- Lancaster Environment Centre, Lancaster University, Lancaster, UK
We have known about the processes of methanogenesis and methanotrophy for over 100 years, since the days of Winogradsky, yet their contributions to the carbon cycle were deemed to be of negligible importance for the majority of that period. It is only in the last two decades that methane has been appreciated for its role in the global carbon cycle, and stable isotopes have come to the forefront as tools for identifying and tracking the fate of methane-derived carbon (MDC) within food webs, especially within aquatic ecosystems. While it is not surprising that chemosynthetic processes dominate and contribute almost 100% to the biomass of organisms residing within extreme habitats like deep ocean hydrothermal vents and seeps, way below the reach of photosynthetically active radiation, it is perhaps counterintuitive to find reliance upon MDC in shallow, well-lit, well-oxygenated streams. Yet, apparently, MDC contributes to varying degrees across the spectrum from point sources to extremely diffuse sources. Certainly a good proportion of the evidence for MDC contributing to freshwater food webs comes from somewhere in the middle of that spectrum; from studies of seasonally stratifying lakes (mono- or dimictic) wherein, there is a defined gradient or boundary at which anoxic meet oxic conditions and consequently allows for close coupling of methanogenesis and methanotrophy. However, even seemingly well-mixed (polymictic) lakes have a contribution of MDC contributing to the benthic biomass, despite an almost continual supply of photosynthetic carbon being delivered from the surface. Aside from the fundamental importance of identifying the carbon sources fuelling biomass production, stable isotopes have been integral in the tool box of palaeolimnologists seeking to identify how contributions from methane have waxed and waned over time. Here, we synthesize the current state of knowledge in the use of stable isotopes to trace MDC in primarily freshwater ecosystems.
A Brief Synopsis on the Global Importance of Methane in Aquatic Systems, and Particularly in Freshwaters
The global carbon cycle was considered, until relatively recently, to be solely the flux and storage of carbon between the atmosphere, and terrestrial and oceanic pools. Within the total carbon budget, it has been noted that despite their relatively small area, inland freshwaters make a considerable contribution to the global methane (CH4) budget with emissions of CH4 from freshwaters being at least comparable to the terrestrial CH4 sink (Battin et al., 2009). However, there is a considerable bias toward data from lakes and other wetlands, and the role of rivers remains poorly defined (Bastviken et al., 2011). Emissions of CH4 may be small in terms of carbon, but one must consider that CH4 is a more potent greenhouse gas than CO2 over century time scales; (Bastviken et al., 2011) estimated that global CH4 emissions expressed as CO2 equivalents correspond to at least 25% of the estimated terrestrial greenhouse gas sink. Our understanding of the global carbon cycle will only be complete if we include the flux of carbon through inland freshwaters (Cole et al., 2007; Battin et al., 2009; Trimmer et al., 2012) getting to grips with methane-fuelling of food webs is an interesting and important component of this. Indeed, (Cole, 2013) noted that “the role of methane in supporting food webs in lakes, and perhaps even beyond their shores, has come as a surprise” and that “the notion that lake methane partially supports higher organisms in surrounding terrestrial environments fundamentally changes our understanding of how aquatic food webs work.”
Methanogenesis is a universal terminal degradation process of organic matter in anoxic aquatic sediments when inorganic oxidants such as nitrate, ferric iron, or sulfate are depleted (Conrad, 2005). Hence, in marine systems where there is typically a high concentration of sulfate, the sulfur cycle tends to dominate chemosynthesis, but in freshwaters where sulfate concentrations are typically lower (Hobbie, 1988) then methanogenesis dominates. Stable isotopes have been an incredibly useful tool in the identification and quantification of methanogenic and methanotrophic pathways (Conrad, 2005) and further identifying the constituents of the complex microbial community that is actively involved via stable isotope probing (SIP; e.g., He et al., 2012), but those aspects are not the focus of this review. Methane may be lost directly from the system via ebullition or the recently hypothesized micro-bubble pathway, stochastic processes notoriously difficult to quantify (Prairie and del Giorgio, 2013) or be effectively “piped” to the surface via plants (Bergstrom et al., 2007; Sanders et al., 2007). Alternatively, or in addition, it may subsequently serve as an energy and C source for methanotrophs (methane oxidizing bacteria; MOB), typically at oxic-anoxic boundaries (if anaerobic CH4 oxidation is excluded) in the sediment, or in the water column (Kankaala et al., 2007). It is essentially from this point in the cycle that stable isotopes have been key in tracing the use of methane-derived carbon (MDC) into, and through, food webs, particularly in freshwaters (Jones and Grey, 2011). A schematic of potential routes by which CH4 produced in anoxic freshwater sediments may either by-pass or become incorporated into food webs is shown in Figure 1.
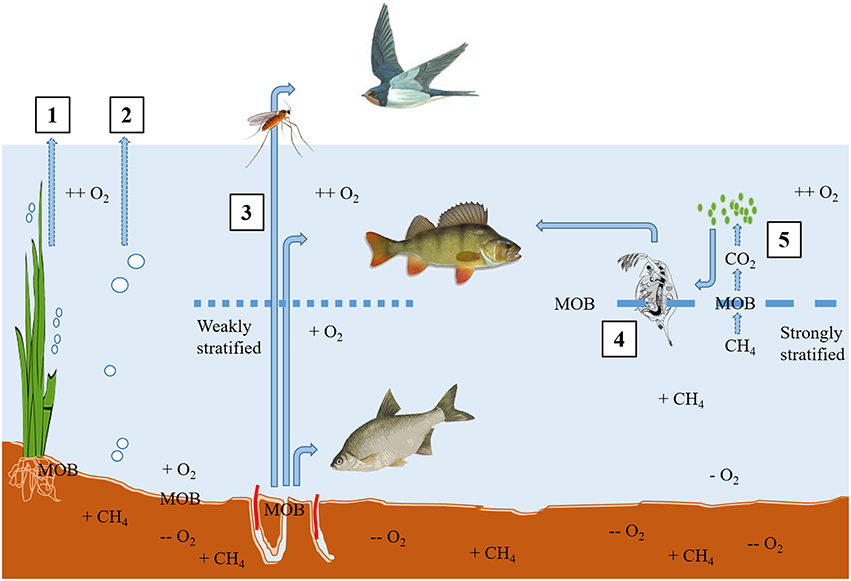
Figure 1. Methane produced in anoxic sediments may be routed through plants (1) or lost from the sediments to the atmosphere via ebullition or micro-bubbles (2). If it reaches a boundary with oxygen at the sediment-water interface (under mixed or weakly stratified conditions), MOB oxidize it and create biomass which routes via benthic macroinvertebrates into benthic, pelagic, and terrestrial predators (3). Under strongly or permanently stratified conditions, methane will diffuse upwards through the water-column, and oxygen (and MOB) might first be encountered at the metalimnion, where zooplankton link MDC into higher predators (4). An indirect route for MDC could be via CO2 derived from the oxidation of CH4 might then be cycled through phytoplankton, and hence on to zooplankton (5), or indeed via sedimentation back down to benthic macroinvertebrates.
Could a Methane Pathway be Important to Secondary Production in Food Webs?
Anoxic water and sediments are typically rich in organic matter compared to the overlying oxic water, and anoxic metabolism may account for a substantial part (20–60%) of the carbon metabolism and the heterotrophic microbial production within freshwater environments (Hessen and Nygaard, 1992). Methanogenesis in lakes has been reported corresponding to 30–80% of the anaerobic mineralization in waters and sediments (Bastviken, 2009). While seasonal variability in CH4 oxidation is known to be considerable, especially in dimictic lakes, between 30–94% of the CH4 reaching oxygenated layers is reputedly oxidized (Casper et al., 2000; Morana et al., 2015). In essence then, CH4 is a major product of the C mineralization in lakes, and a large proportion may be converted to microbial biomass equivalent in some instances to the total C fixation by heterotrophic bacteria and a significant proportion of primary production (Hessen and Nygaard, 1992; Bastviken et al., 2003). Again, data from rivers are lacking, but across 15 rivers, in late summer, i.e., when one might expect the greatest contribution from photosynthesis, (Shelley et al., 2014) conservatively calculated that net methanotrophy was equivalent to between 1 and 46% of benthic net photosynthetic production within the gravel beds of chalkstreams. Couple this to the apparently high (50%) carbon conversion efficiency of methanotrophs (relative to 10–30%, typical for bacteria in detrital-based food webs), regardless of marked spatial and temporal changes in ambient methane concentration, and it suggests that methanotrophs can sustain net production throughout the year (Trimmer et al., 2015).
The importance of a CH4 pathway to food webs might yet increase further under climate change. Increases in temperature forecast for the coming decades may have profound implications for the cycling of carbon in aquatic ecosystems due to the differential temperature dependencies of carbon fixation by gross primary production (GPP) and carbon mineralization by ecosystem respiration (ER). For example, (Yvon-Durocher et al., 2010) showed that warming of 4°C reduced the carbon sequestration capacity of freshwater mesocosms by 13%, shifting them toward net heterotrophy (i.e., net sources of CO2 to the atmosphere) because ER responded more strongly to temperature than GPP. They also found that methanogenesis responded even more strongly than ER or GPP, with 20% more of the GPP being accounted for by CH4 emissions with 4°C of warming (Yvon-Durocher et al., 2011). Benthic community structure and how that contributes to a host of ecosystem processes, including microbial and macrofaunal decomposition rates, was also clearly affected by such warming (Dossena et al., 2012). If it is assumed that delivery of organic matter does not change but temperature increases as predicted, then for example, the increased mineralization will equate to a 4–27% (0.9–6.4 Tg C y−1) decrease in organic carbon burial in boreal lakes (Gudasz et al., 2010). However, very recent work in rivers suggests that methanotrophy has the potential to match methanogenesis enhanced by warming (Shelley et al., 2015). How climate change might impact upon food web mediation of MDC will be returned to later.
Why are Stable Carbon and Hydrogen Such Useful Tracers of Methane?
