
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 15 March 2016
Sec. Behavioral and Evolutionary Ecology
Volume 4 - 2016 | https://doi.org/10.3389/fevo.2016.00022
This article is part of the Research TopicFitness Costs and Benefits of Female SongView all 18 articles
Elaborate plumages and songs in male birds provide classic evidence for Darwinian sexual selection. However, trait elaboration in birds is not gender-restricted: female song has recently been revealed as a taxonomically-widespread trait within the songbirds (oscine Passerines), prompting increased research into likely functions and social/ecological correlates. Here we use phylogenetically-informed comparative analysis to test for an evolutionary association between female song and plumage color elaboration in songbirds. If there is an evolutionary trade-off between signaling modes, we predict a negative correlation between acoustic and visual elaboration. This trade-off hypothesis has been commonly proposed in males but has mixed empirical support. Alternatively, if song and plumage have similar or overlapping functions and evolve under similar selection pressures, we predict a positive correlation between female song and female plumage elaboration. We use published data on female song for 1023 species of songbirds and a novel approach that allows for the reliable and objective comparison of color elaboration between species and genders. Our results reveal a significant positive correlation between female colorfulness and female song presence. In species where females sing, females (but not males) are on average more colorful—with concomitantly reduced average sexual dichromatism. These results suggest that female plumage and female song likely evolved together under similar selection pressures and that their respective functions are reinforcing. We discuss the potential roles of sexual vs. social selection in driving this relationship, and the implications for future research on female signals.
Elaborate male traits often provide evidence for classic sexual selection (Darwin, 1871), but the possession of elaborate traits by females is less well understood. A traditional perspective holds that female trait elaboration is non-adaptive, perhaps detrimental, and results from “shared genetic architecture” with males (Darwin, 1871; Lande, 1980; see Tobias et al., 2012 for review). While current evidence does suggest that female ornamentation is correlated with conspecific male ornamentation to some extent (Bonduriansky and Chenoweth, 2009; Dale et al., 2015a) the view that elaborate female traits are purely non-adaptive pleiotropic effects has been strongly refuted by recent research.
Bird song and elaborate plumage are often considered costly traits (Song: Oberweger and Goller, 2001; Nowicki et al., 2002; Berg et al., 2005; Schmidt et al., 2013; but see Gil et al., 2006. Plumage: McGraw et al., 2002; Walther and Clayton, 2005; Simpson et al., 2015). In females, both traits can be evolutionarily labile with respect to the conspecific male phenotype. First, female plumage changes have played a greater role than male changes in the evolution of dichromatism in New World blackbirds (Icteridae; Irwin, 1994; Hofmann et al., 2008; Price and Eaton, 2014), tanagers (Thraupidae, Burns, 1998) and fairy-wrens (Maluridae, Johnson et al., 2013) and this pattern was shown to hold true for the order Passeriformes as a whole (Dale et al., 2015a).
Second, female song has been recovered as the ancestral condition of songbirds (Odom et al., 2014), indicating that multiple losses of female song have occurred over evolutionary time despite conspecific males retaining the trait (Price, 2015). This shows that female traits are not necessarily tightly constrained by the male phenotype, but are able to evolve rapidly and independently.
Furthermore, female-specific functions of song and plumage ornamentation have been resolved for a growing number of species, revealing these traits to be potentially adaptive for females. For instance, female song has been shown to function in territory and resource defense, mate attraction, mate defense, and pair-bonding (Searcy and Yasukawa, 1995; Langmore et al., 1996; Rogers et al., 2007; Brunton et al., 2008; Templeton et al., 2011; Hall et al., 2015). Female coloration is attractive to mates in some species (Amundsen et al., 1997; Smiseth and Amundsen, 2000; Murphy et al., 2009a,b) and frequently has roles in female-female competition for non-sexual breeding resources (Tobias et al., 2012; Morales et al., 2014).
If elaborated female traits are adaptive, this raises the question: what is the evolutionary relationship between female song and plumage color elaboration? We outline three hypotheses regarding the evolution of multiple elaborate traits: the traits may evolve “in a concerted fashion, in an antagonistic fashion, or in ways unrelated to each other” (Shutler and Weatherhead, 1990). In our context, these options are detailed as follows:
(i) The “trade-off” hypothesis (Darwin, 1871) argues that if two modes of signaling are both costly to produce or maintain, then selection might favor doing one thing well rather than two things badly. This predicts an evolutionary trade-off (an inverse correlation) between signaling modes (Shutler and Weatherhead, 1990). Thus, species with female song are predicted to have less colorful females on average than species lacking female song.
(ii) The “reinforcing signals” hypothesis (inspired by the “redundant signal” hypothesis of Møller and Pomiankowski, 1993) proposes that the two ornamental traits have overlapping, reinforcing functions, acting in concert to convey the condition or status of the signaler. Whereas either trait on its own provides a partial indication of signaler condition or status, in combination the multiple ornaments enable a more accurate assessment by rivals or mates. This predicts a co-evolution of the two traits; that is, a positive correlation between song and plumage color elaboration in females. Thus, species with female song are predicted to have more colorful females on average than species lacking female song.
