- 1Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 2Department of Anatomy, Howard University College of Medicine, Washington, DC, USA
Evolution of serially similar structures has attracted interest since the infancy of comparative morphology and embryology. A long-standing assumption is that the serial patterning reflects ancestral metamerism, which persists in preconceived character polarity from a primitive state of polyisomerism (a series of identical or similar units) to a derived state of anisomerism (a series of differentially specialized parts). We test this assumption against an alternative character polarity—from anisomeric to polyisomeric—in the vertebrate pharyngeal apparatus and paired appendages. We show that, contrary to what is usually assumed, serial similarity represents a derived state in both pharyngeal apparatus and paired appendages: the distinctly patterned structures secondarily assimilated each other. Acquisitions of serial similarity in the pharyngeal apparatus and paired appendages straddle major evolutionary events such as the origin of the jaw and fish-tetrapod transitions. We suggest that: (a) the origin of the jaw coincided with extension of the serial pharyngeal patterning onto the mandibular region; and (b) the pectoral and pelvic appendages have independent origins and their distal portions acquired serial similarities later during the fin-limb transitions.
Introduction
Segmentation—repetition of anteroposteriorly polarized units along the anteroposterior body axis (Hannibal and Patel, 2013)—has been a frequently featured concept in the narrative of vertebrate development and evolution. Serial or iterative homology (homology of parts within a single body) was initially conceived to describe segments and bilateral counterparts in a comparative context (Owen, 1848). In that scheme, the observed segmented pattern corresponded to segments in an idealized archetype (“general homology”), whereas each pair of corresponding segments between different taxa was designated as “special homology” (Owen, 1848). The developmental basis of homology has generated long, convoluted discussions since Owen's time, and numerous schemes and definitions have been proposed (reviewed by De Beer, 1971; Patterson, 1988; Wagner, 1989, 2014; Hall, 1994, 2003; Amundson, 2001; Kuratani, 2009; Faunes et al., 2015). These include historical and biological criteria of homology ranging from trait-specific regulatory networks (“character identity network”; Wagner, 2007, 2014) to the flexibly and neutrally defined “continuity of information” (Van Valen, 1982; Roth, 1994). Specifically in regards to serial homology, gene expression patterns that underlie morphologically repeated structures along the anteroposterior axis, such as collinear Hox expressions, have attained an iconic status in evolutionary developmental biology (Patel et al., 1989; Krumlauf, 1994; Holland and Garcia-Fernàndez, 1996; Gellon and McGinnis, 1998; Manzanares et al., 2000; Trainor and Krumlauf, 2001; Carroll, 2008; Parker et al., 2014). Nevertheless, a problem persists for serial homology: body parts generally considered as segments and thus serially homologous do not necessarily share historical continuity of descent from a common ancestor (Wagner, 1989, 1994; Roth, 1994). We will present notable examples where developmental patterns reveal such decoupling (Figure 1A). This terminological paradox has led to two overlapping criteria in parallel with other categories of homology: (a) identified on the basis of historical continuity of form (phenotype) between similar parts (“evolutionary” serial homology); and (b) identified on the basis of developmental correspondence between parts (“developmental” serial homology; Wagner, 1994; Reno et al., 2013).
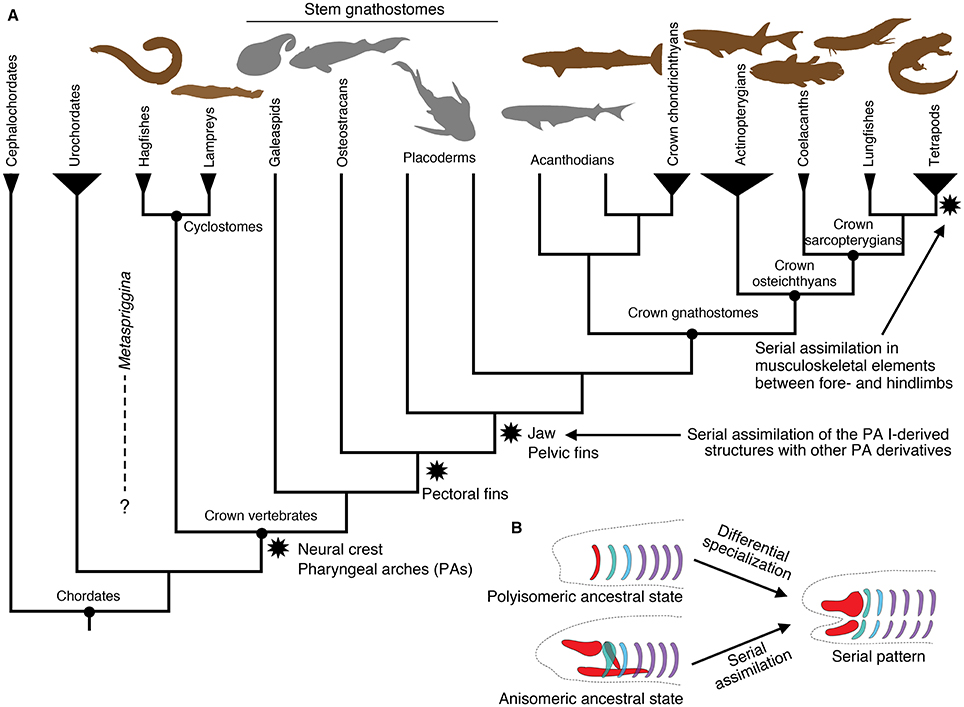
Figure 1. Summary of vertebrate interrelationships and major character transitions in the pharyngeal apparatus and paired appendages. (A) A simplified cladogram of major vertebrate lineages and their outgroups, showing the consensus from the current literature (e.g., Janvier, 2007). Stem gnathostomes include jawless and jawed forms. These and acanthodians are extinct lineages. Cephalochordates and urochordates represent chordate outgroups of vertebrates. Brown silhouettes indicate living representatives, whereas gray silhouettes indicate extinct lineages. (B) Two alternative mechanisms giving rise to a serial pattern, with simplified drawings of the vertebrate pharyngeal skeleton. Polyisomeric ancestral state represents a series of identical or similar units and assumes differential specialization to derive the existing serial pattern. Anisomeric ancestral state represents distinctly patterned regions and assumes serial assimilation to derive the existing serial pattern. The silhouettes in (A) are modified from PhyloPic (http://www.phylopic.org) under either Public Domain Dedication 1.0 or Creative Commons License 3.0. Original drawings: S. Coombs, P. Janvier, G. Monger, L.E. Ray, M. Reinbold, J.H. Richard, N. Tamura, S. Traver, and Y. Wong. Vectorization: T. M. Keesey, and R. D. Sibaja.
In this paper, we recognize serial homology on the basis of historical continuity (“evolutionary” serial homology sensu Wagner, 1994), where the use of serial homology is restricted to those developmentally corresponding parts with shared evolutionary history (arising either simultaneously or via a duplication event). Under this view, serial homology requires phenotypic similarity between corresponding parts as an ancestral state because any congruence not due to such ancestral state then involves some type of phenotypic homoplasy such as convergence and parallelism (Gould, 2002; Hall, 2007). In other words, historical discontinuity in form between parts would violate the pervasive assumption that the serial units originated in a common ancestor simultaneously and in identical forms. Major components of the vertebrate body such as pharyngeal apparatus, axial elements, and paired appendages have each served as a popular example to illustrate how the hypothetical ancestral segmentation underwent differential specialization of its parts (Gegenbaur, 1859; Sewertzoff, 1899, 1931; Goodrich, 1930; De Beer, 1937; Jarvik, 1980). Consequently, these repeating patterns in the vertebrate body have often generated an evolutionary scenario from polyisomerism (a series of identical or similar segments) to anisomerism (a series of differentially specialized units). That is, a series of identical units in an archetypical ancestor (polyisomerism) gave rise to serially organized but distinctly specialized structures (Figure 1B). In this context, the jaw has been long considered as a modified branchial bar (Sewertzoff, 1911, 1913; De Beer, 1937; Jarvik, 1980; Mallatt, 1996), and pectoral and pelvic appendages as serial homologs due to a duplication event (reviewed by Diogo et al., 2013).
In this review, we question the assumption that serial patterns are normally the default ancestral state. To provide a test, this review explores whether two striking cases of serial similarity in vertebrates—within the pharyngeal apparatus and between the paired (pectoral and pelvic) appendages—were gained through assimilation of dissimilar units (serial similarity as a derived state) or through differential modification of originally identical segments (serial similarity due to an ancestral state). When an underlying segmented scheme is extrapolated from observed serial morphological patterns, two possible scenarios exist: (1) segments were differentially modified through secondary specialization (ancestrally polyisomeric state); or (2) distinctly specialized regions secondarily assimilated each other by acquiring serial patterning (ancestrally anisomeric state; Figure 1B). When contrasting these alternative hypotheses, it is critical to not confuse observed or extrapolated serial patterns across different developmental stages. For example, serial organization of the pharyngeal arches at pharyngula stages of vertebrate development should not be confused with serial musculoskeletal patterning of the arch derivatives at later stages of development. Both are hypotheses testable on the basis of phenotypes—such as morphology and gene expression profiles—at that particular stage of development. This is because the mechanisms responsible for serial pattern at an earlier stage (e.g., hindbrain and pharyngeal Hox codes that characterize distinct streams of cranial neural crest cells) can be independent from those that pattern differentiation of the anlagen (e.g., Dlx code that dorsoventrally pattern the crest cells into elements of the pharyngeal apparatus; Hunt et al., 1991a,b; Hunt et al., 1998; Couly et al., 2002; Depew et al., 2002, 2005; Depew and Compagnucci, 2008; Gillis et al., 2013).
The Origin of the Jaws: Mandibular Confinement
During the development of vertebrate embryos, the pharyngeal arches (PAs)—anteroposteriorly arranged columnar structures of mesodermal (MMCs) and neural crest cells (NCCs) set apart by endodermal pouches toward bilateral sides of a pharynx—contain anlagen of a remarkable variety of complex structures (Figure 2; Graham et al., 2005). The pouches begin forming as (a) Tbx1 expression in the mesoderm promotes Wnt11 expression to destabilize the pharyngeal epithelium; and (b) Fgf8 expression zones in the mesoderm guide the destabilized epithelium into pouches (Piotrowski et al., 2003; Crump et al., 2004; Choe et al., 2013; Choe and Crump, 2014; Jandzik et al., 2014). The MMCs are surrounded by NCCs that migrate in position-specific streams corresponding to anteroposterior levels of the hindbrain rhombomeres (Figure 2; Kimmel et al., 2001; McCauley and Bronner-Fraser, 2003; Cerny et al., 2004b; Noden and Trainor, 2005; Hall, 2009). This pattern applies to both of the major living lineages of vertebrates, cyclostomes (hagfish and lampreys) and crown gnathostomes (chondrichthyans and osteichthyans; see Figure 1A for phylogenetic scheme).
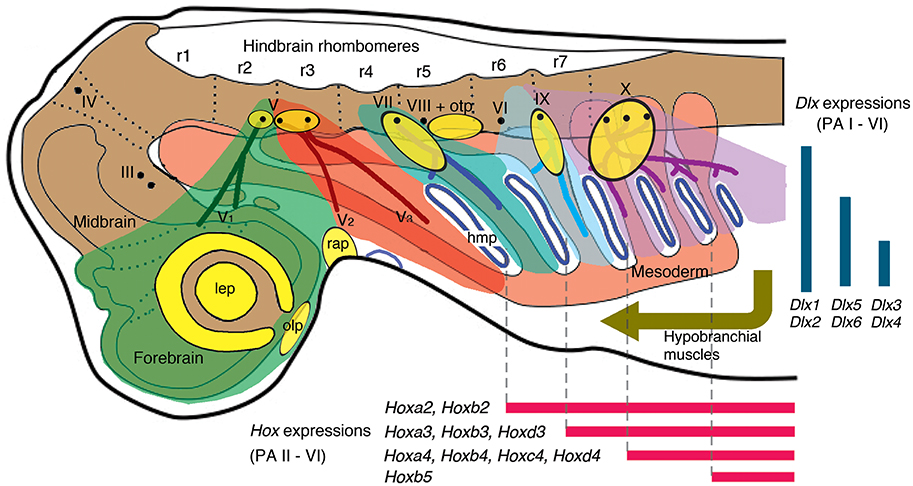
Figure 2. A current scheme of gnathostome head development in Squalus acanthias (dogfish) as an example (modified from Northcutt, 2008; Kuratani, 2012; simplified and corrected [position of V2] from Miyashita, 2015). Color codes: green, NCCs (neural crest cells) of premandibular domain; red, NCCs of PA I (mandibular arch); greenish blue, NCCs of PA II (hyoid arch); light cyan, PA III (branchial arch innervated by the glossopharyngeal nerve); purple, PA IV-VI [all non-glossopharyngeal branchial arches (innervated by the vagus nerve)]; orange, mesoderm; yellow, placodes (lateral line placodes are omitted). Abbreviations: III, oculomotor nerve; IV, trochlear nerve; V, trigeminal nerve; V1, ophthalmic branch of trigeminal nerve; V2, maxillary branch of trigeminal nerve; V3, mandibular branch of trigeminal nerve; VI, abducens nerve; VII, facial nerve; VIII, vestibulocochlear nerve; IX, glossopharyngeal nerve; X, vagus nerve; hmp, hyomandibular pouch; lep, lens placode; olp, olfactory placode; otp, otic placode; prm, premandibular region; r, rhombomeres; rap, Rathke's pouch.
Each PA receives a specific stream of NCCs, and innervations by the cranial nerves (trigeminal, facial, glossopharyngeal, or vagal) follow this arrangement (Figure 2). Importantly, adjacent streams of the migratory NCCs do not mix (Köntges and Lumsden, 1996). The pharyngeal Hox code reflects this specificity of the migratory NCCs to the PAs: the NCCs (ectomesenchyme) in each of the non-mandibular PAs expresses a specific combination of Hox genes (Figure 2; Hunt et al., 1991a,b; Trainor and Krumlauf, 2001; Takio et al., 2007; Minoux and Rijli, 2010). Within respective PAs, NCCs interact with the ectodermal placodes, endodermal epithelium, and the MMCs to form the entire pharyngeal apparatus (Piotrowski and Nüsslein-Volhard, 2000; Couly et al., 2002; Noden and Trainor, 2005; Noden and Francis-West, 2006; Minoux and Rijli, 2010; Frisdal and Trainor, 2014). The differentiated PA structures in a generalized crown gnathostome include the jaw skeleton and branchial bars, jaw and branchiomeric muscles, trunks of cranial nerves, sensory structures like paratympanic organ, pseudobranch and respiratory gills, afferent and efferent vessels, associated ganglia of the cranial nerves, and others (Frisdal and Trainor, 2014). With the exceptions of the paratympanic organ and pseudobranch, these structures are generally repeated from one PA to another as a plesiomorphic condition (see Goodrich, 1930; Hyman, 1992; Shone et al., 2016 for a review of reduction in the PA-derived structures or the PA themselves in various lineages of vertebrates).
By this stage, two critical properties of the PA development are: (a) the initial spatial confinement (compartmentalization) of the NCCs and MMCs to the PAs and (b) the subsequent serial patterning of differentiating tissues (among all the PAs in crown gnathostomes, and among all the PAs except for the PA I in cyclostomes). The NCCs and MMCs in the PAs are spatially delimited in many ways. They are set apart by the pharyngeal pouches (Crump et al., 2004). The NCCs are attracted, repulsed, or maintained competent by various signaling molecules from epithelial structures (such as BMP4, FGF8, and SHH) including the pouches and placodes (Shigetani et al., 2000; Haworth et al., 2004, 2007; Eberhart et al., 2006). The NCCs regulate the MMC differentiation within the same PA into musculature and other connective tissues (Rinon et al., 2007; Grenier et al., 2009; Heude et al., 2010). As a result, it is possible to identify original PA identities of pharyngeal structures even well after tissue differentiation.
Serial Assimilation of the Mandibular Arch
On the basis of the development and anatomy, the vertebrate pharyngeal structures have long been interpreted in a serial pattern between the PAs (Rathke, 1827; Gegenbaur, 1859; Goodrich, 1930; Sewertzoff, 1931; De Beer, 1937). In particular, the jaw has generated more than a century of investigation into its evolutionary origin because its morphology and function clearly depart from those of other PA-derivatives that ancestrally supported gills.
A prevailing idea has been that the jaw evolved as a specialization within the mandibular arch (PA I). Previous jaw-origin hypotheses explicitly or implicitly assume such differential specializations. Some postulate the jaw as a modified branchial bar (Gegenbaur, 1859; Sewertzoff, 1911, 1913; Goodrich, 1930; De Beer, 1937; Mallatt, 1996, 2008). Some premise on correlated shift of character in all of the PAs (Kimmel et al., 2001; Cohn, 2002; Cerny et al., 2004b) and others focus on phenotypic changes required for specialization of the PA I derivatives into a jaw (Forey, 1995; Janvier, 1996; Cerny et al., 2010; Medeiros and Crump, 2012). One hypothesis—the Heterotopy Hypothesis—is free from this assumption (Kuratani et al., 2001, 2013; Shigetani et al., 2002; Kuratani, 2012), but this is discussed in the context of test of the most recently proposed hypothesis. Despite the fact that none of them provides a comprehensive account of all character transitions from the jawless to the jawed vertebrates, it is difficult to reconcile any pair of these hypotheses with each other under the assumption that the vertebrate pharyngeal apparatus has always been serially patterned. Not only is the assumption non-parsimonious on a phylogenetic tree (i.e., taking more character changes than minimally required), it appears to burden each hypothesis with character changes that are either implausible or clearly decoupled from the origin of the jaw (reviewed by Miyashita, 2015). Whether they are based on anatomical or gene expression patterns, the previous hypotheses capture conditions necessary for a jaw to develop in a crown gnathostome, but the proposed phenotypic changes alone are clearly not sufficient for a jaw to evolve.
