- 1Siefferman Lab, Department of Biology, Appalachian State University, Boone, NC, United States
- 2Atkinson Lab, Department of Biological Sciences, The University of Alabama, Tuscaloosa, AL, United States
- 3Gangloff Lab, Department of Biology, Appalachian State University, Boone, NC, United States
- 4Zoological Collections, Department of Biological Sciences, The University of Alabama, Tuscaloosa, AL, United States
- 5Osbourn Lab, Department of Biology, Appalachian State University, Boone, NC, United States
Conspicuous coloration coupled with secondary defensive mechanisms is known as aposematic coloration and is used in predator avoidance and defense. Classic theory argues that aposematic signals tend to be more effective in larger organisms as they are intrinsically more easily detected by predators and are also more profitable prey items. Thus, it has been hypothesized that conspicuous coloration evolves in tandem with increased body size in aposematic prey because this likely increases the visibility and efficacy of the signal. To test this hypothesis, we used a comparative phylogenetic approach to investigate associations between body size and conspicuous coloration in two North American salamander genera: Ambystoma and Plethodon, both of which secrete noxious compounds to deter predators. Our analyses showed that increased conspicuous coloration co-evolved with increased body size in Ambystoma, yet we found the opposite relationship in the Plethodon clade. We speculate this is likely because Ambystoma are generally larger than Plethodon and exhibit gregarious mating behavior. Future studies should explore the toxicity of slimy skin secretions and how this may contribute to aposematic signaling in salamanders.
Introduction
Animals use a variety of visual signals to convey information to one another, including conspicuous coloration (Wallace, 1867). When conspicuous coloration is coupled with a secondary defense, such as venom or poison, color is thought to serve as a signal of unpalatability to potential predators via aposematic signaling (Poulton, 1890). Cott (1940) suggested that aposematic signals typically involve red, yellow, or white patterns on a black background, likely because they are easily detected, recalled, and associated with the defense by predators (Gittleman and Harvey, 1980). It is also hypothesized that larger animals are more likely to evolve conspicuous coloration compared to smaller animals for several reasons. First, larger animals are intrinsically more obvious to predators than smaller animals and are therefore more likely to evolve secondary defensive mechanisms (Hossie et al., 2015), and are less likely to benefit from cryptic coloration (Hagman and Forsman, 2003). Second, because smaller animals are less easily detected, they are less likely to benefit from conspicuous coloration (Hossie et al., 2015). Third, larger prey items are expected to be more profitable than smaller prey items and thus larger prey should experience stronger selection pressure for defensive mechanisms (Penney et al., 2012). Indeed, experimental evidence from a study examining the responses of naïve domestic chicks (Gallus gallus domesticus) to varying sizes of conspicuously colored ground bugs (Lygaeidae) suggests that aposematic signal efficacy increases with increasing body size (Gamberale and Tullberg, 1996a).
There is conflicting evidence in the literature, however, as to whether conspicuous coloration coevolves with increased body size across taxa that employ antipredator coloration. In the caterpillar subfamily Macroglossinae, a phylogenetically-controlled analysis found that conspicuous eye-like markings (eyespots), which are thought to deter predation, are associated with larger body size (Hossie et al., 2015). Using a comparative approach, Hagman and Forsman (2003) found that increased conspicuousness also evolved in tandem with increased body size in poison dart frogs (Family Dendrobatidae). These findings are supported in a subsequent study focusing on a single dendrobatid species, the Strawberry poison dart frog (Oophaga pumilio, Rudh, 2013). Strawberry poison dart frogs exhibit remarkable phenotypic diversity throughout their range, and more conspicuous populations also have a larger mean body size. Moreover, phylogenetic relationships suggest that a loss of conspicuous coloration co-evolved with a decrease in body size in several populations (Rudh, 2013). Similarly, a phylogenetic investigation of camouflage morphology in the crab superfamily Majoidea demonstrated that decreased camouflage decoration behavior is associated with an increase in body size as well as increases in alternative defensive strategies such as color change (Hultgren and Stachowicz, 2009). All the aforementioned studies support the hypothesis that conspicuous coloration is positively associated with body size. However, the opposite pattern occurs in nudibranchs, with conspicuous coloration decreasing with increasing body size (Cheney et al., 2014), despite the presence of secondary chemical defenses (indicating their color is used as an aposematic signal). Finally, in lepidopteran larvae there is no apparent relationship between body size and conspicuousness (Nilsson and Forsman, 2003).
In addition to body size, experimental evidence also suggests that the efficacy of an aposematic signal increases with the size of the conspicuous body pattern (see Gamberale and Tullberg, 1996b), and that avian predators (Parus major) are better able to discriminate between palatable and unpalatable prey when the pattern elements are larger (Lindstrom et al., 1999). Behavioral traits are also expected to influence the efficacy of aposematic signals. The efficacy of aposematic signals should be positively associated with group size for two potential reasons: (1) because there is greater initial unconditioned aversion by predators (Gamberale and Tullberg, 1996b, 1998) and (2) because predators will learn more quickly to avoid unpalatable and conspicuous prey (Gagliardo and Guilford, 1993). Finally, conspicuous behavior may both increase the likelihood that the signal is detected and potentially help protect the prey by increasing the likelihood of engaging the predator with any defensive compounds (Brodie, 1977; Toledo and Jared, 1995). For example, in salamanders, many of the granular (defensive secretion) glands are located in the tail (Toledo and Jared, 1995), and most salamanders, when threatened, will position their bodies so that the tail is closest to the threatening stimuli (e.g., Brodie and Gibson, 1969).
