- 1Department of Biology, College of Staten Island (CSI) CUNY, Staten Island, NY, United States
- 2Department of Life Sciences, Anglia Ruskin University, Cambridge, United Kingdom
There is increasing recognition of the importance of “positive interactions” among species in structuring communities. For seabirds, an important kind of positive interaction is the use of birds of the same species, birds of other species, and other marine predators such as cetaceans, seals and fishes as cues to the presence of prey. The process by which a single bird uses, say, a feeding flock of birds as a cue to the presence of prey is called “local enhancement” or “facilitation.” There are subtly different uses of each of these terms, but the issue we address here is the ubiquity of positive interactions between seabirds and other marine predators when foraging at sea, and whether as a result of their associations the feeding success, and therefore presumably the fitness, of individual seabirds is increased. If this contention is true, then it implies that conservation of any one species of seabird must take into consideration the status and possible conservation of those species that the focal species uses as a cue while foraging. For example, conservation of great shearwaters (Ardenna gravis), which often feed over tuna (e.g., Thunnus) schools, should take in to consideration conservation of tuna. Ecosystem management depends on understanding the importance of such processes; the loss of biodiversity, and the consequent threat to foraging success, may be a substantial threat to the stability of marine ecosystems.
Introduction
Seabirds benefit from positive interactions when foraging at sea; they use cues from other individuals, conspecifics or other taxa whether seabirds, fish or marine mammals, to detect food (Veit, 1999; Grünbaum and Veit, 2003; Silverman et al., 2004; Goyert et al., 2014; Thiebault et al., 2014a,b). This process is often called “local enhancement” (Thorpe, 1956); the same term may refer to birds cooperating in the herding of prey in addition to passively providing cues to the presence of prey. The term “facilitation” means cooperation among species in which only one of the species receives direct benefit (Stachowicz, 2001; Thiebault et al., 2016). It is unclear for most seabird aggregations whether one or more species participating in the interaction derive benefit, however there are important implications; detection of prey may be positively density dependent through local enhancement (Grünbaum and Veit, 2003). In addition to serving as cues to the presence of prey, some marine predators such as dolphins, seals, tunas and other fishes, drive prey to the surface so that these prey become more readily available to birds (“facilitation”; Harrison et al., 1991; International Council for the Exploration of the Seas, 2010; Thiebault et al., 2016). Hence marine predators modify local resource distributions, with implications for the evolution of coexisting species and their interdependence (Laland et al., 1999; Laland and Boogert, 2008).
Positive Interactions among Marine Predators
Mixed-species foraging associations influence the structure of avian communities since attractions among species lead to associations that themselves, in the aggregate, constitute communities (Veit, 1995; Goodale et al., 2010). However, seabirds have been described as occurring only in temporary feeding associations (Munn and Terborgh, 1979; Sridhar et al., 2009) and not influential at the population and community levels. We suggest otherwise. Because of the ubiquity of positive interactions (Stachowicz, 2001; Bruno et al., 2003), implications of associations among marine predators in the open ocean should be re-evaluated. Seabirds observed in foraging associations derive from all major Orders of marine birds (Procellariiformes, Pelecaniformes, Charadriiformes) and there are predictable interspecific associations in different habitats (Hoffman et al., 1981; Veit and Hunt, 1991; Veit, 1995). Our review here shows that interspecific foraging associations among seabirds and other top-level marine predators are an essential component of the life histories of these organisms influence the population growth of the constituent species, and therefore the community structure of the marine systems in which they live.
Most seabirds aggregate in groups. Murphy (1936) observed that in the Southern Ocean the procellariids are more frequently in mixed-species associations than apart from them. Among procellariids and other taxa of seabirds there are interdependencies that vary among species and ecosystems that appear to relate to differences in the foraging behavior, flight dynamics, diving depth and sensory ability, whether vision (Bretagnolle, 1993) or in the case of procellariids, olfaction (Hutchison and Wenzel, 1980; Nevitt et al., 1995; Nevitt, 2008).
The procellariids are unusual among birds in the extent to which they use olfaction to find prey. Nevitt et al. (1995) demonstrated experimentally that they can detect odor fields of DMS, a chemical signature of phytoplankton blooms. There is variation among the procellariids in sensitivity to DMS, related to flight dynamics and plumage coloration. The most sensitive species are smaller, more maneuverable, with darker plumage and more inclined to feed on crustaceans that graze phytoplankton (Nevitt, 2000; Nevitt et al., 2004; Nevitt and Bonadonna, 2005; van Buskirk and Nevitt, 2008; Savoca and Nevitt, 2014). The differing flight behavior and ability to locate patchy and ephemeral ocean blooms, means that some species are providing superior information on changing resource availability. They in turn may benefit from the presence of other species using differing foraging strategies, for example diving species that drive prey to the surface. Surface-feeding storm petrels (Hydrobatidae) or prions (Pachyptila) associate with cetaceans, but also with petrels (e.g., Procellaria) and shearwaters (e.g., Puffinus) which are capable of wing propelled dives to 10s of meters (Weimerskirch and Sagar, 1996). Sooty shearwaters (Ardenna grisea) combine flight efficiency with diving adaptations, typically diving to depths of 40–60 m, and documented to reach 70 m (Weimerskirch and Sagar, 1996). Even highly aerial species such as albatrosses make shallow wing-propelled dives; when pursuit diving light-mantled sooty albatrosses (Phoebetria palpebrata) reach 12 m (Prince et al., 1994). Thus, seabird species that each use different techniques for finding prey can enhance their success by benefitting from the successes of other species. The mixed-species associations of procellariids, and their associations with other predators such as marine mammals, are likely to be of critical importance in structuring the ecosystems in the Southern Ocean, South Atlantic and South Pacific.
