- 1Research Group “Evolution of Communication”, Max Planck Institute for Ornithology, Seewiesen, Germany
- 2Department of Primatology, Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany
- 3Centre for the Advanced Study of Collective Behaviour, University of Konstanz, Konstanz, Germany
- 4Comparative BioCognition, Institute of Cognitive Science, University of Osnabrück, Osnabrück, Germany
- 5Center for Early Childhood Development and Education Research (CEDER), University of Osnabrück, Osnabrück, Germany
The emergence of intentional communication and the intentional presentation of objects have been highlighted as important steps in the ontogeny of cooperative communication in humans. Furthermore, intentional object presentation has been suggested as an extremely rare form of communication evolutionarily. Research on comparable means of communication in non-human species may therefore shed light on the selection pressures that acted upon components of human communication. However, the functions and cognitive mechanisms that underlie object presentation in animals are poorly understood. Here, we addressed these issues by investigating object presentations in wild, cooperative breeding Arabian babblers (Aves: Turdoides squamiceps). Our results showed that individuals presented objects to specific recipients. The recipients most often responded by approaching the signaler and the dyad then moveed jointly to copulate at a hidden location. We provide evidence that object presentations by Arabian babblers (i) do not represent a costly signal, as objects were not costly to acquire; (ii) were not used to trade food for sex, as the presentation of food was not more likely to result in copulation; and (iii) possessed hallmarks of first-order intentionality. These results show that intentional presentation of objects is not restricted to the primate linage and may suggest that the need to engage in cooperative interactions facilitates elaborate socio-cognitive performances.
Introduction
Animals use their body parts in manifold ways to communicate with each other (Bradbury and Vehrencamp, 1998). Some species also employ external tools to produce or modify signals (Smith and Bentley-Condit, 2010). For example, palm cockatoo males (Probosciger aterrimus) drum with sticks, presumably to attract females (Heinsohn et al., 2017). However, only a few species have been yet observed to use the presentation of an object as a communicative signal itself (e.g., bowerbirds, Ptilonorhynchidae; Madden and Balmford, 2004; Amazon river dolphins, Inia geoffrensis; Martin et al., 2008; ravens, Corvus corax; Pika and Bugnyar, 2011).
In contrast, as early as 12 months of age human infants present objects to attract and share attention with an interlocutor to a specific locus (Bates et al., 1979). These communicative presentations of objects involve a two-tiered intentional structure: combining the intention to get something done and the “referential” intention to draw the attention of the recipient to some third entity (Tomasello, 2006). This form of communication has been shown to play a pivotal role during the ontogeny of human cooperative communication by facilitating the learning of novel words (Dunham et al., 1993). In addition, it has been used as an indicator of language development in infants (Bruner, 1975; Morales et al., 2000). In the light of its supposed cognitive complexity and alleged absence in non-human great apes living in natural environments (Leavens et al., 1996; Tomasello, 2006), intentional object presentation has been argued to be an extremely rare form of communication evolutionarily (Tomasello, 2008; Allen et al., 2017). Understanding comparable communicative signals in other animal species is therefore crucial for being able to infer the selection pressures acting upon essential language components (Hauser et al., 2002).
During the last decade, communicative object presentations have been reported in distantly related species such as ravens (Pika and Bugnyar, 2011) and dolphins (Sousa sahulensis, Allen et al., 2017 and Inia geoffrensis, Martin et al., 2008), who present non-edible items mostly to a conspecific of the opposite sex (for an overview of object presentations in other species, see Pika, 2016). These studies have inspired an ongoing debate about whether similar cognitive mechanisms underlie object presentations in human and non-human species (Pika and Bugnyar, 2011; Vail et al., 2013; van Rooijen, 2015; Pika, 2016). In humans, communicative presentation of objects involves the use of intentional communication: the signaler's ability to act with—and the recipient's ability to recognize—the intention to communicate (Carpenter, 2012; Townsend et al., 2016). In non-human animals, however, object presentations have been argued to be based on simpler mechanisms (Vail et al., 2013), such as ritualization (van Rooijen, 2015). Nevertheless, the functions fulfilled by object presentations in animals are poorly understood (e.g., Madden and Balmford, 2004; Allen et al., 2017) and whether they qualify as being intentionally produced or not is almost completely unexplored (but see Pika and Bugnyar, 2011).
Here, we report object presentation behavior in a bird species: the Arabian babbler (Turdoides squamiceps). We investigated the function and whether object presentations qualified as intentionally produced means of communication. To make our results comparable with previous research on humans, we applied comparative methods that were developed to infer intentional communication in pre-linguistic human infants (Piaget, 1952; Bates et al., 1979; Bruner, 1981). Rather than investigating the evolutionary origins of object presentation in a particular species (e.g., whether object presentation by Arabian babblers is a ritualized performance of courtship feeding or not: van Rooijen, 2015), this comparative approach allows for an insightful comparison across species with regards to the cognitive complexity involved (Pika and Bugnyar, 2011; Fröhlich et al., 2016; Townsend et al., 2016). By taking this approach, we aim to facilitate lively exchanges between ethologists and developmental psychologists studying the evolution of communication (Pika, 2016; Townsend et al., 2016).
Arabian babblers are passerine birds that live across the Arabian Peninsula and Israel (IUCN, 2016). They reside year round in cohesive social groups of 2–20 birds (Zahavi, 1989). The dominant pair monopolizes the breeding within the group and produces 95% of the offspring (Lundy et al., 1998). Nonetheless, all group members contribute significantly to the raising of the offspring (Ostreiher, 1997). The species uses an elaborate system of vocal communication in a variety of contexts (e.g., allofeeding: Carlisle and Zahavi, 1986; alarm calls: Sommer et al., 2012). Although the gestural communication of Arabian babblers has not yet been studied systematically, BenMocha et al. (2018) recently reported that males and females often initiate mating by positioning themselves in a location visible to a specific group member only. Simultaneously, they present an object in their beaks. The couple then travels far away and/or hides behind thick vegetation to conceal the copulation from the view of conspecifics. If another group member approaches, the dyad stops its mating behavior (Ben Mocha et al., 2018). While “sneaky copulations” by subordinate animals have been observed in several other species (e.g., Alpine accentors, Prunella collaris; Davies et al., 1996), dominant individuals concealing their copulation from conspecifics is a rare phenomenon (Ford and Beach, 1951). It has thus been suggested that dominant Arabian babblers conceal their mating behavior as a means of preventing within-group social conflicts; and thereby, maintaining alloparental care obtained from helpers (Ben Mocha et al., 2018).
We studied a wild population of individually marked Arabian babblers in Israel (Zahavi, 1989) and documented copulation attempts and all behaviors that preceded them (see Table 1 for definitions). The main hypothesis tested was whether object presentations by Arabian babblers qualify as first-order intentionally produced gesture (i.e., “the signaller intends the signal to produce a response in the recipient, but does not not require that the recipient recognize this”: Townsend et al., 2016, p. 1427; Dennett, 1983; Sperber and Wilson, 1986).
