- 1Laboratory of Socioecology and Social Evolution, KU Leuven, Leuven, Belgium
- 2Laboratório de Comportamento e Ecologia de Insetos Sociais, Departamento de Biologia, FFCLRP, Universidade de São Paulo, Ribeirão Preto, Brazil
- 3Departments of Entomology and Chemistry, University of California, Riverside, Riverside, CA, United States
In several highly eusocial insect species with morphologically distinct castes, queen-characteristic cuticular hydrocarbons (CHCs) have been shown to act as queen signals that suppress the reproduction of nestmate workers. However, it is not known whether such queen pheromones might also play a role in regulating reproductive division of labor in primitively eusocial insects that lack morphologically defined castes. Here, we experimentally tested whether a blend of CHCs which occurred in higher concentrations in the dominant breeding female acts as a queen pheromone, and inhibits reproduction by subordinate females in the primitively eusocial paper wasp Polistes satan. In contrast to earlier findings with highly eusocial species, our results show that although specific compounds were enhanced in dominant, reproductively active females, a blend of five of these compounds did not inhibit development of the ovaries of female nestmates. Instead, the dominant female had to be physically present to prevent subordinate females from reproducing. Our results are in line with earlier evidence suggesting that fertility-linked compounds in primitively eusocial wasps act only as cues and help to regulate reproduction when used in conjunction with aggressive dominance behavior, meaning the physical presence of the dominant female. Alternatively, our results support the hypothesis that queen pheromones in highly eusocial insects were co-opted from fertility cues that were already present in primitively eusocial ancestors, even when initially, such compounds were merely produced as by-products of ovarian activation, without actually serving to signal reproductive status.
Introduction
The reproductive division of labor between fertile queens and sterile workers in highly eusocial insects has been found to be regulated by queen pheromones (Peeters and Liebig, 2009; Holman, 2010; van Zweden, 2010; Kocher and Grozinger, 2011; Richard and Hunt, 2013; Oi et al., 2015; Grüter and Keller, 2016). These pheromones signal the presence of a healthy and fertile queen, to which the workers generally respond by remaining sterile (Monnin, 2006; Le Conte and Hefetz, 2008; Liebig, 2010; Oi et al., 2015). For a long time, the sole species from which queen pheromones had been well-characterized was the honeybee Apis mellifera (Butler et al., 1962; Hoover et al., 2003; Slessor et al., 2005; Le Conte and Hefetz, 2008). In this species, the main queen pheromone component is 9-oxo-(E)-2-decenoic acid (9-ODA)—a semi-volatile compound produced in the queen's mandibular glands (Butler et al., 1962; Hoover et al., 2003; Slessor et al., 2005; Le Conte and Hefetz, 2008). Recently, however, evidence has been mounting that specific non-volatile cuticular hydrocarbons (CHCs) act as conserved constituents of queen pheromone blends in many other social insects, including ants, bumblebees, and wasps [reviewed in Van Oystaeyen et al. (2014), Oi et al. (2015), and Holman (2018)].
The idea that hydrocarbons may act as queen or fertility signals originally was supported by purely correlative evidence, including observations that fertile and non-fertile colony members consistently differed in their CHC profiles (Monnin, 2006; Peeters and Liebig, 2009; Liebig, 2010), that CHC profiles correlated with variation in fecundity within a given caste (d'Ettorre et al., 2004; Heinze, 2004; Endler et al., 2006; Holman et al., 2010a,b; van Zweden et al., 2014), and that workers discriminated between the CHC profiles of fertile and non-fertile individuals (Dietemann et al., 2003; Heinze, 2004). More recently, studies using synthetic versions of queen-characteristic CHCs have provided direct evidence of their roles as mediators of reproductive status by demonstrating experimentally that these compounds reduce worker ovarian activation. This was shown in two species of vespine wasps, Vespula vulgaris, and Dolichovespula saxonica (Van Oystaeyen et al., 2014; Oi et al., 2016), several species of ants (Holman et al., 2010b, 2013, 2016a; Van Oystaeyen et al., 2014; de Narbonne et al., 2016; Smith et al., 2016) and two species of bumblebees (Van Oystaeyen et al., 2014; Holman et al., 2016a). All of the species investigated thus far have morphologically distinct queen and worker castes, which arguably already resolves most of the potential reproductive conflict between them, given that queens are clearly adapted to a reproductive role, being much more fecund than workers, typically having different circulating hemolymph titers of gonadotropic hormones, and normally being the only mated females in the colony (Hartfelder, 2000; Brent and Vargo, 2003; Oliveira et al., 2017). In that sense, queens effectively can be viewed as the germ-line of the superorganism that social insect colonies represent (Boomsma and Gawne, 2018). From this perspective, one might expect that in these species, queen pheromones that signaled the queen's high fecundity would be intrinsically evolutionarily stable, and that the queen would not have to physically aggress workers to be able to maintain her reproductive dominance.
In primitively eusocial species that lack morphologically specialized castes, to date there have been no experimental studies aimed at determining whether chemical fertility signals alone enable breeding females to maintain their reproductive dominion over workers. Earlier research had suggested that reproduction in species with no morphological castes was regulated primarily through aggressive dominance behavior (Pardi, 1948; Reeve, 1991; Ross and Matthews, 1991), which can be correlated with body size or hormonal factors (Tibbetts and Lindsay, 2008). The dominance hierarchy can also be aided by visual cues, so-called “badges of status,” which provide reliable cues of the fighting potential of dominant breeders, and partially remove the necessity for overt conflicts over reproduction (Tibbetts and Dale, 2004; Tibbetts, 2006). In Polistes dominula, dominant females are unable to inhibit ovarian activation of workers by fertility cues or aggression; instead, they use visual inspection of empty cells to detect the presence of new queens (Liebig et al., 2005). Similar to highly eusocial insects, however, dominant breeders express specific fertility- and dominance-linked hydrocarbons on their cuticle (Dapporto et al., 2007a; Bhadra et al., 2010) or in their Dufour's gland (Bonavita-Cougourdan et al., 1991; Layton et al., 1994; Sledge et al., 2004; Dapporto et al., 2007b; Tannure-Nascimento et al., 2008; Gadagkar, 2016). We hypothesized that if these hydrocarbons act as reliable indicators of the females' true fertility or their fighting potential [which could occur for example if reproduction or dominance and the production of those compounds were under shared endocrine control (Holman, 2012; Oliveira et al., 2017)], that these CHCs could provide a mechanism to regulate reproductive division of labor in an analogous way as badges of status, allowing the main breeder(s) to be identified without requiring any overt aggression. If correct, this would predict that these fertility-linked compounds would act as queen pheromones and suppress the reproduction of subordinate females, even in the absence of overt aggression.