Associations of aerial seabirds and diving species, whether birds, fish or mammals, are conspicuous worldwide and likely to include important positive interspecific associations (Evans, 1982; Au and Pitman, 1986; Camphuysen and Webb, 1999; Clua and Grosvalet, 2001; Davoren et al., 2010; Goyert et al., 2014; Boyd et al., 2016). The example of diving predators pushing bait balls of fodder fish to the surface is the most familiar of interspecific associations of seabirds in the open ocean (Cafaro et al., 2016). For some surface feeding species this is a critical source of food, particularly the highly aerial tropical seabird species such as sooty terns (Sterna fuscata) and great frigatebirds (Fregata minor) that are likely obligately dependent on tuna and dolphins for making prey available at the surface (Brewer and Hertel, 2007). The ubiquity of the associations suggests a net benefit to flock-joiners despite inevitable competition. We might quibble about “proving” such net benefit; it seems prudent at this point to accept that many species worldwide join feeding flocks and therefore the benefit of joining such flocks outweighs the potential disadvantage of interference competion and kleptoparasitism, which would appear to represent obstacles to success (e.g., Hoffman et al., 1981). Theoretically there is evidence such associations are beneficial to both diving species and surface feeding species. Lett et al. (2014) developed models which showed predators attacking successively both from above and from the side were most effective in disrupting schooling fish. Their results suggest that both surface-feeding species and diving species should have greater success when foraging together. Furthermore, a higher frequency of attacks, particularly if varied in direction in three-dimensional space, would prevent schooling prey from organizing themselves, and result in higher success rate among all predators (Lett et al., 2014; Thiebault et al., 2016).
Species with different sensory modalities, flight behavior or capacity to search for prey at depth, potentially complement each other in a search for patchy prey. Seabird species differ in their ability to find prey either directly or indirectly, by observing the actions of others (Harrison et al., 1991). These patterns are not unique to marine birds. Many marine predators that would appear to be competitors occur predictably in associations (Veit and Hunt, 1991; Veit, 1995) differ in depths achieved when diving, and modes of sensory perception (e.g., dolphins and tuna).
The associations observed among marine predators are often both ubiquitous and stable. The drivers generating the positive interactions between species result in a gradient of possible interactions (Bronstein, 1994; Stachowicz, 2001), with mutualism at one extreme and competition and a failure to tap resources at the other extreme. Associations of marine predators may not always be profitable, and negative interactions (competition, kleptoparasitism) may occur, as in terrestrial mixed-species foraging flocks of birds (Harrison and Whitehouse, 2011). Natural selection favoring interspecific interactions is likely to vary greatly through years, seasons and age, and depend on the presence or absence of particular taxa in foraging associations. Individual specialization has been documented in many seabird species, in which some individuals for example, forage in interspecific associations whereas others forage independently with implications for relative fitness as resources fluctuate (e.g., Wells et al., 2016). With more diffuse coevolution, there may be selection for facultative foraging associations in many circumstances, but the behavioral patterns observed, and the ubiquity of feeding associations among pelagic predators, indicate that strong selection is likely to be operating, and that there are fitness benefits.
Patterns and Variation in Seabird Associations
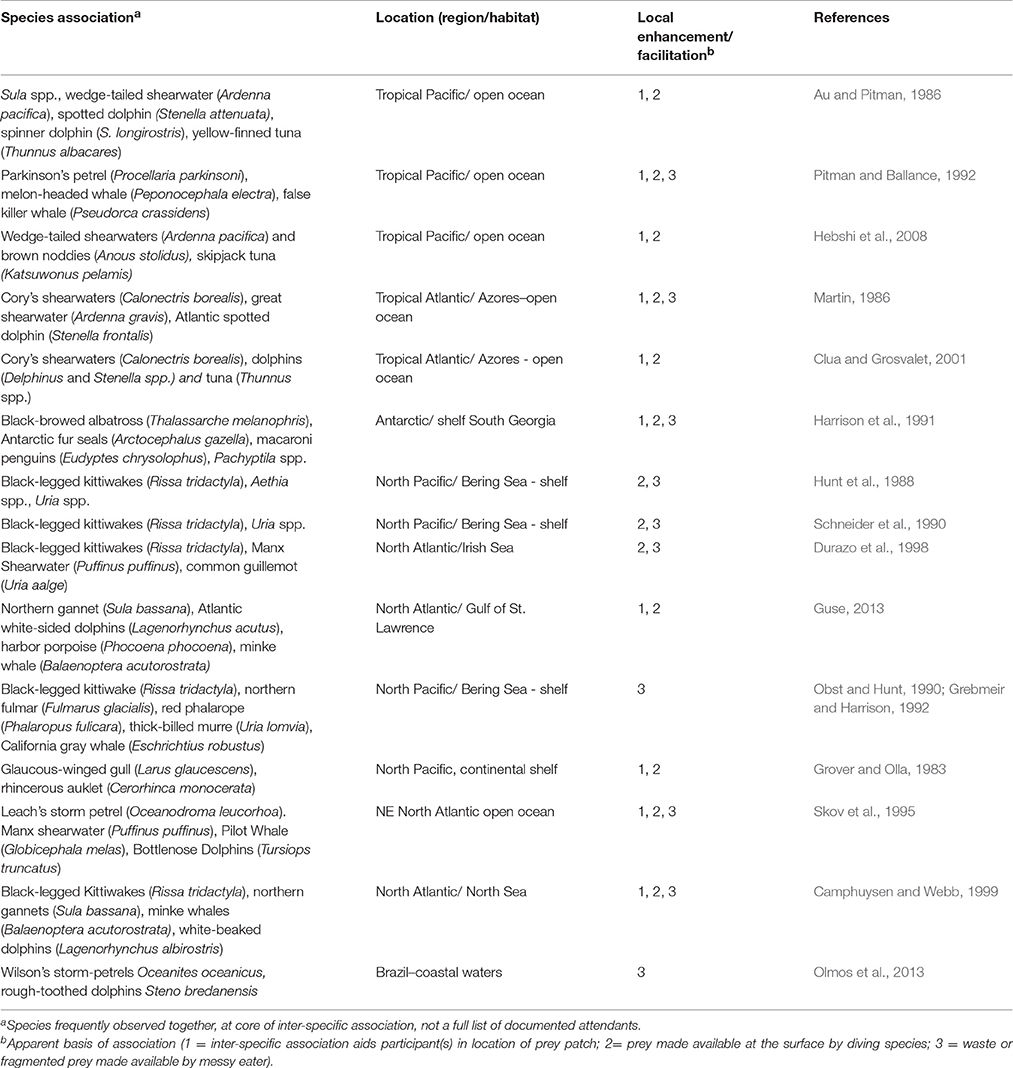
Seabirds occur in different types of feeding associations, reflecting prey availability and the nature of interspecific relationships (Table 1). These interspecific associations have been described and the likely benefits explored (e.g., Ashmole and Ashmole, 1967; Pierotti, 1988; Camphuysen and Webb, 1999). The patterns appear to vary between polar and tropical regions, and between nearshore and offshore habitat, reflecting the constraints on foraging in different marine environments and adaptive responses.