Here, we used a comparative approach to investigate relationships between conspicuous coloration and body size in two genera of North American salamanders: Plethodon (family Plethodontidae) and Ambystoma (family Ambystomatidae). These two genera present an interesting contrast for this type of comparative study: Ambystoma salamanders are generally larger than Plethodon salamanders and nearly all Ambystoma species undertake annual breeding migrations resulting in aggregations, whereas no Plethodon taxa are known to migrate or form large aggregations (Petranka, 1998); referred to hereafter as “gregarious behavior.” Species within both genera display a diversity of color patterns and body sizes (Petranka, 1998) and their integument secretions are unpalatable to some predators (Brodie, 1977).
The aim of this study is to use hypothesized evolutionary relationships in a comparative analysis of independent contrasts to investigate associations between the evolution of body size and conspicuousness in the Ambystoma and Plethodon genera. We predicted that larger-bodied species would have more conspicuous coloration. We expected the positive trends between body size and conspicuousness to be more pronounced in the Ambystoma genus for three reasons: (1) Ambystoma are larger than Plethodon, (2) there is more empirical support for toxicity in Ambystoma than Plethodon, and (3) because Ambystoma aggregate during a discrete breeding season (often as short as 1 month) whereas Plethodon opportunistically breed during a longer portion of the year (Spring to Fall).
Materials and Methods
Data Collection
Our dataset included 50 Plethodon species from Wiens et al. (2006) and 17 Ambystoma species taken from Williams et al. (2013). To obtain information on conspicuousness for each species, we surveyed 43 and 46 undergraduate students for Plethodon and Ambystoma, respectively. We obtained photographs of one individual of each species from Amphibiaweb and removed backgrounds from each photograph. We chose this method for two reasons: (1) to prevent bias in conspicuousness ratings due to background color because salamanders may be found on numerous substrates of varying color (moss, leaf litter, soil, trees, rocks) and no data is available indicating the frequency with which each species may be found on each substrate, and (2) because comparable photographs showing full dorsal coloration for each species were not available on one type of background. We then asked students to rate level of conspicuousness on a scale of 1–10 (1 being least conspicuous and 10 being most conspicuous), then averaged the responses (Summers and Clough, 2001). We acknowledge that attempts to classify coloration as cryptic or conspicuous without accounting for background can be prone to error (Endler, 1978), but felt removing backgrounds was the most objective method given available data and photographs. Although this method uses photographs and human observations to determine conspicuousness rather than the reflectance spectra of animals and visual systems of natural predators and conspecifics, we considered it sufficient for applying the aposematism theory to these taxa because birds are thought to be common predators in North America (Petranka, 1998) and evidence suggests that humans and birds see colors similarly enough that most organisms that appear colorful to humans also appear colorful to birds (Vorobyev et al., 2000). Because birds perceive a greater range of wavelengths (i.e., spectral purity or hue) and are better able to discriminate achromatic differences (i.e., brightness) between two objects (Vorobyev and Osorio, 1998) compared to humans, it is possible this method may underestimate the conspicuousness of integument coloration.
We also compiled a database of maximum and minimum total lengths for adults from each species from published literature (Petranka, 1998; Beane et al., 2010; Mitchell and Gibbons, 2010). We used total lengths rather than snout-vent lengths because tail length is variable between species and because granular cells are most abundant in tail integument tissues. We found that, in both genera, maximum total length and minimum total length were significantly correlated (Ambystoma: r = 0.96, n = 17, p < 0.001; Plethodon: r = 0.82, n = 50, p < 0.001), and thus used average total length in analyses. Appropriate phylogenetic trees were available for each genus and we obtained phylogenetic information for each species from the published literature (Plethodon: Wiens et al., 2006; Ambystoma: Williams et al., 2013).
Statistical Analysis
To investigate general relationships between body size and conspicuousness, we compared the average total length of each species with the average observer response. Because closely related taxa cannot be considered statistically independent because of shared ancestry, we calculated independent contrasts (Felsenstein, 1985) using the computer software Mesquite (Maddison and Maddison, 2011) using the PDAP module (Phenotypic Diversity Analysis Programs, Garland et al., 1992). For both Ambystoma and Plethodon species, we used a gradual model of character evolution and obtained branch length information from Williams et al. (2013) and Wiens et al. (2006), respectively, which was based on the number of substitutions per site in mitochondrial and nuclear DNA sequences. To ensure that the data met the statistical assumptions that body size and coloration evolution are related to phylogenetic data, we then regressed contrasts for conspicuousness on contrasts for body size through the origin for both species and found that the slope did not differ significantly from zero (Harvey and Pagel, 1991; Garland et al., 1992; Pagel, 1993).
Results
A Mann-Whitney Rank Sum Test demonstrated that Ambystoma salamanders are larger than Plethodon salamanders (Mann-Whitney U statistic = 315, T = 774, n(Ambystoma) = 17, n(Plethodon) = 50, p = 0.049; Table 1).
