- 1Instituto Patagónico para el Estudio de los Ecosistemas Continentales, Centro Nacional Patagónico, CONICET, Puerto Madryn, Argentina
- 2Patagonian and Andean Steppe Program, Wildlife Conservation Society, Neuquén, Argentina
- 3Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia San Juan Bosco, Puerto Madryn, Argentina
The southern subspecies of the lesser rhea is distributed through the Argentine Patagonia and southern Chile. Habitat-loss and poaching were identified as the main threats to lesser rhea populations by the IUCN, which classified the species as “Least concern” in 2014. Although Rhea pennata pennata is among the most conspicuous wildlife across the large Patagonian rangelands, the available estimates of abundance are scarce, mostly restricted to reports between 12 and 30 years old, and resulted from different surveying methods. Our purpose in this work was to obtain estimates of R. p. pennata abundance across different sites in the Argentine Patagonia, and to explore the main factors affecting the spatial variation in abundance. We surveyed over 4000 km of line transects across six sites and obtained abundance estimates using the Distance sampling analysis. Also, we fitted restricted mixed models to evaluate the effects of different factors on the variation in R. p. pennata encounter rates among sites. We found that the abundance of R. p. pennata was very low either in the encounter rate (taking until 113 km of survey to detect a group of rheas depending on the site) or population density (between 0.0063 and 0.28 individuals.km−2). The occurrence of sheep ranching (SH) affected negatively the variation in the abundance of R. p. pennata among sites, whereas the availability of grassland (GR) habitat increased the chance of finding groups during the surveys. Line-transect surveys following the Distance sampling procedures are adequate to estimate encounter rates, although the low number of observations requires repeated surveys per site to obtain reliable estimates of animal density. Extended and sustained survey efforts and the implementation of a monitoring program are crucial to assess population trends of lesser rheas. Law enforcement to control poaching, increase in public awareness and an action plan to reduce threats are all necessary steps to conserve this emblematic species in Patagonia.
Introduction
Rheas are large, flightless birds endemic from South America. While the greater rhea Rhea americana spreads across Brazil, Bolivia, Paraguay, Uruguay, and Argentina until 40°S, the lesser Rhea pennata comprise three subspecies. Rhea pennata tarapacensis is found in northern Chile, Rhea pennata garleppi in southern Perú, south-west Bolivia and north-west Argentina, and the southernmost Rhea pennata pennata—known locally as choique—is widely distributed across the arid and semi-arid lands of the Argentine Patagonia and southern Chile (Del Hoyo et al., 1992; BirdLife International, 2011; Figure 1), from the sea level in the Atlantic coast up to 2000 m of altitude in the Andean steppe (Navarro et al., 1999).
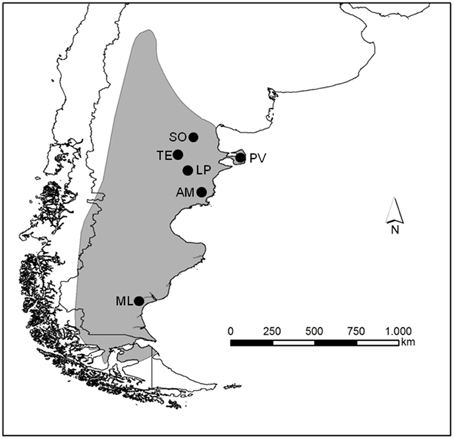
Figure 1. Distributional range of Rhea pennata pennata (gray area) in South America and location of the study sites (black circles do not represent areas but indicate the sites where transects were surveyed).
Habitat loss, egg collection, and poaching have been identified as the main threats that lesser rhea populations are still facing (Bellis et al., 1999; Funes et al., 2000; Barri et al., 2008; Pedrana et al., 2011). Lesser rheas are hunted for subsistence by rural people and poached by people living in Patagonian cities (Funes and Novaro, 1999). There were attempts to develop commercial exploitation through captive breeding (Martella and Navarro, 2006) although the initiatives would not be economically viable. Estimates of abundance of lesser rheas area scarce, they were obtained over a decade ago and resulted from different survey and analyses methodology. While Funes et al. (2000) used line distance sampling to estimate population densities across several sites in the north-west of the range, estimates available for central Patagonia were obtained through strip transect surveys in Chubut 30 years ago (Garrido and Kovacs, 1982). A similar approach was taken by Navarro et al. (1999), combined with interviews to local people to estimate the abundance of lesser rheas at sites across four Patagonian Provinces. Estimates of relative abundance were obtained for southern Patagonia using encounter rates in terms of groups per kilometer traveled (De Lucca, 1996). More recently, Pedrana et al. (2011) mapped the distribution of R. p. pennata in southern Patagonia and concluded that the encounter rate was negatively related to the presence of human habitation, and positively affected by primary productivity, although the probability to find animals was low across most of the area.
