- 1Decision and Policy Analysis Research Area, International Center for Tropical Agriculture, Cali, Colombia
- 2Botanical Research Institute of Texas, Fort Worth, TX, United States
Human management of anthropogenic environments and species is tightly linked to the ecology and evolution of plants gathered by humans. This is certainly the case for wild food plants, which exist on a continuum of human management. Given alarming deforestation rates, wild food plant gathering is increasingly occurring in anthropogenic ecosystems, where farmers actively manage these species in order to ensure their availability and access. This study was conducted in a mestizo village in the Peruvian Amazon deforestation frontier, with the objective of documenting the management practices, including the human-induced movement of wild food plant species across the forest-agriculture landscape, and the motivations that farmers have to manage them using a qualitative ethnobotanical approach. The results of focus group discussions showed that 67% of the 30 “wild” food plant species reported for the village were managed, and almost all plants that were managed have been transplanted. The strongest flow of transplanted material was from forest to agricultural field (11 species), followed by market to field (five species), and field to home garden (four species). Farmers argued that the main reason for transplanting “wild” food plants was to have them closer to home, because they perceived that the abundance of 77% of these species decreased in the last years. Conversely, the most important reason for not transplanting a “wild” plant was the long time it takes to grow, stated for 67% of the species that have not been transplanted. Remarkably, more than half (57%) of the “wild” food plant species, including 76% of the species that are managed, have been classified as weeds by scientific literature. Finally, the “wild” food plant species were classified in six mutually exclusive groups according to management form and perceived abundance. The study concluded that “wild” food plant management, including management of species classified as weeds by scientific literature, is a crucial adaptation strategy of farmers aimed at ensuring their food security in scenarios of increasing deforestation. Finally, the article reflects on the major implications of human management on the ecology and evolution of food plant species.
Introduction
Wild food plant gathering is a deeply rooted component of human heritage, with millions of people gathering these species around the world. From 250,000–300,000 higher plant species known, ~5,000 species have been managed at certain periods of time (Cotton, 1996; Heywood, 1999), but nowadays the diet of humanity largely depends on 53 crop commodities (Khoury et al., 2016). In a global context of increasing dietary homogenization (Khoury et al., 2014), the consumption of thousands of wild food plants and other underutilized food species plays a key role for food and nutritional security (Cruz-Garcia and Ertug, 2014). In addition, it has been documented that wild vegetables and fruits constitute a very important source of vitamins, minerals, and secondary metabolites (Johns, 2007), and many of these species are essential components of the diet during food scarcity periods (Scoones et al., 1992; Heywood, 1999; Cruz-Garcia and Price, 2014a).
Rural families gather wild food plants from highly intervened environments such as agricultural fields, more subsistence environments such as home gardens, and less intervened areas such as forests. They, however, increasingly collect wild food plants from anthropogenic ecosystems, given the alarming loss of natural habitats. For example, it has been documented that families that are more distant from forests (i.e., due to high deforestation rates) prefer to gather in areas closer to home (Price and Ogle, 2008). Ogle and Grivetti (1985) coined the term “botanical dietary paradox” explaining that farmers increasingly depend on agricultural “weeds” when the forest area decreases. For example, they documented in a study conducted in Swaziland that the area with higher management intensity presented a greater number of wild food plants. Likewise, Kosaka et al. (2006a,b) reported from research in Savannakhet (Laos) that households located closer to the forest depended more on forest foods, whereas those far from the forest relied more on wild food plants from agricultural fields to compensate the lack of forest resources.
Wild food plants exist on a continuum of human management from “truly” wild to semi-domesticated and cultivated species (Casas et al., 1996; González-Insuasti and Caballero, 2007). Plant management can be defined as “the set of actions or practices directly or indirectly performed by humans to favor availability of populations or individual phenotypes within populations of useful plant species” (González-Insuasti and Caballero, 2007, p. 303). Certainly, human management is tightly linked to the ecology and evolution of species (Clement et al., 2010). The interactions of humans with plants is clearly contextualized in the continuum model for agricultural (Harris, 1989) and agroforestry systems (Wiersum, 1997b). This model explains that these interactions change in time and space along a gradient that is neither unidirectional nor deterministic. The levels of interaction are not necessarily pre-ordinated steps of increasing management intensity toward domestication; therefore most wild managed species are not necessarily becoming domesticated species (Harlan, 1975; Harris, 1989). In addition, while some plants that used to be intensely managed in the past are only tolerated or slightly protected at present, other wild food species are becoming domesticated ones (Harris, 1989).
Plant species could be grouped into three main categories according to forms of management intensity: (1a) gathered species, (1b) species with incipient management, and (1c) species cultivated ex situ. There is also a gradient within incipient management that includes: (2a) tolerance, (2b) protection, and (2c) promotion. Management practices include those related to protection, such as watering and fertilizing; practices related to promotion, like pruning and weeding; and practices related to ex situ cultivation, such as (trans)planting and sowing (Casas et al., 1996; González-Insuasti and Caballero, 2007). Additionally, incipient management practices can take place in situ, i.e., in the original place occupied by the plant, or ex situ, when transplanted to another place (Casas et al., 1996). In this way, human induced movement of wild food plants across the farming landscape, e.g., transplanting a plant from an agricultural field to a home garden, is a type of management (Cruz-Garcia and Price, 2014b). Domestication processes have (indirectly) promoted management practices such as propagation, protection, transplanting, and selective harvesting, which are important in order to ensure the availability of and access to useful plants that are in risk of decreasing or even disappearing (Price, 1997; Balemie and Kebebew, 2006; Daly, 2014). This plays a key role in the conservation of plant genetic resources particularly in the deforestation frontier.
A species management intensity and the types of management practices associated to the species might vary from place to place (Cotton, 1996; Ogle, 2001; González-Insuasti and Caballero, 2007). Furthermore, local people and scientists might use different classifications for wild and domesticated species. For instance, a species might be classified as wild by a socio-cultural group but classified as domesticated by another group, or by scientists, which has implications for research (Michon and De Foresta, 1997; Clement, 1999; Orwa et al., 2009). This might be the case for the Amazon, where, although plant domestication started earlier than 8,000 years ago (Levis et al., 2017), a substantial portion of the genetic heritage was lost when the indigenous population drastically declined after European contact (Clement, 1999). Nowadays domesticated plant species persist in the forests (Levis et al., 2017), and this might hypothetically imply that some of these species are not managed or present incipient management practices, and newcomers (i.e., mestizo migrants) regard them as “wild” species.
According to Levis et al. (2017, p. 925) “domestication of plant populations is a result of the human capacity to overcome selective pressures of the environment by creating landscapes to manage and cultivate useful species.” In order to better understand the processes of management and domestication it is necessary to incorporate socio-cultural aspects related to the use and valuation of a species (Casas et al., 1996; Blancas et al., 2013), which are distributed inter-culturally and intra-culturally (González-Insuasti et al., 2011). Certainly, the values attributed to species by people will affect their incentives to manage them (Guijt, 1998) and to continue using them (Ogle, 2001). For instance, González-Insuasti and Caballero (2007) and González-Insuasti et al. (2008) demonstrated, from a study conducted in Tehuacán-Cuicatlán (Mexico), that management intensity depends on a species' cultural importance and biology, and these factors, together with land ownership, substantially influence farmer's decisions to intensify management practices. It has also been hypothesized by a number of authors (Stoffle et al., 1990; Cunningham, 1993; Price, 1997) that intensive management of wild food plant species in anthropogenic systems occurs when species have multiple use value and are perceived as rare. Furthermore, Price (1997) concluded from her research in Northeast Thailand that farmers increasingly manage wild food plant species with a high market value that are perceived as rare. Certainly, it has been documented by more authors that the local perception of a species abundance, i.e., perceptions of its rarity, influences the decision to manage the species (Price, 1997; Blancas et al., 2013, 2014).
Domestication is a cultural process (Clement, 1999), therefore the documentation of farmers' motivations to manage “wild” food plant species, taking into account the influence of their perceived abundance and cultural aspects related to these motivations, would contribute to the scientific study of domestication. Farmers' motivations explain why decisions are made and, ultimately, help to understand the distribution of useful species in the anthropogenic landscape. In other words, motivations are the human reasons underlying co-evolutionary domestication processes. The study of the motivations to manage food species is certainly necessary for communities in the deforestation frontier, where families are continuously adapting (or trying to adapt) to the loss of biodiversity, which provides an ideal setting for the study of contemporary domestication processes. This is certainly the case of Ucayali, which, together with Madre de Dios, are the regions with the highest deforestation rate in the country (Oliveira et al., 2007). The perspectives of mestizo farmers are of particular importance in Ucayali, since they constitute 80% of the population in this region (Porro et al., 2015), and have been frequently blamed for contributing to a major extent to the deforestation in the Peruvian Amazon (Alvarez and Naughton-Treves, 2003) as a response to secure tenure rights given a political-ecological context of high demand for land for extractive activities and industrial agriculture (Porro et al., 2015).
This paper presents an ethnobotanical perspective to domestication as an ongoing process, focused on the analysis of human factors influencing artificial selection operating on “wild” food species. In this way, the objective of this study was to document, in a mestizo village of Ucayali, the management practices, including the human-induced movement of “wild” food plant species across the forest-agriculture landscape, and the motivations mestizo farmers have for managing these species. The motivations not only include reasons for managing but also for not managing “wild” food plants. Whereas most research aimed at understanding the factors affecting plant management has been quantitative (e.g., González-Insuasti et al., 2008, 2011; Blancas et al., 2013), this study presents a qualitative approach. In addition, this article compares farmers' motivations to manage “wild” food plants in relation to their perceived abundance, and discusses the findings in a context of deforestation processes.
Given that this study was conducted from an ethnobotanical perspective, the inventory of “wild” food plants was built based on these species classified as “wild” by local people. In this way this study includes species that are not locally classified as domesticated, along a gradient of varying management intensity, from truly wild species (absence of management), wild tolerated, protected, and/or promoted and cultivated species. Management refers to practices and forms. Management forms include incipient management and ex situ cultivation (excluding gathering, unless indicated otherwise). Management practices include transplanting, watering, fertilizing, protecting, pruning, weeding, and mulching (González-Insuasti and Caballero, 2007). Transplanting includes sowing, planting and actual transplanting. Protecting refers to conscious care activities other than watering and fertilizing. Toleration is not considered a management practice per se, given that it does not imply any activity specifically aimed at promoting the growth of a plant; toleration is considered a more incipient type of management form. The motivations focus on transplanting (why farmers transplant a species or not), which was the most common management practice and it is related to the human-induced movement of these plants across the landscape. Finally, the farming landscape, or forest-agriculture landscape includes anthropogenic ecosystems along different degrees of human intervention, encompassing agricultural fields, home gardens and secondary forests (forests in the study site are mainly secondary).
Study Site
This study took place in the village of Pueblo Libre, located in Ucayali, Peruvian Amazon. In 2012, Ucayali had a total of 490,000 inhabitants. Twenty percent of the department is inhabited by indigenous communities, whereas most of the population consists of mestizos. Mestizos, who are migrants from non-Amazonian regions of Peru, are mainly settled along the Federico Basadre highway or the Ucayali river and its tributaries (Porro et al., 2015). The highway was built in 1945 and connects Pucallpa with Lima (860 Km), which is the capital of the country (Pimentel et al., 2004). Sixty percent of the population of Ucayali lives in Pucallpa, which is the capital of the department. Pucallpa is the second most populated capital in the Peruvian Amazon (INEI, 2011a).
