
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 18 June 2019
Sec. Conservation and Restoration Ecology
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00224
This article is part of the Research Topic North American Monarch Butterfly Ecology and Conservation View all 35 articles
The measurement of naturally occurring stable hydrogen (δ2H) and carbon (δ13C) isotopes in wings of the eastern North American monarch butterflies (Danaus plexippus) have proven useful to infer natal origins of individuals overwintering in Mexico. This approach has provided a breakthrough for monarch conservation because it is the only viable means of inferring origins at continental scales. Recently, routine simultaneous analyses of tissue δ2H and δ18O of organic materials has emerged leading to questions of whether the dual measurement of these isotopes could be used to more accurately infer spatial origins even though the two isotopes are expected to be coupled due to the meteoric relationship. Such refinement would potentially increase the accuracy of isotopic assignment of wintering monarchs to natal origin. We measured a sample of 150 known natal-origin monarchs from throughout their eastern range simultaneously for both δ2H and δ18O wing values. Wing δ2H and δ18O values were correlated (r2 = 0.42). We found that wing δ2H values were more closely correlated with amount-weighted growing season average precipitation δ2H values predicted for natal sites (r2 = 0.61) compared to the relationship between wing δ18O values and amount-weighted growing season average precipitation δ18O values (r2 = 0.30). This suggests that monarch wing δ2H values will be generally more useful in natal assignments than δ18O values. Spatial information related to the use of deuterium excess in environmental waters was similarly found to be not useful when applied to monarch wings likely due to the considerable variance in wing δ18O values. Nonetheless, we recommend further testing of monarch wing δ2H and δ18O values from known natal sites with an emphasis on field data across a strong gradient in precipitation deuterium excess.
The monarch butterfly (Danaus plexippus) is an iconic migratory insect that navigates over thousands of kilometers between natal sites in the eastern USA and Canada and overwintering sites in the Oyamel fir (Abies religiosa) forests of central Mexico (Urquhart and Urquhart, 1976; Ries et al., 2015; but see Vander Zanden et al., 2018). This outstanding migration involves multiple generations whereby those individuals returning to well-established, long-term overwintering sites do so without any previous experience of their locations. Despite being a high-profile conservation issue among Mexico, Canada and the USA, monarch butterflies have declined considerably over the last two decades (Semmens et al., 2016). Potential causes for the decline are many but likely factors include loss of milkweed (Asclepia spp.) on the breeding grounds, declining extent and quality of overwintering sites, climate change and the myriad of challenges faced during the migratory passage (Flockhart et al., 2015; Thogmartin et al., 2017). Key to understanding declines of this and other migratory species is the establishment of migratory connections that link breeding, stopover and wintering sites (Hobson and Wassenaar, 2019). However, this is challenging for small insects. Previous attempts have used an impressive mark recapture effort involving tagging Monarchs on breeding and stopover sites with labels affixed to wings (Monarch Watch; https://www.monarchwatch.org/tagmig/index.htm), an approach requiring recovery en route and at the few publicly accessible wintering sites in Mexico. However, during the mid-1990s Hobson et al. (1999) and Wassenaar and Hobson (1998) developed an approach using ratios of naturally occurring stable isotopes in Monarch wings to infer natal origins. Those studies marked a turning point in the conservation of migratory monarchs since it readily identified the US Midwest as the center of production of Monarchs recruited into the overwintering population in Mexico. Since then, the approach has been used by other researchers interested in Monarch patterns of spring recolonization in North America (Miller et al., 2012; Flockhart et al., 2013), the effects of natal origin on parasite loads (Altizer et al., 2015). Other applications have involved the role of wing coloration in flight distance (Hanley et al., 2013) and general conservation concerns related to where most individuals are being produced (Flockhart et al., 2017).
The stable isotope approach to tracking migrant animals is based on the fact that naturally occurring stable isotopes of several elements in nature can provide information on provenance and/or habitat. Spatial patterns of stable isotopes (i.e., “isoscapes”) are ultimately transferred to animal tissues through local foodwebs and so spatial information related to the period of tissue growth can be inferred providing we know the nature of such isoscapes and how stable isotope values change or discriminate from the source to the tissue of interest (Hobson and Wassenaar, 2019). The early studies on stable isotope patterns in eastern monarchs indicated that both stable–carbon (13C/12C expressed as δ13C) and stable-hydrogen (2H/1H, δ2H) isotope ratio measurements of wings could provide information on monarch natal origins in Canada and the USA. However, δ2H measurements provided a more powerful means of inferring origins than δ13C measurements. Hydrogen in foodwebs is ultimately derived from precipitation. The amount-weighted average precipitation δ2H (expressed as δ2Hp) varies across continents according to well-established principles (Clark and Fritz, 1997) and these isoscape patterns are transferred to plants and higher trophic-level organisms (Bowen and West, 2019). Metabolically inert tissues such as animal keratins and chitins are related to local growing season averaged δ2Hp and can be later measured to infer natal origins once the relationship between δ2Hp and tissue δ2H is established (Hobson, 2019). This principle has been used effectively to infer origins of numerous taxa including insects, birds and mammals on several continents (Hobson et al., 2018a; Hobson and Wassenaar, 2019).