This incompatibility questions that the patterning of PA I shares its evolutionary history with that of other PAs prior to the origin of the jaw. The question is two-fold. Were the NCCs and MMCs spatially confined (compartmentalized) in all of the PAs in the last common ancestor of all living vertebrates? Were the PA derivatives (differentiation of the NCCs and MMCs) serially patterned across the pharynx in that ancestor? Miyashita (2015) explored these questions through a review of gene expression patterns, functional analyses, fate-mapping experiments, and adult and embryonic morphology in living jawless and jawed vertebrates (cyclostomes and crown gnathostomes) and through a review of adult morphology in extinct jawless vertebrates (stem gnathostomes; Figure 1A for phylogenetic scheme). In brief, the NCCs and MMCs were likely not spatially confined or delimited in PA I before the jaw evolved. Neither were the PA I derivatives patterned in series with the derivatives of the other PAs in that part of the phylogenetic tree. However, both spatial confinement and serial patterning appear to govern the non-mandibular PAs across all known vertebrates. On the basis of cyclostome development (Figures 3, 4) and morphology of stem gnathostomes, only at the origin of the jaw did the PA I likely acquire both spatial confinement of the NCCs and MMCs and serial patterning of the derivatives. The Mandibular Confinement Hypothesis was generated to explain these predicted character transitions. It proposes: (a) the NCCs and MMCs of the PA I are delineated along interfaces with those of the premandibular, hyoid/hyomandibular (pharyngeal pouch I), and hypobranchial domains in jawed vertebrates; and (b) confinement along these interfaces were acquired in steps as the jaw evolved (Figure 4; Miyashita, 2015).
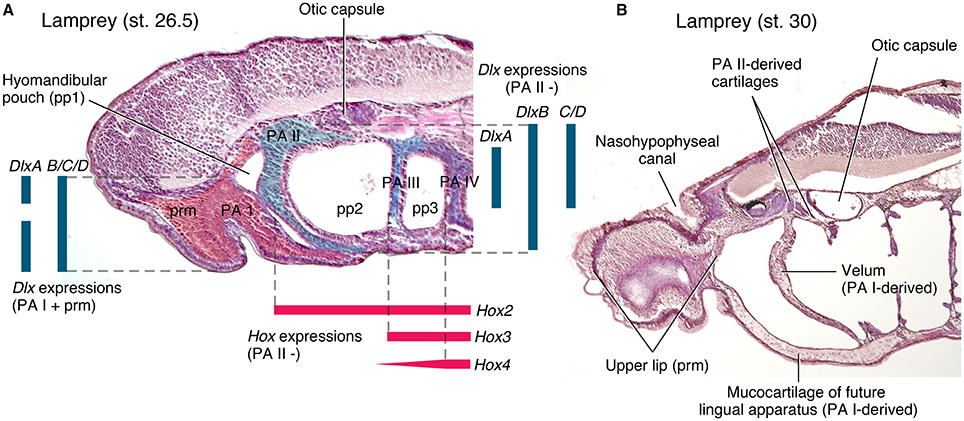
Figure 3. Cyclostome head development in comparison to that of crown gnathostomes (Figure 2). (A) a parasagittal section of Petromyzon marinus at Tahara's stage 26.5, stained with hematoxylin and eosin. The trigeminal NCCs (ectomesenchyme) and mandibular mesoderm is extending into the premandibular region and into the mid-pharyngeal floor, and the hyomandibular pouch is closing to give rise to a velum. These distributions prefigure differentiated structures in B. Ectomesenchyme is shaded in color within each PAs. Color codes: red, PA I and premandibular domain; greenish blue, PA II; light cyan, PA III; purple, PA IV. Color bars represent gene expression domains. Dlx expression patterns are based on Cerny et al. (2010), and Hox on Medeiros and Crump (2012) and Takio et al. (2007). (B) A parasagittal section (slightly oblique toward midline anteriorly) of P. marinus at Tahara's stage 30, stained with hematoxylin and eosin. The structures that correspond in position to the PA I derivatives of crown gnathostomes extend into the premandibular region (upper lip), into the pharynx by contacting the PA II-derived skeleton (velum), and into the mid-pharyngeal floor (lingual apparatus).
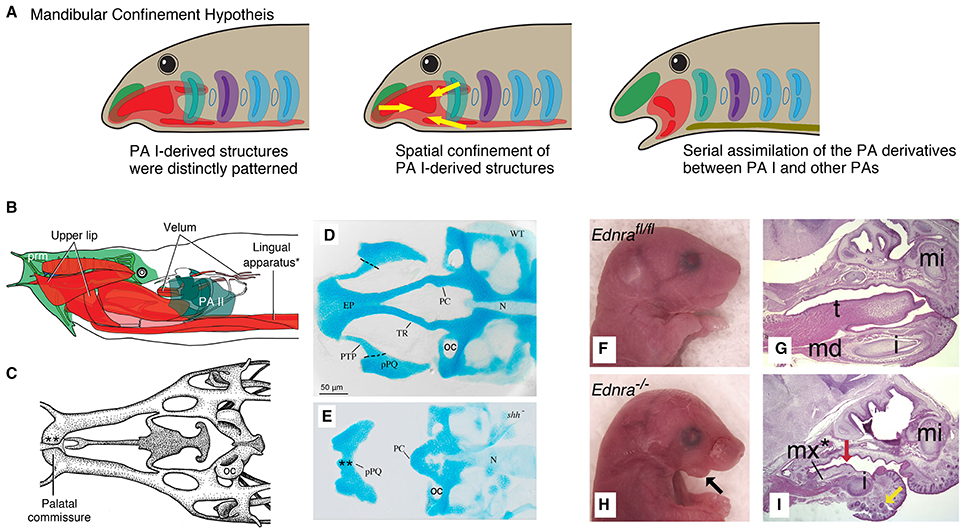
Figure 4. Mandibular Confinement Hypothesis predicts spatial confinement of the PA I-derived structures prior to the origin of the jaw and subsequent serial assimilation to give rise to a jaw. (A) Simple schematics for an evolutionary scenario predicted by the Mandibular Confinement Hypothesis. (B) The head anatomy of hagfish (Eptatretus stoutii) in left lateral view, where the PA I-derived structures are shaded in red and pink (modified from Miyashita, 2015). Color codes follow those of Figures 2, 3. The lingual apparatus (*) is compared with the EdnRA knockout phenotype in H, I. (C) Part of the chondrocranium of E. stoutii in dorsal view (modified from Miyashita, 2012), showing the palatal commissure (**). This midline fusion of the bilateral cartilages that arise from the trigeminal NCCs is compared with the shh mutant phenotype in E. (D) A chondrocranium of wildtype zebrafish in dorsal view (from Eberhart et al., 2006). (E) A chondrocranium of shh− mutant zebrafish in dorsal view. In the absence of SHH signaling from Rathke's pouch (adenohypophyseal placode), the premandibular skeletal elements (e.g., trabecula cranii and ethmoidal plate) do not develop properly, and the PA I-derived cartilages (palatoquadrate) fuse at the midline in a phenotype reminiscent of the cyclostome chondrocranium (palatal commissure, C). This shh− phenotype exemplarily shows that the signaling from the adenohypophyseal placode maintains the boundary—and satisfies differential requirements for skeletogenic differentiation—between the premandibular (pre- and post-optic) and mandibular subpopulations of the trigeminal NCCs in crown gnathostomes. (F) Ednrafl∕fl mouse embryo (E18.5) in right lateral view, showing normal conditions with respect to H (from Ruest and Clouthier, 2009). (G) A sagittal section of Ednrafl∕fl mouse (E18.5) stained with hematoxylin and eosin, showing normal skeletal phenotype with tongue (t) in mid-ventral position (from Ruest and Clouthier, 2009). (H) Ednra−∕− mouse (E18.5) in lateral view, showing defective phenotypes in the mandible. (I) A sagittal section of Ednra−∕− mouse (E18.5) stained with hematoxylin and eosin. The tongue is reduced and the PA I-derived ectopic element forms in the mid-ventral position. This phenotype is reminiscent of the cyclostome condition in which the PA I-derived structures extend into the mid-ventral pharyngeal space (as shown in B). Abbreviations: EP, ethmoidal plate; i, incisor; md, mandible; mi, upper incisor; mx*, ectopic maxilla; N, notochord; OC, otic capsule; PC, polar cartilage; pPQ, palatoquadrate; PTP, pterygoid process; t, tongue; TR, trabecula cranii.
If such scenario is correct, then the jaw evolved through assimilation of an otherwise distinctly patterned region with the rest of the pharyngeal series, and not through specialization of a metameric unit. Many lines of evidence corroborate the scenario of mandibular confinement. This review focuses on those particularly relevant to the developmental aspect of the Mandibular Confinement Hypothesis.
Distinct Features of the Mandibular Arch (PA I)
A striking insight from the Mandibular Confinement Hypothesis is that both the jawless cyclostomes and the jawed gnathostomes—two major lineages of living vertebrates—retain distinct features of PA I patterning from the rest of the PAs, which cannot be easily attributed to secondary modification. Serial patterns between the PA I derivatives and the derivatives of other PAs only occur in jawed gnathostomes, whereas cyclostomes show no such patterns. This contrast is apparent in the morphology of the chondrocranium and pharyngeal muscles (reviewed by Miyashita, 2015; also summarized in Table 1), but is also manifest in embryos.
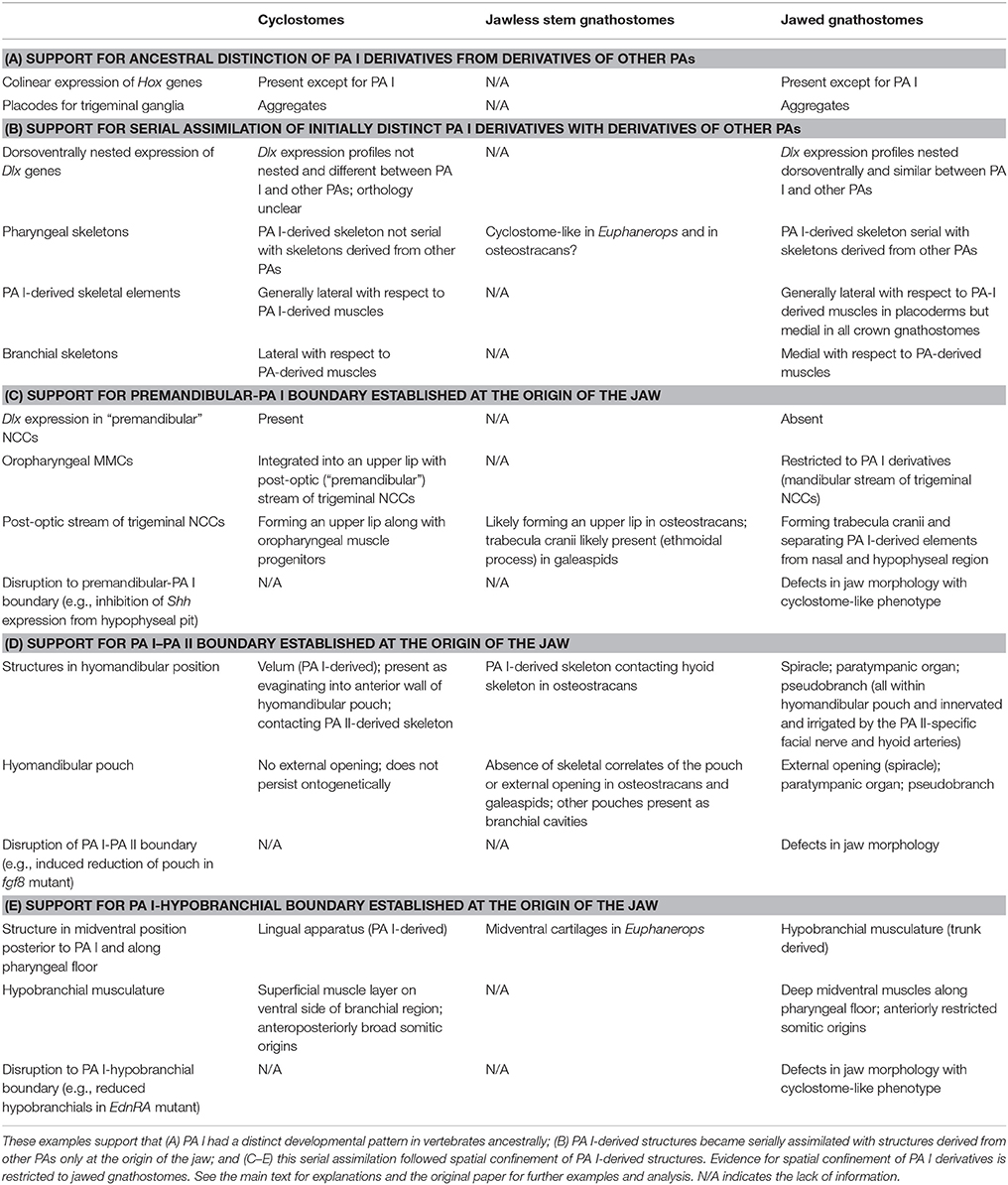
Table 1. Summary of major arguments in the Mandibular Confinement Hypothesis (Miyashita, 2015).
The two conserved gene expression patterns—the pharyngeal Hox and Dlx codes—serve as an illustrating example (Table 1, Figures 2, 3). Both in cyclostomes and gnathostomes, the non-mandibular PAs collinearly express a specific combination of Hox genes, but the PA I stands out in lacking Hox expression in its NCCs (Figure 2; Hunt et al., 1991a,b; Takio et al., 2007). Within crown gnathostomes, the absence of Hox expression in the PA I ectomesenchyme is required for proper patterning of a jaw (Pasqualetti et al., 2000; Couly et al., 2002; Creuzet et al., 2002; Kitazawa et al., 2015). In reverse, Hoxa-deficient gnathostome embryos develop ectopic jaw-like cartilages in posterior PAs (Rijli et al., 1993; Baltzinger et al., 2005; Minoux et al., 2009). These results suggest that expression of the Hox code likely governs serial pattern of the branchial skeleton (PA II and more posterior arches). Because the PA I-derived skeleton requires the absence of the Hox expression, some other mechanism should explain the serial pattern observed between the jaw and the branchial skeleton in gnathostomes.
Consistent with this, the pharyngeal Dlx code—dorsoventrally nested expressions of the Dlx family genes—spans across the PAs in crown gnathostomes. In that clade, the NCCs of all of the PAs (including the PA I) express Dlx1 and Dlx2 at all dorsoventral levels, Dlx5 and Dlx6 from intermediate to ventral levels, and Dlx3 and Dlx4 at ventral levels (Figure 2; Depew et al., 2002, 2005; Medeiros and Crump, 2012; Gillis et al., 2013). Slight differences emerge in the ventral-most Hand2 expression zone both among taxa and between the PA I and other PAs. In zebrafish, dlx1 and dlx2 are expressed in this zone in all of the PAs, but dlx5 expression is also detected for the PA I (Akimenko et al., 1994; Ellies et al., 1997; Kimmel et al., 2003; Talbot et al., 2010). In mice, no Dlx expression is apparent in the equivalent ventral-most zone in all of the PAs (Depew et al., 2002; Ozeki et al., 2004). Despite these minor differences, the Dlx code is an evolutionarily conserved serial patterning mechanism in the pharynx among crown gnathostomes (Compagnucci et al., 2013; Gillis et al., 2013). The pharyngeal Dlx pathway has upstream regulatory components such as the endothelin signaling (upregurating Dlx) and Hand2 (downregulating Dlx) that are expressed ventrally (reviewed by Clouthier and Schilling, 2004; Medeiros and Crump, 2012).
In cyclostomes, however, the Dlx expressions in the NCCs are neither exactly serial across nor restricted to the PAs. Notably, the expression patterns differ between the PA I and others. In the sea lamprey Petromyzon marinus, DlxA has dorsal and ventral expression domains in the PA I (without expression in the intermediate region), whereas its expression in other PAs is restricted to the ventral domain (Figure 3A; Cerny et al., 2010). DlxC and DlxD are expressed throughout in the PA I, but its expression domain in other PAs does not extend to the ventral region (Cerny et al., 2010). Although, initially reported as uniform patterns (Myojin et al., 2001; Shigetani et al., 2002), the Japanese lamprey Lethenteron camtschaticum appears to have differential expressions of Dlx cognates as well. Assuming that the published figures (Kuraku et al., 2010) show representative phenotypes (and assessing them with respect to the description from Cerny et al., 2010), LjDlxA is expressed throughout in the PA I and in the intermediate domain in other PAs; LjDlxB has a weak dorsal expression domain in the PA I but has clear dorsal expression in other PAs; LjDlxC and LjDlxD are expressed throughout in the PA I but clear expression does not extend to dorsal domain in other PAs; LjDlxE has strong expression domain from dorsal to ventral regions of the PA I, but is not expressed so strongly ventrally in other PAs; LjDlxF lacks its dorsal expression domain in the PA I (Kuraku et al., 2010). Whether the Dlx expression patterns in L. camtschaticum are truly nested or uniform, however, interspecific variations in Dlx expression patterns exist among cyclostomes. The analysis of the patterns is further complicated by unclear orthology of Dlx cognates among cyclostomes and with respect to crown gnathostome Dlx family (Kuraku et al., 2010; Fujimoto et al., 2013; Takechi et al., 2013). In addition to this variation, the cyclostome Dlx cognates are expressed in the NCCs of the premandibular region outside the PAs in both hagfish and lampreys (Myojin et al., 2001; Shigetani et al., 2002; Kuraku et al., 2010; Fujimoto et al., 2013). This premandibular expression does not exist in crown gnathostomes. Therefore, Dlx cognates in cyclostomes have dorsoventrally patterned expressions like crown gnathostomes (Cerny et al., 2010; Medeiros and Crump, 2012), but their expression patterns differ (a) from crown gnathostomes, (b) between the PA I and the rest of the pharyngeal series, and (c) from species to species.