(iii) The “multiple messages” hypothesis (adapted from Møller and Pomiankowski, 1993) posits that the two traits reveal different information about their bearers. This implies that the characters are driven by different selective pressures, and therefore elaborate vocal and visual sexual signals should evolve independently. Thus, there should be no difference in female color elaboration between species with female song, and those without female song.
Current evidence is inconclusive regarding the three hypotheses. In line with the trade-off hypothesis, an inverse relationship has been found between male plumage brightness and song complexity in cardueline finches (Badyaev et al., 2002). But in support of the reinforcing signals hypothesis, a positive correlation has been found between song length and number of colored patches among Asian barbets (Gonzalez-Voyer et al., 2013), and a positive correlation between the degree of dichromatism and time spent singing among wood warblers (Shutler and Weatherhead, 1990). Finally, Ornelas et al. (2009) found no relationship between dichromatism and song complexity among trogons, and Mason et al. (2014) found no correlation between song and plumage complexity among the tanagers. These conflicting results likely reflect biological and evolutionary differences among focal taxa, as well as methodological differences in how song and plumage were quantified (Mason et al., 2014). Furthermore, all these studies focus on males. The possibility of a generalized macroevolutionary association between multiple ornamental traits in females has not yet been investigated.
In this study we test for an evolutionary correlation between female song and plumage elaboration (male, female and dichromatism) across the songbirds (i.e., Oscines; order Passeriformes, suborder Passeri). We perform phylogenetically-informed comparative analysis using song and plumage data from repositories supplemented with additional data gleaned from the literature.
We compiled data describing the presence or absence of song in male and female songbirds (Oscines) from Odom et al. (2014) and del Hoyo et al. (2004–2011). We gave each species one of four scores according to the criteria of Odom et al. (2014, full details therein). Scores included: “present,” both males and females of the species sing; “absent,” only the male sings; “songless,” neither sex sings; or “not enough information” if we could not reliably make a designation. (Note that no species where only females sing has been described.) Out of all 4814 songbird species, 1314 had sufficient information on song to reliably score the species. Because the lack of female song in songless species might be the product of different selection pressures than in species with male song, we omitted songless species (291 species) from our analysis. Our final species pool included 1023 singing species comprised of 656 species where both sexes sing (64%), and 367 species where only males sing (36%).
Plumage color scores for the 1023 songbird species were obtained from Dale et al. (2015b). Briefly, for both sexes of each species of passerine (Order: Passeriformes), the mean red, green and blue (RGB) values on 3 dorsal and 3 ventral patches were measured using digital image processing software (Valcu and Dale, 2013) on scanned images from handbook plates. For each patch of each sex of each species, it was determined how “male-like” that patch is by scoring the proportion of males in the nearest 1% of similarly colored patches in other species. The method results in scores where low values correspond to males or females with drab, classically-“female-like” plumage, and high scores correspond to males or females with elaborate, classically-“male-like” plumage. This approach is transferable to other color quantification methods. There is a high correlation between scores determined with handbook plates vs. analogous scores determined with UV-VIS (ultraviolet to visible) reflectance spectra from museum specimens (Dale et al., 2015a). This result provides critical validation of the method because although human and avian vision have considerable overlap (Badyaev and Hill, 2003; Seddon et al., 2010), birds can also see UV light not visible to humans (Cuthill, 2006). See Dale et al. (2015a) for detailed methodology. Sexual dichromatism was calculated for each species as the male plumage color score minus the female plumage score.
To test for an evolutionary correlation between female song and female plumage elaboration, we first performed Pagel's correlation test (Pagel, 1994), in R 3.1.2 (R Development Core Team, 2014) using the “geiger” and “phytools” packages (Harmon et al., 2008; Revell, 2012). The Pagel test controls for phylogenetic relatedness and requires no designation of independent and response variables. We assigned a song character state and a plumage character state to each tip of a phylogeny, and tested the null hypothesis that the two traits had evolved independently. As the Pagel test requires both traits to be binary, plumage scores were binned into binary characters according to an arbitrary cut-off, which was moved in integer increments from 35 (1022 of 1023 species with female plumage elaboration present) to 71 (1 of 1023 species), to study the sensitivity of the correlation test to changes in plumage cutoff value. The phylogenies used for this analysis were obtained from the Hackett backbone (Hackett et al., 2008) supertrees at http://birdtree.org (Jetz et al., 2012).
To estimate the strength of the correlation between female song presence and female plumage elaboration, we fit a multivariate generalized linear mixed model using the “MCMCglmm” package (Monte Carlo Markov Chain generalized linear mixed model; Hadfield, 2010) in R (version 3.1.2). MCMCglmm allowed us to fit a model which had a response vector that contained a mixture of Gaussian and non-Gaussian distributed variables. Female plumage elaboration is a continuous measure (see above); hence we assumed a Gaussian error distribution. For female song, the response vector contained binary presence scores (0 = female song absent, and 1 = female song present) and accordingly we assumed a Bernouli error distribution and used a logit link function. Fixed effects in our model included female plumage elaboration, the presence of female song, and male plumage elaboration as a covariate. Phylogeny was fit as a random effect using the methods described in Hadfield and Nakagawa (2010) to calculate the inverse numerator relationship for phylogenetic effects. For the phylogenetic effects we also allowed separate random intercepts for female song and female plumage elaboration and a non-zero covariance between these two traits by assuming an unstructured variance-covariance structure.
