- 1School of Biosciences, University of Nottingham, Loughborough, United Kingdom
- 2Centre for Analytical Bioscience, School of Pharmacy, University of Nottingham, Nottingham, United Kingdom
- 3Benaki Phytopathological Institute, Attica, Greece
- 4School of Chemistry, University of Nottingham, Nottingham, United Kingdom
Dyadic contests for possession of resources occur across a wide range of animal taxa, with contest outcome often being heavily influenced by the energetic reserves of the competitors. The majority of studied parasitoid wasps lack de novo lipogenesis, with adult lipid reserves being limited to those acquired throughout larval development. Carbohydrate- and lipid-rich diets can increase adult parasitoid lifespan and fecundity by potentially acting as a maintenance energy store. However, the effects of such diets on fat reserve compositions and contest outcome have not been examined. This study assesses the effects of a carbohydrate rich diet (honey) on the longevity, metabolic state and contest performance of Goniozus legneri, a bethylid wasp. The longevity of honey-fed adults was typically more than twice that of starved wasps. Compared to similarly-aged starved wasps, honey feeding increased concentrations of common haemolymph sugars and amino acids in 3-day old and 7-day old wasps, and higher concentrations of stored lipid in 7-day old wasps. However, nutritional status did not affect the outcome of dyadic contests over host possession when both contestants were either 3 days or 7 days old. While contest outcome may be unaffected by diet, it remains possible that an enhancing effect of feeding on contest ability is matched by an effect of higher value being placed on winning by starved wasps.
Introduction
Dyadic contests for possession of indivisible resources are of longstanding and ongoing interest as they occur across a wide range of animal taxa and have provided key examples for the game-theoretic approach to understanding behavioural evolution (Maynard Smith and Price, 1973; Hardy and Briffa, 2013). Two major predictors of contest outcomes are the competitive ability of individuals (resource holding potential, RHP) and the value (V) that contestants place on possession of the resource (Maynard Smith and Parker, 1976; Kokko, 2013): each of these may be affected by the nutritional status of the contestants. For example, individuals that have been well fed may be able to persist for longer during contests (RHP) due to their enhanced energy reserves (Poole, 1989; Kemp and Alcock, 2003; Martinez-Lendech et al., 2007; Briffa et al., 2013b) while individuals with depleted reserves may compete more intensely for a contested resource, due to placing a higher value (V) on its exploitation (Riechert, 1998). Further, the availability of macronutrients (protein, fats and carbohydrates) may promote dominance-related behaviours (Davidson, 1997) while other metabolites may physiologically constrain contest behaviour. For instance, in hermit crabs and other crustaceans, metabolites related to energy reserves (glycogen and glucose) and aerobic capacity (lactic acid) as well as hormones (biogenic amines) influence contestant motivation and contest duration (Briffa and Sneddon, 2007; Briffa et al., 2013b).
In this paper we explore relationships between nutritional status and performance using the parasitoid wasp Goniozus legneri Gordh (Hymenoptera: Bethylidae). Female G. legneri parasitize host larvae that tunnel into crop tissues such as pistachio nuts, almonds and apples (Steffan et al., 2001). Attack by the wasp results in host paralysis and the female then remains in a restricted space with the host before oviposition approx. 24 h later. Additionally, G. legneri exhibits aggressive post-oviposition brood guarding behaviour, during which time a female may spend several days with each host both prior and post oviposition (Hardy et al., 2013). Because of the long time spent with a host, guarding females may encounter a series of conspecific intruders, with whom they compete for host possession (Bentley et al., 2009). A number of prior studies have shown that contest outcomes are affected by components of both RHP (contestant body size) and V (host size and nutritional quality, contestant age and reproductive status) (reviewed in Hardy et al., 2013). Effects of adult nutrition on Goniozus contest performance have, however, not been examined.
In common with other parasitoids (Ellers, 1996; Eijs et al., 1998; Casas et al., 2005; Jervis et al., 2008; Visser and Ellers, 2008), G. legneri adults lack de novo lipogenesis (Visser et al., 2010) and adults are thus limited to utilizing fat reserves accumulated throughout larval development. In many species of parasitoids, adults acquire nutrients from feeding on plant nectar, honey dew and host haemolymph (Jervis et al., 1993; Eijs et al., 1998; Giron et al., 2002; Visser and Ellers, 2008) and decline in lipid reserves can be slower when carbohydrate and lipid-rich diets are available (Ellers, 1996; Heimpel et al., 1997; Lee et al., 2004; Wäckers et al., 2006; Winkler et al., 2006; Desouhant et al., 2010; Ellers et al., 2011; Gómez et al., 2012; Harvey et al., 2012). Feeding on carbohydrates can also enhance adult parasitoid longevity (Heimpel et al., 1997; Pexton and Mayhew, 2002; Lee et al., 2004; Wäckers et al., 2006; Kapranas and Luck, 2008; Gómez et al., 2012) and preliminary laboratory observations (CJPS & AK pers. obs.) indicate that G. legneri fed with honey live longer than starved adults, suggesting that G. legneri can use dietary carbohydrates as a maintenance energy supply. Feeding on honey has the potential to counter the effect of the increased value, V, that older contestants place on host possession (Stockermans and Hardy, 2013, see also Humphries et al., 2006); if so, we might expect starved wasps to win contests against similarly aged but fed wasps. Conversely, honey-fed contestants may have enhanced contest abilities, RHP, due to higher energy reserves (Tsai et al., 2014); if so we might expect starved wasps to lose such contests.
To explore whether feeding on carbohydrates, such as honey, during the adult stage affects the subsequent energy reserves, longevity and contest performance of female G. legneri, we: (1) Employ a metabolomics approach (Snart et al., 2015; Kapranas et al., 2016) to assess how feeding on a carbohydrate-rich honey diet affects the concentrations of lipids, carbohydrates and other metabolites in individual parasitoids; (2) Compare the lifespans of starved wasps with those of wasps provided with carbohydrate-rich honey diet; (3) Stage contests for host possession between pairs of females, either both young or both old, in which one contestant was fed on honey and the other one was left unfed.
Materials and Methods
Parasitoids and Hosts
Goniozus legneri were reared on larvae of Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) by introducing an adult female wasp and a caterpillar in a glass vial (2.5 × 7.5 cm) plugged by gauze and cotton. Culturing and contest experiments were carried out in a climate room at 27°C with a relative humidity of 60–70% maintained by water bath evaporation. The parasitoid strain was obtained from a commercial insectary in the USA, both host and parasitoid strains were the same as those utilised in several previous contest studies (Goubault et al., 2006; Bentley et al., 2009; Stockermans and Hardy, 2013).
In Parts 1 and 3 we respectively examine the metabolomic state and contest behaviour of females aged 3 and 7 days. These ages were chosen because females typically disperse from their natal broods 3 days after eclosion from their cocoons (Hardy et al., 2000) and 7 days is the typical lifespan of unfed females in our laboratory conditions: see Part 2.
Part 1: Effects of Feeding on Metabolomic Profiles
We employed a combined NMR and liquid chromatography–mass spectrometry (LC-MS) metabolomics approach (Kapranas et al., 2016) to assess simultaneously differences in individual lipids across multiple lipid classes and complementary information on polar metabolite concentrations, including haemolymph sugars, amino acids, and organic acids.
