
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Ecol. Evol. , 06 June 2019
Sec. Population, Community, and Ecosystem Dynamics
Volume 7 - 2019 | https://doi.org/10.3389/fevo.2019.00194
This article is part of the Research Topic Arthropod Interactions and Responses to Disturbance in a Changing World View all 20 articles
Habitat loss and degradation due to agricultural intensification and urbanization are key threats facing wild pollinators, especially bees. However, data on the distribution and abundance of most of the world's 20,000+ bee species is lacking, making it difficult to assess the effects of anthropogenic disturbance through time. Moreover, there are geographic biases in the study of bees creating gaps in our understanding of species distributions and regional patterns of diversity. Research efforts are often focused around cities or field stations associated with universities and other research institutions. In this perspectives paper, we provide examples of geographic bias in knowledge regarding bee species distributions using recently collected data from Michigan and Colorado, USA—two states with published species checklists. We illustrate how a limited sampling effort can advance knowledge about bee species distributions, yielding species occurrence records at local and regional scales. Given the implications of geographic biases, we recommend future research efforts focus on poorly sampled geographic regions, especially those affected by anthropogenic disturbance, in order to expand our understanding of human impacts on wild bee species. Sampling across a broader geographic area will provide critical information for taxonomy and predictive models of bee species distributions and diversity. We encourage researchers to plan future studies with consideration of strategies to avoid oversampling local bee populations, the taxonomic expertise required to identify specimens, and resources necessary to voucher specimens.
Pollinators play a key ecological role in terrestrial habitats, contributing to reproduction in more than 85% of flowering plants (Ollerton et al., 2011) and thereby supporting food webs worldwide. Furthermore, pollinators, in particular bees, benefit ~75% of the world's leading crops and contribute significantly to global food production (Klein et al., 2007; Potts et al., 2010). Unfortunately, in recent years, researchers have documented significant declines in the abundances of managed and wild bee species (Goulson et al., 2015). While a number of risk factors threaten bees, habitat loss and degradation due to agricultural intensification and urbanization are leading causes of bee decline (Winfree et al., 2009; Cariveau and Winfree, 2015; Goulson et al., 2015).
Species traits, such as dispersal ability, nesting habits, and diet breadth, influence how bees respond to anthropogenic disturbances (Cariveau and Winfree, 2015; Harrison and Winfree, 2015; Normandin et al., 2017, but see Bartomeus et al., 2018). For example, while cavity-nesting species are often positively influenced by urbanization (e.g., Cane et al., 2006; Bates et al., 2011; Fitch et al., 2019), ground-nesting species are typically more negatively affected (e.g., Kearns and Oliveras, 2009; Geslin et al., 2016). Moreover, some functional and taxonomic groups appear to be especially sensitive to environmental perturbations. Notably, recent studies have shown negative effects of urbanization and agricultural intensification on bumble bee abundance, population size, and geographic range size (e.g., Cameron et al., 2011; Kerr et al., 2015; Glaum et al., 2017; Hamblin et al., 2017; Jacobson et al., 2018). Bumble bee decline is especially concerning due to the ecological and economic importance of these pollinators. The availability of data on bumble bee species' relative abundances and historical distributions have been critical to the detection of their decline.
In recent years, researchers have increasingly recognized the value of pollination services provided by wild bee species in natural and agricultural systems (Losey and Vaughn, 2006; Slagle and Hendrix, 2009; Garibaldi et al., 2013; Klein et al., 2018; Winfree et al., 2018). A recent model of pollinator abundance on the landscape predicted worrisome mismatches between pollinator dependent crops and pollinator abundance (Koh et al., 2016). However, this study, based largely on expert opinion, also modeled large areas of uncertainty in bee abundance in the United States of America (USA). Thus, there is a need to understand how land-use change influences wild bees given continued increases in agricultural intensification and urbanization. The population status of most bee species is uncertain due to a lack of long-term or even baseline data (Bartomeus et al., 2013; Goulson et al., 2015). Relative to other parts of the world, the USA is a well-studied area with respect to pollinators (Archer et al., 2014). In this paper, however, we argue that even in the USA, there are geographic biases in bee research that limit our understanding of population dynamics, taxonomy, species distributions, and regional patterns of species diversity. We propose that limited sampling efforts, especially in understudied geographic regions, can contribute substantially to our knowledge of bees and their responses to anthropogenic disturbances.
Bee research is often centered in areas surrounding universities and research stations, which results in a lack of information in areas beyond these research centers (see e.g., Scott et al., 2011; Gibbs et al., 2017a). In the USA, numerous state checklists have demonstrated a paucity of species occurrence records across many counties and larger regions within these states where certain bee species may be expected to occur (e.g., Donovall and VanEnglesdorp, 2010; Jean, 2010; Scott et al., 2011; Dibble et al., 2017; Gibbs et al., 2017a). Furthermore, published species inventories are lacking for most states in the USA, and available checklists are usually from the eastern half of country (e.g., Michigan, Indiana, New York, Pennsylvania, and Maine). The species richness in these states, however, is substantially less than many states in the western USA (Scott et al., 2011; Carril et al., 2018). Large tracts of the USA, such as the High Plains and Great Plains are poorly sampled, even though they may have some of the greatest habitat loss across the country (Samson et al., 2004). With limited sampling effort, diverse bee communities can be found in both pristine (e.g., Grundel et al., 2011) and in anthropogenically disturbed habitats (e.g., Camilo et al., 2017). For example, a single day of collecting in Bellaire, Michigan added 50 new bee species records to Antrim County, bringing the total known species from 34 to 84 in 2016 (Gibbs unpublished data). Given the variability in bee species' response to anthropogenic change (Cariveau and Winfree, 2015), increased sampling in understudied regions affected by human disturbances is important for understanding which species are most likely at risk of decline (e.g., Bates et al., 2011; Banaszak-Cibicka and Zmihorski, 2012).
