
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Ecol. Evol. , 15 December 2016
Sec. Behavioral and Evolutionary Ecology
Volume 4 - 2016 | https://doi.org/10.3389/fevo.2016.00138
This article is part of the Research Topic Progress in Ecological Stoichiometry View all 34 articles
Pollen rains may temporally mitigate nutritional limitations experienced by terrestrial and aquatic detritivores by supplying stoichiometrically balanced food during periods of detritivore growth and development (spring-summer). This may affect the functioning of food webs and thus influence fundamental processes, e.g., by enabling fungi to decompose nutritionally scarce litter. Nutritional limitation may be studied within the framework of ecological stoichiometry by comparing the stoichiometric mismatches experienced by organisms feeding on various foods. To this end, the elemental compositions of pine pollen, litter and detritivores (fungi, protozoans, worms, insects, mites, millipedes, isopods and slugs) were compared, as were the stoichiometric mismatches experienced by the detritivores feeding on litter and pollen. Additionally, the contribution of pollen to the nutrient flow from the land to aquatic ecosystems was estimated through a literature review. Compared to litter, pine pollen is a stoichiometrically well-balanced food source in terms of its C:N:P ratio but also because of its high concentrations of K, S, and Cu and its favorable Zn:Fe ratio. This characteristic is especially suitable to fungi, which may be responsible for the redistribution of pollen-derived nutrients in food webs, particularly aquatic ones. Pollen rains of various plant species act as temporal pulses of nutrients that are rapidly utilized and quickly introduced into the food web, so calculations of annual biomass input may be misleading. Pollen is an easily available, digestible and nutritious food for fungi, bacteria, protozoans, and various groups of invertebrates, which suggests that pollen plays an important role in within- and cross-ecosystem nutrient cycling.
For organisms feeding on plant materials, the proportion of non-C elements in their food may be more limiting than energy (Pokarzhevskii et al., 2003; Moe et al., 2005) and diet supplementation with high-quality resources may promote the development of animals feeding on dead plant matter (Filipiak and Weiner, 2014, 2016; Filipiak et al., 2016; Horvathova et al., 2016). Hence, it might be advantageous to include in the diet plant matter that is nutritive, easily available in considerable mass and relatively easily digestible, i.e., pollen. Indeed, it was suggested that, in detrital food webs, litter-decomposing fungi are colimited by the scarcity of N, P, and S in litter, which they mitigate by foraging on pollen and which allows them to complete litter decomposition (Stark, 1972; Staaf and Berg, 1982; Hutchison and Barron, 1997). Forests may produce pollen in masses reaching 100 to 1000 kg/ha (Greenfield, 1996), and the bulk of this mass is deposited onto forest floors and in lakes (Richerson et al., 1970; Proctor et al., 1996; Shumilovskikh et al., 2015), thus increasing the productivity of the ecosystem (Graham et al., 2006; Masclaux et al., 2013). The present study considers the ecological stoichiometry framework to relate data on pollen nutritional quality to the role of pollen in nutrient cycling within and between terrestrial and aquatic ecosystems.
The extracellular walls of pollen are difficult to destroy chemically (Brooks et al., 1970), but they can be destroyed through mechanical disruption (crushing by chewing) or osmotic shock, which does not require special adaptations, to make pollen digestible (but see Franchi et al., 1997; Greenfield, 1999; Roulston and Cane, 2000; Johnson and Nicolson, 2001). Bacteria and fungi, however, are able to chemically destroy pollen walls (Brooks et al., 1970; Bradley, 2015; Shumilovskikh et al., 2015) and increase the productivity of the ecosystem through the introduction of pollen-derived nutrients to the food web (Masclaux et al., 2011, 2013; Rösel et al., 2012). Pollen rapidly decomposes in both terrestrial and aquatic ecosystems, liberating large amounts of nutritionally rich matter soon after deposition (Greenfield, 1999; Cho et al., 2003; Webster et al., 2008; Rösel et al., 2012). Ponge (1991) observed pollen in the guts of potworms, earthworms and dipterans, and a wide array of other terrestrial and aquatic organisms has been reported to actively feed on pollen including detritivorous, herbivorous and predatory bacteria, fungi, plankton, insects, arachnids, worms, and gastropods (cf. Supplementary Table 1).