Water contains both hydrogen and oxygen but measurement of the stable isotope ratios in oxygen (18O/16O; δ18O) have not been used extensively to study animal movement (but see Bryant and Froehlich, 1995; Kohn, 1996; Hobson et al., 2004, 2009a, 2012; Bowen et al., 2005; Ehleringer et al., 2008; Chesson et al., 2013; Hobson and Koehler, 2015; Pekarsky et al., 2015). This derives from the fact that oxygen has a compressed isotopic scale compared to hydrogen and that δ2H and δ18O values in environmental waters are highly correlated via the global meteoric water relationship (Craig, 1961). Also, previously, measurement of δ18O in organic materials has been challenging analytically (Hobson and Wassenaar, 2019). Oxygen in animal tissues can be derived from air, drinking water, and diet whereas hydrogen is derived from only diet and drinking water. However there is potential for δ18O when combined with δ2H measurements to provide additional information on provenance of animals because environmental waters can record the degree of evapotranspiration through the evaluation of deuterium excess (defined as δ2H-8*δ18O; Dansgaard, 1964; Rozanski et al., 1993). In highly evaporative systems, we expect departures from the global meteoric water line whereby deuterium excess values are higher than the global average of 10‰, a phenomenon highly associated with low relative humidity. So, again, the relationship between δ2H and δ18O values in insect wing chitin (δ2Hw and δ18Ow) has the potential to contain important environmental and climate information.
The evaluation of both δ2Hw and δ18Ow values of monarch butterflies was first reported by Fourel et al. (1998) as a proof of principle for the measurement of these isotopes via continuous-flow isotope ratio mass spectrometry. That study showed a positive correlation between the two isotopes. However, since then, no studies have further investigated the relationship between these isotopes in monarch wings in particular, and only a few researchers have reported on δ2H and δ18O values among higher-order taxa. Hobson and Koehler (2015) reported that while feathers of American Redstart (Setophaga ruticilla) showed a good relationship between δ2H and mean growing-season precipitation δ2H (r2 = 0.77), the same was not true for feather δ18O (r2 = 0.32). However, for dragonflies, insects that form wings from nutrients derived from an aquatic larval stage, Hobson et al. (2012) found an excellent relationship between δ2Hw and δ18Ow values (r2 = 0.92). The question remains, then, whether δ2Hw and δ18Ow values of terrestrial insects are also well-correlated and indeed reflect underlying relationships in environmental waters that could be used to increase the accuracy of assignment to origin compared to the use of δ2H measurements alone. Here, we report measurements of δ2Hw and δ18Ow values of monarch butterflies sampled at known origin natal sites in eastern North America. Our objective was to determine if a dual isotope approach could provide additional environmental information related to regional patterns in evaporative conditions that could ultimately contribute to more accurate delineation of natal origins of monarchs and other terrestrial insects.
Monarch wing material was obtained from samples reported in Wassenaar and Hobson (1998) and Hobson et al. (1999). Those were monarchs raised on milkweed exposed only to precipitation at 31 known sites throughout the range of the eastern monarch population and which formed the calibration relationship between mean growing season δ2Hp and δ2Hw reported by Hobson et al. (1999) (Table 1). Additionally, monarchs collected from roadkill mortality (n = 92) at a single site in northeast Mexico during the autumn migration of 2015–16 were used to investigate assignment to natal origins using both δ2Hw and δ18Ow values.
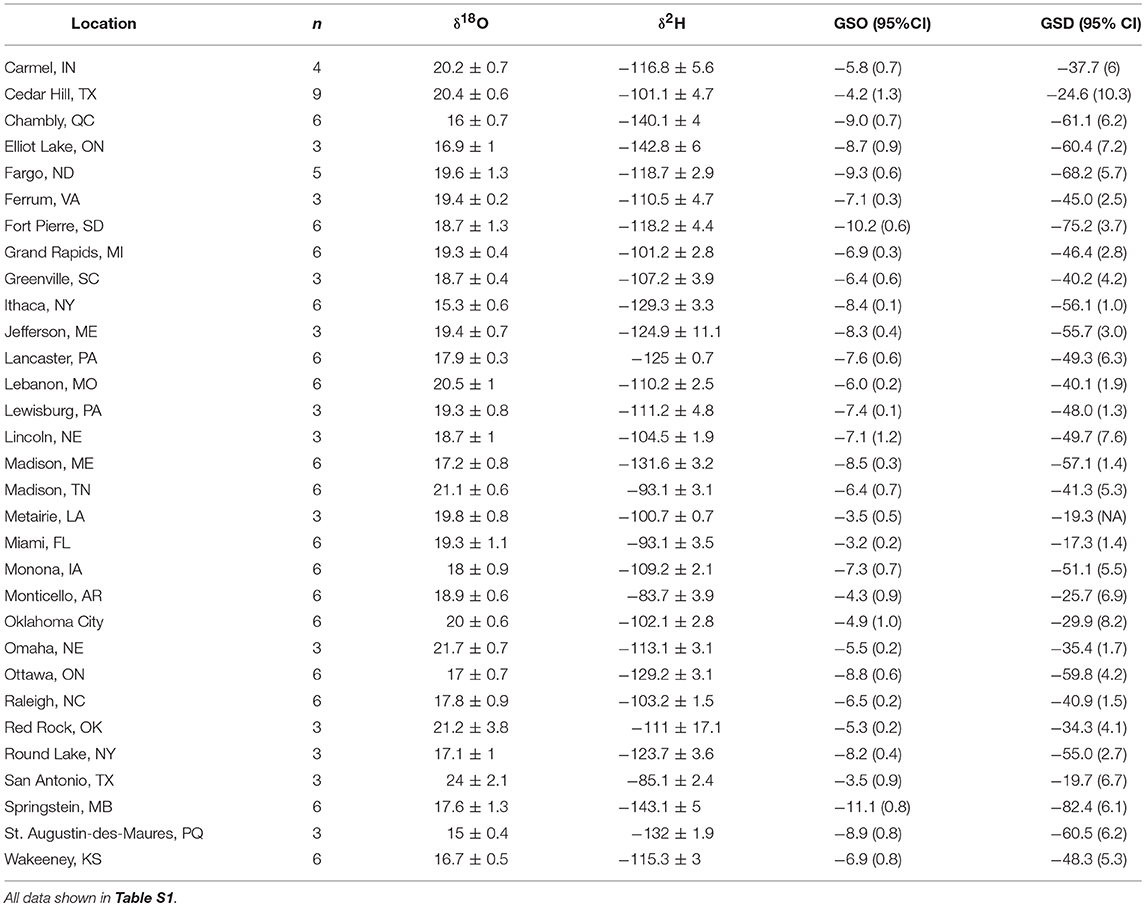
Table 1. Mean isotope values (± 1 SD ‰) of wild reared monarch butterflies from eastern North America and mean growing-season δ18Op (GSO) and δ2Hp (GSD) at each location plus the 95% CI shown in parentheses from Bowen et al. (2005).