Tropical Oceans
In tropical oceans, seabirds have varied adaptive interspecific relationships with other seabird species, with predatory fish such as tuna, and with cetaceans (Au and Pitman, 1986; Hodges and Woehler, 1993; Ballance et al., 1997; Weimerskirch et al., 2004; LeCorre and Jaquemet, 2005; Vaughan et al., 2007; Thiebot and Weimerskirch, 2013). In the eastern tropical Pacific the “tuna-dolphin-seabird assemblage” is a conspicuous feature of the marine community, in which a large diversity of seabirds associate with yellowfin tuna (Thunnus albacares), spotted (Stenella attenuata), and spinner dolphins (S. longirostris) (Ballance et al., 2006). Breeding success and fitness of many aerial tropical species such as sooty tern, almost certainly depend on their association with tuna schools, which drive schooling baitfish to the surface where they can be accessed by the birds (Table 1). Changing oceanic climate seems likely to threaten to shift bigeye tuna (T. obesus) range to areas far removed from seabird colonies and thus threaten the foraging success of birds nesting in these colonies (Polovina et al., 2011).
The open ocean of the tropics may offer particular challenges for aerial predators; hydrographic features do not function to concentrate prey in the same way as on the continental shelf, and the spatial predictability of prey is lower than in high latitude waters (Bost et al., 2009; Assali et al., 2017). The capacity of aerial predators to see each other and interpret the behavior of conspecifics and other seabirds is potentially important in providing cues. The diversity of highly aerial tropical seabirds suggests that there may be an advantage to the efficient coverage of large distances to locate feeding events; the disadvantage is that many of these species are limited in their prey capture to the very surface of the sea. It is a reasonable hypothesis that for many species there is a high level of dependency on other species which function to drive prey to the surface (Ashmole and Ashmole, 1967; Au and Pitman, 1986; Ballance et al., 1997).
In the sometimes enormous and species-rich, mixed-species associations in tropical waters (Au and Pitman, 1986) the participants may differ as to benefits received, and indeed may not always benefit. Thiebot and Weimerskirch (2013) found most seabird species (48 of 71) did not associate with cetaceans, and those that did appeared to be in opportunitic associations, diffusely coevolved, rather than in true commensalisms, but the point we are making is that these positive associations, obligate or temporary, are likely to enhance fitness. Other studies have identified strong interspecific attraction between seabirds and cetaceans (Pitman and Ballance, 1992), and point to the difficulty in studying the behavior of marine predators at sea. Shearwaters have been observed joining non-feeding dolphins; once feeding, dolphin and tuna have been observed to drive bait fish into a dense ball and hold them near the surface where they were available to the birds (Martin, 1986). Some solitary tropical seabirds associate with predatory fish and dolphins but avoid large interspecific feeding frenzies. This is the case with tropicbirds, in which two of the three Pacific species avoid interspecific foraging flocks (Phaethon aethereus and P. rubricauda) and the third (P. lepturus) is only observed foraging in very small foraging flocks; these species plunge dive from considerable height (up to 40 m, through half that height in the case of P. lepturus), and on this basis Spear and Ainley (2005) attribute their solitary feeding to interference when in flocks.
Polar Seas
Species rich, persistent concentrations of top predators have been found associated with ecologically important ocean features such as the Antarctic Circumpolar Current (Santora and Veit, 2013) and South Georgia (Harrison et al., 1991; Silverman and Veit, 2001). The persistent association of multi-species flocks, each containing species with different foraging techniques, implies the importance of local enhancement to the component species of the flocks. Around South Georgia black-browed albatrosses are leaders in mixed-species flocks feeding on Antarctic krill (Harrison et al., 1991); they track the movements of fur seals (Arctocephalus gazella) and penguins, locating ephemeral patches of prey driven to the surface, and they in turn are followed by more than a dozen other seabirds including very large (giant petrels Macronectes spp.) and very small species (Wilson's storm-petrels Oceanites oceanicus).
Grünbaum and Veit (2003) found that, at South Georgia, albatross density had a higher impact on feeding rate than did prey density, indicating, first, the importance of local enhancement (albatrosses responding to albatrosses) and second, that at low densities of prey local enhancement may not be effective. Other evidence suggests that facilitation might be very important at high prey densities (Hunt et al., 1988; Lett et al., 2014). Schneider et al. (1990) identified the importance of the interaction between hydrography and local enhancement as the result of species associations; they found kittiwakes (Rissa spp.) feeding near auks (Uria spp.) on the dead and disoriented euphausiids accumulating in fine-scale convergences near a sub-surface feeding frenzy.
North Atlantic
Associations of seabirds, and seabirds with cetaceans, within European waters sometimes generate large aggregations such as northern gannets (Morus bassanus) and other seabirds with dolphins (Stenella), and Cory's shearwaters (Calonectris borealis) with migrating fin whales (Balaenoptera physalus) in the Bay of Biscay. These mixed-species associations are more common in some sea areas than others—for example gannet associations with marine mammals are more typical of offshore areas (Camphuysen and Webb, 1999; Camphuysen et al., 2012). Bellier et al. (2005) tested patterns of aggregation in gannets in the Bay of Biscay and found evidence for local enhancement. They found that aggregations formed primarily in areas of high gannet density, consistent with findings of Grünbaum and Veit (2003).
