
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol., 30 August 2019
Sec. Conservation and Restoration Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00312
This article is part of the Research TopicHow Prides of Lion Researchers are Evolving to be InterdisciplinaryView all 12 articles
 Yadvendradev V. Jhala1*
Yadvendradev V. Jhala1* Kausik Banerjee1
Kausik Banerjee1 Stotra Chakrabarti1
Stotra Chakrabarti1 Parabita Basu2
Parabita Basu2 Kartikeya Singh3
Kartikeya Singh3 Chittaranjan Dave4
Chittaranjan Dave4 Keshab Gogoi1
Keshab Gogoi1Asiatic lions typify most challenges faced by large carnivores: single population, historical bottlenecks, habitat loss, poaching, and conflict with humans. Their recovery from <50 in a few hundred km2 to >500 occupying 13,000 km2 of agro-pastoral Saurashtra landscape, Gujarat, India is an enigma. We review and evaluate the multidisciplinary aspects of lion conservation-strategy that covers ecology, conflict, community perceptions, economics, management, and politics. The history of modern lions in India dates back to ~4–6,000 BP, but evidence suggests presence as early as 10–15,000 BP. Asiatic lions can be distinguished from African lions by their belly-folds; adult males and females weighing 160 (SE 4.7) and 116 (SE 3.7) kg, respectively. Lion density ranged from 2 to 15/100 km2 in the Saurashtra landscape. Demographic parameters of Asiatic lions were comparable to African lions. Prides were related females and cubs; males lived separately in hierarchical coalitions having overlapping ranges with multiple prides. Lionesses mated with multiple coalitions to reduce infanticide and enhance genetic diversity of their progeny. Few hectares of scrub sufficed as daytime refuges, while >4 km2 patches were required for breeding. Sink populations outside Gir Protected Area (PA) were maintained by immigrants. Lions within PA fed primarily on wild-prey, while scavenging and predation on livestock was the mainstay outside. Monetary compensation for livestock-depredation, legal-protection, lion-related profits, combined with religious and cultural sentiments were major drivers of population recovery. The lion has become a socio-political instrument in Gujarat, which despite a Supreme Court directive, has not parted with founders to establish another population. Threats from epidemics loom large and currently a canine distemper virus outbreak is prevalent. Attacks on humans were rare, however, with increasing lion density the intensity of conflict is increasing. This, coupled with lowered tolerance of communities due to erosion of traditional values sets the stage for retaliation. Future of lions outside PA is uncertain as breeding refuges and their connecting corridors are vanishing rapidly. A human-free National Park of ~1,000 km2 is essential for ensuring a viable population that retains its ecological role and evolutionary potential. Legalizing lion based ecotourism by forming village consortia holds promise to prevent land conversion and promoting lion-human coexistence.
Unprecedented human expansion and consequent resource exploitation in the last two centuries have sheared the range and imperiled the survival of biodiversity globally (Ripple et al., 2014). Large carnivores as a taxa are most affected because by virtue of being apex predators they need large ranges, occur at low densities and compete with humans for space and food, prey on livestock and sometimes on humans; bringing them into direct conflict with human interests (Ceballos and Ehrlich, 2002). In the ushering Anthropocene, the fate of biodiversity depends on how well species and humans adapt to live alongside each other. In densely populated developing countries, conservation of pristine wilderness is a luxury and many protected areas have human habitations within them (Rangarajan and Shahabuddin, 2006). The paradigm of coexistence seems to be the only solution for several carnivore populations wherein local communities either have a high level of tolerance or even encourage carnivore population buildup, while carnivores “learn” to live with people (Ripple et al., 2014). The major challenges faced by mega-biodiverse countries like India in conserving their natural heritage are: high human density (1.2 billion people with an average human density of 382 people/km2; Human Census Report 2011, Government of India available at www.census2011.co.in), poverty, agrarian economy, and rapid development (Karanth and DeFries, 2010). Though 5% of India's geographical area is secured as Protected Areas (PAs), these PAs are small (average size of <300 km2), with several of them having human settlements and varying levels of anthropogenic activities within them (Rodgers et al., 2003). Furthermore, the PA network is severely fragmented by intervening human-modified landscapes, resulting in poor habitat connectivity (Qureshi et al., 2015). Such a scenario creates wildlife populations that are vulnerable to extinction through demographic and environmental stochasticity (Soulé, 1987), and a high potential for human-wildlife conflict (Madhusudan and Mishra, 2003; Banerjee, 2012). It is indeed surprising that despite these odds, with the exception of the Asiatic cheetah (Acinonyx jubatus venaticus) India has not lost its large carnivore assemblage since written history (Divyabhanusinh, 1995). This can primarily be attributed to the historical, religious, and cultural reverence for life forms in majority of the Indians (Gadgil and Thapar, 1990; Dorje, 2011; Renugadevi, 2012). However, with the current escalation of habitat loss due to the “green revolution” in agricultural practices, other developmental activities as well as erosion of traditional values; conservative estimate suggests that 20% of large mammalian fauna in India may face extinction, and several species have already disappeared from over 90% of their original range (Madhusudan and Mishra, 2003; Karanth et al., 2010).
The charisma of lions (Panthera leo) on the human psyche is historical (ingrained in the Vedas and Homer's Iliad) and continues into the modern era. Lions have dominated our association with large carnivores particularly because they represent an elemental survival strategy which is very akin to ours- “living in groups”. From pre-historic war emblems satisfying royal egos to motion pictures catering to young minds like the Lion King, from the notoriously vicious man-eaters of Tsavo to the famed controversy surrounding Cecil getting shot that kindled empathy across the world; lions have seesawed between the notions of charismatic and loved to being hated and persecuted (Macdonell, 1897; Patterson, 1907; Macdonald et al., 2016; Carpenter and Konisky, 2017; Kostuch, 2017).
Asiatic lions (P. l. persica) that once ranged from Persia to eastern India are now restricted to a single population in the Gir-Saurashtra region of the state of Gujarat, Western India. This single population was established from a small founder, shares space with humans across almost all of its range and therefore typifies major challenges that large carnivore conservation can potentially face (Johnsingh et al., 1998). While global debate surrounds the issue of whether lions can be effectively conserved outside PAs (Packer et al., 2013; Stephens, 2015); in India the Gir National Park of only 259 km2 is devoid of human habitation and is exclusively available for free ranging lions. In the remaining ~13,000 km2 (of which in addition to the National Park another ~1,600 km2 are under legal protection as Wildlife Sanctuaries) humans and lions coexist at varying levels of population densities, tolerance toward each other, and magnitude of conflict. Concurrent with a 19.2% rate of human population growth (Human Census Report, Government of India available at www.census2011.co.in) in Gujarat, lions outside PAs (National Park and Wildlife Sanctuaries) have grown by 126% in the last two decades. Consequently, ~30% of the present lion population resides outside the PAs in close proximity to humans (Gujarat Forest Department, 2015; Singh, 2017a).
The exclusive occurrence of Asiatic lions in the small region of the Gir-Saurashtra landscape has created opportunity for lion tourism which is utilized formally by the local government while illegally by local communities for economic gains. These attributes have been exploited by bureaucrats, politicians and local communities to gain mileage and economic privileges, often at the cost of the long-term conservation interests of Asiatic lions.
Gir lions have been a key subject of management and research; managed as a prized trophy prior to late 1800's (Moose, 1957; Divyabhanusinh, 2005) and subsequently conserved as a symbol of regional and national pride (Rangarajan, 2001). Scientific research on this sub-species commenced in the late 1960's with Joslin (1973) and Berwick (1974). It still continues in the form of the longest ecological research project in India under the auspices of the Wildlife Institute of India between 1986 and 2018 (Chellam, 1993; Jhala et al., 1999, 2004, 2011, 2014a, 2016, 2018). Independent researchers have also addressed certain aspects of lion ecology and conservation such as diet (Sinha, 1987), lion recolonization outside the Gir PA (Dharaiya, 2001), and human-lion interface (Meena et al., 2014). Furthermore, many wildlife management- and monitoring- practices that have evolved in Gir (such as pugmark counts; Wynter-Blyth, 1949) were later adopted across India for tigers P. tigris tigris (Choudhury, 1970; Panwar, 1979).
Thus, a unique blend of culture, religion and historical legacy mixes with the science of lion ecology, often being at odds with economics of modernization to create a scenario that necessitates a multidisciplinary approach for conserving the last Asiatic lions. In this paper we review relevant topics related to lion- history, origin, culture, ecology (morphology, demography, behavior, movement, and foraging), conflict, economics, and politics. Our long-term intimate association with the Gir ecosystem provided us with access to information and a better understanding for its interpretation. Information available as reports, guidelines, management plans (that have no Internet access) were systematically reviewed from repositories at the Wildlife Institute of India and the Gir Research Center along with relevant published literature. We evaluate policy and management strategies that have resulted in this conservation success, assess gaps that need to be addressed and highlight impending issues that would need major paradigm shifts for long term survival of these lions.
The work of geneticists, archeologists, and historians have contributed to our understanding of the origin and timing of lions' colonization of the Indian subcontinent. Evidence from the three disciplines do not always corroborate each other and there is still a lot to learn about when and how lions came into India. Two subspecies of extant lions, namely all lions from Africa as P. l. leo and lions from Asia as P. l. persica were recognized (Bauer et al., 2016). These were believed to have diverged sometime between 55,000 and 200,000 BP (O'Brien et al., 1987). Recent investigations on phylogeography of modern lions, based on mitochondrial and nuclear DNA analysis, indicates a single African origin of modern lions (Barnett et al., 2006; Antunes et al., 2008). Extant lions originated from several Pleistocene refugia (324,000–169,000 BP) in East and South Africa (Antunes et al., 2008). Asiatic lions are believed to have originated from an older East African refuge dispersal event some 118,000 BP (95% CI 28,000–208,000 BP) (Antunes et al., 2008). Based on Northern, Western and Central African lions' close genetic proximity to extant Indian lions as compared to Southern and East African lions, Bertola et al. (2011) postulated an alternative explanation, wherein after a Pleistocene extinction event in Western and Central Africa, recolonization occurred from a refugia in the Middle East. More recent analysis of mt-DNA from modern and ancient lion samples (Barnett et al., 2014) shows that lion exodus into Asia started as late as 21,000 BP and probably continued till the late Holocene. Maternal lineage of Gir lions was found to be nested within the clade formed by Northern, Western, and Central African lions (Barnett et al., 2014). Bertola et al. (2015) included nuclear markers along with mt-DNA and found lions from India to form a distinct cluster with little/no admixture with African lions. The IUCN Cat Specialist Group now recognizes two subspecies P. leo leo consisting of lions from India, Central and West Africa and P. leo melanochaita comprised of lions from Eastern and Southern Africa (Kitchener et al., 2017). Fossil records in Sri Lanka (Manamendra-Arachchi et al., 2005) report lion and tiger presence as early as the late quaternary, much before the current estimated arrival of both modern lions and tigers into India. The last land bridge between India and Sri Lanka submerged 5,000–10,000 BP (Yokoyama et al., 2000). Climate and associated vegetation changes are considered as the drivers of extinction of lions, and coupled with hunting by early humans in more recent times arguably caused the extinction of tigers as well in Sri Lanka (Manamendra-Arachchi et al., 2005). However, the possibility of their continued existence in refugia on mainland India prior and during the last glacial maxima cannot be ruled out. Though evidence for such claims are yet to be discovered, such possibilities seem realistic and open up a range of questions that are yet to be answered.
The presence of Neolithic/Chalcolithic cave paintings of lions in Bhimbetka rock shelters of central India (30,000–100,000 BP; Badam and Sathe, 1991) suggest lions to be early entrants into India and lend support to the fossil records from Sri Lanka. But their absence at the peak of the Indus valley civilizations as evidenced from the lack of their appearance in seals, pottery, and terracotta images that abound with representations of other contemporary wildlife like tigers, elephants, and rhinoceros (Divyabhanusinh, 2005) remains a mystery. It is possible that the earlier entrant lions became locally extinct within most/all of India as had happened in Sri Lanka. Lion terracotta art was recovered at Mehrgarh near Bolan Pass (currently in Pakistan), one of the important Neolithic (9,000–4,500 BP) archaeological sites and a lion handle was excavated from Taxila (currently in Pakistan) that dated back to late Harappan period (2,500 BP) (Divyabhanusinh, 2005). While depictions of tigers in Harappan art are widely known, a rare find of a two-headed lion like figurine was also recovered from the Indus valley site (Iyer, 1977). The advent of the Aryans and their influence was marked with an increase in the familiarity with lions. It would be difficult to differentiate if this familiarity was because of lions living in India or by Aryans encountering them in Persia during their migration. Ancient Hindu literature, the Rigveda, which is dated between 3,500 and 4,000 BP mentions the word simha (Sanskrit for lion) at least on 15 different occasions. Based on recorded history, Singh (2007) speculates that modern lions entered India through the western passes of the Hindu Kush and occupied most of Northern and Western India between 2,600 and 3,500 BP. Divyabhanusinh (2005) attributes the entry of modern lions in the Western and North-Western parts of India to the loss of tropical forests caused by environmental changes such as prolonged drought (which is also attributed as a cause for the Aryan migration) and habitat modifications caused by anthropogenic factors like clearing of forests for grazing lands and agriculture. About 3,500 BP the tiger seems to have lost its supremacy to the lion, which was prominently depicted in Indian art, culture, sculpture and literature (Iyer, 1977). Subsequently, by the time Jainism and Buddhism evolved, lions were well-established in India. Contemporaneous ancient Jain and Buddhist literature depicted the lion as a symbol of the 24th Jain tirthankar (spiritual leader) Mahaveer (~2,600 BP); while Gautam Buddha, the son of the Sakya chieftain (born around 2,500 BP) was known as Sakyasimha after achieving enlightenment. Lion capital at Vaishali during pre-Mauryan era (2,100–2,300 BP) symbolized the supreme iconic status of the species as a royal symbol. Lions featured in the ancient Buddhist texts of the Jatakas (~2,400 BP) that depict Buddha as various animal incarnations, often as a noble lion (Choskyi, 1988). The lion was ubiquitous as a symbol of royalty and was given a place of pride in lore and text in Sankrit, Tamil, Pali, and Persian. By the time of the Puranas (~1,000–1,500 BP) and the great epics of the Ramayana and Mahabharata, the lion became the vahana (carrier) of Goddess Durga and was considered an incarnation of God Vishnu as “Narasimha”; and thus became a symbol of worship in Hinduism. In modern Republic India the lion was designated as the national animal (Rangarajan, 2013), a status it subsequently lost to the tiger in 1973 (Rangarajan, 2001). Independent India is often depicted as Bharatmata (mother India) riding a full maned lion (Newell, 2011). The 3rd century BCE Ashoka pillar depicting four lions standing back-to-back, within a Persepolitan style proclaiming the ruler's universal all-encompassing vision of dhamma has now become the national emblem for India and is printed on its currency and official documents. The recent icon adopted by the Indian Government for encouraging local entrepreneurship is a “make-in-India” logo of an Asiatic lion made from mechanized parts.