Species distribution and diversity models are important for documenting, evaluating, and predicting how bees respond to environmental changes, including climate change (e.g., Kerr et al., 2015) and land-use change (e.g., Bennett et al., 2014; Koh et al., 2016). Many regional models could be improved, however, by better sampling across a broader geographic range. Moreover, given that exotic bees may negatively affect native species (Russo, 2016), research evaluating the dispersal and population trends of exotic bees relative to native bees is needed. For example, the introduced bee, Anthidium manicatum, has rapidly spread across North American (Gibbs and Sheffield, 2009) and may competitively exclude native bees in some habitats (Miller et al., 2002, but see Soper and Beggs, 2013). Predictive models have been developed to estimate areas of high abundance for A. manicatum, with variable success (Strange et al., 2011; Graham and MacLean, 2018). Greater sampling across understudied regions could improve predictive habitat models for research examining bee community response to environmental change as well as exotic species spread.
To demonstrate the value of research in historically understudied areas, we summarize new species occurrence records from two recent studies examining the influence of anthropogenic disturbance on bee communities. Both studies occurred in states with published species checklists. We explore how new species records at the local (i.e., county) and regional (i.e., multi-county and statewide) level add to our knowledge of bee species distributions. Furthermore, we propose that research in other understudied areas could find similar or even greater numbers of new records. Finally, we conclude with recommendations for guiding future bee surveys and community studies.
Bee diversity is greater in the western half of the USA as compared with the eastern half, and vast areas of the West have been historically poorly sampled (e.g., Scott et al., 2011; see Carril et al., 2018). Colorado is currently the only state in the West with a recently published statewide checklist of bee species (Scott et al., 2011). Boulder County, home to the University of Colorado, is the best-documented county in the state, with 562 bee species recorded to date (Goldstein and Scott, 2015). Similarly, counties surrounding other universities and research institutions, including Larimer County, home to Colorado State University, have been extensively sampled—with 439 species recorded (Scott et al., 2011). In comparison, the bee community in eastern Colorado has been poorly inventoried relative to the central and western regions of the state where research has been historically focused (Scott et al., 2011; Figure 1A). In the Colorado Eastern Plains, 13 counties had fewer than 100 species recorded as of the 2011 statewide checklist, and most counties had substantially fewer species (mean = 39 ± 27 SD).
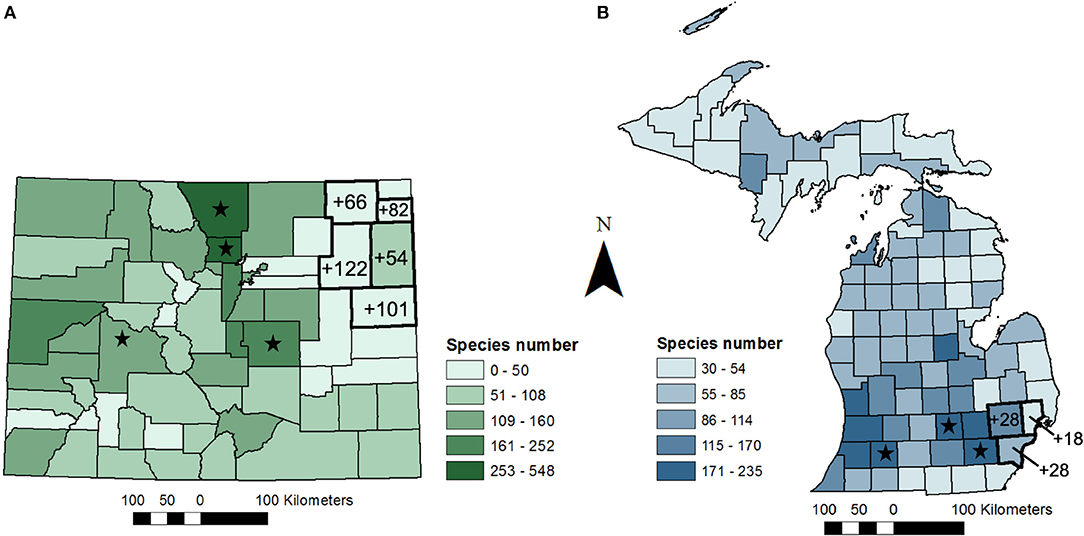
Figure 1. Bee species numbers by county for (A) Colorado (modified from Scott et al., 2011) and (B) Michigan (modified from Gibbs et al., 2017a). Values on maps show the number of new occurrence records for bee species by county from (A) a two-year study in Colorado (N = 32 sites across five counties) and (B) a one-year study (N = 15 sites across three counties). Shades of green and blue reflect species numbers from Scott et al. (2011) and Gibbs et al. (2017a), respectively. Stars highlight counties with universities and research stations where researchers have contributed a significant number of specimens to museum collections.