These observations are consistent with the scenario of assimilation predicted by the Mandibular Confinement Hypothesis (Miyashita, 2015). That is, the distinct evolutionary history of the PA I is consistent with the exclusion of the PA I from the Hox code in both jawless cyclostomes and jawed gnathostomes. Similarly, the Dlx expressions and the musculoskeletal morphology ancestrally differed between the PA I and the rest of the pharyngeal series, as seen in cyclostomes. Only at the origin of the jaw, the PA I derivatives (jaw) assimilated the pattern of the branchial skeleton from the rest of the pharyngeal series. This event can be interpreted as assimilation of the Dlx expression patterns between the PA I and other PAs—in line with the pan-pharyngeal expression of the Dlx code across crown gnathostomes (reviewed by Miyashita, 2015). This scenario of assimilation better explains the serial pattern of the jaw, hyoid, and branchial skeletons in crown gnathostomes than the scenario of differential specialization from the elusive archetypical ancestor with identical skeletons in all the PAs.
Interface with the Premandibular Region
Accepting that elements of the PA I did not ancestrally share serial patterning program that operated in other PAs, what distinct features did the ancestral PA I exhibit? The living cyclostomes provide insights into this question. In cyclostomes, the NCCs and MMCs of the PA I are not spatially confined as in those of other PAs in the same animals or those of the PA I in crown gnathostomes. The boundaries observable in crown gnathostomes between the NCC and MMC populations of the PA I is shifted in position or has different attributes in cyclostomes (Miyashita, 2015). These features are linked to the adult morphology.
The anterior interface for the PA I in crown gnathostomes sits between the premandibular region and the PA I (Table 1, Figures 2, 5). There is no pharyngeal pouch that separates these two domains as between PAs. Instead, the skeleton around the hypophyseal pit mark this boundary (Kuratani et al., 2004, 2013). Earlier during development, the trigeminal NCCs migrate in three distinct subpopulations: pre-optic, post-optic, and mandibular (Kuratani, 2012). The pre-optic and post-optic NCCs fill in the premandibular region and proliferate around the tripartite olfactory and adenohypophyseal placodes to form the trabecula cranii, interorbital septum, and nasal capsule, whereas the mandibular NCCs migrate to the PA I and interact with the mandibular MMCs to form a jaw apparatus (Kuratani et al., 2001; Eberhart et al., 2006; Szabo-Rogers et al., 2009; Wada et al., 2011). In some crown gnathostomes such as axolotls, the subpopulations initially occupy slightly different positions, but the fates are still distinct from one another (Cerny et al., 2004a). The trigeminal NCCs of the mandibular subpopulation are Dlx-positive, whereas those of the pre-optic and post-optic are Dlx-negative (Depew et al., 2002; Shigetani et al., 2002). So in crown gnathostomes, the premandibular-PA I boundary can be recognized by Dlx expression, and the trigeminal NCCs of the mandibular subpopulation give rise to the skeletal derivatives of the PA I.
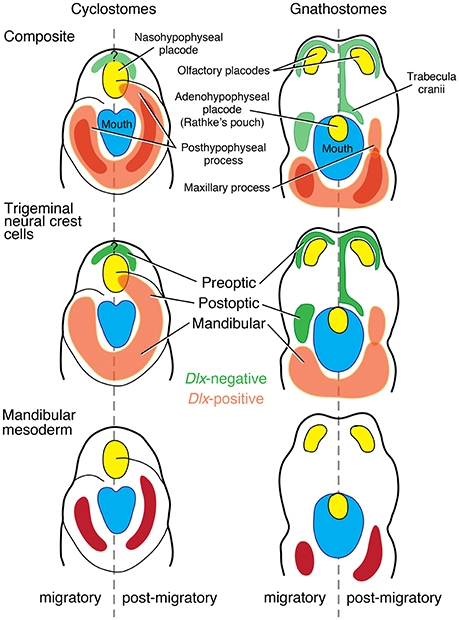
Figure 5. Reinterpretation of the Heterotopy Hypothesis (Kuratani, 2012; Kuratani et al., 2001, 2013; Shigetani et al., 2002) suggests that the premandibular-PA I boundary is not clearly defined in cyclostomes as in crown gnathostomes (modified from Kuratani, 2012; color schemes simplified from review by Miyashita, 2015). In crown gnathostomes, the mandibular trigenminal NCCs can be clearly distinguished from the premandibular (pre- and post-optic) subpopulations of the trigeminal NCCs by the presence of Dlx expressions. This distinction is not clear in cyclostomes, where Dlx expressions broadly mark all the trigeminal ectomesenchyme. In crown gnathostomes, the mandibular ectomesenchyme secondarily extends anteriorly to form a maxillary process. However, the trigeminal ectomesenchyme forms a posthypophyseal process with contribution from the mandibular mesoderm in this position in cyclostomes. Therefore, the structures that broadly correspond to the PA I derivatives of crown gnathostomes have primary anterior extension into the premandibular region in cyclostomes, and the premandibular-PA I distinction is not exactly clear in that latter lineage.
In cyclostomes, however, the premandibular-PA I boundary cannot be clearly delineated (Table 1, Figures 3, 5). Not only is it challenging to delineate three separate subpopulations from each other in histological observations, it is difficult to map identities and fates of the trigeminal NCCs with precision. This is (a) because all three subpopulations of the trigeminal NCCs express Dlx cognates in both lampreys and hagfish (Myojin et al., 2001; Neidert et al., 2001; Cerny et al., 2010; Kuraku et al., 2010) and (b) because the trigeminal NCCs of the post-optic position migrate to what corresponds to the “premandibular” region in crown gnathostomes, and integrate with the MMCs to form a muscular upper lip (Horigome et al., 1999; Kuratani et al., 1999, 2004; McCauley and Bronner-Fraser, 2003; Kuratani, 2012; Oisi et al., 2013b). Medial to this region, the cyclostome counterpart to the olfactory and adenohypophyseal placodes of crown gnathostomes is a single nasohypophyseal placode (Janvier, 1996; Oisi et al., 2013b). Therefore, the skeletogenic proliferation of the pre-optic and post-optic trigeminal NCCs does not occur between the tripartite placodes as in crown gnathostomes, but only surrounds the single placode in cyclostomes (Figure 5).
These features blur a premandibular-PA I boundary in cyclostomes. What would otherwise characterize a PA I in a crown gnathostome—the Dlx-positive NCCs and the MMCs—have different distributions in the cyclostome head. In crown gnathostomes, the PA I derivatives secondarily extend anteriorly later in development, but at the stage of the NCC migration, positions of the NCCs are delimited from each other and tightly linked to distinct skeletal fates (Cerny et al., 2004a; Wada et al., 2011; Kuratani et al., 2013). The maintenance of this boundary is critical in crown gnathostomes. When manipulation disrupts molecular signals expressed in structures that sit at the boundary and thereby delimit the PA I elements in these taxa (e.g., hypophyseal pit), the resulting phenotypes often include jaw defects and even cyclostome-like distribution of the differentiating PA I structures. The shh− and noc− mutant zebrafish show severe reduction of chondrogenic proliferation of the pre-optic and post-optic NCCs (Neuhauss et al., 1996; Eberhart et al., 2006). They also exhibit conditions that parallel cyclostome pattern: fusion of left and right palatoquadrate anterior to the notochord (shh−) and lower labial cartilage upturned to overlap the premandibular region from lateral side (noc−) (Figure 4E; reviewed by Miyashita, 2015).
Interface with the PA II/hyomandibular Pouch
The PA I is delimited posteriorly from the PA II by the hyomandibular pouch (hmp in Figure 2). The pouch sits between the PAs I and II and below the geniculate ganglion of the facial nerve (a branchiomeric nerve for the PA II). The pouch epithelium plays a critical role in patterning the Hox-negative trigeminal NCCs of the PA I (Couly et al., 2002) and often persists into adulthood in crown gnathostomes as the spiracle or paratympanic cavity. A variety of sensory structures develop in the hyomandibular pouch of crown gnathostomes, including the pseudobranch (folded epithelial structure with sensory/thermoregulatory functions) and the spiracular organ (paratympanic organ) (O'Neill et al., 2012). These structures are associated with the PA II. They are innervated by the facial nerve and/or irrigated by the hyoidean arteries (reviewed by Miyashita, 2015).
Cyclostome development shows no evidence for these hyomandibular structures (Figure 3). The hyomandibular pouch never opens externally as in crown gnathostomes. Instead, the trigeminal NCCs and mandibular MMCs of the PA I occupy this position to form an outpocket into the pharynx, which later becomes a ventilation structure called velum (reviewed by Oisi et al., 2013b; Miyashita, 2015). Although, differentiated from elements of the PA I, and although innervated by the trigeminal nerve, the velar skeleton abuts against the skeleton of the PA II in both hagfish and lampreys (Figure 3B; Janvier, 1996).
As such, the hyomandibular pouch persists as a barrier for the NCCs and MMCs of the PA I only among vertebrates with jaws, probably as a functional requirement for the PA II structures that the pouch hosts. Despite considerable spatial overlap between the PA I- and PA II-derived muscles later in ontogeny, the anlagen are spatially confined in crown gnathostomes. The NCCs and MMCs of the PA I do not observe such clear boundary in cyclostomes. To illustrate the importance of the spatial confinement at the hyomandibular pouch in crown gnathostomes, induced reduction of the pouch (e.g., fgf8− and fras1− mutant zebrafish) is linked to severe defective phenotypes in the jaw skeleton (Figure 6B; Crump et al., 2004; Talbot et al., 2012). Conversely, pax1−∕− medaka fish retain the hyomandibular pouch but fail to form segmented pouches for the rest of the pharynx (Okada et al., 2016). Although these pax1 mutants develop jaws without pronounced phenotypic effect, the branchial skeleton is reduced to a severe defective phenotype, and the hyoid elements become fused to each other (Okada et al., 2016).
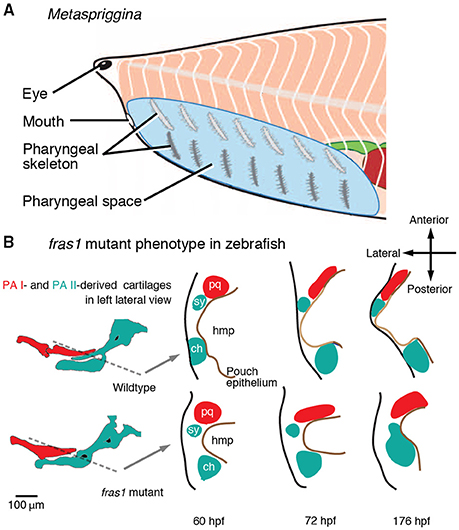
Figure 6. Test of predictions arising from the Mandibular Confinement Hypothesis. (A) Metaspriggina walcotti has dorsoventrally bipartite pharyngeal skeleton (modified from Conway Morris and Caron, 2014). The Mandibular Confinement Hypothesis can be rejected if compelling evidence shows: (A) the most anterior pair is derived of the PA I; and (B) such polyisomeric state is shared across stem gnathostomes up to the origin of the jaw. (B) fras1 mutant zebrafish indicate the role of the pharyngeal epithelium (and signaling from it) as a boundary between distinct skeletogenic NCC condensations (modified from Talbot et al., 2012). The hyomandibular pouch (hmp) does not expand laterally in fras1 mutants. The otherwise separate symplectic cartilage (sy) fuses with the ceratohyal (ch), although the PA I-derived palatoquadrate (pq) is still spatially set apart from the PA II-derived cartilages by the reduced pouch, but the PA I cartilages show defects.
Interface with the Hypobranchial Musculature
In crown gnathostomes, the NCCs and MMCs of the PA I are delimited along a mid-ventral boundary with the hypobranchial musculature, which functions as jaw depressors and/or a tongue (Figures 2, 4G; Sambasivan et al., 2011). The hypobranchial muscle precursors originate in the trunk (they are not part of the head branchiomeric musculature), migrate along a circumpharyngeal path, and extend anteriorly beneath the pharyngeal floor (Lours-Calet et al., 2014). The differentiated muscles become a deep mid-ventral muscle mainly connecting the pectoral girdle and the jaw. Several phenotypes with hypobranchial defects demonstrate confinement of the NCCs and MMCs of the PA I by the hypobranchial muscle precursors. These phenotypes can be induced in knockout/knockdown of the ventrally expressed elements of the endothelin and Dlx pathways (e.g., EdnRA). As the hypobranchial muscle precursors are reduced, the PA I-derived ectopic tissues extend mid-ventrally and develop in the space that would otherwise be occupied by the hypobranchial structures (Figures 4H,I; Abe et al., 2007; Ruest and Clouthier, 2009; Heude et al., 2010; Barron et al., 2011). The hypobranchial defects are linked with jaw defects in edn1 and myoD phenotypes of zebrafish (Miller et al., 2000; Hinits et al., 2011).
On the other hand, cyclostomes appear to have no such boundary between the PA I and the hypobranchial musculature (Figures 3, 4B). In both hagfish and lampreys, the NCCs and MMCs of the PA I extend posteriorly and mid-ventrally to the pharyngeal floor to form a lingual apparatus (Holmgren, 1946; Johnels, 1948; Oisi et al., 2013a,b). Contribution to the lingual apparatus from other PAs in cyclostomes would reject the prediction that the PA I-derived structure in midventral pharyngeal position only became spatially confined at the origin of the jaw. However, no such evidence exists. This cyclostome condition is strikingly similar to the EdnRA mutant phenotype of mice in which the ectopic PA I structures occupy the mid-ventral position (Figures 4H,I; reviewed by Miyashita, 2015). Furthermore, the cyclostome muscles that correspond to the crown gnathostome hypobranchial musculature form the most superficial muscle layer in the ventral region of the pharynx, instead of becoming a deep mid-ventral muscle (Kusakabe and Kuratani, 2005, 2007). These muscles draw their precursors broadly from somites, not just restricted to occipital somites as in crown gnathostomes, and continue to extend anteriorly into the head well after the NCCs occupied the PAs (Kusakabe et al., 2011; Oisi et al., 2015). Therefore, the PA I-hypobranchial interface as observed in crown gnathostomes does not exist in cyclostomes.
Evaluation of New (Counter-)Evidence for Mandibular Confinement
Coupled with fossil and anatomical evidence (reviewed by Miyashita, 2015), the establishment of these interfaces of the PA I appears to have coincided with the origin of the jaw, because only jawed vertebrates exhibit full attributes of spatially confined NCCs and MMCs of the PA I (Figure 1A). The spatial confinement would have allowed the NCCs and MMCs of the PA I—which would otherwise have differentiated into peripheral structures as seen in cyclostomes—to acquire new developmental fates (Figure 4A). A likely scenario is that they co-opted patterning programs that operate in more posterior PAs, such as the pharyngeal Dlx code. Then serial similarities among the PA-derived structures—absent in cyclostomes but present in crown gnathostomes (e.g., jaws and branchial bars)—should reflect incorporation of the PA I into the serial patterning of the PA derivatives at the origin of the jaw. A full scope of this Mandibular Confinement Hypothesis and an evaluation of evidence and counterevidence are provided in the original paper (Miyashita, 2015). The present review looks to new evidence and future directions that were not considered in length in Miyashita (2015).
Spatial confinement is a difficult concept to test, as it ultimately requires precise fate mapping and specific functional analysis. Crucially, the spatial confinement proposed at the three interfaces concerns the undifferentiated NCCs and MMCs of the PA I (Miyashita, 2015). It does not necessarily mean that differentiated structures continue to occupy the same positions as in the embryonic PA I, or that the area occupied by the NCCs and MMCs of the PA I quantitatively decreased in proportion to schematic diagrams. Instead, the confinement implies that the PA I interfaces clearly observed in crown gnathostomes likely had different attributes in jawless ancestors. The NCCs and MMCs “confined” to the PA I as in other PAs—and the resulting serially similar patterns across the pharynx—are a feature specific to vertebrates with jaws. Fate mapping experiments in zebrafish, chick, and Xenopus (and other amphibian models) corroborate delimitation of the NCCs and MMCs of the PA I from three proposed interfaces (trabecula cranii, hyomandibular pouch, and hypobranchial muscles; e.g., Couly et al., 1993; Richman and Lee, 2003; Eberhart et al., 2006; Benouaiche et al., 2008; Gross and Hanken, 2008; Szabo-Rogers et al., 2009; Wada et al., 2011; Talbot et al., 2012; Lours-Calet et al., 2014). Such high-resolution fate mapping remains as a challenge for cyclostomes.
In zebrafish, for example, the premigratory trigeminal NCCs that would contribute to the trabecula cranii and other cartilages of the premandibular region originate more anteriorly than those that would contribute to the jaw cartilages of the PA I (Eberhart et al., 2006). Spatial segregation of the skeletogenic fates among the trigeminal NCCs is maintained through migration, and the “premandibular” trigeminal NCCs require the hedgehog signaling from the hypophyseal pit to chondrify (Eberhart et al., 2006). These observations document the predicted delimitation of the PA I-derived mesenchymal tissues from the premandibular region in crown gnathostomes, even though the elements that occupy this region in cyclostomes are derived from the position of the PA I (reviewed by Miyashita, 2015). Interestingly, the hedgehog-impaired shh− mutant zebrafish have median fusion of the PA I-derived cartilages (Eberhart et al., 2006)—a condition reminiscent of cyclostomes (Figure 4E).
