- 1Department of Behavioural Biology, University of Vienna, Vienna, Austria
- 2Department Biology II, Zoology, Ludwig Maximilian University Munich, Munich, Germany
An astonishing diversity of inner ears and accessory hearing structures (AHS) that can enhance hearing has evolved in fishes. Inner ears mainly differ in the size of the otolith end organs, the shape and orientation of the sensory epithelia, and the orientation patterns of ciliary bundles of sensory hair cells. Despite our profound morphological knowledge of inner ear variation, two main questions remain widely unanswered. (i) What selective forces and/or constraints led to the evolution of this inner ear diversity? (ii) How is the morphological variability linked to hearing abilities? Improved hearing is mainly based on the ability of many fish species to transmit oscillations of swim bladder walls or other gas-filled bladders to the inner ears. Swim bladders may be linked to the inner ears via a chain of ossicles (in otophysans), anterior extensions (e.g., some cichlids, squirrelfishes), or the gas bladders may touch the inner ears directly (labyrinth fishes). Studies on catfishes and cichlids demonstrate that larger swim bladders and more pronounced linkages to the inner ears positively affect both auditory sensitivities and the detectable frequency range, but lack of a connection does not exclude hearing enhancement. This diversity of auditory structures and hearing abilities is one of the main riddles in fish bioacoustics research. Hearing enhancement might have evolved to facilitate intraspecific acoustic communication. A comparison of sound-producing species, however, indicates that acoustic communication is widespread in taxa lacking AHS. Eco-acoustical constraints are a more likely explanation for the diversity in fish hearing sensitivities. Low ambient noise levels may have facilitated the evolution of AHS, enabling fish to detect low-level abiotic noise and sounds from con- and heterosopecifics, including predators and prey. Aquatic habitats differ in ambient noise regimes, and preliminary data indicate that hearing sensitivities of fishes vary accordingly.
Sound Detection in Vertebrates
The inner ear is the primary hearing organ in vertebrates. Typically, tetrapods (amphibians, reptiles, birds, mammals) developed thin membranes on the body surface laterally of the inner ears (tympana or eardrums) to pick up sound pressure changes in the air and transmit these pressure fluctuations via 1-3 tiny auditory ossicles to the inner ear fluids (Ladich, 2010). No basal (e.g., lungfishes, Latimeria) or derived fish taxon developed a tympanum at the outside of the body or a middle ear because no net movement exists between the medium (water) and the animal's body (see discussion in Fritzsch, 1992). Because both fish and water have the same density they move synchronously in the sound field (Hawkins, 1986). Thus, fishes cannot detect sound via an outer tympanum similar to tetrapods but need to detect sound in a fundamentally different way. Fishes analyze the movement of their body in the sound field relative to calcium carbonate structures in the otolith end organs of the ear that have a distinctly greater inertia. These calcareous structures (otoconia and/or otoliths) lag behind in movement relative to the fish in the sound field and thereby stimulate the sensory hair cells by deflecting their ciliary bundles. This physically different process, namely detecting the movement of a tiny calcareous stone, means that fish are unable to detect sound pressure but particle motion instead. Particle motion detection differs from pressure detection in several ways. It limits the detectable frequency range to a few hundred hertz, restricts the detectable sound intensities to higher levels, and also shortens distances over which sounds are detectable (Schuijf and Hawkins, 1976; Fay, 1988; Bradbury and Vehrencamp, 2011).
At least one third of all teleost species developed mechanisms for sound pressure detection similar to tetrapods via tympana. Air-filled cavities within the body such as swim bladders or organs for air-breathing undergo volume changes because air is much more compressible than fluids in any sound field. These volume fluctuations will result in oscillations of the walls, which then function similar to tympana as soon as these membranes transmit their oscillations to the inner ears and improve hearing sensitivities (Alexander, 1966). Structures which enhance hearing in fish by enabling sound pressure detection are termed accessory (ancillary, peripheral) hearing structures, hearing enhancements or hearing specializations. These structures function as pressure-to-particle motion transducers (Hawkins, 1986). Fishes possessing such mechanisms have often been termed “hearing specialists” (Ladich and Popper, 2004; Braun and Grande, 2008; Popper and Fay, 2011). So far, no evidence exists that air-filled cavities evolved purely for sound pressure detection, and therefore we have to assume that sound pressure hearing is a by-product of either buoyancy regulation—which is the primary function of the swim bladder—or air-breathing. Nevertheless, it is quite safe to assume that several taxa of modern bony fishes (teleosts) evolved structures which serve only to connect given gas-filled cavities to the inner ears mechanically (e.g., Weberian ossicles).
Diversity in Auditory Systems in Fish
Cartilaginous (Chondrichthyes) and bony fishes (Osteichthyes) comprise more than one-half of the approximately 55,000 described vertebrate species (Nelson, 2006). Compared to birds and mammals, fishes possess a high diversity in inner ear morphology and accessory hearing structures. These auditory structures result in a diversity of hearing sensitivities, often within members of the same family. In the non-related families Holocentridae (squirrelfishes), Cichlidae, and Sciaenidae (drums and croakers), some genera possess hearing specializations and improved hearing abilities while others lack such auditory enhancements. The functional significance of this diversity is widely unknown and poses one of the main riddles of sensory biology (Ladich and Popper, 2004; Braun and Grande, 2008; Ladich, 2014a,b, 2016; Schulz-Mirbach and Ladich, 2016).
This review provides an overview of the diversity of fish inner ears and accessory hearing structures as well as auditory sensitivities. We further elucidate whether this structural diversity is correlated with hearing abilities. Finally, we discuss three not mutually exclusive hypotheses explaining why enhanced hearing has evolved in modern bony fishes.
Inner Ears
Basic Inner Ear Structure and Function
Despite the high inner ear diversity among cartilaginous (Chondrichthyes) and bony fishes (Osteichthyes), a basic ear structure can be identified: an upper inner ear consisting of three semicircular canals and the utricle (vestibular system), and a lower inner ear comprising the saccule and the lagena (Figures 1–2; Popper, 2011; Popper and Fay, 2011). An endolymphatic duct is present in all fishes (Maisey, 2001). In cartilaginous fishes (Figure 1A) this duct is connected to the surface of the head via a small pore (endolymphatic pore), whereas it ends blindly and may be widely reduced in bony fishes (Maisey, 2001; Lisney, 2010).
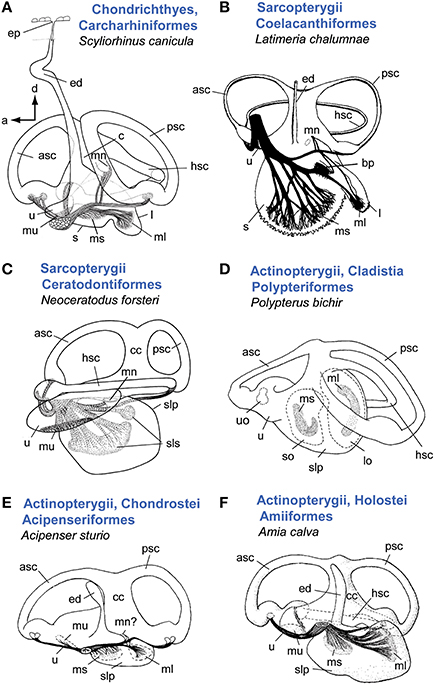
Figure 1. Overview of inner ear diversity in cartilaginous and bony fishes (except teleosts). In sharks (A) and Latimera (B) the saccule and lagena form two sacs, whereas lungfish (C) and non-teleost actinopterygians (D–F) are characterized by a common pouch for these otolith end organs (sacculolagenar pouch). Note, however, that in other species of chondrichthyes (see especially rays and skates and Holocephali; Lisney, 2010), saccule and lagena may be less well-separated (Evangelista et al., 2010). In cartilaginous fishes the endolymphatic duct connects the inner ear to the head surface and thus to the external medium. In Latimeria (B), sturgeon (E), and the bowfin (F), remnants of an endolymphatic duct are visible, but the duct is closed at its dorsal end. A macula neglecta is present in all shown ears except for the bichir (D). Retzius (1881) reported a macula neglecta for sturgeon (E), which was neither confirmed nor refuted in Popper (1978). Note that the macula neglecta in the bowfin is not visible in medial view (see Popper and Northcutt, 1983). In (A, B, D–F), ears are shown in medial view; C in lateral view. Illustrations modified from Retzius (1881), Popper (1978), Popper and Northcutt (1983), Fritzsch (1987), Ladich and Popper (2004). a, anterior; asc, anterior semicircular canal; bp, basilar papilla; c, crus; cc, common canal; d, dorsal; ed, endolymphatic duct; hsc, horizontal semicircular canal; l, lagena; lo, lagenar otolith; ml, macula lagenae; mn, macula neglecta; ms, macula sacculi; mu, macula utriculi; psc, posterior semicircular canal; slp, sacculolagenar pouch; sls, saccular and lagenar striolae, so, saccular otolith; u, utricle; uo, utricular otolith. B with permission from Nature Publishing Group, C, D and A with permission from John Wiley & Sons, Inc.
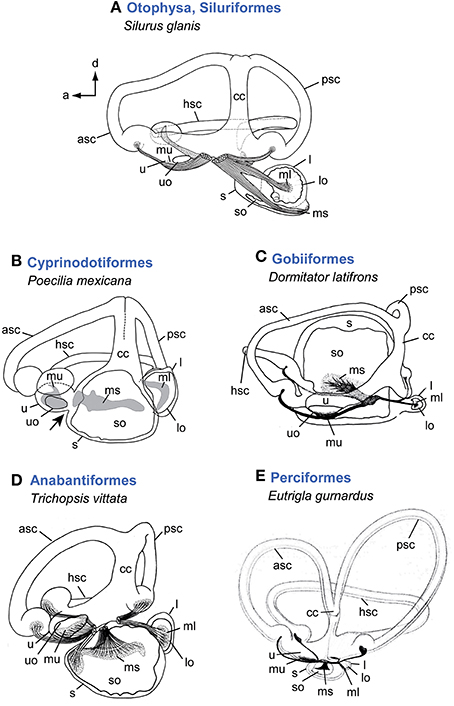
Figure 2. Overview of inner ear diversity in teleost fishes. Otophysan ears have a large round lagena with an asterisk-like otolith and an elongate saccule and a needle-shaped saccular otolith (A). Non-otophysans generally possess a saccule larger than the utricle and lagena (B–E). Cyprinodontiforms (B) show a utricle connected anteriorly to the saccule (indicated by black arrow). In gobiiform fishes (C) the saccule is distinctly large and semicircular canals run around this end organ rather than having an anterodorsal position. The ear of the anabantiform Trichopsis vittata (D) represents a gross morphology found in many non-otophysans. Variation regarding the semicircular canals in teleosts is rare compared to the diversity found in the otolith end organs, especially in the saccule and lagena. Some species, however, such as Eutrigla gurnardus (E) are characterized by distinctly large semicircular canals. All ears are shown in medial view. Illustrations modified from Retzius (1881), Lu and Popper (1998), Ladich and Popper (2001, 2004), and Schulz-Mirbach et al. (2011). For abbreviations see Figure 1.
Each canal and each otolith end organ houses a sensory epithelium. The sensory epithelia in the ampullae of the semicircular canals are termed cristae and are overlain by a gelatinous cupula, whereas those in the otolith end organs (utricle, saccule, lagena) are termed maculae (Popper, 2011).
The maculae of the otolith end organs are overlain by otoconia (except in teleosts) embedded in the otolithic membrane or by a single massive otolith (teleosts), which is connected to the macula via the otolithic membrane (Popper et al., 2005; Casper, 2011; Popper, 2011). In all cartilaginous fishes and some members of the Actinopterygii, a fourth macula, namely the macula neglecta, is present (Casper, 2011). It consists of one or two small patches housing several dozen (e.g., Platt et al., 2004) up to thousands of sensory hair cells (e.g., Corwin, 1981). Similar to the canal cristae, the macula neglecta is overlain by a gelatinous cupula and lacks otoconia or an overlying otolith. The term crista neglecta instead of macula neglecta was therefore suggested by some authors (see Maisey, 2001).
Within the fish's ear, the macula utriculi is mainly oriented horizontally with exception of the lacinia that curves antero-laterally. The macula sacculi and the macula lagenae are both mainly oriented along the vertical plane.
In addition to this differences in the spatial orientation of the whole maculae ciliary bundles of the sensory hair cells are generally arranged in a certain orientation pattern on each macula that is determined according to the position of the eccentrically placed kinocilium within the bundle (Popper, 1976; Hudspeth and Corey, 1977). The orientation pattern of the sensory epithelia of the semicircular canals (= cristae) is similar in all studied vertebrates, and the cristae are thus the most conservative of all sensory epithelia of the inner ear (Mathiesen, 1984). The macula utriculi also shows minimal variation (Platt and Popper, 1981a), indicating that the vestibular part of the inner ear functions similarly in all vertebrates (except perhaps for jawless fishes having just one or two canals; see Ladich and Popper, 2004). The largest diversity in orientation patterns in teleosts occurs on the macula sacculi (Platt and Popper, 1981a; Popper and Coombs, 1982). Different spatial orientation of the whole maculae (“horizontal” vs. “vertical”) as well as different orientation groups of ciliary bundles on the same macula are—among others—hypothesized to enable fish to detect sound emanating from different angles in three-dimensional space (for an overview and discussion of sound source localization in fish see Sisneros and Rogers, 2016).
All fish use the vestibular system to gain information about their body position and motion in three-dimensional space (Straka and Baker, 2011). During head and body motion the movements of the endolymphatic fluid in the semicircular canals deforms the gelatinous cupula which leads to deflection of the ciliary bundles of the sensory hair cells. The canals thus detect body rotation (angular acceleration). The utricle is a highly effective transducer for linear acceleration. The mainly horizontally oriented utricular sensory epithelium senses the inertia provoked by the denser overlying otolith (or otoconial mass) and can thus detect static changes in the position of the head or the body relative to the Earth's gravitation vector (Straka and Baker, 2011). In a few fish taxa such as Clupeidae (herring) it is assumed that the utricle serves in hearing beside its function as gravitation sensor (Popper, 2011). Due to its auditory potential the utricle will be treated as part of the auditory structures.
Diversity in Gross Inner Ear Morphology
Diversity in gross features of the inner ear mainly relates to the (1) size of ears compared to overall size of the fish and the brain, (2) amount of surrounding skull bone or cartilage and potential attachment of the membranous labyrinth to the skull, (3) distance between the two ears and presence/absence of a connection between left and right ears, (4) relative position of upper to lower parts of the ear, i.e., position of the utricle relative to saccule and lagena, (5) size and diameter of the semicircular canals, (6) size ratio among the otolith end organs utricle, saccule, and lagena; and (7) whether saccule and lagena form one or two pouches. For a phylogenetic overview of inner ear diversity and accessory hearing structures see Figures 3, 4.
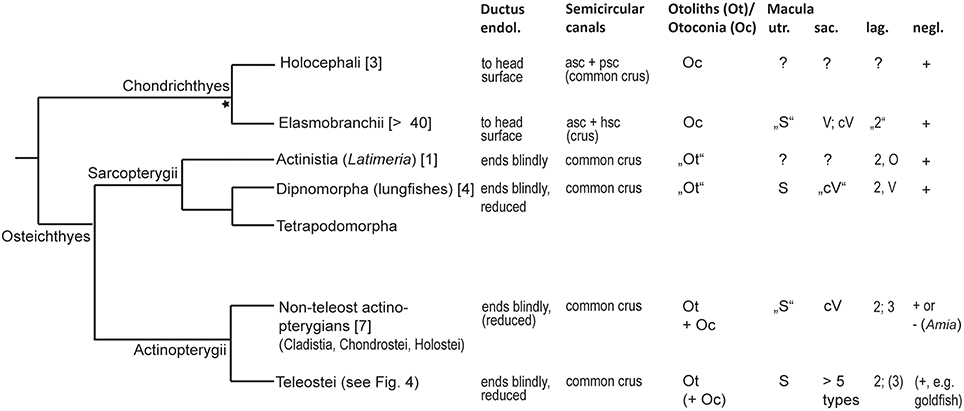
Figure 3. Overview of inner ear characters in cartilaginous and bony fishes. The phylogeny was modified from Betancur et al. (2013), Broughton et al. (2013), and the DeepFin Project (2003-2009), except the split of Chondrichthyes into Holocephali and Elasmobranchii indicated by the asterisk (see Nelson, 2006). For a detailed overview of inner ear characters and species see Schulz-Mirbach and Ladich (2016, Table 2). Numbers given in brackets indicate the number of species studied with respect to inner ear morphology. Ductus endol., ductus endolymphaticus; asc, anterior semicircular canal; psc, posterior semicircular canal; Macula utriculi (utr.): S, standard pattern, i.e., radially oriented ciliary bundles on the cotillus and opposing ciliary bundles in the striola region; Macula sacculi (sac.): cV, curved vertical, i.e., horizontal groups in the anterior portion are due to ciliary bundle orientation that follows the macula curvature. Macula lagenae (lag.): Numbers indicate the number of orientation groups; O, opposing groups of ciliary bundles; V, vertical ciliary bundle orientation. In most species only numbers are given because orientations of ciliary bundles vary to different degrees in a gradual manner along the macula from horizontal, oblique to vertical directions. Macula neglecta (negl.): Note that only presence/absence of this macula type is indicated. Orientation patterns of ciliary bundles are illustrated in Figure 11.