At the species level, R. pennata was assessed as “Near Threatened” according to the IUCN Red List criteria (BirdLife International, 2008), although recently has been classified as “Least Concern” (BirdLife International, 2014). However, the scarce and outdated estimates of abundance and the lack of historical data make difficult to estimate the population trend of R. p. pennata in the Argentine Patagonia.
The purpose of this work was to estimate the abundance of lesser rheas across different sites in the Argentine Patagonia. Additionally, we found relevant to explore some of the factors affecting the spatial variation in the abundance of R. p. pennata. We hypothesize that both ecological and human-related factors affect the abundance of lesser rheas. We expect that human activities at sheep ranches, roads, and populated places negatively affect the abundance of lesser rheas as they are factors associated with hunting and disturbance (Giordano et al., 2010; Pedrana et al., 2011). Whereas, protected areas and remote sites, far from populated places, would favor the presence of lesser rheas. Also, we expect that open-grassland habitats will affect positively the abundance of lesser rheas as they provide forage and allow for early detection of predators (Bellis et al., 2005). At the same time, lesser-rheas could be negatively affected by habitat degradation due to desertification processes which are widespread across the arid Patagonia (del Valle et al., 1998).
Materials and Methods
Study Area
We surveyed six sites distributed across three provinces in the Argentine Patagonia (Figure 1). Somuncura (SO) is a plateau ranging from 900 to 1600 m above the sea level in Río Negro Province. Vegetation is represented by the Monte and the Patagonian provinces, annual precipitation is below 200 mm and mean annual temperature ranges from 10 to 14°C (Beeskow et al., 1987). SO is a provincial protected area, although human activities such as subsistence husbandry do take place. In NE Chubut, Península Valdés (PV) is a provincial protected area and also a UNESCO World Heritage Site since 1999. The Monte vegetation is dominant, with shrublands and grasslands varying in ground cover from 40% in the north of PV up to 60–80% in the south (León et al., 1998) where annual precipitation can reach 250 mm. Extensive sheep ranching (SH) occupies most of the area, while tourism is highly relevant for the local economy although mainly focused toward the coastal wildlife. Telsen (TE) is located in central Chubut, dominated by a Monte sparse, 1–2 m high shrubland where precipitation averages 125 mm per year (León et al., 1998). The land is privately owned and extensive SH is the predominant activity. Las Plumas (LP) site comprises a wide, arid valley along the Chubut river where annual precipitation is below 200 mm. The vegetation was described as Monte-Patagonia ecotone (León et al., 1998). SH by private landowners is the main economic activity in the area. Ameghino (AM) in SE Chubut is formed by a plateau 300 m above the sea level, descending eastward toward the Atlantic. Mean annual rainfall is above 200 mm, and the vegetation is dominated by shrublands of the Patagonian province (León et al., 1998). Extensive SH is very important. Finally, Monte León (ML) is located in SE Santa Cruz. Formerly a large SH property, it was donated to the Government and transformed into a National Park in 2002. All the sheep were removed and the only economic activity is tourism which is restricted to specific parts of the area. Mean annual precipitation is 240 mm, and the vegetation physiognomy (VP) belongs to the Patagonian Province with low shrublands and grasslands (Oliva et al., 2006).
Population Surveys
We conducted ground, line-transect surveys (Buckland et al., 1993; Laake et al., 1993) of lesser rheas between February 2005 and April 2015 (Table 1), along secondary dirt-roads and tracks (see Figure 2 as an example of the distribution of transects). All surveys were conducted from an open pick-up vehicle with two observers standing in the back, using the distance sampling method (Burnham et al., 1980). For every group of lesser rheas encountered, we stopped the vehicle, recorded the number of animals, and the perpendicular distance (using a laser rangefinder) from the transect line to the location where the group was standing at the time it was detected. Early detection of animals is possible at all sites as they comprised wide, predominately flat areas. As we conducted the surveys disregarding the breeding season (which starts in July/August and can spread until February depending on the latitude), we excluded all juveniles (younger than 3 months old) from the analysis in order to reduce variance due to group size and because they are small, highly mimetic, and difficult to detect. Also, high average mortality rates (40% for younger than 2 months old, Funes et al., 2000) would result in high variation in abundance between surveys if juveniles were included.