Thus, the aim of this study was to test if fertility-linked cuticular hydrocarbons act as queen pheromones in a primitively eusocial insect, the paper wasp Polistes satan. Polistine wasps are a key model genus in the study of the evolution of eusociality (Jandt and Toth, 2015) and chemical signaling in this species has been studied extensively, mostly in the context of nestmate recognition [e.g., (Espelie and Hermann, 1990; Panek et al., 2001)] and intraspecific parasitism (Lorenzi et al., 2017). However, to our knowledge, no experimental studies have actually tested the activity of synthetic fertility- or dominance-related chemical signals (Jandt et al., 2014). As part of the studies described here, we identified linear and 3-methylalkanes as being characteristic of reproductive Polistes satan females. These compounds are of the same chemical classes as the hydrocarbon queen pheromones reported from two highly eusocial vespine species, Vespula vulgaris and Dolichovespula saxonica (Van Oystaeyen et al., 2014; Oi et al., 2016) and several ant species [see (Holman, 2018)]. Thus, we hypothesized that these compounds might act as queen pheromones for P. satan, even in the absence of the dominance behavior that normally accompanies the regulation of reproduction in primitively eusocial species (Tannure-Nascimento et al., 2007; Smith et al., 2015). Previous work with the congeneric species P. dominula found no experimental evidence that queen signals or behavioral interactions prevented workers from laying eggs when empty cells in the comb were available (Liebig et al., 2005).
Materials and Methods
Study Species
Polistes satan is a neotropical paper wasp distributed in southeastern Brazil (Carpenter, 1996). Usually, new nests are initiated at the end of the dry season after the winter by an association of a few females, although in most cases only a single female ends up dominating reproduction (Tannure-Nascimento et al., 2008).
Identification of Fertility-Linked Cuticular Hydrocarbons
For the chemical analyses of CHCs, we collected five nests of Polistes satan in Pedregulho (São Paulo state, 20°15′S, 47°27′W) in March 2013, including one in pre-emergence and four in post-emergence. At the time of sampling, only one dominant female was present in each nest, confirmed by later dissections to look for developed eggs, except for one of the nests, from which no dominant breeder was identified. Individual wasps were frozen (−20°C), then extracted in 3 ml pentane (HPLC grade, Sigma-Aldrich) for 10 min, and the carcasses were subsequently kept in the freezer (−20°C) for later dissection to check ovary development. The pentane extracts were left to evaporate at room temperature, then were reconstituted in 250 μl pentane for analysis by coupled gas chromatography-mass spectrometry. This was performed by injecting 2 μl of each sample into a Shimadzu QP2010 GC-MS (Shimadzu Corporation, Tokyo, Japan) equipped with a DB-5ms capillary column (30 × 0.25 mm × 0.25 μm film, J&W Scientific, Folsom CA, USA), using helium as a carrier gas at a flow rate of 1 ml/min. The injection temperature was set to 280°C and the oven temperature was held at 70°C for 1 min, then increased to 220°C at 25°C/min, then to 325°C at 3°C/min followed by a final hold of 15 min at 325°C. We used AMDIS 2.71 software (NIST, Gaithersburg MD, USA) for spectral deconvolution, after which compounds were identified based on a combination of interpretation of mass spectra and use of retention indices (Carlson et al., 1998), and, for one carboxylic acid, a NIST 2014 database search. We identified a total of 56 compounds in the extracts (Figure 1; Table S1), 53 of which were hydrocarbons.
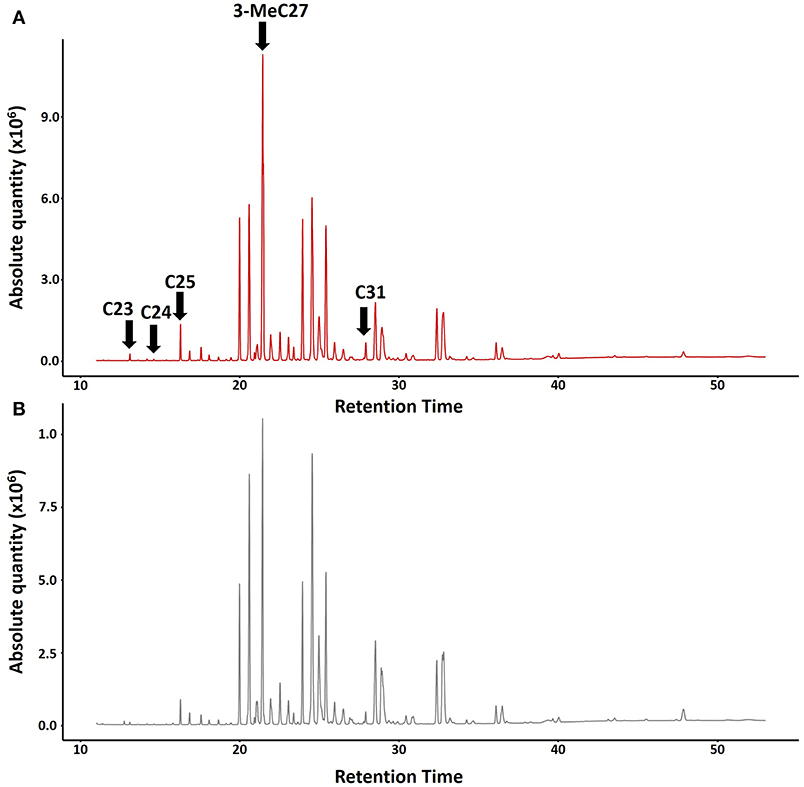
Figure 1. Typical chromatograms showing absolute quantities of CHCs in Polistes satan (A) Dominant queen (top, red) and (B) Subordinate female workers (bottom, gray). Arrows indicate the compounds tested in our bioassays: n-C23, n-C24, n-C25, 3-MeC27, and n-C31.
Peak integration was performed by integrating over total ion chromatograms using in-house developed software in R v.3.0.1 (available upon request from the authors). External alkane standards (C7 to C40 straight-chain alkanes; #49452-U, Supelco Inc., Bellefonte PA, USA), injected at concentrations of 0.1, 0.01, and 0.001 μg/μl, were used to quantify the absolute amounts of compounds present on each individual, as well as to calculate retention indices for all identified compounds based on the cubic spline method (Messadi et al., 1990).
The compounds with a maximal fold difference between reproductive and non-reproductive females were selected as prime candidates for queen pheromones to be tested in bioassays. To shortlist these compounds, we fitted linear mixed models [package lme4 (Bates et al., 2015)] on log2 transformed relative peak areas of each of the compounds present, coding caste as a fixed factor and colony as a random intercept, and ranked compounds based on the t ratio. We further adopted a mean ≥1.5 fold difference in relative abundance of a particular compound in extracts of fertile breeders vs. workers as a threshold (Table S2). Out of the eight compounds that showed the most pronounced differences (Table S2), four were commercially available (tricosane (n-C23), tetracosane (n-C24), pentacosane (n-C25), hentriacontane (n-C31), all purchased from Sigma-Aldrich, St. Louis, MO, USA) and one (3-methylheptacosane (3-MeC27) was synthesized as previously described (Van Oystaeyen et al., 2014) (Table S2). Together, these compounds were tested as a blend in our queen pheromone bioassays, where they were administered at a dose of two dominant foundress equivalents per day. The remaining 3-methylalkanes were not available at the time of the experiment, and so were not included in our experiment. Some earlier studies have shown that queen-characteristic compounds are active even when tested individually in queen pheromone bioassays [e.g., (Van Oystaeyen et al., 2014; Holman et al., 2016b)], whereas other studies have suggested that blends may be required for full activity (Smith et al., 2015; Oi et al., 2016). Although there is no final consensus of the use of blends vs. single compounds in mediating sterility in social insects [see review (Holman, 2018)], due to the number of nests sampled, we proceeded with bioassays using a blend that contained the majority of the compounds characteristic of fertile breeding queens.