Asiatic lions can often be morphologically differentiated from African lions based on (a) skull characteristics, wherein the Asiatic lions have an extra infraorbital foramen, (b) a typical loose fold of skin on the abdomen known as the belly-fold which is absent in African lions (O'Brien, 2003), (c) facial characteristics of Asiatic lions, with a more elongated snout and a more sloping forehead; giving them a longer profile in lateral view in comparison to the African lions and, (d) males having sparser manes, never covering their ears The mane in the adult lion has the typical “mohawk” style look. (Figure 1, Supplementary Material S1). As part of our long-term research project, we combined our field observations of known lions with techniques developed for African lions to develop criteria for estimating the age of individuals (Supplementary Material S2), which helped us construct their population structure and demographic details.

Figure 1. Face and body profiles of Asiatic lions. (A) Adult male, note the sparser mane (than African lions) that does not cover the ear and the top of the head with a Mohawk look, and the prominent belly-fold; and (B) adult female, with a longer sloping snout and side face profile than African lionesses. Also, note the size difference between the male and the female (a consorting pair). Photographs taken by Stotra Chakrabarti.
Between 2001 and 2018, we captured 35 free-ranging lions (including sub-adults that were targeted for understanding dispersal) from different parts of the Gir landscape in order to deploy radio-transmitters or for treatments, and recorded their morphometric details. We found average weights of adult males (n = 7) and females (n = 12) to be 160 (SE 4.7) kg and 116.5 (SE 3.7) kg, respectively (Table 1).
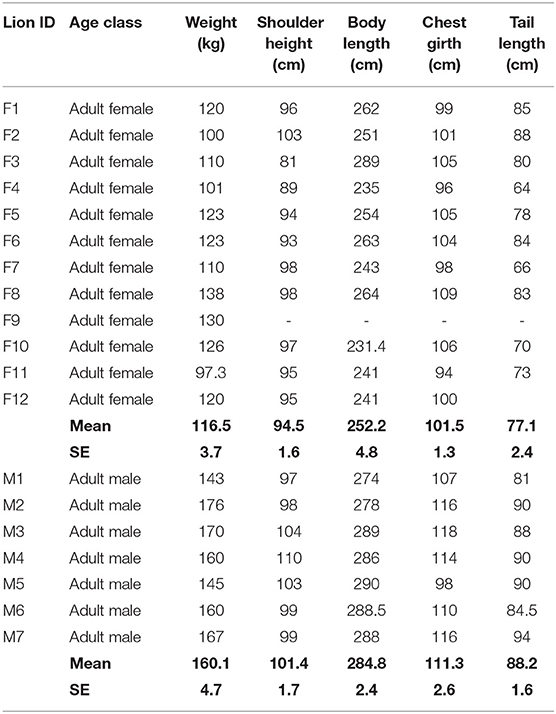
Table 1. Morphometric details of adult Asiatic lions (n = 19; 12F, 7 M) captured between 2001 and 2018 for deploying radio-collars. Body length is measured from nose-tip to tail-tip along the curves.
Like tigers and leopards, several local variations in lions based on their mane size and coloration, and coat texture have been recorded from different parts of India and from within Gir (Divyabhanusinh, 2005). Adult male lions are often grouped by local communities into various categories based on the color of their manes that can range from golden yellow (Pinglo), speckled gray (Bhurio) to black (Kamho) (Divyabhanusinh, 2005).
The erstwhile range of the modern Asiatic lion, reconstructed mainly from paleontological evidence, literature, art, culture, and shikar (hunting) documents suggest an extensive area from Anatolia, Syria across the Middle East to Eastern India (Kinnear, 1920; Caldwell, 1938; Joslin, 1973). Till the mid-1800s, lions in India inhabited the entire northern Indo-Gangetic Basin in North and Central India and were abundant in the modern states of northern and western India, Bihar and Odisha in the east with the river Narmada being the southernmost boundary (Fenton, 1908; Pocock, 1930; Dalvi, 1969). Subsequently by late 1800's they were exterminated from most of their range because of hunting and habitat loss (Divyabhanusinh, 2005). By 1880s lions were restricted as a single free-ranging population in and around the Barda and Alech hills, Mitiyala, Girnar, and Gir forests in the Saurashtra peninsula of Gujarat (Dalvi, 1969). Although some lions continued to survive in isolated habitat pockets of Iran and Iraq, but these were not viable populations and soon became extinct. By 1888–1890, hunting and loss of forests due to agricultural expansion and livestock grazing in Saurashtra restricted the lions to a single population in the Gir forests, a patch of about 2,000 km2 composed of dry deciduous and thorn forest (Divyabhanusinh, 2005).
Driscoll et al. (2002) suggests that about 2,680 (range 1,081–4,279) BP, the Kathiawar Peninsula that contained the Gir forests was separated from mainland India by rising sea levels in the Gulf of Khambhat (Gupta, 1972), causing the first genetic bottleneck that isolated the founders of the present Asiatic lion population, compelling them to inbreed for several generations (O'Brien, 2003). By the time the Gulf water receded and the peninsula became continuous with the mainland, most of the lions from mainland India had become locally extinct providing little chance to the inbred population to enhance their genetic diversity. A second, less-severe but more popularly known bottleneck occurred at the onset of the 19th century when owing to rampant hunting, Gir lions dwindled to around <50 individuals (Edwardes and Fraser, 1907; Kinnear, 1920; Pocock, 1930).
Owing to the timely protection measures taken by the Nawabs of Junagadh who ruled most of the Gir region, lions survived (Divyabhanusinh, 2005) and increased to about 287 by 1936 (Dalvi, 1969). Subsequently, the Government of Independent India enforced a complete ban on lion hunting in 1955 and declared the Gir forests as a Wildlife Sanctuary in 1965. Ensuing protection and habitat management by the Gujarat Forest Department resulted in the lion population increasing steadily (Singh and Kamboj, 1996) to over 500 in the last 2015 total count (Gujarat Forest Department, 2015). The sub-species was also down-listed from the “Critically Endangered” category of the IUCN Red list in 1990s (Nowell and Jackson, 1996) to “Endangered” in 2008 (Breitenmoser et al., 2008). Within the past two decades, lions have dispersed into about 13,000 km2 of agro-pastoral landscape comprising of the Gir Protected Area (Gir PA; 1700 km2), Girnar Wildlife Sanctuary (180 km2) and over 11,000 km2 of human-dominated landscape and coastal scrublands of the surrounding districts of Junagadh, Amreli, Gir Somnath, and Bhavnagar (Ranjitsinh, 2016; Singh, 2017a). Currently, the Saurashtra landscape has a single source population of lions comprised of ~300 adult individuals that live within the Gir National Park and Wildlife Sanctuary, and several patchily distributed small sink populations (Pulliam, 1988) of <50 individuals each in the human dominated agro-pastoral system (Figure 2). Though these small populations do breed and recruit lions, immigrants from the Gir PA are an essential element for their long-term viability (Banerjee et al., 2010). Radio telemetry (Jhala et al., 2014a) has shown extensive movement between these small populations and with the lion population of Gir PA. Lions thus exist in a classical metapopulation framework in the Saurashtra landscape (Hanski and Gilpin, 1997; Cronin, 2003). Consequently, habitat connectivity that facilitates lion movement between populations is vital for long-term lion persistence in the Saurashtra landscape (Banerjee et al., 2010; Banerjee, 2012).
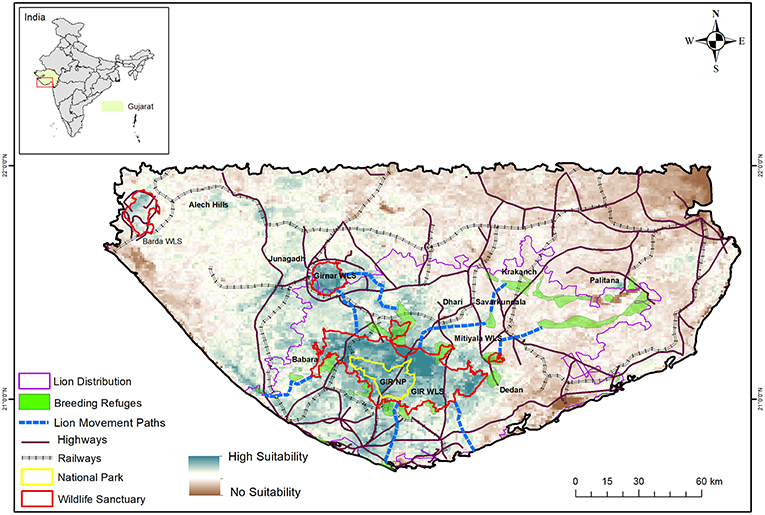
Figure 2. Lion habitat suitability and current lion distribution across Saurashtra landscape overlaid with Protected Areas, breeding refuges outside the protected areas, important lion movement pathways, and linear infrastructure (major roads and railways). Note the location of Barda WLS that is being considered for lion reintroduction.
While the recovery of Gir lions elucidates a conservation success story; it also poses serious challenges for wildlife managers and conservationists in terms of maintaining the future persistence of this subspecies. A population gains security with increasing size and the species becomes secure with increasing number of viable populations (Soulé and Simberloff, 1986). The importance of human free space for large carnivore conservation is undebatable, as conflict with human interests has been the major cause of large carnivore declines worldwide (Woodroffe, 2000). Indeed, lions were often poisoned on livestock carcasses in Gir until recently, when law enforcement became very strict. Currently only 259 km2 of inviolate space (devoid of human habitation and use) is allocated as Gir National Park for lion conservation in Gujarat. The rest of the protected areas are in the form of wildlife sanctuaries (WLS), reserve forests and protected forests with varying levels of human habitation and legally permitted human use of forest resources (Wildlife Protection Act, 1972), including livestock grazing rights of local semi-nomadic pastoral communities, the Maldharis. With land ownership being primarily private in the landscape outside the PAs, creation of new PAs in Saurashtra is difficult. Since the PAs in the landscape have reached carrying capacity for lions with about 300 individuals (Singh, 1997), maintaining the current population of 500 lions or increasing it can only be achieved by ensuring the continued source value of the Gir PA and by providing dispersal corridors to the several small sink-populations in the agro-pastoral landscape. Coexistence with humans thus becomes an inevitable strategy for maintaining a viable lion population in this landscape. However, the Saurashtra landscape is rapidly transforming due to development of linear infrastructure, expanding urban sprawl, agricultural intensification and changing community values. With increasing lion density in this progressively hostile landscape, a multidisciplinary understanding of lion ecology, conflict resolution, and socio-economic underpinnings is required for maintaining lion-human coexistence.
The earliest attempt to estimate lion population based on shikar records were made by William Rice of the Indian Army in 1850s when he concluded that not more than 300 individuals were left in India (Divyabhanusinh, 2005). Subsequent estimates made by forest and army officials under the rule of Junagadh State figured about 20–50 lions in between 1905 and 1913. The first lion census based on pugmark counts at waterholes was conducted in 1936 and reported a total of 287 lions (Wynter-Blyth, 1949). Since 1963, the Gujarat Forest Department has estimated lion numbers about every 5 years by a labor intensive, 3-day total count using livestock bait (Singh, 2017b). In this method, a daily record was kept of all lions that visited the baits. Lions feeding on baits remained localized in the vicinity for 3–4 days. If, however, lions moved away to another bait site a record of the movement was kept and accounted for to minimize double counts while computing total number of lions. The maximum number of lions recorded on any single day was considered to be the total population.
Both pugmark census and total counts depend on unrealistic assumptions, are error prone as they do not address detection issues, require careful identification of duplications, trained field staff and are resource intensive (Williams et al., 2002). To circumvent these issues, we designed and demonstrated lion abundance estimation in a mark-recapture framework (both conventional and spatially explicit) based on individual identification of lions from their vibrissae patterns, ear notches and permanent body marks (Jhala et al., 1999, 2004; Jhala, 2004; Banerjee and Jhala, 2012). Lions >1.5 years were approached within 10–20 m on foot or from vehicles and photographed. Individual lion details (age, gender, identifying features, associated lions, geographic coordinates, photographs, etc.) were then entered in program LION (Jhala et al., 2005) (Supplementary Material S3) for storing, archiving, identifying and comparing with the lion database so as to generate information useful for abundance estimation and long-term monitoring of demographic parameters and movement patterns.
Lion density was found to be the highest in the Gir PA at 15 (SE 0.1) lions/100 km2 followed by Girnar WLS [6 (SE 0.7) lions/100 km2] and the human dominated landscape of Saurashtra [2 (SE 0.1) lions/100 km2] (Jhala et al., 2004; Banerjee, 2012; Banerjee et al., 2013). Spatially explicit density of lions in the western part of the Gir PA was positively correlated with tourism hotspots due to artificial food provisioning at these sites (Gogoi, 2015). Due to vegetarian lifestyles of local communities, dead livestock are dumped outside settlements. These carcasses attract large carnivores including lions and leopards (Panthera pardus). To minimize encounters between large carnivores and humans as well as to enhance sighting of lions by tourists, wildlife managers often retrieve such livestock carcasses from forest settlements and dump them at tourist viewing spots. This assured food source increased pride sizes and reduced their home ranges (Gogoi, 2015; Jhala et al., 2016). This distribution pattern caused by subsidized food resources overrides the influence of natural prey and other ecological factors, resulting in local lion densities that are higher than natural densities. We believe that though this practice would enhance tourist viewing but will have serious implications on the social organization of lions, spread of infectious diseases and might cause enhanced predation pressure on wild prey in small pockets harboring artificially enhanced lion density.
Allozyme and microsatellite studies indicate that the Asiatic lions have low genetic diversity due to an isolated, inbred population with a small founder base (Wildt et al., 1987; O'Brien, 2003). However, random amplified polymorphic DNA analysis showed some levels of polymorphism in Asiatic lions (Shankaranarayanan et al., 1997). O'Brien et al. (1987) and Wildt et al. (1987) found that Asiatic lions and cheetahs showed a high incidence of morphologically abnormal spermatozoa (79 and 71%, respectively) when compared to free-ranging African lions (25–61%) and other species such as bulls Bos spp. and dogs Canis lupus familiaris (20–30%). The serum testosterone (a critical hormone for spermatogenesis) was low and Asiatic lions had lower variability in the major histocompatibility complex gene responsible for immunity (Wildt et al., 1987). Todd (1965) attributed dentition abnormalities in Asiatic lions to inbreeding. Decreased heterozygosity likely diminishes reproductive vigor and long-term survival of a population (O'Brien et al., 1986; Packer et al., 1991).