Generally, the amount of pollen deposited annually by various plants (grasses, trees, and herbs) in different ecosystems varies from several to hundreds of kg/ha, but the majority of studies are only concerned with pine pollen (Richerson et al., 1970; Stark, 1972; Doskey and Ugoagwu, 1989; Greenfield, 1996, 1999; Proctor et al., 1996; Lee et al., 1996a,b; Hicks, 1999; Perez-Moreno and Read, 2001; Cho et al., 2003; Lee and Booth, 2003; Graham et al., 2006; Shumilovskikh et al., 2015). Maggs (1985) showed that pollen constitutes 3.5% of the total mass of the yearly slash pine biomass fall (litterfall plus pollen rain) but accounts for up to 30% of the total amount of deposited N, P, and K, so one could compare the general patterns of mass and nutrient inputs from pollen and litter. In forests, the annual inputs of individual elements from pine pollen rain can reach approximately 0.3–0.5 kg/ha N; 0.04–0.07 kg/ha P; 0.1–0.2 kg/ha K; 0.02 kg/ha S; and 0.01 kg/ha Mg (Lee et al., 1996a; Cho et al., 2003; Lee and Booth, 2003). Read and Perez-Moreno (2003) estimated the annual N and P deposition from pollen in forests as 1.6 and 0.32 kg/ha, respectively, while Webster et al. (2008) estimated the yearly N input from various pollens into soils as 20 kg/ha. Ukonmaanaho et al. (2008) reported mean annual inputs of 9 elements through litterfall; in pine stands, the means (kg/ha) were N: 6.44–23.67, P: 0.19–1.92, K: 1.23–4.39, S: 0.47–0.98, and Mg: 0.56–1.61 and for spruce stands: N: 4.94–58.51, P: 0.55–5.25, K: 0.84–17.10, S: 0.42–4.66, and Mg: 0.51–5.35. The authors also suggested a range of 600–5000 kg/ha of yearly litter production in boreal forests. Bray and Gorham (1964) concluded that annual forest litter production is approximately 1 t/ha in arctic and alpine zones, 3.5–5.5 t/ha in temperate zones and 11 t/ha in equatorial zones. Thus, in forests, the contribution of the pollen mass to the total biomass fall is estimated to be 1–10%, but the contribution of pollen to the total fall of non-C elements should be several-fold higher and may reach 5–50%, because pollen is rich in non-C elements especially if considering P. The N content in the pollen of various plants may reach approximately 0.4–10%, most commonly 2–4%, while the P content may reach 0.05–0.7%, most commonly 0.2–0.5% (Todd and Bretherick, 1942; Nielsen et al., 1955; Stanley and Linskens, 1974; Roulston and Cane, 2000). These values are high compared to those of other plant tissues (e.g., Güsewell, 2004; Marshner, 2012) and especially high compared with those of plant litter (Berg and McClaugherty, 2014). Ignoring pollen outputs, the quality of the matter produced may be more important than its quantity in terms of the biomass flow that is relevant to consumers and ecosystems (Marcarelli et al., 2011; Sitters et al., 2015; Mehner et al., 2016). Due to the high concentrations of non-C elements, the pollen of various taxa may be hypothesized to be stoichiometrically well-balanced, i.e., good quality food for herbivores and detritivores. This would be important since pollen rains affect a wide array of habitats and are produced by a variety of plants, including trees (Betulaceae, Corylaceae, Fagaceae, Salicaceae, Ulmaceae, Oleaceae, Sapindaceae) and herbs, those of which make the greatest contribution to the pollen rain are grasses (Poaceae, also cereals), sedges (Cyperaceae), rushes (Juncaceae), plantains (Plantaginaceae), docks (Polygonaceae), goosefoots (Chenopodiaceae), nettles (Urticaceae), and Asteraceae (including ragweed) as well as many others (Proctor et al., 1996). The total annual pollen production of a ruderal ecosystem was estimated to be 45–590 kg/ha, including species that produce pollen rains (Denisow, 2011), but the amount of pollen that is deposited on the floor of such a habitat is unknown.
Although most pollen remains in the area where it was produced (Koski, 1970), a portion can be moved over distances of hundreds (Proctor et al., 1996; Sitters et al., 2015) or even thousands of kilometers (Campbell et al., 1999), translocating nutrients between ecosystems. The yearly deposition of pollen from a single species into lakes was estimated to range from several to hundreds of kilograms, while the translocation of P in pine pollen from the land to a lake may reach approximately 0.1–10 kg/ha per year, which accounts for half of the yearly external P input for a small oligotrophic lake (Doskey and Ugoagwu, 1989; Cole et al., 1990; Banks and Nighswander, 2000; Graham et al., 2006; Rösel et al., 2012). This input, even if insignificant in terms of annual mass, is short term, and pollen decomposes rapidly, releasing nutrients within a few days of incubation in lake water (Rösel et al., 2012). Therefore, pollen rain may act as a considerable, temporally limited pulse of nutrients. Pollen rains of various species supply food webs with nutritional elements from early spring to late summer, the time when high amounts of these elements are needed to build the bodies of the developing biota (Roulston and Cane, 2000; Beckman and Hurd, 2003; Lundgren, 2009; Wilder, 2011; Eggs and Sanders, 2013). Thus, existing calculations of the nutritional supplementation of ecosystems provided by pollen rain annually may be misleading. Yet, there exist species producing pollen in fall (e.g., Anderson and Hill, 2002).
Pine pollen and pine litter have been studied in sufficient depth to provide data that are appropriate for a comparative analysis of their elemental compositions in various pine species distributed worldwide. To that end, to comprehensively determine how pollen stoichiometry influences the nutritional mismatches experienced by detritivores, the pine forest ecosystem was considered as an exemplary food web in the present study. To determine whether pollen flux may promote the development of detritivores, the elemental compositions and stoichiometry of pollen, litter and detritivores were compared. The available data on the elemental composition of pine litter and pollen of various pine species inhabiting different forests worldwide were collected (details in Supplementary Table 2).