Isotopic signatures of environmental CH4, both 13C/12C and 2H/1H, have been compiled by Whiticar et al. (1986) and Bréas et al. (2001) amongst others. An important characteristic of biogenic methane is that its carbon stable isotope composition is typically markedly 13C-deplete compared to other putative basal resources in a food web. So, for freshwater lakes, CH4 δ13C may be as low as −110 to −50‰ dependent upon formation pathway; (Whiticar, 1999; Deines and Grey, 2006; Taipale et al., 2007) relative to either allochthonous terrestrial plant detritus (δ13C value from C3 plants typically −28 to −26‰; Peterson and Fry, 1987) or autochthonous phytoplankton [δ13C typically between −35 to −25‰; (Grey et al., 2000; Vuorio et al., 2006) but acknowledging that components of the phytoplankton such as their fatty acids may be ~10‰ further 13C-depleted (e.g., Taipale et al., 2015)]. However, CH4 δ13C values reported from sediments are not necessarily linked to the δ13C values of sedimentary organic matter; instead they may be strongly influenced by the quality of the organic matter substrate and/or the predominant methanogenic pathway (Rinta et al., 2015), and of course to a certain extent as to whether some of the CH4 has already been oxidized by MOB prior to analysis (Coleman et al., 1981). In marine hydrocarbon seep communities, δ13C has been the primary isotope value examined, used to differentiate between animals with chemoautotrophic symbionts (–40 to –20‰) from those with methanotrophic symbionts (≤–40‰; Brooks et al., 1987) and to identify the source CH4 pool as either thermogenic (δ13C = −45 to −40‰) or biogenic (δ13C < −45‰) CH4 (Sassen et al., 1999).
Isotopic fractionation during the use of CH4 by MOB typically leads to further 13C-depletion (by 0–30‰; Summons et al., 1994; Templeton et al., 2006). For example, CH4-consuming archaea isolated from anoxic marine sediments have been reported with δ13C values as low as −96‰ (Orphan et al., 2001), while biomarkers (e.g., archaeol and hydroxyarchaeol) from such archaea within a CH4-supported benthic microbial community in cold-seep sediments exhibited δ13C values as low as −111‰ (Werne et al., 2002). Hence, the MOB biomass available to consumers has a strikingly low δ13C and, because stable carbon isotope ratios differ little between consumers and their diets, assuming no selective assimilation or substantial biosynthesis (McCutchan et al., 2003; Grey, 2006), this should allow its contribution to consumer biomass to be rather readily traced.
Hydrogen isotope effects during methanogenesis of methylated substrates can lead to deuterium depletions as large as −531‰, whereas, bacterial D/H discrimination for the CO2-reduction pathway is significantly less (−250 to −170‰; Whiticar, 1999). Very little is known regarding the δD values of MOB. However, when compared to typical values of autochthonous (−290 to −215‰) and allochthonous (−160 to −125‰) resources, there is still great scope for the use of δD to trace CH4-derived production (Estep and Dabrowski, 1980; Doucett et al., 2007), especially when in combination with δ13C (e.g., Deines et al., 2009). The more distinct the sources, and indeed, the more tracers used, the more confidence can be assigned to estimates of contribution to diet derived from any of the recently published mixing models (e.g., Parnell et al., 2013). Problems arise using isotopic tracers when a relatively minor contribution from MDC results in δ values that could be arrived at via alternative pathways (see Section “The Zone of Contention” below).
Methane use Across a Spectrum of Sources
As appreciation of the possibility of MDC providing an alternative energy source to food webs has grown, so the emphasis on research has shifted from point sources to ever more diffuse sources, and less intuitively obvious locations where it might be relevant. The proportion of MDC contributing to food webs at more diffuse sources may well be smaller (but still of significance); as such, there is likely to be greater ambiguity in the stable isotope signal, and so the importance of MDC might have been overlooked in many of these systems (Figure 2).
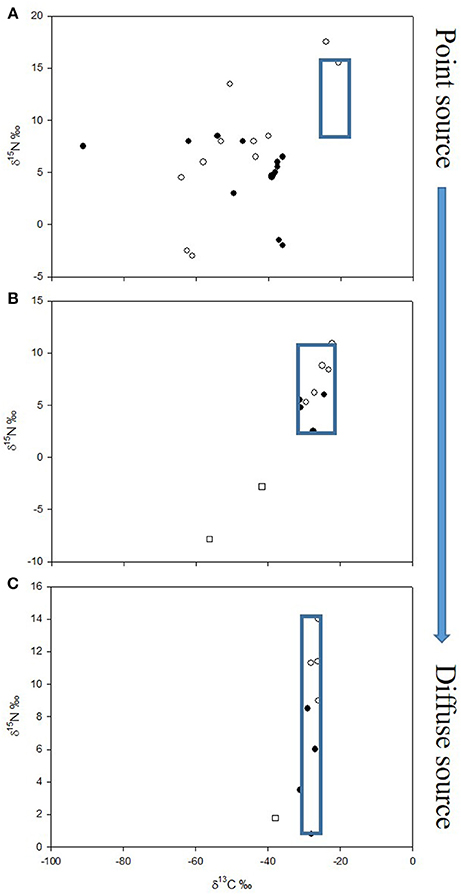
Figure 2. Stable isotope bi-plots of food webs across a spectrum of point to diffuse sources of methane with corresponding decrease in strength of δ13C value as a tracer of methane-derived carbon (MDC); blue boxes indicate components of the food web with small / negligible influence of MDC. (A) Gulf of Alaska (redrawn with permission from Levin and Michener, 2002): solid symbols—pogonophoran field infauna; open symbols—clam field infauna. (B) Plußsee (strongly stratifying small lake, data from Harrod and Grey, 2006): open circles—fish; solid circles—macroinvertebrates; open squares—chironomid larvae. (C) Loch Ness (weakly stratifying large lake, data from Jones and Grey, 2011): open circles—fish; solid circles—invertebrate and basal resources; open squares—chironomid larvae.
It is perhaps unsurprising that the use of CH4 (amongst other chemosynthetic production) is strongly evident at point sources such as deep-sea vents and seeps, whale, kelp and wood falls, and some sewage outflows, typically far beyond the direct reach of photosynthetically active radiation (although of course photosynthetic production can “fall-out” of the water column to benthic communities). The potential for chemosynthesis to fuel entire animal communities in the ocean was first noted around 35 years ago (e.g., Rau and Hedges, 1979). Early attention focused on megafaunal or epifaunal taxa such as molluscs or pogonophorans but there was a broad suite of smaller infaunal deposit feeding and omnivorous invertebrates whose mode of nutrition remained largely ignored until the application of stable isotope analyses by the likes of Van Dover and Fry (1994), Colaço et al. (2002), and Levin and Michener (2002). For example, Levin and Michener (2002) looked at a variety of sites including CH4 seeps in the Gulf of Alaska, on the Oregon margin, and on the northern California slope and found that seep macrofauna exhibited lighter δ13C (and δ15N) values than those in non-seep sediments. Significant contributions were found from MDC to macrofaunal biomass from sediments of pogonophoran fields (32–51%) and clam beds (12–40%) in the Gulf of Alaska, and in microbial mat sediments on the Oregon margin (20–44%). Some polychaetes exhibited extremely low values of δ13C (−90.6‰) at these point sources (see Figure 2A).
Within the last 15 years, research on MDC and food webs primarily focussed on lakes, particularly stratifying lakes in temperate and boreal systems, and much of this work has been extensively reviewed by Jones and Grey (2011). Tube-dwelling chironomid larvae appear key in lake sediments. Field studies from lakes across Alaska, England, Finland, and Germany (amongst others) have demonstrated that chironomids can assimilate MDC extensively (up to 70% of larval biomass; Jones et al., 2008). The degree to which they do may vary within lakes on a temporal (Grey et al., 2004b; Deines et al., 2007b) or spatial (Deines and Grey, 2006; Gentzel et al., 2012) scale, or by taxa (Jones and Grey, 2004; Kelly et al., 2004; Jones et al., 2008), and among lakes with “strength” of stratification (Grey et al., 2004b; Deines et al., 2007a; Hershey et al., 2015). Chironomid larvae are bioengineers; they bioturbate the sediment while “digging” and maintaining their burrows and draw down oxygenated water, bringing it into contact with anoxic sediment. The sediments on the burrow walls have been shown to exhibit higher methane oxidation rates and higher densities of MOB than the surrounding bulk or surficial sediments (Kajan and Frenzel, 1999; Gentzel et al., 2012). Larvae thereby appear to create the perfect micro-niche for the coupling of methanogenesis and methanotrophy (Deines et al., 2007a; Kelly et al., 2004; see route 3 in Figure 1).
It was assumed from field studies that the low δ13C values for taxa such as Chironomus plumosus (e.g., −70 to −50‰; Jones et al., 2008) reflected ingestion of the MOB on their burrow walls (Deines et al., 2007a) akin to “gardening” by trichopteran caddis flies on the biofilms that develop on caddis cases (also studied by using stable isotopes; (Ings et al., 2012). By using 13C-labeled CH4 additions directly into sediments housing chironomid larvae under controlled experimental settings, Deines et al. (2007a) have elegantly demonstrated that larvae assimilate MDC via MOB; this was further supported by phospholipid fatty acids diagnostic for MOB and significantly enriched by the 13C-labeled methane being detected in the larval tissues. In a series of parallel experiments, they showed that larvae could also obtain MDC via 13C-labeled Type II MOB introduced into the water column above sediments. Type I and Type II MOB use different pathways for formaldehyde assimilation (ribulose monophosphate and serine, respectively), and typically favor different environmental conditions; Type I appear to be dominant in environments in which CH4 is limiting and combined nitrogen and copper concentrations are relatively high, whereas Type II appear where there are high CH4 concentrations, low dissolved oxygen, and limiting concentrations of combined nitrogen and/or copper (Hanson and Hanson, 1996). The ability to access MDC via two discrete routes might account for some of the incredible inter-individual variability that has been observed in chironomid stable isotope ratios (e.g., Grey et al., 2004a; Figure 2).
When stratification of the water column becomes too pronounced, generally in duration, and the benthic sediments become inhospitable even for the hypoxic tolerant chironomid larvae, Jones and Grey (2011) hypothesized that MDC is more likely to be taken up in the water column at the oxic-anoxic boundary by zooplankton. Again, evidence for this is mostly derived from the field from small Finnish boreal lakes with marked oxyclines (e.g., Jones et al., 1999; Taipale et al., 2007, 2008; but see Bastviken et al., 2003; Santer et al., 2006; Schilder et al., 2015a). Pelagic zooplankton δ13C values are typically not as low as those reported from similar lakes for benthic chironomids, perhaps again reflecting the more diffuse nature of the source CH4, and/or the more mobile feeding capability of zooplankton in the water column relative to tube dwelling chironomids in the sediments. Some of the lowest values reported are for Daphnia spp. from small, strongly stratifying lakes with anoxic hypolimnia; for example, −47‰ in a kettle lake, Plußsee (Harrod and Grey, 2006), or −46‰ from Mekkojarvi (Taipale et al., 2008). Laboratory support for zooplankton uptake of MOB is sparse, but (Kankaala et al., 2006) measured growth rates of Daphnia in replicated cultures fed microbial suspensions with or without addition of CH4 and found that their δ13C values indicated consumption of 13C-depleted MOB, as have (Deines and Fink, 2011) using 13C-labeling of CH4.