In order to understand the demographic parameters of Gir lions, 68 adult lions, and 91 cubs from 38 litters were intensively monitored using telemetry and individual lion ID profiles (Banerjee and Jhala, 2012). Records of opportunistic mortality events (n = 228) were used to understand mortality causes. Gir lions apparently increased from about 177 in 1968 to about 523 by 2015 with an r = 0.022 (SE 0.001) translated into an annual population growth of 2.2%. Male: female ratio was 0.63 (SE 0.04) while cub: adult lioness ratio was 0.37 (SE 0.02). Though breeding is observed year round, mating peaked in winter while birth peaked in late summer. Average litter size was 2.39 (SE 0.12). Inter-birth interval was 1.37 (SE 0.25) years (n = 7 lionesses) and was higher [2.25 (SE 0.41) years] when cubs of the previous litter survived to independence. Cub (<1 year) survival was 0.57 (SE 0.04) while survival from cub to recruitment age (3 years) was 51% (SE 4%) with infanticide attributing to 30% (SE 7 %) of mortalities. Average annual survival rate of adult lions (>3 years) was 0.9 (SE 0.12). Based on records of 228 lion mortalities recorded between 2007 and 2019, we estimated that 30% of the deaths were caused by diseases (Figure 3). Adult lions died primarily due to natural causes (60%), however, human caused mortality was also substantial (32%). Deaths due to falling in open irrigation wells, electrocution by live wires deployed illegally to prevent crop damage from nilgai (Boselaphus tragocamelus) and wild pigs (Sus scrofa) were a cause of concern in the agro-pastoral landscape. These are being addressed by wildlife authorities by subsidizing the construction of parapets around open wells and pulsating solar-powered wildlife fences to agricultural fields.
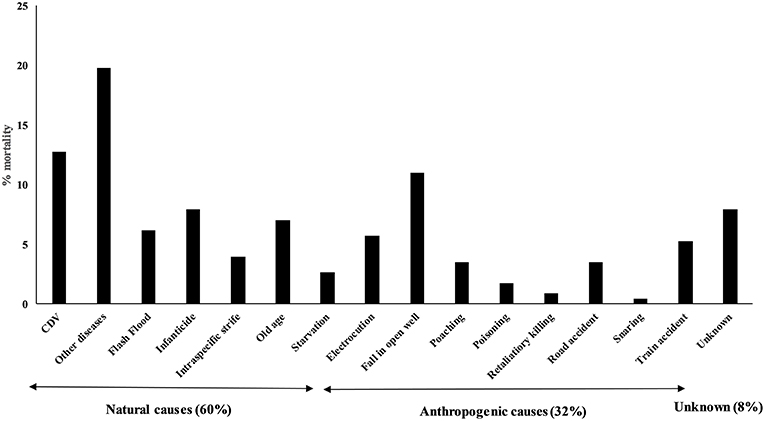
Figure 3. Causes of mortality among free-ranging Asiatic lions (n = 288). Lions primarily died because of natural causes (60%), while anthropogenic reasons for mortalities (32%) were substantial. Among the natural deaths, 33% were due to canine distemper virus and other diseases.
Banerjee and Jhala (2012) had concluded that demographic parameters of Asiatic lions did not differ from those of African lions, and went on to suggest that there was no evidence of inbreeding depression on vital rates. Subsequently, there have been recorded instances where free-ranging lion cubs were detected with missing and malformed limbs, or were born blind (Supplementary Material S4). These are potential indicators of inbreeding (O'Brien, 1990), and in nature such handicapped individuals rarely survive to propagate these traits, thereby purging out deleterious alleles from the population over time (Keller and Waller, 2002). Intensive health care of wild lions as practiced in recent times by wildlife managers (between 2001 and 2010, 501 lions were captured and treated by Gujarat Forest Department, Pathak et al., 2002; Meena and Kumar, 2012) ensures survival of many such unfit individuals. Such tampering with natural selection processes can have serious implications on the future survival of wild lions (Banerjee and Jhala, 2012).
Though biologists have been observing lions in Gir since 1960's (Joslin, 1973), quantitative data on lion social behavior has only just begun to accumulate (Chakrabarti and Jhala, 2017, 2019). In free-ranging Asiatic lions, prides comprise only of females and their dependent cubs, while adult males (singletons or coalitions) form separate units covering the ranges of multiple female prides (Joslin, 1973; Chellam, 1993). However, adjacent female prides were found to have exclusive territories and such territories remained almost constant over the years (Chakrabarti and Jhala, 2019). Females of a pride rear cubs together in a crèche, but estrus synchrony is not as prominent as reported in their Serengeti counterparts (Chakrabarti and Jhala, 2017). Cubs are weaned at 5–6 months of age but remain dependent on their natal pride for food till 2–3 years of age (Joslin, 1973; Banerjee and Jhala, 2012).
Unlike as reported for egalitarian African lion societies (Packer et al., 1988), Asiatic male lions form hierarchical coalitions wherein every coalition has one dominant male who appropriates >70% of all matings and 45% more food from his subordinates from shared kills (Chakrabarti and Jhala, 2017). Owing to such strict linearism in resource appropriation between male partners in the Asiatic lion coalitions, males belonging to coalitions of two acquired higher benefits compared to single and low-ranking males in large coalitions (of >2 males). This has resulted in an optimum coalition size of two males in the Asiatic system (Chakrabarti and Jhala, 2017).
Interactions between the two sexes are limited primarily to mating and occasionally on large kills (Meena, 2008; Chakrabarti and Jhala, 2017). Male lions frequently fend for themselves: hunting on their own, scavenging livestock carcasses and kleptoprasitizing kills made by leopards and lionesses (Chellam, 1993; Meena, 2008; Banerjee et al., 2013). Asiatic lions thus form same-sex groups, where each group behaves more like a solitary carnivore and act as independent entities (Chakrabarti and Jhala, 2019). Group sizes are smaller in the Asiatic system with male and female groups averaging at 1.7 (SE 0.2) and 2.5 (SE 0.4) adults, respectively (Gogoi, 2015). Such operational and functional separation between females and males seem to be in contrast with lion societies reported from the Serengeti and Ngorongoro (Schaller, 1972; Bertram, 1978; Packer et al., 1988). However, degrees of male-female interactions akin to that found in Gir have also been reported from lions in the Luangwa valley in Zambia, where hunting of males have severely reduced their numbers and hence, ability to maintain exclusive and all-round access to female groups (Yamazaki, 1996).
Male coalitions (with ≥2 male partners, n = 7) had an average home range (95% Minimum Convex Polygon) of 120 (SE 19) km2, much larger than single males (n = 4) averaging at 31 (SE 3) km2. Single males had shorter tenures as territorial breeders [14 (SE 3) months) than coalition males [30 (SE 4) months] (Chakrabarti and Jhala, 2019). For reproducing successfully, a male needs to hold tenure for over 24 months so as ensure that cubs sired by him reach recruitment age and are not killed by infanticidal new territorial males (Schaller, 1972). In cases where resident male(s) were ousted by new male(s), cubs and juveniles <18 months of age were mostly killed by the new males or rarely survived when forced to disperse (Chakrabarti and Jhala, 2019).
Chakrabarti and Jhala (2019) hypothesizes that this disparity in group size and male-female association from the lions in the Serengeti can be attributed to plasticity of social behavior in response to the differences in resource availability between the two systems. Asiatic lions subsist on smaller prey (modal prey- chital Axis axis, averaging at around 45 kg) (Meena et al., 2011; Banerjee et al., 2013; Chakrabarti et al., 2016), resulting in heightened intra-group competition for food and ensuing smaller group sizes (Chakrabarti and Jhala, 2017). Furthermore, in the Asiatic system prey species are non-migratory and evenly distributed at reasonably high densities; resulting in smaller and seasonally uniform female pride territories (Jhala et al., 2009) and higher lion density. This possibly allows males to maximize their reproductive potential by encompassing many female prides within their home ranges simultaneously. These arguments pertaining to prey- size and availability are in consonance with circumstances prevailing in West and Central African lion populations, where the lack of large prey has been reported to have resulted in small group sizes in lions (Bauer et al., 2003). Furthermore, it has been reported from the woodlands in Kruger that male lions were often found to be loosely associated with a particular pride of females; and spent more time patrolling territories, hunting on their own and mingling with other female groups (Funston et al., 1998). Such a system somewhat mirrors the degree of male-female association in the Asiatic lion population, and as postulated by Funston et al. (1998), availability of ample cover seems to be one of the driving mechanisms for such a societal regime. With dense cover that aids in concealment, female lions likely require less assistance from their pride males in safeguarding cubs from marauding, infanticidal males (Funston et al., 1998). Following this argument, Chakrabarti and Jhala (2019) opined that dense cover in the deciduous Gir forests may have also prevented male Asiatic lions from controlling the females and retaining exclusive access to a female group.
In the Asiatic system, although male coalitions encompass multiple female groups, none of the female prides remain exclusive to any particular coalition (Meena, 2008; Banerjee, 2012). Such non-exclusivity of female groups to particular males/coalitions have compelled and allowed females to be promiscuous, where lionesses were found to mate with multiple neighboring (rival) coalitions (Chakrabarti and Jhala, 2019). In systems, where male coalitions have mostly exclusive mating rights over pride females (like in the Serengeti), extra coalition paternity are rare (Gilbert et al., 1991). But in land tenure systems where lionesses encounter multiple male coalitions who can potentially kill unfamiliar cubs, promiscuity likely aids females to familiarize with several males and buffer infanticide (Chakrabarti and Jhala, 2019). Furthering this thought, extra-coalition paternity has been reported from lions in Etosha where a genetic assessment has revealed that 41% of the cubs in the population were borne out of multi-male promiscuous matings (Lyke et al., 2013). The social organization and sexual strategies of lions differ across their entire global range of habitats, highlighting resource-mediated and anthropogenically (hunting pressure) driven behavioral plasticity in lions inhabiting diverse eco-regions (Chakrabarti and Jhala, 2019).
We used VHF, GPS, satellite telemetry and long-term monitoring of known individuals to understand ranging patterns, land tenure, habitat use, and activity patterns of lions (n = 97) across the Saurashtra landscape. Besides obtaining regular fixes (locations ranging from one per hour to one in 3 days), we followed each radio-collared lion on foot and/or a four-wheel drive vehicle continuously for 192–360 h sessions and carried out an all-behavior sampling [n = ~6,412 hours of continuous monitoring data from 27 telemetered lions] (details available in Banerjee, 2012; Banerjee et al., 2013; Jhala et al., 2016).
Within the Gir PA, home ranges (95% MCP) of territorial males averaged at 91 (SE 17) km2; which were more than three times the ranges of breeding females [27 (SE 8) km2]. Lion home ranges in the human-dominated landscape outside the Gir PA were much larger than those inside the PA [territorial male = 832 (SE 42) km2; breeding female = 169 (SE 57) km2]. Core area (50% Fixed Kernel) of breeding lionesses inside Gir PA [7 (SE 3) km2] were four times smaller than that of breeding lionesses outside the Gir PA [30 (SE 15) km2] (Banerjee, 2012; Chakrabarti, 2018). Larger home ranges of lions in the outer landscape is in accordance to the Resource Dispersion Hypothesis (Macdonald, 1983) attributable to patchy distribution of resources (prey and suitable habitats) in the landscape, while within the Gir PA these are uniformly available.
Banerjee (2012) found average territorial tenure of males (n = 7) to be 36 (range 18–60) months while average age at dispersal from natal prides for sub-adult males (n = 6) to be 3.9 (SE 0.13) years. We observed an old displaced male to successfully re-establish another territory and even father cubs after spending some time as a nomad. Average shift between successive territories for adult males was 21 (SE 5) km, while dispersal distance of sub-adult males from their natal territories was 16 (SE 4) km (Banerjee, 2012). Contrary to our expectation, activity patterns of lions within and outside PAs differed very little (Figure 4), attributable to the omnipresent human activities in the landscape and within the PAs (tourism, pilgrimage, grazing of livestock, and commercial activities of Maldharis).
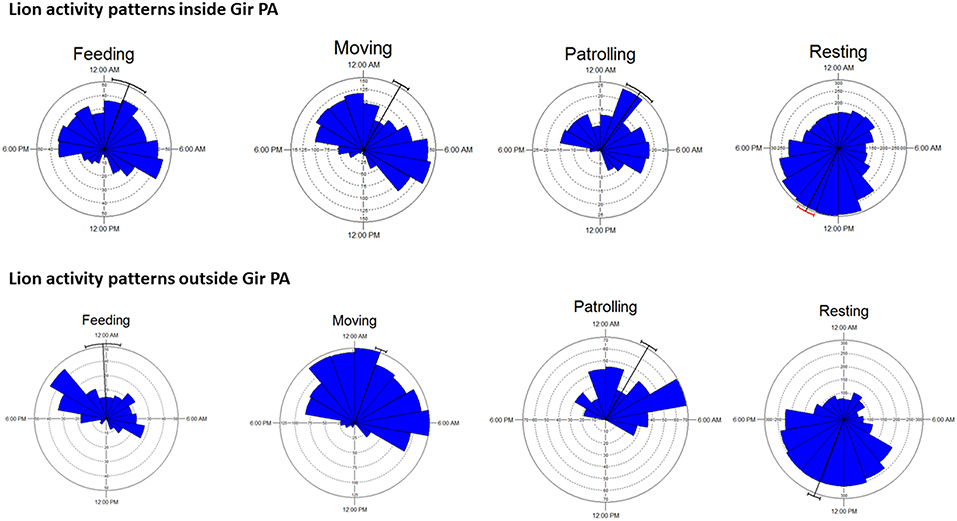
Figure 4. Activity patterns of lions (n = 27 radio-collared lions) inside and outside Gir PA based on continuous (day-night) all behavior sampling data (~6,400 h). Major behavioral states have been depicted.
Gir vegetation primarily comprised of thorn and deciduous forests along with evergreen riverine patches (Qureshi and Shah, 2004). These riverine patches were critical lion habitats that provided respite from the summer heat (Jhala et al., 2009). Creation of the 259 km2 National Park in 1975 after removal of 592 Maldhari families from central part of the Gir PA and recovery of the forest after the cyclone of 1982 has resulted in an increase in shrub (Helicteres isora, Holarrhena antidysenterica etc.) and tree density within the Gir PA (Khan, 1993; Sharma, 1995; Basu, 2013). Wildlife managers believe that this increasing vegetation density makes the habitat unsuitable for lions and their prey (Sinha et al., 2004), and have recommended selective thinning (Singh and Kamboj, 1996). However, wild ungulates of Gir are primarily browsers while domestic livestock are grazers (Dave and Jhala, 2011). Therefore, management interventions of opening habitats (besides removal of exotic invasive weeds like Senna uniflora and Lantana camara that abound in the livestock grazed areas of the PA) should be done only after careful site-specific evaluation.