A comparison of the elemental composition of pine pollen with that of litter (Table 1) demonstrated that the C concentration in pollen is similar to that in litter; the concentrations of N, P, S, K, Mg, and Cu are approximately 2- to 12-fold higher in pollen; and concentrations of Ca, Mn, and Fe are approximately 3- to 19-fold higher in litter. As a detritivore food source, pollen stoichiometry is advantageous compared with that of litter, particularly the C:P ratio (12-fold lower in pollen). P-rich pine pollen is deposited in the spring, when young detritivores develop and have the greatest need for P and the highest vulnerability to suboptimal diets during their ontogeny (Bullejos et al., 2014). Thus, feeding on pine pollen may benefit detritivore development. Other elements supplied to a high degree by pine pollen include K and S. Together, these three elements may be limiting for fungi exploiting pine litter (Staaf and Berg, 1982), so pollen might be a particularly important resource for these organisms. Indeed, the utilization of pollen by forest fungi has been reported previously (Stark, 1972; Hutchison and Barron, 1997; Perez-Moreno and Read, 2001).
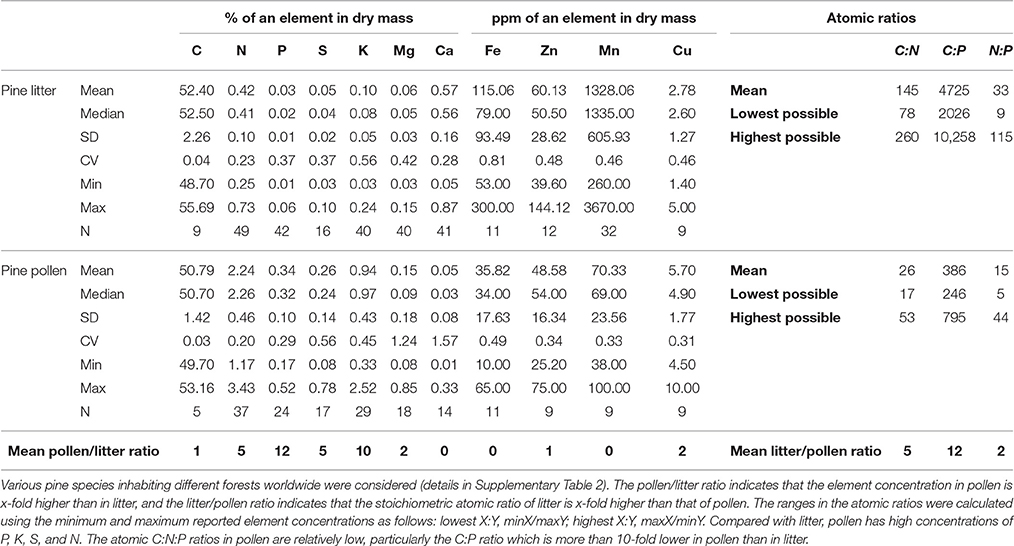
Table 1. Dry mass element concentrations and stoichiometry of pine pollen compared with pine litter.
Nutritionally scarce food is stoichiometrically unbalanced, which is reflected in a stoichiometric mismatch (differences in the concentrations of elements in the food and in the body of the consumer) that limits consumer growth and development (Sterner and Elser, 2002; Denno and Fagan, 2003; Fagan and Denno, 2004; Hessen et al., 2013). To detect potential stoichiometric mismatches and their negative consequences (development limitation), simple comparisons of element ratios are sufficient (Filipiak and Weiner, 2016), so the stoichiometric ratios in food and in the bodies of consumers (C:Xfood/C:Xconsumer, where C is the carbon concentration and X is the concentration of the other element) were calculated to comprehensively detect and compare the stoichiometric mismatches that reflect the variety of detritivores consuming either litter or pollen. Hereafter, these values will be referred to as trophic stoichiometric ratios (TSRs, cf. Filipiak and Weiner, 2016). To this end, data from the literature (means) and newly collected data on the elemental composition of detritivores inhabiting forest litters and soils (means) were used (details in Supplementary Tables 2–4).
Five elements (P, K, N, S, and Cu; Figure 1) were found to be the most limiting to detritivore development. The TSRs calculated for feeding on pine pollen were approximately 10-fold lower than those for feeding on pine litter for P, K, N, and S and 2-times lower for Cu; the mitigating effect was strongest for P and K. The pollen diet might be particularly advantageous for isopods, millipedes and fungi because these organisms have the greatest need for K, P, and S supplementation (Figure 1). Pine pollen might be an excellent source of nutrients for terrestrial detritivores, mitigating stoichiometric mismatches, thereby it might promote detritivore growth and development.
