
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 02 July 2019
Sec. Behavioral and Evolutionary Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00244
This article is part of the Research Topic How Enemies Shape Communication Systems: Sensory Strategies of Prey to Avoid Eavesdropping Predators and Parasites View all 13 articles
Moths have evolved auditory channels under predation pressure from insectivorous bats that emit ultrasonic pulses for capturing prey, including moths. Tympanate moths perform evasive behavior in response to echolocation calls of bats, but they also utilize ultrasonic signals mostly generated by males close to an intended female mate in the context of courtship. Unlike calling songs used to advertise the presence and sexual attractiveness of the signaler, courtship songs need not always be acoustically conspicuous. Male courtship songs are predominantly soft but sufficient for detection by a nearby potential mate. Quiet courtship songs are thought to effectively avoid being eavesdropped by gleaning bats, acoustic parasitoids, and conspecific competitors, i.e., rival males. However, males of some moth species generate loud courtship songs. In the present study, the duration of courtship song, in addition to the sound level of the song was predicted to affect the likelihood of being perceived by eavesdroppers. Loud and lengthy courtship songs, which are easily exploited by eavesdroppers, would be expected to rarely evolve, because a female receiver close to a male emitting a conspicuous song would also be exposed to strong predation pressure. This study explored the relationship between the peak sound level and the duration of single song bouts in 26 moth species from the following families: Noctuidae, Erebidae, Crambidae, Pyralidae, and Geometridae. The softest and loudest songs with mean peak sound levels of 64 and 129 dB peSPL had mean durations of 1,900 and 312 ms, respectively, whereas the shortest and longest songs with mean durations of 110 and 8,839 ms had mean peak sound levels of 102 and 74 dB peSPL, respectively. The peak sound level and duration of courtship song exhibited a significant negative relationship across species. Although the energetic cost of producing song and the size of the sound-producing organ might also affect the relationship, the data support the conclusion that acoustic moths have adaptively evolved ultrasonic courtship songs with properties between “soft-and-long” and “loud-and-short” to avoid eavesdroppers.
Animals have evolved communication signals for mating. During a mating sequence involving emission of a signal to a focal receiver i.e., usually a female, an unintended receiver has a chance to exploit the signal to find, locate, and hunt or parasitize a conspicuous signaler (Zuk and Kolluru, 1998). In addition, conspecific competitors may steal a mating opportunity. Loud calling songs are widespread in acoustic animals because of their usefulness to advertise the presence of the caller and attract mating partners from a long distance, but conspicuous songs simultaneously convey information on the location of the caller to predators, including bats, birds, and reptiles (Tuttle and Ryan, 1981; Sakaluk and Belwood, 1984; Bell, 1985; Tuttle et al., 1985; Bailey and Haythornthwaite, 1998; Igaune et al., 2008; Jones et al., 2011); parasitoids and blood-sucking flies (Cade, 1975; Walker, 1993; Bernal et al., 2006; Bernal and de Silva, 2015); and rival males of the same species (Cade, 1980; Bailey and Field, 2000; Zuk et al., 2006; Bailey et al., 2010).
Calling songs of moths are also prey cues used by insectivorous bats (Alem et al., 2011). Males of the lesser wax moth Achroia grisella (Pyralidae) generate loud ultrasonic clicks to attract female receivers (Jang and Greenfield, 1996; Greenfield, 2014). Bat predators with gleaning strategies, such as the greater horseshoe bat, Rhinolophus ferrumequinum, can exploit a moth's ultrasonic calling songs as a landmark of their prey (Alem et al., 2011). As countertactics to avoid predation by eavesdropping bats, singing males of Achroia grisella, like crickets and katydids (Spangler, 1984; Nolen and Hoy, 1986; Libersat and Hoy, 1991; Faure and Hoy, 2000; Schulze and Schul, 2001), cease emission of their calling songs, and the females stop orientation toward calling males, when they detect ultrasonic echolocation calls of hunting bats (Spangler, 1984; Greenfield and Baker, 2003; Greig and Greenfield, 2004; Rodríguez and Greenfield, 2004;Cordes et al., 2014).
The use of calling songs by moths has been confirmed in only a few species (Conner, 1999; Greenfield, 2014), but it has been increasingly reported that moths communicate acoustically with male courtship songs (Nakano et al., 2015). Male moths produce courtship songs after they have approached close to (within a few centimeters of) a female that has released sex pheromones to attract males from a long distance. For the tiger moth and the lichen moth (Erebidae), it is implied that male courtship song serves as a signal for mate recognition (Conner, 1987; Nakano et al., 2013). However, some noctuid and crambid moths do not discriminate between courtship songs of conspecific male moths and echolocation calls of bat predators (Nakano et al., 2008, 2010a, 2013). Females of these moths show a freezing response in response to the male song as well as to bat cries, which enables the singing male to readily attempt copulation with the stationary female (Nakano et al., 2008, 2010b, 2013).
Males are vulnerable to predation during courtship (Endler, 1987; Alem et al., 2011). Excessive concentration of a male's attention on a female (and subsequent sperm transfer) could cause him to delay perception of the presence or approach of eavesdroppers, including predators. To survive while performing reproductive behavior, males and even females need to maintain multiple multimodal sensory systems to decide between defense and copulation. One solution for this tough choice is to mate “privately” to avoid eavesdropping (Dabelsteen et al., 1998; Dabelsteen, 2004). In singing animals, “soft” song with low-amplitude sound is one of the adaptive courtship behaviors associated with defensive responses to eavesdroppers (Nakano et al., 2009a; Balenger, 2015; Reichard and Anderson, 2015). Low-amplitude signals render a caller inconspicuous to eavesdropping enemies and competitors. Soft songs have the disadvantage of being effective only over a short distance, but the caller can overcome this disadvantage by generating the soft song in close proximity to the intended receiver. Soft courtship songs are found among moths of diverse taxonomic groups, including Noctuidae, Erebidae, Crambidae, Pyralidae, and Geometridae (Conner, 1999; Nakano et al., 2009a,b, 2015). However, courtship songs are not necessarily of low amplitude (e.g., Conner, 1987; Sanderford and Conner, 1995; Nakano et al., 2012a). In this study, we examined how the courtship songs of moths are adaptively balanced between conspicuous and inconspicuous characteristics to enable effective mating while avoiding eavesdroppers.
We studied 26 moth species belonging to the Noctuoidea (five noctuid and three erebid species), Pyraloidea (14 crambid and three pyralid species), and Geometroidea (one geometrid species). In addition to 14 species previously reported (Nakano et al., 2006, 2009a,b, 2012a), male courtship songs were newly recorded for 12 species and analyzed by the procedure described below. Moths of all growth stages were maintained under a 16 h light:8 h dark photo-regime in experimental rooms at 20 ± 1°C for three Canadian species (three crambids: Desmia maculalis, Desmia funeralis, and Nomophila nearctica) and 24 ± 1°C for nine Japanese species [three noctuids (Spodoptera picta, Spodoptera exigua, and Spodoptera pecten), one erebid (Lithosia quadra), three crambids (Ostrinia zealis, Ostrinia palustralis, and Ostrinia latipennis), and two pyralids (Paralipsa gularis and Endotricha icelusalis)]. Larvae were reared on their host plants or an artificial diet (Silkmate-2M; Nosan Corp., Yokohama, Japan). To ensure the virginity of the moths until they were used, each newly emerged male or female adult moth, designated as 0 days old, was separately kept in a nylon mesh cage (30 × 30 × 30 cm) with water or 10% honey solution ad libitum. To minimize the colony artifact associated with inbreeding, we used the generation collected in the field and the next generation.