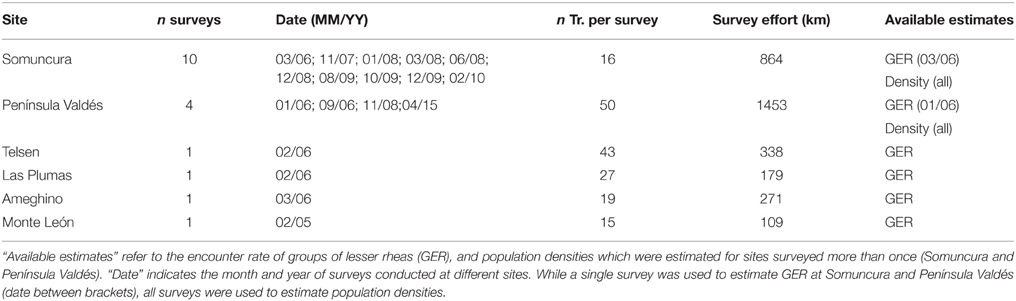
Table 1. Number of surveys (n surveys), transects per survey (n Tr. per survey), and survey effort per site.
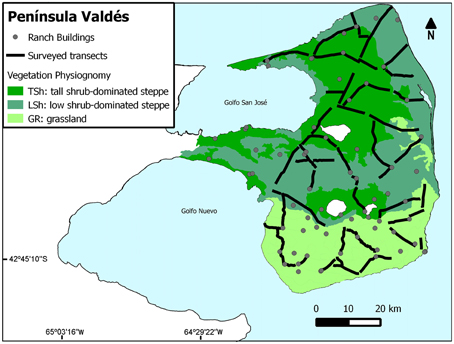
Figure 2. Location of surveyed transects, categories of vegetation physiognomy, and distribution of ranch buildings at one of our study sites (Península Valdés).
Abundance Estimates
Encounter Rates
We calculated group encounter rates (GER) as the number of groups of lesser rheas per km traveled per transect (N = 171 transects). GER were used both to compare the abundance of lesser rheas among sites and to explore the effects of the predictive factors. The study of variation in GER was restricted for the 2005–2006 data-set, when all sites were surveyed once and within a short period of time (Table 1).
Density Estimates
We estimated R. p. pennata densities (animals.km−2) using Distance v. 6.0 (Buckland et al., 1993) which fits the data to a set of theoretical models of probability of detection as a function of the distance to the travel line, and selects the best model following Akaike's (1973) information criterion. A fundamental assumption of this methodology is that objects on the travel line (distance = 0) are always detected. In addition, the objects must be randomly distributed in relation to transects (i.e., the animals should not avoid or be attracted to the travel lines). A limitation of this methodology is the number of observations (60–80) required to obtain acceptable precision (Buckland et al., 1993) As the number of observations of lesser rheas recorded at any single survey was too low (see Table 1) to obtain adequate coefficients of variation (i.e., smaller than 20%), densities were estimated only for the sites surveyed more than once (PV and SO, Table 1). Density and mean group size estimates were obtained using each survey as a stratum in order to improve the precision by increasing the number of observations while decreasing the associated errors (Buckland et al., 1993).
Predictive Factors
We defined and categorized eight factors according to our predictions on the spatial variation in the relative abundance of lesser rheas (Table 2). As the values of the response variable GER were calculated per transect, the levels of each predictive factor were assigned to the individual transects within sites. The information was integrated into a geographic information system using Arc View GIS 3.2. The categories of VP were defined based on the work by Beeskow et al. (1987) and Oliva et al. (2006) and assessed to each transect during the field surveys. Desertification level (DL) was assigned after overlaying the transects to the map published by del Valle et al. (1998). Regarding the occurrence of SH, as it takes place at a larger scale (all sites except SO and ML) all transects within a given site were assigned with the same level. Likewise, as protection (PR) is a management category applied to sites (i.e., SO, PV, and ML are protected areas), all transects within a site were assigned with the same level of PR. We obtained GIS-format data on populated places, rural infrastructure and road network from the Instituto Geográfico Nacional de la República Argentina website (http://www.ign.gob.ar/sig). We overlie a 10 by 10 km grid to maps of rural infrastructure and road network respectively. We defined the density of ranch buildings (BD) per cell as low (0–2 buildings per cell) or high (3–7 buildings per cell) and assessed the level to each transect. The density of roads (RD) was obtained after dividing the total length of roads within a cell by the cell area. The range of road densities varied between 0.059 and 0.692 km−2, and each cell was assessed as of low or high road density according whether its value corresponded to the lower or higher half of the interval. Subsequently, we assessed the level of RD to each transect. The type of road (RT) was assigned to each transect according it was a secondary road or a track (see Table 2). We placed circular, 60 km-radius areas in the midpoint of each transect and assessed the levels defined for the nearest population type (NP, see Table 2). Official data on population numbers were obtained from de Instituto Nacional de Estadística y Censo (INDEC, 2010).

