
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 09 May 2018
Sec. Behavioral and Evolutionary Ecology
Volume 6 - 2018 | https://doi.org/10.3389/fevo.2018.00046
This article is part of the Research Topic What’s Love Got to Do with it: The Evolution of Monogamy View all 17 articles
One major neurobiological substrate regulating social processes is dopamine (DA). DA is implicated in social behavior in species as diverse as fish and birds, and has an established role in regulating relationships between mates in socially monogamous rodents. Marmoset monkeys display traits associated with social monogamy including high rates of affiliation, biparental care, distress upon separation, and aggression toward strangers; several of these behavioral patterns change throughout the development of relationships. This temporal change may represent changing demands, as pairs are likely to jointly face new experiences (e.g., parenthood) throughout pairing. We investigated the role of DA and pairing length on social behavior during reunion after separation from the mate. Marmosets were removed from their home environment and treated with agonists and antagonists for the D1 and D2 receptor subtypes. They were exposed to a novel environment containing an opposite-sex stranger and their pair mate, and then reunited with their mate in the home enclosure. Marmosets in long term pairs exhibited higher levels of food sharing during reunion than marmosets in short term pairs, with females in long term pairs sharing food more than males; no sex difference was observed in short term pairs. Subjects in short term pairs spent more time grooming their mate than receiving grooming during reunion, while marmosets in long term pairs displayed similar amounts of both initiated and received grooming. DA treatment altered pair-level behavior. When females received either a D2 agonist or antagonist, short term pairs spent less time in proximity, compared to when males received the same treatments. In long term pairs, treatment of females with either a D1 agonist or antagonist resulted in pairs spending less time in social proximity than when males were treated. These findings suggest that the function of the DA system in mate behavior may be similar between rodents and primates, with the D1 system modulating the expression of behavior in long term pairs and the D2 system regulating behavior in short term pairs. Furthermore, these results supplement a large body of work suggestive of deep evolutionary roots of the DA system in regulating social behavior.
A close, selective, though not necessarily exclusive, social and sexual relationship with a single partner is a hallmark of social monogamy. There are several behavioral components of social monogamy, all of which reference this relationship between partners. Within the construct of social monogamy there is variability both within, and especially among, species in the specific social and behavioral components that are displayed (Díaz-Muñoz and Bales, 2015; Tecot et al., 2016). In nonhuman primates, social monogamy is associated with a host of behavioral traits, including a selective social preference for the mate over other conspecifics, biparental care, and aggression toward same- or opposite-sex conspecifics (French et al., 2017). Of particular interest are several phenomena associated with separation of the mating pair, which include distress upon separation from the mate, social buffering of stressful experiences while in the presence of the mate, and high rates of affiliative behavior upon reunion with the mate after a period of social separation (French et al., 2017).
Marmoset monkeys (Callithrix spp.) display many characteristics associated with social monogamy. These features include social buffering of stress responses by the presence of the pair mate (Smith et al., 1998; Rukstalis and French, 2005; Cavanaugh et al., 2015), affiliation toward the mate (Ågmo et al., 2012), biparental care (Snowdon, 1996; Ziegler et al., 2009), and aggression toward same-sex conspecifics (Evans, 1983; Ross et al., 2004; Ross and French, 2011). However, marmosets do not display a strong selective social preference for their mate in standard partner preference tests (Smith et al., 2010; Cavanaugh et al., 2014), and they also demonstrate high levels of social and sexual interest in opposite-sex strangers (Cavanaugh et al., 2014; Mustoe et al., 2015). In addition, there is variability in group demography in wild marmosets, ranging from single pair and offspring to multimale/multifemale groups (Digby, 1999; Sousa et al., 2005). Thus, marmosets demonstrate traits that are associated with social monogamy, as well as flexible and conditional social responses in ways that resemble the complexities of human “social monogamy” (Chapais, 2013).
One behavioral trait that may be particularly important to the stability of a mating pair is the degree to which pairs re-establish their relationship during reunion after a period of separation, stress, or disruption. Separation from social partners is often accompanied by behavioral signs of distress, and increased glucocorticoid concentrations (Smith et al., 1998; DeVries et al., 2003; Hennessy et al., 2009; French et al., 2012; Ziegler and Crockford, 2017). Monogamous male titi monkeys demonstrate differential brain activation in regions associated with the regulation of social behavior (limbic and striatal) during separation from a mate, and release of the neuropeptide oxytocin upon reunion with the mate (Hinde et al., 2016), indicating physiological responses to both separation from, and reunion with, a mate. In marmosets, separation from a mating partner and short- or long-term exposure to a novel environment is associated with elevated hormonal stress responses (cortisol increases) (Smith et al., 1998; Cavanaugh et al., 2016). Furthermore, levels of affiliation are higher between mates after a separation, compared to pre-separation levels, suggesting that elevated rates of affiliative behavior upon reunion in marmosets is an important mechanism for down-regulating the negative affect and stress response associated with partner separation (Shepherd and French, 1999; Cavanaugh et al., 2018). High rates of affiliative behavior upon reunion among other classes of social relationships in marmosets is also associated with regulation of the arousal associated with separation from close social contacts. Juvenile marmosets that engage in high levels of affiliation during reunion after social isolation demonstrate a faster return to baseline glucocorticoid levels (Taylor et al., 2015), highlighting the importance of reunion behavior across social contexts in marmosets.
The notion that reunion behavior modulates stress responses to separation is supported by the observation that marmosets experiencing a stressor with their partner exhibit no difference in affiliative behavior upon reunion from pre-stressor levels (Smith et al., 1998). This suggests that in marmosets when a mate is present during a stressor, behavioral and physiological responses during the stressor are reduced (e.g., social buffering), and the need to downregulate stress upon reunion in the home enclosure is hence less important (Cavanaugh et al., 2016). The duration of separation also shapes the nature of affiliative interactions upon reunion. Marmoset pairs separated for an extended period of time (7 days) showed elevated pair-directed affiliation upon reunion, while short separations (5–15 min) did not increase rates of affiliative behavior (Duarte et al., 2017), suggesting that longer separations require larger behavioral responses during reunion, presumably to aid in re-establishment of the social relationship. Together, these data suggest that high rates of affiliative interactions with significant social partners after a period of separation has important consequences for regulating the biobehavioral responses associated with social separation. Thus, reunion after separation represents a time at which individuals in the pair are engaging in behavior to reduce behavioral and endocrine components of the stress response and to re-establish social relationships.