The first study we present to demonstrate the value of a limited sampling effort in understudied regions involved bee surveys at 32 sites located across five counties in northeastern Colorado (study region ≈ 25,000 km2). Our sites covered an area known as the Eastern or High Plains region, and included grassland habitats that were either enrolled in the U.S. Department of Agriculture Conservation Reserve Program or used for low-intensity grazing. The goal of this study was to provide baseline data on bee communities that could be used in the future to evaluate effects of projected land-use change due to increased bioenergy production in the region. In this study, bees were surveyed once per month for a 24-h period using passive and active sampling methods (bowl and vane traps alongside hand-netting) from June-September of 2013 and May-September of 2014.
From 9 days of collecting, this study added 425 new county-level species occurrence records, 97 occurrence records in the five county survey region, and 15 new state records for bees not previously known to occur in Colorado (Table 1A; Table S1; Figure 1A). We increased the number of bees known to occur in Washington county, a relatively large county (6,537 km2) in CO, from 5 to 127 species. One species newly recorded in Colorado, Cemolobus ipomoea, was found ~1,000 km west of its previously known western range limit in Missouri, according to publicly available georeferenced specimens [e.g., DiscoverLife.org, GBIF.org (GBIF, 2018; Carper et al., 2019)]. While not a new species for Colorado, a specimen of Centris ceasalpiniae that we collected from Washington county is only the 3rd record in the state and is now the most northern record for this species in North America. Similarly, specimens collected in this study expanded the known geographic range for at least a few Lasioglossum species. Based on this 2-year study, we conclude that the regional bee species diversity in eastern Colorado, and the broader High Plains Region more generally, is likely much greater than previously documented.
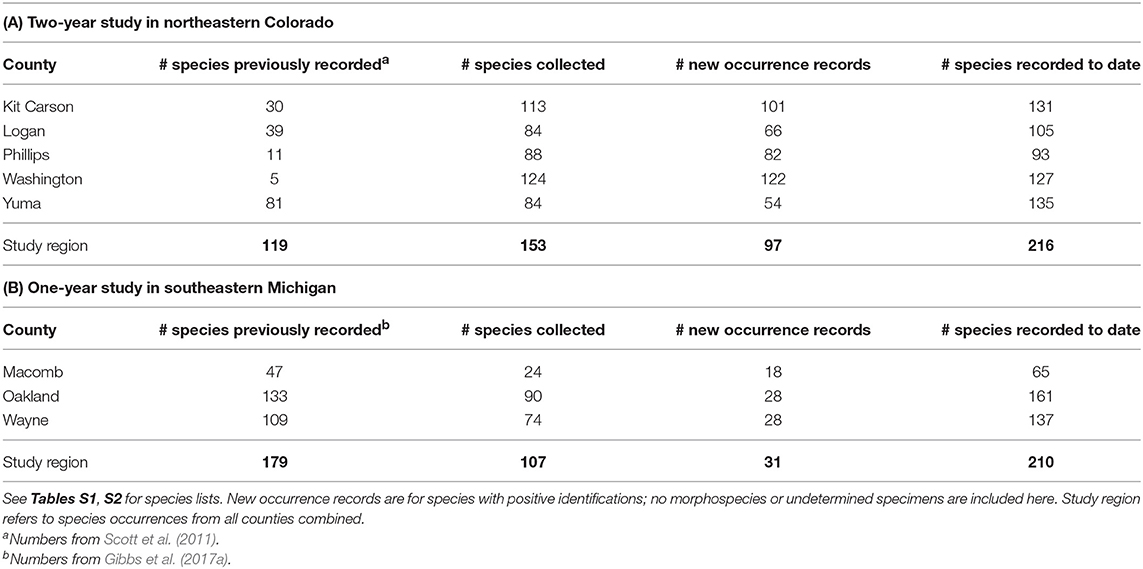
Table 1. New occurrence records for bee species by county and study region for surveys conducted in (A) northeastern Colorado and (B) southeastern Michigan.
This new survey of bees in northeastern Colorado added significantly to our knowledge of bee species that occur in that part of the state. The study added a number of new species occurrence records for the state of Colorado, including multiple species of Perdita, Melissodes, and Lasioglossum (Table S1). Furthermore, this project contributed to expanding the known geographic range limit for several species. It should be noted, however, that there are taxonomic impediments to surveys such as this, particularly in the western USA, as well as many countries throughout the world. Although this survey involved only 9 days of collecting, it took 3 years to get specimens processed and identified by multiple taxonomic specialists, and some specimens remain identified only at a morphospecies level. Other western USA bee surveys have faced similar issues (e.g., Carril et al., 2018). For certain diverse groups (e.g., Nomada, Sphecodes, and some Lasioglossum), taxonomic keys are lacking, making identifications impossible without taxonomic expertise. Even with taxonomic expertise, some groups are not fully resolved and new species are still being described. If we had identifications for all of our collected specimens, we anticipate that this study would contribute many more new species occurrence records for Colorado and some undescribed species. The need for additional series of specimens to support species descriptions underscores the importance of surveying in such undersampled regions.
