- 1Biodiversity Research Centre, Earth and Life Institute, Université Catholique de Louvain, Louvain-la-Neuve, Belgium
- 2Centre National de la Recherche Scientifique, UMR 5321, Station d'Ecologie Théorique et Expérimentale, Université Paul Sabatier Toulouse III, Toulouse, France
- 3Muséum National d'Histoire Naturelle, CNRS, Institut de Systématique, Evolution, Biodiversité, Sorbonne Université, Paris, France
- 4School of Life Sciences and Education, Staffordshire University, Stoke-on-Trent, United Kingdom
- 5Department of Biological and Medical Sciences, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, United Kingdom
A functional definition of the habitat-concept based on ecological resources incorporates three interconnected parameters: composition, configuration, and availability of the resources. The intersection of those parameters represents the functional habitat of a given population or species. Resource composition refers to the co-occurrence of the resources required by each individual to complete its life cycle. Resource configuration refers both to the way individual resources are spatially distributed within the habitat and the way all the resources are organized in the habitat space. Resource availability refers to the accessibility and procureability of resources. Variation in these variables is predicted to influence the demography of the population. To test the suitability of this definition and its transferability across landscapes, we first conducted a very detailed study on habitat and resource use of five butterfly species within a large nature reserve. Second, we conducted a larger-scale study, focusing on metapopulations of two species. We monitored demography for each species and tested whether its variation can be explained by (1) the vegetation type, (2) the vegetation composition or (3) the availability and configuration of the species-specific ecological resources. To confirm that resource availability and configuration reflect habitat quality, we also assessed their impacts on individual morphology. Whatever the investigated spatial scale, our results quantitatively demonstrate the overall better performance of the resource-based habitat approach compared to other most commonly used approaches. Our analysis allowed us to assess the relative importance of each ecological resource in terms of both their availability and organization relative to the species' abundance, demography and individual fitness measures. Resource availability did not play the predominant role in defining habitat quality as it was in most cases overruled by resource organization. Finally, we confirmed the between-population transferability of the habitat definition and quality estimates while adopting a resource-based habitat approach. Our study clearly demonstrates the suitability of the resource-based definition of the habitat. Therefore, we argue that this approach should be favored for species of conservation concern. Although most conclusions so far have emerged from butterfly studies, the resource-based definition of the habitat should also be ecologically relevant to many other organisms.
Introduction
Since the early days of ecology, the habitat has been considered one of the central concepts for the study of the interactions between organisms and their environment, but its unequivocal definition has been debated ever since (e.g., Yapp, 1922; Haskel, 1940; Mitchell, 2005). Much of the debate relates to the question whether the habitat should be defined independent of a particular organism (i.e., vegetation type or biotope), or alternatively, whether the nature of the organism is essential to conceptualize its habitat as a meaningful subsample of the environment. In other words, should we place the emphasis on the habitat in a structural, top-down manner, or rather in a functional, species-specific bottom-up manner? Theoretically, both approaches may generate particular interest depending on the questions being addressed. However, from a species conservation viewpoint, a functional habitat approach that explicitly takes into account the ecological needs and tolerances of the focal organism has been proposed the most adequate method for taking into account ecological relationships between organism and environment (Dennis and Sparks, 2006; Dennis et al., 2006).
Such a functional, species-specific approach of the habitat concept relates to the ecological niche concept (Grinnel, 1917; Elton, 1927; Hutchinson, 1957). The ecological niche is the intersection of the ranges of tolerance for different ecological resources and conditions used or experienced by a species. It is usually conceived as a multidimensional functional space or hypervolume (Hutchinson, 1957). Dennis et al. (2003) proposed a functional, resource-based habitat approach that responds to the real ground spatial projection of the Hutchinson's niche concept. The conservation interest of such an approach has been further developed since (e.g., Dennis et al., 2006; Dennis, 2010) and several species-specific studies adopted and further explored this resource-based habitat approach in a species-conservation context (e.g., Vanreusel and Van Dyck, 2007; Salz and Fartmann, 2009; Turlure et al., 2010b). For a review and detailed discussion, we refer to Dennis et al. (2014). The resource-based habitat approach also connects to the recent developments in the field of sensory ecology as it reflects an organism-centered perception of the environment (i.e., the Umwelt-concept: Van Dyck, 2012). In the meantime, the approach has also stimulated the way habitat is perceived outside the field of conservation biology (e.g., vector-borne disease modeling: Hartemink et al., 2015).
In this article, we propose to model this resource-based habitat approach around three key components that are interconnected: (1) the resource composition referring to the list of ecological resources required by the focal species, (2) the resource configuration related to the concept of resources complementation and supplementation, referring to the distribution of each ecological resource (i.e., resource grain) and their spatial organization (i.e., overlapping degree of all the resources), and (3) the resource availability referring to its variation in quantity and quality. The intersection of these three components represents the functional habitat of the focal species (population) within a particular landscape setting (Figure 1). Although resource composition is implicitly included in the resource availability and configuration, it is still the first required step to recognize a species habitat based on resources. Variation in each of these components is predicted to affect individual behavior and, ultimately, fitness. Therefore, they should in turn relate to the demographic response of the local population and to the evolutionary strategies of resource exploitation and habitat selection. Our previous work offered support to this relationship as the habitat area delineated when adopting a resource-based method provided a reliable proxy for population size in two butterfly species of conservation concern (Turlure et al., 2010b).
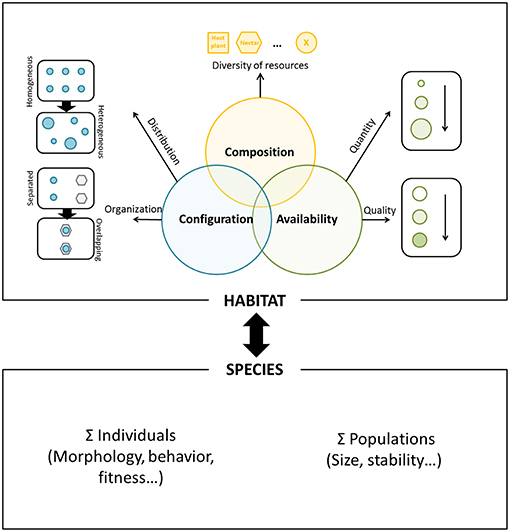
Figure 1. Schematic representation of the resource-based habitat concept. Resource composition (upper part of the figure) refers to the diversity of ecological resources used by the focal species, such as at least host plant and nectar resources for a butterfly species. Resource configuration (left part) refers to the distribution of each ecological resource (i.e., resource grain, from a homogeneous to a heterogeneous distribution of a single resource) and spatial organization (i.e., from fully separated to fully overlapping resources). Resource availability (right part) refers to variation in resource quantity and quality. The intersection of these three components represents the functional habitat of the focal species. Variation in each of these components is likely to affect individual behavior and fitness, in turn affecting both the demographic response of the local population and the underlying evolutionary strategies of resource exploitation and habitat selection.
Here we compared in a quantitative manner the performance of the resource-based habitat approach with the more commonly used vegetation type-based habitat approach for a community of five co-existing butterfly species. The latter and classical approach adopts a habitat definition based on vegetation type, vegetation composition and the presence or abundance of the host plant. The resource-based approach defines the habitat on a series of ecological resources (i.e., consumables and utilities or conditions) specific to the different life stages of the species. All of the study species are food specialists at the larval stage (i.e., each makes use of a single host plant species), but their degree of ecological specialism at the adult stage (including nectar specialism) varies considerably. Hence, this allows us to test whether the relevance of the approach increases with the degree of ecological specialism of the considered species. We predict that overall, the resource-based definition will be more suitable to define habitat and consequently estimate habitat quality than the other definitions, and this will be especially true for more specialized species and for juvenile stages compared to imagoes.
We also assessed the relative importance of each ecological resource in terms of both the availability and configuration relative to the species' demography (i.e., minimum and maximum census population sizes) and individual size as a proxy for individual fitness, for two Boloria species. Contrary to the widespread and common assumption, we predict that host plant abundance does not necessarily play the predominant role in defining the quality of the butterfly's habitat, although the presence of a certain level of host plant abundance is obviously required. This may be particularly true for glacial relict species and species with low mobility for which microclimate and spatial resource configuration are key elements expected to overrule the role of host plant abundance (Turlure et al., 2009, 2010a, 2014).
Finally, we assessed the between-population transferability of the habitat definition and quality estimates adopting a resource-based habitat approach for one species (Boloria aquilonaris) only. In other words, we tested whether we can predict the carrying capacity and then the population size in a given location with reasonable confidence based on the assessment of local resource-based habitat variables. This is a major challenge for the application of the concept. If we can show evidence for such a transferability, it will provide a strong argument in favor of detailed autecological studies on focal species in a limited number of relevant sites in order to identify key resources that affect habitat quality and hence the species' demography over a much wider range of areas or even landscapes.
Methods
Study Species
We studied five butterfly species inhabiting wet meadows and/or peat bogs: Lycaena helle, Lycaena hippothoe, Boloria eunomia, Boloria selene, and B. aquilonaris. In Belgium and in most parts of their European distributional range, those species are specialized on a unique host plant at the caterpillar stage (Bink, 1992; Lafranchis, 2000, 2004). Interestingly, the species form a gradient from highly specialized (B. eunomia) to more generalist species (B. selene), according to (1) the use at the adult stage of a single or multiple nectar resources (as recorded from behavioral observations in different European populations; Turlure et al., 2010c and Turlure and Dubois, personal observations) and (2) their need for other resources (such as microhabitat structures for caterpillars, Turlure et al., 2010b; or tree edges for the adults) We previously demonstrated: (1) that grass tussocks are used by B. eunomia caterpillars to find suitable temperature conditions for thermoregulation and to avoid flooding (Turlure et al., 2009, 2011), (2) that Sphagnum hummocks are used by B. aquilonaris caterpillars to avoid extreme temperature conditions, as the inner part of the hummocks is buffered around 14°C (Turlure et al., 2010a, 2011) and (3) that tree edges are used by L. helle adults as shelter against convective cooling by wind, and males use them also to locate potential mates (Turlure et al., 2014). As such, those five species differ in the resources they need (i.e., habitat composition) and likely in the way the habitat can be defined and its quality assessed. Although host plant use at the caterpillar stage is well documented for most European butterfly species, information on other resources is currently much more scarce; we therefore compiled information we collected from our own field observations (in the present study system and elsewhere), as well as information collected through interactions with practitioners and other researchers across Europe. The resources known to be used by the five species are summarized in Table 1.