Metaspriggina and Tullimonstrum
Serial similarities between the PA derivatives are a relatively tractable concept to test because morphological similarities between the PA derivatives likely reflect serial patterning earlier in development. Here, fossil evidence is relevant. A synthesis of fossil inferences suggests that acquisition of morphological similarities between the PA I-derived and other PA-derived structures coincided with the origin of the jaw (Miyashita, 2015). Metaspriggina—a putative stem vertebrate from the Cambrian Period—displays a mix of features that both contradict and support this scenario of serial assimilation. Metaspriggina has seven bilateral pairs of dorsoventrally bipartite bars in the pharynx, and the most anterior pair may represent the PA I derivatives in superficial resemblance to the traditionally postulated jawless ancestor with a pan-pharyngeal serial pattern (Figure 6A; Sewertzoff, 1931; Jarvik, 1980; Mallatt, 1996; Conway Morris and Caron, 2014). Metaspriggina could reject the Mandibular Confinement Hypothesis if: (a) compelling morphological evidence indicates that the most anterior set of the bipartite pharyngeal bars was derived from the PA I; and (b) the pan-pharyngeal serial pattern should be a shared primitive trait along the stem of gnathostomes.
As for the PA I identity, Metaspriggina has no indication of the trigeminal innervation in the region of the most anterior pharyngeal bars, and the ganglia do not appear to be preserved (Conway Morris and Caron, 2014). Where alternative interpretations (e.g., cephalochordate-like pattern) cannot be ruled out, neither is it certain that each set of the pharyngeal skeletal bars in Metaspriggina were derived from homologs of the vertebrate PAs. The most anterior pharyngeal bars lack branchial filaments and are thicker than the other pairs (Conway Morris and Caron, 2014). On one hand, the morphology does not fit to the traditional hypothetical ancestor with gills in every PA. On the other, the bipartite organization implies serial patterning that operated in all sets of the pharyngeal bars. Additional materials are essential for further comparison, as the specific morphology of the most anterior pair is only preserved in a single specimen (ROM 62933). As for the symplesiomorphic state, the current phylogeny suggests that Metaspriggina is nested outside of crown vertebrates (cyclostomes + gnathostomes) (Figure 1A; Conway Morris and Caron, 2014). Between Metaspriggina and jawed vertebrates lie cyclostomes and several lineages of jawless stem gnathostomes, many of which support that the distinctly patterned PA I derivatives represent a shared primitive trait with respect to the origin of the jaw (Miyashita, 2015). Regardless of whether Metaspriggina has an ancestral state or a uniquely specialized condition, evidence is lacking in lineages closer to crown gnathostomes that the serially patterned PA I derivatives was a shared primitive trait leading to the origin of the jaw.
Recently, Tullimonstrum—an enigmatic fossil bilaterian from the Late Carboniferous Mazon Creek biota—has been reconstructed as a vertebrate or even as a stem lamprey (Clements et al., 2016; McCoy et al., 2016). Tullimonstrum has a prominent proboscis with a hinged feeding apparatus at the anterior end. The support for cyclostome affinity of Tullimonstrum (McCoy et al., 2016) includes the presence of skeletal elements compared to the tectal cartilages and lingual apparatus in lampreys. If these identifications are correct, Tullimonstrum departs from all other vertebrates in the uniquely broad distribution and unusual morphology of the PA I derivatives (e.g., the proposed lingual apparatus). One possible interpretation is that these exceptional features reflect the lack of serial patterning of the PA I derivatives in early branches of vertebrates. However, Tullimonstrum as a stem lamprey (McCoy et al., 2016) informs little about conditions along the gnathostome stem because its unusual morphology would represent a derived state. The alternative phylogenetic positions as a stem vertebrate, stem cyclostome, or stem gnathostome (Clements et al., 2016) would question the skeletal traits identified on the basis of a lamprey model. At any rate, it requires further analysis to constrain the anatomy and phylogenetic position of Tullimonstrum. The hinged apparatus at the anterior end of the proboscis have curious superficial resemblance to a jaw, and the branchial openings remain to be observed. The current information available on Tullimonstrum neither supports nor rejects the Mandibular Confinement Hypothesis.
Transition of the Jaw Cartilages
Kimmel et al. (2001) proposed a solution to the conundrum in vertebrate comparative anatomy: the pharyngeal skeleton forms on the lateral side of the PAs in jawless vertebrates and on the medial side in jawed vertebrates. They postulated that the NCCs migrate to different sides of the PAs. A revised version of this hypothesis posits that skeletal induction site in the migrated NCCs—but not the migration of the NCCs—determine the side of the pharyngeal skeleton in each group (Cerny et al., 2004b). A simultaneous transition of all the PA-derived skeletons from the lateral to medial side would be consistent with the serial nature of the PA-derived skeletons at that part of the phylogenetic tree. However, no such evidence has emerged. In the most primitive jawed vertebrates (placoderms), the branchial skeleton consists of relatively small individual skeletal bars that do not constitute an external branchial basket, indicating medial position relative to the branchial cavities (Stensiö, 1963, 1969; Denison, 1978; Forey and Gardiner, 1986). On the other hand, the jaw cartilages still lie on the lateral side of the major adductor in most placoderms, and the Silurian placoderm Entelognathus documents a gradual transition of the jaw cartilages to the medial side of the adductor (Zhu et al., 2013; reviewed by Miyashita, 2015). This observation provides morphological evidence of the independent patterning of the PA I derivatives from the rest of the pharyngeal apparatus in the earliest jawed gnathostomes.
Quality of Fossil Evidence
Related to Metaspriggina, fossil evidence remains incomplete and difficult to interpret. Although, Miyashita (2015) reviewed available inferences in favor of the cyclostome-like patterning among jawless stem gnathostomes such as osteostracans (Figure 1A for phylogenetic placement), these reconstructions are hypotheses on their own. The fossil evidence is consistent with the PA I derivatives positioned in the premandibular, hyomandibular, and hypobranchial spaces in these fossil taxa as in cyclostomes, and an alternative, crown gnathostome-like pattern is incompatible. Beyond that overall pattern, however, the exact morphology of these structures have yet to be resolved. To complicate the matter further, relative positions of sensory capsules (nasohypophyseal, optic, and otic structures) and oropharyngeal structures (mouth, lips, and prebranchial/branchial cavities) vary considerably among jawless stem gnathostomes (Janvier, 1996, 2007). The Mandibular Confinement Hypothesis does not account for extremely anterior position of the eyes among heterostracomorphs, an exceedingly large number (>45) of branchial cavities in galeaspids, or an anterior shift of orobranchial chambers in osteostracans (Figure 1A for phylogenetic position). It remains unclear how these variations relate to morphological changes toward the origin of the jaw.
“Premandibular” Component of the Upper Jaw
In the presentation of the Mandibular Confinement Hypothesis (Miyashita, 2015), the maxillary process referred to the PA-I derived Dlx-positive ectomesenchyme that forms the anlage of the palatoquadrate. The jaw considered in that review consists of the palatoquadrate and the Meckel's cartilage, along with associated dermal elements where applicable. On the other hand, the maxillary prominence in tetrapod craniofacial development refers to the domain occupied by the post-optic stream of trigeminal NCCs in the literature (Richman et al., 1997; Richman and Lee, 2003; Cerny et al., 2004a). The maxillary prominence gives rise to the functional components of the upper jaw, which intramembranously ossify as dermal elements (Lee et al., 2001, 2004). The functional jaw apparatus thus contains the “premandibular” elements, and such condition is already present in placoderms (Zhu et al., 2013). The maxillary prominence-derived components of the upper jaw do not violate spatial confinement of PA I-specific NCCs and MMCs. The “premandibular” upper jaw elements could be easily integrated into the functional module of the palatoquadrate once having the PA I-derived hinged jaw skeleton. “Confinement” does not necessarily mean the premandibular-PA I decoupling at later developmental stages.
Evolutionary Origin of Extraocular Muscles
The Mandibular Confinement Hypothesis only considers the premandibular region in its oropharyngeal domain that borders the mouth and the foregut and was classically viewed as “visceral” or “splanchnic” (Goodrich, 1930; De Beer, 1937; Jarvik, 1980). For this reason, the premandibular domain of the oropharyngeal region was interpreted as devoid of mesoderm, and the prechordal mesoderm that gives rise to the extraocular muscles were excluded from the context of the Hypothesis (Miyashita, 2015). A clonal analysis has recently shown that the PA I muscles and the extraocular muscles share a common progenitor lineage in mice (Lescroart et al., 2010). It remains to be tested whether this condition in mice represents a broadly conserved pattern among vertebrates as suggested by classical observations (Edgeworth, 1935). If so, the PA I mesoderm would have been already specialized differently from the mesoderm of other PAs well before the origin of the jaw.
Difference from the Heterotopy Hypothesis
The Mandibular Confinement Hypothesis was designed to expand on the scope of the Heterotopy Hypothesis. The latter hypothesis postulates that a tripartite split of the nasohypophyseal placodes into the paired olfactory placodes and orally opened adenohypophyseal placode resulted in diplorhiny (paired nostrils) and heterotopic expression of Dlx genes into the PA I (Dlx-negative post-optic stream of trigeminal NCCs; Figure 5; Kuratani et al., 2001, 2013; Shigetani et al., 2002, 2005; Kuratani, 2012). The Heterotopy Hypothesis associates an important phenotypic difference between cyclostomes and crown gnathostomes with the origin of the jaw, but does not provide any mechanistic scenario to explain how such Dlx heterotopy led to a jaw. As reviewed by Miyashita (2015), the presence of the ethmoidal process in the galeaspid Shuyu has been used as a support for this hypothesis (Gai et al., 2011). However, the jawless stem gnathostome—which does not even represent the most immediate outgroup of jawed vertebrates (Figure 1)—foreshadowing the crown gnathostome condition implies that the jaw did not evolve simultaneously with the acquisition of the heterotopy. Therefore, the Dlx heterotopy is a necessary but not sufficient condition for a jaw to evolve. The Mandibular Confinement Hypothesis interprets the Heterotopy Hypothesis as describing how a diffuse interface between the “premandibular” and PA I derivatives became clearly delineated as in crown gnathostomes, and views it as one of the key steps in the confinement of PA I derivatives before serial assimilation (Figure 4).
Overlap of Structures Derived from Different PAs
In all three interfaces proposed for the PA I, overlap of elements from different domains occurs later in ontogeny. These secondarily extended PA I derivatives in crown gnathostomes include the maxillary process (extending onto the premandibular region) and the intermandibularis (overlapping the hypobranchial muscles; Kuratani, 2004, 2012; Diogo and Abdala, 2010). Nevertheless, the PA identities of the overlapping structures are clearly distinguished on the basis of developmental and morphological correlates such as nerve innervation patterns. In teleosts, an anlage of the symplectic cartilage develops from the dorsal part of the PA II and secondarily extends to the anterior side of the hyomandibular pouch to parallel the upper jaw (palatoquadrate), set apart from the rest of the PA II skeleton by the pouch epithelium (Figure 6B). When the pouch epithelium fails to expand laterally in fras1− mutant zebrafish, the symplectic cartilage fuses to the ceratohyal (a PA II-derived cartilage; Figure 6B; Talbot et al., 2012). This fras1− phenotype indicates: (a) the pouch epithelium is crucial to maintain integrity of the NCC-derived structures it sets apart; and (b) the differentiated chondrocytes maintain the PA identities because the PA I-derived anlage of the palatoquadrate still does not fuse with the PA II-derived anlage of the symplectic cartilage.
Oral Siphon Muscles in Tunicates
Among tunicates—a sister group of vertebrates—ascidians develop an oral siphon following larval settlement and metamorphosis. The muscle precursors for the oral siphon originate in the trunk lateral cells, whereas the muscles of the atrial siphon, heart, and body wall are derived from the trunk ventral cells (Hirano and Nishida, 1997; Tokuoka et al., 2005; Stolfi et al., 2010, 2014). If the oral siphon muscles were treated as a homolog of the PA I-derived muscles of vertebrates (Diogo et al., 2015), it would imply independence of the PA I from the rest of the pharyngeal series in ancestors of vertebrates as predicted by the Mandibular Confinement Hypothesis. However, it is difficult to compare the ascidian oral siphon and the vertebrate PA I except in their superficial topological relationships with the mouth. None of the differentiated larval structures contribute to the oral siphon muscles in adults, and the trunk lateral domain is spatially set apart from the trunk ventral domain (which is often compared to the cardiopharyngeal region of vertebrates; Hirano and Nishida, 1997; Stolfi et al., 2010). Currently, it is no less parsimonious to postulate an independent evolutionary origin of the oral siphon muscles in tunicates (an alternative view in Diogo et al., 2015).
Concluding Remarks: Jaw Origins
Overall, it waits for future tests to determine how robust predictions arising from the Mandibular Confinement Hypothesis are, but its implications are promising. Cumulative evidence suggests ancestral anisomerism in the gnathostome pharynx: the PA I derivatives were patterned differently from those of other PAs (with cyclostomes representing some examples of this), and the serially patterned state of the PA derivatives as observed in crown gnathostomes likely emerged with the origin of the jaw. Otherwise the PA I-specific features in cyclostomes and crown gnathostomes must have evolved independently within the respective lineages, and the cyclostome- or crown gnathostome-like traits identified in stem gnathostomes (such as galeaspids, osteostracans, and placoderms; Figure 1A) must have been misguided. There is no question that cyclostomes and crown gnathostomes have each undergone their own specializations, or that inferences for fossil phenotypes remain challenging. However, alternatives would be best offered through test of predictions arising from the Hypothesis. Currently, it requires far fewer character changes to postulate an anisomeric pharyngeal apparatus in the last common ancestor between cyclostomes and crown gnathostomes than to assume a polyisomeric pharyngeal apparatus.
The Origin of the Paired Appendages: Serial Parallelism and Bottlenecks
The idea that the structures of the forelimb (FL) and hindlimb (HL) are “serial homologues” was first proposed by Vicq-d'Azyr (1774), Oken (1843), and Owen (1849, p. 184). Oken and Owen's ideas were profoundly influenced by Johann Wolfgang Goethe, and so was their notion of polyisomeric archetypes. Interestingly, in their FL and HL comparisons—which were almost exclusively based on skeletal traits—the use of the term “serial homology” referred to what is currently viewed as parallelism (i.e., a subset of homoplasy) and not to true homology. Examples of striking similarity between the FL and HL in Owen (1849) mainly come from tetrapods with highly derived limbs (e.g., bats, horses, and plesiosaurs). Owen used the term parallelism more often than “serial homology” to describe the FL-HL similarity. When he discussed outgroup lineages to tetrapods (e.g., chondrichthyans), he considered that those taxa “confuse” the notion of “archetype” and “serial homology.” Thus, Owen intended homoplasy rather than the concept of serial homology as we understand it today (Diogo et al., 2013).
There is a conceptual difference between developmental biologists and evolutionary biologists over the perception of serial homology, including segmentation. The former tend to focus on the use of similar developmental mechanisms (“developmental serial homology” sensu Wagner, 1994), whereas the latter tend to consider both developmental similarity and evolutionary continuity (“historical serial homology” sensu (Wagner, 1994; Brigandt, 2003). Reno et al. (2013) recently proposed a broadly applicable criterion to identify evolutionary/historical serial homology: “confirmation of its evolutionary continuity throughout a lineage, such that the structure can be shown to have a shared ancestry with segmental duplicates within the same body (paralogues, usually named serial-homologs) or similar structures in other lineages (orthologues, usually named homologs)” (p. 217). By contrast, Goethe's romantic idea of a polyisomeric archetype did not refer to a true ancestral serial pattern, but instead to a theoretical, ideal serial pattern that could be fulfilled through vitalist forces within the body (Richards, 2004). This idea of the vertebrate body composed of segments implies that the pelvic appendage is nothing more than a second pectoral appendage, which was in turn viewed as a derivative of a posterior branchial arch (Gegenbaur, 1859).
Despite the decline of romantic and vitalistic ideas, authors continued to view the structures of the pectoral and pelvic appendages as serial homologs (Diogo et al., 2013). Efforts to work out the origins of the paired appendages have not explicitly questioned the serial homology until recently. One such example is a hypothesis that fins evolved from continuous stripes of competency for appendage formation located ventrally and laterally along the embryonic flank (Shubin et al., 1997; Don et al., 2013). An extension of this hypothesis proposes that the paired appendages evolved with a shift in the zone of competency to the lateral plate mesoderm in conjunction with the establishment of the lateral somitic frontier, thus allowing formation of the limb/fin buds with endoskeletons (Don et al., 2013). The idea that paired appendages are serial homologs assumes that: (a) these appendages were originally similar to each other; and (b) there was a subsequent functional/anatomical divergence between them (Figure 7). In line with these assumptions, Don et al. (2013) proposed: (a) the ancestral Tbx4/5 cluster of vertebrates underwent a duplication event; (b) Tbx4 is related to the HL and Tbx5 with the FL; and (c) pectoral fins evolved first and then were duplicated to form pelvic fins.
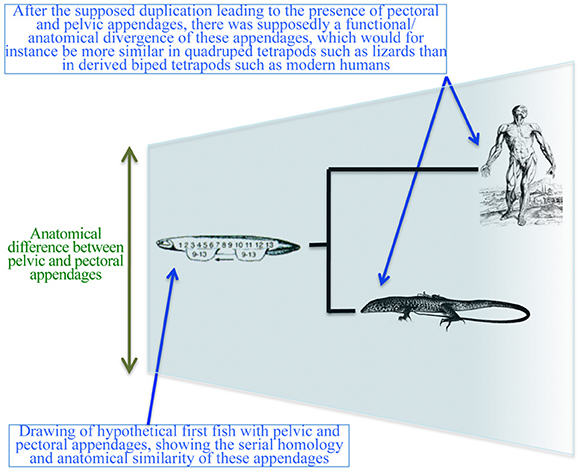
Figure 7. Simplified diagram illustrating the “serial homology followed by functional/anatomical divergence” hypothesis often shown in textbooks and followed in more technical papers, particularly within the fields of developmental biology and evo-devo. Modified from Diogo and Molnar (2014).