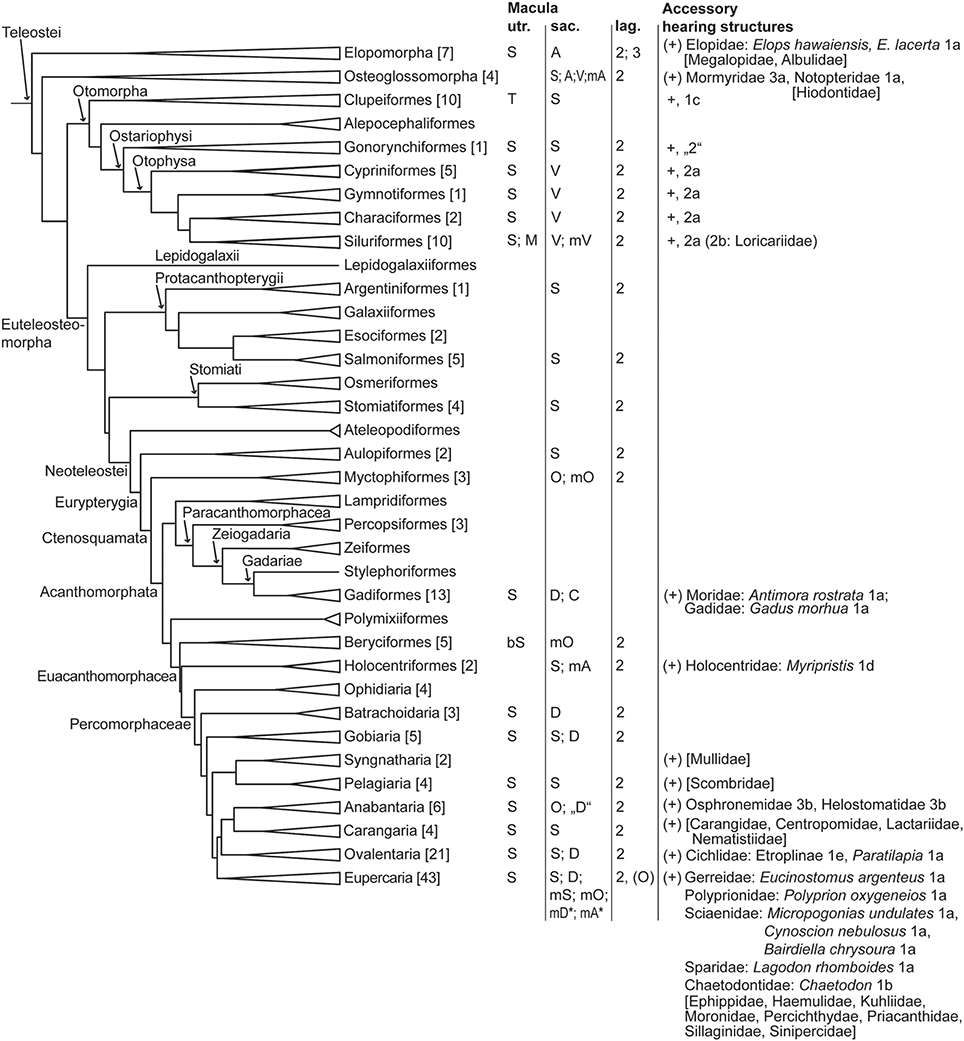
Figure 4. Overview of orientation patterns of ciliary bundles on the maculae and diversity of accessory hearing structures in different teleost groups. The phylogeny was modified from Betancur et al. (2013), Broughton et al. (2013), and the DeepFin Project (2003-2009). For a more detailed overview and list of references see Schulz-Mirbach and Ladich (2016, Table 2). Family names in brackets indicate taxa which possess accessory hearing structure but for which ears and/or hearing abilities have not yet been studied (for details about accessory hearing structures of these taxa see Braun and Grande, 2008). Numbers given in brackets indicate the number of species studied with respect to inner ear morphology. Macula utriculi: S, standard pattern, i.e., radially oriented ciliary bundles on the cotillus and opposing ciliary bundles in the striola region; T, tripartite macula consisting of a macula anterior, macula media and a macula posterior with all three parts showing an opposing pattern of ciliary bundles; M, modified macula shape in Ariopsis felis, the macula forms a narrow band around the utricular otolith, no ventral macula part (cotillus) is present; bSt, in Melamphaidae the macula displays a bilobate striola region. Macula sacculi (see also Figure 8): S, standard pattern; D, dual pattern; A, alternating pattern; O, opposing pattern; V, 2 vertical groups; modified patterns: mS, in Arothron hispidus, 1 vertical group in the anterodorsal part; mA, in Chitala chitala (Figure 7E) and Myripristis murdjan (Figure 7G); mD*, as found in Comephorus baicalensis, Cottocomephorus alexandrae, and Paracottus knerii (lake Baikal; Sapozhnikova et al., 2007); mA* as found in Comephorus dybowski (lake Baikal, Sapozhnikova et al., 2007); mO, in Myctophidae, Melamphaidae and Bairdiella chrysoura (Figure 7H); mV, in Bunocephalus coracoideus and Acanthodoras spinosissimus, 4 vertical groups in the anterior part and 2 vertical groups on the remaining macula; C, complex pattern in Antimora rostrata (Figure 7F). Macula lagenae: Numbers indicate the number of orientation groups; O, opposing groups of ciliary bundles; V, vertical ciliary bundle orientation. In most species only numbers are given because orientations of ciliary bundles vary to different degrees in a gradual manner along the macula from horizontal, oblique to vertical directions. Types of accessory hearing structures (see Figure 12 for types 1-3): 1a-b, anterior swim bladder extensions approach or abut skull in region of ear; 1c, anterior swim bladder extension penetrates skull, contacting the utricle; 1d, anterior swim bladder extension penetrates skull, contacting the saccule; 1e, anterior swim bladder extension penetrates skull, complex etropline type; “2,” Protoweberian coupling?; 2a-b, otophysic connection via Weberian apparatus; 3a, anterior part of swim bladder extension penetrates skull but is separated from the main swim bladder; 3b, suprabranchial chamber close to ear. For morphological details in Polyprion oxygeneios see Caiger et al. (2013). Additional laterophysic connections: 1b-c, 2b.
Some deep-sea or cave fishes (teleosts), for example, show exceptionally large ears compared to the brain (Poulson, 1963; Fine et al., 1987), whereas some epipelagic teleost species have extremely small ears and otoliths (Paxton, 2000; Song et al., 2006). Also the amount of encapsulation and attachment of the ear to the skull differs considerably. Certain teleosts such as poeciliids show rather “free” ears with encapsulation limited to the semicircular canals (Schulz-Mirbach et al., 2011), whereas the non-teleost actinopterygian Amia calva (Popper and Northcutt, 1983) or elasmobranchs display almost full encapsulation of the ears (Maisey, 2001). In other species, attachment of one or several otolith end organs to the skull is associated with the presence of accessory hearing structures as found in the notopterid Chitala chitala, the morid Antimora rostrata, or the cichlid Etroplus maculatus (Coombs and Popper, 1982; Deng et al., 2011; Schulz-Mirbach et al., 2013). This coupling of an otolith end organ to the bone may play a role for effective sound transmission to the ears via the specialized swim or gas bladder (see discussion in Deng et al., 2011).
A connection between left and right ears is known from the otophysans (saccules communicate via the transverse canal; Wohlfahrt, 1932; von Frisch, 1936) and the coelacanth Latimeria (ears are connected at the junction between saccule and lagena to one another via the canalis communicans; Bernstein, 2003). While in otophysans this connection may improve effective sound transmission via the swim bladder and the Weberian apparatus to both ears (von Frisch, 1938; cf. Finneran and Hastings, 2000), it is completely unclear whether the junction between ears plays a role in audition in Latimeria due to the lack of physiological data.
Other aspects of diversity relate to the morphology of the semicircular canals (see especially elasmobranchs), the size ratio of semicircular canals to the otolith end organs, or the size ratio among the three otolith end organs. Unlike in Holocephali and bony fishes, Elasmobranchii do not have a connection between the anterior and posterior semicircular canals (common crus); instead the anterior and horizontal canals are connected to each other via a crus (Figure 1A; Maisey, 2001; Evangelista et al., 2010). According to Maisey (2001) the posterior canal in elasmobranchs is thus rather a circuit than a semicircular canal. Within this group, species display variability in the presence/absence of the canal ducts that connect the semicircular canals to the otolith end organs and thus may differ in whether the semicircular canals are directly connected to the saccule, in the length of the endolymphatic duct, and in the size of the saccule with respect to the utricle (Evangelista et al., 2010). In teleosts, diversity in semicircular canals is restricted to differences in canal thickness and canal radii. Sea horses (Syngnathidae, Syngnathiformes), for example, display “compact” ears with almost rectangular instead of rounded semicircular canals (Retzius, 1881). Moreover, several unrelated species of flying fishes (Dactylopterus volitans, Dactylopteridae, Syngnathiformes; Exocoetus volitans, Beloniformes) show distinctly large semicircular canals and extremely small otolith end organs (Retzius, 1881). Large semicircular canals are also present in the angler Lophius piscatorius (Lophiiformes) and the gray gurnard Eutrigla gurnardus (Perciformes; Figure 2E; Retzius, 1881). The functional meaning of these enlarged semicircular canals remains to be studied (see also discussion in Evangelista et al., 2010).
Whereas the upper inner ear (semicircular canals and utricle) is rather conservative across fishes (but see elasmobranchs), diversity is higher in the lower inner ear (saccule and lagena). In Holocephali (Retzius, 1881; de Burlet, 1934), lungfishes (Retzius, 1881; Platt et al., 2004), and non-actinopterygian teleosts (Popper, 1978; Popper and Northcutt, 1983; Mathiesen and Popper, 1987; Lovell et al., 2005) saccule and lagena form one pouch, whereas in the coelacanth Latimeria (Fritzsch, 1987, 2003), elasmobranchs (e.g., Retzius, 1881; Ladich and Popper, 2004), and teleosts (e.g., Ladich and Popper, 2004; Popper and Schilt, 2008) these otolith end organs form two interconnected sacs. The saccule is often the largest of the three otolith end organs (Figures 1A–B, 2B-E), with teleost orders including Gobiiformes (Figure 2C; e.g., Retzius, 1881; Popper, 1981), Ophidiiformes (e.g., Parmentier et al., 2001, 2002; Kéver et al., 2014), and Batrachoidiformes (e.g., Cohen and Winn, 1967) representing members with one of the largest saccules compared to the tiny utricle and lagena. In these taxa, the semicircular canals run around the large saccule rather than being located dorsally to it. Most otophysans are characterized by having a lagena as large as or larger than the elongate saccule (Figure 2A; Popper and Platt, 1983). In ariid catfishes, however, the utricle is distinctly larger than both saccule and lagena (Popper and Tavolga, 1981).
Otoconia and Otoliths
In cartilaginous fishes, the maculae (except the macula neglecta) are overlain by numerous tiny otoconia embedded in a gelatinous/fibrous matrix (Tester et al., 1972). These otoconia can be exogenous (sand grains) and enter the ear via the endolymphatic duct (see Casper, 2011) and/or endogenous and can be made of calcite, aragonite, vaterite, or calcium carbonate monohydrate in elasmobranchs or solely of aragonite in chimaeras (Carlström, 1963; Gauldie et al., 1987; Mulligan and Gauldie, 1989; Mulligan et al., 1989).
In lungfishes, the single “otolith” (Protopterus, Platt et al., 2004) or the “lapillus” and “sagitta” (Neoceratodus, Gauldie et al., 1986a) consist of a firm aggregation of aragonitic and calcitic otoconia (Carlström, 1963; Gauldie et al., 1986a). The Latimeria ear apparently contains only one large calcitic-aragonitic “saccular otolith” (Carlström, 1963; Rosauer and Redmond, 1985).
Non-teleost actinopterygians have both otoliths and otoconia that overlie the maculae of the otolith end organs (Carlström, 1963; Popper and Northcutt, 1983; Mathiesen and Popper, 1987; Lychakov, 1995). In sturgeons otoliths and otoconia are made up of vaterite. In bichir, bowfin and gar, however, otoliths are aragonitic whereas otoconia are vateritic (Carlström, 1963; Rosauer and Redmond, 1985).
In teleosts, the maculae of the otolith end organs are each overlain by a single massive calcium carbonate biomineralisate, the otolith that apposes material according to a daily rhythm (Pannella, 1971). The otoliths of the utricle and saccule are composed of aragonite, while the lagenar otolith consists of vaterite. Calcite is only rarely found in otoliths (e.g., Gauldie, 1993; Oliveira and Farina, 1996). The simultaneous presence of otoliths and (aragonitic) otoconia in teleosts has been reported for only a few species (Gauldie et al., 1986b). In contrast to the tiny otoconia in non-teleost fishes, otoliths—especially that of the saccule—possess a species-specific shape (e.g., Nolf, 1985). The effects of different shapes on otolith motion relative to the macula are still widely unknown (Popper et al., 2005). The few experimental and theoretical studies, however, indicate that otolith motion differs depending on its shape and is more complex than just a simple forth and backward movement (Sand and Michelsen, 1978; Krysl et al., 2012).
Macula Diversity: Macula Shape and Orientation Patterns of Ciliary Bundles
Generally, the maculae of the otolith end organs are separated. In lungfishes, the macula sacculi and macula lagenae form a continuum, the so-called sacculolagenar macula in which two regions of high hair cell densities (striolas) are separated by areas of lower hair cell densities (Figures 1C, 6C; Platt et al., 2004). Such a sacculolagenar macula is unique among bony fishes. Holocephali apparently possess a similar joint sacculolagenar macula (de Burlet, 1934; Ladich and Popper, 2004; see also illustration of the holocephalid Chimaera monstrosa by Retzius, 1881), but recent detailed studies underpinning this assumption are lacking. Overall, data about orientation patterns of the maculae are scarce for the coelacanth Latimeria (Platt, 1994) as well as for cartilaginous fishes (Lowenstein et al., 1964; Barber and Emerson, 1980; Lovell et al., 2007). Most studies on macula morphology in Chondrichthyes focused on the macula neglecta in Elasmobranchii (Corwin, 1981, 1989; Myrberg, 2001; Casper, 2011). It therefore remains unclear if cartilaginous fishes show variability not only with regard to gross inner ear morphology (Evangelista et al., 2010) and the macula neglecta but also with regard to the maculae of the otolith end organs. Moreover, to our knowledge data on the macula morphology in Holocephali is completely lacking (see Lisney, 2010).
Macula utriculi
Of the three “otolithic” maculae, the macula utriculi is the most conservative one not only across fishes but also across vertebrates in general (see e.g., Figures 5B,D,E; Platt and Popper, 1981a). The macula is bowl shaped displaying (1) the main body—namely the cotillus, which lies on the ventral floor of the utricle—and shows radially oriented ciliary bundles, (2) a striola region in the anterior part, displaying two groups of ciliary bundles with opposing (“face-to-face”) orientation and (3) in some taxa an anterolateral element, the lacinia (Figures 5A,D,E,H,I; Platt and Popper, 1981a).
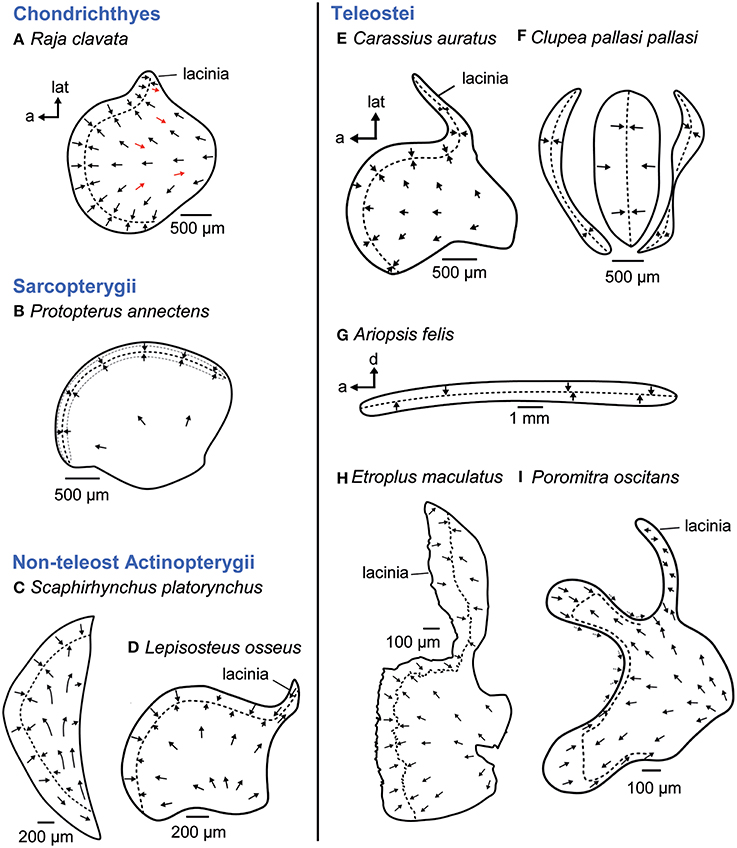
Figure 5. Overview of the diversity of macula shape and the orientation patterns of ciliary bundles on the macula utriculi. Across most fish taxa (B, D, E) and even vertebrates in general, the shape and orientation pattern of the macula utriculi is conserved; it has a bowl shape often including a lacinia and the typical radial orientation of the ciliary bundles on the cotillus as well as opposing (face-to-face) orientated ciliary bundles in the striola region (= region around the stippled line). Cartilaginous fishes, however, display some reversely oriented ciliary bundles on the cotillus (A, see red arrows), and a half-moon shaped macula lacking a lacinia is present in sturgeons (C). In teleost fishes, the goldfish (E) shows the conservative shape and orientation pattern (see also D). Modifications thereof are found in clupeiform fishes (F, tripartite macula) or ariid catfishes, whose ribbon-like macula lacks the cotillus (G). The cichlid Etroplus maculatus (H) has a distinctly enlarged lacinia, while enlargement of the striola region results in a bilobate shape in melamphiid fishes (I). The maculae in (E–H) stem from species that possess accessory hearing structures. Illustrations modified from Barber and Emerson (1980), Deng et al. (2013), Mathiesen and Popper (1987), Platt (1977), Platt et al. (2004), Popper (1978), Popper and Platt (1979), Popper and Tavolga (1981), and Schulz-Mirbach et al. (2014). a, anterior; d, dorsal; lat, lateral.
Among fishes, some exceptions to this shape and orientation pattern of the macula utriculi are found. In cartilaginous fishes, for example, the studies by Lowenstein et al. (1964) and Barber and Emerson (1980) indicated that ciliary bundles with opposing orientation are interspersed in the radial orientation pattern of ciliary bundles on the cotillus (Figure 5A). In addition, the macula utriculi of the lesser spotted dogfish Scyliorhinus canicula seems to lack a striola region (Lovell et al., 2007).
Modified maculae utriculi are also found in the non-teleost actinopterygian shovel nose sturgeon (Scaphirhynchus platorynchus), which displays a half-moon shaped macula lacking a lacinia (Figure 5C; Popper, 1978) or in the ariid catfish Ariopsis felis, whose macula utriculi is reduced to a ribbon-like structure lacking a cotillus and which curves around the exceptionally large utricular otolith like an equatorial band (Figure 5G; Popper and Tavolga, 1981). Further modifications of the macula utriculi in teleosts relate to the striola region, which is uniquely bilobate in Melamphaidae (deep-sea fishes; Figure 5I; Deng et al., 2013). In the cichlid E. maculatus the lacinia is exceptionally large and three-dimensionally curved (Figure 5H; Schulz-Mirbach et al., 2014).
