditions, we observe a significant interaction of feedback with source temperature and experimental temperature [F(1, 60) = 4.97, p = 0.03] (Figure 4B). When the source and experimental temperature was consistent, PSF was negative, but when source and experimental temperature were reversed, PSF was neutral or positive. Specifically, under ambient experimental temperatures, PSF were negative when inoculum soil had also been subjected to ambient temperatures (Figure 4B). However, the direction of the PSF was reversed when ambient inoculum soil had been subjected to elevated temperatures (Figure 4B). When grown under elevated temperatures, PSF were positive when grown with source soils subjected to ambient temperatures, and negative when plants were grown with source soil trained at elevated temperatures (Figure 4B).
We hypothesized that training soil microbial communities would result in environmental stress mitigation; however, our data do not support this a priori hypothesis. No evidence was observed for microbial mediation of drought stress, as we did not detect significant interactions between experimental soil moisture and source soil moisture, though this test was weak because of low survivorship in drought experimental conditions. Furthermore, we did not observe plants growing better when experimental and source temperatures were matched (Supplementary Tables 1–3).
There is increasing evidence suggesting that a rapidly changing climate will impact soil microbial communities (Rillig et al., 2002; Johnson et al., 2013; Schmidt et al., 2018), though little is known about strength or direction of PSF in response to extreme weather events, such as severe drought (Singh et al., 2010; Johnson et al., 2013). Abiotic factors, such as light availability, can influence the strength and direction of feedback interactions (Smith and Reynolds, 2015), but the effects of environmental drivers, such as precipitation and temperature, on the strength and direction of PSF interactions is far less certain. In general, it is thought that elevated temperatures will result in more negative PSF, as increased temperatures are expected to result in increased pathogen prevalence, as well as reduced AM fungal activity (Mohan et al., 2014). However, the results are likely context-dependent, and more research is needed to elucidate any patterns and processes that may exist. In addition, many current and past PSF studies that have tested climate effects have used native species pairings and agricultural crops (Hendriks et al., 2015; Wang et al., 2017; Fry et al., 2018). Consequently, far less research has explored PSF dynamics surrounding climate and non-native invasive species. Our study is one of the first of its kind to test PSF theory with combined environmental factors and non-native invasive plant species.
We found negative feedbacks when the temperature is stable over the training and testing phase of the feedback experiment, but positive or neutral feedback when the training and testing temperature were not matched. As negative pairwise plant soil feedback is a necessary condition for soil microbial dynamics mediating plant species coexistence (Bever et al., 1997; Eppinga et al., 2018), our results suggest soil microbial dynamics can be stabilizing in either ambient or elevated temperatures, but this dynamic is disrupted in variable environments. However, as pairwise negative feedback is not a complete description of the coexistence conditions (Bever et al., 1997; Bever, 2003, Eppinga et al., 2018, Kandlikar et al., 2019), further work is necessary to evaluate whether soil microbial dynamics do determine variation in coexistence patterns across climate stability. We found that elevated temperatures, combined with a soil legacy of elevated temperatures, led to strongly negative PSF between native and non-native warm-season grasses. This was in contrast to the slightly negative to positive feedback exhibited in other experimental temperature and altered inoculum temperature combinations. We observed that PSF was also significantly more negative when the training and testing temperatures were constant in the cool-season grasses when tested under drought conditions. Our findings suggest that changes in environmental drivers can impact the strength and direction of PSFs. Both warm- and cool-season pairings produced negative PSF when experimental temperatures mirrored training phase temperatures. However, the direction of PSF was reversed when experimental and training temperatures were mismatched. These results suggest that coexistence is likely when environmental conditions are similar from year to year, and homogeneity may be promoted when growing conditions are dramatically different than the previous year. These results are in contrast with previous results of van Grunsven et al. (2010), in which direction of PSF detected between pairs of European congeners was largely unaffected by variation in testing temperature, though this study did not manipulate temperature in the testing environment. Our results suggest that across year variation in climate may be one reason why plant-soil feedbacks have been observed to be variable (De Long et al., 2019).