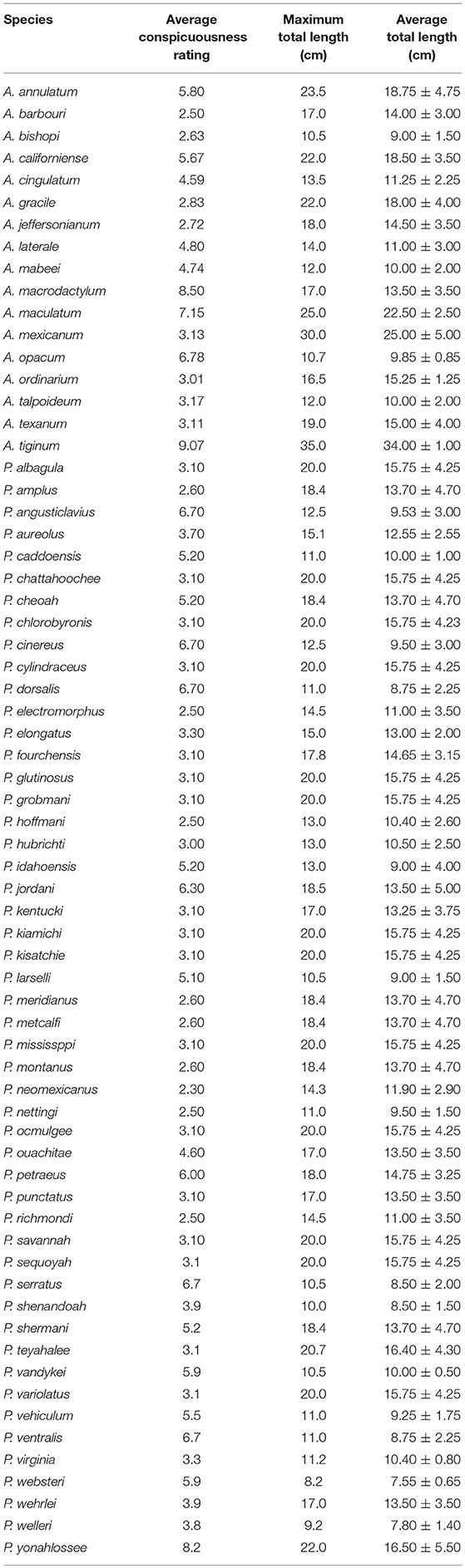
Table 1. Average conspicuousness rating, maximum total length, and average total length ± 1 standard deviation for each species of Ambystoma and Plethodon.
Ambystoma
Regressions of independent contrasts showed that conspicuousness was positively correlated to average total length (R2 = 0.75, n = 16, p < 0.001; Figure 1). Because this analysis used independent contrasts, it is likely that these correlations are not simply the result of phylogenetic inertia.
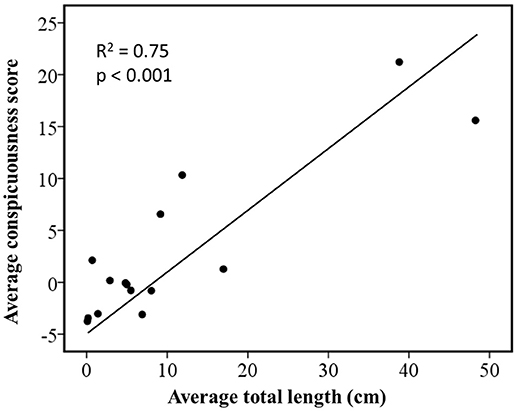
Figure 1. Relationship between coloration and body size in Ambystoma salamanders estimated by phylogenetically independent contrasts using a gradual model of evolutionary change. The figure shows standardized contrasts for average total length against standardized contrasts for average ratings of conspicuousness.
Plethodon
Regression of independent contrasts showed conspicuousness was negatively correlated with average total length (R2 = 0.14, n = 49, p < 0.01; Figure 2). It should be noted that when the largest species is removed (Plethodon yonahlossee), the relationship between average total length and conspicuousness changes from R2 = 0.14 (n = 49), to R2 = 0.29 (n = 48).
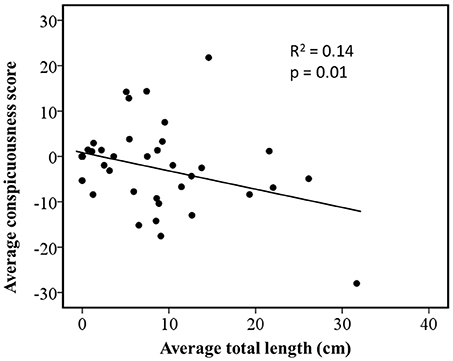
Figure 2. Relationship between coloration and body size in Plethodon salamanders estimated by phylogenetically independent contrasts using a gradual model of evolutionary change. The figure shows standardized contrasts for average total length against standardized contrasts for average ratings of conspicuousness.