While Michigan's bee fauna is relatively well-documented in comparison to some states in the eastern USA, many counties in the northern and eastern parts of the state remain poorly sampled (Gibbs et al., 2017a). Similar to Colorado, two of the most well-studied counties in Michigan (Ingham and Washtenaw Counties) are those that are home to the two major state universities (Figure 1B). Extrapolating from these two counties, most counties in southern Michigan only have one to two-thirds of their bee fauna documented (Gibbs et al., 2017a). Here, we present new county and state records from a recent study conducted across three counties (Wayne, Oakland, and Macomb) in southeastern Michigan to further illustrate how a limited sampling effort can contribute to enhancing knowledge of bee distributions (study region ≈ 5,600 km2). This 3-month study (June-August 2017) involved a once per month 48-h passive and active sampling (bowl traps and hand-netting) of bee communities at 15 farms and community gardens located across a tri-county region (Wilson and Jamieson unpublished data; Figure 1B). The goal of this study was to evaluate bee response to urbanization and floral resource availability in order to better inform urban agriculture practices. Historic records indicate that the three counties surveyed in our study had been undersampled.
Despite the proximity of the surveyed region to counties that are home to two major state universities, this study yielded 74 new county-level species records, with 31 new occurrence records to the overall study region (Table 1B; Figure 1B). Furthermore, this study added two new state-level species records—Chelostoma rapunculi and C. campanularum. Both of these species are cavity-nesting specialists on Campanula (Campanulaceae) that were accidentally introduced into North America (Käpyl, 1978; Eickwort, 1980). These species have previously been reported in central New York State, USA and southern Ontario, Canada (Eickwort, 1980; Buck et al., 2005). To our knowledge, these records are the farthest west these species have been recorded. Results from the study presented here suggest that surveys in these areas have the potential to expand information on bee species distributions and regional species richness.
Efforts to comprehensively document state and county checklists invariably fall short of their ultimate goal. Species distributions fluctuate through time and sampling protocols are always limited in extent and duration. Nonetheless, checklists can invigorate interest in a region and emphasize deficits in our understanding of species distributions. A decade ago, a checklist of Pennsylvanian bees identified 398 species for the state (Donovall and VanEnglesdorp, 2010). The fauna for the state is now known to be at least 450 species (Kilpatrick et al., 2019). Similarly, new records continue to emerge in well-documented states, such as Colorado and Michigan, because bees in some geographic regions of these states have been poorly sampled, as shown above. More recent collection efforts in Michigan continue to find new state and county records (T. Wood in litt.). By surveying bees in undersampled regions as identified in statewide or regional checklists, sampling efforts can be better directed to fill gaps in our knowledge of bee distribution and improve baseline data for future ecological and taxonomic studies.
Continued long-term monitoring efforts in well-studied areas is essential for evaluating changes in bee populations and communities over time. We propose that future research expanding into historically undersampled areas, in particular those undergoing environmental change, is also critical. For example, grasslands in the Great Plains of the USA have experienced significant land-use change due to agricultural intensification (Wright and Wimberly, 2013; Johnston, 2014). Pollination services provided by bees in this area are critical for ecosystems and agriculture in this region. Yet, we know little about bee communities throughout large areas of the Great Plains. When possible, research investigating land-use change effects on bees should include a range of disturbance levels or undisturbed sites for comparison. Likewise, surveys before and after disturbance are ideal for assessing environmental change effects.
In general, standardized protocols for bee sampling and specimen vouchering would help facilitate comparisons across studies. Nationwide surveys could enhance species inventorying and monitoring efforts to detect bee declines. Lebuhn et al. (2013) proposed that a standardized and nationwide survey network could be implemented across the USA to provide a better understanding of bee species trends over time. There are a number of challenges, however, with respect to such large-scale monitoring efforts, including logistical challenges for taxonomists and museums as well as issues with collection biases of pan traps that may not detect rare species or some species sensitive to disturbance (see further discussion in Lebuhn et al., 2015 and Tepedino et al., 2015). Tepedino et al. (2015) recommend that surveys should aim to evaluate how specific anthropogenic disturbances affect bee populations in select areas—in particular, areas undergoing rapid environmental change, such as the oil shale land in the western USA. Specimens collected for such studies could expand our knowledge of species distributions while answering ecological questions and addressing conservation solutions.
To inform conservation efforts, we need to understand how bees interact with their natural and modified habitats—in particular with respect to resource-use and ecological interactions.
In parallel with targeted ecological studies, there is an urgent need to sample more broadly to fill gaps in knowledge regarding species distributions as well as patterns of abundance and diversity over space and time. This type of survey work is more typically associated with taxonomic studies that focus on collecting the maximum number of species rather than repeated collections in a few sites with the goal of collecting species in their relative abundance. We hope examples above help demonstrate that much work remains in order to complete a basic inventory of bee species, let alone the arduous task of monitoring those species. Here we highlight a need for bee research in understudied areas and outline key concerns and considerations for future projects.