Queen Pheromone Bioassays
To conduct bioassays, 20 post-emergent nests of P. satan were collected in February 2014 at the same field site and transported to the laboratory at the University of São Paulo (USP, Campus Ribeirão Preto). We used plastic boxes (18 × 26 × 14 cm) to carefully collect the nests and used a ruler to cut the petiole of the nest without the wasps flying away. Foragers that returned to their nest later on were netted and added to their respective nest. In the laboratory, the wasps were briefly anesthetized with carbon dioxide and the nests were attached with glue to the top of the plastic box. All nests were kept in the laboratory at a temperature of around 28°C. Two holes covered with wire mesh were made for ventilation (one on the top and one on the side of the box). Water and sugar were provided ad libitum on the bottom and pupae of stingless bees were provided as food every day.
Due to the fact that Polistes paper wasps live in open combs, their nests can be readily observed without interfering with the function of the colony, and dominant foundresses could be removed prior to the start of bioassays. Specifically, the dominant females were identified based on their characteristic dominance interactions with other females (such as biting, mounting, boxing, and pushing) [(Tannure-Nascimento et al., 2008)] and on direct observations of egg-laying. Boxing can be characterized by alternating movements of the first two pairs of wasp legs and short advances toward the opponent (as show in Figure 2B) (Tannure-Nascimento, 2006). After removing the dominant egg-layers we also confirmed their identification by dissection, checking that their ovaries were fully developed, and confirmed their mating status by inspecting their spermatheca.
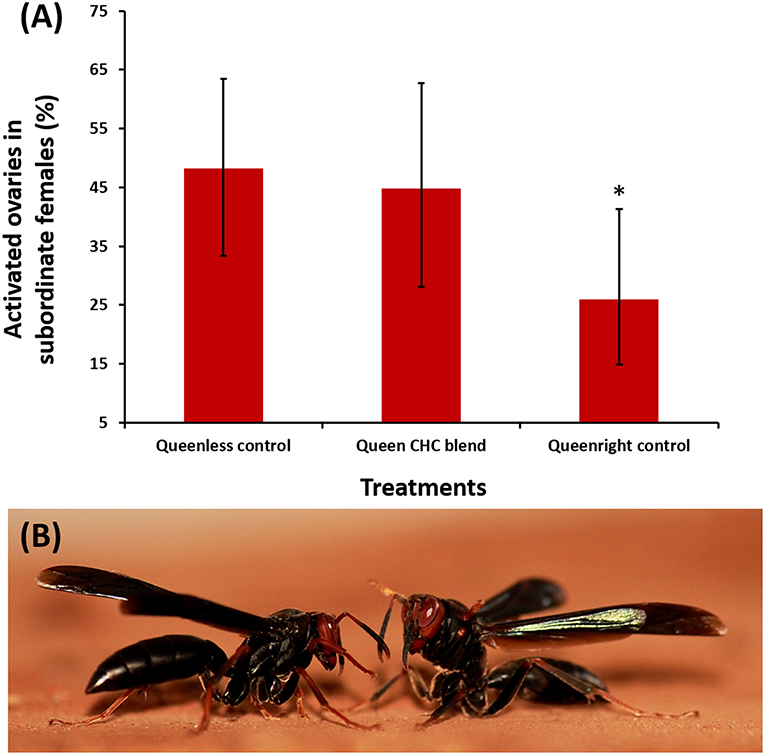
Figure 2. (A) Percentages of subordinate Polistes satan females with developed ovaries, across the different treatments of our queen pheromone bioassays. The proportion of females with developed ovaries in nests which were treated with a blend of compounds characteristic of the dominant breeding females (a mixture of tricosane, tetracosane, pentacosane, hentriacontane, and 3-methylheptacosane at two queen equivalents, middle bar) was not significantly different than that in a queenless solvent-only control (left bar). In contrast, physical presence of the dominant female significantly reduced ovary development among subordinate females (right bar, binomial GLMM, z = −2.05, *p = 0.039) (Control N = 6 nests/n = 81; blend N = 7 nests/n = 58; queenright N = 7 nests/n = 74); (B) Aggressive behavior between two females, characterized as “boxing,” observed during field trials. Boxing is characterized by alternating movements of the first two pairs of wasp legs and short advances toward the opponent (Tannure-Nascimento, 2006). Photo credit: RO.
After removing the single dominant egg-laying female from each nest, the queen pheromone bioassays were performed. Thus, the 13 available nests were randomly allocated to either the queen pheromone blend treatment (n = 7 nests) or a solvent only sham-treated control (n = 6 nests). For the queen pheromone blend treatment, a dose of 2 queen equivalents per compound was administered each day as a 100 μl pentane solution containing 1.04 μg of n-C23 (tricosane), 0.25 μg of n-C24 (tetracosane), 6.44 μg of n-C25 (pentacosane), 4.69 μg of n-C31 (hentriacontane), and 106.82 μg of 3-MeC27 (3-methylheptacosane). From another seven nests we did not remove the dominant females, but instead kept them as queenright controls. Treatment solutions were pipetted daily for 10 days onto the back of the comb through the mesh. At the end of the experiment, all workers were frozen (−20°C) for later dissection to assess ovary activation and insemination status. The dissections were carried out blind. Pictures of the ovaries were taken using a stereomicroscope (Leica MZ 16 coupled with a Leica DFC 500 camera). As in previous studies (Wenseleers and Ratnieks, 2006a; van Zweden et al., 2014) ovaries were considered activated when the largest oocyte was larger than half the size of a laid egg, based on the average length of a developed egg being 2.0 mm (SE±0.13 mm, n = 10 eggs). Fertilized females had a full spermatheca (visible as a whitish mass in the middle of the spermatheca, Figures S1A,B), whereas those of unmated females were empty (Figure S1C).
Statistical Analysis
We used a Generalized Linear Mixed-effect Model (GLMM; package “lme4”) with binomial error distribution and logit link function to analyze the effect of the compound treatments on the probability of workers to have activated ovaries. Treatment was included as a fixed factor, colony size (number of females present in each nest) as a continuous covariate and colony as a random intercept. Wald tests were employed to assess the significance of the fixed effects. The effect sizes were also calculated and converted to Cohen's D, as D = LOR * sqrt(3)/pi. An alternative model in which the number of mated females was included as an additional covariate was also fitted, but returned qualitatively identical results. In addition, we also used a binomial GLMM to test if mated subordinate females were more likely to activate their ovaries and replace the original dominant breeder than unmated subordinates in the queen pheromone blend and queenless control treatments. In this model, treatment and mating status were included as fixed factors and colony was included as a random intercept. All analyses were done in R version 3.5.1. (R Development Core Team, 2012).