Discussion
The results for Ambystoma salamanders are consistent with the hypothesis that conspicuousness is associated with body size; larger species tend to be more conspicuous than smaller species. Moreover, phylogenetic patterns suggest relatively recent evolution of conspicuousness in some species (Figure 3) and the co-evolution of larger body size and more conspicuous coloration in Ambystoma, likely as a consequence of ecological selection pressures. The results for Plethodon salamanders, however, are not consistent with this hypothesis; smaller species tend to be more conspicuous. The phylogeny suggests that the ancestral Plethodon state is more conspicuous and smaller in body size than current taxa (Figure 4), suggesting that evolution has favored increasing body size but not conspicuousness. Although initially these results suggest equivocal support for the hypothesis of coevolution between body size and conspicuousness in salamanders, differences between the genera may explain these discrepancies.
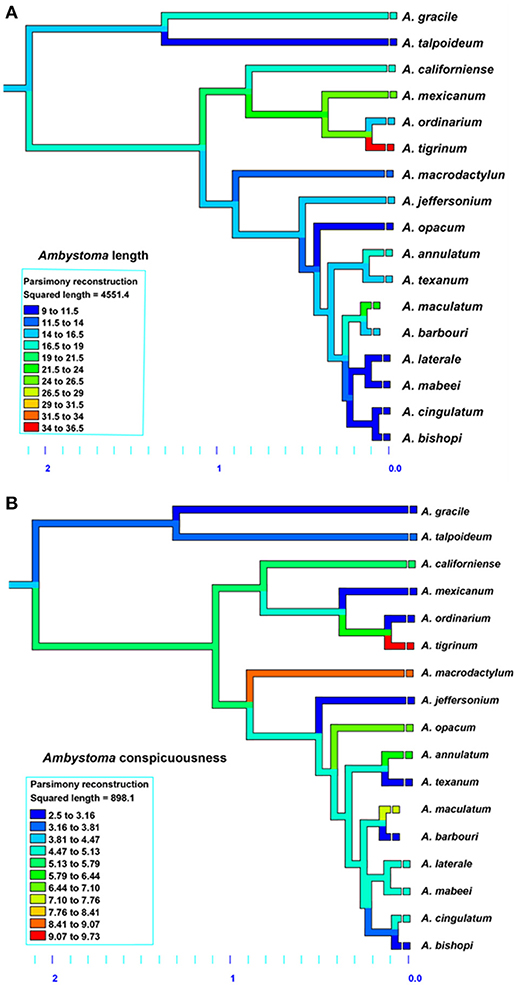
Figure 3. Evolution of (A) average total length and (B) conspicuous coloration in Ambystoma salamanders using the squared-change parsimony Mesquite version 2.75. Orange and red shades represent a larger body size (top) and more conspicuous coloration (bottom). Phylogeny adapted from Williams et al. (2013).
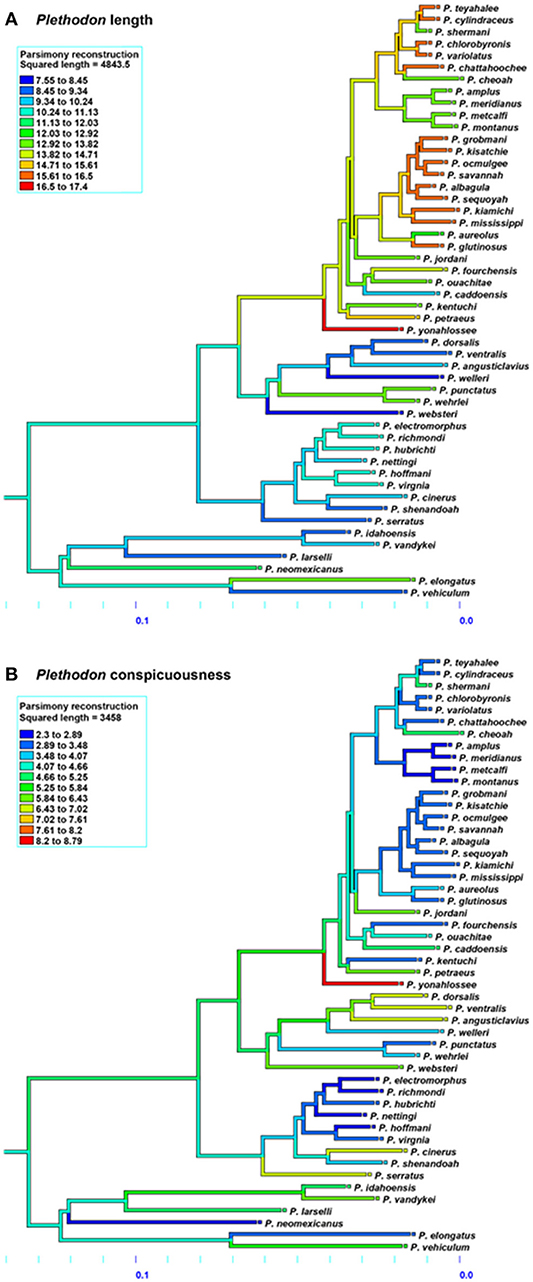
Figure 4. Evolution of (A) average total length and (B) conspicuous coloration in Plethodon salamanders using the squared-change parsimony (Mesquite version 2.75). Orange and red shades represent a larger body size (top) and more conspicuous coloration (bottom). Phylogeny adapted from Wiens et al. (2006).
