- 1Department of Anthropology, East Carolina University, Greenville, NC, United States
- 2Department of Anthropology, University of Utah, Salt Lake City, UT, United States
Despite a long history of study, consensus on a human-typical mating system remains elusive. While a simple classification would be useful for cross-species comparisons, monogamous, polyandrous, and polygynous marriage systems exist across contemporary human societies. Moreover, sexual relationships occur outside of or in tandem with marriage, resulting in most societies exhibiting multiple kinds of marriage and mating relationships. Further complicating a straightforward classification of mating system are the multiple possible interpretations of biological traits typical of humans used to indicate ancestral mating patterns. While challenging to characterize, our review of the literature offers several key insights. 1) Although polygyny is socially sanctioned in most societies, monogamy is the dominant marriage-type within any one group cross-culturally. 2) Sex outside of marriage occurs across societies, yet human extra pair paternity rates are relatively low when compared to those of socially monogamous birds and mammals. 3) Though the timing of the evolution of certain anatomical characteristics is open to debate, human levels of sexual dimorphism and relative testis size point to a diverging history of sexual selection from our great ape relatives. Thus, we conclude that while there are many ethnographic examples of variation across human societies in terms of marriage patterns, extramarital affairs, the stability of relationships, and the ways in which fathers invest, the pair-bond is a ubiquitous feature of human mating relationships. This may be expressed through polygyny and/or polyandry but is most commonly observed in the form of serial monogamy.
Introduction
How best to characterize the human mating system is a subject of intense and polarized debate. On the one hand, sex differences in reproductive investment, and resultant differing potential reproductive rates, are argued to favor elevated mating effort behavior in males (i.e., a short-term, multiple mate seeking orientation; Symons, 1979) and polygyny. However, on the other hand, an evolved sexual division of labor, with offspring dependence on paternal care, is argued to generate overlapping interests in long-term, monogamous relationships for both men and women (Washburn and Lancaster, 1968; Lancaster and Lancaster, 1987; Kaplan et al., 2000). Given the varied sources of support for both approaches, disagreement exists on how best to describe mating patterns in humans. Particularly challenging is to generate an agreed upon definition of a species-typical strategy often used in comparative studies. This review is focused on an attempt to offer resolution regarding the current debate. After reviewing the literature on marriage and mating systems in humans, we present a cross-cultural examination as well as comparative and evolutionary evidence for and against particular lines of inquiry.
What is the Human Mating System?
Confusion and debate describing a human-typical mating pattern are warranted given the diversity of strategies both across and within cultures. For example, data from the Standard Cross-Cultural Sample (Murdock and White, 1969), a representative global sample of primarily pre-industrial societies, indicates that polygynous marriage (one male, multiple females) is sanctioned in nearly 85% of societies (Figure 1). This figure is often used to support claims of the mating effort intensive nature of males given that most societies allow men to have multiple wives. However, upon closer inspection, within a small-scale polygynous society, the majority of marriages are monogamous (Murdock and White, 1969; Flinn and Low, 1986; Binford, 2001). For example, among the Savanna Pumé (South American hunter-gatherers) while polygyny occurs (20% of women and 11% of men are polygynously married at some point during their lives), most marriages are monogamous, consistent with other foraging groups (Marlowe and Berbesque, 2012; Kramer et al., 2017).
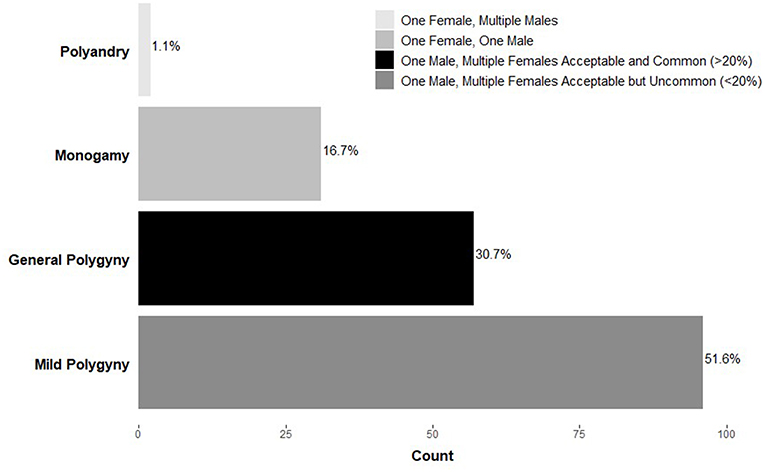
Figure 1. Frequency of marriage systems across societies (n = 186) in the Standard Cross-Cultural Sample (Murdock and White, 1969). Adapted from Marlowe (2000).
Although most marriages are monogamous at any one point in time, over the life course individuals may reenter the marriage market more than once. Among hunter-gatherers, industrializing societies, and many contemporary Western populations, remarriage is common after spousal death and/or divorce, resulting in serial monogamy where both men and women have multiple partners over their reproductive careers (Fisher, 1989; Hill and Hurtado, 1996; Borgerhoff Mulder, 2009; Jokela et al., 2010). Nonetheless, while individuals may have more than one partner across their life, sexual fidelity within a marriage is generally expected. Marriage is common to all human societies and publicly acknowledges who has sexual access to whom, with divorce often resulting from extramarital relationships (Irons, 1983; Marlowe, 2003; Kramer and Greaves, 2011). However, typical of the range of behavioral variation expressed by humans, many exceptions exist, and sex is found outside of marriage both cross-culturally and among individuals in any one society (Box 1: Sex outside of the pairbond across human societies). Yet, while engaging in sex outside of marriage likely occurs to some extent in all societies, because men and women typically live in long-term pairbonds within the same residential unit, they have been described as practicing social monogamy (Reichard, 2003; Strassmann, 2003). While human patterns are distinct from genetic monogamy, defined as two individuals who only reproduce with one another, levels of extra pair paternity are relatively low compared to other socially monogamous species. Estimates of non-paternity rates range from 0-11% across societies (Simmons et al., 2004; Anderson, 2006; with median values falling between 1.7–3.3%) while among birds these rates regularly exceed 20% (Griffith et al., 2002).
Box 1. Sex outside of the pairbond across human societies.
While humans form long-term pair bonds that are recognized as marriages in all societies, sexual relations also occur outside of marriage. In some societies and incidences these relations are clandestine and considered transgressions with punishments that range in severity. But in other cases, uncommitted sexual liaisons are socially permissible, and generally fall under two well-documented ethnographic contexts. The first occurs prior to first marriage when adolescent girls are in a life stage when they have a low probability of conceiving and are given freedom to explore different premarital relationships (Mead, 1928; Irons, 1983; Parker, 1985; Gregor, 1987). For example, among the Makushi of Guyana, recently sexual mature individuals receive parental support to engage in pre-marital sex (Schacht, 2013). The stated purpose of this mating behavior is to allow for mutual mate choice and the identification of a possible long-term mate. However, once married, copulation outside of the pair-bond is expected to cease. A second socially sanctioned form of sex outside marriage occurs in the context of either partible paternity or wife sharing during prescribed situations. For example, among some lowland South American groups, women regularly have several sexual partners in addition to their husband (Beckerman and Valentine, 2002; Walker et al., 2010). This practice is common where the contribution of multiple men is thought to be required for fetal development. While women do not formalize additional relationships through marriage (i.e., polyandry is not institutionalized), these men are expected to provide protection for and investment in children as they develop – a long-term commitment (Beckerman et al., 1998). In other societies, wife sharing may occur during publicly acknowledged situations. A well-described example comes from many different ethnographic sources of the Inuit, where monogamous couples engage in “wife-swapping” (Boas, 1907; Rubel, 1961; Hennigh, 1970; although husband-swapping may be more accurate). This exchange was reportedly agreed upon by all parties, and often, though not always, resulted in long-term social (and sexual) relationships. Other extrapair relationships are more clandestine, likely because of penalties that may follow (e.g., violence in response to sexual jealousy). Nonetheless, there are many examples of men offering food and other resources in exchange for extramarital sex (Holmberg, 1969; Gregor, 1987; Hill and Hurtado, 1996; Pollock, 2002).