The expression of reunion behavior with a pair mate may be dependent on the phase of the relationship. The underlying relationship between mating partners in socially monogamous rodents has been delineated into two phases: formation and maintenance. Each of these phases is associated with distinct behavioral traits, as well as neurobiology, in rodent models of social monogamy (i.e., prairie voles) (Young and Wang, 2004; Curtis et al., 2006; Lim and Young, 2006; Young et al., 2008, 2011; Aragona and Wang, 2009). Social preference for the mate is a key marker of pair bond formation, and in voles is observed after 24 h of cohabitation (Williams et al., 1992), while selective aggression toward conspecifics is considered a marker of pair bond maintenance and is tied to onset of mating with a mate (Carter et al., 1995). While the phases of pair formation and maintenance in prairie voles appear to follow a strict and short timeline, primates tend to show longer and more variable transitions in relationships (Maninger et al., 2017). Patterns of behavior between mates in marmosets shift across time, including levels of sexual behavior (typically, high in the beginning and lower with increased length of pairing) and affiliative social behavior across pairing (typically, lower in the beginning and increasing with pairing length) (Schaffner et al., 1995; Ågmo et al., 2012; c.f. Evans and Poole, 1984). Thus, while there are differences in affiliative behavior in marmosets between short and long term pairs, there is no clear behavioral marker of an “established” pair. Furthermore, social monogamy is theorized to have evolved from an environment in which females were highly dispersed and males experienced fitness benefits from guarding and maintaining a relationship with a single female (Lukas and Clutton-Brock, 2013). Thus, male and female mates experience a suite of different evolutionary pressures in terms of mating success. While males may benefit from engaging in as many sexual encounters as possible given the minimal cost associated with sperm production, they also benefit from continuous presence near their female mates (e.g., mate guarding, proximity maintenance) to reduce the risk of her engaging in extra pair copulations. Especially in species that exhibit paternal care, it is critically important, from an energetic perspective, that males raise offspring genetically related to them. It is therefore likely that males and females will display different behavioral profiles from one another at both initial and later stages of a relationship.
The neurotransmitter dopamine (DA) has been identified as a key player in decision making and social behavior in species as diverse as invertebrate leeches, fish, birds, and mammals (O'Connell and Hofmann, 2011). Of particular note is the role that DA plays in the formation and maintenance of bonds between mating partners in rodents. DA is a neurobiological regulator of the reward system, and its role in associative learning may facilitate its involvement in social behavior (Aragona et al., 2003; Curtis et al., 2006; Brom et al., 2014). The DA system has five receptor types that can be divided into two subfamilies of receptors: D1-like (D1 and D5), and D2-like (D2, D3, and D4). These subtypes differentially regulate bond formation and maintenance in prairie vole pairs (Curtis et al., 2006; Aragona and Wang, 2009): activation of the D2 system facilitates selective social preferences and thereby formation (Wang et al., 1999; Gingrich et al., 2000; Aragona et al., 2003, 2006; Edwards and Self, 2006) and activation of the D1 system regulates selective social aggression associated with bond maintenance (Aragona et al., 2006). In prairie voles, bond formation is accompanied by an upregulation of D1 receptors in the nucleus accumbens, a brain region central to the reward system (Aragona et al., 2006). To date, there has not been a systematic assessment of the role of the D1 and D2 system in regulating the formation and maintenance of attachments in socially monogamous nonhuman primates.
There is, however, some evidence for the importance of the DA system in mediating social relationships in primates. D1 receptor binding in monogamous male titi monkeys is increased after pairing with a female in the lateral septum, a brain region associated with motivation, reward, and reinforcement (Hostetler et al., 2016), suggesting that pairing with a social partner may alter the expression of D1 receptors. Genetic variation in a DA receptor in humans is associated with variability in measures of fidelity and sexual promiscuity (Garcia et al., 2010), and brain regions associated with DA show increased activation in human males treated with oxytocin in response to viewing images of a romantic partner (Scheele et al., 2013), indicating that other neural systems may be working with, or through, the DA system to induce social effects. General cooperative behaviors even outside of a pairing context in humans appears to be mediated through activation of brain regions rich in DA and involved in reward processing (Rilling et al., 2002). Furthermore, blocking either D1 or D2 receptor types in macaques, a polygynous primate, reduced attention toward a social stimulus (Yamaguchi et al., 2017), indicating that both the D1 and D2 systems function in assessing social stimuli in nonhuman primates. While there is evidence indicating that the DA system influences social behavior in primates, it is unclear to what extent the DA system has a conserved role in regulating relationships in socially monogamous primates in relatively new vs. well-established pairs.
The current study assessed the ways in which reunion behavior after separation in marmosets was influenced by manipulation of the D1 and D2 signaling systems, and whether the effects of DA manipulation differed as a function of the length of the social relationship between pair mates. Marmoset pairs cohabiting for 8 weeks (short-term) or 3 years (long-term) were physically separated from pair mates and housed in a novel environment for 60–75 min, and affiliative behavior upon reunion was quantified. D1 and D2 receptor activation was pharmacologically manipulated with selective receptor agonists and antagonists. If the role of the DA system in relationship dynamics in marmosets is similar to voles, then we expected that manipulation of the D2 system would alter reunion behavior in short term, but not long term pairs, while manipulation of the D1 system would alter reunion behavior in long term, but not short term pairs.
A total of 20 marmosets (Callithrix jacchus) were used in this study. Subjects included animals in short term (n = 7 pairs, average pair length = 8.76 weeks, SD = 3.07 weeks), and long term (n = 5 pairs, average pairing length = 3.07 years, SD = 1.25 years) pairs. None of the pairs had parental experience with their current mate, and one female in the study had previous parental experience. Four animals were repaired and studied both in a short term and a long term pair context, but the remaining subjects were studied in only one pairing context. Both members of a pair served as subjects on different days of testing, with a minimum of 3 days between tests as a treated focal animal. Marmosets were housed at the Callitrichid Research Center at the University of Nebraska at Omaha. Animals were housed in enclosures with minimum dimensions of 101 × 76 × 160 cm.
Marmosets received a daily diet of a prepared commercial marmoset food (Zupreem®) supplemented by fresh fruits, vegetables, yogurt, apple sauce, eggs, and mealworms. The production of offspring was prevented by either surgical vasectomy of the male or monthly treatment of the female with the luteolytic agent Estrumate® (Merck). Hormonal states of the subjects are not anticipated to be highly impacted by these procedures and treatments. Surgical vasectomy is not known to reduce testosterone levels, and estrumate produces a normative nonconceptive ovarian cycle, with post-ovulatory progesterone levels equivalent to those of untreated females (Hodges et al., 1988; Mustoe et al., 2012). Additional information regarding animal care can be found in Schaffner et al. (1995). This study was carried out in accordance with the PHS Policy on the Humane Care and Use of Laboratory Animals. The protocol was approved by the University of Nebraska Medical Center/University of Nebraska at Omaha Institutional Animal Care and Use Committee (protocol # 15-033-05-FC).