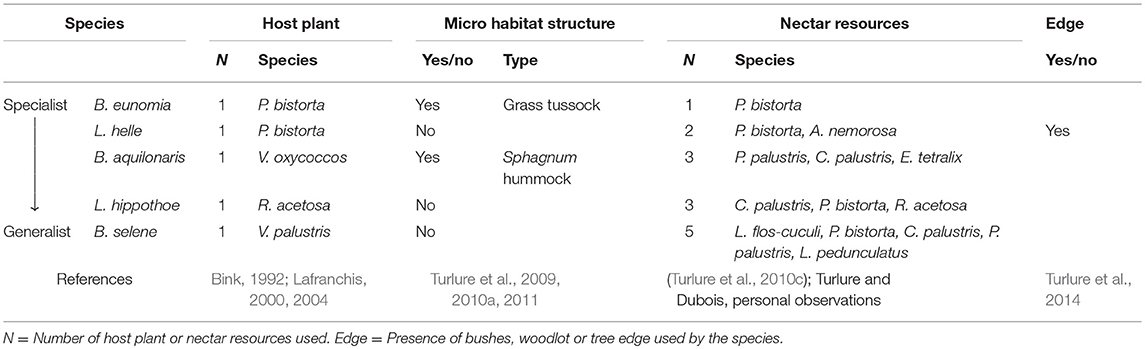
Table 1. List of the resources used by the five study species along the specialist-generalist gradient.
Study Systems
All study sites are located in south-eastern Belgium, in the Belgian Ardenne (Figure 2). Our study took place at three spatial levels. First, we conducted a very detailed, local scale study on the five species within the Fange de Pisserotte nature reserve (50°13′N, 5°47′E, 56 ha). The site has been characterized as a mosaic of 40 zones (17 ha), each being a homogeneous area of specific peat bog vegetation as previously defined in Turlure et al. (2009). Second, we conducted a study at the metapopulation scale on two of the species: B. eunomia (10 populations, 2009–2014, later referred as to B. eunomia metapopulation) and B. aquilonaris (15 populations, 2009–2015, later referred as to B. aquilonaris metapopulation). Finally, we studied 13 extra B. aquilonaris populations spread across southeast Belgium (2013–2014).
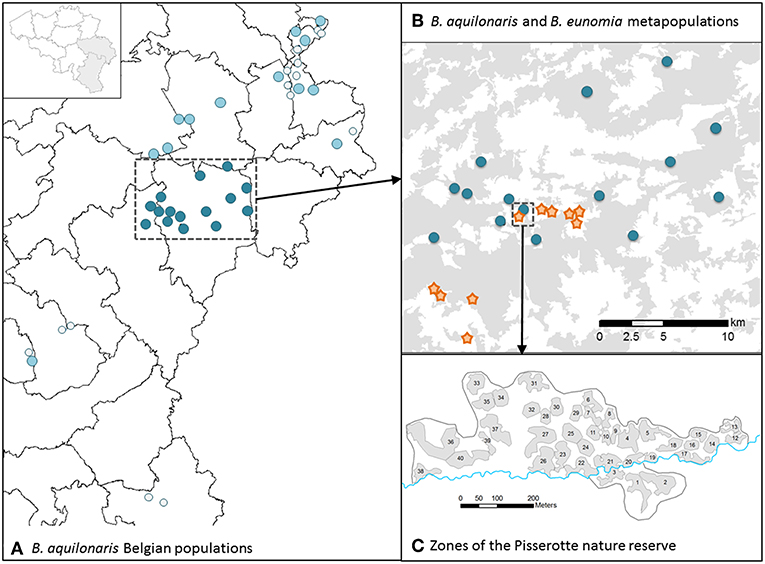
Figure 2. Maps of the study sites. (A) Location of all Belgian B. aquilonaris populations in the Ardenne region (SE-Belgium). Dark blue dots: studied populations in the plateau des Tailles area. Light blue dots: other studied populations in Belgium. Light gray dots: other known populations. (B) Location of the studied populations for the B. aquilonaris (dark blue dots) and B. eunomia (orange stars) metapopulations in the plateau des Tailles area. (C) Location of the 40 zones (gray areas) in the Pisserotte nature reserve, where the five species were studied.
Vegetation and Resource Characterization
Habitat quality was computed using three habitat definitions: habitat (1) based on vegetation type, (2) based on vegetation composition or (3) based on species-specific ecological resources. This was done for each zone of the Pisserotte reserve and for each other site hosting B. eunomia and B. aquilonaris populations. This was done as follows.
(1) The first habitat definition is based on vegetation type. The vegetation type (Supplementary Figure 1A) was assigned to each zone and site using the defined vegetation associations occurring in this area, namely wet meadows (two types: the strict Deschampsia-Bistort association and other wet meadows), bogs (including floating mats and humid bogs, drier bogs with heathland plant species and raised bogs), and (degraded) heathlands. In the Pisserotte reserve, the 40 preselected zones were classified as wet meadows (12 zones), bogs (19 zones), and (degraded) heathlands (9 zones). The 10 sites forming the B. eunomia metapopulation were classified as the strict Deschampsia-Bistort association (commonly defined as the preferred vegetation type for the species, 5 populations) and other wet meadows where the host plant occurs but believed to be less suitable for the species (5 populations). The 15 sites forming the B. aquilonaris metapopulation were classified as floating mats and humid bogs (6 populations), drier bogs with heathland plant species (7 populations), and raised bogs (2 populations).
(2) The second habitat definition is based on vegetation composition. The vegetation composition (Supplementary Figure 1B) was characterized based on randomly placed 1 m2 vegetation samples in which we recorded the abundance of each plant species. Each 1 m2 sample was divided in a 5*5 grid, forming 25 squares (each 20*20 cm) to estimate the abundance of each plant species on the basis of their presence in each square, i.e., on a zero to 25 scale (Supplementary Figure 2A). In total, we recorded 10 vegetation samples in each zone of the Pisserotte reserve (totaling 400 vegetation samples, as described in Turlure et al., 2009), and a number adjusted to the area and the heterogeneity for each other site (10 to 53 for B. eunomia populations with a total of 247 vegetation samples, Supplementary Figure 2B; 7 to 80 for B. aquilonaris populations with a total of 759 vegetation samples, Supplementary Figure 2C). Three Principal Component Analyses (one for the Pisserotte reserve, one for B. eunomia sites and one for B. aquilonaris sites) were then used to summarize plant species abundances and define the vegetation composition based on the first two PCA axes: PIS-1 and PIS-2 for the Pisserotte reserve (Supplementary Figure 2D), BEU-1 and BEU-2 for the B. eunomia metapopulation (Supplementary Figure 2E), and BAQ-1 and BAQ-2 for the B. aquilonaris metapopulation (Supplementary Figure 2F).
For the Pisserotte reserve, PIS-1 separated three main vegetation types, with lowest values for bog vegetation dominated by common heather Calluna vulgaris; then wet bogs and floating mats, and higher values for marshes and meadow-like vegetation. Small cranberry V. oxycoccos abundance increased with lower values of PIS-1, while marsh violet V. palustris, bistorta P. bistorta and sorrel R. acetosa abundances increased with higher values of PIS-1. PIS-2 separated the Deschampsia-Bistort association (lower values) from the flower-rich areas (higher values).
For the B. eunomia metapopulation, BEU-1 corresponded to a gradient of eutrophication, and separated Deschampsia-Bistort association (lower values) from rushes and meadows (higher values). BEU-2 represented a gradient of degradation (i.e., increasing abundance of moor grass Molinia with increasing values of BEU-2).
For the B. aquilonaris metapopulation, BAQ-1 separated strictly bog species (lower values) from wet meadow species (higher values). BAQ-2 represents a gradient of humidity. Nectar plant species were more abundant for higher values of both BAQ-1 and BAQ-2.
(3) The third habitat definition is based on species-specific ecological resources, extracted from the vegetation samples described above. We specifically extracted information on both juvenile and adult resources (i.e., the host and nectar plants). Additionally, we counted the number of grass tussocks, Sphagnum hummocks and P. bistorta flowers (only for B. eunomia populations) in each vegetation sample. Table 1 lists the resources used for each species. Resource quantity considered the abundance (averaged across zone/site vegetation samples) of (1) the host plant, (2) the important elements of microhabitat structure (i.e., Sphagnum hummocks for B. aquilonaris, grass tussocks for B. eunomia), and (3) nectar plant species. Also, for the Pisserotte zones, we assessed (4) the percentage of perimeter (edge) surrounded by trees (on a three value scale: 0 for no tree edge, 0.5 for partial tree surrounding and 1 for total tree surrounding). Resource configuration was estimated by two variables; resource distribution and resource organization. Resource distribution was assessed using a classical niche breadth measure (Edwards et al., 1998), computed according to Ricklefs' equation:
where Pi is the proportion of vegetation samples that contained the ith abundance level of the considered resource (in this case either host plant or nectar). B ranges from 1 (i.e., homogeneous distribution: all samples contain the same resource abundance) to the number of abundance levels (i.e., heterogeneous distribution: samples are equally frequent for all abundance levels). For the assessment of the juvenile resource distribution in B. eunomia and B. aquilonaris, we considered the abundance of the host plant only when grass tussocks or Sphagnum hummocks were present, as previously we have shown the importance of those two resources for the functional habitat of B. eunomia and B. aquilonaris, respectively (Turlure et al., 2009, 2010a). Resource organization was quantified by the percentage of overlap (spatial dimensions) shared by juvenile and adult resources, estimated using Schoener's index of niche overlap:
where Pil and Pia represented the proportion in the ith vegetation sample of either juvenile or adult resources (Linton et al., 1981). Some resource variables were highly correlated in a number of the datasets. To prevent multicollinearity issues, we excluded the following descriptors: (1) juvenile resource distribution (correlated with host abundance) and adult resource distribution (correlated with nectar abundance) in Pisserotte; (2) nectar abundance (correlated with nectar distribution) and micro-habitat structure abundance (correlated with resource organization) in the B. eunomia metapopulation; and (3) nectar abundance (correlated with nectar distribution and resource organization) in the B. aquilonaris metapopulation.
We also assessed the area of each study zone/site. In the Pisserotte reserve, area was computed as the total surface of each zone. In the B. eunomia and B. aquilonaris metapopulations, area was computed as the functional area (i.e., the area containing the resources for the species) to avoid including non-habitat area. A connectivity index was computed using all 53 known Belgian populations of B. aquilonaris as , where α is a constant setting the survival rate of dispersers over distance Dij, which is the distance between populations i and j, and Aj is the area of the population j (Hanski, 1999); here we used α = 2, as often used for butterflies (Hanski, 1994) when this formula is used to compute relative connectivity metrics of a series of sites. We did not compute such a connectivity index for the B. eunomia metapopulation as we do not have an exhaustive record of the populations in the considered area, and missing populations could strongly bias any connectivity measure.
Butterfly Abundance and Population Size
We monitored butterfly populations using a Mark–Release–Recapture (MRR) approach and estimated adult abundance in each Pisserotte zone and the population size in each site separately. The study zones and sites were visited every 2 days if weather permitted (i.e., no strong wind, few clouds and air temperature > 15°C). Adult butterflies were individually marked and released on the spot of capture. At each (re)capture, we recorded the marking code, species, sex, date and time, and location (i.e., one of the 40 predefined vegetation zones in Pisserotte or the site).