Gannets in the North Atlantic are strongly associated with other species which serve as facilitators, driving prey toward the surface. As in the tropics, tuna (Thunnus alalunga) have associated predators including dolphins and seabirds, including gannets (Rogan and Mackey, 2007). Gannets associate with cetaceans, particularly dolphins, in the productive waters of the Gulf of St. Lawrence; in an analysis of the relative importance of various drivers, cetacean abundance was most important, indicating local enhancement and facilitation is important for foraging gannets (Guse, 2013). As in the tropical Pacific and polar oceans, gannets foraging in North Atlantic waters have a hierarchical search pattern: they occupy physical environment defined by the ocean currents and oceanographic features such as hydrographic frontal systems, and then use local enhancement to detect prey patches (Bellier et al., 2005; Guse, 2013). Cory's Shearwaters similarly use a hierarchical pattern of search strategies while switching between longer and shorter foraging trips (Paiva et al., 2010).
Strong tidal fronts are found around European coasts, and gannets, shearwaters, kittiwakes and alcids converging on these good foraging areas may also be benefitting from local enhancement, as described above in the Bering Sea. At a tidal front in the Irish Sea surface-feeding species (mostly kittiwakes Rissa tridactyla) were found feeding in surface convergences on the accumulating debris resulting from a subsurface feeding frenzy by Manx shearwaters (Puffinus puffinus), common guillemots (Uria aalge) and razorbills (Alca torda) (Durazo et al., 1998).
Future Research
The challenge is to establish how a bird's fitness increases through local enhancement. More achievable would be data showing a positive relationship between feeding rate (as a proxy for fitness) and size of flock. Even the latter is difficult, but with the advent of bird mounted cameras (Tremblay et al., 2014) and GPS tracking this goal is more and more achievable. Since large feeding flocks seem to last longer than smaller flocks (pers. obs.; Hunt et al., 1988; Harrison et al., 1991; Veit et al., 1993), prey capture probably increases over some range of flock sizes. If this is true, then certainly population growth rates of seabirds that depend on finding feeding flocks to find sufficient food need to be linked to the presence, frequency and size of those flocks (Thiebault et al., 2016). Irons (1998) found that breeding black-legged kittiwakes returned to the same feeding areas, and selectively joined flocks in preferred feeding areas—with preference shown for large flocks, which were typically associations with diving auks (Uria).
The existence of attractions between species—when other species do not have similar tendencies toward interspecific association—represents important evidence in itself. For example it is likely to be important but rather poorly emphasized that birds such as kittiwakes are attracted to each other, and to other marine predators such as cetaceans and predatory fishes. There is need for greater focus on feeding behavior, and a greater understanding of requirements for successful foraging (Camphuysen et al., 2012). In particular, how does enhancement affect the energetics of seabirds provisioning young? What is required for recruitment and does enhancement dramatically improve survival probabilities of some species in the first years of life? It is counterintuitive that seabirds would benefit in foraging associations with competitors, and it may be that such associations are not always profitable. However, such foraging associations are ubiquitous, sometimes involving enormous numbers of individual seabirds. It should be possible with modern technology to quantify the drivers of profitability.
There is merit in the study of patterns in interspecific associations and evidence of inherent attraction between species (even out of context of a foraging event), and description of the foraging behavior of the species (e.g., sensory modalities, flight or diving behavior). New technology for tracking movements, recording behavior at sea (e.g., dive depth) and the use of fatty acid signatures or stable isotopes for evaluating diet offer opportunities to research the relationships between marine predators, and differences in trophic flow when they are feeding with or apart from interspecific associations (e.g., Das et al., 2000; Weimerskirch et al., 2004; Käkelä et al., 2007; Cherel et al., 2008; Bost et al., 2009; Phillips et al., 2009; Young et al., 2010; Ceia et al., 2014).
Comparisons of the foraging behavior of populations of the same species across different marine communities are of particular value. If the immediate concern is the conservation of populations, then the description of species associations, their frequency and persistence is of immediate value, and such data are not difficult to acquire from dedicated ship-board observations (Camphuysen and Webb, 1999; Veit, 1999; Thiebot and Weimerskirch, 2013; Santora and Sydeman, 2015). We need additional data on interactions among seabirds and other marine predators. Understanding patterns in the aggregation of birds have important implications for designation of protected areas, and management of species, particularly management of populations for recovery (Assali et al., 2017).
Niche variation is frequently observed in seabirds, in which some individuals of a population feed in interspecific associations and others forage independently (Ceia and Ramos, 2015; Wells et al., 2016), and can have a frequency dependent effect with profound implications for population stability (Bolnick et al., 2003). Further research is merited measuring the degree of specialization (Bolnick et al., 2002) and the dependence on interspecific associations—and the vulnerability of populations if specialized feeding associations are lost.
Conservation
The frequency and apparent importance of positive interactions between species across taxa and marine communities provides a compelling argument for a more ecosystem-level approach to protecting marine habitats. Ecosystem management depends on understanding the importance of such processes (Savoca and Nevitt, 2014). If seabirds are worth protecting, then certainly other animals that contribute to their acquisition of resources require protection as well. Seabirds which are dependent mainly on others as cues for finding food, whether other predators or conspecifics, may be highly vulnerable with declining populations; such declines may trigger a rapid, nonlinear crash below some threshold where they are no longer useful to one another as cues. The drivers of foraging success are implied by the behavior of seabirds and other marine predators, but the vulnerability of populations to changes in the relative abundance of conspecifics and other apex predators is obscure.