We directly observed the mating behavior of 2–4 days old previously unmated moths confined in the cubical mesh cages, which were placed in a soundproof box (90 × 65 × 65 cm) with one side opened in the scotophase (dark phase) under a dim red light at 0.6 lux. Male courtship ultrasounds were individually recorded with a 1/4-inch condenser microphone (type 4939; Brüel and Kjær, Nærum, Denmark) connected to a preamplifier (type 2670; Brüel and Kjær) and a customized conditioning amplifier (Nexus type 2690, 0.02–140 kHz bandpass filter; Brüel and Kjær). The acoustic signals were digitized with an analog-to-digital converter, Wavebook 512A (12-bit; IOtech, OH, USA) or USB-1604HS (16-bit; Measurement Computing, MA, USA) at a sampling rate of 300 kHz. The microphone was hand-held and approximately kept 10 mm from the singing male, and the membrane was directed to the intended individual. The recorded courtship songs were stored as.wav format files.
To determine the relationships among loudness, duration, and peak frequency of male courtship ultrasounds, we extracted peak equivalent sound pressure levels (dB peSPL; re. 20 μPa), the longest duration of a single song bout, and dominant frequencies from the recorded sound files using the software BatSound 4.03 (Pettersson Elektronik, Uppsala, Sweden). The data were individually obtained from each singing male without replication. Sound pressure levels were calculated with reference to the known signal voltage of the sound calibrator (type 4231, 94 dB SPL, 1 kHz; Brüel and Kjær). Song duration was measured using both oscillogram and spectrogram. Because each recording was performed for a maximum of 10 s, the duration of a song that was continuously emitted over the length of the recording was recorded as 10 s for two of 20 songs of Ostrinia furnacalis (Crambidae), four of nine songs of Ostrinia nubilalis (Crambidae), one of seven songs of Ostrinia scapulalis (Crambidae), and four of seven songs of Ostrinia zealis (Crambidae). Dominant frequencies were determined by computing power spectra on a Hanning window with a fast Fourier transformation size of 1,024 points. These analyses were performed after high-pass filtering at 10 kHz to eliminate low-frequency background noise.
In statistical analyses of peak sound level, song duration and dominant frequency among species and families, we performed likelihood ratio (LR) test in generalized linear model (GLM) with Gamma error distribution. Additionally, we examined if significant relationships among the song characteristics were found within the species. Because of limited sample sizes (n < 3) for Palpita nigropunctalis (Crambidae) and Herminia tarsicrinalis (Noctuidae), we used 24 species out of 26 species for the within-species analysis. Relationships among the peak sound level, the song duration and the dominant frequency were analyzed by generalized additive model (GAM) (Wood, 2008). Coefficients in the relationships obtained from each species were used for the random effects meta-analyses using the restricted maximum likelihood (REML) estimation (“metafor” package; Viechtbauer, 2010) which tests the significance for 24 species. These analyses were done using R version 3.4.3 (R Core Team, 2017).
For phylogenetic analysis of the 26 moth species, we used the nucleotide sequences deposited at GenBank® (http://www.ncbi.nlm.nih.gov). We tried to incorporate all the available nucleotide sequences of nuclear and mitochondria genes of the 26 species on the International Nucleotide Sequence Database into the construction of a phylogenetic tree. However, because only cytochrome oxidase subunit I (COI), cytochrome oxidase subunit II (COII), and NADH dehydrogenase subunit 5 (ND5) genes of mtDNA were listed for our moths, we searched the homologs of these three genes in the Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) with the nucleotide sequences of COI, COII, and ND5 of Ostrinia furnacalis as the query (Table S1). The homologous sequences of Hyphantria cunea (Erebidae), Paraona staudingeri (Erebidae), Nomophila noctuella (Crambidae), Palpita quadristigmalis (Crambidae), Corcyra cephalonica (Pyralidae), Endotricha consocia (Pyralidae), and Ectropis obliqua (Geometridae) were substituted for those of Spilosoma punctarium (Erebidae), Eilema japonica (Erebidae), Nomophila nearctica (Crambidae), Palpita nigropunctalis (Crambidae), Paralipsa gularis (Pyralidae), Endotricha icelusalis (Pyralidae), and Ascotis selenaria cretacea (Geometridae), respectively, because these genes are not available in the present database (Table S1). Alignments were performed with the Clustal W program (Thompson et al., 1994) in MEGA7 (Kumar et al., 2016) with default values, and gap sequences were manually removed.
We estimated the phylogenetic relationships among the 26 species by four steps described below. In all steps, selection of the best-fit models of nucleotide substitutions was based on the Bayesian information criterion in MEGA7. The focal phylogenetic relationships, for which a geometrid moth Ascotis selenaria cretacea was treated as a root of the tree, were reconstructed by the maximum likelihood method. After bootstrap tests with 1,000 resamplings, we used branches with a bootstrap value of >60.
First, we drew an outline tree using 15 sequences of combinations of COI genes (1,514 bp) and ND5 genes (1,632 bp) for two noctuids (Spodoptera litura and Spodoptera exigua), two erebids [Hyphantria cunea (instead of Spilosoma punctarium) and Paraona staudingeri (instead of Eilema japonica)], seven crambids [Ostrinia furnacalis, Ostrinia nubilalis, Glyphodes pyloalis, Spoladea recurvalis, Conogethes punctiferalis, Nomophila noctuella (instead of Nomophila nearctica), and Chilo suppressalis], three pyralids [Galleria mellonella and Corcyra cephalonica (instead of Paralipsa gularis), and Endotricha consocia (instead of Endotricha icelusalis)], and a geometrid moth [Ectropis obliqua (instead of Ascotis selenaria cretacea)] based on the GTR+G model to estimate phylogenetic relationships at the interfamily level (Figures S1A, S2A).
Second, three intrafamily trees were constructed with six sequences of COI genes (1,423 bp) for five noctuids (Spodoptera litura, Spodoptera picta, Spodoptera exigua, Spodoptera pecten, and Herminia tarsicrinalis) and a geometrid moth [Ectropis obliqua (instead of Ascotis selenaria cretacea)] according to the GTR+G model (Figures S1B, S2B), with four sequences of COI genes (658 bp) for three erebids [Hyphantria cunea (instead of Spilosoma punctarium), Paraona staudingeri (instead of Eilema japonica), and Lithosia quadra] and a geometrid moth [Ectropis obliqua (instead of Ascotis selenaria cretacea)] according to the TN93+G model (Figures S1C, S2C), and with 12 sequences of COII genes (674 bp) for 12 crambids [Ostrinia furnacalis, Ostrinia nubilalis, Ostrinia scapulalis, Ostrinia zealis, Ostrinia palustralis, Ostrinia latipennis, Glyphodes pyloalis, Spoladea recurvalis, and Palpita quadristigmalis (instead of Palpita nigropunctalis), Conogethes punctiferalis, Nomophila noctuella (instead of Nomophila nearctica), and Chilo suppressalis] according to the TN93+G model (Figures S1D, S2D).
Third, the phylogenetic relationships among Desmia maculalis, Desmia funeralis, another five crambids [Glyphodes pyloalis, Spoladea recurvalis, Palpita quadristigmalis, Conogethes punctiferalis, and Nomophila noctuella (instead of Nomophila nearctica)] and a geometrid species [Ectropis obliqua (instead of Ascotis selenaria cretacea)] were estimated with the eight sequences of COI genes (657 bp) according to the GTR+G+I model (Figures S1E, S2E).
Fourth, the interfamily trees were combined with the outline tree.