The most derived macula utriculi characterizes the whole order Clupeiformes (see Platt and Popper, 1981a,b). The unique tripartite macula (Figure 5F; Popper and Platt, 1979; Platt and Popper, 1981b; Higgs et al., 2004) is in part (middle and posterior macula) overlain by an also highly modified utricular otolith (Wohlfahrt, 1936; O'Connell, 1955). This otolith has a tetrahedral shape and thin extensions in anterolateral and ventral directions instead of the “stone-like” appearance present in most teleosts (Wohlfahrt, 1936; Assis, 2005).
Macula sacculi
The macula sacculi in cartilaginous fishes is elongate without a distinction into a wider ostial and a narrower caudal macula region that is otherwise typical of many teleost species. Mainly two vertical groups of ciliary bundles are present. In the anterior portion these vertically oriented bundles are brought into a new horizontal orientation by upwards curving of the macula in this region (Figure 6B; Lowenstein et al., 1964; Corwin, 1981; Lovell et al., 2007; but see Figure 6A; Barber and Emerson, 1980).
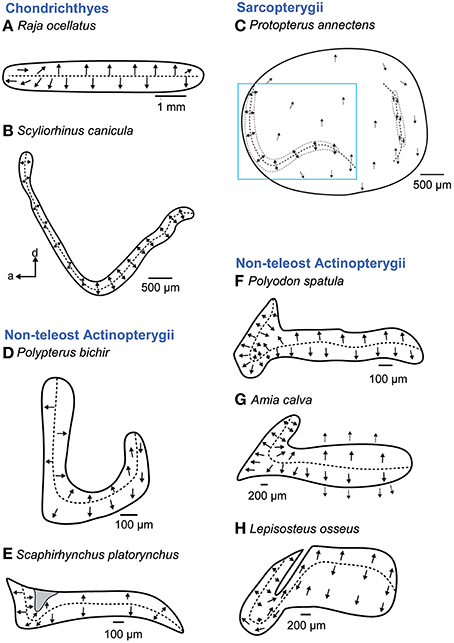
Figure 6. Overview of the diversity of macula shape and the orientation patterns of ciliary bundles on the macula sacculi in non-teleost fishes. The macula sacculi in non-teleost fishes shows two main orientation groups of ciliary bundles which are brought into a horizontal orientation by upwards curving of the anterior macula portion (B–H) except in rays (A). The shaded gray area in (E) depicts a special area of supporting cells. Arrows around the saccular and lagenar striola regions in (C) and around the macula in (G) indicate the orientation of ciliary bundles in regions with low densities of sensory hair cells. The rectangle in C indicates the striola region of the macula sacculi. Illustrations modified from Barber and Emerson (1980), Lovell et al. (2005, 2007), Mathiesen and Popper (1987), Platt et al. (2004), Popper (1978), and Popper and Northcutt (1983). a, anterior; d, dorsal.
A similar transition from a ventral to a more horizontal orientation pattern of ciliary bundles in the anterior macula region is also characteristic in non-teleost actinopterygians (Popper and Fay, 1993). In these fishes, the macula sacculi is hook shaped (Polypterus bichir; Figure 6D) or has a hook-shaped anterior part (Figures 6E–H). In the anterior portion, ciliary bundle orientation follows the curvature of the closest macula margin, thereby creating horizontal groups. In the bowfin Amia calva (Figure 6G), the anterior portion of the macula sacculi has a distinct 3D curvature bringing the ciliary bundles in a new spatial orientation (Popper and Northcutt, 1983).
In teleosts, five main orientation patterns have been described (Figure 7; Popper and Coombs, 1982). Four of them show vertical and “true” horizontal orientation groups and are termed standard, dual, opposing, or alternating patterns; the fifth pattern type is characterized by vertical orientation groups only (Popper and Coombs, 1982). The standard (Figures 7B,I,J) and the dual patterns are mainly typical of species that lack accessory auditory structures (Platt and Popper, 1981b; Popper and Coombs, 1982) or in which these structures are not connected to the saccule; the standard pattern of the macula sacculi, for example, is found in clupeiform fishes (Figure 7B), whereas a highly modified macula utriculi in these fishes (Figure 5F) is associated with the connection of the gas bladder to the utricle (e.g., Denton and Gray, 1979; Platt and Popper, 1981b). In contrast, some species whose accessory auditory structures approach the saccule show a dual pattern such as the cichlid E. maculatus (Schulz-Mirbach et al., 2014) or the standard pattern like some sciaenid species (Figures 7I,J). In some teleost groups, however, the presence of accessory auditory structures correlates with modified orientation patterns. Examples include Notopteridae and Mormyridae (both Osteoglossiformes) or otophysans, which have highly modified maculae sacculi, displaying the vertical pattern in mormyrids (Figure 7A) and otophysans (Figure 7C) or a complex trilobate macula sacculi with a modified alternating pattern in the Clown knifefish C. chitala (Notopteridae; Figure 7E).
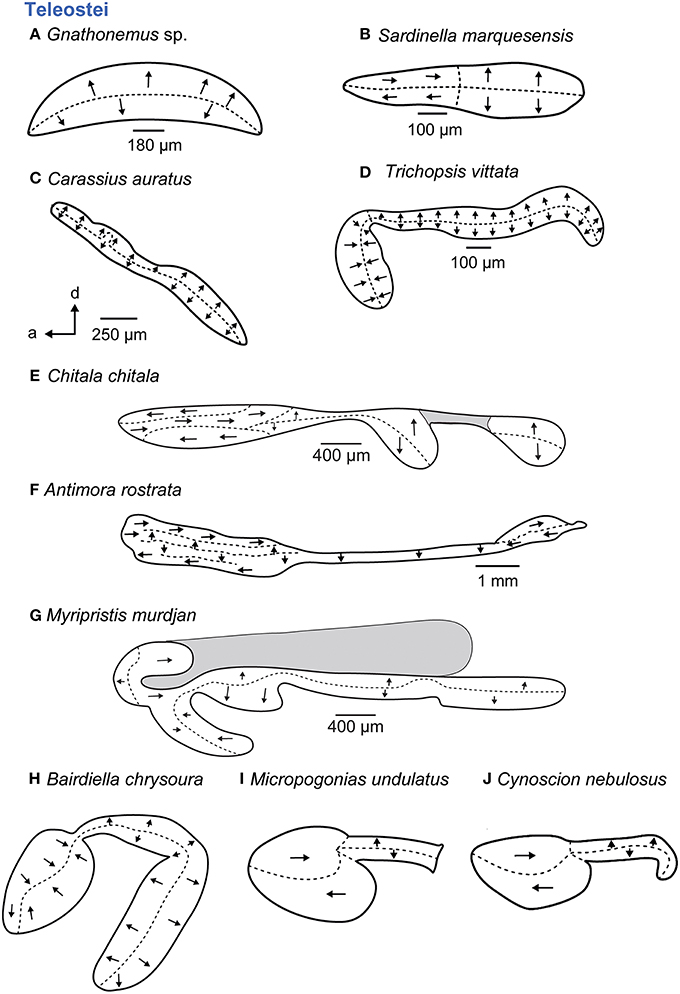
Figure 7. Overview of the diversity of macula shape and the orientation patterns of ciliary bundles on the macula sacculi in teleost fishes. The shaded gray areas in (E,G) depict special areas of supporting cells. All maculae stem from species that possess accessory hearing structures (A–J). For the maculae in (H–J) no scale bars were given in the original publications (Ramcharitar et al., 2001, 2004). Illustrations modified from Coombs and Popper (1982), Deng et al. (2011), Ladich and Popper (2001), Platt (1977), Popper (1977, 1981), Platt and Popper (1981a), Ramcharitar et al. (2001, 2004), and Platt et al. (2004). a, anterior; d, dorsal.
Members of deep-sea fishes (Myctophidae, Bregmacerotidae, Macrouridae, Moridae, Gadidae, Melamphaidae, Opisthoproctidae, Gonostomatidae, Melanocetidae, or Holocentridae) show some of the most remarkable modifications, especially with respect to the maculae (Popper, 1977, 1980; Deng, 2009; Deng et al., 2011, 2013). Several species are marked by complex (“unique”) orientation patterns on the macula sacculi (Figures 7F,G) and also possess accessory auditory structures such as anterior swim bladder extensions, for example in A. rostrata (Deng et al., 2011) and species of the genus Myripristis (Nelson, 1955; Popper, 1977).
Given the diversity of orientation patterns on the teleost macula sacculi the question arises what the macula sacculi looked like in the ancestor of the teleosts (Popper and Fay, 1993). Tetrapods have only two “vertical” groups on the macula sacculi and this may also hold true for cartilaginous fishes, non-teleost actinopterygians and lungfishes: the horizontal groups in these fishes are classified to be no “true” horizontal groups because originally vertically oriented ciliary bundles simply follow the curvature of the closest macula margin, gradually leading to an increased horizontal-like orientation (Figure 8; Popper and Platt, 1983, Popper and Fay, 1993). Two alternative hypotheses have been discussed (Popper and Platt, 1983). First, the vertical pattern is an ancestral pattern that was retained in otophysans and mormyrids, whereas in the remaining teleosts true horizontal groups evolved at least seven times independently. The second hypothesis assumes that the ancestral teleost condition is the pattern including vertical and horizontal groups and that horizontal groups were lost twice, in otophysans and mormyrids. If the second hypothesis applies—which is the more parsimonious one—the vertical pattern in otophysans and mormyrids may have convergently evolved due to similar selection pressures (Popper and Platt, 1983). The vertical pattern is the constant element in each of the five different orientation patterns on the macula sacculi in teleosts (Popper, 1981), and the vertical pattern is also found in Chondrichthyes, lungfishes, and non-teleost actinopterygians (see above; Popper and Fay, 1977; 1993). Accordingly, it may further be assumed that the vertical pattern on the macula sacculi is the basic vertebrate pattern on this sensory epithelium (Mathiesen and Popper, 1987): it did not experience diversification—including the “invention” of true horizontal groups—before the emergence and diversification of the teleosts.
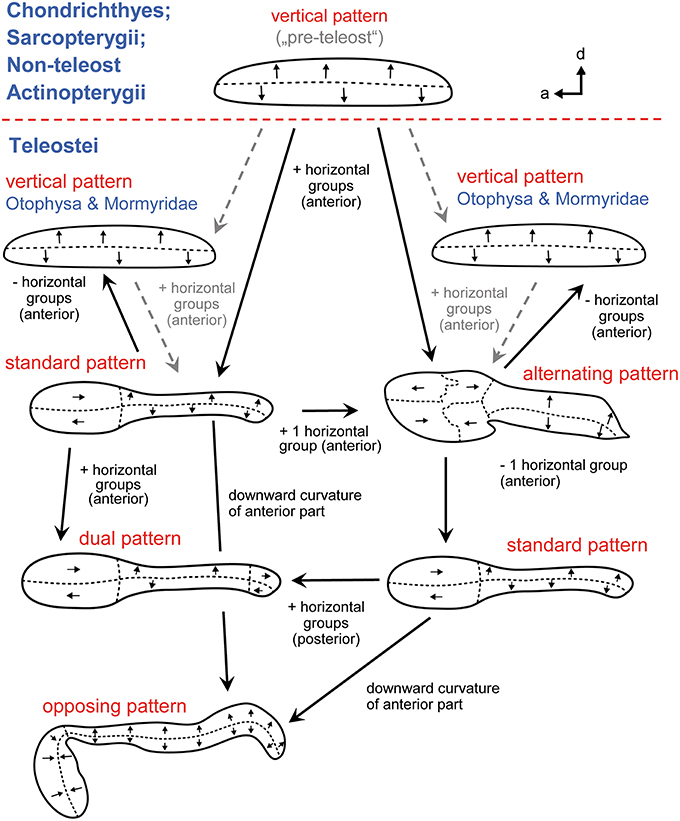
Figure 8. The main ciliary bundle orientation patterns on the macula sacculi in teleosts and how the patterns may be derived from one another (see also Popper and Fay, 1993). Arrow tips point in the direction of the kinocilia, indicating the orientation of the ciliary bundles in the respective area; the dashed lines separate different orientation groups. Addition of two or three horizontally oriented groups of ciliary bundles results in the standard or alternating patterns, respectively. From the standard pattern the dual pattern can be derived by adding horizontal groups in the posterior region; in the opposing pattern the anterior macula portion is ventrally bent while the orientation of the horizontal groups is retained. The standard pattern may also be obtained by removing one horizontal group from the alternating pattern. The vertical patterns in otophysans and mormyrids may be derived by removing the horizontal groups from the standard or the alternating patterns. The five patterns are modified from Popper and Coombs (1982) and Popper and Schilt (2008). a, anterior; d, dorsal.
The five orientation groups can be derived from one another if one either adds two or three horizontal groups to the vertical pattern (resulting in the standard or the alternating pattern) or removing the horizontal groups, leading to the vertical pattern (Figure 8). From the standard pattern (1) the dual pattern can be obtained by adding two horizontal groups in the posterior portion and (2) the opposing pattern can be created by bending the anterior macula downwards in ventral direction while ciliary bundles retain their horizontal orientation in this area. Alternatively, the standard pattern can emerge from an alternating pattern when one (the most anterodorsal) horizontal group is lost. Only genetic studies could unravel how orientation groups form during ontogeny, leading to the different orientation patterns. Knowledge about underlying genetic processes of pattern formation is increasing (Duncan and Fritzsch, 2012; Sienknecht et al., 2014) and is likely to shed new light on the evolution of different orientation patterns in different lineages.
Macula lagenae
In cartilaginous fishes (Figures 9A–B), sarcopterygians (Figure 9C; Platt, 1994; Platt et al., 2004), non-teleost actinopterygians (Figures 9D–H; Popper, 1978; Popper and Northcutt, 1983; Mathiesen and Popper, 1987; Lovell et al., 2005), and teleosts (Figures 10A–H; for an overview see Platt and Popper, 1981b), the macula lagenae is crescent or half-moon shaped and contains two main orientation groups. In rays, these differently orientated ciliary bundles are less strictly organized into two separate groups (Figure 9A; Barber and Emerson, 1980; Lowenstein et al., 1964), whereas sharks seem to show two distinct groups on their macula lagenae (Figure 9B; Lovell et al., 2007). In contrast to bony fishes, the few studies on the macula lagenae in cartilaginous fishes (Barber and Emerson, 1980; Lovell et al., 2007) indicate that the posterior “orientation group” on the macula shows ciliary bundles oriented in anterodorsal direction while in bony fishes ciliary bundles of the posterior orientation group mainly point in posteroventral direction (compare Figures 9A,B with Figures 9C–E, G–H, 10A,C,E–H).
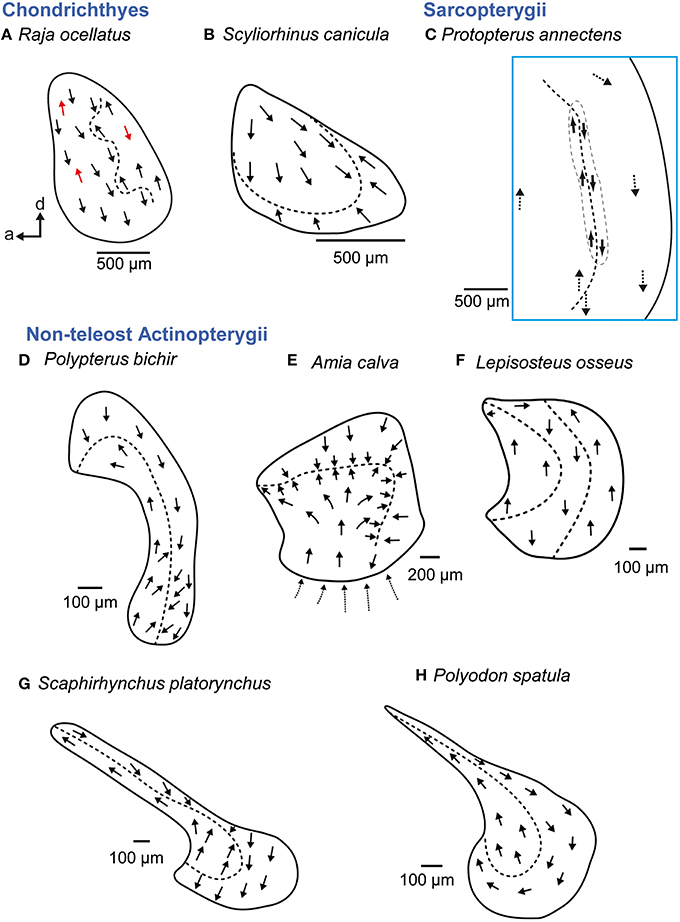
Figure 9. Overview of the diversity of macula shape and the orientation patterns of ciliary bundles on the macula lagenae in non-teleost fishes. The macula lagena is characterized by two main orientation groups of ciliary bundles (B–E, G–H) except in rays (A), whose ciliary bundles show opposing directions across the whole macula (indicated by red arrows) or in gar (F), which have three orientation groups on the macula. Arrows around the saccular and lagenar striola regions in (C) and around the macula in (E) indicate the orientation of ciliary bundles in regions with low densities of sensory hair cells. Illustrations modified from Popper (1978), Barber and Emerson (1980), Popper and Northcutt (1983), Mathiesen and Popper (1987), Platt et al. (2004), and Lovell et al. (2005, 2007). a, anterior; d, dorsal.
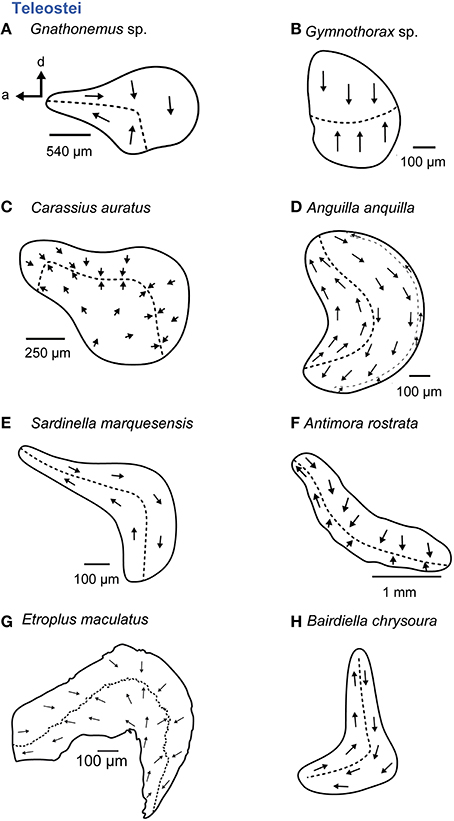
Figure 10. Overview of the diversity of macula shape and the orientation patterns of ciliary bundles on the macula lagenae in teleost fishes. The macula lagena is characterized by two main orientation groups of ciliary bundles (A–C, E–H) with exception of the eel (D) having three orientation groups on the macula. (A,C, E–H) These maculae stem from species that possess accessory auditory structures. For the macula in P no scale bar was given in the original publication (Ramcharitar et al., 2004). Illustrations modified from Platt (1977), Popper (1979; 1981), Platt and Popper (1981a), Mathiesen (1984), Ramcharitar et al. (2004), and Deng et al. (2011) and Schulz-Mirbach et al. (2014). a, anterior; d, dorsal.