Evidence of MDC contributions to biomass in polymictic (permanently mixed) lakes is rarer. Such lakes are often shallow and contain considerable stands of macrophytes; while methanogenesis is certainly proceeding in the sediments, much of the CH4 produced might be routed via the plant stems and via ebullition (routes 1&2 in Figure 1) and hence, side-step incorporation into the food web [although see reference to Agasild et al. (2014), below]. Since the whole water column is well oxygenated, there is no distinct boundary where MOB will accumulate and thus it is unlikely that zooplankton will feed heavily upon MOB (Jones and Grey, 2011). In the benthos, there is also typically a more consistent supply of 13C-enriched phytoplankton production from above which will “swamp” the lower δ13C values from MOB. Examples of such lakes with permanently oxic sediment surface layers in which MDC has been shown to make only a limited (maximum ~20%) or negligible contribution to chironomid biomass include Großer Binnensee and Schöhsee in north Germany (Grey et al., 2004b; Deines et al., 2007b), Lough Neagh and Rostherne Mere in the UK (Kelly et al., 2004), Izunuma in Japan (Yasuno et al., 2012), and Võrtsjärv in Estonia (Agasild et al., 2014; Cremona et al., 2014). Interestingly, the latter lake was sampled at various sites and it was only at one particular site dominated by vegetation that low δ13C values were recorded in both zooplankton and chironomids. Agasild et al. (2014) postulated that the stands of macrophytes prevented wind mixing from disturbing the sediments, and that dissolved oxygen in the water column was reduced by the restricted circulation of water and gas exchange between the water surface and the atmosphere and by increased oxygen demand from the decomposition of organic matter; all processes which would lead to greater MDC being available to the food web.
Within the last 5 years has come the first convincing evidence of MDC contributing to food webs in free-flowing, well oxygenated streams and rivers, where because of the turbulent nature, the source of CH4 could be considered to be most diffuse. One of the first studies claiming a river food web to be fuelled by MDC was by Kohzu et al. (2004) who reported Helodes sp. beetle larvae and adults with δ13C values as low as −69.8‰ but these were from stagnant backwater pools akin to stratifying lakes, and while these may be important habitats on some lotic systems, they were not from the free flowing, main-stem river food web. Since then, considerable research on the chalk streams of the UK, highly productive, ground water fed systems has revealed that trichopteran larvae may play a similar role to chironomids in lakes, the main conduit for MDC to route into the wider food web (e.g., Trimmer et al., 2009, 2010). In contrast, Mbaka et al. (2014) studied small inline impoundments with extremely short residence times on a river system in Germany but could find negligible evidence of MDC contributing to chironomids from the sediments there. How MDC might contribute significantly to river food webs clearly requires more research.
Unless there is almost 100% trophic transfer of MDC higher into the food web, then obviously mixing with non-MDC food sources results in a dilution of the indicator isotope in question, and the ability to trace MDC further using stable isotopes alone is weakened (see below). An apparent gradient is thus evident from point to diffuse source of methane. For example, on a species-specific basis, some mobile benthic predators (eels, sea stars, and predatory snails) have been shown on the basis of their low δ13C (and δ15N & δ34S) values to obtain close to 100% of their nutrition from CH4 seep production in the Gulf of Mexico (MacAvoy et al., 2002). From stratifying lakes, (Harrod and Grey, 2006) and (Ravinet et al., 2010) have found isotopic evidence of MDC contributing (up to ~12%) to bream (Abramis brama) and to ruffe (Gymnocephalus cernuus), respectively, while in a shallow, well-mixed Pantanal (tropical) wetland lake (Sanseverino et al., 2012) could trace MDC into various fish species. Even from the very shallow lake Võrtsjärv, Agasild et al. (2014) reported that at sites amongst the macrophytes where zooplankton and chironomid larvae were most 13C-deplete, there was a corresponding decrease in δ13C for roach (Rutilus rutilus), perch (Perca fluviatilis), and the apex predator, pike (Esox lucius), indicative of trophic transfer of MDC to the very top of the food web. To date, evidence from rivers has not been reported, but given the extremely abundant nature of the primary consumers (particularly cased caddis flies) that appear key to linking MOB into the food web in such systems, the pathway is certainly in place (Trimmer et al., 2012). Evidence of the transfer of MDC across ecosystem boundaries is still limited. Aquatic invertebrates such as Helodes sp., Chloroperlidae spp., Leuctridae spp., and Sialis sp. have all been recorded from Malaise traps on stream banks, i.e., post emergence, with δ13C values from −69.8 to −51.8‰ (Kohzu et al., 2004) but there has still been only one study quantifying transfer of MDC and that was into riparian spiders (up to 18% of their biomass) mediated by emerging chironomid imagos from stratifying lakes (Jones and Grey, 2011). The potential is clear to see for vertebrate predators as well, such as barn swallows (Hirundo rustica) which, using stable isotopes, have been identified as prioritizing such abundant prey at specific times of the year (Parnell et al., 2013). Of course, we should also consider how alteration of a food web, for example by introduction of a top predator for recreation or as a function of range expansion might cause cascading effects down to biogeochemical cycling near the base of a food web. By experimentally manipulating fish density in a previously fish-less lake, (Devlin et al., 2015) showed that a trophic cascade from fish to microbes affected methane efflux to the atmosphere and reduced the amount of MDC assimilated into the biomass of zooplankton that remained (assessed from Daphnia δ13C values). It may well be that such improved quantitative understanding of the influence of higher trophic consumers on carbon budgets creates future opportunity for management and policy to identify and implement new options for mitigating greenhouse gas release at regional scales (Schmitz et al., 2014).
The Zone of Contention
Various authors (e.g., Deines et al., 2009) have acknowledged that confidence in the use of isotopic tracers of MDC from field studies must be tempered where/when alternative explanations for such isotope values can arise. The “zone of contention” for δ13C from consumers in freshwater lakes for example typically occurs between −40 and −30‰. Chironomid larvae could exhibit such a value if they assimilated: (a) a small percentage from very low δ13C MOB and a greater percentage from relatively high δ13C phytoplankton (e.g., Grey et al., 2004a); (b) alternative chemosynthetic sources of carbon such as sulfur bacteria (e.g., Deines et al., 2009; Roach et al., 2011); or (c) phytoplankton with very low δ13C. It should be remembered that these scenarios are not mutually exclusive. Scenario c may arise because a substantial part of the dissolved CO2 pool may originate from respiration of autochthonous and allochthonous organic matter and have low δ13C (from −20 to −15‰; Lennon et al., 2006; Kankaala et al., 2010). The degree of fractionation of that CO2 by phytoplankton is uncertain and extremely variable, but in lakes might range from 0 to 15‰ (with values near the upper end of the range probably most widespread; (Bade et al., 2006). Therefore, it is not uncommon to find δ13C values for lake phytoplankton of < −30‰ (e.g., Grey et al., 2000; Vuorio et al., 2006), and anything feeding selectively on 13C-depleted phytoplankton (or assimilating selectively from components thereof such as fatty acids) will show correspondingly low δ13C values (Pel et al., 2003). The same has been shown for rivers (Finlay et al., 1999). The situation is even more complex when a proportion of the low δ13C values for CO2 could have originated from the oxidation of CH4, and hence in effect, be an indirect contribution from MDC (Route 5 in Figure 1). Further dilution of the MDC signal with trophic transfer up the food web has already been mentioned.
In such scenarios, only with the addition of alternative but complementary tracers can the assimilation of MDC be assigned with confidence. Hence, the addition of further stable isotopes such as δD [e.g., (Deines et al., 2009; Belle et al., 2015; van Hardenbroek et al., 2016), δ34S (Grey and Deines, 2005), and to a certain extent δ15N (Stephen et al., 2002; Grey et al., 2004a); see later discussion], have proved useful in ascertaining the use of MDC. Radio isotopes might offer some support under certain situations; for example. (Opsahl and Chanton, 2006) studied the food webs of troglobitic organisms in the Upper Floridian aquifer and found that crayfish trapped from remote sinkhole conduits were not only on average ~10‰ 13C-depleted relative to their counterparts at accessible springs at the surface but that there was a strong correlation with radiocarbon (Δ14C) depletion relative to modern values, indicative of a chemosynthetic food source. Concurrent analysis of phospholipid fatty acids (PLFAs) which are diagnostic for MOB, as well as compound-specific analysis of the isotope ratios of those PLFAs has also been invaluable. For example, (Taipale et al., 2009) demonstrated a strong relationship between the δ13C values of Daphnia and the proportion of MOB-specific PLFAs in Daphnia. These methods have also highlighted the indirect route via methane-oxidation and uptake of the resulting 13C-depleted CO2 by autotrophs (Route 5 in Figure 1). For bog-pool food webs in Estonia, (Duinen et al., 2013) suggested that the most parsimonious explanation for relatively low δ13C values of algae-derived polyunsaturated fatty acids found in insects was that MOB were creating CO2 from the oxidation of CH4 which was assimilated by their direct algal “neighbors” within the biofilm community. (Sanseverino et al., 2012) used the presence of MOB-diagnostic PLFAs in various benthivorous and detritivorous fishes to support claims of MDC assimilation in Brazilian wetlands as the fish δ13C values were <−36‰; low relative to the other food web components in question but clearly not the marked 13C-depletion classically associated with CH4. Further correlative evidence may be provided by concurrent assessment of the methanogen/methanotroph community by molecular methods. Eller et al. (2005) reported zones of aerobic and anaerobic CH4 oxidation in the water column of a strongly stratifying lake, where high cell numbers of MOB were also detected by fluorescence in situ hybridization techniques. It was around this depth in the same lake that (Santer et al., 2006) found that one of the cyclopoid copepod species, Diacyclops bicuspidatus, consistently maintained highest density and exhibited δ13C values ~10‰ lower than epilimnetic species and the photosynthetic particulate organic matter sampled during the same time interval, and proposed the role of MDC in its diet.