To take account of species' non-independence due to phylogenetic relatedness (Felsenstein, 1985), we first estimated phylogenetic signals of Pagel's λ (Pagel, 1999) and Blomberg's K (Blomberg et al., 2003). We then applied the phylogenetic generalized least square (PGLS) models with the maximum likelihood method to analysis of relationships among the song characteristics (sound level, duration, and frequency) take into account of the reconstructed phylogenetic tree for our 26 moth species (Pagel, 1999). The PGLS approach includes a variance–covariance matrix with Pagel's λ correlation structure, which is derived from the Brownian motion model expecting a random walk, based on the phylogenetic relationships of species. We also fitted the Ornstein-Uhlenbeck process model expecting a random walk around a central tendency under stabilizing selection in PGLS (Martins and Hansen, 1997). Our data points in some cases seemed to better fit a nonlinear relationship. Therefore, in addition to PGLS models, we statistically analyzed relationships among the three parameters by generalized additive mixed models (GAMM) into which the taxonomic family was incorporated as a random effect (Bradshaw et al., 2008; Wood, 2008).
All analyses were done in R version 3.4.3. We calculated the phylogenetic signals and tested the null hypothesis of no phylogenetic signal using the “phytools” package (Revell, 2012). PGLS models and GAMMs were built with packages “nlme” (Pinheiro et al., 2019) and “gamm4” (Wood, 2008), respectively. A.nexus format file for phylogeny was read through the package “ape” (Paradis and Schliep, 2018). For GAMMs, we used gamma error distribution with log-link function to treat positive continuous variables showing the non-normal distribution and examined the significance of each explanatory variable by the LR test in the analysis of deviance.
The courtship songs of the male moths had highly diverse acoustic characteristics (Figure 1). Peak sound level, song duration, and frequency components varied among species even within the same family, Crambidae. Male songs of Ostrinia palustralis and Ostrinia zealis (Figures 1A,B) had lower amplitudes, longer durations, and lower frequency ranges than those of Desmia funeralis, Palpita nigropunctalis, and Glyphodes pyloalis (Figures 1C–E). The minimal sound units generated by one tentative cycle of the sound-producing movement were also different among species; some species generated consecutive pulses (bursts) and others generated several transient clicks. Details are given in subsection courtship song parameters.
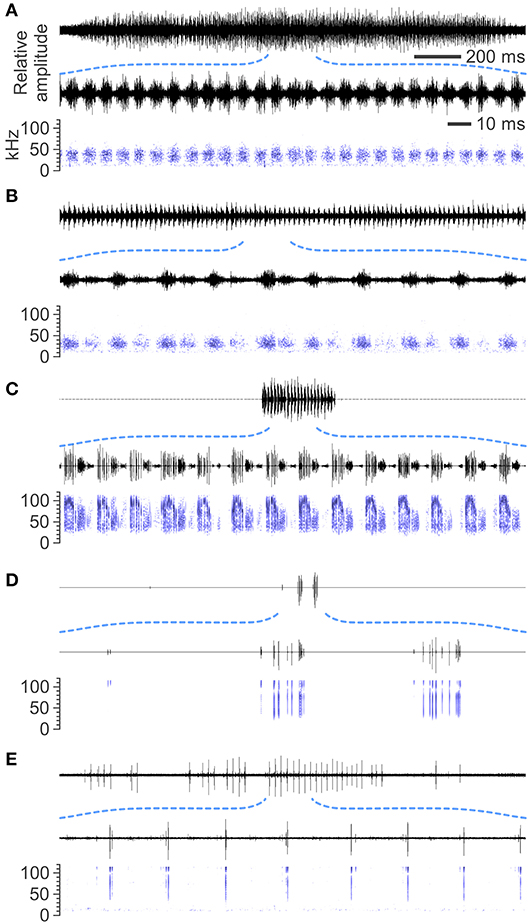
Figure 1. Examples of male courtship songs in crambid moths. Representative oscillograms and spectrograms are shown. (A) Ostrinia palustralis, (B) Ostrinia zealis, (C) Desmia funeralis, (D) Palpita nigropunctalis, and (E) Glyphodes pyloalis.
As in previous sophisticated molecular studies of Lepidoptera (Regier et al., 2013; Kawahara and Breinholt, 2014), superfamilies of Noctuoidea and Pyraloidea formed independent clades on a constructed phylogenetic tree (Figure 2A, Table S1). Five noctuid and three erebid species and 14 crambid and three pyralid species were classified into the monophyletic groups Noctuoidea and Pyraloidea, respectively. Thus, the 26 species belonging to five families (Noctuidae, Erebidae, Crambidae, Pyralidae, and Geometridae) formed five clusters of each taxonomic family on the phylogenetic tree. For the Noctuoidea, Spodoptera spp. (Noctuidae) and the two Lithosiini species (Erebidae) individually converged on single clades. For the Pyraloidea, the seven Spilomelinae, six Pyraustinae species (Crambidae), and two Galleriinae species (Pyralidae) each formed a single cluster.
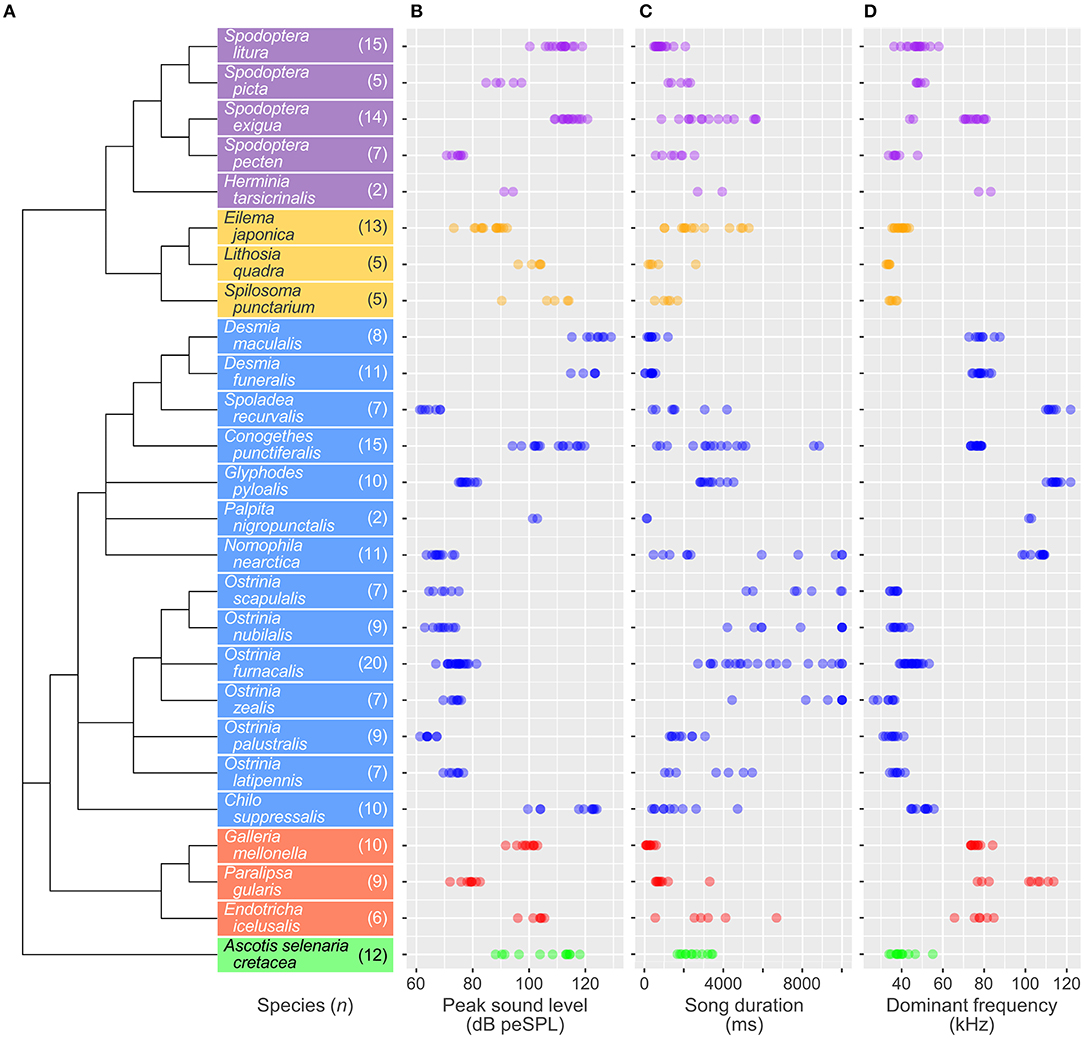
Figure 2. Phylogenetic relationship of the 26 moth species and acoustic characteristics of male ultrasonic courtship songs in each species. (A) The phylogenetic tree was constructed by the maximum likelihood method. Colors indicate taxonomic families: purple, Noctuidae (Noctuoidea); orange, Erebidae (Noctuoidea); blue, Crambidae (Pyraloidea); red, Pyralidae (Pyraloidea); green, Geometridae (Geometroidea). The sample sizes used for acoustic analysis are shown in parentheses after the species names. Distribution of peak sound level (B), song duration (C), and dominant frequency (D) of male songs. Each circle denotes a value extracted from an individual singing male.
