
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 07 March 2017
Sec. Behavioral and Evolutionary Ecology
Volume 5 - 2017 | https://doi.org/10.3389/fevo.2017.00008
This article is part of the Research TopicThe Importance of Olfaction in Intra- and Interspecific CommunicationView all 7 articles
Parental care is costly enough that species exist which exploit the parental care of other individuals to rear their own brood, as social parasites do among social insects. Intraspecific, facultative social parasites use the nest and worker force of another colony of the same species to rear their own young as a reproductive strategy alternative to independent nest foundation. Intraspecific parasites face barriers similar to those of interspecific social parasites: they must bypass host nestmate recognition abilities and keep host workers under control. In the present study, we investigate the cuticular chemical signature of Polistes biglumis paper wasps when they behave as intraspecific social parasites and invade conspecific colonies. We performed our analysis on three geographically separated populations, which differ in their social structure; in one population foundresses regularly produce workers, whereas in the others they rarely do. We tested whether the chemical signature of females which parasitize conspecific colonies resembles that of females who found their colonies, and if this effect is similar among populations with high and low numbers of workers. Our results show that in the two populations where foundresses produce virtually no workers, the hydrocarbon signatures of intraspecific social parasites were not chemically distinct from those of the foundresses. In contrast, in the population where foundresses usually produce workers, the hydrocarbon signatures of intraspecific social parasites had a significantly larger proportion of long-chained and branched hydrocarbons than those of foundresses. These characteristics may have evolved in that population to facilitate parasite exploitation of the host workforce, as long-chained and branched hydrocarbons are relevant as recognition and fertility cues. The lack of workers in the other populations may have relaxed the selection pressure that host workers impose on the chemical signature of intraspecific social parasites.
Parental care is costly enough that species exist which exploit the parental care of other individuals to rear their own brood (i.e., brood parasites; Clutton-Brock, 1991; Royle et al., 2012). In social insects, where parental care is usually performed by workers, brood parasites exploit the social organization of host colonies (hence the name social parasites), and force the host worker caste to care for their brood, which is often composed of reproductive individuals only, as many species of social parasites produce no workers (Wilson, 1971; Hölldobler and Wilson, 1990). In this way, social parasites spare themselves not only the time and resources needed to build the nest and care for their young, but also those necessary to produce workers.
Studies on brood and social parasites have flourished in recent years, providing fascinating, novel examples of coevolution between parasites and hosts. These studies investigate reciprocal adaptations and counter-adaptations in the perspective of an arms race between parasites and hosts (Davies et al., 1989; Davies, 2011; Kilner and Langmore, 2011). For example, among bird brood parasites, cuckoos lay eggs which are nearly identical in shape, size and color to those of their hosts (Brooke and Davies, 1988; Davies, 2011; Spottiswoode et al., 2012). In host populations infested by cuckoos, hosts are often able to discriminate between their own and parasite eggs, and reject eggs dissimilar to their own; this ability declines when parasites become rarer (Thorogood and Davies, 2013) and does not exist in non-parasitized populations (Rothstein, 1990).
Whereas in birds the arms race between brood parasites and hosts mainly involves the visual mode, in social insects it is mainly based on olfactory cues (Kilner and Langmore, 2011). Social parasites and their offspring escape host detection and eventually coopt hosts into caring for them using chemical deception, which emerges through the selection pressures that hosts impose on social parasites by using chemical cues to discriminate against intruders (Lorenzi, 2006; Bagnères and Lorenzi, 2010). Nestmate/non-nestmate discrimination in social insects is mainly based on cuticular blends of hydrocarbons and is highly effective (Van Zweden and d'Ettorre, 2010), but social parasites circumvent it in different ways (Lenoir et al., 2001; Lorenzi, 2006; Bagnères and Lorenzi, 2010). For example, among primitively eusocial insects, such as Polistes social wasps, Polistes semenowi, and Polistes atrimandibularis parasites are poor in chemical recognition cues (Lorenzi and Bagnères, 2002; Lorenzi et al., 2004) and the same characteristic was found among eusocial insects, such as Polyergus slave making ant queens (Lenoir et al., 2001). It is likely that the scarcity of recognition cues makes it difficult for hosts to detect parasites, as the defensive responses of social insects are affected by the amount of recognition cues on the cuticle of intruders (the smaller the amount, the lower the aggressive response, Cini et al., 2009; Ichinose and Lenoir, 2010). Social parasites may also mimic host recognition cues as a way to bypass host detection, and again we have examples of such a deception trait in both eusocial and primitively eusocial insects. For example, both social wasp P. atrimandibularis and P. semenowi parasites, and the parasite queen of Acromyrmex insinuator ants match their host recognition cues (Bagnères et al., 1996; Lorenzi et al., 2004; Nehring et al., 2015), and this is likely to make it difficult for hosts to identify social parasites as intruders. Similar strategies have been reported in other inquiline ants (Guillem et al., 2014). Workers of Harpagoxenus sublaevis slave-making ants mimic the recognition cues of the different host species they enslave (Kaib et al., 1993; Bauer et al., 2010) and Solenopsis picea ants mimic their hosts, although to a limited extent (Emery and Tsutsui, 2016). In contrast, among the rare facultative slave-making ants (D'Ettorre and Heinze, 2001), Formica sanguinea ants integrate slaves in their colonies by contaminating them with the parasite odor (Wlodarczyk and Szczepaniak, 2014, 2017). Other social parasites use appeasement substances which reduce attacks by hosts. For example, a newly discovered special class of compounds, crematenones, enables Crematogaster ants to avoid aggression by Camponotus ants when they live in the same nest (parabiosis; Menzel et al., 2013).
Consistently with reports on how bird populations diverge locally in the reciprocal adaptations of hosts and parasites (Rothstein, 1990; Thorogood and Davies, 2013), the chemical strategies of integration into host nests may differ between populations of social parasites (Nash et al., 2008; Ruano et al., 2011). This is due to the uniqueness in the gene pools of both local parasites and local hosts, and to the uniqueness of the quality of the biological interactions which occur locally, both between hosts and parasites and with hosts, parasites and other interacting organisms (Thompson, 2005). In this perspective, studying multiple populations sheds light on the variety of evolutionary outcomes in the conflict of interests between hosts and parasites. However, reconstructing what pathways have led to the evolution of the specialized chemical integration strategies of social parasites has been challenging up to now. In this perspective, identifying intermediate steps along this route may increase our understanding of the evolution of deception mechanisms. Intraspecific social parasitism—where parasites use the nest and worker force of another individual of the same species to rear their own young—may represent the first step toward the evolution of obligate interspecific parasitism (Taylor, 1939; Cervo, 2006) and is widespread among insects (Field, 1992).
In birds, where intraspecific parasitism is a common female reproductive strategy, a recent review discusses the adaptive value of conspecific nest parasitism (Lyon and Eadie, 2008).
According to Lyon and Eadie (2008), intraspecific parasites may be females doing the best-of-a-bad-job when independent nesting is limited or restrained by environmental or phenotypic factors (e.g., when nesting sites are limited, or females emerge late from hibernation). Alternatively, intraspecific parasites may have lost their previous nests, e.g., to predators. Yet, they may be “lifelong specialist parasites,” i.e., females who rely exclusively on others to rear their own brood. The best-of-a-bad-job and the nest-loss hypotheses are conditional tactics and are maintained in the population even if they yield lower fitness payoffs than independent nesting (parasitizing conspecific nests is the only option to females who would not gain any fitness otherwise). In contrast, the lifelong-specialist-hypothesis implies that intraspecific parasitism is frequency-dependent, as it yields higher fitness payoffs than nesting when it is rare (Lyon and Eadie, 2008). Detailed studies are lacking that clarify which of these hypotheses explains intraspecific parasitism in social insects.