Within the agro-pastoral landscape outside the PA, core areas of lion home ranges were composed of agriculture and thorn forests (Banerjee, 2012). Home range cores were observed to be farther from villages and townships but were closer to drainage and PAs (Banerjee, 2012; Jhala et al., 2016). Lions were active at night in this human dominated landscape, often venturing into villages and townships to hunt livestock. However, with advent of human activities during the day, lions sought concealment in vegetation cover. Average day time refuge patch size of lions in the human dominated landscape outside the PA was 7.5 km2 (SE 0.74) but even small patches of vegetation (5–7 ha) were used. However, successful breeding by lionesses in this landscape required habitat patches of >4 km2 (Banerjee, 2012). These findings through radio-telemetry highlight the importance of small interspersed vegetation patches characterized by thickets of Prosopis juliflora and Acacia senegal for lion persistence in the larger agro-pastoral landscape of Saurashtra (Figure 2). Remotely sensed time-series data on land cover changes suggests that this agro-pastoral landscape is rapidly being converted into urban setup with increasing development of linear infrastructure (Basu, 2013). Such infrastructure are detrimental for continued lion occupancy of the landscape as they will remove breeding and day-time refuges, as well as hinder dispersal routes between lion populations in the landscape and the PA. If lions are to continue to persist in this landscape, urgent changes in land policy and infrastructure development are required to safeguard these critical habitat patches and their connectivity.
A successful strategy for conserving large carnivores is to maintain a metapopulation structure (Hanski, 1994) within the landscape wherein one to many populations, that are demographically and genetically connected, act as source populations (Chapron et al., 2008; Walston et al., 2010). Preferably the source population habitat(s) for a large carnivore should be inviolate, wherein carnivores can subsist on natural prey and perform their ecological role. For Asiatic lions, such an area is a small National Park (259 km2), that can at best accommodate 25 lions which are demographically not viable by themselves (Banerjee et al., 2010). For tiger reserves in India, a minimum population of 20 breeding females is considered to be viable (Gopal et al., 2007; Chapron et al., 2008). To achieve this, an area of 800–1,000 km2 is required, and has been legally mandated to be made inviolate by incentivized voluntary relocation of human settlements from Tiger Reserves to delineate core areas (Gopal et al., 2007). A similar approach is required for Asiatic lions and an additional area of the Gir WLS needs to be legally demarcated and augmented to the existing National Park so as to cover a total of about 800–1,000 km2. Land ownership outside the PA is predominantly private and the Gujarat Forest Department has little control over changes in land-use patterns. Therefore, after securing a viable lion population within an inviolate space, protected areas under less stringent categories like conservation and community reserves that permit uses by local communities and safeguard their livelihoods [Wildlife (Protection) Act 1972 (2006 amendment)] should be used to conserve habitat patches within the larger human dominated landscape. Currently, radio-telemetry has shown that lions move across the landscape freely using certain land use categories and topographical features like drainage systems (Banerjee, 2012). However, the expansion of existing roads into heavy traffic highways, railways, and other linear infrastructure is likely to severely curtail such movement. Using lion locations from telemetry (>9,000), a habitat suitability map using an ecological niche factor analysis (Hirzel et al., 2002) was prepared and optimal connectivity between lion habitat patches modeled using PATHMATRIX (Basu, 2013; Jhala et al., 2016; Figure 2). These habitat corridors are the minimal requirements for lions to move between habitat patches and maintain the landscape scale metapopulation structure. Infrastructure that cuts through lion habitat patches and corridors needs to be made lion friendly and permeable using wildlife under- and over-passes (Jhala et al., 2016). The only legal provision available to regulate land use conversions in such lion habitats is by the provision of declaring Ecosensitive Zones under the Environment Protection Act (1986). Identified habitat patches and corridors (Figure 2) should be made part of the ecosensitive zone of the Gir PA. Such a declaration by the Government of Gujarat would enable authorities to reduce further losses of these areas to industry, mining and infrastructure while permitting uses that are conducive to lion conservation and local livelihoods. Currently, the Gujarat Forest Department is primarily responsible for lion conservation across the landscape, a responsibility that needs to be shared with various stakeholder agencies including roadways, railways, electricity, and civil administration. Such a multi-collaborative approach would ensure that development and conservation go hand-in-hand and are not always at loggerheads.
Until early 1970s, Gir PA was dotted with about 300 Maldhari settlements (nesses) having over 40,000 livestock that formed the staple prey of lions (75% of their diet, Joslin, 1973), while wild ungulate numbers in the PA were few (5,600, Berwick, 1974). In 1975, when Gujarat was under the federal Government rule, about 190 Maldhari families along with their livestock were resettled outside Gir PA. In 1982 Gir experienced a major cyclone that uprooted ~2.5 million large trees, resulting in the opening of the canopy and increased browse availability for ungulates (Dave and Jhala, 2011). Reduction in competition from livestock (Khan, 1993; Sharma, 1995) coupled with increased food availability by the cyclone and better law enforcement that checked poaching are believed to have resulted in the recovery of wild prey (Dave and Jhala, 2011). Regular monitoring of prey using line transect based distance sampling compared with data on prey estimates from Joslin (1973) and Berwick (1974) show that wild ungulates increased in their numbers till early 2000, and since then have reached stable densities (Jhala et al., 2016). Consequently, proportion of domestic livestock in lions' diet within the PA declined to 52% by the 1980's (Sinha, 1987) and further to 25% (Chellam, 1993; Meena et al., 2011; Banerjee et al., 2013) during the next three decades.
We investigated lion foraging ecology through direct continuous observations on radio-collared lions to record feeding events (>6,000 h observation on 27 lions), and through scat analysis (n = 495). The Saurashtra landscape supports a large livestock population (~6.4 million, Junagadh Agricultural University, 2016). With majority of the people being vegetarian combined with the religious sentiment of Hinduism and Jainism, cattle are not consumed for meat. Several charitable cattle camps (locally known as Gaushalas and Panjrapoles) that house old and unproductive cattle are distributed across the landscape. Livestock carcasses are usually dumped at specific locations called haddakhodis outside villages and such Panjrapoles. Carnivores including wolves (Canis lupus pallipes) and striped hyenas (Hyena hyena hyena) within agro-pastoral landscapes rely predominantly on this assured food source (Jhala, 2002) across India. This factor has played a major role in promoting and sustaining the dispersal of lions outside of the PA. Lions are opportunistic feeders and rely both on predation and scavenging. Occurrences of food remains in scats are unable to distinguish between predation and scavenging, and if used alone can overestimate livestock-lion conflict. By using both direct observation on feeding events and scat analysis, Chakrabarti et al. (2016) was able to quantify contribution of dead livestock to lion diet. Chakrabarti et al. (2016) further developed models for estimating biomass consumption from prey occurrences in scats by conducting feeding experiments on lions, correcting previous diet estimates from lion scats that were fraught with considerable biases owing to the use of an incompatible model developed by Ackerman et al. (1984) for pumas (Puma concolor). Optimal foraging models developed by Chakrabarti et al. (2016) suggest that due to constraints of gut fill, passage time and carcass decomposition; medium-sized prey like chital comprise of the principal prey for large carnivores, including lions, in tropical systems. Lion diet outside the PA was composed of 25% wild prey and 75% livestock (Banerjee, 2012). However, telemetry data demonstrated that among the total consumption of livestock, 35% was from actual predation while 65% from scavenging (Banerjee, 2012). Rarely were prized productive livestock killed by lions due to the husbandry practice of stall feeding and corralling such livestock during the night (Banerjee, 2012). Farmers were tolerant toward lions in their vicinity and property due to lions acting as effective predators for nilgai and wild pigs that caused substantial crop damage in this landscape.
The Gir forests have been inhabited by the Maldharis for the past 200 years (Casimir, 2001). Maldharis have strong ethics and sentiments toward nature and natural resources. They are primarily vegetarian and their major livelihood is livestock husbandry for sale of dairy products. This religious and social background makes them tolerant toward lions, a powerful figure in their folklore and culture. Yet, Maldharis persecute lions to deter them from attacking their stock with sling shots, axes, and staffs. In the past, lions have also been poisoned on livestock kills. The Nawab of Junagadh recognized this threat early on and commenced a livestock depredation compensation scheme to the owners of livestock killed by lions. This scheme has been continued by the Gujarat Forest department and is revised regularly to keep pace with livestock market prices (Supplementary Material S5). Lions loath Maldharis and keep their distance when detected by them and their livestock. The water buffalo (Bubalus bubalis), that constitutes the majority of the livestock (78%) kept by Maldharis, herd together and defend themselves against lions (Banerjee et al., 2013). The husbandry practices of Maldharis are honed over years of experience to minimize losses to predation. Livestock are grazed in forests during the day and corralled in thorn bomas during the night. The herd leaves the boma much after sunrise with one to three herdsmen (depending on the size of the herd) and returns back around sundown. The grazing herd structure is composed of cattle and juvenile male buffalos at the front with prized buffalos in the middle, and herdsmen at leading and trailing ends. During lion attacks, the cattle and juvenile livestock (least expensive) scamper and run, becoming most vulnerable. Adult buffalos form a protective ring, often attacking lions in this formation under directions of the herdsmen, and rarely get killed (Banerjee et al., 2013). Dead livestock of Maldharis are dumped at specific sites and lions use this resource extensively. Radio-collared lions within the PA were observed to make regular excursions to these dump-sites near the nesses in search of free food (Jhala et al., 2016). Therefore, lions do benefit from Maldhari livestock through scavenging opportunities and occasional predation, only when strict law enforcement along with a fair livestock depredation compensation scheme control for lethal retaliation against them. The Maldharis that live in lion habitats benefit from getting free access to forest resources for themselves and their livestock. We found that Maldharis living within the Gir forests made 76 (SE 0.05) % more profits than livestock herders living outside the Gir forests (Banerjee et al., 2013). Thus, the relation between Maldharis and lions is far from harmonious coexistence, it is more of co-occurrence with benefits to both parties that are maintained by a delicate balance through cultural attitudes, strict law enforcement, fair compensation scheme for livestock kills, livelihood benefits to Maldharis and rare attacks on humans by lions. A total 190 lion attacks on humans have been recorded between 2007 and 2016 in the Gir landscape, of which a small proportion (n = 12, 4%; 1.3 attacks/year) resulted in human fatalities. While attacks by leopards on humans in the same landscape were 383 between 2011 and 2016, out of which 41 were lethal (~7/year). Elephants (Elephas maximus) and tigers cause higher losses to human lives (408 and 34 human deaths/year respectively between 2013 and 2015) across India (answer to un-starred question no. 2581, The Lok Sabha, Government of India, 2017; accessible at http://www.indiaenvironmentportal.org.in/files/file/Human-Wildlife%20Conflict_0.pdf). Attacks on humans by lions were observed to increase during years of extreme droughts that caused large livestock populations to enter and graze within PAs (Saberwal et al., 1994). Data from telemetered lions show that lions were mostly non-hostile to humans (one in ten thousand encounters translated into an attack, Jhala et al., 2016). Attacks were mostly accidental: lions rarely stalked or targeted humans as prey, but usually attacked in self-defense or when spooked (Banerjee, 2012).
Livestock densities within a PA beyond a threshold were detrimental to native vegetation communities and wild ungulates (Dave and Jhala, 2011). Profuse growth of weeds and unpalatable vegetation were found to grow in the vicinity of ness sites (Dave, 2008). Lions, on the other hand can do well without livestock in their diet and will adjust their densities to natural levels based on the availability of wild ungulates (Schaller, 1972; Van Orsdol et al., 1985) which are reasonably high in Gir PA (63/km2; Jhala et al., 2016). Therefore, creating additional inviolate space within the Gir PA by relocating the remaining nesses to increase the area under the National Park would not only benefit lions but the entire native biota of the region. At the least, Maldhari ness sites should be rotated every 4–5 years to allow native vegetation to recover from the heavy grazing and trampling effects of livestock (Dave and Jhala, 2011).
Banerjee (2012) interviewed 680 local residents in the landscape using structured interviews to gain an understanding on their attitudes toward lions. Besides the common factor of culture and religion that helped foster lion presence in the human dominated landscape, factors related to livelihood benefits differed from those that operate inside the PA. Pastoralists, on the contrary, were not tolerant toward lions because of the losses they incur from lion predation on their livestock and occasional attacks on them when they attempted to deter lion predation on their livestock (Figure 5). Analysis of last 5-year data on livestock kills by lions across the entire Saurashtra landscape (914 villages) suggests an increasing trend in the intensity of depredation (Figure 6). Livestock kills were compensated by the Government, and these helped ameliorate retribution. However, pastoral communities outside the Gir PA were not satisfied with the Government compensation scheme (Banerjee, 2012) since there were no free resources (like for Maldharis inside the PA) and there was a significant deficit between the market rate for livestock and compensation paid for lion predation (Jhala et al., 2018). Rarely do compensation schemes take into account the “lost opportunity cost” and therefore even when compensated at market rates, predation does take a toll on livelihoods (Banerjee et al., 2013).
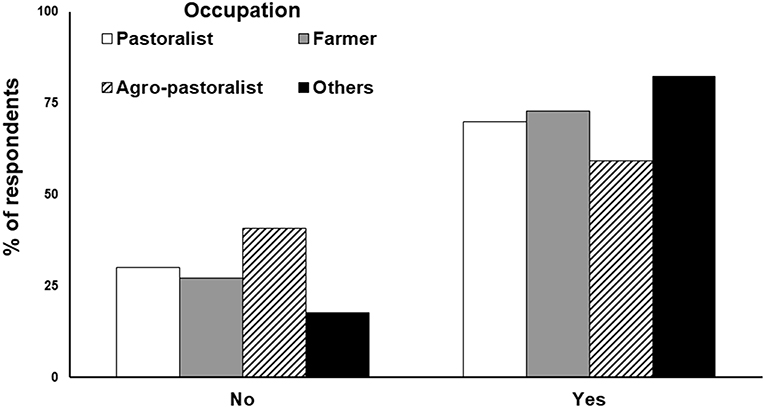
Figure 5. Attitudes of local people (n = 680 respondents) from 254 villages in the landscape outside the Gir PA regarding the continued presence of lions in their neighborhood. The respondents were categorized based on their livelihoods. The category “others” primarily represent individuals associated with the tourism industry like hoteliers, safari-vehicle providers, etc.