Subjects were treated with one of five treatments: D1 agonist (SKF 38393, 0.05 mg/kg), D1 antagonist (SCH 23390, 0.01 mg/kg), D2 agonist (Quinpirole, 0.05 mg/kg), D2 antagonist (Raclopride 0.03 mg/kg), or saline vehicle. Treatment order was counterbalanced among subjects. Thus, each marmoset received five treatments and served as an untreated pair mate five times for a total of 10 exposures to the testing paradigm. Treatment doses were selected based on a systematic dose-response study conducted in our lab that identified doses at which motoric side effects were not observed (Carp, unpublished data). Injections were given intramuscularly in a volume of 0.5 mL/kg. These compounds have been documented to cross the blood brain barrier. Both raclopride (Farde et al., 1986) and SCH 23390 (Hostetler et al., 2016) are regularly utilized in PET imaging studies. Quinpirole is able to cross the blood brain barrier (Kostrzewa et al., 1993). It is unclear how well SKF 38393 penetrates the blood brain barrier (Kamien and Woolverton, 1985), however, intramuscular administration does alter neuronal activity (Boraud et al., 2001). Therefore, peripheral administration of all compounds are anticipated to produce effects through central activation. All treatments were purchased from Sigma Aldrich and prepared in sterile saline and kept frozen at −20°C until day of treatment. As per recommendations from the manufacturer, D1 and D2 antagonist treatments were reconstituted from stock every 30 days, while the D1 and D2 agonist treatment solutions were reconstituted every 90 days.
Subjects were removed from their home enclosure and administered a treatment, housed alone in a small transport cage in an isolated room for 30 min during the drug uptake period, and then placed in a novel T-shaped enclosure in a separate room for 30–45 min. During the time in the T-enclosure, the marmoset had simultaneous visual, auditory, olfactory, and limited tactile access to their untreated mate and to an untreated unfamiliar opposite-sex marmoset in stimulus cages at each end of the T-portion of the cage [for more details on the preference testing apparatus, see (Cavanaugh et al., 2014) and (Smith et al., 2010)]. The T maze is a novel environment for subjects, and simultaneous separation from a mate, and exposure to a novel environment results in a reliable stress response in marmosets (Smith et al., 1998). After this procedure, the treated marmoset and mate were reunited in their home enclosure. Interactions between males and females were recorded for 10 min (long-term pairs) or 15 min (short-term pairs), and included rates of food sharing, approaches to partner, instances of initiating and receiving grooming, and durations of grooming and time spent in social proximity. Definitions for the behavioral patterns are found in Table 1. Duration of separation and reunion were different because data were collected for two projects, one with short term and one with long term pairs. To correct for the different observation lengths, all behaviors were converted to duration or count per hour of observation for data analysis purposes. This correction assumes that the rates of recorded behavior did not vary across the observation period.
To assess the effects of treatment, sex, and pair type (short term or long term) we used a Linear Mixed Model analysis that nested marmoset ID within Pair ID. This nesting allowed us to account for the non-independence of marmosets being tested in both the short term and long term pairing context. Because age at testing varied between social conditions, this measure was included as a covariate. For behavior that could be exhibited by either the treated marmoset or the untreated mate, factors were added to include behavior initiated and received. As such, our template model is as follows: Behavior = DA Treatment × Sex × Pair Type × Initiate/Receive + Age + error(Pair ID) + error(Subject ID) + error(residual), with those factors in bold as the tests of our hypotheses, and those in italics added when grooming behavior was analyzed. We calculated a Hinde index for approach and leave behavior of subjects using the following equation: [Number of Subject Approaches/(Number of Subject Approaches + Number of Pair Mate Approaches)]–[Number of Subject Leaves/(Number of Subject Leaves + Number of Pair Mate Leaves)]. This index allows for a simultaneous measure of responsibility of both initiation (approach) of proximity and breaking proximity (leave) by the subject. Post hoc probing was conducted only if significant main effects or interactions were obtained. Statistical tests were conducted with Fisher's tests and a Satterthwaite approximation for degrees of freedom. Cohen's d effect sizes for post hoc Fisher's t-tests were calculated using model estimated marginal means and standard errors, and standard deviations were calculated from standard errors using Satterthwaite approximated degrees of freedom +1 to estimate n. ANOVA tables for all reported analyses can be found in Supplementary Tables S1–S4.
After a social stressor involving partner separation and exposure to an opposite-sex stranger, marmosets in short and long term pairs behaved differentially upon reunion. Marmosets in both short and long term pairs spent similar amounts of time in proximity during reunion, Figure 1; main effect: F(1, 14.11) = 2.69, p = 0.123, but differed in the specific behavior patterns associated with close spatial proximity.
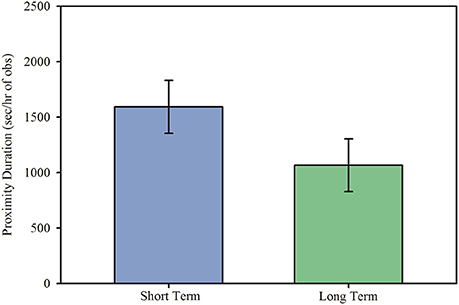
Figure 1. Duration in proximity (model estimates ± SEM) to the opposite sex mate during reunion among short and long term marmoset pairs. There was no significant difference in time spent in proximity during reunion between short and long term pairs of marmosets.
Marmosets in long term pairs engaged in higher levels of food sharing upon reunion than did marmosets in short term pairs, Figure 2A; main effect: F(1, 14.90) = 6.17, p = 0.025. There was also a sex difference observed in long term, but not short term, pairs of marmosets in frequency of food sharing, interaction effect: F(1, 11.98) = 5.84, p = 0.032. In long term pairs of marmosets, females engaged in higher levels of food sharing during reunion than did male marmosets, Figure 2B; post hoc: t(12.1) = 2.59, p = 0.024, 95% CI [0.009, 0.103], d = 0.695. However, in short term pairs of marmosets females and males displayed no difference in their rate of food sharing, Figure 2B; post hoc: t(12.2) = −0.66, p = 0.519, 95% CI [−0.052, 0.028], d = −0.181.
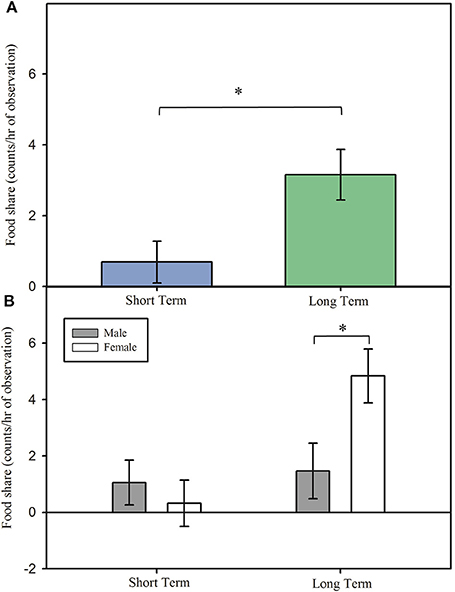
Figure 2. Food sharing (model estimates ± SEM) among short and long term marmoset pairs. (A) Long term marmoset pairs engaged in more food sharing during reunion than did short term marmoset pairs. (B) Females in long term pairs engaged in more food sharing during reunion than did males in long term pairs. There was no significant difference in food sharing between males and females in short term pairs. *Indicates significant difference at p < 0.05.