Non-teleost actinopterygians show a considerable diversity in the shape of the macula lagenae (Figures 9D–H). The macula lagenae is almost as large as or even larger than the macula sacculi (except in Amia), which contrasts the condition in many teleost species (Platt and Popper, 1981a; Ladich and Popper, 2004). In addition, Amia calva exhibits a striola-like region that resembles that of the utricular maculae (Popper and Northcutt, 1983), and Lepisosteus osseus displays three instead of two orientation groups (Mathiesen and Popper, 1987). Three groups are also found in some members of the Elopomorpha (Anguilla anguilla; Figure 10D; Mathiesen, 1984), especially in some deep-sea elopomorphs (Buran et al., 2005) or the chaetodontid Chaetodon miliaris (Popper, 1977); but in these teleosts the third orientation group is restricted to a very narrow band at the posterior margin of the macula lagenae (Mathiesen and Popper, 1987).
Some teleost taxa with accessory auditory structures such as mormyrids (Popper, 1981), otophysans (e.g., Popper and Platt, 1983), and the cichlid E. maculatus (Schulz-Mirbach et al., 2014) possess a large macula lagenae that may be even larger than the maculae sacculi (Popper et al., 2003). In addition, the maculae lagenae of otophysans tend to be oriented more along the antero-posterior axis than stretching along a dorso-ventral or posteroventral to anterodorsal axis (compare Figures 10A,C,G with Figures 10E,H).
Macula neglecta
In cartilaginous fishes a macula neglecta is always present. It contains one patch with “randomly” orientated ciliary bundles in benthic species (Figure 11A) or two patches with a preferred orientation on each of the patches in more pelagic species (Figure 11B; Corwin, 1981; 1989; Myrberg, 2001). In bony fishes, the macula neglecta—if present—is smaller than in cartilaginous fishes (Corwin, 1989). Latimeria (Fritzsch, 1987) and lungfishes (maybe except Neoceratodus) possess a macula neglecta: it is a single patch in Protopterus, with ciliary bundles uniformly oriented along the antero-posterior axis (Figure 11C; Platt et al., 2004). In non-teleost actinopterygians and teleosts possessing a macula neglecta, it consists of two patches with a preferred orientation of ciliary bundles on each patch (Figures 11D–F; Platt, 1977; Mathiesen, 1984; Mathiesen and Popper, 1987). Thus, if a macula neglecta is present in Actinopterygii, the macula structure and orientation patterns seem to be constant across different species (Mathiesen and Popper, 1987).
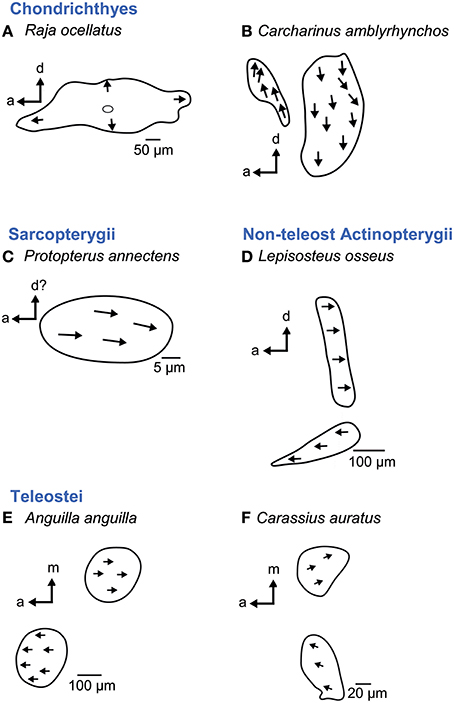
Figure 11. Overview of the diversity of the macula neglecta in cartilaginous (A–B) and bony fishes (C–F). This macula consists of either one patch (A, C) or two patches (B, D–F). Ciliary bundles show no preferred orientation (A) or are oriented in one direction on the patch (C) or on either patch if two patches are present (B, D–F). Note that if a macula neglecta is present in Actinopterygii, the macula structure and orientation patterns seem to be constant across different species (D–F). For the macula in B no scale bar was given in the original publication (Corwin, 1989). Illustrations modified from Platt (1977), Mathiesen (1984), Barber et al. (1985), Mathiesen and Popper (1987), Corwin (1989), and Platt et al. (2004). a, anterior; d, dorsal; m, medial.
Interestingly, the macula neglecta in elasmobranchs is located in the canal duct of the posterior semicircular canal dorsal to the saccule (e.g., Corwin, 1989; Casper, 2011), whereas it is situated in the posterior part of the utricle near the common crus in holocephalans and actinopterygians (Maisey, 2001).
Accessory Hearing Structures and Auditory Sensitivities
Fishes possess a large variety of gas-filled cavities within the body and the swim bladder is certainly the most widespread among these. Swim bladders primarily help to generate the buoyancy necessary for fishes to hover at particular water depths (Alexander, 1966). Only cartilaginous fishes (sharks, rays, chimaeras) and bottom-dwelling fishes such as flatfish or sculpins lack swim bladders. These groups therefore lack a pressure-to-particle motion transducer, which limits their hearing sensitivities accordingly (Figure 12E). Nevertheless, experimental studies on the lemon shark Negaprion brevirostris indicate that sharks may detect sound in parallel in two different ways, giving them more directional information. The non-otolithic channel enables detecting sound directly via loose tissue covering dorsal openings in the skull (parietal fossa) and stimulating the macula neglecta. The otolithic channel enables sound detection indirectly via relative motion between the otoconial mass and ciliary bundles in the saccule (Corwin, 1981, 1989).
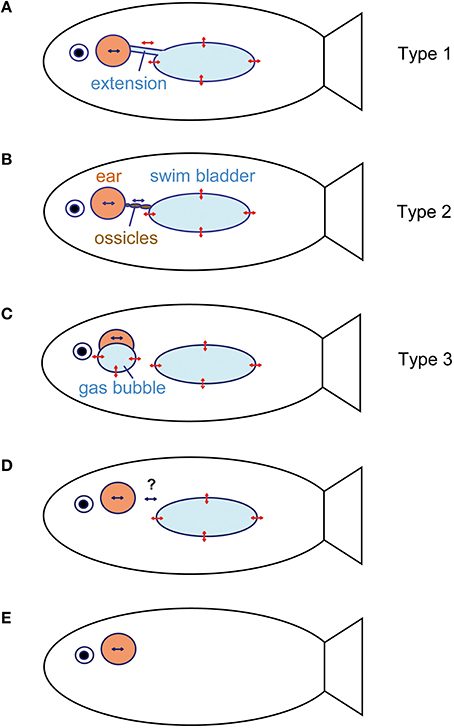
Figure 12. Schematic relationship between the inner ears and accessory hearing structures that enhance hearing in fishes. (A) Anterior swim bladder extensions (see e.g., Etroplus maculatus, Myripristis sp., or Chitala chitala). The extensions may bear an anterior enlargement such as in clupeids. (B) Direct connection between the swim bladder and inner ears via a chain of ossicles (Weberian ossicles), which transmits swim bladder vibrations to the ear in otophysans. (C) Air-filled cavities directly attached to the inner ears without connection to the swim bladder (mormyrids and labyrinth fishes). (D) No connection between a gas bladder (swim bladder, lungs) and inner ear. In the latter, the bladder may (lungfish, damselfish) or may not (toadfish) have an auditory function (see question marks). (E) No gas-filled cavity (swim bladder) and subsequently no accessory structure to improve hearing (sharks, flatfishes, sculpins). Double-headed red arrows indicate oscillations of gas bladder walls due to sound pressure fluctuations in a sound field. Blue arrows indicate particle motion within the inner ear endolymph due to the movement of the entire fish in the sound field. This particle motion may be enhanced by additional oscillations of air-filled cavities connected in various ways to the inner ear. Modified from Ladich and Popper (2004) and Ladich (2016).
All taxa possessing gas-filled cavities may utilize these for hearing (Popper and Fay, 2011). There are three main ways to connect the gas bladder directly to the inner ear (Figures 12A–C) and to detect sound pressure. Pressure detection may even take place in the absence of such a direct connection, most likely because tissues between the bladder and inner ear transmit bladder oscillations (e.g., Myrberg and Spires, 1980; see Section Cichlidae; Figure 12D).
Otophysan Fishes
The Weberian apparatus connecting the swim bladder to the inner ears characterizes the Otophysa, which comprise four orders with approximately 8000 species. Otophysans possess a chain of 1-4 Weberian ossicles that function in analogy to the middle ear bones in mammals and transmit vibrations of the anterior swim bladder wall (which can be regarded as an “internal tympanum”) to the inner ears (Figure 12B). These ossicles were first described by Weber almost 200 years ago, who postulated that they conduct sounds from the swim bladder to the ears (Figure 13A; Weber, 1819, 1820).
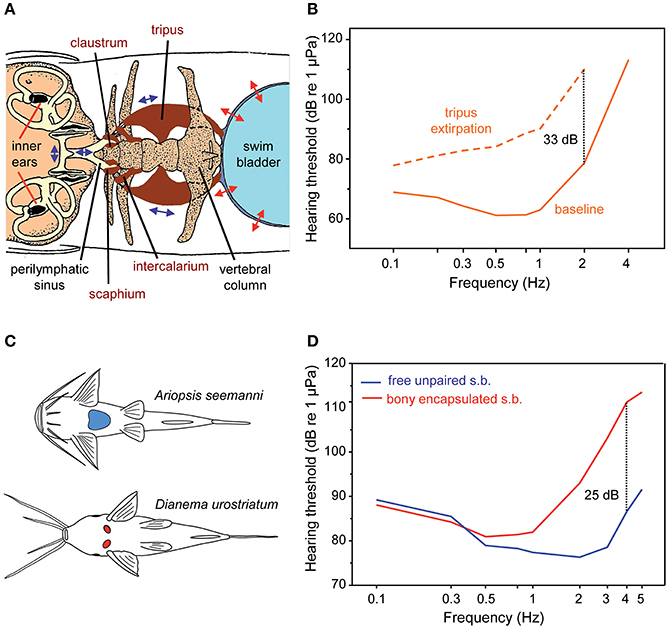
Figure 13. Accessory hearing structures and auditory sensitivity in otophysans. (A) Swim bladder, Weberian ossicles (tripus, intercalarium, scaphium, and claustrum) and inner ears in the minnow Phoxinus phoxinus (Otophysa) in dorsal view. The otolith end organs of the inner ears (utricle, saccule, and lagena) and their otoliths (black structures) are shown. Double headed arrows indicate the oscillations of the swim bladder wall, the Weberian ossicles and the fluids within the perilymphatic sinus and the inner ears. Modified after von Frisch and Stetter (1932). (B) Mean AEP-sound pressure audiograms of the goldfish Carassius auratus before (baseline) and after bilateral extirpation of the tripus (dotted line)—the largest Weberian ossicle—to indicate hearing improvement by the Weberian ossicles. Dotted line: hearing loss at 2 kHz in C. auratus. Redrawn after Ladich and Wysocki (2003). (C) Diversity in swim bladders in catfishes. Ventral view of swim bladders and ossicles of representatives possessing free, large unpaired swim bladders (blue structure; Ariopsis seemanni, family Ariidae) or small, paired and encapsulated swim bladders (red structures; Dianema urostriatum, family Callichthyidae). (D) Mean AEP-sound pressure audiograms of six catfish species out of six families with free unpaired swim bladders (s.b.) and of five species out of two families with bony encapsulated swim bladders. Dotted line: difference in sensitivity at 4 kHz. Adapted after Lechner and Ladich (2008).
Several experimental studies which either filled the swim bladder with fluids or removed its gas or which extirpated the tripus—the largest Weberian ossicle—showed a drop in hearing sensitivity, thereby underpinning Weber's hypothesis of sound conduction via the Weberian ossicles (von Frisch and Stetter, 1932; Poggendorf, 1952; Fay and Popper, 1974). Ladich and Wysocki (2003) demonstrated that bilateral extirpation of the tripus in the goldfish Carassius auratus resulted in a decline in hearing sensitivity of 7 dB at 100 Hz up to 33 dB at 2 kHz and a loss of detection of frequencies above 2 kHz (Figure 13B). Unilateral tripus extirpation did not result in any hearing loss, which is easily explained by the fact that both chains of Weberian ossicles transmit swim bladder oscillations to an unpaired perilymphatic sinus (see Figure 13A; Ladich, 2014a).
Otophysans do not exhibit a standard morphology of swim bladders and Weberian ossicles as illustrated by von Frisch and Stetter (1932) (Figure 13A) but a large diversity, especially in siluriforms (Chranilov, 1927, 1929; Alexander, 1962, 1964; Chardon, 1968; Lechner and Ladich, 2008). Members of numerous catfish families have large unpaired and free swim bladders and one up to four ossicles. In contrast, several groups have tiny and paired swim bladders located directly behind the cranium (Figure 13C). These tiny bladders are surrounded by bony capsules formed by the skull and anterior vertebrae (Chranilov, 1929). The small size of these bladders indicates that they no longer function as buoyancy organs but were most likely retained for hearing purposes (Lechner and Ladich, 2008).
How do these differences in swim bladder size and Weberian ossicle number affect hearing in catfishes? Ladich (1999) observed that members of the families Pimelodidae and Doradidae are more sensitive to sound than a member of the family Callichthyidae with reduced bladders. In order to determine whether this is a common difference between these two catfish groups, Lechner and Ladich (2008) investigated swim bladders, Weberian ossicles and hearing sensitivities in eleven species from eight different catfish families. Representatives of the Ariidae, Pseudopimelodidae, Malapteruridae, Heptapteridae, Mochokidae, and Auchenipteridae possess large, unpaired and free swim bladders and 1–4 ossicles, whereas members of the Loricariidae and Callichthyidae have significantly smaller swim bladders (3–5 vs. 8–13% of fish length), just 1–2 ossicles and thus a significantly shorter ossicular chain. Mean auditory thresholds of six species having large bladders and of all five species having tiny paired bladders revealed significant differences in hearing sensitivity between both groups between 1 and 5 kHz but not at lower frequencies (Figure 13D). Moreover, a longer ossicular chain and more ossicles resulted in better hearing at 3 to 5 kHz (for details see Lechner and Ladich, 2008; Ladich, 2016).
Non-Otophysan Fishes
Anterior extensions of the swim bladder directly contacting the auditory region (bullae) of the skull constitute the second type of a direct connection between the bladder and the inner ears (Figure 12A). Such extensions are apparently characteristics of three unrelated taxa, namely the order Clupeiformes (herrings), the families Notopteridae (knifefishes; order Osteoglossiformes) and Moridae (deep-sea cods, order Gadiformes; Nelson, 2006; Braun and Grande, 2008). Such linkages are furthermore found in several genera of non-related families such as the genus Myripristis (family Holocentridae, order Holocentriformes, Nelson, 1955) or the genus Etroplus (family Cichlidae, order Cichliformes, Dehadrai, 1959; Schulz-Mirbach et al., 2012). Families in which only some genera evolved swim bladder extensions and other members lack extensions or possess intermediate stages are particularly interesting for comparative studies.
Osteoglossomorpha: Notopteridae (Knifefishes) and Clupeidae (Herrings)
In notopterids, anterior projections of the swim bladder are attached to the bony auditory bullae, which are thinner than other regions of the skull (Coombs and Popper, 1982). Chitala chitala is able to detect sound up to 1000 Hz and has best sensitivities at 500 Hz (67 dB; all threshold values are referenced to 1 μPa in this review), similar to goldfish (Coombs and Popper, 1982).
Clupeiforms possess a quite different connection. The swim bladder extensions widen anteriorly and form large prootic bullae in which the gas is separated only by a bulla membrane from the inner ear fluid. These bullae are additionally in contact with the lateral line, forming a laterophysic connection (Blaxter et al., 1981). Mann et al. (1997, 2001) showed that all clupeiforms detect sounds up to 4 kHz and the members of the subfamily Alosinae (Alosa sapidissima, Brevoortia patronus) can detect ultrasound with frequencies up to 180 kHz. Note, however, that clupeids are, despite their high-frequency hearing, rather insensitive to sound because their lowest thresholds are about 100 dB and thus at least 40 dB above those of goldfish. Higgs et al. (2004) found that the middle macula of the utricle (see Figure 5F) is more loosely connected to the rest of the utricle in the American shad A. sapidissima and presumably vibrates more compared with species that do not detect ultrasound. Wilson et al. (2009) showed experimentally that the gas-filled bullae and their attachment to the lateral line are responsible for ultrasonic hearing in the Gulf menhaden B. patronus. The prootic bullae are positioned closer to the body surface in B. patronus. Thus, both studies indicate—although in different ways—that anatomical differences between members of the subfamily Alosinae and members of other subfamilies explain why the latter are unable to detect ultrasound. Clupeids demonstrate that small anatomical differences may extend the detectable frequency range considerably.
Cichlidae
Cichlids are a speciose family of freshwater fishes comprising more than 1000 species (Figure 14B; McMahan et al., 2013). They exhibit a large diversity in swim bladder size and in the relationship between swim bladder and inner ears (Dehadrai, 1959; Schulz-Mirbach et al., 2012, 2013). Swim bladders can be directly connected to the inner ears in the basal Etroplinae such as the orange chromide E. maculatus from India and Sri Lanka in which a bipartite swim bladder extension contacts the upper as well as the lower parts of each inner ear, a condition not observed in any other teleost species studied so far (Schulz-Mirbach et al., 2013). In the Malagasy species Paratilapia polleni, the anterior extensions of the swim bladder abut the posterior skull and thus come close to the inner ears but without contacting them directly (Schulz-Mirbach et al., 2012). In species that lack anterior swim bladder extensions, the bladders may be normal sized like in the jewel cichlid Hemichromis guttatus or may be reduced (vestigial) in some rheophilic representatives such as Steatocranus tinanti (Figure 14A).