In sum, a simple classification of a human-typical mating system is challenging given the variety of pairing strategies observed. Monogamous, polyandrous, polygynous, and short-term mating patterns are found across contemporary human societies, with most societies exhibiting multiple kinds of marriages and mating relationships (Marlowe, 2000; Fortunato, 2015). What can be most simply distilled from this is that humans form long-term pairbonds. However, while polygynous and polyandrous marriages are found in many societies, ethnographic evidence indicates that most individuals within a society live in monogamous marriages that are generally, but not always, sexually exclusive. It is important also to emphasize that these unions are commonly serially monogamous, and that regardless of divorce rates, this likely would have been the case in the past due to high rates of spousal mortality under premodern mortality schedules (Gurven and Kaplan, 2007).
Ancestral Mating System in Humans
Although cross-cultural information may illuminate contemporary variation in mating patterns, it tells us less about their antiquity. To seek additional support to characterize the human mating system, we turn to indicators of ancestral mating patterns. Sexual selection is a widely recognized force influencing behavioral and physical traits across animal taxa (Andersson, 1994). Differences between males and females within and across species can offer insight into both historical and contemporary selection pressures. Mating systems are amazingly diverse across mammals generally, and primates in particular (Dixson, 1997; Kappeler and van Schaik, 2002). Given human placement in the primate order, here we approach human mating from a comparative perspective to better understand behavioral and physical traits that either are shared or distinguish us from our closest living relatives. We target three commonly examined traits in reference to predicting primate breeding systems: sexual dimorphism, testis size, and concealed ovulation (Dixson, 2009). We review each of these and discuss whether the evidence supports a human monogamous past that may serve to explain the mating system's current prevalence.
Sexual Dimorphism
Sexual dimorphism exists within a species when, in addition to differences between the sexual organs themselves, males and females differ in size or appearance (Andersson, 1994). Across primates, minimal levels of sexual dimorphism in body weight and canine size are generally associated with monogamy and low rates of male antagonistic competition (e.g., gibbons; Harcourt, 1981). Size differences are expected to be most pronounced within single-male/multi-female polygynous species where male competition can be intense, and stakes high, because winners have much to gain. For example, among mountain gorillas (Gorilla beringei beringei) dominant males monopolize sexual access to a group of females and perform up to 70% of all copulations (Stoinski et al., 2009). Unsurprisingly, gorillas exhibit high levels of reproductive skew and males are nearly twice the size of females (Leigh and Shea, 1995). However, for species that live in multi-male/multi-female groups, such as chimpanzees, body size dimorphism tends to be intermediary between monogamous and polygynous species (Dixson, 2009). Given these patterns, what evidence of sexual dimorphism do we see in our hominin line (i.e., the phylogenetic group consisting of all modern humans, extinct human species, and our immediate ancestors) and what inferences can be drawn of ancestral mating systems?
Determining size dimorphism from the fossil record is fraught with debate due to interpretations that vary across researchers (Lockwood et al., 2007; Gordon et al., 2008; Reno et al., 2010; Plavcan, 2012). However, the general consensus is that dimorphism was greater in our past and has diminished over time. This is often interpreted to suggest that male mating competition decreased in intensity over the course of hominin evolution in conjunction with a rise in monogamy. When this transition occurred, however, is debated. Some researchers speculate that dimorphism was fairly modest around 4 million years ago among australopithicines and place monogamy and male provisioning deep in the hominin line (Lovejoy, 1981; Reno et al., 2003, 2010). Others contend that australopithecines were highly dimorphic; therefore, monogamy had yet to become established (Lockwood et al., 1996, 2007; Gordon et al., 2008). Nonetheless, because of the fragmentary nature of fossil remains, difficulties in assigning sex, and the number of different species and subspecies, the fossil record may be an unreliable indicator of mating behavior in extinct species (Plavcan, 2000, 2012; Churchhill et al., 2012). For example, male competition may be expressed in many ways besides physical aggression (e.g., sperm competition, social status, and wealth), and so size dimorphism may underestimate male competition (Puts, 2010; Marlowe and Berbesque, 2012).
Regardless of the timing of the reduction in sexual dimorphism, humans today express only slight differences in body size by sex compared to closely-related promiscuous and polygynous species. For example, human body size dimorphism by weight averages about 1.15 (i.e., males are 15% heavier), with chimpanzees at 1.3 and orangutans and gorillas near 2 or more (Willner, 1989; Plavcan and van Schaik, 1992; Dixson, 2009). Humans fit more neatly in the range of variation typical of monogamous gibbons (e.g., Hylobates lar) who exhibit very little difference in body size by sex (1.07; Willner, 1989; Box 2: Which living ape is the best model for the breeding system of our last common ancestor?).
Box 2. Which living ape is the best model for the breeding system of our last common ancestor?
Which ape mating system best serves as the baseline from which directionality in the fossil record should be interpreted? Chimpanzees have long been used as the behavioral model assumed to best resemble our last common ancestor. However, this has more recently given way to debate about whether past hominins (our bipedal ancestors) lived in multimale/multifemale groups like chimps (Hrdy, 2009; van Schaik and Burkart, 2010; Gavrilets, 2012) or were instead organized in polygynous, gorilla-like harems (Dixson, 2009; Chapais, 2011; Grueter et al., 2012) or had a hamadryas baboon-like structure with multiple single-male groups living together within a larger population. While this debate is ongoing, most researchers agree that ancient hominins were a group living animal, and that these groups were organized in nested multi-level societies (e.g., biological families, extended families, bands, tribes, etc.) with multiple breeding females, who commonly lived within socially recognized long-term pairbonds (Chapais, 2008; Grueter et al., 2012). Thus, whether pairbonds developed in the context of a polygynous or polygynandrous breeding system remain ambiguous. What we can say with certainty is that if our last common ancestor were “gorilla like,” we have become less dimorphic and less polygynous. And if it were more “chimpanzee like,” we have reduced body-size dimorphism only slightly, but have become much less promiscuous.