All monarch wing samples were soaked and rinsed in a 2:1 chloroform:methanol solution and air dried. Subsamples were cut from the same region of the hindwing to reduce inter-sample variance due to isotopic effects from pigmentation (Hobson et al., 2017) and weighed (0.35 ± 0.02 mg) into silver capsules. All samples were prepared for δ2H and δ18O analysis at the Stable Isotope Laboratory of Environment Canada, Saskatoon, Canada. Our approach involved the analysis of both δ2H and δ18O on the same analytical run (i.e., both H2 and CO gases were analyzed from the same pyrolysis) from samples and standards. All measurements were performed on a HTC system (Thermo Finnigan, Bremen, Germany) equipped with a Costech Zero-Blank autosampler. The helium carrier gas rate was set to 120 ml/min through a 0.6 m ¼-inch 5-Å molecular sieve (80–100 mesh) GC column. The HTC reactor was operated at a temperature of 1,400°C and the GC column temperature was set to 90°C. After separation, the gases were introduced into a Delta V plus isotope-ratio mass spectrometer via a ConFlo IV interface (Thermo Finnigan, Bremen, Germany). The eluted N2 was flushed to waste by withdrawing the CF capillary from the ConFlo interface. We used keratin reference standards CBS and KHS to calibrate sample δ2H (−197 and −54.1‰, respectively) and δ18O (+2.50 and +21.46‰, respectively; Qi and Coplen, 2011). These standard values were used vs. the newly reported values of Soto et al. (2017) simply to maintain consistency with our earlier published work for monarch assignments and we note that this will not affect the assignment per se (providing appropriate calibration algorithms are used). Based on replicate (n = 5) within-run measurements of keratin standards, sample measurement error was estimated at ±2‰ for δ2H and ±0.4‰ for δ18O. All H results are reported for non-exchangeable H and for both H and O in typical delta notation, in units of per mil (‰), and normalized on the Vienna Standard Mean Ocean Water – Standard Light Antarctic Precipitation (VSMOW-SLAP) standard scale.
We created δ18Ow and δ2Hw isoscapes based on derived transfer functions using wing stable isotope values of known origin monarchs found in this study. This was accomplished by calibrating amount-weighted growing-season average precipitation δ18O and δ2H (δ18Op and δ2Hp) (Terzer et al., 2013; Hobson et al., 2014) surfaces into separate δ2Hw and δ18Ow isoscapes. We then depicted potential origins of a sample of roadkilled monarchs salvaged during their autumn migration through northeastern Mexico. That depiction was done using techniques described previously (Hobson et al., 2018b). Briefly, we used a likelihood-based assignment method (Hobson et al., 2009b; Wunder, 2010; Van Wilgenburg et al., 2012) to assign individual monarchs using δ2Hw and δ18Ow to each calibrated wing isoscape (see results), separately. We used the residual SD error of 9.33‰ for δ2Hw and 1.50‰ for δ18Ow from regressions in our assignments. We limited assignments to the current known geographic range for the eastern monarch population and used this as a spatial mask (i.e., clip) to limit our analysis. We estimated the likelihood that a cell (pixel) within the δ2Hw and δ18Ow isoscapes represented a potential origin for a sample by using a normal probability density function (pdf) based on the observed δ2Hw and δ18Ow, and thus depicted the likely origins of each monarch by assigning individuals to the δ2Hw and δ18Ow isoscapes, separately. We arbitrarily chose a 2:1 odds ratio to include only those pixels (coded 1) with at least a 67% probability of origin vs. all others (coded 0). This resulted in a binary map per assigned individual of presence and absence. We then summed the results of individual assignments by stacking the surfaces for a final depiction. We conducted geographic assignments to origin using functions within the R statistical computing environment (R Core Team., 2016) employing the “raster” (Hijmans, 2016) and “maptools” (Bivand and Lewin-Koh, 2016) packages. Thus, the final assignment surface depicted the number of individuals co-assigned at each pixel based on the odds criteria. We also conducted assignment to origin analysis for individual Monarch samples using a dual-isotope (δ2Hw, δ18Ow) approach applied with a multivariate normal probability density function (mvnpdf; see Hobson et al., 2014 for details). Similar to the univariate pdf approach, the mvnpdf method calculates the probability that a particular spatially referenced cell represents a potential origin in calibrated raster isoscape space.