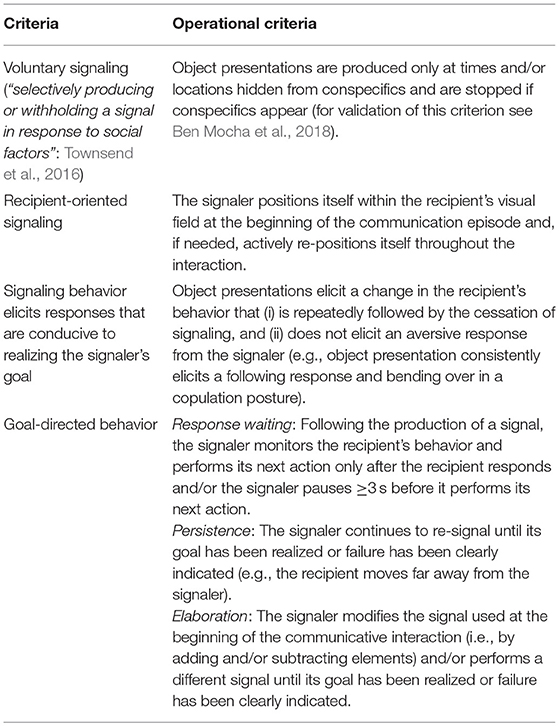
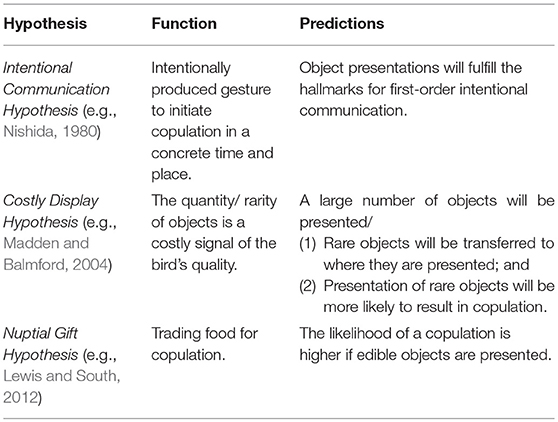
According to the Intentional Communication hypothesis, the presentation of an object is an intentionally produced gesture (Nishida, 1980). In some chimpanzee populations (e.g., Mahale in Tanzania, Nishida, 1980; Bossou in Guinea, Sugiyama, 1981) males and females clip leafs from detached branches to attract the attention of a recipient and invite him for copulation (“leaf-clipping” gesture). Matsumoto-Oda and Tomonaga (2005) suggested that this communicative tool use (Smith and Bentley-Condit, 2010) qualifies as intentional since it is selectively produced and withheld in response to audience composition. To test this hypothesis, we applied a cross-species framework (Townsend et al., 2016) for the inference of first-order intentional communication and examined whether the production of object presentation is (1) voluntarily; (2) recipient-oriented; (3) goal-directed; and (4) elicits responses that are conducive to realizing the signaler's goal (see Table 2 for operational criteria).
In addition, we tested alternative hypotheses that attribute no mentality (i.e., zero-order intentionality, Dennett, 1983; Sperber and Wilson, 1986) nor sophisticated cognition for the integration of objects within sexual interactions (Table 3). The Costly Display hypothesis posits that objects are costly components and thereby honestly advertise the signaler's quality. Object-use may impose significant search time either due to the high number of objects collected (e.g., black wheatear, Oenanthe leucura; Moreno et al., 1994) or the rarity of the presented objects in the habitat (e.g., bowerbirds, Madden and Balmford, 2004). Mates' responses to such a distinct collection of objects may thus be innate (e.g., generated by sexual selection: Uy and Borgia, 2000) and the underlying cognition may not be very complex (Vail et al., 2013). If object presentation is a costly signal, we predicted that birds would present a high number of objects. Alternatively, we predicted that (1) rare objects would be carried from where they had been discovered to the recipient or to a special arena; and (2) the presentation of rare, rather than abundant, objects would be more likely to result in copulation.
The Nuptial Gift hypothesis postulates that males trade food for sex (Lewis and South, 2012). This hypothesis assumes no communicative intentions at all. Rather, one bird may be attracted to a food item carried by the other, while the latter takes advantage and copulates with her. If this hypothesis were true, we expected that the presentation of edible, rather than non-edible, objects would be more likely to result in copulation.
Materials and Methods
Study Site and Population
Observations were conducted in the Shezaf Nature Reserve (30.718N/35.266E) and its surrounding areas located in Israel. The Arabian babbler population at the site has been studied since 1971 (Zahavi, 1989). Individuals are habituated to researchers and are each marked with colored rings in a way that provides comprehensive information on the life history, family relationships and dominance rank of most individuals (Ben Mocha, 2014). Research permission was obtained from the Israel Nature and Parks Authority (number: 2014/40304).
Behavioral Observations
Behavioral observations were carried out during three breeding seasons (January-June 2010, August 2011-July 2012, and February-June 2014). Observations took place over 4 h beginning when the group left the roosting tree at first light and were conducted from a distance of 2–20 m. During the first two breeding seasons, we studied the social behavior of the species (Ben Mocha, 2014) and opportunistically observed a total of 16 object presentations. These observations were documented in the study's logbook and via a series of still photos taken with a digital camera (Nikon D90; 4.5 frames per second) equipped with a telephoto lens (Nikkor 18–200 mm VR II). During the breeding season of 2014, we systematically followed the alpha female throughout her egg-laying period (i.e., when most copulations occurs: Perel, 1996), and documented a total of 57 object presentation (a total of 45 observation sessions/ 78.4 h). We filmed object presentations with a digital high-definition video camera (Canon LEGRIA HFM41) and recorded narration of the observed behaviors simultaneously to the visual recording.
Data Coding
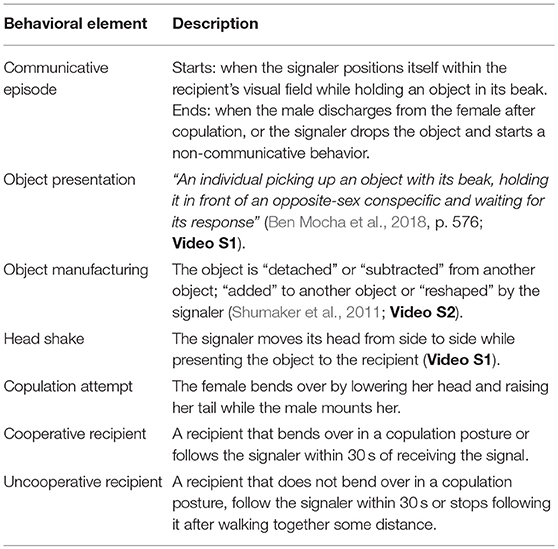
We used the programs Adobe Premiere Pro CS6 and Excel to code the photographs and videos following established coding schemes (Pika and Bugnyar, 2011). To create an ethogram of the object presentation behavior, we coded any descriptive element of this behavior (e.g., whether the signaler shook its head while presenting the object and whether the object was manufactured by the signaler before its presentation; see Table 1 for definitions).