Firstly, in addition to considering the statistical power needed for addressing research questions, researchers should consider the effect of sampling large numbers of bees on local bee populations. Active netting and pan traps are unlikely to have strong effects on bee populations when used for targeted surveys (Gezon et al., 2015). However, the increased use of blue vane traps in regular surveys may be more problematic (Gibbs et al., 2017b). Blue vane traps are larger, more apparent, and may collect certain taxa, in particular larger apid bees, at levels that are potentially harmful on a local scale (Gibbs et al., 2017b). It would be foolhardy to limit collection efforts given the critical need to inventory and monitor bee populations; however, we suggest that blue vane traps may be best deployed for short periods (i.e., 24 h or less), especially in ecologically sensitive regions or seasons.
Secondly, bee species of some genera are notoriously difficult to accurately identify, and large monitoring projects could create a tremendous amount of work for a small number of bee taxonomists (Tepedino et al., 2015). As funding for bee research continues to increase worldwide, we hope to see greater funding and training opportunities for emerging bee taxonomists in addition to continued advances in DNA barcoding and molecular tools for species identifications (Packer et al., 2009; see Gonzalez et al., 2013; and Sheffield et al., 2017 for further discussion). DNA barcoding entire collections or representative samples as standard practice in ecological surveys could have two potential benefits: (1) reducing the pressure on taxonomic experts for routine identifications and (2) contributing data for taxonomists to further revise species concepts. It should be noted that DNA barcodes are not foolproof. Not all specimens will barcode successfully and DNA barcodes do not always discriminate closely related species (Meyer and Paulay, 2005; Meier et al., 2006; Gibbs, 2018), but neither does morphology (Packer et al., 2009). A helpful step that researchers can take prior to planning a community survey is to contact the appropriate taxonomists in advance of a project and write support for taxonomic expertise or DNA barcoding into grant applications. Since DNA barcoding databases may not be comprehensive or may require interpretation, taxonomic expertise is always recommended.
Finally, prior to planning a new study, researchers must consider where and how bee specimens will be vouchered. Large samples of bee specimens can require significant time, space, and resources to voucher in museums. Thus, researchers should discuss plans for vouchering specimens with collections managers. Museum specimens are the backbone of bee research—vital to the compilation of state, regional, and worldwide species lists as well as the development of taxonomic revisions, identification keys, and as reference material for specimen identification. Given that some bee taxa require revisions or present problems for accurate identification, vouchering of specimens in museum collections is imperative. Furthermore, museum specimens are good resources for documenting species declines over time (Colla et al., 2012b; Bartomeus et al., 2013, 2018; Burkle et al., 2013; Jacobson et al., 2018), or lack there of (Colla et al., 2012a), and are thus invaluable for directing conservation efforts. Colla and Packer (2008) used museum specimens to document the decline in Bombus affinis Cresson in southern Ontario over an ~30-year period. Burkle et al. (2013) also used historic data to evaluate changes in plant-pollinator networks in Illinois, USA. Resampling an area after 120 years, Burkle et al. (2013) found that 50% of bee species from the regional study were locally extirpated. They attributed this effect to land-use and climate change that resulted in spatial and temporal mismatches between forbs and bees, in particular rare and specialized bee species. Such longitudinal studies are critical for assessing changes in bee communities, and these studies benefit from surveys over broad geographic areas.
We conclude that current gaps in knowledge and research biases make it difficult to monitor changes in bee communities over time and space, which presents challenges in evaluating and modeling the effects of anthropogenic disturbances on bees. We contend, however, that targeted sampling efforts in understudied areas have the potential to substantially improve our knowledge of bee species distributions, range limits, and geographic patterns of species diversity. A better understanding of such patterns can aid in identifying species of concern, such as declining or introduced species, and help direct future conservation efforts.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
All authors contributed to writing and development of perspectives presented in this paper. MJ, AC, and CW designed experiments, implemented field research, and conducted data analyses. VS and JG contributed occurrence data and identifications for CO and MI bee species.
Research was supported by funding from the Foundation for Food and Agriculture (Award No. 430876) and the USDA National Institute for Food and Agriculture (Award No. 2013-67009-20375) provided to MJ in addition to an Oakland University Graduate Student Provost Award to CW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00194/full#supplementary-material
Archer, C. R., Pirk, C. W. W., Carvalheiro, L. G., and Nicolson, S. W. (2014). Economic and ecological implications of geographic bias in pollinator ecology in the light of pollinator declines. Oikos 123, 401–407. doi: 10.1111/j.1600-0706.2013.00949.x
Banaszak-Cibicka, W., and Zmihorski, M. (2012). Wild bees along an urban gradient: winners and losers. J. Insect Conserv. 16, 331–343. doi: 10.1007/s10841-011-9419-2
Bartomeus, I., Ascher, J. S., Gibbs, J., Danforth, B. N., Wagner, D. L., Hedtke, S. M., et al. (2013). Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. U.S.A. 110, 4656–4660. doi: 10.1073/pnas.1218503110
Bartomeus, I., Cariveau, D. P., Harrison, T., and Winfree, R. (2018). On the inconsistency of pollinator species traits for predicting either response to land-use change or functional contribution. Oikos 127, 306–315. doi: 10.1111/oik.04507
Bates, A. J., Sadler, J. P., Fairbrass, A. J., Falk, S. J., Hale, J. D., and Matthews, T. J. (2011). Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE 6:23459. doi: 10.1371/journal.pone.0023459
Bennett, A. B., Meehan, T. D., Gratton, C., and Isaacs, R. (2014). Modeling pollinator community response to contrasting bioenergy scenarios. PLoS One 9:e110676. doi: 10.1371/journal.pone.0110676
Buck, M., Paiero, S. M., and Marshall, S. A. (2005). New records of native and introduced aculeate hymenoptera from ontario, with keys to eastern canadian species of Cerceris (Crabronidae) and Eastern Nearctic Species of Chelostoma (Megachilidae). J. Entomol. Soc. Ontario. 136, 37–52.