The main economic activities of Ucayali are agriculture, livestock farming and timber industry, contributing altogether to almost 20% of the gross domestic product (MINEM-GOREU, 2007; INEI, 2011b). Certainly, Ucayali is the main center of the Peruvian timber industry (Ramos Delgado, 2009). The staple crops are cassava, maize, plantain, rice, and beans. However, during the last decade, the region experienced an increase in palm oil and cacao plantations (Salisbury and Fagan, 2013). The mean annual rainfall in Ucayali ranges from 1,800 to 3,000 mm (Fujisaka et al., 2000), whereas the mean annual temperature is 25.7°C with 80% of relative humidity (Lojka et al., 2008).
Peru, after Brazil, is the country with the highest extension of Amazonian forest (Lu, 2009). However, Peru has an average of 64,500 ha deforested every year, which are mainly located in the departments of Ucayali and Madre de Dios (Oliveira et al., 2007). Deforestation and land degradation are prevalent in Ucayali mainly due to the expansion of legal and illegal logging, land clearing and road construction (Galarza and La Serna, 2005; Miranda et al., 2014). For instance, by 2010 about 9% of the original forest area of Ucayali, which was 8.7 million ha, had been deforested (Porro et al., 2015). Ucayali's increasing rate of deforestation goes back to 2002, when half of the forest was declared fit for permanent production and given as concessions by the Instituto Nacional de Recursos Naturales (INRENA; INEI, 2011c). After 2002, the proportion of illegal logging increased when most forest concessions lost their licenses due to a change in regulations (Smith et al., 2006). Certainly, 80–95% of logging in Peru is illegal (Sears and Pinedo-Vasquez, 2011; Cossío et al., 2014).
Pueblo Libre (174 m. asl) is a mestizo upland village situated at km. 60 of the Federico Basadre highway, followed by 22 km. of dirt road (Figures 1, 2). Pueblo Libre is inhabited by mestizos who migrated from the Peruvian highlands and coast, as well as people from other regions of the Amazon. It has a population of ~75 families encompassing more than 350 inhabitants. Most of the houses have electricity and access to drinking water. There is telephone signal and there is an internet service in town, which was built in 2013 by a project funded by the United States Agency for International Development (USAID). The main source of income consists of the production of palm oil, cacao, plantain and, to a lesser degree, livestock. There is no communally owned land in the village, and the forest, which is privately owned, is fragmented and scattered across the different land properties of the families (Vael, 2015). It has been reported that villagers from Pueblo Libre consume “wild” food plants, which are mainly gathered from agricultural fields, forests and home gardens. People mostly consume “wild” fruits (mainly gathered by men), followed by tubers and roots (mainly collected by women; Cruz-Garcia and Vael, 2017).

Figure 1. Photograph of Pueblo Libre village, Ucayali, Peru. Source: Photograph taken by G. S. Cruz-Garcia.
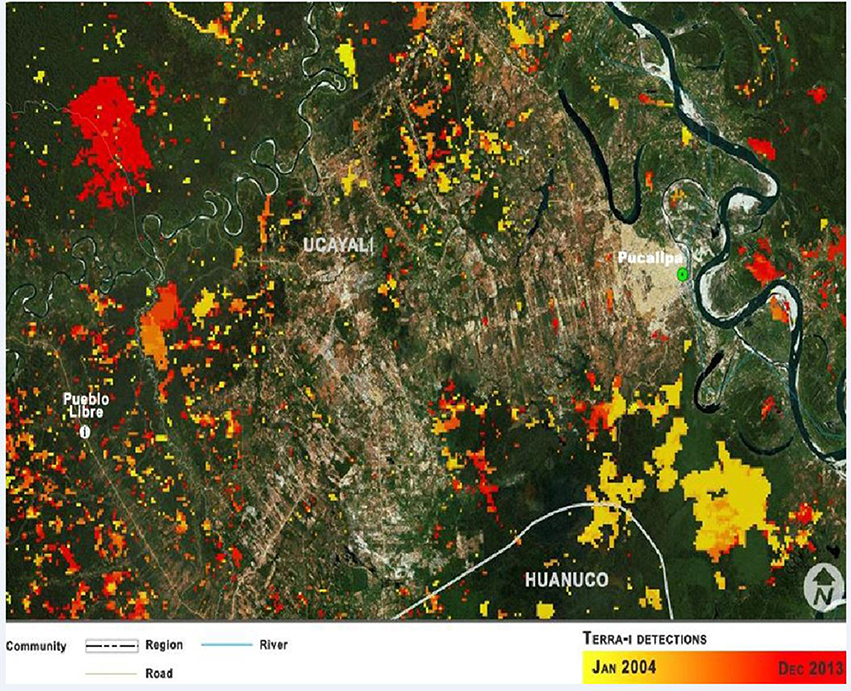
Figure 2. Location of the mestizo village Pueblo Libre, in the Ucayali department, Peruvian Amazon. The map indicates the changes in land use from January 2004 to December 2013 in a color gradient, according to Terra-i (CIAT, 2015). The map also shows the deforestation that occurred before 2004 along the Federico Basadre highway and surrounding roadsides. Source: Terra-i; map prepared by Paula Paz.
Methods
This study took as a baseline a list of 30 “wild” food plant species belonging to 18 botanical families reported by Cruz-Garcia and Vael (2017) in a study conducted in Pueblo Libre. This list was constructed during focus group elicitations using local names of plants in Spanish, which is the main language spoken in the village. The villagers use the term planta silvestre alimenticia (wild food plant) as a cultural domain; for instance, they identified the species of the list based on their local knowledge. According to Borgatti (1999), a cultural domain is a set of items that belong to the same category corresponding to a socio-cultural group. In this way, the informants explained that “wild” food plants are plants from the forest or transplanted from the forest near the house, as well as plants that grow in the home garden or agricultural field and do not require much care (Cruz-Garcia and Vael, 2017). The botanical identification of plant species was conducted by a local taxonomist from the Universidad Intercultural de la Amazonía Peruana in Pucallpa. Herbarium specimens of most identified species are on repository in the Herbarium of the University.
Fieldwork was conducted from August to September 2014 in Pueblo Libre, and encompassed three focus group discussion sessions in order to cover information for all 30 species. Focus groups were conducted with men and women ranging from 23 to 52 years of age, identified by the villagers themselves as knowledgeable about contemporarily gathered food plants. Each session lasted about 2 h and consisted of five to six informants, following Bernard's recommendations on the number of participants per focus group (Bernard, 2002). During focus groups mestizo farmers were asked if people in the village manage each “wild” food plant species from the list (“do you transplant the species?” “do you water it,” “do you protect it?” “do you fertilize it?” “do you prune it?” “do you weed it?” and “do you mulch it?”). They were also asked the origin of planting material when a species was transplanted (“from where did you bring the planting material?”); the motivations for transplanting (“why do you transplant the species?” if the species was not transplanted “why you did not transplant it?”); and perceived abundance (“the species was more abundant, less abundant, or had the same abundance as 10 years ago?”). This study was carried out in accordance with the recommendations of the guidelines of the International Society of Ethnobiology Code of Ethics. Participation was voluntary and all participants provided oral, informed consent, in accordance with the Code of Ethics.
Focus groups have been well-established in the area of development studies (Rifkin and Pridmore, 2001; Desai and Potter, 2006; Chambers, 2012). Focus groups are particularly useful when a study focuses on the everyday use of culture for a particular socio-cultural group (Morgan and Kreuger, 1993). In this study, focus groups did not aim to analyze how many people carry out a management practice, nor to quantify the proportion of farmers that share a particular opinion. Rather, the implementation of focus groups aimed at providing an exploratory assessment of practices and perceptions, and the results obtained were neither suitable for estimations of statistical significance or accuracy, nor for generalization to larger populations (Kumar, 2002; Chambers, 2012). The preference for this approach was driven by the lack of information on “wild” food plant management in mestizo villages and, consequently is exploratory. In this way, the present study paves the road for future in-depth and quantitative studies on this topic.
The list of “wild” food plants was compared to the Global Compendium of Weeds (HEAR, 2007). Growth form and endemicity were determined for each species with literature review (USDA, 2015a; United States Department of Agriculture (USDA), 2015b). The species were classified based on their associated management practices in different types of non-agricultural management forms following González-Insuasti and Caballero (2007) and Casas et al. (1996). A data matrix was prepared in Microsoft Excel where each row was a species and each column a variable (management practices, motivations, perceived abundance, weed category, growth form, and endemicity). Data analysis consisted of comparing frequencies of species in relation to the studied variables, and was conducted with Microsoft Excel.
Results
Management Practices and Movement of Planting Material across the Landscape
The results of the focus group discussions showed that more than two-thirds (67%) of the “wild” food plant species have associated management practices (Figure 3). Almost all plants that have associated management practices have been transplanted (18 out of 20 species, see Table 1), with the exception of Mauritia flexuosa and Manilkara bidentata that are only pruned. Sixty-one percent of all transplanted species did not include additional management practices. Among those species that included additional management practices were Artocarpus altilis, Passiflora quadrangularis, and Solanum sessiliflorum var. sessiliflorum that were transplanted and weeded; Bactris gasipaes and Myrciaria dubia that were transplanted, watered, fertilized and weeded; Theobroma cacao that was transplanted, fertilized, weeded and pruned; and Matisia cordata, which is a native fruit tree that not only was transplanted, watered, fertilized and weeded, but also protected from chickens by placing a little fence around it. None of the species was mulched. Contrarily, species like Attalea phalerata and Oenocarpus bataua did not present any management practices (Table 2).
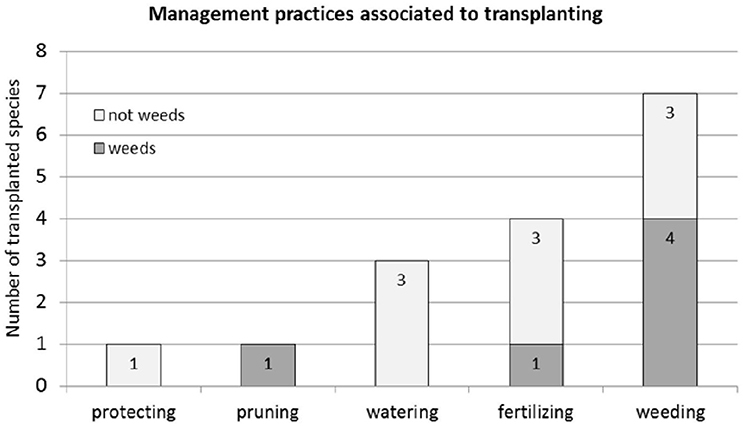
Figure 3. Additional management practices associated with transplanting “wild” food plant species (n = 18).
