
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 12 December 2018
Sec. Behavioral and Evolutionary Ecology
Volume 6 - 2018 | https://doi.org/10.3389/fevo.2018.00205
This article is part of the Research Topic Contributions of Behavior and Physiology to Conservation Biology View all 13 articles
 Rachel A. Settle1†
Rachel A. Settle1† Jeffery A. Ettling2†
Jeffery A. Ettling2† Mark D. Wanner2
Mark D. Wanner2 Chawna D. Schuette2
Chawna D. Schuette2 Jeffrey T. Briggler3
Jeffrey T. Briggler3 Alicia Mathis1*
Alicia Mathis1*Understanding behaviors associated with reproductive events is vital to management of captive breeding programs for threatened and endangered species. The Ozark hellbender (Cryptobranchus alleganiensis bishopi) is a federally endangered aquatic salamander with only one successful captive breeding program (the Saint Louis Zoo's Ron Goellner Center for Hellbender Conservation). Although anecdotal observations have been reported for hellbender reproductive behavior from field observations, no quantitative assessments have been made. We quantified hellbender behavior from video-recordings of three successful breeding events at the Saint Louis Zoo that occurred in 2012, including aggressive, sexual, social, and locomotory behaviors. We used transition matrices to organize these data into kinematic diagrams that illustrated behavioral sequences for five time periods: pre-oviposition (2 nights), first oviposition night, inter-oviposition night, second oviposition night, and post-oviposition. General activity and agonistic behaviors increased moderately through the first oviposition night, peaked during inter-oviposition, and declined abruptly following the second oviposition night. Agonistic behavior included bites, charges, chases, and flight. Female-female aggression was common. Surfacing (presumably for accessory air breathing) followed intense activity. Presumed courtship behaviors (tail swishing and circling) occurred at low rates. During oviposition, females remained in the nest box for 1–2+ h. We encourage managers of captive breeding programs to use quantitative behavioral analyses to pin-point critical time periods and conditions for successful reproduction.
Captive breeding and subsequent reintroduction can be an important tool in conservation of declining populations (Griffiths and Pavajeau, 2008), particularly when the reason for the decline is unclear or unresolved. Zoos, aquariums, and other ex situ breeding facilities frequently do an excellent job of developing ethograms (lists and descriptions of behavior), which can be helpful in design and implementation of captive breeding programs (e.g., Stanton et al., 2015). Quantitative studies of behavior of animals in captivity are less common (Maple and Segura, 2015), but these detailed analyses can lead to improved captive breeding success. For example, due to expense, space, and availability of reproductive adults, captive breeding efforts often are made only between assigned pairs of males and females, but quantitative behavioral studies showed that mating can be enhanced when females are allowed to choose their mating partners (Martin-Wintle et al., 2015; Hartnett et al., 2018). Behavioral studies have also helped to define receptivity periods for species in captivity (e.g., duck-billed platypus Ornithorhynchus anatinus, Hawkins and Battaglia, 2009; collared peccary, Pecari tajacu, da Silva et al., 2016), which helps program directors to better target breeding efforts.
Historically, captive breeding efforts have focused on large, charismatic species, particularly mammals (Leader-Williams and Dublin, 2000). As species are added to threatened and endangered lists at an unprecedented rate, captive breeding efforts are expanding to include many nontraditional species, including fishes, amphibians and invertebrates, which have added benefits of often requiring less space, having higher birth rates and being easier to reintroduce than larger fauna (Keulartz, 2015). Amphibians, in particular, have received increasing attention (Griffiths and Pavajeau, 2008; Harding et al., 2015; Murphy and Gratwicke, 2017) due to the rapid widespread severity of their population declines (41% of amphibian species listed as threatened with extinction by the IUCN: https://www.iucn.org/theme/species/our-work/amphibians). However, studies of behavior related to captive breeding of amphibians are not as well developed as for taxa with a longer history of ex situ breeding efforts.
About half of salamanders (Amphibia: Urodela) are considered by the IUCN to be threatened or extinct. One family of particular conservation concern is the Cryptobranchidae, which contains the world's largest extant salamanders and which is represented by only two genera, Andrias in Asia and Cryptobranchus in the United States. All species of these fully aquatic salamanders are threatened or endangered (Browne et al., 2014). Generally, captive breeding efforts have been more successful for Andrias (Kuwabara et al., 1989) than for Cryptobranchus, which has had only one known successful breeding program (the Ron Goellner Center for Hellbender Conservation at the Saint Louis Zoo: Ettling et al., 2013).
Two subspecies are currently recognized within the genus Cryptobranchus, the Eastern (Cryptobranchus alleganiensis alleganiensis) and Ozark (C. a. bishopi) hellbenders, although both are paraphyletic (Crowhurst et al., 2011; Tonione et al., 2011). The Ozark subspecies is listed as federally endangered in the United States (USFWS, 2011), and the Eastern hellbender (Cryptobranchus alleganiensis alleganiensis) is currently petitioned to be listed as threatened or endangered under the Endangered Species Act (USFWS, https://ecos.fws.gov/ecp0/profile/speciesProfile?spcode=D043). A Population and Viability Assessment indicated a high probability of extinction within 75 years without significant intervention, including captive propagation (Briggler et al., 2007; Ettling et al., 2017).
Hellbenders are exceptionally long-lived for amphibians, with a lifespan of over 50 years (Nickerson and Mays, 1973). These large salamanders are habitat specialists, requiring clear, cool, fast-flowing water with rocky substrates (Nickerson and Mays, 1973). During a short breeding season (several weeks), males aggressively defend spawning sites under rocks or within bedrock, court females, and guard eggs after spawning. The cause(s) for the decline have not been specifically identified, although numerous factors have been suggested, including river sedimentation/siltation and changes in electrical conductivity due to deforestation, pollution from run-off, increased predation from introduced or reintroduced species, amphibian chytrid fungus infections, and over-collection (Nickerson and Briggler, 2007; Briggler et al., 2008; Gall and Mathis, 2011; Nickerson et al., 2017; Pitt et al., 2017).
As part of a strategy to combat the decline of hellbenders, captive rearing efforts were initiated at the Saint Louis Zoo's (SLZ) Ron Goellner Center for Hellbender Conservation (RGCHC) and the Missouri Department of Conservation's (MDC) Shepherd of the Hills Fish Hatchery in Branson, Missouri. Both programs have successfully hatched eggs collected from naturally-occurring nests and reared larvae for release in the wild (Briggler, 2007; Briggler et al., 2011; Crowhurst et al., 2011; Bodinof et al., 2012).
Captive breeding of hellbenders proved to be more difficult. In 2011, a conservation milestone was reached when the RGCHC, in collaboration with MDC, reported the first successful breeding of Ozark hellbenders in captivity (Ettling et al., 2013). The SLZ continued to successfully breed Ozark hellbenders each year between 2011 and 2016 (Briggler, 2007; Briggler et al., 2011; Ettling et al., 2017).
