- 1Faculty of Health, Department of Cardiology, Örebro University, Örebro, Sweden
- 2School of Health and Medical Sciences, Örebro University, Örebro, Sweden
- 3The institute of Environmental Health, Karolinska Institutet, Stockholm, Sweden
Ipsilateral retinal projections (IRP) in the optic chiasm (OC) vary considerably. Most animal groups possess laterally situated eyes and no or few IRP, but, e.g., cats and primates have frontal eyes and high proportions of IRP. The traditional hypothesis that bifocal vision developed to enable predation or to increase perception in restricted light conditions applies mainly to mammals. The eye-forelimb (EF) hypothesis presented here suggests that the reception of visual feedback of limb movements in the limb steering cerebral hemisphere was the fundamental mechanism behind the OC evolution. In other words, that evolutionary change in the OC was necessary to preserve hemispheric autonomy. In the majority of vertebrates, motor processing, tactile, proprioceptive, and visual information involved in steering the hand (limb, paw, fin) is primarily received only in the contralateral hemisphere, while multisensory information from the ipsilateral limb is minimal. Since the involved motor nuclei, somatosensory areas, and vision neurons are situated in same hemisphere, the neuronal pathways involved will be relatively short, optimizing the size of the brain. That would not have been possible without, evolutionary modifications of IRP. Multiple axon-guidance genes, which determine whether axons will cross the midline or not, have shaped the OC anatomy. Evolutionary change in the OC seems to be key to preserving hemispheric autonomy when the body and eye evolve to fit new ecological niches. The EF hypothesis may explain the low proportion of IRP in birds, reptiles, and most fishes; the relatively high proportions of IRP in limbless vertebrates; high proportions of IRP in arboreal, in contrast to ground-dwelling, marsupials; the lack of IRP in dolphins; abundant IRP in primates and most predatory mammals, and why IRP emanate exclusively from the temporal retina. The EF hypothesis seams applicable to vertebrates in general and hence more parsimonious than traditional hypotheses.
Introduction
The proportion of ipsilateral retinal projections (IRP), the uncrossed fibers in the optic chiasm (OC), varies considerably with species. Most animal groups have laterally situated eyes and no or few IRP. However, for example cats and primates have frontal eyes and high proportions of IRP. The traditional assumption is that the latter configuration is due to the selective advantage of accurate depth perception through stereopsis (Pettigrew, 1986a; Heesy, 2009). Due to the position of the eyes, binocular vision creates two slightly different images, and this disparity provides information that the brain uses to estimate depth in the visual scene (Wheatstone, 1838; Barlow et al., 1967; Heesy, 2009). Binocular vision has been associated with ecological/behavioral factors such as predatory behavior, nocturnalism, and living in trees (Tigges, 1970; Heesy, 2009). It is functionally related to changes in the visuomotor system, high visual acuity, specializations of visual pathways in the brain, elaboration and differentiation of the visual cortex, and with orbital convergence (Heesy, 2009). Many features of primates, including specializations of the visual system, have been explained as adaptations to the occupation of an arboreal niche (Heesy, 2009). Collins (1921) proposed that binocular vision and stereopsis, i.e., reconstructing a three-dimensional world in the cortex using binocular visual information, is necessary for accurate judgment of distance in arboreal locomotion, not least during leaping, where a miscalculation may be deadly. Similarly, the fine-branch niche hypothesis of primate origins postulates that visual specializations for judgment of depth during acrobatic locomotion in a network of typically small branches was vital (Martin, 1979).