Aposematic colors signal information about unpalatability to potential predators; conventional theory posits that conspicuousness should be positively correlated with unprofitability (Summers and Clough, 2001). There is evidence to suggest that both Ambystoma and Plethodon are unpalatable to certain predators and that they exhibit behavior consistent with possession of noxious integument secretions (Brodie, 1977). Moreover, Ambystoma may be less profitable prey than Plethodon. Species of Ambystoma are known to produce integument secretions which have strong adhesive properties (Evans and Brodie, 1994), contain neurotoxic components (Hamning et al., 2000) and may be lethal to animals such as mice and voles (Brodie and Gibson, 1969). Although Plethodon species are typically avoided in feeding trials in comparison with other salamanders in Plethodontidae, particularly Desmognathus species, their integument secretions have not been shown to be lethal to any potential predators (Hensel and Brodie, 1976; Brodie et al., 1979). For aposematic traits to be adaptive, the benefit of conspicuousness must outweigh the costs. Perhaps, among Ambystoma, greater unpalatability coupled with conspicuousness was more adaptive, while, among Plethodons, reduced unpalatability coupled with conspicuousness was less adaptive and thus conspicuousness was lost in Plethodons. Future work should focus on quantifying unpalatability of these groups.
The efficacy of warning signals increases with increasing body size, thus larger animals should be more likely to evolve conspicuous coloration than smaller animals (Gamberale and Tullberg, 1996a; Hagman and Forsman, 2003; Penney et al., 2012; Hossie et al., 2015). Additionally, larger prey items are associated with greater nutritional benefit and thus may need to allocate more resources to defense mechanisms than smaller prey items (Smith et al., 2016; Briolat et al., 2018). Generally, Ambystoma are larger than Plethodon (average Ambystoma length = 15.9 cm, average Plethodon length = 12.7 cm); Ambystoma typically have stout bodies with broad heads and tails, while Plethodon tend to be slender with narrow heads and tails (Petranka, 1998). Thus, the difference in direction of the covariation between body size and conspicuousness between Ambystoma and Plethodon genera may be influenced by differences in body size of species in these genera. Perhaps there is a size threshold wherein most Plethodon salamanders are too small to benefit from conspicuous coloration while larger Ambystoma benefit more from conspicuous coloration and secondary defense mechanisms as they are more distinguishable and likely more profitable prey. This idea is supported by the one exception to the negative relationship in Plethodon between body size and conspicuousness: Plethodon yonahlossee. The Yonahlossee Salamander (P. yonahlossee) is the largest species in the genus, as well as the most conspicuous, and, when removed from the analysis, changes the results rather dramatically. Though the data show that selection in Plethodon currently favors large body size and inconspicuous coloration, P. yonahlossee has either not undergone the same selection pressures as other Plethodon species, or is large enough or has acquired/maintained a defense mechanism potent enough to benefit from conspicuous coloration.
Ambystoma and Plethodon also have important lifestyle differences; Ambystoma species are largely fossorial with aquatic larvae (n = 17) and a few taxa that are fully aquatic (A. mexicanum, A. ordinarium, and A. dumerilii-a data deficient species excluded from analyses) and inconspicuous (Petranka, 1998). Terrestrial Ambystoma exhibit explosive annual breeding migrations where large numbers of individuals aggregate above ground at least once per year (Petranka, 1998). One exception is the inconspicuously-colored A. barbouri, a streamside species that does not travel long distances to breed. Gregarious behavior is thought to amplify aposematic signals, as large numbers of organisms gathered in one place increases the likelihood of being seen and encountered by predators (Poulton, 1890). Models of the evolution of aposematic coloration and gregariousness suggest that they should evolve in tandem in insects (Sillen-Tullberg, 1988), and that gregarious behavior evolved with both chemical defenses and warning coloration in Macrolepidopteran larvae (Tullberg and Hunter, 1996). This is further supported by studies using artificial prey (Alatalo and Mappes, 1996; Mappes and Alatalo, 1997; Riipi et al., 2001).
Species in the Plethodon clade, in contrast, are solitary and terrestrial throughout their life cycle but may occur in high densities. Most are active on warm, rainy nights, as well as under leaf litter and cover objects during the day (Brandon and Huheey, 1975; Petranka, 1998). Though they are rarely seen on the surface during the day, some potential predators (such as grouse and turkeys) scratch in the leaf litter and could potentially uncover individuals. Moreover, despite nocturnal activity patterns, species could have evolved warning coloration through selection pressure from being uncovered during the day or from nocturnal predators like owls. Among the largely fossorial caecilians (Amphibia: Gymnophiona), a slight propensity to surface activity coincided with the evolution of bright yellow integument patterns in three separate evolutionary events (Wollenberg and Measey, 2009).
In addition to aposematism, conspicuous coloration can evolve via mimicry of unprofitable organisms (Rudh and Qvarnstrom, 2013). There is evidence of mimicry in other plethodontid species; using clay model simulations, Kuchta (2005) found that the plethodontid salamander Ensatina eschscholtzii xanthoptica is likely a mimic of the highly toxic Taricha newts. Mimicry of the red eft stage of the eastern newt, Notophthalmus viridescens viridescens is the most often explored hypothesis for conspicuous coloration in Plethodon (Howard and Brodie, 1971; Brodie and Howard, 1973). Red coloration is common among the Plethodon species that received high conspicuousness ratings, and, though most species with red coloration are not entirely red (N. viridescens is entirely red), a red patch could be sufficient to induce avoidance in predators that have an established innate or learned avoidance response of N. viridescens efts.