To test the intentional communication hypothesis several parameters successfully applied in previous studies on intentional vocal and gestural signaling we coded: (1) whether signaling behavior was directed toward a specific recipient (see Table 2); (2) whether the recipient demonstrated a “voluntary response” to object presentation (yes/no). Namely, following signal production, the recipient stopped its previous behavior and started a new one without being physically manipulated by the signaler. For instance, the recipient stopped foraging and approached the signaler. This definition allowed us to exclude cases when the recipient continued his previous behavior (i.e., did not respond to the signal), but later responded to a physical act from the signaler (e.g., the recipient closed her beak and moved away to avoid being fed); (3) the first voluntary response of the recipient to object presentation; (4) the recipient's behavior at the moment when the signaler stopped signaling. Following previous research (Hobaiter and Byrne, 2014; Moore, 2014), we used this parameter as an approximation for the signaler's goal, which we defined as a change in the recipient's behavior that (i) is repeatedly followed by the cessation of signaling (Schel et al., 2013) and (ii) does not elicit an aversive response from the signaler (Hobaiter and Byrne, 2014). An example for a response that was coded as fulfilling the desired goal of the signaler' is the bending over for copulation posture. An example for a response that was not considered as a desired goal for the signaler was the recipient moving away from the signaler (since the signaler persisted with signaling and continued to reposition itself within the recipient visual field).
To infer goal-directed signaling, we coded (5) whether the recipient was cooperative or uncooperative (see Table 1), whether the signaler demonstrated response waiting, persistence of signaling, and elaboration of signaling (see Table 2 for definitions) and the duration of signaling behavior. Duration measurements were extracted from video recordings with an accuracy of 1 s. Although all interactions were observed from the moment the signaler positioned itself within the recipient's visual field while holding an object, video recordings occasionally started 1–5 s after. The reported durations are therefore underestimates.
To test the costly display and the nuptial gift hypotheses we coded: (6) the type of object presented (up to the species level when applicable); (7) whether the object was found <15/>15 m from where it was presented; (8) whether the presented object was abundant (if numerous similar objects were present where the presentation occurred, n = 32) or rare (if similar objects were not observed where the presentation occurred, n = 4). Edible objects that were consumed and could not be identified to the species level were coded as unidentifiable in term of abundance (n = 23, see Table S1 for a list of presented objects); (9) whether the object presentation ended with a copulation attempt/ no cooperation from the recipient/ an interruption by conspecifics; and (10) what happened to the object at the end of the social interaction.
Inter-observer Reliability
A second observer, blind to the hypotheses tested, coded 22% of the interactions. Inter-observer reliability was tested using Cohen's Kappa for categorical variables (Fleiss et al., 1981) and Spearman's rank correlation for the duration of signaling. The latter was calculated using a R function (R Core Team, 2017) provided by Roger Mundry. This function calculates the coefficient (using the R function cor.test), but determines an exact P-value based on enumeration of all possible outcomes for sample sizes up to eight, and an approximate permutation test (n = 1,000) for larger samples. We used this function since it reveals a more reliable P-value in the presence of tied observations. All reliability tests results were significant (P ≤ 0.002). Cohen's K ranged from 0.81 to 1.00 and the correlation coefficient for the duration of signaling behavior was 0.96 (see Table S2 for full results).
Statistical Analysis
We used R version 3.3.3 with the packages irr (Gamer et al., 2012), parallel (R Core Team, 2017), and lme4 (version 1.1-11; Bates et al., 2015) for statistical analyses. The significance level was set to α = 0.05 and all tests were two-tailed. Due to the elusive nature of Arabian babblers' mating behavior (Ben Mocha et al., 2018) and the fact that some objects were eaten by the animals, data for some of the tested criteria were not always available. Therefore, for some analyses we report a different sample size. To avoid pseudo-replication (Waller et al., 2013), we used Linear Mixed Models (LMM) and Generalized Linear Mixed Models (GLMM) (Baayen, 2008) with the signaler, recipient and group identities as random effects. To avoid a multiple testing issue due to several predictors of interest in a model (Forstmeier and Schielzeth, 2011), we conducted a full-null model comparison for each model. That is, the full version of each model (i.e., containing the intercept and all fixed and random effects) was compared to a respective null model (containing the intercept and random effects only). To test whether these two models differed significantly we used a likelihood ratio test (R function “anova” with argument test “Chisq”: Dobson and Barnett, 2008; Barr et al., 2013). Only full models that were significantly different from their null versions were considered further and all predictors included in them were discussed.
Model stability was assessed by excluding each level of the random effects (the signaler, recipient and group identities), one at a time, from the dataset and comparing the model estimates derived from these data with those derived from the entire data set (Nieuwenhuis et al., 2012). We found no influential levels of random effects to exist. To keep type I error rate at the nominal level of 5%, we included random slopes which were likely to be identifiable (e.g., with sufficient variation of the fixed effects predictor per level of the random effect: Schielzeth and Forstmeier, 2009; Barr et al., 2013. See Table S3 for the random slops included in each model).
The Recipient's Response to Object Presentation
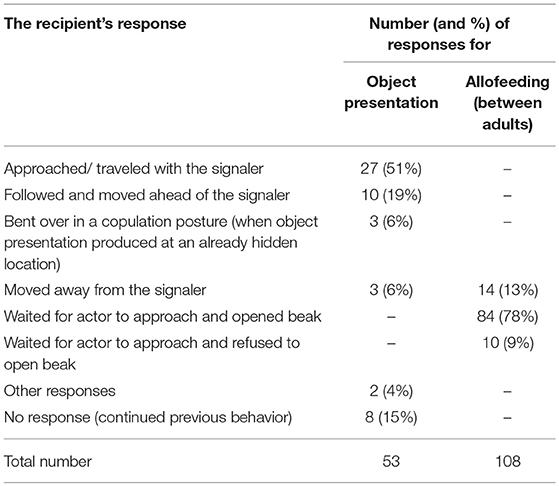
To examine whether the behaviors of recipients following object presentations were indeed responses to these signals (rather than being fixed responses to the sight of a conspecific holding an object), they were compared to the behaviors of Arabian babblers observing a group member performing a physically similar behavior. As object presentations involve the individual standing and moving while holding an object in its beak, responses obtained for this behavior were compared with those obtained toward allofeeding attempts between adult birds (i.e., when an adult bird (> 1 year old) moved toward another adult while holding a food item in its beak). Allofeeding attempts, which is a common behavior among Arabian babblers (Kalishov et al., 2005), were documented throughout our second research period (August 2011-July 2012). Data were coded such that for each object presentation and allofeeding attempt the data included one row for each of the eight possible response behaviors that could happen (see Table 4 for list of observed responses). We used a GLMM with a binomial error structure and logit link function (McCullagh and Nelder, 1996; Baayen, 2008). The response variable indicated “one” for the behavior that was actually performed by the recipient in an episode and “zero” for the other seven possible behaviors (i.e., the recipient could only show one behavior per signaling event). The model included the fixed effect of condition (object presentation/allofeeding), a random intercept for the particular episode and a random intercept for possible response behaviors (factor with eight levels). The key term in this model was the random slope (Schielzeth and Forstmeier, 2009; Barr et al., 2013) of condition within the response type. This term accounts for the possibility that certain types of response would be more likely to occur in one of the conditions. It was considered the key term of the model since it is conceptually equivalent to the interaction between condition and behavior type. The random slope tests the hypothesis in question (whether certain behaviors would be particularly common in one condition whereas others would be common in the other) since we included behavior type as random effect (the alternative, which is to set the factor as a fixed effect, would have been resulted in a unnecessary complex model with seven estimates for seven dummy variables plus seven variables for the interaction between behavior and condition, see Table S4 for the model's code). We estimated the significance level by permutation test (Adams and Anthony, 1996) to account for the fact that the possible choices of responses within an episode were not independent (i.e., that only one could be performed). To do so, we randomized the behavior performed by the recipient within each object presentation and allofeeding attempt. Next, we performed a thousand permutations into which we included the original data as one permutation. Finally, we estimated the p-value as the proportion of permutations that revealed a test statistic at least as large as that of the original data (as a test statistic we used the standard deviation estimated for the random slope of condition-within-responses-type). Sample size for the model was 1,288 possible response behaviors (i.e., 8 behaviors multiplied by 161 object presentations and allofeeding attempts) involving 31 actors, 29 recipients and 9 social groups.