Whatever the adaptive benefits they gain, intraspecific parasites in social insects have been selected to find evolutionary solutions to the same proximate problems as those faced by obligate social parasites. Since they are usually unrelated to host workers (e.g., Seppä et al., 2011), intraspecific parasites have to conceal their identity to host workers, which have no fitness gains in rearing unrelated brood. However, the evolutionary outcomes of the conflict of interests between hosts and parasites may differ between intra and interspecific parasitism. Unlike interspecific social parasites, intraspecific parasites use exactly the same communication code as their hosts (i.e., cues, signals) which may facilitate enslaving hosts. Moreover, they have the same genes as their hosts (Lyon and Eadie, 2008). This is especially true if intraspecific parasitism is a conditional reproductive strategy, i.e., foundresses switch to invading conspecific nests after the loss of their nests. In this case, hosts and parasites share both the genes for founding nests and producing workers and those for parasitizing conspecific nests and enslaving host workers.
Intraspecific, social parasitism is common in Polistes wasps and it is often the only breeding option besides nest-founding in wasps with an annual colony cycle (Reeve, 1991; Cervo and Dani, 1996; Cervo, 2006).
Toward the end of the founding phase (that is, before workers emerge), fertile Polistes females can invade conspecific colonies and evict the foundresses (Reeve, 1991). Such females (often referred to as “usurpers” in social wasp literature) are unrelated with the foundress they evict, as documented by DNA microsatellite analyses (Seppä et al., 2011) and behave as social parasites (Cervo, 2006). In general, after violently entering the host colony and killing the legitimate foundress(es) (or inducing her—or them—to flee), intraspecific social parasites kill part of the foundresses' brood (eggs and young larvae) and begin laying their own eggs; the host workers accept the parasite and care for its brood, although the intraspecific parasites' reproductive success is usually poor (Cervo and Lorenzi, 1996a; Cervo, 2006; Seppä et al., 2011). Laboratory studies documented that foundresses forced to adopt alien colonies and to behave as intraspecific social parasites do not exhibit any chemical mimicry with host nests and mark them with their own signature (Lorenzi et al., 2007, 2011; Costanzi et al., 2013). However, we do not know whether these simulations of parasitism reliably describe the integration strategies used by intraspecific social parasites in the wild, nor whether such strategies are consistent among populations.
In the present study, we combine field work and laboratory analyses to investigate what chemical strategy paper wasps use when they behave as intraspecific social parasites as a strategy opposed to independent nest founding, and if this effect is similar in the different populations.
Our study model is Polistes biglumis, a social wasp with solitary nest foundation that inhabits open meadows in mountain areas (>1,000 m a.s.l.). This species is peculiar in many respects, including its social structure. P. biglumis populations are geographically separated, as the areas surrounding each population are comprised of mountain barriers and areas covered by forests or rocks, which are unsuitable for nesting (Lorenzi and Turillazzi, 1986). Previous work has shown that these populations have largely diverged, as they differ between each other in several life-history traits, including worker production (Fucini et al., 2009; Lorenzi and Thompson, 2011). Usually, workers are the first female offspring to emerge in Polistes colonies; they are indistinguishable from fertile females in their external morphology, but they have no fat bodies in their abdomen and forage actively. In general, they do not lay eggs. Fertile females emerge at the end of colony cycle; they have fat bodies, they spend most of the time resting at the natal colony, they mate, hibernate and found their own colony next year (Reeve, 1991). In P. biglumis, there are populations where even the very first female offspring have fat bodies in the abdomen, and thus cannot be classified as workers (measured in the first three females emerged, Fucini et al., 2009; Lorenzi and Thompson, 2011). However, in other populations, only one or two females per colony (on average) are workers (in some colony no workers are produced; Fucini et al., 2009; Lorenzi and Thompson, 2011). This was interpreted as the result of the combined effects of severe climatic conditions—which make the colony cycle very short and worker production less profitable—and high rates of interspecific social parasitism—which make worker production even less profitable (Fucini et al., 2009; Lorenzi and Thompson, 2011). The diversity in social structure between populations offers a unique opportunity to test whether the chemical signature of intraspecific social parasites differs from that of foundresses depending on whether host workers are, or are not, present. Indeed, we focused on one population where all colonies produced workers, and on two others where foundresses produced, on average, 1–2 workers only. Under the hypothesis that intraspecific social parasites are exposed to selection pressures imposed by host workers (namely, host workers' ability to discriminate between nestmates and non-nestmates), we expect that intraspecific social parasites are under stronger selection to exhibit chemical deception strategies in populations where host workers are more abundant.
We sampled foundresses and intraspecific social parasites in three geographically isolated populations. Obligate, interspecific parasites who parasitize the same host employ a double concealing strategy, composed of chemical insignificance and mimicry. They are poor in cuticular hydrocarbons and mimic their host signature, as they lose all parasite-specific hydrocarbons and become enriched in long-chained hydrocarbons, which are typical of their hosts (Bagnères et al., 1996; Lorenzi and Bagnères, 2002; Uboni et al., 2012). As a result, they are tolerated as nestmates by host workers (Lorenzi, 2003). Therefore, we asked whether intraspecific social parasites employ similar deception strategies.
P. biglumis intraspecific social parasites evict host foundresses and are the only adults on the host nests until host brood will emergence some weeks later (Lorenzi and Cervo, 1995). Therefore, chemically mimicking the hosts may be unlikely. However, other chemical changes might favor parasite integration in host colonies: compared to legitimate foundresses, the chemical signature of intraspecific social parasites might be poorer in recognition cues—so that they will go undetected—and/or might have enhanced proportions of branched or long-chained hydrocarbons, as occurs in the obligate social parasites which use the same host (Uboni et al., 2012).
The field work was done during summer 2009 and 2010 in three geographically isolated populations of P. biglumis (Carì, Switzerland; Ferrere, Italy; Montgenèvre, France; the two closest populations are about 70 km apart). Foundations are strictly solitary in all three populations. Foundresses (who mated soon after emergence the previous summer) emerge from a 7–8 months-long hibernation and found their colonies in late spring (end of May–June). Worker production is poor in these populations, as foundresses produce 1–2 workers (on average) in Montgenèvre and Ferrere, whereas all colonies in Carì contain at least three workers (Fucini et al., 2009; Lorenzi and Thompson, 2011). Intraspecific social parasites invade colonies about 1 month after their foundation, and 10–20 days before host brood emergence (as reported by Lorenzi and Turillazzi (1986) for Montgenèvre). Prevalence of intraspecific social parasite varies among populations (Carì: 11% of breeding attempts; Ferrere: 4%; Montgenèvre: 1%; Lorenzi and Thompson, 2011).