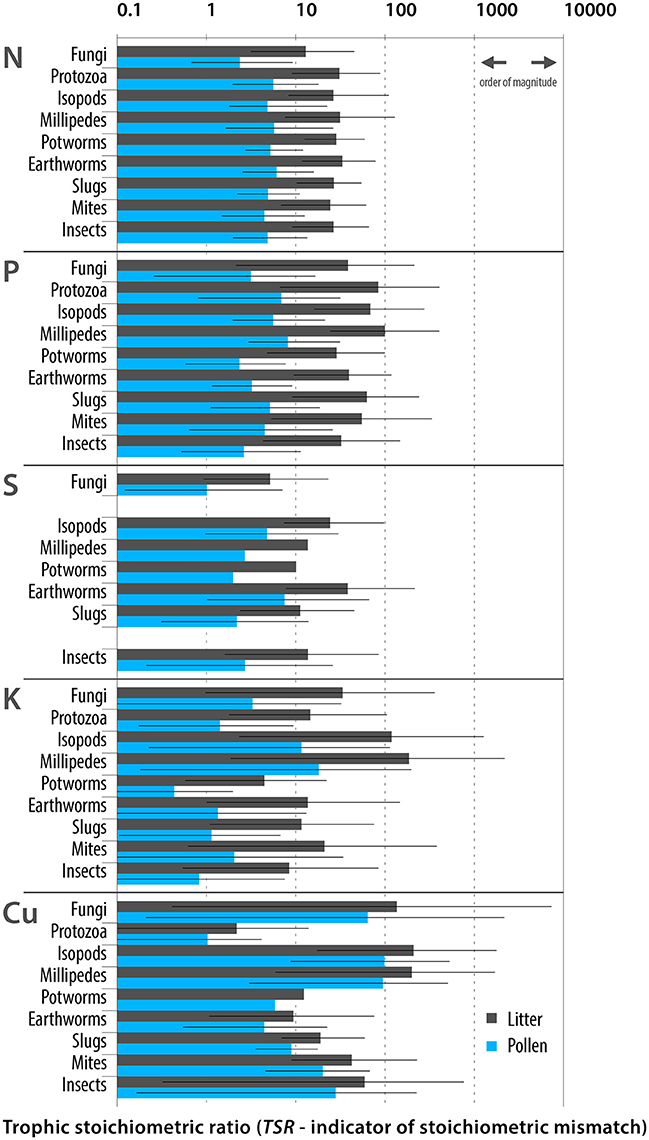
Figure 1. Stoichiometric mismatches (TSRs) calculated for detritivorous soil and litter dwellers that may utilize pollen as a supplementary food. TSR values were calculated for the two sources of food: pine litter and pine pollen. Bars denote means; whiskers denote minima and maxima. Various pine species inhabiting different forests in diverse locations were considered. The scale of the Y-axis is logarithmic. After lowering any mismatches by approximately one order of magnitude, feeding on pollen mitigated N, P, S, and K nutritional mismatches in a variety of organisms.
Pollen has been suggested to be an important source of nutrients for aquatic organisms and a factor that influences nutrient cycling in aquatic ecosystems (Masclaux et al., 2011; Rösel et al., 2012), and the stoichiometric characteristics of pollen are consistent with this suggestion. Pine pollen C:N:P stoichiometry is more similar to that of freshwater than terrestrial autotrophs; i.e., pollen is relatively more stoichiometrically balanced for aquatic consumers compared with other types of plant matter (Elser et al., 2000). Pine pollen has markedly lower C:P and N:P ratios compared with the other matter that flows in large masses from terrestrial to aquatic ecosystems, e.g., foliage (Elser et al., 2000). This may promote the growth of P-limited aquatic detritivores (Sterner and Hessen, 1994; Sterner and Elser, 2002; Sardans et al., 2012). It is possible that pollen does not act as a direct source of nutrients for aquatic herbivores but is first utilized by aquatic fungi, consequently increasing the productivity of the entire system (Masclaux et al., 2011, 2013).
In aquatic food webs, fungal action may be more important to the introduction of pollen-derived nutrients than in terrestrial ecosystems. Although pollen is nutritive and digestible, recent studies have shown that pollen alone is insufficient to promote zooplankton growth (Masclaux et al., 2011, 2013), suggesting that pollen walls are highly resistant to the action of zooplankton and that saprotrophic fungi are responsible for weakening and degrading these walls, thereby introducing pollen-derived nutrients into the food web and boosting ecosystem productivity (Goldstein, 1960; Masclaux et al., 2011, 2013; Rösel et al., 2012; Wurzbacher et al., 2014). Pine pollen is an attractive source of nutrients for fungi due to its C:N:P ratio, but it may also provide other limiting elements, especially K, S, and Cu (Figure 1).
Knowledge of the ecological stoichiometry of aquatic fungi is lacking, and specific predictions or comparisons with food stoichiometry are currently impossible (Danger et al., 2016). The limited data on elemental compositions are either for terrestrial fungi consumed by humans and consider only a finite number of elements, excluding C (Rudawska and Leski, 2005; Dursun et al., 2006), or are related to fungal strains cultured in laboratories on artificial media, which are not relevant to natural situations (Mouginot et al., 2014). A study on the mineral requirements of aquatic fungi, which covered other elements besides C, N, and P (Schoenlein-Crusius et al., 1999), showed that aquatic hyphomycetes may be sensitive to the stoichiometry of nutrients, specifically the contents of Ca, S, K, Mg, Mn, Na, and Zn (positively correlated) and Fe and Al (negatively correlated). Among these elements, S and K are highly concentrated in pine pollen (Table 1), and the positive effect of Zn and the negative effect of Fe on fungi might be associated with competition among these elements for absorption sites, as the excess of one could induce a deficiency of the other. This reported effect occurred with food with a low Zn:Fe atomic ratio (approximately 0.01–0.007); the mean Zn:Fe atomic ratio in pine pollen is 1.2, thus neutral and 24-fold higher than that in pine litter and thus relatively favorable. Pine pollen is also relatively high in Cu, another element that is rich in fungal tissues.