Testis Size
Testis size is another commonly used metric of mating system as it indicates, generally, female multiple mating, such that large testis relative to body size is positively correlated with the frequency of females mating with multiple males simultaneously (Harcourt et al., 1981; Kenagy and Trombulak, 1986; Moller, 1988; Parker, 2016). Adjusting for body size, human testes are smaller than would be predicted, and, when compared to our closest living relatives, are considerably smaller than those of chimpanzees (Harcourt et al., 1981; Figure 2). Together this provides evidence of relatively low rates of sex outside of a pairbond. However, human testes are somewhat larger than those of other monogamous primates, leading some to argue that this hints at a measure of extrapair copulation not expected in a monogamous species. Yet studies employing genetic methods find that rates of non-paternity are low among humans (~2%) when compared to those of socially monogamous birds (~20%) and mammals (~5%; Anderson, 2006; Box 1), casting doubt on claims of relatively high rates of extrapair engagement in human males compared to males in other monogamous species.
While testis size is a predictor of the extent to which females multiply mate, it is often mistakenly used as an indicator of monogamy. Testis size cannot discriminate between monogamy and polygyny because, in both cases, females mate with a single male for each offspring, resulting in relatively low sperm competition (Martin and May, 1981; Dixson, 2009). Thus, testis to body size complicates a simple story of ancestral mating derived from sexual dimorphism alone because human values are encompassed within the range of variation found among gorillas and orangutans—great ape species with polygynous mating systems. Therefore, we can only say that human values are consistent with pair-bonded polygynous species, but not with species where females mate multiply.
Concealed Ovulation
Human females lack obvious visible signals of ovulation, particularly in comparison to the conspicuous sexual swellings of, for example, chimpanzees and baboons (Strassmann, 1981; Dixson, 1983; Sillén-Tullberg and Moller, 1993; Rooker and Gavrilets, 2018). As a result, human ovulation is argued to be concealed, with several functional arguments put forward to explain this phenomenon. Commonly claimed is that concealed ovulation and constant sexual receptivity of human females facilitates social monogamy (Morris, 1967; Campbell, 1974; Lovejoy, 1981) by limiting information available to males regarding fertility, thereby promoting monogamy through mate guarding and/or paternal care (Alexander and Noonan, 1979). Specifically, given that humans live in multi male/multi female groups, concealed ovulation is argued to minimize male-male competition and allow for stable, monogamous unions (Marlowe and Berbesque, 2012). However, more recently this association has been rethought as it is increasingly apparent from comparative study that concealed ovulation is not only characteristic of humans and other monogamous primates, but species from other mating systems as well. Many polygynous primates do not have overt signs of ovulation (Sillén-Tullberg and Moller, 1993). While human ovulatory cycles are indeed particularly concealed, what appears to be more remarkable are cycles that are particularly conspicuous. For example, chimpanzee females' estrus swellings are unambiguous and concentrate attention from multiple males during a short window of fertility (Hrdy, 1988; Smuts and Smuts, 1993; Gowaty, 1997; Nunn, 1999).
The traits discussed above, when interpreted singly, allow for different perspectives on ancestral mating in humans. For example, while men are larger on average than women, weight and canine dimorphism are slight compared to that of polygynous gorillas and more comparable to monogamous gibbons (Plavcan, 2012). This relative lack of dimorphism suggests diverging histories of sexual selection among the great apes regarding male reliance on contest competition for reproductive success (Dixson, 2009; Marlowe and Berbesque, 2012). Yet, while size dimorphism suggests a more monogamous past, relative testis size implies the extent to which females mate with multiple partners is higher than would be predicted for a monogamous primate. Human testes to body size values are lower than chimpanzees, higher than that of other monogamous primates, but not significantly different from gorillas. And, while concealed ovulation was once thought to be a human adaptation to promote monogamy, it is common among anthropoid primates, highlighting that what is notable are more conspicuous displays of fertility (e.g., sexual swellings) rather than their absence.
What becomes clear when the traits above are viewed collectively is that humans fall within the range of variation typical of pairbonded species. The lack of exaggerated sexual dimorphism or testis size seems to rule out a history of elevated reproductive skew typical of highly promiscuous or polygynous mating systems. Instead, biological indicators suggest a mating system where both sexes form a long-term pairbond with a single partner (Møller, 2003). And while polygyny was likely present in the human past, as it is across contemporary human societies, the weight of evidence seems to support social monogamy. This does not preclude males and females from taking multiple partners through serial monogamy, or by occasionally engaging in uncommitted sexual relationships (as indicated by testis to body size values). However, while extra-pair paternity (EPP) varies across socially monogamous animals, human rates of non-paternity are comparatively low.
Causes and Consequences of Monogamy
The human life history pattern (i.e., short birth intervals, relatively high child survival, and a long period of juvenile dependence) means that mothers are often in the position of supporting multiple dependents of various ages simultaneously. Because infants, juveniles, and adolescents each require different kinds of time and energy investments, mothers are posed with an allocation problem throughout much of their reproductive career: how to care for infants and small children without compromising time spent in activities that provide food and other resources for older children (Lancaster, 1991; Hurtado et al., 1992; Hrdy, 1999; Kaplan et al., 2000; Kramer, 2005b, 2010; Kramer and Veile, 2018). How mothers resolve this trade-off to support a rapid reproductive pace has long been theoretically tied to monogamy and the cooperation of fathers, siblings, and others to help mothers raise dependents.
Cooperative Breeding
Humans are typically described as cooperative breeders (although see Bogin et al., 2014), which in addition to male parental investment, is a key defining aspect of human sociality, cognition, and demographic success (Hrdy, 2005, 2009; Kramer, 2010; van Schaik and Burkart, 2010; Kramer and Greaves, 2011). Several recent phylogenetic analyses provide compelling evidence that cooperative breeding in bird, insect, and mammalian taxa was preceded by an ancestry of monogamy (Hughes et al., 2008; Cornwallis et al., 2010; Lukas and Clutton-Brock, 2012). The logic is that in a non-monogamous mating system, a sexually mature individual is likely to be more closely related to his or her own offspring (r = 0.5) than to siblings who may have a different parent (r between siblings = 0.25). Consequently, after sexual maturity, individual fitness is generally maximized by investing in one's own offspring rather than helping to raise siblings. In a monogamous mating system, however, the value for a sexually mature sibling to stay in his/her natal group and help full siblings is equal to that of rearing one's own offspring (r = 0.5 for both) (Boomsma, 2007, 2009; Lukas and Clutton-Brock, 2012, 2013). Because kin-based benefits are diluted under female multiple mating, monogamy is hypothesized to be a critical step to raise relatedness within groups and sibships and thus to favor the evolution of kin-biased cooperative breeding (Boomsma, 2007, 2009; Hughes et al., 2008; Lukas and Clutton-Brock, 2012).