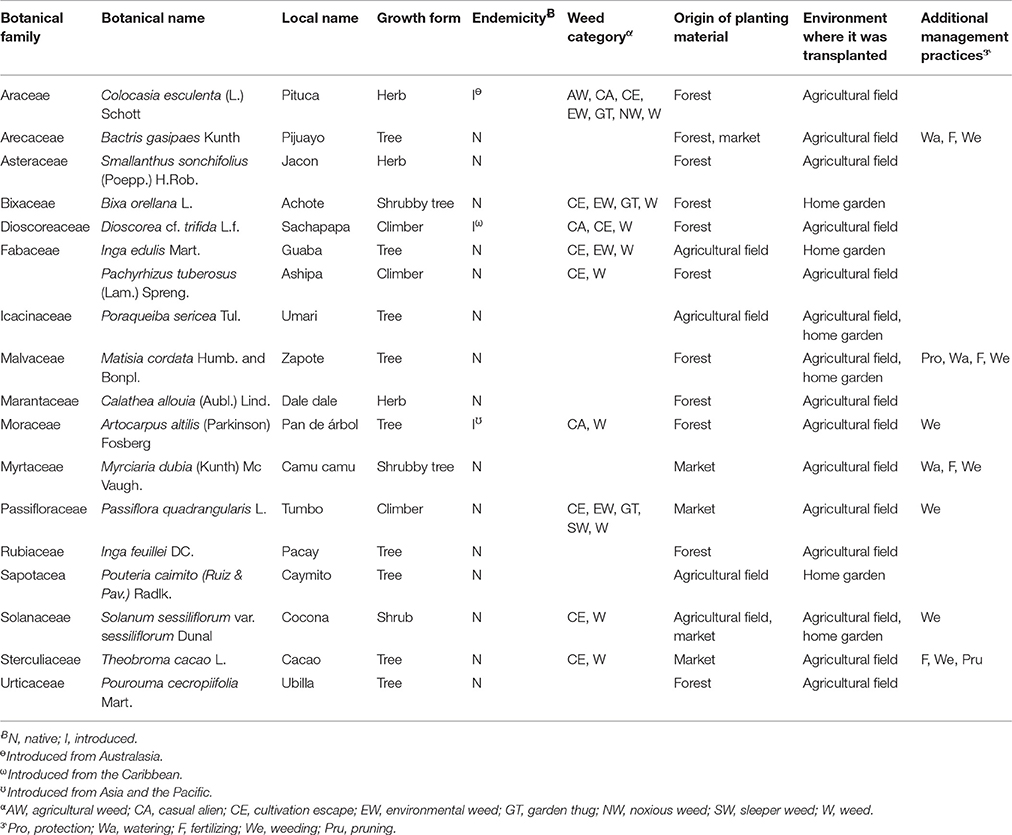
Table 1. List of transplanted “wild” food plant species indicating botanical name, local name, growth form, endemicity, weed category, origin of planting material, environment where the species was transplanted, and additional management practices (n = 18).
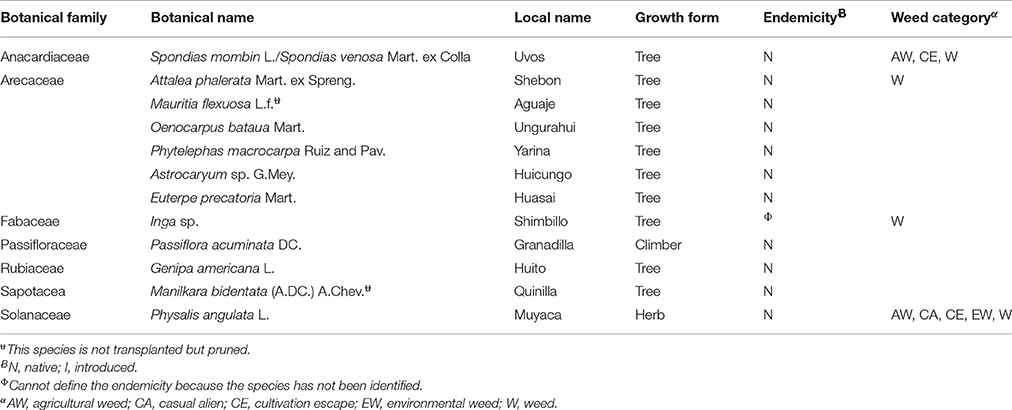
Table 2. List of “wild” food plant species that are not transplanted indicating botanical name, local name, growth form, endemicity, and weed category (n = 12).
More than half (57%) of the “wild” food plant species, including 76% of the species that have associated management practices, have been classified as weeds by scientific literature. In addition, weeds such as the introduced tree A. altilis, native climber P. quadrangularis and native shrub S. sessiliflorum var. sessiliflorum are weeded by the local population.
The most important origin of planting material was the forest, with 61% of transplanted species, followed by the market (28%) and agricultural fields (22%). The most common environments where species were transplanted were the agricultural field (83% of transplanted species), followed by the home garden (33%). This was reflected in the flows of transplanted material. For instance, the strongest flow of transplanted material was from forest to farm, encompassing 11 species; followed by market to agricultural field with five species, and agricultural field to home garden with four. Flows also included species that were transplanted from one place to another within the same environment (Figure 4).
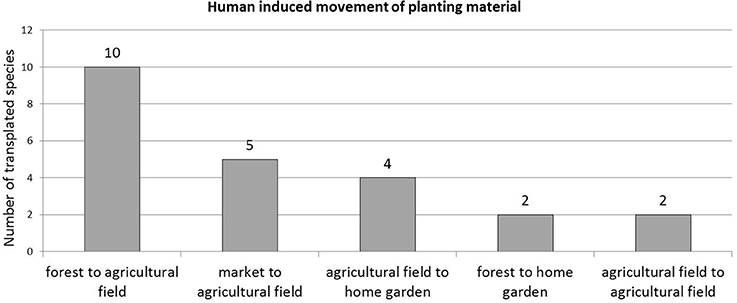
Figure 4. Human-induced movement of “wild” food planting material in the farming landscape (n = 18).
Non-agricultural Management Forms for “Wild” Food Plants
A total of four species are managed in situ and 18 species are managed ex situ (Table 3). From the species managed in situ only two presented associated management practices (pruning, i.e., M. flexuosa and M. bidentata), whereas the other two were tolerated. The tolerated species were Passiflora acuminata and Physalis angulata, which are spared within agricultural fields and are not taken out when weeding the crop. Species cultivated ex situ include these transplanted from one environment to other (e.g., from forest to farm), within the same environment (e.g., from farm to farm) and from the market to an environment (e.g., from market to farm). All management forms, including incipient management in situ and management ex situ, include species classified as weeds by scientific literature.
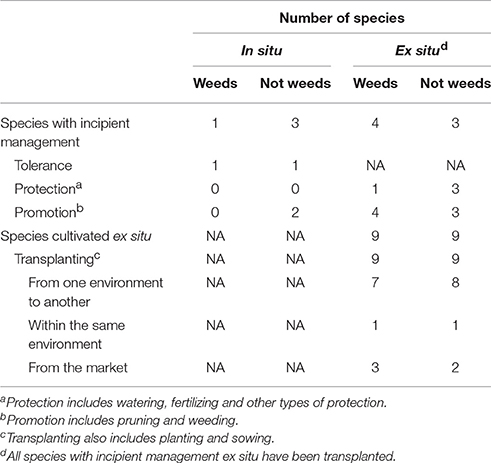
Table 3. Number of “wild” food plant species according to different non-agricultural management forms (n = 22).
Farmers' Perceived Abundance and Motivations to Transplant “Wild” Food Plant Species
Farmers perceived that the abundance of 77% of the “wild” food plants decreased during the last decade, whereas the abundance of 20% of the species increased, and the abundance of Pourouma cecropiifolia remained the same, because, as farmers clarified, they did not cut it down given that it takes too long to grow. Instead, they transplanted P. cecropiifolia from the forest to their farms to have it nearer to their homes. Fifty-seven percent of the plants that are less abundant, have been transplanted, two are pruned but not transplanted, and the remaining are not managed. Four species that are more abundant, have been transplanted and two have not. The most important motivation for transplanting a “wild” food plant was to have it close to home, mentioned for 72% of the species that have been transplanted (n = 18). Conversely, the most important reason for not transplanting a “wild” plant was the long time it takes to grow (cannot make immediate use of it), stated for 67% of the species that have not been transplanted (n = 12; Tables 4, 5).
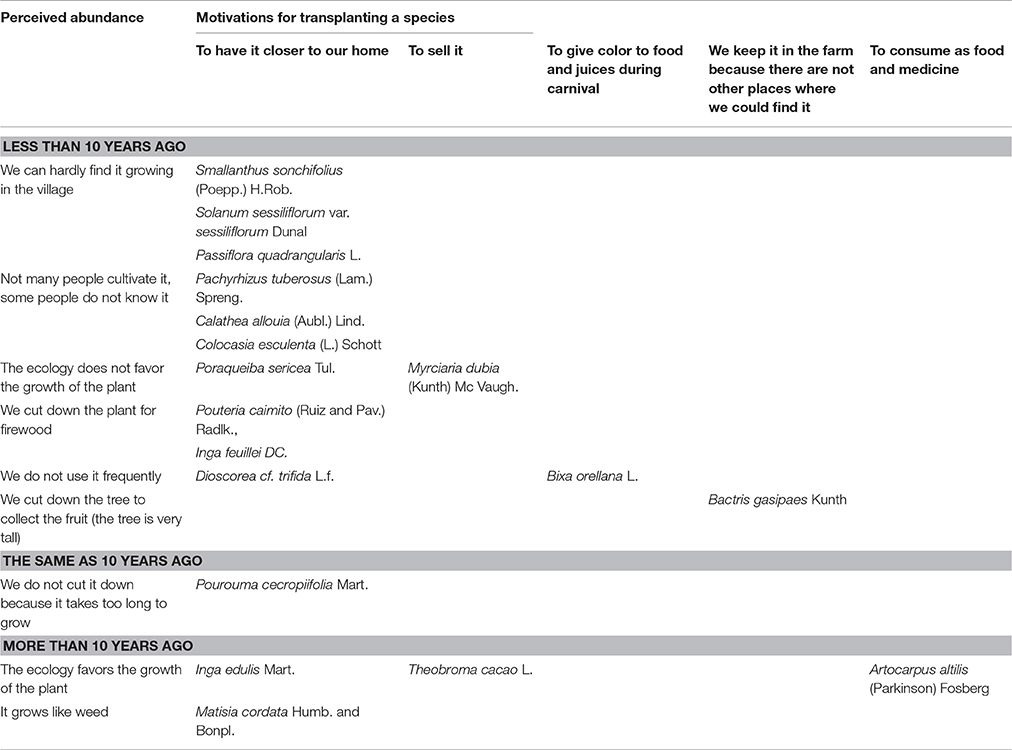
Table 4. Farmers' motivations for transplanting a “wild” food plant species in relation to perceived abundance (n = 18).