The success of the captive breeding program at RGCHC appears to be largely attributable to use of artificial breeding streams that closely mimic natural conditions, including temperature, photoperiod, precipitation, water quality, and prey availability (Ettling et al., 2013). Adjusting the ionic composition (total dissolved solids) and the introduction of artificial nest boxes were likely major contributing factors to the success of fertilized clutches (Ettling et al., 2013). At the time of the first successful breeding events, an indoor artificial stream was outfitted with a four-camera surveillance system that recorded hellbender activity around the clock.
In this study, we provide an analysis of the video recordings of the behavior of the hellbenders during the three successful sequential oviposition events of 2012, culminating in kinematic diagrams of sequences of behavior that occurred before, during, between, and immediately after the successful reproductive events. Although there have been numerous anecdotal descriptions of reproductive events in the wild (Smith, 1907; Huheey and Stupka, 1967; Floyd and Unger, 2016), there has not been a systematic ethological analysis of the steps involved in courtship and mating. Herein, we (a) describe the behaviors that we observed during pre-oviposition, oviposition, inter-oviposition and post-oviposition periods, (b) quantify the frequency of each behavior during each period, and (c) use transition matrices to describe sequences of behavior. These observations will help to identify social interactions and other behaviors that contributed to successful captive breeding and help to identify behaviors that signal that reproduction is imminent and that signal transitions between sequential breeding periods.
The successful breeding events occurred at the RGCHC in an indoor artificial stream (9.7 m × 1.7 m × 0.6 m; Figure 1) containing five male and three female adult Ozark hellbenders. Broodstock (Table 1), collected from the North Fork of the White River, Ozark County, Missouri, were added to the indoor artificial stream at the time of collection and were kept in the stream until after the successful breeding events reported in this study. The excess number of males vs. females was used to provide increased opportunities for mate selection by females. For details about quarantine and husbandry protocols, see Ettling et al. (2013). All males had the typical donut cloacal swelling that indicates reproductive condition. The range of male sizes (Table 1) was chosen to maximize the probability of healthy sperm by including a range of individuals from small/young (near lower-end of sexual maturity) to large/old (near high end of size range) (e.g., Peterson et al., 1983). Females all exhibited abdominal swellings consistent with egg production.
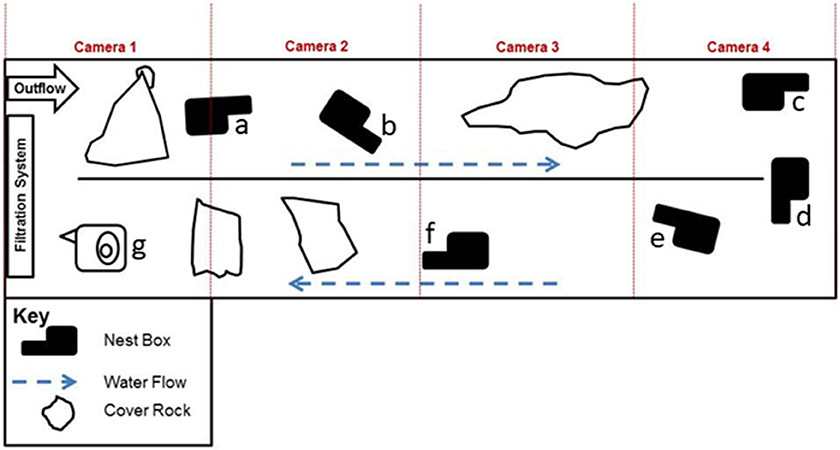
Figure 1. Diagram of the artificial indoor stream (9.7 m × 1.7 m × 0.6 m), including nest box (a–g) and cover rock locations, at the Saint Louis Zoo Herpetarium. For boot-shaped nest boxes (a–f), the neck of the boot is the entrance chamber and the foot of the boot is the nest chamber. Nest box (g) is of an older, non-boot, design and was not used during any oviposition events.
The stream was a closed recirculating system with water flow in a circular direction at 227 L/min at an average depth of 0.3 m. Mechanical and biological filtration together with ultraviolet sterilization helped to maintain water quality, and reconstituted reverse osmosis water was used for water changes. Year-round light:dark cycles, water temperature, water quality and precipitation events were selected to mimic values that occurred in natural habitats in the river of origin. A chiller was used to manually adjust temperatures each day to match data recorded by data loggers in the river of origin; annual temperatures ranged from 4.4 to 22.2°C. Total dissolved solids were also kept similar to natural river water at 175–300 mg/L because related characteristics, such as salinity and osmolality, can influence sperm motility in some aquatic species (Alavi and Cosson, 2006; Bonislawska et al., 2015). Data for other measures of water quality (pH, nitrates, nitrites, ammonia, phosphates, dissolved oxygen) are provided by Ettling et al. (2013). A manual sprinkler system plus adjustment of water levels was used to mimic natural precipitation, and photoperiods were adjusted daily via an automatic timer. The floor of the artificial stream was covered with river gravel (10.2–15.2 cm), and a variety of large (approximately 0.2–0.7 m) sandstone and moss-covered rocks were scattered over the gravel. Crayfish (Orconectes spp., Procambarus spp.), darters (Etheostoma spp.), sculpins (Cottus bairdi, Cottus carolinae), and shiners (Notropis spp.) were collected from various streams near the St. Louis, MO, area and introduced into the stream as a source of natural forage.
Artificial nest boxes (n = 7) were positioned in the stream (Figure 1) with the open end of the entrance tunnel of the boxes facing downstream of water flow. As described in Briggler and Ackerson (2012), nest boxes were constructed with a chicken-wire base frame covered with hardware cloth and a concrete/sand mixture. All but one of the nest boxes were a modified “boot” design, with an entrance tunnel (“leg” of the boot; ~27 tunnel length × 7.3 entrance height × 10 entrance width cm) connected to a nesting chamber (“foot” of the boot, ~ 39 × 31 cm). An opening with a removable lid was made on the surface of the nesting chamber so that eggs deposited inside the chamber could be monitored periodically with minimal disturbance. The seventh box (Figure 1, g) was an older non-“boot” design and was not used during any of the oviposition events.
A four-camera (Figure 1) infrared video recording system positioned directly above the stream monitored the hellbenders between 20:00 and 08:00 h daily because hellbenders are primarily nocturnal (Noeske and Nickerson, 1979; Coatney, 1982). Video recordings were archived to computer hard drives at the RGCHC.
Our analyses are based on video data collected from 21 to 26 September 2012, during which time three oviposition events occurred (Ettling et al., 2013). We quantified the behavior of the hellbenders on the night before the first oviposition to illustrate “pre-oviposition behavior”; qualitatively, the behavior on this night was similar to the behavior on the preceding three nights (personal observations). The first oviposition night (two oviposition events) occurred on 22 September 2012 and the second oviposition night (one oviposition event) occurred on 24 September 2012. The night between the two oviposition events (23 September 2012) was categorized as “Inter-oviposition” behavior. Post-oviposition behavior was quantified for 2 days following the last oviposition event.