Adult female wasps that had emerged from their cocoons within 12 h were isolated in 0.5 ml Eppendorf vials. Half of the vials, chosen randomly, had been streaked on the inside wall with a raw honey droplet as a source of carbohydrates. Honey droplets were weighed to approximately 1 ± 0.1 mg prior to provision. The remaining vials contained no dietary provision (including water). Wasps were maintained in isolation for either 3 or 7 days after eclosion. Observations of the tube at the end of this period indicated that no wasp had successfully exhausted its honey droplet, as such honey provision was considered to have been ad infinitum. At the end of this time individual wasps were weighed to an accuracy of 0.01 mg and then snap frozen in liquid nitrogen and stored at −80°C prior to solvent extraction. There were 20 replicates (individual females) for each of the starved and honey-fed treatments. In the 7-day treatments, four starved wasps died prior to snap freezing; these replicates were excluded from further analysis.
Polar and non-polar extracts of intact, individual wasps were obtained using a modified Bligh and Dyer (1959) biphasic extraction approach (Kapranas et al., 2016). An additional reference sample was generated alongside wasp extracts by replacing the wasp with 1 mg of raw honey to aid spectral comparisons. Polar phases were transferred to a sterile 1.5 ml solvent resistant Eppendorf tube (Biopur) and non-polar phases were transferred to a sterile 2 ml borosilicate glass vial. Sample order was randomised prior to extraction and again before sample analysis. Solvent phases were dried and reconstituted for experimental analysis (Kapranas et al., 2016). A pooled quality control sample was generated using 10 μl of each reconstituted sample. All solvents used were of high LC-MS grade purity (CHROMASOLV Sigma-Aldrich) and were kept chilled during sample extraction. Reconstituted polar phases were analysed using proton nuclear magnetic resonance (1H NMR) spectroscopy, whereas reconstituted non-polar phases were analysed by LC-MS.
NMR Spectroscopy
1D NMR spectra were generated from individual parasitoid polar phases using a Bruker Advance 800 MHz III spectrometer equipped with a 5 mm QCI cyroprobe. A spectral width of 13 ppm was utilised for spectral acquisition; the spectral signal was averaged over a total of 512 transients. A NOSEY pre-saturation experiment selectively suppressed any water resonances throughout spectral acquisition; frequency and power of the pre-saturation experiment was determined prior to experimental analysis using a set of representative wasp samples generated under the same extraction protocol. The internal D2O signal was utilised for sample locking, with a secondary DSS signal (0.5 mM) referenced to 0.0 ppm. Total recycling delay was 4.7 s. An exponential window function was applied to give line broadening of 0.3 Hz before zero filling and Fourier transformation. To further establish metabolite identity 2D NMR spectra were generated from a pooled extract of 20 wasps produced by the same method as previous extracts. These spectra were acquired using the method described by Kapranas et al. (2016); briefly a heteronuclear single-quantum correlation (HSQC) experiment was employed. Spectral signal was averaged over a range of 40–160 transients with between 400 and 512 points in t1 and 4,096 points in t2. Spectra covered a 1H width of 8484 Hz and a 13C width of 33339 Hz.
LC-MS Analysis
Lipidomics analysis of non-polar extracts used an Accela LC coupled to a Thermo Scientific Exactive MS. An Ace Excel 2 Super C18 (2 μm particle size, 2.1 × 50 mm) column (Hichrom, UK) was maintained at 40°C throughout the analysis. The pooled quality control (QC) sample was analysed throughout the experimental timeframe to assess the quality of the analysis. LC gradient program and MS scan parameters were as described by Kapranas et al. (2016), briefly: ion range, m/z 100–1,500; ESI voltage, 3,500 V; capillary temperature, 350°C; scan rate, 250 ms).
Further MS-MS analysis was undertaken to identify lipids which were found to change significantly between groups (see section on data analysis). For this purpose, extracts were pooled prior to LC-MS/MS analysis to improve sensitivity. Analysis was performed utilising an Accela LC coupled with an LTQ Velos Pro Dual-Pressure linear ion trap mass spectrometer (Thermo Fisher Scientific, USA) as described by Kapranas et al. (2016). Briefly, an ion range of m/z 100–1,500 was monitored (ESI voltage, 3,000; capillary temperature, 275°C; scan rate, 50 ms) and a collision energy of 40 V was employed during fragmentation.
The quality of the lipidomics analysis was confirmed by assessing the relative standard deviations (RSD) of the peak areas and retention times of a range of key lipids in the pooled QC samples. An RSD acceptability threshold of < 30% for a minimum of 70% of key ions was considered acceptable to establish LC-MS stability (see Supplementary Material).
Part 2: Effects of Feeding on Longevity
A total of 100 newly emerged wasps were weighed to an accuracy of 0.01 mg then isolated in 0.5 ml Eppendorf vials, half of which contained a droplet of honey, as above. Eppendorf lids were pierced to provide ventilation. Wasps were then maintained in isolation until death. Each vial was inspected daily for signs of life (movement of the body in response to gentle shaking of the vial, movement of the mandibles or legs).
Part 3: Effects of Feeding on Contest Outcomes
Newly emerged female wasps were weighed to an accuracy of 0.01 mg then isolated for either 3 or 7 days in 0.5 ml Eppendorf vials, half of which contained honey, as above. Pairs of females were chosen for staged contests on the basis of closely matching weight (the largest difference between a pair of contests 0.04 mg) and within each dyad one female had been fed and one starved. Further, females within dyads were the same age (either 3 days or 7 days old), were non-siblings that had emerged from different broods (Lizé et al., 2012) and were distinguishable by red or yellow acrylic paint dotted onto the dorsal surface of their thoraxes (Petersen and Hardy, 1996). There were 80 contests set up between pairs of females aged 3 days and 60 between pairs of 7-day old females.
Contests were staged in a plastic contest block consisting of three chambers connected by a slot filled with movable barriers and covered with a transparent Plexiglas lid (Petersen and Hardy, 1996; Goubault et al., 2006). A host of known weight (range: 30.11–38.95 mg) that had been stung and paralysed by a female wasp (which was then discarded) was placed into the central chamber. Contestants were placed separately in the lateral chambers, with the barriers closed. Thus, neither contestant had encountered or taken possession of the host prior to the contest. We staged such “intruder-intruder” contest setups (following Lizé et al., 2012) rather than “owner-owner” or “owner-intruder” setups (Hardy et al., 2013) to prevent females from being able to feed on host haemolymph prior to the contest. After a 60 min acclimatisation period, the barriers were withdrawn sufficiently to connect the three chambers. Parasitoid contests were recorded from above for 60 min using a SONY HDR-CX 190 digital camcorder. A contest winner was defined as the individual that remained within the immediate vicinity of host at the end of the observation period. Aggressive contest behaviour was additionally confirmed to have occurred by reviewing the video footage (Petersen and Hardy, 1996). For the purposes of this study, aggressive behaviour was considered to include any of the following categories: chasing, biting, stinging and fighting (mutual grappling), as outlined by Stockermans and Hardy (2013). Contest trials that did not result in aggressive behaviour were considered not to be clearly resolved.
Data Analysis
Metabolomics data were analysed using a combination of multivariate and non-parametric data analysis as outlined in Kapranas et al. (2016). Briefly, generated NMR bins were baseline-corrected and normalised to wasp dry weight prior to multivariate analysis, whilst LC-MS data was framed using Sieve 2.0 (Thermofisher Scientific) and normalised to total chromatogram ion count. Principle components analysis (PCA) was employed to highlight any differences between dietary treatments, identified key frames/bins were evaluated by Kruskall-Wallis tests using GenStat v.15 (VSN International, Hemel Hempstead). Multiple-comparisons were accounted for by Bonferroni correction (Quinn and Keough, 2002); an adjusted P-value of 0.05 and an arbitrary fold change of >1.5 were used as significance between treatments. Lipid identities were assigned through comparisons with the Lipidmaps, Metlin, Lipid blast (Kind et al., 2013), and Human Metabolome databases (HMDB), along with previously generated LC-MS/MS datasets (Kapranas et al., 2016). NMR bins were identified through comparisons with 2D NMR spectra generated alongside 1D NMR spectra (Kapranas et al., 2016).