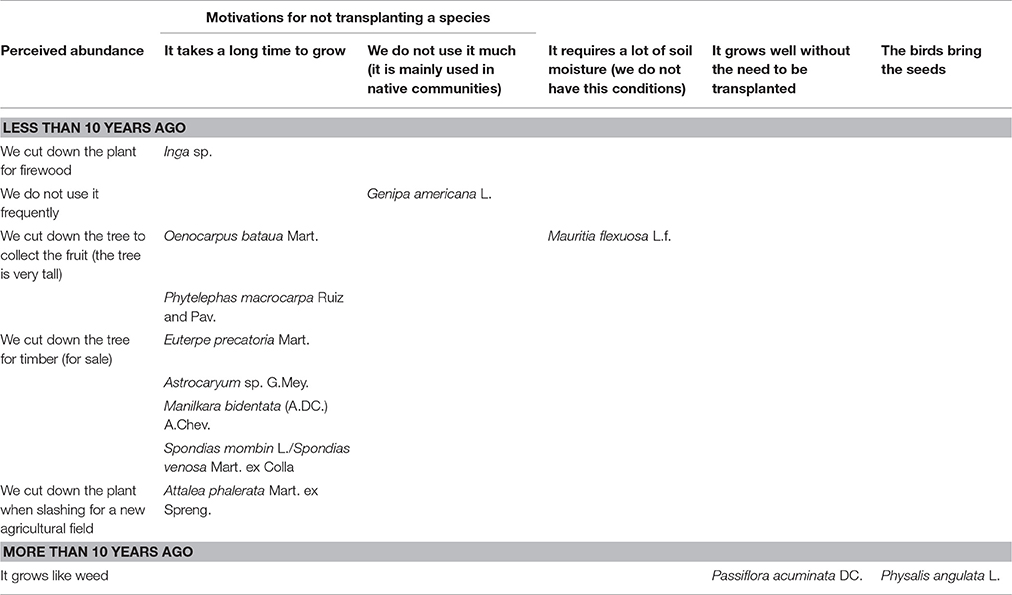
Table 5. Farmers' motivations for not transplanting a “wild” food plant species in relation to perceived abundance (n = 12).
Villagers explained that transplanting is a local strategy to ensure the presence of species that are less abundant than a decade ago, because nowadays they are unusually cultivated in the village, used as firewood, have difficulties to grow due to their ecological requirements, or are decreasing in availability, because they are not frequently used. For example, farmers explained that they have transplanted Bixa orellana from the forests to their home gardens to give color to food and juices during carnival. They highlighted that they have transplanted B. gasipaes in their farms, because they cannot find it in other environments within the village anymore, given that they cut down the trees, which were very tall, to collect the fruits. Likewise, Pouteria caimito and Inga feuillei have been transplanted near the house to be used as firewood. Regarding the “wild” food plants that are more abundant than 10 years ago, villagers explained that although some species are characterized by their favorable ecological requirements, they have been transplanted to have them close to home. For example, Inga edulis has been transplanted from farms to home gardens, and A. altilis has been transplanted from forests to farms (Table 4).
There are species like P. acuminata and P. angulata that are perceived to be more abundant than 10 years ago, and have not been transplanted by villagers because, as they stated, these plants grow like “weeds” and, in the case of P. angulata, the birds bring the seeds so there is no need to propagate them. Conversely, there are species that despite their decreased abundance, have not been transplanted. Farmers explained that their abundance has decreased, because they cut down the trees to sell the timber, use the firewood, collect the fruit, or make a new agricultural field. They argued that these species, with the exemption of Genipa americana and M. flexuosa, have not been transplanted because it takes them too much time to grow. They also indicated that G. americana is not commonly used by the mestizos but mainly utilized by indigenous communities. Although they cut down the trees of M. flexuosa to collect the fruits, they explained that they cannot transplant it because it requires a lot of soil moisture, which they do not have in the village (Table 5).
Groups of “Wild” Food Plants According to Management and Local Perceptions
The “wild” food plants gathered in Pueblo Libre (n = 30) could be classified into six groups, according to management forms and local perceptions (mainly in relation to species abundance). Four groups present species classified as weeds by scientific literature (Figure 5).
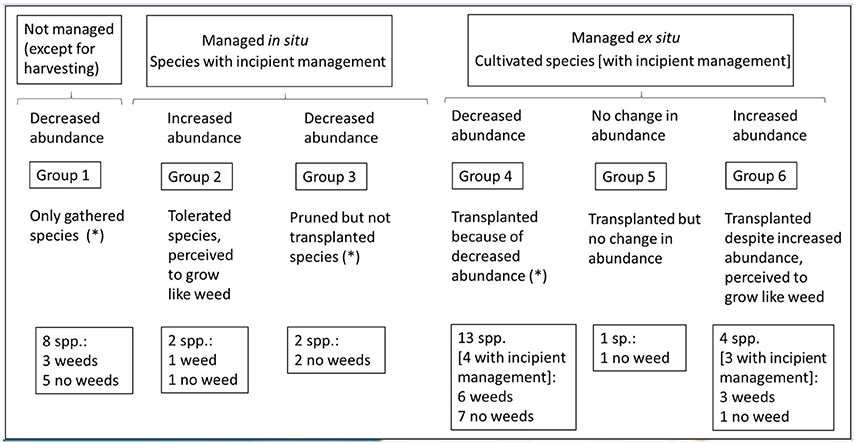
Figure 5. Groups of “wild” food plant species according to management and local perceptions (n = 30).
Group 1 includes species that are not managed although famers perceived that they have decreased in abundance. Informants reported that the abundance of all species from this group, except for G. americana, has decreased because they cut down the trees to sell timber, to collect fruits, for firewood or when slashing for a new agricultural field; but they do not transplant them because it takes them too much time to grow. For example, farmers cut down the trees of O. bataua and Phytelephas macrocarpa to collect the fruits. Farmers mentioned that although G. americana decreased in numbers, it is not transplanted, because they do not use it frequently. The other species that belong to this group, are Inga sp., Euterpe precatoria, Astrocaryum sp., Spondias mombin/Spondias venosa, and A. phalerata. This group includes three species classified as weeds (I. sp., S. mombin /S. venosa and A. phalerata). All species in this group are native.
Group 2 encompasses species with non-agricultural incipient management, managed in situ and with a perceived increased abundance. This group includes the two species that are tolerated (P. acuminata and P. angulata), which farmers mentioned as growing like “weeds.” P. angulata has also been classified as weed by the literature. Both species in this group are native.
Group 3 includes species with non-agricultural incipient management, managed in situ and with a perceived decreased abundance. This group includes the two species that are pruned but not transplanted (M. flexuosa and M. bidentata). Informants stated that they have decreased in abundance because they cut down the trees to collect the fruit or to sell the timber; and they do not transplant them because M. flexuosa requires a lot of soil moisture and M. bidentata takes a long time to grow. None of these species has been classified as weed. Both species in this group are native.
Group 4 comprises species cultivated ex situ that farmers perceived having a decreased abundance. The reasons for decreasing in numbers include explanations related to little use or knowledge about the uses of the plant, unfavorable ecological conditions, and cutting down the trees for firewood or to collect the fruit. The main motivations to transplant them are related to use-value (having the plant close to home, to sell it and to give color to juices). The species belonging to this group are B. orellana, M. dubia, Smallanthus sonchifolius, P. caimito, I. feuillei, Poraqueiba sericea, S. sessiliflorum var. sessiliflorum, P. quadrangularis, Dioscorea cf. trifida, Pachyrhizus tuberosus, C. allouia, Colocasia esculenta, and B. gasipaes. All the species from this group have been transplanted, and four of them presented additional incipient management practices (M. dubia, S. sessiliflorum var. sessiliflorum, P. quadrangularis, and B. gasipaes). Six species from this group have been classified as weeds (B. orellana, S. sessiliflorum var. sessiliflorum, P. quadrangularis, D. cf. trifida, P. tuberosus, and C. esculenta). C. esculenta and D. cf. trifida are the only species that have been introduced to the region (from Australasia and the Caribbean respectively).
Group 5 includes a native species, i.e., P. cecropiifolia, which is cultivated ex situ and has a perceived unchanged abundance. In contrast to the species from the previous groups, as mentioned before, this is the only plant that farmers do not cut down because it takes a long time to grow. This plant has not been classified as weed.
Group 6 encompasses species that farmers cultivated ex situ although they perceived an increased abundance. They explained that the abundance of these plants increased because of favorable ecological conditions. The motivations for transplanting these species are related to use-value. The species that belong to this group are T. cacao, I. edulis, A. altilis, and M. cordata. All species, except I. edulis, presented additional incipient management practices. M. cordata is the only species that has not been classified as weed. All species are native, except for A. altilis, which has been introduced from Asia and the Pacific.
Discussion
General Reflections on Management of “Wild” Food Plants
This study, which was based on focus group discussions conducted with mestizo farmers, showed that more than two-thirds of the “wild” food plant species in the study village are managed, and most of them have been transplanted ex situ. Certainly, humans have intervened in the populations of wild species throughout the world, for example, changing the diversity and density of food plants by transplanting or introducing new species to an environment (Wiersum, 1997a; Daly, 2014; Parrotta et al., 2015). The use and management of wild food plants have already been reported in Latin America, for example, among the Nahua and Mixtec communities (Casas et al., 1996) and in Santa Maria Tecomavaca (González-Insuasti and Caballero, 2007) in Mexico, among the Mapuche in Chile (Daly, 2014), in the Monte region in Argentina (Ladio and Lozada, 2009), in the Bolivian Amazon (Reyes García et al., 2005; Thomas, 2012) and Andes (Vandebroek and Sanca, 2007), in Pernambuco in Brazil (Cruz et al., 2013), and in Cuba (Volpato and Godinez, 2007). Furthermore, the management of wild food plants has also been documented in Africa, for example in the Collines region in Central Benin (Avohou et al., 2012) and Central Shewa of Ethiopia (Feyssa et al., 2012); and Asia, for example in Northeastern Thailand (Cruz-Garcia and Price, 2014b), among others.
Defour and Wilson (1994) reported a total of 131 wild food plant species for all Amazonia, which mainly include trees and palms, consumed by indigenous communities. Certainly, the study of management and domestication of food plants in the Amazonia has largely focused on fruit trees (Clement and Villachica, 1994; Clement, 2006; Miller and Nair, 2006); and the documentation of food plant management in this region has mainly focused on indigenous communities (e.g., Reyes-García et al., 2006; Thomas, 2012), but not on mestizo villages. The Botanical Garden—Arboretum El Huayo (JBAH) from the Universidad Nacional de la Amazonía Peruana (National University of the Peruvian Amazon) located in Iquitos, Peru, possess a collection of 46 species of edible fruit plants reported in surrounding communities (Freyre, 2003). Taking into account these numbers, the amount of food plants documented by using focus group discussions for Pueblo Libre village (n = 30 species) might seem low. However, compared to other studies conducted in the Amazonia, the number of “wild” food plants documented for Pueblo Libre is higher than those reported by Reyes-García et al. (2006) for the Tsimane' communities of the Bolivian Amazon (n = 18), and very similar to the number of food species reported by Vásquez and Peláez (2015) for the inhabitants of Berlín village in Bagua Grande, Peru (n = 29).