Each night's videos (4 videos × 12 h) were viewed in their entirety using Milestone XProtect® Smart Client 2013 R2 – Player v. 8.1b. The hellbender keepers at the zoo developed a list of behaviors that they observed during their daily surveys, and this list formed the basis of the ethogram (list of species-specific behavior describing the elements and putative function of each behavior) (Table 2) we used in this study. Behaviors were categorized as “agonistic,” “solitary/locomotory,” “sexual,” or “social.” We recorded every occurrence of any of the defined behaviors, the location of the behavior (camera number, nest box number, etc.), and, when possible, the sex of the individual. An individual's sex was identified based on physical features unique to that individual, and these features were not always visible on the video; we estimate that we were unable to identify the individuals, and, thus, their sex, for about 10% of observations.
We defined a behavioral sequence as beginning when one or multiple individuals performed any of the defined behaviors (Table 2) and ending when the hellbender(s) was/were inactive for a period of 5 min, began a new defined behavior, or when the individual(s) entered a next box or other cover object (i.e., natural rock). We calculated transitional probabilities (the probability that one behavioral pattern follows another) through the use of transition matrices (Martin and Bateson, 2007), which were calculated for all individuals combined. The columns and rows of the matrix consisted of all behavioral patterns, and the numbers in each cell were the percentage of times that the first behavioral pattern (rows) was followed by the second behavioral pattern (columns). We illustrated the transition probabilities using kinematic graphs (flow diagrams) (Lehner, 1996). Separate transitional matrices and kinematic diagrams were made for the periods of pre-oviposition, first oviposition night, inter-oviposition, second oviposition night, and post-oviposition (2 nights).
Consider the following two examples of sequence scenarios. The first example is one sequence comprised of four sequential behaviors: Hellbender A (1) Walked out of a nest box onto a rock. He (2) Approached and (3) Bit Hellbender B, while Hellbender B (4) Fled. The second example is comprised of two sequences, with the first consisting of one behavior only and the second consisting of four behaviors: Hellbender A (1) Walked, rested for 5 or more min, (1) Walked, (2) Approached Hellbender B, (3) Bit Hellbender B and caused Hellbender B to (4) Flee.
Transitional sequences between any two specific behaviors did not occur with sufficient frequencies for statistical analysis. However, we increased sample sizes by combining behaviors into functional categories so that we could address two questions. First, does Approach lead to a higher proportion of interactive behaviors (e.g., combined Bite, Flee, Swim, Oviposition, additional Approaches) than non-interactive behaviors (walk)? Second, does Surfacing follow a greater proportion of high-activity behaviors (e.g., combined Swim, Chase, Flee) than low-activity behaviors (e.g., Walk). These two comparisons were made via two-tailed Binomial tests (Minitab, v. 16). Note that each event was treated as a unique data point even though the same individual hellbender may have initiated multiple events.
In our artificial streams, individuals commonly shared cover objects, including nest boxes, prior to the breeding season, but exclusive residency occurred as the breeding period approached (Ettling et al., 2013). The reproductively successful males in our study had become established in their nest boxes before we began our observations. However, all males did not engage in nest box defense, and nest boxes were sometimes occupied by single females. Hellbenders were generally most active during 01:00–07:00 h, and oviposition occurred between 02:00 and 07:00 h, with females remaining in the nest box with the male for 65 min to over 2 h. For the results below, the transitional matrices used to construct kinematic diagrams are in Supplementary Material.
On the night before oviposition (21 September), most behaviors were Solitary/locomotory (Figure 2A). Almost all (97%) of these locomotory movements were Walking, with the rest being Swimming (Figure 2A). Only one agonistic sequence was recorded during the pre-oviposition period. This sequence was initiated by a hellbender Approaching another hellbender and Biting it, resulting in the bitten hellbender Fleeing. No sexual behaviors (Tail swish, Circle) were observed during the quantified pre-oviposition period (21 September 2012) or during our observations of the videos for 18–20 September 2012, which are not included in Figure 2A.
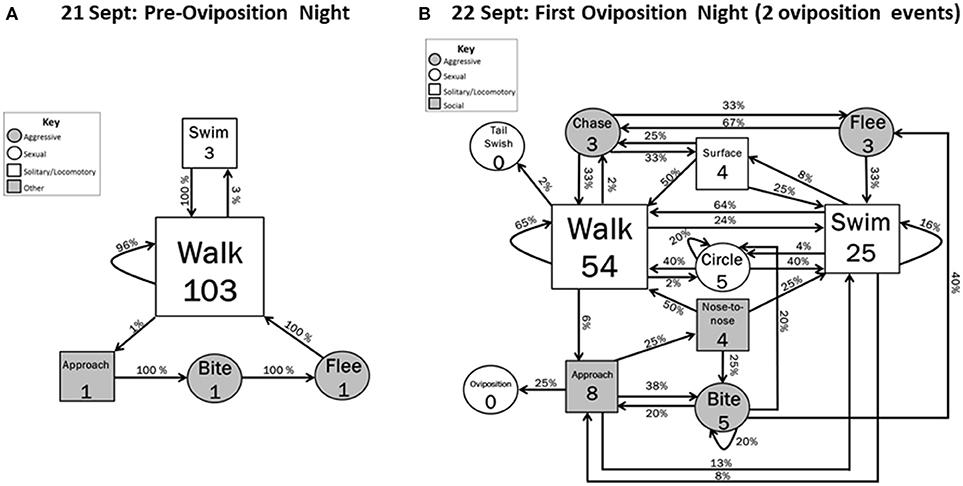
Figure 2. Behavioral transitions during (A) the night before the oviposition events occurred and (B) the first night of oviposition when 2 oviposition events occurred. Frequency of initiating behavioral actions (inside boxes) and % time initiated action was followed (arrow) by other actions are indicated. Zeros indicate that the behavior occurred but did not initiate a sequence within the 5 min designated time frame. Shape sizes are indicators of the relative frequency in which the initiating behavior occurred (e.g., larger boxes indicate the behavior occurred more often than smaller boxes).
During the first oviposition night (22 September), the overall level of activity was higher and the diversity of behavior increased to include all behavioral categories (agonistic, solitary/locomotory, reproductive and social) (Figure 2B). Solitary/locomotory behavior continued to be the most frequently performed behavior, but, in comparison to the previous night, the frequency of Walking decreased by about 50%, from 103 to 54 instances, and Swimming behavior increased by a factor of 8 (from 3 to 25 instances). Agonistic behavior also increased in frequency, with Biting occurring five times, Fleeing occurring three times, and the first occurrences of Chasing. Surfacing behavior was also observed for the first time during this event (four times).
Some patterns in behavioral sequences were apparent (Figure 2B). Although Bites sometimes (1/5) led to Circling behavior, Circling did not lead directly to escalated agonistic or sexual interactions, but only to more circling (1/5) or locomotory behaviors (4/5). Flight resulted only from either Bites (2/3) or Chases (1/3). Approach led to swimming (1/8) or the intense social interactions of Biting (3/8), Nose-to-nose (2/8), and Oviposition (2/8) (Interactive vs. Noninteractive, Z = 1.76, P = 0.078). Surfacing events only followed the high-activity behaviors of Swimming (2/4) and Chasing (2/4).