We checked nest foundations each 3–7 days from the beginning of the founding period (late May) to its end in late July. Each time we discovered a nest, the foundress was individually marked with enamel paint, her nest numbered, and the brood counted (eggs, small larvae, large larvae, and pupae). In case the marked foundress was absent at one of the next checks, and a non-marked female was on the nest, the non-marked female was classified as an intraspecific social parasite and individually marked. If a nest was discovered late during the founding season, the status of the female on the nest (either foundress or parasite) was assigned after checking whether brood instars were all present. Intraspecific social parasites destroy all eggs and small larvae when they takeover nests and before they lay their own eggs (Cervo and Lorenzi, 1996a). As a result, small larvae are missing soon after usurpaion and other brood instars are missing later on [in this study, the average number of small larvae (± s.e) was 3.9 ± 0.7 in foundress colonies and 2.0 ± 0.6 in parasitized colonies]. In the present work, if small larvae were present in the nest, the female was classified as a foundress; if they were missing, the female was classified as an intraspecific social parasite. [In order to check whether we correctly classified females, we built a Generalized Linear Model (GZLM, for binomially distributed data, logit link) where the proportion of small larvae to old brood (large larvae and pupae) in the nests was the response variable, and we entered the following effects: female reproductive strategy (foundress or intraspecific parasite), decision criterion (whether the female strategy was observed or estimated), year and day of the year. We also included in the preliminary model the interaction between the female reproductive strategy and the decision criterion, as we might have misclassified only one of the two strategies. After removing non-significant interactions and factors, the reduced model showed that foundress colonies had significantly larger proportions of small larvae than parasitized colonies and there was no significant effect of the decision criterion (GZLM, factor: female reproductive strategy, Wald χ2 = 10.345, df = 1, P = 0.001; factor: decision criterion, Wald χ2 = 1.156, df = 1, P = 0.282; sample size n = 48, see below)].
At the end of the founding phase, we collected 48 females from free-living and usurped colonies (n = 30 foundresses and n = 18 intraspecific social parasites; evicted foundresses were not on nests; sampling dates: July 8–20, 2009 and July 13–22, 2010). Each female was put in a separate glass vial and kept in a freezer-bag during the trip to the laboratory, where females were killed by freezing.
We analyzed the chemical profiles of the 48 females after weighing the wasps with a precision balance (Precisa 125A; average foundress weight ± s.e: 74.90 ± 2.10 mg; average intraspecific social parasite weight: 74.06 ± 3.48 mg). We extracted the cuticular hydrocarbons by dipping each wasp separately into 1 ml of pentane for 60 s. We added to each extract n-C20 as an internal standard to quantify the concentration of hydrocarbons.
We injected 2 μl of each extract into an Agilent 6850 Network capillary gas-chromatography system with a flame-ionization detector and a 30 m-Chrompack capillary column CPSil5 WCOT (internal diameter: 320 μm; stationary phase: 0.25 μm), spilt/splitless injection method (15 s). Helium was the carrier gas (50 ml/min, 1 bar). Oven temperature program was as follows: from 70 to 150°C at a rate of 30°/min, and from 150 to 320°C at a rate of 5°/min. The final temperature was 320°C and was kept for 10 min (total time of each run: 46.7 min).
Peak areas were integrated using the GC ChemStation software and corrected manually. Compounds were identified by injecting a blend of standards (C20-C40) in the GC and by calculating the Equivalent Chain Length of the peak in the cuticular hydrocarbon extracts. Additionally, GC-MS analyses were done on a pool of eight foundresses and another of eight intraspecific parasites using an Agilent 5890 GC system coupled with a 5989A Mass Spectrometer (HP Chemstation software; temperature program as above). Finally, we validated peak identity with identifications from previous work (Bagnères et al., 1996; Lorenzi et al., 1997; Uboni et al., 2012).
GC data were analyzed focusing on the concentration of hydrocarbons on the female cuticle, on the ratio of branched to linear hydrocarbons and the ratio of long-chained to short hydrocarbons.
First, we calculated the relative proportion of branched to linear hydrocarbons as the sum of peak areas of branched hydrocarbons divided by the total integration area of hydrocarbons in that extract. Second, we calculated the relative proportion of long-chained to short-chained hydrocarbons as the sum of peak area of long-chained hydrocarbons divided by the total integration area of hydrocarbons in the extract. We classified hydrocarbons as short-chained if they eluted as peaks 1–26 (peak 26 was n-C29) and as long-chained if they eluted as peaks 27–63 [This classification is based on analyses on P. atrimandibularis, obligate parasite of P. biglumis wasps; the chemical signature of P. atrimandibularis has/has no peaks with a retention time larger than that of peak 26 (n-C29) depending on whether we analyze the pre- or the post-invasion signatures of the parasites].
Finally, we calculated the concentration of hydrocarbons on the cuticle of each female (ng of hydrocarbons per mg of wasp) as follows: the overall sum of peak areas was multiplied by 800 ng (i.e., the concentration of the internal standard C20 in 2 μl of extract) and divided by the peak area of C20 in that extract; the resulting value was divided by the weight of the wasp (in mg).
We analyzed the differences in the ratio of branched to linear hydrocarbons and the ratio of long- to short-chained hydrocarbons on the female cuticle by running two different Generalized Linear Models GZLMs (for normally distributed data, identity link), one on the proportion of branched hydrocarbons and one on the proportion of long-chained hydrocarbons. In both analyses, proportion data were arcsine-square-root-transformed to account for normality (Parallel GZLMs on non-transformed data were also run because our data fell in the middle of the range—between 0.2 and 0.7—where the relationship between data and predictors is approximately linear; such models yielded results substantially similar to those run on arcsine-sqrt-transformed data.) We used female reproductive strategy (two levels: foundress or intraspecific parasite), population (three levels), and year (two levels) as fixed factor effects and the date of collection (as the day of the year) and the number of brood in the nest as covariates (to account for differences due to date and/or colony size).
We analyzed the differences in the concentration of hydrocarbons on the female cuticle using GZLMs (for normally distributed data, log link) on the total hydrocarbon concentration, and using, as above, female reproductive strategy, population, and year as fixed factor effects and the date of collection and the number of brood as covariates.
In all analyses, the interactions between population and female reproductive strategy were non-significant or marginally so. However, post-hoc tests revealed significant differences between reproductive strategies within populations, suggesting that the slopes of the regression lines between the response variables and the female reproductive strategy were similar in the three populations, but only in one population the slope was significantly different from 0. We took into account the likelihood of incorrectly rejecting a null hypothesis (i.e., making a Type I error) because of the multiple comparisons; testing our hypotheses at a significance level of α = 0.017 (i.e., after Bonferroni correction) yielded substantially similar results.
Finally, we checked whether the proportions of branched and long-chained hydrocarbons covaried within population, as melting points may be differently affected by these two categories of compounds.
In the graphs, predicted means, that is, means adjusted to covariates and factors are shown (un-corrected means are provided in Table 1).
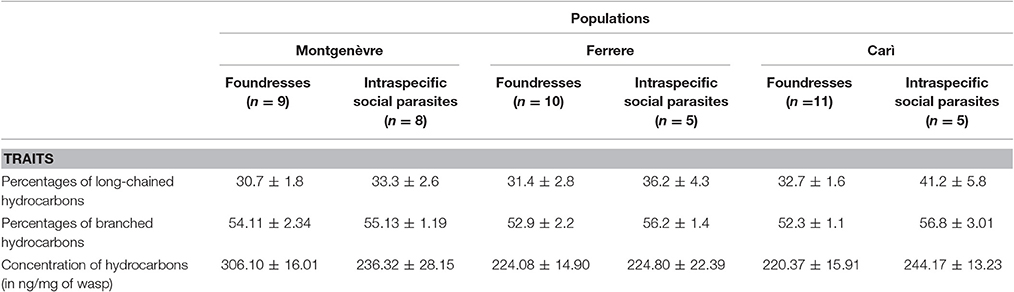
Table 1. Descriptive statistics of the percentages of long-chained and branched hydrocarbons, and of the concentration of hydrocarbons on the cuticle of intraspecific social parasites and foundresses in Carì, Ferrere, and Montgenèvre (mean ± s.e.).
The blend of cuticular hydrocarbons of P. biglumis females consisted of more than 70 peaks which were homologous series of linear and methyl- branched alkanes, as previously reported (Lorenzi et al., 1997; Uboni et al., 2012). There was no qualitative difference between populations and years (i.e., foundress chemical signatures were composed of the same compounds, whatever the population they came from).