Goal Directed Signaling
We tested whether signaling behavior is goal directed from three perspectives. First, we compared the duration of signaling behavior toward cooperative vs. uncooperative recipients. An LMM was set with the duration of signaling (in seconds) as the response variable and the recipient behavior (cooperative/uncooperative) as a fixed effect. The duration of signaling had a right skewed distribution. It was thus log transformed to meet the assumptions of normally distributed and homogeneous residuals and to avoid influential cases. Sample size for the model was a total of 52 object presentations involving 9 signalers, 8 recipients, and 5 social groups.
Second, we used two separate GLMMs (hereafter the “persistence” model and the “elaboration” model) to compare the probability that the signaler would persist and elaborate its signaling behavior toward cooperative vs. uncooperative recipients. Both models had a binomial error structure and logit link function. As a response variable, the persistence model included the signaler's behavior (continue signaling/quit signaling) and the elaboration model included the signaler's behavior (elaborated/did not elaborate the signal). The fixed effect in both models was the recipient's behavior (cooperative/uncooperative). Since the response variable in both models did not include cases in which the signaler persisted or elaborated its signaling behavior toward a cooperative recipient, the models suffered from complete separation (Field, 2005). To cope with this we used the approach proposed by Goodale et al. (2014). For the persistence model: we modified the data to make it include all possible cases (i.e., also cases of persistence toward cooperative recipient). To this end, we altered one of the episodes in which the recipient was cooperative and the signaler quit signaling to indicate a signaler that persisted with signaling. We then fitted a model to this modified dataset and conducted a full-null model comparison. Note that this is a conservative approach since the difference between the two conditions (i.e., the number of cases in which the signaler persisted when communicating with a cooperative vs. uncooperative recipient) is made slightly smaller than it actually was. However, altering only one episode may be sensitive to exceptional cases. We thus repeated this procedure for each of the 15 object presentations in which the signaler met a cooperative recipient and quit signaling. This resulted in 15 model results (i.e., estimates, standard errors, and p-values), for which we examined the entire range of P-values. To simplify the presentation of the results, we present the range of P values and number of models with P < 0.05. To allow full account of the analysis' robustness, we present the results of all “persistence” models in Table S5. We used the same procedure for the elaboration model (see also Table S7 for the results of all “elaboration” models). Sample size for both models was 49 object presentations involving 10 signalers, 9 recipients, and 6 social groups.
Did the Presentation of Edible Objects Affect Mating Success?
To examine whether the presentation of edible objects affected mating success, we used a GLMM with a binomial error structure, logit link function and a response variable indicating whether the object presentation resulted in a copulation attempt or not. All cases when object presentation was interfered by conspecifics were excluded from the data. The model had two fixed effects (i) object type (edible/non-edible) and (ii) the signaler's dominance rank (coded as dominant for alpha males and females and as subordinate for all others). The model also included the dyad identity as an additional random effect. Since the model tests what objects were more likely to cause the recipient to bend over for copulation or to move away from the signaler, in episodes when the signaler presented several objects (n = 7) we only considered the last object presented (i.e., the object that had been presented when the female bent over or moved away from the signaler). Sample size for the model was 62 object presentations that involved 13 signalers and 14 recipients forming 15 different dyads from 10 social groups.
Results
Sex and Dominance Rank of Signalers
We recorded a total of 73 object presentations, which were performed by eight alpha males (n = 58), two alpha females (n = 3), and six subordinate males (n = 12) from eleven social groups.
Forty-seven object presentations (64%) resulted in copulation. We recorded five additional copulations that were not initiated by object presentation. These copulations occurred after the alpha male suddenly left the group (e.g., to chase after an intruder) and seem to have been initiated by the bending over of the alpha female in front of a subordinate male (for an account of these observations see Ben Mocha et al., 2018).
Preparation for Presentation
At least 23 different types of objects were presented (Figure 1 and Table S1 in the Supplementary Materials). Eighteen percent of objects were manufactured before they were presented (n = 49): Live prey was usually killed (10%) and in some cases vegetative parts were detached from a larger plant (8%; Video S2).

Figure 1. Object presentations of (A) a twig; (B) a Nitraria retusa fruit; (C) an Acacia raddiana leaf (next to the bird's left leg) that was dropped to the ground and replaced by a Loranthus acacia flower; and (D) an eggshell.
Intentional Communication Hypothesis
Do Object Presentations Qualify as Intentional Communication?
Recipient-oriented signaling
After the signaler had positioned itself to be within the visual field of a specific recipient (see Ben Mocha et al., 2018), it often subtly shook its head while holding the object in its beak (86%, n = 44. Video S1). Signalers demonstrated then “response waiting” by pausing for more than 3 s, performing their next action only after the recipient had responded (e.g., starting to move toward a hiding mating location) or by fulfilling both of these criteria (11, 13, and 74% of object presentations respectively, n = 53. Videos S1,S2).
Furthermore, as the recipient approached the signaler, the latter moved further away toward a hidden location. During their joint travel, signalers frequently moved out of the recipients' sight due to vegetation cover. In all cases when visual contact between interlocutors was interfered, the signaler actively returned to re-signal within the recipient's visual field. Thereby demonstrating sensitivity to the recipient's attentional state (100% of object presentations, n = 40. Video S2).