Table 2. Description of specific hypotheses, predictions, and factors defined to analyze the spatial variation in the abundance of lesser rheas.
Model Fitting
Differences between the log of GER among sites were studied by fitting a generalized linear model (GLM) with normally distributed errors and identity link function. We tested the statistical significance of differences between sites using t-tests with a significance level = 0.05 (Crawley, 1993). GenStat v. 7.2 (Lawes Agricultural Trust–VSN International, Rothamstead, UK) was used for all statistical analyses.
We fitted REML (restricted maximum likelihood) mixed models to the log of GER to evaluate the effects of different factors on the GER of R. p. pennata. Predictive factors were considered as fixed effects while “site” was set as the random factor. All the individual factors which affected GER significantly were included into a single REML model. Subsequently, we tested the statistical significance of differences in GER for each individual factor using a Wald test (Payne et al., 2003).
Results
Abundance of Lesser Rheas Across Sites
Encounter Rates
Average encounter rates calculated per site were below 0.25 groups of lesser rheas observed per km traveled (Table 3) during 2005–2006, when we completed a single survey per site (Table 1). While it took on average to travel 4.8 km to sight a group of lesser rheas in SO, it was necessary to travel on average 113.7 km to observe a group in TE. The number of observations per site ranged from 3 to 28 groups, while the coefficients of variation associated to GER varied between 72% for TE and 23% for SO (Table 3).
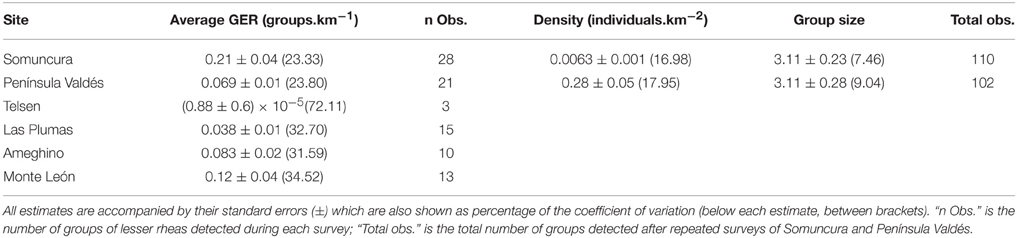
Table 3. Abundance of lesser rheas expressed as average group encounter rate per site (Average GER) and population densities for Somuncura and Península Valdés, where the average number of adults per group (Group size) was also estimated.
Comparisons Among Sites
GERs were significantly different among sites [F(5, 170) = 6.32; p < 0.001]. GER for SO was significantly higher than for the rest of the sites except ML (Table 4). Also, GER for TE was significantly lower than for the other sites except LP (Table 4).
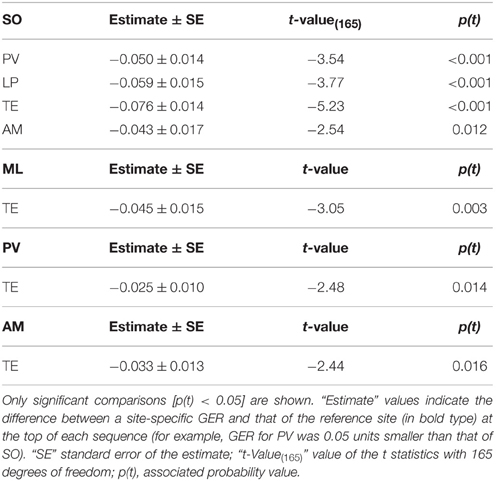
Table 4. Differences in group encounter rate (GER) among sites according to the model log (GER) ≈ Constant + Site + error.