The two oviposition behaviors during the first oviposition night occurred as follows. After two females Approached a nest box (Figure 1), nest box e containing a male, one female Bit the other, and the bitten hellbender Fled away from the nest box while being chased. The female that initiated the bite then slowly entered the nest box (~02:00 h). She stayed inside the nest box for approximately 120 min and exited without any indication of coercion by the male. After approximately 90 min, the second female Approached and entered the nest box (~06:00 h) and stayed inside the nest box until the video stopped recording (08:00 h). The male did not leave the nest box after oviposition occurred.
During the Inter-oviposition period (23 September), locomotory behavior occurred at the highest frequencies of the entire data collection period. Walking initiated behavioral sequences 330 times, and Swimming initiated sequences 110 times (Figure 3A). In addition, an increased number of interactions between hellbenders were observed. Approach (n = 30) almost always led to interactive events (20 Bites, 5 Flees, 2 additional Approaches; interactive vs. noninteractive: Z = 4.20, P < 0.001) (Figure 3A). Agonistic behaviors also occurred at the highest frequencies during the Inter-oviposition period: 20 Bites, 23 Flees, 23 Chases, and 2 Charges. Charges always led to Walking and Biting always led to Fleeing. Female-female aggression occurred in 55% of the aggressive acts, with male-male (30%) and female-male (15%) aggression explaining the remainder. Tail swishing (n = 1) and Circling (n = 6) were the only sexual behaviors to occur, and both of these behaviors led only to locomotory behaviors. As in the previous night, Surfacing followed high-activity behaviors (Flee, Swim) or other Surfacing (high-activity vs. low activity: Z = 2.12, P = 0.034). During this period, the male defending the nest box with eggs briefly emerged a few times, but generally did not engage in aggressive acts.
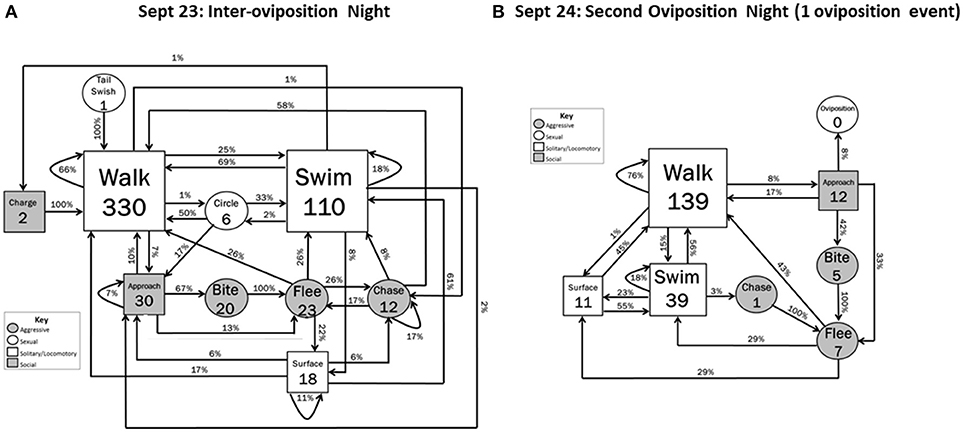
Figure 3. Behavioral transitions during (A) the night between the two oviposition and (B) the second oviposition night when 1 oviposition event occurred. Frequency of initiating behavioral actions (inside boxes) and % time initiated action was followed (arrow) by other actions are indicated. Zeros indicate that the behavior occurred but did not initiate a sequence within the 5 min designated time frame. Shape sizes are indicators of the relative frequency in which the initiating behavior occurred (e.g., larger boxes indicate the behavior occurred more often than smaller boxes).
Although locomotory behaviors were not as frequent during the second oviposition night (24 September) as during the Inter-oviposition period, locomotory behavior was still moderately frequent and initiated behavioral sequences at a higher rate than on the first oviposition night (Walk, increase of 257%; Swim, increase of 72%) (Figure 3B). Agonistic sequences occurred, but at a lower frequency than the previous night and similar to that during the first oviposition night. Behavioral transition sequences showed some similar patterns as observed during the first oviposition night. With one exception, Surfacing events only followed the high-activity behaviors of Fleeing and Chasing (High-activity vs. Low-activity: Z = 2.41, P = 0.020). Flight continued to result only from Bites or Chases. The most intense interactions of Biting and Oviposition followed from Approach behavior (Interactive vs. Noninteractive: Z = 1.76; 0.042). In general, sexual behavioral transitions were less complex than those occurring in the first oviposition night; neither Circling nor Nose-to-nose behaviors were observed during this oviposition event.
The oviposition activity during the second oviposition night was less complex than in the first oviposition night. The remaining non-spent female approached a separate nest box (Figure 1, nest box a) that was occupied by a different male than the male that fertilized both clutches on the first oviposition night. After Approach, the sequence of behavior by the female was: Walk, Walk, Walk, Swim, Surface, Walk, Swim, Swim, Walk, Walk, Walk, Approach, Walk, Approach, Oviposition (06:00 h). The female stayed within the nest box for approximately 65 min and slowly exited after Oviposition. The male remained within the nest box, and so we could not observe his behavior.
The post-oviposition period began on 25 September 2012 and ended on 26 September 2012. This period was characterized by an abrupt decrease in frequency of all behaviors, with only Solitary/locomotory behavior exhibited. On the first night post-oviposition, Walking was the only behavior exhibited, and it occurred only four times (Figure 4A). To determine whether this very low level of activity continued, we also quantified behavior on the second night post-oviposition; 14 instances of Walking and one of Swimming occurred (Figure 4B).
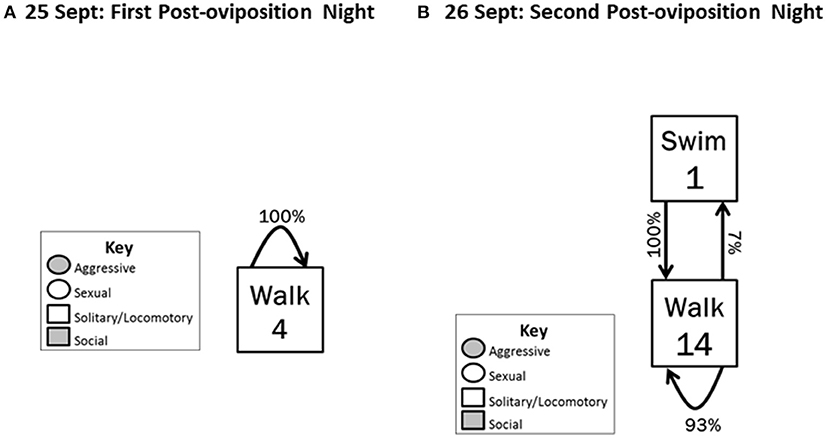
Figure 4. Behavioral transitions during (A) the first night post-oviposition and (B) the second night post-oviposition. Locomotory behaviors were followed (arrows) by only other locomotory behaviors. Frequency of initiating behavioral actions (inside box) and the % time the initiated action was followed (arrow) by another action are indicated.