We found a positive relationship between monarch δ2Hw and δ18Ow values for known-origin, outdoor-raised individuals (δ2Hw = 5.21 * δ18Ow – 211, r2 = 0.42; Figure 1). The relationship between δ18Ow and δ18Op was positive (δ18Ow = 0.54 * δ18Op + 22.5; Figure 2) but relatively weak (r2 = 0.30, p < 0.001). In contrast, the relationship between δ2Hw and δ2Hp was positive (δ2Hw = 0.78 * δ2Hp – 77.42; Figure 3) and stronger (r2 = 0.61, p < 0.001).
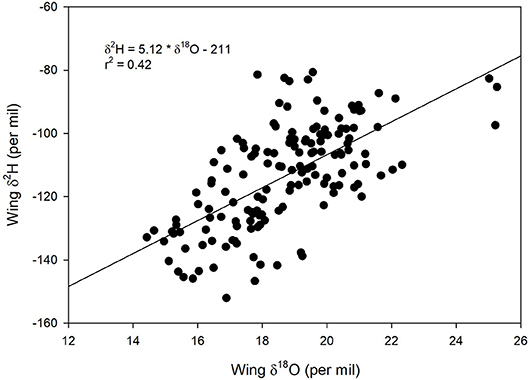
Figure 1. Relationship between δ2Hw and δ18Ow for monarchs raised outdoors at 31 known sites (Table 1) throughout their range in 1996. Sample originally reported for δ2Hw and δ13Cw by Hobson et al. (1999) but these are new δ2Hw values measured simultaneously with δ18Ow.
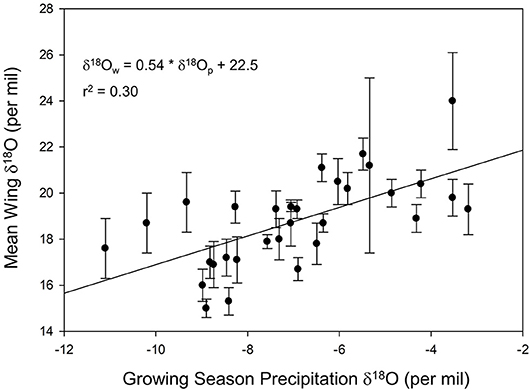
Figure 2. Relationship between monarch δ18Ow and amount-weighted mean growing-season average δ18Op (Bowen et al., 2005) for monarchs raised outdoors at 31 known sites (Table 1) throughout their range in 1996.
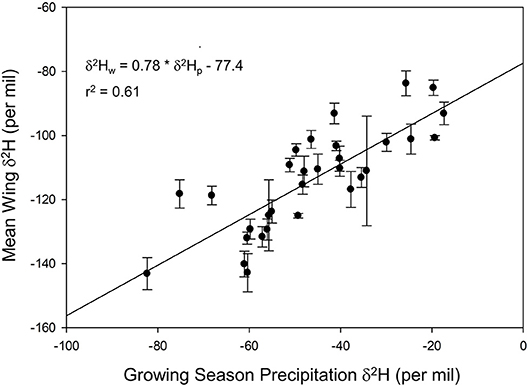
Figure 3. Relationship between monarch δ2Hw and amount-weighted mean growing-season average δ2Hp (Bowen et al., 2005) for monarchs raised outdoors at 31 known sites (Table 1) throughout their range in 1996.
Using the relationships established between δ18Ow and δ2Hw values and δ18Op and δ2Hp values, respectively, we created predicted monarch wing isoscapes for each isotope (Figures 4A,B). These surfaces are superficially similar due to the correlation between isotopes. We then used these surfaces to assign origins of a sample of monarchs from roadkill mortality during their fall migration. That sample included monarchs from across their range and we contrasted depictions of origins of these individuals using only δ2Hw values, only δ18Ow values and both δ18Ow and δ2Hw values in a multivariate normal assignment. These depictions were similar in that they showed highest probability in the southwestern portion of the range but differences were also apparent (Figures 5A–C). The dual isotope approach constrained the assignment compared to the use of δ2Hw values alone.
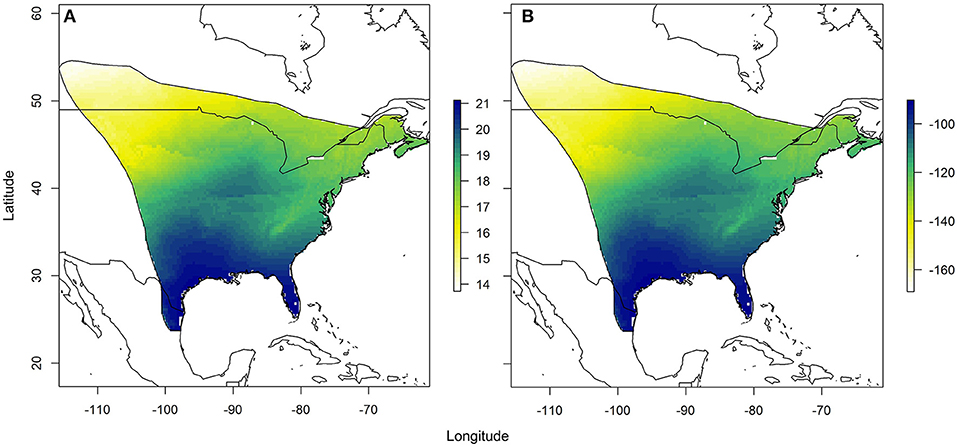
Figure 4. Depiction of (A) the expected monarch δ18Ow isoscape based on the relationship derived between δ18Ow and amount-weighted mean growing season average δ18Op (Bowen et al., 2005) shown in Figure 2 and (B) the expected monarch δ2Hw isoscape based on the relationship derived between δ2Hw and amount-weighted mean growing-season average δ2Hp (Bowen et al., 2005) shown in Figure 3. Legend is the number of individuals assigned to a pixel based on the odds ratio criterion used.