Sexual signaling is another possible use of conspicuous coloration (Rudh and Qvarnstrom, 2013) and sexual dimorphism is a general indicator of sexual selection (reviewed in Andersson, 1994). Although there is some evidence of sexual dichromatism in Ambystoma (Morgan et al., 2014), there is no evidence in Plethodon (Petranka, 1998). Thus, sexual signaling among Plethodons seems unlikely. Many Plethodon species have evolved an “olfactory” approach to sexual communication, with males using a proteinaceous pheromone stored and dispensed from an enlarged mental gland to increase female receptivity during courtship (Palmer et al., 2005). Finally, there may be physiological selection pressure for darker (less conspicuous) coloration in amphibians. Melanin pigmentation gives the body a darker color and aids in thermoregulation and UV protection (Trullas et al., 2007). Perhaps evolution favored the loss of conspicuousness in larger bodied Plethodon species via physiological pressures to increase melanin content in the integument.
Although salamanders are often conspicuously colored, to date, very little empirical work has addressed the signaling function of elaborate coloration in this group (but see Mochida, 2009, 2010; Kraemer et al., 2015). Using a comparative study, we show that increased body size and increased conspicuous coloration have evolved in tandem in Ambystoma salamanders, while increased body size has co-evolved with a loss of conspicuous coloration in Plethodon species. Differences between the genera help explain these trends; Ambystoma are larger, presumably more noxious, and exhibit gregarious behavior during annual breeding migrations. Plethodon are smaller, with evidence indicating they are less noxious than Ambystoma, are not known to exhibit gregarious behavior, and several species could potential be imperfect mimics of the toxic N. viridescens. More information on toxicity in each genus, and among salamanders in general, will increase understanding of antipredator defense mechanisms as well as the signal function of conspicuous coloration.
Author Contributions
MW is the Master's student who compiled data for genus Plethodon, completed data analysis for both genera, and prepared the manuscript. MP assisted in data compilation and editing the manuscript. MO and MG assisted in the initial stages of the project design. LS is the Master's advisor and assisted in project design as well as manuscript edits.
Funding
This project was funded by Appalachian State University Department of Biology as well as the Office of Student Research. Funding was also provided by the Chicago Herpetological Society.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the Biology Department at Appalachian State University for funding this research via the Office of Student Research and the Graduate Student Association Senate. We also would like to thank the Chicago Herpetological Society for providing funding. Finally, we would also like to acknowledge John Harwood, an undergraduate student who assisted in data compilation for the genus Ambystoma and the development of methods.
References
Alatalo, R. V., and Mappes, J. (1996). Tracking the evolution of warning signals. Nature 382, 708–710. doi: 10.1038/382708a0
Beane, J. C., Braswell, A. L, Mitchell, J. C, and Palmer, W. M (2010). Amphibians and Reptiles of the Carolinas and Virginia. Chapel Hill, NC: University of North Carolina Press.
Brandon, R. A., and Huheey, J. E. (1975). Diurnal activity, avian predation, and the question of warning coloration and cryptic coloration in salamanders. Herpetologica 31, 252–255.
Briolat, E. S., Zagrobelny, M., Olsen, C. E., Blount, J. D., and Stevens, M. (2018). Sex differences but no evidence of quantitative honesty in the warning signals of six-spot burnet moths (Zygaena filipendulae L.). Evolution 72, 1460–1474. doi: 10.1111/evo.13505
Brodie, E. D. Jr. (1977). Salamander antipredator postures. Copeia 1977, 523–535. doi: 10.2307/1443271
Brodie, E. D. Jr., and Gibson, L. S. (1969). Defensive behavior and integument glands of the Northwestern Salamander, Ambystoma gracile. Herpetologists League 25, 187–194.