Figure 5 shows a clear pattern of behavioral changes across the six nights of the study. Frequencies of all behavioral categories were relatively low during pre-oviposition. Although the total number of behavioral events remained relatively low on the first oviposition night, there was a shift to include more agonistic and sexual/social behaviors. By far, the highest level of all activity was on the inter-oviposition night, with an approximately 5-fold increase in all categories of behavior from the previous night. On the second oviposition night, the frequency of behavioral events decreased to only about 2 × that of the first oviposition night. On the two post-oviposition nights, the frequency of behavior dropped abruptly to below that of the pre-oviposition night, and the only behavior that occurred was solitary/locomotory (mostly walking).
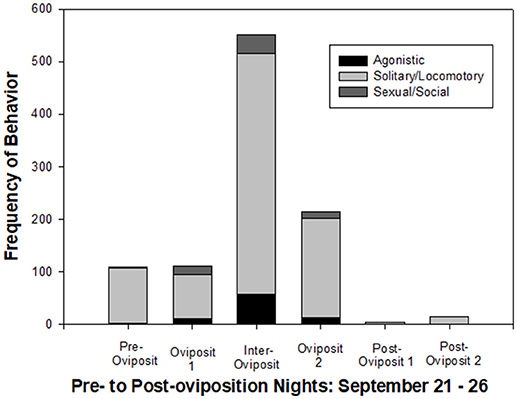
Figure 5. Summary of frequencies of behavioral transitions across the six nights of the study, which included one night each, including pre-oviposition, oviposition night 1 (2 breeding events), inter-oviposition, oviposition night 2 (1 breeding event), and two nights post-oviposition. Agonistic behaviors were Bites, Charges, Chases and Flight. Solitary/Locomotory behaviors were Walking, Swimming, and Surfacing. Sexual and Social behaviors were Ovipositions, Circling, Tail Swishes, Approach, and Nose-to-Nose.
Descriptions of the reproductive behavior of hellbenders is limited both in natural habitats due to their secretive nature, and in captivity, where the first successful reproductive event occurred relatively recently (Ettling et al., 2013). This study provides the first quantitative ethological analysis of the behavior of hellbenders immediately prior to, during and after an oviposition event. These data, which were collected from video recordings of the captive reproductive events reported in Ettling et al.'s (2013) study, help to fill in the details of sequences of behavior previously reported in anecdotal field observations. Studies of captive breeding events for threatened and endangered species, including our study, often suffer from low sample sizes due to availability of reproductive individuals and appropriate-sized of enclosures (Snyder et al., 1996). In our study, the minimal information on individual variation of the behaviors due to low sample size limits the strengths of the inferences that can be drawn. However, behavior surrounding our three observed reproductive events were generally consistent, and there were several similarities with some anecdotal observations from nature.
As reported in numerous previous studies (Smith, 1907; Bishop, 1941; Peterson, 1988), males began defending nesting sites prior to the oviposition period. In nature, males typically defend a “den” site consisting of a depression located under a flat cover rock, or within crevices or holes in the bedrock (Bishop, 1941; Pfingsten and Downs, 1989). Although flat rocks were available, the hellbenders in our study defended only the boot-shaped nest boxes. The same type of nest boxes have been successfully used for oviposition by hellbenders in the wild, with success likely due primarily to the ease of defensibility provided by the single, narrow neck opening and the spacious chamber for eggs (Briggler and Ackerson, 2012).
Our findings are consistent with other studies that suggest that aggression increases during the breeding period (Smith, 1907; Peterson, 1988; Foster et al., 2009). Although our data span a limited period (after initial establishment of den sites by males), the increase in aggression we observed was abrupt, with increases in both number and types of overt acts. The night before the first oviposition included only three agonistic acts (one each of approach, bite and flee), whereas the night of the first oviposition included 38 acts of six overt behaviors (chase, flee, nose-to-nose, approach, circle, bite). Locomotory activity also became more intense, with swimming (as opposed to walking) comprising only 3% of pre-oviposition locomotory movements, increasing to 28% on the night of oviposition. It is not known whether the observed increase in aggression and movement intensity is as abrupt in the field or whether the very low level of aggression and movement intensity on the night before oviposition is typical of a more extended pre-oviposition period under natural conditions. In any case, we recommend that managers of captive breeding facilities carefully monitor hellbenders for increased aggression and swimming activity as a possible indicator of imminent oviposition. Continued high levels of activity, including aggression, after one oviposition event, could indicate that additional oviposition events are forthcoming.
Most previously-reported anecdotal field observations of aggression and the apparent territorial spacing of males in the field suggest that aggression has three primary functions: male-to-male competition for breeding sites (Alexander, 1927; Hillis and Bellis, 1971; Nickerson and Mays, 1973), (2) male attempts to coerce females to enter or leave their nest sites or (3) male attempts to protect their eggs from oophagy (Smith, 1907). However, the aggressive acts that we observed in the artificial stream were mostly (55%) female-female, with females apparently competing to occupy the oviposition sites. Female-female aggression associated with reproduction may be more common than previously thought; relatively few overt aggressive acts have been observed in the field, and the contestants are rarely definitively identified with respect to sex (e.g., Nickerson and Mays, 1973). Alternatively, female-female aggression could be a result of the specific conditions/densities within the artificial stream, which could be tested with artificial streams with varying sizes and densities if sufficient numbers of adults in breeding condition were available. The consequences of aggression may be severe. After this breeding period, both males and females in our study had severe lacerations on the limbs, bite marks along the lateral folds, and even lost limbs (Ettling et al., 2013).
It is possible that the dramatic reduction of population sizes of Ozark hellbenders in recent decades (e.g., Wheeler et al., 2003) has also resulted in alterations in the frequency or intensity of aggressive behavior in natural habitats. For example, limitation of available receptive females or fertile males (see Unger and Mathis, 2013) may have resulted in more intense male-male or female-female competition. Alternatively, lower population densities may have led to an overall reduction in aggressive encounters in the wild. The latter seems unlikely since fresh wounds, in at least some cases resembling conspecific bite marks, have been reported in post-decline (~ early 1980's: Wheeler et al., 2003) populations (Pfingsten, 1990; Wheeler et al., 2003; Miller and Miller, 2005; Williams and Groves, 2014).
The kinematic analysis also allows for inferences about whether there are consistent transitions from one behavior to the next. Although variability of transitions was high, some general patterns were apparent from the data. Not surprisingly, Flight was typically the result of being bitten or chased. Both Swimming and Chasing appear to be energetically costly because they were frequently followed by surfacing behavior, presumably for accessory air breathing. Strenuous activity can lead to respiratory and metabolic acidosis in hellbenders (Boutilier et al., 1980); although hellbenders rely primarily on cutaneous respiration (Guimond and Hutchison, 1973), lung-based respiration may be important for maintenance of sufficient blood oxygen levels during stressful periods. Although we did not measure levels of stress hormones, we hypothesize that corticosterone may increase during reproductive events to mobilize energy for high activity levels, as has been reported for some other salamanders (Reedy et al., 2014). Overall, hellbenders have very low plasma corticosterone levels, but levels rise during periods of acute stress (restraint), and, at least during the early breeding season, males have higher corticosterone levels than females (Hopkins and DuRant, 2011).