Ucayali has been highly affected by high deforestation rates (Smith et al., 2006; Miranda et al., 2014; Porro et al., 2015). Land use change, deforestation, and unsustainable management of natural resources are the main drivers of loss of biodiversity and, consequently, decrease of wild food plants (Daly, 2014) and other underutilized species. This decline affects the food security of rural families directly (e.g., availability and accessibility to food) and indirectly (e.g., modifying the ecological conditions where species grow) (Van Noordwijk et al., 2014; Agarwal et al., 2015). Management practices: (a) allow rural families to have sufficient food and nutritional diversity by increasing the availability and access to wild food plants and other underutilized species, and (b) favors the conservation of these species in highly intervened anthropogenic environments when forests decline (i.e., by bringing planting material from forests to farms and home gardens). Certainly, the dietary diversity and nutritional quality of the diet of hundreds of millions of people in the world rely on the consumption of wild food plants and other underutilized edible plants (Grivetti and Ogle, 2000; Johns and Eyzaguirre, 2006; Heywood, 2011).
There are two points of recommendations for future research in relation to management and domestication of food plants by mestizo villagers (or immigrant communities). The first is to understand the historical process of knowledge acquisition about Amazonian flora by mestizos from indigenous peoples and/or by experimentation. The second is to assess the historical management of these species by mestizo villages. For instance, are the practices described in this study new ones that emerged as a response to deforestation and loss of biodiversity? Or are they practices that existed among mestizo villages before deforestation rates started to increase, and were adjusted to the new settings (i.e., intensifying human induced movement of biodiversity from forests to more intervened anthropogenic environments)? Such additional studies would shed light on understanding historical processes of management and domestication by non-indigenous societies.
Human Induced Movement of “Wild” Food Plants across the Farming Landscape as a Management Strategy
The findings of this study provide evidence that mestizo farmers transplant “wild” food plant species across different environments within the farming landscape. This emphasizes the spatial and seasonal complementarity of different anthropogenic ecosystems for food provision, which is particularly important for families living in the forest-agriculture interface. The importance of this complementarity for the food security of rural families has been also reported in other regions of the world, for example in Northeastern Thailand (Cruz-Garcia and Price, 2014a) and West Java in Indonesia (Abdoellah and Marten, 1986). Certainly, Frison et al. (2011) emphasized that a major basis of dietary diversity is the diversity of environments within farming landscapes, which was also illustrated by this study in Ucayali.
The key role played by forests, agricultural fields, and home gardens for provisioning food to rural households has been highlighted by various studies around the world. For instance, the importance of forests for food security has largely been acknowledged in scientific literature (e.g., Arnold et al., 2011; FAO, 2011; Sunderland et al., 2014); as well as the importance of multiple habitats, ranging from terrestrial to aquatic ones, that diversified agricultural fields encompass to facilitate the growth of multiple food species (e.g., Altieri and Anderson, 1987; Cruz-Garcia et al., 2016); and the importance of home gardens not only as source of food but also for processes of domestication and biodiversity conservation (e.g., Miller and Nair, 2006; Galluzzi et al., 2010; Cruz-Garcia and Struik, 2015; Freedman and Stoilova, 2015). Additionally, it is important to highlight that not only the farming landscape but also the market plays a key role in providing planting material, which has also been reported in other regions of the world (e.g., Ruiz-Pérez et al., 2004).
Ogle and Grivetti (1985) in their botanical dietary paradox highlighted that wild food plant gathering increasingly occurs in more intervened anthropogenic environments rather than less disturbed environments, i.e., forests, in the face of deforestation and land use change. However, the results of this study go beyond the botanical dietary paradox, showcasing that humans have a more active role in ensuring the availability of food species, for instance supporting the flows of planting material from less to more intervened environments. This was observed in the following findings: (a) the human induced movement of “wild” food plants across the landscape occurred for all transplanted species in the study site; (b) the most common source of planting material was the forest (for 61% of transplanted species); (c) informants constantly highlighted the need to have “wild” food plant species closer to home (for 72% of transplanted species), and (d) villagers usually bring planting material from less to more intervened environments, i.e., from forests to agricultural fields, from forests to home gardens, from agricultural fields to home gardens. Certainly Zohary (2004) highlighted that human selection leads to the dispersal of plant populations toward more disturbed anthropogenic ecosystems. The movement of planting material is necessary in order to ensure the availability of wild food plants and other underutilized species given scenarios of increasing deforestation. Undoubtedly, the importance of agricultural fields and home gardens as recipients of plant genetic material has to be taken into account in agricultural interventions.
Reflections on the Definitions of “Wild” Food Plants
The use of cultural domains, capturing the emic or native's point of view, is a starting point of ethnobotanical research (Borgatti, 1999) that can be very useful for the study of wild food plants and domestication, given that different socio-cultural groups perceive and conceive the world differently according to their own social, historical, cultural and environmental conditions and experiences (Brosius et al., 1986). Through comparing the list of native “wild” food plants from Pueblo Libre with the results of Clement (1999) and Levis et al. (2017), it was possible to see that 10 species that were categorized as “wild” by villagers, were domesticated before European contact, whereas nine were either semi-domesticated or presented incipient domestication by that time (see Table 6). Clearly, villagers and scientists have different classifications for “wild” and “domesticated,” as previously highlighted by Michon and De Foresta (1997) and Clement (1999).
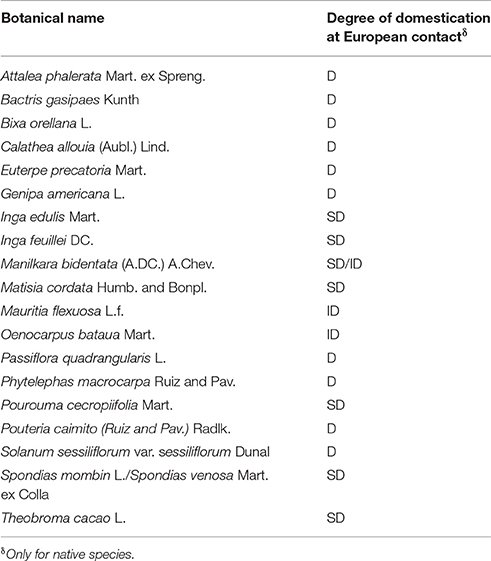
Table 6. “Wild” food plant species that were reported as domesticated (D), semi-domesticated (SD), and with incipiently domesticated populations (ID) in Amazonia at European contact (Clement, 1999; Levis et al., 2017).
Interestingly, some species of “wild” food plants that were domesticated before European contact, are nowadays treated as “wild,” as emphasized in the mestizo's emic conceptualization of “wild” food plants where “wilderness” is associated with species that “do not require much care” (Cruz-Garcia and Vael, 2017). This was expected, given that a significant part of the crop genetic resources was lost—alongside traditional knowledge—with the eradication of 90–95% of the Amazonian population after European contact (Clement, 1999), and consequently it is still possible to see that domesticated species are nowadays dominant across the Amazon forest (Levis et al., 2017). Certainly, the domesticated species reported by this study, did not exhibit management patterns in an intensity expected for domesticates, for instance fully depending on human intervention for survival (González-Insuasti and Caballero, 2007). For example, although B. gasipaes was domesticated during pre-Colombian times (Clement and Urpí, 1987), it was listed as a “wild” species by the informants. They transplant B. gasipaes to their agricultural fields, where they collect the chonta (inner core of the stem) and fruits for food (Cruz-Garcia and Vael, 2017), bringing the planting material not only from the market but also from the forest. This species is watered, fertilized, and weeded, but neither pruned nor protected.
Conversely, T. cacao was semi-domesticated before European contact, but nowadays most populations are domesticated. Families from Pueblo Libre, however, not only manage the species in the agricultural field with the objective to sell the seeds in the market, but also gather and consume the fruits of non-transplanted, non-managed individuals of T. cacao that are growing in the forest (Cruz-Garcia and Vael, 2017). Another example is I. edulis, which was semi-domesticated before European contact, but nowadays is domesticated throughout Amazonia for its fruits and wood (Clement et al., 2010). Undoubtedly, González-Insuasti and Caballero (2007) explained that a species could be managed differently in different places and times, and Cruz-Garcia and Price (2014b) stated that domestication is a locally differentiated concept and process. Clement et al. (2010) also explained that domestication as a process occurs at population level (not at species level); therefore it is not correct to say that a species is a domesticate unless all its wild populations are extinct, which is unusual. Instead they recommended to affirm that a species “exhibits domesticated populations” that, for this study in Ucayali, might be the case of T. cacao and I. edulis.
It would be interesting if future studies evaluate the management and motivations to manage wild food plants for the different indigenous communities in Ucayali, for instance, to assess how do they conceptualize the cultural domain of “wild food plant,” which species do they classify as part of this cultural domain, and which management practices and forms do they exhibit. Do they classify the species of this study as wild or as domesticated? Do they recognize different species as wild food plants? Do they manage those species that have been domesticated before European contact (according to Clement, 1999; Levis et al., 2017) with higher intensity than mestizos?
Reflections on the Definitions of “Weeds”
Another term that deserves further discussion is “weed,” which has historically been defined as “a plant growing where it is not wanted” (Mortimer, 1990), or, contrarily as “a plant whose virtues are yet to be discovered” (Perrins et al., 1992). Although most agronomists and agricultural extension officers recommend their eradication to favor crop production, 89% of the most aggressive weeds in the world are edible (Rapoport et al., 1995) and several of them are highly nutritional (Duke, 1992). Certainly, the consumption of weeds has been reported throughout the world (Grivetti et al., 1987; Duke, 1992; Tanji and Nassif, 1995; Casas et al., 1996; Díaz-Betancourt et al., 1999; Pieroni, 1999; Turner et al., 2011; Cruz-Garcia and Price, 2012). Likewise, the results of this study showed that more than half of food plant species reported in the village have been classified as weeds by scientific literature (HEAR, 2007). In addition, 76% of these “weeds” have been transplanted by the villagers in order to increase their availability and to have them closer to home. Indeed, “weeds” exhibited different management forms: (a) incipient management in situ (toleration) despite their increased abundance (group 2), (b) managed ex situ due to their decreased abundance (group 4), and (c) managed ex situ despite their increased abundance (group 6). Therefore, none of both definitions of “weed” adjusts to the species documented by this study: they are tolerated or managed in the place where they are growing, and their virtues have already been discovered (i.e., as food).
It might also sound somehow contradictory that species that have been classified as domesticates by scientists—as some food plants reported by this study—have also been classified as weeds by other scientists. However, on the one hand, this can be explained by the fact that weed classifications are controversial, given that the way scientists define weeds and classify species as weeds depend on their disciplinary background (Perrins et al., 1992). On the other hand, crops might become weeds in other parts of the world, depending on the biological characteristics of the species, the environment where it is growing and the associated management practices (Harlan, 1965).
Farmers' Motivations to Manage “Wild” Food Plants
Mestizo farmers' motivations to manage “wild” food plant species affect management practices and forms. Management, including human induced movement of planting material across the landscape, influences: (a) artificial selection and, consequently, evolution; and (b) the availability and distribution of species across the farming landscape and, consequently, their ecology. The results of this study, based on focus group discussions, showed that farmers' motivations belong to two major groups: motivations related to cultural importance, particularly in relation to use-value, and motivations related to perceived abundance. These will be discussed in the following paragraphs.