To add a bit of complexity, while monogamy may motivate the evolution of cooperative breeding and explain why reproductive-aged individuals help, non-reproductive individuals are able to realize kin-based benefits regardless of mating system. In many human societies, juvenile siblings and older females constitute much of the childrearing work force, contributing not only to childcare but also to resource provisioning (Flinn, 1988; Ivey, 2000; Lee and Kramer, 2002; Lahdenpera et al., 2004; Kramer, 2005b; Leonetti et al., 2005; Hrdy, 2009; Kramer and Veile, 2018). This help is empirically associated with improved maternal fertility and offspring outcomes (Turke, 1988; Blurton Jones et al., 1994; Hawkes et al., 1995a; Bliege Bird and Bird, 2002; Ivey et al., 2005; Kramer, 2005a, 2010). Among cooperative breeding mammals and eusocial insects, juveniles and subadults make important contributions to rearing and ensuring the survival of other's offspring Clutton-Brock, 2002, 2009; Russell, 2004; Gilchrist and Russell, 2007; Boomsma, 2013. And, while grandmothering is rare in other species (McAuliffe and Whitehead, 2005), it is well-documented in humans (Hawkes et al., 1998). As a general point, while monogamy may facilitate the cooperation of sexually mature siblings, cooperation between a mother and juvenile, and a grandmother and her daughter can be favored irrespective of breeding system because of high coefficients of relatedness and low opportunity costs (reviewed in Kramer and Russell, 2014, 2015).
Paternal Care
Established claims in the anthropological literature posit that human mothers can support a rapid reproductive pace compared to our other ape relatives because fathers provide investment to both a partner and children (e.g., calories, protection). This argument hinges on an assertion that during human evolution, the increased need for paternal investment (due to big brains and expensive children) generated selective pressure for long-term pair bonds and a sexual division of labor (Washburn and Lancaster, 1968; Lancaster and Lancaster, 1987). However, phylogenetic analyses suggest that paternal care evolves only after monogamy becomes established in a population (Brotherton and Komers, 2003). Because male investment likely would have resulted in male absence (e.g., through resource provisioning), caring males would have faced potential fitness costs due to freerider males who are liable to steal paternity (Hawkes et al., 1995b; Gavrilets, 2012). Specifically, males that do not care benefit directly from caring males' investments in offspring that are not theirs. As a consequence, the assumption that paternal care drives monogamy is likely overly simplistic (Mathews, 2003; Fromhage et al., 2005). For example, a recent survey found that over 40% of socially monogamous species exhibit no indication of male care (Lukas and Clutton-Brock, 2013).
While paternal care is rare across animal taxa, it is generally present across human societies. However, if the needs of offspring did not drive the evolution of male care, how did it come to be? Under certain circumstances, monogamy can increase male fitness more than deserting a partner and remating (Grafen and Sibly, 1978; Yamamura and Tsuji, 1993; Fromhage et al., 2005; Schacht and Bell, 2016). Social and ecological factors that reduce male mating opportunities, such as females being dispersed or rare, reduce opportunity costs associated with monogamy and allow for selection to act on male paternal investment. Under these conditions, selection is expected to favor paternal investment if this investment improves offspring survival or quality, particularly when payoffs to desertion are low and paternity certainty is high (Dunbar, 1976; Thornhill, 1976; Perrone and Zaret, 1979; Clutton-Brock, 1991; Westneat and Sherman, 1993). Once biparental care becomes established, specialization of care tasks by males and females may serve to stabilize the pair-bond. The modal pattern cross-culturally is a life history characterized by specialization in child care by females (i.e., direct investment) and resource provisioning by males (i.e., indirect investment; Murdock and Provost, 1973). This specialization can result from and further lead to synergistic fitness benefits tied to offspring success (Leonetti and Chabot-Hanowell, 2011; Barta et al., 2014). These payoffs both constrain the behavioral options available to a parent and decrease sex-biased asymmetries in the costs of performing a parental investment task. Thus, task specialization can serve to strengthen biparental care once it emerges against invasion by other strategies.
Human fathers regularly provide care to dependent offspring well into the second decade of their life, and often care for multiple children at the same time (e.g., Kaplan et al., 2000; Gurven and Hill, 2009; Gray and Anderson, 2010). However, men still regulate the time and energy they allocate between mating and parental effort (Kaplan and Lancaster, 2003; Ross et al., 2016). Human paternal investment, while often substantial in relation to other mammals, is facultative rather than obligatory, and the anthropological record indicates considerable cross-cultural variability in how and how much fathers invest in their children (Marlowe, 2000; Lamb, 2004; Gray and Anderson, 2010; Shwalb et al., 2013). A key variable found associated with male investment is paternity certainty. Often males invest less where extra-pair relationships are more common (Gaulin and Schlegel, 1980).
Thus, while a gender division of labor appears to be a human universal, paternal investment is sensitive to a variety of conditions and seems to be regulated, at least in part, by testosterone. Testosterone is an androgenic steroid hormone that supports many aspects of male mating effort, including the development and maintenance of sexually dimorphic musculature and bone structure as well as courtship and male-male aggression (Archer, 2006; Bribiescas et al., 2012). Accordingly, testosterone levels are argued to reflect a male's allocation to reproductive effort at a particular point in time. Levels of circulating testosterone in males are thus reasoned to reflect the evolved hormonal regulation of investment in mating vs. parenting effort (Wingfield et al., 1990). In support of this claim, cross-sectional and longitudinal evidence indicates that married men have lower testosterone levels than unmarried men, and that married men with children have the lowest levels. These results suggest that partnered men, and in particular fathers, are hormonally primed to invest more time and energy into parenting rather than mating effort (Gettler et al., 2011; Gray, 2011).
Kin Discrimination
While mammalian mothers are certain of their maternity, fathers may be uncertain of their paternity. Monogamy ensures relatedness between fathers and their purported children, and permits for both the paternity confidence and relatedness necessary to favor investment by fathers. Because cooperation among close relatives increases the fitness benefit gained by cooperators, mechanisms for discriminating between kin and non-kin, and between close and more distant kin, are critical for its evolution (Hatchwell et al., 2001; Griffin and West, 2003; Chapais, 2008, 2009). If fathers and siblings are able to identify one another, relative payoffs to investment vs. desertion increase for fathers, as do the payoffs for cooperative breeding among siblings. For humans, language and the ability to identify a range of relations through kin classificatory systems likely amplified payoffs to kin-biased cooperation by allowing distinctions in relatedness among group members to be recognized (Kramer and Greaves, 2011; Kramer and Russell, 2014). Complex kin systems are highly developed in traditional human societies and permit distinguishing classificatory from biological kin and close kin from distant kin. This allows individuals to selectively identify and cooperate with close kin, and to make decisions about when and how much to help. In the case of fathers, kin discrimination allows for a range of paternal relationships (e.g., biological, social, and/or stepfathers), all of which have societally prescribed roles.
One interesting implication of language-based kin classificatory systems found in all human societies is that, even in the absence of monogamy, they allow children to identify their siblings and father and fathers to identify their children. Because serial monogamy was likely the norm throughout human history due to long breeding careers and high rates of spousal death and divorce, kin terms allow parents and children to identify each other and close relatives despite not cohabiting or living in proximity. Moreover, kinship classificatory systems attenuate the requirement of monogamy for the maintenance of cooperation between mothers, fathers, and siblings by facilitating payoffs to investing in kin outside of a current household (Kramer and Russell, 2015). Thus, the range of breeding systems that we see across and within human societies may be an outcome of our ability to identify close relatives and preferentially invest in them even in the absence of monogamy.