Response to object presentation
Eighty six percent of object presentations elicited a voluntary response from the recipient (n = 73). The majority of the first responses consisted of traveling with the signaler and bending over in a copulation posture (76%; Table 4). These responses to object presentation were not fixed responses toward an individual holding an object, as a physically similar behavior which involves the holding and moving with an object (i.e., allofeeding attempts) elicited significantly different responses (Permutation test: standard deviation of the random slope of condition within behavior = 6.367, P = 0.002; Table 4 and Table S4).
The signaler's goal
The signaler quit signaling by dropping the object after the recipient bent over in a copulation posture (66% of object presentations), moved far away from the signaler (23% of object presentations), or after another group member appeared (11% of object presentations, n = 73). We thus considered the signaler's goal as to be followed for copulation.
Goal-directed behavior
Signaling behavior was significantly shorter toward a cooperative than an uncooperative recipient (LMM, estimate ± SE = −0.66 ± 0.26, χ2 = 5.17, df = 1, P = 0.02; Figure 2A and Table S9).
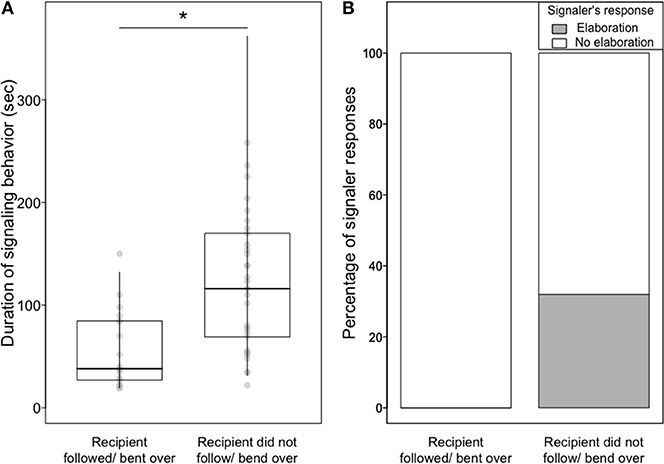
Figure 2. (A) Median, quartiles, and quantiles (0.025 and 0.975) of the duration of object presentations according to whether the recipient followed the signaler/bent over in a copulation posture or did not, (B) percentage of episodes in which a signaler elaborated the signal and continued with the same signal according to whether the recipient followed the signaler/bent over in a copulation posture or did not.
Furthermore, signalers were less likely to persist in, and to elaborate, their signaling with a cooperative recipient rather than with an uncooperative recipient (Persistence (n = 15 GLMMs): range of P = 0.0003–0.02, number of P < 0.05 = 15; see Tables S5, S6 for all models' results and Video S2. Elaboration (n = 15 GLMMs): range of P = 0.03–0.17, number of P < 0.05 = 10; see Tables S7, S8 for all models' results and Figure 2B). Elaboration of object presentation included one or more of the following: adding soft vocalizations (4 episodes), replacing the initial object with another (6 episodes; Video S1), adding an additional object (1 episode, Video S1), bending over (2 episodes), and feeding the recipient (1 episode).
Costly Display Hypothesis
Do Birds Present a High Number of Objects?
In 90% of object presentations only one object was presented (n = 73). In nine percent of presentations the signaler replaced the initial object with another. Only a single episode involved four objects that were replaced one after the other.
Are the Presented Objects Rare in the Natural Habitat?
In 95% of object presentations the signaler used an object that was found in its close vicinity (<15 m; n = 62). In 89% of presentations similar objects to the one presented were abundant in the immediate surrounding area of the signaler and recipient (n = 38 objects). For example, signalers presented a leaf, a twig, a seedpod or an inflorescence of the most common tree in the habitat—twisted acacia (Acacia raddiana: Ridley, 2007)—while standing under this tree species (see Figures 1A,C and Video S1). The presentation of abundant (n = 32) and rare objects (n = 4) had a similar probability to result in copulation (71 and 75%, respectively), although the number of rare objects was too small for powerful statistical analysis.
Nuptial Gift Hypothesis
Does the Presentation of an Edible Object Increase the Likelihood of Copulation?
Fifty-seven percent of objects were non-edible (n = 79; see Table S1 for the type of objects presented). With a single exception, all edible objects were consumed by the signaler, the recipient or, in three cases when the recipient did not responded, they were given to a dependent fledgling (see Table S10 for post-presentation usage of objects). Whether the presented object was edible or not did not affect the probability that an object presentation would result in copulation (GLMM: χ2 = 1, df = 2, P = 0.6; Table S11). No post-presentation usage was documented for non-edible objects.
Discussion
The present study investigated the function of object presentations by Arabian babblers and whether their use qualifies as first-order intentional communication. We describe a behavior in which male and female Arabian babblers present an object within the visual field of a specific recipient. Recipients often responded by approaching the signaler and the pair then traveled together to copulate at a location hidden from the view of other group members.
The dependency on external objects is disadvantageous in comparison to being able to signal with one's own body. We thus tested three alternative hypotheses about the function of object presentations in Arabian babblers. We found that most episodes involved the presentation of a single object. The majority of these objects were collected within a few meters from where the presentation took place and while similar objects were abundant in the immediate environmental surrounding of the signaler and recipient. These findings contradict the predictions implied by the costly display hypothesis (Madden and Balmford, 2004), which suggests that the presentation of a high number of objects or rare objects is time-consuming and thus represents a honest signal of the bird's quality. Our results also contradict the nuptial gift hypothesis (Lewis and South, 2012), which predicts that edible or useful objects would be traded for sex. In contrast to this prediction, our data showed that the presentation of edible objects did not affect the probability that copulations would occur and non-edible objects were not used after presentation.
In many of the species that use costly displays or deliver food gifts during courting, males and females have little previous experience with each other (e.g., bowerbirds: Borgia, 1985; spiders: Lewis and South, 2012). In such cases, presentation of costly objects can signal mate quality honestly to an unfamiliar potential mate (Zahavi and Zahavi, 1997). However, Arabian babblers live in stable social groups where individuals have daily opportunities to assess each other's qualities directly (e.g., by observing foraging success; Keynan et al., 2014) and object presentation is used to initiate copulation with a familiar group member. Hence, it is very unlikely that Arabian babblers use this signal for mate selection.
According to the intentional communication hypothesis object presentations are, primarily, gestural means of communication with a concrete function (Nishida, 1980). In the Arabian babblers their function is to invite a recipient for copulation at a given time and location. This hypothesis is supported by our findings that object presentations possess key hallmarks used to infer first-order intentional communication across species (Townsend et al., 2016): (i) efforts are made to produce a signal when it cannot be detected by adult group members and to withhold it when privacy is interrupted (i.e., voluntary signaling: Ben Mocha et al., 2018); (ii) signalers flexibly positioned themselves within the recipients' visual fields throughout the communicative episode (i.e., recipient-oriented signaling); (iii) object presentations consistently elicted following and bending-over responses, resulting in termination of signaling (i.e., the signal elicts responses that are conducive to realizing the signaler's goal), and (iv) signalers persisted and elaborated their signaling behavior toward uncooperative recipients (i.e., goal-directed signaling).