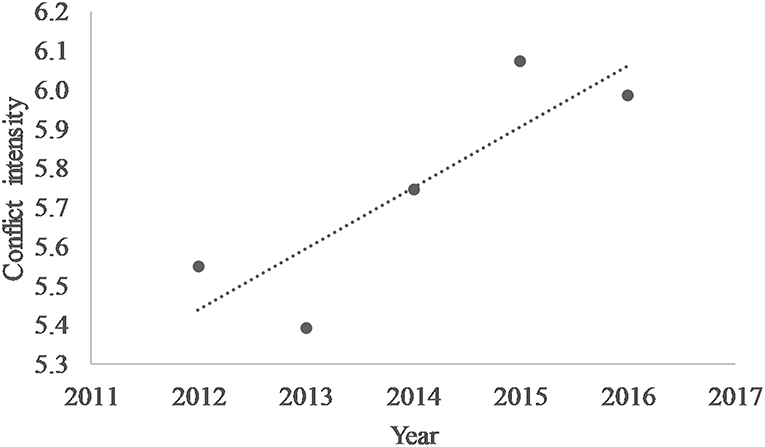
Figure 6. Trend in the intensity of livestock predation (number of predation events/number of villages with predation) by lions in between 2012–2016 within the Saurashtra landscape. The data on livestock kill by lions encompasses a total of 914 villages across the entire range of Asiatic lions.
Economic reasons were found to be the most significant factor shaping people's tolerance toward lions in the landscape. Communities making direct or indirect profit from lions were more tolerant toward them (Figure 5). The two important economic benefits from lions were: (a) their propensity to predate nilgai and wild pigs, both considered as agricultural pests. With no hunting allowed in India, these ungulates can achieve high densities and cause severe local economic losses to livelihoods, (b) presence of lions offered an opportunity for tourism and employment. The Gir PA has a tourism zone where wildlife enthusiasts can visit for a safari, which has encouraged tourist resorts and correlates to flourish in Western Gir. This has economically benefited the local communities residing in this region of Gir. However, not all tourists get to see lions, and the PA management has imposed several restrictions on limited number of vehicles, on-foot access, baiting of lions, etc. Such restrictions are difficult to enforce on private lands across the 13,000 km2 of lion occupied Saurashtra landscape. Local communities avail this opportunity and conduct “lion shows” outside the PA (Singh, 2017b). Such shows primarily comprise of lions being attracted on private lands through subsidized food (baits/carcasses), while tourists pay the owners of these farmlands to watch lions in action. The tourists often pay exorbitant amounts for these shows as they are guaranteed sightings of lions and granted liberties with them (night photography, watching lions on foot and/or from close proximity) that can be dangerous for tourists as well as lions. However, the profits from such shows are not shared equitably and monopolized by few powerful members of the community. Though considered “illegal,” such lion shows are difficult to control and are a major source of lucrative and easy income for locals across the agro-pastoral landscape. Thus, in our assessment, lion-human coexistence in the human dominated landscape has been possible due to: (a) low lion density (about 2–3 lions per 100 km2); (b) low levels of conflict, lions subsist by scavenging dead livestock, predate unproductive cattle (that are reasonably compensated), and rarely attack humans. Problem lions are immediately removed by management; (c) economic benefits to local communities through removal of crop pests and revenue generation via lion tourism; (d) high level of tolerance of local communities due to religious and cultural attitudes; and (e) strict laws and their enforcement against killing of lions. Changes in any of these factors can disrupt the current coexistence. However, since lions continue to increase in density and occupancy across Saurashtra, it is a matter of time before they exceed social tolerance limits. There is a perceived shift in the values of local communities from those of tolerance and reverence toward direct economic gains (Banerjee, 2012). Although attacks on humans are rare, the psychological (Löe and Röskaft, 2004) and socio-economic consequences of these attacks can be dire for future lion-human coexistence in Saurashtra.
Also, recently, the wildlife authorities have seriously implemented measures to curb “lion shows” by local communities. This may have serious consequences on continued lion persistence in the human dominated landscape, if indeed this action manages to stop such shows. Communities that cannot have direct profits from having lions in their backyards may not be willing to have them there anymore.
Majority of the people in the agro-pastoral landscape of Saurashtra have a positive attitude toward lions (Banerjee, 2012; Meena et al., 2014). This is vital, but a positive attitude by the majority does not necessarily translate into tolerant coexistence, since it is the behavior of the few but resentful people that ultimately determines the dynamics of human-lion interface (Kansky and Knight, 2014). Such behavior is largely determined by a combination of factors relating to their personal situation and experiences, psychological factors and value judgement (Barr, 2003). Understanding biological and social carrying capacities (threshold for human tolerance) for lions thus becomes important in managing coexistence in this multiple-use landscape of Saurashtra. For example, ranches adjacent to Kenya's Tsavo East National Park, lose 3% of their herd's total economic value to lions; nonetheless, the ranchers are prepared to tolerate a population of ~26 adult lions whose diet consists of 6% livestock, costing the ranches US$290/lion/year (Patterson et al., 2004). We suggest that lion density outside the Gir PA should be maintained below social carrying capacity and problem lions should be removed immediately from the vicinity of the people. Guidelines for such removals can be adopted from the Standard Operating Procedures developed for tigers and leopards in India (National Tiger Conservation Authority, 2013), keeping in mind the social dynamics of lions (Whitman et al., 2004). Thus, a futuristic and multifaceted policy is required to permit this delicate balance of human-lion coexistence to continue.
A single population of an endangered species is susceptible to extinction events caused by environmental and demographic stochasticity (Soulé, 1987). The 1994 outbreak of canine distemper virus (CDV) in the Serengeti killed an estimated 33% of the lion population (Roelke-Parker et al., 1996). An epidemic of such magnitude in Gir could potentially put the Asiatic lion at a high risk of extinction. Gir lions have tested positive for CDV, feline parvovirus, feline herpesvirus, feline immunodeficiency virus and peste des petits ruminants virus (Sabapara, 2002; Ramanathan et al., 2007; Balamurugan et al., 2012). Lions move regularly between habitat patches in the landscape and share space with feral dogs, cats, and other carnivores, creating a condition for the spread of epidemics. A recent infection of canine distemper virus killed a minimum of 28 lions in 2018 as per official records in the eastern part of the PA. However, the actual death toll could be of epidemic proportions, but remains unknown, since many carcasses remain undetected in the wild and investigations were limited only to park authorities.
The threat of extinction due to disease and natural calamities to this single population of lions was recognized early on by the Executive Committee of the Indian Board of Wildlife during a meeting held in Gir in 1956. The first attempt to establish a second population in Chandraprabha in the state of Uttar Pradesh was undertaken in 1957 (Negi, 1969). Though these reintroduced lions initially bred and increased to 11 individuals from the founding population of five, they were subsequently poached out by 1965 (Negi, 1969). After initiation of modern scientific studies on Asiatic lions, Joslin (1985) and Sale (1986) emphasized the need for establishing a second population away from Gir. This was followed by a population-habitat viability analysis workshop in 1993, wherein all stakeholders, including the Government of Gujarat agreed to the need of establishing a second lion population as an insurance against extinction (Ashraf et al., 1995). The Wildlife Institute of India was mandated with the task of identifying a site for establishing this insurance population. From the three potential sites surveyed (Sitamata, Darrah-Jawaharsagar, and Kuno) within the recent historical range of the lion, the area of Kuno Wildlife Sanctuary (345 km2) in the central Indian state of Madhya Pradesh was found most suitable since it was located within an intact forested landscape of about 3,300 km2 (Chellam et al., 1995).
Substantial efforts were made by both the Government of India and Madhya Pradesh Forest Department in preparing Kuno for lion reintroduction (Johnsingh et al., 2007; Khudsar et al., 2008). Currently Kuno has been declared as an inviolate National Park (700 km2) after the resettlement of 24 villages (1,547 families). A financial investment of about Rs 15 crores (US$ ca. 3.2 million) was done by the Government of India until 2005 for resettlement and management of Kuno (Johnsingh et al., 2006) and an equal amount invested by the Government of Madhya Pradesh. Subsequently, a buffer area of 1,280 km2 has been added to the Kuno National Park as Kuno Wildlife Division. Better protection, habitat management, and relocation of human settlement along with majority of their livestock, resulted in a substantial recovery of the wild ungulate population. The chital population have exponentially increased from a density of 5 to 68/km2 within the past 10 years (Banerjee, 2005; Bipin et al., 2013).
Gujarat monopolized Gir lions after they were stripped off their status as India's National Animal in 1973. Lions were promoted as a Gujarat State icon which soon became engrained as a symbol of the pride of the people of Gujarat (Rangarajan, 2001). Indeed, it was due to the efforts of the people of Gujarat that lions have shown an extraordinary recovery for any large carnivore. The local media exemplified and promoted this monopoly (Rahmani, 2013) which was subsequently used as an instrument of political and bureaucratic gain (Dutta, 2019). This new found exclusive ownership of the lions by Gujarat State and its bearing on the public psyche resulted in the Gujarat Government's reluctance to provide a founder stock of wild lions to the State of Madhya Pradesh (Kuno). The Gujarat Forest Department, which is the technical arm of the State Government in matters of wildlife, posed trivial arguments against reintroduction of lions in Kuno (Singh, 2007). However, a landmark judgement was passed by the Supreme Court of India in 2013 [IA No.100 in W.P (C) No.337/1995, accessible at http://www.conservationindia.org/wp-content/files_mf/Lion-judgment-SC-Apr-2013.pdf] which directed the Governments of India, Gujarat and Madhya Pradesh to reintroduce lions in Kuno despite contrary arguments of Gujarat. Although this landmark verdict by the apex court was primarily directed toward lion reintroduction, it recognized conservation as an integral part of civilized development and beckoned for applying the “species' best interest standard” for conservation of lions and other endangered species. This judgement strongly places the responsibility on the national and state governments, together with the citizens, to view development through an eco-centric approach and not just with an anthropocentric perspective.
As per the assessment of the committee for lion reintroduction appointed by the Supreme Court through their court order, Kuno National Park can currently hold about 40 lions. The larger forested landscape of about 3,000 km2 around Kuno, has the potential to support a viable lion population for the long-term. The Kuno lion reintroduction action plan (Ministry of Environment Forests and Climate Change [MoEFCC], 2016) is in consonance with the IUCN/SSC reintroduction group guidelines (IUCN/SS, 2013) and provides operational guidelines to the wildlife managers of Gujarat and Madhya Pradesh States to implement the reintroduction and subsequent management of the lion population. Despite the direction of the Supreme Court in 2013 and an action plan (Ministry of Environment Forests and Climate Change [MoEFCC], 2016) with a clear vision, the reintroduction program is facing a socio-political deadlock for the past 6 years. The program implementation is still being debated between the Ministry of Environment, Forests and Climate Change, Government of India; Gujarat Forest Department; and the Madhya Pradesh Forest Department.
While lion reintroduction in Kuno was being debated, the Gujarat Forest Department mandated the Wildlife Institute of India to evaluate the potential of Barda Wildlife Sanctuary (Figure 2) as another reintroduction site for lions within Saurashtra, Gujarat. Barda and its adjacent Alech hills (Barda landscape) had lions until the late nineteenth century after which they were locally extirpated (Divyabhanusinh, 2005). Subsequent conversion of forest and grazing lands to agriculture separated Barda from Gir (~80 km). This less permeable habitat matrix along with the initial policy of the Gujarat Forest Department to capture dispersing lions and relocate them back to Gir, prevented recolonization (Ranjitsinh, 2016). The assessment of Barda (Jhala et al., 2014b) suggested that the landscape (410 km2 comprising of 190 km2 of Barda WLS, Alech hills and coastal forest patches) could sustain about 25 lions after creating an inviolate area of about 100 km2 within the Barda WLS, restocking prey, enhancing protection, and restoring habitats. Currently the sanctuary is inhabited by about 1,325 families of Maldharis in 62 nesses and 98% of them are willing to resettle outside Barda (Jhala et al., 2014b). The costs of incentivised, voluntary relocation (Narain et al., 2005) would be close to Rs 200 crore (US$ ca. 28 million). Current wild prey density in Barda is very low and inadequate for sustaining even a single lion pride, but livestock and scavenging opportunities abound. Resettlement of human habitation and building up wild prey is likely to take several years. Establishing a lion population in Barda landscape would be beneficial for lion conservation as well as help conserve the native flora and fauna of this region which is threatened by intense human exploitation. However, a lion population in Barda cannot be considered as a security from catastrophic events like disease epidemics in the Gir landscape due to the geographic proximity of both areas and continuous presence of feral dogs, cats, and livestock in the intervening habitat. Barda, therefore cannot be an alternative solution to lion reintroduction in Kuno (Jhala et al., 2014b). Efforts of the Gujarat Forest Department in conserving a representative lion gene pool through conservation breeding programs (Meena and Kumar, 2012) are important initiatives to pre-empt a catastrophic extinction event within the Gir landscape. However, carnivores bred in captivity over several generation are usually unfit for reintroductions into the wild (Jule et al., 2008). Therefore, we submit that the “species best interest” strategy for securing Asiatic lions in the long-term would be to establish as many free ranging populations as possible within the historic range of the Asiatic lions. Founders for such populations should be sourced from wild Gir lions to capture their existing genetic diversity and subsequently managed as a metapopulation with the Gir lion population (Ministry of Environment Forests and Climate Change [MoEFCC], 2016).
The contribution of wildlife managers has been the most vital ingredient for the conservation of Asiatic lions. Wildlife management in India is done by the respective State Forest Departments. Their primary role is to manage the PAs in terms of administration, law-enforcement, wildlife conflict mitigation, habitat management, and management of wildlife tourism. Other aspects include community participation through incentives by sharing park revenues in the form “eco-development projects,” sensitization of local communities through awareness and education camps, treatment and rescue of wildlife. In this section we succinctly portray the management arena for Gir PA and discuss their strengths and weaknesses under the larger gambit of lion ecology and conservation, tethering with our previous sections.
The total strength of the wildlife department of Gir PA in 2012 was about 688 (Meena and Kumar, 2012). Modern amenities in the form wireless service, good road network, 4- and 2-wheel drive vehicles and arms are available and used by the wildlife authorities. Regular patrolling on-foot and by vehicles has controlled poaching within PAs. However, snaring and electrocution continue to be a major concern for wildlife authorities in the larger landscape. Eight lions were poached in 2007 for meeting the illegal demand of lion bone trade (Singh, 2017b). The wildlife authorities successfully nabbed the poachers and got them convicted in the court of law under the Wildlife (Protection) Act 1972 setting an example that has deterred poaching of lions to a great extent.