Human management practices usually focus on the preservation of culturally important species. Although, as part of this study we did not directly ask informants about the cultural value of each species, culture was captured through the study of motivations to manage “wild” food plants, particularly in relation to species use-value. This is reflected in the following: (a) villagers transplanted almost three-fourths of the species to have them close to home, which facilitates their availability and access for frequent use; (b) informants also emphasized the use of species as food and medicine, or their use for coloring food and drinks, and for firewood; (c) the most common reason for not transplanting a species, accounting for more than two-thirds of the species that have not been transplanted, was the long time it takes to grow, what implies that villagers cannot make immediate use of it. This is aligned to the findings of various authors that have documented the influence of cultural importance, including use-value, on farmers' incentives to manage a species (e.g., Casas et al., 1996; Guijt, 1998; Ogle, 2001; González-Insuasti et al., 2008, 2011; Blancas et al., 2013).
Human management practices also focused on the preservation of species perceived to decrease in abundance. For instance, more than half of the species that were perceived to be less abundant, were transplanted, plus two species that were not transplanted but presented incipient management practices. Likewise, it has been reported by other studies that decreased abundance (or perceptions of a species rarity) are directly related with a farmers' decisions to manage a species (e.g., Stoffle et al., 1990; Cunningham, 1993; Price, 1997; Blancas et al., 2013, 2014).
Although the results of this study showed that human adaptation to rapidly changing environments (i.e., under scenarios of deforestation) promotes the management of food species and their movement across the farming landscape, the findings also reported the presence of destructive harvesting practices. For instance, villagers cut down trees to collect fruits (four species), for timber (four species), for firewood (two species) and when slashing and burning (one species), accounting for almost half of the species that have decreased in abundance. Whereas some of these species are transplanted to home gardens (but cut down in forests and agricultural fields), others are not transplanted because of—as villagers mentioned—the long time it takes them to grow. This is not surprising in the deforestation frontier, where multiple stakeholders, including villagers themselves, contribute to forest loss. For instance, it has been documented that timber production is facilitated by legal and illegal channels of trade, which are more available for mestizo upland villages in Ucayali than for mestizos living in lowlands or indigenous communities (Porro et al., 2015). The presence of unsustainable management practices related to useful wild plants has also been reported in other regions (e.g., González-Insuasti et al., 2011; Blancas et al., 2013). In these cases, it is necessary to promote sustainable management practices that favor the conservation of important wild food plants and other underutilized species. For example, local organizations are teaching the communities situated in the buffer zone of the Cordillera Azul National Park (Peruvian Amazonia) sustainable practices to collect the fruits of M. flexuosa without cutting down the trees (CIMA, 2012). This kind of initiatives is very important and necessary for ensuring the conservation of valuable food species, particularly those whose populations are decreasing in the Amazon deforestation frontier. Furthermore, it is necessary to implement interventions that simultaneously aim at environmental conservation, social equity, and sustainable livelihoods (Porro et al., 2015).
Limitations of this Study
The main constraint of this study was that the results obtained were not suitable to generalization for larger populations, given that the data collection was based on focus group discussions. For instance, a limitation of participatory methods, like focus groups, is that their outputs do not allow statistical estimations (Kumar, 2002; Chambers, 2012). However, focus groups capture group perspectives and provide reliable information on topics that are significant for marginalized communities (Bernard, 2002). It is important to emphasize that the results from this study should not constitute the endpoint for decision-support processes, but should rather constitute the exploratory and hypothesis generating stage of future quantitative scientific projects. In this way, this study opens the possibilities for future in-depth and quantitative studies on management perspectives, for example aimed at understanding how motivations to manage wild food plants are shared within and among socio-cultural groups. In addition, future studies should take into consideration issues related to gender, education, wealth and geographical location, which affect the ways people interact with plants (and, consequently, people's motivations to manage them). This is certainly necessary, given that the study of people's motivations contributes to understanding the reasons behind management decisions and, ultimately, domestication.
Conclusions
The results of this study on management practices, including the human-induced movement of “wild” food plant species across the forest-agriculture landscape, and the motivations that mestizo farmers have to manage them, magnify the already acknowledged importance of “wild” food plant management, including weed management, for ensuring the availability of “wild” food species. In this way, management practices see to rural families having sufficient food and nutritional diversity, and to these species being preserved in highly intervened anthropogenic environments when forests decline.
This research, conducted in a village in the Peruvian Amazon, provides empirical evidence that mestizo farmers transplant food plant species across different environments within the farming landscape, bringing planting material from less to more disturbed anthropogenic environments. This emphasizes the spatial and seasonal complementarity of different anthropogenic ecosystems for food provision, which is particularly important for families living in the forest-agriculture interface. The findings of this study showed that farmers' motivations to manage “wild” food plant species are related to their cultural importance, particularly in relation to use-value, and to their perceived abundance. However, the presence of unsustainable management practices was also reported, therefore initiatives that support the conservation and sustainable use of these species are increasingly needed in the region.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I am very grateful to Maria Elena Chuspe Zans from the Herbarium of the Universidad Intercultural de la Amazonía Peruana who did the taxonomical identification of the plants. I would like to thank Lore Vael and Madeleine Hancco who collected the data, José Sanchez-Choy who coordinated data collection in Ucayali, and Paula Paz from the Terra-i team from CIAT who prepared the map. I am grateful to Hub Peters who revised the English of the manuscript. I would like to extend my thanks to the villagers from Pueblo Libre who participated in the study. Funding was partly provided by the International Center for Tropical Agriculture (CIAT). This work was associated with the ‘Attaining Sustainable Services from Ecosystems using Trade-off Scenarios’ project (ASSETS; http://espa-assets.org/; NE-J002267-1), and partly funded with support from the United Kingdom's Ecosystem Services for Poverty Alleviation (ESPA) programme. ESPA receives its funding from the Department for International Development (DFID), the Economic and Social Research Council (ESRC) and the Natural Environment Research Council (NERC).
References
Abdoellah, O. S., and Marten, G. G. (1986). “The complementary roles of homegardens, upland fields, and rice fields for meeting nutritional needs in West Java,” in Traditional Agriculture in Southeast Asia. A Human Ecology Perspective, ed G. E. Marten (London: Westview Press), 293–325.
Agarwal, B., Jamnadass, R., Kleinschmit, D., McMullin, S., Mansourian, S., Neufeldt, H., et al. (2015). “Introduction. Forests, trees and landscapes for food security and nutrition,” in Forests, Trees and Landscapes for Food Security and Nutrition. A global Assessment Report, Vol 33. IUFRO World Series, eds B. Vira, C. Wildburger, and S. Mansourian (Vienna: CPF and IUFRO), 13–24.
Altieri, M. A., and Anderson, M. K. (1987). Peasant agriculture and the conservation of crop and wild plant resources. Conserv. Biol. 1, 49–58. doi: 10.1111/j.1523-1739.1987.tb00008.x
Alvarez, N. L., and Naughton-Treves, L. (2003). Linking national agrarian policy to deforestation in the Peruvian Amazon: a case study of Tambopata, 1986–1997. Ambio 32, 269–274. doi: 10.1579/0044-7447-32.4.269
Arnold, M., Powell, B., Shanley, P., and Sunderland, T. C. H. (2011). EDITORIAL: Forests, Biodiversity and Food Security (International Forestry Review). Int. Forest. Rev. 13, 259–264. doi: 10.1505/146554811798293962
Avohou, H. T., Vodouhe, R. S., Dansi, A., Bellon, M., and Kpeki, B. (2012). Ethnobotanical factors influencing the use and management of wild edible plants in agricultural environments in Benin. Ethnobot. Res. Appl. 10, 571–592. doi: 10.17348/era.10.0.571-592
Balemie, K., and Kebebew, F. (2006). Ethnobotanical study of wild edible plants in Derashe and Kucha Districts, South Ethiopia. J. Ethnobiol. Ethnomed. 2:53. doi: 10.1186/1746-4269-2-53
Bernard, H. R. (2002). Research Methods in Anthropology. Qualitative and Quantitative Approaches. Walnut Creek, CA; London; New Delhi: Altamira Press.
Blancas, J., Casas, A., Pérez-Salicrup, D., Caballero, J., and Vega, E. (2013). Ecological and socio-cultural factors influencing plant management in Náhuatl communities of the Tehuacán Valley, Mexico. J. Ethnobiol. Ethnomed. 9:39. doi: 10.1186/1746-4269-9-39
Blancas, J., Pérez-Salicrup, D., and Casas, A. (2014). Evaluando la incertidumbre en la disponibilidad de recursos vegetales. Gaia Sc. 8, 137–160. doi: 10.21707/gs.v8i2.22419
Borgatti, S. (1999). “Elicitation techniques for cultural domain analysis,” in Enhanced Ethnographic Methods: Audiovisual Techniques, Focussed Group Interviews and Elicitation Techniques, eds J. J. Schensul, M D. L. Compte, B. K. Nastasi, and S. P. Borgatti (Walnut Creek, CA: Altamira Press), 115–151.
Brosius, J. P., Lovelace, G. W., and Marten, G. G. (1986). “Ethnoecology: an approach to understanding traditional agricultural knowledge.” in Traditional Agriculture in Southeast Asia. A Human Ecology Perspective, ed G. E. Marten (London: Westview Press), 187–198.
Casas, A., Del Carmen Vázquez, M., Viveros, J. L., and Caballero, J. (1996). Plant management among the Nahua and the Mixtec in the Balsas River Basin, Mexico: an ethnobotanical approach to the study of plant domestication. Hum. Ecol. 24, 455–478. doi: 10.1007/BF02168862
CIAT (2015). Terra-i. An Eye on Habitat Change. International Center for Tropical Agriculture (CIAT). Available online at: http://www.terra-i.org/terra-i.html
CIMA (2012). Revalorización e importancia del aguaje en la zona de amortiguamiento del Parque Nacional Cordillera Azul. Centro de Conservación, Investigación y Manejo de Areas Naturales (CIMA), Lima.
Clement, C. R. (1999). 1492 and the loss of Amazonian crop genetic resources. I. The relation between domestication and human population decline. Econ. Bot. 53, 188–202. doi: 10.1007/BF02866498
Clement, C. R. (2006). “Fruit trees and the transition to food production in Amazonia,” in Time and Complexity in Historical Ecology: Studies in the Neotropical Lowlands, eds W. Balée and C. Erickson (New York, NY: Columbia University Press), 165–185.
Clement, C. R., and Urpí, J. E. M. (1987). Pejibaye palm (Bactris gasipaes, Arecaceae): multi-use potential for the lowland humid tropics. Econ. Bot. 41, 302–311. doi: 10.1007/BF02858977
Clement, C. R., and Villachica, J. H. (1994). “Amazonian fruits and nuts: potential for domestication in various agroecosystems,” in Tropical Trees: The Potential for Domestication and the Rebuilding of Forest Resources, eds A. C. Newton and R. R. B. Leakey (London: H.M.S.O.), 230–238.
Clement, C. R., de Cristo-Araújo, M., Coppens D'Eeckenbrugge, G., Alves Pereira, A., and Picanço-Rodrigues, D. (2010). Origin and domestication of native Amazonian crops. Diversity 2, 72–106. doi: 10.3390/d2010072
Cossío, R., Menton, M., Cronkleton, P., and Larson, A. (2014). Community Forest Management in the Peruvian Amazon: A Literature Review, Vol 136. Bogor: CIFOR.