Results
Our treatment blend contained five compounds that were characteristic of dominant breeders: tricosane (n-C23), tetracosane (n-C24), pentacosane (n-C25), 3-methylheptacosane (3-MeC27), and hentriacontane (n-C31) (Figure 1; Table S2). However, daily treatment with the blend for 10 days did not significantly inhibit ovary activation in the subordinate females compared to the queenless control (Table 1, z = −0.28, p = 0.78, binomial GLMM with colony as as random factor, raw data at Table S3). The effect size (as log of odds ratio) was calculated as −0.14 (SE = 0.48), with upper CI = 0.80 and lower CI = −1.01. Converting to Cohen's D, the confidences limits were considered large, with D ranging from −0.60 to +0.40. In fact, the estimated percentage of females with developed ovaries was virtually identical across both treatments (Figure 2A; 45% in the queen pheromone treatment, N = 7 nests, n = 58 individuals vs. 48% in the queenless control, N = 6 nests, n = 81 individuals). The physical presence of a dominant breeder, however, significantly reduced ovary activation among the subordinate females, as can be seen from the significant difference between the queenright control and the queenless control (Table 1, z = −2.05, p = 0.039) and the contrast in the percentage of females with activated ovaries across both treatments (Figure 2A; 48% in the queenless control, N = 6 nests, n = 81 individuals vs. 26 % in the queenright control, N = 7 nests, n = 74 individuals). Dissection revealed that in all nests from which the dominant inseminated female was removed, 36 % (N = 13 nests, 50 females from 139) of the subordinate females were also inseminated. There was no evidence that the presence of higher numbers of inseminated females affected the mean probability of subordinate females to activate their ovaries (binomial GLMM with number of mated females coded as an additional covariate, z = −1.42, p = 0.16, Table S4). As expected, however, inseminated females were more likely to activate their ovaries than non-inseminated females after removal of the original dominant foundress (binomial GLMM, z = 2.99, **p = 0.002, Table S4).
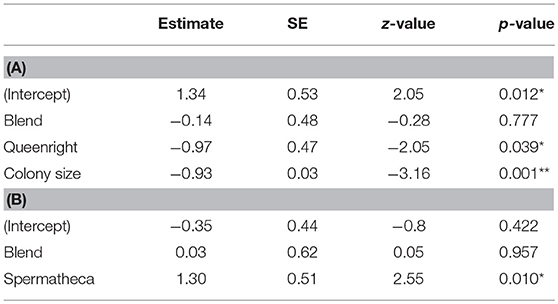
Table 1. (A) Binomial generalized linear mixed model output testing for differences in the proportion of Polistes satan females with activated ovaries across our different treatments. In this model, colony, treatment, and colony size were included as a random intercept, a fixed factor, and a continuous covariate. Significance levels are based on Wald z tests for the difference between the treatment and the queenless control (intercept, shown between brackets). (B) Binomial generalized linear mixed model output testing for differences in the proportion of mated Polistes satan females with developed ovaries where the dominant breeder had been removed. *p < 0.05; **p < 0.01.
Discussion
In the primitively eusocial paper wasp Polistes satan, ovary activation of subordinate females was not inhibited by exposure to a mixture of the hydrocarbons n-C23, n-C24, n-C25, n-C31, and 3-MeC27, all of which were more abundant in dominant reproductives than in subordinates. These findings contrast with our earlier studies on two highly eusocial vespid species, Vespula vulgaris and Dolichovespula saxonica, where queen-characteristic hydrocarbons significantly inhibited worker ovary development (Van Oystaeyen et al., 2014; Oi et al., 2016). Thus, our current results suggest that primitively eusocial wasps rely on other factors, such as aggressive dominance behavior, to inhibit reproduction by nestmates (Reeve, 1991; Röseler, 1991; Tibbetts and Dale, 2004), which necessitates physical contact with the dominant female. This was true despite the fact that the quantitative differences in chemical profiles between reproductive and non-reproductive females in Polistes satan were analogous, and perhaps only slightly less pronounced, than the queen-worker differences in chemical profiles observed in highly eusocial wasps such as Vespula vulgaris and Dolichovespula saxonica (Van Oystaeyen et al., 2014; van Zweden et al., 2014; Oi et al., 2016).
In another Polistes species, P. dominula, reproduction has been suggested to be regulated via chemical signals (Sledge et al., 2001, 2004) after the initial dominance interactions among co-foundresses diminishes (Pardi, 1948; Gamboa and Dropkin, 1979; Gamboa and Stump, 1996). In contrast, in our study the blend did not affect ovary development in subordinates, suggesting that the chemicals associated with the dominant are not used as queen pheromones. This suggests several possibilities. First, the compounds may not be detected, but this seems unlikely in an animal which uses CHC profiles for recognition of nestmates. Second, the tested blend may have been incomplete or incorrect; a different or more complete blend might indeed signal reproductive dominance, and so at this point we cannot state categorically that chemical signals are not used for reproductive dominance in this, and by implication, other primitively eusocial species. Third, the effect sizes indicated that an increase in the sample size may be required in order to accurately determine any possible subtle effects of the CHC blend. Fourth, the tested compounds may not be involved in regulation of reproduction, or only have an effect when used in combination with physical aggression and dominance behavior. This later explanation, which is perhaps the most likely, suggests that the compounds which are enhanced in dominant individuals act as chemical badges of status, which are used by subordinates to assess their position so as to avoid aggression. Such badges of status would work in a somewhat different way than an actual queen pheromone, and yet might still serve as its predecessor.
One possible explanation for the discrepancy between highly and primitively eusocial insects might be that highly eusocial insects are characterized by the presence of a morphologically specialized queen caste which is specifically adapted to egg-laying, in some cases becoming totally dependent on workers for nutrition, grooming, and other vital functions. These more clearly defined castes align the evolutionary interests of the queen and workers, thereby making aggression and dominance behavior to regulate reproduction unnecessary. In other words, the evolution of morphological castes may have coincided with the coevolution of queen pheromones that evolved to regulate reproduction without requiring aggression, simply through the fact that such pheromones signal the presence of a fertile egg-layer, to which the workers would be selected to respond by remaining sterile (Wenseleers et al., 2004; Oi et al., 2015). A slight caveat with this scenario is that there are also a few examples of primitively eusocial wasps, including Ropalidia marginata and some Epiponine wasps (e.g., Metapolybia aztecoides, Asteloeca ujhelyii, and Synoeca surinama), which lack morphological castes and where dominance behavior is absent or ritualized, thereby suggesting the presence of a chemical queen signal (West-Eberhard, 1978; Nascimento et al., 2004; Mitra and Gadagkar, 2011; Mitra et al., 2011; Kelstrup et al., 2014). Further experimental work with synthetic compounds, however, would be required to prove that chemical signals alone regulate reproduction in these primitively social species.
Surprisingly, our dissections revealed that 36% of the subordinate females in P. satan colonies were inseminated, and that inseminated females were more likely to develop their ovaries than unmated females after removal of the original dominant foundress. This suggests that a significant part of the benefit of cooperation in Polistes paper wasps could be due to colony inheritance and delayed direct reproductive benefits (Cant et al., 2006; Leadbeater et al., 2011; Field and Leadbeater, 2016), where subordinates wait for an opportunity to become a queen at some point in the future. In P. satan, although there is no information of lifespan of a foundress, queens can be replaced due to mortality factors, as was shown experimentally whereby removing dominant females resulted in fights for 2 weeks to establish a new hierarchy of dominance (Tannure-Nascimento, 2006).