Density Estimates
Repeated surveys at SO and PV resulted in a higher number of observations (110 and 102 groups of lesser rheas detected across all surveys per site, Table 3). Density estimates obtained for both SO and PV across all surveys were associated with acceptable errors (around 17–19% in terms of the coefficient of variation, Table 3). Monotonically decreasing functions of detection probability fitted for both sites showed that the animals did not avoid or were attracted to the transects (Figure 3).
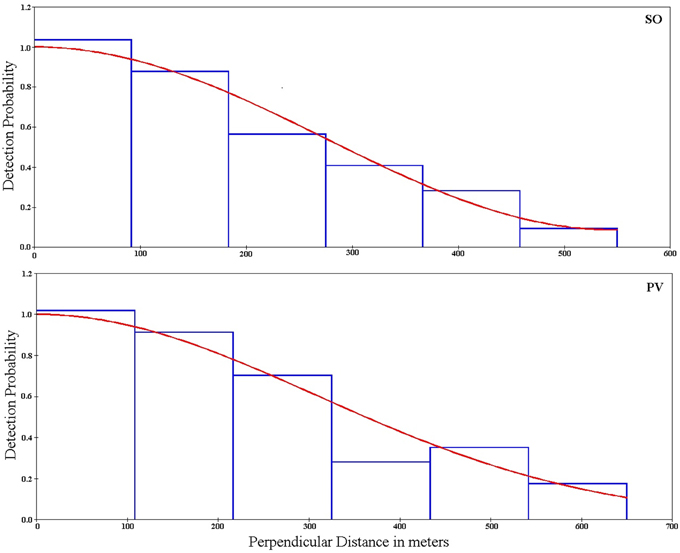
Figure 3. Probability of detection as a function to the distance to the survey line for Somuncura (SO) and Península Valdés (PV). Bars represent grouped data of lesser-rhea groups observed, while the lines represent the fitted, monotonically decreasing functions (Uniform and Half-normal for SO and PV respectively).
Factors Affecting the Spatial Variation in Lesser Rhea Abundance
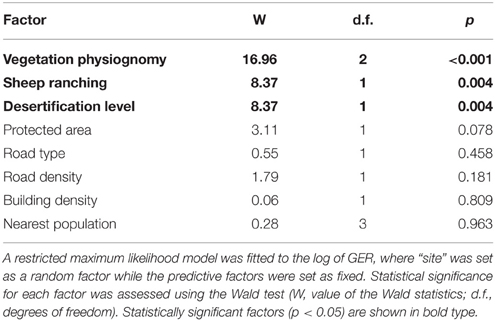
The predictive factors VP, occurrence of SH and DL, did all affect significantly the encounter rate of groups of R. p. pennata (Table 5). GER was on average three times higher in grasslands (GR) than either in tall or short shrublands (GR = 0.0675 vs. TSh = 0.0243 and LSh = 0.0236 groups.km−1). Also, GER was higher at sites without sheep (0.0206 groups.km−1) than where sheep were present (0.0637 groups.km−1), and sites where DLs were light or moderate (0.0637 groups.km−1) compared to sites more severely affected (0.0206 groups.km−1). The rest of the factors did not have significant effects on lesser rheas' GER (Table 5). Significant predictive factors (VP, SH, and DL) were used to fit the GER which was better predicted by VP as the first term, followed either by SH or DL independently of the term. As the third term was not significant, GER = VP + SH + Site or else GER = VP + DL + Site, where GER is the response variable, VP, SH, and DL are fixed effects and Site the random factor.
Discussion
Spatial Variation in Abundance of Lesser Rheas
Across all sites, sighting lesser rheas was a rare event. During the 2005–2006 season we observed 90 groups, around 300 animals in total, after surveying 1300 km. Likewise, 110 groups were observed throughout nine additional surveys in SO (between 2007 and 2010), and 102 groups after four surveys in PV (between 2006 and 2015).