For the five species in Pisserotte, the classical approach to estimate population size (see below) was not suitable because demographic parameters are estimated at the whole population level; the frequent movements among zones within Pisserotte did not allow considering each zone as an independent population whose size can be estimated independently. We therefore estimated the local abundance per species and sex in each of the 40 Pisserotte zones as the number of (re)capture events (pooled for the 2 years of available MRR data). Frequency of (re)captures was then used as a proxy of the local butterfly abundance assuming the probability of capture was similar among the zones, which is a reasonable assumption (Schtickzelle and Baguette, 2004). The total abundance of all local abundances (captures and recaptures) in the Pisserotte site was 2376 B. eunomia, 917 L. helle, 254 B. aquilonaris, 186 L. hippothoe and 1038 B. selene.
For several populations of the B. eunomia (10 populations sampled in 2009–2014) and B. aquilonaris (15 populations sampled in 2009–2015) metapopulations, as well as for the additional B. aquilonaris populations (13 populations sampled in 2013–2014), MRR data were analyzed to estimate demographic parameters (i.e., survival and recapture rates, total population size, for each species, sex and year separately) using constrained linear models implemented in Mark software (White and Burnham, 1999) following the procedure described in Schtickzelle et al. (2002). In cases where the low number of captures prevented us from adopting such a modeling approach, we computed the population size using a conversion function from the number of marked individuals based on the strong relationships existing in the two species between the number of marked individuals and population size in the two species (Turlure et al., 2018). The mean yearly metapopulation size was estimated to 2212 B. eunomia individuals and 7322 B. aquilonaris individuals, with 2533 B. aquilonaris individuals in the additional populations.
In the Pisserotte reserve, we also recorded the abundance of juvenile stages (caterpillars for the three Boloria species and eggs for the two Lycaena species). We surveyed Boloria caterpillars prior to the flying period of 2005 and 2006 in all the zones where the specific host plants were present (Total searching time with one to three persons: 70 h for B. eunomia caterpillars and 27 h for B. aquilonaris caterpillars; B. selene caterpillars were searched simultaneously with the two other species). The search effort was proportional to the host plant coverage in the zone (Relation between searching time ST in min and host plant area HPA in m2: B. eunomia, ST = 34 + 0.17*HPA, R2 = 53% –B. aquilonaris, ST = 91 + 0.13*HPA, R2 = 51%). Recorded abundances per zone in both years were pooled for further analysis. In total, we found 514 B. eunomia, 86 B. aquilonaris and 106 B. selene caterpillars. At the end of L. hippothoe flight period in 2005, we counted eggs in all the zones on all R. acetosa plants, as described in Turlure and Van Dyck (2009). At the end of the L. helle flight period in 2005, we counted eggs in all zones with P. bistorta plants as described in Turlure et al. (2014). Searching time by one to three persons was 37 h for L. hippothoe and 77 hours for L. helle eggs. We found 181 L. hippothoe and 692 L. helle eggs.
Morphological Measures of B. aquilonaris and B. eunomia
During the flight period of the two species in summer 2010 and 2011, we took morphological measures in 10 different individuals per species, sex and site (562 B. aquilonaris and 349 B. eunomia individuals in total). To do so, butterflies were captured and measured alive with calipers. We recorded thorax length TL along the center line and width TW at the widest part, abdomen length AL along the center line and width AW at widest part, and length of the upper edge of forewing (FW). From these measures, we estimated the volume of the abdomen (AV) and the volume of the thorax (TV), both approximated as an ellipsoid volume with height equal to width (as in Turlure et al., 2010c). Since both Boloria species are legally protected, we could only apply non-invasive methods. This was done on freshly emerged individuals (i.e., with entire and brightly colored wings and often a drop of pink liquid expelled from the abdomen at the time of capture), to limit differences in morphology due to individual age.
Statistical Analysis
Modeling Spatial Variation in Abundance of Five Species in the Pisserotte Reserve
We used linear models to analyze variation in butterfly species abundance in the 40 Pisserotte zones adopting the three habitat definitions for each species, adult sex (male and female) and juvenile stage (egg or caterpillar), separately. We first fitted linear models implemented in SAS (Genmod procedure, with a Poisson distribution and a log link function) corresponding to all the combinations of the descriptors, i.e., area, and descriptors defining one of the three definitions of habitat quality (i.e., either vegetation type, vegetation composition, or ecological resources). Then we used AICc based multimodel averaging (considering models with ΔAICc ≤ 10 only; Burnham and Anderson, 2002) to obtain a global estimate of the slope associated with each descriptor. Descriptors were standardized prior to analysis, so that slope estimates can be compared within each model to assess their relative effect size.
Modeling Spatial Variation in Population Size for B. eunomia and B. aquilonaris Metapopulations
For each population of the B. eunomia and B. aquilonaris metapopulations, we summarized the time series of population size by its minimum and maximum. This was done in order to buffer the variation among sites due to the non-negligible and asynchronous temporal variations in these butterfly populations (Thomas, 1991). This was crucial to avoid that spatial variation, which we attempt to explain, are biased by asynchronous temporal variation. We then analyzed spatial variation in minimum and maximum population sizes among sites relative to all the combinations of the descriptors, i.e., functional area, descriptors defining one of the three definitions of habitat quality, and connectivity (only for the B. aquilonaris metapopulation) using linear models implemented in SAS (Genmod procedure with a Poisson distribution and a log link function) with multimodel averaging as previously described.
Transferability of Habitat Quality
To test for the transferability of the habitat quality assessment, we used the models estimated on the 15 populations in the B. aquilonaris metapopulation to make predictions of the minimum and maximum expected population sizes. This was done for the three habitat definitions, the habitat based on vegetation type, vegetation composition and ecological resources. We measured the accuracy of the predictions at two spatial levels: (1) in the metapopulation by cross-validation, where we predicted the minimum and the maximum population sizes for each site by removing it from the dataset used to fit the model for each of these response variable; (2) in 13 additional Belgian populations (i.e., an external set of populations that were not used to fit the model) by assessing whether the observed population size (assessed in two consecutive years, 2013 and 2014) fell within the range predicted by the models. Also, we calculated the relative error RE in prediction for each population as follows: , Npred being the predicted population size and Nobs being the observed population size computed as means over 2013 and 2014.
Habitat Quality and Butterfly Morphology
Finally, we analyzed the quality of a sample of adult individuals, using morphological descriptors as proxies. For each species and sex separately, we tested the effects of habitat descriptors (functional area, resource abundance, distribution and organization, and connectivity for B. aquilonaris only) on the forewing length, thorax and abdomen volumes of B. eunomia and B. aquilonaris adults using mixed models implemented in SAS (Glimmix procedure) with year as a random intercept and a Gauss-Hermite quadrature approximation.
Results
Spatial Variation in Abundance: Five Species in the Pisserotte Reserve
Variation in local abundance among the 40 Pisserotte zones was best explained by a combination of resource variables for juvenile stages of the five species and the adult stages of the two more specialized species (B. eunomia and L. helle) (Table 2). It was best explained by the vegetation composition for B. aquilonaris, L. hippothoe, and B. selene adults. The vegetation type presented a very weak explanatory power in all cases.
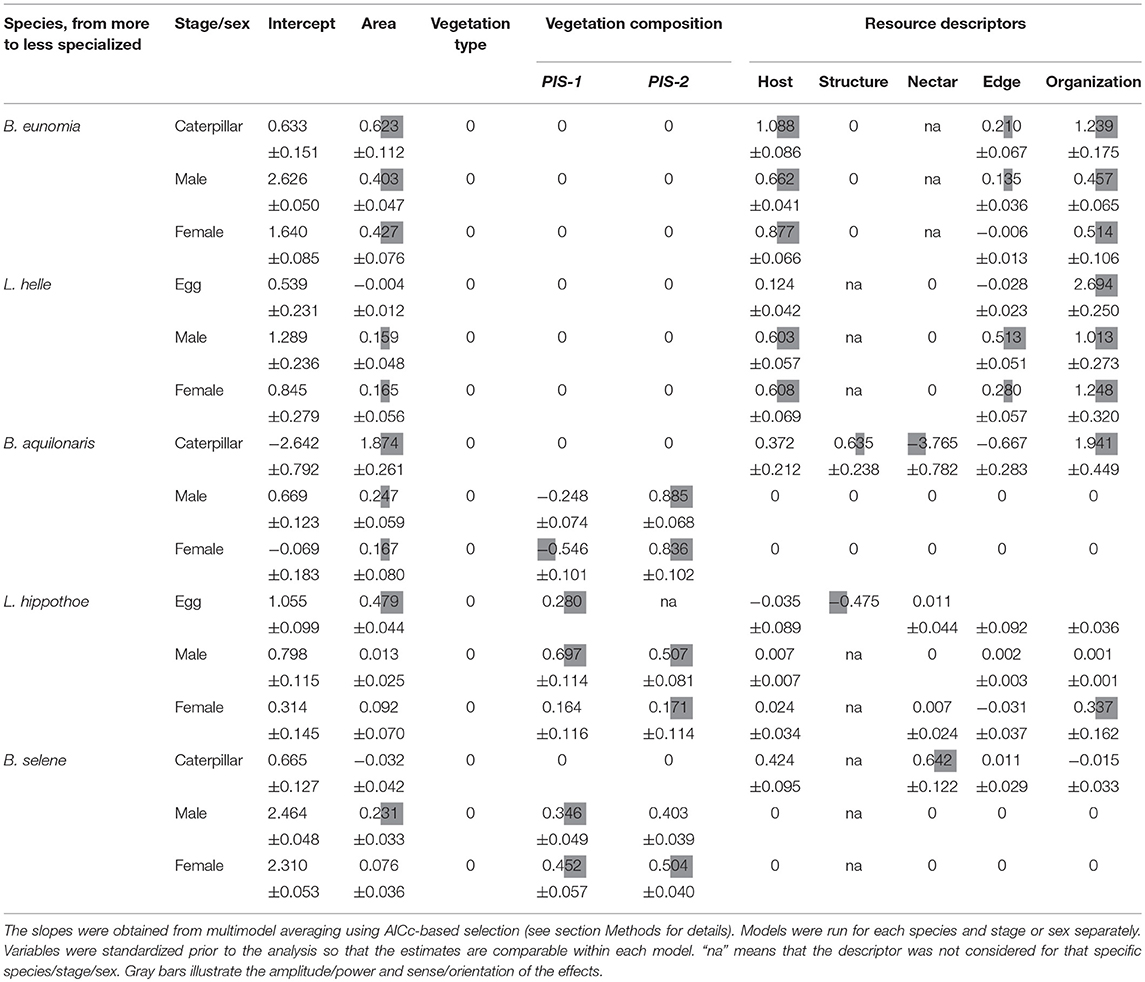
Table 2. Relationship between local species abundance and habitat descriptors represented by slope estimates of linear models.
Caterpillars and both adult males and females of B. eunomia were more abundant in zones with stronger spatial overlap between adult and juvenile resources, higher host plant abundance and of larger size. Tree edges had a positive effect on caterpillar and male abundance only.
For L. helle, egg abundance increased with a greater spatial overlap between adult and juvenile resources and a higher host plant abundance. Adults were also more abundant in zones with a greater overlap between adult and juvenile resources, in larger zones with more host plants and in zones surrounded by trees; the latter effect was more pronounced in males.