Conclusion
Consensus on a human-typical mating system has remained elusive in the literature. Across human societies today, monogamous, polyandrous, polygynous, and short-term mating patterns are present, with most societies exhibiting multiple types of marriages and mating relationships. Further complicating a straightforward classification of mating system are the multiple possible interpretations of biological traits typical of humans used to indicate ancestral mating patterns. While challenging, our review of the literature offers several key insights. 1) Although polygyny is socially sanctioned in most societies, monogamy is the dominant marriage-type within any one group cross-culturally. 2) Sex outside of marriage occurs across societies, yet human extra pair paternity rates are relatively low when compared to those of socially monogamous birds and mammals. 3) While the timing of the evolution of certain anatomical characteristics is open to debate, human levels of sexual dimorphism and relative testis size point to a diverging history of sexual selection from our great ape relatives.
In sum, we conclude that while there are many ethnographic examples of variation across human societies in terms of mating patterns, the stability of relationships, and the ways in which fathers invest, the residential pair-bond is a ubiquitous feature of human mating relationships. This, at times, is expressed through polygyny and/or polyandry, but is most commonly observed in the form of monogamous marriage that is serial and characterized by low levels of extra-pair paternity and high levels of paternal care.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexander, R. D., and Noonan, K. M. (1979). “Concealment of ovulation, parental care, and human social evolution,” in Evolutionary Biology and Human Social Organization, eds N. A. Chagnon and W.G. Irons. (Massachusetts: Duxbury North Scituate), 436–453.
Anderson, K. (2006). How well does paternity confidence match actual paternity? Evidence from worldwide nonpaternity rates. Curr. Anthropol. 47, 513–520. doi: 10.1086/504167
Archer, J. (2006). Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 30, 319–345. doi: 10.1016/j.neubiorev.2004.12.007
Barta, Z., Székely, T., Liker, A., and Harrison, F. (2014). Social role specialization promotes cooperation between parents. Am. Nat. 183, 747–761. doi: 10.1086/676014
Beckerman, S., and Valentine, P. (2002). Cultures of Multiple Fathers: The Theory and Practice of Partible Paternity in Lowland South America. Gainesville, GA: University Press of Florida.
Beckerman, S., Lizarralde, R., Ballew, C., Schroeder, S., Fingelton, C., Garrison, A., et al. (1998). The barí partible paternity project: preliminary results. Curr. Anthropol. 39, 164–167. doi: 10.1086/204706
Binford, L. R. (2001). Constructing Frames of Reference: An Analytical Method for Archaeological Theory Building Using Ethnographic and Environmental Data Sets. Berkeley, CA: University of California Press.
Bliege Bird, R., and Bird, D. (2002). Constraints of knowing or constraints of growing? Fishing and collecting by the children of Mer. Hum. Nat. 13, 239–267. doi: 10.1007/s12110-002-1009-2
Blurton Jones, N., Hawkes, K., and Draper, P. (1994). Foraging returns of !Kung adults and children: why didn't !Kung children forage? J. Anthropol. Res. 50, 217–248. doi: 10.1086/jar.50.3.3630178
Boas, F. (1907). The Eskimo of Baffin Land and Hudson Bay. New York, NY: American Museum of Natural History; Order of the Trustees.
Bogin, B., Bragg, J., and Kuzawa, C. (2014). Humans are not cooperative breeders but practice biocultural reproduction. Ann. Hum. Biol. 41, 368–380. doi: 10.3109/03014460.2014.923938
Boomsma, J. J. (2007). Kin selection versus sexual selection why the ends do not meet. Curr. Biol. 17, R673–R683. doi: 10.1016/j.cub.2007.06.033
Boomsma, J. J. (2009). Lifetime monogamy and the evolution of eusociality. Philos. Trans. R. Soc. B 364, 3191–3207. doi: 10.1098/rstb.2009.0101
Boomsma, J. J. (2013). Beyond promiscuity: mate-choice commitments in social breeding. Philos. Trans. R. Soc. B. 368:20120050. doi: 10.1098/rstb.2012.0050
Borgerhoff Mulder, M. (2009). Serial monogamy as polygyny or polyandry? marriage in the Tanzanian Pimbwe. Hum. Nat. 20, 130–150. doi: 10.1007/s12110-009-9060-x
Bribiescas, R. G., Ellison, P. T., and Gray, P. B. (2012). Male life history, reproductive effort, and the evolution of the genus Homo: new directions and perspectives. Curr Anthropol. 53, S424–S435. doi: 10.1086/667538
Brotherton, P. N., and Komers, P. E. (2003). “Mate guarding and the evolution of social monogamy in mammals,” Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals eds Reichard, H. Ulrich, and Christophe Boesch (Cambridge University Press, Cambridge), 42–58.
Chapais, B. (2009). “The deep structure of human society: primate origins and evolution,” in Mind the Gap: Tracing the Origins of Human Universals, eds P. M. Kappeler and J. B. Silk (Berlin; Heidelberg: Springer), 19–51.
Chapais, B. (2011). “The evolutionary history of pair-bonding and parental collaboration,” in In the Oxford Handbook of Evolutionary Family Psychology, eds T. K. Shackelford and C. A. Salmon (Oxford: Oxford University Press).
Churchhill, S. E., Berger, L. R., Hartstone-Rose, A., and Zondo, B. H. (2012). “Body size in African Middle Pleistocene Homo,” in African Genesis: Perspectives on Hominin Evolution, eds S. C. Reynolds, A. Gallagher (Cambridge: Cambridge University Press), 319–346.
Clutton-Brock, T. (2002). Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. doi: 10.1126/science.296.5565.69
Clutton-Brock, T. (2009). Structure and function in mammalian societies. Phil. Trans. R. Soc. B. 364, 3229–3242. doi: 10.1098/rstb.2009.0120
Clutton-Brock, T. H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton University Press.
Cornwallis, C. K., West, S. A., Davis, K. E., and Griffin, A. S. (2010). Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972. doi: 10.1038/nature09335
Dixson, A. F. (1983). Observations on the evolution and behavioral significance of “sexual skin” in female primates. Adv. Study Behav (Elsevier), 13, 63–106.
Dixson, A. F. (1997). Evolutionary perspectives on primate mating systems and behavior. Ann. NY. Acad. Sci. 807, 42–61. doi: 10.1111/j.1749-6632.1997.tb51912.x
Dixson, A. F. (2009). Sexual Selection and the Origins of Human Mating Systems. Oxford: Oxford University Press.
Dunbar, R. I. M. (1976). Some aspects of research design and their implications in the observational study of behavior. Behaviour 58, 58–78. doi: 10.1163/156853976X00244
Fisher, H. E. (1989). Evolution of human serial pairbonding. Am. J. Phys. Anthropol. 78, 331–354. doi: 10.1002/ajpa.1330780303
Flinn, M. V. (1988). “Parent-offspring interactions in a Caribbean village: daughter guarding,” in Human Reproductive Behavior: A Darwinian Perspective, eds L. Betzig, M. Borgerhoff Mulder, P. Turke (Cambridge: Cambridge University Press), 189–200.
Flinn, M. V., and Low, B. S. (1986). Resource distribution, social competition, and mating patterns in human societies. Ecol. Aspects Soc. Evol. 14, 217–243.
Fortunato, L. (2015). “Evolution of marriage systems,” in International Encyclopedia of the Social & Behavioral Sciences, 2nd Ed, Vol. 14, eds N. J. Smelser and P. B. Baltes (Oxford: Elsevier), 611–619.