Also Crompton (1995) supported that primates' stereoscopic depth perception and orbit convergence developed for accurate judge distance, but added that it may also have been for locating food, such as fruit and insects, in an obscure and complex arboreal environment. Aforementioned hypotheses have in common the necessity for accurate judgment of distance in arboreal substrates to avoid a fatal estimation of distance. However, Heesy (2009) argued that arboreality is not exclusive to primates; some other arboreal mammals, e.g., rodents, have divergent orbits and panoramic visual fields, including flying squirrels, which may fly over 100 m between trees. Cartmill (1992) suggested that high orbit convergence and binocular visual field overlap are adaptations to the nocturnal visual predatory habits of the last common ancestor of all primates. He demonstrated that many predatory mammals relying on vision to follow and grab prey have comparatively high orbit convergence, and postulated “the visual predation hypothesis” i.e., that several characteristics of the primate visual system might have been due to alterations serving in visually based predation. Allman (1977) and Pettigrew (1978) developed the visual predation hypothesis further, suggesting that orbit convergence is associated with the development of suitable optical, and visual axes, in nocturnal taxa. The optical axis is the axis of symmetry through the cornea and lens; the visual axis means the line that fits through the point of fixation, nodal points, and area centralis (or fovea); the orbital axis is the line of symmetry of the orbit (Heesy, 2008). If visual and optic axes are more and more brought into line, the result will be that light from the object of interest can pass through the center of the lens, and close alignment of the optic and visual axes will permit nocturnal animals to view prey and other objects within binocular fields without compromising light-gathering capabilities (Heesy, 2009).
Pettigrew (1986a) expanded his avian hypothesis to include mammals. Regardless of panoramic or binocular visual fields arboreal animals are likely to use multiple cues to perceive depth during locomotion, examples are interposition (an object is partly obscuring another object), perspective, motion parallax, vergence eye movement cues, accommodation, and optic flow (Heesy, 2009). Heesy (2009) proposed that large binocular visual fields, give at least three unique potential visual advantages: enhanced light sensitivity, expanded stereoscopic depth perception, and contrast discrimination. The latter is made possible by summation of two similar images presented to each eye, which helps the brain to distinguish unwanted “noise” from useful information. Stereopsis means the computation of object solidity and depth based on binocular disparity cues (Poggio and Poggio, 1984; Cumming and DeAngelis, 2001). Stereoscopic depth perception is founded on neurons sensitive to binocular disparities. These “binocular neurons” need contribution from each eye (Poggio, 1984) and are responsible for the perception of depth, object solidity, and binocular fusion (Heesy, 2009). Stereoscopic processing in the cortex integrates/fuses percepts of retinal images from each eye into a singular mental visual image (Poggio, 1984; Poggio and Poggio, 1984; Cumming and DeAngelis, 2001). Stereoscopic depth perception precludes binocular visual field overlap since that is the portion of the visual field from which binocular parallax cues are composed; binocular parallax is generated by the slightly different views of objects that are projected onto each retina; the stereopsis hypothesis suggests that these slight discrepancies, termed binocular disparities, provide the cues from which stereoscopic depth is computed (Heesy, 2009).
Parallax is a function of the interocular distance, which limits the functional utility of stereopsis. The maximum range in amphibians and birds is less than 1 m, and mammals also have a short range over which stereopsis functions (Collett, 1977). In humans vergent eye movements occur most frequently in the space corresponding to the arm's length (Viguier et al., 2001). Since the earliest true primates were likely to be very small, with small interocular distance, their stereoscopic range was probably much shorter than their maximal leaping distance, which contradicts that primate binocularity evolved to judge distance for arboreal leaping (Heesy, 2009).
Archeological evidence have suggest that grasping and visual adaptations evolved asynchronously in primates, i.e., adaptations for manual and pedal grasping evolved long before primates' typical visual characteristics (Silcox et al., 2007; Kirk et al., 2008). Silcox et al. (2007) proposed that a shift to increased herbivory diet characterized the origin of primates, which would contradict the visual predation hypothesis. Moreover, modern phylogenies have united tarsiers with anthropoid primates (monkeys, apes, and humans) (Jameson et al., 2011). Studies in tarsiers (Tan and Li, 1999; Melin et al., 2013) have suggested that many hallmarks of the anthropoid visual system had already evolved in the last common ancestor of tarsiers and anthropoid primates, which also challenges the visual predation hypothesis.