Lesser rheas were more abundant, in terms of encounter rates, at sites without SH like SO and ML. Although both are protected areas, SO holds a number of occupants owning small herds of goats for subsistence in fiscal lands, while ML is a National Park. PV is also a protected area but extensive SH takes place, and the encounter rate of lesser rheas was not significantly different from other areas where SH is the predominant human activity. It is unlikely that lesser rheas were less frequent at SH sites due to interspecific competition for food, as diet overlap between both species has been reported as very low (Somlo, 1997). Instead, low encounter rates at sites where SH takes place might be the result of human presence, as poaching is still common and widespread (Funes and Novaro, 1999; Baldi, pers. comm.). Considering habitat-related variables, the relative abundance of R. p. pennata was positively associated with GR and slight DLs. These results are coincident with the finding by Bellis et al. (2005) who observed that lesser rheas preferred open habitats at a Monte-Patagonian Steppe ecotone in Río Negro, combining the availability of food and good visibility to detect predators. It has been suggested that the loss of habitat, together with poaching and egg collection are the main causes of population decline (Bellis et al., 1999; Funes et al., 2000; Barri et al., 2008). Contrary to our findings, Pedrana et al. (2011) found that R. p. pennata was more likely to occur in areas with high sheep stock abundance across Santa Cruz, in southern Patagonia. However, the authors found a positive association between lesser rheas and primary productivity which is typically associated to open, flat areas favoring the “watch and run” anti-predator strategy (Pedrana et al., 2011). Additionally, the negative effect of human dwellings on the occurrence of lesser rheas reported in that study suggests that they are more disturbed near those areas, while competition with sheep has no noticeable effects (Pedrana et al., 2011).
In terms of encounter rates, previous estimates were obtained for Santa Cruz by De Lucca (1996) who observed one group of lesser rheas every 90.5 km traveled (0.011 groups.km−1), comparable to the lowest estimates in our study, while Pedrana et al. (2011) observed 795 groups along 8000 km traveled (0.099 groups.km−1), a lower rate than our highest estimate (see Table 3). Regarding the densities of R. p. pennata, our estimates were close to the range but smaller than the average 0.44 animals.km−2 (range 0.06–1.5 animals.km−2) reported by Funes et al. (2000) using the same methodology in NW Patagonia. It is difficult to compare our estimates to others which used different methodology of survey and analysis. For example, Navarro et al. (1999) reported densities of 2.06 and 2.93 lesser rheas.km−2 for sites in Río Negro and Santa Cruz, although the estimates were calculated for fixed-width strip transects. For Chubut and Neuquén, Navarro et al. (1999) reported densities of 2.52 and 1.94 lesser rheas.km−2 respectively, based on interviews to local settlers.
Conservation Implications
Although protected by all Provincial laws in Argentina, R. p. pennata is very scarce across all sites surveyed. Over a decade ago, Funes et al. (2000) reported that lesser rhea populations declined in NW Patagonia, while Navarro et al. (1999) suggested an overall population increase. The densities of lesser rheas are markedly low compared to guanacos (Lama guanicoe), the other large native herbivore sharing the range in Patagonia. Burgi (2013) estimated densities ranging from 2 to 12 guanacos.km−2 across the same sites surveyed in Río Negro and Chubut. Also, while guanacos were the main prey consumed by the puma Puma concolor in Chubut (Fernández and Baldi, 2014), Río Negro and Neuquén (Funes et al., 2000), lesser rheas were considered “ecologically extinct” in terms of their functionality as prey, given that they were consumed occasionally by the native carnivores (Novaro et al., 2000).
The lack of historic data on the abundance of R. p. pennata makes difficult to assess the population trend. However, there is indirect evidence suggesting that they were more abundant in the past. Lesser rheas, as well as guanacos, were a crucial resource to the indigenous people in historical times. The Tehuelches hunted lesser rheas to obtain meat, fat, feathers and tendons. But the hunting pressure increased dramatically in 1866 when the Tehuelches initiated the commercial trade of feathers with the Welsh colonists settled in the Chubut valley (Gavirati, 2003). The feather trade has been very important as in 1882 it made up 65% of the total value exported from the colony to Buenos Aires. Accordingly, the growing demand of feathers obligated the Tehuelches to increase the hunting pressure on lesser rheas to satisfy both their own needs and the external market. As on average one lesser rhea provides 0.227 kg of feathers (half a pound, Cox, 1999), around 66,000 animals should have been hunted only in 1882 to export 15 tons of feathers from Chubut (Gavirati, 2003). Using the global encounter rate resulting from our data (around 300 lesser rheas observed in 1300 km traveled), it would take to travel over 285,000 km with a 100% efficiency to hunt 66,000 lesser rheas within 1 year. According to records from the Ministry of Economy of Argentina, the Welsh colony sold 71,401 kg of feathers between 1866 and 1893, representing a harvest of around 315,000 lesser rheas to trade the feathers from the Chubut colony only, as there were other trading sites in Patagonia. For all of Argentina, between 30,000 and 70,000 kg of lesser and greater rhea feathers were traded per year (Gavirati, 2003).
