
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol., 07 April 2017
Sec. Behavioral and Evolutionary Ecology
Volume 5 - 2017 | https://doi.org/10.3389/fevo.2017.00024
This article is part of the Research TopicBehavioural and Ecological Consequences of Urban Life in BirdsView all 31 articles
Intentional feeding of wild birds in gardens or backyards is one of the most popular forms of human–wildlife interactions in the developed world, especially in urban environments. The scale and intensity of bird feeding are enormous with mainly birdseed consumed daily by a range of species. This represents a subsidy to natural diets of birds attracted to the feeders and typically involves novel dietary components. Yet, relatively little is known about how it influences the behavior and ecology of the species visiting feeders. In part, research has been hampered by logistical difficulties of working in urban areas but studies have demonstrated powerful influences on behavior and phenology of avian breeding, the spread of disease, and the structure of avian communities. Here, we compare bird feeding between Northern and Southern Hemispheres as a means of exploring how similarities and differences in avian responses might inform knowledge of this global urban phenomenon. We start by tracing its origins to north-western Europe and how its expansion has occurred before considering how geographical differences in feeding practices and attitudes map onto bird feeding “on the ground.” We explore some of the major emerging themes of recent interest, including why citizens are motivated to feed birds, whether birds become fully dependent on food supplements, the role of feeding in avian disease transmission, and how feeding changes urban bird communities. By proposing that scientists work in collaboration with the public providing food to birds, we pose key research questions that need to be answered urgently and suggest accompanying experimental approaches to do so. These approaches are essential if we are to improve our understanding of how bird feeding shapes the behavior, ecology, movements, and community structure of urban birds. Our hope is that through such citizen science we will be able to provide advice as to location-relevant practices that should maximize benefits to both urban biodiversity and human well-being, and minimize potential adverse impacts. We demonstrate that bird feeding is important for urban biodiversity conservation, community engagement, and in establishing personal connections with nature and their associated benefits.
The world is urbanizing rapidly (United Nations, Department of Economic and Social Affairs, Population Division, 2014) and, as a result, human–wildlife interactions will become ever more commonplace. One of the most popular and globally common of such interactions is the feeding of wild birds in residential gardens or backyards (hereafter referred to as “garden bird feeding”) that is widespread across many parts of the developed world (Jones, 2017). This pastime is increasingly becoming the subject of scientific and societal scrutiny (Jones and Reynolds, 2008; Robb et al., 2008a). Bird feeding is variously advocated as an essential conservation activity, a simple way for people to connect with nature in an urbanizing world, and a means for enhancing environmental awareness and psychological well-being (Schoech et al., 2008; Davies et al., 2012; Cox and Gaston, 2016). However, it has also been implicated in the spread of catastrophic avian diseases (e.g., Dhondt et al., 2007; Robinson et al., 2010), altering ecosystem structure (Galbraith et al., 2015), benefiting invasive species (Galbraith et al., 2015), changing predator-prey dynamics (Malpass et al., 2017), and even contributing to rapid evolutionary change (Bearhop et al., 2005). Furthermore, the possibility that birds may become dependent on anthropogenic food is a primary concern of both opponents to, and proponents of, bird feeding (e.g., Howard and Jones, 2004; Jones, 2011). These issues constitute a far from exhaustive list and clearly a more complete understanding of this interaction between birds and humans should be a significant research priority. This is especially pertinent within the context of urban ecology (Robb et al., 2008a) as it is becoming clear that many animal and plant populations exhibit “phenotypic signatures” associated with the urban areas in which they live (Alberti et al., 2017). We have previously described bird feeding as a supplementary feeding experiment on a global scale but without due consideration of its effects on the behavioral, community, and population ecology of the birds consuming supplements (Jones and Reynolds, 2008). However, despite the above concerns, scientific investigations into most aspects of the practice are fragmentary and geographically limited.
Here, we employ a comparison between Northern and Southern Hemispheres to compare and contrast the impacts of bird feeding on the biology of birds, and on avian populations and communities. We identify gaps in knowledge that require bridging as a matter of urgency because bird feeding appears to be growing in popularity (Jones, 2017). Ultimately, we will identify key research priorities that should be targeted by researchers and citizen scientists with the ultimate aim of promoting avian conservation efforts globally.
Our first task is to define what we mean by garden or backyard “bird feeding.” The focus of this review is the intentional feeding (i.e., food supplementation) through the provision of food to free-living birds (Figure 1). It does not include the incidental feeding of birds congregating at locations where food sources are available as a result of disposal (e.g., landfill, fish discards from fishing vessels) or feeding of non-avian companion taxa such as dogs Canis lupus familiaris and cats Felis catus silvestris. We considered the growing interest in bird feeding in the scientific literature by performing a literature search (Figure 2) of the ISI Web of Science in January 2017 entering “food supplement* AND bird*” as a search term. We defined the start of this literature search as being the year when the first major review was published in the primary scientific literature by Boutin (1990) who considered the impacts of feeding on free-living terrestrial vertebrates, including birds. The search included articles, proceedings papers, reviews, notes, and editorial materials, and was restricted to the “Ornithology” Web of Science category; this search yielded 206 items. Figure 2 reveals a slow but steady increase in the scientific outputs related to bird feeding, demonstrating that the subject is of increasing interest.

Figure 1. Examples of bird species feeding on food supplements in city gardens in (A) New Zealand (tui Prosthemadura novaeseelandiae) (Photo: J. Galbraith), (B) the US (house finch Carpodacus mexicanus) (Photo: B. Vuxinic), (C) the UK (blue tits Cyanistes caeruleus) (Photo: J. Galbraith), and (D) Australia (rainbow lorikeet Trichoglossus moluccanus) (Photo: D. Jones).
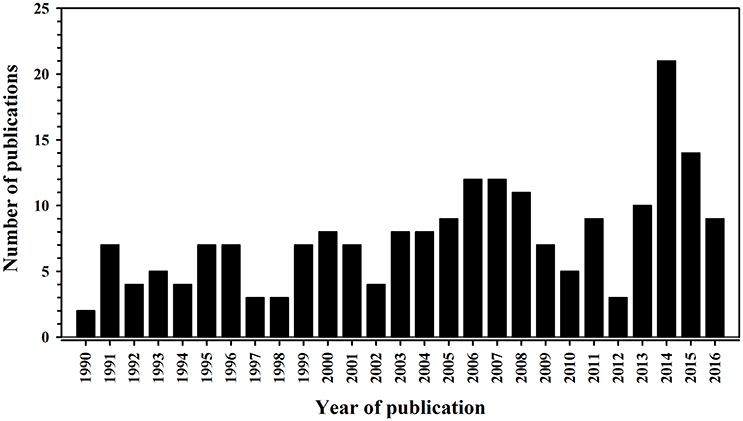
Figure 2. Number of publications (including articles, proceedings papers, reviews, notes, and editorial materials) from a search of the ISI Web of Science conducted in January 2017 about food supplementation of birds. Note that the literature search ended in 2016 because 2017 was less than a month old when the search was performed.
The scale of bird feeding by a range of different criteria is remarkable. In the United Kingdom (UK), various studies have found that more than 60% of households regularly feed birds in their gardens, spending US$188–226 million on 60,000 tons of birdseed annually (Fuller et al., 2008). Robb et al. (2008a) estimated that sufficient seed was distributed for blue tits Cyanistes caeruleus alone to support five times the national population of the species. In the United States (US), the 2011 National Survey of Fishing, Hunting, and Wildlife-Associated Recreation reported that 52.8 million households practiced some form of bird feeding, giving a countrywide rate of 73% (U.S. Fish Wildlife Service and U.S. Census Bureau, 2011) spending a total of US$4 billion on birdseed and an additional US$10 billion on related hardware and peripherals, annually. The annual amount of bird food supplied in the US is ~500,000 tons, enough to feed 300 million chickadees Poecile spp. if they consumed nothing else (Robb et al., 2008a.
Although bird feeding is commonly practiced through much of North America and parts of Europe, relatively little is known about its extent and scale in countries other than Germany, Norway, Sweden, the UK, and the US (Jones, 2017). Anecdotally, there has long been a general impression that it is a far more established practice in the more northern European countries, a conclusion confirmed by a recent informal qualitative survey (Jones, 2017). The widespread practice of bird feeding is directly linked to geographical areas that experience prolonged or extreme climatic conditions during winter. Indeed, apart from the notable exceptions of the UK (Cox and Gaston, 2015) and Germany (Berthold and Mohr, 2006), bird feeding in northern Europe remains almost entirely a winter-only activity. In contrast, bird feeding is rarely practiced throughout the countries of southern Europe, although there are some enclaves—notably areas with residents from more northern European countries—where some bird feeding does occur (Jones, 2017).
It is noteworthy that bird feeding—at least as practiced in much of the Northern Hemisphere—is not just the preserve of “northern” countries but also of the “Western World”; bird feeding is apparently virtually unknown in most of Asia, including China, Korea, Japan, and most countries of the south-east (Jones, 2017). An important exception occurs in many parts of the Indian subcontinent and beyond where daily offerings, traditionally of rice cakes, are made to birds (and other animals) in the practice of Bhuta-Yajna, a ritual observed by orthodox Hindus (Jones, 2017). Undoubtedly, ethno-biology, and more specifically ethno-ornithology (Tidemann and Gosler, 2010), are increasingly providing further insights into practices such as feeding of wildlife and how they explain the prominent role that animals play in human culture (Cocker, 2013). However, in the context of non-ritualistic feeding of birds within our towns and cities, it is important to remember that while we regard it as commonplace and familiar, it appears to be confined to parts of the world populated by people originally from north-western Europe (Jones, 2017).