Although overall duration of grooming did not differ in reunion between short term and long term pairs, Figure 3A; main effect: F(1, 14.798) = 0.33, p = 0.576, the duration of grooming initiated by subjects or mates differed by pair length, interaction effect: F(1, 215.99) = 4.87, p = 0.028. Regardless of DA treatment, treated marmosets in short term pairs spent longer grooming their mate than their mate spent grooming them upon reunion, Figure 3B; post hoc: t(216) = 2.72, p = 0.007, 95% CI [0.154, 0.967], d = 0.450. However, treated subjects and their mates in long term pairs displayed no difference in the duration of grooming during reunion after separation, Figure 3B; post hoc: t(216) = −0.59, p = 0.556, 95% CI [−0.625, 0.337], d = −0.095.
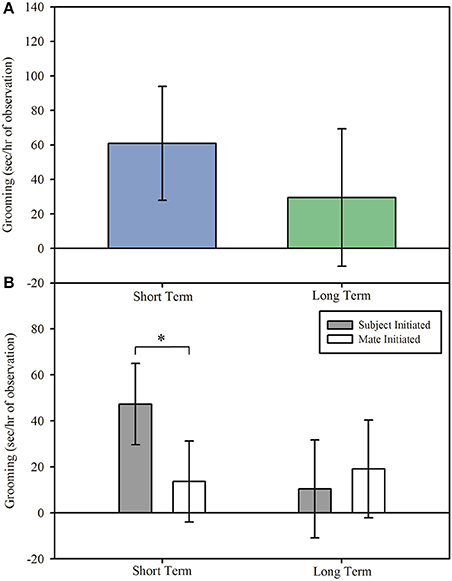
Figure 3. Grooming (model estimates ± SEM) among short and long term marmoset pairs. (A) There was no difference in time spent grooming during reunion between short and long term pairs of marmoset monkeys. (B) Treated marmosets in short term pairs initiated grooming more often than they received grooming, regardless of treatment, whereas treated marmosets in long term pairs initiated grooming equally as often as they received grooming. *Indicates significant difference at p < 0.05.
During reunion, marmoset pairs spent similar amounts of time in proximity regardless of DA treatment, main effect: F(4, 96.00) = 1.91, p = 0.114. However, time in proximity differed between short and long term pairs based on DA treatment and whether the male or female received the treatment, interaction effect: F(4, 96.00) = 2.50, p = 0.047. During reunion, male and female marmosets in short term pairs spent similar amounts of time in proximity when they received a saline treatment, Figure 4A; post hoc: t(70.70) = −0.29, p = 0.772, 95% CI [−11.471, 8.556], d = −0.047. However, marmosets in short term pairs spent less time in proximity during reunion when females were treated with either a D2 receptor agonist, Figure 4A; post hoc: t(70.70) = −2.04, p = 0.045, 95% CI [−20.237, −0.213], d = −0.333, or a D2 receptor antagonist, Figure 4A; post hoc: t(70.70) = −2.18, p = 0.032, 95% CI [−20.973, −0.946], d = −0.356, compared to when males received the same treatment. Additionally, pairs spent less time in proximity when females were treated with a D2 receptor agonist compared to when females were treated with saline, post hoc: t(96.0) = −2.29, p = 0.024, 95% CI [−19.530, −1.399], d = −0.336. No other treatments were significantly different from saline. Short term pairs spent similar amounts of time in proximity when either males or females were treated with a D1 agonist, Figure 4A; post hoc: t(70.8) = 0.48, p = 0.634, 95% CI [−7.607, 12.407], d = 0.078, or D1 antagonist, Figure 4A; post hoc: t(70.8) = −0.37, p = 0.716, 95% CI [−11.842, 8.175], d = −0.060.
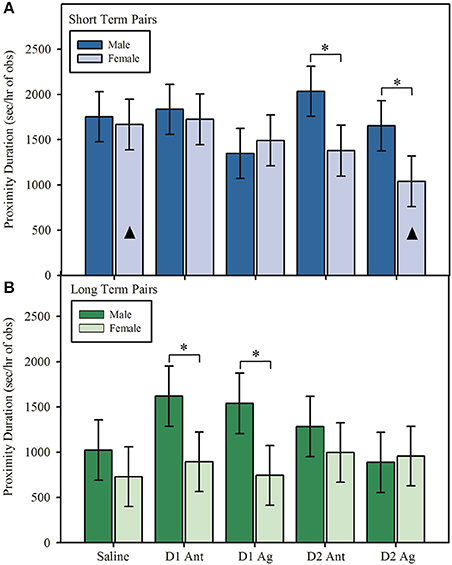
Figure 4. Duration in proximity (model estimates ± SEM) to the opposite sex partner during reunion. (A) Short term marmoset pairs spent less time in proximity during reunion when the female was treated with a dopamine receptor subtype 2 agonist or antagonist compared to when the male was treated with the same compound. Duration in proximity during reunion did not differ when males or females in short term pairs were treated with a dopamine receptor subtype 1 agonist or antagonist or saline. Bars marked with ▴ denote significant within sex differences from saline. Pairs spent less time in proximity during reunion when females were treated with a D2 agonist compared to when females received saline. There were no significant effects within males compared to saline. (B) Long term marmoset pairs spent less time in proximity during reunion when the female was treated with a dopamine receptor subtype 1 agonist or antagonist compared to when the male was treated with the same compound. Duration in proximity during reunion did not differ when males or females in long term pairs were treated with a dopamine receptor subtype 2 agonist or antagonist or saline (significant sex × pair type × treatment interaction). There were no significant within sex treatment effects for either males or females. *Indicates significant difference at p < 0.05. D1 Ant = D1 antagonist, D1 Ag = D1 agonist, D2 Ant = D2 antagonist, D2 Ag = D2 agonist.
A different pattern of treatment effects was observed in long term pairs. There was no sex difference in time spent in proximity in long term pairs when males and females were treated with saline, Figure 4B; post hoc: t(71.1) = −0.83, p = 0.412, 95% CI [−16.704, 6.925], d = −0.134. However, male and female marmosets in long term pairs spent less time in proximity when females received treatment with either a D1 agonist, Figure 4B; post hoc: t(71.00) = −2.23, p = 0.029, 95% CI [−25.061, −1.415], d = −0.365, or D1 antagonist, Figure 4B; t(71.00) = −2.03, p = 0.046, 95% CI [−23.880, −0.238], d = −0.329, compared to when males received the same treatment. Long term marmoset pairs spent similar amounts of time in proximity upon reunion when males and females were treated with a D2 agonist, Figure 4B; post hoc: t(71.00) = 0.19, p = 0.846, 95% CI [−10.663, 12.971], d = 0.031, or D2 antagonist, Figure 4B; post hoc: t(71.00) = −0.81, p = 0.421, 95% CI [−16.615, 7.028], d = −0.131.
The Hinde index revealed no effect of DA treatment on the rate at which treated subjects were initiating and breaking proximity with their mates, main effect: F(4, 96.00) = 0.88, p = 0.477, suggesting that the observed differences in proximity behavior were not attributable to changes in approach or leave behavior by the subject.