Longevity and contest outcome data were analysed using generalised linear models in GenStat. The effect of feeding on longevity was assessed using parametric cohort survival analysis (Crawley, 1993) which also evaluated age-dependent survival rates and the influence of female weight. Logistic analyses of covariance were used to explore influences on the probability of a contest being clearly resolved, fitting the age of the contestant females as a factor with two levels and the magnitude of the weight difference between contestants and the weight of the contested host as covariates. Dietary treatment was not included in this analysis as it was invariant across replicates: contests were always between one fed and one starved female. For resolved contests, influences on the probability of a given female (nominally, the red-marked individual) winning a contest were explored by fitting dietary treatment and contestant age as factors (each with two levels) and the size difference between contestants (calculated as red wasp weight minus yellow wasp weight: Goubault et al., 2006; Bentley et al., 2009; Lizé et al., 2012) as a covariate. Host weight was not included as an explanatory variable as the same host was contested by both females and neither had had the opportunity to assess its quality prior to the contest. All possible interactions between fitted main effects were included in the initial statistical models of contest outcomes. Simplification to the minimal adequate model was achieved by backward stepwise procedures (Crawley, 1993; Hardy and Field, 1998; Briffa, 2013a).
Results
Part 1: Effects of Feeding on Metabolomic Profiles
NMR Spectroscopy
Raw spectral comparisons of different treatment groups identified a consistent sugar signature between 4.15 and 3.2 ppm across the majority of both 3-day old and 7-day old honey-fed wasps. There was a high degree of overlap with the equivalent region of the spectra collected from raw honey samples (Figure S1); this resonance overlap is most likely due to detection of honey within the intestinal tract of honey-fed wasps. G. legneri were observed directly feeding on the provided honey throughout isolation, additionally females displayed extensive self-cleaning behaviour after accidental honey contact, allowing us to exclude the possibility that the observed honey resonance was a result of widespread surface contamination (CJPS & AK pers. obs.). Two 3-day old and three 7-day old honey-fed females did not display these resonances, moreover these samples clustered alongside starved wasps in PCA score plots, likely due to the lack of ingestion of honey by these individuals and thus these replicates were removed from further NMR and LC-MS data analysis.
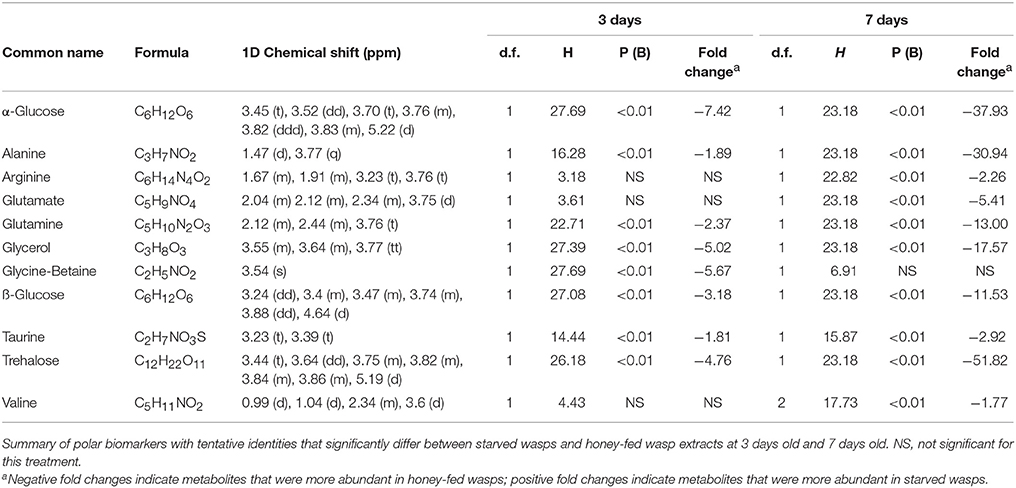
NMR PCA scores plots showed clear separation between honey-fed and starved treatments for both 3-day and 7-day old wasps (Figure 1). Validation of weighted scores plots for 3-day old wasps by Kruskal-Wallis tests (d.f. = 1, P < 0.05) found that 166 bins differed between treatments. Of these spectral identities were assignable to 44 bins, comprising of 15 unique metabolites associated with honey-fed wasps and 3 associated with starved wasps. Further validation of pooled bins associated with these metabolites by Kruskal-Wallis tests (d.f. = 1) found a total of 8 metabolites that were significantly elevated in honey-fed wasps (Figure 2A), whilst no metabolite associated with starved wasps met our criteria for significance (P < 0.05, fold change >1.5).
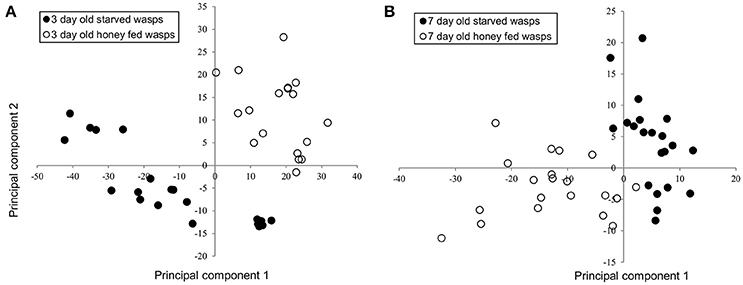
Figure 1. PCA analysis of 3-day and 7-day old G. legneri NMR spectra (A) PCA of NMR samples of 3-day old starved and honey-fed (PC1 = 53.3%, PC2 = 14.3%, PC3 = 6.07%, R2X = 0.803) (B) PCA of NMR samples of 7-day old starved and honey-fed wasps (PC1 = 19.1%, PC2 = 7.7%, PC3 = 7.3%, R2X = 0.582).
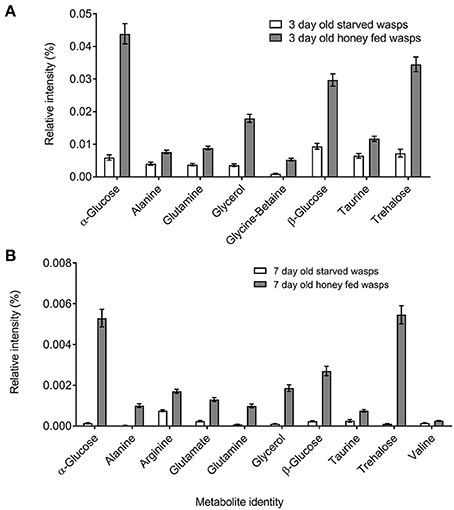
Figure 2. Polar metabolite differences between starved and honey-fed G. legneri (A) metabolite differences between 3-day old starved and 3-day old honey-fed G. legneri (B) metabolite differences between 7-day old starved and 7-day old honey-fed G. legneri. The displayed values consist of the mean normalised metabolite area, error bars show 1 standard error.
For 7-day old females, a total of 79 bins differed between treatments (d.f. = 1, P < 0.05). Of these spectral identities were assignable to 32 bins, comprising of 11 unique metabolites associated with honey-fed wasps. Further validation of pooled bins associated with these metabolites by Kruskal-Wallis tests (d.f. = 1) found a total of 10 metabolites that were significantly elevated in honey-fed wasps (Figure 2B). All bins associated with starved wasp treatments were identified as baseline fluctuations.
Bins associated with honey feeding for both 3-day and 7-day old wasps included common insect haemolymph sugars, along with amino acids that varied between 3-day and 7-day treatments. Further information regarding specific spectral identities is outlined in Table 1.