Brodie, E. D. Jr., and Howard, R. R. (1973). Experimental study of Batesian mimicry in the salamanders Plethodon jordani and Desmognathus ochrophaeus. Am. Midl. Nat. 90, 38–46. doi: 10.2307/2424264
Brodie, E. D. Jr., Nowak, R. T., and Harvey, W. R. (1979). The effectiveness of antipredator secretions and behavior of selected salamanders against shrews. Copeia 1979,270–274. doi: 10.2307/1443413
Cheney, K. L., Cortesi, F., How, M. J., Wilson, N. G., Blomberg, S. P, and Marshall, N. J. (2014). Conspicuous visual signals do not coevolve with increased body size in marine sea slugs. J. Evol. Biol. 27, 676–687. doi: 10.1111/jeb.12348
Endler, J. A. (1978). A predator's view of animal color patterns. Evol. Biol. 11, 319–364. doi: 10.1007/978-1-4615-6956-5_5
Evans, C. M., and Brodie, E. D. Jr (1994). Adhesive strength of amphibian skin secretions. J. Herpetol. 28, 499–502. doi: 10.2307/1564965
Felsenstein, J. (1985). Phylogenies and the comparative method. Am. Nat. 125, 1–15. doi: 10.1086/284325
Gagliardo, A., and Guilford, T. (1993). Why do warning-colored prey live gregariously? Proc. R. Soc. Lond. B Biol. Sci. 251, 69–74. doi: 10.1098/rspb.1993.0010
Gamberale, G., and Tullberg, B. S. (1996a). Evidence for a peak-shift in predator generalization among aposematic prey. Proc. R. Soc. Lond. B Biol. Sci. 263, 1329–1334. doi: 10.1098/rspb.1996.0195
Gamberale, G., and Tullberg, B. S. (1996b). Evidence for a more effective signal in aggregated aposematic prey. Anim. Behav. 52, 597–601. doi: 10.1006/anbe.1996.0200
Gamberale, G., and Tullberg, B. S. (1998). Aposematism and gregariousness: the combined effect of group size and coloration on signal repellence. Proc. R. Soc. Lond. B Biol. Sci. 265, 889–894. doi: 10.1098/rspb.1998.0374
Garland, T., Harvey, P. H., and Ives, A. R. (1992). Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32. doi: 10.1093/sysbio/41.1.18
Gittleman, J. L., and Harvey, P. H. (1980). Why are distasteful prey not cryptic? Nature 286, 149–150. doi: 10.1038/286149a0
Hagman, M., and Forsman, A. (2003). Correlated evolution of conspicuous coloration and body size in poison frogs (Dendrobatidae). Evolution 57, 2904–2910. doi: 10.1111/j.0014-3820.2003.tb01531.x
Hamning, V. K., Yanites, H. L., and Peterson, N. L. (2000). Characterization of adhesive neurotoxic components in skin granular gland secretions of Ambystoma tigrinum. Copeia 2000, 856–859. doi: 10.1643/0045-8511(2000)000[0856:COAANC]2.0.CO;2
Harvey, P. H., and Pagel, M. D. (1991). The Comparative Method in Evolutionary Biology. Oxford: Oxford University Press.
Hensel, J. L. Jr., and Brodie, E. D. Jr (1976). An experimental study of aposematic coloration in the salamander Plethodon jordani. Copeia 1, 59–65. doi: 10.2307/1443772
Hossie, T. J., Skelhorn, J., Breinholt, J. W., Kawahara, A. Y., and Sherratt, T. N. (2015). Body size affects the evolution of eyespots in caterpillars. Proc. Natl. Acad. Sci. U.S.A. 112, 6664–6669. doi: 10.1073/pnas.1415121112
Howard, R. R., and Brodie, E. D. Jr (1971). Experimental study of mimicry in salamanders involving Notopthalmus viridescens and Pseudotriton ruber schencki. Nature 233: 277. doi: 10.1038/233277a0
Hultgren, K. M., and Stachowicz, J. J (2009). Evolution of decoration in Majoid crabs: a comparative phylogenetic analysis of the role of body size and alternative defensive strategies. Am. Nat. 173, 566–578. doi: 10.1086/597797
Kraemer, A. C., Serb, J. M., and Adams, D. C. (2015). Batesian mimics influence the evolution of conspicuousness in an aposematic salamander. J. Evol. Biol. 28, 1016–1023. doi: 10.1111/jeb.12622
Kuchta, S. R. (2005). Experimental support for aposematic coloration in the salamander Ensatina eschscholtzii xanthoptica: implications for mimicry of Pacific Newts. Copeia 2005, 265–271. doi: 10.1643/CH-04-173R
Lindstrom, L., Alatalo, R. V., Mappes, J., Riipi, M., and Vertainen, L. (1999). Can aposematic signals evolve by gradual change? Nature 397, 249–251. doi: 10.1038/16692
Maddison, W. P., and Maddison, D. R. (2011). Mesquite: a Modular System for Evolutionary Analysis. Version 2.75. Available online at: http://mesquiteproject.org
Mappes, J., and Alatalo, R. V. (1997). Effects of novelty and gregariousness in survival of aposematic prey. Behav. Ecol. 8, 174–177. doi: 10.1093/beheco/8.2.174
Mitchell, J. C., and Gibbons, J. W. (2010). Salamanders of the Southeast. Athens: University of Georgia Press.
Mochida, K. (2009). A parallel geographic mosaic of morphological and behavioural aposematic traits of the newt, Cynops pyrrhogaster (Urodela: Salamandridae). Biol. J. Linn. Soc. 97, 613–622. doi: 10.1111/j.1095-8312.2008.01182.x
Mochida, K. (2010). Temerature-dependent aposematic behavior in the newt Cynops pyrrhogaster. Zool. Sci. 27, 555–558. doi: 10.2108/zsj.27.555
Morgan, S. K., Pugh, M. W., Gangloff, M. M., and Siefferman, L. (2014). The spots of the spotted salamander are sexually dimorphic. Copeia 2014, 251–256. doi: 10.1643/CE-13-085
Nilsson, M., and Forsman, A. (2003). Evolution of conspicuous colouration, body size and gregariousness: a comparative analysis of lepidopteran larvae. Evol. Ecol. 17, 51–66. doi: 10.1023/A:1022417601010
Pagel, M. (1993). Seeking the evolutionary regression coefficient: an analysis of what comparative methods measure. J. Theor. Biol. 164:191–205. doi: 10.1006/jtbi.1993.1148
Palmer, C. A., Watts, R. A., Gregg, R. G., McCall, M. A., Houck, L. D., and Arnold, S. J. (2005). Lineage-specific differences in evolutionary mode in a salamander courtship pheromone. Mol. Biol. Evol. 22, 2243–2256. doi: 10.1093/molbev/msi219
Penney, H. D., Hassall, C., Skevington, J. H., Abbott, K. R., and Sherratt, T. N. (2012). A comparative analysis of the evolution of imperfect mimicry. Nature 483, 461–464. doi: 10.1038/nature10961
Petranka, J. W. (1998). Salamanders of the United States and Canada. Washington, DC: Smithsonian Institution Press.