Fromhage, L., Elgar, M. A., and Schneider, J. M. (2005). Faithful without care: the evolution of monogyny. Evolution 59, 1400–1405. doi: 10.1111/j.0014-3820.2005.tb01790.x
Gaulin, S. J., and Schlegel, A. (1980). Paternal confidence and paternal investment: a cross cultural test of a sociobiological hypothesis. Ethol. Sociobiol. 1, 301–309. doi: 10.1016/0162-3095(80)90015-1
Gavrilets, S. (2012). Human origins and the transition from promiscuity to pair-bonding. Proc. Nat. Acad. Sci. 109, 9923–9928. doi: 10.1073/pnas.1200717109
Gettler, L. T., McDade, T. W., and Feranil, A. B. (2011). Longitudinal evidence that fatherhood decreases testosterone in human males Proc. Nat. Acad. Sci. 27, 16194–16199. doi: 10.1073/pnas.1105403108
Gilchrist, J. S., and Russell, A. F. (2007). Who cares? Individual contributions to pup care by breeders versus nonbreeders in the cooperatively breeding banded mongoose (Mungos mungo). Behav. Ecol. Sociobiol. 61, 1053–1060. doi: 10.1007/s00265-006-0338-2
Gordon, A. D., Green, D. J., and Richmond, B. G. (2008). Strong postcranial size dimorphism in Australopithecus afarensis: results from two new resampling methods from multivariate data sets with missing data. Am. J. Phys. Anthropol. 135, 311–328. doi: 10.1002/ajpa.20745
Gowaty, P. A. (1997). “Sexual dialectics, sexual selection, and variation in reproductive behaviour,” in Feminism and Evolutionary Biology (Boston, MA: Springer), 351–384.
Grafen, A., and Sibly, R. (1978). A model of mate desertion. Anim. Behav. 26, 645–652. doi: 10.1016/0003-3472(78)90131-8
Gray, P. B. (2011). The descent of a man's testosterone. Proc. Nat. Acad. Sci. 108, 16141–16142. doi: 10.1073/pnas.1113323108
Gray, P. B., and Anderson, K. G. (2010). Fatherhood: Evolution and Human Paternal Behavior. Cambridge: Harvard University Press.
Gregor, T. (1987). Anxious Pleasures: The Sexual Lives of an Amazonian People. Chicago, IL: University of Chicago Press.
Griffin, A. S., and West, S. A. (2003). Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636. doi: 10.1126/science.1089402
Griffith, S. C., Owens, I. P., and Thuman, K. A. (2002). Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212. doi: 10.1046/j.1365-294X.2002.01613.x
Grueter, C. C., Chapais, B., and Zinner, D. (2012). Evolution of multilevel social systems in nonhuman primates and humans. Int. J. Primatol. 33, 1002–1037. doi: 10.1007/s10764-012-9618-z
Gurven, M., and Hill, K. (2009). Why do men hunt? A reevaluation of “man the hunter” and the sexual division of labor. Curr. Anthropol. 50, 51–74. doi: 10.1086/595620
Gurven, M., and Kaplan, H. (2007). Longevity among hunter-gatherers: a cross-cultural examination. Popul. Dev. Rev. 33, 321–365. doi: 10.1111/j.1728-4457.2007.00171.x
Harcourt, A. H. (1981). “Intermale competition and the reproductive behavior of the great apes,” in Reproductive Biology of the Great Apes, ed Graham, C. E. (Cambridge: Academic Press), 301–318.
Harcourt, A. H., Harvey, P. H., Larson, S. G., and Short, R. V. (1981). Testis weight, body weight and breeding system in primates. Nature, 293:55. doi: 10.1038/293055a0
Hatchwell, B. J., Ross, D. J., Fowlie, M. K., et al. (2001). Kin discrimination in cooperatively breeding long-tailed tits. Proc. Royal Soc. B 268, 885–890. doi: 10.1098/rspb.2001.1598
Hawkes, K., O'Connell, J., and Blurton Jones, N. (1995a). Hadza children's foraging: Juvenile dependency, social arrangements, and mobility among hunter-gatherers. Curr. Anthropol. 36, 688–700. doi: 10.1086/204420
Hawkes, K., O'Connell, J. F., Jones, N. B., Alvarez, H., and Charnov, E. L. (1998). Grandmothering, menopause, and the evolution of human life histories. Proc. Nat. Acad. Sci. 95, 1336–1339. doi: 10.1073/pnas.95.3.1336
Hawkes, K., Rogers, A. R., and Charnov, E. L. (1995b). The male's dilemma: Increased offspring production is more paternity to steal. Evol. Ecol. 9, 662–677. doi: 10.1007/BF01237661
Hennigh, L. (1970). Functions and limitations of Alaskan Eskimo wife trading. Arctic 24–34. doi: 10.14430/arctic3151
Hill, K., and Hurtado, A. M. (1996). Ache Life History: The Ecology and Demography of a Foraging People. NewYork, NY: Aldine de Gruyter.
Holmberg, A. R. (1969). Nomads of the Long Bow: The Siriono of eastern Bolivia. New York, NY: American Museum of Natural History; Natural History Press.
Hrdy, S. B. (1988). “The primate origins of human sexuality,” in The Evolution of Sex (San Francisco, CA: Harper & Row), 101–132.
Hrdy, S. B. (1999). Mother Nature: A History of Mothers, Infants, and Natural Selection. New York, NY: Pantheon Books.
Hrdy, S. B. (2005). “Comes the child before the man: how cooperative breeding and prolonged postweaning dependence shaped human potential,” in Hunter Gatherer Childhoods: Evolutionary, Developmental and Cultural Perspectives, eds B. Hewlett, M. Lamb (New Brunswick. NJ: Transaction Publishers), 65–91.
Hrdy, S. B. (2009). Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Belknap Press of Harvard University Press.
Hughes, W. O., Oldroyd, B. P., and Beekman, M. (2008). Ancestral monogamy shows kin selection in key to the evolution of eusociality. Science 320, 1213–1216. doi: 10.1126/science.1156108
Hurtado, A. M., Hill, K., Hurtado, I., and Kaplan, H. (1992). Trade-offs between female food acquisition and child care among Hiwi and Ache foragers. Hum. Nat. 3, 185–216. doi: 10.1007/BF02692239
Irons, W. (1983). “Human female reproductive strategies,” in Social. Behaviour female Vertebrates, ed S. K. Wasser (New York, NY), 169–213.
Ivey, P. K. (2000). Cooperative reproduction in Ituri Forest hunter-gatherers: who cares for Efe infants? Curr. Anthropol. 41, 856–866. doi: 10.1086/317414
Ivey, P. K., Morrelli, G. A., and Tronick, E. Z. (2005). “Child caretakers among Efe foragers of the Ituri Forest,” in Hunter-Gatherer Childhoods, eds B. S. Hewlett and M. E. Lamb (New Brunswick: Aldine Transaction), 191–213.