LC-MS Analysis
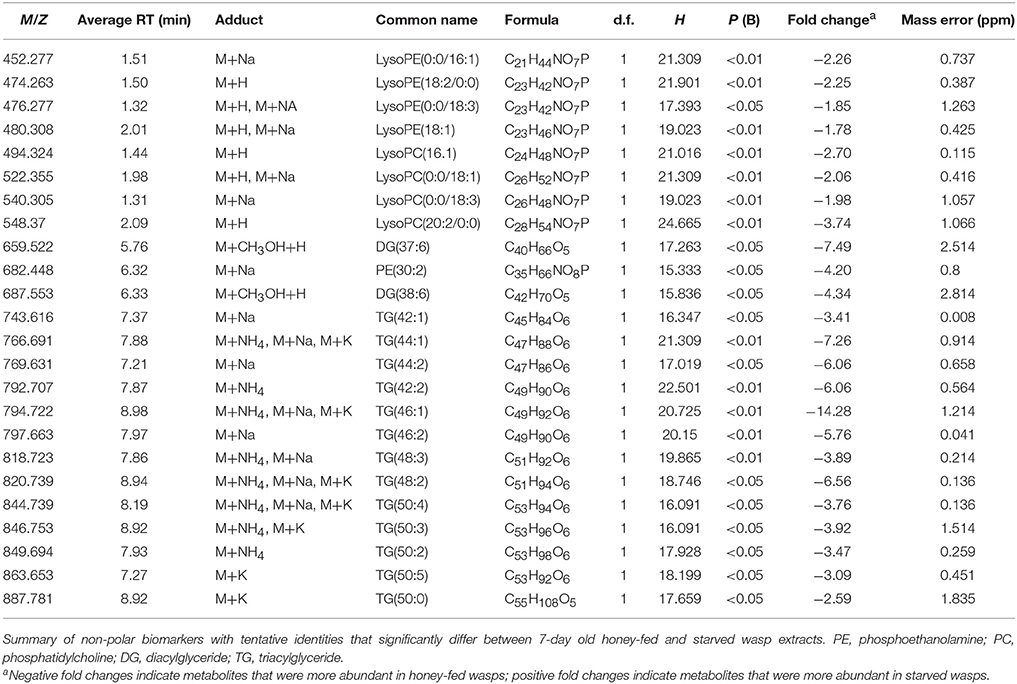
PCA plots of LC-MS data indicated poor separation between the fed and starved treatments at 3 days (Figure 3A) but greater separation at 7 days (Figure 3B). Further validation of weighted PCA scores plots found that 63 ions differed significantly between 7-day old starved and fed wasps (Kruskal-Wallis tests, d.f. = 2, P < 0.05, fold change > 1.5). Tentative identities were assigned to 40 ions through metabolite database comparisons and 37 of these were validated by LC-MS/MS; due to the presence of multiple adducts this corresponded to 24 unique lipids. Lipid identities comprised eight lysophospholipids, two diacylglycerides, one phospholipid and 13 triacylglycerides. All 24 lipids were elevated in honey-fed wasps (Figure 4). Specific lipid identities are detailed in Table 2.
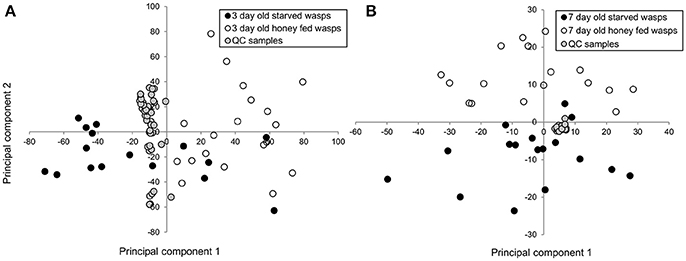
Figure 3. Principle components analysis of 3-day and 7-day old G. legneri LC-MS chromatograms (A) PCA of LC-MS samples of 3-day old starved and honey-fed wasps (PC1 = 25%, PC2 = 13.8%, PC 3 = 11.3%, R2X = 0.856) (B) PCA of LC-MS samples of 7-day old starved and honey-fed wasps (PC1 = 37.1%, PC2 = 16.2%, PC 3 = 5.86%, R2X = 0.718). QC, quality control.
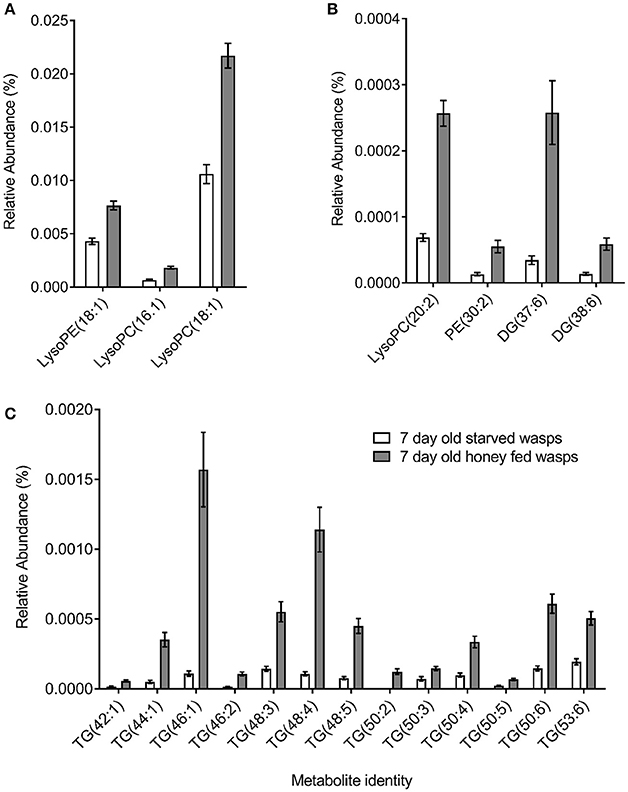
Figure 4. Non-polar metabolite differences between 7-day old starved and 7-day old honey-fed G. legneri (A) lysophospholipids (B) lysophospholipids and (C) glycerolipids. PE, phosphoethanolamine; PC, phosphatidylcholine; DG, diglyceride; TG, triglyceride. The displayed values consist of the mean normalised metabolite area, error bars show 1 standard error. Honey-fed wasps (PC1 = 37.1%, PC2 = 16.2%, PC 3 = 5.86%, R2X = 0.718).
Part 2: Effects of Feeding on Longevity
Survival data were initially assessed by comparing two models of longevity: an exponential model (estimating the rate of mortality) and a Weibull model (additionally estimating variation in mortality rate over time). The Weibull model resulted in a significantly better fit (G1 = 58.3, P < 0.001), indicating that the daily mortality rate increases with age. Adding the weights of individual wasp weights to the Weibull model showed that longevity was positively related to size (G1 = 4.91, P < 0.05). Diet also affected longevity (G1 = 533.72, P < 0.001): the majority of starved females died aged around seven days and none lived beyond 10 days. In contrast, honey-fed wasps lived at least 17 days and some lived beyond 30 days (Figure 5).