Caterpillars of B. aquilonaris were more abundant in (1) zones with fewer nectar resources, (2) with a greater overlap between adult and juvenile resources, (3) large zones, (4) open zones, less surrounded by trees, and (5) zones with abundant Sphagnum hummocks. Contrary to caterpillars, adult local abundance was best described by the vegetation composition: males and females were more abundant in larger flower-rich zones (higher values of VEG-2) and in bog vegetation zones where the host plant was more abundant (lower VEG-1 values, especially for females).
Eggs of L. hippothoe were more abundant in larger open zones (i.e., not surrounded by trees) containing more host plants. Variation in adult local abundance was best described by the vegetation composition. Males were more abundant in meadows, flower-rich zones and when the host plant was abundant (higher values of VEG-1 and VEG-2). It was also the case for females, although with a predominant effect of resource organization; females being more abundant in zones with a greater overlap between adult and juvenile resources.
Caterpillars of B. selene were more abundance in zones rich in nectar resources and host plants. Adult local abundance was best described by the vegetation composition: abundance of males and females increased with increasing values of VEG-1 (i.e., in meadow-like vegetation) and with increasing values of VEG-2 (i.e., in flower rich zones).
Spatial Variation in Population Size for Metapopulations of B. eunomia and B. aquilonaris
Variation in minimum and maximum population sizes in the sites of the B. eunomia and B. aquilonaris metapopulations were best explained by a combination of resource variables (Table 3). The explanatory power of vegetation composition and of vegetation type was clearly inferior.
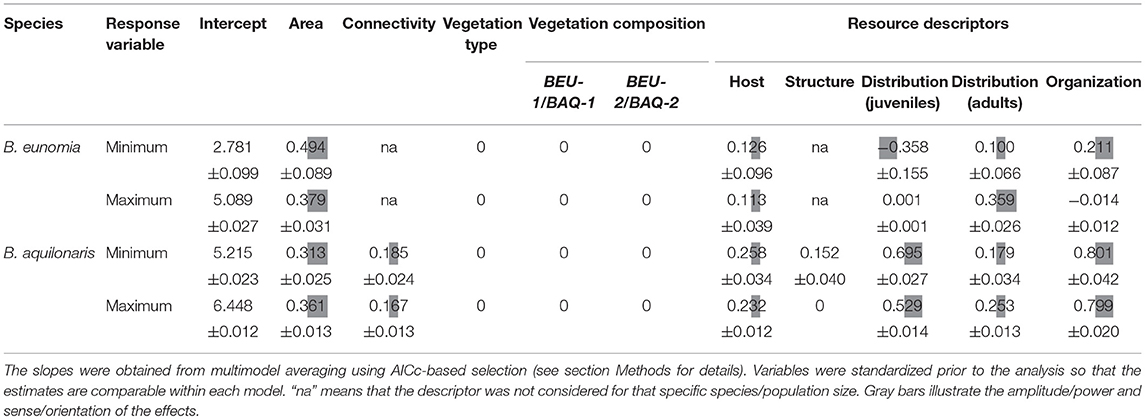
Table 3. Relationship between minimum and maximum population sizes (for B. eunomia and B. aquilonaris) to habitat parameters represented by slope estimates of linear models.
In B. eunomia, minimum population size observed in each site was positively related to a larger functional area, a homogeneous distribution of resources for caterpillars but a heterogeneous distribution of nectar resources for adults (i.e., nectar resources being abundant and scattered at some places but absent in other, nearby places within the same habitat space), a greater overlap between juvenile and adult resources, and a high abundance of the host plant. Maximum population size was greater in larger sites with a higher abundance of the host plant. The effect of the distribution of adult resources was much larger, whereas the effect of juvenile resource distribution and resource organization was limited.
In B. aquilonaris, minimum and maximum B. aquilonaris population sizes were influenced by similar effects: adults were more numerous in sites showing a greater overlap between juvenile and adult resources, a heterogeneous distribution of resources for caterpillars, a large functional area, and abundant host plants. Greater connectivity and a heterogeneous distribution of nectar resources for adults also had a positive effect. The abundance of Sphagnum hummocks had a positive effect on minimum population size only.
Transferability of Habitat Quality Assessment
The predictive power of the resource-based habitat quality model estimated at the metapopulation level for B. aquilonaris was high: the cross validation analysis indicated that the observed population size (i.e., averaged over time to remove temporal variation) fell 73% of the sites of the metapopulation between the predicted values for minimum population size and maximum population size. For the models with the two other habitat definitions, the rate of correct cross validation dropped to 47%.
A stronger test consisted in performing the same predictive power test on independent data, i.e., the other Belgian populations of B. aquilonaris. The rate of correct prediction (i.e., observed population size fell between predicted minimum and maximum population sizes) was also high (62%) when using the resource-based habitat model. It dropped to 46% when using the vegetation type model and 19% when using the vegetation composition model (Figures 3A–C). Also, relative prediction error was not related to population size, whatever the habitat model use, although there is a trend of larger errors in smaller populations (Figure 3D).
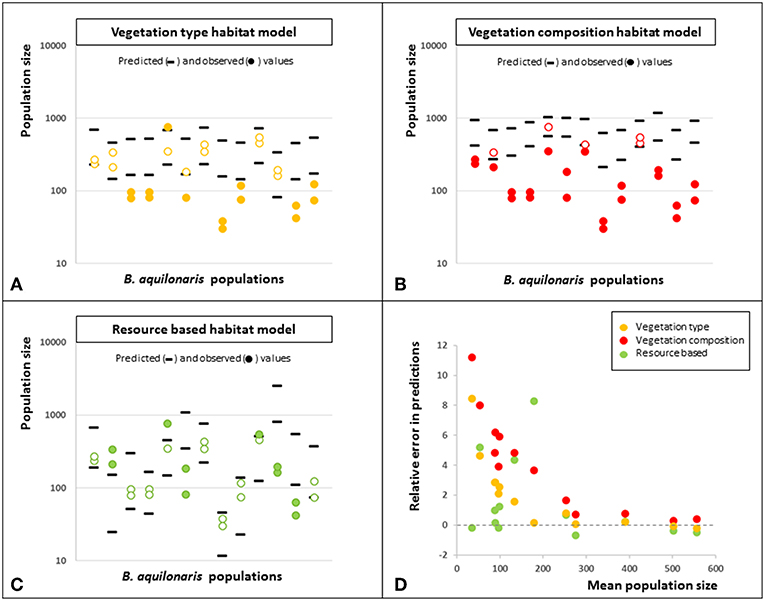
Figure 3. Predictive power of the three habitat quality models. In panels (A–C) are shown the predicted minimum and predicted maximum population sizes (black ticks) using the models based on vegetation type (panel A), vegetation composition (panel B) and ecological resources (panel C) fitted on the original metapopulation data, as well as the observed population sizes (2013–2014, dots). Plain dots indicate the observed population sizes that did not fall between the predicted minimum and maximum population sizes. Predictions were incorrect for 14 couples of population and year when using the vegetation type habitat model, for 21 couples when using the vegetation composition habitat model and for 10 couples when using the resource based habitat model. (D) Relative error in the predictions according to the mean population size for the three habitat type models. Errors are lower for predictions made with the resource based habitat model. There is no relationship between the error and the mean population size, whatever the model considered, although relative errors are larger for smaller population sizes.
Habitat Quality and Butterfly Morphology
B. eunomia males had significantly smaller thoraxes in case of greater overlap between caterpillar and adult resources and more homogeneous distribution of the adult nectar resources (Table 4). We did not find any significant effect of the resource variables on the other morphological traits in B. eunomia males, and on all morphological traits in B. eunomia females.
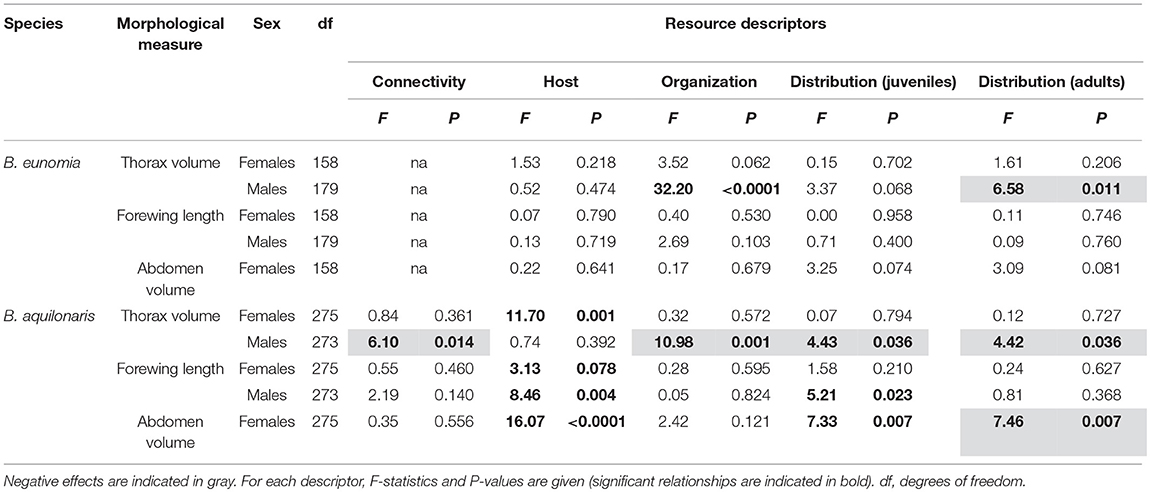
Table 4. Relationship between habitat quality and adult morphology in males and females of both B. eunomia and B. aquilonaris.
B. aquilonaris females had significantly bigger thoraxes, bigger abdomens and larger wings in sites with a great abundance of the host plant. Their abdomen was also bigger in sites with a more homogeneous distribution of the juvenile resources and a more heterogeneous distribution of the adult nectar resources. Effects on B. aquilonaris male morphology were more diverse, with (1) smaller thoraxes in case of greater between-population connectivity, greater overlap between juvenile and adult resources, and more homogeneous distribution of the caterpillar and adult resources, and (2) larger wings in sites with a high abundance of the host plant and a more heterogeneous distribution of the caterpillar resources (Table 4).
Discussion
The Resource-Based Habitat Approach: A Templet for Species Conservation?
Whatever the investigated spatial scale (local population and metapopulation), our quantitative results show an overall better performance of the resource-based habitat approach to represent the relationships between the organism and the environment compared to the most commonly used vegetation-based habitat approaches, including the “host plant only” approach. As predicted, differences are most pronounced at the juvenile stages (eggs or caterpillars) compared to adults whatever the degree of ecological specialism of the species. In the case of the most specialized species, we have also shown significant effects at the adult stage. Local abundance within the Pisserotte site was best explained by a combination of resource variables in 70% of the cases, namely for (1) the juvenile stages of the five study species, and (2) the adults of B. eunomia and L. helle. At the metapopulation scale, it was also the case for the adults of the two species, B. eunomia and B. aquilonaris.