This first, systematic assessment of different markers of first-order intentionality in the same signal and species adds to the growing evidence that intentional communication is not restricted to the primate lineage (Pika and Bugnyar, 2011; Vail et al., 2013). The finding of intentionally produced object presentations in distantly related species that rely on cooperative interactions (e.g., humans, Arabian babblers, ravens, and grouper fish) provides further support for the hypothesis that the need to engage in cooperation facilities social-cognitive performances (Vygotsky, 1978; Burkart et al., 2009; Pika, 2016). To test this hypothesis further, it will be crucial to investigate intentional communication and object presentations in closely related species that present different degrees of cooperation.
The discreet performance of Arabian babblers' object presentation points to an intriguing aspect of the phenomenon. Animals often use distinct signals to initiate copulation. For example, male zebra finches (Taeniopygia guttata) sing and dance (Birkhead et al., 1988), while Alpine accentor and dunnock (Prunella modularis) females employ a distinct body posture in front of males (Davies et al., 1996). Distinct signals do not overlap with other environmental stimuli and are thus easly detected against back-ground noise (Rendall et al., 2009). Nevertheless, Arabian babblers subtly present objects that are regularly held during non-communcative behaviors. Such a non-distinct signal should provide an advantage that overcomes its presumed dificulty to be detected. Nishida (1980) suggested that leaf-clipping in the Mahale chimpanzee population (Tanzania) resembles daily behaviors in order to conceal it from an unintended audience: For those who see only part of the leaf-clipping, the leaf-clipper may appear to be engaging in a non-communicative behavior (e.g., eating leafs). Unintended observers would then be less likely to attack the signaler than if the latter had used a distinct courtship display (Nishida, 1980). In our study, alpha males appeared during three presentations by subordinate males. The subordinate males then dropped their objects and the alpha males showed no aggressive behaviors (e.g., an attack or display). In three other episodes, a more dominant individual appeared when a subordinate male was in close proximity to the alpha female after they had copulated opportunistically without using an object presentation. Yet in these episodes the dominant male acted to separate the couple by physical aggression or by producing dominance vocalizations (termed “reprimand calls”: Zahavi, 1988; see also Video S3 in Ben Mocha et al., 2018). Although further data are needed to test this aspect statistically, these observations suggest that object presentations may be less likely to be detected as a signal by an unintended audience. They may therefore be used as an additional means of concealing the initiation of mating in this species.
From the recipient's perspective, signals that resemble non-communicative behaviors pose a challenge since it must differentiate communicative from non-communicative performances of a certain behavior. For example, object presentations and leaf-clipping gestures are similar to other frequent behaviors of Arabian babblers and chimpanzees, respectively: Arabian babblers hold and carry food items and nesting materials during sentinel replacements (Ben Mocha, 2014), while chimpanzees manipulate leaves for food-intake (Nishida, 1980). Both species also initiate copulation using other signals (Goodall, 1986; Hobaiter and Byrne, 2014). Hence, a reliable association between object holding/ leaf manipulation per se and mating initiation is less likely to be established (Scott-Phillips, 2015). Associative learning is thus not likely to underlie the proper response of recipients (Seed and Byrne, 2010; Sievers and Gruber, 2016). Instead, recipients may use an ensemble of contextual cues to infer the concrete meaning of object holding and leaf manipulation (e.g., the timing of communication, the identity of the signaler). An additional explanation may be that signalers facilitate the recognition of their communicative intentions by “overt intentional” acts (Grice, 1957). For instance, by gently shaking their heads from side to side while holding the object (see Video S1). Overtly intentional acts play a crucial role in how humans identify behaviors as communicative by calling attention to the very fact that communicative intention is being expressed (Grice, 1957; Scott-Phillips, 2015). Interestingly, humans from different cultures often supplement their sexual communication with overtly intentional acts to make communication accessible to a certain recipient only. For instance, when gardening in the presence of others, a husband from the Malekula people of Melanesia may invite his wife for intercourse by “break[ing] a stick purposely so that she could see…when the others were not looking” (Deacon and Wedgwood, 1934, p. 551). Breaking a stick is a functional act for a gardener. But doing this “purposely” visible only for a specific recipient makes it a communicative act. Future studies in animal communication should therefore investigate subtle modifications of signalers' behavior to determine whether overtly intentional acts are being used (Scott-Phillips, 2015; Sievers and Gruber, 2016).
Conclusions
Here, we present the first systematic investigation of object presentation behavior in the Arabian babbler. We provide evidence that this behavior is used to initiate concealed copulation and that its production fulfills the criteria of first-order intentionality. Our results support the view that first-order intentional presentation of objects is not uniquely human. To test the role cooperation may have played in the evolution of communication, future studies should investigate object presentations in species that exhibit different degrees of cooperation. We call for greater research attention to tackle how recipients recognize the communicative intention of signalers when non-distinct signals are used.
Author Contributions
YBM and SP designed the study and wrote the manuscript. YBM collected and analyzed the data.
Funding
DAAD (The German Academic Exchange Service); IMPRS for Organismal Biology; The DFG Center of Excellence 2117: Center for the Advanced Study of Collective Behaviuor (ID: 422037984).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Roger Mundry for statistical advice and comments on earlier drafts. We thank Amotz Zahavi and Avishag Zahavi for introducing YBM to Arabian babblers and enabling research at their site. We are thankful to Yael Alon, Arnon Datner, Oded Keynan, Dorit Narisna, Yonatan Narisna, Roni Ostreiher, and Peter Santema for invaluable assistance in the field and to Manuela Jäger for the reliability coding. We thank Christophe Boesch for providing us with a stimulating working environment at the Max Planck Institute for Evolutionary Anthropology.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00087/full#supplementary-material
References
Adams, D. C., and Anthony, C. D. (1996). Using randomization techniques to analyse behavioural data. Anim. Behav. 51, 733–738. doi: 10.1006/anbe.1996.0077
Allen, S. J., King, S. L., Krützen, M., and Brown, A. M. (2017). Multi-modal sexual displays in Australian humpback dolphins. Sci. Rep. 7:13644. doi: 10.1038/s41598-017-13898-9
Baayen, R. H. (2008). Analyzing Linguistic Data: A Practical Introduction to Statistics Using R. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511801686
Barr, D. J., Levy, R., Scheepers, C., and Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 68, 255–278. doi: 10.1016/j.jml.2012.11.001
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bates, E., Benigni, L., Bretherton, I., Camaioni, L., and Volterra, V. (1979). The Emergence of Symbols: Cognition and Communication in Infancy. New York, NY: Academic Press.
Ben Mocha, Y. (2014). The Social and Functional Role of Sentinel Behavior in the Arabian Babbler Dominant Arabian Babblers (Turdoides squamiceps). MSc thesis, Tel-Aviv University.