Burkle, L. A., Marlin, J. C., and Knight, T. M. (2013). Plant-pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339, 1611–1615. doi: 10.1126/science.1232728
Cameron, S. A., Lozier, J. D., Strange, J. P., Koch, J. B., Cordes, N., Solter, L. F., et al. (2011). Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U.S A. 108, 662–667. doi: 10.1073/pnas.1014743108
Camilo, G. R., Muñiz, P. A., Arduser, M. S., Spevak, M., and Louis, S. (2017). A checklist of the bees (Hymenoptera: Apoidea) of St. Louis. J. Kansas Entomol. Soc. 90, 175–188. doi: 10.2317/0022-8567-90.3.175
Cane, J. H., Minckley, R. L., Kervin, L. J., Roulston, T. H., and Neal, M. (2006). Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 16, 632–644. doi: 10.1890/1051-0761(2006)016[0632:CRWADB]2.0.CO;2
Cariveau, D. P., and Winfree, R. (2015). Causes of variation in wild bee responses to anthropogenic drivers. Curr. Opin. Insect Sci. 10, 104–109. doi: 10.1016/j.cois.2015.05.004
Carper, A. L., Schwantes, C. J., and Jamieson, M. A. (2019). A new state record of the rare bee, Cemolobus ipomoeae (Hymenoptera, Apidae), from Colorado, USA. J. Kansas Entomol. Soc. 91, 171–175. doi: 10.2317/0022-8567-91.2.171
Carril, O. M., Griswold, T., Haefner, J., and Wilson, J. S. (2018). Wild bees of Grand Staircase-Escalante National Monument: richness, abundance, and spatio-temporal beta-diversity. PeerJ 6, e5867. doi: 10.7717/peerj.5867
Colla, S. R., Ascher, J. S., Arduser, M., Cane, J., Deyrup, M., Droege, S., et al. (2012a). Documenting persistence of most Eastern North American bee species (Hymenoptera: Apoidea: Anthophila) to 1990 – 2009. J. Kansas Entomol. Soc. 85, 14–22. doi: 10.2317/JKES110726.1
Colla, S. R., Gadallah, F., Richardson, L., Wagner, D., and Gall, L. (2012b). Assessing declines of North American bumble bees (Bombus spp.) using museum specimens. Biodivers. Conserv. 21, 3585–3595. doi: 10.1007/s10531-012-0383-2
Colla, S. R., and Packer, L. (2008). Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers. Conserv. 17, 1379–1391. doi: 10.1007/s10531-008-9340-5
Dibble, A. C., Drummond, F. A., Stubbs, C., Veit, M., and Ascher, J. S. (2017). Bees of maine, with a state species checklist. Northeast. Nat. 24, 1–48. doi: 10.1656/045.024.m1503
Donovall, L. R. I., and VanEnglesdorp, D. (2010). A checklist of the bees (Hymenoptera: Apoidea) of Pennsylvania. J. Kansas Entomol. Soc. 83, 7–24. doi: 10.2317/JKES808.29.1
Eickwort, G. C. (1980). Two european species of Chelostoma established in New York State (Hymenoptera: Megachilidae). Psyche 87, 315–323.
Fitch, G., Glaum, P., Simao, M. C, Vaidya, C., and Matthijs, J. (2019). Changes in adult sex ratio in wild bee communities are linked to urbanization. Sci. Rep. 9:3767. doi: 10.1038/s41598-019-39601-8
Garibaldi, L. A., Steffan-Dewenter, I., Winfree, R., Aizen, M. A., Bommarco, R., Cunningham, S. A., et al. (2013). Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. doi: 10.1126/science.1230200
GBIF (2018). GBIFO ccurrence Download, Cemolobus ipomoea. Available online at: https://doi.org/10.15468/dl.f9fcnx (accessed March 1, 2019).