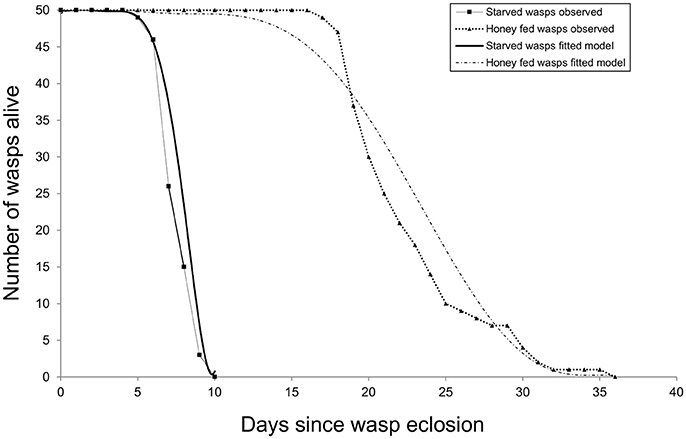
Figure 5. Cohort survival of starved and honey-fed G. legneri females. After 1 week over 50% of starved individuals had died, honey-fed individuals lived around 2 weeks longer.
Part 3: Effects of Feeding on Contest Outcomes
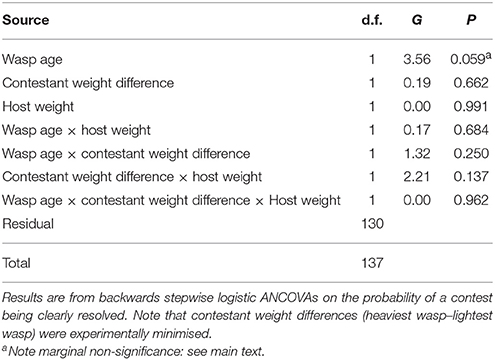
Out of the 80 contests between 3-day old wasps, 54 resulted in a clear contest outcome (a winner and a loser). For females aged 7 days, 31 of 60 contests were resolved. The probability of a contest being resolved was not significantly affected by host weight or the difference in weight between contestant wasps (Table 3). The age of the contestant wasps had a marginally non-significant effect on the probability of contest resolution (G1 = 3.56, P = 0.059, %Deviance explained 1.93, Table 3): as probability estimates in logistic analyses are inexact (Crawley, 1993) and as over-reliance on P-values be generally inadvisable (Nakagawa and Cuthill, 2007), we interpret this as suggestive that contests between younger wasps are typically more likely to be resolved than those between older wasps.
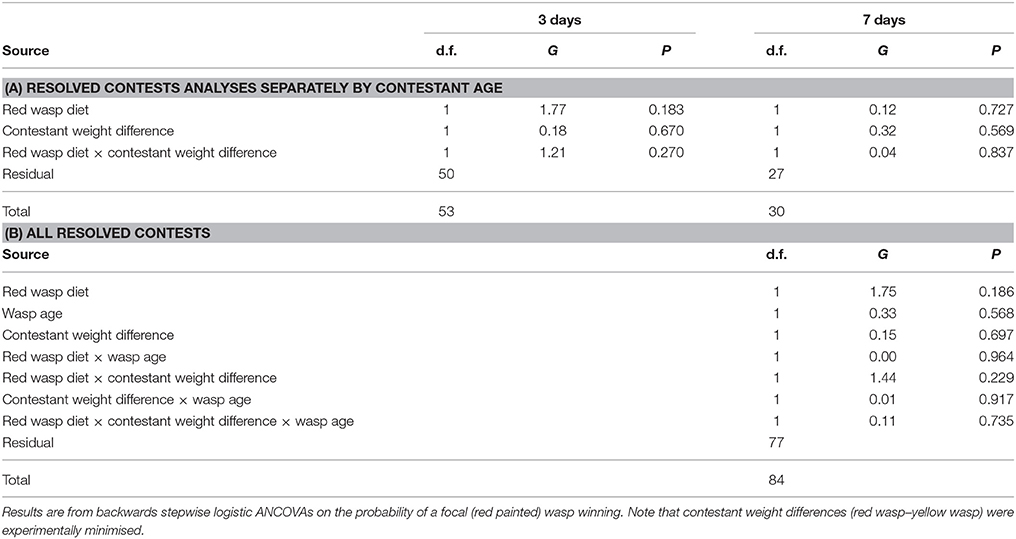
Among contests that were resolved, the probability of the focal (red marked) was winning was not significantly affected by diet, wasp age or the size difference between contestants (Table 4). Similar results were obtained whether analysing data from contests between 3-day old females and between females aged 7 days separately or in combination (Table 4). The proportion of contests won by the fed wasp was 0.42 (± SE = 0.05) overall and there were no significant effects of any of the measured variables on the probability of the contest being won by the wasp that had been fed honey.
Discussion
While several previous studies have considered the metabolomic effects of dietary intake in a range of organisms (German et al., 2002, 2003; Davis and Milner, 2004; Zeisel et al., 2005; Wang et al., 2009; Ametaj et al., 2010; Primrose et al., 2011), ours is the first to consider dietary effects on the metabolome in the context of animal resource competition. Results from our dyadic contest setup indicate that dietary intake does not influence the outcome of contests between G. legneri females, while we also find that feeding enhances female longevity considerably and that a large number of polar and non-polar metabolites associated with energy storage and metabolism were elevated in older fed wasps. There are a number of possible implications of these findings for the understanding of parasitoid behaviour and life-histories and we discuss these in turn along with suggestions for further investigations.
The energetic costs of animal contests have previously been examined in red deer (Clutton-Brock et al., 1979), cichlid fish (Neat et al., 1998a,b), hermit crabs (Briffa and Elwood, 2000, 2001, 2004, 2007), shore crabs (Sneddon et al., 1999), swimming crabs (Thorpe et al., 1994), and spiders (Prestwich, 1983a,b, 1988; DeCarvalho et al., 2004; Elwood and Prenter, 2013). These studies have predominantly focused on the depletion of immediately available energy reserves, such as glycogen, and the accumulation of muscle waste products, such as lactic acid (Briffa and Sneddon, 2007). Although fed wasps contained higher levels of polar-metabolites associated with the rapid mobilization of energy (e.g., glucose, trehalose, glutamate, glycerol) than did starved wasps, this does not appear to have enhanced their resource holding potentials (RHP); an affect that might be expected if contest interactions are energetically demanding. In a study on dyadic contests between male parasitoid wasps, Tasi et al. (2014) also found no effect of honey feeding on outcome when wasps were young (1 day old) but they did find that feeding by 4-day old males enhanced their contest success (although it was not possible to conclude whether this likely effect on RHP was operating via increased body mass after ingestion or improved energetic reserves). It may be that the Goniozus contest interactions we observed were too brief (typically < 60 s of agonistic interactions, including just a few seconds of fully escalated fighting, Petersen and Hardy, 1996) for energetic constraints on RHP to become apparent. Experiments that force G. legneri females into more sustained interactions, or that use bethylid species in which contests appear to be of naturally longer durations (Batchelor et al., 2005), may prove useful to explore this possibility further.
Our study also assessed the levels of non-polar metabolites, typically associated with long-term energy storage. As for the carbohydrates, the higher levels of glycerolipids (storage lipids) and diacylglycerides (the major form of fat mobilisation in insect haemolymph (Arrese and Soulages, 2010) and one of the primary energy sources for insect flight (Vogt et al., 2000; Harrison and Fewell, 2002) of 7-day old honey-fed wasps suggest that these contestants could have greater available energy and thus higher RHPs than their starved opponents. However, starved wasps could potentially place a greater value (V) on winning possession of the contested host than would honey-fed wasps, due to their lower life-expectancy (Humphries et al., 2006; Stockermans and Hardy, 2013) and/or the opportunity to host-feed, whereas fed wasps may perceive a host as having lower value due to their more abundant reserves. Thus, it remains possible that diet influences G. legneri contests between starved and fed wasps, but via opposing and coincidentally equal effects.