Looking Back: Hindcasting
A particular area of research related to CH4-fuelling of food webs that has emerged most recently aims to identify or determine past “methane environments,” predominantly in lakes. Insight into past variations of CH4 availability in lakes would further our understanding of the timing and magnitude of the response of lake CH4 production and emissions to changing environmental conditions. Palaeolimnologists have long recognized that recalcitrant remains of organisms such as the strongly sclerotized head capsules of chironomids or the ephippia of daphniids, can be found in abundance and well preserved in most lake sediment records. Chironomid remains, especially the larval head capsules, can be found abundantly in lake sediments. Indeed, exoskeleton fragments originating from molting and deceased larvae, or zooplankton resting eggs, are preserved for tens to hundreds of thousands of years at a quality which allows microscopic identification usually to genus, or species morphotype, but sometimes also to species level (van Hardenbroek et al., 2011). Since lake sediments can be dated using radiometric and/or other dating methods, these remains can be used to reconstruct historical community composition and by inference the water quality, ambient temperature, or a particular habitat structure (Eggermont and Heiri, 2012). Head capsules and exoskeletons comprise mainly chitin and proteins and, on the basis that their chemical composition does not seem to be strongly affected by decomposition processes, they can be used to develop palaeo-environmental reconstructions based upon stable isotope composition (Perga, 2010, 2011; Heiri et al., 2012).
Heiri et al. (2012) recently reviewed the available stable isotope studies based on fossil chironomids (which had mainly examined the elements C, N, H, and O), and identified four key areas: (1) developing the methodology for preparing samples for isotopic analysis, (2) studies rearing chironomid larvae under controlled laboratory conditions to determine those factors affecting stable isotope composition, (3) ecosystem-scale studies relating stable isotope measurements of fossil chironomid assemblages to environmental conditions, and (4) developing the first down-core records describing past changes in the stable isotope composition of chironomid assemblages. In a relatively short period of time since that review, a number of publications have arisen expanding upon those areas, further demonstrating the usefulness of the technique, and including other complementary tracer evidence to support suppositions when the stable isotopes alone might prove ambiguous.
Firstly, it is important to determine whether there is any isotopic offset between the recalcitrant parts of organisms recovered from palaeolimnological samples and the whole body that is typically analyzed for the study of contemporary relationships in food webs. It is also important to determine whether the “clean up” protocols that palaeo-samples typically require have any significant effect upon isotopic integrity. To answer both of these questions for chironomid head capsules, (van Hardenbroek et al., 2010) trialed various commonly used chemical methods for sediment processing and found that treatment with 10% KOH, 10% HCl, or 40% HF showed no detectable effect on δ13C, whereas, perhaps unsurprisingly, a combination of boiling, accelerated solvent extraction and heavy chemical oxidation resulted in a small but statistically significant decrease in δ13C values (0.2‰). Then, using a modification of the culturing experimental protocol by Deines et al. (2007a), they demonstrated with MOB grown on 13C-labeled methane, that methanogenic carbon is transferred into chironomid head capsules (van Hardenbroek et al., 2010). Frossard et al. (2013) have also looked at head capsule to whole organism isotopic offsets for chironomid larvae and reported from experimental rearing on three different diets that the head capsules were 13C-depleted by 0.9‰ relative to whole biomass. For zooplankton, Perga (2010) has shown that the C and N stable isotope compositions of the daphniid exoskeleton and those of the whole body are strongly correlated. Exoskeleton δ13C values were similar to those of the whole body but were strongly depleted in 15N (−7.9‰), reflecting its derivation from excretory ammonia of dietary origin, known to be 15N-depleted compared with dietary organic nitrogen (Schimmelmann, 2011). Further elegant experiments have shown that the stable isotopic composition of Daphnia ephippia provides information on that of the parent Daphnia, and of the food and water they were exposed to during formation. Schilder et al. (2015b) demonstrated that there were only small offsets between Daphnia and ephippia relative to the range of variation in Daphnia stable isotopic composition reported from down-core studies. Interestingly however, their work also indicated that temperature may have a minor influence on the δ13C, δ15N, and δ18O values of Daphnia body tissue and ephippia which has implications for water temperature reconstruction work using oxygen isotopes, as well as highlighting the care with which controlled feeding experiments need to be conducted (sensu Perga and Grey, 2010). The suite of organism remains has been further extended recently, as it now appears bryozoan statoblasts and zooids have the potential to act as indicators of MDC (van Hardenbroek et al., 2016).
Prior to the interest in palaeo-reconstruction, site-specific, and hence, differing CH4 production potential and oxidation had only been linked to living chironomid larvae (e.g., Deines and Grey, 2006). More confidence in the potential of recalcitrant remains to provide information about past changes in CH4 availability in lakes using sediment records has arisen since studies have been conducted across lake types and actually using remains from surficial sediments i.e., reflecting the most recent CH4 history that can be measured concurrently. In a study of seven Swedish lakes, (van Hardenbroek et al., 2012) observed significant negative correlations between the δ13C of Chironomini and both CH4 fluxes at the lake surface, and CH4 releases from the sediment. That dataset was built upon by incorporating samples from 10 Siberian lakes and expanding the suite of remains to include those of Daphnia and Tanytarsini; the δ13C of all three groups were correlated significantly with diffusive CH4 flux in the combined Siberian and Swedish dataset suggesting that δ13C in the biomass of these invertebrates was affected by CH4 availability (van Hardenbroek et al., 2013). Schilder et al. (2015a) measured Daphnia ephippial δ13C values from the surface sediments of 15 small European lakes, and found a strong correlation to the late summer aqueous CH4 concentration in both the surface water and above the sediment.
Down-core work is providing some tantalizing evidence of past CH4 variability over time. Adding to their proof-of-concept work on which invertebrate remains are useful tracers of MDC (van Hardenbroek et al., 2013) went on to measure the δ13C of invertebrate remains from a sediment record (covering the past ~1000 years) of a shallow thermokarst lake in northeast Siberia. Those taxa most sensitive to CH4 availability (Chironomini, Tanytarsini, and Daphnia) exhibited the lowest δ13C values in sediments deposited from ca AD 1250 to ca AD 1500, and after AD 1970, which coincided with periods of warmer climate (indicated by an independent local temperature record). As a consequence, the discrepancy in δ13C between CH4-sensitive taxa and bulk organic matter was higher in these sections than in other parts of the core, whereas the δ13C of other invertebrate taxa did not show the same trend. They concluded that there was higher CH4 availability in the study lake during warmer periods and that the energy sources of some key benthic invertebrates changed accordingly. Wooller et al. (2012) managed to reconstruct the CH4 history of Qalluuraq Lake, a shallow Alaskan tundra lake, over a period ~12,000 years in this manner, and similar work has been conducted on large, deep sub-alpine lakes, particularly in France. A change from oligotrophic status associated with anthropogenic nutrient enrichment over the last 150 years was examined for associated shifts in the basal resources available to the benthic food web (Frossard et al., 2015). Chironomid head capsule δ13C values started to decrease with the onset of eutrophication in both Lake Annecy and Lake Bourget; the estimates of the MDC contribution to chironomid biomass ranged from <5% prior to the 1930s to nearly 30% in recent years.
To date, values for chironomid head capsules have not been reported as 13C-depleted as for live organisms. This is in part a frustrating function of the requirement for multiple head capsules to be pooled to provide sufficient material for elemental and isotopic analysis. It is also likely associated with the fact that the sampling of the remains of organisms at a specific location (depth) might not truly reflect the location where the animal assimilated its diet, due perhaps to resuspension of sediments and/or focussing of material (Battarbee, 1999). Hence, the “strength” of a MDC signal that one can find in a contemporary sample derived from fresh larvae with values for individuals <−70‰, will always be dampened (i.e., less 13C-depleted) by pooling and/or dilution effects in palaeolimnological samples. As a consequence, the usefulness of δ13C alone as a tracer deteriorates (see Section The Zone of Contention above). One very promising approach is the analysis of ancient DNA (aDNA) from the methanotroph community. Belle et al. (2014) has elegantly demonstrated how aDNA can be used to complement stable isotopes in a study of a sediment core from the deepest zone of Lake Narlay, representing the last 1500 years of sediment accumulation. A significant change was noted since ca AD1600, with an increase in the proportion of MOB in the total bacteria community, and a corresponding decrease in chironomid head capsule δ13C. These trends suggest that assimilation of MOB may account for up to 36% of chironomid biomass, with evidence for preferential assimilation of methanotroph type I and the NC10 phylum. Parallel strands of evidence are clearly required whenever there is ambiguity in stable isotope data, and the development of aDNA will surely grow in this particular field.
Looking Forward: Knowledge Gaps
To date, the majority of studies on CH4 in food webs have solely concentrated on the stable carbon isotopes as a tracer. However, equally evident to the very low and varying δ13C values in consumers part-fuelled by biogenic CH4 have been low and highly variable δ15N values; indeed, one of the most striking patterns to emerge from studies involving chironomids and CH4 is the strong, positive relationship between δ13C and δ15N (Grey et al., 2004a) which appears to have some species-specific basis (Kelly et al., 2004). These relationships appear consistent and widespread (Figure 3) and while most likely linked to assimilation of MOB, a test of the potential mechanisms underpinning such low δ15N values in consumer tissues is currently lacking.
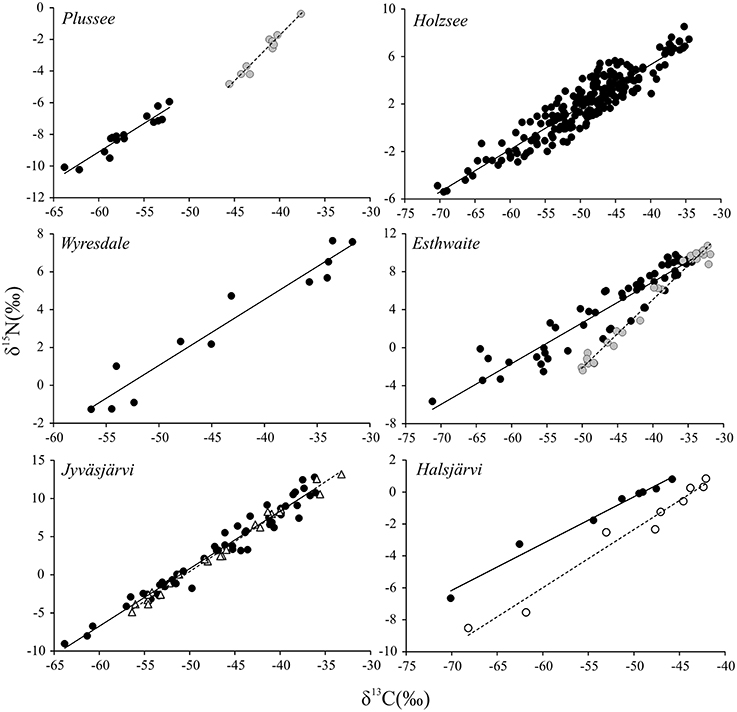
Figure 3. Stable carbon and nitrogen isotope ratios of benthic chironomid larvae collected from stratifying lakes in Germany, England and Finland [data derived from Grey et al. (2004a), Deines et al. (2007a), Ravinet et al. (2010)]. Individuals were collected from a specific depth in each lake and on one date (except for Holzsee where the data are compiled from 12 sampling events in 1year). Species are Chironomus plumosus (filled black markers, solid line), Chironomus anthracinus (filled gray markers, dashed line), Propsilocerus jacuticus (Jyväsjärvi only; open triangle, dashed line), and Chironomus teniustylus (Halsjärvi only; open marker, dashed line). Lines are least squares regressions for illustrative purposes only.