Of the 56 compounds identified from the whole-body extracts, the CHCs that appeared to differ most between fertile and non-fertile Polistes satan individuals were the linear alkanes n-C23, n-C24, n-C25, and n-C31 and the 3-methylalkanes 3-MeC27, 3-MeC23, 3-MeC25, and 3-MeC26. These results were similar to those obtained by Tannure-Nascimento et al. (2008), who characterized 28 cuticular hydrocarbons in this species from a different field site, and also reported linear and methyl-branched alkanes, including n-C25, n-C27, n-C29, n-C31, and 3-MeC29 to be most characteristic of dominant breeding females. Likewise, in P. dominula, 3-MeC29 together with the alkenes C29:1 and C31:1 were found to be most characteristic of the dominant foundresses (Sledge et al., 2004), whereas the linear alkanes n-C23, n-C27, n-C29, n-C31, and n-C33 were characteristic of dominant foundresses in P. metricus (Layton et al., 1994). Furthermore, odd-numbered long-chain linear and 3-methylalkanes have frequently been reported as queen-characteristic compounds in vespine wasps (van Zweden et al., 2014) and in other groups of social Hymenoptera (Holman et al., 2010b, 2013, 2016a; Van Oystaeyen et al., 2014; Oi et al., 2015, 2016; de Narbonne et al., 2016; Smith et al., 2016). This suggests that the overproduction of these linear and 3-methylalkanes may be linked to fertility (Liebig et al., 2000; Dietemann et al., 2003; Monnin, 2006; Peeters and Liebig, 2009; Liebig, 2010), perhaps due to intrinsic physiological links (Toth et al., 2014; Oi et al., 2015; Oliveira et al., 2017). If so, this would explain why these compounds tend to be associated so often with signaling queen presence and fertility across different taxa. Recently, it was also shown that interspecific Polistes parasites mimic the chemical profile of foundresses and the specific long-chain and branched CHCs that they produce (Lorenzi et al., 2017). In yet another species, Polistes gallicus, long-chain hydrocarbons secreted by the sternal glands have been suggested to signal reproductive dominance by correlative evidence (Dapporto et al., 2007b).
The degree of relatedness among the nestmates in Polistes satan is not yet known, but this may affect the behaviors and motivations of the workers to cooperate or act selfishly. For example, females within a nest could all be unrelated, thus leading to a strong motivation to oviposit whenever possible, whereas if at least some are related, then the motivation would be lower. Further details of the mating biology of this primitively eusocial species would be informative because an unexpectedly large percentage of the workers were mated, unlike in highly eusocial species, which would again affect the motivation to invest in their own reproduction rather than remaining subordinate to the queen. The results of our bioassays were not unexpected, given that factors other than chemical signaling have been shown to play a key role in suppressing the reproduction of subordinates in primitively eusocial species, including dominance interactions (Reeve, 1991; Röseler, 1991) (e.g., in Polistes dominula (Pardi, 1948; Gamboa and Dropkin, 1979; Gamboa and Stump, 1996), P. fuscatus (Downing and Jeanne, 1985), P. gallicus (Röseler et al., 1985; Röseler and Röseler, 1989) [changed later to P. dominula (O'Donnell, 1998)], P. satan (Tannure-Nascimento et al., 2008), and Ropalidia cyathiformis (Gadagkar and Joshi, 1984)], differential oophagy [e.g., in P. chinensis (Saigo and Tsuchida, 2004; Wenseleers and Ratnieks, 2006b)], the presence of empty cells [e.g., in P. dominula (Liebig et al., 2005)], or visual marks on the clypeus that have been shown to act as badges of status [e.g., in Polistes dominula (Tibbetts and Dale, 2004; Tibbetts, 2006; Tibbetts and Lindsay, 2008) and in P. satan (Tannure-Nascimento et al., 2008)]. In some primitively eusocial species though, such as P. fuscatus wasps (Downing and Jeanne, 1985), Euglossa melanotricha orchid bees (Andrade-Silva and Nascimento, 2015), and Dinoponera quadriceps ants (Monnin and Peeters, 1999), it has been suggested that a partial transition occurs from reproductive regulation entirely via dominance interactions to one that is at least partially based on chemical signaling later on in the colony cycle. Likewise, in Ropalidia marginata, reproduction has been suggested to be regulated primarily by caste-specific chemicals derived from the Dufour's gland (Kardile and Gadagkar, 2002). Overall, however, it seems that in the small nests of primitively eusocial species, the queen or dominant foundress exerts control over the reproduction of nestmates primarily through aggression and physical intimidation (van Zweden, 2010), although experimental evidence for aggression needs to be confirmed, and chemical signals have never been shown to act without the physical presence of a dominant individual. Also, it may reflect one extreme of the possible continuum from reproductive dominance solely by physical aggression, through to reproductive dominance solely by chemical signaling. Thus, reproductive regulation via queen pheromone signaling may be restricted mainly to the more eusocial species, where queens are morphologically distinct from workers and the large colony size prevents the queen from dominating the workers via physical aggression (Ratnieks, 1988; Ratnieks et al., 2006). Our findings are in agreement with the prediction of the absence of queen pheromones that inhibit worker ovarian activation in primitively eusocial species like Polistes satan (Smith and Liebig, 2017), in which CHCs cues would function as signature mixtures due to the limitation of colony size, but still would show differences between reproductive females. Indeed, this is likely one of the main reasons that queen pheromones had a strong effect on worker ovary activation in the highly eusocial wasps Vespula vulgaris (Van Oystaeyen et al., 2014), less so in the moderately eusocial Dolichovespula saxonica (Oi et al., 2016), and none in the primitively eusocial P. satan.
Data Availability
The datasets for this manuscript are not publicly available because the dataset is available on the Supplementary Material. Requests to access the datasets should be directed to Y2ludGlhYWtlbWlvaUBnbWFpbC5jb20=.
Ethics Statement
All material was collected in accordance with the ethical standards and laws of the institution and country in which the studies were conducted.
Author Contributions
CO, JvZ, FN, and TW designed and contributed to all aspects of this study. CO, RO, and SM collected nests and performed bioassays. CO, FN, and TW designed the experiments. JM synthetized the methyl-branched hydrocarbon. CO, JvZ, and TW analyzed the results. CO, JvZ, JM, and TW wrote the manuscript.