We are not the first to consider the impacts of bird feeding on avian biology (see Boutin, 1990; Reynolds et al., 2004; Robb et al., 2008a), but we break new ground in considering comparative spatial perspectives. Traditionally, attempts to explore the origins of bird feeding have emphasized the significance of harsh winter weather on the origins of the practice, with the humane response to the apparent suffering of birds being an obvious motivation (Fuller et al., 2008). However, this perspective has limited relevance to bird feeding in several major countries of the Southern Hemisphere (Chapman, 2015). In general, the main population centers of Australia, New Zealand, and South Africa do not experience the often prolonged and severe periods of cold typical of much of the Northern Hemisphere. Despite such differences in climate, the feeding of wild birds in Australia and New Zealand is on a similar scale to that of the Northern Hemisphere; recent surveys have revealed participation rates of households of 36–57% in New Zealand (Galbraith et al., 2014), and 63% in Australia (Chapman, 2015) (No similar studies have been conducted in South Africa despite the practice being popular among Europeans in cities such as Cape Town and Durban). Although there was evidence of a slightly higher frequency of feeding in winter in these two countries, by far the largest proportion of participants did so throughout the year and most provided food items daily. Nonetheless, many Australians also indicated that they were especially likely to feed or increase the amount of food supplements during challenging times for birds such as heat waves, and extended periods of drought and cold (Galbraith et al., 2014; Chapman, 2015).
Perhaps the most unexpected contrast in bird feeding characteristics between the hemispheres is provided by the bird species visiting feeders (Table 1; Figure 1). While species richness at feeders is high in all countries where garden bird feeding is a common pastime, by far the most frequent visitors in North America are black-capped chickadees Poecile atricapillus and Carolina chickadees Poecile carolinensis, and blue and great tits Parus major in the UK, all being members of the family Paridae (Toms and Sterry, 2008; Baicich et al., 2015). These species are typically small, weighing between 10 and 19 g (all body masses reported from Dunning, 2008). Extending the list to the top five species that are the most frequent feeder visitors, the heaviest in the US is the mourning dove Zenaida macroura (119 g) and in the UK it is the common blackbird Turdus merula (113 g). In contrast, the two most frequent species at feeders in Australia, the Australian magpie Gymnorhina tibicen and the rainbow lorikeet Trichoglossus moluccanus, weigh 212–360 and 84–169 g, respectively. Therefore, Australian feeder birds are far larger than those typically found at Northern Hemisphere feeders. In New Zealand, the situation is strongly influenced by the abundance of introduced species with the most common being house sparrows Passer domesticus (20–35 g), common blackbirds and common mynas Acridotheres tristis (82–140 g) with the only native species feeding on grain-based supplements being the silvereye Zosterops lateralis (9–17 g; Galbraith et al., 2015). In South Africa, while the diversity of birds visiting gardens is remarkably large—from diminutive waxbills (Estrildidae) to huge hornbills (Bucerotidae)—we could find no reliable data with which to compile a comparative list of the most common species observed at bird feeders.
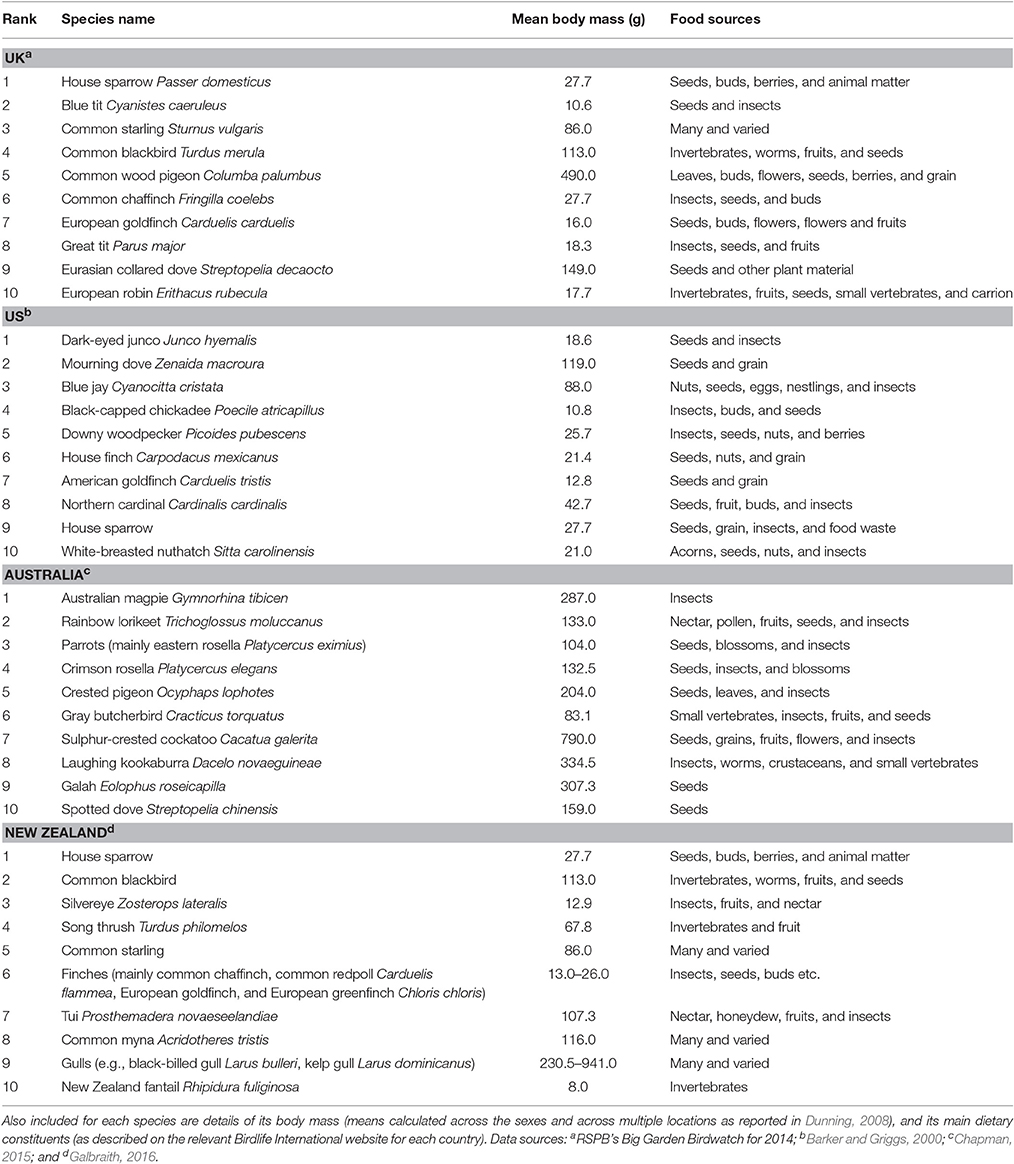
Table 1. The top 10 bird species visiting feeders in selected countries of the Northern and Southern Hemispheres.
While the predominant food supplements provided to wild birds in garden feeders throughout the world are seeds, especially sunflower Helianthus spp. and millet Panicum spp., nyjer Guizotia abyssinica, and various cereals, as well as peanuts Arachis hypogaea, several other supplements are frequently used, often triggered by changes in local weather (Berthold and Mohr, 2006; Toms and Sterry, 2008; Baicich et al., 2015). For example, various fat-rich items (typically suet balls, often with added peanuts) are commonly fed in winter. In areas with nectar-feeding species such as hummingbirds (Trochilidae), silvereyes, honeybirds (Indicatoridae), and lorikeets (Psittacidae), a wide variety of sugar- or honey-based solutions is provided in drinkers. Bread is also an extremely common food source provided in all countries where bird feeding is widespread (Jones, 2017).
As well as differences in avian community composition at feeders of Northern and Southern Hemispheres (Table 1), there are major differences in the types of food supplements provided, especially in Australia where meat is routinely fed to populous species such as Australian magpies, butcherbirds Cracticus spp., and kookaburras (Alcedinidae) that are all relatively large species and abundant in urban landscapes (O'Leary and Jones, 2006; Jones, 2011). The most commonly provisioned form of meat is raw beef mince (or ground beef), presumably because it is relatively inexpensive and readily available, although pieces of sausage, salami, ham, bacon, and cooked chicken are also provided (Ishigame and Baxter, 2007). This raises many concerns including the impacts on bird communities dominated by large and often predatory species, the hygiene of feeding structures, and the nutritional impacts if these food supplements were to constitute a large proportion of their diets. Rainbow lorikeets, for example, that are specialized consumers of pollen and nectar have learned to consume meat supplements too; this recent expansion of their foraging niche is resulting in much consternation among participants and ecologists (Gillanders et al., in press).