In Grey et al. (2004a), it was postulated that nitrogen within chironomid tubes may be continuously cycled between the larva and microbial consortia; for example, chironomids excrete nitrogen in the form of ammonium directly into their tubes and the overlying water (Devine and Vanni, 2002), and via essential fractionation of ammonia, any microbial community taking up that nitrogen source would be 15N-depleted (Macko et al., 1987; Ings et al., 2012). More specifically, both Type I and II MOB can fix atmospheric N2 into ammonium and share similar pathways to oxidize ammonia/ammonium as autotrophic ammonium oxidizing bacteria (Lee and Childress, 1994) and thus, are likely to exhibit correspondingly low δ15N values. However, ammonium oxidation rates are typically low and high ammonium concentrations may inhibit CH4 oxidation. In addition, some MOB can convert nitrate back to N2 and such denitrifying methanotrophs may outcompete other MOB in nitrogen-rich, low oxygen environments (Stein and Klotz, 2011), which are characteristic of many of the lakes where low δ13C and δ15N values in chironomids have been found (Jones et al., 2008). To examine the underlying causal mechanisms for the strong, consistent, and widespread relationship between chironomid δ13C and δ15N values, more research is required to characterize the stable isotope values of potential nitrogen sources, to measure potential N-fractionation by MOB, and to use complementary methods such as molecular biomarker profiling (PLFAs and 16S rRNA genes) of chironomid gut contents.
While δ13C values for dissolved CH4 are relatively easily measured in the lab as well as in the field nowadays, and hence are available from a wide range of aquatic environments, more robust end-member values for MOB are required if we are to improve estimates for the quantitative contribution of CH4-carbon to total carbon budgets and production figures for different ecosystems. To date, such estimates have relied on some of the earliest simple two-source mixing models (i.e., only using one stable isotope: carbon) by applying a range of trophic fractionation factors for MOB (reported from a very small number of laboratory experiments) to values of CH4 gas to derive one end-member. Direct measures of MOB δ13C from aquatic environments are badly needed. Currently, it is possible to measure the δ13C of MOB-specific PLFAs extracted from aquatic sediments, but how these relate to the values from whole MOB cells still needs to be established. More laboratory studies of how carbon isotope fractionation between CH4 and MOB may vary with different environmental and cell growth conditions would be extremely useful, acknowledging that “controlling” every parameter even in the lab can be extremely difficult (e.g., Perga and Grey, 2010).
The geographic range of studies of MDC in food webs is still rather limited. Within freshwaters, Jones et al. (2008) is the only paper to synthesize data from across a wide latitudinal gradient and a distinct knowledge gap exists for the lower latitudes. Tropical regions are responsible for approximately half of the estimated CH4 emissions from freshwater ecosystems to the atmosphere, although they have been consistently under-sampled (Bastviken et al., 2011). Indeed, the permanently stratified (meromictic) Lake Kivu, within the western branch of the East African Rift, is one of the largest freshwater reservoirs of dissolved methane (CH4) on Earth. Given the relatively high magnitude of MOB production integrated over the entire water column reported by Morana et al. (2015) (equivalent to 16–60% of the average photosynthetic primary production), and the substantial contribution of MDC to the overall biomass in the oxycline, suggest that MOB could potentially sustain a significant fraction of the pelagic food web in this lake. With few exceptions (like Lake Kivu), it should also be noted that the majority of studies have focused upon relatively small stratifying stillwaters with strong oxygen gradients. The use of MDC in river food webs—substantial quantities of CH4 are oxidized in large riverine systems, including the Amazon and the Hudson River (de Angelis and Scranton, 1993; Melack et al., 2004)—may prove to be a more widespread and significant ecosystem process than given credit at present (Trimmer et al., 2012). Whilst acknowledging that other chemosynthetic processes tend to dominate in marine systems, the use of MDC at pelagic boundaries, such as above the oxygen minimum zones of the various oceans, might well be locally important (but over vast areas) to zooplankton as it is in stratifying lakes subject to similar chemical gradients. There is very recent evidence for substantial oxidation of CH4 within the water column above seeps off Svalbard, and carbon isotopic evidence that atmospheric methane above those seeps is not influenced by contributions from the seafloor source (Graves et al., 2015). Clearly then there must be MOB biomass accruing between the sediment and the surface that could be incorporated into food webs, a pathway that is only likely to increase in importance if gas hydrate destabilization is promoted by warming of bottom waters.
Analyses of long-term data series from lakes demonstrate that many are subject to increasing average water temperature (Schindler et al., 1990; Hampton et al., 2008). While temperature exerts a strong control on CH4 efflux via the physiological stimulation of microbial metabolism (Gedney et al., 2004; Yvon-Durocher et al., 2011), increasingly warm summer surface water temperatures may also increase the duration of stratification, Schmidt stability and hypolimnetic oxygen depletion (e.g., Jankowski et al., 2006), all of which will have ramifications for CH4 dynamics and the routing of MDC into biomass (Jones and Grey, 2011). Some limited yet tantalizing empirical evidence for this arose from the physical manipulation of the depth of the thermocline in a lake (compared to a nearby reference lake) by installation of an impellor system (Forsius et al., 2010). As a consequence of deepening the thermocline, the dominant fish species, perch (Perca fluviatilis) were observed to become more 13C-depleted; a function of increased surface area of sediment adjacent to oxygenated water ideal for chironomid uptake of MOB (route 3 in Figure 1), and the oxygenated water allowing perch to forage on the benthos (Rask et al., 2010). Further manifestations of climate change, such as an increase in both the frequency and severity of storms, could affect both the strength and duration of stratification in lakes, and increase the flux of carbon from the catchment. Not only might erosion from the terrestrial ecosystem provide the substrate for methanogenesis in aquatic ecosystems (e.g., Sanders et al., 2007), but increased concentration and use of dissolved organic and inorganic carbon in lakes and rivers (Schindler et al., 1997; Jones et al., 2001; Worrall et al., 2004; Evans et al., 2005) will shift the balance toward heterotrophic rather than autotrophic functioning. Stable isotope tracers will remain key to unraveling the extent of MDC use in such food webs in future research.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I have had many fruitful discussions on this topic over the past 15 years or so, but particular thanks must be made to my long term collaborators Roger I. Jones and Mark Trimmer, as well as Peter Deines, Jari Syväranta, Oliver Heiri, Maarten van Hardenbroek, Jos Schilder and Matt Wooller. This work was funded by the Natural Environment Research Council, UK (NE/H02235X/1).
References
Agasild, H., Zingel, P., Tuvikene, L., Tuvikene, A., Timm, H., Feldmann, T., et al. (2014). Biogenic methane contributes to the food web of a large, shallow lake. Freshw. Biol. 59, 272–285. doi: 10.1111/fwb.12263
Bade, D., Pace, M., Cole, J., and Carpenter, S. (2006). Can algal photosynthetic inorganic carbon isotope fractionation be predicted in lakes using existing models? Aquat. Sci. 68, 142–153. doi: 10.1007/s00027-006-0818-5
Bastviken, D. (2009). “Methane,” in Encyclopedia of Inland Waters, ed G. E. Likens (Oxford: Academic Press), 783–805.
Bastviken, D., Ejlertsson, J., Sundh, I., and Tranvik, L. (2003). Methane as a source of carbon and energy for lake pelagic food webs. Ecology 84, 969–981. doi: 10.1890/0012-9658(2003)084[0969:MAASOC]2.0.CO;2
Bastviken, D., Tranvik, L. J., Downing, J. A., Crill, P. M., and Enrich-Prast, A. (2011). Freshwater methane emissions offset the continental carbon sink. Science 331, 50–50. doi: 10.1126/science.1196808
Battarbee, R. (1999). “The importance of palaeolimnology to lake restoration,” in The Ecological Bases for Lake and Reservoir Management Developments in Hydrobiology, eds D. Harper, B. Brierley, A. D. Ferguson, and G. Phillips (Springer), 149–159.
Battin, T. J., Luyssaert, S., Kaplan, L. A., Aufdenkampe, A. K., Richter, A., and Tranvik, L. J. (2009). The boundless carbon cycle. Nat. Geosci. 2, 598–600. doi: 10.1038/ngeo618
Belle, S., Parent, C., Frossard, V., Verneaux, V., Millet, L., Chronopoulou, P.-M., et al. (2014). Temporal changes in the contribution of methane-oxidizing bacteria to the biomass of chironomid larvae determined using stable carbon isotopes and ancient DNA. J. Paleolimnol. 52, 215–228. doi: 10.1007/s10933-014-9789-z
Belle, S., Verneaux, V., Millet, L., Parent, C., and Magny, M. (2015). A case study of the past CH4 cycle in lakes by the combined use of dual isotopes (carbon and hydrogen) and ancient DNA of methane-oxidizing bacteria: rearing experiment and application to Lake Remoray (eastern France). Aquat. Ecol. 49, 279–291. doi: 10.1007/s10452-015-9523-6
Bergstrom, I., Makela, S., Kankaala, P., and Kortelainen, P. (2007). Methane efflux from littoral vegetation stands of southern boreal lakes: an upscaled regional estimate. Atmos. Environ. 41, 339–351. doi: 10.1016/j.atmosenv.2006.08.014
Bréas, O., Guillou, C., Reniero, F., and Wada, E. (2001). The global methane cycle: isotopes and mixing ratios, sources and sinks. Isotopes Environ. Health Stud. 37, 257–379. doi: 10.1080/10256010108033302
Brooks, J. M., Kennicutt, M. C., Fisher, C. R., Macko, S. A., Cole, K., Childress, J. J., et al. (1987). Deep-sea hydrocarbon seep communities - evidence for energy and nutritional carbon-sources. Science 238, 1138–1142.