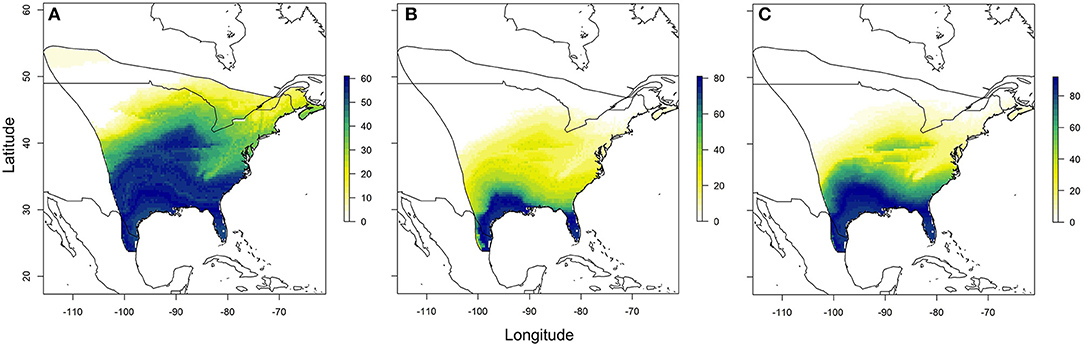
Figure 5. Depiction of origins of a subsample of roadkilled monarchs during fall migration near Monterrey, Mexico, using (A) only δ2Hw values, (B) only δ18Ow values, and (C) both δ2Hw and δ18Ow values in a multivariate normal likelihood approach. Legend is the number of individuals assigned to a pixel based on the odds ratio criterion used.
We contrasted deuterium excess values calculated for individual monarch wings and compared these to the growing-season average deuterium excess calculated for each natal site. No correlation was found between these values (Figure 6, r2 = 0.02). This suggests that little information associated with precipitation environmental deuterium excess was transferred to monarchs per se.
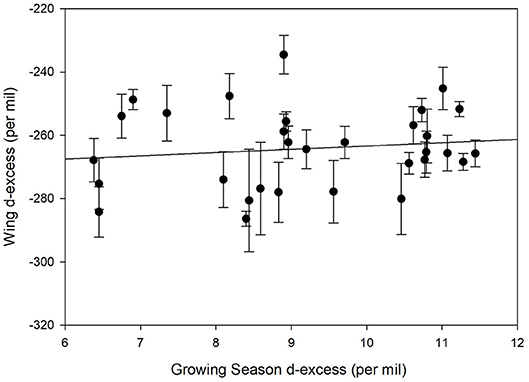
Figure 6. Relationship between measured deuterium excess in known-origin monarch wings (Table 1) and predicted growing season precipitation deuterium excess for these locations.
Our results confirm a strong relationship between wing chitin δ2H values and precipitation δ2H values at known natal sites. This result underlines the strong assignment power of using Monarch wing δ2H values to infer origins and confirms that even though the earlier investigations of Monarch δ2H values to infer origins used different analytical techniques (i.e., offline zinc reduction and steam equilibration, Wassenaar and Hobson, 1998; Hobson et al., 1999), the power of that isotopic assignment holds. The different analytical approaches resulted in different calibrations between wing chitin δ2H (δ2Hw) and predicted amount-weighted growing season δ2Hp (earlier method: δ2Hw = 0.62 * δ2Hp – 79; r2 = 0.69; recent method: δ2Hw = 0.78 * δ2Hp – 77.4; r2 = 0.61) and we recommend that from now on authors use the recent calibration relationship reported here. The δ2Hw results contrasts with the much weaker relationship we found between δ18Ow and δ18Op. Thus, while the measurement of δ18Ow possibly confers additional information on origin, the strong relationship between δ2Hw and δ18Ow values likely precludes the effective use of a dual isotope approach in most cases. This result has been seen previously in assignment exercises involving several taxa (reviewed by Hobson and Koehler, 2015). Our comparison of deuterium excess values calculated for monarch wings and those of the mean growing season average deuterium excess for natal sites confirms that little environmental information related to evaporative conditions was available from our sample by running δ18O analyses. Nonetheless, we recognize the potential utility of using both δ18Ow and δ2Hw values, especially for areas corresponding to potential origins involving high deuterium excess values that were not included in our study. It may be possible then, to identify migrant individuals raised in xeric vs. humid environments and we encourage further work in this area for migrant insects in general. Clearly, further dual isotope analyses of a large sample of monarchs from known natal origins that spans large isotopic gradients in North America will be useful in testing situations where this more extensive analytical approach might be justified.