Poulton, E. B. (1890). The Colours of Animals: Their Meaning and use, Especially Considered in the Case of Insects. New York, NY: Appleton D.
Riipi, M., Alatalo, R. V., Lindstrom, L., and Mappes, J. (2001). Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413, 512–514. doi: 10.1038/35097061
Rudh, A. (2013). Loss of conspicuous coloration has co-evolved with decreased body size in populations of poison dart frogs. Evol. Ecol. 17, 755–767. doi: 10.1007/s10682-013-9649-8
Rudh, A., and Qvarnstrom, A. (2013). Adaptive colouration in amphibians. Semin. Cell Dev. Biol. 24, 553–561. doi: 10.1016/j.semcdb.2013.05.004
Sillen-Tullberg, B. (1988). Evolution of gregariousness in aposematic butterfly larvae: a phylogenetic analysis. Evolution 42, 293–305. doi: 10.1111/j.1558-5646.1988.tb04133.x
Smith, K. E., Halpin, C. G., and Rowe, C. (2016). The benefits of being toxic to deter predators depends on prey body size. Behav. Ecol. 27, 1650–1655. doi: 10.1093/beheco/arw086
Summers, K., and Clough, M. E. (2001). The evolution of coloration and toxicity in the poison frog family (Dendrobatidae). Proc. Natl Acad. Sci. U.S.A. 98, 6227–6232. doi: 10.1073/pnas.101134898
Toledo, R. C., and Jared, C. (1995). Cutaneous granular glands and amphibian venoms. Comp. Biochem. Physiol. 111, 1–29. doi: 10.1016/0300-9629(95)98515-I
Trullas, S. C., Wyk, J. H., and Spotila, J. R. (2007). Thermal melanism in ectotherms. J. Therm. Biol. 32, 235–245. doi: 10.1016/j.jtherbio.2007.01.013
Tullberg, B. S., and Hunter, A. F. (1996). Evolution of larval gregariousness in relation to repellent defences and warning coloration in tree-feeding Macrolepidoptera: a phylogenetic analysis based on independent contrasts. Biol. J. Linn. Soc. 57, 253–276. doi: 10.1111/j.1095-8312.1996.tb00312.x
Vorobyev, M., Marshall, J., Osorio, D. N., de Ibarra, H., and Menzel, R. (2000). Colourful objects through animal eyes. Color Res. Appl. 26, S214–S217. doi: 10.1002/1520-6378(2001)26:1+<::AID-COL45>3.0.CO
Vorobyev, M., and Osorio, D. (1998). Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B Biol. Sci. 265, 351–358. doi: 10.1098/rspb.1998.0302
Wallace, A. R. (1867). Mimicry and Other Protective Resemblances Among Animals. Worcestershire: Read Books Limited.
Wiens, J. J., Engstrom, T. N., and Chippindale, P. T. (2006). Rapid diversification, incomplete isolation, and the “speciation clock” in North American salamanders (genus Plethodon): testing the hybrid swarm hypothesis of rapid radiation. Evolution 60, 2585–2603. doi: 10.1111/j.0014-3820.2006.tb01892.x
Williams, J., Niedzwiecki, J., and Weisrock, D. (2013). Species tree reconstruction of a poorly resolved clade of salamanders (Ambystomatidae) using multiple nuclear loci. Mol. Phylogenet. Evol. 68, 671–682. doi: 10.1016/j.ympev.2013.04.013
Keywords: aposematism, comparative analysis, independent contrasts, Mesquite, phylogeny
Citation: Winebarger MM, Pugh MW, Gangloff MM, Osbourn MS and Siefferman L (2018) Body Size Is Positively Correlated With Conspicuous Coloration in Ambystoma Salamanders, but Negatively Correlated With Conspicuous Coloration in Plethodon Salamanders. Front. Ecol. Evol. 6:143. doi: 10.3389/fevo.2018.00143
Received: 18 May 2018; Accepted: 03 September 2018;
Published: 25 September 2018.
Edited by:
Martin Stevens, University of Exeter, United KingdomReviewed by:
Bibiana Rojas, University of Jyväskylä, FinlandDavid W. Kikuchi, University of Arizona, United States
Copyright © 2018 Winebarger, Pugh, Gangloff, Osbourn and Siefferman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monica M. Winebarger, bW13aW5lYmFyZ2VyQGNyaW1zb24udWEuZWR1
 Monica M. Winebarger
Monica M. Winebarger M. Worth Pugh3,4
M. Worth Pugh3,4 Michael M. Gangloff
Michael M. Gangloff Lynn Siefferman
Lynn Siefferman