Despite our hypothesis that experimental drought conditions would result in strong positive feedbacks, we found the opposite occurred. Our hypothesis was based on a combination of observations that non-native B. ischaemum currently invades into grasslands, and Duell et al. (2016) reported elevated temperatures and reduced soil moisture did not affect biomass production of the species. We observed the alternative scenario, in that the biomass of the non-native was not influenced, while the biomass of S. scoparium was greater in non-native soil compared to when grown in conspecific soil. While we do not suggest that the non-native soil generally promotes native growth, we propose that these results may result from two mechanisms. The first mechanism is that the changes in the AM fungal community contributed to the negative PSF observed (Bever, 2002). In the event of elevated temperatures, AM fungi may decrease activity (Mohan et al., 2014), which in turn weakens positive PSF (De Long et al., 2019), which could further explain our observed negative feedbacks. Changes to the fungal community were likely more pronounced due to the greater growth of the non-native grass relative to the native grass in the training phase of the experiment (Duell et al., 2016) under elevated temperatures (Supplementary Tables 4–6; Supplementary Figure 1). Warm-season grasses, such as B. ischaemum, readily associate with AM fungi (Wilson and Hartnett, 1998) and can alter the soil community. Native S. scoparium might have taken advantage of the changes in the fungal community composition more effectively than the non-native species. Alternatively, while not assessed in our current study, the accumulation of host specific pathogens could explain the increase in S. scoparium biomass in B. ischaemum soil in elevated temperatures with soil from elevated temperature. Plants in their native communities can accumulate host specific pathogens that contribute to negative feedback and to community succession (Bauer et al., 2015; Wang et al., 2017, Crawford et al., 2019). These host specific pathogens inhibit the growth of the host paving the way for colonization of other plant species. The release of S. scoparium from its host-specific pathogens would also result in the increase in S. scoparium growth in the non-native soil that led to the observed negative PSF. Either mechanism indicates that the native grass is able to utilize soil communities altered by the non-native more effectively relative to the non-native grass, when grown under elevated temperatures or following a soil legacy of elevated temperatures. We observed similar reversal of PSF direction in our cool-season species. The weaker PSF in cool-season grasses was driven by smaller, but consistent improvements in growth of both plant species in each other's soil communities. Given that these species are not strongly responsive to AM fungi (Wilson and Hartnett, 1998), we expect that these feedbacks are likely due to other soil biota, as pathogens and rhizobacteria are known to affect plant performance (Bever et al., 2012, Pineda et al., 2013; Rubin et al., 2017, Crawford et al., 2019). The impacts of pathogens, in particular, are likely to depend upon climate (Bever et al., 2015). This is supported by the consistent growth in each other's soils, as B. inermis was introduced into North America in the 1800's, and this length of time may be sufficient to adapt to local soil pathogens.
Similar to findings by Duell et al. (2016), various combinations of soil moisture and temperature did not affect biomass production of non-native B. ischaemum, and it consistently produced significantly greater biomass compared to native S. scoparium. This is not surprising, as B. ischaemum was introduced into the Great Plains as an improved forage, producing substantially greater biomass than many native grasses of similar stature. Additionally, while native P. smithii produced overall greater total biomass relative to non-native B. inermis, no differences were detected when analyzing by source training species, source temperature, and experimental temperature when grown under well-watered conditions. While we expected that both plant species would perform best when the climate legacy of a soil was matched with the current environment, but we did not see any evidence of microbial mediation of plant adaptation to the environment.
Our findings suggest that plant responses to warming temperatures and drought will be species-specific, and some invasives, such as B. ischaemum, will continue to produce large amounts of biomass relative to native species. We suspect that the presence of native plants will have a greater influence on the inhibition of non-native growth and establishment under predicted climate change scenarios. More research is required to confirm the extent to which soil environmental legacy affects the following year's PSF, especially in the context of moderate and severe drought. Furthermore, our study consisted of two pairings of functionally-similar native and non-native invasive prairie grasses, and additional species should be assessed to further our knowledge of the role of environmental soil training on invasive species PSF dynamics. There are still many questions surrounding plant invasion dynamics, and research such as our current study provide key insight into plant-soil-microbial interactions under projected climate regimes. Nevertheless, results from our two experiments suggest that when climate is consistent across years, soil microbes can contribute to coexistence of native and non-native plant species, while this does not occur when climate is variable across years. Further work on other plant species pairs and other environmental dimensions is required to test whether this is a general result.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
KZ, JB, and GW conceived and designed the research. ED conducted Experiment 1 and KZ conducted Experiment 2. KZ and JB analyzed the results. KZ and ED wrote the manuscript. All authors contributed the editing and preparation of the final product.