Funding
We thank CNPq-Brazil for the scholarships to CO (201959/2012-7), RO (238127/2012-5) and a grant to TW and FN (402661/2012-5). Additional funding was provided by the Research Foundation Flanders to CO (postdoctoral fellowship FWO-12V6318N and international mobility grant V449117N), JvZ (postdoctoral fellowship FWO-12Q7615N), RO (postdoctoral fellowship 12R9619N and international mobility grant V406714N), and TW (FWO grant G.0A51.15, KU Leuven Center of Excellence grant PF/2010/007), and to CO (PDM/16/098 KU Leuven). Also, funding provided by Bilateral grant FWO-FAPESP to TW, CO, and FN; FWO: GOF8319N FAPESP: 2018/10996-0).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to D. A. Alves, A. Vollet-Neto, I. C. Tannure-Nascimento, J. P. Figueiredo, and M. Nanzer for assistance with experiments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00199/full#supplementary-material
References
Andrade-Silva, A. C. R., and Nascimento, F. S. (2015). Reproductive regulation in an orchid bee: social context, fertility and chemical signaling. Anim. Behav. 106, 43–49. doi: 10.1016/j.anbehav.2015.05.004
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Statist. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Bhadra, A., Mitra, A., Deshpande, S. A., Chandrasekhar, K., Naik, D. G., Hefetz, A., et al. (2010). Regulation of reproduction in the primitively eusocial wasp Ropalidia marginata: on the trail of the queen pheromone. J. Chem. Ecol. 36, 424–431. doi: 10.1007/s10886-010-9770-x
Bonavita-Cougourdan, A., Theraulaz, G., Bagnères, A. G., Roux, M., Pratte, M., Provost, E., et al. (1991). Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 100, 667–680. doi: 10.1016/0305-0491(91)90272-F
Boomsma, J. J., and Gawne, R. (2018). Superorganismality and caste differentiation as points of no return: how the major evolutionary transitions were lost in translation. Biol Rev. 93, 28–54. doi: 10.1111/brv.12330
Brent, C. S., and Vargo, E. L. (2003). Changes in juvenile hormone biosynthetic rate and whole body content in maturing virgin queens of Solenopsis invicta. J. Insect Physiol. 49, 967–974. doi: 10.1016/S0022-1910(03)00166-5
Butler, C. G., Callow, R. K., and Johnston, N. C. (1962). The isolation and synthesis of queen substance, 9-oxodec-trans-2-enoic acid, a honeybee pheromone. Proc. R. Soc. Lond. B Biol. Sci. 155, 417–432. doi: 10.1098/rspb.1962.0009
Cant, M., English, S., Reeve, H., and Field, J. (2006). Escalated conflict in a social hierarchy. Proc. R. Soc. B Biol. Sci. 273, 2977–2984. doi: 10.1098/rspb.2006.3669
Carlson, D. A., Bernier, U. R., and Sutton, B. D. (1998). Elution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 24, 1845–1865. doi: 10.1023/A:1022311701355
Carpenter, J. M. (1996). Distributional checklist of species of the genus Polistes (Hymenoptera: Vespidae: Polistinae: Polistini). Am. Museum Nat. Hist. Libr. 9196:39.
Dapporto, L., Dani, F., and Turillazzi, S. (2007b). Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr. Biol. 17, R504–R505. doi: 10.1016/j.cub.2007.05.002
Dapporto, L., Santini, A., Dani, F. R., and Turillazzi, S. (2007a). Workers of a Polistes paper wasp detect the presence of their queen by chemical cues. Chem. Senses 32:795. doi: 10.1093/chemse/bjm047
de Narbonne, M. M., van Zweden, J. S., Bello, J. E., Wenseleers, T., Millar, J. G., and d'Ettorre, P. (2016). Biological activity of the enantiomers of 3-methylhentriacontane, a queen pheromone of the ant Lasius niger. J. Exp. Biol. 219(Pt 11):1632–8. doi: 10.1242/jeb.136069
d'Ettorre, P., Heinze, J., Schulz, C., Francke, W., and Ayasse, M. (2004). Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 207, 1085–1091. doi: 10.1242/jeb.00865
Dietemann, V., Peeters, C., Liebig, J., Thivet, V., and Hölldobler, B. (2003). Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl. Acad. Sci. U.S.A. 100, 10341–10346. doi: 10.1073/pnas.1834281100
Downing, H., and Jeanne, R. (1985). Communication of status in the social wasp Polistes fuscatus (Hymenoptera: Vespidae). Zeitschrift für Tierpsychol. 67, 78–96. doi: 10.1111/j.1439-0310.1985.tb01380.x
Endler, A., Liebig, J., and Hölldobler, B. (2006). Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav. Ecol. Sociobiol. 59, 490–499. doi: 10.1007/s00265-005-0073-0
Espelie, K. E., and Hermann, H. R. (1990). Surface lipids of the social wasp Polistes annularis (L.) and its nest and nest pedicel. J. Chem. Ecol. 16, 1841–1852. doi: 10.1007/BF01020498
Field, J., and Leadbeater, E. (2016). Cooperation between non-relatives in a primitively eusocial paper wasp, Polistes dominula. Philos. Trans. R. Soc. Lond. Ser B Biol. Sci. 371:93. doi: 10.1098/rstb.2015.0093
Gadagkar, R. (2016). Evolution of social behaviour in the primitively eusocial wasp Ropalidia marginata: do we need to look beyond kin selection? Phil. Trans. R. Soc. B 371, 20150094. doi: 10.1098/rstb.2015.0094
Gadagkar, R., and Joshi, N. V. (1984). Social organisation in the Indian wasp Ropalidia cyathiformis (Fab.) (Hymenoptera: Vespidae). Zeitschrift für Tierpsychol. 64, 15–32. doi: 10.1111/j.1439-0310.1984.tb00350.x
Gamboa, G. J., and Dropkin, J. A. (1979). Comparisons of behaviors in early vs. late foundress associations of the paper wasp, Polistes metricus (Hymenoptera: Vespidae). Can. Entomol. 111, 919–926. doi: 10.4039/Ent111919-8
Gamboa, G. J., and Stump, K. A. (1996). The timing of conflict and cooperation among cofoundresses of the social wasp Polistes fuscatus (Hymenoptera: Vespidae). Can. J. Zool. 74, 70–74. doi: 10.1139/z96-009
Grüter, C., and Keller, L. (2016). Inter-caste communication in social insects. Curr. Opin. Neurobiol. 38, 6–11. doi: 10.1016/j.conb.2016.01.002
Hartfelder, K. (2000). Insect juvenile hormone: from “status quo” to high society. Braz. J. Med. Biol. Res. 33, 157–177. doi: 10.1590/S0100-879X2000000200003
Heinze, E. (2004). Reproductive conflict in insect societies. Adv. Study Behav. 34, 1–57. doi: 10.1016/S0065-3454(04)34001-5
Holman, L. (2010). Queen pheromones: the chemical crown governing insect social life. Commun. Integr. Biol. 3, 558–560. doi: 10.4161/cib.3.6.12976
Holman, L. (2012). Costs and constraints conspire to produce honest signalling: Insights from an ant queen pheromone. Evolution 66, 2094–2105. doi: 10.1111/j.1558-5646.2012.01603.x
Holman, L. (2018). Queen pheromones and reproductive division of labor: a meta-analysis. Behav. Ecol. 29, 1199–1209. doi: 10.1093/beheco/ary023
Holman, L., Dreier, S., and d'Ettorre, P. (2010a). Selfish strategies and honest signalling: reproductive conflicts in ant queen associations. Proc. R. Soc. Lond. B Biol. Sci. 277, 2007–2015. doi: 10.1098/rspb.2009.2311