It is interesting to speculate why tissue δ18O values do not seem to add much additional information on origins of Monarch Butterflies and other terrestrial taxa based on δ2H analyses alone. The typical response to this question is that oxygen enters metabolic pathways from more diverse origins than hydrogen (Hobson and Koehler, 2015). Fundamentally, oxygen is derived metabolically from diet, drinking water and air whereas hydrogen is derived only from diet and drinking water. Atmospheric oxygen is added to the body water pool during metabolism and respiration and has a relatively constant isotopic composition (Luz and Barkan, 2011). Therefore, the oxygen isotopic coupling between tissues and environmental waters is dependent on the relative contribution of respirative processes to the oxygen isotopic composition of body water. Additionally, with insects, atmospheric oxygen for respiration is obtained primarily by diffusion through the spiricules and tracheal pathways (Chown et al., 2006) which may further alter its isotopic composition. It is worth noting that while the hydrogen isotopic compositions of chitin are broadly similar to those of keratins and other tissues of birds or mammals (Ehleringer et al., 2008; Hobson and Koehler, 2015), the δ18O values of chitin are generally more positive. This, along with the relatively poor fit between oxygen isotopic compositions of wing chitin and precipitation, likely indicates that slightly different sources, processes or pathways are utilized for oxygen in terrestrial insects than in other taxa. This would not necessarily preclude the use of both isotopes in typical assignments to origin but such assignment models require isotopes to be relatively orthogonal. For oxygen isotopes, current analytical methods result in a much higher relative measurement error than for hydrogen isotopes (0.4 vs. 2‰ for hydrogen). To use both isotopes to determine an inferred deuterium excess of environmental waters requires the Meteoric Water Line multiplicative factor of 8, so that currently we can only determine the deuterium excess values to precisions of about 3.2‰. Precipitation varies in deuterium excess from ~0 to +10‰ which means that such poor precisions will require large numbers of samples depending on which populations are being examined. This is not to say that measurement of both isotopes in monarch tissue will not be useful, but that, in general, the use of wing chitin δ2H is the most preferred for single isotope assignments.
In addition to the earlier proof of concept papers on the use of both wing δ18O and δ2H values to investigate natal origins of monarch butterflies (Fourel et al., 1998), our investigation has underlined the fact that there is much future work required to move the field forward. For example, we stress again that monarchs deriving from natal sites in areas of high deuterium excess (e.g., southern states) might still produce wings which reflect these relationships and so to improve our statistical power more sampling in those areas would be desirable.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Only dead monarch butterflies were examined in this study. These were derived from collections, natural overwinter mortality and roadkills, so no animal care or ethics approval was necessary.
KH and GK conceived of the idea and performed stable isotope measurements. KK performed isotope assignments and assisted with figures. All authors contributed to the writing of the manuscript.
This study was funded by Environment and Climate Change Canada operating funds to KH and GK and by an NSERC Discovery Grant to KH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Blanca X. Mora Alvarez for assistance with preparing samples for stable isotope analyses. Two reviewers provided useful comments on an earlier draft of the paper.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00224/full#supplementary-material
Altizer, S. M., Hobson, K. A., Davis, A. K., de Roode, J. C., and Wassenaar, L. I. (2015). Do healthy monarchs migrate farther? Tracking natal origins of parasitized vs. uninfected monarch butterflies overwintering in Mexico. PLoS ONE 10:e0141371. doi: 10.1371/journal.pone.0141371
Bivand, R. S., and Lewin-Koh, N. (2016). maptools: Tools for Reading and Handling Spatial Objects. Version 0.8-39. Available online at: http://CRAN.R-project.org/package=maptools (accessed May 15, 2019).
Bowen, G. J., Wassenaar, L. I., and Hobson, K. A. (2005). Application of stable hydrogen and oxygen isotopes to wildlife forensic investigations at global scales. Oecologia 143, 337–348. doi: 10.1007/s00442-004-1813-y
Bowen, G. J., and West, J. B. (2019). ”Isoscapes for terrestrial migration research,” in Tracking Animal Migration with Stable Isotopes, 2nd Edn, eds K. A. Hobson and L. I. Wassenaar (London: Academic Press, 53–84.