This supposed polyisomeric to anisomeric polarity links two distinct hypothetical phenomena: (a) duplication of the Tbx4/5 cluster and subsequent co-option associated with the ontogeny of the different paired appendages; and (b) morphological duplication of the appendages at the levels of individual musculoskeletal elements. As we will argue below, (a) is true but (b) is not. Gene expressions may facilitate an outgrowth that gives rise to different limbs. However, these limbs do not necessarily represent serial homologs. An alternative explanation is that some genes and regulatory cascades/networks have been recruited due to homoplasy between FLs and HLs to coordinate limb development (Willmer, 2003). At least some of these genes/networks have also been recruited to coordinate the development of other appendages that are clearly not serial homologs with pectoral or pelvic appendages (sexual organs and head barbels; Archambeault et al., 2014).
Serial Homology of the Paired Appendages: Predictions
If the paired appendages represent evolutionary (historical) serial homology, four testable predictions must be met:
(1) The pectoral and pelvic appendages resulted from a duplication event.
(2) Serially corresponding structures between the FLs and HLs of tetrapods (e.g., humerus vs. femur or adductor pollicis vs. adductor hallucis) have counterparts in more basal gnathostomes, which can be rooted to the supposed pectoral-pelvic duplication event. For example, Humphry (1872) suggested that sharks have homologs of muscles such as the latissimus dorsi and pectoralis of tetrapods, as well as serial homologs of these muscles in their pelvic appendages.
(3) The pectoral and pelvic appendages are more similar to each other in adults of earlier/more plesiomorphic taxa than in adults of later/more derived taxa (Wilder, 1871).
(4) Symplesiomorphic structures of the pelvic and pectoral appendages are more similar to each other than are new structures acquired during independent evolutionary history of each appendage (Wyman, 1860). Examples of such innovations are the tetrapod zeugopodia (arm/leg) and autopodia (hand/foot). Even if tetrapod digits are considered to be derived from fish distal radials (Johanson et al., 2007), at least some tetrapod wrist/ankle bones are neomorphic structures (Don et al., 2013).
Anatomy and Development of the Paired Appendages
Recent anatomical, developmental, and functional studies raise intriguing questions about the serial homology concept. Among them are marked differences in the development of muscles between the pectoral and pelvic appendage in both tetrapods and non-tetrapod gnathostomes (Cole et al., 2011; Don et al., 2013). These differences are clearly more marked in phylogenetically older structures (e.g., girdles and pectoral-arm and pelvic-thigh muscles attached to them) than in structures that are derived. The hypothetical pectoral-pelvic duplication event in early gnathostomes cannot explain this pattern. In no known extant tetrapod does topological correspondence exist between the muscles attached to the pectoral girdle (i.e., pectoral and arm muscles) and those attached to the pelvis (i.e., gluteal and thigh muscles) in configurations in adults or through ontogeny (Figure 8; Diogo and Tanaka, 2014; Diogo and Ziermann, 2014; Diogo et al., 2014a,b). In the zeugopodial and autopodial muscles, however, there are numerous topological correspondences (e.g., 19 in most salamanders) between FLs and HLs in embryonic development of many tetrapods. Some are lost later in development, whereas others persist in adults (Figure 8; see numbers in Figure 10).
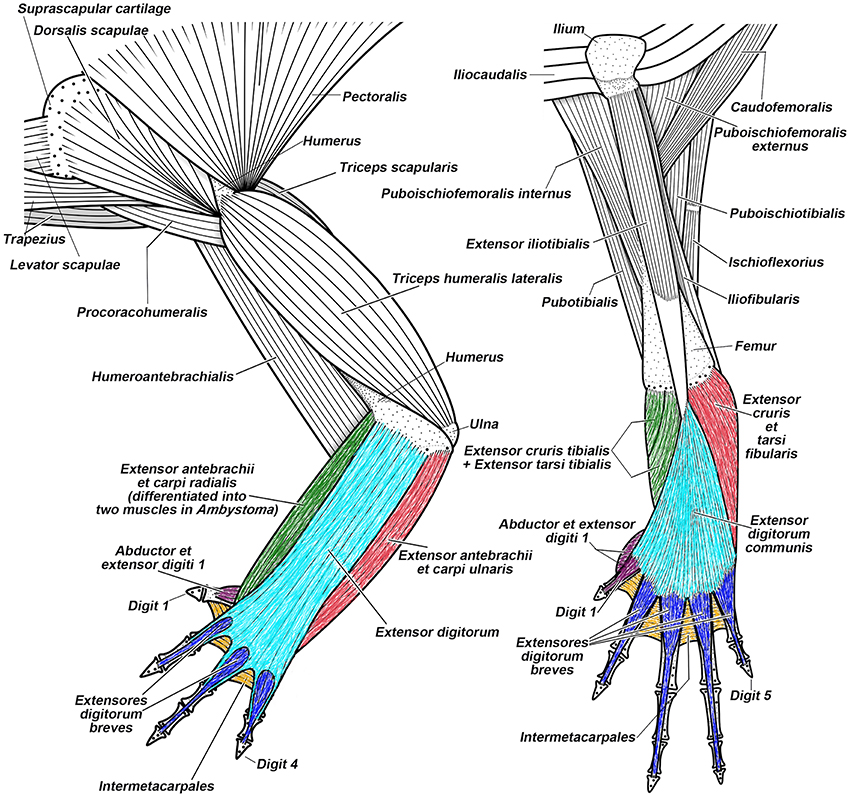
Figure 8. Superficial musculature of the forelimb (on the left) and the hindlimb (on the right) of the salamander Taricha torosa, seen in dorsal view. Striking similarities between forearm-hand muscles and leg-foot muscles (shown by using similar colors), as well as striking differences between the pectoral-arm muscles and the pelvic-thigh muscles, are evident in salamanders (modified from Diogo et al., 2013; N.B.).
The FLs and HLs of humans and other tetrapods reveal further heterogeneity from early development to the adult stage (Bardeen, 1906; Lewis, 1910; Čihák, 1972). There are marked differences between the configuration of the arteries of the human FL and HL in the stylopodial (arm vs. tight) and girdle (pectoral vs. pelvic) regions, not only in adults but also in early development (Figure 9). By contrast, remarkable similarities exist between the hands and feet in humans and some other tetrapods (e.g., salamanders) in the patterns of bones, muscles, nerves, and blood vessels (Diogo et al., 2013). An analysis of development regulation of the pectoral and pelvic appendages in tetrapods and some fish helps explain these morphological patterns (Sears et al., 2015). Sears et al. provided the first detailed review of the developmental programs and gene networks of the pectoral and pelvic girdles and of the HL and FL of tetrapods. This analysis revealed that embryological origins are more similar between the zeugo- and autopodia of the FLs and HLs than between the pectoral and pelvic girdles. Known genes and regulatory gene interactions differ greatly between the pectoral and pelvic girdles, with few genes regulating patterning of both girdles, whereas there are many similar genes involved in patterning the FL and HL zeugo- and autopodia. Importantly, only some of the genes with known roles in pectoral girdle development have roles in development of the pelvic girdle, and vice versa. In these few mutually expressed genes, their functions, interacting partners, and tissue domains of expression may be different in each of the girdles. Overall, the networks that regulate patterning of the pectoral and pelvic girdles differ to a large degree, when the number of genes that are present in both networks is used as the metric. Specifically, they identified 10 genes with a role in pectoral girdle development that could be placed in the network, and 10 genes for the pelvic girdle; fewer than half (4 of 10) of the 10 genes in the pectoral girdle network are also present in the pelvic girdle network. In contrast, while the gene network regulating initial specification and outgrowth of the FL and HL fields differ in many respects, the networks regulating later stages of FL and HL outgrowth and patterning are more similar to each other. Namely, the networks regulating initial specification and outgrowth of the FL and HL share about half or slightly more than half of their genes (3 out of 5 for the FL and 3 out of 6 for the HL), whereas the networks regulating later stages of FL and HL outgrowth and patterning share almost all of their genes (27 out of 28 for the FL and 27 out of 29 for the HL). Sear et al. thus concluded that patterning of the regions of the FL and HL that represent major tetrapod innovations (i.e., the zeugo- and autopodia) occurred through the sequential (homoplastic) deployment of a similar developmental program.
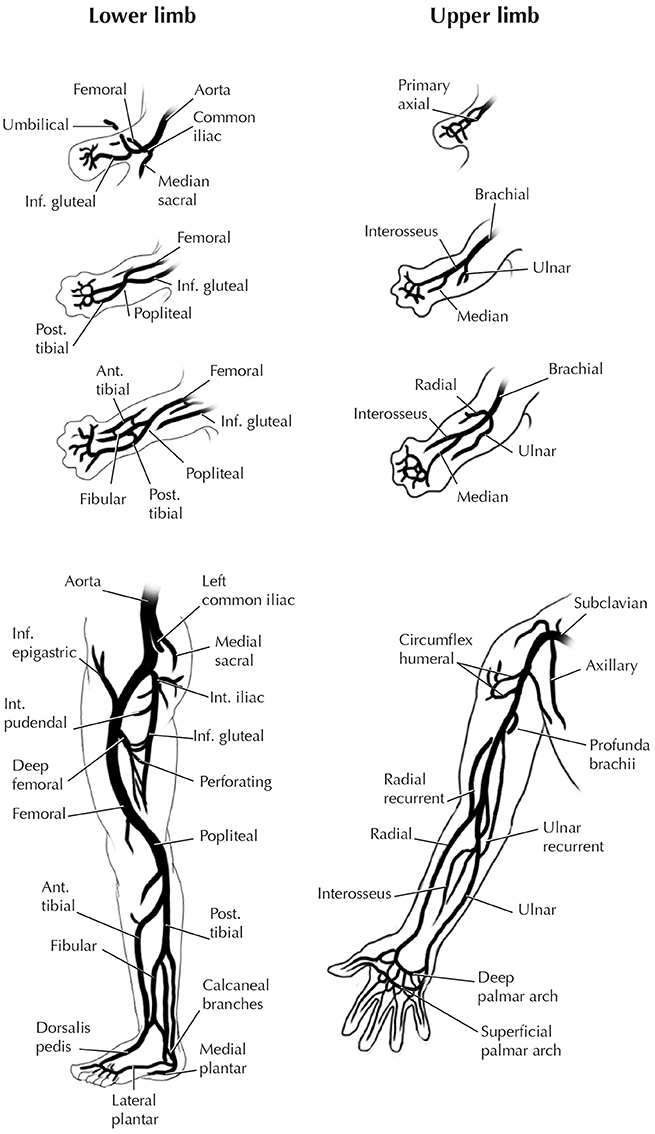
Figure 9. Development of the arteries of human FL and HL. Modified from Hinchliffe and Johnson 1980; for more details see that book and references therein).
Other authors have also previously proposed that similar genes were co-opted (“gene piracy”) during the transition from fish to tetrapods (e.g., Tbx4 and Tbx5 for the development of the HL and FL, respectively), rather than being ancestrally present in earlier vertebrates with pectoral and pelvic appendages (Roth, 1994; Willmer, 2003; Diogo et al., Hinchliffe and Johnson (2013). Specifically, Roth (1988, 1994) proposed that because of this “gene piracy,” the hindlimb and forelimb became anatomically more similar to each other than were the pectoral and pelvic appendages of non-tetrapod gnathostomes. This idea is defended by Diogo et al. (2013): the striking similarity between the forearm/hand and leg/foot of tetrapods is due to derived (homoplastic) events that occurred during the fin-limb transitions, and not to ancestral serial similarity between the pectoral and pelvic appendages. As such, prediction 4 is contradicted.
To test prediction 3, Diogo and Ziermann (2015) compared the musculoskeletal structures of the paired appendages of actinopterygians and chondrichthyans. They suggest that many of the strikingly similar FL-HL muscles of extant tetrapods evolved independently in each appendage because the ancestors of crown gnathostomes are predicted to have possessed only an adductor and an abductor in each fin. This is inconsistent with the idea that at least some muscles present in the tetrapod FLs and HLs were already present in the first vertebrates with pectoral and pelvic appendages. They propose that the origin of the pectoral girdle was instead likely related to head evolution, as illustrated by the cucullaris of gnathostomes such as chondrichthyans inserting onto both the branchial arches and pectoral girdle. Only later in evolution the cucullaris became differentiated into the levatores arcuum branchialium and protractor pectoralis, which gave rise to the amniote neck muscles trapezius and sternocleidomastoideus.
There are numerous evolutionary and functional reasons for the deep spatial relation between the skull and pectoral girdle in early gnathostomes: the girdle forms the rear wall of the internal branchial chamber—a shield for the pericardial cavity and a secure insertion for the pectoral fins (Coates and Cohn, 1998; Matsuoka et al., 2005). The pectoral appendage is deeply associated to the head developmentally through the use of strikingly similar developmental mechanisms, which include a Shh-dependence in both this appendage and the PAs in chondrichthyans, a commonality also seen in the pelvic appendage (Gillis et al., 2009; Gillis and Hall, 2016). It is interesting that these observations are consistent with Gegenbaur's (1859) old—and now often discredited—idea that the pectoral appendage may be a derivative of the posterior PA region (see below).
Fossils and the Evolution of the Paired Appendages
The existing data about the evolution of both fossil and extant gnathostomes contradict prediction 4. The pectoral appendages appeared earlier than the pelvic appendages: the former is present in jawless stem gnathostomes (Janvier et al., 2004; Janvier, 2007; Wilson et al., 2007), whereas the latter appears to be restricted to jawed gnathostomes (Zhu et al., 2012; Brazeau and Friedman, 2014). One could argue that the absence of evidence is not evidence of absence. For a long time it was thought that antiarch placoderms lack pelvic appendages, but a dermal pelvic girdle was reported recently in the antiarch Parayunnanolepis, which has also a mainly dermal pectoral girdle that consists partly of perichondral elements (e.g., a scapulocoracoid; Zhu et al., 2012; Figure 10). Therefore, it is not unreasonable to predict co-occurrence of pelvic and pectoral appendages in the deeper stem of gnathostomes. However, any proposition for simultaneous appearance of both appendages would require multiple events of secondary losses in the lineages without pelvic appendages (Wilson et al., 2007).
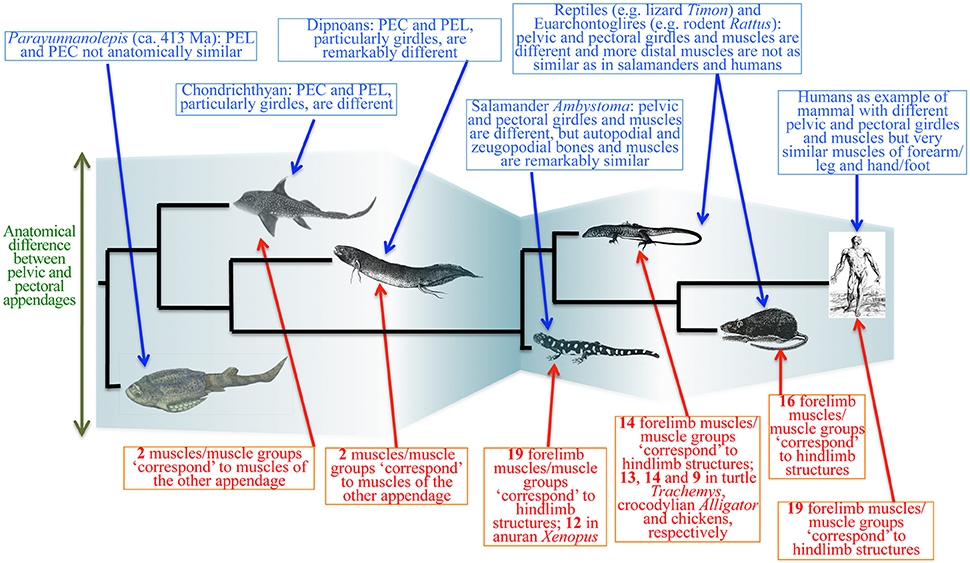
Figure 10. Simplified diagram of the evolutionary transitions in muscle anatomy leading to modern humans. The evolutionary history of the pelvic and pectoral appendages is much more complex than the “serial homology followed by functional/anatomical divergence” hypothesis suggests. Complex interplay between ontogenetic, functional, topological and phylogenetic constraints leads to cases of anatomical divergence followed by convergence (“similarity bottlenecks”). Modified from Diogo and Molnar (2014). PEL, PEC: pelvic and pectoral appendages.
Instead of secondary loss, the tendency appears to be independent acquisition of different types of appendages in several early vertebrate clades. This is borne out by complex taxonomic distribution of not only the pectoral and pelvic appendages, but also of paired reproductive organs and “anal” fins in the stem of gnathostomes (Sansom et al., 2013; Brazeau and Friedman, 2014; Trinajstic et al., 2014; Long et al., 2015). Co-options of gene expression may explain the homoplastic acquisitions of these appendages. Patterns of Hox and Shh expression are similar in body appendages as diverse as the head barbels of paddlefishes and the sexual claspers of sharks (Archambeault et al., 2014). These Hox patterns are probably part of an ancient module that provided a shared genetic program that was co-opted in the independent (homoplastic) formation of appendages such as genitalia, barbels, and the vent of some fishes (a medial structure that is analogous to a urethra). Similar Hox patterns also govern the development of the pelvic and pectoral, as well as the medial, fins of sharks (Freitas et al., 2006). Even in lampreys, the formation of medial fins involves Hox genes, suggesting that paired appendages originated when gene expression patterns for the median fin were redeployed in lateral plate mesoderm (Freitas et al., 2006). As such, similar developmental mechanisms are at play to form appendages as diverse as sexual organs, vents, barbels, and median, pectoral, and pelvic appendages. Accordingly, it is interesting that both the intromittent organs and paired appendages of placoderms consist of dermal and perichondral components (e.g., Trinajstic et al., 2014; Long et al., 2015). This is also the case for the pectoral appendage in osteostracans (Janvier et al., 2004). Furthermore, similar mechanisms are also used in the formation of the PAs and both the pectoral and pelvic appendages, and this similarity has led Gillis and colleagues to resuscitate Gegenbaur's idea that the pectoral appendage might be derived from the PAs, or at least be associated to a co-option of some of the mechanisms used in the formation of these arches (Gillis et al., 2009; Gillis and Hall, 2016).