A vegetation-based habitat approach could work in cases where all the resources needed by the species are encompassed in a particular vegetation type, but such conditions are rather the exception than the rule. In our study, it was only the case for adults of the less specialized species (B. aquilonaris, L. hippothoe, and B. selene) at a local scale, for which adult resources are present in some vegetation association at a given site. However, we draw attention to the fact that there is a discrepancy in the results obtained at local and larger spatial scales for adults of B. aquilonaris. So, although the vegetation-based habitat approach can be suitable under singular conditions or for specific sites, our study supports the broader and default use of the resource-based habitat approach to establish the functional habitat of a species for the purpose of conservation and habitat restoration. Therefore, we conclude that the resource-based habitat approach should be preferred over a vegetation-based habitat approach to properly define the habitat of a species. Also we argue that fieldwork should be conducted on several life stages (Radchuk et al., 2013) and over several sites or populations rather than at one given location to provide widely applicable guidelines toward species conservation.
As certain amounts of host plant are needed to feed a population at any site (i.e., host plant is a limiting resource), host plant presence and abundance are widely used to predict butterfly population occurrence within a landscape (see examples in Sharp et al., 1974 for Plebejus saepiolus and in Bauerfeind et al., 2009 for L. helle). Although host abundance was present in most of our selected regression models, it was not sufficient to predict local butterfly abundance or population size. Knowledge on the use of specific or different nectar resources and their significance for species distribution is much less complete compared to host plant use, because butterflies have long been considered opportunistic nectar feeders. However, Tudor et al. (2004) observed that butterfly species differed in the nectar resources they used and specialist flower users were more frequently of conservation concern than generalist flower users. Loertscher et al. (1995) found a positive relationship between the distributions of adults and their preferred nectar sources in several butterfly species (i.e., Melanargia galathea, Polyommatus coridon, and Ochlodes sylvanus). Our multi-species study showed that both host and nectar resources do play a role in defining a species' functional habitat.
Structural resources are often neglected although they are of high functional significance as they generally relate to micro-climatic conditions. In line with our previous work (Turlure et al., 2009, 2010a,b), we confirmed that structures provided by grass tussocks or Sphagnum hummocks are key elements to the larval habitat of B. eunomia or B. aquilonaris, respectively. The presence of grass tussocks was also recognized as an important element in the composition of the larval habitat of Coenonympha tullia as these structures favor caterpillar survival during periods of flooding (Dennis and Eales, 1997; Joy and Pullin, 1997). Adult butterflies make use of trees and shrubs to shelter, to roost or to mate (Dover et al., 1997; Dennis, 2004; Pywell et al., 2004; Binzenhofer et al., 2005). We demonstrated the significance of the presence of edges with trees as they provide shelter for L. helle adults (both males and females; Turlure et al., 2014) and perching sites for the territorial males of L. hippothoe (Turlure and Van Dyck, 2009). The significance of such structural elements may also vary with local weather conditions as has been shown in Plebejus argus: adults were more abundant near scrub under wind exposed conditions (Dennis and Sparks, 2006).
Adopting the resource-based habitat approach, the functional habitat of a butterfly consists of (1) nectar feeding resources, mate location sites, roosting sites and egg-laying sites for adults, (2) a substrate and appropriate microclimate for eggs, (3) host plants providing larval feeding resources that occur in an appropriate structure and microclimate for caterpillar growth, and (4) appropriate structure and microclimate for the pupal stage and hibernation (aestivation) (see more details in Dennis et al., 2006; Dennis, 2010). The number of resources involved in habitat composition may vary, with generalist species likely using a wider range of more numerous resources, while more exigent species likely needing less but more specific resources. Not all ecological resources were included in our study and certainly we still lack knowledge on habitat resource composition for our study species. Vegetation type and composition may indeed have additional explanatory power. But even with incomplete ecological information, we have demonstrated the suitability of the resource-based approach of the species' habitat and argue for its standard use in conservation.
Although the terminology used to describe a species habitat seems to be taxonomic-specific, many examples of key resource elements whose distribution does not coincide with a unique vegetation type can be found in the literature for a wide range of organisms other than butterflies. For example, the seasonal use of different vegetation types providing suitable food resources has been illustrated in the lilac-crowned parrot Amazona finschi (Katherine, 2001) and in the wildebeest Connochaetes taurinus (Yoganand and Owen-Smith, 2014). Not only the species' needs in terms of food resources were investigated. Long ago, Magnuson et al. (1979) proposed that temperature be recorded as an ecological resource, as well as other chemical and physical factors, and illustrated how thermal needs differ for several freshwater fish species. In the same way, light conditions have been identified as an important ecological resource for the damselfly Megalagrion nigrohamatum nigrolineatum, whose individuals use dark locations to perch (Henry et al., 2017). Structural elements can also be considered as resources, as they may serve as roosts (Weber et al., 2015; Paramanantha Swami and Nagarajan-Radha, 2017), or as shelters (Lucherini et al., 2009; Bruton et al., 2014). Even time used for foraging the same food resources could be considered as a resource, as exemplified with sympatric cormorants species (Mahendiran, 2016). In some cases, however, vegetation type or composition may be an efficient proxy for a (part of) species habitat, with (some) resources being encompassed in this given vegetation type or composition. This seems the case for some butterfly species such as Lopinga achine, a forest species for which canopy closure limits the availability of host plants (Konvicka et al., 2008), Maculinea arion, a species thriving in small pastures that are not mown or intensively grazed (Spitzer et al., 2009), or Melitaea aurelia, a species whose habitat resources coexist in low disturbed calcareous grasslands (Eichel and Fartmann, 2008), among other examples. Nevertheless, a resource-based approach describing a species habitat may theoretically suit any organism and requires detailed auto-ecological study to assess species resource needs in details. Its use may help to guide conservation programs (Parentoni Martins, 2017) and to understand the pattern of species coexistence in relation to the effects of inter-specific competition (Cloyed and Eason, 2017; Estevo et al., 2017; Matley et al., 2017).
Resource Availability or Resource Configuration: What Is the Key Element to Define Habitat Quality?
At the individual level, variation in availability and quality of host plant resources (stored nutrients) and nectar resources (incoming nutrients) were shown to affect adult morphology, longevity, reproduction and, ultimately, butterfly fitness (Hill, 1992; Boggs and Freeman, 2005; Jervis and Boggs, 2005). In our study, populations of B. aquilonaris with more abundant host plants had adults with longer forewings, and in females both thoraxes and abdomens were relatively bigger. Variation in availability and quality of host plant and nectar resources also had an effect at the population level. Population size has been suggested to be strongly linked with the abundance of host plant and nectar resources (Schultz and Dlugosch, 1999). Several studies on different butterfly species have illustrated such a relationship. Population density of Polyommatus coridon was, for example, largely explained by its larval food plant quantity (Krauss et al., 2005); population size of Pseudophilotes sinaicus was affected by resource area and habitat quality (James et al., 2003); and the probability of regional extinction of Hamaeris lucina was related to the reduction of habitat quantity and increasing isolation of habitat (Leon-Cortes et al., 2003). Here, we have also demonstrated that the availability of larval resources is significantly related to adult population size in two species (i.e., host plant for B. eunomia; host plant and vegetation structure for B. aquilonaris).
Resource organization is likely to affect habitat exploitation, and hence individual distribution and movements. For example, the configuration of host and nectar resources used by Parnassius apollo impacted on adult distribution as females are more abundant in host plant patches close to nectar resources, independently of the host plant abundance (Fred et al., 2006). Here, we observed our study species to be locally more abundant in zones with a greater overlap between juvenile and adult resources. In Coenonympha tullia, the most suitable conditions for population persistence were defined as the overlap (contiguity) of larval host plant and nectar resources (Dennis and Eales, 1997). Similarly, higher minimum population sizes were observed in the case of a greater overlap between juvenile and adult resources in B. eunomia and B. aquilonaris. Organization of host plant in patches of homogeneous abundance had a positive influence on minimum population size in B. eunomia, whereas in B. aquilonaris larger population sizes should be favored by the heterogeneous distribution of juvenile and adult resources. A conflict of interest may occur for females when host and nectar resources do not overlap spatially: they have to choose between meeting their own requirements and those of their offspring. Hence, this represents a case of the concept known as the parent-offspring conflict (Trivers, 1974). Females of some species were observed to prefer staying at nectar rich zones (Grossmueller and Lederhouse, 1987; Brommer and Fred, 2001) while others avoid feeding at the cost of longevity (e.g., most moth species). Here, we only observed females of B. eunomia and L. hippothoe to be locally more abundant in open zones compared to males. The open zones probably offer more constant sun exposure and warmer microclimates needed to mature eggs.
Baguette and Van Dyck (2007) and Dennis and Hardy (2007) proposed that in the process of resource finding, resource grain may affect the types of movement (i.e., “direct linear flight” or “searching flight”) and consequently morphology. When resources are spatially separated, the associated cost of exploitation may lead to either more sedentary individuals through selection against high mobility (Komonen et al., 2004), or adaptation through morphological characteristics favoring higher mobility for resource exploitation. Here, we have demonstrated that the grain and organization of resources were associated with morphology in several ways. Adult males of B. aquilonaris had, for example, a larger thorax when key resources were spatially distributed in a more heterogeneous way.
Our analysis has allowed us to assess the relative importance of each ecological resource in terms of both availability and organization relative to the species' abundance, demography and individual fitness. As predicted, resource availability, and especially host plant abundance, does not necessarily play the predominant role in defining habitat quality. Although the host plant is a necessary resource whose presence is required for an area to qualify as habitat, it is in most cases overruled by the resource organization to define habitat quality. More generally, this shows that understanding behavior relative to resource distribution can be of key significance to define species-specific habitat.
Similar conclusions can be drawn for other taxonomic groups. Habitat quality has previously been defined as the ability of the environment to provide conditions appropriate for individual and population persistence (Hall et al., 1997). In their review paper, Mortelliti et al. (2010) pointed out that habitat size is not necessarily equivalent to habitat quality and that although measuring key resources for invertebrate species is highly feasible, it might be more complex for vertebrate species with poorly known ecological requirements. However, numerous papers exist reporting links between pattern of occupancy, population growth rate, population size or individual fitness and resource availability. For examples, (1) optimal vs. suboptimal habitat quality impacted on species presence in the European Nuthatch (Verboom et al., 1991) and (2) food resource availability determined the pattern of patch use in the badger Meles meles and the beech marten Martes foina (Mortelliti and Boitani, 2008). Habitat quality (from resource composition and availability) predicted density or population size in the Great Crested Newt Triturus cristatus (Unglaub et al., 2018) and the Grizzly bear Ursus arctos (Lamb et al., 2018). In the Cap mountain zebra Equus zebra zebra, lower resource availability influenced individual physiology, in turn affecting population growth rate (Lea et al., 2018). In the mud crab Panopeus herbstii, habitat quality (reef height, in the field) and the diet (in the lab) impacted on the reproductive performance of females (Griffen and Norelli, 2015). Although the influence of habitat patch distribution in the landscape on population dynamics and persistence has received support from several studies (e.g., in the rainbow trout Oncorhynchus mykiss in Jacobson et al. (2015); in the crayfish Procambarus fallax in van der Heiden and Dorn (2017), only a few illustrates the influence of local resource organization on population dynamics and size (but see Kempe et al., 2016). However, resource distribution has been (theoretically and experimentally) shown to influence resource utilization, feeding behaviors, social organization and mating systems in many organisms (e.g., Dell'Arte and Leonardi, 2005; Reluga and Shaw, 2015; Vincenot et al., 2015; Fernandez-Duque, 2016; Halliwell et al., 2017). In particular, the Resources Dispersion Hypothesis (RDH, Johnson et al., 2002; Revilla, 2003) has been used to explain how resource distribution affects group living in non-cooperative animals (Grouping behavior, Territory, and Home range size; e.g., McClintic et al., 2014; Kittle et al., 2015; Koenig and Walters, 2015). The list of examples quoted here is obviously not exhaustive, but illustrates that habitat quality using the resource-based approach may provide a functional basis for assessing the population status of any species from different taxonomic groups.