Nutrients that are incorporated into the ecosystem with pollen rain might either be directly utilized by invertebrates, thus mitigating their stoichiometric mismatches (Figure 1), or introduced into the food web via microorganisms (mainly fungi). Pollen on the forest floor decomposes rapidly, losing approximately 20–70% of its initial mass in a month solely as a result of microbial action (Greenfield, 1999; Webster et al., 2008). The majority of the water-extractable macronutrients in pollen (more than 80%) can leach within a few hours in both land (Lee et al., 1996a) and water (Rösel et al., 2012) ecosystems. These nutrients might subsequently be incorporated, redistributed and recycled by microorganisms (Stark, 1972; Hutchison and Barron, 1997; Davidson et al., 1999; Perez-Moreno and Read, 2001; Van Mourik, 2003).
The contribution of pollen to litter decomposition is underrated. It was reported that the N, P, and S supplied by pine pollen to the forest floor enabled fungi to decompose nutritionally scarce pine litter (Stark, 1972; Staaf and Berg, 1982; Hutchison and Barron, 1997), but pine pollen may be even more important, supplying decomposers with sufficient amounts of K and Cu and having a desirable Zn:Fe atomic ratio.
Data on the elemental content of pollen are scarce. Surprisingly, data concerning the concentration of C in litter, pollen and detritivores are also scarce, so studies of particular food webs that utilize a set of elements and allow C:X ratios to be calculated are needed to understand the role of pollen in nutrient cycling. These studies should move beyond traditional C:N:P stoichiometry and incorporate other physiologically important elements, of which K, S, Zn, Fe, and Cu might be the most important.
Even if the annual contribution of pollen-derived nutrients is not significant in terms of the mass of the elements input to the ecosystem, pollen rains may act as a temporal pulse of nutrients. Pollen is cycled through ecosystems during short periods from early spring (primarily from trees) to late summer (primarily from herbs; Lee et al., 1996a; Proctor et al., 1996; Cho et al., 2003). Cole et al. (1990) claimed that summer pollen rains do not contribute to enriching aquatic ecosystems, but data show that spring pollen rains dominated by anemophilous trees relocate considerable amounts of nutrients from terrestrial to aquatic ecosystems (Graham et al., 2006; Rösel et al., 2012). Rösel et al. (2012) suggested that algal blooms may be connected with short-duration but massive pollen deposition from trees. The present study indicates that pine pollen stoichiometry and the ability of pollen rains to rapidly introduce nutrients into food webs makes pollen an ideal agent for triggering algal-blooms.
Future studies could (1) track the pathways of pollen-derived nutrients within and between ecosystems, (2) undertake feeding experiments that supplement the diets of detritivores with various amounts of pollen and evaluate their physiological responses and life history traits (e.g., growth rate, larval development time, size at maturity, assimilation rates of limiting elements, etc.), (3) experimentally manipulate natural food webs by preventing pollen deposition during pollen rain periods and observing the impact on ecosystem productivity. Also needed are studies of the seasonal variations in pollen stoichiometry and deposition that consider various pollen species in different ecosystems. In the case of aquatic ecosystems, the factors affecting the quantity of deposited pollen (e.g., surrounding flora, length of shoreline/lake area ratio, distance from the shore, etc.) should be acknowledged as well as the potential power of a pollen enrichment effect (e.g., trophic state of the pollen-receiving ecosystem). Further suggestions for merging ecological stoichiometry and cross-ecosystem material flows in a spatial context were presented by Sitters et al. (2015).
The author confirms being the sole contributor of this work and approved it for publication.
This study was supported by grants from the Polish Ministry of Science and Higher Education (Grant No. DS/WBiNoZ/INoŚ/DS 756) and the National Science Centre (Grant No. DEC-2013/11/N/NZ8/00929).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author is indebted to Zuzanna Świątek and January Weiner for their constructive critical comments and thanks American Journal Experts (AJE) for English language editing.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2016.00138/full#supplementary-material
Anderson, G. J., and Hill, J. D. (2002). Many to flower, few to fruit: the reproductive biology of Hamamelis virginiana (Hamamelidaceae). Am. J. Bot. 89, 67–78. doi: 10.3732/ajb.89.1.67
Banks, H. H., and Nighswander, J. E. (2000). “Relative contribution of hemlock pollen to the phosphorus loading of the Clear Lake Ecosystem near Minden, Ontario,” in Proceedings: Symposium on Sustainable Management of Hemlock Ecosystems in Eastern North America, eds K. A. McManus, K. S. Shields, and D. Souto (Delaware, OH: U.S.D.A. Forest Service), 167–174.