All these structures provide an illustrative example of deep homology (Shubin et al., 2009), which overlaps with the developmental criterion of serial homology (sensu Wagner, 1994) and assumes at least some degree of parallelism (homoplasy) under the evolutionary criterion of serial homology (Shubin et al., 1997; Hall, 2003; Wake et al., 2011). These iterative correspondences are broken by historical (evolutionary) discontinuity of phenotype. That is, there might be continuity of some genes/developmental mechanisms that account for the similarity between the pectoral and pelvic appendages. However, there is no historical continuity in phenotype in the sense that ancestrally these appendages were not similar to each other. There likely was no true morphological duplication of the whole appendages (Diogo et al., 2013). Within the context of the idea propagated by Gillis and colleagues, the evolutionarily later acquisition of the pelvic appendage could mainly be seen in two different ways. Firstly, it could be related to a co-option of some of the mechanisms shared by both the pectoral appendage and the PAs. In this regard, it is interesting that the pelvic fin converts into a large gill-like organ during breeding season in males of the lungfish Lepidosiren paradoxa (Foxon, 1933). A non-mutually exclusive alternative is a co-option of some of the mechanisms shared by other appendages, such as the medial (unpaired) appendages. In this sense, it is worthy to note that various authors have pointed out that both the embryologic development and adult configuration of the pelvic appendage in various fishes (e.g., Acipenser, Polyodon) are much more similar to those seen in the dorsal and anal fins than in the pectoral appendages (Danforth, 1913; Mabee and Noordsy, 2004). A different, third hypothesis was proposed by Tabin and Laufer (1993), but this hypothesis does not match the temporal sequence according to the fossil record available so far. Namely, after recognizing that the similarity between the structures of the fore- and hindlimbs of tetrapods is very likely the result of parallel (homoplasic) evolution, they suggested that hox genes were originally expressed only in the pelvic appendage. Then, later in the evolutionary history of gnathostomes, the subsequent ectopic activation of Hox genes in the developing pectoral appendage resulted in a homeotic transformation, imposing the pattern of the pelvic appendage on the pectoral appendage by initiating signaling centers originally specific to the hindlimb, such as the zone of polarizing activity.
Aside from this latter idea, fossil evidence also rejects prediction 3. In the antiarch Parayunnanolepis—the earliest and plesiomorphic stem gnathostome with both pectoral and pelvic appendages—these appendages are different anatomically (Figure 10; Zhu et al., 2012). This goes in line with a detailed, long-term work on the paired appendages of early vertebrates and their evolution in gnathostomes, which show that the pectoral and pelvic appendages are primitively distinct from each other in the stem of gnathostomes and only became more similar later, in osteichthyans and particularly in tetrapods (Coates and Cohn, 1998, 1999). Specifically, 1999, p. 678) stated that “pelvic fins therefore neither originate as simple copies, nor as identical serial homologs of the pectorals […] Patterns of primitive fin phylogeny therefore provide little evidence of parallel (or concerted) evolution between pectoral and pelvic appendages […] close similarity between pectoral and pelvic fins is therefore a specialized feature which is developed most clearly within sarcopterygian (lobe-finned) osteichthyans; but even in this taxon, which includes tetrapods and their closest fish-like relatives, pectoral and pelvic fins primitively differ.” Janvier et al. (Coates and Cohn (2004) also pointed out that while the pectoral appendages of osteostracans are likely homologous with those of gnathostomes, the pelvic fins of the gnathostomes likely had a “rather different history”; in the gnathostome stem, the pelvic endoskeleton does not show the same process of radial formation that the pectoral appendages do (Janvier et al., 2004).
Serial Parallelism and Bottlenecks in the Macroevolution of the Paired Appendages
In summary, the four predictions of the serial homology model (1–4) are rejected on the basis of developmental, comparative, and fossil evidence. We support the “serial parallelism and bottleneck” model as an alternative (Figure 10; Diogo et al., 2013; Diogo and Molnar, 2014). The pectoral appendages appeared earlier than the pelvic ones, and were anatomically and functionally part of a cohesive structural unit that may have included the head. This link was likely developmental as well, as indicated by the Shh dependence (Gillis et al., 2009) and by the cucullaris—a muscle connecting the head and pectoral girdle in placoderms and chondrichthyans (Trinajstic et al., 2013)—as part of the cardiopharyngeal field (Diogo et al., 2015). The pectoral and pelvic girdles and the muscles attached to them remained markedly different across gnathostomes. In all tetrapod clades listed in Figure 10, no pelvic-thigh muscle corresponds topologically to a pectoral-arm muscle.
The FL-HL similarities in zeugopodial and autopodial bones and muscles were therefore acquired secondarily across the fish-tetrapod transitions (Figure 10). The zeugopodia and autopodia of tetrapods have ossified skeletons, and the developmental patterns are in general quite different from those in distal elements of the pectoral and pelvic fins in fishes (Don et al., 2013). The zeugopodia- and autopodia-specific patterns include cooption of paralogues (e.g., Tbx4 and Tbx5) as a result of “gene piracy” (Roth, 1994), and these cooptions explain similarity between the distal regions of these limbs in tetrapods (a “similarity bottleneck”: Figure 10). In salamanders such as axolotls, 19 muscles/muscle groups of the leg-foot clearly seem to “correspond” topologically to forearm-hand structures (Figure 4).
However, gene piracy alone is not sufficient to explain the striking similarity between the leg-foot and forearm-hand muscles of some derived tetrapods such as horses or humans (Figure 10). In humans, 19 muscles/muscle groups of the leg-foot have clear counterparts in the forearm-hand structures. However, the number is only 16 in quadrupedal mammals (e.g., rats) and 14 in reptiles (e.g., lizards), respectively (Figure 10). Importantly, many of the human FL muscles/muscle groups with clear counterparts in the HL were acquired independently within primates (Diogo et al., 2013). For example, the adductor hallucis and adductor pollicis are similar because they have well-differentiated transverse and oblique heads. However, the heads of the adductor pollicis only became well-differentiated in the node leading to catarrhines (Old World monkeys + hominoids), whereas those of the adductor hallucis are already well-differentiated in primitive primates such as lemurs.
The 'similarity bottlenecks' leading to derived taxa such as humans are also influenced by topological and functional factors. For example, the only way to have a functional abductor hallucis or pollicis brevis (both muscles were acquired as homoplasies during tetrapod evolution) is to have a muscle lying on the radial or tibial side and then having it insert onto the radial or tibial side of the first digit of the hand or foot. Another crucial point is that various leg-foot muscles that are strikingly similar topologically to forearm-hand muscles in derived taxa do not develop from similar anlagen. For example, the FL muscle extensor pollicis longus and the HL extensor hallucis longus of humans are remarkably similar topologically, but the former derives from the anlage of the short extensors of the hand, whereas the latter derives from the anlage of the long extensors of the leg (Diogo et al., 2013; Diogo and Wood, 2015). In summary, during the evolutionary history of the tetrapod FLs and HLs, there are cases of evolutionary divergence leading to differences, whereas substantial evolutionary parallelism occurred in subsequent “similarity bottlenecks.” The strikingly similar FL and HL muscles are therefore the result of a complex interplay between ontogenetic, topological, functional factors, and not due to serial homology between the appendages in non-tetrapod ancestors.
Concluding Remarks
Serial pattern can arise via assimilation between structures that have different evolutionary histories. We present two case studies in the origin of the jaw and serial patterning of the paired appendages among vertebrates. These two structures have long been considered in the context of differential specialization of initially polyisomeric (identically or similarly patterned) units, but we suggest an alternative mechanism: serial assimilation of anisomeric (dissimilarly patterned) structures. At the level of musculoskeletal patterning, the pharyngeal apparatus are serially patterned only in jawed vertebrates. The mandibular and non-mandibular PA derivatives have separate evolutionary histories and have paralleled each other since the origin of the jaw. Spatial confinement of the PA I-derived structures likely allowed serial assimilation of the PA derivatives via acquisition of the Dlx code across the pharynx. A similar pattern is found in the evolution of the paired appendages: they appeared at different times, and were significantly different morphologically in early gnathostomes, at which stage the pectoral appendages were anatomically and functionally more closely related to the head than to the pelvic appendages. Many of the similarities between these appendages were secondarily acquired in parallel during and after the fin-limb transition (likely due, at least in part, to co-option or “gene piracy”).
These two examples reveal a break in historical continuity of serial pattern shared between corresponding parts. Although the concept of serial homology has generated a controversy over different definitions and criteria, the decoupling of historical continuity and developmental correspondence is real in the cases of the vertebrate pharyngeal apparatus and paired appendages, and a polyisomeric ancestral phenotype likely is not. Under the criterion of historical continuity, differential specializations of serial homologs are an untenable explanation for serial patterns observed among these structures. We suggest two antidotes to assuming a polyisomeric ancestral phenotype a priori. First, anisomeric patterns should be tested as an alternative hypothesis. Second, similarity can only be assessed in particular context, such as at specific developmental stages or within certain clades. It remains to be explored whether or not serial assimilation helps account for the evolution of other serial structures such as vertebrae, but polyisomeric archetypes needs not serve as a default ancestral state.
Author Contributions
TM drafted the section on jaws, Introduction, and Concluding Remarks. RD drafted the section on paired appendages.
Funding
Howard University, National Sciences and Engineering Research Council (Canada).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Alessandro Minelli for an invitation to contribute this review. TM acknowledges Michael Coates (University of Chicago), Philippe Janvier (Muséum national d'Histoire naturelle), Brian Hall (Dalhousie University), Stephen Green and Marianne Bronner (California Institute of Technology), and Shigeru Kuratani (RIKEN Center for Developmental Biology) for extensive discussion. TM thanks Jean-Bernard Caron (Royal Ontario Museum) for access to original materials of Metaspriggina. Kesia Miyashita provided logistical support. TM was supported by Vanier CGS, Michael Smith Foreign Study Supplement (NSERC), and I.W. Killam Memorial Scholarship. RD acknowledges Karen Sears (University of Illinois), Terry Capellini (Harvard University), Gunter Wagner (Yale University), Zerina Johanson (Natural History Museum London), Renata Freitas (IMCB Porto), and Virginia Abdala (Universidad de Tucuman) for discussions on fin/limb evolution and serial homology. We thank two referees for comments on the submitted manuscript and three anonymous colleagues for critiques on earlier drafts. Phylopic (http://phylopic.org/) was the source of silhouettes used for cladograms.
References
Abe, M., Ruest, L.-B., and Clouthier, D. E. (2007). Fate of cranial neural crest cells during craniofacial development in endothelin-A receptor-deficient mice. Int. J. Dev. Biol. 51, 97–105. doi: 10.1387/ijdb.062237ma
Akimenko, M. A., Ekker, M., Wegner, J., Lin, W., and Westerfield, M. (1994). Combinatorial expression of three zebrafish genes related to distal- less: part of a homeobox gene code for the head. J. Neurosci. 14, 3475–3486.
Amundson, R. (2001). Homology and homoplasy: a philosophical perspective, in eLS (John Wiley & Sons, Ltd). Available online at: http://onlinelibrary.wiley.com/doi/10.1002/9780470015902.a0003445.pub2/abstract (Accessed on: July 9, 2015).
Archambeault, S., Taylor, J. A., and Crow, K. D. (2014). HoxA and HoxD expression in a variety of vertebrate body plan features reveals an ancient origin for the distal Hox program. EvoDevo 5, 44. doi: 10.1186/2041-9139-5-44
Baltzinger, M., Ori, M., Pasqualetti, M., Nardi, I., and Rijli, F. M. (2005). Hoxa2 knockdown in Xenopus results in hyoid to mandibular homeosis. Dev. Dyn. 234, 858–867. doi: 10.1002/dvdy.20567
Bardeen, C. R. (1906). Development and variation of the nerves and the musculature of the inferior extremity and of the neighboring regions of the trunk in man. Am. J. Anat. 6, 259–390. doi: 10.1002/aja.1000060108
Barron, F., Woods, C., Kuhn, K., Bishop, J., Howard, M. J., and Clouthier, D. E. (2011). Downregulation of Dlx5 and Dlx6 expression by Hand2 is essential for initiation of tongue morphogenesis. Development 138, 2249–2259. doi: 10.1242/dev.056929
Benouaiche, L., Gitton, Y., Vincent, C., Couly, G., and Levi, G. (2008). Sonic hedgehog signalling from foregut endoderm patterns the avian nasal capsule. Development 135, 2221–2225. doi: 10.1242/dev.020123
Brazeau, M. D., and Friedman, M. (2014). The characters of Palaeozoic jawed vertebrates. Zool. J. Linn. Soc. 170, 779–821. doi: 10.1111/zoj.12111
Brigandt, I. (2003). Homology in comparative, molecular, and evolutionary developmental biology: the radiation of a concept. J. Exp. Zoolog. B Mol. Dev. Evol. 299B, 9–17. doi: 10.1002/jez.b.36
Carroll, S. B. (2008). Evo-Devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36. doi: 10.1016/j.cell.2008.06.030
Cerny, R., Cattell, M., Sauka-Spengler, T., Bronner-Fraser, M., Yu, F., and Medeiros, D. M. (2010). Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc. Natl. Acad. Sci. U.S.A. 107, 17262–17267. doi: 10.1073/pnas.1009304107
Cerny, R., Lwigale, P., Ericsson, R., Meulemans, D., Epperlein, H.-H., and Bronner-Fraser, M. (2004a). Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular.” Dev. Biol. 276, 225–236. doi: 10.1016/j.ydbio.2004.08.046
Cerny, R., Meulemans, D., Berger, J., Wilsch-Bräuninger, M., Kurth, T., Bronner-Fraser, M., et al. (2004b). Combined intrinsic and extrinsic influences pattern cranial neural crest migration and pharyngeal arch morphogenesis in axolotl. Dev. Biol. 266, 252–269. doi: 10.1016/j.ydbio.2003.09.039
Choe, C. P., Collazo, A., Trinh, L. A., Pan, L., Moens, C. B., and Crump, J. G. (2013). Wnt-Dependent epithelial transitions drive pharyngeal pouch formation. Dev. Cell 24, 296–309. doi: 10.1016/j.devcel.2012.12.003
Choe, C. P., and Crump, J. G. (2014). Tbx1 controls the morphogenesis of pharyngeal pouch epithelia through mesodermal Wnt11r and Fgf8a. Development 141, 3583–3593. doi: 10.1242/dev.111740
Čihák, R. (1972). Ontogenesis of the skeleton and intrinsic muscles of the human hand and foot. Adv. Anat. Embryol. Cell Biol. 46, 1–194.
Clements, T., Dolocan, A., Martin, P., Purnell, M. A., Vinther, J., and Gabbott, S. E. (2016). The eyes of Tullimonstrum reveal a vertebrate affinity. Nature 532, 500–503. doi: 10.1038/nature17647
Clouthier, D. E., and Schilling, T. F. (2004). Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish. Birth Defects Res. Part C Embryo Today Rev. 72, 190–199. doi: 10.1002/bdrc.20007
Coates, M. I., and Cohn, M. J. (1998). Fins, limbs, and tails: outgrowths and axial patterning in vertebrate evolution. BioEssays 20, 371–381. doi: 10.1002/(SICI)1521-1878(199805)20:5<371::AID-BIES4>3.0.CO;2-R
Coates, M. I., and Cohn, M. J. (1999). Vertebrate axial and appendicular patterning: the early development of paired appendages. Am. Zool. 39, 676–685. doi: 10.1093/icb/39.3.676
Cohn, M. J. (2002). Evolutionary biology: lamprey Hox genes and the origin of jaws. Nature 416, 386–387. doi: 10.1038/416386a
Cole, N. J., Hall, T. E., Don, E. K., Berger, S., Boisvert, C. A., Neyt, C., et al. (2011). Development and evolution of the muscles of the pelvic fin. PLoS Biol. 9:e1001168. doi: 10.1371/journal.pbio.1001168
Compagnucci, C., Debiais-Thibaud, M., Coolen, M., Fish, J., Griffin, J. N., Bertocchini, F., et al. (2013). Pattern and polarity in the development and evolution of the gnathostome jaw: both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula. Dev. Biol. 377, 428–448. doi: 10.1016/j.ydbio.2013.02.022
Conway Morris, S., and Caron, J.-B. (2014). A primitive fish from the Cambrian of North America. Nature 512, 419–422. doi: 10.1038/nature13414
Couly, G., Creuzet, S., Bennaceur, S., Vincent, C., and Douarin, N. M. L. (2002). Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 129, 1061–1073.
Couly, G. F., Coltey, P. M., and Douarin, N. M. L. (1993). The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117, 409–429.
Creuzet, S., Couly, G., Vincent, C., and Douarin, N. M. L. (2002). Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development 129, 4301–4313.
Crump, J. G., Maves, L., Lawson, N. D., Weinstein, B. M., and Kimmel, C. B. (2004). An essential role for Fgfs in endodermal pouch formation influences later craniofacial skeletal patterning. Development 131, 5703–5716. doi: 10.1242/dev.01444
Danforth, C. H. (1913). The myology of polyodon. J. Morphol. 24, 107–146. doi: 10.1002/jmor.1050240104
Depew, M. J., and Compagnucci, C. (2008). Tweaking the hinge and caps: testing a model of the organization of jaws. J. Exp. Zoolog. B Mol. Dev. Evol. 310B, 315–335. doi: 10.1002/jez.b.21205
Depew, M. J., Lufkin, T., and Rubenstein, J. L. R. (2002). Specification of jaw subdivisions by Dlx genes. Science 298, 381–385. doi: 10.1126/science.1075703
Depew, M. J., Simpson, C. A., Morasso, M., and Rubenstein, J. L. R. (2005). Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J. Anat. 207, 501–561. doi: 10.1111/j.1469-7580.2005.00487.x
Diogo, R., and Abdala, V. (2010). Muscles of Vertebrates: Comparative Anatomy, Evolution, Homologies and Development. Oxford: CRC Press.