Whether in Northern or Southern Hemispheres, at its most superficial level the simple act of feeding birds in one's private garden may appear to be little more than a way to see birds close at hand and to promote their persistence and welfare. However, several detailed and recent studies have expanded our knowledge of motives further. For example, in the UK, Cox and Gaston (2016) found that most participants felt relaxed and connected with nature, feelings that increased positively with the frequency of bird feeding activity. Furthermore, public perception of decreasing natural food supplies resulted in increased intensity of bird feeding. A recent investigation of the motivations of participants within the south-east of England (Clark, 2013) identified a complex suite of influences and drivers, with pleasure, enhancing survival, and a desire to nurture all being significant. These results suggest a strong relationship between perceptions—regardless of their veracity—and practice among people feeding birds, and illustrate the potential role this activity may play in promoting mental wellness. The physical, psychological, educational, and social benefits from interaction with nature through a wide variety of means are being increasingly recognized (Horvath and Roelands, 1991; Beck et al., 2001; Shanahan et al., 2014).
The above conclusion may be appropriate and relevant in a cultural setting in which garden bird feeding is popular and promoted, as is the case in the UK. However, in contrast, in Australia where although bird feeding occurs at similar levels to that in the UK, there is widespread antipathy toward the practice among many environmental and conservation groups, a stance broadly recognized (although largely ignored) by most participants (Howard and Jones, 2004; Jones, 2011, 2014). This opposition generates concern among participants about the potential impacts of feeding birds and a common reluctance to discuss it publicly (Jones, 2014). Nonetheless, the potential welfare benefits that garden bird feeding provides for humans connecting with nature are presumably equivalent between Australia and the UK. Given these contrasting societal contexts, exploring the salient motivations of participants from the two countries is sure to yield valuable insights. Adapting Kellert's widely used “wildlife values” to discern themes among participants, Chapman (2015) found unexpected similarities between the two groups. Among UK participants, the theme associated with care and responsibility for the birds they feed was by far the most predominant. Given the more equitable climate of Australia, this featured highly too for Australian participants and was second only to the enjoyment that they derived from the activity. Interestingly, enjoyment was the second most important motivator for feeding in the UK too. Participants from both countries were also motivated by reasons associated with a meaningful connection with nature as well as the more objective goal of observation (Chapman, 2015). Thus, despite seemingly significant differences in the status of the pastime between the two countries, their citizens share many of the same motivations for bird feeding.
The obvious popularity and widespread practice of garden bird feeding in the Northern Hemisphere is often acknowledged to have a generally positive value for both human participants and birds (e.g., see Baicich et al., 2015) with sometimes forceful arguments based on apparent welfare and conservation benefits being advanced in favor of the practice (Berthold and Mohr, 2006). Similar claims are also made by feeding proponents in the Southern Hemisphere (see Jones, 2011 for further details). Yet, Australia is notable in the extent to which the practice is opposed (Jones, 2017). While reasons given for this opposition are largely similar to concerns expressed elsewhere—potential dependency on anthropogenic foods, the spread of disease, inadequate nutrition, attracting predators and vermin, for example—despite their ubiquity, most are not based on robust empirical data (Jones, 2011; Murray et al., 2016). What little has been published has tended to demonstrate that these concerns, while justified, are often less straightforward than was initially hypothesized (Robb et al., 2008a). This can be illustrated with reference to the first three of these well-known issues. Numerous studies have found that the possibility of birds becoming dependent upon anthropogenic food supplements is a primary concern for both advocates and opponents of bird feeding (e.g., Rollinson et al., 2003; Jones and Reynolds, 2008). There are certainly examples of avian populations that are entirely reliant on supplementary food in winter, including tits in Finland (Jansson et al., 1981) and Anna's hummingbirds Calypte anna in British Columbia, Canada, that feed from heated feeders supplying sugar solution to them even in the coldest weather (Jones, 2017). In these cases, birds exhibit full dependence on food supplements with their survival through the winter not being possible without access to them.
Such examples also illustrate another unforeseen outcome increasingly being attributed to an abundance of, and dependency of birds on, anthropogenic food: the tendency of some individuals or groups to overwinter or alter their migration route (Courter et al., 2013). However, even among the well-studied examples such as Eurasian blackcaps Sylvia atricapilla (Plummer et al., 2015) and white storks Ciconia ciconia (Massemin-Challet et al., 2006), it is difficult to disentangle various other potential influences such as the effects of climate change.
In the few studies where dependency of supplementary food has been explicitly investigated in resident species, the predicted outcomes did not eventuate. In their study of wintering black-capped chickadees in Wisconsin in the US, Brittingham and Temple (1992) found that a population supplied with supplementary food for 25 years had an identical survivorship to that of an unfed (control) population nearby. In Australia, adult Australian magpies continued to provide natural foods to their nestlings even when large supplies of favored foods were readily available (O'Leary and Jones, 2006). There are numerous other studies from a wide variety of species that strongly suggest that in the vast majority of cases individuals that visit feeders do so in rather a sporadic fashion with the diet comprising mainly natural food sources (e.g., Harrison, 2010; Robb et al., 2011; but see Sauter et al., 2006). Nevertheless, the proportion of the diet constituted by food supplements can vary seasonally (e.g., Chamberlain et al., 2007).
One of the most obvious characteristics of bird feeding is that, unlike natural food sources, food supplements are typically made available regularly, in a surfeit, and in the same location. This has a consequence of concentrating many birds in one place, often including species that are unlikely to interact when foraging naturally, as they compete closely for access to food. As well as increasing aggressive interactions (e.g., Wojczulanis-Jakubas et al., 2015; Le Louarn et al., 2016; and presumably stress levels), these aggregations also provide ideal conditions under which infectious agents persist and spread. Perhaps the best-studied example is provided by the so-called House Finch Disease, a particularly virulent form of conjunctivitis spread by mycoplasmal bacteria (e.g., Dhondt et al., 2005). Within a few months of this disease's appearance among house finches Carpodacus mexicanus in the mid-1990s near Washington DC in the US it was reported by participants in the Cornell Lab of Ornithology's Project FeederWatch. This citizen science program already had a large and active network of members and, having been informed about the disease outbreak, they were able to provide real-time information on the spread of the epidemic as it moved rapidly through the eastern US (Bonney and Dhondt, 1997). Within just a few years, the house finch population in the eastern US had declined by a third, partly because of the gregariousness of the species, but especially because of the bacteria's capacity to remain viable on damp feeding structures (Adelman et al., 2015). Although there is evidence that access to feeders enabled some infected birds to survive for longer (by being able to access food despite being sight-impaired), their tendency to remain for prolonged periods on or near feeders undoubtedly increased the likelihood of infection (Adelman et al., 2015). No cure or antidote has been developed for this disease and incidents are still reported.
A similar disease phenomenon occurred in the UK in 2005–2006 involving a trichomoniasis epidemic among several finch (Fringillidae) species but primarily impacting European greenfinches Chloris chloris. This species had been one of the chief beneficiaries of the increase in the popularity of bird feeding over the preceding decades, its considerable increase in abundance having been attributed at least in part to its attraction to nyjer seed provided as a food supplement (Lawson et al., 2012a). Within a single year of the outbreak of this extremely contagious bacterial infection, the British greenfinch population declined by 35% (Robinson et al., 2010). A detailed picture of the spatio-temporal dynamics of the disease was only made possible through the network of participants in the British Trust for Ornithology's (BTO's) Garden BirdWatch citizen science program (Robinson et al., 2010). As for the House Finch Disease outbreak, the role of feeders as sites for disease transmission was called into question for the trichomoniasis outbreak. Prior to this, the disease had been unknown among finches; in mainland Europe, it was primarily associated with rural-dwelling columbids such as common wood pigeons Columba palumbus (Höfle et al., 2004). However, in recent decades this species traditionally found in the countryside, has increasingly become a resident of towns and cities, an unexpected move attributed both to declining food resources in rural areas and the proliferation of seed feeders within urban areas (Table 1; Lawson et al., 2012a). Although as yet unconfirmed, this suggests a potential source of cross-species transfer of infection that previously would have been unlikely.
These two disease outbreaks are highlighted mainly because of their scale and impact, but also because of the possible role bird feeding plays in increasing rates of infection in avian populations. Moreover, numerous other diseases may be similarly spread because of the interactions of birds at feeders. These include salmonellosis and Avian Pox, highlighting the importance of thorough and frequent cleaning of feeders as best practice when feeding birds (Lawson et al., 2012b). Despite the severity of these outbreaks, and the relatively high level of publicity associated with them, relationships between feeders and disease remain remarkably under-studied. In a rare exception, the presence of pathogens was investigated among common garden bird species in New Zealand, comparing individuals frequenting feeders with those that were not (Galbraith et al., 2017). All pathogens of interest—Salmonella, Chlamydophila and Avian Pox Virus—were detected in at least one of the species, with Salmonella enterica being present on ~7% of all feeding structures examined. In addition, birds using feeders carried greater parasite loads than those that did not, with common blackbirds having more helminths and house sparrows more feather lice (Phthiraptera) (Galbraith et al., 2017).
The importance of food resources in all aspects of the lives of animals is fundamental to understanding population and community dynamics. These complex interactions have been investigated experimentally in a vast number of supplementary feeding studies on many different species and in many biomes. However, remarkably little empirical data exist for comparison between cities and species. In part, this is due to the many challenges associated with undertaking scientifically robust studies in environments unavoidably occupied by high densities of people. Nonetheless, a growing number of important pioneering investigations are shedding light on the ecological influence of food supplements on the local community of birds. One recent study compared the changes in composition and abundance of the suburban bird community in Auckland, New Zealand over an 18-month period during which food was provided by householders, followed by its withdrawal (Galbraith et al., 2015). The results were dramatic for several of the species involved, with increased abundance of house sparrows and spotted doves Streptopelia chinensis—both introduced species—during the provisioning phase, while that of the native gray gerygone Gerygone igata was significantly lower. The influence of feeding in shaping avian communities was further emphasized when the community returned to its pre-supplementary feeding structure within only a few weeks of the cessation of feeding. An important (but rarely achieved) aspect of this study was the willing compliance of the participants to engage with the scientific objectives in stopping feeding birds when instructed to do so to allow the role of feeding to be investigated rigorously.