Beckman, N., and Hurd, L. E. (2003). Pollen feeding and fitness in praying mantids: the vegetarian side of a tritrophic predator. Environ. Entomol. 32, 881–885. doi: 10.1603/0046-225X-32.4.881
Bray, J. R., and Gorham, E. (1964). Litter production in forests of the world. Adv. Ecol. Res. 2, 101–157. doi: 10.1016/S0065-2504(08)60331-1
Brooks, J., Grant, P. R., and Muir, M. (eds.). (1970). “Sporopollenin,” in Proceedings of a Symposium Held at the Geology Department, Imperial College, (London: Elsevier Ltd).
Bullejos, F. J., Carrillo, P., Gorokhova, E., Medina-Sánchez, J. M., Balseiro, E. G., and Villar-Argaiz, M. (2014). Shifts in food quality for herbivorous consumer growth: multiple golden means in the life history. Ecology 95, 1272–1284. doi: 10.1890/13-0410.1
Campbell, I. D., McDonald, K., Flannigan, M. D., and Kringayark, J. (1999). Long-distance transport of pollen into the Arctic. Nature 399, 29–30. doi: 10.1038/19891
Cho, Y.-J., Sung Kim, I., Kim, P., and Ju Lee, E. (2003). Deposition of airborne pine pollen in a temperate pine forest. Grana 42, 178–182. doi: 10.1080/00173130310016158
Cole, J. J., Caraco, N. F., and Likens, G. E. (1990). Short-range atmospheric transport: a significant source of phosphorus to an oligotrophic lake. Limnol. Oceanogr. 35, 1230–1237. doi: 10.4319/lo.1990.35.6.1230
Danger, M., Gessner, M. O., and Bärlocher, F. (2016). Ecological stoichiometry of aquatic fungi: current knowledge and perspectives. Fungal Ecol. 19, 100–111. doi: 10.1016/j.funeco.2015.09.004
Davidson, D. A., Carter, S., Boag, B., Long, D., Tipping, R., and Tyler, A. (1999). Analysis of pollen in soils: processes of incorporation and redistribution of pollen in five soil profile types. Soil Biol. Biochem. 31, 643–653. doi: 10.1016/S0038-0717(98)00123-0
Denisow, B. (2011). Pollen Production of Selected Ruderal Plant Species in the Lublin Area. Lublin: University of Life Sciences in Lublin Press [Wydawnictwo Uniwersytetu Przyrodniczego].
Denno, R. F., and Fagan, W. F. (2003). Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology 84, 2522–2531. doi: 10.1890/02-0370
Doskey, P. V., and Ugoagwu, B. J. (1989). Atmospheric deposition of macronutrients by pollen at a semi-remote site in northern Wisconsin. Atmos. Environ. 23, 2761–2766. doi: 10.1016/0004-6981(89)90556-8
Dursun, N., Özcan, M. M., Kaşık, G., and Öztürk, C. (2006). Mineral contents of 34 species of edible mushrooms growing wild in Turkey. J. Sci. Food Agric. 86, 1087–1094. doi: 10.1002/jsfa.2462
Eggs, B., and Sanders, D. (2013). Herbivory in spiders: the importance of pollen for orb-weavers. PLoS ONE 8:e82637. doi: 10.1371/journal.pone.0082637
Elser, J. J., Fagan, W. F., Denno, R. F., Dobberfuhl, D. R., Folarin, A., Huberty, A., et al. (2000). Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580. doi: 10.1038/35046058
Fagan, W. F., and Denno, R. F. (2004). Stoichiometry of actual vs. potential predator-prey interactions: insights into nitrogen limitation for arthropod predators. Ecol. Lett. 7, 876–883. doi: 10.1111/j.1461-0248.2004.00641.x
Filipiak, M., Sobczyk, Ł., and Weiner, J. (2016). Fungal transformation of tree stumps into a suitable resource for xylophagous beetles via changes in elemental ratios. Insects 7:13. doi: 10.3390/insects7020013
Filipiak, M., and Weiner, J. (2014). How to make a beetle out of wood: multi-elemental stoichiometry of wood decay, xylophagy and fungivory. PLoS ONE 9:e115104. doi: 10.1371/journal.pone.0115104
Filipiak, M., and Weiner, J. (2016). Nutritional dynamics during the development of xylophagous beetles related to changes in the stoichiometry of 11 elements. Physiol. Entomol. doi: 10.1111/phen.12168. [Epub ahead of print].
Franchi, G. G., Franchi, G., Corti, P., and Pompella, A. (1997). Microspectrophotometric evaluation of digestibility of pollen grains. Plant Foods Hum. Nutr. 50, 115–126. doi: 10.1007/BF02436031
Goldstein, S. (1960). Degradation of pollen by phycomycetes. Ecology 41, 543–545. doi: 10.2307/1933329
Graham, M. D., Vinebrooke, R. D., and Turner, M. (2006). Coupling of boreal forests and lakes: effects of conifer pollen on littoral communities. Limnol. Oceanogr. 51, 1524–1529. doi: 10.4319/lo.2006.51.3.1524
Greenfield, L. (1996). Plant pollen production in selected tree species. Canterbury Bot. Soc. J. 31, 10–13.