Reunion in marmoset pairs constitutes an important time for assessing affiliative behavior. We noted several differences in the ways in which pairs housed together for differing lengths of time interact upon reunion. Marmosets in long term pairs engaged in higher levels of food sharing than did marmosets in short term pairs. Females in long term pairs demonstrated higher rates of food sharing than their mates, while males and females shared food at equal rates in short term pairs. It has been hypothesized that in social settings in which females have multiple mating partner options, males should share food with females at high rates in order to enhance the chance for mating opportunities (Jaeggi and Van Schaik, 2011). However, in our study females in long term pairs were observed to share food more often than males. Though the social context in the current study differs from that in Jaeggi and Van Schaik (2011), females were reunited with their mate after exposure to a strange male. Presumably, males are cognizant of the odors/sounds of the other marmoset in the testing room and therefore may be more likely to behave during reunion as if their female mate had additional mating opportunities. In a comparative analysis of food sharing in primate species whose groups are composed of a single adult male, high rates of male-to-female food sharing predominantly occurs in socially monogamous species (Jaeggi and Van Schaik, 2011), however, that study did not measure female-to-male instances of food sharing. Food sharing is a behavior that is sensitive to the context of the pair (i.e., pregnancy). Monogamous male and female owl monkeys share food at equal rates, however, male-to-female food sharing increases when females are lactating compared to when they are cycling or pregnant, while female-to-male food sharing occurs at equal rates regardless of reproductive status (Wolovich et al., 2010). Though male-to-female food sharing may facilitate reduced energy expenditure by females and shortened interbirth interval (Wolovich et al., 2010), underlying proximate and ultimate causes of female-to-male food sharing are less clear.
Marmosets in short term pairs displayed a different pattern of grooming compared to that observed in long term pairs. Grooming behavior was altered such that rather than initiating and receiving comparable levels of grooming, as in long term pairs, treated marmosets in short term pairs spent more time grooming their partners upon reunion than their partner spent grooming them. This difference in behavior may indicate that while in long term pairs the context of the separation (e.g., experience as the treated subject vs experience as the untreated mate) does not change the expression of grooming, members of short term pairs are sensitive to the experimental context. Thus, in short term pairs, treated subjects engaged in longer duration of grooming than did untreated mates during reunion, thereby displaying a behavioral difference dependent on experimental experience. In prairie voles, the opposite effect is found: separated voles receive, rather than initiate, higher rates of grooming from their partners (Burkett et al., 2016). These contrasts point to potentially important species differences in the roles of initiated vs. received sociality upon reunion in regulating and reestablishing relationships after separation.
There was no difference in the total amount of time spent in proximity between short term and long term pairs. Though there are documented differences in normative levels of affiliation as measured by sexual behavior, time in proximity, and overall time spent grooming in marmoset pairs dependent on length of pairing (Evans and Poole, 1984; Schaffner et al., 1995; Ågmo et al., 2012), we did not find these differences expressed during reunion. Thus, while marmosets in short and long term pairs may use slightly different strategies to reestablish their relationship upon reunion (e.g., initiation of grooming and rates of food sharing), they spent similar amounts of time in proximity. The pattern of change in behavior dependent on the actions of both members of the pair is largely consistent across findings in the current study. Both food sharing and grooming (behaviors that differed between short and long term pairs in reunion) can only occur when both members of the pair engage in the appropriate dyadic interaction. This dependence on both partners highlights the complexities of studying pair-level interactions and the behavioral richness of dyads.
The DA system appears to be involved in the way that pairs behave during reunion, with DA treatment affecting pair-level interactions. Marmoset pairs, of both short and long term, spent similar amounts of time in proximity upon reunion under saline conditions regardless of whether the male or female was treated. However, alteration of the D2 system, either through receptor agonism or antagonism, altered this pattern in short term pairs, with pairs spending less time in proximity when females received treatment compared to when males received the same treatment. D1 treatments produced different effects in long term pairs, such that pairs spent less time in proximity when the female was treated with either a D1 receptor agonist or antagonist compared to when males received the same treatment. Furthermore, short term pairs spent less time in proximity when females were treated with a D2 agonist compared to when females were treated with saline, indicating that the sex difference observed between males and females was likely due to a decrease in proximity when females were treated. Though other treatments did not produce significant differences from saline, this suggests that at least in short term pairs, treatment of females may yield differences in the way in which pairs regulate social proximity. Male marmosets increase proximity regulation as their mate progresses through pregnancy (Evans and Poole, 1984), suggesting that pairs may be primed to be sensitive to alterations in female physiology, and our data suggest that DA signaling in females may be included in changes in female physiology that alter pair social dynamics. DA, particularly D2 agonists, also reduce prolactin release in marmoset monkeys (Almond et al., 2006), indicating the potential for DA treatments to have off-target effects on other hormonal systems. Given the known role of prolactin (PRL) in facilitating parental behavior (Ziegler et al., 2009) and parent-infant bonding, it is likely that DA-PRL interactions may have meaningful implications for behavior between mates. This may provide another mechanism through which marmoset females are more sensitive to manipulation of the DA system than males. While overall proximity duration was affected by both DA treatment and sex, other measures, such as initiating and breaking proximity did not differ among DA treatment conditions. Future research should evaluate aspects of individual behavior that may underlie the observed differences in pair-level behavior. In the current study individual initiation and breaking of proximity (the Hinde index) was not able to explain observed differences of time spent in proximity. However, other measures of social interest, such as social gaze, a measure known to be important in directing human social behavior (Frischen et al., 2007), may help to illuminate the behavioral mechanisms through which pairs are altering interactions in response to DA treatment.
It is also worth noting that the same pattern of change in social proximity to the partner during reunion is observed when marmosets are treated with either the agonist or antagonist, suggesting that pairs may be responding to an alteration of DA signaling rather than enhancement or inhibition of the system. The similarity in agonist and antagonist effects stands in contrast to research in other species indicating differential effects of agonist and antagonist treatment. One potential explanation for the similarity in effect is differential efficacy of some of the compounds. The D1 receptor agonist (SKF 38393) has been documented to have a lower efficacy in primate than in rodent brain tissue, and in both rodents and primates the efficacy of the agonist is lower than that of DA itself (Arnt et al., 1988; Pifl et al., 1991). Additionally, there is evidence of a U shaped dose-response curve for agonists of the D1 system (Cai and Arnsten, 1997), indicating the potential that observed effects are dose-dependent. Therefore, at least within the D1 system, it is possible that the effects of the agonist and antagonist are both producing effects less potent than if DA were acting alone.
The consistency of findings across pair types (D2 manipulation altering behavior in short term pairs, and D1 manipulation altering behavior in long term pairs) indicates that there may be dynamic shifts in behavior that affect the dyadic nature of pair interactions dependent on pairing length and DA subsystem. Research in prairie voles has indicated a role for the D2 system in measures of relationship formation (Gingrich et al., 2000; Aragona et al., 2003, 2006), and the D1 system in measures of relationship maintenance (Aragona et al., 2006). Thus, it is interesting to note that social proximity in short term pairs of marmosets is changed by manipulation of the D2 system, and social proximity in long term pairs of marmosets is changed by manipulation of the D1 system, as would be predicted if the DA system has a conserved role in pair behavior during reunion.