Priors for the location effects were diffuse about zero and had a large variance (108). For the variance components we used priors conforming to a scaled non-central F-distribution (Gelman, 2006) with the location parameter equal to zero. The scale parameter for female color elaboration was equal to half of the phenotypic variation in female color elaboration, and for female song the scale parameter was equal to p(1−p), where p is the mean probability of female song across the dataset. For the residual covariance matrix we assumed an inverse-Wishart distributed prior for female coloration. For female song (which is a binary trait), it is not possible to estimate a residual variance, so we fixed the prior at a value of 1 (Hadfield, 2014). The MCMC chain had 20,600,000 iterations, with a burn-in of 600,000 and a thinning interval of 20,000, resulting in ~1000 samples of the posterior distribution of the parameters. Model fit was confirmed by ensuring that autocorrelation was low and the trait means lay within the 95% highest posterior density (HPD) intervals of the posterior predictive distribution of each trait. To incorporate some of the uncertainty in the phylogenetic relationships among bird species, we applied the statistical model described above to 10 different phylogenetic trees randomly selected from http://birdtree.org (Jetz et al., 2012). Finally, we examined the convergence of the phylogenetic variances and covariances estimated from the 10 models (each using different trees, and therefore with different numerator relationship matrices for the phylogenetic effects), with the Gelman and Rubin (1992) diagnostic, R. For these 10 trees, the point estimate was R = 1.2 indicating moderate convergence. This phylogenetic uncertainly is incorporated in all the estimates of the posterior means and the HPD intervals we present.
To assess the significance of the phylogenetic correlation between female song (FS) and female plumage elaboration (FP), we first calculated the posterior distribution of the correlation using
If the 95% HPD intervals of the posterior distribution of the correlation did not overlap zero, we interpreted this as evidence for a significant phylogenetic correlation between female plumage elaboration and female song. Similarly, to assess the significance of the association between male color elaboration and our two female traits, we tested whether the 95% HPD intervals of the fixed interactions between male plumage and female plumage elaboration, as well as between male plumage and female song, overlapped zero.
For many species in our sample, males and females have similar plumage elaboration scores (Figure 1A, points lying along the diagonal). However, there are also many sexually dichromatic species where the male is more colorful and the female is more drab (Figure 1A, points in the upper left of the distribution). Overlaying the density of female song presence on this plumage elaboration scatter (Figure 1B) reveals that female song presence is most concentrated amongst species with high female plumage scores and reduced average sexual dichromatism.
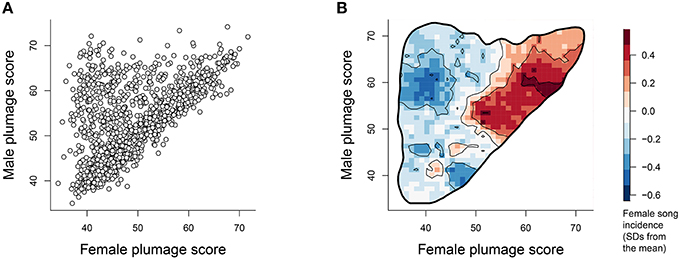
Figure 1. (A) Male plumage score vs. female plumage score for each species (N = 1023). (B) Contour map depicting the average of the (scaled) female song values overlaid within the 99.8% volume contour of the male vs. female plumage score distribution. Values within the plot were calculated by superimposing a 50 × 50 grid over the scatter occurring between 30 and 80 and then calculating at each grid point the mean female song scores of the closest 3% of species. The units represent standard deviations away from the mean incidence of song, with redder values indicating increasingly higher occurrence of female song.
The phylogenetic distribution of the co-occurrence of female elaboration traits is visualized in Figure 2, where color-coded species tips correspond to the presence/absence of female song and female plumage elaboration. Instances where both traits co-occur (257 species, 25% of the total sample) are not concentrated within one or a few clades but are dispersed throughout the tree, suggesting many separate origins of the co-evolution of these traits (Figure 2). Wide phylogenetic dispersion persists when higher plumage elaboration cutoff values or different trees from Jetz et al. (2012) are used. Also numerous and widely dispersed around the tree are species with only one trait or the other: female song but not female plumage elaboration (399 species, 39% of the total sample); female plumage elaboration but not female song (72 species, 7% of the total sample), and neither trait present (295 species, 29% of the total sample).
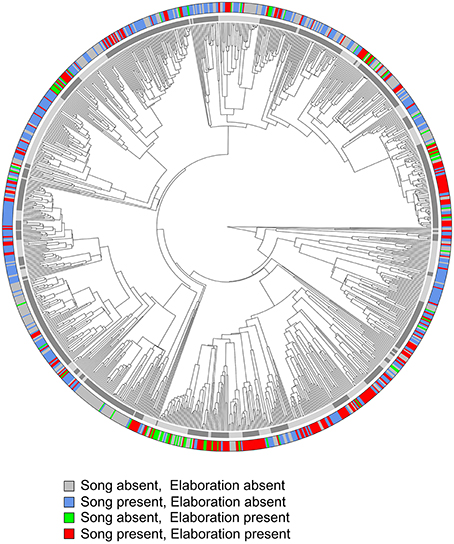
Figure 2. Phylogenetic distribution of female song and female plumage elaboration. Plumage elaboration is here treated as a binary character with cutoff = plumage score of 50. Red labels represent species where female song and female plumage elaboration (plumage score >50) co-occur. The inner ring of alternating gray tones depicts boundaries of the 86 oscine families in our sample.