At the species level, the mean peak sound levels ranged from 64 dB peSPL, emitted by Ostrinia palustralis (Crambidae; minimum–maximum, 59–67 dB peSPL; n = 9 males; Figure 1A), to 129 dB peSPL, emitted by Desmia funeralis (Crambidae; 115–134 dB peSPL, n = 11; Figure 1C). The sound level was significantly different among species (LR test in GLM with gamma error distribution; χ2 = 11.30; p < 0.0001; Figure 2B). The mean song duration ranged from 110 ms, emitted by Palpita nigropunctalis (Crambidae; 95–124 ms; n = 2; Figure 1D), to 8,839 ms, emitted by Ostrinia zealis (Crambidae; 4,430 to >10,000 ms; n = 7; Figure 1B). Song duration also significantly differed among species (χ2 = 181.90; p < 0.0001; Figure 2C). The mean dominant frequency ranged from 33 kHz, emitted by Ostrinia zealis (Crambidae; 26–37 kHz; n = 7; Figure 1B), to 115 kHz, emitted by Glyphodes pyloalis (Crambidae; 110–122 kHz; n = 10; Figure 1E). The peak frequency differed significantly among species (χ2 = 39.16; p < 0.0001; Figure 2D).
At the family level, the mean peak sound levels were 107 dB peSPL (Noctuidae, n = 5 species), 104 dB peSPL (Erebidae, n = 3), 112 dB peSPL (Crambidae, n = 14), 98 dB peSPL (Pyralidae, n = 3), and 110 dB peSPL (Geometridae, n = 1) (Figure 3A). The sound level did not significantly differ among families (LR test in GLM with gamma error distribution, χ2 = 0.11, p = 0.75). The mean song duration was 2,174 ms (Noctuidae, n = 5), 1,609 ms (Erebidae, n = 3), 3,736 ms (Crambidae, n = 14), 1,538 ms (Pyralidae, n = 3), and 2,484 ms (Geometridae, n = 1) (Figure 3B). Song duration did not significantly differ among families (χ2 = 3.23, p = 0.24). The mean dominant frequency was 57 kHz (Noctuidae, n = 5), 36 kHz (Erebidae, n = 3), 68 kHz (Crambidae, n = 14), 84 kHz (Pyralidae, n = 3), and 40 kHz (Geometridae, n = 1) (Figure 3C). The frequency did not significantly differ among families (χ2 = 1.32, p = 0.079).
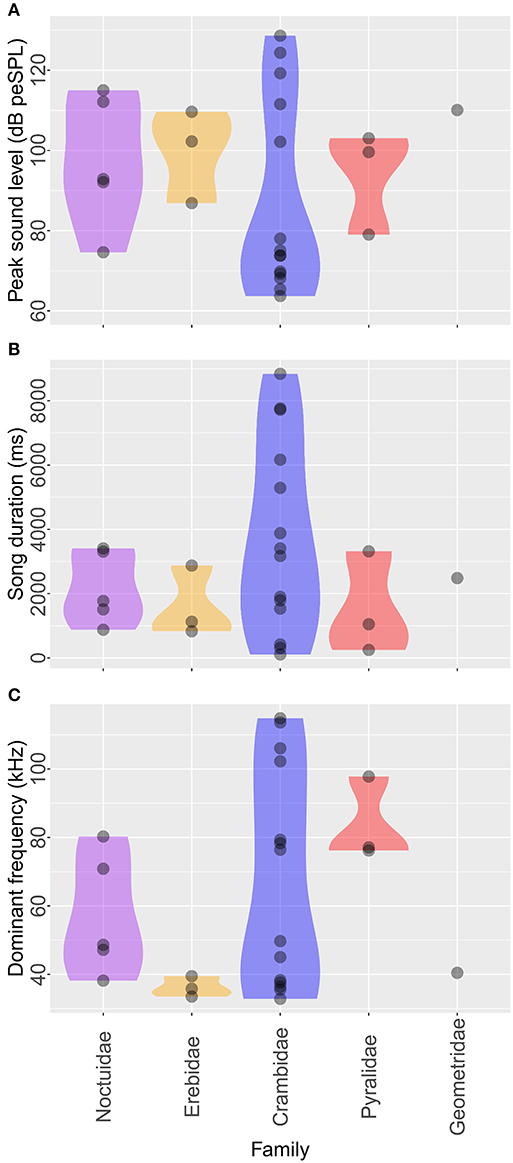
Figure 3. Acoustic characteristics of male ultrasonic courtship songs in each moth family. Peak sound level (A) and song duration (B) do not differ among the five moth families but dominant frequency (C) differs among them. In these violin plots, the upper and lower limits indicate the minimum-to-maximum range, and the width indicates the relative frequencies of the data points.
In the within-species analyses (Table S2), estimated mean of the coefficient in the relationship between the song duration and the peak sound level was −0.086 (95% CI: −0.20–0.027) with no significant difference from 0 (z = −1.49, p = 0.14). For the relationship between the duration and the dominant frequency, estimated mean was −0.0053 (95% CI: −0.094–0.083) and was not significantly different from 0 (z = −0.12, p = 0.91). Estimated mean of the coefficient between the frequency and the sound level was −0.0021 (95% CI: −0.013–0.0086) and was not significantly different from 0 (z = −0.38, p = 0.70).
For the peak sound level, phylogenetic signals of Pagel's λ and Blomberg's K were 0.76 (p = 0.0038) and 0.92 (p = 0.0020), respectively. For the dominant frequency, λ and K were 0.52 (p = 0.15) and 0.66 (p = 0.062), and those for the song duration were 0.76 (p = 0.0047) and 0.90 (p = 0.0050), respectively.
Considering phylogenetic relatedness (see Construction of phylogenetic tree and Phylogenetic comparative analysis), we found a significant negative linear relationship between song duration and peak sound level (Brownian motion model in PGLS, t = −2.23, p = 0.035, AIC = 474.49; Ornstein-Uhlenbeck model in PGLS, t = −2.11, p = 0.045, AIC = 474.92). The results of statistical analyses with GAMM also indicated that song duration was significantly associated with peak sound level (LR test in GAMM, χ2 = 8.83, p = 0.012; Figure 4A).