Greenfield, L. (1999). Weight loss and release of mineral nitrogen from decomposing pollen. Soil Biol. Biochem. 31, 353–361. doi: 10.1016/S0038-0717(98)00134-5
Güsewell, S. (2004). N : p ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266. doi: 10.1111/j.1469-8137.2004.01192.x
Hessen, D. O., Elser, J. J., Sterner, R. W., and Urabe, J. (2013). Ecological stoichiometry: an elementary approach using basic principles. Limnol. Oceanogr. 58, 2219–2236. doi: 10.4319/lo.2013.58.6.2219
Hicks, S. (1999). The relationship between climate and annual pollen deposition at northern tree-lines. Chemosph. Glob. Change Sci. 1, 403–416. doi: 10.1016/S1465-9972(99)00043-4
Horvathova, T., Babik, W., and Bauchinger, U. (2016). Biofilm feeding: microbial colonization of food promotes the growth of a detritivorous arthropod. Zookeys 577, 25–41. doi: 10.3897/zookeys.577.6149
Hutchison, L. J., and Barron, G. L. (1997). Parasitism of pollen as a nutritional source for lignicolous Basidiomycota and other fungi. Mycol. Res. 101, 191–194. doi: 10.1017/S095375629600233X
Johnson, S. A., and Nicolson, S. W. (2001). Pollen digestion by flower-feeding Scarabaeidae: protea beetles (Cetoniini) and monkey beetles (Hopliini). J. Insect Physiol. 47, 725–733. doi: 10.1016/S0022-1910(00)00166-9
Koski, V. (1970). A study of pollen dispersal as a mechanism of gene flow in conifers. Commun. Inst. For. Fenn. 70, 1–78.
Lee, E. J., and Booth, T. (2003). Macronutrient input from pollen in two regenerating pine stands in southeast Korea. Ecol. Res. 18, 423–430. doi: 10.1046/j.1440-1703.2003.00566.x
Lee, E. J., Kenkel, N., and Booth, T. (1996a). Atmospheric deposition of macronutrients by pollen in the boreal forest. Ecoscience 3, 304–309. doi: 10.1080/11956860.1996.11682347
Lee, E. J., Kenkel, N., and Booth, T. (1996b). Pollen deposition in the boreal forest of west-central Canada. Can. J. Bot. 74, 1265–1272. doi: 10.1139/b96-153
Lundgren, J. G. (2009). Relationships of Natural Enemies and Non-Prey Foods. Dordrecht: Springer Netherlands.
Maggs, J. (1985). Litter fall and retranslocation of nutrients in a refertilized and prescribed burned Pinus elliottii plantation. For. Ecol. Manage. 12, 253–268. doi: 10.1016/0378-1127(85)90094-5
Marcarelli, A. M., Baxter, C. V., Mineau, M. M., and Hall, R. O. (2011). Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92, 1215–1225. doi: 10.1890/10-2240.1
Masclaux, H., Bec, A., Kagami, M., Perga, M., Sime-Ngando, T., Desvilettes, C., et al. (2011). Food quality of anemophilous plant pollen for zooplankton. Limnol. Oceanogr. 56, 939–946. doi: 10.4319/lo.2011.56.3.0939
Masclaux, H., Perga, M.-E., Kagami, M., Desvilettes, C., Bourdier, G., and Bec, A. (2013). How pollen organic matter enters freshwater food webs. Limnol. Oceanogr. 58, 1185–1195. doi: 10.4319/lo.2013.58.4.1185
Mehner, T., Attermeyer, K., Brauns, M., Brothers, S., Diekmann, J., Gaedke, U., et al. (2016). Weak response of animal allochthony and production to enhanced supply of terrestrial leaf litter in nutrient-rich lakes. Ecosystems 19, 311–325. doi: 10.1007/s10021-015-9933-2
Moe, S. J., Stelzer, R. S., Forman, M. R., Harpole, W. S., Daufresne, T., and Yoshida, T. (2005). Recent advances in ecological stoichiometry: insights for population and community ecology. Oikos 109, 29–39. doi: 10.1111/j.0030-1299.2005.14056.x
Mouginot, C., Kawamura, R., Matulich, K. L., Berlemont, R., Allison, S. D., Amend, A. S., et al. (2014). Elemental stoichiometry of fungi and bacteria strains from grassland leaf litter. Soil Biol. Biochem. 76, 278–285. doi: 10.1016/j.soilbio.2014.05.011
Nielsen, N., Grömmer, J., and Lunden, R. (1955). Investigations on the chemical composition of pollen from some plants. Acta Chem. Scand. 9, 1100–1106. doi: 10.3891/acta.chem.scand.09-1100
Perez-Moreno, J., and Read, D. J. (2001). Exploitation of pollen by mycorrhizal mycelial systems with special reference to nutrient recycling in boreal forests. Proc. R. Soc. B Biol. Sci. 268, 1329–1335. doi: 10.1098/rspb.2001.1681
Pokarzhevskii, A. D., van Straalen, N. M., Zaboev, D. P., and Zaitsev, A. S. (2003). Microbial links and element flows in nested detrital food-webs. Pedobiologia 47, 213–224. doi: 10.1078/0031-4056-00185
Ponge, J. F. (1991). Food resources and diets of soil animals in a small area of Scots pine litter. Geoderma 49, 33–62. doi: 10.1016/0016-7061(91)90090-G
Proctor, M., Yeo, P., and Lack, A. (1996). The Natural History of Pollination. London: Harper Collins New Naturalist.