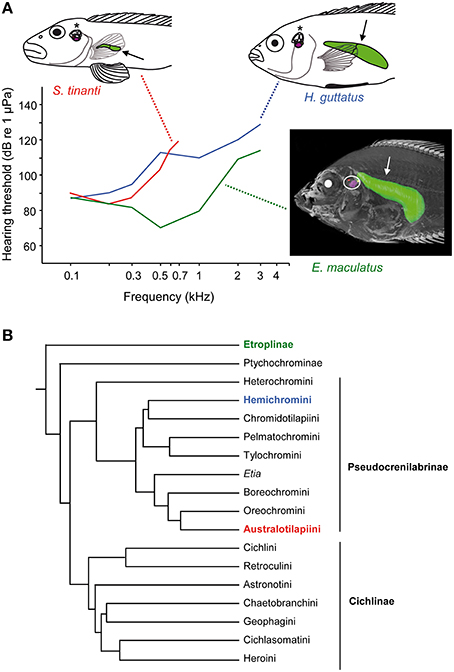
Figure 14. Diversity in swim bladder morphology, auditory sensitivities and the phylogeny of cichlids. (A) Lateral view of three cichlid species including their swim bladders (arrows) and inner ears (asterisks, white circle). Steatocranus tinanti and Hemichromis guttatus both lack anterior swim bladder extensions but differ widely in swim bladder size (see line drawings). In contrast, E. maculatus possesses anterior swim bladder extensions that directly contact the inner ears (see 3D reconstruction based on microCT imaging). Mean AEP-audiograms are shown for all three species. Note that only sound pressure thresholds are given due to the similarity of sound pressure and particle acceleration thresholds in the study by Schulz-Mirbach et al. (2012). Modified from Schulz-Mirbach et al. (2012). (B) The illustrated phylogeny is modified from McMahan et al. (2013). Identical colors used in species names, audiogram and the phylogeny indicate the subfamily or tribe to which the studied species belong.
The structural diversity in swim bladders is paralleled by differences in hearing abilities between species. As expected for species whose swim bladder directly contacts or comes close to the inner ears, hearing sensitivities are significantly better than in taxa lacking such accessory auditory structures (Schulz-Mirbach et al., 2012). Etroplus maculatus and P. polleni responded to frequencies up to 3 kHz and showed the lowest thresholds of approximately 70 dB at 0.5 kHz (Figure 14A). Species lacking a close swim bladder-inner ear relationship are less sensitive, clearly depending on swim bladder size. In H. guttatus and S. tinanti, auditory sensitivity decreases steeply above 0.3 kHz. This results in sensitivity differences of 20–40 dB between species. S. tinanti, having the smallest swim bladder, did not respond to sounds above 0.7 kHz (Figure 14A).
The relationship between swim bladder morphology and hearing sensitivity in cichlids allows several conclusions. Those species which have a large bladder but no connection to the inner ears display intermediate hearing abilities. They can detect frequencies up to 3 kHz, similar to E. maculatus, but the absolute sensitivity is low and similar to S. tinanti. This indicates that the large swim bladder in H. guttatus contributes to high-frequency hearing despite the lack of a direct connection to the inner ears.
Considering the hearing abilities in H. guttatus the question arises of whether swim bladders without connection to the ears affect hearing and enable fish to detect sound pressure? According to our current data the answer to the latter question must be “yes” although the experimental design does not enable differentiating between particle motion and pressure hearing. Sound detection up to 3 kHz can be explained only when a species is sound pressure sensitive. Prior studies in other taxa demonstrated that fishes can detect sound pressure in the absence of a clear connection (Figure 12D). This has been shown in the genus Stegastes (family Pomacentridae, damselfishes; Myrberg and Spires, 1980), Gadus (family Gadidae, cods; Sand and Enger, 1973), and recently in the African lungfish Protopterus (family Protopteridae, lungfishes, Christensen et al., 2015). It is assumed that in these families bladder wall oscillations are transmitted to the inner ears via the interjacent tissue (Hawkins, 1986). This, however, is not a general rule. Yan et al. (2000) demonstrated that, in three spot gourami Trichopodus trichopterus (formerly Trichogaster trichopterus) and in the oyster toadfish Opsanus tau, removal of gas from the swim bladder did not affect hearing; this indicates that the bladder plays no role in audition.
Holocentridae (Squirrelfishes)
Holocentrids represent the second family in which the diversity in hearing sensitivities can be correlated to differences in swim bladder structures. Swim bladder morphology and presence/absence of auditory bullae led to the classification into the two subfamilies Myripristinae with a sophisticated swim bladder inner ear connection and Holocentrinae possessing short anterior swim bladder extensions (Holocentrus) or lacking any anterior extensions (e.g., Sargocentron; Nelson, 1955). This classification is also confirmed by recent phylogenetic analyses based on nuclear and mitochondrial DNA (Figure 15C; Dornburg et al., 2012).
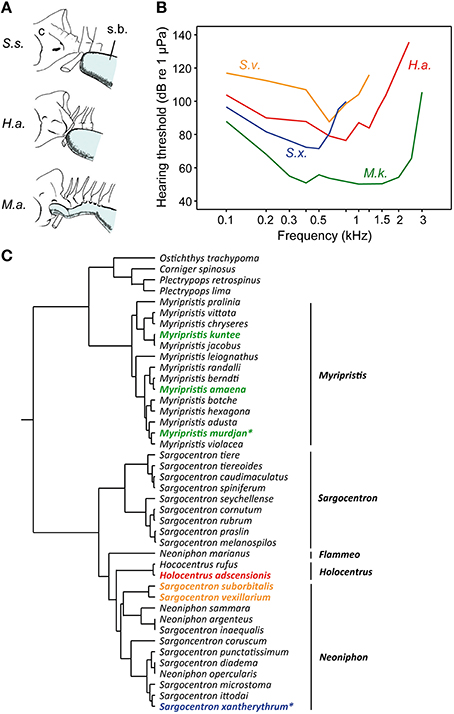
Figure 15. Diversity in swim bladders, hearing thresholds and the phylogeny of squirrelfishes (family Holocentridae). (A) Relationship between posterior cranium (C) and the anterior portion of the swim bladder (s.b.) in three species. Anatomical drawings from Nelson (1955) modified by Hawkins (1986). From above Sargocentron suborbitalis (S.s.), Holocentrus adscensionis (H.a.), and Myripristis amaena (M.a). (B) Hearing thresholds of Myripristis kuntee (M.k.) and Sargocentron xantherythrum (S.x.) in terms of sound pressure levels are depicted from Coombs and Popper (1979), those of Sargocentron vexillarium (S.v.) and of H. adscensionis (H.a.) from Tavolga and Wodinsky (1963). (C) The illustrated phylogeny is modified from Dornburg et al. (2012). Identical colors used in audiograms and the phylogeny indicate the subfamily or tribe to which the studied species belong. Asterisks highlight those species of which the macula sacculi and macula lagenae have been studied by Popper (1977).
The functional morphological comparison within the family is, however, somewhat limited because morphological and physiological data were, with one exception, gained in different species using different techniques for threshold determination (Nelson, 1955; Tavolga and Wodinsky, 1963; Coombs and Popper, 1979; Hawkins, 1986).
Coombs and Popper (1979) determined that the shoulderbar soldierfish Myripristis kuntee detects sounds up to 3 kHz, whereas the Hawaiian squirrelfish Sargocentron xantherythrum (formerly Adioryx xantherythrus) detects frequencies only up to 800 Hz at much higher sound levels (Figure 15B). This difference is paralleled by differences between genera in swim bladder morphology (note that the swim bladder morphology of M. kuntee and S. xantherythrum is unknown). Nelson (1955) showed that the brick soldierfish Myripristis amaena (formerly M. argyromus) has an anterior swim bladder extension that extends forward and covers the auditory bullae (Figure 15A). In contrast, the bladder of the tinsel soldierfish Sargocentron suborbitalis (formerly Holocentrus suborbitalis) is not attached to the skull. Holocentrus adscensionis represents an intermediate stage in terms of the swim bladder attachment and hearing ability (i.e., in the frequency range detectable but not in absolute thresholds; Tavolga and Wodinsky, 1963). In the genus Sargocentron, differences in hearing occur because S. vexillarius is much less sensitive than S. xantherythrum. This could be due to differences in swim bladder morphology (which is unknown in both species) or different methodologies to measure hearing (Hawkins, 1981). Nevertheless, a shorter distance between the swim bladder and the inner ears in holocentrids results in improved hearing sensitivities. The effects of bladder size on hearing cannot be analyzed because of insufficient data.
Sciaenidae (Drums and Croakers)
Numerous morphological and physiological studies have been conducted on the auditory systems in sciaenids (order Perciformes) and revealed a large diversity in swim bladder structures and hearing sensitivities, but the situation is less straightforward than that in cichlids and holocentrids (Ramcharitar et al., 2004, 2006; Horodysky et al., 2008; Wysocki et al., 2009). Ramcharitar et al. (2006) showed that the swim bladder in the weakfish Cynoscion regalis has anterior horns that terminate close to the ears and that this species detects sound frequencies up to 2 kHz. The spot Leiostomus xanthurus, on the other hand, has no extensions and detects frequencies only up to 700 Hz. Surprisingly, both species do not differ in absolute sensitivity, which is rather low (90 dB). In contrast, the silver perch Bairdiella chrysoura has an anterior swim bladder chamber that surrounds the otic capsule and hears up to 4 kHz at thresholds close to that of goldfish (74 dB at 600 Hz; Ramcharitar et al., 2004). Furthermore, Horodysky et al. (2008) reported no significant difference in hearing thresholds in species with (Cynoscion regalis, Cynoscion nebulosus, Micropogonias undulatus) and without swim bladder specializations (Sciaenops ocellatus, Leiostomus xanthurus; for a comparison of audiograms see review by Ladich and Fay, 2013). In summary, the form-function relationship in sciaenids is less consistent than in catfishes, holocentrids and cichlids. This difference may partly be explained by potential differences in techniques applied (maximum frequency measured by Horodysky et al. was 1.2 kHz) or by differences in the attachment of swim bladder extensions to the ears. These factors, however, cannot explain the lack of sensitivity differences within the same studies (Ramcharitar et al., 2006; Horodysky et al., 2008).
Mormyridae (Elephantfishes) and Anabantiformes (Labyrinth fishes).
The weakly electric mormyrids from African freshwaters (order Osteoglossiformes) and the mainly Southeast Asian labyrinth fishes (order Anabantiformes) possess gas bladders attached to the inner ears; these gas bladders are entirely separated from the swim bladder (Figure 12C).
The otic (tympanic) gas bladder in mormyrids constitutes an anterior extension of the swim bladder, which became completely separated and improves hearing sensitivity up to 3 kHz (Stipetić, 1939; McCormick and Popper, 1984). Elimination experiments showed that the otic bladder improves hearing in mormyrids by 15–30 dB between 0.5 and 1 kHz, whereas no change in the detectable frequency range was observed (Yan and Curtsinger, 2000; Fletcher and Crawford, 2001).
The non-related labyrinth fishes (order Anabantiformes) have a suprabranchial chamber (labyrinth organ) which derives from the first gill arch and serves in air-breathing (Bader, 1937). This air-filled chamber is in direct contact with the saccule and enhances hearing (Schneider, 1941; Yan, 1998). Schneider (1941) showed that the upper hearing range dropped from 4.5 kHz down to 800 Hz when the suprabranchial organ was filled with water. Yan (1998) observed a decline in sensitivity between 16 dB in the dwarf gourami Colisa lalia and up to 32 dB in the blue gourami T. trichopterus when deflating the organ.
Does Inner Ear Diversity Correlate with Hearing Abilities?
The Role of the Macula Neglecta in Elasmobranchii
Several physiological studies in elasmobranchs suggest a main auditory role of the macula neglecta together with the macula sacculi (e.g., Corwin, 1981, 1989; Myrberg, 2001; Casper, 2011). This may explain why the macula neglecta is generally larger in elasmobranchs than in bony fishes and larger in pelagic than in more benthic elasmobranch species (cf. Corwin, 1989; Myrberg, 2001). In holocephalans and especially in bony fishes, which possess a macula neglecta, its function remains elusive (Popper, 2011).
Modified Otolith End Organs in Teleosts
In contrast to elasmobranch fishes, in which it is rather clear that the macula neglecta together with the macula sacculi represent the main auditory organs, the role of the otolith end organs in audition and the vestibular sense in bony fishes is less well-understood. The saccule is assumed to be the main auditory organ in modern bony fishes (e.g., von Frisch and Stetter, 1932; Fay and Edds-Walton, 1997; Lu and Xu, 2002; Lu et al., 2002), which is supported by the fact that when connections or close relationships exist between accessory auditory structures and ears, the saccule is generally contacted by these structures. Nonetheless, several studies provide support for an auditory role of the lagena (e.g., Lu et al., 2003) as well as the utricle (e.g., Lu et al., 2004; Maruska and Mensinger, 2015).
Certain modified orientation patterns—mainly on the macula sacculi—may have evolved to enhance hearing together with accessory auditory structures. Apparently, species with accessory auditory structures, which mostly correlate with improved hearing (Ladich and Popper, 2004; Braun and Grande, 2008; Ladich and Fay, 2013; Ladich, 2014a), often display modified orientation patterns on the maculae, mainly on the macula sacculi (Platt and Popper, 1981a). This is evident in the vertical pattern of otophysans and mormyrids (Figures 7A,C), the opposing pattern of anabantiform fishes (Figure 7D) or “unique” patterns (see Antimora; Figure 7F) that cannot be assigned to one of the five patterns. Conceivably, the inner ear in such species and accessory auditory structures coevolved to some degree to guarantee fine-tuning between these two units to improve audition.
In some cases, however, accessory structures and modified orientation patterns—deviating from the standard or dual patterns—are present but without distinctly improved hearing compared to species that lack accessory structures. The clown knifefish C. chitala, for example, does not show an expanded hearing bandwidth or higher auditory sensitivities (Coombs and Popper, 1982), and the sciaenid species Micropogonias undulates and Cynoscion nebulosus show a slightly expanded bandwidth but similar auditory sensitivities as species without anterior swim bladder extensions (Horodysky et al., 2008). Moreover, accessory auditory structures and improved auditory abilities do not necessarily correlate with modified (more complex) orientation patterns on the maculae. This is demonstrated for the Hawaiian ladyfish Elops hawaiensis (Elopidae; Popper, 1981) and the cichlid Etroplus maculatus: they have “standard” patterns on all three macula types (when analyzing artificially flattened maculae (Figures 5H, 10G; Schulz-Mirbach et al., 2014). A distinct 3D curvature bringing the ciliary bundles in a new spatial orientation without modifications of the orientation patterns in 2D is present in E. maculatus. The anterior arm of its macula lagenae and the lacinia of the macula utriculi are strongly curved. The wider range of directions of ciliary bundles based on the 3D curvature—a condition also found in the macula sacculi of the silver perch Bairdiella chrysoura (Sciaenidae; Figure 7H)—might translate into a wider range of directional stimuli being detectible, and thus may play a role in localizing sound sources (Schulz-Mirbach et al., 2014). Finally, species such as the cod Gadus morhua that lack a direct morphological connection between the swim bladder and the inner ears (see Hawkins, 1986) and that display a dual pattern on the macula sacculi (Dale, 1976) were shown to be pressure sensitive (Chapman and Hawkins, 1973).
Though we have a solid knowledge about the diversity of inner ear morphology (otoliths, gross ear anatomy, sensory epithelia) and accessory auditory structures in fishes (see chapters above), our understanding of the ontogenetic development of ears and accessory auditory structures, as well as of the underlying genetic basis and molecular mechanisms for formation of sensory epithelia, is restricted to a few model organisms such as the otophysan Danio rerio or the batrachoidid plainfin midshipman Porichthys notatus. This hardly covers the tremendous morphological diversity in fishes (see e.g., Baxendale and Whitfield, 2014; Alderks and Sisneros, 2013). Another issue is to unravel the linkage between ear morphology and ear function. In most species, data about hearing abilities still only refer to hearing bandwidth and auditory sensitivities (see Fay, 1988; Ladich and Fay, 2013). To date it remains elusive how certain inner ear modifications are correlated to certain aspects of auditory abilities. Moreover, only few studies successfully disentangle the detection of the amounts of particle motion and sound pressure in fishes (e.g., Myrberg and Spires, 1980; Christensen et al., 2015).
Why Hearing Enhancement in Fishes?
As illustrated above, fishes, especially teleosts, exhibit a considerable variation in the auditory system including inner ears, accessory hearing structures and auditory sensitivities (Popper, 2011; Schulz-Mirbach and Ladich, 2016; Ladich, 2016). So far, we do not know why mechanisms to detect sound pressure, have evolved in taxonomically unrelated species or only in a few genera within entire families. Testable hypotheses have seldom been posed and the evolution of this diversity remains a field of much theoretical consideration (Ladich, 2014a,b; Lugli, 2015a,b).
Accessory hearing structures improve auditory sensitivities in several ways. They may e.g., expand the distance, the frequency range or sound level range (or other auditory abilities) over which fishes are able to detect sound. Accessory hearing structures do not necessarily improve all auditory abilities at the same time (Fay, 1988; Ladich and Fay, 2013). Comparison of baseline audiograms (recorded under quiet lab conditions) reveal that expansion of the detectable frequency range is not always paralleled by an enhanced absolute sensitivity, i.e., lower sound levels necessary to get a response either behaviorally or physiologically. Clupeids are able to detect ultrasound up to 180 kHz but their sensitivity to low level sounds is low in contrast to otophysans (Ladich and Fay, 2013). Thus, the diversity in hearing enhancement even in closely related taxa may help to fulfil different auditory tasks or similar tasks at different sound frequencies or levels.
In general, accessory hearing structures enable fish to detect acoustic information at frequencies and/or sound levels which would not be possible without these structures as demonstrated in numerous elimination experiments (Ladich and Wysocki, 2003). In order to detect such low level or high frequency sound, it is important that the relevant sound is not masked by ambient (background noise of different origin) noise at the sound frequencies but that the sound is loud enough so that there exists a reasonable signal to noise ratio (Fay, 1974). Relevant acoustic information for fish includes abiotic noise (e.g., water falls, coastal surf, reef noise) as well as biotic sound. The latter includes vocalizations from con- and hetero-specifics produced for intraspecific communication but also unintentional sound such as feeding or swimming noise. All of this constitutes the auditory scene (or soundscape, (Fay, 2009)) and provides important information for migration, reproductive activities as well as predator avoidance or prey detection. It needs to be mentioned that such acoustic information may be important for all fish species independent of their hearing abilities and that we have still limited knowledge of what fish hear besides conspecific sounds in vocalizing species (Fay, 2011).
The evolution of the detection of low level or high frequency sounds (or both) as compared to limited hearing in non-specialized taxa may be advantageous in many ways. In the following, we discuss potential factors responsible for the evolution of hearing enhancement in fishes, review current data and formulate as far as possible testable hypotheses.