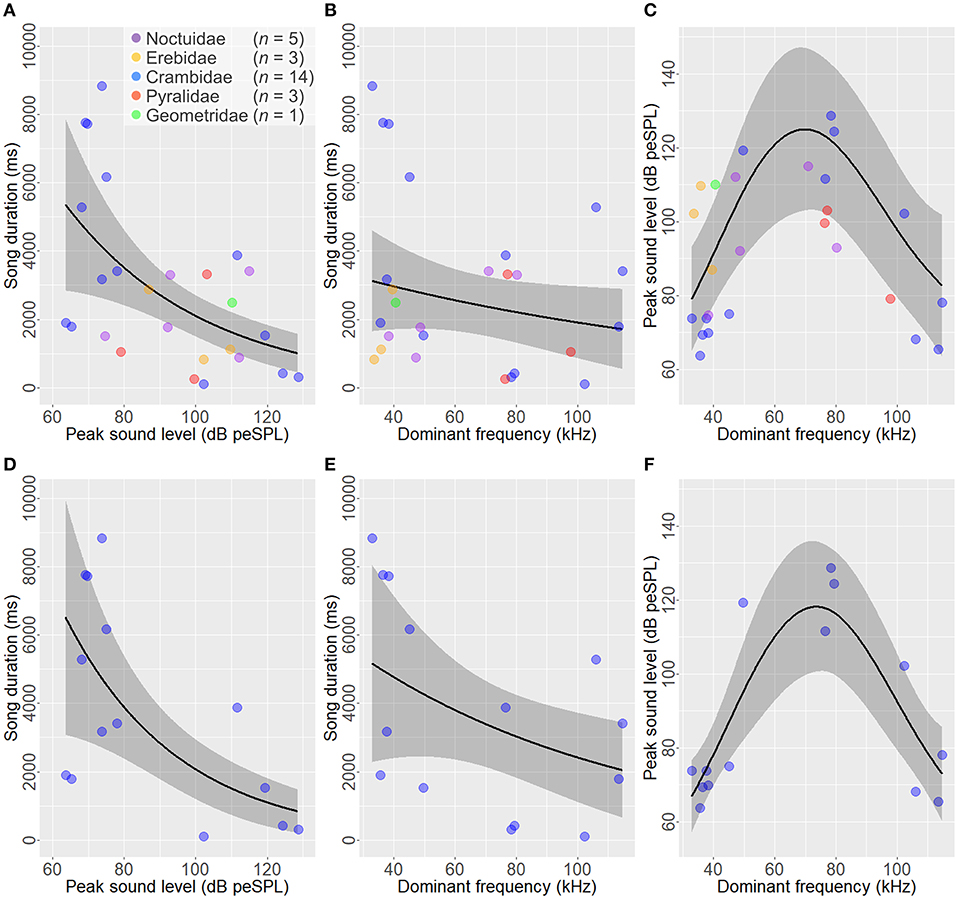
Figure 4. Relationships among song duration, peak sound level, and dominant frequency in male ultrasonic courtship songs of moths. For the 26 moth species, there is a negative relationship between song duration and peak sound level (A), whereas no relation is found between song duration and dominant frequency (B). A significant nonlinear relationship is found between peak sound level and dominant frequency (C). When considering only the 14 crambid moths, a negative relationship between song duration and peak sound level is prominent (D), there is no relation between song duration and dominant frequency (E), and a nonlinear relationship is significant between peak sound level and dominant frequency (F). Black lines and gray bands are means and 95% confidence intervals estimated by GAMM. Colored circles are means for each species; purple, Noctuidae (Noctuoidea); orange, Erebidae (Noctuoidea); blue, Crambidae (Pyraloidea); red, Pyralidae (Pyraloidea); green, Geometridae (Geometroidea).
There was no significant relationship between song duration and dominant frequency (Brownian motion model in PGLS, t = 1.04, p = 0.31, AIC = 476.34; Ornstein-Uhlenbeck model in PGLS, t = −0.21, p = 0.84, AIC = 478.61). GAMM supported the results shown above. There was no significant relationship between song duration and dominant frequency (χ2 = 1.49, p = 0.47; Figure 4B).
No significant relationship was detected between peak sound level and dominant frequency (Brownian motion model in PGLS, t = −1.29, p = 0.21, AIC = 234.35; Ornstein-Uhlenbeck model in PGLS, t = −0.80, p = 0.43, AIC = 233.80). In contrast, GAMM indicated that peak sound level was significantly associated with dominant frequency (χ2 = 14.77, p = 0.00062; Figure 4C).
In the present study, we analyzed the greatest number of species of crambid moths among the five families. Male courtship song in crambids showed a high diversity (Figure 1), ranging from soft to loud songs (60–130 dB peSPL at 10 mm) and from short to long songs (20 to >10,000 ms) (Figures 2B–D). Even when focusing on the single taxonomic family Crambidae, we again corroborated the negative relationship between song duration and peak sound level (LR test in GAMM, χ2 = 5.32, p = 0.0025; Figure 4D), the absence of a significant relationship between song duration and dominant frequency (χ2 = 3.06, p = 0.058; Figure 4E), and the negative relationship between peak sound level and dominant frequency (χ2 = 0.73, p < 0.0001; Figure 4F).
We have shown that a negative relationship between loudness and duration exists in the ultrasonic courtship songs of male moths. Because loud-and-long songs are conspicuous to unintended receivers as well as to potential mating partners, we propose that the acoustical tradeoff in moth song is a consequence of evolutionary adaptation relevant to avoidance of location by eavesdroppers. Males of some field crickets are known to generate “soft” courtship songs after attracting a female by calling songs (Alexander, 1961; Balenger, 2015). The courtship songs of the field crickets last only a few seconds, suggesting that the softness and the shortness of the songs evolved for the avoidance of eavesdropping by predators and parasites. Successful copulation (genital coupling) in insects generally requires the absence of interference from other males. Courtship songs are likely to evolve to be soft and short to reduce the opportunity for a rival male to interrupt the courtship behavior of a singing male and, in some cases, to steal an intended mate (Balsby and Dabelsteen, 2005; Balenger, 2015; Reichard and Anderson, 2015).
In moths, multiple males may gather around a single female that is releasing a sex pheromone in advance of a mating bout (Baker, 1983; Schlaepfer and McNeil, 2000; Nakano et al., 2014). Hence, singing males would gain the benefit of avoidance of eavesdropping by emitting low-amplitude courtship songs that can be detected only by a female in close proximity to the singer. Lengthy courtship songs, such as those of Ostrinia lasting for >10 s, might be perceived by predacious gleaning bats, which can even perceive the rustling sounds of small moving insects (Fuzessery et al., 1993; Goerlitz et al., 2008; Jones et al., 2011; Siemers et al., 2012). However, the longer the duration of a moth song, the lower is the peak sound level. Taking account of atmospheric attenuation of high-frequency ultrasonic courtship songs, the opportunity for eavesdropping long-and-soft songs of moths would be limited for bats as well as for male moth competitors. In this study, we focused on comparison of peak sound level and duration of courtship song among various species that emit ultrasounds ranging from transient clicks to consecutive bursts (Figure 1). It is hard to perform a direct comparison of total acoustic power, consisting of sound amplitude and song duration, among the 26 species we studied, but the energetic cost of production of courtship song also could contribute to the negative relationship between peak sound level and song duration (Figures 4A,D) (Hoback and Wagner, 1997; Reinhold et al., 1998; Oberweger and Goller, 2001; Clark, 2012). In terms of explaining the obtained results of the acoustical tradeoff, hypotheses of physical constraints on the energetic cost and the sound-producing mechanism are not mutually exclusive to our hypothesis that moths evolved hidden courtship songs for avoiding eavesdroppers. The within-species analyses, however, supported no significant relationships among the song characteristics, implying that the energetic constraint may not affect the acoustical tradeoff in the courtship songs of each moth species. To corroborate the adaptive function of the countertactic courtship song, we need to confirm that insectivorous bats more often (1) approach louder-and-longer courtship songs and (2) attack male moths singing louder-and-longer songs in a future study. For the significant nonlinear relationship between peak sound level and dominant frequency (Figures 4C,F), we speculate that the mechanical constraint in the ultrasound production influences this relationship. It is generally because the stridulation and percussion organs do not generate extremely high-frequency ultrasounds, whereas ultrasonic songs produced by the tymbal organs include high-frequency components of >50 kHz (Nakano et al., 2015). Taking the damping of high-frequency ultrasounds in the air into consideration, the dominant frequency of courtship songs might be related to the eavesdropping; however, the hypothesis that courtship songs with higher frequency have higher sound levels is not supported by our data.