Cruz, M. P., Peroni, N., and Albuquerque, U. P. (2013). Knowledge, use and management of native wild edible plants from a seasonal dry forest (NE, Brazil). J. Ethnobiol. Ethnomed. 9:79. doi: 10.1186/1746-4269-9-79
Cruz-Garcia, G. S., and Ertug, F. (2014). “Introduction: wild food plants in the present and past,” in Plants and People. Choices and Diversity through Time, Vol 1, Early Agricultural Remnants and Technical Heritage (EARTH): 8,000 years of resilience and innovation, eds A. Chevalier, E. Marinova, and L. Pe-a-Chocarro (Oxford, Philadelphia, PA: Oxbow Books), 211–215.
Cruz-Garcia, G. S., and Price, L. L. (2012). Weeds as important vegetables for farmers. Acta Societatis Botanicorum Poloniae 814, 397–403. doi: 10.5586/asbp.2012.047
Cruz-Garcia, G. S., and Price, L. L. (2014a). Gathering of wild food plants in anthropogenic environments across the seasons: implications for poor and vulnerable farm households. Ecol. Food Nutr. 53, 1–24. doi: 10.1080/03670244.2013.808631
Cruz-Garcia, G. S., and Price, L. L. (2014b). Human-induced movement of wild food plant biodiversity across farming systems is essential to ensure their availability. J. Ethnobiol. 34, 68–83. doi: 10.2993/0278-0771-34.1.68
Cruz-Garcia, G. S., and Struik, P. C. (2015). Spatial and seasonal diversity of wild food plants in home gardens of Northeast Thailand. Econ. Bot. 69, 99–113. doi: 10.1007/s12231-015-9309-8
Cruz-Garcia, G. S., and Vael, L. (2017). “El manejo de plantas silvestres alimenticias en escenarios de deforestación, ilustrado por una comunidad mestiza de la Amazonía Peruana,” in Domesticación en el Continente Americano. Volumen 2. Investigación y Manejo Sustentable de Recursos Genéticos en el Nuevo Mundo. A. Casas, A., Torres-Guevara J, and F. Parra (Morelia; Lima: Universidad Nacional Autonoma de México and Universidad Nacional Agraria La Molina), 327–344.
Cruz-Garcia, G. S., Struik, P. C., and Johnson, D. E. (2016). Wild harvest: distribution and diversity of wild food plants in rice ecosystems of Northeast Thailand. NJAS Wageningen J. Life Sci. 78, 1–11. doi: 10.1016/j.njas.2015.12.003
Cunningham, A. B. (1993). African medicinal plants: setting priorities at the interface between conservation and primary healthcare. Working Paper 1, People and Plants Online. People and Plants Initiative, UNESCO.
Daly, B. A. (2014). Narrating Changing Foodways: Wild Edible Plant Knowledge and Traditional Food Systems in Mapuche Lands of the Andean Temperate Forests, Chile. Ph.D. thesis, University of British Columbia, Vancouver.
Defour, D. L., and Wilson, W. M. (1994). “Characteristics of “wild” plant foods used by indigenous populations in Amazonia,” in Eating on the Wild Side, ed N. Etkin (Tucson, AZ; London: University of Arizona Press), 114–142.
Díaz-Betancourt, M., Ghermandi, L., Ladio, A., López-Moreno, I. R., Raffaele, E., and Rapoport, E. H. (1999). Weeds as a source for human consumption. A comparison between tropical and temperate Latin America. Rev. Biol. Trop. 47, 329–338.
FAO (2011). Forests for Improved Nutrition an Food Security. Rome: Food and Agriculture Organization.
Feyssa, D. H., Njoka, J. T., Asfaw, Z., and Nyangito, M. M. (2012). Comparative analysis of indigenous knowledge on use and management of wild edible plants: the case of central East Shewa of Ethiopia. Ethnobot. Res. Appl. 10, 287–304. doi: 10.17348/era.10.0.287-304
Freedman, B., and Stoilova, T. (2015). Home gardens bolstered by diversity from indigenous, semi-domesticated and wild vegetable species for improved health and income (Sub- Saharan Africa). Acta Horticult. 1102. International Society for Horticultural Science.
Freyre, H. V. (2003). Plantas de Importancia Económica y Ecológica en el Jardín Botánico-Arboretum el Huayo, Iquitos, Perú. Folia Amazónica. Available online at: http://revistas.iiap.org.pe/index.php/foliaamazonica/
Frison, E. A., Cherfas, J., and Hodgkin, T. (2011). Agricultural biodiversity is essential for a sustainable improvement in food and nutrition security. Sustainability 3, 238–253. doi: 10.3390/su3010238
Fujisaka, S., Escobar, G., and Veneklaas, E. J. (2000). Weedy fields and forests: interactions between land use and the composition of plant communities in the Peruvian Amazon. Agric. Ecosyst. Environ. 78, 175–186. doi: 10.1016/S0167-8809(99)00122-X
Galarza, E., and La Serna, K. (2005). “Las Concesiones Forestales en el Perú: Cómo Hacerlas Sostenibles,” in La política forestal en la Amazonía y Los Andes Estudios de caso, Bolivia, Ecuador y Perú (Lima, OH: Universidad del Pacífico).
Galluzzi, G., Eyzaguirre, P. B., and Negri, V. (2010). Home gardens: neglected hotspots of agro-biodiversity and cultural diversity. Biodivers. Conserv. 19, 3635–3654. doi: 10.1007/s10531-010-9919-5
González-Insuasti, M. S., and Caballero, J. (2007). Managing plant resources: how intensive can it be? Hum. Ecol. 35, 303–314. doi: 10.1007/s10745-006-9063-8
González-Insuasti, M. S., Casas, A., Méndez-Ramírez, I., Martorell, C., and Caballero, J. (2011). Intra-cultural differences in the importance of plant resources and their impact on management intensification in the Tehuacán Valley, Mexico. Hum. Ecol. 39, 191–202. doi: 10.1007/s10745-010-9369-4
González-Insuasti, M. S., Martorell, C., and Caballero, J. (2008). Factors that influence the intensity of non-agricultural management of plant resources. Agrofor. Syst. 74, 1–15. doi: 10.1007/s10457-008-9148-z
Grivetti, L. E., and Ogle, B. M. (2000). Value of traditional foods in meeting macro- and micronutrient needs: the wild plant connection. Nutr. Res. Rev. 13, 31–46. doi: 10.1079/095442200108728990
Grivetti, L. E., Frentzel, C. J., Ginsberg, K. E., Howell, K. L., and Ogle, B. M. (1987). “Bush foods and edible weeds of agriculture: perspectives on dietary use of wild plants in Africa, their role in maintaining human nutritional status and implications for agricultural development,” in Health and Disease in Tropical Africa. Geographical and Medical Viewpoints, ed R. Akhtar (London: Harwood), 51–81.
Guijt, I. (1998). “Valuing wild plants with economics and participatory methods: an overview of the Hidden Harvest methodology,” in Plants for Food and Medicine, eds H. D. V. Prendergast, N. L. Etkin, D. R. Harris, and P. J. Houghton (Kew: Royal Botanical Gardens Kew), 223–235.
Harlan, J. (1975). Crops and Man. Madison, WI: American Society of Agronomy and Crop Science Society of America.
Harlan, J. R. (1965). The possible role of weed races in the evolution of cultivated plants. Euphytica 14, 173–176. doi: 10.1007/BF00038984
Harris, D. R. (1989). “The evolutionary continuum of people-plant interactions,” in Foraging and Farming: The Evolution of Plant Exploitation, eds D. R. Harris and G. C. Hillman (London: Unwin Hyman), 11–26.
HEAR (2007). Global Compendium of Weeds Hawaiian Ecosystems at Risk Project (HEAR). Avaialble online at: http://www.hear.org/gcw/ (Accessed December 8, 2010).
Heywood, V. (1999). Use and Potential of Wild Plants in Farm Households, Vol. 15. FAO Farm Systems Management Series. Rome: FAO.
Heywood, V. (2011). Ethnopharmacology, food production, nutrition and biodiversity conservation: towards a sustainable future for indigenous peoples. J. Ethnopharmacol. 137, 1–15. doi: 10.1016/j.jep.2011.05.027
INEI (2011a). Perú: Perfil de la Pobreza por Departamentos, 2001–2010. Lima: Instituto Nacional de Estadística e Informática (INEI).
INEI (2011b). Producto Bruto Interno por Departamentos, 2001–2010. Lima: Instituto Nacional de Estadística e Informática (INEI).
INEI (2011c). Ucayali: Compendio Estadístico Departamental, 2010. Lima: Instituto Nacional de Estadística e Informática (INEI).
Johns, T. (2007). “Agrobiodiversity, diet and human health,” in Managing Biodiversity in Agricultural Ecosystems, eds D. I. Jarvis, C. Padoch, and D. Cooper (New York, NY: Columbia University Press), 382–406.
Johns, T., and Eyzaguirre, P. B. (2006). Linking biodiversity, diet and health in policy and practice. Proc. Nutr. Soc. 65, 182–189. doi: 10.1079/PNS2006494
Khoury, C. K., Achicanoy, H. A., Bjorkman, A. D., Navarro-Racines, C., Guarino, L., Flores-Palacios, X., et al. (2016). Origins of food crops connect countries worldwide. Proc. R. Soc. B Biol. Sci. 283:20160792. doi: 10.1098/rspb.2016.0792
Khoury, C. K., Bjorkman, A. D., Dempewolf, H., Ramirez-Villegas, J., Guarino, L., Jarvis, A., et al. (2014). Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. U.S.A. 111, 4001–4006. doi: 10.1073/pnas.1313490111
Kosaka, Y., Takeda, S., Prixar, S., Sithirajvongsa, S., and Xaydala, K. (2006a). Species composition, distribution and management of trees in rice paddy fields in central Lao, P. D. R. Agrofor. Syst. 67, 1–17. doi: 10.1007/s10457-005-1109-1
Kosaka, Y., Takeda, S., Sithirajvongsa, S., and Xaydala, K. (2006b). Plant diversity in paddy fields in relation to agricultural practices in Savannakhet Province, Laos. Econ. Bot. 60, 49–61. doi: 10.1663/0013-0001(2006)60[49:PDIPFI]2.0.CO;2
Kumar, S. (2002). Methods for Community Participation: A Complete Guide for Practitioners. New Delhi: Vistaar Publications.
Ladio, A. H., and Lozada, M. (2009). Human ecology, ethnobotany and traditional practices in rural populations inhabiting the Monte region: resilience and ecological knowledge. J. Arid Environ. 73, 222–227. doi: 10.1016/j.jaridenv.2008.02.006
Levis, C., Costa, F. R., Bongers, F., Pe-a-Claros, M., Clement, C. R., Junqueira, A. B., et al. (2017). Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 355, 925–931. doi: 10.1126/science.aal0157
Lojka, B., Lojkova, J., Banout, J., Polesny, Z., and Preininger, D. (2008). Performance of an improved fallow system in the Peruvian Amazon—modelling approach. Agrofor. Syst. 72, 27–39. doi: 10.1007/s10457-007-9079-0
Lu, G. M. M. (2009). The Corrientes River Case: Indigenous People's Mobilization in Response to Oil Development in the Peruvian Amazon. Oregon: University of Oregon.