Aggregations of seabirds occur at a number of spatial scales, indicating the scale of their oceanic habitat, and then within that aggregations forming as the result of local enhancement and facilitation (e.g., Weimerskirch et al., 2004; Bost et al., 2009; Thiebot and Weimerskirch, 2013; Cafaro et al., 2016). In the case of the first, it is within our power to establish habitat associations, and define the habitat of a species of seabird at sea. However local enhancement and facilitation are the products of the communities, the characteristic combination of species and their relative abundances. The importance of interspecific interdependencies represents an obstacle to our ability to define at sea areas important for seabirds. The importance of local enhancement and facilitation varies between species (or sometimes populations), and in some cases may be a fundamental characteristic of the species foraging ecology. Understanding this is important for protecting these species.
It is convincing that populations of cetaceans are important for foraging seabirds (Evans, 1982; Au and Pitman, 1986; Hodges and Woehler, 1993; Ballance et al., 1997; Vaughan et al., 2007; Cafaro et al., 2016); their demise has represented degradation of seabird foraging habitat. The apex predator guild is important to the community structure in the tropics and is affected by fisheries on skipjack (Katsuwonus pelamis) and yellowfin tuna (Hunsicker et al., 2012), and the ranges of these fishes are likely to change with climate (Polovina et al., 2011; Furness, 2016). In locations such as Northern European waters there are many species which once would have been important in the marine ecosystem as facilitators that are now missing. The recovery of great whales regionally in European waters will be a significant development improving foraging opportunities of species such as gannets and various procellariids.
Managers of marine resources and conservation biologists share an interest in predicting the distribution of seabirds, and in particular establishing what factors are most influential in attracting birds. In this paper we have considered how positive interactions result in local enhancement and facilitation among seabirds and other marine predators, and how these interactions are of fundamental importance in understanding survival and reproductive success and distribution at sea. There are unknown consequences of biodiversity loss (Worm et al., 2006) and dismissing the importance of these interspecific associations could have profound consequences in terms of ecosystem function and ecosystem services.
Author Contributions
RV originally conceived of the review paper. RV and NH outlined the structure of the review and jointly wrote the manuscript.
Funding
Review funded through institutions employing authors. RV's participation was in part supported by a National Science Foundation grant to John H. Steele.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This paper benefitted from discussions within the Working Group on Seabird Ecology of ICES, the International Council for the Exploration of the Seas (Copenhagen, October 2013).
References
Ashmole, N. P., and Ashmole, M. J. (1967). Comparative feeding essscology of seabirds of a tropical oceanic island. Bull., Peabody Mus. Nat. Hist. 24, 1–131.
Assali, C., Bez, N., and Tremblay, Y. (2017). Seabird distribution patterns observed with fishing vessel's radar reveal previously undescribed sub-meso-scale clusters. Sci. Rep. 7:7364. doi: 10.1038/s41598-017-07480-6
Au, D. W. K., and Pitman, R. L. (1986). Seabird associations with dolphins and tuna in the Eastern Tropical Pacific. Condor 88, 304–317. doi: 10.2307/1368877
Ballance, L. T., Pitman, R. L., and Fiedler, F. C. (2006). Oceanographic influences on seabirds and cetaceans of the eastern tropical Pacific: a review. Prog. Oceanogr. 69, 360–390. doi: 10.1016/j.pocean.2006.03.013
Ballance, L. T., Pitman, R. L., and Reilly, S. B. (1997). Seabird community structure along a productivity gradient: importance of competition and energetic constraint. Ecology 78, 1502–1518. doi: 10.1890/0012-9658(1997)078[1502:SCSAAP]2.0.CO;2
Bellier, E., Certain, G., Chadoeuf, J., Monestiez, P., and Bretagnolle, V. (2005). Spatial Pattern in Seabirds' Distribution: Testing for Influence of Foraging Strategies. The Case of Northern Gannets in the Bay of Biscay. Copenhagen: ICES CM 2005/L:13
Bolnick, D. I., Svanbäck, R., Fordyce, J. A., Yang, L. H., Davis, J. M., Hulsey, C. D., et al. (2003). The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28. doi: 10.1086/343878
Bolnick, D. I., Yang, L. H., Fordyce, J. A., Davis, J. M., and Svanbäck, R. (2002). Measuring individual-level resource specialization. Ecology 83, 2936–2941. doi: 10.1890/0012-9658(2002)083[2936:MILRS]2.0.CO;2
Bost, C. A., Cotté, C., Bailleul, F., Cherel, Y., Charrassin, J. B., Guinet, C., et al. (2009). The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Syst. 78, 363–376. doi: 10.1016/j.jmarsys.2008.11.022
Boyd, C., Grünbaum, D., Hunt, G. L. Jr., Punt, A. E., Weimerskirch, H., and Bertrand, S. (2016). Effects of variation in the abundance and distribution of prey on the foraging success of central place foragers. J. Appl. Ecol. 54, 1362–1372. doi: 10.1111/1365-2664.12832
Bretagnolle, V. (1993). Adaptive significance of seabird coloration: the case of Procellariiformes. Am. Nat. 142, 141–173. doi: 10.1086/285532
Brewer, M. L., and Hertel, F. (2007). Wing morphology and flight behavior of Pelecaniform seabirds. J. Morph. 268, 866–877. doi: 10.1002/jmor.10555
Bronstein, J. L. (1994). Conventional outcomes in mutualistic interactions. TREE 9, 214–217. doi: 10.1016/0169-5347(94)90246-1
Bruno, J. F., Stachowicz, J. J., and Bertness, M. D. (2003). Inclusion of facilitation into ecological theory. TREE 18, 119–126. doi: 10.1016/S0169-5347(02)00045-9
van Buskirk, R. W., and Nevitt, G. A. (2008). The influence of developmental environment on the evolution of olfactory foraging behaviour in procellariiform seabirds. J. Evol. Biol. 21, 67–76. doi: 10.1111/j.1420-9101.2007.01465.x
Cafaro, V., Angeletti, D., Bellisario, B., and Macali, A. (2016). Habitat overlap between bottlenose dolphins and seabirds: a pilot study to identify high-presence coastal areas in the Tyrrhenian Sea. J. Mar. Biol. Assoc. UK 96, 891–901. doi: 10.1017/S0025315415001447
Camphuysen, C. J., Shamoun-Baranes, J., Bouten, W., and Garthe, S. (2012). Identifying ecologically important marine areas for seabirds using behavioural information in combination with distribution patterns. Biol. Cons. 156, 22–29. doi: 10.1016/j.biocon.2011.12.024
Camphuysen, C. J., and Webb, A. (1999). Multi-species feeding associations in North Sea seabirds: jointly exploiting a patchy environment. Ardea 87, 177–198.