This work was funded by the United States Department of Defense Strategic Environmental Research and Development Program (SERDP) grant no. RC-2330; NSF DEB-1556664, DEB-1738041, and OIA 1656006; Konza Prairie Long-Term Ecological Research Program, and the Department of Natural Resource Ecology and Management at Oklahoma State University.
KZ is employed by Ecologic LLC, a restoration company based out of Bloomington, IN, United States.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank J. Nolan Craun and Jeremy Schallner for their work maintaining the watering regimes of this experiment. Additionally, we would like to thank Oklahoma State University Department of Natural Resource Ecology and Management for the use of greenhouse space and facilities. The authors would also like to acknowledge that this manuscript contains research that is part of KZ's thesis work, which is archived and available online.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00419/full#supplementary-material
Allen, W. J., Meyerson, L. A., Flick, A. J., and Cronin, J. T. (2018). Intraspecific variation in indirect plant-soil feedbacks influences a wetland plant invasion. Ecology 99, 1430–1440. doi: 10.1002/ecy.2344
Ba, L., Facelli, E., and Facelli, J. M. (2018). Plant-mycorrhizal fungi feedbacks: potential accomplices of Avena barbata's high invasiveness. Plant Ecol. 219, 1045–1052. doi: 10.1007/s11258-018-0857-8
Bauer, J. T., Mack, K. M. L., and Bever, J. D. (2015). Plant-soil feedbacks as drivers of succession: evidence from remnant and restored tallgrass prairies. Ecosphere 6, 1–12. doi: 10.1890/ES14-00480.1
Bever, J. D. (1994). Feedback between plants and their soil communities in an old field community. Ecology 75, 1965–1977. doi: 10.2307/1941601
Bever, J. D. (2002). Host-specificity of AM fungal population growth rates can generate feedback on plant growth. Plant Soil 244, 281–290. doi: 10.1023/A:1020221609080
Bever, J. D. (2003). Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol. 157, 465–473. doi: 10.1046/j.1469-8137.2003.00714.x
Bever, J. D., Dickie, I. A., Facelli, E., Facelli, J. M., Klironomos, J., Moora, M., et al. (2010). Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 25, 468–478. doi: 10.1016/j.tree.2010.05.004
Bever, J. D., Mangan, S., and Alexander, H. (2015). Pathogens maintain plant diversity. Annu. Rev. Ecol. Syst. 46, 305–325. doi: 10.1146/annurev-ecolsys-112414-054306
Bever, J. D., Platt, T. G., and Morton, E. R. (2012). Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 66, 265–283. doi: 10.1146/annurev-micro-092611-150107
Bever, J. D., Westover, K. M., and Antonovics, J. (1997). Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J. Ecol. 85, 561–573. doi: 10.2307/2960528
Bowles, T. M., Jackson, L. E., and Cavagnaro, T. R. (2018). Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Change Biol. 24, e171–e182. doi: 10.1111/gcb.13884
Celarier, R. P., and Harlan, J. P. (1955). Studies on Old World Bluestems. Oklahoma Agr Exp Sta Tech Bull T-58.