DA itself is an evolutionarily conserved neurotransmitter with widespread effects on regulating behavior. Though commonly recognized as having a role in motoric function, DA also has known roles in decision making and social behavior (O'Connell and Hofmann, 2011). DA receptor distribution has been characterized in cichlid fish and D1 and D2 receptors are found in regions homologous to those associated with social behavior in other vertebrate species (O'Connell et al., 2011). The effect of DA on mate behavior is not limited to mammals. DA has been associated with male courtship and pairing behavior in monogamous zebra finch birds (Huang and Hessler, 2008; Goodson et al., 2009), and higher levels of DA and its metabolites are found in paired than unpaired zebra finches (Banerjee et al., 2013). Furthermore, immediately early gene markers indicated increased neuronal activity of dopamine rich brain regions after pairing compared to unpaired finches (Banerjee et al., 2013). Together, these studies highlight the conserved nature of DA in facilitating both social behavior generally, and regulating interactions between mates.
There is further evidence for the role of DA in primate pair interactions from studies on humans. Subjects looking at pictures of a partner with whom they considered themselves to be in love showed increased activation of brain regions rich in DA, compared to looking at pictures of an acquaintance (Fisher et al., 2005). This indicates that the DA system may be selectively important in romantic attachments rather than overall sociality. Additionally, genetic variability in the DA system has been linked to differences in human sexual behavior. Variation in a D2-like receptor has been associated with human male sexual desire and arousal (Ben Zion et al., 2006), as well as self-reported levels of fidelity and promiscuity (Garcia et al., 2010). Though DA has a role in modulating sociosexual relationships, it is not the only regulatory neurotransmitter that impacts sociality in mammals. Other neural systems, especially oxytocin and vasopressin, interact with dopaminergic signaling to facilitate social behavior (Johnson and Young, 2015). Thus, research on the specific roles of the D1 and D2 systems in primate pair behavior is necessary in order to inform not only the independent role of the dopaminergic system, but the potential co-modulatory effects with other neural systems. Specifically, the current study indicates that DA subsystems do maintain a role in regulating reunion behavior in a primate species displaying social monogamy. These findings fit into the larger emerging evolutionary notion that there are neurobiological systems, including those involving dopamine signaling, conserved across vertebrate species that may play crucial roles in regulating sociality.
SC: designed the study, carried out experiments, organized the dataset, and wrote the first draft of the manuscript; JF and SC: obtained funding for the project; JT and SC: performed statistical analyses and interpreted analyses; JT, SW, and JF: contributed to and revised the manuscript. All authors read, revised and approved the final version of the submitted manuscript.
The research in this manuscript was supported by the National Institutes of Health (HD089147 awarded to JF) and the University of Nebraska at Omaha's Graduate Research and Creative Activity Award (awarded to SC, title: The Role of Social Context and Dopamine in Partner Fidelity in Marmoset Monkeys).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank members of the French lab for their comments on study design and feedback during manuscript preparation. We would also like to thank Heather Jensen, Haley Hassenstab, Jacqueline Muellner, and Drs. Liz Gunkelman and Noel Johnson for providing excellent care of the marmoset colony. We greatly appreciate research assistance by the following volunteers: Kathy Barnett, Haley Classe, Brianna Martinie, Amber Park, Mariah Wulf, and Colton Wulfekuhl.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00046/full#supplementary-material
Ågmo, A., Smith, A. S., Birnie, A. K., and French, J. A. (2012). Behavioral characteristics of pair bonding in the black tufted-ear marmoset (Callithrix penicillata). Behaviour 149, 407–440. doi: 10.1163/156853912X638454
Almond, R. E. A., Brown, G. R., and Keverne, E. B. (2006). Suppression of prolactin does not reduce infant care by parentally experienced male common marmosets (Callithrix jacchus). Horm. Behav. 49, 673–680. doi: 10.1016/j.yhbeh.2005.12.009
Aragona, B. J., Liu, Y., Curtis, J. T., Stephan, F. K., and Wang, Z. (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J. Neurosci. 23, 3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003
Aragona, B. J., Liu, Y., Yu, Y. J., Curtis, J. T., Detwiler, J. M., Insel, T. R., et al. (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139. doi: 10.1038/nn1613
Aragona, B. J., and Wang, Z. (2009). Dopamine regulation of social choice in a monogamous rodent species. Front. Behav. Neurosci. 3:15. doi: 10.3389/neuro.08.015.2009
Arnt, J., Bøgeso, K. P., Hyttel, J., and Meier, E. (1988). Relative dopamine D1 and D2 receptor affinity and efficacy determine whether dopamine agonists induce hyperactivity or oral stereotypy in rats. Pharmacol. Toxicol. 62, 121–130. doi: 10.1111/j.1600-0773.1988.tb01859.x
Banerjee, S. B., Dias, B. G., Crews, D., and Adkins-Regan, E. (2013). Newly paired zebra finches have higher dopamine levels and immediate early gene Fos expression in dopaminergic neurons. Eur. J. Neurosci. 38, 3731–3739. doi: 10.1111/ejn.12378
Ben Zion, I. Z., Tessler, R., Cohen, L., Lerer, E., Raz, Y., Bachner-Melman, R., et al. (2006). Polymorphisms in the dopamine D4 receptor gene (DRD4) contribute to individual differences in human sexual behavior: desire, arousal and sexual function. Mol. Psychiatry 11, 782–786. doi: 10.1038/sj.mp.4001832
Boraud, T., Bezard, E., Bioulac, B., and Gross, C. E. (2001). Dopamine agonist-induced dyskinesias are correlated to both firing pattern and frequency alterations of pallidal neurones in the MPTP-treated monkey. Brain 124, 546–557. doi: 10.1093/brain/124.3.546
Brom, M., Both, S., Laan, E., Everaerd, W., and Spinhoven, P. (2014). The role of conditioning, learning and dopamine in sexual behavior: a narrative review of animal and human studies. Neurosci. Biobehav. Rev. 38, 38–59. doi: 10.1016/j.neubiorev.2013.10.014
Burkett, J. P., Andari, E., Johnson, Z. V., Curry, D. C., de Waal, F. B. M., and Young, L. J. (2016). Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378. doi: 10.1126/science.aac4785
Cai, J. X., and Arnsten, A. F. T. (1997). Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J. Pharmacol. Exp. Ther. 283, 183–189.