Intraspecific social parasites had significantly higher percentages of long-chained hydrocarbons than foundresses in one population (Carì) but not in Ferrere or Montgenèvre (Figure 1, Tables 1, 2). Intraspecific social parasites from the three populations had significantly different proportions of long-chained hydrocarbons, whereas foundresses had not (Figure 1, Table 3). This trait was also significantly associated with colony size in Carì and Montgenèvre (females in larger colonies had a larger proportion of long-chained hydrocarbons) and with year in Carì and Ferrere (possibly as a result of the effect of local climatic conditions on these annual colonies).
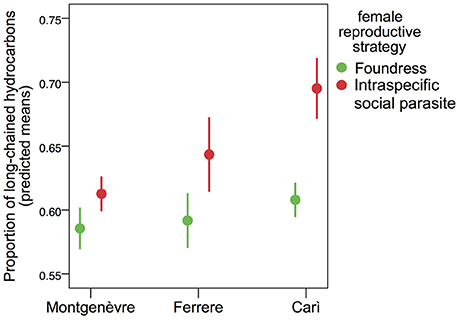
Figure 1. The percentage of long-chained hydrocarbons on the cuticle of foundresses and intraspecific social parasites. Intraspecific social parasites had significantly higher percentages than foundresses in Carì, but not in Ferrere and Montgenèvre (mean adjusted to covariates ± s.e.).
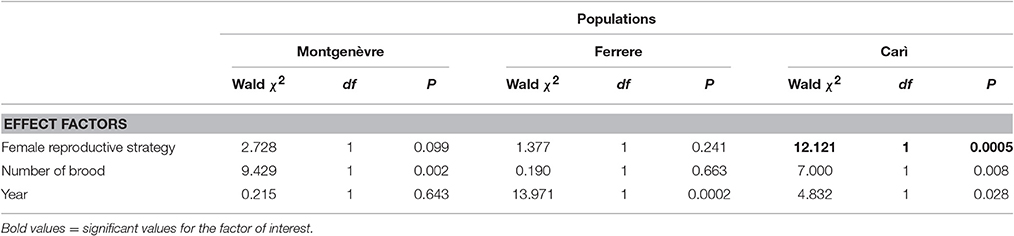
Table 2. Within-population comparisons in the percentages of long-chained hydrocarbons as a function of female reproductive strategy (intraspecific social parasites vs. foundresses), number of brood and year (GZLMs).
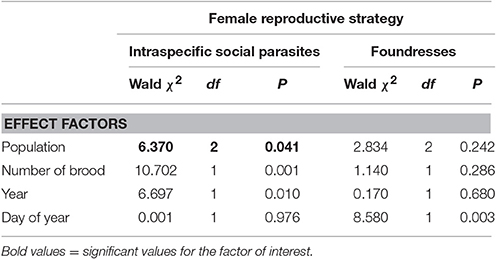
Table 3. Between population comparisons in the percentages of long-chained hydrocarbons among intraspecific social parasites and among foundresses as a function of population, number of brood, date of collection, and year (GZLMs).
Intraspecific social parasites had significantly larger proportion of branched hydrocarbons than foundresses in Carì, but not in Ferrere or Montgenèvre (Figure 2, Tables 1, 4). This was due to the fact that, despite intraspecific social parasites not differing between populations, foundresses did (Figure 2, Table 5). The proportion of branched hydrocarbons was also significantly associated with colony size in Carì and Montgenèvre (again, females in larger colonies had a larger proportion of long-chained hydrocarbons) and with year in Carì.
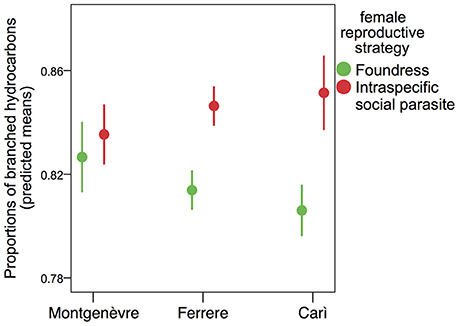
Figure 2. The percentage of branched hydrocarbons on the cuticle of foundresses and intraspecific social parasites. Intraspecific social parasites had significantly higher percentages than foundresses in Carì, but not in Ferrere and Montgenèvre (mean adjusted to covariates ± s.e.).
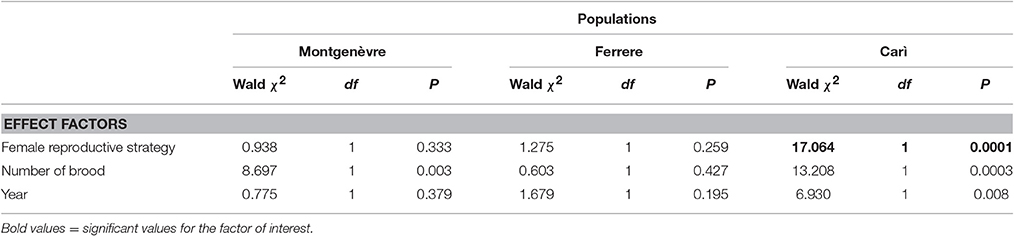
Table 4. Intraspecific social parasites vs. foundresses: within-population comparisons in the percentages of branched hydrocarbons (GZLMs; the day of the year was a non-significant factor and was removed from the models).
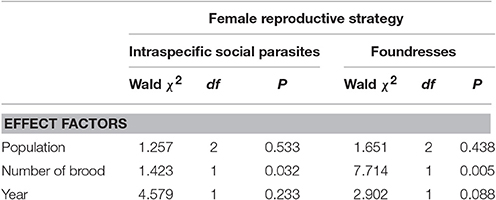
Table 5. Between population comparisons in the percentages of branched hydrocarbons among intraspecific social parasites and among foundresses as a function of population, number of brood and year (GZLMs; the fixed factor day of the year was non-significant and was removed from the models).
Intraspecific social parasites in Montgenèvre had ~1/5 the concentration of cuticular hydrocarbons of foundresses, and this difference was marginally significant (after Bonferroni correction, see Section Methods), whereas there was no significant difference in the other two populations (Figure 3, Tables 1, 6). However, the difference in Montgenèvre was not due to intraspecific social parasites having low concentration of cuticular hydrocarbons but to Montgenèvre foundresses having high amount of hydrocarbons. Indeed, there was no significant difference among intraspecific social parasites, whereas foundresses differed significantly between populations in this trait (Figure 3, Table 7).
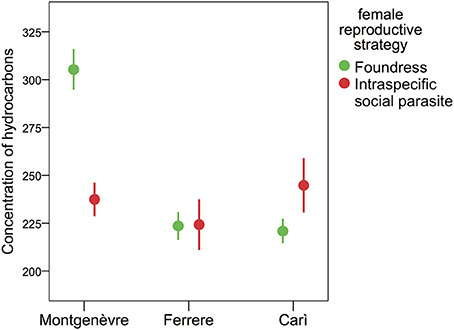
Figure 3. The concentration of cuticular hydrocarbons on the cuticle of foundresses and intraspecific social parasites. Intraspecific social parasites had less cuticular hydrocarbons than foundresses in Montgenèvre, but not in Ferrere and Carì. Data are shown as ng/mg of wasp (mean adjusted to covariates ± s.e.).
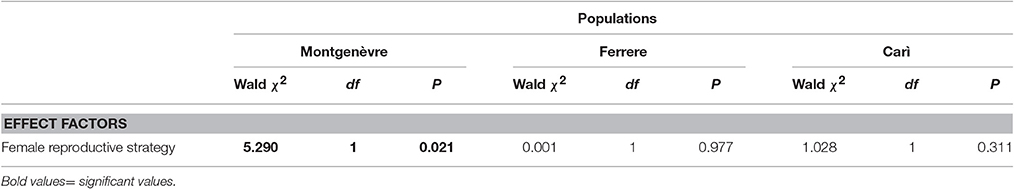
Table 6. Intraspecific social parasites vs. foundresses: within-population comparisons in the concentration of hydrocarbons on the cuticle (GZLMs; brood number, day of the year and year were non-significant factors and were removed from the models).