The Pagel test provides strong support for correlated evolution of female song presence and female plumage elaboration for all plumage cut-off values between 41 and 54 (Figure 3, P < 0.0001). This is a large range by comparison to the entire range of female plumage scores (35–71), and even at a cut-off as high as 60, the correlation is still statistically significant at the 5% level. The presence of an evolutionary association between female song and female color elaboration is therefore highly robust to alternative cut-off values used to categorize female coloration as elaborated.
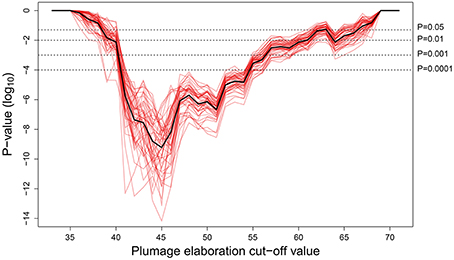
Figure 3. Pagel correlation test P-values (Pagel, 1994) at all possible plumage elaboration cutoff values, for each of 50 phylogenetic trees from http://birdtree.org (Jetz et al., 2012). The black line indicates the mean of the 50 runs (red lines). Evidence of correlated evolution is strong (<0.0001) for all plumage cut-off values between 41 and 54.
Despite the phylogenetic uncertainty introduced by using 10 randomly selected phylogenetic trees from Jetz et al. (2012), we found a strongly significant evolutionary correlation between female plumage elaboration and the presence of female song (Figure 4, mean cor(FS, FP) = 0.402, 95% HPD = 0.220–0.583). Accounting for evolutionary relationships among bird species we found that male and female color elaboration were positively associated (posterior mean = 0.472, 95% HPD = 0.422–0.527), confirming the apparent pattern seen in Figure 1. In addition there was a positive, but not statistically significant, relationship between the degree of male color elaboration and the presence of female song (posterior mean = 0.044, 95% HPD = −0.014–0.102).
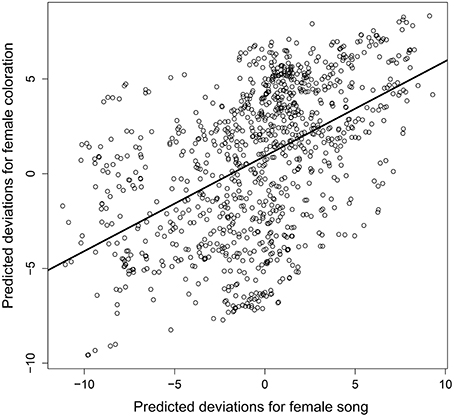
Figure 4. Plot of best linear unbiased predictions of the phylogenetic association between female song and female plumage color elaboration. Points indicate the predicted phylogenetic deviations of each species (i.e., the 1023 species tips within the pruned phylogenetic tree) for the two female display traits, and solid line denotes the posterior mean phylogenetic correlation between female song with female plumage.
We used phylogenetically-informed analysis to reveal the relationship between female song presence and plumage elaboration across the songbirds. Our study shows that (1) female song is more concentrated amongst species with elaborated (classically “male-like”) female plumage, (2) the co-occurrence of female song and female plumage elaboration is widely dispersed across the songbird phylogeny, suggesting many independent origins of this evolutionary association, (3) Pagel correlation tests demonstrate clear support for an evolutionary correlation between female song and female plumage color elaboration, and (4) estimates from MCMCglmm models suggest that the presence of female song accounts for 16.2% (R = 0.402) of the variation in female plumage elaboration (and vice versa).
Mason et al. (2014) provided the largest comparative study of multimodal sexual signaling to date, finding no relationship between plumage elaboration and song elaboration across the males of 301 tanager species (Thraupidae). In contrast, our results show a clear positive relationship between female song and female plumage color elaboration across the songbirds. The difference between our results and those of Mason et al. could stem from a number of factors. First, Mason et al. consider only male elaboration. The selective pressures acting on males and females are not necessarily equivalent, and so female traits may exhibit a different relationship to each other than do male traits (Tobias et al., 2012). Second, we use different methods of scoring song and plumage elaboration; notably, the song data of Mason et al. are quantitative, whereas our data are presence/absence. Finally, there are differences in scale between our studies. The presence of negative or non-significant relationships within some families such as the Thraupidae does not preclude a positive correlation at the broader taxonomic level of the songbirds as a whole. These considerations apply equally to other studies of multimodal signaling which find a negative relationship or no relationship between song and plumage (Badyaev et al., 2002; Ornelas et al., 2009).