The Resource-Based Habitat Approach: Is It Widely Applicable?
Finally, we have confirmed the between-population transferability of the habitat definition and habitat quality estimates by adopting a resource-based habitat approach. In B. aquilonaris the predictive power of the resource-based habitat quality model (by cross validation and use of additional independent data on other Belgian populations) was assessed to be very good (73% and 62% of correct predictions). Although performed on a single species, this demonstration provides a strong argument in favor of detailed autecological studies to identify (1) key resources defining the functional habitat, and (2) how resource availability and organization influence habitat quality. Many species conservation plans need to quantify habitat quality at more or less large spatial scales. A resource-based approach is likely to prove an efficient way to do so from the presence and abundance of a series of key resources. Using a vegetation type based definition of the habitat fails in such a situation because of its habitat vs. non-habitat binary view that fails to provide estimates of habitat quality. Note that the transferability has been investigated here at a rather small geographical scale (i.e., tenths of km). If applied at much larger scales, like continental scales, changes in resources used by a species, as well as local adaptations, may hamper the transferability. However, conservation plans are rarely designed at such large scales.
Given the number of variables included in the resource-based definition such as previously described, it is not a restrictive definition. Its philosophy can be applied to a wide range of species. In theory, at the intra-specific level, resource composition should remain much the same. Variation in the availability and distribution of resources should occur more frequently, leading, in turn, to local behavioral and life history adaptations. Availability of food resources have been invoked as the main determinant of population size (Pianka, 1974; Pollard, 1981) and has led to the concept of carrying capacity (i.e., the maximum population size an organism can reach in absence of enemies and catastrophes). But the sole availability of resources does not necessarily reflect quality per se. The examples quoted in this discussion, among others, indicate the importance, co-occurrence, composition, quality, and organization of resources (consumables and utilities) to determine the quality of any given location for supporting population of a given species (Maes et al., 2006; Dennis and Hardy, 2007).
Deterioration of habitat quality plays an important role in the extinction risk faced by local populations (Thomas, 1984; Thomas et al., 1992). Inferring habitat quality based on quantitative and reliable parameters is then of utmost interest. Such habitat quality estimates can be integrated in metapopulation models (Hanski and Gilpin, 1991; Dennis and Eales, 1997; Hanski and Simberloff, 1997; Schtickzelle and Baguette, 2009), and will make it possible to identify where resources are lacking in specific regions resulting in clear guidelines for landscape restoration and identifying potential locations for species to occur (e.g., Vanreusel and Van Dyck, 2007). Although most of these issues emerged from butterfly studies, the resource-based definition of the habitat is likely to be relevant for many other organisms (Maes et al., 2006). Finally, we want to stress that such thorough resource-based habitat studies aiming at managing threatened species one by one are indeed not feasible on the ever-growing numbers of imperiled taxa in the current era of global extinction. We advocate for an alternative approach to a species-based strategy of biodiversity conservation in those areas that are relatively or totally sheltered from strong human impact. In such areas that are still close to their natural state, a sanctuary-based approach aiming at maintaining those eco-evolutionary processes that shape biodiversity at the landscape scale should be privileged. The meta-climax concept coined by Blondel (1987) could be used as a framework to maintain landscapes in such a way that they contain many habitats for numerous species. A meta-climax can be defined as the whole set of simultaneous co-occurring successional vegetation stages offering a range of ecological key resources for all the species within the landscape. It is, however, often assumed that in many industrialized or urbanized areas, the human pressure is way too strong for applying this sanctuary-based approach. Nevertheless, even in our study area where large forests remain, the re-introduction of landscape engineers like beavers coupled with natural disturbances (storms, flooding) will create open areas that would be maintained by other engineers, i.e., large ungulates like moose, bison or elks. Various successional stages will thus coexist in a patchy and spatially dynamic meta-climax characteristic of the primeval vegetation that was present after the last Ice Age before human driven large deforestation (Vera, 2002). Such a landscape will meet the two basic conditions of a sanctuary-based approach to conservation, i.e., to allow the persistence of all the species by providing all of them with their resources, and to maintain those eco-evolutionary processes that shape biodiversity.
Author Contributions
CT designed the study, together with NS and MB applying ideas developed by RD and HV. CT and QD collected the data. CT and NS analyzed the data in interaction with HV; all authors commented, interpreted and participated to the improvement of these analyses. CT and HV wrote the first draft of the manuscript, and all authors contributed substantially to revisions. All authors read and approved the final version of the paper.
Funding
CT (post-doctoral fellow), QD (PhD-student), and NS (senior research associate) acknowledge financial support from the F.R.S.-FNRS. MB is part of the Laboratoire d'Excellence (LABEX) entitled TULIP (ANR-10-LABX-41), and acknowledges support by the TenLamas project (EU FP6 BiodivERsA Eranet). CT and MB acknowledge support by the EU FP7 project SCALES (project #226852).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Julie Choutt, Christophe Lebigre, Séverin Pierret, Guillaume Senterre, Manhattan Solheid, and Sofie Vandewoestijne for their valuable help with field work. We thank the two reviewers for their in-depth constructive comments, which helped us to further improve this manuscript. Site access and a permission to study the species in the field were granted by the Ministère de la Région Wallonne (now Service Public de Wallonie). This is publication no. BRC436 of the Biodiversity Research Centre–Earth and Life Institute of UCLouvain.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00127/full#supplementary-material
Supplementary Figure 1. Summary table on how vegetation type, vegetation composition and ecological resources were assessed in this study. (A) A vegetation type was assigned to each zone of the Pisserotte reserve and each site hosting a population of B. eunomia and B. aquilonaris. (B) Vegetation composition was summarized separately by the two first axes of Principal Component Analysis (PCAs) using vegetation samples collected in Pisserotte (400 samples summarized in PIS-1 and PIS-2), in B. eunomia populations (247 samples summarized in BEU-1 and BEU-2) and B. aquilonaris populations (759 sites summarized in BAQ-1 and BAQ-2). (C) Ecological resources measured from vegetation samples and extra measurements in each zones and sites. As some resources variables were highly correlated (see section Methods), some were discarded from the analysis.
Supplementary Figure 2. Additional information on vegetation samples. (A) Schematic representation of a vegetation sample, considering two plant species (black dot and star). The 1 m2 sample was divided in a 5*5 grid, forming 25 squares (each 20*20 cm) to estimate the abundance of each plant species on the basis of their presence in each square, i.e., on a zero to 25 scale. In this example, abundance of the black dot species equals 11 and abundance of the black star species equals 5. (B) The number of vegetation samples collected (Y-axis) in sites hosting B. eunomia populations increased with the site host plant area (X-axis). (C) The number of vegetation samples collected (Y-axis) in sites hosting B. aquilonaris populations increased with the site host plant area (X-axis). Note that the relationship is not perfectly linear due to the heterogeneity in the vegetation of within each site (not illustrated here). (D–F) Results of the PCAs performed with the vegetation samples collected in each zone and site.
References
Baguette, M., and Van Dyck, H. (2007). Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landsc. Ecol. 22, 1117–1129. doi: 10.1007/s10980-007-9108-4
Bauerfeind, S., Theisen, A., and Fischer, K. (2009). Patch occupancy in the endangered butterfly Lycaena helle in a fragmented landscape: effects of habitat quality, patch size and isolation. J. Insect Conserv. 13, 271–277. doi: 10.1007/s10841-008-9166-1
Bink, F. A. (1992). Ecologische Atlas van de Dagvlinders van Noordwest-Europa. Haarlem: Schuyt and Co.
Binzenhofer, B., Schroder, B., Strauss, B., Biedermann, R., and Settele, J. (2005). Habitat models and habitat connectivity analysis for butterflies and burnet moths-the example of Zygaena carniolica and Coenonympha arcania. Biol. Conserv. 126, 247–259. doi: 10.1016/j.biocon.2005.05.009
Blondel, J. (1987). From biogeography to life history theory: a multithematic approach illustrated by the biogeography of vertebrates. J. Biogeogr. 14, 405–422. doi: 10.2307/2844972
Boggs, C. L., and Freeman, K. D. (2005). Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia 144, 353–361. doi: 10.1007/s00442-005-0076-6
Brommer, J. E., and Fred, M. S. (2001). Movement of the Apollo butterfly Parnassius apollo related to host plant and nectar plant patches. Ecol. Entomol. 24, 125–131. doi: 10.1046/j.1365-2311.1999.00190.x
Bruton, M. J., McAlpine, C. A., Smith, A. G., and Franklin, C. E. (2014). The importance of underground shelter resources for reptiles in dryland landscapes: a woma python case study. Austral. Ecol. 39, 819–829. doi: 10.1111/aec.12150
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer-Verlag.