Ceia, F. R., Paiva, V. H., Garthe, S., Marques, J. C., and Ramos, J. A. (2014). Can variations in the spatial distribution at sea and isotopic niche width be associated with consistency in the isotopic niche of a pelagic seabird species? Mar. Biol. 161, 1861–1872. doi: 10.1007/s00227-014-2468-9
Ceia, F. R., and Ramos, J. A. (2015). Individual specialization in the foraging and feeding strategies of seabirds: a review. Mar. Biol. 162, 1923–1938. doi: 10.1007/s00227-015-2735-4
Cherel, Y., Le Corre, M., Jaquemet, S., Menard, F., Richard, P., and Weimerskirch, H. (2008). Resource partitioning within a tropical seabird community: new information from stable isotopes. Mar. Ecol. Prog. Ser. 366, 281–291. doi: 10.3354/meps07587
Clua, E., and Grosvalet, F. (2001). Mixed-species feeding aggregations of dolphins, large tunas and seabirds in the Azores. Aquat. Living Resour. 14, 11–18. doi: 10.1016/S0990-7440(00)01097-4
Das, K., Lepoint, G., Loizeauà, V., Debacker, V., Dauby, P., and Bouquegneau, J. M. (2000). Tuna and dolphin associations in the North-East Atlantic: evidence of different ecological niches from stable isotope and heavy metal measurements. Mar. Pollut. Bull. 40, 102–109. doi: 10.1016/S0025-326X(99)00178-2
Davoren, G. K., Garthe, S., Montevecchi, W. A., and Benvenuti, S. (2010). Influence of prey behaviour and other predators on the foraging activities of a marine avian predator in a Low Arctic ecosystem. Mar. Ecol. Prog. Ser. 404, 275–287. doi: 10.3354/meps08370
Durazo, R., Harrison, N. M., and Hill, A. E. (1998). Seabird observations at a tidal mixing front in the Irish Sea. Estuar. Coast. Shelf Sci. 47, 153–164. doi: 10.1006/ecss.1998.0339
Evans, P. G. H. (1982). Associations between seabirds and cetaceans: a review. Mamm. Rev. 12, 187–206. doi: 10.1111/j.1365-2907.1982.tb00015.x
Furness, R. W. (2016). “Impacts and effects of ocean warming on seabirds,” in Explaining Ocean Warming: Causes, Scale, Effects and Consequences, eds D. Laffoley and J. M. Baxter (Gland: IUCN), 271–288.
Goodale, E., Beauchamp, G., Magrath, R. D., Nieh, J. C., and Ruxton, G. D. (2010). Interspecific information transfer influences animal community structure. TREE 25, 354–361. doi: 10.1016/j.tree.2010.01.002
Goyert, H. F., Manne, L. L., and Veit, R. R. (2014). Facilitative interactions among the pelagic community of temperate migratory terns, tunas and dolphins. Oikos 11, 1400–1408. doi: 10.1111/oik.00814
Grebmeir, J. M., and Harrison, N. M. (1992). Seabird feeding on benthic amphipods facilitated by gray whale activity in the northern Bering Sea. Mar. Ecol. Prog. Ser. 80, 125–133. doi: 10.3354/meps080125
Grover, J. J., and Olla, B. L. (1983). The role of the rhinoceros auklet (Cerorhinca monocerata) in mixed-species feeding assemblages of seabirds in the Strait of Juan de Fuca, Washington. Auk 100, 979–982.
Grünbaum, D., and Veit, R. R. (2003). Black-browed albatrosses foraging on Antarctic krill: density-dependence through local enhancement? Ecology 84, 3265–3275. doi: 10.1890/01-4098
Guse, N. (2013). Habitat Selection and Foraging Ecology of Seabirds in the Gulf of St Lawrence. Dissertation. Christian-Albrechts-Universität zu Kiel (Keil).
Harrison, N. H., Whitehouse, M. J., Heinemann, D., Prince, P. A., Hunt, G. L. Jr., and Veit, R. R. (1991). Observations of multi-species seabird flocks around South Georgia. Auk 108, 801–810.
Harrison, N. M., and Whitehouse, M. J. (2011). Mixed-species flocks: an example of niche construction? Anim. Behav. 81, 675–682. doi: 10.1016/j.anbehav.2011.01.013
Hebshi, A. J., Duffy, D. C., and Hyrenbach, K. D. (2008). Associations between seabirds and subsurface predators around Oahu, Hawaii. Aquat. Biol. 4, 89–98. doi: 10.3354/ab00098
Hodges, C. L., and Woehler, E. J. (1993). Associations between seabirds and cetaceans in the Australian sector of the southern Indian Ocean. Mar. Ornith. 22, 205–212.
Hoffman, W., Heinemann, D., and Wiens, J. A. (1981). The ecology of seabird feeding flocks in Alaska. Auk 98, 437–456.