Our results are most consistent with the “reinforcing signals” hypothesis; a positive correlation between female song and female plumage elaboration is expected if the two signaling modes tend to have reinforcing, overlapping functions, and thus have co-evolved together in response to similar selective pressures. Females may use song and plumage as a multimodal signal to reinforce the reliability and/or potency of the message to receivers.
A central tenet of sexual selection theory is that males and females often differ in their routes to reproductive success; for males, competition for mates is paramount, whereas for females, access to resources that affect fecundity is thought to be of greater importance (Rosvall, 2011). Therefore, the primary selection operating on females is likely to be non-sexual “social selection” for ecological or social resources, such as foraging territories, nest sites and paternal investment (West-Eberhard, 1979, 1983; Tobias et al., 2012). Such a view would suggest that a key function of both female song and plumage color elaboration may be signaling the female status (or resource-holding potential: Searcy and Nowicki, 2005) to competitive rivals, a prediction that is borne out in a number of studies (Tobias et al., 2012). This is not to deny that sexual selection for elaborate female traits may also be important (Clutton-Brock, 2007; Rosvall, 2011; Clutton-Brock and Huchard, 2013); indeed, the same ornaments can perform both sexual and (non-sexual) social functions (Kraaijeveld et al., 2004).
The co-occurrence of both visual and acoustic indicators of status (or resource-holding potential) likely reinforces the overall message and facilitates effective communication under different signaling scenarios. Song can be communicated over longer distances, without a clear line of sight; and as sound is propagated radially (Fahy and Gardonio, 2007), vocal communication does not depend on precise directionality of the signaler in relation to the receiver. Song is amenable to rapid temporal changes, conveying the short-term intentions of the individual and encoding complex information about signaler identity. Plumage coloration, by contrast, is a more permanent feature (though birds may be able to mediate plumage display behaviorally). In general, plumage coloration is a more direction-sensitive close-range signal than song. Thus, to signal quality and ward off rivals, selection could favor song for long-range broadcasting and plumage elaboration for close encounters, even if the message and intended receiver are the same.
If differing signaling scenarios promote female song or plumage elaboration differentially, this might explain the many species in our sample with only one trait or the other. Given that 16.2% of variation in female plumage elaboration is explained by female song presence (and vice versa), a remaining 83.8% of variation in each trait is thus attributable to other factors. That is, the relationship between female song and female plumage elaboration is complex and likely mediated by additional effects of, for example, habitat type, predation risk, territory size, and social structure. Closed habitat might strongly favor female song for effective communication if the range and efficacy of visual signaling is impaired. Or, for species experiencing high predation, elaborate female plumage may impose too great a risk around the nest (Martin and Badyaev, 1996) and be selected against. Or, in colony-living species where individuals remain in close proximity, close-range visual signaling may be sufficient for female signaling needs, making female song redundant. In short, there is much work left to do in identifying and quantifying the factors that contribute to visual and acoustic ornamentation in female songbirds.
If song and elaborate plumage are both costly (e.g., Oberweger and Goller, 2001; McGraw et al., 2002), as expected if the signals are “honest” indicators of aspects of female quality, then why have we not observed a trade-off between signaling modes? There are a number of potential explanations. First, while our results demonstrate that presence of female song does not trade off with female plumage elaboration, we lack information on the degree of female song elaboration (e.g., in terms of repertoire size, vocal agility, and time spent singing) and thus we cannot evaluate whether there is some degree of trade-off of resource allocation between modes. As more studies focus on quantifying the vocal performance of female birds, addressing whether singing females with bright plumage have “cheaper” songs than those with drab plumage will be practicable. It is also conceivable that when comparing across species, trade-offs might not manifest if different species are selected to invest different levels of resources into the overall message.
Another possibility is that resources involved in song and plumage development are not limiting for species where status signaling is strongly favored by selection. Rather than functioning as condition-dependent indices or handicaps, the honesty of these signals may instead be socially enforced, with cheaters (i.e., individuals who signal having greater status than they actually have) being punished through increased aggression by conspecifics (Tibbetts and Dale, 2004; Tibbetts and Izzo, 2010). The social cost in such cases will depend on how accurately the signal reflects true quality of the individual, rather than the number of ornaments involved in the signal per se. That is, under social costs, female song and plumage elaboration may be no more costly to produce than either trait alone, in which case we would not expect a trade-off between traits.
We have demonstrated strong evidence for a positive co-evolutionary relationship between plumage elaboration and song in female songbirds, a result which supports an overlapping function of the two traits (i.e., the reinforcing signals hypothesis). We have suggested, in light of current selection theory (Tobias et al., 2012), that the primary context for this multimodal signaling is non-sexual social competition for ecological or social resources, and that the different signaling ranges of plumage and song may have favored the evolutionary maintenance of both traits. Our finding raises several questions for future research, including: (1) Does this pattern hold for the sub-oscines? Though generally poorer singers than the songbirds, and lacking vocal learning (but see Kroodsma et al., 2013), many sub-oscines vocalize for mate attraction and territorial defense (Chelén et al., 2005) and thus may be subject to similar evolutionary pressures. (2) Are female ornaments gained and lost more frequently than male ornaments (Kraaijeveld, 2014) and is there a consistent order of female trait evolution? That is, do gains (or losses) of song follow gains (or losses) in plumage elaboration, or vice versa? And (3), what are the social and ecological drivers of the evolutionary association of female song and female plumage elaboration? It is our hope that the patterns reported in this study contribute to future research on the functions of both visual and acoustic ornamentation in females.