Circling behavior, which occurred 11 times, has been reported during courtship in a taxonomically wide range of salamanders (e.g., Plethodontidae: Cupp, 1971; Salamandridae: Bruni and Romano, 2011), frequently leading to oviposition; however, in our observations, circling consistently led only to locomotory behavior or more circling. Approach typically led to physical interactions (bite, nose-to-nose, and oviposition). Tail Swishing by the male was observed on only two occasions and so may not play as strong a role as the tail undulations that are a part of courtship of some other salamander taxa (Houck and Arnold, 2003).
Our set-up had an excess of males to allow females opportunities for mate choice. However, no particular feature stands out as a basis for success. The two successful males were intermediate in size (SVL), and the male that fertilized the third clutch was missing both hind limbs (Table 1; see Nickerson et al., 2011 for discussion of recent increases in hellbender abnormalities). Oxygen concentration (see Settle et al., 2018) or other features of the nest box might also be important, but the successful nest boxes were at opposite sides of the artificial stream (Figure 1, nest boxes a and e), suggesting general nest box location was not a critical factor.
Two females in our study laid eggs in the same nest box, with fertilization by the same male, and clutches of multiple females in the same nest has also been reported for hellbenders in nature (Nickerson and Mays, 1973). Spawning of several females in one nest site also occurs in the other species in this family, the Asian giant salamanders (Andrias sp.: Browne et al., 2014), but the function of this behavior is not known. Generally, such spawning decisions by females could result either from preferred characteristics of the nest site or preferred characteristics of the male (e.g., Refsnider and Janzen, 2010). In any case, we recommend that managers provide females with multiple nest sites and multiple males during the spawning season [see also details in (Ettling et al., 2013)]. In addition to mate choice opportunities, multiple individuals could provide increased concentrations of potential pheromones or reproductive hormones that are released into the water. For example, in lampreys, Petromyzon marinus, odors from mature males facilitate sexual maturation for both sexes, attract females, and are important for nest construction and gamete release (review in Buchinger et al., 2015).
The use of quantitative behavioral data to predict timing of potential reproduction should be useful in captive breeding programs for a wide range of species. In addition, understanding the sequence of events that lead to copulation/oviposition can help managers to pinpoint the point at which failure occurs so that problems can be more effectively addressed. For example, detailed behavioral observations of Giant Pandas at a breeding center near Wolong, China, led to the conclusion that copulation failure was due to lack of motivation by the male (Zhang et al., 2004). Even though mounting successfully occurred, unsuccessful males frequently had improper mounting positions, low persistence, and low penetration success. Mitigation efforts could then be focused on steps to increase the motivation of the male.
For hellbenders, in combination with husbandry details described by Ettling et al. (2013), close monitoring of hellbender behavior during the breeding season can provide clues to the imminent onset of oviposition. The most striking result was the rapid on-set of behavioral changes. We recommend that the behavior of hellbenders in captive breeding programs be monitored closely each night during the breeding season. An increase in surfacing events is easy for even staff with minimal training to detect. Closer observation should reveal increased aggression and other social interactions as well as a substantial increase in the proportion of locomotory events involving swimming as opposed to walking. Such observations allow managers to detect newly deposited eggs early and to intervene if aggression levels are high enough to endanger the lives of the adults. Although the presence of a guarding male undoubtedly increases survival of eggs in natural habitats, we recommend removal of the eggs from the nest for rearing; at the RGCHC, we remove the eggs 14 days after oviposition. Separate rearing allows for the elimination of potential predation, including by the guarding male, and allows for close control of water quality, maintenance of high levels of oxygenation, and removal of eggs that become infected with disease.
Surprisingly, much of the observed aggression in this study of captive individuals was among females, so it is important that females are provided with multiple males and multiple nest sites. Even so, two females in this study spawned in the same nest with the same male. A relatively large space is required for captive-breeding of this species, and having more than one gravid female per breeding stream increases the probability of at least one successful mating event. Moreover, it is not known whether female-female social interactions are important to maintaining normal behavior. However, managers should be aware of the potential cost of the high level of aggression (female-female, male-male, male-female) during reproductive activities, and carefully examine individuals for injuries post-reproduction.
Handling and maintenance associated with captive rearing were performed in entirety by the staff and interns of the Saint Louis Zoo according to Association of Zoos and Aquariums accredited institutional protocol.
RS and AM conceived and conducted the quantification and analyses of data from the video recordings. JB made field collections of the adult broodstock. JE, MW, CS, and JB developed housing and maintenance procedures, cared for study animals, and provided videos. CS contributed to observations that formed the basis of the ethogram.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Support was provided by the Ron Goellner Center for Hellbender Conservation and the Saint Louis Zoo, the Missouri Department of Conservation, and the Graduate College and Biology Department at Missouri State University. Protocols were approved by the Saint Louis Zoo's Institutional Animal Care and Use Committee. Ruthie Carter worked with CS on development of the initial ethogram and provided training on identification of behaviors and use of software for this project. We thank the late Ron Goellner for designing and building the runway, and Ron and his wife, Karen Goellner, for their work in initiating the captive-breeding program for hellbenders at the Saint Louis Zoo. We appreciate the help of the many Zoo staff who assisted with captive-breeding efforts over the years. The manuscript was considerably improved by the thoughtful comments of three reviewers.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00205/full#supplementary-material
Alavi, S. M. H., and Cosson, J. (2006). Sperm motility in fishes: (II) Effects of ions and osmolality. Cell. Biol. Int. 30, 1–14. doi: 10.1016/j.cellbi.2005.06.004
Bodinof, C. M., Briggler, J. T., Junge, R. E., Beringer, J., Wanner, M. D., Schuette, C. D., et al. (2012). Postrelease movements of captive-reared Ozark hellbenders (Cryptobranchus alleganiensis bishopi). Herpetologica 68, 160–173. doi: 10.1655/HERPETOLOGICA-D-11-00033.1
Bonislawska, M., Szulc, J., and Formicki, K. (2015). The effect of water salinity on the motility of spermatozoa of the brook trout, Salvelinus fontinalis (Actinopterygii: Salmoniformes: Salmonidae). Acta Ichthyol. Piscat. 45:143. doi: 10.3750/AIP2015.45.2.04
Boutilier, R. G., McDonald, D. G., and Toews, D. P. (1980). The effects of enforced activity on ventilation, circulation and blood acid-base balance in the aquatic gill-less urodele, Cryptobranchus alleganiensis; a comparison with the semi-terrestrial anuran, Bufo marinus. J. Exp. Biol. 84, 289–302.
Briggler, J. J., Utrup, C., Davidson, J., Humphries, J., and Groves, T., Johnson, et al. (eds.). (2007). Hellbender Population and Viability Assessment: Final Report. Apple Valley, MN: IUCN/SSC Conservation Breeding Specialist Group.