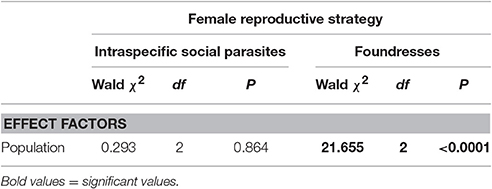
Table 7. Between population comparison in the concentration of hydrocarbons on the cuticle of intraspecific social parasites and on that of foundresses as a function of population (GZLMs; brood number, day of the year and year were non-significant factors and were removed from the models).
Branched and long-chained hydrocarbons were significantly and positively correlated in Carì and Ferrere (Carì: Spearman's rho = 0.756, P = 0.001; Ferrere: rho = 0.574, P = 0.025) and marginally so in Montgenèvre (rho = 0.471, P = 0.057).
These results show that the chemical integration strategies of intraspecific social parasite wasps—i.e., females who invade the colony of unrelated conspecific foundresses in the field—differ from those of obligate interspecific social parasites. Whereas the latter chemically mimic their hosts and/or are poor in recognition cues, intraspecific social parasites have hydrocarbon abundance similar to conspecific foundresses. However, we could chemically distinguish them from foundresses in one population, where intraspecific social parasites had hydrocarbon signatures characterized by significantly larger proportion of long-chained and branched hydrocarbons (in the other two populations such differences were not significant). Such signatures might have evolved in that population to facilitate the integration of intraspecific social parasites into host colonies and the exploitation and control of host workforce, because colonies consistently produce workers there. In contrast, we could not detect any specific signature in intraspecific social parasites in the two populations where colonies produce few workers, if any.
It may appear counterintuitive that intraspecific social parasites express changes in their cuticular signatures, which may consistently reveal themselves as parasites to host workers. However, social wasp workers learn their colony recognition odor at emergence from the paper nest (Gamboa, 2004), i.e., they generally use the recognition cues deposited by foundresses on the surface of the paper nest as a template for recognition processes. Intraspecific social parasites, which have recognition cues different from those of the unrelated foundresses they evict, will be unmasked by host workers if they do not interfere in such a process. Indeed, there is evidence that hosts unmask social parasites and their eggs, in P. biglumis (Cervo and Lorenzi, 1996a; Lorenzi and Filippone, 2000; Lorenzi, 2006) and in other social insects (e.g., Achenbach and Foitzik, 2009; Pamminger et al., 2013). We expect that this ability selects for deception strategies in parasites, which might include changes in the chemical signature such as higher proportion of long-chained and branched hydrocarbons.
Indeed, long-chained and branched hydrocarbons play special roles in social interactions in social insects. Menzel and Schmitt (2011) and Emery and Tsutsui (2013) showed that large amounts of long-chained and methyl-branched hydrocarbons (branched alkenes) are typical of parabiotic species of Camponotus ants—which nest within the nests of other species—when compared to free-living species of the same genus—that is, long-chained and branched hydrocarbons were relatively more abundant in species which were tolerated by other species. Of course, the conflict of interests between counterparts is much stronger in systems involving social parasites and their hosts than in those involving parabiotic ants, whose relationship is mutualistic. However, both systems imply overcoming aggressive responses to unrelated individuals, and might imply convergent chemical adaptations. There are also antagonistic interactions which involve social parasites shifting their chemical signature toward higher proportions of branched and long-chained hydrocarbons after settling in host colonies. Among paper wasps, the signature of the obligate social parasite P. atrimandibularis shift to a larger proportion of both branched and long-chained hydrocarbons after infiltrating host nests (Bagnères et al., 1996; Uboni et al., 2012). These parasites, initially violently attacked by hosts (Cervo et al., 1990), are accepted as nestmates after these changes have occurred (Lorenzi, 2003); in the meantime, foundresses regress their ovary development (Cervo and Lorenzi, 1996b). Similarly, among ants, the queens of Polyergus breviceps and P. rufescens exhibit a shift from linear to branched hydrocarbons (although not to long-chained hydrocarbons) as they enter host colonies (Johnson et al., 2001 and d'Ettorre and Errard, 1998, respectively). Therefore, several parasite and parabiotic species increase their proportions of long-chained and methyl-branched hydrocarbons when they enter their host colonies, which suggest that these compounds may play a role in deception mechanisms and/or in host control. However, these compounds might also increase for other functions, which might explain why they were relatively abundant in the signature of intraspecific social parasites. In the socially parasitic A. insinuator ants, for example, the abundance of unusually long-chained hydrocarbons (n-alkenes) “might help to hide the information from the shorter hydrocarbons” or might serve as appeasing substances (Lambardi et al., 2007). Intraspecific social parasites might hide their identity in similar ways.
In fact, an increase in branched hydrocarbons occurs at a cost, as it lowers the melting point of the hydrocarbon blends and diminishes the waterproofing properties of the cuticular layer, which are especially associated to linear hydrocarbons (Gibbs and Rajpurohit, 2010). Chain length appears to be less relevant to waterproofing, although longer chain-length hydrocarbons melt at relatively higher temperatures (Gibbs and Pomonis, 1995) and thus may contrast water loss caused by the increase proportions of branched hydrocarbons. In our study, the proportions of branched and long-chained hydrocarbons covaried in Carì and Ferrere (and were marginally correlated in Montgenèvre); such a correlation suggests that the increase in the proportion of branched hydrocarbons in intraspecific social parasites may drive a parallel increase in the proportion of longer chain-length hydrocarbons as a compensation for increased water loss. We do not know whether longer chained hydrocarbons are there for merely physiological reasons or whether they serve other functions.
Both branched and long-chained hydrocarbons may also act as fertility cues in social insects. For example, there is a shift to long-chained and branched cuticular hydrocarbons as females become fertile in the drywood termite Cryptotermes secundus (Weil et al., 2009) and in the ant Gnamptogenys striatula (Lommelen et al., 2006). A shift toward long-chained hydrocarbons also occurs in females of the ponerine ants Pachycondyla inversa (Heinze et al., 2002) and Harpegnathos saltator (Liebig et al., 2000) and it is positively associated with fertility. Recently, it has been shown that some hydrocarbons regulate worker reproduction (“queen pheromones,” Van Oystaeyen et al., 2014), and the rest of the blend may contribute to this effect (Smith et al., 2015). Indeed, obligate social parasites take over the dominant position in social wasp colonies and their chemical signatures match that of the most dominant host female in the colony (Dapporto et al., 2004), supporting the hypothesis that the changes in the chemical signature of parasites are driven by selection for regulating worker reproduction as well as for bypassing host detection.