Carter, C. S., DeVries, A. C., and Getz, L. L. (1995). Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev. 19, 303–314. doi: 10.1016/0149-7634(94)00070-H
Cavanaugh, J., Carp, S. B., Rock, C. M., and French, J. A. (2016). Oxytocin modulates behavioral and physiological responses to a stressor in marmoset monkeys. Psychoneuroendocrinology 66, 22–30. doi: 10.1016/j.psyneuen.2015.12.027
Cavanaugh, J., Huffman, M. C., Harnisch, A. M., and French, J. A. (2015). Marmosets treated with oxytocin are more socially attractive to their long-term mate. Front. Behav. Neurosci. 9:251. doi: 10.3389/fnbeh.2015.00251
Cavanaugh, J., Mustoe, A. C., Taylor, J. H., and French, J. A. (2014). Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology 49, 1–10. doi: 10.1016/j.psyneuen.2014.06.020
Cavanaugh, J., Mustoe, A., and French, J. A. (2018). Oxytocin regulates reunion affiliation with a pairmate following social separation in marmosets. Am. J. Primatol. doi: 10.1002/ajp.22750. [Epub ahead of print].
Chapais, B. (2013). Monogamy, strongly bonded groups, and the evolution of human social structure. Evol. Anthropol. Issues News Rev. 22, 52–65. doi: 10.1002/evan.21345
Curtis, J. T., Liu, Y., Aragona, B. J., and Wang, Z. (2006). Dopamine and monogamy. Brain Res. 1126, 76–90. doi: 10.1016/j.brainres.2006.07.126
DeVries, A. C., Glasper, E. R., and Detillion, C. E. (2003). Social modulation of stress responses. Physiol. Behav. 79, 399–407. doi: 10.1016/S0031-9384(03)00152-5
Díaz-Muñoz, S. L., and Bales, K. L. (2015). “Monogamy” in primates: variability, trends, and synthesis: introduction to special issue on primate monogamy. Am. J. Primatol. 78, 283–287. doi: 10.1002/ajp.22463
Digby, L. J. (1999). Sexual behavior and extragroup copulations in a wild population of common marmosets (Callithrix jacchus). Folia Primatol. 70, 136–145. doi: 10.1159/000021686
Duarte, R. B., Maior, R. S., and Barros, M. (2017). Behavioral and cortisol responses of adult marmoset monkeys (Callithrix penicillata) to different home-cage social disruption intervals. Appl. Anim. Behav. Sci. 201, 117–124. doi: 10.1016/j.applanim.2017.12.005
Edwards, S., and Self, D. W. (2006). Monogamy: dopamine ties the knot. Nat. Neurosci. 9, 7–8. doi: 10.1038/nn0106-7
Evans, S. (1983). The pair-bond of the common marmoset, Callithrix jacchus jacchus: an experimental investigation. Anim. Behav. 31, 651–658. doi: 10.1016/S0003-3472(83)80220-6
Evans, S., and Poole, T.B. (1984). Long-term changes and maintenance of the pair-bond in common marmosets, Callithrix jacchus jacchus. Folia Primatol. 42, 33–41. doi: 10.1159/000156142
Farde, L., Hall, H., Ehrin, E., and Sedvall, G. (1986). Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 231, 258–261. doi: 10.1126/science.2867601
Fisher, H., Aron, A., and Brown, L. L. (2005). Romantic love: an fMRI study of a neural mechanism for mate choice. J. Comp. Neurol. 493, 58–62. doi: 10.1002/cne.20772
French, J. A., Cavanaugh, J., Mustoe, A. C., Carp, S. B., and Womack, S. L. (2017). Social monogamy in nonhuman primates: phylogeny, phenotype, and physiology. J. Sex Res. 55, 1–25. doi: 10.1080/00224499.2017.1339774
French, J. A., Smith, A. S., Gleason, A. M., Birnie, A. K., Mustoe, A., and Korgan, A. (2012). Stress reactivity in young marmosets (Callithrix geoffroyi): ontogeny, stability, and lack of concordance among co-twins. Horm. Behav. 61, 196–203. doi: 10.1016/j.yhbeh.2011.12.006
Frischen, A., Bayliss, A. P., and Tipper, S. P. (2007). Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol. Bull. 133, 694–724. doi: 10.1037/0033-2909.133.4.694
Garcia, J. R., MacKillop, J., Aller, E. L., Merriwether, A. M., Wilson, D. S., and Lum, J. K. (2010). Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. PLoS ONE 5:e14162. doi: 10.1371/journal.pone.0014162
Gingrich, B., Liu, Y., Cascio, C., Wang, Z., and Insel, T. R. (2000). Dopamine D2 receptors in thenucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav. Neurosci. 114, 173–183. doi: 10.1037/0735-7044.114.1.173
Goodson, J. L., Kabelik, D., Kelly, A. M., Rinaldi, J., and Klatt, J. D. (2009). Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc. Natl. Acad. Sci. U.S.A. 106, 8737–8742. doi: 10.1073/pnas.0811821106
Hennessy, M. B., Kaiser, S., and Sachser, N. (2009). Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470–482. doi: 10.1016/j.yfrne.2009.06.001
Hinde, K., Muth, C., Maninger, N., Ragen, B. J., Larke, R. H., Jarcho, M. R., et al. (2016). Challenges to the pair bond: neural and hormonal effects of separation and reunion in a monogamous primate. Front. Behav. Neurosci. 10:221. doi: 10.3389/fnbeh.2016.00221
Hodges, J. K., Green, D. I., Cottingham, P. G., Sauer, M. J., Edwards, C., and Lightman, S. L. (1988). Induction of luteal regression in the marmoset monkey (Callithrix jacchus) by a gonadotrophin-releasing hormone antagonist and the effects on subsequent follicular development. J. Reprod. Fertil. 82, 743–752. doi: 10.1530/jrf.0.0820743
Hostetler, C. M., Hinde, K., Maninger, N., Mendoza, S. P., Mason, W. A., Rowland, D. J., et al. (2016). Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). Am. J. Primatol. 79, 1–9. doi: 10.1002/ajp.22612
Huang, Y.-C., and Hessler, N. A. (2008). Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE 3:e3281. doi: 10.1371/journal.pone.0003281
Jaeggi, A. V., and Van Schaik, C. P. (2011). The evolution of food sharing in primates. Behav. Ecol. Sociobiol. 65, 2125–2140. doi: 10.1007/s00265-011-1221-3
Johnson, Z. V., and Young, L. J. (2015). Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci. 3, 38–44. doi: 10.1016/j.cobeha.2015.01.009
Kamien, J. B., and Woolverton, W. L. (1985). The D 1 dopamine agonist SKF 38393 functions as a discriminative stimulus in rats. Psychopharmacology 87, 368–370. doi: 10.1007/BF00432723
Kostrzewa, R. M., Brus, R., Rykaczewska, M., and Plech, A. (1993). Low-dose quinpirole ontogenically sensitizes to quinpirole-induced yawning in rats. Pharmacol. Biochem. Behav. 44, 487–489. doi: 10.1016/0091-3057(93)90496-G
Lim, M. M., and Young, L. J. (2006). Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 50, 506–517. doi: 10.1016/j.yhbeh.2006.06.028
Lukas, D., and Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677