The wildlife department has developed competence in veterinary facilities for treating animals in distress at eight facilities across the Gir landscape. Also, lions in conflict or individuals straying into human-habitation too often are captured and rehabilitated. Perception of the public and media to an ailing/injured lion forces wildlife authorities to capture and treat such animals. Within the past decade, medical interventions for treating even minor injuries and ailments in lions have become the norm. As discussed earlier, such actions can tamper with the process of natural selection, and should be undertaken judiciously. The reluctance of wildlife authorities in seeking expert advice on dealing with dangerous situations like CDV outbreaks can have disastrous impacts on the long-term survival of this single population of Asiatic lions.
Gir being a dry deciduous forest tract, water is a major factor that limits the abundance and distribution of animals. Wildlife authorities manage the availability of water in the landscape through provisioning by regular maintenance and filling of artificial waterholes. Weed and invasive species removal is done across the PA, and an area of over 270 km2 was prescribed for treatment (Meena and Kumar, 2012). Gir PA is prone to fires and the regular management of ~1,900 km of fire-lines is done annually to contain accidental fires (Meena and Kumar, 2012).
Lion centric tourism within Gir PA is an important source of revenue for the Gujarat Forest Department and about 0.12 million tourists visited Gir PA annually in between 1995 and 2010 (Meena and Kumar, 2012). The number of tourists has substantially gone up in the recent years (0.533 million in 2015 and 0.522 million in 2016). The Forest Department permits tourism in a part of the western Gir WLS by allowing tourist vehicles (accompanied by trained nature guides) to ply over forest roads in eight pre-fixed routes (ranging from 22 to 45 km) after obtaining online permits. In order to reduce tourism pressure inside the Gir PA and to provide tourists with guaranteed opportunities of viewing lions and other wildlife, two safari parks (each of about 4 km2, enclosed by chain-link fences); at Devalia (western Gir) and Ambardi (eastern Gir) have been created that house semi-free ranging wildlife including lions. All these activities generate a substantial amount of revenue. For example, in 2016 revenue generated from gate fees was Rs. 102.5 million (~ 1.5 million US$, https://timesofindia.indiatimes.com/city/ahmedabad/gir-sanctuary-collects-its-highest-ticket-revenue-ever/articleshow/61108927.cms). However, as mentioned earlier, practice of luring lions with artificial subsidies such as carcasses to maximize lion viewing by tourists (Gogoi, 2015) should be discontinued.
With the increase in the extent and magnitude of lion-tourism, hospitality industry has flourished along the periphery of the Gir PA. Within a six-km radius of the tourism circuit of the PA, there are nearly 100 resorts, hotels and guesthouses catering to the needs of tourists. Many such facilities have been totally or partially shut down following a suo motu Gujarat High Court order against illegal and haphazard construction around Gir PA. Meanwhile, the Government of Gujarat has submitted an eco-tourism policy to the High Court proposing to: (i) decline new licenses for hotels and resorts within 1 km of the Gir PA and (ii) levy a new tax known as “eco development fee” for conversion of agricultural land to commercial tourism purposes.
With the objective to sensitize the younger generation toward wildlife conservation, nature camps are conducted by the Gujarat Forest Department since 1976. Students from local schools and colleges camp at five designated sites in the PA and are taken on field excursions with trained nature interpreters, interact with wildlife managers through illustrated talks and field demonstrations, and are shown wildlife documentaries (Meena and Kumar, 2012). Additionally, eco-clubs have been established in about 300 schools within the Gir landscape with the aim of spreading awareness related to nature conservation in their localities. The Government tourism facility at Gir has a good interactive interpretation center that is popular amongst the visitors of the park.
In order to garner public support for lion conservation, an “eco-development” scheme was initiated in Gir along with six other PAs in India under the India Eco-development Project funded by World Bank's Global Environment Facility. A total of 193 villages have so far been covered in the landscape under this scheme (Singh, 2017a). Under this scheme, repair of village roads, support for self-employment, construction of structures for harvesting water and preventing soil erosion, facilities for education, drinking water, sanitation and improvement of houses are provided and linked to wildlife conservation. Parapets were constructed for about 25,000 open wells that otherwise act as death traps to lions and other wildlife. Members of the local community that have demonstrated wildlife skills are designated as vanya prani mitra (friends of wildlife) and paid a nominal remuneration for assisting with wildlife management activities of the park managers (including information on poaching, fire management, and wildlife conflict resolution). Besides the monetary remuneration, the enhanced social status of the vanya prani mitra is an incentive for community members to strive to become “wildlife friends.” An average amount of Rs. 8.36 million (~122,139 US$) was spent by the Forest Department for accomplishing various activities under this scheme between 2006 and 2010.
Another major activity undertaken by wildlife managers is the 5 yearly periodic population enumeration of lions. This exercise is commendable in its extent, effort, and regularity. However, as previously mentioned, the archaic approach of population census through total counts needs to be replaced with modern scientific approaches of animal abundance estimation that explicitly address the issue of detectability and double counts. Such a scientific assessment by an independent agency would also preclude the potential of distorting numbers to create political populations (Darimont et al., 2018) as was done earlier with tiger populations in India (Karanth et al., 2003).
The recovery of the Asiatic lion in the Saurashtra landscape is a success story and lauds the efforts of the native rulers and the republic Governments of India and Gujarat. The exemplary coexistence between lions and the people of Saurashtra provides the world with a plausible model and after addressing the caveats mentioned above, can be replicated in other areas. The most important ingredient that sets the stage for this coexistence is the traditional value of reverence toward lions and other life forms. Yet, in a changing world of values, economic profits related to livelihoods derived directly or indirectly from lions played a significant role in promoting coexistence. The current stand of authorities in accordance with the Wildlife (Protection) Act 1972, is to ban lion shows, since there are currently no mechanisms for regulating them. However, these shows are a major source of direct profits for local communities. Despite the negative aspects of lion shows, we believe that they provide a window of opportunity for a paradigm shift in the conservation ethos in India. We propose that wildlife authorities should take the initiative to work with elected representatives of the local community (village panchayats) to form consortia of one to several villages across the landscape to form “Community Lion Conservancies (CLCs).” These CLCs would then formulate guidelines for lion based ecotourism in accordance with the Wildlife (Protection) Act 1972, in consultation with the Forest Department and NGO's. The principles for profit sharing from lion shows should be equitable unlike the present despotic system where profits are monopolized by socially powerful elements of the community. A potential mechanism for equitable sharing of profits while retaining individual incentives would be to use majority of the revenue in community upliftment activities (such as better health-care, sanitation, education etc.), while a small fee is paid to the land owners who manage their lands in a lion-friendly way to promote such ecotourism opportunities. Lion ecotourism operated and regulated on such principles would ensure that existing laws are not violated, lions are not harassed, allow wildlife authorities to keep check on such activities on private lands, while profits are shared amongst the entire community thereby promoting goodwill and support for lion conservation across all sections of the society. Once legally recognized and benefits are directly associated with lions, we believe that communities will protect and promote lions and their habitats in CLCs and can also locally pay compensation for livestock predation events to bridge the gap between Government compensation and market price. In densely populated countries like India where creating large PAs is difficult, conserving lions as well as other wildlife in human dominated landscapes is essential to house viable populations. Co-occurrence is bound to create conflict. Good innovative management practices to promptly address these conflicts along with economic incentives to local communities is the only way to ensure continued tolerance toward large carnivores in such landscapes. Formalizing and legally recognizing profits from wildlife with appropriate controls through the proposed CLCs will be a major paradigm shift for conservation in India.
The only source population of Asiatic lions is within the Gir PA. To preserve the lions' ecological role and evolutionary potential it is important that a substantial population is maintained in its natural setting. Thus, it is important to expand the National Park to create an inviolate habitat of 800–1,000 km2 for lions, as is recommended for tiger reserves in India. In the human dominated landscape of Saurashtra, all habitat patches larger than 4 km2 (Figure 2) should be targeted for protection and conservation, as these serve as breeding refuges and are vital elements for lion persistence in the landscape. Development within the identified lion movement corridors should be curtailed and linear infrastructure traversing such corridors must be mitigated with animal passageways.
Establishing a second free-ranging lion population away from Gir should be the most important conservation priority for the species. Kuno is an ideal option in a state that has a proven track record for tiger conservation. Substantial investments have already been made in Kuno which is ready to receive the founding stock of lions. It is unfortunate that due to overly enthusiastic zeal of ownership and monopoly of the people of Gujarat, the Asiatic lions are caught in a socio-political deadlock that prevents this essential reintroduction program, despite a clear directive of the Supreme Court of India.
Conservation of Asiatic lions is thus a conundrum with an admixture of contradictions and improvisations. Based on information accrued from our long-term research added to past knowledge, we demonstrate that conservation of a species so deeply engrained in human ethos and psyche not only requires appropriate scientific knowledge of its ecology but also a multidimensional understanding that encompasses history, culture, economics, and politics for its holistic management. We reiterate that only through the continued nurture of Asiatic lions and other wildlife would their nature be fully safeguarded in a country like India that teems with people and biodiversity.
Author sequence is in order of their contribution to this research and review. YJ conceived the research project and raised resources, supervised field work, logistics, and data collection. YJ, KB, SC, PB, KS, CD, and KG collected and analyzed primary data pertaining to different components of the long-term research project. YJ, KB, and SC reviewed pertinent literature and wrote the manuscript. YJ, KB, SC, and KG prepared the maps and figures for the manuscript. All authors read and commented on the initial drafts of the manuscript.
Between 1995 and 2018, the funding for our long-term project was from multiple organizations. The Wildlife Institute of India provided major funds. Department of Science and Technology, Government of India (SERB/F/0601/2013-2016; between 2013 and 2016), Gujarat Forest Department (WLP/B/TS/WII; partial funding between 2000 and 2003, 2011 and 2013, 2016 and 2018), and US Fish and Wildlife Service (98210-2-G458; partial funding between 2006 and 2009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Ministry of Environment Forests and Climate Change, India, Chief Wildlife Warden, Gujarat State and Chief Conservator of Forests, Junagadh for granting permissions and facilitation of the study. Deputy Conservators of Forests, Gir are deeply acknowledged for their facilitation of field work and data collection through the last two and half decades. The Wildlife Institute of India, Gujarat Forest Department, US Fish and Wildlife Service, National Geographic Society and Department of Science and Technology, India are thanked for funding various components of our long-term research project in Gir. We thank our field assistants: Late Taj, Osman, Ismail, Hamal, Late Guga, Bhola, Bhupat, Kanti, Mannu, Hanif, Sameer, and Iqbal for their hard work and skill in working with lions. We also thank the wildlife guards and trackers of Gir Management Unit for their dedicated lion search and sharing of information. Swati Saini, Nupur Rautela, and Adarsh Kulkarni are thanked for their help with preparing the maps. We thank Luke T. B. Hunter, Michael J. Somers, and Matt W. Hayward for their constructive comments on earlier versions of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00312/full#supplementary-material
Ackerman, B. B., Lindzey, F. G., and Hemker, T. P. (1984). Cougar food habits in Southern Utah. J. Wildlife Manag. 48, 147–155. doi: 10.2307/3808462
Antunes, A., Troyer, J. L., Roelke, M. E., Pecon-Slattery, J., Packer, C., Winterbach, C., et al. (2008). The evolutionary dynamics of the lion Panthera leo revealed by host and viral population genomics. PLoS Genet. 4:e1000251. doi: 10.1371/journal.pgen.1000251
Ashraf, N. V. K., Chellam, R., Molur, S., Sharma, D., and Walker, S. (1995). Population and Habitat Viability Assessment Workshops for Asiatic Lion, Panthera Leo Persica, Report July 1995. Conservation Breeding Specialist Group, Apple Valley, MN, U.S.A. 113 p.
Badam, G. L., and Sathe, V. G. (1991). “Animal depictions in rock art and palaeoecology—a case study at bhimbetka, Madhya Pradesh, India”, in Rock Art—The Way Ahead: South African Rock Art Research Association First International Conference Proceedings, eds S. A. Pager, B. K. Swatrz Jr., and A. R. Willcox (Natal), 196–208.
Balamurugan, V., Sen, A., Venkatesan, G., Bhanot, V., Yadav, V., Bhanuprakash, V., et al. (2012). Peste des petits ruminants virus detected in tissues from an Asiatic lion (Panthera leo persica) belongs to Asian lineage IV. J. Vet. Sci. 13, 203–206. doi: 10.4142/jvs.2012.13.2.203
Banerjee, K. (2005). Estimating the ungulate abundance and developing the habitat specific effective strip width models in Kuno Wildlife Sanctuary, Madhya Pradesh (Dissertation/Master's thesis). India: Forest Research Institute University, Dehradun, India, 170.
Banerjee, K. (2012). Ranging patterns, habitat use and food habits of the satellite lion populations (Panthera leo persica) in Gujarat, India (Dissertation/Ph.D. thesis). India: Forest Research Institute Deemed University, Dehradun, India, 435.
Banerjee, K., and Jhala, Y.V. (2012). Demographic parameters of endangered Asiatic lions (Panthera leo persica) in Gir forests, India. J. Mammal. 93, 1420–1430. doi: 10.1644/11-MAMM-A-231.1
Banerjee, K., Jhala, Y. V., Chauhan, K. S., and Dave, C. V. (2013). Living with lions: economics of coexistence in the Gir forests, India. PLoS ONE 8:e49457. doi: 10.1371/journal.pone.0049457
Banerjee, K., Jhala, Y. V., and Pathak, B. (2010). Demographic structure and abundance of Asiatic lions (Panthera leo persica) in Girnar Wildlife Sanctuary, Gujarat, India. Oryx 44, 248–251. doi: 10.1017/S0030605309990949
Barnett, R., Yamaguchi, N., Barnes, I., and Cooper, A. (2006). The origin, current diversity and future conservation of the modern lion Panthera leo. Proc. R. Soc. B. 273, 2119–2125. doi: 10.1098/rspb.2006.3555
Barnett, R., Yamaguchi, N., Shapiro, B., Ho, S. Y. W., Barnes, I., Sabin, R., et al. (2014). Revealing the maternal demographic history of Panthera leo using ancient DNA and a spatially explicit genealogical analysis. Evol. Biol 14:70. doi: 10.1186/1471-2148-14-70
Barr, A. (2003). Trust and expected trustworthiness: experimental evidence from Zimbabwean villages. Econ. J. 113, 614–630. doi: 10.1111/1468-0297.t01-1-00150
Basu, P. (2013). Assessment of landscape pattern for modelling habitat suitability for lions and prey species in Gir Protected Area, Gujarat (Dissertation/Ph.D. thesis). India: Forest Research Institute Deemed University, Dehradun, India, 288.
Bauer, H., De Iongh, H. H., and Di Silvestre, I. (2003). Lion (Panthera leo) social behaviour in the West and Central African savannah belt. Mamm. Biol. 68, 239–243. doi: 10.1078/1616-5047-00090
Bauer, H., Packer, C., Funston, P. F., Henschel, P., and Nowell, K. (2016). Panthera leo. The IUCN Red List of Threatened Species 2016 e.T15951A115130419.