Bryant, J. D., and Froehlich, P. N. (1995). A model of oxygen isotope fractionation in body water of large mammals. Geochim. Cosmochim. Acta 59, 4523–4537. doi: 10.1016/0016-7037(95)00250-4
Chesson, L. A., Tipple, B. J., Erkilla, B. R., and Ehleringer, J. R. (2013). Hydrogen and oxygen stable isotope analysis of pollen collected from honey. Grana 52, 305–315. doi: 10.1080/00173134.2013.841751
Chown, S. L., Gibbs, A. G., Hetz, S. K., Klok, J. C., Lighton, J. R. B., and Marais, E. (2006). Discontinuous gas exchange in insects: a clarification of hypotheses and approaches. Physiol. Biochem. Zool. 79, 333–343. doi: 10.1086/499992
Craig, H. (1961). Isotopic variations in meteoric waters. Science 133, 1833–1834. doi: 10.1126/science.133.3467.1833
Ehleringer, J. R., Bowen, G. J., Chesson, L. A., West, A. G., Podlesak, D. W., and Cerling, T. E. (2008). Hydrogen and oxygen isotope ratios in human hair are related to geography. Proc. Nat. Acad. Sci. U.S.A. 105, 2788–2793. doi: 10.1073/pnas.0712228105
Flockhart, D. T., Brower, L. P., Ramirez, M. I., Hobson, K. A., Wassenaar, L. I., Altizer, S., et al. (2017). Regional climate on the breeding grounds predicts variation in the natal origin of monarch butterflies overwintering in Mexico over 38 years. Glob. Chang. Biol. 23, 2565–2576. doi: 10.1111/gcb.13589
Flockhart, D. T., Pichancourt, J.-B., Norris, D. R., and Martin, T. G. (2015). Unravelling the annual cycle in a migratory animal:breeding-season habitat loss drives population declines of monarch butterflies. J. Anim Ecol. 84, 155–165. doi: 10.1111/1365-2656.12253
Flockhart, T., Wassenaar, L. I., Martin, T., Hobson, K. A., Wunder, M., and Norris, D. R. (2013). Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proc. Roy. Soc. Lond. B 280:20131087. doi: 10.1098/rspb.2013.1087
Fourel, F., Merren, T., Morrison, J., Wassenaar, L. I., and Hobson, K. A. (1998). Application of EA-Pyrolysis – IRMS δD and δ18O Analysis to Animal Migration Patterns. Application Note 300. Manchester: Micromass UK Ltd.
Hanley, D., Miller, N. G., Flockhart, D. T., and Norris, D. R. (2013). Forewing pigmentation predicts migration distance in wild-caught migratory monarch butterflies. Behav. Ecol. 24, 1108–1113. doi: 10.1093/beheco/art037
Hijmans, R. J. (2016). raster: Geographic Data Analysis and Modeling. Version 2.5-8. Available online at: http://CRAN.R-project.org/package=raster (accessed May 15, 2019).
Hobson, K. A. (2019). “Applicatoin of isotopic methods to tracking animal movements,” in Tracking Animal Migration with Stable Isotopes, 2nd Edn, eds K. A. Hobson and L. I. Wassenaar (London: Academic Press, 85–116.
Hobson, K. A., Bowen, G., Wassenaar, L. I., Ferrand, Y., and Lormee, H. (2004). Using stable hydrogen isotope measurements of feathers to infer geographical origins of migrating European birds. Oecologia 141, 477–488. doi: 10.1007/s00442-004-1671-7
Hobson, K. A., DeMent, S. H., Van Wilgenburg, S. L., and Wassenaar, L. I. (2009a). Origins of American Kestrels wintering at two southern U.S. sites: an investigation using stable-isotope (δD, δ18O) methods. J. Raptor Res. 43, 325–337. doi: 10.3356/JRR-08-74.1
Hobson, K. A., Doward, K., Kardynal, K., and McNeil, J. (2018a). Inferring origins of migrating insects using isoscapes: a case study using the true armyworm, Mythimna unipuncta, in North America. Ecol. Entomol. 43, 332–341. doi: 10.1111/een.12505
Hobson, K. A., and Koehler, G. (2015). On the use of stable-oxygen isotope (δ18O) measurements for tracking avian movements in North America. Ecol. Evol. 5, 799–806. doi: 10.1002/ece3.1383
Hobson, K. A., Norris, D. R., Kardynal, K., and Yohannes, E. (2018b). “Animal migration: a context for using new techniques and approaches,” in Tracking Animal Migration with Stable Isotopes, 2nd Edn, eds K. A. Hobson and L. I. Wassenaar (London: Academic Press, 1–24.
Hobson, K. A., Plint, T., Garcia Ramirez, E., Mora Alvarez, B. X., Ramirez, I., and Longstaffe, F. J. (2017). Within-wing isotopic (δ2H, δ13C, δ15N) variation of monarch butterflies: implications for studies of migratory origins and diet. Anim. Migr. 4, 8–14. doi: 10.1515/ami-2017-0002
Hobson, K. A., Soto, D. X., Paulson, D. R., Wassenaar, L. I., and Matthews, J. (2012). A dragonfly (δ2H) isoscape for North America: a new tool for determining natal origins of migratory aquatic emergent insects. Methods Ecol. Evol. 3, 766–772. doi: 10.1111/j.2041-210X.2012.00202.x
Hobson, K. A., Van Wilgenburg, S. L., Wesolowski, T., Maziarz, M., Biljsma, A. R., Grendelmeier, G., et al. (2014). A multi-isotope (δ2H, δ13C, δ15N) approach to establishing migratory connectivity in Palearctic-Afrotropical migrants: an example using Wood Warblers Phylloscopus sibilatrix. Acta Ornithol. 49, 57–69.
Hobson, K. A., and Wassenaar, L. I. (2019). Tracking Animal Migration with Stable isotopes, 2nd Edn. London: Academic Press.