Ben Mocha, Y., Mundry, R., and Pika, S. (2018). Why hide? Concealed sex in dominant Arabian babblers (Turdoides squamiceps) in the wild. Evol. Hum. Behav. 39, 575–582. doi: 10.1016/j.evolhumbehav.2018.05.009
Birkhead, T. R., Clarkson, K., and Zann, R. (1988). Extra-pair courtship, copulation and mate guarding in wild zebra finches Taeniopygia guttata. Anim. Behav. 36, 1853–1855. doi: 10.1016/S0003-3472(88)80133-7
Borgia, G. (1985). Bower quality, number of decorations and mating success of male satin bowerbirds (Ptilonorhynchus violaceus): an experimental analysis. Anim. Behav. 33, 266–271. doi: 10.1016/S0003-3472(85)80140-8
Bradbury, J. W., and Vehrencamp, S. L. (1998). Principles of Animal Communication. Sunderland: Sinauer Associates.
Bruner, J. S. (1975). The ontogenesis of speech acts. J. Child Lang. 2, 1–19. doi: 10.1017/S0305000900000866
Bruner, J. S. (1981). “Intention in the structure of action and interaction,” in Advances in Infancy Research, Vol. 1, ed L. P. Lipsitt (Ablex; Norwood), 41–56.
Burkart, J. M., Hrdy, S. B., and van Schaik, C. P. (2009). Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186. doi: 10.1002/evan.20222
Carlisle, T. R., and Zahavi, A. (1986). Helping at the nest, allofeeding and social status in immature arabian babblers. Behav. Ecol. Sociobiol. 18, 339–351. doi: 10.1007/BF00299665
Carpenter, M. (2012). Joint attention in humans and animals. Encycl. Sci. Learn. 1663–1664. doi: 10.1007/978-1-4419-1428-6_802
Davies, N. B., Hartley, I. R., Hatchwell, B. J., and Langmore, N. E. (1996). Female control of copulations to maximize male help: a comparison of polygynandrous alpine accentors, Prunella collaris, and dunnocks, P. modularis. Anim. Behav. 51, 27–47. doi: 10.1006/anbe.1996.0003
Deacon, B., and Wedgwood, C. (1934). Malekula: A Vanishing People in the New Hebrides. London: George Routledge and Sons. Available online at: http://ehrafworldcultures.yale.edu/document?id=oo12–004
Dennett, D. C. (1983). Intentional systems in cognitive ethology: the ‘Panglossian paradigm' defended. Behav. Brain Sci. 6, 343–390.
Dobson, A. J., and Barnett, A. (2008). An Introduction to Generalized Linear Models. Boca Raton, FL: CRC press.
Dunham, P., Dunham, F., and Curwin, A. (1993). Joint attentional statements and lexical acquisition at 18 months. Dev. Psychol. 29, 827–831. doi: 10.1037/0012-1649.29.5.827
Fleiss, J., Levin, B., and Cho Paik, M. (1981). Statistical Methods for Rates and Proportions. 3rd Edn. New Jersey, NJ: John Wiley and Sons.
Forstmeier, W., and Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner's curse. Behav. Ecol. Sociobiol. 65, 47–55. doi: 10.1007/s00265-010-1038-5
Fröhlich, M., Kuchenbuch, P., Müller, G., Fruth, B., Furuichi, T., Wittig, R. M., et al. (2016). Unpeeling the layers of language: bonobos and chimpanzees engage in cooperative turn-taking sequences. Sci. Rep. 6. doi: 10.1038/srep25887
Gamer, M., Lemon, J., and Singh, I. F. P. (2012). irr: Various Coefficients of Interrater Reliability and Agreement. Available online at: https://cran.r-project.org/package=irr
Goodale, E., Ratnayake, C. P., and Kotagama, S. W. (2014). Vocal mimicry of alarm-associated sounds by a drongo elicits flee and mobbing responses from other species that participate in mixed-species bird flocks. Ethology 120, 266–274. doi: 10.1111/eth.12202
Goodall, J. (1986). The Chimpanzees of Gombe: Patterns of Behaviour. Cambridge: Belknap Press of Harvard University Press.
Hauser, M. D., Chomsky, N., and Fitch, W. T. (2002). The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579. doi: 10.1126/science.298.5598.1569
Heinsohn, R., Zdenek, C. N., Cunningham, R. B., Endler, J. A., and Langmore, N. E. (2017). Tool-assisted rhythmic drumming in palm cockatoos shares key elements of human instrumental music. Sci. Adv. 3:e1602399. doi: 10.1126/sciadv.1602399
Hobaiter, C., and Byrne, R. W. (2014). The meanings of chimpanzee gestures. Curr. Biol. 24, 1601–1605. doi: 10.1016/j.cub.2014.05.066
IUCN (2016). Arabian Babbler. Available online at: http://www.iucnredlist.org/details/22716364/0 (Accessed April 2, 2016).
Kalishov, A., Zahavi, A., and Zahavi, A. (2005). Allofeeding in Arabian babblers (Turdoides squamiceps). J. Ornithol. 146, 141–150. doi: 10.1007/s10336-005-0073-x
Keynan, O., Ridley, A. R., and Lotem, A. (2014). Social foraging strategies and acquisition of novel foraging skills in cooperatively breeding Arabian babblers. Behav. Ecol. 26, 1–8. doi: 10.1093/beheco/aru181
Leavens, D. A., Hopkins, W. D., and Bard, K. A. (1996). Indexical and referential pointing in chimpanzees (Pan troglodytes). J. Comp. Psychol. 110, 346–353. doi: 10.1037/0735-7036.110.4.346
Lewis, S., and South, A. (2012). The evolution of animal nuptial gifts. Adv. Study Behav. 44, 53–97. doi: 10.1016/B978-0-12-394288-3.00002-2
Lundy, K. J., Parker, P. G., and Zahavi, A. (1998). Reproduction by subordinates in cooperatively breeding Arabian babblers is uncommon but predictable. Behav. Ecol. Sociobiol. 43, 173–180. doi: 10.1007/s002650050478
Madden, J. R., and Balmford, A. (2004). Spotted bowerbirds Chlamydera maculata do not prefer rare or costly bower decorations. Behav. Ecol. Sociobiol. 55, 589–595. doi: 10.1007/s00265-003-0737-6
Martin, A. R., da Silva, V. M. F., and Rothery, P. (2008). Object carrying as socio-sexual display in an aquatic mammal. Biol. Lett. 4, 243–245. doi: 10.1098/rsbl.2008.0067
Matsumoto-Oda, A., and Tomonaga, M. (2005). “Intentional” control of sound production found in leaf-clipping display of Mahale chimpanzees. J. Ethol. 23, 109–112. doi: 10.1007/s10164-004-0133-3
Moore, R. (2014). Ape gestures: interpreting chimpanzee and bonobo minds. Curr. Biol. 24, R645–R647. doi: 10.1016/j.cub.2014.05.072
Morales, M., Mundy, P., Delgado, C. E. F., Yale, M., Messinger, D., Neal, R., et al. (2000). Responding to joint attention across the 6- through 24-month age period and early language acquisition. J. Appl. Dev. Psychol. 21, 283–298. doi: 10.1016/S0193-3973(99)00040-4
Moreno, J., Soler, M., Møller, A. P., and Linden, M. (1994). The function of stone carrying in the black wheatear, Oenanthe leucura. Anim. Behav. 47, 1297–1309. doi: 10.1006/anbe.1994.1178
Nieuwenhuis, R., te Grotenhuis, M., and Pelzer, B. (2012). influence.ME: tools for detecting influential data in mixed effects models. R J. 4, 38–47. doi: 10.32614/RJ-2012-011
Nishida, T. (1980). The leaf-clipping display: a newly-discovered expressive gesture in wild chimpanzees. J. Hum. Evol. 9, 117–128. doi: 10.1016/0047-2484(80)90068-8
Ostreiher, R. (1997). Food division in the Arabian babbler nest: adult choice or nestling competition? Behav. Ecol. 8, 233–238. doi: 10.1093/beheco/8.2.233
Perel, J. (1996). Competition for Breeding Between Arabian Babbler Males. MSc thesis, Tel-Aviv University.