Hunsicker, M. E., Olson, R. J., Essington, T. E., Maunder, M. N., Duffy, L. M., and Kitchell, J. F. (2012). Potential for top-down control on tropical tunas based on size structure of predator—prey interactions. Mar. Ecol. Prog. Ser. 445, 263–277. doi: 10.3354/meps09494
Hunt, G. L. Jr., Harrison, N. M., Hamner, W. M., and Obst, B. S. (1988). Observations of a mixed-species flock of birds foraging on euphausiids near St Matthew Island Bering Sea. Auk 105, 345–349. doi: 10.2307/4087500
Hutchison, L. V., and Wenzel, B. M. (1980). Olfactory guidance in foraging by Procellariiforms. Condor 82, 314–319. doi: 10.2307/1367400
International Council for the Exploration of the Seas (2010). Report of the Working Group on Seabird Ecology (WGSE). Copenhagen: ICES CM 2010/SSGEF (Accessed Mar 15-19, 2010).
Irons, D. B. (1998). Foraging area fidelity of individual seabirds in relation to tidal cycles and flock feeding. Ecology 79, 647–655. doi: 10.1890/0012-9658(1998)079[0647:FAFOIS]2.0.CO;2
Käkelä, A., Furness, R. W., Kelly, A., Strandberg, U., Waldron, S., and Käkelä, R. (2007). Fatty acid signatures and stable isotopes as dietary indicators in North Sea seabirds. Mar. Ecol. Prog. Ser. 342, 291–301. doi: 10.3354/meps342291
Laland, K. N., and Boogert, N. J. (2008). Niche construction, co-evolution and biodiversity. Ecol. Econ. 69, 731–736. doi: 10.1016/j.ecolecon.2008.11.014
Laland, K. N., Odling-Smee, J., and Feldman, M. W. (1999). Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl. Acad. Sci. U.S.A. 96, 10242–10247. doi: 10.1073/pnas.96.18.10242
LeCorre, M., and Jaquemet, S. (2005). Assessment of the seabird community of the Mozambique Channel and its potential use as an indicator of tuna abundance. Estuar. Coast. Shelf Sci. 63, 421–428. doi: 10.1016/j.ecss.2004.11.013
Lett, C., Semeria, M., Thiebault, A., and Tremblay, Y. (2014). Effects of successive predator attacks on prey aggregations. Theor. Ecol. 7, 239–252. doi: 10.1007/s12080-014-0213-0
Martin, A. R. (1986). Feeding association between dolphins and shearwaters around the Azores Islands. Can. J. Zool. 64, 1372–1374. doi: 10.1139/z86-205
Munn, C. A., and Terborgh, J. W. (1979). Multi-species territoriality in Neotropical foraging flocks. Condor 81, 338–347. doi: 10.2307/1366956
Murphy, R. C. (1936). Oceanic Birds of South America. New York, NY: American Museum of Natural History.
Nevitt, G. A. (2000). Olfactory foraging by Antarctic procellariiform seabirds: life at high Reynolds numbers. Biol. Bull. 198, 245–253. doi: 10.2307/1542527
Nevitt, G. A. (2008). Sensory ecology on the high seas: the odor world of the procellariiform seabirds. J. Exp. Biol. 211, 1706–1713. doi: 10.1242/jeb.015412
Nevitt, G. A., and Bonadonna, F. (2005). Seeing the world through the nose of a bird: new developments in the sensory ecology of Procellariiform seabirds. Mar. Ecol. Prog. Ser. 287, 292–295. doi: 10.1098/rsbl.2005.0350
Nevitt, G. A., Reid, K., and Trathan, P. (2004). Testing olfactory foraging strategies in an Antarctic seabird assemblage. J. Exp. Biol. 207, 3537–3544. doi: 10.1242/jeb.01198
Nevitt, G. A., Veit, R. R., and Kareiva, P. (1995). Dimethyl sulphide as a foraging cue for Antarctic Procellariiform seabirds. Nature 376, 680–682. doi: 10.1038/376680ao
Obst, B. S., and Hunt, G. L. Jr. (1990). Marine birds feed at gray whale mud plumes in the Bering Sea. Auk 107, 678–688. doi: 10.2307/4087998
Olmos, F., Rotenberg, E., and Muscat, E. (2013). A feeding association between between Wilson's Storm-petrels Oceanites oceanicus and Rough-toothed Dolphins Steno bredanensis. Biota Neotrop. 13, 303–307. doi: 10.1590/S1676-06032013000200030
Paiva, V. H., Geraldes, P., Ramírez, I., Garthe, S., and Ramos, J. A. (2010). How area restricted search of a pelagic seabird changes while preforming a dual foraging strategy. Oikos 119, 1423–1434. doi: 10.1111/j.1600-0706.2010.18294.x
Phillips, R. A., Bearhop, S., Mcgill, R. A. R., and Dawson, D. A. (2009). Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160, 795–806. doi: 10.1007/s00442-009-1342-9
Pierotti, R. (1988). “Associations between marine birds and mammals in the northwest Atlantic ocean,” in Seabirds and Other Marine Vertebrates: Competition, Predation and Other Interactions, ed J. Burger (New York, NY: Columbia University Press), 31–58.
Pitman, R. L., and Ballance, L. T. (1992). Parkinson's petrel distribution and foraging ecology in the Eastern Pacific: aspects of an exclusive feeding relationship with dolphins. Condor 94, 825–835. doi: 10.2307/1369280
Polovina, J. J., Dunne, J. P., Woodworth, P. A., and Howell, E. A. (2011). Projected expansion of the subtropical biome and contraction of the temperate and equatorial upwelling biomes in the North Pacific under global warming. ICES J. Mar. Sci. 68, 986–995. doi: 10.1093/icesjms/fsq198
Prince, P., Huin, N., and Weimerskirch, H. (1994). Diving depths of albatrosses. Ant. Sci. 6, 353–354. doi: 10.1017/S0954102094000532
Rogan, E., and Mackey, M. (2007). Megafauna bycatch in drift nets of albacore tuna in the NE Atlantic. Fish. Res. 86, 6–14. doi: 10.1016/j.fishres.2007.02.013
Santora, J. A., and Sydeman, W. J. (2015). Persistence of hotspots and variability of seabird species richness and abundance in the southern California Current. Ecosphere 6:214. doi: 10.1890/ES14-00434.1
Santora, J. A., and Veit, R. R. (2013). Spatio-temporal persistence of top predator hotspots near the Antarctic Peninsula. Mar. Ecol. Prog. Ser. 487, 287–304. doi: 10.3354/meps10350
Savoca, M. S., and Nevitt, G. A. (2014). Evidence that dimethyl sulphide facilitates a tritrophic mutualism between marine primary producers and top predators. Proc. Natl. Acad. Sci. U.S.A. 111, 4157–4161. doi: 10.1073/pnas.1317120111
Schneider, D. C., Harrison, N. M., and Hunt, G. L. Jr. (1990). Seabird diet at a front near the Pribilof Islands, Alaska. Stud. Avian Biol. 14, 61–66.