We may hypothesize that when P. biglumis intraspecific social parasites take over the host colonies and begin laying eggs, they may keep host workers more efficiently under control if they signal both that they belong to the colony and that they are dominant, fertile females. We know that P. biglumis intraspecific social parasites exhibit an intense marking behavior when they enter host nests (Cervo and Lorenzi, 1996a) which results in overmarking the nest-foundress signature with their own hydrocarbon signature and in shifting the original nest odor toward their own signature (Lorenzi et al., 2011). Eventually, newly emerged (host) wasps will learn their colony odor as the odor of their intraspecific social parasites and will erroneously accept them as their mothers (Lorenzi et al., 2011). This is the first step to get access to the host colony, but intraspecific social parasites need also to be recognized as the dominant, fertile females by resident workers. P. biglumis intraspecific social parasites might have enhanced proportions of branched and long-chained hydrocarbons in their signature as a means to regulate host worker reproduction through dishonest signaling, as expected for social parasites (Heinze and d'Ettorre, 2009). Up to now, little is known about fertility signals in Polistes wasps (but see Dapporto et al., 2004; Oi, 2016), and we do not know whether single compounds, or blends (such as increased proportions of branched or long-chained hydrocarbons), act as fertility signals in P. biglumis. Although we might expect that the fertility of intraspecific social parasites is larger than that of foundresses (e.g., they might spare time and resources for nest construction), data on reproductive success depict a different scenario. P. biglumis intraspecific social parasites produced 25–50% the brood produced by foundresses, according to two separate studies (Lorenzi and Cervo, 1995; Seppä et al., 2011). However, these data described the success of intraspecific parasites in the populations of Montgenèvre and Ferrere, and we lack data for Carì.
Recent genetic analyses have confirmed that these wasp populations belong to the same species (Bonelli et al., 2015). However, P. biglumis populations are geographically separated and live at relatively high elevation in the Alps. Geographic distance, mountain barriers, and local biotic and abiotic factors have imposed distinct selective pressures on each population. We know that P. biglumis populations have diverged in several relevant traits, such as body size, behavioral, and life history traits (Fucini et al., 2004, 2009; Lorenzi and Thompson, 2011). For example, foundresses from different populations have quantitatively distinct cuticular chemical profiles (Bonelli et al., 2015), distinct behavioral profiles and different colony productivity (Fucini et al., 2014; Mignini and Lorenzi, 2015). More relevant to the scope of this study, foundresses produce workers in some populations, including Carì, but not in others (Fucini et al., 2009; Lorenzi and Thompson, 2011). Therefore, it does not come as a surprise that in the present research we found that intraspecific social parasites exhibit a special chemical signature in Carì, whereas their chemical signature is similar to that of foundresses in the other two populations (Ferrere and Montgenèvre), where foundresses produce almost no workers. We interpret these population differences as the result of the diversity of selection pressures acting on intraspecific social parasites in the different populations: where workers are present, as occurs in Carì, intraspecific social parasites have been selected—by host workers—to change their cuticular signals in ways that possibly conceal their identity, and/or advertise their fertility and manipulate workers. These pressures are not at play in Ferrere and Montgenèvre, where intraspecific social parasites rarely have host workers.
The divergence among phenotypic traits between populations may also be associated with the presence or absence of obligate social parasites. Obligate social parasites are absent in Carì, but intraspecific social parasites are relatively common there, whereas in the others two populations, the opposite occurs: obligate social parasites are relatively common and intraspecific intraspecific social parasites relatively rare. These differences have resulted in different selection gradients on foundress traits depending on the population of origin (Lorenzi and Thompson, 2011) and were associated with a divergence between populations in some features of the chemical signatures (Lorenzi et al., 2014). Intraspecific social parasites being more common in Carì, selection may be faster and traits can spread more rapidly; additionally, the concomitant selective pressures on chemical signatures imposed by obligate social parasites in Montgenèvre and Ferrere may have prevented the spread of intraspecific-parasite strategies. Indeed, foundresses from Montgenèvre themselves already have the highest proportions of branched hydrocarbons among the three populations (possibly as a consequence of the high prevalence of the obligate parasite P. atrimandibularis in that population, Lorenzi et al., 2014). It is reasonable that branched hydrocarbons do not further increase in intraspecific parasites because their increase would imply high fitness costs, at least in terms of waterproofing effects (Gibbs and Rajpurohit, 2010). Foundresses in Montgenèvre were special also in respect to the striking abundance of cuticular hydrocarbons—possibly another outcome of high, local, obligate social-parasite pressure—and intraspecific parasites appeared as chemical insignificant there relative to foundresses, as a byproduct of hydrocarbon abundance in foundresses.
It is not clear whether parasitizing colonies is a reproductive option conditional on nest loss or whether intraspecific social parasites are a genetically separated subset of the population, in which case even other hypotheses might be made to explain our results. Lorenzi et al. (2011) showed that foundresses behaved as intraspecific social parasites when experimentally deprived of their own nests and offered an alien one in the lab, which stands against the hypothesis that intraspecific parasites are genetically distinct from foundresses. They adopted the alien nest, marked it with their own signature and changed the host nest signature. The amount of branched hydrocarbons increased significantly in females forced to behave as intraspecific social parasites similarly to what the present data show about naturally occurring intraspecific parasites. There is both evidence that intraspecific social parasites invade host nest after nest loss (Lorenzi and Cervo, 1995) and that fertile females “sit and wait” for nest to invade without any prior nest founding attempt (Starks, 2001), so that we do not know whether intraspecific social parasites followed a conditional strategy or relied exclusively on conspecific nest usurpation to establish their colonies. In any case, the present results suggest that adaptations to social parasitism might emerge in free-living species and intraspecific social parasites display sophisticated chemical strategies which consist of adjusting their chemical signature likely in relation to the pressures imposed by host workers.
The collection of colonies and the experiments performed comply with the current laws in Italy and France. No specific permits were required for the collection neither for collection location. The species used in the experiments was not endangered or protected in these countries.
MCL conceived the study, MCL and LA designed the experiment, LA collected samples and field data and LA and AGB acquired chemical data. LA, AGB, and MCL analyzed the data, and MCL wrote the manuscript. All authors read, commented, and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to thanks Patrizia d'Ettorre and two referees for their helpful comments and valuable criticisms on earlier versions of the manuscript and Jack Coggins for linguistic revision.
Achenbach, A., and Foitzik, S. (2009). First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075. doi: 10.1111/j.1558-5646.2009.00591.x
Bagnères, A.-G., and Lorenzi, M. C. (2010). “Chemical deception/mimicry using cuticular hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. Blomquist and A.-G. Bagnères (Cambridge, MA: Cambridge University Press), 282–324.
Bagnères, A.-G., Lorenzi, M. C., Clément, J.-L., Dusticier, G., and Turillazzi, S. (1996). Chemical usurpation of a nest by paper wasp parasites. Science 272, 889–892. doi: 10.1126/science.272.5263.889
Bauer, S., Bohm, M., Witte, V., and Foitzik, S. (2010). An ant social parasite in-between two chemical disparate host species. Evol. Ecol. 24, 317 3332. doi: 10.1007/s10682-009-9308-2
Bonelli, M., Lorenzi, M. C., Christidès, J.-P., Dupont, S., and Bagnères, A.-G. (2015). Population diversity in cuticular hydrocarbons and mtDNA in a mountain social wasp. J. Chem. Ecol. 41, 22–31. doi: 10.1007/s10886-014-0531-0
Brooke, M. de. L., and Davies, N. B. (1988). Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335, 630–632. doi: 10.1038/335630a0
Cervo, R. (2006). Polistes wasps and their social parasites: an overview. Ann. Zool. Fenn. 43, 531–549.
Cervo, R., and Dani, F. R. (1996). “Social parasitism and its evolution in Polistes,” in Natural History and Evolution of Paper Wasps, eds S. Turillazzi and M. J. West-Eberhard (Oxford: Oxford University Press), 98–112.