WW and DB were supported by a Marsden Fund Grant (13-MAU-004) from the Royal Society of New Zealand.
Conceived of the study: WW, JD, and DB; collected the data: JD, MV, WW; analyzed the data: WW, JD, JA, DT; wrote the paper: WW, JD, and JA with input from the other authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Guest Associate Editors Michelle Hall and Naomi Langmore for initiating this special issue on female song, and the many ornithologists and scientists who have contributed data which made this study possible. Thanks to Aaron Harmer for his helpful comments on the manuscript.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2016.00022
Amundsen, T., Forsgren, E., and Hansen, L. T. T. (1997). On the function of female ornaments: male bluethroats prefer colourful females. Proc. Biol. Sci. 264, 1579–1586. doi: 10.1098/rspb.1997.0220
Badyaev, A. V., and Hill, G. E. (2003). Avian sexual dichromatism in relation to phylogeny and ecology. Annu. Rev. Ecol. Evol. Syst. 34, 27–49. doi: 10.1146/annurev.ecolsys.34.011802.132441
Badyaev, A. V., Hill, G. E., and Weckworth, B. V. (2002). Species divergence in sexually selected traits: increase in song elaboration is related to decrease in plumage ornamentation in finches. Evolution 56, 412–419. doi: 10.1111/j.0014-3820.2002.tb01350.x
Berg, M. L., Beintema, N. H., Welbergen, J. A., and Komdeur, J. (2005). Singing as a handicap: the effects of food availability and weather on song output in the Australian reed warbler Acrocephalus australis. J. Avian Biol. 36, 102–109. doi: 10.1111/j.0908-8857.2005.03285.x
Bonduriansky, R., and Chenoweth, S. F. (2009). Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288. doi: 10.1016/j.tree.2008.12.005
Brunton, D. H., Evans, B., Cope, T., and Ji, W. (2008). A test of the dear enemy hypothesis in female New Zealand bellbirds (Anthornis melanura): female neighbors as threats. Behav. Ecol. 19, 791–798. doi: 10.1093/beheco/arn027
Burns, K. J. (1998). A phylogenetic perspective on the evolution of sexual dichromatism in tanagers (Thraupidae): the role of female versus male plumage. Evolution 52, 1219–1224. doi: 10.2307/2411252
Chelén, A. A. R., Garcia, C. M., and Riebel, K. (2005). Variation in the song of a sub-oscine, the vermilion flycatcher. Behaviour 142, 1115–1132. doi: 10.1163/156853905774405326
Clutton-Brock, T. (2007). Sexual selection in males and females. Science 318, 1882–1885. doi: 10.1126/science.1133311
Clutton-Brock, T. H., and Huchard, E. (2013). Social competition and selection in males and females. Philos. Trans. R. Soc. B Biol. Sci. 368, 20130074. doi: 10.1098/rstb.2013.0074
Cuthill, I. C. (2006). “Mechanisms and Measurements, Vol. 1,” in Bird Coloration, eds G. E. Hill and K. J. McGraw (Cambridge, MA: Harvard University Press), 3–40.
Dale, J., Dey, C. J., Delhey, K., Kempenaers, B., and Valcu, M. (2015a). The effects of life-history and sexual selection on male and female plumage coloration. Nature. 527, 367–370. doi: 10.1038/nature15509
Dale, J., Dey, C. J., Delhey, K., Kempenaers, B., and Valcu, M. (2015b). Data from: the effects of life history and sexual selection on male and female plumage colouration. Dryad Digit. Repository. doi: 10.5061/dryad.1rp0s
del Hoyo, J., Elliott, A., and Christie, D. (2004–2011). Handbook of the Birds of the World, Vol. 9–16. Barcelona: Lynx Edicions.
Fahy, F. J., and Gardonio, P. (2007). Sound and Structural Vibration: Radiation, Transmission and Response. Oxford, UK; Burlington, MA: Academic Press.