The detection of high frequency sounds may be advantageous in detecting sound sources in shallow water. Low frequencies with long wavelengths do not propagate in shallow water due to the cut-off frequency phenomenon (Fine and Lenhardt, 1983; Rogers and Cox, 1988). High frequency hearing may have evolved to detect conspecifics or predators at larger distances in such habitats. In order to prove this notion it needs to be shown that fish with hearing specialization communicate or eavesdrop important acoustic information at higher distances in shallow waters than other taxa. Unfortunately, our knowledge on communication distances in fishes proven by playback experiments in the field is very limited and comparative data between fishes possessing different hearing abilities are entirely missing. Successful playback experiments were seldom carried out in the field. Myrberg et al. (1986) showed that female bicolor damselfish Stegastes partitus (see Figure 12D) were attracted to speakers playing back male chirp sound over distances of maximally 10 meters. However, communication distances in fish are usually much shorter. Typically, fish respond to conspecifics at distances of a few body lengths after an opponent or mate was detected visually (Ladich and Myrberg, 2006; Amorim et al., 2015). Present data do not provide unambiguous support that shallow water acoustics was an important selective force in the evolution of accessory hearing structures in any taxon.
A further potential factor in the evolution of hearing improvement may have been the detection of predators. Numerous insects evolved ultrasonic hearing abilities to detect echolocation clicks of bats, their main predators (Hoy, 1992). Similarly, certain clupeids such as the American shad Alosa sapidissima undergo an escape response when they detect ultrasonic clicks (Mann et al., 1998). Despite a lack of field data, it seems likely that predator avoidance was a main selective pressure driving the evolution of accessory hearing structures (type 1, Figure 12A) and ultrasonic hearing in several but not all clupeids (see Section Osteoglossomorpha). Ultrasonic hearing is an ideal candidate to test the predator avoidance hypothesis because the ultrasonic frequency range does not overlap with frequencies potentially used for acoustic communication. Yet, the ability to detect ultrasound has only been demonstrated for some members of the clupeid subfamily Alosinae (Mann et al., 2001, 2005). The observation by Astrup and Mohl (1993) that cod (Gadus morhua) detect ultrasound at 38 kHz could not be confirmed by studies. Schack et al. (2008) showed that unconditioned cods do not respond to ultrasound and thus will not react to toothed whale vocalizations. Further support for the predator avoidance hypothesis comes from the observation that many fish species avoid predators using a C-like startle behavior (C-start; Canfield and Eaton, 1990; Canfield and Rose, 1996). This escape response is mediated by the ability to detect sound pressure waves of rapidly approaching predators which coevolved with the addition of hearing to the swim bladder function. Thus, fish with hearing specializations could detect predators earlier and initiate escape responses more effectively.
Interestingly, fish species which lack hearing specialization or have only limited hearing improvements such as Holocentrus (Figure 15B) can detect vocalizations of dolphins as well and respond accordingly. Gulf toadfish Opsanus beta and longspine squirrelfish Holocentrus rufus reduced calling in the field when low frequency vocalizations but not ultrasound of bottlenose dolphins were played back (Remage-Healey et al., 2006; Luczkovich and Keusenkothen, 2007). Vasconcelos et al. (2011) demonstrated that the auditory system of the Lusitanian toadfish Halobatrachus didactylus detects dolphin sounds. These latter species are vocal and their ability to detect predators overlaps with the frequency range used for acoustic communication.
It was also hypothesized that hearing enhancement may have evolved for optimization of intraspecific acoustic communication (Ladich, 1999, 2000). Fishes show a large variety of mechanisms for producing sounds (sonic organs; for recent reviews see Ladich and Fine, 2006; Ladich and Bass, 2011; Fine and Parmentier, 2015). Sonic organs and sound communication are found in taxa with (mormyrids, catfish, piranhas, some labyrinth fishes) and without accessory hearing structures. Sound-producing taxa lacking accessory hearing structures can either be mainly particle motion sensitive such as toadfishes (Batrachoidiformes), sculpins (Cottiformes), and gobies (Gobiidae, Perciformes), or also display sound pressure sensitivity such as damselfish (Pomacentridae) and cods (Gadidae; Sand and Enger, 1973; Myrberg and Spires, 1980). Comparative studies among labyrinth fishes show that closely related genera may be vocal or non-vocal—such as croaking gouramis (genus Trichopsis) and Siamese fighting fish (genus Betta)—without differing in inner ear ultrastructure or accessory hearing organs (Ladich and Popper, 2001). The fact that sonic organs and/or sound production often evolved in only a few genera within taxa with hearing specializations (labyrinth fishes, weakly electric mormyrids, or cyprinids; Figure 4) raises the question how this can be explained at a phylogenetic level. It is possible that these taxa had vocal ancestors which evolved a particular sonic organ and that the majority of genera lost this sonic mechanism. This explanation is unlikely i.e., in labyrinth fishes because the vocal genera possess different sonic mechanisms (Kratochvil, 1985). Therefore, Ladich (2014b) proposed that vocal organs and sound production evolved under a different selection regime namely territory defense and mate attraction.
Coevolution of vocal communication and hearing enhancement is rather unlikely. In otophysans for example all members of this group share the same basic basic structure for hearing enhancement (Weberian apparatus, Figures 12B, 13) whereas vocal groups evolved a large diversity of sonic organs and do not share a common sonic mechanism (Ladich and Fine, 2006). It is therefore unlikely that the ancestor of otophysans was vocal. Acoustic communication as a main driver for the evolution of hearing enhancements is contrasted by the presence of numerous vocal taxa which lack any hearing improvement such as toadfishes (Batrachoidiformes), gobiids (Gobioidei), and sculpins (Cottoidei; Ladich, 2014b),
All potential factors facilitating the evolution of hearing enhancements (shallow water sound propagation, predator avoidance, acoustic communication) are based on the notion that the relevant sound is detectable against the background noise at particular frequencies. This notion requires an analysis of the acoustic conditions of the fish's habitats.
Several comparative studies described that ambient noise conditions vary considerably in the habitats of freshwater and marine fishes. Wysocki et al. (2007) analyzed 12 aquatic habitats in central Europe, Lugli (2010) five different habitats in northern Italy and the Mediterranean, and Speares et al. (2011) different places in creeks in Alabama. All these studies reported differences in spectral levels of 40–60 dB, with highest levels in rapidly moving waters such as creeks, large streams, and rocky shores. Kennedy et al. (2010) recorded the ambient noise at 40 reefs of the Las Perlas archipelago in the Gulf of Panama and compared these to offshore sites while the sea was calm. Spectral profiles between different reefs were rather similar, in contrast to offshore recordings in which spectral noise levels were about 20–30 dB lower, mainly due to lack of vocalizing animals such as shrimp. The diversity in ambient noise spectra raises the question whether the high auditory sensitivities of some species are adapted to low ambient noise conditions. To verify this assumption hearing sensitivities have been measured and compared under quiet laboratory and under ambient noise conditions. Chapman (1973) and Chapman and Hawkins (1973) demonstrated in field experiments that cod hearing is unmasked under calm sea conditions and that hearing sensitivity decreases (thus hearing was masked) when ambient noise levels rose. Amoser and Ladich (2005) measured hearing in two common non-vocal European freshwater fish, the carp Cyprinus carpio (family Cyprinidae, an otophysine) and the European perch Perca fluviatilis (family Percidae, no specializations) in the presence of ambient noise of four different habitats. Carps were moderately masked by the quiet noise of standing waters but heavily affected by the river noise in their best hearing range (0.5–1 kHz). In contrast, perch were only slightly masked by the highest noise levels presented. This raises the question if the diversity in ambient noise levels affected the evolution of particular hearing sensitivities. Ladich (2014b) argued that hearing evolved in adaptation to the acoustical conditions in the fishes' habitats (“eco-acoustical constraints hypothesis”). Hence, hearing thresholds are assumed to be as low as possible without being masked by the ambient (background) noise in their environment. If the ambient noise varies in aquatic environments, it would inevitably result in a large variety of hearing abilities in fishes. Low noise levels would then facilitate the evolution of accessory hearing structures and the detection of low-level sound against low level background noise, whereas high ambient noise levels likely render such structures meaningless.
Importantly, the eco-acoustical constraints hypothesis does not explain to which sound sources fishes are listening to. Besides vocalizations from con- and hetero-specifics (e.g., predators), fish may also listen to habitat noise built up of numerous abiotic and biotic sound sources. Pelagic coral reef fish larvae (of the families Trypterigiidae, Pomacentridae, Apogonidae, Gobiidae, Lethrinidae) orient to loudspeakers playing back reef noise (Tolimieri et al., 2000; Simpson et al., 2008; Radford et al., 2011). Our knowledge on the importance of acoustic orientation in prey detection or food finding is still in its infancy, but this factor should not be underestimated. The attractiveness of artificial underwater sounds to fish has been exploited by indigenous people all over the world for hundreds of years (see Wolff, 1966). Rattling coconut shells underwater, for instance, is very attractive for sharks (shark rattle) and was used in the South Seas. It remains to be clarified what triggers the catch success in such non-vocal species, i.e., whether it is attraction to potential food sources or startling. Markl (1972) observed that the red piranha Pygocentrus nattereri, a representative of the otophysan order Characiformes, attacked prey producing splashing noise more often than silent prey.
Summary and Conclusions
Fishes have evolved an enormous diversity of inner ears and accessory hearing structures. While the accessory hearing structures enhance hearing, the diversity of inner ears remains mostly unexplained. They may be adaptations to various ecological conditions and/or and auditory tasks such as improvement of hearing. The latter may be the case in otophysans, in which major changes in inner ear structure (maculae, needle-like saccular otolith) are associated with the presence of the unique Weberian apparatus.
The occurrence of accessory hearing structures and enhanced hearing in fishes does not reflect the phylogenetic relationships among the groups in which these specializations evolved.
On the contrary, these specialized structures evolved several times independently and are either characteristic of a whole group with a high taxonomic rank (e.g., superorder Otophysa; order Anabantiformes; families Notopteridae, Mormyridae) or appear in only a few species or genera within a (speciose) family such as cichlids, holocentrids, or sciaenids. Hearing enhancement may be a simple by-product of other functions such as air-breathing (labyrinth fishes, lungfishes) or buoyancy (damselfish), or it may have evolved solely for hearing enhancement as seems to be the case in otophysans. This interpretation is supported by the observation that the Weberian ossicles have not been entirely lost in any species even though swim bladders were reduced considerably and certainly lost their function in buoyancy control. This leaves us with the question why certain non-related taxa such as the genera Etroplus and Myripristis, the family Mormyridae or the order Cypriniformes evolved accessory hearing structures and others did not.
We propose several factors which may explain the evolution of hearing enhancements in fishes. They are based on a limited number of observations but need rigorous testing in order to prove their validiy in some taxa. Ultrasonic hearing in some herrings, for example, most likely evolved to detect echolocating clicks of dolphins. To test this assumption elimination of the accessory hearing structure and analysis of the behavior under field conditions will be necessary. The “eco-acoustical constraints hypothesis” could be tested by recording the ambient noise in the habitats of closely related species which differ considerably in hearing abilities. Representatives of catfishes, cichlids or holocentrids may provide ideal candidates for testing as they cover a large variety of auditory sensitivities (Figures 13–15; Ladich, 2014a,b). According to this hypothesis, ambient noise levels and spectra in the habitats of the non-related genera Myripristis and Etroplus should differ as compared to that of other representatives of the same families. Results of these experiments will help to gain deeper insights into the evolution of hearing specializations and enhanced hearing in fishes.
Author Contributions
All authors listed, have made substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This article was supported by the Open Access Publishing Fund of the University of Vienna.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Michael Stachowitsch for scientific English proofreading and two reviewers for their valuable comments on an earlier version of the manuscript.
References
Alderks, P. W., and Sisneros, J. A. (2013). Development of the acoustically evoked behavioral response in larval plainfin midshipman fish Porichthys notatus. PLoS ONE 8:e82182. doi: 10.1371/journal.pone.0082182
Alexander, R. M. N. (1962). The structure of the Weberian apparatus in the Cyprini. Proc. Zool. Soc. London 139, 451–473. doi: 10.1111/j.1469-7998.1962.tb01839.x
Alexander, R. M. N. (1964). The structure of the Weberian apparatus in the Siluri. Proc. Zool. Soc. London 142, 419–440. doi: 10.1111/j.1469-7998.1964.tb04507.x
Alexander, R. M. N. (1966). Physical aspects of swimbladder function. Biol. Rev. 41, 141–176. doi: 10.1111/j.1469-185X.1966.tb01542.x
Amorim, M. C. P., Vasconcelos, R. O., and Fonseca, P. J. (2015). “Fish sounds and mate choice,” in Sound Communication in Fishes, ed F. Ladich (Wien: Springer-Verlag), 1–33.
Amoser, S., and Ladich, F. (2005). Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J. Exp. Biol. 208, 3533–3542. doi: 10.1242/jeb.01809
Assis, C. A. (2005). The utricular otoliths, lapilli, of teleosts: their morphology and relevance for species identification and systematics studies. Sci. Mar. 69, 259–273. doi: 10.3989/scimar.2005.69n2259
Astrup, J., and Mohl, B. (1993). Detection of intense ultrasound by the cod Gadus morhua. J. Exp. Biol. 182, 71–80.
Bader, R. (1937). Bau Entwicklung und Funktion des akzessorischen Atmungsorgans der Labyrinthfische. Z. wiss. Zool. Leipzig 149, 323–401.
Barber, V. C., and Emerson, C. J. (1980). Scanning electron microscopic observations on the inner ear of the skate, Raja ocellata. Cell Tissue Res. 205, 199–215. doi: 10.1007/BF00234680
Barber, V. C., Yake, K. I., Clark, V. F., and Pungur, J. (1985). Quantitative analyses of sex and size differences in the macula neglecta and ramus neglectus in the inner ear of the skate, Raja ocellata. Cell Tissue Res. 241, 597–605. doi: 10.1007/BF00214581
Baxendale, S., and Whitfield, T. T. (2014). “Zebrafish Inner Ear Development and Function,” in Development of Auditory and Vestibular Systems, eds R. Romand, and I. Varela-Nieto (San Diego, CA: Academic Press), 63–106.
Bernstein, P. (2003). The ear region of Latimeria chalumnae: functional and evolutionary implications. Zoology 106, 233–242. doi: 10.1078/0944-2006-00119
Betancur, -R. R., Broughton, R. E., Wiley, E. O., Carpenter, K., López, J. A., Li, C., et al. (2013). The tree of life and a new classification of bony fishes. PLoS Curr. 5:ecurrents.tol.53ba26640df0ccaee75bb165c8c26288. doi: 10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288
Blaxter, J. H. S., Denton, E. J., and Gray, J. A. B. (1981). “Acousticolateralis system in clupeid fishes,” in Hearing and Sound Communication in Fishes, eds W. N. Tavolga, A. N. Popper, and R. R. Fay (New York, NY: Springer-Verlag), 39–56.
Bradbury, J. W., and Vehrencamp, S. L. (2011). Principles of Animal Communication, 2nd Edn. Sunderland: Sinauer Associates Inc. Publishers.
Braun, C. B., and Grande, T. (2008). “Evolution of peripheral mechanisms for the enhancement of sound reception,” in Fish Bioacoustics, eds J. F. Webb, A. N. Popper, and R. R. Fay (New York, NY: Springer-Verlag), 99–144.
Broughton, R. E., Betancur, -R. R., Li, C., Arratia, G., and Ortí, G. (2013). Multi-locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution. PLoS Curr. 5:ecurrents.tol.2ca8041495ffafd0c92756e75247483e. doi: 10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e
Buran, B. N., Deng, X. H., and Popper, A. N. (2005). Structural variation in the inner ears of four deep-sea elopomorph fishes. J. Morphol. 265, 215–225. doi: 10.1002/jmor.10355
Caiger, P. E., Montgomery, J. C., Bruce, M., Lu, J., and Radford, C. A. (2013). A proposed mechanism for the observed ontogenetic improvement in the hearing ability of hapuka (Polyprion oxygeneios). J. Comp. Physiol. A 199, 653–661. doi: 10.1007/s00359-013-0820-z
Canfield, J. G., and Eaton, R. C. (1990). Swimbladder acoustic pressure transduction initiates Mauthner-mediated escape. Nature 347, 760–762. doi: 10.1038/347760a0
Canfield, J. G., and Rose, G. J. (1996). Hierarchical sensory guidance of Mauthner-mediated escape response in goldfish (Carassius auratus) and cichlids (Haplochromis burtoni). Brain Behav. Evol. 48, 137–156. doi: 10.1159/000113193
Carlström, D. (1963). A crystallographic study of vertebrate otoliths. Biol. Bull. 125, 441–463. doi: 10.2307/1539358
Casper, B. M. (2011). “The Ear and Hearing in Sharks, Skates, and Rays” in Encyclopedia of Fish Physiology: From Genome to Environment, ed A. P. Farrell (San Diego, CA: Academic Press), 262–269.
Chapman, C. J. (1973). Field studies of hearing in teleost fish. Helgol. Wiss. Meeresunters. 24, 371–390. doi: 10.1007/BF01609527
Chapman, C. J., and Hawkins, A. D. (1973). A field study of hearing in the cod, Gadus morhua L. J. Comp. Physiol. A 85, 147–167. doi: 10.1007/BF00696473
Chardon, M. (1968). Anatomie Comparée de l'appareil de Weber et des Structures Connexes chez les Siluriformes. Musée Royal de l'Afrique Centrale - Tervuren, Belgique Annales, Serie in 8, Sciences Zoologiques. Tervuren: Musee Royal de l'Afrique Centrale.
Chranilov, N. S. (1927). Beiträge zur Kenntnis des Weber'schen Apparates der Ostariophysi 1. Vergleichend-anatomische Übersicht der Knochenelemente des Weber'schen Apparates bei Cypriniformes. Zool. Jahrb. (Anatomie) 49, 501–597.
Chranilov, N. S. (1929). Beiträge zur Kenntnis des Weber'schen Apparates der Ostariophysi: 2. Der Weber'sche Apparat bei Siluroidea. Zool. Jahrb. (Anatomie) 51, 323–462.
Christensen, C. B., Christensen-Dalsgaard, J., and Madsen, P. T. (2015). Hearing of the African lungfish (Protopterus annectens) suggests underwater pressure detection and rudimentary aerial hearing in early tetrapods. J. Exp. Biol. 218, 381–387. doi: 10.1242/jeb.116012
Cohen, M. J., and Winn, H. E. (1967). Electrophysiological observations on hearing and sound production in the fish, Porichthys notatus. J. Exp. Zool. 165, 355–369. doi: 10.1002/jez.1401650305
Coombs, S., and Popper, A. N. (1979). Hearing differences among Hawaiian squirrelfish (family Holocentridae) related to differences in the peripheral auditory system. J. Comp. Physiol. 132, 203–207. doi: 10.1007/BF00614491
Coombs, S., and Popper, A. N. (1982). Structure and function of the auditory system in the clown knifefish, Notopterus chitala. J. Exp. Biol. 97, 225–239.