Briggler, J. T., and Ackerson, J. R. (2012). Construction and use of artificial shelters to supplement habitat for hellbenders (Cryptobranchus alleganiensis). Herpetol. Rev. 43, 412–416.
Briggler, J. T., Larson, K. A., and Irwin, K. J. (2008). Presence of the amphibian chytrid fungus (Batrachochytrium dendrobatidis) on hellbenders (Cryptobranchus alleganiensis) in the Ozark highlands. Herpetol. Rev., 39, 443–444.
Briggler, J. T., Wanner, M., and Civiello, J. (2011). Hellbender propagation efforts. MDC Res. Sci. 6, 1–2.
Browne, R. K., Li, H., Wang, Z. G., Okada, S., Hime, P., McMillan, A., et al. (2014). The giant salamanders (Cryptobranchidae): Part B. biogeography, ecology and reproduction. Amphib. Rept. Conserv. 5, 30–50.
Bruni, G., and Romano, A. (2011). Courtship behaviour, mating season and male sexual interference in Salamandrina perspicillata (Savi, 1821) Amphib. Rept. 32, 63–76. doi: 10.1163/017353710X541878
Buchinger, T. J., Siefkes, M. J., Zielinski, B. S., Brant, C. O., and Li, W. (2015). Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front. Zool. 12:32. doi: 10.1186/s12983-015-0126-9
Coatney, C. E. (1982). Home Range and Nocturnal Activity of the Ozark Hellbender. Master's thesis, Springfield, Southwest Missouri State University.
Crowhurst, R. S., Faries, K. M., Collantes, J., Briggler, J. T., Koppelman, J. B., and Eggert, L. S. (2011). Genetic relationships of hellbenders in the Ozark highlands of Missouri and conservation implications for the Ozark subspecies (Cryptobranchus alleganiensis bishopi). Conserv. Genet. 12, 637–646. doi: 10.1007/s10592-010-0170-0
Cupp, P. V. Jr. (1971). Fall courtship of the green salamander, Aneides aeneus. Herpetologica 27, 308–310.
da Silva, S. S., Le Pendu, Y., Ohashi, O. M., Oba, E., de Albuquerque, N. I., Garcia, A. R., et al. (2016). Sexual behavior of Pecari tajacu (Cetartiodactyla: Tayassuidae) during periovulatory and early gestation periods. Behav. Proc. 131, 68–73. doi: 10.1016/j.beproc.2016.08.008
Ettling, J. A., Wanner, M. D., Pedigo, A. S., Kenkel, J. L., Noble, K. R., and Briggler, J. T. (2017). Augmentation programme for the endangered Ozark hellbender Cryptobranchus alleganiensis bishopi in Missouri. International. Zoo Yearbook 51, 79–86. doi: 10.1111/izy.12162
Ettling, J. A., Wanner, M. D., Schuette, C. D., Armstrong, S. L., Pedigo, A. S., and Briggler, J. T. (2013). Captive reproduction and husbandry of adult Ozark hellbenders Cryptobranchus alleganiensis bishopi. Herpetol. Rev. 44, 605–610.
Floyd, T. M., and Unger, S. (2016). Cryptobranchus alleganiensis (Eastern Hellbender) reproductive behavior and habitat. Herpetol. Rev. 47, 273–274.
Foster, R. L., McMillan, A. M., and Roblee, K. J. (2009). Population status of hellbender salamanders (Cryptobranchus alleganiensis) in the Allegheny River drainage of New York State. J. Herpetol. 43, 579–588. doi: 10.1670/08-156.1
Gall, B. G., and Mathis, A. (2011). Innate predator recognition and the problem of introduced trout. Ethology 116, 47–58. doi: 10.1111/j.1439-0310.2009.01718.x
Griffiths, R. A., and Pavajeau, L. (2008). Captive breeding, reintroduction, and the conservation of amphibians. Conserv. Biol. 22, 852–861. doi: 10.1111/j.1523-1739.2008.00967.x
Guimond, R. W., and Hutchison, V. H. (1973). Aquatic respiration: an unusual strategy in the hellbender Cryptobranchus alleganiensis alleganiensis (Daudin). Science 182, 1263–1265. doi: 10.1126/science.182.4118.1263
Harding, G., Griffiths, R. A., and Pavajeau, L. (2015). Developments in amphibian captive breeding and reintroduction programs. Conserv. Biol. 30, 340–349. doi: 10.1111/cobi.12612
Hartnett, C. M., Parrott, M. L., Mulder, R. A., Coulson, G., and Magrath, M. J. L. (2018). Opportunity for female mate choice improves reproductive outcomes in the conservation breeding program of the eastern barred bandicoot (Perameles gunnii). Appl. Anim. Behav. Sci. 199, 67–74. doi: 10.1016/j.applanim.2017.10.008
Hawkins, M., and Battaglia, A. (2009). Breeding behaviour of the platypus (Ornithorhynchus anatinus) in captivity. Austral. J. Zool. 57, 283–229. doi: 10.1071/ZO09090
Hillis, R. E., and Bellis, E. D. (1971). Some aspects of the ecology of the hellbender, Cryptobranchus alleganiensis alleganiensis, in a Pennsylvania stream. J. Herpetol. 5, 121–126. doi: 10.2307/1562734
Hopkins, W. A., and DuRant, S. A. (2011). Innate immunity and stress physiology of eastern hellbenders (Cryptobranchus alleganiensis) from two stream reaches with differing habitat quality. Gen. Comp. Endocrinol. 174, 107–115. doi: 10.1016/j.ygcen.2011.08.006
Houck, L. D., and Arnold, S. J. (2003). “Courtship and mating behavior,” in Reproductive Biology and Phylogeny of Urodela, ed D. M. Sever (Enfield, NH: Science Publishers), 383–424.
Huheey, J. E., and Stupka, A. (1967). Amphibians and Reptiles of Great Smoky Mountains National Park. Knoxville, TN: University of Tennessee Press.
Keulartz, J. (2015). Captivity for conservation? Zoos at a crossroads. J. Agr. Environ. Ethic. 28, 335–351. doi: 10.1007/s10806-015-9537-z
Kuwabara, K., Suzuki, N., Wakabayashi, F., Ashikaga, H., Inoue, T., and Kobara, J. (1989). Breeding the Japanese giant salamander Andrais japonicus at Asa Zoological Park. Internat. Zoo Yearbook 28, 22–31. doi: 10.1111/j.1748-1090.1989.tb03249.x
Leader-Williams, N., and Dublin, H. T. (2000). “Charismatic megafauna as ‘flagship species,”’ in Has the Panda Had its Day? Priorities for the Conservation of Mammalian Diversity, eds A. Entwistle and N. Dunstone (Cambridge: Cambridge University Press), 53–81.