Silverman, E. D., and Veit, R. R. (2001). Associatons among Antarctic seabirds in mixed-species feeding flocks. Ibis 143, 51–62. doi: 10.1111/j.1474-919X.2001.tb04169.x
Silverman, E. D., Veit, R. R., and Nevitt, G. A. (2004). Nearest neighbors as foraging cues: information transfer in a patchy environment. Mar. Ecol. Prog. Ser. 277, 25–36. doi: 10.3354/meps277025
Skov, H., Durinck, J., Danielsen, F., and Bloch, D. (1995). Co-occurrence of cetaceans and seabirds in the northeast Atlantic. J. Biog. 22, 71–88. doi: 10.2307/2846074
Spear, L. B., and Ainley, D. G. (2005). At-sea behaviour and habitat use by tropicbirds in the eastern Pacific. Ibis 147, 391–407. doi: 10.1111/j.1474-919x.2005.00418.x
Sridhar, H., Beauchamp, G., and Shanker, K. (2009). Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. An. Beh. 78, 337–347. doi: 10.1016/j.anbehav.2009.05.008
Stachowicz, J. J. (2001). Mutualism, facilitation and the structure of ecological communities. Bioscience 51, 235–246. doi: 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2
Thiebault, A., Mullers, R., Pistorius, P., and Tremblay, Y. (2014a). Local enhancement in a seabird: reaction distances and foraging consequence of predator aggregations. Beh. Ecol. 25, 1302–1310. doi: 10.1093/beheco/aru132
Thiebault, A., Mullers, R., Pistorius, P., Meza-Torres, M., Dubroca, L., Green, D., et al. (2014b). From colony to first patch: processes of prey searching and social information in Cape gannets. Auk 131, 595–609. doi: 10.1642/AUK-13-209.1
Thiebault, A., Semeria, M., Lett, C., and Tremblay, Y. (2016). How to capture fish in a school? Effect of successive predator attacks on seabird feeding success. J. Anim. Ecol. 85, 157–167. doi: 10.1111/1365-2656.12455
Thiebot, J.-B., and Weimerskirch, H. (2013). Contrasted associations between seabirds and marine mammals across four biomes of the southern Indian Ocean. J. Ornith. 154, 441–453. doi: 10.1007/s10336-012-0909-0
Tremblay, Y., Thiebault, A., Mullers, R., and Pistorius, P. (2014). Bird-borne video-cameras show that seabird movement patterns relate to previously unrevealed proximate environment, not prey. PLoS ONE 9:e0088424. doi: 10.1371/journal.pone.0088424
Vaughan, R. L., Shelton, D. E., Timm, L. L., Watson, L. A., and Würsig, B. (2007). Dusky dolphin (Lagenorhynchus obscurus) feeding tactics and multi-species associations. New Zeal. J. Mar. Freshw. Res. 41, 391–400. doi: 10.1080/00288330709509929
Veit, R. R. (1995). Pelagic communities of seabirds in the South Atlantic Ocean. Ibis 137, 1–10. doi: 10.1111/j.1474-919X.1995.tb03213.x
Veit, R. R. (1999). Behavioral responses by foraging petrels to swarms of Antarctic krill. Ardea 87, 41–50.
Veit, R. R., and Hunt, G. L. Jr. (1991). Broadscale density and aggregation of pelagic birds from a circumnavigational survey of the Antarctic Ocean. Auk 108, 790–800.
Veit, R. R., Silverman, E. D., and Everson, I. (1993). Aggregation patterns of pelagic predators and their principal prey, Antarctic krill, near South Georgia. J. Anim. Ecol. 62, 551–564. doi: 10.2307/5204
Weimerskirch, H., and Sagar, P. M. (1996). Diving depths of sooty shearwaters Puffinus griseus. Ibis 138, 786–788. doi: 10.1111/j.1474-919X.1996.tb08837.x
Weimerskirch, J., LeCorre, M., Jaquemet, S., Potier, M., and Marsac, F. (2004). Foraging strategy of a top predator in tropical waters: great frigatebirds in the Mozambique Channel. Mar. Ecol. Prog. Ser. 275, 297–308. doi: 10.3354/meps275297
Wells, M. R., Angel, L. P., and Arnould, J. P. Y. (2016). Habitat-specific foraging strategies in Australasian gannets. Biol. Open. 4, 1298–1305. doi: 10.1242/bio.018085
Worm, B., Barbier, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S., et al. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science 314, 787–790. doi: 10.1126/science.1132294
Keywords: coevolution, conservation, facilitation, foraging behavior, interspecific associations, local enhancement, marine predators, seabird
Citation: Veit RR and Harrison NM (2017) Positive Interactions among Foraging Seabirds, Marine Mammals and Fishes and Implications for Their Conservation. Front. Ecol. Evol. 5:121. doi: 10.3389/fevo.2017.00121
Received: 30 June 2017; Accepted: 21 September 2017;
Published: 06 October 2017.
Edited by:
Jean-Yves Georges, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Yann Tremblay, Institut de Recherche pour le Développement (IRD), FranceMarcelo Bertellotti, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2017 Veit and Harrison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard R. Veit, cnJ2ZWl0MjNAZ21haWwuY29t
 Richard R. Veit
Richard R. Veit Nancy M. Harrison
Nancy M. Harrison