Casper, P., Maberly, S. C., Hall, G. H., and Finlay, B. J. (2000). Fluxes of methane and carbon dioxide from a small productive lake to the atmosphere. Biogeochemistry 49, 1–19. doi: 10.1023/A:1006269900174
Colaço, A., Dehairs, F., and Desbruyères, D. (2002). Nutritional relations of deep-sea hydrothermal fields at the Mid-Atlantic Ridge: a stable isotope approach. Deep Sea Res. I Oceanogr. Res. Papers 49, 395–412. doi: 10.1016/S0967-0637(01)00060-7
Cole, J. J., Prairie, Y. T., Caraco, N. F., McDowell, W. H., Tranvik, L. J., Striegl, R. G., et al. (2007). Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10, 172–185. doi: 10.1007/s10021-006-9013-8
Coleman, D. D., Risatti, J. B., and Schoell, M. (1981). Fractionation of carbon and hydrogen isotopes by methane-oxidizing bacteria. Geochim. Cosmochim. Acta 45, 1033–1037.
Conrad, R. (2005). Quantification of methanogenic pathways using stable carbon isotopic signatures: a review and a proposal. Organ. Geochem. 36, 739–752. doi: 10.1016/j.orggeochem.2004.09.006
Cremona, F., Timm, H., Agasild, H., Tonno, I., Feldmann, T., Jones, R. I., et al. (2014). Benthic foodweb structure in a large shallow lake studied by stable isotope analysis. Freshw. Sci. 33, 885–894. doi: 10.1086/677540
de Angelis, M. A., and Scranton, M. I. (1993). Fate of methane in the Hudson River and Estuary. Global Biogeochem. Cycles 7, 509–523. doi: 10.1029/93GB01636
Deines, P., Bodelier, P. L. E., and Eller, G. (2007a). Methane-derived carbon flows through methane-oxidizing bacteria to higher trophic levels in aquatic systems. Environ. Microbiol. 9, 1126–1134. doi: 10.1111/j.1462-2920.2006.01235.x
Deines, P., and Fink, P. (2011). The potential of methanotrophic bacteria to compensate for food quantity or food quality limitations in Daphnia. Aquat. Microb. Ecol. 65, 197–206. doi: 10.3354/ame01542
Deines, P., and Grey, J. (2006). Site-specific methane production and subsequent midge mediation within Esthwaite Water, UK. Archiv. Hydrobiol. 167, 317–334. doi: 10.1127/0003-9136/2006/0167-0317
Deines, P., Grey, J., Richnow, H. H., and Eller, G. (2007b). Linking larval chironomids to methane: seasonal variation of the microbial methane cycle and chironomid delta C-13. Aquat. Microb. Ecol. 46, 273–282. doi: 10.3354/ame046273
Deines, P., Wooller, M. J., and Grey, J. (2009). Unravelling complexities in benthic food webs using a dual stable isotope (hydrogen and carbon) approach. Freshw. Biol. 54, 2243–2251. doi: 10.1111/j.1365-2427.2009.02259.x
Devine, J. A., and Vanni, M. J. (2002). Spatial and seasonal variation in nutrient excretion by benthic invertebrates in a eutrophic reservoir. Freshw. Biol. 47, 1107–1121. doi: 10.1046/j.1365-2427.2002.00843.x
Devlin, S. P., Saarenheimo, J., Syvaranta, J., and Jones, R. I. (2015). Top consumer abundance influences lake methane efflux. Nat. Commun. 6:8787. doi: 10.1038/ncomms9787
Dossena, M., Yvon-Durocher, G., Grey, J., Montoya, J. M., Perkins, D. M., Trimmer, M., et al. (2012). Warming alters community size structure and ecosystem functioning. Proc. R. Soc. B Biol. Sci. 279, 3011–3019. doi: 10.1098/rspb.2012.0394
Doucett, R. R., Marks, J. C., Blinn, D. W., Caron, M., and Hungate, B. A. (2007). Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88, 1587–1592. doi: 10.1890/06-1184
Duinen, G. A. V., Vermonden, K., Bodelier, P. L. E., Hendriks, A. J., Leuven, R. S. E. W., Middelburg, J. J., et al. (2013). Methane as a carbon source for the food web in raised bog pools. Freshw. Sci. 32, 1260–1272. doi: 10.1899/12-121.1
Eggermont, H., and Heiri, O. (2012). The chironomid-temperature relationship: expression in nature and palaeoenvironmental implications. Biol. Rev. 87, 430–456. doi: 10.1111/j.1469-185X.2011.00206.x
Eller, G., Kanel, L. K., and Kruger, M. (2005). Cooccurrence of aerobic and anaerobic methane oxidation in the water column of lake plusssee. Appl. Environ. Microbiol. 71, 8925–8928. doi: 10.1128/AEM.71.12.8925-8928.2005
Estep, M. F., and Dabrowski, H. (1980). Tracing food webs with stable hydrogen isotopes. Science 209, 1537–1538. doi: 10.1126/science.209.4464.1537
Evans, C. D., Monteith, D. T., and Cooper, D. M. (2005). Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ. Pollut. 137, 55–71. doi: 10.1016/j.envpol.2004.12.031
Finlay, J. C., Power, M. E., and Cabana, G. (1999). Effects of water velocity on algal carbon isotope ratios: implications for river food web studies. Limnol. Oceanogr. 44, 1198–1203.
Forsius, M., Saloranta, T., Arvola, L., Salo, S., Verta, M., Ala-Opas, P., et al. (2010). Physical and chemical consequences of artificially deepened thermocline in a small humic lake - a paired whole-lake climate change experiment. Hydrol. Earth Syst. Sci. 14, 2629–2642. doi: 10.5194/hess-14-2629-2010
Frossard, V., Belle, S., Verneaux, V., Millet, L., and Magny, M. (2013). A study of the δ13C offset between chironomid larvae and their exuvial head capsules: implications for palaeoecology. J. Paleolimnol. 50, 379–386. doi: 10.1007/s10933-013-9732-8
Frossard, V., Verneaux, V., Millet, L., Magny, M., and Perga, M.-E. (2015). Changes in carbon sources fueling benthic secondary production over depth and time: coupling Chironomidae stable carbon isotopes to larval abundance. Oecologia 178, 603–614. doi: 10.1007/s00442-015-3225-6
Gedney, N., Cox, P. M., and Huntingford, C. (2004). Climate feedback from wetland methane emissions. Geophys. Res. Lett. 31:L20503. doi: 10.1029/2004GL020919
Gentzel, T., Hershey, A. E., Rublee, P. A., and Whalen, S. C. (2012). Net sediment production of methane, distribution of methanogens and methane-oxidizing bacteria, and utilization of methane-derived carbon in an arctic lake. Inland W. 2, 77–88.
Graves, C. A., Steinle, L., Rehder, G., Niemann, H., Connelly, D. P., Lowry, D., et al. (2015). Fluxes and fate of dissolved methane released at the seafloor at the landward limit of the gas hydrate stability zone offshore western Svalbard. J. Geophys. Res. 120, 6185–6201. doi: 10.1002/2015JC011084
Grey, J. (2006). The use of stable isotope analyses in freshwater ecology: Current awareness. Pol. J. Ecol. 54, 563–584.
Grey, J., and Deines, P. (2005). Differential assimilation of methanotrophic and chemoautotrophic bacteria by lake chironomid larvae. Aquat. Microb. Ecol. 40, 61–66. doi: 10.3354/ame040061
Grey, J., Jones, R. I., and Sleep, D. (2000). Stable isotope analysis of the origins of zooplankton carbon in lakes of differing trophic state. Oecologia 123, 232–240. doi: 10.1007/s004420051010
Grey, J., Kelly, A., and Jones, R. (2004a). High intraspecific variability in carbon and nitrogen stable isotope ratios of lake chironomid larvae. Limnol. Oceanogr. 49, 239–244. doi: 10.4319/lo.2004.49.1.0239
Grey, J., Kelly, A., Ward, S., Sommerwerk, N., and Jones, R. I. (2004b). Seasonal changes in the stable isotope values of lake-dwelling chironomid larvae in relation to feeding and life cycle variability. Freshw. Biol. 49, 681–689. doi: 10.1111/j.1365-2427.2004.01217.x
Gudasz, C., Bastviken, D., Steger, K., Premke, K., Sobek, S., and Tranvik, L. J. (2010). Temperature-controlled organic carbon mineralization in lake sediments. Nature 466, 478–481. doi: 10.1038/nature09186
Hampton, S. E., Izmest'Eva, L. R., Moore, M. V., Katz, S. L., Dennis, B., and Silow, E. A. (2008). Sixty years of environmental change in the world's largest freshwater lake – Lake Baikal, Siberia. Glob. Change Biol. 14, 1947–1958. doi: 10.1111/j.1365-2486.2008.01616.x
Harrod, C., and Grey, J. (2006). Isotopic variation complicates analysis of trophic relations within the fish community of Plu beta see: a small, deep, stratifying lake. Archiv. Hydrobiol. 167, 281–299. doi: 10.1127/0003-9136/2006/0167-0281
He, R., Wooller, M. J., Pohlman, J. W., Catranis, C., Quensen, J., Tiedje, J. M., et al. (2012). Identification of functionally active aerobic methanotrophs in sediments from an arctic lake using stable isotope probing. Environ. Microbiol. 14, 1403–1419. doi: 10.1111/j.1462-2920.2012.02725.x
Heiri, O., Schilder, J., and van Hardenbroek, M. (2012). Stable isotopic analysis of fossil chironomids as an approach to environmental reconstruction: state of development and future challenges. Fauna Norvegica 31, 7–18. doi: 10.5324/fn.v31i0.1436
Hershey, A. E., Northington, R. M., Hart-Smith, J., Bostick, M., and Whalen, S. C. (2015). Methane efflux and oxidation, and use of methane-derived carbon by larval Chironomini, in arctic lake sediments. Limnol. Oceanogr. 60, 276–285. doi: 10.1002/lno.10023
Hessen, D. O., and Nygaard, K. (1992). Bacterial transfer of methane and detritus; implications for the pelagic carbon budget and gaseous release. Arch. Hydrobiol. Beih. Ergebn. Limnol. 37, 139–148.