Geslin, B., Le Feon, V., Folschweiller, M., Flacher, F., Carmignac, D., Motard, E., et al. (2016). The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol. Evol. 6, 6599–6615. doi: 10.1002/ece3.2374
Gezon, Z. J., Wyman, E. S., Ascher, J. S., Inouye, D. W., and Irwin, R. E. (2015). The effect of repeated, lethal sampling on wild bee abundance and diversity. Methods Ecol. Evol. 6, 1044–1054. doi: 10.1111/2041-210X.12375
Gibbs, J. (2018). DNA barcoding a nightmare taxon: assessing barcode index numbers and barcode gaps for sweat bees. Genome 61, 21–31. doi: 10.1139/gen-2017-0096
Gibbs, J., Ascher, J. S., Rightmyer, M. G., and Isaacs, R. (2017a). The bees of Michigan (Hymenoptera: Apoidea: Anthophila), with notes on distribution, taxonomy, pollination, and natural history. Zootaxa 4352, 1−160. doi: 10.11646/zootaxa.4352.1.1
Gibbs, J., Joshi, N. K., Wilson, J. K., Rothwell, N. L., Powers, K., Haas, M., et al. (2017b). Does passive sampling accurately reflect the bee (Apoidea: Anthophila) communities pollinating apple and sour cherry orchards? Environ. Entomol. 46, 579–588. doi: 10.1093/ee/nvx069
Gibbs, J., and Sheffield, C. S. (2009). Rapid range expansion of the wool-carder bee, Anthidium manicatum (Linnaeus) (Hymenoptera : Megachilidae), in North America. J. Kansas Entomol. Soc. 82, 21–29. doi: 10.2317/JKES805.27.1
Glaum, P., Simao, M. C., Vaidya, C., Fitch, G., and Iulinao, B. (2017). Big city Bombus : using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. R. Soc. Open Sci. 4:170156. doi: 10.1098/rsos.170156
Goldstein, P. Z., and Scott, V. L. (2015). Taxonomic and behavioral components of faunal comparisons over time: the bees (Hymenoptera: Anthophila) of boulder county, colorado, past and present. Proc. Entomol. Soc. Washingt. 117, 290–346. doi: 10.4289/0013-8797.117.3.290
Gonzalez, V. H., Griswold, T., and Engel, M. S. (2013). Obtaining a better taxonomic understanding of native bees: where do we start? Syst. Entomol. 38, 645–653. doi: 10.1111/syen.12029
Goulson, D., Nicholls, E., Botías, C., and Rotheray, E. L. (2015). Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957. doi: 10.1126/science.1255957
Graham, K. K., and MacLean, M. G. (2018). Presence-only modeling is ill-suited for a recent generalist invader, Anthidium manicatum. Ecol. Indic. 89, 56–62. doi: 10.1016/j.ecolind.2018.02.002
Grundel, R., Jean, R. P., Frohnapple, K. J., Gibbs, J., Glowacki, G. A., and Pavlovic, N. B. (2011). A survey of bees (Hymenoptera: Apoidea) of the indiana dunes and Northwest Indiana, USA. J. Kansas Entomol. Soc. 84, 105–138. doi: 10.2317/JKES101027.1
Hamblin, A. L., Youngsteadt, E., López-Uribe, M. M., and Frank, S. D. (2017). Physiological thermal limits predict differential responses of bees to urban heat-island effects. Biol. Lett. 13:20170125. doi: 10.1098/rsbl.2017.0125
Harrison, T., and Winfree, R. (2015). Urban drivers of plant-pollinator interactions. Funct. Ecol. 29, 879–888. doi: 10.1111/1365-2435.12486
Jacobson, M. M., Tucker, E. M., Mathiasson, M. E., and Rehan, S. M. (2018). Decline of bumble bees in northeastern North America, with special focus on Bombus terricola. Biol. Conserv. 217, 437–445. doi: 10.1016/j.biocon.2017.11.026
Jean, R. P. (2010). Studies of bee diversity in indiana the influence of collection methods on species capture and a state checklist based on museum collections. (Dissertation). Indiana State University, Terre Haute, IN, United States.
Johnston, C. A. (2014). Agricultural expansion: Land use shell game in the U.S. Northern Plains. Landsc. Ecol. 29, 81–95. doi: 10.1007/s10980-013-9947-0
Käpylä, M. (1978). Bionomics of five wood-nesting solitary species of bees (Hym., Megachilidae), with emphasis on flower relationships. Biol. Rev. Rep. Univ. 5, 3–89.
Kearns, C. A., and Oliveras, D. M. (2009). Environmental factors affecting bee diversity in urban and remote grassland plots in Boulder, Colorado. J. Insect. Conserv. 13, 655–665. doi: 10.1007/s10841-009-9215-4
Kerr, J. T., Pindar, A., Galpern, P., Packer, L., Potts, S. G., Roberts, S. M., et al. (2015). Climate change impacts on bumblebees converge across continents. Science 349, 177–180. doi: 10.1126/science.aaa7031
Kilpatrick, S. K., Gibbs J Mikulas, M. M., Spichiger, S., Ostiguy, N., Biddinger, D., et al. (2019). Checklist of the Bees of Pennsylvania. Available online at: https://lopezuribelab.com/checklist-bees-pennsylvania/ (accessed March 1, 2019).