Cervo, R., and Lorenzi, M. C. (1996a). Behaviour in usurpers and joiners of Polistes biglumis bimaculatus (Hymenoptera Vespidae). Insect. Soc. 43, 255–266. doi: 10.1007/BF01242927
Cervo, R., and Lorenzi, M. C. (1996b). Inhibition of host queen reproductive capacity by the obligate social parasite Polistes atrimandibularis (Hymenoptera Vespidae). Ethology 102, 1042–1047. doi: 10.1111/j.1439-0310.1996.tb01180.x
Cervo, R., Lorenzi, M. C., and Turillazzi, S. (1990). Nonaggressive usurpation of the nest of Polistes biglumis bimaculatus by the social parasite Sulcopolistes atrimandibularis (Hymenoptera Vespidae). Insect. Soc. 37, 333–347. doi: 10.1007/BF02225996
Cini, A., Gioli, L., and Cervo, R. (2009). A quantitative threshold for nest-mate recognition in a paper social wasp. Biol. Lett. 5, 459–461. doi: 10.1098/rsbl.2009.0140
Clutton-Brock, T. H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton University Press.
Costanzi, E., Bagnères, A.-G., and Lorenzi, M. C. (2013). Nestmate recognition in social wasp is based on the relative proportions of cuticular hydrocarbons within species-specific ranges of hydrocarbon concentrations. PLoS ONE 8:e65107. doi: 10.1371/journal.pone.0065107
d'Ettorre, P., and Errard, C. (1998). Chemical disguise during colony founding in the dulotic ant Polyergus rufescens Latr. (Hymenoptera, Vespidae). Insect. Soc. Life 2, 71–77.
D'Ettorre, P., and Heinze, J. (2001). Sociobiology of slave-making ants. Acta Ethol. 3, 67–82. doi: 10.1007/s102110100038
Dapporto, L., Theodora, P., Spacchini, C., Pieraccini, G., and Turillazzi, S. (2004). Rank and epicuticular hydrocarbons in different populations of the paper wasp Polistes dominulus (Christ) (Hymenoptera, Vespidae). Insect. Soc. 51, 279–286. doi: 10.1007/s00040-004-0738-0
Davies, N. B. (2011). Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14. doi: 10.1111/j.1469-7998.2011.00810.x
Davies, N. B., Bourke, A. F., and de L Brooke, M. (1989). Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Tree 4, 274–278. doi: 10.1016/0169-5347(89)90202-4
Emery, V. J., and Tsutsui, N. D. (2013). Recognition in a social symbiosis: chemical phenotypes and nestmate recognition behaviors of neotropical parabiotic ants. PLoS ONE 8:e56492. doi: 10.1371/journal.pone.0056492
Emery, V. J., and Tsutsui, N. D. (2016). Differential sharing of chemical cues by social parasites versus social mutualists in a three-species symbiosis. J. Chem. Ecol. 42, 277–285. doi: 10.1007/s10886-016-0692-0
Field, J. (1992). Intraspecific parasitism as an alternative reproductive tactic in nest-building wasps and bees. Biol. Rev. 67, 79–126. doi: 10.1111/j.1469-185X.1992.tb01659.x
Fucini, S., Cavallo, M. V., Di Bona, V., and Lorenzi, M. C. (2004). Local adaptations in Polistes biglumis expression of sociality (Hymenoptera Vespidae). Redia 87, 177–178.
Fucini, S., Di Bona, V., Mola, F., Piccaluga, C., and Lorenzi, M. C. (2009). Social wasps without workers: geographic variation of caste expression in the paper wasp Polistes biglumis. Insect. Soc. 56, 347–358. doi: 10.1007/s00040-009-0030-4
Fucini, S., Uboni, A., and Lorenzi, M. C. (2014). Geographic variation in air temperature leads to intraspecific variability in the behavior and productivity of a eusocial insect. J. Insect Behav. 27, 403–410. doi: 10.1007/s10905-013-9436-y
Gibbs, A. G., and Pomonis, J. G. (1995). Physical properties of insect cuticular hydrocarbons: the effect of chain length, methyl-branching and unsuturation. Comp. Biochem. Physiol. 112B, 243–249. doi: 10.1016/0305-0491(95)00081-X
Gibbs, A. G., and Rajpurohit, S. (2010). “Cuticular lipids and water balance,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. Blomquist and A.-G. Bagnères (Cambridge: Cambridge University Press), 100–120.
Guillem, R. M., Drijfhout, F., and Martin, S. J. (2014). Chemical deception among ant social parasites. Curr. Zool. 60, 62–75. doi: 10.1093/czoolo/60.1.62
Heinze, J., and d'Ettorre, P. (2009). Honest and dishonest communication in social Hymenoptera. J. Exp. Biol. 212, 1775–1779. doi: 10.1242/jeb.015008
Heinze, J., Stengl, B., and Sledge, M. (2002). Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52, 59–65. doi: 10.1007/s00265-002-0491-1
Ichinose, K., and Lenoir, A. (2010). Hydrocarbon detection levels in ants. Insect. Soc. 57, 453–455. doi: 10.1007/s00040-010-0103-4
Johnson, C., Vander Meer, R. K., and Lavine, B. (2001). Changes in the cuticular hydrocarbon profile of the slave-maker ant queen, Polyergus breviceps, after killing a Formica queen. J. Chem. Ecol. 27, 1787–1804. doi: 10.1023/A:1010456608626
Kaib, K., Heinze, J., and Ortius, D. (1993). Cuticular hydrocarbon profiles in the slave-making ant Harpagoxenus sublaevis and its hosts. Naturwiss. 80, 281–285. doi: 10.1007/BF01135915
Kilner, R. M., and Langmore, N. E. (2011). Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. Camb. Philos. Soc. 86, 836–852. doi: 10.1111/j.1469-185X.2010.00173.x
Lambardi, D., Dani, F. R., Turillazzi, S., and Boomsma, J. J. (2007). Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 61, 843–851. doi: 10.1007/s00265-006-0313-y
Lenoir, A., D'Ettorre, P., Errard, C., and Hefetz, A. (2001). Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. doi: 10.1146/annurev.ento.46.1.573
Liebig, J. J., Peeters, C., Oldham, N. J., Markstadter, C., and Holldobler, B. (2000). Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl. Acad. Sci. U.S.A. 97, 4124–4131. doi: 10.1073/pnas.97.8.4124
Lommelen, E., Johnson, C. A., Drijfhout, F. P., Billen, J., Wenseleers, T., and Gobin, B. (2006). Cuticular hydrocarbons provide reliable cues. J. Chem. Ecol. 32, 2023–2034. doi: 10.1007/s10886-006-9126-8
Lorenzi, M. C. (2003). Social wasp parasites affect the nestmate recognition abilities of their hosts (Polistes atrimandibularis and P. biglumis, Hymenoptera: Vespidae). Insect. Soc. 50, 82–87. doi: 10.1007/s000400300013
Lorenzi, M. C. (2006). The result of an arms race: the chemical strategies of Polistes social parasites. Ann. Zool. Fenn. 43, 550–563.