Read, D. J., and Perez-Moreno, J. (2003). Mycorrhizas and nutrient cycling in ecosystems - a journey towards relevance? New Phytol. 157, 475–492. doi: 10.1046/j.1469-8137.2003.00704.x
Richerson, P. J., Moshiri, G. A., and Godshalk, G. L. (1970). Certain ecological aspects of pollen dispersion in Lake Tahoe (California–Nevada). Limnol. Oceanogr. 15, 149–153. doi: 10.4319/lo.1970.15.1.0149
Rösel, S., Rychła, A., Wurzbacher, C., and Grossart, H. (2012). Effects of pollen leaching and microbial degradation on organic carbon and nutrient availability in lake water. Aquat. Sci. 74, 87–99. doi: 10.1007/s00027-011-0198-3
Roulston, T., and Cane, J. (2000). Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 222, 187–209. doi: 10.1007/BF00984102
Rudawska, M., and Leski, T. (2005). Macro- and microelement contents in fruiting bodies of wild mushrooms from the Notecka forest in west-central Poland. Food Chem. 92, 499–506. doi: 10.1016/j.foodchem.2004.08.017
Sardans, J., Rivas-Ubach, A., and Pe-uelas, J. (2012). The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: a review and perspectives. Biogeochemistry 111, 1–39. doi: 10.1007/s10533-011-9640-9
Schoenlein-Crusius, I. H., Pires-Zottarelli, C. L. A., Milanez, A. I., and Humphreys, R. D. (1999). Interaction between the mineral content and the occurrence number of aquatic fungi in leaves submerged in a stream in the Atlantic rainforest, São Paulo, Brazil. Rev. Bras. Botânica 22, 133–139. doi: 10.1590/S0100-84041999000200004
Shumilovskikh, L. S., Schlütz, F., Achterberg, I., Kvitkina, A., Bauerochse, A., and Leuschner, H. H. (2015). Pollen as nutrient source in Holocene ombrotrophic bogs. Rev. Palaeobot. Palynol. 221, 171–178. doi: 10.1016/j.revpalbo.2015.07.001
Sitters, J., Atkinson, C. L., Guelzow, N., Kelly, P., and Sullivan, L. L. (2015). Spatial stoichiometry: cross-ecosystem material flows and their impact on recipient ecosystems and organisms. Oikos 124, 920–930. doi: 10.1111/oik.02392
Staaf, H., and Berg, B. (1982). Accumulation and release of plant nutrients in decomposing Scots pine needle litter. Long-term decomposition in a Scots pine forest II. Can. J. Bot. 60, 1561–1568. doi: 10.1139/b82-199
Stanley, R. G., and Linskens, H. F. (1974). Pollen Biology Biochemistry Management. Berlin, Heidelberg: Springer.
Stark, N. (1972). Nutrient cycling pathways and litter fungi. Bioscience 22, 355–360. doi: 10.2307/1296341
Sterner, R. W., and Elser, J. J. (2002). Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton, NJ: Princeton University Press.
Sterner, R. W., and Hessen, D. O. (1994). Algal nutrient limitation and the nutrition of aquatic herbivores. Annu. Rev. Ecol. Syst. 25, 1–29. doi: 10.1146/annurev.es.25.110194.000245
Todd, F. E., and Bretherick, O. (1942). The composition of pollens. J. Econ. Entomol. 35, 312–317. doi: 10.1093/jee/35.3.312
Ukonmaanaho, L., Merilä, P., Nöjd, P., and Nieminen, T. M. (2008). Litterfall production and nutrient return to the forest floor in Scots pine and Norway spruce stands in Finland. Boreal Environ. Res. 13, 67–91.
Van Mourik, J. M. (2003). Life cycle of pollen grains in mormoder humus forms of young acid forest soils: a micromorphological approach. CATENA 54, 651–663. doi: 10.1016/S0341-8162(03)00116-4
Webster, E. A., Tilston, E. L., Chudek, J. A., and Hopkins, D. W. (2008). Decomposition in soil and chemical characteristics of pollen. Eur. J. Soil Sci. 59, 551–558. doi: 10.1111/j.1365-2389.2008.01022.x
Wilder, S. M. (2011). Spider nutrition: an integrative perspective. Adv. Insect Physiol. 40, 87–136. doi: 10.1016/B978-0-12-387668-3.00002-7
Keywords: ecological stoichiometry, food chain, nutritional ecology, ecosystem ecology, trophic ecology, trophic interactions, nitrogen, phosphorous
Citation: Filipiak M (2016) Pollen Stoichiometry May Influence Detrital Terrestrial and Aquatic Food Webs. Front. Ecol. Evol. 4:138. doi: 10.3389/fevo.2016.00138
Received: 10 August 2016; Accepted: 30 November 2016;
Published: 15 December 2016.
Edited by:
James Joseph Elser, University of Montana, USAReviewed by:
Shawn M. Wilder, Oklahoma State University, USACopyright © 2016 Filipiak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michał Filipiak, bWljaGFsMGZpbGlwaWFrQGdtYWlsLmNvbQ==, bWljaGFsLmZpbGlwaWFrQHVqLmVkdS5wbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.