Although we have claimed that bird feeding is extremely popular and effectively ubiquitous, the timing, duration, and intensity of the practice can be markedly heterogeneous even over short spatial scales. For example, Lepczyk et al. (2004) found almost twice as many feeders in suburban as in rural areas of Michigan in the US. In the UK, Davies et al. (2012) attempted to discern patterns of participation in feeding at a national scale and found considerable variation, although the prevalence increased with the more detached house types and participant age. In a more detailed investigation within the city of Sheffield in the UK, Fuller et al. (2008) found a clear negative relationship between socioeconomic deprivation and the proportion of households participating in feeding. This study also found a notably robust positive correlation between the density of feeders and the overall abundance of birds. However, there was no such relationship between feeder density and species richness (i.e., feeding increases numbers of birds but not their diversity).
The remainder of this piece focuses on research priorities that should concern all of us with interests in bird feeding, whether researchers investigating the biological effects of providing food supplements to birds to address targeted research questions, or members of the general public feeding birds over long temporal and wide spatial scales. The world is urbanizing ever more rapidly (United Nations, Department of Economic and Social Affairs, Population Division, 2014) and as a consequence our interactions with wildlife generally, and birds more specifically, are likely to increase in frequency and, in the case of bird feeding, intensity. That urbanization will inevitably influence all aspects of avian life has not gone unnoticed by ornithologists; research examining how urbanization influences the behavior and ecology of birds has been summarized in a number of books over the last 20 years (e.g., Bird et al., 1996; Marzluff et al., 2001; Lepczyk and Warren, 2012; Gil and Brumm, 2014; Marzluff, 2014; Jones, 2017). Many provide invaluable accounts of how urbanization (and sub-urbanization) impacts birds in terms of their behavior, ecology, physiology, abundance, and distribution. The next major challenge, however, is to determine how food availability, especially through the provision of food supplements, influences the biology of birds in our urban centers while contemporaneously being able to control extrinsic factors that influence the biology of urban birds equally strongly (e.g., predation—Gering and Blair, 1999; temperature—Stager et al., 2016; light pollution—Kempenaers et al., 2010; noise pollution—Arroyo-Solís et al., 2013).
In the case of garden bird feeding, we think that it is highly unlikely that the practice will decline in popularity in countries where it is well-established. In fact, there is evidence to suggest that it may intensify as human populations are ever more concentrated in cities in the future where feeder density will inevitably increase (Fuller et al., 2008). Therefore, it may be timely to harness further the power of citizen science (Dickinson and Bonney, 2012) to investigate how feeding influences individual birds, and avian populations and communities in a concerted and structured way. Citizen science has significantly advanced our understanding of various aspects of the breeding biology of birds (e.g., phenology, clutch size, productivity; reviewed in Cooper et al., 2015) and promises significant accumulations of further knowledge through carefully planned and coordinated research projects (Greenwood, 2007). We see no reason why those engaged in bird feeding would not embrace the opportunity to carry out similar projects that improve our understanding of its impacts on urban birds.
Below, we retain the comparative perspectives offered by ongoing research in the Northern and Southern Hemispheres to explain what we consider to be the key future research priorities. Such an approach allows us to compare and contrast the responses to feeding of species between different ecological (and avian) communities (Table 1), under different seasonal conditions and under different patterns for introduced species as well as native species sometimes in competition for food at feeders. Here, we pose a number of research questions that will allow us to gain further and new insights into how individuals, populations, and communities respond to bird feeding.
Modern field ornithology has access to an increasing number of methodologies that allow this question to be answered effectively. They include, for example, the marking of individual birds with devices such as Passive Integrated Transponder (PIT) tags that quantify visitation rates to feeders where receivers have been incorporated into feeder access points (e.g., Aplin et al., 2013). However, the number of feeder visits that a bird makes may reflect intense defense of food, and therefore the value that the bird places on this resource, but it may not indicate levels of food consumption. More invasive protocols involving tissue sampling enable methods such as fatty acid signature characterization to be carried out (Andersson et al., 2015) while stable isotope analysis (SIA; Inger and Bearhop, 2008) allows comparisons of biomarkers within samples with dietary reference material. Such approaches have revealed that there is much variability in dietary intake of food supplements within populations of blue tits visiting feeders in the winter in Northern Ireland (Robb et al., 2011). They were also used to determine the breeding diet of adult great tits and blue tits in central England that contained only a small percentage (~9%) of food supplements, suggesting that birds were only “snacking” during visits to feeders (Harrison, 2010). Furthermore, food supplements were estimated to constitute only ~9% of nestling diet suggesting that adults were feeding natural foods such as Lepidopteran larvae to their offspring (Harrison, 2010). Statistical approaches that allow dietary reconstruction from SIA outputs using Bayesian modeling are growing ever more sophisticated (e.g., Parnell et al., 2013) and now extend to multiple food (reference) sources. These approaches promise much in improving knowledge of urban birds' dependence on food supplements.
Examination of the patterns of feeder use by birds exposed to long-term food supplementation and how they relate to over winter-survival, recruitment into the breeding population, investment of energy, time, etc. to current vs. future breeding attempts. This could be achieved through surveys combining monitoring efforts of both feeders and nests, and intense tissue sampling of birds at feeders and of birds and/or their eggs at nests to describe diet composition. The challenge will be directly relating dietary composition to reproductive and life-history traits of birds when we know that they are sensitive to so many intrinsic and extrinsic factors.
Feeding studies of birds across entire cities are well-suited to citizen science approaches and, indeed, many citizens have contributed winter observations of feeder-visiting birds to the BTO through the Garden Bird Feeding Survey (GBFS; 1970/1971 to the present day) in the UK (Chamberlain et al., 2005) and to the Cornell Lab of Ornithology through Project FeederWatch (1987/1988 to the present day) in the US (Bonter, 2012). Both surveys provide invaluable long-term species richness and abundance data over large spatial scales but neither is targeted specifically at urban areas and food availability is not manipulated in the sense that provisioning is not experimentally prescriptive. Manipulative feeding studies have attempted to mimic urban habitats by providing food supplements over long periods from feeders in high density. One such study (Robb et al., 2008b) was carried out over the winter and, despite feeding finishing 6 weeks before the breeding season began, blue tits produced on average one extra fledgling per breeding attempt compared with unsupplemented (control) birds. Harrison et al. (2010) supplemented birds in a UK deciduous woodland over the spring and early summer and found that brood sizes of both blue tits and great tits were reduced compared with unsupplemented (control) birds. We know that some urban bird species demonstrate reduced clutch size and productivity in urban landscapes (Chamberlain et al., 2009), but the findings of Harrison et al. (2010) were unexpected because it took place in a preferred breeding habitat for the focal species and typically food supplementation advances laying onset and either increases or has no statistically significant effect on clutch size (see Table 2 of Robb et al., 2008a).
Examination of whether birds exposed to long-term food supplementation over-commit in defense of food sources and/or breeding habitat resulting in reduced investment of energy, time etc. in breeding attempts. This could be achieved through surveys combining monitoring efforts of both feeders and nests. The challenge will be recruiting sufficient participants to undertake monitoring of both nests and feeder use by breeding birds.
Carry-over effects are defined as the outcome of processes experienced by an organism in one season that subsequently influence its performance in the following season. Harrison et al. (2011) reviewed the potential mechanisms through which such effects might be mediated, focusing especially on macronutrient availability and downstream effects such as reproductive success and survival. The feeding of urban birds appears to be an ideal “study system” to test some of their ideas. For example, much could be learned from an investigation of the differences in how birds access and use food resources in the pre-breeding season, how they translate into “state” differences between birds that make the transition into the breeding season, and ultimately whether these map onto fitness differences between birds (e.g., Bearhop et al., 2004; Gunnarsson et al., 2005; see Table 1 in Harrison et al., 2011 for further examples). Robb et al. (2008b) found evidence for such carry-over effects in blue tits fed over the winter that exhibited increased fledging success the following breeding season compared with unsupplemented (control) birds. More investigations need to take place within the urban context as a matter of urgency. Of course, it is one thing to examine how protracted feeding of birds influences their breeding performance within one annual cycle; it is quite another to study how food supplementation throughout the life of an urban bird influences its lifetime reproductive success (Newton, 1991).
Examination of whether birds exposed to long-term food supplementation exhibit carry-over effects that result in their improved breeding performance and survival. This could be achieved through surveys combining monitoring efforts of both feeders and nests. The challenge will be controlling for major sources of variation between birds in breeding experience, age, and onset of senescence.
Food supplementation is a potent tool in applied conservation of endangered species when it is employed to promote the establishment of new populations in areas that have been ecologically restored (e.g., Florida scrub-jays Aphelocoma coerulescens—Schoech et al., 2008), augment the nutritional status of a non-breeding fraction of a population to the point where more individuals recruit to the breeding population (e.g., kakapo Strigops habroptilus—Powlesland and Lloyd, 1994), and increase the productivity of translocated populations (e.g., stitchbirds [or “hihi”] Notiomystis cincta—Castro et al., 2003). However, it is not a panacea and in the case of kakapo, as well as promoting recruitment, food supplementation resulted in unpredicted adverse effects such as breeding adult obesity (Powlesland and Lloyd, 1994) and an unwanted skew toward a male-biased sex ratio in offspring (Clout et al., 2002).