For the peak sound level and the song duration, phylogenetic signals (Pagel's λ and Blomberg's K) which significantly differed from no signal indicated that the two traits were not independent of the species relatedness, but more divergent than expected under the Brownian motion models of evolution (random drift). By contrast, the phylogenetic signals of the dominant frequency of song supported the independence of the phylogeny, and less similar than expected under random drift. PGLS and GAMM approaches suggest that the correlation between sound level and duration evolved under a random walk and the negative relationship was affected by directional selection (Figure 4). Moderately strong phylogenetic relatedness in the peak sound level and song duration supports the conserved ultrasound-producing mechanisms in erebid and pyralid moths and the recent independent evolution of diverse ultrasound-producing mechanisms in noctuid and crambid moths (Conner, 1999; Nakano et al., 2015). In the family Erebidae, tiger and lichen moths have similar organs on the lateral side of the metathorax (Conner, 1987, 1999; Nakano et al., 2013), and in the family Pyralidae, Galleriinae moths have corrugated tymbals on the tegulae covering the base of the forewings (Spangler, 1986; Conner, 1999; Kindl et al., 2011). In the family Noctuidae, Spodoptera moths have tymbal membranes on the ventral side of the metathorax (Nakano et al., 2009b, 2010a), Hecatesia moths have alar castanets on the forewings (Bailey, 1978), and Rileyana fovea has stridulation organs on the hindwings and hind legs (Surlykke and Gogala, 1986). In the family Crambidae, Conogethes punctiferalis has smooth tymbal organs on the lateral side of the mesothorax (Nakano et al., 2012b), and Ostrinia moths have specific stridulation scales on the mesothorax and forewings (Nakano et al., 2008). While the ultrasound-producing mechanisms for courtship songs vary among genera (or families for Erebidae and Pyralidae), the peak sound level and duration of moth courtship songs showed a significant negative relationship among the 26 species for five moth families that we used. The negative relationship, therefore, has possibly originated from selective forces from eavesdroppers and female receivers.
Females may evolve a preference for acoustically conspicuous courtship songs, similar to the calling songs generated by high-quality or good-condition males (Jang and Greenfield, 1996; Simmons et al., 2013; Cordes et al., 2014; Balenger et al., 2016). Among acoustic moths in which the function and detailed acoustic characteristics of the songs have been analyzed to date, courtship songs relevant to mate recognition or mate preference are found only in the erebid Eilema japonica (Nakano et al., 2013), the crambid Conogethes punctiferalis (Nakano et al., 2012a, 2014), and the pyralid Galleria mellonella (Spangler, 1985, 1986). Males of these species emit courtship songs at average peak sound levels of 87, 112, and 100 dB peSPL and average durations of 2,872, 3,880, and 249 ms, respectively, indicating that they do not produce loud-and-long courtship songs. This finding suggests that the preference of female receivers for male courtship song is also affected by negative selection pressures. A female close to a male that is emitting exaggerated loud-and-long songs would be exposed to predation from eavesdropping bats that are hunting singing males (Pocklington and Dill, 1995; Candolin, 1997; Alem et al., 2011). In moth species, in which the males use “deceptive” courtship songs toward females, i.e., Spodoptera litura and Ostrinia spp. (Nakano et al., 2008, 2010a,b), such a risk of predation on silent females by eavesdropping bats would increase if these males generated conspicuous lengthy songs in close proximity to focal females. We suggest that both avoiding eavesdroppers and being detected by intended receivers drive the current tradeoff between loudness and duration of male courtship song. A similar relationship in song characteristics may be found in other singing animals as well.
RN conceptualized the study, performed acoustic recordings and measurements, performed statistical analyses, and wrote the original draft. KN carried out and wrote the phylogenetic analyses.
RN was supported by the Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellowships for Research Abroad 26132 (http://www.jsps.go.jp/english/index.html), JSPS KAKENHI JP 17K07581 and the Council for Science, Technology and Innovation (CSTI) Cross-Ministerial Strategic Innovation Promotion Program (Technologies for creating next-generation agriculture, forestry and fisheries) (http://www8.cao.go.jp/cstp/panhu/sip_english/sip_en.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Takuma Takanashi, Yukio Ishikawa, Sadahiro Tatsuki, Sugihiko Hoshizaki, Annemarie Surlykke, Niels Skals, Fumio Ihara, and Andrew C. Mason for helpful suggestions and encouragement in a series of this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00244/full#supplementary-material
Alem, S., Koselj, K., Siemers, B. M., and Greenfield, M. D. (2011). Bat predation and the evolution of leks in acoustic moths. Behav. Ecol. Sociobiol. 65, 2105–2116. doi: 10.1007/s00265-011-1219-x
Alexander, R. D. (1961). Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17, 130–223. doi: 10.1163/156853961X00042
Bailey, N. W., Gray, B., and Zuk, M. (2010). Acoustic experience shapes alternative mating tactics and reproductive investment in male field crickets. Curr. Biol. 20, 845–849. doi: 10.1016/j.cub.2010.02.063
Bailey, W. J. (1978). Resonant wing systems in the Australian whistling moth Hecatesia (Agarasidae, Lepidoptera). Nature 272, 444–446. doi: 10.1038/272444a0
Bailey, W. J., and Field, G. (2000). Acoustic satellite behaviour in the Australian bushcricket Elephantodeta nobilis (Phaneropterinae, Tettigoniidae, Orthoptera). Anim. Behav. 59, 361–369. doi: 10.1006/anbe.1999.1325
Bailey, W. J., and Haythornthwaite, S. (1998). Risks of calling by the field cricket Teleogryllus oceanicus; potential predation by Australian long-eared bats. J. Zool. 244, 505–513. doi: 10.1111/j.1469-7998.1998.tb00056.x
Baker, T. C. (1983). Variations in male oriental fruit moth courtship patterns due to male competition. Experientia 39, 112–114. doi: 10.1007/BF01960660
Balenger, S. L. (2015). Stridulated soft song by singing insects. Anim. Behav. 105, 275–280. doi: 10.1016/j.anbehav.2015.03.024
Balenger, S. L., Lara, L. M., and Zuk, M. (2016). Relative amplitude of courtship song chirp and trill components does not alter female Teleogryllus oceanicus mating behavior. Ethology 123, 168–173. doi: 10.1111/eth.12583
Balsby, T. J. S., and Dabelsteen, T. (2005). Simulated courtship interactions elicit neighbor intrusions in the whitethroat, Sylvia communis. Anim. Behav. 69, 161–168. doi: 10.1016/j.anbehav.2004.01.021
Bell, G. P. (1985). The sensory basis of prey location by the California leaf-nosed bat Macrotus californicus (Chiroptera: Phyllostomidae). Behav. Ecol. Sociobiol. 16, 343–347. doi: 10.1007/BF00295547
Bernal, X. E., and de Silva, P. (2015). Cues used in host-seeking behavior by frog-biting midges (Corethrella spp. Coquillet). J. Vector Ecol. 40, 122–128. doi: 10.1111/jvec.12140
Bernal, X. E., Rand, A. S., and Ryan, M. J. (2006). Acoustic preferences and localization performance of blood-sucking flies (Corethrella Coquillett) to túngara frog calls. Behav. Ecol. 17, 709–715. doi: 10.1093/beheco/arl003
Blomberg, S. P., Garland, T. Jr., and Ives, A. R. (2003). Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x
Bradshaw, C. J. A., Giam, X., Tan, H. T. W., Brook, B. W., and Sodhi, N. S. (2008). Threat or invasive status in legumes is related to opposite extremes of the same ecological and life-history attributes. J. Ecol. 96, 869–883. doi: 10.1111/j.1365-2745.2008.01408.x
Cade, W. (1975). Acoustically orienting parasitoids: fly phonotaxis to cricket song. Science 190, 1312–1313. doi: 10.1126/science.190.4221.1312
Cade, W. (1980). Alternative male reproductive behaviors. Florida Entomol. 63, 30–45. doi: 10.2307/3494654
Candolin, U. (1997). Predation risk affects courtship and attractiveness of competing threespine stickleback males. Behav. Ecol. Sociobiol. 41, 81–87. doi: 10.1007/s002650050367
Clark, C. J. (2012). The role of power versus energy in courtship: what is the ‘energetic cost’ of a courtship display? Anim. Behav. 84, 269–277. doi: 10.1016/j.anbehav.2012.04.012
Conner, W. E. (1987). Utrasound: its role in the courtship of the arctiid moth, Cycnia tenera. Experientia 43, 1029–1031. doi: 10.1007/BF01952230
Conner, W. E. (1999). ‘Un chant d'appel amoureux’: acoustic communication in moths. J. Exp. Biol. 202, 1711–1723.