Hobson, K. A., Wassenaar, L. I., and Taylor, O. R. (1999). Stable isotopes (δD and δ13C) are geographic indicators of natal origins of monarch butterflies in eastern North America. Oecologia 120, 397–404. doi: 10.1007/s004420050872
Hobson, K. A., Wunder, M. B., Van Wilgenburg, S. L., Clark, R. G., and Wassenaar, L. I. (2009b). A method for investigating population declines of migratory birds using stable isotopes: origins of harvested lesser scaup in North America. PLoS ONE 4:e7915. doi: 10.1371/journal.pone.0007915
IAEA/WMO (2015). Global Network of Isotopes in Precipitation. The GNIP Database. Available online at: https://nucleus.iaea.org/wiser
Kohn, M. J. (1996). Predicting animal δ18O: accounting for diet and physiological adaptation. Geochim. Cosmochim. Acta 60, 4811–4829. doi: 10.1016/S0016-7037(96)00240-2
Luz, B., and Barkan, E. (2011). The isotopic composition of atmospheric oxygen. Global Biogeochem. Cycles 25:GB3001. doi: 10.1029/2010GB003883
Miller, N. G., Wassenaar, L. I., Hobson, K. A., and Norris, D. R. (2012). Migratory connectivity of the monarch butterfly (Danaus plexippus): patterns of spring re-colonization in eastern North America. PLoS ONE 7:e31891. doi: 10.1371/journal.pone.0031891
Pekarsky, S., Angert, A., Haese, B., Werner, M., Hobson, K. A., and Nathan, R. (2015). Enriching the isotopic toolbox for migratory connectivity analysis: a new approach for migratory species breeding in remote or unexplored areas. Divers. Distrib. 21, 416–427. doi: 10.1111/ddi.12306
Qi, H., and Coplen, T. B. (2011). Investigation of preparation techniques for δ2H analysis of keratin materials and a proposed analytical protocol. Rapid Commun. Mass Spectrom 25, 2209–2222. doi: 10.1002/rcm.5095
R Core Team. (2016). R: A Language and Environment for Statistical Computing, Version 3.3.2. ISBN 3-900051-07-0. Vienna: The R Foundation for Statistical Computing.
Ries, L., Taron, D. J., Rendon-Salinas, E., and Oberhauser, K. (2015). “Connecting eastern monarch population dynamics across their migratory cycle,” in Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly, eds K. S. Oberhauser, K. R. Nail and S. Altizer (Itahca, NY: Cornell University Press, 268–282.
Rozanski, K., Araguas-Araguas, L., and Gonfiantini, R. (1993). “Isotopic patterns in modern global precipitation,” in Continental Isotope Indicators of Climate, eds P. K. Swart, K. C. Lohmann, J. McKenzie, and S. Savin (Washington, DC: American Geophysical Union Monograph, 1–36.
Semmens, B. X., Semmens, D. J., Thogmartin, W. E., Wiederholt, R., López-Hoffman, L., Diffendorfer, J. E., et al. (2016). Quasi extinction risk and population targets for the eastern migratory population of monarch butterflies (Danaus plexippus). Sci. Rep. 6:23265. doi: 10.1038/srep23265
Soto, D. X., Koehler, G., Wassenaar, L. I., and Hobson, K. A. (2017). Determination of stable hydrogen isotopic compositions of complex organic materials: contrasting the role of exchangeable hydrogen and residual moisture. Rapid Comm. Mass Spectrom. 31, 1193–1203.
Terzer, S., Wassenaar, L. I., Araguás-Araguás, L. J., and Aggarwal, P. K. (2013). Global isoscapes for δ18O and δ2H in precipitation: improved prediction using regionalized climatic regression models. Hydrol. Earth Syst. Sci. Discuss. 10, 7351–7393. doi: 10.5194/hessd-10-7351-2013
Thogmartin, W.E., López-Hoffman, L., Rohweder, J., Diffendorfer, J., Drum, R., Semmens, D., et al. (2017). Restoring monarch butterfly habitat in the Midwestern US: all hands on deck. Environ. Res. Lett. 12:074005. doi: 10.1088/1748-9326/aa7637
Urquhart, F. A., and Urquhart, N. R. (1976). The overwintering site of the eastern population of the monarch butterfly (Danaus p. Plexippus: Danaidae) in Southern Mexico. J. Lepidopt. Soc. 30, 153–158.
Van Wilgenburg, S. L., Hobson, K. A., Brewster, K. R., and Welker, J. M. (2012). Assessing dispersal in threatened migratory birds using stable hydrogen isotope (δD) analysis of feathers. Endang. Sp. Res. 16, 17–29. doi: 10.3354/esr00383
Vander Zanden, H. B., Chaffee, C. L., Gonzalez-Rodriguez, A., Flockhart, D. Y., Norris, D. R., and Wayne, M. L. (2018). Alternate migration strategies of eastern monarch butterflies revealed by stable isotopes. Anim. Migr. 5, 74–83. doi: 10.1515/ami-2018-0006
Wassenaar, L., and Hobson, K. A. (1998). Natal origins of migratory monarch butterflies at wintering colonies in Mexico: new isotopic evidence. Proc. Natl. Acad. Sci. U.S.A. 95, 15436–15439. doi: 10.1073/pnas.95.26.15436
Keywords: deuterium, stable isotopes, oxygen-18, natal origins, wing chitin, assignment
Citation: Hobson KA, Kardynal KJ and Koehler G (2019) Expanding the Isotopic Toolbox to Track Monarch Butterfly (Danaus plexippus) Origins and Migration: On the Utility of Stable Oxygen Isotope (δ18O) Measurements. Front. Ecol. Evol. 7:224. doi: 10.3389/fevo.2019.00224
Received: 27 February 2019; Accepted: 29 May 2019;
Published: 18 June 2019.
Edited by:
Jay E. Diffendorfer, United States Geological Survey, United StatesReviewed by:
David Nelson, University of Maryland Center for Environmental Science (UMCES), United StatesCopyright © 2019 Hobson, Kardynal and Koehler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keith A. Hobson, a2hvYnNvbjZAdXdvLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.