In light of the negative (as well as the positive) effects of intentional feeding of birds outlined above, we feel that it is critical that research be directed urgently toward understanding the impacts of feeding on the mating behaviors of urban birds. Our focus in this context is on the role that feeding plays in connecting bird populations within towns and cities through the movements of individual birds. After all, such connectivity may be of major importance in the processes of natural selection (i.e., survival), sexual selection (i.e., mate choice and, hence, mating systems), and gene flow. In turn, it has downstream effects on population dynamics, the distribution of species, the spatial distribution of genetic diversity, and urban ecosystem functioning (Unfried et al., 2013; LaPoint et al., 2015). Ultimately, it is essential for the long-term viability of metapopulations since it reduces local extinctions, accelerates recolonization, and controls the detrimental effects of inbreeding (Unfried et al., 2013).
One mechanism that would bring about increased genetic diversity in urban compared with rural populations of birds would be changes in mating systems of birds as a result of feeding. One could envisage that feeding might result in males guarding their social mates more intensely, resulting in reduced incidents of broods containing extra-pair young (EPY) and reduced proportions of broods containing EPY (e.g., O'Brien and Dawson, 2011); equally, the opposite may be just as likely with feeding resulting in better-fed males and females of social pairs seeking out more extra-pair copulations (EPCs) with the outcome being greater incidents of broods containing EPY and greater proportions of broods comprising EPY (e.g., Hoi-Leitner et al., 1999). To the best of our knowledge, no urban study has tested such hypotheses although Smith (2011) provided food supplements to a woodland population of birds throughout the spring and early summer. This study mimicked urban bird feeding and found that feeding had no direct effect on the proportion of EPY within broods (Figure 3). Clutch size had a significant effect on the incidence of EPY with the direction of this relationship influenced by supplementary feeding; the proportion of EPY increased with clutch size in broods raised in unfed (control) areas of the woodland, but decreased in areas where supplementary food was available (Figure 3). Although this suggests that food availability may influence the mating decisions of birds, supplementary feeding throughout the breeding season appears to have no direct effect on extra-pair paternity (EPP) rate (Møller, 1986). Clearly, many mechanisms by which feeding influences the mating strategies and gene flow in urban bird populations remain unresolved.
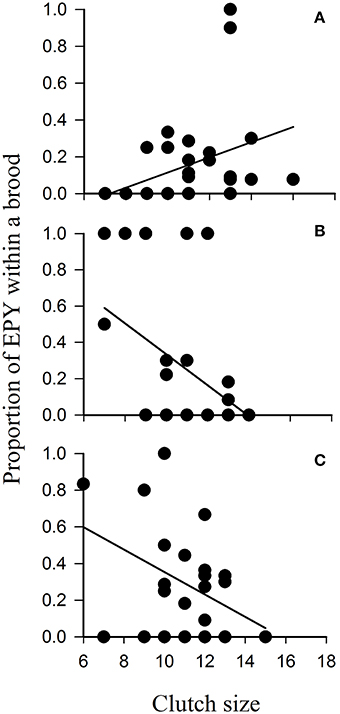
Figure 3. Proportion of extra-pair young (EPY) within each brood in relation to clutch size of blue tits Cyanistes caeruleus breeding in Chaddesley Woods National Nature Reserve, Worcs., UK in 2008 and 2009 that were (A) unfed controls, (B) supplemented with peanut cake, or (C) supplemented with peanut cake and live mealworms Tenebrio molitor. Adapted from Smith (2011).
Examination of whether birds exposed to long-term food supplementation exhibit changes in mating behavior that play out through changes in movements across cityscapes and in the degree of EPP found across and within broods. This could be achieved through heavy investments in banding (i.e., “ringing”) in combination with intensive resighting programs across cities and in banding-retrapping studies at nests where both social adults and nestlings are all tissue sampled to provide genetic samples for parentage analyses. The challenge will be banding enough birds to generate meaningful movement and parentage data.
Artificially increasing food availability through feeding birds in urban centers has the potential to act as an ecological trap (Schlaepfer et al., 2002). Feeding may provide a false environmental cue of habitat quality that invokes behaviors (e.g., choice of foraging locations, sedentariness) and life-history strategies (e.g., timing of breeding, clutch and brood sizes) that may prove to be maladaptive. In fact, bird feeding can be viewed more broadly as an evolutionary trap that can result in local extinctions because birds are unable to keep pace through adaptation with rapidly changing environmental conditions. The idea of adverse effects on urban bird populations mediated by ecological traps can be extended also to consider the idea of cities as habitat mosaics, some habitat types producing more birds than they lose through mortality (so-called “source” areas) while others lose more birds through local mortality than they gain through local breeding success (so-called “sink” areas; Pulliam, 1988). Dias (1996) described how source-sink dynamics can be played out in population regulation, outlining processes that might result in a re-distribution of birds across a cityscape driven by movements of birds from source to sink habitats. Importantly, such ideas explain why maladaptations persist in urban bird populations but also how local extinctions can occur rapidly. An appreciation of source and sink habitats driven by food availability (i.e., feeding in this context) informs conservation managers in assessing the importance, and the need for conservation, of source habitats within cities (Leston and Rodewald, 2006) because if only sink habitats are prioritized, local populations may be at risk of extirpation.
Examination of whether feeding of urban birds creates ecological traps resulting in differential production, mortality, and migration between different areas of cities. This could be undertaken through heavy investments in banding-resighting programs across cities, in nest and feeder monitoring to assess annual productivity and to define potential source and sink habitats, respectively, and in estimating turnover of birds across cityscapes. The challenge will be investing sufficiently in banding and nest monitoring to generate a city-wide assessment of source-sink dynamics.
Perhaps the most concerning impact of bird feeding is its potential to spread disease. Although there is often strong defense of feeding in response to this concern (see Jones, 2017), numerous studies have strongly implicated feeders as mediating infection and exacerbating the virulence of outspread, most markedly in the case of house finch mycoplasma (Hartup et al., 2001) and trichomoniasis (Lawson et al., 2012a). The implications of this potential link between likelihood of epidemics and bird feeders are sufficiently significant to warrant a high level of concern and action among all people engaged in the pastime. The response may be as simple as regular hygienic practices (Cleary et al., 2016). However, the best such approaches are not “second nature” to the bird feeding public, and thus much remains to be developed both in terms of adding to knowledge and effective communication of pertinent research outputs to the “end user” (Galbraith et al., 2014). Moreover, the relationship between feeders and transmission of disease is far from clear or straightforward.
Widespread surveillance of feeding platforms to assess the background levels of a range of common diseases and parasites, potentially through the existing networks of citizen scientists already engaged in programs such as Project FeederWatch and Garden BirdWatch. Simple protocols are already suitable for rolling out to address this research question (see Galbraith et al., 2017 for further details). The challenge will be building sufficiently strong relationships with the bird feeding public that they will buy into this research aspiration, one that could markedly change the way that birds are fed depending upon research findings.
We know that in the UK feeding in cities results in peaks in numbers of farmland bird species such as the yellowhammer Emberiza citrinella in the late spring in response to food deficits on farmland caused by changes in agricultural practise such as the loss of winter stubbles (Chamberlain et al., 2005). Such transient incursions into urban areas from surrounding countryside typically result in temporary changes to urban bird community structure. In contrast, changes in migration patterns of some species such as the Eurasian blackcap have led to more enduring changes to community structure because birds are now present throughout the winter in the UK. This is a trend that has grown in blackcaps in the last 60 years and appears to be closely related to the increased availability of feeders in gardens (Plummer et al., 2015). We expect that urban bird communities will continue to be sensitive to anthropogenic feeding practices resulting in foods being available year-round. This will increase the carrying capacity of urban habitats despite also potentially increasing the creation of many more ecological/evolutionary traps and sink habitats (Leston and Rodewald, 2006).
As well as changes in short-distance movements and seasonal migratory patterns of native species, feeding in urban centers has also favored the predominance of introduced species in New Zealand. Galbraith et al. (2015) found greater abundances of introduced house sparrows and spotted doves at feeders in Auckland and reduced abundances of native species such as gray gerygones. While feeders clearly attract greater numbers of the species using them, they may also reduce species richness in the avian community because of the predominance of introduced species. Certainly, in the UK and the rest of temperate Europe the introduced rose-ringed parakeet Psittacula krameri has established in many cities with its success at least in part associated with access to urban bird feeders (Clergeau and Vergnes, 2011). This species not only outcompetes many native species for food but also for nest sites (e.g., Strubbe and Matthysen, 2009). However, we urge caution in uncritically assuming that introduced avian species always have negative implications. For example, despite many local studies suggesting that common starlings Sturnus vulgaris, one of the most abundant introduced birds in the US, negatively impact native species, Koenig (2003) could find no evidence for this contention in a nationwide analysis of bird census data. Moreover, while many people feeding birds do not enjoy the presence of parakeets, others value them as exotic and colorful additions to the local avifauna (e.g., Menchetti et al., 2016).