Cordes, N., Engqvist, L., Schmoll, T., and Reinhold, K. (2014). Sexual signaling under predation: attractive moths take the greater risks. Behav. Ecol. 25, 409–414. doi: 10.1093/beheco/art128
Dabelsteen, T. (2004). Strategies that facilitate or counter eavesdropping on vocal interactions in songbirds. An. Acad. Bras. Ciênc. 76, 274–278. doi: 10.1590/S0001-37652004000200014
Dabelsteen, T., McGregor, P. K., Lampe, H. M., Langmore, N. E., and Holland, J. (1998). Quiet song in song birds: an overlooked phenomenon. Bioacoustics 9, 89–105. doi: 10.1080/09524622.1998.9753385
Endler, J. A. (1987). Predation, light intensity and courtship behaviour in Poecilia reticulata (Pisces: Poeciliidae). Anim. Behav. 35, 1376–1385. doi: 10.1016/S0003-3472(87)80010-6
Faure, P. A., and Hoy, R. R. (2000). The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviors in the katydid Neoconocephalus ensiger (Orthoptera; Tettigoniidae). J. Comp. Physiol. A 186, 129–142. doi: 10.1007/s003590050013
Felsenstein, J. (1985). Phylogenies and the comparative method. Am. Nat. 125, 1–15. doi: 10.1086/284325
Fuzessery, Z. M., Buttenhoff, P., Andrews, B., and Kennedy, J. M. (1993). Passive sound localization of prey by the pallid bat (Antrozous p. pallidus). J. Comp. Physiol. A 171, 767–777. doi: 10.1007/BF00213073
Goerlitz, H. R., Greif, S., and Siemers, B. M. (2008). Cues for acoustic detection of prey: insect rustling sounds and the influence of walking substrate. J. Exp. Biol. 211, 2799–2806. doi: 10.1242/jeb.019596
Greenfield, M. D. (2014). “Acoustic communication in the nocturnal Lepidoptera,” in Insect Hearing and Acoustic Communication, Vol. 1, ed B. Hedwig (Heidelberg: Germany Springer-Verlag, 81–100.
Greenfield, M. D., and Baker, M. (2003). Bat avoidance in non-aerial insects: the silence response of signaling males in an acoustic moth. Ethology 109, 427–442. doi: 10.1046/j.1439-0310.2003.00886.x
Greig, E. I., and Greenfield, M. D. (2004). Sexual selection and predator avoidance in an acoustic moth: discriminating females take fewer risks. Behaviour 141, 799–815. doi: 10.1163/1568539042265626
Hoback, W. W., and Wagner, W. E. Jr. (1997). The energetic cost of calling in the variable field cricket, Gryllus lineaticeps. Physiol. Entomol. 22, 286–290. doi: 10.1111/j.1365-3032.1997.tb01170.x
Igaune, K., Krams, I., Krama, T., and Bobkova, J. (2008). White storks Ciconia ciconia eavesdrop on mating calls of moor frogs Rana arvalis. J. Avian Biol. 39, 229–232. doi: 10.1111/j.2008.0908-8857.04180.x
Jang, Y., and Greenfield, M. D. (1996). Ultrasonic communication and sexual selection in wax moths: female choice based on energy and asynchrony of male signals. Anim. Behav. 51, 1095–1106. doi: 10.1006/anbe.1996.0111
Jones, P. L., Page, R. A., Hartbauer, M., and Siemers, B. M. (2011). Behavioral evidence for eavesdropping on prey song in two Palearctic sibling bat species. Behav. Ecol. Sociobiol. 65, 333–340. doi: 10.1007/s00265-010-1050-9
Kawahara, A. Y., and Breinholt, J. W. (2014). Phylogenomics provides strong evidence for relationships of butterflies and moths. Proc. R. Soc. B 281:20140970. doi: 10.1098/rspb.2014.0970
Kindl, J., Kalinová, B., Cervenka, M., Jílek, M., and Valterová, I. (2011). Male moth songs tempt females to accept mating: the role of acoustic and pheromonal communication in the reproductive behaviour of Aphomia sociella. PLoS ONE 6:e26476. doi: 10.1371/journal.pone.0026476
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Libersat, F., and Hoy, R. R. (1991). Ultrasonic startle behavior in bushcrickets (Orthoptera; Tettigoniidae). J. Comp. Physiol. A 169, 507–514. doi: 10.1007/BF00197663
Martins, E. P., and Hansen, T. F. (1997). Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667. doi: 10.1086/286013
Nakano, R., Ihara, F., Mishiro, K., and Toyama, M. (2012b). Male courtship ultrasound produced by mesothoracic tymbal organs in the yellow peach moth Conogethes punctiferalis (Lepidoptera: Crambidae). Appl. Entomol. Zool. 47, 129–135. doi: 10.1007/s13355-012-0099-5
Nakano, R., Ihara, F., Mishiro, K., Toyama, M., and Toda, S. (2014). Double meaning of courtship song in a moth. Proc. R. Soc. B. 281:20140840. doi: 10.1098/rspb.2014.0840
Nakano, R., Ishikawa, Y., Tatsuki, S., Skals, N., Surlykke, A., and Takanashi, T. (2009a). Private ultrasonic whispering in moths. Commun. Integr. Biol. 2, 123–126. doi: 10.4161/cib.7738
Nakano, R., Ishikawa, Y., Tatsuki, S., Surlykke, A., Skals, N., and Takanashi, T. (2006). Ultrasonic courtship song in the Asian corn borer moth, Ostrinia furnacalis. Naturwissenschaften 93, 292–296. doi: 10.1007/s00114-006-0100-7
Nakano, R., Skals, N., Takanashi, T., Surlykke, A., Koike, T., Yoshida, K., et al. (2008). Moths produce extremely quiet ultrasonic courtship songs by rubbing specialized scales. Proc. Natl Acad. Sci. U.S.A. 105, 11812–11817. doi: 10.1073/pnas.0804056105
Nakano, R., Takanashi, T., Fujii, T., Skals, N., Surlykke, A., and Ishikawa, Y. (2009b). Moths are not silent, but whisper ultrasonic courtship songs. J. Exp. Biol. 212, 4072–4078. doi: 10.1242/jeb.032466
Nakano, R., Takanashi, T., Ihara, F., Mishiro, K., Toyama, M., and Ishikawa, Y. (2012a). Ultrasonic courtship song of the yellow peach moth, Conogethes punctiferalis (Lepidoptera: Crambidae). Appl. Entomol. Zool. 47, 87–93. doi: 10.1007/s13355-012-0092-z
Nakano, R., Takanashi, T., Skals, N., Surlykke, A., and Ishikawa, Y. (2010a). To females of a noctuid moth, male courtship songs are nothing more than bat echolocation calls. Biol. Lett. 6, 582–584. doi: 10.1098/rsbl.2010.0058
Nakano, R., Takanashi, T., Skals, N., Surlykke, A., and Ishikawa, Y. (2010b). Ultrasonic courtship songs of male Asian corn borer moths assist copulation attempts by making the females motionless. Physiol. Entomol. 35, 76–81. doi: 10.1111/j.1365-3032.2009.00712.x
Nakano, R., Takanashi, T., Skals, N., Surlykke, A., and Ishikawa, Y. (2013). Evolution of deceptive and true courtship songs in moths. Sci. Rep. 3:2003. doi: 10.1038/srep02003
Nakano, R., Takanashi, T., and Surlykke, A. (2015). Moth hearing and sound communication. J. Comp. Physiol. A 201, 111–121. doi: 10.1007/s00359-014-0945-8
Nolen, T. G., and Hoy, R. R. (1986). Phonotaxis in flying crickets. I. Attraction to the calling song and avoidance of bat-like ultrasound are discrete behaviors. J. Comp. Physiol. A 159, 423–439. doi: 10.1007/BF00604163
Oberweger, K., and Goller, F. (2001). The metabolic cost of birdsong production. J. Exp. Biol. 204, 3379–3388.