Further investigations could assess individual contest behaviours, rather than contest outcome. Older G. legneri females behave more aggressively during contests (Stockermans and Hardy, 2013), due to RV increasing as females age; thus increased aggression in contests between two starved wasps compared to two honey-fed wasps may similarly indicate that starved wasps value hosts more greatly, while higher aggression among fed wasps may indicate that diet affects RHP, and both influences may interact with female age. Preliminary observations on 3-day old female G. legneri, however, suggest that feeding does not affect behavioural interactions during contests (A.K. unpublished data). In other words, starved wasps could be on one hand weaker, due to lack of energetic reserves (lower RHP), but may on the other hand be bolder due to their limited future reproductive opportunities, and as a result fight more fiercely than honey fed wasps (higher RV). Similarly, Tasi et al. (2014) found no effect of honey feeding on the degree of agonism during male-male contests, despite feeding by older males affecting the outcome of contests.
While we have found no overall relationships between metabolome of wasps and contest outcomes, we have found relationships between wasp age and metabolomic state. At 3 days, the lipid profiles of honey-fed and starved wasps were similar but differed clearly among 7-day old wasps. This indicates that effects of dietary provision only become important as individuals approach the life-expectancy of unfed wasps. The greatly increased longevity of honey-fed wasps compared to starved wasps suggests that fed wasps are capable of preserving long-term lipid stores by using dietary carbohydrates as a maintenance source for energy metabolism. However, the presence of higher levels of diacylglycerides in 7-day old honey-fed females than in starved females suggests that carbohydrate-rich dietary feeding did not entirely supress lipid reserve mobilisation throughout the experimental time-frame. This result suggests that lipid store deterioration is merely delayed, rather than prevented, in honey-fed wasps.
Parasitoid fecundity has not been assessed in this study, but it is likely that the use of dietary carbohydrates to maintain vital body processes would allow greater allocation of lipid reserves toward egg production and maturation, as demonstrated by a number of studies on other parasitoid species (Jervis and Kidd, 1986; Heimpel et al., 1997; Winkler et al., 2006; Kapranas and Luck, 2008; Harvey et al., 2012). Indeed, it has already been shown for the congener Goniozus nephantidis that, as adult wasps aged, the egg loads of honey-fed females rose higher and declined later than those of starved females (Stokkebo and Hardy, 2000). Provision of such a diet could also conceivably aid parasitoid dispersion, as two of the three main circulating metabolites used during flight (trehalose and lipids) were found to be elevated in wasps that had consumed a honey diet (Beenakkers et al., 1984; Vogt et al., 2000; Harrison and Fewell, 2002). As such it is plausible that a parasitoid with access to a carbohydrate rich diet could exploit a larger field range in search of potential host larvae, and additionally achieve greater exploitation through decreased egg resorption.
Although it has antecedents in contest studies that focussed on a small sub-set of metabolites (e.g., Briffa and Elwood, 2007) and in studies of the effect of feeding on parasitoid contest behaviour without the associated metabolic analysis (Tsai et al., 2014), ours is the first study to explicitly couple the untargeted metabolomics approach (Snart et al., 2015) with the study of dyadic animal contests (Hardy and Briffa, 2013). Despite the small size of the organisms involved, this methodology is capable of reliably producing identifiable NMR spectra associated with different diet treatments (Kapranas et al., 2016) and as such has potential for application to a wide range of studies of the behaviour and ecology of small insects and other organisms. The approach can identify experimental outliers, in this instance individuals that had not ingested the provided diet were detected by absence of the highly conserved honey signal in the NMR spectra of fed wasps. However, as the whole insect is sampled, the method cannot distinguish between resonances generated from insect haemolymph from those generated from diet within an insect's digestive tract (similarly, Tsai et al., 2014 were unable to determine whether mass gained by honey-fed wasps indicated metabolism or simply ingestion). Hence, further experimentation, such as our longevity study, is required to confirm that parasitoids are capable of utilising a particular dietary resource other than merely ingesting it.
Conclusions
This is the first study to explicitly couple the untargeted metabolomics approach with the study of dyadic animal contests. Provision of a carbohydrate-rich diet affected the metabolomic state of G. legneri and enhanced its longevity but did not affect its performance in dyadic contests. While honey-fed wasps had higher levels of energy rich metabolites, these may not have been sufficient to enhance RHP substantially. Alternatively, an effective increase in RHP among fed females may have been countered by an increase in the host's perceived value, V, by starved competitors.
Ethics Statement
The research reported here using insects is in full compliance with the principles of Replacement, Reduction and Refinement as set by ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines and enforced by the University of Nottingham Animal Welfare and Ethical Review Body (AWERB).
Author Contributions
CS, AK, IH, and DB: Conceived and designed the experiments; CS, AK, IH and HW: Performed the experiments; CS, AK, IH, and HW: Analysed the data; IH, DB, and HW: Provided resources; CS, AK, DB, and IH: Wrote the paper; All authors contributed to final draft approval.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council [studentship number 1099415], a BBSRC studentship supporting CS. AK was supported by a Marie Curie Fellowship (FP7-PEOPLE-2010-IEF 273431). We thank Clare Daykin for her initial input to this project, Catherine Ortori for help with instrument method development, maintenance and operation, and Srinivasarao Ravipati for input on method development and data analysis. We thank two referees for useful comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2018.00012/full#supplementary-material
References
Ametaj, B. N., Zebeli, Q., Saleem, F., Psychogios, N., Lewis, M. J., Dunn, S. M., et al. (2010). Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows. Metabolomics 6, 583–594. doi: 10.1007/s11306-010-0227-6
Arrese, E. L., and Soulages, J. L. (2010). Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225. doi: 10.1146/annurev-ento-112408-085356
Batchelor, T. P., Hardy, I. C. W., Barrera, J. F., and Pérez-Lachaud, G. (2005). Insect gladiators II: competitive interactions within and between bethylid parasitoid species of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Biol. Control 33, 194–202. doi: 10.1016/j.biocontrol.2005.02.010
Beenakkers, A. M. T., van der Horst, D. J., and van Marrewijk, W. J. A. (1984). Insect flight metabolism. Insect Biochem. 14, 243–260. doi: 10.1016/0020-1790(84)90057-X
Bentley, T., Hull, T. T., Hardy, I. C. W., and Goubault, M. (2009). The elusive paradox: owner-intruder roles, strategies, and outcomes in parasitoid contests. Behav. Ecol. 20, 296–304. doi: 10.1093/beheco/arp007
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/y59-099
Briffa, M., Bridger, D., and Biro, P. A. (2013a). How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Anim. Behav. 86, 47–54. doi: 10.1016/j.anbehav.2013.04.009
Briffa, M., and Elwood, R. W. (2000). Cumulative or sequential assessment during hermit crab shell fights: effects of oxygen on decision rules. Proc. Biol. Sci. 267, 2445–2452. doi: 10.1098/rspb.2000.1304
Briffa, M., and Elwood, R. W. (2001). Decision rules, energy metabolism and vigour of hermit-crab fights. Proc. Biol. Sci. 268, 1841–1848. doi: 10.1098/rspb.2001.1752
Briffa, M., and Elwood, R. W. (2004). Use of energy reserves in fighting hermit crabs. Proc. R. Soc. B 271, 373–379. doi: 10.1098/rspb.2003.2633
Briffa, M., and Elwood, R. W. (2007). Monoamines and decision making during contests in the hermit crab Pagurus bernhardus. Anim. Behav. 73, 605–612. doi: 10.1016/j.anbehav.2006.06.008
Briffa, M., Hardy, I. C. W., Gammell, M. P., Jennings, D. J., Clarke, D. D., and Goubault, M. (2013b). “Analysis of animal contest data,” in Animal Contests, eds I. C. W. Hardy and M. Briffa (Cambridge: Cambridge University Press), 47–85.