Crawford, K. M., Bauer, J. T., Comita, L. S., Eppinga, M. B., Johnson, D. J., Mangan, S. A., et al. (2019). When and where plant-soil feedback may promote plant coexistence: a meta-analysis. Ecol Lett. 22, 1274–1284. doi: 10.1111/ele.13278
Crawford, K. M., and Knight, T. M. (2017). Competition overwhelms the positive plant-soil feedback generated by an invasive plant. Oecologia 183, 211–220. doi: 10.1007/s00442-016-3759-2
De Long, J. R., Fry, E. L., Veen, G. F., and Kardol, P. (2019). Why are plant-soil feedbacks so unpredictable, and what to do about it? Funct. Ecol. 33, 118–128. doi: 10.1111/1365-2435.13232
Delavaux, C. S., Smith-Ramesh, L. M., and Kuebbing, S. E. (2017). Beyond nutrients: a meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 98, 2111–2119. doi: 10.1002/ecy.1892
Dillemuth, F. P., Rietschier, E. A., and Cronin, J. T. (2009). Patch dynamics of a native grass in relation to the spread of invasive smooth brome (Bromus inermis). Biol. Invasions 11, 1381–1391. doi: 10.1007/s10530-008-9346-7
Duell, E. B., Wilson, G. W. T., and Hickman, K. R. (2016). Above- and below-ground responses of native and invasive prairie grasses to future climate scenarios. Botany 94, 471–479. doi: 10.1139/cjb-2015-0238
Eppinga, M. B., Bausena, M., Johnson, D. J., Jiang, J., Mack, K. M. L., Strand, A. E., et al. (2018). Frequency-dependent feedback constrains plant community coexistence. Nat. Ecol. Evol. 2, 1403–1407. doi: 10.1038/s41559-018-0622-3
Fry, E. F., Johnson, G. N., Hall, A. L., Pritchard, W. J., Bullock, J. M., and Bardgett, R. D. (2018). Drought neutralizes plant-soil feedback of two mesic grassland forbs. Oecologia 186, 1113–1125. doi: 10.1007/s00442-018-4082-x
Gabbard, B. L., and Fowler, N. L. (2007). Wide ecological amplitude of a diversity-reducing invasive grass. Biol. Invasions 9, 149–160. doi: 10.1007/s10530-006-9012-x
Grove, S., Haubensak, K. A., Gehring, C., and Parker, I. M. (2017). Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption. J. Ecol. 105, 1496–1508. doi: 10.1111/1365-2745.12853
Heinze, J., Gensch, S., Weber, E., and Joshi, J. (2017). Soil temperature modifies effects of soil biota on plant growth. J. Plant Ecol. 10, 808–821. doi: 10.1093/jpe/rtw097
Hendriks, M., Visser, E. J. W., Visschers, I. G. S., Aarts, B. H. J., de Caluwe, H., Smit-Tiekstra, A. E., et al. (2015). Root responses of grassland species to spatial heterogeneity of plant-soil feedback. Funct. Ecol. 29, 177–186. doi: 10.1111/1365-2435.12367
Hetrick, B. A. D., Wilson, G. W. T., and Todd, T. C. (1990). Differential responses of C3 and C4 grasses to mycorrhizal symbiosis, phosphorous fertilization, and soil microorganisms. Can. J. Bot. 68, 461–467. doi: 10.1139/b90-061
Hickman, K. R., Farley, G. H., Channell, R., and Steier, J. E. (2006). Effects of Old World bluestem (Bothriochloa ischaemum) on food availability and avian community composition within the mixed-grass prairie. Southwest. Nat. 51, 524–530. doi: 10.1894/0038-4909(2006)51[524:EOOWBB]2.0.CO;2
Inderjit, and van der Putten, W. H. (2010). Impacts of soil microbial communities on exotic plant invasion. Trends Ecol. Evol. 25, 512–519. doi: 10.1016/j.tree.2010.06.006
Intergovernmental Panel on Climate Change (IPCC) (2014). “Climate change 2014: synthesis report. Contribution of Working Groups I, II and II to the fifth assessment report of the Intergovernmental Panel on Climate Change,” in Climate Change 2014, eds R. K. Pachauri and L. A Meyer (Geneva: IPCC, 7–12.