Monitoring of the community of birds that routinely forage at urban feeders to determine whether long-term feeding results in changes in the avian community. This could be achieved through heavy investments in feeder monitoring not only to record species richness but also to undertake behavioral observations between introduced and native species to detect early warning signs of competitive exclusion of the latter (Grarock et al., 2012). The challenge will be investing sufficiently in behavioral data collection to define adverse effects of feeding from the perspective of the avian community.
The research questions posed here are framed by the comparison of the impacts of feeding on urban birds between the Northern and Southern Hemispheres. Rather than establishing lines of investigation in countries where bird feeding is not well-established, we suggest that such research should be carried out in countries where many households are engaged in bird feeding activities. All of the research questions raised require sustained engagement with the bird feeding public who we feel would be readily recruited to citizen science programs to provide banding capacity, food supplements at urban feeders, and feeder monitoring (Fuller et al., 2012; Amrhein, 2014).
Target countries in the Northern Hemisphere include Belgium, Canada, Germany, Sweden, the UK, and the US that already have well-established banding programs (Balmer et al., 2008). In fact, banding takes place in most European countries with the European Union for Bird Ringing (EURING) coordinating banding schemes to ensure consistency in data collection and overseeing the exchange of banding data between different countries (Balmer et al., 2008). Furthermore, the UK, Canada, and the US also have well-established feeder monitoring programs that form a solid foundation for integration of banding and feeding activities into a comprehensive and rigorous urban citizen science network for data collection (Dickinson and Bonney, 2012).
There are fewer countries in the Southern Hemisphere in which banding and feeder monitoring programs run in parallel so the first step would be to establish a garden bird watch scheme in a country such as South Africa where banding is well established (Jones, 2017) but is not paralleled by a systematic survey of feeding practices by its citizens. South Africa could follow in the footsteps of New Zealand where the New Zealand Garden Bird Survey was started in 2007 (Spurr, 2012). This survey was modeled on the Royal Society for the Protection of Birds' (RSPB's) Big Garden Birdwatch that has taken place in the UK since 1979. Spurr (2012) provided an informative account of results from the first 4 years of the New Zealand scheme and found that citizens have a strong appetite for engaging in citizen science programs. For example, he found that 66% of survey returns came from gardens in which food supplements were provided and that 76% came from urban, as opposed to rural, locations. Of course, the latter observation probably simply reflects how the human population is distributed across the country but it also highlights the fact that there is potential in New Zealand to carry out research that we envisage. In Australia the Birds in Backyards program run by BirdLife Australia has recently included surveys among its members focused on feeding and watering of birds. It is now discussing research partnerships with several of the country's universities.
Much has been written about how to establish, coordinate, and collate research outputs from citizen science programs (e.g., Greenwood, 2007; Dickinson and Bonney, 2012). Therefore, it is not necessary here to “go over old ground.” However, it is clear that our research questions cannot be answered by scientists alone. By taking a north-south perspective and working with urban-dwelling citizen scientists, as we propose, we believe that we will maximize our understanding of bird feeding, a phenomenon that is showing absolutely no signs of waning.
Conceived the review and wrote the paper: SR, JG, JS, and DJ. Contributed funding: SR and DJ. Wrote the paper: SR, JG, JS, and DJ.
This research was funded in part through Ph.D. studentships to JG and JS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are most grateful to the three topic editors—Caroline Isaksson, Diego Gil, and Amanda Rodewald—for establishing this research topic, particularly the former for encouraging us to pursue the direction this paper has taken. We would also like to extend thanks to Jon Sadler, Victoria Pattison-Willits, and two reviewers for critical comments on earlier drafts of the manuscript. We also thank Robyn Bailey and Chelsea Benson at the Cornell Lab of Ornithology and Oliver Smart of Smart Images for assistance in sourcing images of birds at feeders as shown in Figure 1.
Adelman, J. S., Moyers, S. C., Farine, D. R., and Hawley, D. M. (2015). Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. R. Soc. B 282:20151429. doi: 10.1098/rspb.2015.1429
Alberti, M., Correa, C., Marzluff, J. M., Hendry, A. P., Palkovacs, E. P., Gotanda, K. M., et al. (2017). Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl. Acad. Sci. U.S.A. doi: 10.1073/pnas.1606034114. [Epub ahead of print].
Amrhein, V. (2014). “Wild bird feeding (probably) affects avian urban ecology,” in Avian Urban Ecology: Behavioural and Physiological Adaptations, eds D. Gil and H. Brumm (Oxford: Oxford University Press), 29–38.
Andersson, M. N., Wang, H.-L., Nord, A., Salmón, P., and Isaksson, C. (2015). Composition of physiologically important fatty acids in great tits differs between urban and rural populations on a seasonal basis. Front. Ecol. Evol. 3:93. doi: 10.3389/fevo.2015.00093
Aplin, L. M., Farine, D. R., Morand-Ferron, J., Cole, E. F., Cockburn, A., and Sheldon, B. C. (2013). Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecol. Lett. 16, 1365–1372. doi: 10.1111/ele.12181
Arroyo-Solís, A., Castillo, J. M., Figueroa, E., López-Sánchez, J. L., and Slabbekoorn, H. (2013). Experimental evidence for an impact of anthropogenic noise on dawn chorus timing in urban birds. J. Avian Biol. 44, 288–296. doi: 10.1111/j.1600-048X.2012.05796.x
Baicich, P. J., Barker, M. A., and Henderson, C. L. (2015). Feeding Wild Birds in America: Culture, Commerce, and Conservation. College Station, TX: Texas A&M University Press.
Balmer, D., Coiffait, L., Clark, J., and Robinson, R. (2008). Bird Ringing: A Concise Guide. Thetford: British Trust for Ornithology.
Barker, M. A., and Griggs, J. (2000). The FeederWatcher's Guide to Bird Feeding. New York, NY: HarperCollins.
Bearhop, S., Fiedler, W., Furness, R. W., Votier, S. C., Waldron, S., Newton, J., et al. (2005). Assortative mating as a mechanism for rapid evolution of a migratory divide. Science 310, 502–504. doi: 10.1126/science.1115661
Bearhop, S., Hilton, G. M., Votier, S. C., and Waldron, S. (2004). Stable isotope ratios indicate that body condition in migrating passerines is influenced by winter habitat. Proc. R. Soc. Lond. B 271(Suppl. 4), S215–S218. doi: 10.1098/rsbl.2003.0129
Beck, A. M., Melson, G. F., da Costa, P. L., and Liu, T. (2001). The educational benefits of a ten-week home-based wild bird feeding program for children. Anthrozoös 14, 19–28. doi: 10.2752/089279301786999599
Bird, D. M., Varland, D. E., and Negro, J. J. (1996). Raptors in Human Landscapes: Adaptation to Built and Cultivated Environments. London: Academic Press.
Bonney, R., and Dhondt, A. A. (1997). “FeederWatch: an example of a student-scientist partnership,” in Internet Links for Science Education, ed K. C. Cohen (New York, NY: Plenum Press), 31–53.
Bonter, D. N. (2012). “From backyard observations to continent-wide trends: lessons from the first twenty-two years of Project FeederWatch,” in Citizen Science: Public Participation in Environmental Research, eds J. L. Dickinson and R. Bonney (Ithaca, NY: Comstock Publishing Associates), 27–35.
Boutin, S. (1990). Food supplementation experiments with terrestrial vertebrates: patterns, problems, and the future. Can. J. Zool. 68, 203–220. doi: 10.1139/z90-031
Brittingham, M. C., and Temple, S. A. (1992). Does winter bird feeding promote dependency? J. Field Ornithol. 63, 190–194.
Castro, I., Brunton, D. H., Mason, K. M., Ebert, B., and Griffiths, R. (2003). Life history traits and food supplementation affect productivity in a translocated population of the endangered Hihi (Stitchbird, Notiomystis cincta). Biol. Conserv. 114, 271–280. doi: 10.1016/S0006-3207(03)00046-6
Chamberlain, D. E., Cannon, A. R., Toms, M. P., Leech, D. I., Hatchwell, B. J., and Gaston, K. J. (2009). Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151, 1–18. doi: 10.1111/j.1474-919X.2008.00899.x
Chamberlain, D. E., Toms, M. P., Cleary-McHarg, R., and Banks, A. N. (2007). House sparrow (Passer domesticus) habitat use in urbanized landscapes. J. Ornithol. 148, 453–462. doi: 10.1007/s10336-007-0165-x
Chamberlain, D. E., Vickery, J. A., Glue, D. E., Robinson, R. A., Conway, G. J., Woodburn, R. J. W., et al. (2005). Annual and seasonal trends in the use of garden feeders by birds in winter. Ibis 147, 563–575. doi: 10.1111/j.1474-919x.2005.00430.x
Chapman, R. A. (2015). Why Do People Feed Wildlife? An International Comparison. Ph.D. thesis, Griffith University, Nathan.
Clark, D. (2013). A Study of the Motivation of the General Public in Feeding Birds in Their Gardens. M.Sc. thesis, University of Birmingham, Birmingham.