Gelman, A. (2006). Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper). Bayesian Anal. 1, 515–534. doi: 10.1214/06-BA117A
Gelman, A., and Rubin, D. B. (1992). Inference from iterative simulation using multiple sequences. Stat. Sci. 7, 457–472. doi: 10.1214/ss/1177011136
Gil, D., Naguib, M., Riebel, K., Rutstein, A., and Gahr, M. (2006). Early condition, song learning, and the volume of song brain nuclei in the zebra finch (Taeniopygia guttata). J. Neurobiol. 66, 1602–1612. doi: 10.1002/neu.20312
Gonzalez-Voyer, A., den Tex, R.-J., Castelló, A., and Leonard, J. A. (2013). Evolution of acoustic and visual signals in Asian barbets. J. Evol. Biol. 26, 647–659. doi: 10.1111/jeb.12084
Hackett, S. J., Kimball, R. T., Reddy, S., Bowie, R. C., Braun, E. L., Braun, M. J., et al. (2008). A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768. doi: 10.1126/science.1157704
Hadfield, J. D. (2010). MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. doi: 10.18637/jss.v033.i02
Hadfield, J. D. (2014). MCMCglmm Course Notes. Available online at: https://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf
Hadfield, J. D., and Nakagawa, S. (2010). General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508. doi: 10.1111/j.1420-9101.2009.01915.x
Hall, M. L., Rittenbach, M. R. D., and Vehrencamp, S. L. (2015). Female song and vocal interactions with males in a neotropical wren. Behav. Evol. Ecol. 3, 12. doi: 10.3389/fevo.2015.00012
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E., and Challenger, W. (2008). GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. doi: 10.1093/bioinformatics/btm538
Hofmann, C. M., Cronin, T. W., and Omland, K. E. (2008). Evolution of sexual dichromatism. 1. Convergent losses of elaborate female coloration in New World orioles (Icterus spp.). Auk 125, 778–789. doi: 10.1525/auk.2008.07112
Irwin, R. E. (1994). The evolution of plumage dichromatism in the New World blackbirds: social selection on female brightness. Am. Nat. 144, 890–907. doi: 10.1086/285717
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., and Mooers, A. O. (2012). The global diversity of birds in space and time. Nature 491, 444–448. doi: 10.1038/nature11631
Johnson, A. E., Jordan Price, J., and Pruett-Jones, S. (2013). Different modes of evolution in males and females generate dichromatism in fairy-wrens (Maluridae). Ecol. Evol. 3, 3030–3046. doi: 10.1002/ece3.686
Kraaijeveld, K. (2014). Reversible trait loss: the genetic architecture of female ornaments. Annu. Rev. Ecol. Evol. Syst. 45, 159–177. doi: 10.1146/annurev-ecolsys-120213-091550
Kraaijeveld, K., Gregurke, J., Hall, C., Komdeur, J., and Mulder, R. A. (2004). Mutual ornamentation, sexual selection, and social dominance in the black swan. Behav. Ecol. 15, 380–389. doi: 10.1093/beheco/arh023
Kroodsma, D., Hamilton, D., Sánchez, J. E., Byers, B. E., Fandiño-Mariño, H., Stemple, D. W., et al. (2013). Behavioral evidence for song learning in the suboscine bellbirds (Procnias spp.; Cotingidae). Wilson J. Ornithol. 125, 1–14. doi: 10.1676/12-033.1
Lande, R. (1980). Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305. doi: 10.2307/2407393
Langmore, N. E., Davies, N. B., Hatchwell, B. J., and Hartley, I. R. (1996). Female song attracts males in the alpine accentor Prunella collaris. Proc. R. Soc. Lond. B. Biol. Sci. 263, 141–146. doi: 10.1098/rspb.1996.0022
Martin, T. E., and Badyaev, A. V. (1996). Sexual dichromatism in birds: importance of nest predation and nest location for females versus males. Evolution 50, 2454–2460. doi: 10.2307/2410712
Mason, N. A., Shultz, A. J., and Burns, K. J. (2014). Elaborate visual and acoustic signals evolve independently in a large, phenotypically diverse radiation of songbirds. Proc. R. Soc. Lond. B Biol. Sci. 281, 20140967. doi: 10.1098/rspb.2014.0967
McGraw, K. J., Mackillop, E. A., Dale, J., and Hauber, M. E. (2002). Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 205, 3747–3755.
Møller, A. P., and Pomiankowski, A. (1993). Why have birds got multiple sexual ornaments? Behav. Ecol. Sociobiol. 32, 167–176. doi: 10.1007/BF00173774
Morales, J., Gordo, O., Lobato, E., Ippi, S., Martínez-de la Puente, J., Tomás, G., et al. (2014). Female-female competition is influenced by forehead patch expression in pied flycatcher females. Behav. Ecol. Sociobiol. 68, 1195–1204. doi: 10.1007/s00265-014-1730-y
Murphy, T. G., Hernández-Muciño, D., Osorio-Beristain, M., Montgomerie, R., and Omland, K. E. (2009a). Carotenoid-based status signaling by females in the tropical streak-backed oriole. Behav. Ecol. 20, 1000–1006. doi: 10.1093/beheco/arp089
Murphy, T. G., Rosenthal, M. F., Montgomerie, R., and Tarvin, K. A. (2009b). Female American goldfinches use carotenoid-based bill coloration to signal status. Behav. Ecol. 20, 1348–1355. doi: 10.1093/beheco/arp140
Nowicki, S., Searcy, W., and Peters, S. (2002). Brain development, song learning and mate choice in birds: a review and experimental test of the “nutritional stress hypothesis.” J. Comp. Physiol. A 188, 1003–1014. doi: 10.1007/s00359-002-0361-3
Oberweger, K., and Goller, F. (2001). The metabolic cost of birdsong production. J. Exp. Biol. 204, 3379–3388.