Bertola, L. D., Tensen, L., van Hooft, P., White, P. A., Driscoll, C. A., Henschel, P., et al. (2015). Autosomal and mtDNA markers affirm the distinctiveness of lions in West and Central Africa. PLoS ONE 10:e0137975. doi: 10.1371/journal.pone.0137975
Bertola, L. D., van Hooft, W. F., Vrieling, K., de Weerd, D. R. U., York, D. S., Bauer, H., et al. (2011). Genetic diversity, evolutionary history and implications for conservation of the lion (Panthera leo) in West and Central Africa. J. Biogeogr. 38, 1356–1367. doi: 10.1111/j.1365-2699.2011.02500.x
Berwick, S. (1974). The community of wild ruminants in Gir ecosystem (Dissertation/Ph.D. thesis). New Haven, CT: Yale University, 225.
Bipin, C. M., Bhattacharjee, S., Shah, S., Sharma, V. S., Mishra, R. K., Ghose, D., et al. (2013). Status of prey in Kuno Wildlife Sanctuary, Madhya Pradesh. Wildlife Institute of India, Dehradun.
Breitenmoser, U., Mallon, D. P., Khan, J. A., and Driscoll, C. (2008). “Panthera leo ssp. persica,” in IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. Available online at: www.iucn.org (accessed January 21, 2019).
Carpenter, S., and Konisky, D. (2017). The killing of Cecil the lion as an impetus for policy change. Oryx 1–9. doi: 10.1017/S0030605317001259
Casimir, M. J. (2001). Of lions, herders and conservationists: brief notes on the Gir Forest National Park in Gujarat (Western India). Nomadic Peoples 5, 154–161. doi: 10.3167/082279401782310808
Ceballos, G., and Ehrlich, P. R. (2002). Mammal population losses and the extinction crisis. Science 296, 904–907. doi: 10.1126/science.1069349
Chakrabarti, S. (2018). Sociality in Asiatic lions (Dissertation/Ph.D. thesis). India: Forest Research Institute Deemed University, Dehradun. India, 123 p.
Chakrabarti, S., and Jhala, Y. V. (2017). Selfish partners: resource partitioning in male coalitions of Asiatic lions. Behav. Ecol. 28, 1532–1539. doi: 10.1093/beheco/arx118
Chakrabarti, S., and Jhala, Y. V. (2019). Battle of the sexes: a multi-male mating strategy helps lionesses win the gender war of fitness. Behav. Ecol. 30, 1050–1061. doi: 10.1093/beheco/arz048
Chakrabarti, S., Jhala, Y. V., Dutta, S., Qureshi, Q., Kadivar, R. F., and Rana, V. J. (2016). Adding constraints to predation through allometric relation of scats to consumption. J. Anim. Ecol. 85, 660–670. doi: 10.1111/1365-2656.12508
Chapron, G., Miquelle, D. G., Lambert, A., Goodrich, J. M., Legendre, S., and Clobert, J. (2008). The impact on tigers of poaching versus prey depletion. J. Appl. Ecol. 45, 1667–1674. doi: 10.1111/j.1365-2664.2008.01538.x
Chellam, R. (1993). Ecology of the Asiatic lion (Panthera leo persica) (Dissertation/Ph.D. thesis). India: Saurashtra University, Rajkot, India.
Chellam, R., Joshua, J., Williams, C. A., and Johnsingh, A. J. T. (1995). Survey of Potential Sites for Reintroduction of Asiatic Lions. Technical Report, Wildlife Institute of India, Dehradun, 39 p.
Choskyi, V. J. (1988). Symbolism of Animals in Buddhism. Buddhist Himalaya, Vol. I, no. I, SUMMER 1988. Tokyo: Gakken Co. Ltd.
Cronin, J. T. (2003). Movement and spatial population structure of a prairie planthopper. Ecology 84, 1179–1188. doi: 10.1890/0012-9658(2003)084[1179:MASPSO]2.0.CO;2
Darimont, C. T., Paquet, P. C., Treves, A., Artelle, K. A., and Chapron, G. (2018). Political populations of large carnivores. Conserv. Biol. 32, 747–749. doi: 10.1111/cobi.13065
Dave, C. (2008). Ecology of chital (Axis axis) in Gir (Dissertation/Ph.D. thesis). India: Saurashtra University, Rajkot, India, 262 p.
Dave, C., and Jhala, Y. (2011). Is competition with livestock detrimental for native wild ungulates?—A case study of chital (Axis axis) in Gir forest, India. J. Trop. Ecol. 27, 239–247. doi: 10.1017/S0266467410000738
Dharaiya, N. (2001). A study on the ecology of the satellitic lion metapopulation outside Gir, P. A., and its conservation (Dissertation/Ph.D. thesis). India: Saurashtra University, Rajkot, India, 119 p.
Divyabhanusinh, C. (1995). The End of a Trail: The Cheetah in India. Banyan Books, the University of Michigan, MI, United States, 248 p.
Dorje, O. T. (2011). Walking the path of environmental Buddhism through compassion and emptiness. Conserv. Biol. 25, 1094–1097. doi: 10.1111/j.1523-1739.2011.01765.x
Driscoll, C. A., Menotti-Raymond, M., Nelson, G., Goldstein, D., and O'Brien, S. J. (2002). Genomic microsatellites as evolutionary chronometers: a test in wild cats. Genome Res. 12, 414–423. doi: 10.1101/gr.185702
Dutta, R. (2019). Reintroduction of the Asiatic lion: precedence of politics over rule of law. Econ. Political Weekly 54, 13–16.
Edwardes, S. M., and Fraser, L. G. (1907). “The Gir forests and its lions”, in The Ruling Princes of India, Junagadh: Being a Historical, Archaeological, Political and Statistical Account of the Premier State of Kathiawar (Bombay: Times of India Press), 202.
Funston, P. J., Mills, M. G. L., Biggs, H. C., and Richardson, P. R. K. (1998). Hunting by male lions: ecological influences and socioecological implications. Anim. Behav. 56, 1333–1345. doi: 10.1006/anbe.1998.0884
Gadgil, M., and Thapar, R. (1990). Human ecology in India some historical perspective. Interdiscipl. Sci. Rev. 15, 209–223. doi: 10.1179/030801890789797365
Gilbert, D. A., Packer, C., Pusey, A. E., Stephens, J. C., and O'Brien, S. J. (1991). Analytical DNA fingerprinting in lions: parentage, genetic diversity, and kinship. J. Heredity 82, 378–386. doi: 10.1093/oxfordjournals.jhered.a111107
Gogoi, K. (2015). Factors governing the spatial distribution and density of Asiatic lions (Panthera leo persica) in Gir Protected Area (Dissertation/Master's thesis). India: Saurashtra University, Rajkot, India. 81 p.
Gopal, R., Sinha, P. R., Mathur, V. B., Jhala, Y. V., and Qureshi, Q. (2007). Guidelines for Preparation of Tiger Conservation Plan. The National Tiger Conservation Authority, Ministry of Environment and Forests, Government of India, New Delhi, India.
Gujarat Forest Department (2015). 14th Lion Population Estimation Report−2015. Gujarat Forest Department, Gandhinagar, Gujarat, India; Gujarat Forest Department, Sasan Gir, Junagadh, India, 242 p.
Gupta, S. K. (1972). Chronology of the raised beaches and inland coral reefs of the Saurashtra Coast. J. Geol. 80, 357–361. doi: 10.1086/627738
Hanski, I. (1994). A practical model of metapopulation dynamics. J. Anim. Ecol. 63:151–162. doi: 10.2307/5591
Hanski, I. A., and Gilpin, M. E. (1997). Metapopulation Biology: Ecology, Genetics and Evolution. San Diego, CA: Academic Press. 512 p.
Hirzel, A. H., Hausser, J., Chessel, D., and Perrin, N. (2002). Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology 83, 2027–2036. doi: 10.1890/0012-9658(2002)083[2027:ENFAHT]2.0.CO;2
IUCN/SS (2013). Guidelines for Reintroductions and Other Conservation Translocations. Version 1.0. Gland. Switzerland: IUCN Species Survival Commission, viiii + 57.
Jhala, Y. V. (2002). Cattle and Carnivores. The National Wildlife Federation. Available online at: https://www.nwf.org/en/Magazines/National-Wildlife/2002/Cattle-and-Carnivores (accessed April 15, 2019).
Jhala, Y. V. (2004). Monitoring of Gir. Technical Report, Wildlife Institute of India, Dehra Dun, India, RR -04/002.
Jhala, Y. V., Banerjee, K., and Basu, P. (2014a). Ecology of Lion in Agro-pastoral Gir Landscape, Gujarat - Final Project Report. Technical Report, Wildlife Institute of India, Dehra Dun, India, pp xvi + 374. TR-2014/TR-2006.
Jhala, Y. V., Banerjee, K., Basu, P., Chakrabarti, S., and Gayen, S. (2014b). Assessment of Barda Landscape for Reintroduction of Asiatic Lions. Technical Report, Wildlife Institute of India, Dehra Dun, India, pp xiii + 146. TR-2014/TR-2003.
Jhala, Y. V., Banerjee, K., Basu, P., Chakrabarti, S., Gayen, S., Gogoi, K., et al. (2016). Ecology of Asiatic Lions in Gir, P. A., and Adjoining Human-Dominated Landscape of Saurashtra, Gujarat: Technical Report. Dehradun, India: Wildlife Institute of India.
Jhala, Y. V., Chellam, R., Pathak, B., Qureshi, Q., Meena, V., Chauhan, K., et al. (2011). Ecology of lions in Greater Gir landscape. A technical report (TR-2011/001), Wildlife Institute of India, Dehra Dun, 439 pp.
Jhala, Y. V., Mukherjee, S., Shah, N., Chauhan, K. S., Dave, C., and Zala, Y. P. (2004). “Monitoring lions”, in Monitoring of Gir, ed. Y. V. Jhala. Technical Report, Wildlife Institute of India, Dehra Dun, India, RR−04/002, 55-71.
Jhala, Y. V., Mukherjee, S., Shah, N., Chauhan, K. S., Dave, C. V., Meena, V., et al. (2009). Home range and habitat preference of female lions (Panthera leo persica) in Gir forests, India. Biodivers. Conserv.18, 3383–3394. doi: 10.1007/s10531-009-9648-9
Jhala, Y. V., Qureshi, Q., Bhuva, V., and Sharma, L. N. (1999). Population estimation of Asiatic lions. J. Bombay Nat. Hist. Soc. 96, 3–15.
Jhala, Y. V., Qureshi, Q., and De, P. (2005). Lion: a Software to Identify Individual Lion and Database Management, Wildlife Institute of India. Available online at: http://wii.gov.in/lion_id (accessed May 01, 2019).
Jhala, Y. V., Singh, A. P., Gogoi, K., Chakrabarti, S., Singh, P., Nala, R. R., et al. (2018). Spatial Analysis of Livestock Predation by Lions in the Greater Gir Landscape. Technical report of Gujarat Forest Department & Wildlife Institute of India, Dehradun, India, TR 2018/18.
Johnsingh, A. J. T., Chellam, R., and Sharma, D. (1998). Prospects for conservation of Asiatic lions in India. Biosphere Conserv. 1, 81–89.
Johnsingh, A. J. T., Goyal, S. P., and Qureshi, Q. (2007). Preparations for the reintroduction of Asiatic lion Panthera leo persica into Kuno Wildlife Sanctuary, Madhya Pradesh, India. Oryx 41, 93–96. doi: 10.1017/S0030605307001512
Johnsingh, A. J. T., Qureshi, Q., and Goyal, S. P. (2006). Assessment of Prey Populations for Lion Re-introduction in Kuno Wildlife Sanctuary, Central India. Report Submitted to Government of India and Government of Madhya Pradesh. Wildlife Institute of India, Dehradun, 32 p.
Joslin, P. (1973). The Asiatic lion: a study of ecology and behavior (Dissertation/Ph.D. thesis). Department of Forestry and Natural Resources, University of Edinburgh, UK, 249 p.
Joslin, P. (1985), The environmental limitations and future of the Asiatic lion. J. Bombay Natural History Soc. 81, 648–664.
Jule, K. R., Leaver, L. A., and Lea, S. E. (2008). The effects of captive experience on reintroduction survival in carnivores: a review and analysis. Biol. Cons. 141, 355–363. doi: 10.1016/j.biocon.2007.11.007
Junagadh Agricultural University (2016). Status Report of Junagadh Agricultural University (2014-15 and 2015-16). Director of Research, Junagadh Agricultural University, Gujarat. Available online at: http://www.jau.in/attachments/book/Status_Report_2016.pdf. (accessed February 8, 2019).
Kansky, R., and Knight, A. T. (2014). Key factors driving attitudes towards large mammals in conflict with humans. Biol. Conserv. 179, 93–105. doi: 10.1016/j.biocon.2014.09.008
Karanth, K. K., and DeFries, R. (2010). Conservation and management in human-dominated landscapes: case studies from India. Biol. Cons. 143, 2865–2869. doi: 10.1016/j.biocon.2010.05.002
Karanth, K. K., Nichols, J. D., Karanth, K. U., Hines, J. E., and Christensen, N. L. (2010). The shrinking ark: patterns of large mammal extinctions in India. Proc. R. Soc. Lond. B. 277, 1971–1979. doi: 10.1098/rspb.2010.0171
Karanth, K. U., Nichols, J. D., Seidensticker, J., Dinerstein, E., Smith, J. L. D., McDougal, C., et al. (2003), Science deficiency in conservation practice: the monitoring of tiger populations in India. Anim. Conserv. 6, 141–146. doi: 10.1017/S1367943003003184
Keller, L. K., and Waller, D. M. (2002). Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. doi: 10.1016/S0169-5347(02)02489-8
Khan, J. A. (1993). Ungulate-habitat relationships in Gir forest ecosystem and its management implications (Dissertation/Ph.D. thesis). India: Aligarh Muslim University, India, 277 p.
Khudsar, F. A., Sharma, K., Rao, R. J., and Chundawat, R. S. (2008). Estimation of prey base and its implications in Kuno wildlife sanctuary. J. Bombay Nat. Hist. Soc. 105, 42–48.
Kinnear, N. B. (1920). The past and present distribution of the lion in south eastern Asia. J. Bombay Nat. Hist. Soc. 27, 34–39.
Kitchener, A. C., Breitenmoser-Wursten, C.h., Eizirik, E., Gentry, A., Werdelin, L., Wilting, A., et al. (2017). A revised taxonomy of the Felidae. The final report of the Cat Classification Task Force of the IUCN/SSC Cat Specialist Group. Cat News Special Issue 11:80.