Pika, S. (2016). Response to: commentary: the use of referential gestures in ravens (Corvus corax) in the wild. Front. Ecol. Evol. 4:121. doi: 10.3389/fevo.2016.00121
Pika, S., and Bugnyar, T. (2011). The use of referential gestures in ravens (Corvus corax) in the wild. Nat. Commun. 2:560. doi: 10.1038/ncomms1567
R Core Team (2017). R: A Language and Environment for Statistical Computing. Available online at: http://www.r-project.org/
Rendall, D., Owren, M. J., and Ryan, M. J. (2009). What do animal signals mean? Anim. Behav. 78, 233–240. doi: 10.1016/j.anbehav.2009.06.007
Ridley, A. R. (2007). Factors affecting offspring survival and development in a cooperative bird: social, maternal and environmental effects. J. Anim. Ecol. 76, 750–760. doi: 10.1111/j.1365-2656.2007.01248.x
Schel, A. M., Townsend, S. W., Machanda, Z., Zuberbühler, K., and Slocombe, K. E. (2013). Chimpanzee alarm call production meets key criteria for intentionality. PLoS ONE 8:e76674. doi: 10.1371/journal.pone.0076674
Schielzeth, H., and Forstmeier, W. (2009). Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. doi: 10.1093/beheco/arn145
Scott-Phillips, T. C. (2015). Meaning in animal and human communication. Anim. Cogn. 18, 801–805. doi: 10.1007/s10071-015-0845-5
Seed, A., and Byrne, R. (2010). Animal tool-use. Curr. Biol. 20, R1032–R1039. doi: 10.1016/j.cub.2010.09.042
Shumaker, R. W., Walkup, K. R., and Beck, B. B. (2011). Animal Tool Behaviour: the Use and Manufacture of Tools by Animals. Baltimore, MD: JHU Press.
Sievers, C., and Gruber, T. (2016). Reference in human and nonhuman primate communication : what does it take to refer? Anim. Cogn. 19, 759–768. doi: 10.1007/s10071-016-0974-5
Smith, E. O., and Bentley-Condit, V. (2010). Animal tool use: current definitions and an updated comprehensive catalog. Behaviour 147:185. doi: 10.1163/000579509X12512865686555
Sommer, C., Todt, D., Ostreiher, R., and Mundry, R. (2012). Urgency-related alarm calling in Arabian babblers, Turdoides squamiceps: predator distance matters in the use of alarm call types. Behaviour 149, 755–773. doi: 10.1163/1568539X-00003003
Sperber, D., and Wilson, D. (1986). Relevance: Communication and Cognition. Cambridge, MA: Harvard University Press.
Sugiyama, Y. (1981). Observations on the population dynamics and behavior of wild chimpanzees at Bossou, Guinea, in 1979-1980. Primates 22, 435–444. doi: 10.1007/BF02381236
Tomasello, M. (2006). “Why don't apes point?,” in Roots of Human Sociality: Culture, Cognition and Interaction, eds N. J. Enfield and S. C. Levinson (London: Berg), 506–524.
Tomasello, M. (2008). Origins of Human Communication. Cambridge, MA: MIT Press. doi: 10.7551/mitpress/7551.001.0001
Townsend, S. W., Koski, S. E., Byrne, R. W., Slocombe, K. E., Bickel, B., Boeckle, M., et al. (2016). Exorcising Grice's ghost: an empirical approach to studying intentional communication in animals. Biol. Rev. 92, 1427–1433. doi: 10.1111/brv.12289
Uy, J. A. C., and Borgia, G. (2000). Sexual selection drives rapid divergence in bowerbird display traits. Evolution 54, 273–278. doi: 10.1111/j.0014-3820.2000.tb00027.x
Vail, A. L., Manica, A., and Bshary, R. (2013). Referential gestures in fish collaborative hunting. Nat. Commun. 4:1765. doi: 10.1038/ncomms2781
van Rooijen, J. (2015). Commentary: the use of referential gestures in ravens (Corvus corax) in the wild. Front. Ecol. Evol. 3:113. doi: 10.3389/fevo.2015.00113
Vygotsky, L. S. (1978). Mind in Society: The Development of Higher Psychological Processes, eds M. Cole, V. John-Steiner, S. Scribner, and E. Souberman. London: Harvard University Press.
Waller, B. M., Warmelink, L., Liebal, K., Micheletta, J., and Slocombe, K. E. (2013). Pseudoreplication: a widespread problem in primate communication. Anim. Behav. 86, 483–488. doi: 10.1016/j.anbehav.2013.05.038
Zahavi, A. (1988). “Mate guarding in the Arabian babbler, a group-living songbird,” in Proceedings of the 19th International Ornithological Congress, 420–427.
Zahavi, A. (1989). “Arabian babbler,” in Lifetime Reproduction in Birds, ed I. Newton (London: Academic Press), 253–275.
Keywords: Arabian babblers, gestures, intentional communication, mating behavior, object presentation, overt intentionality, referential communication, Turdoides squamiceps
Citation: Ben Mocha Y and Pika S (2019) Intentional Presentation of Objects in Cooperatively Breeding Arabian Babblers (Turdoides squamiceps). Front. Ecol. Evol. 7:87. doi: 10.3389/fevo.2019.00087
Received: 28 June 2018; Accepted: 07 March 2019;
Published: 09 April 2019.
Edited by:
Elise Huchard, UMR5554 Institut des Sciences de l'Evolution de Montpellier (ISEM), FranceReviewed by:
Anindita Bhadra, Indian Institute of Science Education and Research Kolkata, IndiaJordi Figuerola, Estación Biológica de Doñana (EBD), Spain
Copyright © 2019 Ben Mocha and Pika. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yitzchak Ben Mocha, eWl0emNoYWtibUBnbWFpbC5jb20=
 Yitzchak Ben Mocha
Yitzchak Ben Mocha Simone Pika
Simone Pika