Odom, K. J., Hall, M. L., Riebel, K., Omland, K. E., and Langmore, N. E. (2014). Female song is widespread and ancestral in songbirds. Nat. Commun. 5:3379. doi: 10.1038/ncomms4379
Ornelas, J. F., González, C., and Espinosa De Los Monteros, A. (2009). Uncorrelated evolution between vocal and plumage coloration traits in the trogons: a comparative study. J. Evol. Biol. 22, 471–484. doi: 10.1111/j.1420-9101.2008.01679.x
Pagel, M. (1994). Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. Biol. Sci. 255, 37–45. doi: 10.1098/rspb.1994.0006
Price, J. J. (2015). Rethinking our assumptions about the evolution of bird song and other sexually dimorphic signals. Behav. Evol. Ecol. 3:40. doi: 10.3389/fevo.2015.00040
Price, J. J., and Eaton, M. D. (2014). Reconstructing the evolution of sexual dichromatism: current color diversity does not reflect past rates of male and female change. Evolution 68, 2026–2037. doi: 10.1111/evo.12417
R Development Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: http://www.R-project.org
Revell, L. J. (2012). phytools: an R package for phylogenetic comparative biology (and other things): phytools: R package. Methods Ecol. Evol. 3, 217–223. doi: 10.1111/j.2041-210X.2011.00169.x
Rogers, A. C., Langmore, N. E., and Mulder, R. A. (2007). Function of pair duets in the eastern whipbird: cooperative defense or sexual conflict? Behav. Ecol. 18, 182–188. doi: 10.1093/beheco/arl070
Rosvall, K. A. (2011). Intrasexual competition in females: evidence for sexual selection? Behav. Ecol. 22, 1131–1140. doi: 10.1093/beheco/arr106
Schmidt, K. L., Moore, S. D., MacDougall-Shackleton, E. A., and MacDougall-Shackleton, S. A. (2013). Early-life stress affects song complexity, song learning and volume of the brain nucleus RA in adult male song sparrows. Anim. Behav. 86, 25–35. doi: 10.1016/j.anbehav.2013.03.036
Searcy, W. A., and Nowicki, S. (2005). The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. Princeton, NJ: Princeton University Press.
Searcy, W. A., and Yasukawa, K. (1995). Polygyny and Sexual Selection in Red-Winged Blackbirds. Princeton, NJ: Princeton University Press.
Seddon, N., Tobias, J. A., Eaton, M., and Ödeen, A. (2010). Human vision can provide a valid proxy for avian perception of sexual dichromatism. Auk 127, 283–292. doi: 10.1525/auk.2009.09070
Shutler, D., and Weatherhead, P. J. (1990). Targets of sexual selection: song and plumage of wood warblers. Evolution 44, 1967–1977. doi: 10.2307/2409607
Simpson, R. K., Johnson, M. A., and Murphy, T. G. (2015). Migration and the evolution of sexual dichromatism: evolutionary loss of female coloration with migration among wood-warblers. Proc. R. Soc. Lond. B Biol. Sci. 282, 20150375. doi: 10.1098/rspb.2015.0375
Smiseth, P. T., and Amundsen, T. (2000). Does female plumage coloration signal parental quality? A male removal experiment with the bluethroat (Luscinia s. svecica). Behav. Ecol. Sociobiol. 47, 205–212. doi: 10.1007/s002650050657
Templeton, C. N., Rivera-Cáceres, K. D., Mann, N. I., and Slater, P. J. B. (2011). Song duets function primarily as cooperative displays in pairs of happy wrens. Anim. Behav. 82, 1399–1407. doi: 10.1016/j.anbehav.2011.09.024
Tibbetts, E. A., and Dale, J. (2004). A socially enforced signal of quality in a paper wasp. Nature 432, 218–222. doi: 10.1038/nature02949
Tibbetts, E. A., and Izzo, A. (2010). Social punishment of dishonest signalers caused by mismatch between signal and behavior. Curr. Biol. 20, 1637–1640. doi: 10.1016/j.cub.2010.07.042
Tobias, J. A., Montgomerie, R., and Lyon, B. E. (2012). The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2274–2293. doi: 10.1098/rstb.2011.0280
Valcu, M., and Dale, J. (2013). colorZapper: A Platform for Scoring Biological Coloration. R package version 1.0. Available online at: https://github.com/valcu/colorZapper
Walther, B. A., and Clayton, D. H. (2005). Elaborate ornaments are costly to maintain: evidence for high maintenance handicaps. Behav. Ecol. 16, 89–95. doi: 10.1093/beheco/arh135
West-Eberhard, M. J. (1979). Sexual selection, social competition, and evolution. Proc. Am. Philos. Soc. 123, 222–234.
Keywords: female song, multimodal signaling, oscine, Passeriformes, plumage coloration, trade-off, trait correlation
Citation: Webb WH, Brunton DH, Aguirre JD, Thomas DB, Valcu M and Dale J (2016) Female Song Occurs in Songbirds with More Elaborate Female Coloration and Reduced Sexual Dichromatism. Front. Ecol. Evol. 4:22. doi: 10.3389/fevo.2016.00022
Received: 06 November 2015; Accepted: 29 February 2016;
Published: 15 March 2016.
Edited by:
Peter Schausberger, University of Natural Resources and Life Sciences Vienna, AustriaReviewed by:
Ken Kraaijeveld, Vrije Universiteit Amsterdam, NetherlandsCopyright © 2016 Webb, Brunton, Aguirre, Thomas, Valcu and Dale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wesley H. Webb, dy53ZWJiQG1hc3NleS5hYy5ueg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.