Johnson, N. C., Angelard, C., Sanders, I. R., and Kiers, E. T. (2013). Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecol. Lett. 16, 140–153. doi: 10.1111/ele.12085
Johnson, N. C., Wilson, G. W. T., Bowker, M. A., Wilson, J. A., and Miller, R. M. (2010). Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. U.S.A. 107, 2093–2098. doi: 10.1073/pnas.0906710107
Kaisermann, A., de Vries, F. T., Griffiths, R. I., and Bardgett, R. D. (2017). Legacy and effects of drought on plant-soil feedbacksand plant-plant interactions. New Phyt. 215, 1413–1424. doi: 10.1111/nph.14661
Kandlikar, G. S., Johnson, C. A., Yan, X., Kraft, N. J. B., and Levine, J. M. (2019). Winning and losing with microbes: how microbially mediated fitness differences influence plant diversity. Ecol. Lett. 22, 1178–1191. doi: 10.1111/ele.13280
Kivlin, S. N., Emery, S. M., and Rudgers, J. A. (2013). Fungal symbionts alter plant responses to global change. Am. J. Bot. 100, 1445–1457. doi: 10.3732/ajb.1200558
Kulmatiski, A. (2018). Community-level plant-soil feedbacks explain landscape distribution of native and non-native plants. Ecol. Evol. 8, 2041–2049. doi: 10.1002/ece3.3649
Kulmatiski, A., Beard, K. H., Stevens, J. R., and Cobbold, S. M. (2008). Plant-soil feedbacks: a meta-analytical review. Ecol. Lett. 11, 980–992. doi: 10.1111/j.1461-0248.2008.01209.x
Larson, D. L., Anderson, P. J., and Newton, W. (2001). Alien plant invasion in mixed-grass prairie: effects of vegetation type and anthropogenic disturbance. Ecol. Appl. 11, 128–141. doi: 10.1890/1051-0761(2001)011[0128:APIIMG]2.0.CO;2
Lau, J. A., and Lennon, J. T. (2011). Evolutionary ecology of plant-microbe interactions: soil microbial structure alters selection of plant traits. New Phytol. 192, 215–224. doi: 10.1111/j.1469-8137.2011.03790.x
Lau, L. A., and Lennon, J. T. (2012). Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc. Natl. Acad. Sci. U.S.A. 35, 14058–14062. doi: 10.1073/pnas.1202319109
Mangan, S. A., Schnitzer, S. A., Herre, E. A., Mack, K. M. L., Valencia, M. C., Sanchez, E. I., et al. (2010). Negative plant-soil feedback predicts tree-species relative abundance in a tropical forest. Nature 7307, 752–755. doi: 10.1038/nature09273
Meiners, S. J., Phipps, K. K., Pendergrast, I. V., T. H, Canam, T., and Carson, W. P. (2017). Soil microbial communities alter leaf chemistry and influence allelopathic potential among coexisting plant species. Oecologia 183, 1155–1165. doi: 10.1007/s00442-017-3833-4
Mohan, J. E., Cowden, C. C., Baas, P., Dawadi, A., Frankson, P. T., Helmick, K., et al. (2014). Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol. 10, 3–19. doi: 10.1016/j.funeco.2014.01.005
Pineda, A., Dicke, M., Pieterse, C. M., and Pozo, M. J. (2013). Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Funct. Ecol. 27, 574–586. doi: 10.1111/1365-2435.12050
Pringle, A., Bever, J. D., Gardes, M., Parrent, J. L., Rillig, M. C., and Klironomos, J. N. (2009). Mycorrhizal symbioses and plant invasions. Annu. Rev. Ecol. Evol. Syst. 40, 699–715. doi: 10.1146/annurev.ecolsys.39.110707.173454
Reinhart, K. O., and Callaway, R. M. (2006). Soil biota and invasive plants. New Phyt. 170, 445–457. doi: 10.1111/j.1469-8137.2006.01715.x
Rillig, M. C., Treseder, K. K., and Allen, M. F. (2002). “Global change and mycorrhizal fungi,” in Mycorrhizal Ecology, eds M. G. A. van der Heijden and I. R. Sanders (Berlin; Heidelberg: Springer Press), 135–160. doi: 10.1007/978-3-540-38364-2_6
Rubin, R. L., van Groenigen, K. J., and Hungate, B. A. (2017). Plant growth promoting rhizobacteria are more effective under drought: a meta-analysis. Plant Soil 416, 309–323. doi: 10.1007/s11104-017-3199-8
Schmidt, P. A., Schmitt, I., Otte, J., Bandow, C., Römbke, J., and Bálint, M. (2018). Season-long experimental drought alters fungal community composition but not diversity in a grassland soil. Microb. Ecol. 75, 468–478. doi: 10.1007/s00248-017-1047-2