R. pennata was globally classified as “Near Threatened” in 2008 (BirdLife International, 2008) but re-classified as “Least Concern” in 2014 according to the IUCN Red List criteria, which considers the global range and population size, yet an alleged population decrease (BirdLife International, 2014). Recommended conservation actions such as to prevent illegal hunting and egg-collecting (BirdLife International, 2014) require reliable information on abundance and distribution parameters to monitor the effects of management on R. p. pennata. Our work provides evidence that lesser rheas are scarce, at least across large expanses of land through central and Eastern Patagonia. Therefore, the dissemination of results, increase in public awareness, planning of extended and sustained population surveys, law enforcement and the implementation of a monitoring program are necessary steps to take in the near future to conserve this emblematic—though uncommon—wild species in Patagonia.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the Centro Nacional Patagónico, an institute of CONICET (the Argentine Research Council), and the Wildlife Conservation Society for financial and logistic support. We thank to V. Alonso Roldán, P. Raposo, A. Marino, M. Zamero, D. Udrízar-Sauthier, R. D'Agostino, and Fundación Vida Silvestre Argentina for their assistance in the field. We thank two reviewers for their constructive comments and suggestions which helped to improve this manuscript.
References
Akaike, H. (1973). “Information theory and an extension of the maximum likelihood principle,” in International Symposium on Information Theory, 2nd Edn., eds B. N. Petran and F. Csaaki (Budapest: Akadeemiai Kiadi), 267–281.
Barri, F. R., Martella, M. B., and Navarro, J. (2008). Effects of hunting, egg harvest and livestock grazing intensities on density and reproductive success of lesser rhea Rhea pennata pennata in Patagonia: implications for conservation. Oryx 42, 607–610. doi: 10.1017/S0030605307000798
Beeskow, A. M., del Valle, H. F., and Rostagno, C. M. (1987). Los Sistemas Fisiográficos de la Región Árida y Semiárida de la Provincia de Chubut. Buenos Aires: SECyT.
Bellis, L. M., Martella, M. B., Navarro, J. L., and Vignolo, P. (1999). “Experience of release of yearlings of greater rhea reproduced artificially,” in Proceedings of the IV Neotropical Ornithological Congress (Monterrey), 55–56.
Bellis, L. M., Navarro, J. L., Vignolo, P., and Martella, M. B. (2005). Habitat preferences of lesser rheas in Argentine Patagonia. Biodivers. Conserv. 15, 3065–3075. doi: 10.1007/s10531-005-5398-5
BirdLife International (2008). Rhea Pennata. The IUCN Red List of Threatened Species. Available online at: http://www.iucnredlist.org/details/22678081/0
BirdLife International (2011). Distribution Maps of Birds of the World. Cambridge, MA: BirdLife International.
BirdLife International (2014). Rhea Pennata. The IUCN Red List of Threatened Species. Version 2014.3. Available online at: http://www.iucnredlist.org/details/22728199/0
Buckland, S. T., Anderson, D. R., Burnham, K. P., and Laake, J. L. (1993). Distance Sampling: Estimating Abundance of Biological Populations. London: Chapman & Hall.
Burgi, M. V. (2013). Distribución Espacial y Estructura Genética de las Poblaciones de Guanaco en la Patagonia Central. Ph D. dissertation, Universidad Nacional del Comahue.
Burnham, K. P., Anderson, D. R., and Laake, J. L. (1980). Estimation of density from line transect sampling of biological populations. Wildl. Monogr. 72, 3–202.
Cox, G. (1999). Viaje en las Regiones Septentrionales de la PATAGONIA (1862–1863). Buenos Aires: Elefante Blanco.
Del Hoyo, J., Elliot, A., and Sargatal, J. (1992). Handbook of the Birds of the World, Vol. 1. Barcelona: Lynx Editions.
De Lucca, E. R. (1996). Censos de choiques (Pterocnemia pennata pennata) en el sur patagónico. Hornero 14, 74–77.
del Valle, H. F., Elissalde, N. O., Gagliardini, D. A., and Milovich, J. (1998). Status of desertification in the Patagonian region: assessment and mapping from satellite imagery. Arid Land Res. Manage. 12, 95–121. doi: 10.1080/15324989809381502
Fernández, C., and Baldi, R. (2014). Hábitos alimentarios del puma (Puma concolor) e incidencia de la depredación en la mortandad de guanacos (Lama guanicoe) en el noreste de la Patagonia. Mastozool. Neotrop. 21, 331–338. Available online at: http://www.sarem.org.ar/wp-content/uploads/2014/12/SAREM_MastNeotrop_21-2_13_Fernandez.pdf
Funes, M. C., and Novaro, A. J. (1999). Rol de la fauna silvestre en la economía del poblador rural, provincia del neuquén, argentina. Rev. Argent. Producción Anim. 19, 265–271.