Maple, T. L., and Segura, V. D. (2015). Advancing behavior analysis in zoos and aquariums. Behav. Analyst 38, 77–91. doi: 10.1007/s40614-014-0018-x
Martin-Wintle, M. S., Shepherdson, D., Zhang, G., Zhang, H., Li, D., Zhou, X., et al. (2015). Free mate choice enhances conservation breeding in the endangered giant panda. Nat. Comm. 6:10125. doi: 10.1038/ncomms10125
Miller, B. T., and Miller, J. L. (2005). Prevalence of physical abnormalities in eastern hellbender (Cryptobranchus alleganiensis alleganiensis) populations of middle Tennessee. Southeast. Natural. 4, 513–520. doi: 10.1656/1528-7092(2005)004[0513:POPAIE]2.0.CO;2
Murphy, J. B., and Gratwicke, B. (2017). History of captive management and conservation amphibian programs mostly in zoos and aquariums. Part II—salamanders and caecilians. Herpetol. Rev. 48, 474–486.
Nickerson, M. A., and Briggler, J. T. (2007). Harvesting as a factor in population decline of a long-lived salamander; the Ozark hellbender, Cryptobranchus alleganiensis bishopi Grobman. Appl. Herpetol. 4, 207–216. doi: 10.1163/157075407781268354
Nickerson, M. A., and Mays, C. E. (1973). The hellbenders: North American “giant salamanders.” Milwaukee Publ. Mus. Spec. Publ. Biol. Geol. 1, 1–106.
Nickerson, M. A., Ott, C. M., Castro, S. L., Garcia, V. M., Molina, T. C., Briggler, J. T., et al. (2011). Evaluation of microorganisms cultured from injured and repressed tissue regeneration sites in endangered giant aquatic Ozark hellbender salamanders. PLoS ONE 6:e28906. doi: 10.1371/journal.pone.0028906
Nickerson, M. A., Pitt, A. L., Tavano, J. J., Hecht, K. A., and Mitchell, J. C. (2017). Forest removal and the cascade of effects corresponding with an Ozark Hellbender population decline. Bull. Fla. Mus. Nat. Hist. 54, 147–164.
Noeske, T. A., and Nickerson, M. A. (1979). Diel activity rhythms in the hellbender, Cryptobranchus alleganiensis (Caudata: Cryptobranchidae). Copeia 1979, 92–95. doi: 10.2307/1443733
Peterson, C. L. (1988). Breeding activities of the hellbender in Missouri. Herpetol. Rev. 19, 28–29.
Peterson, C. L., Metter, D. E., Miller, B. T., Wilkinson, R. F., and Topping, M. S. (1983). Am. Midl. Nat. 119, 291–303. doi: 10.2307/2425812
Pfingsten, R. A. (1990). The status and distribution of the hellbender, Cryptobranchus alleganiensis, in Ohio. Herpetol. Rev. 21, 48–51.
Pfingsten, R. A., and Downs, F. L. (1989). Salamanders of Ohio. Columbus, OH: Ohio State University College of Biological Sciences.
Pitt, A. L., Sinskie, J. L., Tavano, J. J., Hartzell, S. M., Delahunty, T., and Spear, S. F. (2017). Decline of a giant salamander assessed with historical records, environmental DNA and multi-scale habitat data. Freshwater Biol. 62, 967–976. doi: 10.1111/fwb.12917
Reedy, A. M., Edwards, A., Pendle bury, C., Murdaugh, L., Avery, R., Seidenberg, J., et al. (2014). An acute increase in the stress hormone corticosterone is associated with mating behavior in both male and female red-spotted newts, Notophthalmus viridescens. Gen. Comp. Endocrinol. 208, 57–63. doi: 10.1016/j.ygcen.2014.08.008
Refsnider, J. M., and Janzen, F. J. (2010). Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu. Rev. Ecol. Evol. Syst. 41, 39–57. doi: 10.1146/annurev-ecolsys-102209-144712
Settle, R. A., Briggler, J. T., and Mathis, A. (2018). A quantitative field study of paternal care in Ozark hellbenders, North America's giant salamander. J. Ethol. 36, 235–242. doi: 10.1007/s10164-018-0553-0
Smith, B. G. (1907). The life history and habits of Cryptobranchus allegheniensis. Biol. Bull. 13, 5–39. doi: 10.2307/1535594
Snyder, N. F. R., Scott, R. D., Beissinger, S. R., Wiley, J. W., Smith, T. B., Toone, W. D., et al. (1996). Limitations of captive breeding in endangered species recovery. Cons. Biol. 10, 338–348. doi: 10.1046/j.1523-1739.1996.10020338.x
Stanton, L. A., Sullivan, M. S., and Fazio, J. M. (2015). A standardized ethogram for the felidae: a tool for behavioral researchers. Appl. Anim. Behav. Sci. 173, 3–16. doi: 10.1016/j.applanim.2015.04.001
Tonione, M., Johnson, J. R., and Routman, E. J. (2011). Microsatellite analysis supports mitochondrial phylogeography of the hellbender (Cryptobranchus alleganiensis). Genetica 139, 209–219. doi: 10.1007/s10709-010-9538-9
U.S. Fish and Wildlife Service (USFWS) (2011). Endangered and Threatened Wildlife and Plants; Endangered Status for the Ozark Hellbender Salamander. Fed. Reg. 76, 50. CFR Part 17.
Unger, S. D., and Mathis, A. (2013). Larval growth and the potential for head-starting of Eastern and Ozark hellbenders (Cryptobranchus alleganiensis alleganiensis and C. a. bishopi). Herpetol. Rev. 44, 547–550.
Wheeler, B. A., Prosen, E., Mathis, A., and Wilkinson, R. F. (2003). Population declines of a long-lived salamander: a 20+-year study of hellbenders, Cryptobranchus alleganiensis. Biol. Conserv. 109, 151–156. doi: 10.1016/S0006-3207(02)00136-2
Williams, L. A., and Groves, J. D. (2014). Prevalence of the amphibian pathogen Batrachochytrium dendrobatidis in eastern hellbenders (Cryptobranchus a. alleganiensis) in western North Carolina, USA. Herpetol. Conserv. Biol. 9, 454–467.
Keywords: reproduction, kinematic analysis, captive breeding, endangered species, hellbender
Citation: Settle RA, Ettling JA, Wanner MD, Schuette CD, Briggler JT and Mathis A (2018) Quantitative Behavioral Analysis of First Successful Captive Breeding of Endangered Ozark Hellbenders. Front. Ecol. Evol. 6:205. doi: 10.3389/fevo.2018.00205
Received: 19 July 2018; Accepted: 14 November 2018;
Published: 12 December 2018.
Edited by:
Caitlin R. Gabor, Texas State University System, United StatesReviewed by:
Beate Anna Apfelbeck, Technische Universität München, GermanyCopyright © 2018 Settle, Ettling, Wanner, Schuette, Briggler and Mathis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alicia Mathis, YWxpY2lhbWF0aGlzQG1pc3NvdXJpc3RhdGUuZWR1
†Present Address: Rachel A. Settle, Florida Fish and Wildlife Conservation Commission, Naples, FL, United States
Jeffery A. Ettling, Sedgwick County Zoo, Wichita, KS, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.