Singh, B. K., Bardgett, R. D., Smith, P., and Reay, D. S. (2010). Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 8, 779–790. doi: 10.1038/nrmicro2439
Smith, L. M., and Reynolds, H. L. (2015). Plant-soil feedbacks shift from negative to positive with decreasing light in forest understory species. Ecology 96, 2523–2532. doi: 10.1890/14-2150.1
Stotz, G. C., Gianoli, E., Patchell, M. J., and Cahill, J. F. (2017). Differential responses of native and exotic plant species to an invasive grass are driven by variation in biotic and abiotic factors. J. Veg. Sci. 28, 325–336. doi: 10.1111/jvs.12499
van der Putten, W. H., Bargett, R. D., Bever, J. D., Bezemer, T. M., Casper, B. B., and Fukami, T. (2013). Plant-soil feedbacks: the past, the present and future challenges. J. Ecol. 101, 265–276. 10.1111/1365-2745.12054. doi: 10.1111/1365-2745.12054
van Grunsven, R. H. A., van der Putten, W. H., Benzemer, T. M., and Veenendaal, E. M. (2010). Plant—soil feedback of native and range-expanding plant species is insensitive to temperature. Oecologia 162, 1059–1069. doi: 10.1007/s00442-009-1526-3
Vogelsang, K. M., and Bever, J. D. (2009). Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 90, 399–407. doi: 10.1890/07-2144.1
Wang, G. Z., Li, H. G., Christie, P., Zhang, F. S., Zhang, J. L., and Bever, J. D. (2017). Plant-soil feedback contributes to intercropping overyielding by reducing the negative effect of take-all on wheat and compensating the growth of faba bean. Plant Soil 415, 1–12. doi: 10.1007/s11104-016-3139-z
Wilson, G. W. T., and Hartnett, D. C. (1997). Effects of mycorrhizae on plant growth and dynamics in experimental tallgrass prairie micrcosms. Am. J. Bot. 84:478–482. doi: 10.2307/2446024
Wilson, G. W. T., and Hartnett, D. C. (1998). Interspecific variation in plant responses to mycorrhizal colonization in tallgrass prairie. Am. J. Bot. 85, 1732–1738. doi: 10.2307/2446507
Wilson, G. W. T., Hickman, K. R., and Williamson, M. M. (2012). Invasive warm-season grasses reduce mycorrhizal root colonization and biomass production of native prairie grasses. Mycorrhiza 22, 327–336. doi: 10.1007/s00572-011-0407-x
Xi, N., Chu, C., and Bloor, J. M. G. (2018). Plant drought resistance is mediated by soil microbial co munity structure and plant-soil feedbacks in a savanna tree species. J. Exp. Bot. 155, 695–701. doi: 10.1016/j.envexpbot.2018.08.013
Yu, H., Ma, Q., Liu, X., Xu, Z., Zhou, G., and Shi, Y. (2018). Short- and long-term warming alters soil microbial community and relates to soil traits. Agric. Ecosyst. Environ. Appl. Soil Ecol. 131, 22–28. doi: 10.1016/j.apsoil.2018.07.006
Zaiger, K. L. (2016). Environmental extremes drive plant and soil community dynamics of native and disturbed grasslands (master's thesis). Oklahoma State University, Stillwater, OK, United States.
Zhang, P., Li, B., Wu, J., and Hu, S. (2019). Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol. Lett. 22, 200–210. doi: 10.1111/ele.13181
Zubek, S., Majewska, M. L., Blaszkowski, J., Stefanowicz, A. M., Nobis, M., and Kapusta, P. (2016). Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol. Fertil. Soils 52, 879–893. doi: 10.1007/s00374-016-1127-3
Keywords: Bothriochloa ischaemum, Bromus inermis, climate, invasive species, Pascopyrum smithii, plant-soil feedback, Schizachyrium scoparium, soil training
Citation: Duell EB, Zaiger K, Bever JD and Wilson GWT (2019) Climate Affects Plant-Soil Feedback of Native and Invasive Grasses: Negative Feedbacks in Stable but Not in Variable Environments. Front. Ecol. Evol. 7:419. doi: 10.3389/fevo.2019.00419
Received: 15 June 2019; Accepted: 18 October 2019;
Published: 05 November 2019.
Edited by:
Johannes Heinze, University of Potsdam, GermanyReviewed by:
Rachel Wooliver, North Carolina State University, United StatesCopyright © 2019 Duell, Zaiger, Bever and Wilson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric B. Duell, ZXJpYy5kdWVsbEBva3N0YXRlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.