Hobbie, J. E. (1988). A comparison of the ecology of planktonic bacteria in fresh and salt water. Limnol. Oceanogr. 33, 750–764. doi: 10.4319/lo.1988.33.4_part_2.0750
Ings, N. L., Hildrew, A. G., and Grey, J. (2012). ‘House and garden’: larval galleries enhance resource availability for a sedentary caddisfly. Freshw. Biol. 57, 2526–2538. doi: 10.1111/fwb.12025
Jankowski, T., Livingstone, D. M., Bührer, H., Forster, R., and Niederhauser, P. (2006). Consequences of the 2003 European heat wave for lake temperature profiles, thermal stability, and hypolimnetic oxygen depletion: implications for a warmer world. Limnol. Oceanogr. 51, 815–819. doi: 10.4319/lo.2006.51.2.0815
Jones, R., and Grey, J. (2004). Stable isotope analysis of chironomid larvae from some Finnish forest lakes indicates dietary contribution from biogenic methane. Boreal Environ. Res. 9, 17–23.
Jones, R. I., Carter, C. E., Kelly, A., Ward, S., Kelly, D. J., and Grey, J. (2008). Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 89, 857–864. doi: 10.1890/06-2010.1
Jones, R. I., and Grey, J. (2011). Biogenic methane in freshwater food webs. Freshw. Biol. 56, 213–229. doi: 10.1111/j.1365-2427.2010.02494.x
Jones, R. I., Grey, J., Quarmby, C., and Sleep, D. (2001). Sources and fluxes of inorganic carbon in a deep, oligotrophic lake (Loch Ness, Scotland). Global Biogeochem. Cycles 15, 863–870. doi: 10.1029/2001GB001423
Jones, R. I., Grey, J., Sleep, D., and Arvola, L. (1999). Stable isotope analysis of zooplankton carbon nutrition in humic lakes. Oikos 86, 97–104. doi: 10.2307/3546573
Kajan, R., and Frenzel, P. (1999). The effect of chironomid larvae on production, oxidation and fluxes of methane in a flooded rice soil. FEMS Microbiol. Ecol. 28, 121–129. doi: 10.1111/j.1574-6941.1999.tb00567.x
Kankaala, P., Taipale, S., Grey, J., Sonninen, E., Arvola, L., and Jones, R. I. (2006). Experimental delta C-13 evidence for a contribution of methane to pelagic food webs in lakes. Limnol. Oceanogr. 51, 2821–2827. doi: 10.4319/lo.2006.51.6.2821
Kankaala, P., Taipale, S., Li, L., and Jones, R. I. (2010). Diets of crustacean zooplankton, inferred from stable carbon and nitrogen isotope analyses, in lakes with varying allochthonous dissolved organic carbon content. Aquat. Ecol. 44, 781–795. doi: 10.1007/s10452-010-9316-x
Kankaala, P., Taipale, S., Nykaenen, H., and Jones, R. I. (2007). Oxidation, efflux, and isotopic fractionation of methane during autumnal turnover in a polyhumic, boreal lake. J. Geophys. Res. Biogeosci. 112:G02033. doi: 10.1029/2006jg000336
Kelly, A., Jones, R., and Grey, J. (2004). Stable isotope analysis provides fresh insights into dietary separation between Chironomus anthracinus and C-plumosus. J. N. Am. Benthol. Soc. 23, 287–296. doi: 10.1899/0887-3593(2004)023<0287:SIAPFI>2.0.CO;2
Kohzu, A., Kato, C., Iwata, T., Kishi, D., Murakami, M., Nakano, S., et al. (2004). Stream food web fueled by methane-derived carbon. Aquat. Microb. Ecol. 36, 189–194. doi: 10.3354/ame036189
Lee, R. W., and Childress, J. J. (1994). Assimilation of inorganic nitrogen by marine invertebrates and their chemoautotrophic and methanotrophic symbionts. Appl. Environ. Microbiol. 60, 1852–1858.
Lennon, J. T., Faiia, A. M., Feng, X., and Cottingham, K. L. (2006). Relative importance of CO2 recycling and CH4 pathways in lake food webs along a dissolved organic carbon gradient. Limnol. Oceanogr. 51, 1602–1613. doi: 10.4319/lo.2006.51.4.1602
Levin, L. A., and Michener, R. H. (2002). Isotopic evidence for chemosynthesis-based nutrition of macrobenthos: the lightness of being at Pacific methane seeps. Limnol. Oceanogr. 47, 1336–1345. doi: 10.4319/lo.2002.47.5.1336
MacAvoy, S. E., Carney, R. S., Fisher, C. R., and Macko, S. A. (2002). Use of chemosynthetic biomass by large, mobile, benthic predators in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 225, 65–78. doi: 10.3354/meps225065
Macko, S. A., Fogel, M. L., Hare, P. E., and Hoering, T. C. (1987). Isotopic fractionation of nitrogen and carbon in the synthesis of amino acids by microorganisms. Chem. Geol. 65, 79–92. doi: 10.1016/0168-9622(87)90064-9
Mbaka, J. G., Somlai, C., Köpfer, D., Maeck, A., Lorke, A., and Schäfer, R. B. (2014). Methane-derived carbon in the benthic food web in stream impoundments. PLoS ONE 9:e111392. doi: 10.1371/journal.pone.0111392
McCutchan, J. H. Jr., Lewis, W. M. Jr., Kendall, C., and McGrath, C. C. (2003). Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. doi: 10.1034/j.1600-0706.2003.12098.x
Melack, J. M., Hess, L. L., Gastil, M., Forsberg, B. R., Hamilton, S. K., Lima, I. B. T., et al. (2004). Regionalization of methane emissions in the Amazon Basin with microwave remote sensing. Glob. Change Biol. 10, 530–544. doi: 10.1111/j.1365-2486.2004.00763.x
Morana, C., Borges, A. V., Roland, F. A. E., Darchambeau, F., Descy, J. P., and Bouillon, S. (2015). Methanotrophy within the water column of a large meromictic tropical lake (Lake Kivu, East Africa). Biogeosciences 12, 2077–2088. doi: 10.5194/bg-12-2077-2015
Opsahl, S. P., and Chanton, J. P. (2006). Isotopic evidence for methane-based chemosynthesis in the upper floridan aquifer food web. Oecologia 150, 89–96. doi: 10.1007/s00442-006-0492-2
Orphan, V. J., Hinrichs, K. U., Ussler, W., Paull, C. K., Taylor, L. T., Sylva, S. P., et al. (2001). Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl. Environ. Microbiol. 67, 1922–1934. doi: 10.1128/AEM.67.4.1922-1934.2001
Parnell, A. C., Phillips, D. L., Bearhop, S., Semmens, B. X., Ward, E. J., Moore, J. W., et al. (2013). Bayesian stable isotope mixing models. Environmetrics 24, 387–399. doi: 10.1002/env.2221
Pel, R., Hoogveld, H., and Floris, V. (2003). Using the hidden isotopic heterogeneity in phyto- and zooplankton to unmask disparity in trophic carbon transfer. Limnol. Oceanogr. 48, 2200–2207. doi: 10.4319/lo.2003.48.6.2200
Perga, M.-E. (2010). Potential of delta C-13 and delta N-15 of cladoceran subfossil exoskeletons for paleo-ecological studies. J. Paleolimnol. 44, 387–395. doi: 10.1007/s10933-009-9340-9
Perga, M.-E. (2011). Taphonomic and early diagenetic effects on the C and N stable isotope composition of cladoceran remains: implications for paleoecological studies. J. Paleolimnol. 46, 203–213. doi: 10.1007/s10933-011-9532-y
Perga, M.-E., and Grey, J. (2010). Laboratory measures of isotope discrimination factors: comments on Caut, Angulo & Courchamp (2008,2009). J. Appl. Ecol. 47, 942–947. doi: 10.1111/j.1365-2664.2009.01730.x
Peterson, B. J., and Fry, B. (1987). Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 18, 293–320. doi: 10.1146/annurev.es.18.110187.001453
Prairie, Y., and del Giorgio, P. (2013). A new pathway of freshwater methane emissions and the putative importance of microbubbles. Inland W. 3, 311–320. doi: 10.5268/IW-3.3.542
Rask, M., Verta, M., Korhonen, M., Salo, S., Forsius, M., Arvola, L., et al. (2010). Does lake thermocline depth affect methyl mercury concentrations in fish? Biogeochemistry 101, 311–322. doi: 10.1007/s10533-010-9487-5
Rau, G. H., and Hedges, J. I. (1979). Carbon-13 depletion in a hydrothermal vent mussel: suggestion of a chemosynthetic food source. Science 203, 648–649. doi: 10.1126/science.203.4381.648
Ravinet, M., Syvaranta, J., Jones, R. I., and Grey, J. (2010). A trophic pathway from biogenic methane supports fish biomass in a temperate lake ecosystem. Oikos 119, 409–416. doi: 10.1111/j.1600-0706.2009.17859.x
Rinta, P., Bastviken, D., van Hardenbroek, M., Kankaala, P., Leuenberger, M., Schilder, J., et al. (2015). An inter-regional assessment of concentrations and δ13C values of methane and dissolved inorganic carbon in small European lakes. Aquat. Sci. 77, 667–680. doi: 10.1007/s00027-015-0410-y
Roach, K. A., Tobler, M., and Winemiller, K. O. (2011). Hydrogen sulfide, bacteria, and fish: a unique, subterranean food chain. Ecology 92, 2056–2062. doi: 10.1890/11-0276.1
Sanders, I. A., Heppell, C. M., Cotton, J. A., Wharton, G., Hildrew, A. G., Flowers, E. J., et al. (2007). Emission of methane from chalk streams has potential implications for agricultural practices. Freshw. Biol. 52, 1176–1186. doi: 10.1111/j.1365-2427.2007.01745.x
Sanseverino, A. M., Bastviken, D., Sundh, I., Pickova, J., and Enrich-Prast, A. (2012). Methane carbon supports aquatic food webs to the fish level. PLoS ONE 7:e42723. doi: 10.1371/journal.pone.0042723
Santer, B., Sommerwerk, N., and Grey, J. (2006). Food niches of cyclopoid copepods in eutrophic PluBsee determined by stable isotope analysis. Archiv. Hydrobiol. 167, 301–316. doi: 10.1127/0003-9136/2006/0167-0301
Sassen, R., Joye, S., Sweet, S. T., DeFreitas, D. A., Milkov, A. V., and MacDonald, I. R. (1999). Thermogenic gas hydrates and hydrocarbon gases in complex chemosynthetic communities, Gulf of Mexico continental slope. Organ. Geochem. 30, 485–497. doi: 10.1016/S0146-6380(99)00050-9
Schilder, J., Bastviken, D., van Hardenbroek, M., Leuenberger, M., Rinta, P., Stoetter, T., et al. (2015a). The stable carbon isotopic composition of Daphnia ephippia in small, temperate lakes reflects in-lake methane availability. Limnol. Oceanogr. 60, 1064–1075. doi: 10.1002/lno.10079
Schilder, J., Tellenbach, C., Möst, M., Spaak, P., van Hardenbroek, M., Wooller, M. J., et al. (2015b). The stable isotopic composition of Daphnia ephippia reflects changes in δ13C and δ18O values of food and water. Biogeosciences 12, 3819–3830. doi: 10.5194/bg-12-3819-2015
Schimmelmann, A. (2011). “Carbon, nitrogen and oxygen stable isotope ratios in Chitin,” in Chitin Topics in Geobiology, ed N.S. Gupta (Springer), 81–103.