Lorenzi, M. C., and Bagnères, A.-G. (2002). Concealing identity and mimicking hosts: a dual chemical strategy for a single social parasite? (Polistes atrimandibularis, Hymenoptera: Vespidae). Parasitology 125, 507–512. doi: 10.1017/S003118200200238X
Lorenzi, M. C., and Cervo, R. (1995). Usurpations and late associations in the solitary founding social wasp, Polistes biglumis bimaculatus. J. Ins. Behav. 8, 443–451. doi: 10.1007/BF01995318
Lorenzi, M. C., and Filippone, F. (2000). Opportunistic discrimination of alien eggs by social wasps (Polistes biglumis, Hymenoptera Vespidae): a defense against social parasitism? Behav. Ecol. Sociobiol. 48, 402–406. doi: 10.1007/s002650000251
Lorenzi, M. C., and Thompson, J. N. (2011). The geographic structure of selection on a coevolving interaction between social parasitic wasps and their hosts hampers social evolution. Evolution 65, 3527–3542. doi: 10.1111/j.1558-5646.2011.01403.x
Lorenzi, M. C., and Turillazzi, S. (1986). Behavioural and ecological adaptations to the high mountain environment of Polistes biglumis bimaculatus. Ecol. Entomol. 11, 199–204. doi: 10.1111/j.1365-2311.1986.tb00295.x
Lorenzi, M. C., Azzani, L., and Bagnères, A.-G. (2014). Evolutionary consequences of deception: complexity and informational content of colony signature are favored by social parasitism. Curr. Zool. 60, 137–148. doi: 10.1093/czoolo/60.1.137
Lorenzi, M. C., Bagnères, A.-G., Clément, J.-L., and Turillazzi, S. (1997). Polistes biglumis bimaculatus epicuticular hydrocarbons and nestmate recognition (Hymenoptera, Vespidae). Insect. Soc. 44, 123–138. doi: 10.1007/s000400050035
Lorenzi, M. C., Caldi, M., and Cervo, R. (2007). The chemical strategies used by Polistes nimphus social wasp usurpers (Hymenoptera Vespidae). Biol. J. Linn. Soc. 91, 505–512. doi: 10.1111/j.1095-8312.2007.00815.x
Lorenzi, M. C., Cervo, R., and Bagnères, A.-G. (2011). Facultative social parasites mark host nests with branched hydrocarbons. Anim. Behav. 82, 1143–1149. doi: 10.1016/j.anbehav.2011.08.011
Lorenzi, M. C., Cervo, R., Zacchi, F., Turillazzi, S., and Bagnères, A.-G. (2004). Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera Vespidae). Parasitology 129, 643–651. doi: 10.1017/S0031182004005992
Lyon, B. E., and Eadie, J. McA. (2008). Conspecific brood parasitism in birds: a life history perspective. Annu. Rev. Ecol. Evol. Syst. 39, 343–363. doi: 10.1146/annurev.ecolsys.39.110707.173354
Menzel, F., Blüthgen, N., Tolasch, T., Conrad, J., Beifuß, U., Beuerle, T., et al. (2013). Crematoenones – a novel substance class exhibited by ants – functions as appeasement signal. Front. Zool. 10:32. doi: 10.1186/1742-9994-10-32
Menzel, F., and Schmitt, T. (2011). Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66, 896–904. doi: 10.1111/j.1558-5646.2011.01489.x
Mignini, M., and Lorenzi, M. C. (2015). Vibratory signals predict rank and offspring caste ratio in a social insect. Behav. Ecol. Sociobiol. 69, 1739–1748. doi: 10.1007/s00265-015-1986-x
Nash, D. R., Als, T. D., Maile, R., Jones, G. R., and Boomsma, J. J. (2008). A mosaic of chemical coevolution in a large blue butterfly. Science 319, 88–90. doi: 10.1126/science.1149180
Nehring, V., Dani, F. R., Turillazzi, S., Boomsma, J. J., and d'Ettorre, P. (2015). Integration strategies of a leaf-cutting ant social parasite. Anim. Behav. 108, 55–65. doi: 10.1016/j.anbehav.2015.07.009
Oi, C. A. (2016). The Chemical Crown of Social Insect Life: the Origin and Evolution of Queen Pheromones in Social Wasps. Ph.D. thesis, University of Leuven, Belgium.
Pamminger, T., Leingärtner, A., Achenbach, A., Kleeberg, I., Pennings, P. S., and Foitzik, S. (2013). Geographic distribution of the anti-parasite trait “slave rebellion.” Evol. Ecol. 27, 39–49. doi: 10.1007/s10682-012-9584-0
Reeve, H. K. (1991). “Polistes,” in The Social Biology of Wasps, eds K. G. Ross and R. W. Matthews (Ithaca, NY: Cornell Univ. Press), 99–148.
Rothstein, S. I. (1990). A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. doi: 10.1146/annurev.es.21.110190.002405
Royle, N. J., Smiseth, P. T., and Köelliker, M. (2012). Evolution of Parental Care. Oxford: Oxford University Press.
Ruano, F., Devers, S., Sanllorente, O., Errard, C., Tinaut, A., and Lenoir, A. (2011). A geographical mosaic of coevolution in a slave-making host–parasite system. J. Evol. Biol. 24, 1071–1079. doi: 10.1111/j.1420-9101.2011.02238.x
Seppä, P., Fogelqvist, J., Gyllenstrand, N., and Lorenzi, M. C. (2011). Colony kin structure and breeding patterns in the social wasp, Polistes biglumis. Insect. Soc. 58, 345–355. doi: 10.1007/s00040-011-0149-y
Smith, A. A., Millar, J. G., and Suarez, A. V. (2015). A social insect fertility signal is dependent on chemical context. Biol. Lett. 11:20140947. doi: 10.1098/rsbl.2014.0947
Spottiswoode, C. N., Kilner, R. M., and Davies, N. B. (2012). “Brood parasitism,” in Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Köelliker (Oxford: Oxford University Press) 226–243.
Starks, P. T. (2001). Alternative reproductive tactics in the paper wasp Polistes dominulus with specific focus on the sit-and-wait tactic. Ann. Zool. Fenn. 38, 189–199.
Taylor, L. H. (1939). Observations on social parasitism in the genus Vespula Thomson. Ann. Entomol. Soc. Am. 32, 304–315. doi: 10.1093/aesa/32.2.304
Thorogood, R., and Davies, N. B. (2013). Reed warbler hosts fine-tune their defenses to track three decades of cuckoo decline. Evolution 67, 3545–3556. doi: 10.1111/evo.12213
Uboni, A., Bagnères, A.-G., Christidès, J.-P., and Lorenzi, M. C. (2012). Cleptoparasites, social parasites and a common host: chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 58:1259–1264. doi: 10.1016/j.jinsphys.2012.06.013
Van Oystaeyen, A., Oliveira, R. C., Holman, L., van Zweden, J. S., Romero, C., Oi, C. A., et al. (2014). Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287–290. doi: 10.1126/science.1244899
Van Zweden, J., and d'Ettorre, P. (2010). “Nestmate recognition in social insects and the role of hydrocarbons,” in Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, eds G. Blomquist and A.-G. Bagnères (Cambridge: Cambridge University Press), 222–243.
Weil, T., Hoffmann, K., Kroiss, J., Strohm, E., and Korb, J. (2009). Scent of a queen - cuticular hydrocarbons specific for female reproductives in lower termites. Naturwiss 96, 315. doi: 10.1007/s00114-008-0475-8
Wlodarczyk, T., and Szczepaniak, L. (2014). Incomplete homogenization of chemical recognition labels between Formica sanguinea and Formica rufa Ants (Hymenoptera: Formicidae) living in a mixed colony. J. Insect Sci. 14:214. doi: 10.1093/jisesa/ieu076
Keywords: Polistes, nest usurpation, social parasitism, intraspecific parasitism, hydrocarbons, mosaic evolution, social wasps
Citation: Lorenzi MC, Azzani L and Bagnères A-G (2017) Divergence in Cuticular Chemical Signatures between Isolated Populations of an Intraspecific Social Parasite. Front. Ecol. Evol. 5:8. doi: 10.3389/fevo.2017.00008
Received: 16 September 2016; Accepted: 09 February 2017;
Published: 07 March 2017.
Edited by:
Luisa Amo, Consejo Superior de Investigaciones Científicas, SpainReviewed by:
David Richard Nash, University of Copenhagen, DenmarkCopyright © 2017 Lorenzi, Azzani and Bagnères. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Cristina Lorenzi, Y3Jpc3RpbmEubG9yZW56aUBsZWVjLnVuaXYtcGFyaXMxMy5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.