Holman, L., Hanley, B., and Millar, J. G. (2016b). Highly specific responses to queen pheromone in three Lasius ant species. Behav. Ecol. Sociobiol. 70, 387–392. doi: 10.1007/s00265-016-2058-6
Holman, L., Jørgensen, C. G., Nielsen, J., and d'Ettorre, P. (2010b). Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. Lond. B Biol. Sci. 277, 3793–3800. doi: 10.1098/rspb.2010.0984
Holman, L., Lanfear, R., and d'Ettorre, P. (2013). The evolution of queen pheromones in the ant genus Lasius. J. Evol. Biol. 26, 1549–1558. doi: 10.1111/jeb.12162
Holman, L., van Zweden, J. S., Oliveira, R. C., Van Oystaeyen, A., and Wenseleers, T. (2016a). Conserved queen pheromones in bumblebees: a reply to Amsalem et al. PeerJ 4, e2003ve2001. doi: 10.7287/peerj.preprints.2003
Hoover, S. E. R., Keeling, C. I., Winston, M. L., and Slessor, K. N. (2003). The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90, 477–480. doi: 10.1007/s00114-003-0462-z
Jandt, J. M., Tibbetts, E. A., and Toth, A. L. (2014). Polistes paper wasps: a model genus for the study of social dominance hierarchies. Insectes Soc. 61, 11–27. doi: 10.1007/s00040-013-0328-0
Jandt, J. M., and Toth, A. L. (2015). Physiological and genomic mechanisms of social organization in wasps (Family: Vespidae). Adv. Insect Physiol. 48:95–130. doi: 10.1016/bs.aiip.2015.01.003
Kardile, S. P., and Gadagkar, R. (2002). Docile sitters and active fighters in paper wasps: a tale of two queens. Naturwissenschaften 89, 176–179. doi: 10.1007/s00114-002-0306-2
Kelstrup, H. C., Hartfelder, K., Nascimento, F. S., and Riddiford, L. M. (2014). The role of juvenile hormone in dominance behavior, reproduction and cuticular pheromone signaling in the caste-flexible epiponine wasp, Synoeca surinama. Front. Zool. 11:5. doi: 10.1186/s12983-014-0078-5
Kocher, S. D., and Grozinger, C. M. (2011). Cooperation, conflict, and the evolution of queen pheromones. J. Chem. Ecol. 37, 1263–1275. doi: 10.1007/s10886-011-0036-z
Layton, J. M., Camann, M. A., and Espelie, K. E. (1994). Cuticular lipid profiles of queens, workers, and males of social wasp Polistes metricus Say are colony specific. J. Chem. Ecol. 20, 2307–2321. doi: 10.1007/BF02033205
Le Conte, Y., and Hefetz, A. (2008). Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 53, 523–542. doi: 10.1146/annurev.ento.52.110405.091434
Leadbeater, E., Carruthers, J. M., Green, J. P., Rosser, N. S., and Field, J. (2011). Nest inheritance is the missing source of direct fitness in a primitively eusocial insect. Science 333, 874–876. doi: 10.1126/science.1205140
Liebig, J. (2010). “Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies,” in Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology, eds G. Blomquist and A. Bagnères (New York, NY: Cambridge University Press), 282–324.
Liebig, J., Monnin, T., and Turillazzi, S. (2005). Direct assessment of queen quality and lack of worker suppression in a paper wasp. Proc. R. Soc. Lond. B. Biol. Sci. 272, 1339–1344. doi: 10.1098/rspb.2005.3073
Liebig, J., Peeters, C., Oldham, N. J., Markstadter, C., and Hölldobler, B. (2000). Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl. Acad. Sci. U.S.A. 97, 4124–4131. doi: 10.1073/pnas.97.8.4124
Lorenzi, M. C., Azzani, L., and Bagnères, A.-G. (2017). Divergence in cuticular chemical signatures between isolated populations of an intraspecific social parasite. Front. Ecol. Evol. 5:8. doi: 10.3389/fevo.2017.00008
Messadi, D., Helaimia, F., Ali-Mokhnache, S., and Boumahraz, M. (1990). Accurate determination of retention indices in programmed temperature gas chromatography. Chromatographia 29, 429–434. doi: 10.1007/BF02261389
Mitra, A., and Gadagkar, R. (2011). Can Dufour's gland compounds honestly signal fertility in the primitively eusocial wasp Ropalidia marginata? Naturwissenschaften 98, 157–161. doi: 10.1007/s00114-010-0749-9
Mitra, A., Saha, P., Chaoulideer, M. E., Bhadra, A., and Gadagkar, R. (2011). Chemical communication in Ropalidia marginata: Dufour's gland contains queen signal that is perceived across colonies and does not contain colony signal. J. Insect Physiol. 57, 280–284. doi: 10.1016/j.jinsphys.2010.11.014
Monnin, T. (2006). Chemical recognition of reproductive status in social insects. Ann. Zool. Fenn. 43, 515–530.
Monnin, T., and Peeters, C. (1999). Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 10, 323–332. doi: 10.1093/beheco/10.3.323
Nascimento, F. S., Tannure-Nascimento, I. C., and Zucchi, R. (2004). Behavioral mediators of cyclical oligogyny in the Amazonian swarmfounding wasp Asteloeca ujhelyii (Vespidae, Polistinae, Epiponini). Insectes Soc. 51, 17–23. doi: 10.1007/s00040-003-0696-y
O'Donnell, S. (1998). Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu. Rev. Entomol. 43, 323–346. doi: 10.1146/annurev.ento.43.1.323
Oi, C. A., Millar, J. G., van Zweden, J. S., and Wenseleers, T. (2016). Conservation of queen pheromones across two species of vespine wasps. J. Chem. Ecol. 42, 1175–1180. doi: 10.1007/s10886-016-0777-9
Oi, C. A., van Zweden, J. S., Oliveira, R. C., Van Oystaeyen, A., Nascimento, F. S., and Wenseleers, T. (2015). The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. Bioessays 37, 808–821. doi: 10.1002/bies.201400180
Oliveira, R. C., Vollet-Neto, A., Oi, C. A., van Zweden, J. S., Nascimento, F., Sullivan Brent, C., et al. (2017). Hormonal pleiotropy helps maintain queen signal honesty in a highly eusocial wasp. Sci. Rep. 7:1654. doi: 10.1038/s41598-017-01794-1
Panek, L. M., Gamboa, G. J., and Espelie, K. E. (2001). The effect of a wasp's age on its cuticular hydrocarbon profile and its tolerance by nestmate and non-nestmate conspecifics (Polistes fuscatus, Hymenoptera: Vespidae). Ethology 107, 55–63. doi: 10.1046/j.1439-0310.2001.00633.x
Pardi, L. (1948). Dominance order in Polistes wasps. Physiol. Zool. 1948, 1–13. doi: 10.1086/physzool.21.1.30151976
Peeters, C., and Liebig, J. (2009). “Fertility signaling as a general mechanism of regulating reproductive division of labor in ants,” in Organization of Insect Societies: From Genome to Sociocomplexity, eds J. Gadau and J. Fewell (Cambridge, MA: Harvard University Press), 220–242.
R Development Core Team (2012). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ratnieks, F. L. W. (1988). Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 132, 217–236. doi: 10.1086/284846
Ratnieks, F. L. W., Foster, K. R., and Wenseleers, T. (2006). Conflict resolution in insect societies. Annu. Rev. Entomol. 51, 581–608. doi: 10.1146/annurev.ento.51.110104.151003
Reeve, H. (1991). “Polistes,” in The Social Biology of Wasps, eds K. Ross and R. Matthews (Ithaca, NY: Cornell University Press), 99–148.