Briffa, M., and Sneddon, L. U. (2007). Physiological constraints on contest behaviour. Funct. Ecol. 21, 627–637. doi: 10.1111/j.1365-2435.2006.01188.x
Casas, J., Pincebourde, S., Mandon, N., Vannier, F., Poujol, R., and Giron, D. (2005). Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 86, 545–554. doi: 10.1890/04-0812
Clutton-Brock, T. H., Albon, S. D., Gibson, R. M., and Guinness, F. E. (1979). Logical stag - adaptive aspects of fighting in red deer (Cervus elaphus L). Anim. Behav. 27, 211–225. doi: 10.1016/0003-3472(79)90141-6
Davidson, D. W. (1997). The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol. J. Linnean Soc. 61, 153–181. doi: 10.1111/j.1095-8312.1997.tb01785.x
Davis, C. D., and Milner, J. (2004). Frontiers in nutrigenomics, proteomics, metabolomics and cancer prevention. Mutat. Res. 551, 51–64. doi: 10.1016/j.mrfmmm.2004.01.012
DeCarvalho, T. N., Watson, P. J., and Field, S. A. (2004). Costs increase as ritualized fighting progresses within and between phases in the sierra dome spider, Neriene litigiosa. Anim. Behav. 68, 473–482. doi: 10.1016/j.anbehav.2003.08.033
Desouhant, E., Lucchetta, P., Giron, D., and Bernstein, C. (2010). Feeding activity pattern in a parasitic wasp when foraging in the field. Ecol. Res. 25, 419–428. doi: 10.1007/s11284-009-0671-9
Eijs, I. E. M., Ellers, J., and van Duinen, G. (1998). Feeding strategies in drosophilid parasitoids: the impact of natural food resources on energy reserves in females. Ecol. Entomol. 23, 133–138. doi: 10.1046/j.1365-2311.1998.00117.x
Ellers, J. (1996). Fat and eggs: an alternative method to measure the tradeoff between survival and reproduction in insect parasitoids. Netherlands J. Zool. 46, 227–235. doi: 10.1163/156854295X00186
Ellers, J., Ruhe, B., and Visser, B. (2011). Discriminating between energetic content and dietary composition as an explanation for dietary restriction effects. J. Insect Physiol. 57, 1670–1676. doi: 10.1016/j.jinsphys.2011.08.020
Elwood, R. W., and Prenter, J. (2013). “Aggression in spiders,” in Animal Contests, eds I. C. W. Hardy and M. Briffa (Cambridge: Cambridge University Press), 113–133.
German, J. B., Roberts, M. A., Fay, L., and Watkins, S. M. (2002). Metabolomics and individual metabolic assessment: the next great challenge for nutrition. Journal of Nutrition, 132, 2486–2487. doi: 10.1093/jn/132.9.2486
German, J. B., Roberts, M. A., and Watkins, S. M. (2003). Personal metabolomics as a next generation nutritional assessment. J. Nutr. 133, 4260–4266. doi: 10.1093/jn/133.12.4260
Giron, D., Rivero, A., Mandon, N., Darrouzet, E., and Casas, J. (2002). The physiology of host feeding in parasitic wasps: implications for survival. Funct. Ecol. 16, 750–757. doi: 10.1046/j.1365-2435.2002.00679.x
Gómez, J., Barrera, J. F., Liedo, P., and Valle, J. (2012). Influence of age and diet on the performance of Cephalonomia stephanoderis (Hymenoptera, Bethylidae) a parasitoid of the coffee berry borer, Hypothenemus hampei (Coleoptera, Curculionidae). Rev. Bras. Entomol. 56, 95–100. doi: 10.1590/S0085-56262012005000017
Goubault, M., Batchelor, T. P., Linforth, R. S. T., Taylor, A. J., and Hardy, I. C. W. (2006). Volatile emission by contest losers revealed by real-time chemical analysis. Proc. R. Soc. B 273, 2853–2859. doi: 10.1098/rspb.2006.3655
Hardy, I. C. W., and Field, S. A. (1998). Logistic analysis of animal contests. Anim. Behav. 56, 787–792. doi: 10.1006/anbe.1998.0833
Hardy, I. C. W., Goubault, M., and Batchelor, T. P. (2013). “Hymenopteran contests and agonistic behaviour,” in Animal Contests, eds I. C. W. Hardy and M. Briffa (Cambridge: Cambridge University Press), 147–177.
Hardy, I. C. W., Stokkebo, S., Bønløkke-Pedersen, J., and Sejr, M. K. (2000). Insemination capacity and dispersal in relation to sex allocation decisions in Goniozus legneri (Hymenoptera: Bethylidae): why are there more males in larger broods? Ethology 106, 1021–1032. doi: 10.1046/j.1439-0310.2000.00621.x
Harrison, J. F., and Fewell, J. H. (2002). Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 133, 323–333. doi: 10.1016/S1095-6433(02)00163-0
Harvey, J. A., Gols, R., Vet, L. E., and Kruidhof, H. M. (2012). Development of a hyperparasitoid wasp in different stages of its primary parasitoid and secondary herbivore hosts. J. Insect Physiol. 58, 1463–1468. doi: 10.1016/j.jinsphys.2012.08.013
Heimpel, G. E., Rosenheim, J. A., and Kattari, D. (1997). Adult feeding and lifetime reproductive success in the parasitoid Aphytis melinus. Entomol. Exp. Appl. 83, 305–315. doi: 10.1046/j.1570-7458.1997.00185.x
Humphries, E. L., Hebblethwaite, A. J., Batchelor, T. P., and Hardy, I. C. W. (2006). The importance of valuing resources: host weight and contender age as determinants of parasitoid wasp contest outcomes. Anim. Behav. 72, 891–898. doi: 10.1016/j.anbehav.2006.02.015
Jervis, M. A., Ellers, J., and Harvey, J. A. (2008). Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 53, 361–385. doi: 10.1146/annurev.ento.53.103106.093433
Jervis, M. A., and Kidd, N. A. C. (1986). Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. Camb. Philos. Soc. 61, 395–434. doi: 10.1111/j.1469-185X.1986.tb00660.x
Jervis, M. A., Kidd, N. A. C., Fitton, M. G., Huddleston, T., and Dawah, H. A. (1993). Flower-visiting by hymenopteran parasitoids. J. Nat. Hist. 27, 67–105. doi: 10.1080/00222939300770051
Kapranas, A., and Luck, R. F. (2008). Egg maturation, host feeding, and longevity in two Metaphycus species parasitoids of soft scale insects. Biol. Control 47, 147–153. doi: 10.1016/j.biocontrol.2008.08.002
Kapranas, A., Snart, C. J. P., Williams, H., Hardy, I. C. W., and Barrett, D. A. (2016). Metabolomics of aging assessed in individual parasitoid wasps. Sci. Rep. 6:34848. doi: 10.1038/srep34848
Kemp, D. J., and Alcock, J. (2003). Lifetime resource utilization, flight physiology, and the evolution of contest competition in territorial insects. Am. Natur. 162, 290–301. doi: 10.1086/376890
Kind, T., Liu, K. H., Lee, D. Y., Defelice, B., Meissen, J. K., and Fiehn, O. (2013). LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 10, 755–788. doi: 10.1038/nmeth.2551
Kokko, H. (2013). “Dyadic contests: modelling fights between two individuals,” in Animal Contests, eds I. C. W. Hardy and M. Briffa (Cambridge: Cambridge University Press), 5–52.