Diogo, R., Kelly, R. G., Christiaen, L., Levine, M., Ziermann, J. M., Molnar, J. L., et al. (2015). A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 520, 466–473. doi: 10.1038/nature14435
Diogo, R., Linde-Medina, M., Abdala, V., and Ashley-Ross, M. A. (2013). New, puzzling insights from comparative myological studies on the old and unsolved forelimb/hindlimb enigma. Biol. Rev. 88, 196–214. doi: 10.1111/j.1469-185X.2012.00247.x
Diogo, R., and Molnar, J. (2014). Comparative anatomy, evolution, and homologies of tetrapod hindlimb muscles, comparison with forelimb muscles, and deconstruction of the forelimb-hindlimb serial homology hypothesis. Anat. Rec. 297, 1047–1075. doi: 10.1002/ar.22919
Diogo, R., Murawala, P., and Tanaka, E. M. (2014a). Is salamander hindlimb regeneration similar to that of the forelimb? Anatomical and morphogenetic analysis of hindlimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative and developmental studies. J. Anat. 224, 459–468. doi: 10.1111/joa.12150
Diogo, R., Nacu, E., and Tanaka, E. M. (2014b). Is salamander limb regeneration really perfect? anatomical and morphogenetic analysis of forelimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative, developmental, and evolutionary studies. Anat. Rec. 297, 1076–1089. doi: 10.1002/ar.22906
Diogo, R., and Tanaka, E. M. (2014). Development of fore- and hindlimb muscles in GFP-transgenic axolotls: morphogenesis, the tetrapod bauplan, and new insights on the Forelimb-Hindlimb Enigma. J. Exp. Zoolog. B Mol. Dev. Evol. 322, 106–127. doi: 10.1002/jez.b.22552
Diogo, R., and Wood, B. (2015). Origin and evolution of primate and human muscles, anatomical variations and anomalies, and evolutionary developmental biology, in Evolutionary Developmental Anthropology, eds J. Boughner and C. Rolian (Hoboken, NJ: John Wiley & Sons), 167–204.
Diogo, R., and Ziermann, J. M. (2014). Development of fore-and hindlimb muscles in frogs: Morphogenesis, homeotic transformations, digit reduction, and the forelimb–hindlimb enigma. J. Exp. Zoolog. B Mol. Dev. Evol. 322, 86–105. doi: 10.1002/jez.b.22549
Diogo, R., and Ziermann, J. M. (2015). Muscles of chondrichthyan paired appendages: comparison with Osteichthyans, deconstruction of the fore–hindlimb serial homology dogma, and new insights on the evolution of the vertebrate neck. Anat. Rec. 298, 513–530. doi: 10.1002/ar.23047
Don, E. K., Currie, P. D., and Cole, N. J. (2013). The evolutionary history of the development of the pelvic fin/hindlimb. J. Anat. 222, 114–133. doi: 10.1111/j.1469-7580.2012.01557.x
Eberhart, J. K., Swartz, M. E., Crump, J. G., and Kimmel, C. B. (2006). Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development 133, 1069–1077. doi: 10.1242/dev.02281
Edgeworth, F. H. (1935). The Cranial Muscles of the Vertebrates, 1st Edn. Cambridge: Cambridge University Press.
Ellies, D. L., Langille, R. M., Martin, C. C., Akimenko, M.-A., and Ekker, M. (1997). Specific craniofacial cartilage dysmorphogenesis coincides with a loss of dlx gene expression in retinoic acid-treated zebrafish embryos. Mech. Dev. 61, 23–36. doi: 10.1016/S0925-4773(96)00616-8
Faunes, M., Francisco Botelho, J., Ahumada Galleguillos, P., and Mpodozis, J. (2015). On the hodological criterion for homology. Evol. Psychol. Neurosci. 9:223. doi: 10.3389/fnins.2015.00223
Forey, P. L. (1995). Agnathans recent and fossil, and the origin of jawed vertebrates. Rev. Fish Biol. Fish. 5, 267–303.
Forey, P. L., and Gardiner, B. G. (1986). Observations on Ctenurella (Ptyctodontida) and the classification of placoderm fishes. Zool. J. Linn. Soc. 86, 43–74. doi: 10.1111/j.1096-3642.1986.tb01807.x
Freitas, R., Zhang, G., and Cohn, M. J. (2006). Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature 442, 1033–1037. doi: 10.1038/nature04984
Frisdal, A., and Trainor, P. A. (2014). Development and evolution of the pharyngeal apparatus. Wiley Interdiscip. Rev. Dev. Biol. 3, 403–418. doi: 10.1002/wdev.147
Fujimoto, S., Oisi, Y., Kuraku, S., Ota, K. G., and Kuratani, S. (2013). Non-parsimonious evolution of hagfish Dlx genes. BMC Evol. Biol. 13:15. doi: 10.1186/1471-2148-13-15
Gai, Z., Donoghue, P. C. J., Zhu, M., Janvier, P., and Stampanoni, M. (2011). Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature 476, 324–327. doi: 10.1038/nature10276
Gellon, G., and McGinnis, W. (1998). Shaping animal body plans in development and evolution by modulation of Hox expression patterns. BioEssays 20, 116–125. doi: 10.1002/(SICI)1521-1878(199802)20:2<116::AID-BIES4>3.0.CO;2-R
Gillis, J. A., Dahn, R. D., and Shubin, N. H. (2009). Shared developmental mechanisms pattern the vertebrate gill arch and paired fin skeletons. Proc. Natl. Acad. Sci. U.S.A. 106, 5720–5724. doi: 10.1073/pnas.0810959106
Gillis, J. A., and Hall, B. K. (2016). A shared role for sonic hedgehog signalling in patterning chondrichthyan gill arch appendages and tetrapod limbs. Development 143, 1313–1317. doi: 10.1242/dev.133884
Gillis, J. A., Modrell, M. S., and Baker, C. V. H. (2013). Developmental evidence for serial homology of the vertebrate jaw and gill arch skeleton. Nat. Commun. 4, 1436. doi: 10.1038/ncomms2429
Goodrich, E. S. (1930). Studies on the Structure and Development of Vertebrates. London: Dover Publishing.
Graham, A., Okabe, M., and Quinlan, R. (2005). The role of the endoderm in the development and evolution of the pharyngeal arches. J. Anat. 207, 479–487. doi: 10.1111/j.1469-7580.2005.00472.x
Grenier, J., Teillet, M.-A., Grifone, R., Kelly, R. G., and Duprez, D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 4:e4381. doi: 10.1371/journal.pone.0004381
Gross, J. B., and Hanken, J. (2008). Segmentation of the vertebrate skull: neural-crest derivation of adult cartilages in the clawed frog, Xenopus laevis. Integr. Comp. Biol. 48, 681–696. doi: 10.1093/icb/icn077
Hall, B. K. (ed.). (1994). Homology: The Hierarchial Basis of Comparative Biology. San Diego, CA: Academic Press.
Hall, B. K. (2003). Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biol. Rev. 78, 409–433. doi: 10.1017/S1464793102006097
Hall, B. K. (2007). Homoplasy and homology: Dichotomy or continuum? J. Hum. Evol. 52, 473–479. doi: 10.1016/j.foodchem.2016.05.044
Hall, B. K. (2009). The Neural Crest and Neural Crest Cells in Vertebrate Development and Evolution. New York, NY: Springer. Available online at: http://www.springer.com/life+sciences/evolutionary+%26+developmental+biology/book/978-0-387-09845-6 (Accessed on: December 14, 2014).
Hannibal, R. L., and Patel, N. H. (2013). What is a segment? EvoDevo 4:35. doi: 10.1161/JAHA.115.001945
Haworth, K. E., Healy, C., Morgan, P., and Sharpe, P. T. (2004). Regionalisation of early head ectoderm is regulated by endoderm and prepatterns the orofacial epithelium. Development 131, 4797–4806. doi: 10.1242/dev.01337
Haworth, K. E., Wilson, J. M., Grevellec, A., Cobourne, M. T., Healy, C., Helms, J. A., et al. (2007). Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Dev. Biol. 303, 244–258. doi: 10.1016/j.ydbio.2006.11.009
Heude, É., Bouhali, K., Kurihara, Y., Kurihara, H., Couly, G., Janvier, P., et al. (2010). Jaw muscularization requires Dlx expression by cranial neural crest cells. Proc. Natl. Acad. Sci. U.S.A. 107, 11441–11446. doi: 10.1073/pnas.1001582107
Hinchliffe, J. R., and Johnson, D. R. (1980). The Development of the Vertebrate Limb: An Approach Through Experiment, Genetics and Evolution. Oxford: Clarendon Press.
Hinits, Y., Williams, V. C., Sweetman, D., Donn, T. M., Ma, T. P., Moens, C. B., et al. (2011). Defective cranial skeletal development, larval lethality and haploinsufficiency in Myod mutant zebrafish. Dev. Biol. 358, 102–112. doi: 10.1016/j.ydbio.2011.07.015
Hirano, T., and Nishida, H. (1997). Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. I. Origin of mesodermal tissues of the juvenile. Dev. Biol. 192, 199–210. doi: 10.1006/dbio.1997.8772
Holland, P. W. H., and Garcia-Fernàndez, J. (1996). Hox Genes and Chordate Evolution. Dev. Biol. 173, 382–395. doi: 10.1006/dbio.1996.0034
Horigome, N., Myojin, M., Ueki, T., Hirano, S., Aizawa, S., and Kuratani, S. (1999). Development of cephalic neural crest cells in embryos of lampetra japonica, with special reference to the evolution of the jaw. Dev. Biol. 207, 287–308. doi: 10.1006/dbio.1998.9175
Humphry, G. M. (1872). The disposition of muscles in vertebrate animals. J. Anat. Physiol. 6, 293–376.
Hunt, P., Clarke, J. D. W., Buxton, P., Ferretti, P., and Thorogood, P. (1998). Stability and plasticity of neural crest patterning and branchial arch hox code after extensive cephalic crest rotation. Dev. Biol. 198, 82–104. doi: 10.1006/dbio.1998.8886
Hunt, P., Gulisano, M., Cook, M., Sham, M.-H., Faiella, A., Wilkinson, D., et al. (1991a). A distinct Hox code for the branchial region of the vertebrate head. Nature 353, 861–864. doi: 10.1038/353861a0
Hunt, P., Whiting, J., Nonchev, S., Sham, M. H., Marshall, H., Graham, A., et al. (1991b). The branchial Hox code and its implications for gene regulation, patterning of the nervous system and head evolution. Dev. Camb. Engl. (Suppl. 2), 63–77.
Hyman, L. H. (1992). Hyman's Comparative Vertebrate Anatomy, 3rd Edn. Chicago, IL: University of Chicago Press.
Jandzik, D., Hawkins, M. B., Cattell, M. V., Cerny, R., Square, T. A., and Medeiros, D. M. (2014). Roles for FGF in lamprey pharyngeal pouch formation and skeletogenesis highlight ancestral functions in the vertebrate head. Development 141, 629–638. doi: 10.1242/dev.097261
Janvier, P. (2007). Homologies and evolutionary transitions in early vertebrate history, in Major Transitions in Vertebrate Evolution, eds J. S. Anderson and H.-D. Sues (Bloomington, IN: Indiana University Press), 57–121.
Janvier, P., Arsenault, M., and Desbiens, S. (2004). Calcified cartilage in the paired fins of the osteostracan Escuminaspis laticeps (Traquair 1880), from the Late Devonian of Miguasha (Québec, Canada), with a consideration of the early evolution of the pectoral fin endoskeleton in vertebrates. J. Vertebr. Paleontol. 24, 773–779. doi: 10.1671/0272-4634(2004)024[0773:CCITPF]2.0.CO;2
Johanson, Z., Joss, J., Boisvert, C. A., Ericsson, R., Sutija, M., and Ahlberg, P. E. (2007). Fish fingers: digit homologues in sarcopterygian fish fins. J. Exp. Zoolog. B Mol. Dev. Evol. 308B, 757–768. doi: 10.1002/jez.b.21197
Johnels, A. G. (1948). On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool. 29, 139–279. doi: 10.1111/j.1463-6395.1948.tb00030.x
Kimmel, C. B., Miller, T. C., and Keynes, J. R. (2001). Neural crest patterning and the evolution of the jaw. J. Anat. 199, 105–119. doi: 10.1017/S0021878201008068
Kimmel, C. B., Ullmann, B., Walker, M., Miller, C. T., and Crump, J. G. (2003). Endothelin 1-mediated regulation of pharyngeal bone development in zebrafish. Development 130, 1339–1351. doi: 10.1242/dev.00338
Kitazawa, T., Fujisawa, K., Narboux-Nême, N., Arima, Y., Kawamura, Y., Inoue, T., et al. (2015). Distinct effects of Hoxa2 overexpression in cranial neural crest populations reveal that the mammalian hyomandibular-ceratohyal boundary maps within the styloid process. Dev. Biol. 402, 162–174. doi: 10.1016/j.ydbio.2015.04.007
Köntges, G., and Lumsden, A. (1996). Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122, 3229–3242.
Krumlauf, R. (1994). Hox genes in vertebrate development. Cell 78, 191–201. doi: 10.1016/0092-8674(94)90290-9
Kuraku, S., Takio, Y., Sugahara, F., Takechi, M., and Kuratani, S. (2010). Evolution of oropharyngeal patterning mechanisms involving Dlx and endothelins in vertebrates. Dev. Biol. 341, 315–323. doi: 10.1016/j.ydbio.2010.02.013
Kuratani, S. (2004). Evolution of the vertebrate jaw: comparative embryology and molecular developmental biology reveal the factors behind evolutionary novelty. J. Anat. 205, 335–347. doi: 10.1111/j.0021-8782.2004.00345.x
Kuratani, S. (2009). Modularity, comparative embryology and evo-devo: developmental dissection of evolving body plans. Dev. Biol. 332, 61–69. doi: 10.1016/j.ydbio.2009.05.564
Kuratani, S. (2012). Evolution of the vertebrate jaw from developmental perspectives. Evol. Dev. 14, 76–92. doi: 10.1111/j.1525-142X.2011.00523.x
Kuratani, S., Adachi, N., Wada, N., Oisi, Y., and Sugahara, F. (2013). Developmental and evolutionary significance of the mandibular arch and prechordal/premandibular cranium in vertebrates: revising the heterotopy scenario of gnathostome jaw evolution. J. Anat. 222, 41–55. doi: 10.1111/j.1469-7580.2012.01505.x
Kuratani, S., Horigome, N., and Hirano, S. (1999). Developmental morphology of the head mesoderm and reevaluation of segmental theories of the vertebrate head: evidence from embryos of an agnathan vertebrate, Lampetra japonica. Dev. Biol. 210, 381–400. doi: 10.1006/dbio.1999.9266
Kuratani, S., Murakami, Y., Nobusada, Y., Kusakabe, R., and Hirano, S. (2004). Developmental fate of the mandibular mesoderm in the lamprey, Lethenteron japonicum: comparative morphology and development of the gnathostome jaw with special reference to the nature of the trabecula cranii. J. Exp. Zoolog. B Mol. Dev. Evol. 302B, 458–468. doi: 10.1002/jez.b.21011
Kuratani, S., Nobusada, Y., Horigome, N., and Shigetani, Y. (2001). Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Philos. Trans. R. Soc. Lond. Ser. B 356, 1615–1632. doi: 10.1098/rstb.2001.0976
Kusakabe, R., Kuraku, S., and Kuratani, S. (2011). Expression and interaction of muscle-related genes in the lamprey imply the evolutionary scenario for vertebrate skeletal muscle, in association with the acquisition of the neck and fins. Dev. Biol. 350, 217–227. doi: 10.1016/j.ydbio.2010.10.029
Kusakabe, R., and Kuratani, S. (2005). Evolution and developmental patterning of the vertebrate skeletal muscles: perspectives from the lamprey. Dev. Dyn. 234, 824–834. doi: 10.1002/dvdy.20587
Kusakabe, R., and Kuratani, S. (2007). Evolutionary perspectives from development of mesodermal components in the lamprey. Dev. Dyn. 236, 2410–2420. doi: 10.1002/dvdy.21177
Lee, S.-H., Bédard, O., Buchtová, M., Fu, K., and Richman, J. M. (2004). A new origin for the maxillary jaw. Dev. Biol. 276, 207–224. doi: 10.1016/j.ydbio.2004.08.045
Lee, S.-H., Fu, K. K., Hui, J. N., and Richman, J. M. (2001). Noggin and retinoic acid transform the identity of avian facial prominences. Nature 414, 909–912. doi: 10.1038/414909a
Lescroart, F., Kelly, R. G., Garrec, J.-F. L., Nicolas, J.-F., Meilhac, S. M., and Buckingham, M. (2010). Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137, 3269–3279. doi: 10.1242/dev.050674
Lewis, W. H. (1910). The development of the muscular system, in Manual of Embryology, eds F. Keibel and F. P. Mall (Philadelphia, PA: Lippincott), 455–522.