Richard, F.-J., and Hunt, J. H. (2013). Intracolony chemical communication in social insects. Insectes Soc. 60, 275–291. doi: 10.1007/s00040-013-0306-6
Röseler, P. (1991). “Reproductive competition during colony establishment,” in The Social Biology of Wasps, eds K. Ross and R. Matthews (Ithaca, NY: Cornell University Press), 309–35. doi: 10.7591/9781501718670-012
Röseler, P.-F., Röseler, I., and Strambi, A. (1985). Role of ovaries and ecdysteroids in dominance hierarchy establishment among foundresses of the primitively social wasp, Polistes gallicus. Behav. Ecol. Sociobiol. 18, 9–13.
Röseler, P. F., and Röseler, I. (1989). Dominance of ovariectomized foundresses of the paper wasp, Polistes gallicus. Insectes Soc. 36, 219–234. doi: 10.1007/BF02226305
Ross, K. G., and Matthews, R. W. (1991). The Social Biology of Wasps. Ithaca, NY: Cornell University Press.
Saigo, T., and Tsuchida, K. (2004). Queen and worker policing in monogynous and monandrous colonies of a primitively eusocial wasp. Proc. R. Soc. Lond. B. Biol. Sci. 271, S509–S512. doi: 10.1098/rsbl.2004.0238
Sledge, M. F., Boscaro, F., and Turillazzi, S. (2001). Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav. Ecol. Sociobiol. 49, 401–409. doi: 10.1007/s002650000311
Sledge, M. F., Trinca, I., Massolo, A., Boscaro, F., and Turillazzi, S. (2004). Variation in cuticular hydrocarbon signatures, hormonal correlates and establishment of reproductive dominance in a polistine wasp. J. Insect Physiol. 50, 73–83. doi: 10.1016/j.jinsphys.2003.10.001
Slessor, K. N., Winston, M. L., and Le Conte, Y. (2005). Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 31, 2731–2745. doi: 10.1007/s10886-005-7623-9
Smith, A. A., and Liebig, J. (2017). The evolution of cuticular fertility signals in eusocial insects. Curr. Opin. Ins. Sci. 22, 79–84. doi: 10.1016/j.cois.2017.05.017
Smith, A. A., Millar, J. G., and Suarez, A. V. (2015). A social insect fertility signal is dependent on chemical context. Biol. Lett. 11:20140947. doi: 10.1098/rsbl.2014.0947
Smith, A. A., Millar, J. G., and Suarez, A. V. (2016). Comparative analysis of fertility signals and sex-specific cuticular chemical profiles of Odontomachus trap-jaw ants. J. Exp. Biol. 219, 419–430. doi: 10.1242/jeb.128850
Tannure-Nascimento, I. (2006). Sócio-etologia dos Agregados Coloniais de Polistes Satan (Bequaert, 1940): Fenologia, Mediadores Comportamentais e Sinalização química (Hymenoptera, Vespidae, Polistinae). Ribeirão Preto: Universidade de São Paulo.
Tannure-Nascimento, I. C., Nascimento, F. S., Turatti, I. C., Lopes, N. P., Trigo, J. R., and Zucchi, R. (2007). Colony membership is reflected by variations in cuticular hydrocarbon profile in a Neotropical paper wasp, Polistes satan (Hymenoptera, Vespidae). Gen. Mol. Res. 6, 390–396.
Tannure-Nascimento, I. C., Nascimento, F. S., and Zucchi, R. (2008). The look of royalty: visual and odour signals of reproductive status in a paper wasp. Proc. R. Soc. Lond. B Biol. Sci. 275:2555. doi: 10.1098/rspb.2008.0589
Tibbetts, E. A. (2006). Badges of status in worker and gyne Polistes dominulus wasps. Ann. Zool. Fenn. 43, 575–582.
Tibbetts, E. A., and Dale, J. (2004). A socially enforced signal of quality in a paper wasp. Nature 432, 218–222. doi: 10.1038/nature02949
Tibbetts, E. A., and Lindsay, R. (2008). Visual signals of status and rival assessment in Polistes dominulus paper wasps. Biol. Lett. 4, 237–239. doi: 10.1098/rsbl.2008.0048
Toth, A. L., Tooker, J. F., Radhakrishnan, S., Minard, R., Henshaw, M. T., and Grozinger, C. M. (2014). Shared genes related to aggression, rather than chemical communication, are associated with reproductive dominance in paper wasps (Polistes metricus). BMC Genomics 15:75. doi: 10.1186/1471-2164-15-75
Van Oystaeyen, A., Oliveira, R. C., Holman, L., Van Zweden, J. S., Romero, C., Oi, C. A., et al. (2014). Conserved class of queen pheromones stops social insect workers from reproducing. Science 287, 287–290. doi: 10.1126/science.1244899
van Zweden, J. S. (2010). The evolution of honest queen pheromones in insect societies. Commun. Integr. Biol. 3, 50–52. doi: 10.4161/cib.3.1.9655
van Zweden, J. S., Bonckaert, W., Wenseleers, T., and d'Ettorre, P. (2014). Queen signalling in social wasps. Evolution 68, 976–986. doi: 10.1111/evo.12314
Wenseleers, T., Hart, A., and Ratnieks, F. (2004). When resistance is useless: policing and the evolution of reproductive acquiescence in insect societies. Am. Nat. 164, E154–E167. doi: 10.1086/425223
Wenseleers, T., and Ratnieks, F. L. W. (2006a). Enforced altruism in insect societies. Nature 444:50. doi: 10.1038/444050a
Wenseleers, T., and Ratnieks, F. L. W. (2006b). Comparative analysis of worker reproduction and policing in eusocial Hymenoptera supports relatedness theory. Am. Nat. 168, E163–E179. doi: 10.1086/508619
Keywords: fertility cues, dominance behavior, queen pheromones, Polistes satan, aggressive behavior
Citation: Oi CA, Oliveira RC, van Zweden JS, Mateus S, Millar JG, Nascimento FS and Wenseleers T (2019) Do Primitively Eusocial Wasps Use Queen Pheromones to Regulate Reproduction? A Case Study of the Paper Wasp Polistes satan. Front. Ecol. Evol. 7:199. doi: 10.3389/fevo.2019.00199
Received: 26 March 2019; Accepted: 15 May 2019;
Published: 11 June 2019.
Edited by:
Qing-He Zhang, Sterling International, Inc., Spokane, WA, United StatesReviewed by:
Robert L. Jeanne, University of Wisconsin-Madison, United StatesJohannes Stökl, University of Giessen, Germany
Copyright © 2019 Oi, Oliveira, van Zweden, Mateus, Millar, Nascimento and Wenseleers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cintia A. Oi, Y2ludGlhYWtlbWkub2lAa3VsZXV2ZW4uYmU=
 Cintia A. Oi
Cintia A. Oi Ricardo C. Oliveira
Ricardo C. Oliveira Jelle S. van Zweden
Jelle S. van Zweden Sidnei Mateus2
Sidnei Mateus2 Jocelyn G. Millar
Jocelyn G. Millar Fabio S. Nascimento
Fabio S. Nascimento