Corwin, J. T. (1981). Peripheral auditory physiology in the Lemon shark: evidence of parallel otolithic and non-otolithic sound detection. J. Comp. Physiol. A 142, 379–390. doi: 10.1007/BF00605450
Corwin, J. T. (1989). Functional anatomy of the auditory system in sharks and rays. J. Exp. Zool. 252, 62–74. doi: 10.1002/jez.1402520408
Dale, T. (1976). The labyrinthine mechanoreceptor organs of the cod Gadus morhua L. (Teleostei: Gadidae). Norweg. J. Zool. 24, 85–128.
de Burlet, H. M. (1934). “Vergleichende Anatomie des stato-akustischen Organs. a) Die innere Ohrsphäre,” Handbuch der Vergleichenden Anatomie der Wirbeltiere, eds L. Bolk, E. Göppert, E. Kallius, and W. Lubosch (Berlin; Vienna: Urban & Schwarzenberg), 1293–1380.
DeepFin Project (2003-2009). Phylogenetic Classification of Bony Fishes - Version 3. Available online at: http://deepfin.ou.edu/classification_v3.htm (Access January 12 2016).
Dehadrai, P. V. (1959). On the swimbladder and its connection with the internal ear in family Cichlidae. Proc. Natl. Inst. Sci. India B 25, 54–261.
Deng, X. H. (2009). Comparative Studies on the Structure of the Ears of Deep-Sea Fishes Dissertation, University of Maryland, College Park, Maryland, USA. 190.
Deng, X. H., Wagner, H.-J., and Popper, A. N. (2011). The inner ear and its coupling to the swim bladder in the deep-sea fish Antimora rostrata (Teleostei: Moridae). Deep Sea Res. 58, 27–37. doi: 10.1016/j.dsr.2010.11.001
Deng, X. H., Wagner, H.-J., and Popper, A. N. (2013). Interspecific variations of inner ear structure in the deep-sea fish family Melamphaidae. Anat. Rec. 296, 1064–1082. doi: 10.1002/ar.22703
Denton, E. J., and Gray, J. A. B. (1979). The analysis of sound by the sprat ear. Nature 282, 406–407. doi: 10.1038/282406a0
Dornburg, A., Moore, J. A., Webster, R., Warren, D. L., Brandley, M. C., Iglesias, T. L., et al. (2012). Molecular phylogenetics of squirrelfishes and soldierfishes (Teleostei: Beryciformes: Holocentridae): reconciling more than 100 years of taxonomic confusion. Mol. Phylogen. Evol. 65, 727–738. doi: 10.1016/j.ympev.2012.07.020
Duncan, J. S., and Fritzsch, B. (2012). Evolution of sound and balance perception: innovations that aggregate single hair cells into the ear and transform a gravistatic sensor into the organ of Corti. Anat. Rec. 295, 1760–1774. doi: 10.1002/ar.22573
Evangelista, C., Mills, M., Siebeck, U. E., and Collin, S. P. (2010). A comparison of the external morphology of the membranous inner ear in elasmobranchs. J. Morphol. 271, 483–495. doi: 10.1002/jmor.10812
Fay, R. R. (1974)., Masking of tones by noise for the goldfish (Carassius auratus). J. Comp. Physiol. Psychol. 87, 708–716. doi: 10.1037/h0037002
Fay, R. R. (1988). Hearing in Vertebrates: A Psychophysics Databook. Winnetka, IL: Hill-Fay Associates.
Fay, R. R. (2009). Soundscapes and the sense of hearing in fishes. Integr. Zool. 4, 26–32. doi: 10.1111/j.1749-4877.2008.00132.x
Fay, R. R. (2011). “Psychoacoustics: what fish hear,” in Encyclopedia of Fish Physiology: From Genome to Environment, Vol. 1, ed A.P. Farrell, (San Diego, CA: Academic press), 276–282.
Fay, R. R., and Edds-Walton, P. L. (1997). Diversity in frequency response properties of saccular afferents of the toadfish, Opsanus tau. Hear. Res. 113, 235–246. doi: 10.1016/S0378-5955(97)00148-2
Fay, R. R., and Popper, A. N. (1974). Acoustic stimulation of the ear of the goldfish, (Carassius auratus). J. Exp. Biol. 61, 243–260
Fine, M. L., Horn, M. H., and Cox, M. (1987). Acanthonus armatus, a deep-sea teleost fish with a minute brain and large ears. Proc. R. Soc. London B Biol. Sci. 230, 257–265. doi: 10.1098/rspb.1987.0018
Fine, M. L., and Lenhardt, M. L. (1983). Shallow water propagation of the toadfish mating call. Comp. Biochem. Physiol. 76, 225–231. doi: 10.1016/0300-9629(83)90319-5
Fine, M. L., and Parmentier, E. (2015). “Mechanisms of fish sound production,” in Sound Communication in Fishes, ed F. Ladich (Wien: Springer-Verlag), 77–126.
Finneran, J. J., and Hastings, M. C. (2000). A mathematical analysis of the peripheral auditory system mechanics in the goldfish (Carassius auratus). J. Acoust. Soc. Am. 108, 1308–1321. doi: 10.1121/1.1286099
Fletcher, L. B., and Crawford, J. D. (2001). Acoustic detection by sound-producing fishes (Mormyridae): the role of gas-filled tympanic bladders. J. Exp. Biol. 204, 175–183.
Fritzsch, B. (1987). Inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature 327, 153–154. doi: 10.1038/327153a0
Fritzsch, B. (1992). “The water-to-land transition: evolution of the tetrapod basilar papila, middle ear and auditory nuclei,” in The Evolutionary Biology of Hearing, eds D. B. Webster, R. R. Fay, and A. N. Popper (New York, NY: Springer-Verlag), 351–375.
Fritzsch, B. (2003). The ear of Latimeria chalumnae revisited. Zoology 106, 243–248. doi: 10.1078/0944-2006-00120
Gauldie, R. W. (1993). Polymorphic crystalline structure of fish otoliths. J. Morphol. 218, 1–28. doi: 10.1002/jmor.1052180102
Gauldie, R. W., Dunlop, D., and Tse, J. (1986a). The remarkable lungfish otolith. New Zealand J. Mar. Freshw. Res. 20, 81–92. doi: 10.1080/00288330.1986.9516132
Gauldie, R. W., Dunlop, D., and Tse, J. (1986b). The simultaneous occurrence of otoconia and otoliths in four teleost fish species. New Zealand J. Mar. Freshw. Res. 20, 93–99. doi: 10.1080/00288330.1986.9516133
Gauldie, R. W., Mulligan, K. P., and Thompson, R. K. (1987). The otoliths of a chimaera, the New Zealand elephant fish Callorhynchus milii. New Zealand J. Mar. Freshw. Res. 21, 275–280. doi: 10.1080/00288330.1987.9516223
Hawkins, A. D. (1981). “The hearing abilities of fish,” in Hearing and Sound Communication in Fishes, eds W. N. Tavolga, A. N. Popper, and R. R. Fay (New York, NY: Springer-Verlag), 109–133.
Hawkins, A. D. (1986). “Underwater sound and fish behaviour,” in The Behaviour of Teleost Fishes, ed T. J. Pitcher (London; Sydney, NSW: Croom Helm), 114–151.
Higgs, D. M., Plachta, D. T. T., Rollo, A. K., Singheiser, M., Hastings, M. C., and Popper, A. N. (2004). Development of ultrasound detection in American shad (Alosa sapidissima). J. Exp. Biol. 207, 155–163. doi: 10.1242/jeb.00735
Horodysky, A. Z., Brill, R. W., Fine, M. L., Musick, J. A., and Latour, R. J. (2008). Acoustic pressure and particle thresholds in six sciaenid fishes. J. Exp. Biol. 211, 1504–1511. doi: 10.1242/jeb.016196
Hoy, R. R. (1992). “The evolution of hearing in insects as an adaptation to predation from bats,” in The Evolutionary Biology of Hearing, eds D. B. Webster, R. R. Fay, and A. N. Popper (New York, NY: Springer-Verlag), 115–129. doi: 10.1007/978-1-4612-2784-7_8
Hudspeth, A. J., and Corey, D. P. (1977). Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc. Natl. Acad. Sci. U.S.A. 74, 2407–2411. doi: 10.1073/pnas.74.6.2407
Kennedy, E. V., Holderied, M. W., Mair, J. M., Guzman, H. M., and Simpson, S. D. (2010). Spatial patterns in reef-generated noise relate to habitats and communities: evidence from a Panamanian case study. J. Exp. Mar. Biol. Ecol. 395, 85–92. doi: 10.1016/j.jembe.2010.08.017
Kéver, L., Colleye, O., Herrel, A., Romans, P., and Parmentier, E. (2014). Hearing capacities and otolith size in two ophidiiform species (Ophidion rochei and Carapus acus). J. Exp. Biol. 217, 2517–2525. doi: 10.1242/jeb.105254
Kratochvil, H. (1985). Beiträge zur Lautbiologie der Anabantoidei - Bau, Funktion und Entwicklung von lauterzeugenden Systemen. Zool. Jahrb (Physiologie) 89, 203–255.
Krysl, P., Hawkins, A. D., Schilt, C., and Cranford, T. W. (2012). Angular oscillation of solid scatterers in response to progressive planar acoustic waves: do fish otoliths rock? PLoS ONE 7:e42591. doi: 10.1371/journal.pone.0042591
Ladich, F. (1999). Did auditory sensitivity and vocalization evolve independently in otophysan fishes? Brain, Behav. Evol. 53, 288–304. doi: 10.1159/000006600
Ladich, F. (2000). Acoustic communication and the evolution of hearing in fishes. Phil. Trans. R. Soc. B Biol. Sci. 355, 1285–1288. doi: 10.1098/rstb.2000.0685
Ladich, F. (2010). “Hearing: Vertebrates,” in Encyclopedia of Animal Behaviour, Vol. 2, eds M. D. Breed, and J. Moore (Oxford: Academic Press), 54–60.
Ladich, F. (2014a). “Diversity in hearing in fishes: Ecoacoustical, communicative, and developmental constraints,” in Insights from Comparative Hearing Research, eds C. Koeppl, G. A. Manley, A. N. Popper, and R. R. Fay (New York, NY: Springer Science +Business Media), 289–321.
Ladich, F. (2014b). Fish bioacoustics. Curr. Opin. Neurobiol. 28, 121–127. doi: 10.1016/j.conb.2014.06.013
Ladich, F. (2016). “Peripheral hearing structures in fishes: diversity and sensitivity of catfishes and cichlids,” in Fish Hearing and Bioacoustics: An Anthology in Honor of Arthur N. Popper and Richard R. Fay. v. Exp. Med. Biol. 877, ed J. A. Sisneros (Cham: Springer International Publishing AG), 323–342. doi: 10.1007/978-3-319-21059-9_15
Ladich, F., and Bass, A. H. (2011). “Vocal behavior of fishes: anatomy and physiology,” in Encyclopedia of Fish Physiology: From Genome to Environment, Vol. 1, ed A. P. Farrell (San Diego, CA: Academic Press), 321–329.
Ladich, F., and Fay, R. R. (2013). Auditory evoked potential audiometry in fish. Rev. Fish Biol. Fish 23, 317–364. doi: 10.1007/s11160-012-9297-z
Ladich, F., and Fine, M. L. (2006). “Sound-generating mechanisms in fishes: a unique diversity in vertebrates,” in Communication in Fishes, Vol. 1, eds F. Ladich, S. P. Collin, P. Moller, and B. G. Kapoor (Enfield: Science Publishers), 3–43.
Ladich, F., and Myrberg, A. A. (2006). “Agonistic behaviour and acoustic communication,” in Communication in Fishes, eds F. Ladich, S. P. Collin, P. Moller, and B. G. Kapoor (Enfield: Science Publisher), 122–148.
Ladich, F., and Popper, A. N. (2001). Comparison of the inner ear ultrastructure between teleost fishes using different channels for communication. Hear. Res. 154, 62–72. doi: 10.1016/S0378-5955(01)00217-9
Ladich, F., and Popper, A. N. (2004). “Parallel evolution in fish hearing organs,” in Evolution of the Vertebrate Auditory System, eds G. Manley, R. R. Fay, and A. N. Popper (New York, NY: Springer-Verlag), 95–127. doi: 10.1007/978-1-4419-8957-4_4
Ladich, F., and Wysocki, L. E. (2003). How does tripus extirpation affect auditory sensitivity in goldfish? Hear. Res. 182, 119–129. doi: 10.1016/S0378-5955(03)00188-6
Lechner, W., and Ladich, F. (2008). Size matters: diversity in swimbladders and Weberian ossicles affects hearing in catfishes. J. Exp. Biol. 211, 1681–1689. doi: 10.1242/jeb.016436
Lisney, T. J. (2010). A review of the sensory biology of chimaeroid fishes (Chondrichthyes; Holocephali). Rev. Fish Biol. Fish 20, 571–590. doi: 10.1007/s11160-010-9162-x
Lovell, J. M., Findlay, M. M., Harper, G. M., and Moate, R. M. (2007). The polarization of hair cells from the inner ear of the lesser spotted dogfish Scyliorhinus canicula. J. Fish Biol. 70, 362–373. doi: 10.1111/j.1095-8649.2006.01304.x
Lovell, J. M., Findlay, M. M., Moate, R. M., Nedwell, J. R., and Pegg, M. A. (2005). The inner ear morphology and hearing abilities of the Paddlefish (Polyodon spathula) and the Lake Sturgeon (Acipenser fulvescens). Comp. Biochem. Physiol. A 142, 286–296. doi: 10.1016/j.cbpa.2005.07.018
Lowenstein, O., Osborne, M. P., and Wersäll, J. (1964). Structure and innervation of the sensory epithelia of the labyrinth in the Thornback ray (Raja clavata). Proc. R. Soc. London Ser. B. Biol. Sci. 160, 1–12. doi: 10.1098/rspb.1964.0026
Lu, Z., and Popper, A. N. (1998). Morphological polarizations of sensory hair cells in the three otolithic organs of a teleost fish: fluorescent imaging of ciliary bundles. Hear. Res. 126, 47–57. doi: 10.1016/S0378-5955(98)00149-X
Lu, Z., and Xu, Z. (2002). Effects of saccular otolith removal on hearing sensitivity of the sleeper goby (Dormitator latifrons). J. Comp. Physiol. A 188, 595–602. doi: 10.1007/s00359-002-0334-6
Lu, Z., Xu, Z., and Buchser, W. J. (2003). Acoustic response porperties of lagenar nerve fibers in the sleeper goby, Dormitator latifrons. J. Comp. Physiol. A 189, 899–905. doi: 10.1007/s00359-003-0462-7
Lu, Z., Xu, Z., and Buchser, W. J. (2004). Coding of acoustic particle motion by utricular fibers in the sleeper goby, Dormitator latifrons. J. Comp. Physiol. A 190, 923–928. doi: 10.1007/s00359-004-0550-3
Lu, Z., Xu, Z., and Stadler, J. H. (2002). Roles of the saccule in directional hearing. Bioacoustics 12, 205–207. doi: 10.1080/09524622.2002.9753696
Luczkovich, J. J., and Keusenkothen, M. (2007). “Behavior and sound production by Longspine Squirrelfish Holocentrus rufus during playback of predator and conspecific sounds,” Proceedings of the American Academy of Underwater Sciences 26th Symposium. (Dauphin Island: AL), 127–134.
Lugli, M. (2010). Sound of shallow water fishes pitch within the quiet window of the habitat noise. J. Comp. Physiol. A 196, 439–451. doi: 10.1007/s00359-010-0528-2
Lugli, M. (2015a). “Habitat acoustics and the low-frequency communication of shallow water fishes,” in Sound Communication in Fishes, ed F. Ladich (Wien: Springer-Verlag), 175–206.
Lugli, M. (2015b). The tradeoff between signal detection and recognition rules auditory sensitivity under variable background noise conditions. J. Theoret. Biol. 386, 1–6. doi: 10.1016/j.jtbi.2015.08.033
Lychakov, D. V. (1995). Investigation of the otolithic apparatus in the Acipenser fry. J. Evol. Biochem. Physiol. 31, 333–341.
Maisey, J. G. (2001). Remarks on the inner ear of elasmobranchs and its interpretation from skeletal labyrinth morphology. J. Morphol. 250, 236–264. doi: 10.1002/jmor.1068
Mann, D. A., Higgs, D. M., Tavolga, W. N., Souza, M. J., and Popper, A. N. (2001). Ultrasound detection by clupeiform fishes. J. Acoust. Soc. Am. 109, 3048–3054. doi: 10.1121/1.1368406
Mann, D. A., Lu, Z., Hastings, M., and Popper, A. N. (1998). Detection of ultrasonic tones and simulated dolphin echolocation clicks by a teleost fish, the American shad (Alosa sapidissima). J. Acoust. Soc. Am. 104, 562–568 doi: 10.1121/1.423255
Mann, D. A., Lu, Z., and Popper, A. N. (1997). A clupeid fish can detect ultrasound. Nature 389:341. doi: 10.1038/38636
Mann, D. A., Popper, A. N., and Wilson, B. (2005). Pacific herring hearing does not include ultrasound. Bio. Lett. 1, 158–161. doi: 10.1098/rsbl.2004.0241
Markl, H. (1972). Aggression und Beuteverhalten bei Piranhas (Serrasalminae, Charaidae). Z. Tierpsychol. 30, 190–216.
Maruska, K. P., and Mensinger, A. F. (2015). Directional sound sensitivity in utricular afferents in the toadfish, Opsanus tau. J. Exp. Biol. 218, 1759–1766. doi: 10.1242/jeb.115345
Mathiesen, C. (1984). Structure and innervation of inner ear sensory epithelia in the European eel (Anguilla anguilla L.). Acta Zool. 65, 189–207. doi: 10.1111/j.1463-6395.1984.tb01041.x
Mathiesen, C., and Popper, A. N. (1987). The ultrastructure and innervation of the ear of the gar, Lepisosteus osseus. J. Morphol. 194, 129–142. doi: 10.1002/jmor.1051940203
McCormick, C. A., and Popper, A. N. (1984). Auditory sensitivity and psychophysical tuning curves in the elephant nose fish, Gnathonemus petersii. J. Comp. Physiol. A 155, 753–761. doi: 10.1007/BF00611592
McMahan, C. D., Chakrabarty, P., Sparks, J. S., Smith, W. L., and Davis, M. P. (2013). Temporal patterns of diversification across global cichlid biodiversity (Acanthomorpha: Cichlidae). PLoS ONE 8:e71162. doi: 10.1371/journal.pone.0071162
Mulligan, K. P., and Gauldie, R. W. (1989). The biological significance of the variation in crystalline morph and habit of otoconia in elasmobranchs. Copeia 1989, 856–871. doi: 10.2307/1445969
Mulligan, K. P., Gauldie, R. W., and Thomson, R. (1989). Otoconia from four New Zealand Chimaeriformes. US Fish Wildlife Serv. Fish. Bull. 87, 923–934.