Maninger, N., Mendoza, S. P., Williams, D. R., Mason, W. A., Cherry, S. R., Rowland, D. J., et al. (2017). Imaging, behavior and endocrine analysis of “jealousy” in a monogamous primate. Front. Ecol. Evol. 5:119. doi: 10.3389/fevo.2017.00119
Mustoe, A. C., Cavanaugh, J., Harnisch, A. M., Thompson, B. E., and French, J. A. (2015). Do marmosets care to share? Oxytocin treatment reduces prosocial behavior toward strangers. Horm. Behav. 71, 83–90. doi: 10.1016/j.yhbeh.2015.04.015
Mustoe, A. C., Jensen, H. A., and French, J. A. (2012). Describing ovarian cycles, pregnancy characteristics, and the use of contraception in female white-faced marmosets, Callithrix geoffroyi: marmoset ovarian cycles and pregnancy. Am. J. Primatol. 74, 1044–1053. doi: 10.1002/ajp.22058
O'Connell, L. A., Fontenot, M. R., and Hofmann, H. A. (2011). Characterization of the dopaminergic system in the brain of an African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol. 519, 75–92. doi: 10.1002/cne.22506
O'Connell, L. A., and Hofmann, H. A. (2011). Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Front. Neuroendocrinol. 32, 320–335. doi: 10.1016/j.yfrne.2010.12.004
Pifl, C., Reither, H., and Hornykiewicz, O. (1991). Lower efficacy of the dopamine D1 agonist, SKF 38393, to stimulate adenylyl cyclase activity in primate than in rodent striatum. Eur. J. Pharmacol. 202, 273–276. doi: 10.1016/0014-2999(91)90304-9
Rilling, J. K., Gutman, D. A., Zeh, T. R., Pagnoni, G., Berns, G. S., and Kilts, C. D. (2002). A neural basis for social cooperation. Neuron 35, 395–405. doi: 10.1016/S0896-6273(02)00755-9
Ross, C., French, J., and Patera, K. (2004). Intensity of aggressive interactions modulates testosterone in male marmosets. Physiol. Behav. 83, 437–445. doi: 10.1016/j.physbeh.2004.08.036
Ross, C. N., and French, J. A. (2011). Female marmosets' behavioral and hormonal responses to unfamiliar intruders. Am. J. Primatol. 73, 1072–1081. doi: 10.1002/ajp.20975
Rukstalis, M., and French, J. A. (2005). Vocal buffering of the stress response: exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav. 47, 1–7. doi: 10.1016/j.yhbeh.2004.09.004
Schaffner, C. M., Shepherd, R. E., Santos, C. V., and French, J. A. (1995). Development of heterosexual relationships in wied's black tufted-ear marmosets (Callithrix kuhli). Am. J. Primatol. 36, 185–200. doi: 10.1002/ajp.1350360303
Scheele, D., Wille, A., Kendrick, K. M., Stoffel-Wagner, B., Becker, B., Güntürkün, O., et al. (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl. Acad. Sci. U.S.A. 110, 20308–20313. doi: 10.1073/pnas.1314190110
Shepherd, R. E., and French, J. A. (1999). Comparative analysis of sociality in lion tamarins (Leontopithecus rosalia) and marmosets (callithrix kuhli): responses to separation from long-term pairmates. J. Comp. Psychol. 113, 24–32. doi: 10.1037/0735-7036.113.1.24
Smith, A. S., Ågmo, A., Birnie, A. K., and French, J. A. (2010). Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 57, 255–262. doi: 10.1016/j.yhbeh.2009.12.004
Smith, T. E., McGreer-Whitworth, B., and French, J. A. (1998). Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing inblack tufted-ear marmosets (Callithrix kuhli). Horm. Behav. 34, 211–222. doi: 10.1006/hbeh.1998.1469
Snowdon, C. T. (1996). Infant care in cooperatively breeding species. Adv. Stud. Behav. 25, 643–689. doi: 10.1016/S0065-3454(08)60345-9
Sousa, M. B. C., Albuquerque Fda, S., Araujo, A., Yamamoto, M. E., and Arruda, Mde, F. (2005). Behavioral strategies and hormonal profiles of dominant and subordinate common marmoset (Callithrix jacchus) females in wild monogamous groups. Am. J. Primatol. 67, 37–50. doi: 10.1002/ajp.20168
Taylor, J. H., Mustoe, A. C., Hochfelder, B., and French, J. A. (2015). Reunion behavior after social separation is associated with enhanced HPA recovery in young marmoset monkeys. Psychoneuroendocrinology 57, 93–101. doi: 10.1016/j.psyneuen.2015.03.019
Tecot, S. R., Singletary, B., and Eadie, E. (2016). Why “monogamy” isn't good enough: pair-living, pair-bonding, and monogamy. Am. J. Primatol. 78, 340–354. doi: 10.1002/ajp.22412
Wang, Z., Yu, G., Cascio, C., Liu, Y., Gingrich, B., and Insel, T. R. (1999). Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav. Neurosci. 113:602. doi: 10.1037/0735-7044.113.3.602
Williams, J. R., Catania, K. C., and Carter, C. S. (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm. Behav. 26, 339–349. doi: 10.1016/0018-506X(92)90004-F
Wolovich, C. K., Evans, S., and Green, S. M. (2010). Mated pairs of owl monkeys (Aotus nancymaae) exhibit sex differences in response to unfamiliar male and female conspecifics. Am. J. Primatol. 72, 942–950. doi: 10.1002/ajp.20858
Yamaguchi, Y., Atsumi, T., Poirot, R., Lee, Y.-A., Kato, A., and Goto, Y. (2017). Dopamine-dependent visual attention preference to social stimuli in nonhuman primates. Psychopharmacology (Berl) 234, 1113–1120. doi: 10.1007/s00213-017-4544-6
Young, K. A., Gobrogge, K. L., Liu, Y., and Wang, Z. (2011). The neurobiology of pair bonding:Insights from a socially monogamous rodent. Front. Neuroendocrinol. 32, 53–69. doi: 10.1016/j.yfrne.2010.07.006
Young, K. A., Liu, Y., and Wang, Z. (2008). The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 148, 401–410. doi: 10.1016/j.cbpc.2008.02.004
Young, L. J., and Wang, Z. (2004). The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054. doi: 10.1038/nn1327
Ziegler, T. E., and Crockford, C. (2017). Neuroendocrine control in social relationships in non-human primates: field based evidence. Horm. Behav. 91, 107–121. doi: 10.1016/j.yhbeh.2017.03.004
Keywords: social monogamy, pairing length, social relationships, formation, mating system
Citation: Carp SB, Taylor JH, Womack SL and French JA (2018) Dopamine Modulation of Reunion Behavior in Short and Long Term Marmoset Pairs. Front. Ecol. Evol. 6:46. doi: 10.3389/fevo.2018.00046
Received: 15 January 2018; Accepted: 04 April 2018;
Published: 09 May 2018.
Edited by:
Nancy G. Solomon, Miami University, United StatesReviewed by:
Zachary V. Johnson, Georgia Institute of Technology, United StatesCopyright © 2018 Carp, Taylor, Womack and French. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah B. Carp, c2NhcnBAdW5vbWFoYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.