Jokela, M., Rotkirch, A., and Rickard, I. J. (2010). Serial monogamy increases reproductive success in men but not in women. Behav. Ecol. 21, 906–912. doi: 10.1093/beheco/arq078
Kaplan, H., Hill, K., Lancaster, J., and Magdalena Hurtado, A. (2000). A theory of human life history evolution: diet, intelligence, and longevity. Evolut. Anthropol. 9, 156–185. doi: 10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7
Kaplan, H. S., and Lancaster, J. B. (2003). “An evolutionary and ecological analysis of human fertility, mating patterns, and parental investment,” in Offspring: Human fertility Behavior in Biodemographic Perspective (Washington, DC: National Academies Press), 170–223.
Kappeler, P. M., and van Schaik, C. P. (2002). Evolution of primate social systems. Int. J. Primatol. 23, 707–740. doi: 10.1023/A:1015520830318
Kenagy, G. J., and Trombulak, C. (1986). Size and function of mammalian testes in relation to body size. J. Mammal. 67, 1–22. doi: 10.2307/1380997
Kramer, K. L. (2005a). Children's help and the pace of reproduction: cooperative breeding in humans. Evolut. Anthropol. 14, 224–237. doi: 10.1002/evan.20082
Kramer, K. L. (2010). Cooperative breeding and its significance to the demographic success of humans. Ann. Rev. Anthropol. 39, 414–436. doi: 10.1146/annurev.anthro.012809.105054
Kramer, K. L., and Greaves, R. D. (2011). Postmarital residence and bilateral kin associations among hunter-gatherers. Hum. Nat. 22:41. doi: 10.1007/s12110-011-9115-7
Kramer, K. L., and Russell, A. F. (2014). Kin-selected cooperation without lifetime monogamy: Human insights and animal implications. Trends Ecol. Evol. 29, 600–606. doi: 10.1016/j.tree.2014.09.001
Kramer, K. L., and Russell, A. F. (2015). Was monogamy a key step on the hominin road? Reevaluation of the monogamy hypothesis. Evolut. Anthropol. 24, 73–83. doi: 10.1002/evan.21445
Kramer, K. L., Schacht, R., and Bell, A. V. (2017). Adult sex ratios and partner scarcity among hunter-gatherers: implications for dispersal patterns and the evolution of human sociality. Philos. Trans. R Soc. London Series B 372:20160316 doi: 10.1098/rstb.2016.0316
Kramer, K. L., and Veile, A. (2018). Infant allocare in traditional societies. Physiol. Behav. 193, 117–126. doi: 10.1016/j.physbeh.2018.02.054
Lahdenpera, M., Lummaa, V., Helle, S., Tremblay, M., Russell, A. F., et al. (2004). Fitness benefits of prolonged postreproductive lifespan in women. Nature 428, 178–181. doi: 10.1038/nature02367
Lancaster, J. B. (1991). A feminist and evolutionary biologist looks at women. Am. J. Phys. Anthropol. 34, 1–11. doi: 10.1002/ajpa.1330340603
Lancaster, J. B., and Lancaster, C. S. (1987). “The watershed: change in parental-investment and family-formation strategies in the course of human evolution,” in Parenting Across the Life Span: Biosocial Dimensions, eds J. B. Lancaster, J. Altmann, A. S. Rossi, and L. R. Sherrod (Hawthorne, NY: Aldine Publishing Co), 187–205.
Lee, R. D., and Kramer, K. L. (2002). Children's economic roles in the Maya family life cycle: Cain, Caldwell and Chayanov revisited. Popul. Dev. Rev. 28, 475–499.
Leigh, S. R., and Shea, B. T. (1995). Ontogeny and the evolution of adult body size dimorphism in apes. Am. J. Primatol. 36, 37–60. doi: 10.1002/ajp.1350360104
Leonetti, D. L., and Chabot-Hanowell, B. (2011). The foundation of kinship: households. Hum. Nat. 22, 16–40. doi: 10.1007/s12110-011-9111-y
Leonetti, D. L., Nath, D. C., Heman, N. S., et al. (2005). “Kinship organization and the impact of grandmothers on reproductive success among the matrilineal Khasi and patrilineal Bengali of Northeast India,” in Grandmotherhood: The Evolutionary Significance of the Second Half of Life, eds E. Voland, A. Chasiotis, W. Schiefenhovel (New Brunswick, NJ: Rutgers University Press, 194–214
Lockwood, C. A., Menter, C. G., Moggi-Cecchi, J., et al. (2007). Extended male growth in a fossil sample hominin species. Science 318, 1443–1446. doi: 10.1126/science.1149211
Lockwood, C. A., Richmond, B. G., Jungers, W. L., and Kimbel, W. (1996). Randomization procedures and sexual dimorphism in Australopithecus afarensis. J. Hum. Evol. 31, 537–548. doi: 10.1006/jhev.1996.0078
Lukas, D., and Clutton-Brock, T. (2012). Cooperative breeding and monogamy in mammalian societies. Proc. R. Soc. B. 259, 2151–2156. doi: 10.1098/rspb.2011.2468
Lukas, D., and Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677
Marlowe, F. (2000). Paternal investment and the human mating system. Behav. Proc. 51, 45–61. doi: 10.1016/S0376-6357(00)00118-2
Marlowe, F. W. (2003). A critical period for provisioning by Hadza men: implications for pair bonding. Evol. Hum. Behav. 24, 217–229. doi: 10.1016/S1090-5138(03)00014-X
Marlowe, F. W., and Berbesque, J. C. (2012). The human operational sex ratio: effects of marriage, concealed ovulation, and menopause on mate competition. J. Hum. Evolut. 63, 834–842. doi: 10.1016/j.jhevol.2012.09.004
Martin, R. D., and May, R. M. (1981). Outward signs of breeding. Nature 293, 7–9 doi: 10.1038/293007a0
Mathews, L. M. (2003). Tests of the mate-guarding hypothesis for social monogamy: male snapping shrimp prefer to associate with high-value females. Behav. Ecol. 14, 63–67. doi: 10.1093/beheco/14.1.63
McAuliffe, K., and Whitehead, H. (2005). Eusociality, menopause and information in matrilineal whales. Trends Ecol. Evol. 20, 650. doi: 10.1016/j.tree.2005.09.003
Mead, M. (1928). Coming of age in Samoa: A Psychological Study of Primitive Youth for Western Civilization. New York, NY: William Morrow.
Moller, A. P. (1988). Testis size, ejaculate quality and sperm competition in birds. Biol. J. Linnean. Soc. 33, 273–283. doi: 10.1111/j.1095-8312.1988.tb00812.x
Møller, A. P. (2003). “The evolution of monogamy: mating relationships, parental care and sexual selection,” in Monogamy: Mating strategies and partnerships in birds, humans and other mammals, eds R. H. Ulrich, and C. Boesch (Cambridge: Cambridge University Press), 29–41.