Cloyed, C. S., and Eason, P. K. (2017). Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. R. Soc. Open Sci. 4:170060. doi: 10.1098/rsos.170060
Dell'Arte, G. L., and Leonardi, G. (2005). Effects of habitat composition on the use of resources by the red fox in a semi arid environment of North Africa. Acta Oecol. 28, 77–85. doi: 10.1016/j.actao.2004.12.003
Dennis, R. L. H. (2004). Just how important are structural elements as habitat components? Indications from a declining lycaenid butterfly with priority conservation status. J. Insect. Conserv. 8, 37–45. doi: 10.1023/B:JICO.0000027496.82631.4b
Dennis, R. L. H. (2010). A Resource-Based Habitat View for Conservation: Butterflies in the British Landscape. Wiley-Blackwell. doi: 10.1002/9781444315257
Dennis, R. L. H., Dapporto, L., and Dover, J. W. (2014). Ten years of the resource-based habitat paradigm: biotope habitat issues and implications for conserving butterfly biodiversity. J. Insect. Biodivers. 2, 1–32. doi: 10.12976/jib/2014.2.8
Dennis, R. L. H., and Eales, H. T. (1997). Patch occupancy in Coenonympha tullia (Muller, 1764) (Lepidoptera: Satyridae): habitat quality matters as much as patch size and isolation. J. Insect. Conserv. 1, 167–176. doi: 10.1023/A:1018455714879
Dennis, R. L. H., and Hardy, P. B. (2007). Support for mending the matrix: resource seeking by butterflies in apparent non-resource zones. J. Insect. Conserv. 11, 157–168. doi: 10.1007/s10841-006-9032-y
Dennis, R. L. H., Shreeve, T. G., and Van Dyck, H. (2003). Towards a functional resource-based concept for habitat : a butterfly biology viewpoint. Oikos 102, 417–426. doi: 10.1034/j.1600-0579.2003.12492.x
Dennis, R. L. H., Shreeve, T. G., and Van Dyck, H. (2006). Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers. Conserv. 15, 1943–1966. doi: 10.1007/s10531-005-4314-3
Dennis, R. L. H., and Sparks, T. H. (2006). When is a habitat not a habitat? Dramatic resource use changes under differing weather conditions for the butterfly Plebejus argus. Biol. Conserv. 129, 291–301. doi: 10.1016/j.biocon.2005.10.043
Dover, J. W., Sparks, T. H., and Greatorex, D. (1997). The importance of shelter for butterflies in open landscapes. J. Insect. Conserv. 1, 89–97. doi: 10.1023/A:1018487127174
Edwards, J. W., Heckel, D. G., and Guynn, D. C. (1998). Niche overlap in sympatric populations of fox and gray squirrels. J. Wildlife Manage. 62, 354–363. doi: 10.2307/3802299
Eichel, S., and Fartmann, T. (2008). Management of calcareous grasslands for Nickerl's fritillary (Melitaea aurelia) has to consider habitat requirements of the immature stages, isolation, and patch area. J. Insect. Conserv. 12, 677–688. doi: 10.1007/s10841-007-9110-9
Estevo, C. A., Nagy-Reis, M. B., and Nichols, J. D. (2017). When habitat matters: habitat preferences can modulate co-occurrence patterns of similar sympatric species. PLOS ONE 12:e0179489. doi: 10.1371/journal.pone.0179489
Fernandez-Duque, E. (2016). Social monogamy in wild owl monkeys (Aotus azarae) of Argentina: the potential influences of resource distribution and ranging patterns. Am. J. Primatol. 78, 355–371. doi: 10.1002/ajp.22397
Fred, M. S., O'Hara, R. B., and Brommer, J. E. (2006). Consequences of the spatial configuration of resources for the distribution and dynamics of the endangered Parnassius apollo butterfly. Biol. Conserv. 130, 183–192. doi: 10.1016/j.biocon.2005.12.012
Griffen, B. D., and Norelli, A. P. (2015). Spatially variable habitat quality contributes to within-population variation in reproductive success. Ecol. Evol. 5, 1474–1483. doi: 10.1002/ece3.1427
Grinnel, J. D. (1917). The niche relationship of the California Thrasher. The Auk 34, 427–433. doi: 10.2307/4072271
Grossmueller, D. W., and Lederhouse, R. C. (1987). The role of nectar source distribution in the habitat use and oviposition by the tiger swallowtail butterfly. J. Lepidopterist's Soc. 41, 159–165.
Hall, L. S., Krausman, P. R., and Morrison, M. L. (1997). The habitat concept and a plea for standard terminology. Wildlife Soc. Bull. 25, 173–182.
Halliwell, B., Uller, T., Wapstra, E., and While, G. M. (2017). Resource distribution mediates social and mating behavior in a family living lizard. Behav. Ecol. 28, 145–153. doi: 10.1093/beheco/arw134
Hanski, I. (1994). A practical model of metapopulation dynamics. J. Anim. Ecol. 63, 151–162. doi: 10.2307/5591
Hanski, I. (1999). Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 87, 209–219. doi: 10.2307/3546736
Hanski, I., and Gilpin, M. (1991). Metapopulation dynamics : brief history and conceptual domain. Biol. J. Linn. Soc. 42, 3–16. doi: 10.1111/j.1095-8312.1991.tb00548.x
Hanski, I., and Simberloff, D. (1997). “The metapopulation approach, its history, conceptual domain, and application to conservation,” in Metapopulation Biology. Ecology, Genetics, and Evolution, eds I. Hanski and M. Gilpin (San Diego, CA: Academic Press), 5–26.
Hartemink, N., Vanwambeke, S. O., Purse, B. V., Gilbert, M., and Dyck, H. V. (2015). Towards a resource-based habitat approach for spatial modelling of vector-borne disease risks. Biol. Rev. 90, 1151–1162. doi: 10.1111/brv.12149
Haskel, E. F. (1940). Mathematical systematization of “environment”, “organism” and “habitat”. Ecology 21, 1–16. doi: 10.2307/1930613
Henry, E. R., Rivera, J. A., Linkem, C. N., Scales, J. A., and Butler, M. A. (2017). Damselflies that prefer dark habitats illustrate the importance of light as an ecological resource. Biol. J. Linn. Soc. 123, 144–154. doi: 10.1093/biolinnean/blx122
Hill, C. J. (1992). Temporal changes in abundance of two lycaenid butterflies (Lycaenidae) in relation to adult food resources. J. Lepidopter. Soc. 46, 174–182.
Hutchinson, G. E. (1957). Concluding remarks. Cold Spring Harbor Symp. Quantitat. Biol. 22, 415–427. doi: 10.1101/SQB.1957.022.01.039
Jacobson, B., Grant, J. W. A., Peres-Neto, P. R., and Amarasekare, P. (2015). The interaction between the spatial distribution of resource patches and population density: consequences for intraspecific growth and morphology. J. Anim. Ecol. 84, 934–942. doi: 10.1111/1365-2656.12365
James, M., Gilbert, F., and Zalat, S. (2003). Thyme and isolation for the Sinai baton blue butterfly (Pseudophilotes sinaicus). Oecologia 134, 445–453. doi: 10.1007/s00442-002-1123-1
Jervis, M. A., and Boggs, C. L. (2005). Linking nectar amino acids to fitness in female butterflies. Trends Ecol. Evol. 20, 585–587. doi: 10.1016/j.tree.2005.08.015
Johnson, D. D. P., Kays, R., Blackwell, P. G., and Macdonald, D. W. (2002). Does the resource dispersion hypothesis explain group living? Trends Ecol. Evol. 17, 563–570. doi: 10.1016/S0169-5347(02)02619-8
Joy, J., and Pullin, A. S. (1997). The effects of flooding on the survival and behaviour of overwintering large heath butterfly Coenonympha tullia larvae. Biol. Conserv. 82, 61–66. doi: 10.1016/S0006-3207(97)00006-2
Katherine, R. (2001). Lilac-crowned parrot diet and food resource availability: resource tracking by a parrot seed predator. Condor 103, 62–69. doi: 10.1650/0010-5422(2001)103[0062:LCPDAF]2.0.CO;2
Kempe, C., Nowicki, P., Harpke, A., Schweiger, O., and Settele, J. (2016). The importance of resource distribution: spatial co-occurrence of host plants and host ants coincides with increased egg densities of the Dusky Large Blue Maculinea nausithous (Lepidoptera: Lycaenidae). J. Insect Conserv. 20, 1033–1045. doi: 10.1007/s10841-016-9937-z
Kittle, A. M., Anderson, M., Avgar, T., Baker, J. A., Brown, G. S., Hagens, J., et al. (2015). Wolves adapt territory size, not pack size to local habitat quality. J. Anim. Ecol. 84, 1177–1186. doi: 10.1111/1365-2656.12366
Koenig, W. D., and Walters, E. L. (2015). Temporal variability and cooperative breeding: testing the bet-hedging hypothesis in the acorn woodpecker. Proc. R. Soc. B. Biol. Sci. 282:20151742. doi: 10.1098/rspb.2015.1742
Komonen, A., Grapputo, A., Kaitala, V., Kotiaho, J. S., and Päivinen, J. (2004). The role of niche breadth, resource availability and range position on the life history of butterfly. Oikos 105, 41–54. doi: 10.1111/j.0030-1299.2004.12958.x
Konvicka, M., Novak, J., Benes, J., Fric, Z., Bradley, J., Keil, P., et al. (2008). The last population of the Woodland Brown butterfly (Lopinga achine) in the Czech Republic: habitat use, demography and site management. J. Insect Conserv. 12, 549–560. doi: 10.1007/s10841-007-9087-4
Krauss, J., Steffan-Dewenter, I., Muller, C. B., and Tscharntke, T. (2005). Relative importance of resource quantity, isolation and habitat quality for landscape distribution of a monophagous butterfly. Ecography 28, 465–474. doi: 10.1111/j.0906-7590.2005.04201.x
Lafranchis, T. (2000). Les Papillons de Jour de France, Belgique et Luxembourg et Leurs Chenilles. Méze: Parthenope Collection-Biotope.
Lamb, C. T., Mowat, G., Reid, A., Smit, L., Proctor, M., McLellan, B. N., et al. (2018). Effects of habitat quality and access management on the density of a recovering grizzly bear population. J. Appl. Ecol. 55, 1406–1417. doi: 10.1111/1365-2664.13056
Lea, J. M. D., Walker, S. L., Kerley, G. I. H., Jackson, J., Matevich, S. C., Shultz, S., et al. (2018). Non-invasive physiological markers demonstrate link between habitat quality, adult sex ratio and poor population growth rate in a vulnerable species, the Cape mountain zebra. Funct. Ecol. 32, 300–312. doi: 10.1111/1365-2435.13000
Leon-Cortes, J. L., Lennon, J. J., and Thomas, C. D. (2003). Ecological dynamics of extinct species in empty habitat networks. 1. The role of habitat pattern and quantity, stochasticity and dispersal. Oikos 102, 449–464. doi: 10.1034/j.1600-0706.2003.12129.x
Linton, L. R., Davies, R. W., and Wrona, F. J. (1981). Resource utilization indices: an assessment. J. Anim. Ecol. 50, 283–292. doi: 10.2307/4045
Loertscher, M., Erhardt, A., and Zettel, J. (1995). Microdistribution of butterflies in a mosaic-like habitat: the role of nectar sources. Ecography 18, 15–26. doi: 10.1111/j.1600-0587.1995.tb00115.x
Lucherini, M., Lovari, S., and Crema, G. (2009). Habitat use and ranging behaviour of the red fox (Vulpes vulpes) in a Mediterranean rural area: is shelter availability a key factor? J. Zool. 237, 577–591. doi: 10.1111/j.1469-7998.1995.tb05016.x
Maes, D., Shreeve, T. G., and Dennis, R. L. H. (2006). A special issue on insect habitats. J. Insect Conserv. 10, 1089–1093. doi: 10.1007/s10841-006-6285-4
Magnuson, J. J., Croder, L. B., and Medvick, P. A. (1979). Temperature as an ecological resource. Am. Zool. 19, 331–343. doi: 10.1093/icb/19.1.331
Mahendiran, M. (2016). Coexistence of three sympatric cormorants (Phalacrocorax spp.); partitioning of time as an ecological resource. R. Soc. Open Sci. 3:160175. doi: 10.1098/rsos.160175
Matley, J. K., Heupel, M. R., Fisk, A. T., Simpfendorfer, C. A., and Tobin, A. J. (2017). Measuring niche overlap between co-occurring Plectropomus spp. using acoustic telemetry and stable isotopes. Mar. Freshw. Res. 68, 1468–1478. doi: 10.1071/MF16120
McClintic, L. F., Taylor, J. D., Jones, J. C., Singleton, R. D., and Wang, G. (2014). Effects of spatiotemporal resource heterogeneity on home range size of American beaver. J. Zool. 293, 134–141. doi: 10.1111/jzo.12128
Mitchell, S. C. (2005). How useful is the concept of habitat?-A critique. Oikos 110, 634–638. doi: 10.1111/j.0030-1299.2005.13810.x
Mortelliti, A., Amori, G., and Boitani, L. (2010). The role of habitat quality in fragmented landscapes: a conceptual overview and prospectus for future research. Oecologia 163, 535–547. doi: 10.1007/s00442-010-1623-3
Mortelliti, A., and Boitani, L. (2008). Interaction of food resources and landscape structure in determining the probability of patch use by carnivores in fragmented landscapes. Landsc. Ecol. 23, 285–298. doi: 10.1007/s10980-007-9182-7
Paramanantha Swami, D. D., and Nagarajan-Radha, V. (2017). Male resource defence behaviour strengthens harem size in promiscuously mating fruit bats. Acta Chiropterol. 19, 329–336. doi: 10.3161/15081109ACC2017.19.2.009
Parentoni Martins, R. (2017). To what degree are philosophy and the ecological niche concept necessary in the ecological theory and conservation? Eur. J. Ecol. 3, 42–54. doi: 10.1515/eje-2017-0005
Pianka, E. R. (1974). Niche overlap and diffuse competition. Proc. Natl. Acad. Sci. U.S.A. 71, 2141–2145. doi: 10.1073/pnas.71.5.2141
Pollard, E. (1981). Aspects of the ecology of the meadow brouw butterfly Maniola jurtina (L.) (Lepidoptera : Satyridae). Entomol. Gazette 32, 67–74.