Long, J. A., Mark-Kurik, E., Johanson, Z., Lee, M. S. Y., Young, G. C., Min, Z., et al. (2015). Copulation in antiarch placoderms and the origin of gnathostome internal fertilization. Nature 517, 196–199. doi: 10.1038/nature13825
Lours-Calet, C., Alvares, L. E., El-Hanfy, A. S., Gandesha, S., Walters, E. H., Sobreira, D. R., et al. (2014). Evolutionarily conserved morphogenetic movements at the vertebrate head–trunk interface coordinate the transport and assembly of hypopharyngeal structures. Dev. Biol. 390, 231–246. doi: 10.1016/j.ydbio.2014.03.003
Mabee, P. M., and Noordsy, M. (2004). Development of the paired fins in the paddlefish, Polyodon spathula. J. Morphol. 261, 334–344. doi: 10.1002/jmor.10253
Mallatt, J. (1996). Ventilation and the origin of jawed vertebrates: a new mouth. Zool. J. Linn. Soc. 117, 329–404. doi: 10.1111/j.1096-3642.1996.tb01658.x
Mallatt, J. (2008). The origin of the vertebrate jaw: neoclassical ideas versus newer, development-based ideas. Zoolog. Sci. 25, 990–998. doi: 10.2108/zsj.25.990
Manzanares, M., Wada, H., Itasaki, N., Trainor, P. A., Krumlauf, R., and Holland, P. W. H. (2000). Conservation and elaboration of Hox gene regulation during evolution of the vertebrate head. Nature 408, 854–857. doi: 10.1038/35048570
Matsuoka, T., Ahlberg, P. E., Kessaris, N., Iannarelli, P., Dennehy, U., Richardson, W. D., et al. (2005). Neural crest origins of the neck and shoulder. Nature 436, 347–355. doi: 10.1038/nature03837
McCauley, D. W., and Bronner-Fraser, M. (2003). Neural crest contributions to the lamprey head. Development 130, 2317–2327. doi: 10.1242/dev.00451
McCoy, V. E., Saupe, E. E., Lamsdell, J. C., Tarhan, L. G., McMahon, S., Lidgard, S., et al. (2016). The “Tully monster” is a vertebrate. Nature 532, 496–499. doi: 10.1038/nature16992
Medeiros, D. M., and Crump, J. G. (2012). New perspectives on pharyngeal dorsoventral patterning in development and evolution of the vertebrate jaw. Dev. Biol. 371, 121–135. doi: 10.1016/j.ydbio.2012.08.026
Miller, C. T., Schilling, T. F., Lee, K., Parker, J., and Kimmel, C. B. (2000). Sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch. Development 127, 3815–3828.
Minoux, M., Antonarakis, G. S., Kmita, M., Duboule, D., and Rijli, F. M. (2009). Rostral and caudal pharyngeal arches share a common neural crest ground pattern. Development 136, 637–645. doi: 10.1242/dev.028621
Minoux, M., and Rijli, F. M. (2010). Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial. Development 137, 2605–2621. doi: 10.1242/dev.040048
Miyashita, T. (2012). Comparative Analysis of the Anatomy of the Myxinoidea and the Ancestry of Early Vertebrate Lineages. Unpublished M.Sc thesis, The University of Alberta, Edmonton.
Miyashita, T. (2015). Fishing for jaws in early vertebrate evolution: a novel hypothesis of mandibular confinement. Biol. Rev. doi: 10.1111/brv.12187. [Epub ahead of print].
Myojin, M., Ueki, T., Sugahara, F., Murakami, Y., Shigetani, Y., Aizawa, S., et al. (2001). Isolation of Dlx and Emx gene cognates in an agnathan species, Lampetra japonica, and their expression patterns during embryonic and larval development: conserved and diversified regulatory patterns of homeobox genes in vertebrate head evolution. J. Exp. Zool. 291, 68–84. doi: 10.1002/jez.6
Neidert, A. H., Virupannavar, V., Hooker, G. W., and Langeland, J. A. (2001). Lamprey Dlx genes and early vertebrate evolution. Proc. Natl. Acad. Sci. U.S.A. 98, 1665–1670. doi: 10.1073/pnas.98.4.1665
Neuhauss, S. C., Solnica-Krezel, L., Schier, A. F., Zwartkruis, F., Stemple, D. L., Malicki, J., et al. (1996). Mutations affecting craniofacial development in zebrafish. Development 123, 357–367.
Noden, D. M., and Francis-West, P. (2006). The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. 235, 1194–1218. doi: 10.1002/dvdy.20697
Noden, D. M., and Trainor, P. A. (2005). Relations and interactions between cranial mesoderm and neural crest populations. J. Anat. 207, 575–601. doi: 10.1111/j.1469-7580.2005.00473.x
Northcutt, R. G. (2008). Historical hypotheses regarding segmentation of the vertebrate head. Integr. Comp. Biol. 48, 611–619. doi: 10.1093/icb/icn065
O'Neill, P., Mak, S.-S., Fritzsch, B., Ladher, R. K., and Baker, C. V. H. (2012). The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nat. Commun. 3, 1041. doi: 10.1038/ncomms2036
Oisi, Y., Fujimoto, S., Ota, K. G., and Kuratani, S. (2015). On the peculiar morphology and development of the hypoglossal, glossopharyngeal and vagus nerves and hypobranchial muscles in the hagfish. Zool. Lett. 1, 6. doi: 10.1186/s40851-014-0005-9
Oisi, Y., Ota, K. G., Fujimoto, S., and Kuratani, S. (2013a). Development of the chondrocranium in hagfishes, with special reference to the early evolution of vertebrates. Zoolog. Sci. 30, 944–961. doi: 10.2108/zsj.30.944
Oisi, Y., Ota, K. G., Kuraku, S., Fujimoto, S., and Kuratani, S. (2013b). Craniofacial development of hagfishes and the evolution of vertebrates. Nature 493, 175–180. doi: 10.1038/nature11794
Okada, K., Inohaya, K., Mise, T., Kudo, A., Takada, S., and Wada, H. (2016). Reiterative expression of pax1 directs pharyngeal pouch segmentation in medaka. Development 143, 1800–1810. doi: 10.1242/dev.130039
Owen, R. (1848). On the Archetype and Homologies of the Vertebrates Skeleton. London: John van Voorst.
Ozeki, H., Kurihara, Y., Tonami, K., Watatani, S., and Kurihara, H. (2004). Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech. Dev. 121, 387–395. doi: 10.1016/j.mod.2004.02.002
Parker, H. J., Bronner, M. E., and Krumlauf, R. (2014). A Hox regulatory network of hindbrain segmentation is conserved to the base of vertebrates. Nature 514, 490–493. doi: 10.1038/nature13723
Pasqualetti, M., Ori, M., Nardi, I., and Rijli, F. M. (2000). Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development 127, 5367–5378.
Patel, N. H., Martin-Blanco, E., Coleman, K. G., Poole, S. J., Ellis, M. C., Kornberg, T. B., et al. (1989). Expression of engrailed proteins in arthropods, annelids, and chordates. Cell 58, 955–968. doi: 10.1016/0092-8674(89)90947-1
Piotrowski, T., Ahn, D., Schilling, T. F., Nair, S., Ruvinsky, I., Geisler, R., et al. (2003). The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development 130, 5043–5052. doi: 10.1242/dev.00704
Piotrowski, T., and Nüsslein-Volhard, C. (2000). The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev. Biol. 225, 339–356. doi: 10.1006/dbio.2000.9842
Rathke, H. (1827). Bemerkungen über den innern Bau des Qerders (Ammocoetes branchialis) und des kleinen Neunauges (Petromyzon planeri), in Neueste Schriften der Naturforschenden Gesellschaft in Danzig Bd. II.
Reno, P. L., Horton, W. E., and Lovejoy, C. O. (2013). Metapodial or Phalanx? An Evolutionary and Developmental Perspective on the Homology of the First Ray's Proximal Segment. J. Exp. Zoolog. B Mol. Dev. Evol. 320, 276–285. doi: 10.1002/jez.b.22506
Richards, R. J. (2004). The Romantic Conception of Life: Science and Philosophy in the Age of Goethe, 1st Edn. Chicago, IL: University Of Chicago Press.
Richman, J. M., Herbert, M., Matovinovic, E., and Walin, J. (1997). Effect of fibroblast growth factors on outgrowth of facial mesenchyme. Dev. Biol. 189, 135–147. doi: 10.1006/dbio.1997.8656
Richman, J. M., and Lee, S.-H. (2003). About face: signals and genes controlling jaw patterning and identity in vertebrates. BioEssays 25, 554–568. doi: 10.1002/bies.10288
Rijli, F. M., Mark, M., Lakkaraju, S., Dierich, A., Dollé, P., and Chambon, P. (1993). A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75, 1333–1349. doi: 10.1016/0092-8674(93)90620-6
Rinon, A., Lazar, S., Marshall, H., Büchmann-Møller, S., Neufeld, A., Elhanany-Tamir, H., et al. (2007). Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 134, 3065–3075. doi: 10.1242/dev.002501
Roth, V. L. (1988). The biological basis of homology, in Ontogeny and Systematics, ed C. J. Humphries (New York, NY: Columbia University Press), 1–26.
Roth, V. L. (1994). Within and between organisms: replicators, lineages, and homologues, in Homology: the Hierarchical Basis of Comparative Biology, ed B. K. Hall (San Diego, CA: Academic Press), 301–337.
Ruest, L.-B., and Clouthier, D. E. (2009). Elucidating timing and function of endothelin-A receptor signaling during craniofacial development using neural crest cell-specific gene deletion and receptor antagonism. Dev. Biol. 328, 94–108. doi: 10.1016/j.ydbio.2009.01.005
Sambasivan, R., Kuratani, S., and Tajbakhsh, S. (2011). An eye on the head: the development and evolution of craniofacial muscles. Development 138, 2401–2415. doi: 10.1242/dev.040972
Sansom, R. S., Gabbott, S. E., and Purnell, M. A. (2013). Unusual anal fin in a Devonian jawless vertebrate reveals complex origins of paired appendages. Biol. Lett. 9:20130002. doi: 10.1098/rsbl.2013.0002
Sears, K. E., Capellini, T. D., and Diogo, R. (2015). On the serial homology of the pectoral and pelvic girdles of tetrapods. Evolution 69, 2543–2555. doi: 10.1111/evo.12773
Sewertzoff, A. N. (1899). Studien zur Entwicklungsgeschichte der Wirbeltierkopfes. I. Die Metamerie des Kopfes des elektrischen Rochen. Bull. Société Impériale Nat. Moucou 1899, 197–263.
Shigetani, Y., Nobusada, Y., and Kuratani, S. (2000). Ectodermally derived FGF8 defines the maxillomandibular region in the early chick embryo: epithelial–mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev. Biol. 228, 73–85. doi: 10.1006/dbio.2000.9932
Shigetani, Y., Sugahara, F., Kawakami, Y., Murakami, Y., Hirano, S., and Kuratani, S. (2002). Heterotopic shift of epithelial-mesenchymal interactions in vertebrate jaw evolution. Science 296, 1316–1319. doi: 10.1126/science.1068310
Shigetani, Y., Sugahara, F., and Kuratani, S. (2005). A new evolutionary scenario for the vertebrate jaw. BioEssays 27, 331–338. doi: 10.1002/bies.20182
Shone, V., Oulion, S., Casane, D., Laurenti, P., and Graham, A. (2016). Mode of reduction in the number of pharyngeal segments within the sarcopterygians. Zool. Lett. 2:6. doi: 10.1186/s40851-016-0043-6
Shubin, N., Tabin, C., and Carroll, S. (1997). Fossils, genes and the evolution of animal limbs. Nature 388, 639–648. doi: 10.1038/41710
Shubin, N., Tabin, C., and Carroll, S. (2009). Deep homology and the origins of evolutionary novelty. Nature 457, 818–823. doi: 10.1038/nature07891
Stensiö, E. A. (1963). Anatomical studies on the arthrodiran head. Part I. Preface, geological and geographical distribution, the organization of the head in the Dolichothoraci, Coccosteomorphi and Pachyosteomorphi. Taxonomic appendix. K. Sven. Ventenskapsakademiens Handl. 9, 1–419.
Stensiö, E. A. (1969). Elasmobranchiomorphi placodermata arthrodires, in Traité de Paléontologie, ed J. Piveteau (Paris: Masson et Cie), 71–692.
Stolfi, A., Gainous, T. B., Young, J. J., Mori, A., Levine, M., and Christiaen, L. (2010). Early chordate origins of the vertebrate second heart field. Science 329, 565–568. doi: 10.1126/science.1190181
Stolfi, A., Lowe, E. K., Racioppi, C., Ristoratore, F., Brown, C. T., Swalla, B. J., et al. (2014). Divergent mechanisms regulate conserved cardiopharyngeal development and gene expression in distantly related ascidians. eLife 3:e03728. doi: 10.7554/eLife.03728
Szabo-Rogers, H. L., Geetha-Loganathan, P., Whiting, C. J., Nimmagadda, S., Fu, K., and Richman, J. M. (2009). Novel skeletogenic patterning roles for the olfactory pit. Development 136, 219–229. doi: 10.1242/dev.023978
Tabin, C., and Laufer, E. (1993). Hox genes and serial homology. Nature 361, 692–693. doi: 10.1038/361692a0
Takechi, M., Adachi, N., Hirai, T., Kuratani, S., and Kuraku, S. (2013). The Dlx genes as clues to vertebrate genomics and craniofacial evolution. Semin. Cell Dev. Biol. 24, 110–118. doi: 10.1016/j.semcdb.2012.12.010
Takio, Y., Kuraku, S., Murakami, Y., Pasqualetti, M., Rijli, F. M., Narita, Y., et al. (2007). Hox gene expression patterns in Lethenteron japonicum embryos—Insights into the evolution of the vertebrate Hox code. Dev. Biol. 308, 606–620. doi: 10.1016/j.ydbio.2007.05.009
Talbot, J. C., Johnson, S. L., and Kimmel, C. B. (2010). hand2 and Dlx genes specify dorsal, intermediate and ventral domains within zebrafish pharyngeal arches. Development 137, 2507–2517. doi: 10.1242/dev.049700
Talbot, J. C., Walker, M. B., Carney, T. J., Huycke, T. R., Yan, Y.-L., BreMiller, R. A., et al. (2012). fras1 shapes endodermal pouch 1 and stabilizes zebrafish pharyngeal skeletal. Development 139, 2804–2813. doi: 10.1242/dev.074906
Tokuoka, M., Satoh, N., and Satou, Y. (2005). A bHLH transcription factor gene, Twist-like1, is essential for the formation of mesodermal tissues of Ciona juveniles. Dev. Biol. 288, 387–396. doi: 10.1016/j.ydbio.2005.09.018
Trainor, P. A., and Krumlauf, R. (2001). Hox genes, neural crest cells and branchial arch patterning. Curr. Opin. Cell Biol. 13, 698–705. doi: 10.1016/S0955-0674(00)00273-8
Trinajstic, K., Boisvert, C., Long, J., Maksimenko, A., and Johanson, Z. (2014). Pelvic and reproductive structures in placoderms (stem gnathostomes). Biol. Rev. 90, 467–501. doi: 10.1111/brv.12118
Trinajstic, K., Sanchez, S., Dupret, V., Tafforeau, P., Long, J., Young, G., et al. (2013). Fossil musculature of the most primitive jawed vertebrates. Science 341, 160–164. doi: 10.1126/science.1237275
Van Valen, L. M. (1982). Homology and causes. J. Morphol. 173, 305–312. doi: 10.1002/jmor.1051730307
Wada, N., Nohno, T., and Kuratani, S. (2011). Dual origins of the prechordal cranium in the chicken embryo. Dev. Biol. 356, 529–540. doi: 10.1016/j.ydbio.2011.06.008
Wagner, G. P. (1989). The biological homology concept. Annu. Rev. Ecol. Syst. 20, 51–69. doi: 10.1146/annurev.es.20.110189.000411
Wagner, G. P. (1994). Homology and the mechanisms of development, in Homology: The Hierarchical Basis of Comparative Biology, ed B. K. IHall (San Diego, CA: Academic Press), 273–299.
Wagner, G. P. (2007). The developmental genetics of homology. Nat. Rev. Genet. 8, 473–479. doi: 10.1038/nrg2099
Wagner, G. P. (2014). Homology, Genes, and Evolutionary Innovation. Princeton, NJ: Princeton University Press.
Wake, D. B., Wake, M. H., and Specht, C. D. (2011). Homoplasy: from detecting pattern to determining process and mechanism of evolution. Science 331, 1032–1035. doi: 10.1126/science.1188545
Wilder, B. G. (1871). The correspondence of the anterior and posterior limbs of vertebrates. Proc. Boston Soc. Nat. Hist. 13, 154–420.
Willmer, P. (2003). Convergence and Homoplasy in the Evolution of Organismal Form, in Origination of Organismal Form - Beyond the Genes in Develpmental and Evolutionary Biology, eds G. Muller and S. Newman (Cambridge: MIT Press), 40–49.
Wilson, M. V. H., Hanke, G. F., and Märss, T. (2007). Paired fins of jawless vertebrates and their homologies across the ‘agnathan’-gnathostome transition, in Major Transitions in Vertebrate Evolution, eds J. S. Anderson and H.-D. Sues (Bloomington, IN: Indiana University Press), 122–149.
Wyman, J. (1860). On anterior and posterior symmetry in the limbs of mammals. Proc. Boston Soc. Nat. Hist. 36, 246–278.
Zhu, M., Yu, X., Ahlberg, P. E., Choo, B., Lu, J., Qiao, T., et al. (2013). A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502, 188–193. doi: 10.1038/nature12617
Keywords: serial homology, segmentation, pharyngeal arch, jaw, fins, limbs, co-option, archetype
Citation: Miyashita T and Diogo R (2016) Evolution of Serial Patterns in the Vertebrate Pharyngeal Apparatus and Paired Appendages via Assimilation of Dissimilar Units. Front. Ecol. Evol. 4:71. doi: 10.3389/fevo.2016.00071
Received: 16 April 2016; Accepted: 02 June 2016;
Published: 16 June 2016.
Edited by:
Stefano Tiozzo, Pierre and Marie Curie University, FranceReviewed by:
Zerina Johanson, Natural History Museum, UKMaja Adamska, Australian National University, Australia
Copyright © 2016 Miyashita and Diogo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Diogo, cnVpLmRpb2dvQGhvd2FyZC5lZHU=
 Tetsuto Miyashita
Tetsuto Miyashita Rui Diogo
Rui Diogo