Funes, M. C., Rosauer, M. M., Sánchez Aldao, G., Monsalvo, O. B., and Novaro, A. J. (2000). Manejo y Conservación del Choique en la Patagonia: Análisis de los Relevamientos Poblacionales. Neuquén: Centro PYME.
Garrido, J. L., and Kovacs, Z. (1982). Distribución de Herbívoros en Chubut. Afinidad Ambiental de Guanaco, Ñandúy Mara. CENPAT Technical Paper 63. Buenos Aires: CONICET.
Gavirati, M. (2003). Un negocio liviano?: la importancia del comercio de plumas de avestruz para la colonia galesa, la patagonia y argentina. Pueblos y Fronteras 4, 4–16.
Giordano, P. F., Navarro, J. L., and Martella, M. B. (2010). Building large-scale spatially explicit models to predict the distribution of suitable habitat patches for the Greater rhea (Rhea americana), a near-threatened species. Biol. Conserv. 143, 357–365. doi: 10.1016/j.biocon.2009.10.022
INDEC (2010). Censo 2010. Available online at: http://www.indec.mecon.ar/nivel2_default.asp?seccion=P&id_tema=2
Laake, J. L., Buckland, S. T., Anderson, D. R., and Burnham, K. P. (1993). DISTANCE User's Guide V2.0. Fort Collins, CO: Colorado State University.
León, R. J. C., Bran, D., Collantes, M., Paruelo, J. M., and Soriano, A. (1998). Grandes unidades de vegetación de la patagonia extra andina. Ecología Austral 8, 125–144.
Martella, M. B., and Navarro, J. L. (2006). “Proyecto ñandú. manejo de rhea americana y rhea pennata en argentina,” in Manejo de Fauna Silvestre en la Argentina: Programas de uso Sustentable, eds M. L. Bolkovick and D. Ramadori (Buenos Aires: SAyDS), 39–50.
Novaro, A. J., Funes, M. C., and Walker, R. S. (2000). Ecological extinction of native prey of a carnivore assemblage in Argentine Patagonia. Biol. Cons. 92, 25–33. doi: 10.1016/S0006-3207(99)00065-8
Navarro, J. L., Cardón, R., Manero, A., and Clarke, R. (1999). Estimación de la Abundancia Poblacional de Choique en Vida Silvestre. Technical Paper. Buenos Aires: SAyDS.
Oliva, G., Humano, G., Rial, P., González, L., and Paredes, P. (2006). Estudio de Línea de Base y Plan de Monitoreo de la Biodiversidad Vegetal del Parque Nacional Monte León. Technical Paper. Río Gallegos: INTA Argentina.
Payne, R., Murray, D., Harding, S., Baird, D., Soutar, D., and Lane, P. (2003). GenStat for Windows, 7th Edn. Oxford: VSN International.
Keywords: Rhea pennata pennata, lesser rhea, abundance estimation, spatial variation, Patagonia Argentina, conservation
Citation: Baldi R, Pirronitto A, Burgi MV and Antún M (2015) Abundance Estimates of the Lesser Rhea Rhea pennata pennata in the Argentine Patagonia: Conservation Implications. Front. Ecol. Evol. 3:135. doi: 10.3389/fevo.2015.00135
Received: 17 April 2015; Accepted: 17 November 2015;
Published: 08 December 2015.
Edited by:
Enrique Martínez-Meyer, Universidad Nacional Autónoma de México, MexicoReviewed by:
Jose F. Gonzalez-Maya, Universidad Nacional Autónoma de México, MexicoCarlos Alberto Lopez Gonzalez, Universidad Autónoma de Querétaro, Mexico
Copyright © 2015 Baldi, Pirronitto, Burgi and Antún. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ricardo Baldi, cmJhbGRpQGNlbnBhdC1jb25pY2V0LmdvYi5hcg==
 Ricardo Baldi
Ricardo Baldi Analía Pirronitto3
Analía Pirronitto3