Klein, A.-M., Vaissière, B. E., Cane, J. H., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Klein, A. M., Boreux, V., Fornoff, F., Mupepele, A. C., and Pufal, G. (2018). Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 26, 82–88. doi: 10.1016/j.cois.2018.02.011
Koh, I., Lonsdorf, E. V., Williams, N. M., Brittain, C., Isaacs, R., Gibbs, J., et al. (2016). Modeling the status, trends, and impacts of wild bee abundance in the United States. Proc. Natl. Acad. Sci. U. S. A. 113, 140–145. doi: 10.1073/pnas.1517685113
Lebuhn, G., Droege, S., Connor, E. F., Gemmill-Herren, B., Potts, S. G., Minckley, R. L., et al. (2013). Detecting insect pollinator declines on regional and global scales. Conserv. Biol. 27, 113–120. doi: 10.1111/j.1523-1739.2012.01962.x
Lebuhn, G., Droege, S., Connor, E. F., Gemmill-Herren, B., Potts, S. G., Minckley, R. L., et al. (2015). Evidence-based conservation: reply to Tepedino et al. Conserv. Biol. 29, 283–285. doi: 10.1111/cobi.12438
Losey, J. E., and Vaughn, M. (2006). The economic value of ecological services provided by insects. Bioscience 56, 311–323. doi: 10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2
Meier, R., Shiyang, K., Vaidya, G., and Ng, P. K. L. (2006). DNA barcoding and taxonomy in diptera: a tale of high intraspecific variability and low identification success. Syst. Biol. 55, 715–728. doi: 10.1080/10635150600969864
Meyer, C. P., and Paulay, G. (2005). DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 3, e422. doi: 10.1371/journal.pbio.0030422
Miller, S. R., Gaebel, R., Mitchell, R. J., and Arduser, M. (2002). Occurrence of two species of old world bees, Anthidium manicatum and A. oblongatum (Apoidea: Megachilidae), in northern Ohio and southern Michigan. Gt. Lakes Entomol. 35, 65–69.
Normandin, É., Vereecken, N. J., Buddle, C. M., and Fournier, V. (2017). Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 5:e3051. doi: 10.7717/peerj.3051
Ollerton, J., Winfree, R., and Tarrant, S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. doi: 10.1111/j.1600-0706.2010.18644.x
Packer, L., Gibbs, J., Sheffield, C., and Hanner, R. (2009). DNA barcoding and the mediocrity of morphology. Mol. Ecol. Resour. 9, 42–50. doi: 10.1111/j.1755-0998.2009.02631.x
Potts, S. G., Biesmeijer, J. C., Kremen, C., Neumann, P., Schweiger, O., and Kunin, W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Russo, L. (2016). Positive and negative impacts of non-native bee species around the world. Insects 7:E69. doi: 10.3390/insects7040069
Samson, F. B., Knopf, F. L., and Ostlie, W. R. (2004). Great Plains ecosystems: past, present, and future. Wildl. Soc. Bull. 32, 6–15. doi: 10.2193/0091-7648(2004)32[6:GPEPPA]2.0.CO;2
Scott, V. L., Ascher, J. S., Griswold, T. L., and Nufio, C. R. (2011). The bees of colorado. Nat. Hist. Invent. Color. 23, vi–100. doi: 10.1016/S1472-6483(10)60037-5
Sheffield, C. S., Heron, J., Gibbs, J., Onuferko, T. M., Oram, R., Best, L., et al. (2017). Contribution of DNA barcoding to the study of the bees (Hymenoptera: Apoidea) of Canada: progress to date. Can. Entomol. 149, 736–754. doi: 10.4039/tce.2017.49
Slagle, M. W., and Hendrix, S. D. (2009). Reproduction of Amorpha canescens (Fabaceae) and diversity of its bee community in a fragmented landscape. Oecologia 161, 813–823. doi: 10.1007/s00442-009-1429-3
Soper, J., and Beggs, J. R. (2013). Assessing the impact of an introduced bee, Anthidium manicatum, on pollinator communities in New Zealand. New Zeal. J. Bot. 51, 213–228. doi: 10.1080/0028825X.2013.793202
Strange, J. P., Koch, J. B., Gonzalez, V. H., Nemelka, L., and Griswold, T. (2011). Global invasion by Anthidium manicatum (Linnaeus) (Hymenoptera: Megachilidae): Assessing potential distribution in North America and beyond. Biol. Invasions 13, 2115–2133. doi: 10.1007/s10530-011-0030-y
Tepedino, V. J., Durham, S., Cameron, S. A., and Goodell, K. (2015). Documenting bee decline or squandering scarce resources. Conserv. Biol. 29, 280–282. doi: 10.1111/cobi.12439
Winfree, R., Aguilar, R., Vásquez, D. P., LeBuhn, G., and Aizen, M. A. (2009). A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 93, 2068–2076.
Winfree, R., Reilly, J. R., Bartomeus, I., Cariveau, D. P., Williams, N. M., and Gibbs, J. (2018). Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science 359, 791–793. doi: 10.1126/science.aao2117
Keywords: bee decline, land-use change, pollinator conservation, global change, bee communities
Citation: Jamieson MA, Carper AL, Wilson CJ, Scott VL and Gibbs J (2019) Geographic Biases in Bee Research Limits Understanding of Species Distribution and Response to Anthropogenic Disturbance. Front. Ecol. Evol. 7:194. doi: 10.3389/fevo.2019.00194
Received: 07 March 2019; Accepted: 13 May 2019;
Published: 06 June 2019.
Edited by:
Shannon Murphy, University of Denver, United StatesReviewed by:
Ruben Martin-Blazquez, University of Illinois at Urbana-Champaign, United StatesCopyright © 2019 Jamieson, Carper, Wilson, Scott and Gibbs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mary A. Jamieson, bWphbWllc29uQG9ha2xhbmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.