Kostuch, L. (2017). Do animals have a homeland? Ancient Greeks on the cultural identity of animals. Humanimalia 9, 69–87.
Löe, J., and Röskaft, E. (2004). Large carnivores and human safety: a review. Ambio 33, 283–288. doi: 10.1579/0044-7447-33.6.283
Lyke, M. M., Dubach, J., and Briggs, M. B. (2013). A molecular analysis of African lion (Panthera leo) mating structure and extra-group paternity in Etosha National Park. Mol. Ecol. 22, 2787–2796. doi: 10.1111/mec.12279
Macdonald, D. W. (1983). The ecology of carnivore social behaviour. Nature 301, 379–384. doi: 10.1038/301379a0
Macdonald, D. W., Jacobsen, K. S., Burnham, D., Johnson, P. J., and Loveridge, A. J. (2016). Cecil: a moment or a movement? analysis of media coverage of the death of a lion, Panthera leo. Animals 6:26. doi: 10.3390/ani6050026
Madhusudan, M. D., and Mishra, C. (2003). “Why big, fierce animals are threatened: conserving large mammals in densely populated landscapes” in Battles Over Nature: Science and Politics of Conservation, eds V. K. Saberwal, and M. Rangarajan (New Delhi: Permanent Black, 31–55.
Manamendra-Arachchi, K., Pethiyagoda, R., Dissanayake, R., and Meegaskumbura, M. (2005). A second extinct big cat from the late quaternary of Sri Lanka. Raffles Bull. Zool. Suppl. 12, 423–434.
Meena, R. L., and Kumar, S. (2012). Management Plan for Gir Protected Areas, Vol. 1. Gujarat Forest Department, Gujarat, India, 290 p.
Meena, V. (2008). Reproductive strategy and behaviour of male Asiatic lions (Dissertation/Ph.D. thesis). India: Forest Research Institute University, Dehra Dun, 179 p.
Meena, V., Jhala, Y. V., Chellam, R., and Pathak, B. (2011). Implications of diet composition of Asiatic lions for their conservation. J. Zool. 284, 60–67. doi: 10.1111/j.1469-7998.2010.00780.x
Meena, V., Macdonald, D. W., and Montgomery, R. A. (2014). Managing success: Asiatic lion conservation, interface problems and peoples' perceptions in the Gir Protected Area. Biol. Conserv. 174, 120–126. doi: 10.1016/j.biocon.2014.03.025
Ministry of Environment Forests and Climate Change [MoEFCC] (2016). Action Plan for the Reintroduction of the Asiatic Lions (Panthera leo persica) in Kuno Wildlife Sanctuary, Madhya Pradesh. Lion reintroduction expert committee report, Ministry of Environment, Forests and Climate Change, New Delhi.
Narain, S., Panwar, H. S., Gadgil, M., Thapar, V., and Singh, S. (2005). Joining the Dots: The Report of the Tiger Task Force. Project Tiger, Ministry of Environment and Forests, Government of India, New Delhi. Available online at: http://projecttiger.nic.in/TTF2005/pdf/full_report.pdf. (accessed January 20, 2019).
National Tiger Conservation Authority (2013). Standard Operating Procedure to Deal With Emergency Arising Due to Straying of Tigers in Human Dominated Landscapes. National Tiger Conservation Authority, Ministry of Environment, Forests and Climate Change, Government of India, New Delhi, India.
Newell, Z. M. (2011). Picturing the goddess: bazaar images and the imagination of modern Hindu religious identity (Dissertation/Ph.D. thesis). Vanderbilt University, Tennessee, USA.
Nowell, K., and Jackson, P. (1996). Wild Cats: Status Survey and Conservation Action Plan. Gland: IUCN, 382.
O'Brien, S. J. (1990). Genetic introgression within the Florida panther Felis concolor coryi. Nat. Geogr. Res. 6, 485–494.
O'Brien, S. J. (2003). “Prides and prejudice”, in Tears of the Cheetah and Other Tales from the Genetic Frontier: The Genetic Secrets of our Animal Ancestors, ed S. J. O'Brien, and T. D. Books (New York, NY: St. Martin's Griffin, 35–55.
O'Brien, S. J., Joslin, P., Smith, G. L. III., Wolfe, R., Schaffer, N., Heath, E, et al. (1987). Evidence for African origins of founders of the Asiatic lion species survival plan. Zoo Biol. 6, 99–116. doi: 10.1002/zoo.1430060202
O'Brien, S. J., Wildt, D. E., and Bush, M. (1986). The African cheetah in genetic peril. Sci. Am. 254, 84–92. doi: 10.1038/scientificamerican0586-84
Packer, C., Herbst, L., Pusey, A., Bygott, J. D., Hanby, J. P., Cairns, S. J., et al. (1988). “Reproductive success of lions,” in Reproductive Success, eds T. H. Clutton-Brock (Chicago, IL: University of Chicago Press, 363–383.
Packer, C., Loveridge, A., Canney, S., Caro, T., Garnett, S. T., Pfeifer, M., et al. (2013). Conserving large carnivores: dollars and fence. Ecol. Lett. 16, 635–641. doi: 10.1111/ele.12171
Packer, C., Pusey, A. E., Rowley, H., Gilbert, D. A., Martenson, J., and O'Brien, S. J. (1991). Case study of a population bottleneck: lions of the Ngorongoro Crater. Conserv. Biol. 5, 219–230. doi: 10.1111/j.1523-1739.1991.tb00127.x
Panwar, H. S. (1979). A Note on Tiger Census Technique Based on Pugmark Tracings. Indian Forester. Dehradun, 70–77.
Pathak, B., Pati, B. P., Kumar, R., Kumar, A., Raval, P. P., Patel, V. S., et al. (2002). Biodiversity Conservation Plan for Gir (A Supplementary Management Plan, 2002-03 to 2006-07). Wildlife Circle, Junagadh. Gujarat Forest Department, India, 407 p.
Patterson, B. D., Kasiki, S. M., Selempo, E., and Kays, R. W. (2004). Livestock predation by lions (Panthera leo) and other carnivores on ranches neighbouring Tsavo National Parks, Kenya. Biol. Conserv. 119, 507–16. doi: 10.1016/j.biocon.2004.01.013
Patterson, J. H. (1907). The Man-Eaters of Tsavo and Other East African Adventures. London: Macmillan, 338.
Pulliam, H. R. (1988). Sources, sinks, and population regulation. Am. Nat. 132, 652–661. doi: 10.1086/284880
Qureshi, Q., Saini, S., Basu, P., Gopal, R., Raza, R., and Jhala, Y. (2015). Connecting the Tiger Populations for Long Term Conservation. National Tiger Conservation Authority (New Delhi) and Wildlife Institute of India, Dehradun, TR-2014/R-2002.
Qureshi, Q., and Shah, N. (2004). “Vegetation and habitat monitoring,” in Monitoring of Gir, ed Y. V. Jhala (Dehra Dun: Technical Report, Wildlife Institute of India, RR-04/002, 8–14.
Rahmani, A. (2013). Asiatic lion: reintroduction or assisted dispersal? J. Bombay Nat. Hist. Soc. 110, 93–94.
Ramanathan, A., Malik, P. K., and Prasad, G. (2007). Seroepizootiological survey for selected viral infections in captive Asiatic lions (Panthera leo persica) from western India. J. Zoo Wildl. Med. 38, 400–408. doi: 10.1638/2007-0006.1
Rangarajan, M. (2001). “From princely symbol to conservation icon: a political history of the lion in India,” in The Unfinished Agenda: Nation Building in South Asia, eds M. Hasan and N. Nakazato (New Delhi: Manohar Publishers and Distributors, 399–442.
Rangarajan, M. (2013). Animals with rich histories: the case of the lions of Gir forest, Gujarat, India. Hist. Theory Theme Issue 52, 109–127. doi: 10.1111/hith.10690
Rangarajan, M., and Shahabuddin, G. (2006). Displacement and relocation from Protected Areas: towards a biological and historical synthesis. Conservat. Soc. 4, 359–378. Available online at: www.conservationandsociety.org/text.asp?2006/4/3/359/49270
Ranjitsinh, M. K. (2016). Reoccupation of former territories by the Asiatic lion Panthera leo persica, Meyer, 1826, in southern Saurashtra, Gujarat, India: a vision for future management. J. Bombay Nat. Hist. Soc. 111, 161–171. doi: 10.17087/jbnhs/2014/v111i3/82338
Renugadevi, R. (2012). Environmental ethics in the Hindu Vedas and Puranas in India. Afr. J. Hist. Cult. 4, 1–3. doi: 10.5897/AJHC11.042
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. U., Hebblewhite, M., et al. (2014). Status and ecological effect of the world's largest carnivores. Science 343:1241484. doi: 10.1126/science.1241484
Rodgers, A., Hartley, D., and Bashir, S. (2003). “Community approaches to conservation: some comparison of Africa and India,” in Battles Over Nature: Science and Politics of Conservation, eds V. K. Saberwal, and M. Rangarajan (New Delhi: Permanent Black,, 324–382.
Roelke-Parker, M. E., Munson, L., Packer, C., Kock, R., Cleveland, S., Carpenter, M., et al. (1996). A canine distemper virus epidemic in Serengeti lions. Nature 379, 411–445. doi: 10.1038/379441a0
Sabapara, R. H. (2002). Survey of the health status and development of health monitoring system for captive large felids (Dissertation/Master's thesis). India: Anand Veterinary College, Gujarat.
Saberwal, V., Gibbs, J. P., Chellam, R., and Johnsingh, A. J. T. (1994). Lion-human conflict in the Gir forest, India. Conserv. Biol. 8, 501–507. doi: 10.1046/j.1523-1739.1994.08020501.x
Shankaranarayanan, P., Banerjee, M., Kacker, R. K., Aggarwal, R. K., and Singh, L. (1997). Genetic variation in Asiatic lions and Indian tigers. Electrophoresis 18, 1693–1700. doi: 10.1002/elps.1150180938
Sharma, D. (1995). Ecology and management of lion and ungulate habitats in Gir (Dissertation/Ph.D. thesis). India: Saurashtra University, Rajkot, 178 p.
Singh, H. S. (1997). Population dynamics, group structure and natural dispersal of the Asiatic Lion Panthera leo persica. J. Bombay Nat. Hist. Soc. 94, 65–70.
Singh, H. S. (2007). The Gir Lion Panthera leo Persica- A Natural History, Conservation Status and Future Prospect. Ahmedabad: Pugmark Qumulus Consortium, 320.
Singh, H. S. (2017a). Dispersion of the Asiatic lion Panthera leo persica and its survival in human-dominated landscape outside the Gir forest, Gujarat, India. Curr. Sci. 112, 933–940. doi: 10.18520/cs/v112/i05/933-940
Singh, H. S., and Kamboj, R. D. (1996). Biodiversity Conservation Plan for Gir (A Management Plan for Gir Sanctuary and National Park), Vol. I. Junagadh: Sasan Gir Wildlife Division, Gujarat Forest Department, Sasan Gir, 242.
Sinha, S. P. (1987). Ecology of wildlife with special reference to the lion (Panthera leo persica) in Gir wildlife sanctuary, Saurashtra, Gujarat (Dissertation/Ph.D. thesis). India: Saurashtra University, Rajkot, 291.
Sinha, S. P., Pathak, B. J., and Rawal, P. P. (2004)., Man-animal conflicts in and around protected areas–a case study on Gir national park/wildlife sanctuary, Junagadh, Gujarat, India. Tigerpaper 31, 27–32.
Soulé, M. E. (ed). (1987). Viable Populations for Conservation. Cambridge, UK: Cambridge University Press, 189. doi: 10.1017/CBO9780511623400
Soulé, M. E., and Simberloff, D. (1986). What do genetics and ecology tell us about the design of nature reserves? Biol Conserv. 35, 19–40. doi: 10.1016/0006-3207(86)90025-X
Stephens, P. A. (2015). Land sparing, land sharing, and the fate of Africa's lions. Proc. Natl. Acad. Sci. U.S.A. 112, 14753–14754. doi: 10.1073/pnas.1520709112
Todd, N. B. (1965). Metrical and non-metrical variation in the skulls of Gir lions. J. Bombay Nat. Hist. Soc. 62, 507–520.
Van Orsdol, K. G., Hanby, J. P., and Bygott, J. D. (1985). Ecological correlates of lion social organization (Panthera leo). J. Zool. 206, 97–112.
Walston, J., Robinson, J. G., Bennett, E. L., Breitenmoser, U., da Fonseca, G. A., Goodrich, J., et al. (2010). Bringing the tiger back from the brink—the six percent solution. PLoS Biol. 8:e1000485. doi: 10.1371/journal.pbio.1000485
Whitman, K., Starfield, A. M., Quadling, H., and Packer, C. (2004), Sustainable trophy hunting of African lions. Nature 428, 175–178. doi: 10.1038/nature02395
Wildlife Protection Act (1972). Wildlife Protection Act, Act Number 53 of 1972. 9th September, 1972, Ministry of Law, Justice and Company, New Delhi, India. Available online at: http://moef.nic.in/downloads/public-information/Annexure-IV-NBA.pdf. (accessed January 26, 2019).
Wildt, D. E., Bush, M., Goodrowe, K. L., Packer, C., Pusey, A. E., Brown, J. L., et al. (1987). Reproductive and genetic consequences of founding isolated lion populations. Nature. 329, 328–331. doi: 10.1038/329328a0
Williams, B. K., Nichols, J. D., and Conroy, M. J. (2002). Analysis and Management of Animal Populations. London: Academic Press, 817 p.
Woodroffe, R. (2000). Predators and People: Using Human Densities to Interpret Declines of Large World Conservation Union, Gland, Switzerland. Available online at: www.iucn.org (accessed January 21, 2019).
Yamazaki, K. (1996). Social variation of lions in a male-depopulated area in Zambia. J. Wildl. Manag. 60, 490–497. doi: 10.2307/3802066
Keywords: conservation policy, Gir, human-carnivore conflict, long-term research, reintroduction
Citation: Jhala YV, Banerjee K, Chakrabarti S, Basu P, Singh K, Dave C and Gogoi K (2019) Asiatic Lion: Ecology, Economics, and Politics of Conservation. Front. Ecol. Evol. 7:312. doi: 10.3389/fevo.2019.00312
Received: 14 February 2019; Accepted: 05 August 2019;
Published: 30 August 2019.
Edited by:
Matt W. Hayward, University of Newcastle, AustraliaReviewed by:
Luke T. B. Hunter, Panthera Corporation, United StatesCopyright © 2019 Jhala, Banerjee, Chakrabarti, Basu, Singh, Dave and Gogoi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yadvendradev V. Jhala, amhhbGF5QHdpaS5nb3YuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.