Cleary, G. O., Coleman, B. R., Davis, A. D., Jones, D. N., Miller, K. K., and Parsons, H. (2016). Keeping it clean: bird bath hygeine in urban and rural areas. J. Urban Ecol. 2, 1–4. doi: 10.1093/jue/juw005
Clergeau, P., and Vergnes, A. (2011). Bird feeders may sustain feral rose-ringed parakeets Psittacula krameri in temperate Europe. Wildl. Biol. 17, 248–252. doi: 10.2981/09-092
Clout, M. N., Elliott, G. P., and Robertson, G. P. (2002). Effects of supplementary feeding on the offspring sex ratio of kakapo: a dilemma for the conservation of a polygynous parrot. Biol. Conserv. 107, 13–18. doi: 10.1016/S0006-3207(01)00267-1
Cooper, C. B., Bailey, R. L., and Leech, D. I. (2015). “The Role of Citizen Science in Studies of Avian Reproduction,” in Nests, Eggs, and Incubation: New Ideas about Avian Reproduction, eds D. C. Deeming and S. J. Reynolds (Oxford: Oxford University Press), 208–220.
Courter, J. R., Johnson, R. J., Bridges, W. C., and Hubbard, K. G. (2013). Assessing migration of Ruby-throated Hummingbirds (Archilocus colubris) at broad spatial and temporal scales. Auk 130, 107–117. doi: 10.1525/auk.2012.12058
Cox, D. T., and Gaston, K. J. (2015). Likeability of garden birds: importance of species knowledge & richness in connecting people to nature. PLoS ONE 10:e0141505. doi: 10.1371/journal.pone.0141505
Cox, D. T., and Gaston, K. J. (2016). Urban bird feeding: connecting people with nature. PLoS ONE 11:e0158717. doi: 10.1371/journal.pone.0158717
Davies, Z. G., Fuller, R. A., Dallimer, M., Loram, A., and Gaston, K. J. (2012). Household factors influencing participation in bird feeding activity: a national scale analysis. PLoS ONE 7:e39692. doi: 10.1371/journal.pone.0039692
Dhondt, A. A., Altizer, S., Cooch, E. G., Davis, A. K., Dobson, A., Driscoll, M. J., et al. (2005). Dynamics of a novel pathogen in an avian host: mycoplasmal conjunctivitis in house finches. Acta Trop. 94, 77–93. doi: 10.1016/j.actatropica.2005.01.009
Dhondt, A. A., Dhondt, K. V., Hawley, D. M., and Jennelle, C. S. (2007). Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol. 36, 205–208. doi: 10.1080/03079450701286277
Dias, P. C. (1996). Sources and sinks in population biology. Trends Ecol. Evol. 11, 326–330. doi: 10.1016/0169-5347(96)10037-9
Dickinson, J. L., and Bonney, R. (2012). Citizen Science: Public Participation In Environmental Research. Ithaca, NY: Comstock Publishing Associates.
Fuller, R. A., Irvine, K. N., Davies, Z. G., Armsworth, P. R., and Gaston, K. J. (2012). “Interactions between people and birds in urban landscapes,” in Urban Bird Ecology and Conservation, eds C. Lepczyk and P. Warren (Berkeley, CA: University of California Press), 249–266.
Fuller, R. A., Warren, P. H., Armsworth, P. R., Barbosa, R., and Gaston, K. J. (2008). Garden bird feeding predicts the structure of urban avian assemblages. Diversity Distrib. 14, 131–137. doi: 10.1111/j.1472-4642.2007.00439.x
Galbraith, J. A. (2016). Ecological Impacts of Supplementary Feeding on Urban Bird Communities in New Zealand. Ph.D. thesis, University of Auckland, Auckland.
Galbraith, J. A., Beggs, J. R., Jones, D. N., McNaughton, E. J., Krull, C. R., and Stanley, M. C. (2014). Risks and drivers of wild bird feeding in urban areas of New Zealand. Biol. Conserv. 180, 64–74. doi: 10.1016/j.biocon.2014.09.038
Galbraith, J. A., Beggs, J. R., Jones, D. N., and Stanley, M. C. (2015). Supplementary feeding restructures urban bird communities. Proc. Natl. Acad. Sci. U.S.A. 112, 1–10. doi: 10.1073/pnas.1501489112
Galbraith, J. A., Stanley, M. C., Jones, D. N., and Beggs, J. R. (2017). Experimental feeding regime influences urban bird disease dynamics. J. Avian Biol. doi: 10.1111/jav.01076. [Epub ahead of print].
Gering, J. C., and Blair, R. B. (1999). Predation on artifical bird nests along an urban gradient: predatory risk or relaxation in urban environments? Ecography 22, 532–541. doi: 10.1111/j.1600-0587.1999.tb00542.x
Gil, D., and Brumm, H. (2014). Avian Urban Ecology: Behavioural and Physiological Adaptations. Oxford: Oxford University Press.
Gillanders, R., Awasthy, M., and Jones, D. N. (in press). Extreme dietary switching: widespread consumption of meat by rainbow lorikeets at garden bird feeders in Australia. Corella.
Grarock, K., Tidemann, C. R., Wood, J., and Lindenmayer, D. B. (2012). Is it benign or is it a pariah? Empirical evidence for the impact of the common myna (Acridotheres tristis) on Australian birds. PLoS ONE 7:e40622. doi: 10.1371/journal.pone.0040622
Greenwood, J. J. D. (2007). Citizens, science and bird conservation. J. Ornithol. 148, S77–S124. doi: 10.1007/s10336-007-0239-9
Gunnarsson, T. G., Gill, J. A., Newton, J., Potts, P. M., and Sutherland, W. J. (2005). Seasonal matching of habitat quality and fitness in a migratory bird. Proc. R. Soc. B 272, 2319–2323. doi: 10.1098/rspb.2005.3214
Harrison, T. J. E. (2010). A curate's egg: Feeding Birds during Reproduction is ‘Good in Parts’. A Study of Blue Tits Cyanistes caeruleus and Great Tits Parus major. Ph.D. thesis, University of Birmingham, Birmingham.
Harrison, T. J. E., Smith, J. A., Martin, G. R., Chamberlain, D. E., Bearhop, S., Robb, G. N., et al. (2010). Does food supplementation really enhance productivity of breeding birds? Oecologia 164, 311–320. doi: 10.1007/s00442-010-1645-x
Harrison, X. A., Blount, J. D., Inger, R., Norris, D. R., and Bearhop, S. (2011). Carry-over effects as drivers of fitness differences between animals. J. Anim. Ecol. 80, 4–18. doi: 10.1111/j.1365-2656.2010.01740.x
Hartup, B. K., Bickal, J. M., Dhondt, A. A., Ley, D. H., and Kollias, G. V. (2001). Dynamics of conjunctivitis and Mycoplasma gallisepticum infections in house finches. Auk 118, 327–333. doi: 10.1642/0004-8038(2001)118[0327:DOCAMG]2.0.CO;2
Höfle, U., Gortazar, C., Ortíz, J. A., Knispel, B., and Kaleta, E. F. (2004). Outbreak of trichomoniasis in a woodpigeon (Columba palumbus) wintering roost. Eur. J. Wildlife Res. 50, 73–77. doi: 10.1007/s10344-004-0043-2
Hoi-Leitner, M., Hoi, H., Romero-Pujante, M., and Valera, F. (1999). Female extra-pair behaviour and environmental quality in the serin (Serinus serinus): a test of the “constrained female hypothesis.” Proc. R. Soc. Lond. B 266, 1021–1026. doi: 10.1098/rspb.1999.0738
Horvath, T., and Roelands, A. M. (1991). Backyard feeders: not entirely for the birds. Anthrozoös 4, 232–236. doi: 10.2752/089279391787057080
Howard, P., and Jones, D. N. (2004). “A qualitative study of wildlife feeding in south-east Queensland,” in Urban Wildlife: More than Meets the Eye, eds D. Lunney and S. Burgin (Sydney: Royal Zoological Society of NSW), 55–62. doi: 10.7882/FS.2004.081
Inger, R., and Bearhop, S. (2008). Applications of stable isotope analyses to avian ecology. Ibis 150, 447–461. doi: 10.1111/j.1474-919X.2008.00839.x
Ishigame, G., and Baxter, G. S. (2007). Practice and attitudes of suburban and rural dwellers to feeding wild birds in Southeast Queensland, Australia. Ornithol. Sci. 6, 11–19. doi: 10.2326/1347-0558(2007)6[11:PAAOSA]2.0.CO;2
Jansson, C., Ekman, J., and Bromssen, A. (1981). Winter mortality and food supply in tits Parus spp. Oikos 37, 313–322. doi: 10.2307/3544122
Jones, D. (2011). An appetite for connection: Why we need to understand the effect and value of feeding wild birds. Emu 111, i–vii. doi: 10.1071/muv111n2_ed
Jones, D. (2014). “It's time to talk about feeding,” in Australian Birdlife (Carlton, VIC: Birdlife Australia), 8.