Murdock, G. P., and Provost, C. (1973). Factors in the division of labor by sex: a cross-cultural analysis. Ethnology 12, 203–225. doi: 10.2307/3773347
Murdock, G. P., and White, D. R. (1969). Standard cross-cultural sample. Ethnology 8, 329–369. doi: 10.2307/3772907
Nunn, C. L. (1999). The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim. Behav. 58, 229–246. doi: 10.1006/anbe.1999.1159
Parker, G. A. (2016). The evolution of expenditure on testes. J. Zool. 298, 3–19. doi: 10.1111/jzo.12297
Parker, R. (1985). Anxious anthropologists: the study of sex. Anthropol. Human. 10, 130–131. doi: 10.1525/ahu.1985.10.4.130
Perrone, M., and Zaret, T. M. (1979). Parental care patterns of fishes. Am Nat. 113, 351–361. doi: 10.1086/283394
Plavcan, J. M. (2000). Inferring social behavior from sexual dimorphism in the fossil record. J. Hum. Evol. 39, 327–344. doi: 10.1006/jhev.2000.0423
Plavcan, J. M. (2012). Sexual size dimorphism, canine dimorphism, and male-male competition in primates. Hum. Nat. 23, 45–67. doi: 10.1007/s12110-012-9130-3
Plavcan, J. M., and van Schaik, C. P. (1992). Intrasexual competition and canine dimorphism in anthropoid primates. Am. J. Phy. Anthropol. 87, 461–477. doi: 10.1002/ajpa.1330870407
Pollock, D. (2002). “Partible paternity and multiple maternity among the Kulina,” in Cultures of Multiple Fathers: The Theory and Practice of Partible Paternity in Lowland South America (Gainesville, FL: University Press of Florida), 42–61.
Puts, D. A. (2010). Beauty and the beast: mechanisms of sexual selection in humans. Evol. Hum. Behav. 31, 157–175. doi: 10.1016/j.evolhumbehav.2010.02.005
Reichard, U. H. (2003). “Monogamy: past and present,” in Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals, eds R. H. Ulrich and C. Boesch (Cambridge: Cambridge University Press), 3–25.
Reno, P. L., McCollum, M. A., Meindl, R. S., and Lovejoy, C. O. (2010). An enlarged postcranial sample confirms Australopithecus afarensis dimorphism was similar to modern humans. Philos. Trans. R. Soc. B. 365, 3355–3363. doi: 10.1098/rstb.2010.0086
Reno, P. L., Meindl, R. S., McCollum, M. A., and Lovejoy, C. O. (2003). Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc. Natl. Acad. Sci. U.S.A. 100, 9404–9409. doi: 10.1073/pnas.1133180100
Rooker, K., and Gavrilets, S. (2018). On the evolution of visual female sexual signaling. Proc. R. Soc. B. 285:20172875. doi: 10.1098/rspb.2017.2875
Ross, C. T., Mulder, M. B., Winterhalder, B., et al. (2016). Evidence for quantity–quality trade-offs, sex-specific parental investment, and variance compensation in colonized Agta foragers undergoing demographic transition. Evolut. Hum. Behav. 37, 350–365. doi: 10.1016/j.evolhumbehav.2016.02.005
Rubel, A. J. (1961). Partnership and Wife-Exchange Among the Eskimo and Aleut of Northern North America. Fairbanks, AK: University of Alaska.
Russell, A. F. (2004). “Mammals: comparisons and contrasts,” in Ecology and Evolution of Cooperative Breeding in Birds, eds W. D. Koenig and J. L. Dickinson (New York, NY: Cambridge University Press), 210–227.
Schacht, R. (2013). “Cassava and the Makushi: a shared history of resiliency and transformation,” in Food and Identity in the Caribbean, ed H. Garth (London: Berg Publishers), 15–29.
Schacht, R., and Bell, A. (2016). The evolution of monogamy in response to partner scarcity. Nat. Sci. Commun. 6:32472. doi: 10.1038/srep32472
Shwalb, D. W., Shwalb, B. J., and Lamb, M. E. (2013). Fathers in Cultural Context. New York, NY: Routledge.
Sillén-Tullberg, B., and Moller, A. P. (1993). The relationship between concealed ovulation and mating systems in anthropoid primates: a phylogenetic analysis. Am. Nat. 141, 1–25. doi: 10.1086/285458
Simmons, L. W., Firman, R. C., and Rhodes, G. (2004). Human sperm competition: Testis size, sperm production and rates of extrapair copulations. Anim. Behav. 68, 297–302. doi: 10.1016/j.anbehav.2003.11.013
Smuts, B. B., and Smuts, R. W. (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Adv. Study Behav. 22, 1–63. doi: 10.1016/S0065-3454(08)60404-0
Stoinski, T. S., Rosenbaum, S., and Ngaboyamahina, T. (2009). Patterns of male reproductive behaviour in multi-male groups of mountain gorillas: Examining theories of reproductive skew. Behaviour 146, 1193–1215. doi: 10.1163/156853909X419992
Strassmann, B. I. (1981). Sexual selection, paternal care, and concealed ovulation in humans. Ethol. Sociobiol. 2, 31–40. doi: 10.1016/0162-3095(81)90020-0
Strassmann, B. I. (2003). “Social monogamy in a human society: marriage and reproductive success among the Dogon,” in Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals, eds R. H. Ulrich and C. Boesch (Cambridge: Cambridge University Press), 177–189.
Thornhill, R. (1976). Sexual selection and paternal investment in insects. Am. Nat. 110, 153–163. doi: 10.1086/283055
Turke, P. W. (1988). “Helpers at the nest: childcare networks on Ifaluk,” in Human Reproductive Behavior: A Darwinian Perspective, eds L. Betzig, M. B. Mulder, and P. Turke (Cambridge: Cambridge University Press), 173–188.
van Schaik, C. P., and Burkart, J. M. (2010). “Mind the gap: cooperative breeding and the evolution of our unique features,” in Mind the Gap, eds P. Kappeler and J. Silk (Berlin; Heidelberg: Springer).
Walker, R. S., Flinn, M. V., and Hill, K. R. (2010). Evolutionary history of partible paternity in lowland South America. Proc. Nat. Acad. Sci. 107, 19195–19200. doi: 10.1073/pnas.1002598107
Washburn, S., and Lancaster, C. (1968). “The evolution of hunting,” in Man the Hunter, eds R. B. Lee and I. Devore (Chicago, IL: Aldine), 293–303.
Westneat, D. F., and Sherman, P. W. (1993). Parentage and the evolution of parental behavior. Behav. Ecol. 4, 66–77. doi: 10.1093/beheco/4.1.66
Wingfield, J. C., Hegner, R. E., Dufty, A. M. Jr., and Ball, G. F. (1990). The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846. doi: 10.1086/285134
Keywords: monogamy, mating system, sexual selection, anthropology, evolution
Citation: Schacht R and Kramer KL (2019) Are We Monogamous? A Review of the Evolution of Pair-Bonding in Humans and Its Contemporary Variation Cross-Culturally. Front. Ecol. Evol. 7:230. doi: 10.3389/fevo.2019.00230
Received: 24 September 2018; Accepted: 04 June 2019;
Published: 17 July 2019.
Edited by:
Alexander G. Ophir, Cornell University, United StatesReviewed by:
Francisco Garcia-Gonzalez, Estación Biológica de Doñana (EBD), SpainMary Katherine Shenk, Pennsylvania State University, United States
Copyright © 2019 Schacht and Kramer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan Schacht, c2NoYWNodHIxOEBlY3UuZWR1
 Ryan Schacht
Ryan Schacht Karen L. Kramer2
Karen L. Kramer2