Myrberg, A. A. (2001). The acoustical biology of elasmobranchs. Environ. Biol. Fishes 60, 31–45. doi: 10.1023/A:1007647021634
Myrberg, A. A., Mohler, M., and Catala, J. D. (1986). Sound production by males of a coral reef fish (Pomacentrus partitus): its significance to females. Anim. Behav. 34, 913–923. doi: 10.1016/S0003-3472(86)80077-X
Myrberg, A. A., and Spires, J. Y. (1980). Hearing in damselfishes: an analysis of signal detection among closely related species. J. Comp. Physiol. A 140, 135–144. doi: 10.1007/BF00606305
Nelson, E. M. (1955). The morphology of the swim bladder and auditory bulla in the Holocentridae. Fieldiana Zool. 37, 121–130. doi: 10.5962/bhl.title.2938
Nolf, D. (1985). Otolithi Piscium. Handbook of Paleoichthyology 10. Stuttgart, New York, NY: Gustav Fischer.
O'Connell, C. P. (1955). The gas bladder and its relation to the inner ear in Sardinops caerulea and Engraulis mordax. Fish. Bull. 56, 505–533.
Oliveira, A. M., and Farina, M. (1996). Vaterite, calcite, and aragonite in the otoliths of three species of piranha. Naturwissenschaften 83, 133–135. doi: 10.1007/BF01142180
Pannella, G. (1971). Fish otoliths: daily growth layers and periodical patterns. Science 173, 1124–1127. doi: 10.1126/science.173.4002.1124
Parmentier, E., Lagardere, F., and Vandewalle, P. (2002). Relationships between inner ear and sagitta growth during ontogenesis of three Carapini species, and consequences of life-history events on the otolith microstructure. Mar. Biol. 141, 491–501. doi: 10.1007/s00227-002-0853-2
Parmentier, E., Vandewalle, P., and Lagardere, F. (2001). Morpho-anatomy of the otic region in carapid fishes: eco-morphological study of their otoliths. J. Fish Biol. 58, 1046–1061. doi: 10.1111/j.1095-8649.2001.tb00554.x
Paxton, J. R. (2000). Fish otoliths: do sizes correlate with taxonomic group, habitat and/or luminescence? Phil. Trans. R. Soc. London Ser. B Biol. Sci. 355, 1299–1303. doi: 10.1098/rstb.2000.0688
Platt, C. (1977). Hair cell distribution and orientation in goldfish otolith organs. J. Comp. Neurol. 172, 283–297. doi: 10.1002/cne.901720207
Platt, C. (1994). Hair cells in the lagenar otolith organ of the coelacanth are unlike those in amphibians. J. Morphol. 220, 381.
Platt, C., Jørgensen, J. M., and Popper, A. N. (2004). The inner ear of the lungfish Protopterus. J. Comp. Neurol 471, 277–288. doi: 10.1002/cne.20038
Platt, C., and Popper, A. N. (1981a). “Fine structure and function of the ear,” in Hearing and Sound Communication in Fishes, eds W. N. Tavolga, A. N. Popper, and R. R. Fay (New York, NY: Springer-Verlag), 3–38.
Platt, C., and Popper, A. N. (1981b). “Otolith organ receptor morphology in herring-like fishes,” in The Vestibular System: Function and Morphology, ed T. Gualtierotti (New York, NY: Springer-Verlag), 64–76.
Poggendorf, D. (1952). Die absolute Hörschwelle des Zwergwelses (Amiurus nebulosus) und Beiträge zur Physik des Weberschen Apparates der Ostariophysen. Z. Vergl. Physiol. 34, 222–257. doi: 10.1007/BF00298202
Popper, A. N. (1976). Ultrastructure of auditory regions in inner ear of Lake whitefish. Science 192, 1020–1023. doi: 10.1126/science.1273585
Popper, A. N. (1977). Scanning electron microscopic study of sacculus and lagena in ears of fifteen species of teleost fishes. J. Morphol. 153, 397–417. doi: 10.1002/jmor.1051530306
Popper, A. N. (1978). Scanning electron microscopic study of the otolithic organs in the Bichir Polypterus bichir and Shovel-nose sturgeon Scaphirhynchus platorynchus. J. Comp. Neurol. 181, 117–128. doi: 10.1002/cne.901810107
Popper, A. N. (1979). Ultrastructure of the sacculus and lagena in a moray eel (Gymnothorax sp.). J. Morphol. 161, 241–256. doi: 10.1002/jmor.1051610302
Popper, A. N. (1980). Scanning electron microscopic study of the sacculus and lagena in several deep-sea fishes. Am. J. Anat. 157, 115–136. doi: 10.1002/aja.1001570202
Popper, A. N. (1981). Comparative scanning electron microscopic investigation of the sensory epithelia in the teleost sacculus and lagena. J. Comp. Neurol. 200, 357–374. doi: 10.1002/cne.902000306
Popper, A. N. (2011). “Auditory system morphology,” in Encyclopedia of Fish Physiology: From Genome to Environment, Vol. 1. ed A. D. Farrell (Amsterdam: Academic Press), 252–261.
Popper, A. N., and Coombs, S. (1982). The morphology and evolution of the ear in actinopterygian fishes. Am. Zool. 22, 311–328. doi: 10.1093/icb/22.2.311
Popper, A. N., and Fay, R. R. (1977). Structure and function of elasmobranch auditory system. Am. Zool. 17, 443–452. doi: 10.1093/icb/17.2.443
Popper, A. N., and Fay, R. R. (1993). Sound detection and processing by fish - critical review and major research questions. Brain Behav. Evol. 41, 14–38. doi: 10.1159/000113821
Popper, A. N., and Fay, R. R. (2011). Rethinking sound detection by fishes. Hear. Res. 273, 25–36. doi: 10.1016/j.heares.2009.12.023
Popper, A. N., Fay, R. R., Platt, C., and Sand, O. (2003). “Sound detection mechanisms and capabilities of teleost fishes,” in Sensory Processing in Aquatic Environments, eds S. P. Collin and N. J. Marshall (New York, NY: Springer), 3–38.
Popper, A. N., and Northcutt, R. G. (1983). Structure and innervation of the inner ear of the bowfin, Amia calva. J. Comp. Neurol. 213, 279–286. doi: 10.1002/cne.902130304
Popper, A. N., and Platt, C. (1979). Herring has a unique receptor pattern. Nature 280, 832–833. doi: 10.1038/280832a0
Popper, A. N., and Platt, C. (1983). Sensory surface of the saccule and lagena in the ears of ostariophysan fishes. J. Morphol. 176, 121–129. doi: 10.1002/jmor.1051760202
Popper, A. N., Ramcharitar, J. U., and Campana, S. E. (2005). Why otoliths? Insights from inner ear physiology and fisheries biology. Mar. Freshw. Res. 56, 497–504. doi: 10.1071/MF04267
Popper, A. N., and Schilt, C. R. (2008). “Hearing and acoustic behavior: basic and applied considerations,” in Fish Bioacoustics, eds J. F. Webb, R. R, Fay, and A. N. Popper (New York, NY: Springer Science and Business Media), 17–48.
Popper, A. N., and Tavolga, W. N. (1981). Structure and function of the ear in the marine catfish, Arius felis. J. Comp. Physiol. A 144, 27–34. doi: 10.1007/BF00612794
Poulson, T. L. (1963). Cave adaptation in amblyopsid fishes. Am. Midl. Nat. 70, 257–290. doi: 10.2307/2423056
Radford, C., Stanley, J. A., Simpson, S. D., and Jeffs, A. G. (2011). Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30, 295–305. doi: 10.1007/s00338-010-0710-6
Ramcharitar, J. U., Deng, X., Ketten, D., and Popper, A. N. (2004). Form and function in the unique inner erar of the teleost: silver perch (Bairdiella chrysoura). J. Comp. Neurol. 475, 571–539. doi: 10.1002/cne.20192
Ramcharitar, J. U., Higgs, D. M., and Popper, A. N. (2001). Sciaenid inner ears: a study in diversity. Brain Behav. Evol. 58, 152–162. doi: 10.1159/000047269
Ramcharitar, J. U., Higgs, D. M., and Popper, A. N. (2006). Audition in sciaenid fishes with different swim bladder-inner ear configurations. J. Acoust. Soc. Am. 119, 439–443. doi: 10.1121/1.2139068
Remage-Healey, L., Nowacek, D. P., and Bass, A. H. (2006). Dolphins foraging sounds suppress calling and elevate stress hormone levels in prey species, the Gulf toadfish. J. Exp. Biol. 209, 4444–4451. doi: 10.1242/jeb.02525
Retzius, G. (ed.). (1881). “Das Gehörorgan der Fische und Amphibien,” in Das Gehörorgan der Wirbelthiere, Vol. 1. Stockholm: Samson & Wallin.
Rogers, P. H., and Cox, H. (1988). “Underwater sound as a biological stimulus,” in Sensory Biology of Aquatic Animals, eds J. Atema, R. R. Fay, and A. N. Popper (New York, NY:Springer), 131–149.
Rosauer, E. A., and Redmond, J. R. (1985). Comparative crystallography of vertebrate otoconia. J. Laryng. Otol. 99, 21–28. doi: 10.1017/S0022215100096249
Sand, O., and Enger, P. S. (1973). Evidence for an auditory function of the swimbladder in the cod. J. Exp. Biol. 59, 405–414.
Sand, O., and Michelsen, A. (1978). Vibration measurements of perch saccular otolith. J. Comp. Physiol. A 123, 85–89. doi: 10.1007/BF00657346
Sapozhnikova, Y. P., Klimenkov, I. V., and Melnik, N. G. (2007). Peculiarities of morphological polarization of sensor elements of hearing saccular epithelium in Baikal Cottoidei. Sens. Syst. 21, 140–146.
Schack, H. B., Malte, H., and Madsen, P. T. (2008). The response of the Atlantic cod (Gadus morhua L.) to ultrasound-emitting predators: stress, behavioural changes or debilitation. J. Exp. Biol. 211, 2079–2086. doi: 10.1242/jeb.015081
Schneider, H. (1941). Die Bedeutung der Atemhöhle der Labyrinthfische für ihr Hörvermögen. Z. Vergl. Physiol. 29, 172–194. doi: 10.1007/BF00304447
Schuijf, A., and Hawkins, A. D. (eds.). (1976). Sound Reception in Fish. Amsterdam; Oxford; NewYork, NY: Elsevier Scientific Publishing Company.
Schulz-Mirbach, T., Heß, M., Metscher, B. D., and Ladich, F. (2013). A unique swim bladder-inner ear connection in a teleost fish revealed by a combined high-resolution microCT and 3D histological study. BMC Biol. 11:75. doi: 10.1186/1741-7007-11-75
Schulz-Mirbach, T., Heß, M., and Plath, M. (2011). Inner ear morphology in the Atlantic molly Poecilia mexicana - first detailed microanatomical study of the inner ear of a cyprinodontiform species. PLoS ONE 6:e27734. doi: 10.1371/journal.pone.0027734
Schulz-Mirbach, T., and Ladich, F. (2016). “Diversity of inner ears in fishes: possible contribution towards hearing improvements and evolutionary considerations,” in Fish Hearing and Bioacoustics: An Anthology in Honor of Arthur N. Popper and Richard R. Fay. ed J. A. Sisneros (Cham: Springer International Publishing AG), 343–394. doi: 10.1007/978-3-319-21059-9_16
Schulz-Mirbach, T., Ladich, F., Plath, M., Metscher, B. D., and Heß, M. (2014). Are accessory hearing structures linked to inner ear morphology? Insights from 3D orientation patterns of ciliary bundles in three cichlid species. Front. Zool. 11:25. doi: 10.1186/1742-9994-11-25
Schulz-Mirbach, T., Metscher, B. D., and Ladich, F. (2012). Relationship between swim bladder morphology and hearing abilities–A case study on Asian and African cichlids. PLoS ONE 7:e42292. doi: 10.1371/journal.pone.0042292
Sienknecht, U. J., Köppl, C., and Fritzsch, B. (2014). Evolution and development of hair cell polarity and efferent function in the inner ear. Brain Behav. Evol. 83, 150–161. doi: 10.1159/000357752
Simpson, S. D., Meekan, M. G., Jeffs, A., Montgomery, J. C., and McCauley, R. D. (2008). Settlement-stage coral reef fish prefer the higher frequency invertebrate-generated audible component of reef noise. Anim. Behav. 75, 1861–1868. doi: 10.1016/j.anbehav.2007.11.004
Sisneros, J. A., and Rogers, P. H. (2016). “Directional hearing ans sound source localization in fishes,” in Fish Hearing and Bioacoustics: An Anthology in Honor of Arthur N. Popper and Richard R. Fay. ed J.A. Sisneros (Cham, Switzerland: Springer International Publishing AG), 121–156. doi: 10.1007/978-3-319-21059-9_7
Song, J., Mathieu, A., Soper, R. F., and Popper, A. N. (2006). Structure of the inner ear of bluefin tuna Thunnus thynnus. J. Fish Biol. 68, 1767–1781. doi: 10.1111/j.0022-1112.2006.01057.x
Speares, P., Holt, D., and Johnston, C. (2011). The relationship between ambient noise and dominant frequency of vocalization in two species of darters (Percidae: Etheostoma). Environ. Biol. Fish 90, 103–110. doi: 10.1007/s10641-010-9722-x
Stipetić, E. (1939). Über das Gehörorgan der Mormyriden. Z. Vergl. Physiol. 26, 740–752. doi: 10.1007/BF00341099
Straka, H., and Baker, R. (2011). “Vestibular system anatomy and physiology,” in Encyclopedia of Fish Physiology, From Genome to Environment, Vol. 1, ed A.P. Farrell (Amsterdam: Elsevier Academic Press), 244–251.
Tavolga, W. N., and Wodinsky, J. (1963). Auditory capacities in fishes. Pure tone thresholds in nine species of marine teleosts. Bull. Am. Mus. Nat. Hist. 126, 177–240.
Tester, A. L., Kendall, J. I., and Milisen, W. B. (1972). Morphology of the ear of the shark genus Carcharhinus, with particular reference to the macula neglecta. Pacific Sci. 26, 264–274.
Tolimieri, N., Jeffs, A., and Montgomery, J. C. (2000). Ambient sound as a cue for navigation by the pelagic larvae of reef fishes. Mar. Ecol. Progr. Ser. 207, 219–224. doi: 10.3354/meps207219
Vasconcelos, R. O., Fonseca, P. J., Amorim, M. C. P., and Ladich, F. (2011). Representation of complex vocalizations in the Lusitanian toadfish auditory system: evidence of fine temporal, frequency and amplitude discrimination. Proc. R. Soc. London Ser. B. Biol. Sci. 278, 826–834. doi: 10.1098/rspb.2010.1376
von Frisch, K. (1936). Über den Gehörsinn der Fische. Biol. Rev. 11, 210–246. doi: 10.1111/j.1469-185X.1936.tb00502.x
von Frisch, K., and Stetter, H. (1932). Untersuchungen über den Sitz des Gehörsinnes bei der Elritze. Z. vergl. Physiol. 17, 687–801. doi: 10.1007/BF00339067
Weber, E. H. (1819). Vergleichende Anatomie der Gehörwerkzeuge. Deutsches Archiv für die Physiologie 5, 323–332.
Weber, E. H. (1820). De Aure et Auditu Hominis et Animalium. Part I. De aure Animalium Aquatilium. Lipsiae: Apud Gerhardum Fleischerum.
Wilson, M., Montie, E. W., Mann, K. A., and Mann, D. A. (2009). Ultrasound detection in the Gulf menhaden requires gas-filled bullae and an intact lateral line. J. Exp. Biol. 212, 3422–3427. doi: 10.1242/jeb.033340
Wohlfahrt, T. A. (1932). Anatomische Untersuchungen über das Labyrinth der Elritze (Phoxinus laevis L.). Z. Vergl. Physiol. 17, 659–685. doi: 10.1007/BF00339066
Wohlfahrt, T. A. (1936). Das Ohrlabyrinth der Sardine (Clupea pilchardus Walb.) und seine Beziehungen zur Schwimmblase und Seitenlinie. Zoomorphology 31, 371–410. doi: 10.1007/bf00547260
Wolff, D. L. (1966). Akustische Untersuchungen zur Klapperfischerei und verwandter Methoden. Z. Fisch. Hilfswiss. 14, 277–315.
Wysocki, L. E., Amoser, S., and Ladich, F. (2007). Diversity in ambient noise in European freshwater habitats: noise levels, spectral profiles, and impact on fishes. J. Acoust. Soc. Am. 121, 2559–2566. doi: 10.1121/1.2713661
Wysocki, L. E., Codarin, A., Ladich, F., and Picciulin, M. (2009). Sound pressure and particle acceleration audiograms in three marine fish species from the Adriatic Sea. J. Acoust. Soc. Am. 126, 2100–2107. doi: 10.1121/1.3203562
Yan, H. Y. (1998). Auditory role of the suprabranchial chamber in gourami fish. J. Comp. Physiol. A. 183, 325–333. doi: 10.1007/s003590050259
Yan, H. Y., and Curtsinger, W. S. (2000). The otic gasbladder as an ancillary structure in a mormyrid fish. J. Comp. Physiol. A 186, 595–602. doi: 10.1007/s003590000114
Keywords: inner ears, hearing, accessory auditory structures, weberian ossicles, audiograms, swim bladder
Citation: Ladich F and Schulz-Mirbach T (2016) Diversity in Fish Auditory Systems: One of the Riddles of Sensory Biology. Front. Ecol. Evol. 4:28. doi: 10.3389/fevo.2016.00028
Received: 30 October 2015; Accepted: 10 March 2016;
Published: 31 March 2016.
Edited by:
Shaun Collin, The University of Western Australia, AustraliaCopyright © 2016 Ladich and Schulz-Mirbach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Friedrich Ladich, ZnJpZWRyaWNoLmxhZGljaEB1bml2aWUuYWMuYXQ=
 Friedrich Ladich
Friedrich Ladich Tanja Schulz-Mirbach
Tanja Schulz-Mirbach