Jones, D. N., and Reynolds, S. J. (2008). Feeding birds in our towns and cities: a global 966 research opportunity. J. Avian Biol. 39, 265–271. doi: 10.1111/j.2008.0908-9678857.04271.x
Kempenaers, B., Borgström, P., Loës, P., Schlicht, E., and Valcu, M. (2010). Artificial night light affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. doi: 10.1016/j.cub.2010.08.028
Koenig, W. D. (2003). European Starlings and their effect on native cavity-nesting birds. Conserv. Biol. 17, 1134–1140. doi: 10.1046/j.1523-1739.2003.02262.x
LaPoint, S., Balkenhol, N., Hale, J., Sadler, J., and van der Ree, R. (2015). Ecological connectivity research in urban areas. Funct. Ecol. 29, 868–878. doi: 10.1111/1365-2435.12489
Lawson, B., Lachish, S., Colvile, K. M., Durrant, C., Peck, K. M., Toms, M. P., et al. (2012b). Emergence of a novel avian pox disease in British tit species. PLoS ONE 7:e40176. doi: 10.1371/journal.pone.0040176
Lawson, B., Robinson, R. A., Colvile, K. M., Peck, K. M., Chantrey, J., Pennycott, T. W., et al. (2012a). The emergence and spread of finch trichomonosis in the British Isles. Phil. Trans. R. Soc. B 367, 2852–2863. doi: 10.1098/rstb.2012.0130
Le Louarn, M., Couillens, B., Deschamps-Cottin, M., and Clergeau, P. (2016). Interference competition between an invasive parakeet and native bird species at feeding sites. J. Ethol. 34, 291–298. doi: 10.1007/s10164-016-0474-8
Lepczyk, C. A., Mertig, A. G., and Liu, J. (2004). Assessing landowner activities related to birds across rural-to-urban landscapes. Environ. Manage. 33, 110–125. doi: 10.1007/s00267-003-0036-z
Lepczyk, C. A., and Warren, P. S. (2012). Urban Bird Ecology and Conservation. Studies in Avian Biology No. 45. Berkeley, CA: University of California Press.
Leston, L. F., and Rodewald, A. D. (2006). Are urban forests ecological traps for understory birds? An examination using Northern Cardinals. Biol. Conserv. 131, 566–574. doi: 10.1016/j.biocon.2006.03.003
Malpass, J. S., Rodewald, A. D., and Matthews, S. N. (2017). Species-dependent effects of bird feeders on nest predators and nest survival of urban American Robins and Northern Cardinals. Condor 119, 1–16. doi: 10.1650/CONDOR-16-72.1
Marzluff, J. M. (2014). Welcome to Subirdia: Sharing Our Neighborhoods with Wrens, Robins, Woodpeckers, and Other Wildlife. New Haven, CT: Yale University Press.
Marzluff, J. M., Bowman, R., and Donnelly, R. (2001). Avian Ecology and Conservation in an Urbanizing World. Boston, MA: Kluwer Academic Publishers.
Massemin-Challet, S., Gendner, J.-P., Samtmann, S., Pichegru, L., Wulgué, A., and Le Maho, Y. (2006). The effect of migratory strategy and food availability on White Stork Ciconia ciconia breeding success. Ibis 148, 503–508. doi: 10.1111/j.1474-919X.2006.00550.x
Menchetti, M., Mori, E., and Angelici, F. M. (2016). “Effects of the recent world invasion by ring-necked parakeets Psittacula krameri,” in Problematic Wildlife, ed F. M. Angelici (Cham: Springer International Publishing), 253–266.
Møller, A. P. (1986). Mating systems among European passerines: a review. Ibis 128, 234–250. doi: 10.1111/j.1474-919x.1986.tb02671.x
Murray, M. H., Becker, D. J., Hall, R. J., and Hernandez, S. M. (2016). Wildlife health and supplemental feeding: a review and management recommendations. Biol. Conserv. 204, 163–174. doi: 10.1016/j.biocon.2016.10.034
O'Leary, R., and Jones, D. N. (2006). The use of supplementary foods by Australian magpies Gymnorhina tibicen: Implications for wildlife feeding in suburban environments. Aust. Ecol. 31, 208–216. doi: 10.1111/j.1442-9993.2006.01583.x
O'Brien, E. L., and Dawson, R. D. (2011). Plumage color and food availability affect male reproductive success in a socially monogamous bird. Behav. Ecol. 22, 66–72. doi: 10.1093/beheco/arq167
Parnell, A. C., Phillips, D. L., Bearhop, S., Semmens, B. X., Ward, E. J., Moore, J. W., et al. (2013). Bayesian stable isotope mixing models. Environmetrics 24, 387–399. doi: 10.1002/env.2221
Plummer, K. E., Siriwardena, G. M., Conway, G. J., Risely, K., and Toms, M. P. (2015). Is supplementary feeding in gardens a driver of evolutionary change in a migratory bird species? Glob. Change Biol. 21, 4353–4363. doi: 10.1111/gcb.13070
Powlesland, R. G., and Lloyd, B. D. (1994). Use of supplementary feeding to induce breeding in free-living kakapo Strigops habroptilus in New Zealand. Biol. Conserv. 69, 97–106. doi: 10.1016/0006-3207(94)90332-8
Pulliam, H. R. (1988). Sources, sinks, and population regulation. Am. Nat. 132, 652–661. doi: 10.1086/284880
Reynolds, S. J., Mänd, R., and Tilgar, V. (2004). Calcium supplementation of breeding birds: directions for future research. Ibis 146, 601–614. doi: 10.1111/j.1474-919x.2004.00298.x
Robb, G. N., McDonald, R. A., Chamberlain, D. E., and Bearhop, S. (2008a). Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. doi: 10.1890/060152
Robb, G. N., McDonald, R. A., Chamberlain, D. E., Reynolds, S. J., Harrison, T. J. E., and Bearhop, S. (2008b). Winter feeding of birds increases productivity in the subsequent breeding season. Biol. Lett. 4, 220–223. doi: 10.1098/rsbl.2007.0622
Robb, G. N., McDonald, R. A., Inger, R., Reynolds, S. J., Newton, J., and McGill, R. A. R. (2011). Using stable-isotope analysis as a technique for determining consumption of supplementary foods by individual birds. Condor 113, 475–482. doi: 10.1525/cond.2011.090111
Robinson, R. A., Lawson, B., Toms, M. P., Peck, K. M., Kirkwood, J. K., Chantrey, J., et al. (2010). Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 5:e12215. doi: 10.1371/journal.pone.0012215
Rollinson, D. J., O'Leary, R., and Jones, D. N. (2003). The practice of wildlife feeding in suburban Brisbane. Corella 27, 52–58.
Sauter, A., Bowman, R., Schoech, S. J., and Pasinelli, G. (2006). Does optimal foraging theory explain why suburban Florida scrub-jays (Aphelocoma coerulescens) feed their young human-provided food? Behav. Ecol. Sociobiol. 60, 465–474. doi: 10.1007/s00265-006-0187-z
Schlaepfer, M. A., Runge, M. C., and Sherman, P. W. (2002). Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. doi: 10.1016/S0169-5347(02)02580-6
Schoech, S. J., Bridge, E. S., Boughton, R. K., Reynolds, S. J., Atwell, J. W., and Bowman, R. (2008). Food supplementation: a tool to increase reproductive output? A case study in the threatened Florida Scrub-Jay. Biol. Conserv. 141, 162–173. doi: 10.1016/j.biocon.2007.09.009
Shanahan, D. F., Lin, B. B., Gaston, K. J., Bush, R., and Fuller, R. A. (2014). Landscape and urban planning socio-economic inequalities in access to nature on public and private lands: a case study from Brisbane, Australia. Landscape Urban Plan. 130, 14–23. doi: 10.1016/j.landurbplan.2014.06.005
Smith, J. A. (2011). From Nest Building to Life-History Patterns: Does Food Supplementation Influence Reproductive Behaviour of Birds? Ph.D. thesis, University of Birmingham, Birmingham.
Spurr, E. B. (2012). New Zealand Garden Bird Survey - analysis of the first four years. N. Z. J. Ecol. 36, 1–13.
Stager, M., Pollock, H. S., Benham, P. M., Sly, N. D., Brawn, J. D., and Cheviron, Z. A. (2016). Disentangling environmental drivers of metabolic flexibility in birds: the importance of temperature extremes versus temperature variability. Ecography 39, 787–795. doi: 10.1111/ecog.01465
Strubbe, D., and Matthysen, E. (2009). Experimental evidence for nest-site competition between invasive ring-necked parakeets (Psittacula krameri) and native nuthatches (Sitta europaea). Biol. Conserv. 142, 1588–1594. doi: 10.1016/j.biocon.2009.02.026
Tidemann, S., and Gosler, A. (2010). Ethno-Ornithology: Birds, Indigenous Peoples, Culture, and Society. London: Earthscan.
Unfried, T. M., Hauser, L., and Marzluff, J. M. (2013). Effects of urbanization on Song Sparrow (Melospiza melodia) population connectivity. Conserv. Genet. 14, 41–53. doi: 10.1007/s10592-012-0422-2
United Nations, Department of Economic Social Affairs, Population Division. (2014). World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352). New York, NY: The United Nations.
U.S. Fish Wildlife Service U.S. Census Bureau (2011). National Survey of Fishing, Hunting, and Wildlife-Associated Recreation. Washington, DC; Suitland, MD: U.S. Fish and Wildlife Service; U.S. Census Bureau.
Keywords: carry-over effects, citizen science, community structure, dependency, disease transmission, food supplements, human well-being, source-sink dynamics
Citation: Reynolds SJ, Galbraith JA, Smith JA and Jones DN (2017) Garden Bird Feeding: Insights and Prospects from a North-South Comparison of This Global Urban Phenomenon. Front. Ecol. Evol. 5:24. doi: 10.3389/fevo.2017.00024
Received: 31 January 2017; Accepted: 21 March 2017;
Published: 07 April 2017.
Edited by:
Caroline Isaksson, Lund University, SwedenReviewed by:
Jose I. Aguirre, Complutense University of Madrid, SpainCopyright © 2017 Reynolds, Galbraith, Smith and Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. James Reynolds, Si5SZXlub2xkcy4yQGJoYW0uYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.