Schindler, D. W., Beaty, K. G., Fee, E. J., Cruikshank, D. R., DeBruyn, E. R., Findlay, D. L., et al. (1990). Effects of climatic warming on lakes of the central boreal forest. Science 250, 967–970. doi: 10.1126/science.250.4983.967
Schindler, D. W., Curtis, P. J., Bayley, S. E., Parker, B. R., Beaty, K. G., and Stainton, M. P. (1997). Climate-induced changes in the dissolved organic carbon budgets of boreal lakes. Biogeochemistry 36, 9–28. doi: 10.1023/A:1005792014547
Schmitz, O., Raymond, P., Estes, J., Kurz, W., Holtgrieve, G., Ritchie, M., et al. (2014). Animating the carbon cycle. Ecosystems 17, 344–359. doi: 10.1007/s10021-013-9715-7
Shelley, F., Abdullahi, F., Grey, J., and Trimmer, M. (2015). Microbial methane cycling in the bed of a chalk river: oxidation has the potential to match methanogenesis enhanced by warming. Freshw. Biol. 60, 150–160. doi: 10.1111/fwb.12480
Shelley, F., Grey, J., and Trimmer, M. (2014). Widespread methanotrophic primary production in lowland chalk rivers. Proc. R. Soc. B Biol. Sci. 281:20132854. doi: 10.1098/rspb.2013.2854
Stein, L. Y., and Klotz, M. G. (2011). Nitrifying and denitrifying pathways of methanotrophic bacteria. Biochem. Soc. Trans. 39, 1826. doi: 10.1042/BST20110712
Stephen, E. M., Robert, S. C., Charles, R. F., and Stephen, A. M. (2002). Use of chemosynthetic biomass by large, mobile, benthic predators in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 225, 65–78. doi: 10.3354/meps225065
Summons, R. E., Jahnke, L. L., and Roksandic, Z. (1994). Carbon isotopic fractionation in lipids from methanotrophic bacteria: relevance for interpretation of the geochemical record of biomarkers. Geochim. Cosmochim. Acta 58, 2853–2863. doi: 10.1016/0016-7037(94)90119-8
Taipale, S. J., Peltomaa, E., Hiltunen, M., Jones, R. I., Hahn, M. W., Biasi, C., et al. (2015). Inferring phytoplankton, terrestrial plant and bacteria bulk delta c-13 values from compound specific analyses of lipids and fatty acids. PLoS ONE 10:e0133974. doi: 10.1371/journal.pone.0133974
Taipale, S., Kankaala, P., Hamalainen, H., and Jones, R. I. (2009). Seasonal shifts in the diet of lake zooplankton revealed by phospholipid fatty acid analysis. Freshw. Biol. 54, 90–104. doi: 10.1111/j.1365-2427.2008.02094.x
Taipale, S., Kankaala, P., and Jones, R. I. (2007). Contributions of different organic carbon sources to Daphnia in the pelagic foodweb of a small polyhumic lake: results from mesocosm (DIC)-C-13-additions. Ecosystems 10, 757–772. doi: 10.1007/s10021-007-9056-5
Taipale, S., Kankaala, P., Tiirola, M., and Jones, R. I. (2008). Whole-lake dissolved inorganic C-13 additions reveal seasonal shifts in zooplankton diet. Ecology 89, 463–474. doi: 10.1890/07-0702.1
Templeton, A. S., Chu, K.-H., Alvarez-Cohen, L., and Conrad, M. E. (2006). Variable carbon isotope fractionation expressed by aerobic CH4-oxidizing bacteria. Geochim. Cosmochim. Acta 70, 1739–1752. doi: 10.1016/j.gca.2005.12.002
Trimmer, M., Grey, J., Heppell, C. M., Hildrew, A. G., Lansdown, K., Stahl, H., et al. (2012). River bed carbon and nitrogen cycling: State of play and some new directions. Sci. Total Environ. 434, 143–158. doi: 10.1016/j.scitotenv.2011.10.074
Trimmer, M., Hildrew, A. G., Jackson, M. C., Pretty, J. L., and Grey, J. (2009). Evidence for the role of methane-derived carbon in a free-flowing, lowland river food web. Limnol. Oceanogr. 54, 1541–1547. doi: 10.4319/lo.2009.54.5.1541
Trimmer, M., Maanoja, S., Hildrew, A. G., Pretty, J. L., and Grey, J. (2010). Potential carbon fixation via methane oxidation in well-oxygenated riverbed gravels. Limnol. Oceanogr. 55, 560–568. doi: 10.4319/lo.2009.55.2.0560
Trimmer, M., Shelley, F. C., Purdy, K. J., Maanoja, S. T., Chronopoulou, P.-M., and Grey, J. (2015). Riverbed methanotrophy sustained by high carbon conversion efficiency. ISME J. 9, 2304–2314. doi: 10.1038/ismej.2015.98
Van Dover, C. L., and Fry, B. (1994). Microorganisms as food resources at deep-sea hydrothermal vents. Limnol. Oceanogr. 39, 51–57. doi: 10.4319/lo.1994.39.1.0051
van Hardenbroek, M., Heiri, O., Grey, J., Bodelier, P. L. E., Verbruggen, F., and Lotter, A. F. (2010). Fossil chironomid delta(13) C as a proxy for past methanogenic contribution to benthic food webs in lakes? J. Paleolimnol. 43, 235–245. doi: 10.1007/s10933-009-9328-5
van Hardenbroek, M., Heiri, O., Parmentier, F. J. W., Bastviken, D., Ilyashuk, B. P., Wiklund, J. A., et al. (2013). Evidence for past variations in methane availability in a Siberian thermokarst lake based on delta C-13 of chitinous invertebrate remains. Quat. Sci. Rev. 66, 74–84. doi: 10.1016/j.quascirev.2012.04.009
van Hardenbroek, M., Heiri, O., Wilhelm, M. F., and Lotter, A. F. (2011). How representative are subfossil assemblages of Chironomidae and common benthic invertebrates for the living fauna of Lake De Waay, the Netherlands? Aquat. Sci. 73, 247–259. doi: 10.1007/s00027-010-0173-4
van Hardenbroek, M., Leuenberger, M., Hartikainen, H., Okamura, B., and Heiri, O. (2016). Bryozoan stable carbon and hydrogen isotopes: relationships between the isotopic composition of zooids, statoblasts and lake water. Hydrobiologia 765, 209–223. doi: 10.1007/s10750-015-2414-y
van Hardenbroek, M., Lotter, A. F., Bastviken, D., Duc, N. T., and Heiri, O. (2012). Relationship between d13C of chironomid remains and methane flux in Swedish lakes. Freshw. Biol. 57, 166–177. doi: 10.1111/j.1365-2427.2011.02710.x
Vuorio, K., Meili, M., and Sarvala, J. (2006). Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshw. Biol. 51, 807–822. doi: 10.1111/j.1365-2427.2006.01529.x
Werne, J. P., Baas, M., and Sinninghe Damsté, J. S. (2002). Molecular isotopic tracing of carbon flow and trophic relationships in a methane-supported benthic microbial community. Limnol. Oceanogr. 47, 1694–1701. doi: 10.4319/lo.2002.47.6.1694
Whiticar, M. J. (1999). Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 161, 291–314. doi: 10.1016/S0009-2541(99)00092-3
Whiticar, M. J., Faber, E., and Schoell, M. (1986). Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 50, 693–709. doi: 10.1016/0016-7037(86)90346-7
Wooller, M., Pohlman, J., Gaglioti, B., Langdon, P., Jones, M., Walter Anthony, K., et al. (2012). Reconstruction of past methane availability in an Arctic Alaska wetland indicates climate influenced methane release during the past ~12,000 years. J. Paleolimnol. 48, 27–42. doi: 10.1007/s10933-012-9591-8
Worrall, F., Harriman, R., Evans, C. D., Watts, C. D., Adamson, J., Neal, C., et al. (2004). Trends in dissolved organic carbon in UK rivers and lakes. Biogeochemistry 70, 369–402. doi: 10.1007/s10533-004-8131-7
Yasuno, N., Shikano, S., Muraoka, A., Shimada, T., Ito, T., and Kikuchi, E. (2012). Seasonal increase of methane in sediment decreases δ13C of larval chironomids in a eutrophic shallow lake. Limnology 13, 107–116. doi: 10.1007/s10201-011-0360-6
Yvon-Durocher, G., Jones, J. I., Trimmer, M., Woodward, G., and Montoya, J. M. (2010). Warming alters the metabolic balance of ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 365, 2117–2126. doi: 10.1098/rstb.2010.0038
Keywords: methanotrophy, chironomids, zooplankton, carbon, food webs, greenhouse gas, biogeochemical cycling, trophic transfer
Citation: Grey J (2016) The Incredible Lightness of Being Methane-Fuelled: Stable Isotopes Reveal Alternative Energy Pathways in Aquatic Ecosystems and Beyond. Front. Ecol. Evol. 4:8. doi: 10.3389/fevo.2016.00008
Received: 31 October 2015; Accepted: 25 January 2016;
Published: 11 February 2016.
Edited by:
Jason Newton, Scottish Universities Environmental Research Centre, UKReviewed by:
David Bastviken, Linköping University, SwedenBlake Matthews, Eawag: Swiss Federal Institute of Aquatic Science and Technology, Switzerland
Copyright © 2016 Grey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Grey, ai5ncmV5QGxhbmNhc3Rlci5hYy51aw==
 Jonathan Grey
Jonathan Grey