Pywell, R. F., Warman, E. A., Sparks, T. H., Greatorex-Davies, J. N., Walker, K. J., Meek, W. R., et al. (2004). Assessing habitat quality for butterflies on intensively managed arable farmland. Biol. Conserv. 118, 313–325. doi: 10.1016/j.biocon.2003.09.011
Radchuk, V., Turlure, C., and Schtickzelle, N. (2013). Each life stage matters: the importance of assessing the response to climate change over the complete life cycle in butterflies. J. Anim. Ecol. 82, 275–285. doi: 10.1111/j.1365-2656.2012.02029.x
Reluga, T. C., and Shaw, A. K. (2015). Resource distribution drives the adoption of migratory, partially migratory, or residential strategies. Theor. Ecol. 8, 437–447. doi: 10.1007/s12080-015-0263-y
Revilla, E. (2003). What does the resource dispersion hypothesis explain, if anything? Oikos 101, 428–432. doi: 10.1034/j.1600-0706.2003.12087.x
Salz, A., and Fartmann, T. (2009). Coastal dunes as important strongholds for the survival of the rare Niobe fritillary (Argynnis niobe). J. Insect Conserv. 13, 643–654. doi: 10.1007/s10841-009-9214-5
Schtickzelle, N., and Baguette, M. (2004). Metapopulation viability analysis of the bog fritillary butterfly using RAMAS/GIS. Oikos 104, 277–290. doi: 10.1111/j.0030-1299.2004.12825.x
Schtickzelle, N., and Baguette, M. (2009). “(Meta)population viability analysis: a crystal ball for the conservation of endangered butterflies?,” in Ecology of Butterflies in Europe, eds J. Settele, T. G. Shreeve, M. Konvicka and H. Van Dyck (Cambridge: Cambridge University Press), 339–352.
Schtickzelle, N., Le Boulengé, E., and Baguette, M. (2002). Metapopulation dynamics of the bog fritillary butterfly: demographic processes in a patchy population. Oikos 97, 349–360. doi: 10.1034/j.1600-0706.2002.970305.x
Schultz, C. B., and Dlugosch, K. M. (1999). Nectar and hostplant scarcity limit populations of an endangered Oregon butterfly. Oecologia 119, 231–238. doi: 10.1007/s004420050781
Sharp, M. A., Parks, D. R., and Ehrlich, P. R. (1974). Plant resouces and butterfly habitat selection. Ecology 55:875.
Spitzer, L., Benes, J., Dandova, J., Jaskova, V., and Konvicka, M. (2009). The Large Blue butterfly, Phengaris [Maculinea] arion, as a conservation umbrella on a landscape scale: the case of the Czech Carpathians. Ecol. Indicat. 9, 1056–1063. doi: 10.1016/j.ecolind.2008.12.006
Thomas, C. D. (1991). Spatial and temporal variability in a butterfly population. Oecologia 87, 577–580. doi: 10.1007/BF00320423
Thomas, C. D., Thomas, J. A., and Warren, M. S. (1992). Distributions of occupied and vacant butterfly habitats in fragmented landscapes. Oecologia 92, 563–567. doi: 10.1007/BF00317850
Thomas, J. A. (1984). “The conservation of butterflies in temperate countries : past efforts and lessons for the future,” in The Biology of Butterflies, 11th symposium of the Royal Entomological Society of London, eds R. I. Vane-Wright and P. R. Ackery (London: Academic Press), 333–353.
Tudor, O., Dennis, R. L. H., Greatorex-Davies, J. N., and Sparks, T. H. (2004). Flower preferences of woodland butterflies in UK: nectaring specialists are species of conservation concern. Biol. Conserv. 119, 397–403. doi: 10.1016/j.biocon.2004.01.002
Turlure, C., Choutt, J., Baguette, M., and Van Dyck, H. (2010a). Microclimate buffering and resource-based habitat of a relict butterfly species: significance for conservation under climate change. Glob. Change Biol. 16, 1883–1893. doi: 10.1111/j.1365-2486.2009.02133.x
Turlure, C., Choutt, J., Van Dyck, H., Baguette, M., and Schtickzelle, N. (2010b). Functional habitat area as a reliable proxy for population size: case study using two butterfly species of conservation concern. J. Insect Conserv. 14, 379–388. doi: 10.1007/s10841-010-9269-3
Turlure, C., Pe'er, G., Baguette, M., and Schtickzelle, N. (2018). A simplified mark release recapture protocol to improve the cost-effectiveness of repeated population size quantification. Methods Ecol. Evol. 9, 645–656. doi: 10.1111/2041-210X.12900
Turlure, C., Radchuk, V., Baguette, M., Van Dyck, H., and Schtickzelle, N. (2011). On the significance of structural vegetation elements for caterpillar thermoregulation in two peat bog butterflies: Boloria eunomia and B. aquilonaris. J. Ther. Biol. 36, 173–180. doi: 10.1016/j.jtherbio.2011.02.001
Turlure, C., Schtickzelle, N., and Baguette, M. (2010c). Resource grain scales mobility and adult morphology in butterflies. Landsc. Ecol. 25, 95–108. doi: 10.1007/s10980-009-9403-3
Turlure, C., and Van Dyck, H. (2009). On the consequences of agressive male mate locating behaviour and microclimate for female host plant use in the butterfly Lycaena hippothoe. Behav. Ecol. Sociobiol. 63, 1581–1591. doi: 10.1007/s00265-009-0753-2
Turlure, C., Van Dyck, H., Goffart, P., and Schtickzelle, N. (2014). “Resource-based habitat use in Lycaena helle: significance of a functional, ecological niche-oriented approach,” in Jewels in the Mist: A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle, eds J. C. Habel, M. Meyer and T. Schmitt (Pentsoft press), 67–85.
Turlure, C., Van Dyck, H., Schtickzelle, N., and Baguette, M. (2009). Resource-based definition of the habitat, niche overlap and conservation of two glacial relict butterflies. Oikos 118, 950–960. doi: 10.1111/j.1600-0706.2009.17269.x
Unglaub, B., Steinfartz, S., Kühne, D., Haas, A., and Schmidt, B. R. (2018). The relationships between habitat suitability, population size and body condition in a pond-breeding amphibian. Basic Appl. Ecol. 27, 20–29. doi: 10.1016/j.baae.2018.01.002
van der Heiden, C. A., and Dorn, N. J. (2017). Benefits of adjacent habitat patches to the distribution of a crayfish population in a hydro-dynamic wetland landscape. Aquat. Ecol. 51, 219–233. doi: 10.1007/s10452-016-9612-1
Van Dyck, H. (2012). Changing organisms in rapidly changing anthropogenic landscapes: the significance of the ‘Umwelt'-concept and functional habitat for animal conservation. Evol. Appl. 5, 144–153. doi: 10.1111/j.1752-4571.2011.00230.x
Vanreusel, W., and Van Dyck, H. (2007). When functional habitat does not match vegetation types: a resource-based approach to map butterfly habitat. Biol. Conserv. 135, 202–211. doi: 10.1016/j.biocon.2006.10.035
Vera, F. W. M. (2002). The dynamic European forest. Arboricult. J. 26, 179–211. doi: 10.1080/03071375.2002.9747335
Verboom, J., Schotman, A., Opdam, P., and Johan, A. J. M. (1991). European Nuthatch metapopulations in a fragmented agricultural landscape. Oikos 61, 149–156. doi: 10.2307/3545332
Vincenot, C. E., Mazzoleni, S., Moriya, K., Cartenì, F., and Giannino, F. (2015). How spatial resource distribution and memory impact foraging success: a hybrid model and mechanistic index. Ecol. Complex. 22, 139–151. doi: 10.1016/j.ecocom.2015.03.004
Weber, N., Duengkae, P., Fahr, J., Dechmann, D. K. N., Phengsakul, P., Khumbucha, W., et al. (2015). High-resolution GPS tracking of Lyle's flying fox between temples and orchards in central Thailand. J. Wildlife Manage. 79, 957–968. doi: 10.1002/jwmg.904
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139. doi: 10.1080/00063659909477239
Keywords: butterflies, Lycaena helle, Lycaena hippothoe, Boloria eunomia, Boloria aquilonaris, Boloria selene, resource-based habitat, ecological niche
Citation: Turlure C, Schtickzelle N, Dubois Q, Baguette M, Dennis RLH and Van Dyck H (2019) Suitability and Transferability of the Resource-Based Habitat Concept: A Test With an Assemblage of Butterflies. Front. Ecol. Evol. 7:127. doi: 10.3389/fevo.2019.00127
Received: 31 January 2019; Accepted: 28 March 2019;
Published: 03 May 2019.
Edited by:
Ricardo Baldi, Centro Nacional Patagónico, ArgentinaReviewed by:
Piotr Nowicki, Jagiellonian University, PolandMartin Konvicka, University of South Bohemia, Czechia
Copyright © 2019 Turlure, Schtickzelle, Dubois, Baguette, Dennis and Van Dyck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Camille Turlure, Y2FtaWxsZS50dXJsdXJlQHVjbG91dmFpbi5iZQ==
 Camille Turlure
Camille Turlure Nicolas Schtickzelle
Nicolas Schtickzelle Quentin Dubois
Quentin Dubois Michel Baguette
Michel Baguette Roger L. H. Dennis
Roger L. H. Dennis Hans Van Dyck
Hans Van Dyck