Michon, G., and De Foresta, H. (1997). Agroforests: pre-domestication of forest trees or true domestication of forest ecosystems? Netherlands J. Agric. Sci. 45, 451–462.
Miller, R., and Nair, P. (2006). Indigenous agroforestry systems in Amazonia: from prehistory to today. Agrofor. Syst. 66, 151–164. doi: 10.1007/s.10457-005-6074-1
MINEM-GOREU (2007). Caracterización del Departamento de Ucayali, Con Fines de Ordenamiento territorial. Pucallpa: Ministerio de Energía y Minas (MINEM) y Gobierno Regional de Ucayali (GOREU).
Miranda, J. J., Corral, L., Blackman, A., Asner, G., and Lima, E. (2014). Effects of Protected Areas on Forest Cover Change and Local Communities: Evidence from the Peruvian Amazon. Washington, DC: Inter-American Development Bank.
Morgan, D. L., and Kreuger, R. A. (1993). “When to use focus groups and why,” in Successful Focus Groups, ed D. L. Morgan (London: Sage), 3–19.
Mortimer, A. (1990). The Biology of Weeds in Weed Control Handbook. Oxford: Principales Blackwel Scientific publications.
Ogle, B. M. (2001). Wild Vegetables and Micronutrient Nutrition. Studies on the Significance of Wild Vegetables in Women's Diets in Vietnam. Uppsala: Uppsala University.
Ogle, B. M., and Grivetti, L. E. (1985). Legacy of the chameleon: edible wild plants in the kingdom of Swaziland, Southern Africa. A cultural, ecological, nutritional study. Part III - Cultural and ecological analysis. Ecol. Food Nutr. 17, 31–40. doi: 10.1080/03670244.1985.9990880
Oliveira, P. J., Asner, G. P., Knapp, D. E., Almeyda, A., Galván-Gildemeister, R., Keene, S., et al. (2007). Land-use allocation protects the Peruvian Amazon. Science 317, 1233–1236. doi: 10.1126/science.1146324
Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., and Simons, A. (2009). Psidium Guajava. Agroforestry Database: A Tree Reference and Selection Guide Version 4.0. Retrieved 18 November 2013. Available online at: http://www.worldagroforestry.org/treedb2/AFTPDFS/Psidium_guajava.pdf
Parrotta, J. A., de Pryck, J. D., Obiri, B. D., Padoch, C., Powell, B., Sandbrook, C., et al. (2015). “The historical, environmental and socio-economic context of forests and tree-based systems for food security and nutrition,” in Forests, Trees and Landscapes for Food Security and Nutrition. A Global Assessment Report, Vol. 33, IUFRO World Series, eds B. Vira, C. Wildburger, and S. Mansourian (Vienna: CPF; IUFRO), 51–86.
Perrins, J., Williamson, M., and Fitter, A. (1992). A survey of differing views of weed classification: implications for regulation of introductions. Biol. Conserv. 60, 47–56. doi: 10.1016/0006-3207(92)90798-R
Pieroni, A. (1999). Gathered wild food plants in the upper valley of the Serchio River (Garfagnana), Central Italy. Econ. Bot. 53, 327–341. doi: 10.1007/BF02866645
Pimentel, L. V., Rengifo, E. B., and Portomisnski, R. L. (2004). The Great Encyclopedia of the Ucayali Region: Peruvian Amazon: Regional Identity. Lima: El Comercio.
Porro, R., Lopez-Feldman, A., and Vela-Alvarado, J. W. (2015). Forest use and agriculture in Ucayali, Peru: livelihood strategies, poverty and wealth in an Amazon frontier. For. Policy Econ. 51, 47–56. doi: 10.1016/j.forpol.2014.12.001
Price, L. L. (1997). Wild plant food in agricultural environments: a study of occurrence, management, and gathering rights in Northeast Thailand. Hum. Organ. 56, 209–221. doi: 10.17730/humo.56.2.6572033n03673640
Price, L., and Ogle, B. M. (2008). “Gathered indigenous vegetables in Mainland Southeast Asia: a gender asset,” in Gender and Natural Resource Management: Livelihoods, Mobility and Interventions, eds B. P. Resurreccion and R. Elmhirst (London: Earthscan), 213–242.
Ramos Delgado, N. (2009). Impacto de la Producción Forestal Maderable en la Economía de la Región Ucayali, Perú. Universidad Nacional Agraria La Molina, Lima (Perú). Escuela de Postgrado. Especialidad de Bosques y Gestión de Recursos Forestales.
Rapoport, E. H., Raffaele, E., Ghermandi, L., and Margutti, L. (1995). Edible weeds: a scarcely used resource. Bull. Ecol. Soc. Am. 76, 163–166.
Reyes García, V., Vadez, V., Huanca, T., Leonard, W., and Wilkie, D. (2005). Knowledge and consumption of wild plants: a comparative study in two Tsimane' villages in the Bolivian Amazon. Ethnobot. Res. Appl. 3, 201–207. doi: 10.17348/era.3.0.201-208
Reyes-García, V., Huanca, T., Vadez, V., Leonard, W., and Wilkie, D. (2006). Cultural, practical, and economic value of wild plants: a quantitative study in the Bolivian Amazon. Econ. Bot. 60, 62–74. doi: 10.1663/0013-0001(2006)60[62:CPAEVO]2.0.CO;2
Rifkin, S., and Pridmore, P. (2001). Partners in Planning: Information, Participation and Empowerment. London: Macmillan.
Ruiz-Pérez, M., Belcher, B., Achdiawan, R., Alexiades, M., Aubertin, C., Caballero, J., et al. (2004). Markets drive the specialization strategies of forest peoples. Ecol. Soc. 9:4.
Salisbury, D. S., and Fagan, C. (2013). Coca and conservation: cultivation, eradication, and trafficking in the Amazon borderlands. Geojournal 78, 41–60. doi: 10.1007/s10708-011-9430-x
Scoones, I., Melnyk, M., and Pretty, J. N. (1992). The Hidden Harvest: Wild Foods and Agricultural Systems: A Literature Review and Annotated Bibliography. London: International Institute for Environment and Development.
Sears, R. R., and Pinedo-Vasquez, M. (2011). Forest policy reform and the organization of logging in Peruvian Amazonia. Dev. Change 42, 609–631. doi: 10.1111/j.1467-7660.2011.01697.x
Smith, J., Colan, V., Sabogal, C., and Snook, L. (2006). Why policy reforms fail to improve logging practices: the role of governance and norms in Peru. For. Policy Econ. 8, 458–469. doi: 10.1016/j.forpol.2005.08.001
Stoffle, R. W., Halmo, D. B., Evans, M. J., and Olmsted, J. E. (1990). Calculating the cultural significance of American Indian plants: Paiute and Shoshone ethnobotany of Yucca Mountain Nevada. Am. Anthropol. 2, 416–432. doi: 10.1525/aa.1990.92.2.02a00100
Sunderland, T., Achdiawan, R., Angelsen, A., Babigumira, R., Ickowitz, A., Paumgarten, F., et al. (2014). Challenging perceptions about men, women and forest product use: a global comparative study. World Dev. 64, S56–S66. doi: 10.1016/j.worlddev.2014.03.003
Thomas, E. (2012). The impact of traditional lifestyle, provenance and contact history on plant use knowledge and management: a cross-cultural comparison of two small-scale societies from the Bolivian Amazon. Hum. Ecol. 40, 355–368. doi: 10.1007/s10745-012-9488-1
Turner, N. J., Łuczaj, Ł. J., Migliorini, P., Pieroni, A., Dreon, A. L., Sacchetti, L. E., et al. (2011). Edible and tended wild plants, traditional ecological knowledge and agroecology (critical reviews in plant sciences). CRC Crit. Rev. Plant Sci. 30, 198–225. doi: 10.1080/07352689.2011.554492
USDA (2015a). Germplasm Resources Information Network (GRIN). Beltsville, MD: United States Department of Agriculture (USDA); Agricultural Research Service. Available online at: http://www.ars-grin.gov/cgi-bin/npgs/html/taxgenform.pl?language=es (Accessed September, 2015).
USDA (2015b). Natural Resources Conservation Service. United States Department of Agriculture (USDA). Available online at: http://plants.usda.gov/java/ (Accessed September, 2015).
Vael, L. (2015). Ethnobotanical Study of the Plant Use in the Natural Landscape of Two Mestizo Communities in the Ucayali Region of the Peruvian Amazon. Ghent: Universiteit Ghent.
Van Noordwijk, M., Bayala, J., Hairiah, K., Lusiana, B., Muthuri, C., Khasanah, N., et al. (2014). “Agroforestry solutions for buffering climate variability and adapting to change,” in Climate Change Impact and Adaptation in Agricultural Systems, Vol. 5, eds J. Fuhrer and P. Gregory (Oxford; Boston, MA: CAB International), 216–232. doi: 10.1079/9781780642895.0216
Vandebroek, I., and Sanca, S. (2007). “Food medicines in the Bolivian Andes (Apillapampa, Cochabamba Department),” in Eating and Healing: Traditional Food as Medicine, eds A. Pieroni and L. L. Price (New York, NY: Food Products Press, an imprint of The Haworth Press, Inc.), 273–295.
Vásquez, M. G., and Peláez, F. P. (2015). Especies vegetales utilizadas por pobladores de Berlín, Bagua Grande (Amazonas, Perú) 2011-2012. Revista Rebiolest 2, 61–75. Available online at: http://www.revistas.unitru.edu.pe/index.php/ECCBB/article/view/754
Volpato, G., and Godinez, D. (2007). “Medicinal foods in Cuba: promoting health in the household,” in Eating and Healing: Traditional Food as Medicine, eds A. Pieroni and L. L. Price (New York, NY: Food Products Press, an imprint of The Haworth Press, Inc.), 213–236.
Wiersum, K. F. (1997a). From natural forest to tree crops, co-domestication of forests and tree species, an overview. Netherlands J. Agric. Sci. 45, 425–438.
Wiersum, K. F. (1997b). Indigenous exploitation and management of tropical forest resources: an evolutionary continuum in forest-people interactions. Agric. Ecosyst. Environ. 63, 1–16. doi: 10.1016/S0167-8809(96)01124-3
Keywords: Peru, domestication, transplanting, perceived abundance, wild food plant
Citation: Cruz-Garcia GS (2017) Management and Motivations to Manage “Wild” Food Plants. A Case Study in a Mestizo Village in the Amazon Deforestation Frontier. Front. Ecol. Evol. 5:127. doi: 10.3389/fevo.2017.00127
Received: 12 June 2017; Accepted: 28 September 2017;
Published: 26 October 2017.
Edited by:
Alejandro Casas, Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México, MexicoReviewed by:
Jay Howard Samek, Michigan State University, United StatesMilton Kanashiro, Embrapa Amazonia Oriental (Embrapa Easter Amazon), Brazil
Copyright © 2017 Cruz-Garcia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gisella S. Cruz-Garcia, Zy5zLmNydXpAY2dpYXIub3Jn
 Gisella S. Cruz-Garcia
Gisella S. Cruz-Garcia