Lee, J. C., Heimpel, G. E., and Leibee, G. L. (2004). Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol. Exp. Appl. 111, 189–199. doi: 10.1111/j.0013-8703.2004.00165.x
Lizé, A., Khidr, S. K., and Hardy, I. C. W. (2012). Two components of kin recognition influence parasitoid aggression in resource competition. Anim. Behav. 83, 793–799 doi: 10.1016/j.anbehav.2012.01.001
Martinez-Lendech, N., Cordoba-Aguilar, A., and Serrano-Meneses, M. A. (2007). Body size and fat reserves as possible predictors of male territorial status and contest outcome in the butterfly Eumaeus toxea Godart (Lepidoptera: Lycaenidae). J. Ethol. 25, 195–199. doi: 10.1007/s10164-007-0040-5
Maynard Smith, J., and Parker, G. A. (1976). The logic of asymmetric contests. Anim. Behav. 24, 159–175. doi: 10.1016/S0003-3472(76)80110-8
Maynard Smith, J., and Price, G. R. (1973). The logic of animal conflict. Nature 246, 15–18. doi: 10.1038/246015a0
Nakagawa, S., and Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. doi: 10.1111/j.1469-185X.2007.00027.x
Neat, F. C., Huntingford, F. A., and Beveridge, M. M. C. (1998a). Fighting and assessment in male cichlid fish: the effects of asymmetries in gonadal state and body size. Anim. Behav. 55, 883–891. doi: 10.1006/anbe.1997.0669
Neat, F. C., Taylor, A. C., and Huntingford, F. A. (1998b). Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Anim. Behav. 55, 875–882. doi: 10.1006/anbe.1997.0668
Petersen, G., and Hardy, I. C. W. (1996). The importance of being larger: parasitoid intruder-owner contests and their implications for clutch size. Anim. Behav. 51, 1363–1373. doi: 10.1006/anbe.1996.0139
Pexton, J. J., and Mayhew, P. J. (2002). Siblicide and life-history evolution in parasitoids. Behav. Ecol. 13, 690–695. doi: 10.1093/beheco/13.5.690
Poole, J. H. (1989). Announcing intent: the aggressive state of musth in African elephants. Anim. Behav. 37, 140–152. doi: 10.1016/0003-3472(89)90014-6
Prestwich, K. N. (1983a). Anaerobic metabolism in spiders. Physiol. Zool. 56, 112–121. doi: 10.1086/physzool.56.1.30159972
Prestwich, K. N. (1983b). The roles of aerobic and anaerobic metabolism in active spiders. Physiol. Zool. 56, 122–132. doi: 10.1086/physzool.56.1.30159973
Prestwich, K. N. (1988). The constraints on maximal activity in spiders. 1. Evidence against the fluid insufficiency hypothesis. J. Comp. Physiol. B 158, 437–447. doi: 10.1007/BF00691141
Primrose, S., Drape, J., Elsom, R., Kirkpatrick, V., Mathers, J. C., Seal, C., et al. (2011). Metabolomics and human nutrition. Br. J. Nutr. 105, 1277–1283. doi: 10.1017/S0007114510004812
Quinn, G. P., and Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press.
Riechert, S. E. (1998). “Game theory and animal contests,” in Game Theory and Animal Behaviour, eds L. A. Dugatkin and H. K. Reeve (Oxford: Oxford University Press), 64–93.
Snart, C. J. P., Hardy, I. C. W., and Barrett, D. A. (2015). Entometabolomics: applications of modern analytical techniques to insect studies. Entomol. Exp. Appl. 155, 1–17. doi: 10.1111/eea.12281
Sneddon, L. U., Taylor, A. C., and Huntingford, F. A. (1999). Metabolic consequences of agonistic behaviour: crab fights in declining oxygen tensions. Anim. Behav. 57, 353–363. doi: 10.1006/anbe.1998.0982
Steffan, S. A., Daane, K. M., and Mahr, D. L. (2001). 15N-enrichment of plant tissue to mark phytophagous insects, associated parasitoids, and flower-visiting entomophaga. Entomol. Exp. Appl. 98, 173–180. doi: 10.1046/j.1570-7458.2001.00772.x
Stockermans, B. C., and Hardy, I. C. W. (2013). Subjective and objective components of resource value additively increase aggression in parasitoid contests. Biol. Lett. 9:20130391. doi: 10.1098/rsbl.2013.0391
Stokkebo, S., and Hardy, I. C. W. (2000). The importance of being gravid: egg load and contest outcome in a parasitoid wasp. Anim. Behav. 59, 1111–1118. doi: 10.1006/anbe.2000.1407
Thorpe, K. E., Huntingford, F. A., and Taylor, A. C. (1994). Relative size and agonistic behavior in the female velvet swimming crab, Necora puber (L) (Brachyura, Portunidae). Behav. Processes 32, 235–246. doi: 10.1016/0376-6357(94)90045-0
Tsai, Y. J. J., Barrows, E. M., and Weiss, M. R. (2014). Why do larger and older males win contests in the parasitoid wasp Nasonia vitripennis? Anim. Behav. 91, 151–159. doi: 10.1016/j.anbehav.2014.03.010
Visser, B., and Ellers, J. (2008). Lack of lipogenesis in parasitoids: a review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 54, 1315–1322. doi: 10.1016/j.jinsphys.2008.07.014
Visser, B., Le Lann, C., den Blanken, F. J., Harvey, J. A., van Alphen, J. J. M., and Ellers, J. (2010). Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. U.S.A. 107, 8677–8682. doi: 10.1073/pnas.1001744107
Vogt, J. T., Appel, A. G., and West, M. S. (2000). Flight energetics and dispersal capability of the fire ant, Solenopsis invicta Buren. J. Insect Physiol. 46, 697–707. doi: 10.1016/S0022-1910(99)00158-4
Wäckers, F. L., Lee, J. C., Heimpel, G. E., Winkler, K., and Wagenaar, R. (2006). Hymenopteran parasitoids synthesize 'honeydew-specific' oligosaccharides. Funct. Ecol. 20, 790–798. doi: 10.1111/j.1365-2435.2006.01158.x
Wang, J., Wu, G., Zhou, H., and Wang, F. (2009). Emerging technologies for amino acid nutrition research in the post-genome era. Amino Acids 37, 177–186. doi: 10.1007/s00726-008-0193-8
Winkler, K., Wäckers, F. L., Bukovinszkine-Kiss, G., and van Lenteren, J. (2006). Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl. Ecol. 7, 133–140. doi: 10.1016/j.baae.2005.06.001
Keywords: parasitoid diet, metabolomics, lipidomics, nuclear magnetic resonance, mass spectrometry, longevity, contest performance
Citation: Snart CJP, Kapranas A, Williams H, Barrett DA and Hardy ICW (2018) Sustenance and Performance: Nutritional Reserves, Longevity, and Contest Outcomes of Fed and Starved Adult Parasitoid Wasps. Front. Ecol. Evol. 6:12. doi: 10.3389/fevo.2018.00012
Received: 14 October 2017; Accepted: 23 January 2018;
Published: 07 February 2018.
Edited by:
Carlos Alonso Alvarez, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Shawn M. Wilder, Oklahoma State University, United StatesFloria Mora-Kepfer Uy, University of Miami, United States
Copyright © 2018 Snart, Kapranas, Williams, Barrett and Hardy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles J. P. Snart, Yy5qLnAuc25hcnRAbGVlZHMuYWMudWs=
Apostolos Kapranas, YS5rYXByYW5hc0BicGkuZ3I=
†These authors have contributed equally to this work and are co-senior authors.
 Charles J. P. Snart
Charles J. P. Snart Apostolos Kapranas
Apostolos Kapranas Huw Williams4
Huw Williams4 Ian C. W. Hardy
Ian C. W. Hardy