Pagel, M. (1999). Inferring the historical patterns of biological evolution. Nature 401, 877–884. doi: 10.1038/44766
Paradis, E., and Schliep, K. (2018). ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. doi: 10.1093/bioinformatics/bty633
Pinheiro, J., Bates, D., DebRoy, S., and Sarkar, D. R Core Team. (2019). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–139. Available online at: https://CRAN.R-project.org/package=nlme
Pocklington, R., and Dill, L. M. (1995). Predation on females or males: who pays for bright male traits? Anim. Behav. 49, 1122–1124. doi: 10.1006/anbe.1995.0141
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Regier, J. C., Mitter, C., Zwick, A., Bazinet, A. L., Cummings, M. P., Kawahara, A. Y., et al. (2013). A large-scale, higher-level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS ONE 8:e58568. doi: 10.1371/journal.pone.0058568
Reichard, D. G., and Anderson, R. C. (2015). Why signal softly? The structure, function and evolutionary significance of low-amplitude signals. Anim. Behav. 105, 253–265. doi: 10.1016/j.anbehav.2015.04.017
Reinhold, K., Greenfield, M. D., Jang, Y., and Broce, A. (1998). Energetic cost of sexual attractiveness: ultrasonic advertisement in wax moths. Anim. Behav. 55, 905–913. doi: 10.1006/anbe.1997.0594
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. doi: 10.1111/j.2041-210X.2011.00169.x
Rodríguez, R., and Greenfield, M. D. (2004). Behavioural context regulates dual function of ultrasonic hearing in lesser waxmoths: bat avoidance and pair formation. Physiol. Entomol. 29, 159–168. doi: 10.1111/j.1365-3032.2004.00380.x
Sakaluk, S. K., and Belwood, J. J. (1984). Gecko phonotaxis to cricket calling song: a case of satellite predation. Anim. Behav. 32, 659–662. doi: 10.1016/S0003-3472(84)80141-4
Sanderford, M. V., and Conner, W. E. (1995). Acoustic courtship communication in Syntomeida epilais Wlk. (Lepidoptera: Arctiidae, Ctenuchinae). J. Insect Behav. 8, 19–31. doi: 10.1007/BF01990967
Schlaepfer, M. A., and McNeil, J. N. (2000). Are virgin male lepidopterans more successful in mate acquisition than previously mated individuals? A study of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). Can. J. Zool. 78, 2045–2050. doi: 10.1139/z00-147
Schulze, W., and Schul, J. (2001). Ultrasound avoidance behaviour in the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae). J. Exp. Biol. 204, 733–740.
Siemers, B. M., Kriner, E., Kaipf, I., Simon, M., and Greif, S. (2012). Bats eavesdrop on the sound of copulating flies. Curr. Biol. 22, R563–R564. doi: 10.1016/j.cub.2012.06.030
Simmons, L. W., Thomas, M. L., Simmons, F. W., and Zuk, M. (2013). Female preferences for acoustic and olfactory signals during courtship: male crickets send multiple messages. Behav. Ecol. 24, 1099–1107. doi: 10.1093/beheco/art036
Spangler, H. G. (1984). Silence as a defense against predatory bats in two species of calling insects. Southwest. Nat. 29, 481–488. doi: 10.2307/3671001
Spangler, H. G. (1985). Sound production and communication by the greater wax moth (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Am. 78, 54–61. doi: 10.1093/aesa/78.1.54
Spangler, H. G. (1986). Functional and temporal analysis of sound production in Galleria mellonella L. (Lepidoptera: Pyralidae). J. Comp. Physiol. A 159, 751–756. doi: 10.1007/BF00603728
Surlykke, A., and Gogala, M. (1986). Stridulation and hearing in the noctuid moth Thecophora fovea (Tr.). J. Comp. Physiol. A 159, 267–273. doi: 10.1007/BF00612309
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Tuttle, M. D., and Ryan, M. J. (1981). Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214, 677–678. doi: 10.1126/science.214.4521.677
Tuttle, M. D., Ryan, M. J., and Belwood, J. J. (1985). Acoustical resource partitioning by two species of Phyllostomid bats (Trachops cirrhosus and Tonatia sylvicola). Anim. Behav. 33, 1369–1371. doi: 10.1016/S0003-3472(85)80204-9
Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. doi: 10.18637/jss.v036.i03
Walker, T. J. (1993). Phonotaxis in female Ormia ochracea (Diptera: Tachinidae), a parasitoid of field crickets. J. Insect Behav. 6, 389–410. doi: 10.1007/BF01048119
Wood, S. N. (2008). Fast stable direct fitting and smoothness selection for generalized additive models. J. R. Stat. Soc. B. 70, 495–518. doi: 10.1111/j.1467-9868.2007.00646.x
Zuk, M., and Kolluru, G. R. (1998). Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438. doi: 10.1086/420412
Keywords: bat-predator, courtship song, eavesdropper, moth-prey, ultrasonic communication
Citation: Nakano R and Nagamine K (2019) Loudness–Duration Tradeoff in Ultrasonic Courtship Songs of Moths. Front. Ecol. Evol. 7:244. doi: 10.3389/fevo.2019.00244
Received: 06 December 2018; Accepted: 12 June 2019;
Published: 02 July 2019.
Edited by:
Ximena E. Bernal, Purdue University, United StatesReviewed by:
Tom Tregenza, University of Exeter, United KingdomCopyright © 2019 Nakano and Nagamine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryo Nakano, cm5ha2Fub0BhZmZyYy5nby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.