Hypotheses about the evolution of bifocal vision/stereopsis are largely restricted to mammals and/or birds. Ward et al. (1995) stated that the variation in IRP among non-mammalian vertebrates is mysterious and lacks a relationship with nocturnalism or predatory lifestyle. A recent eye-forelimb (EF) hypothesis proposed that receiving visual feedback of limb movements in the limb-steering hemisphere was fundamental in the evolution of vertebrate visual systems (Larsson, 2011) and the primary basis for the substantial variation in proportion of crossed and uncrossed retinal ganglion cells (RGC). The aim of this review was to examine OC anatomy and retinal specializations in a variety of vertebrates with respect to the EF hypothesis and to explore associations with motor behavior.
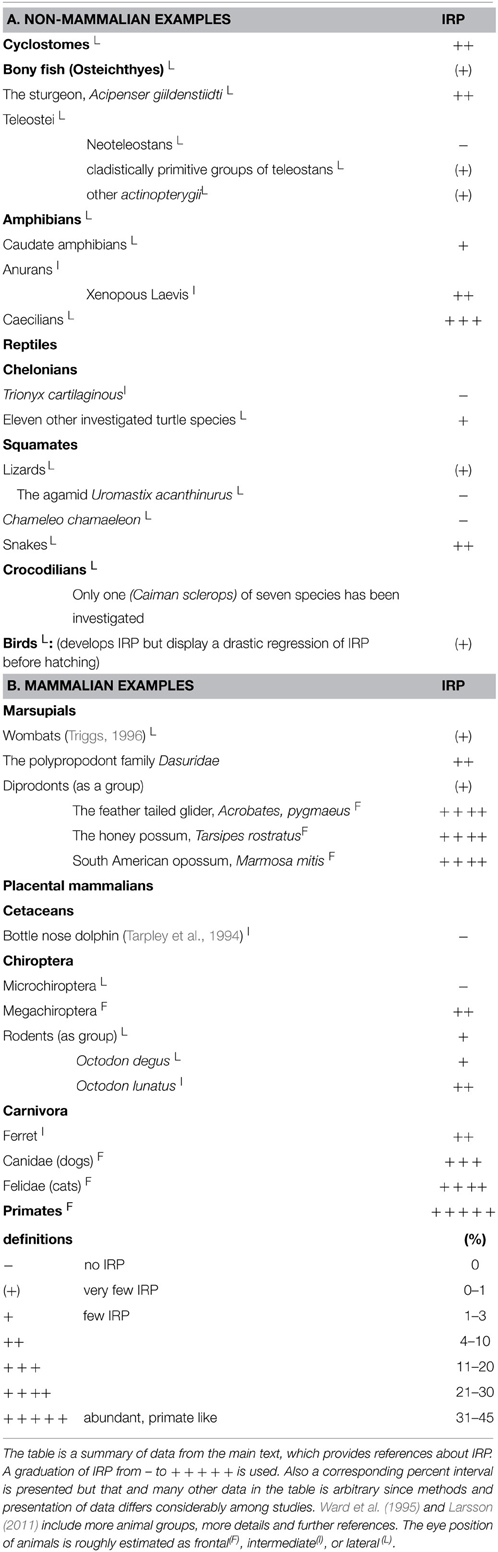
The OC is the structure of the ventral diencephalon midline where RGC axons either cross the midline or return to the ipsilateral hemisphere (Figure 1). Thus, fibers destined for the right or left hemispheres are separated here. The arrangement of the OC is not consistent among species, and shows notable differences in gross architecture (Figure 2) (Jeffery and Erskine, 2005). Developmental mechanisms shaping this region may vary among closely related animals (Jeffery and Erskine, 2005; Herrera and Mason, 2007).
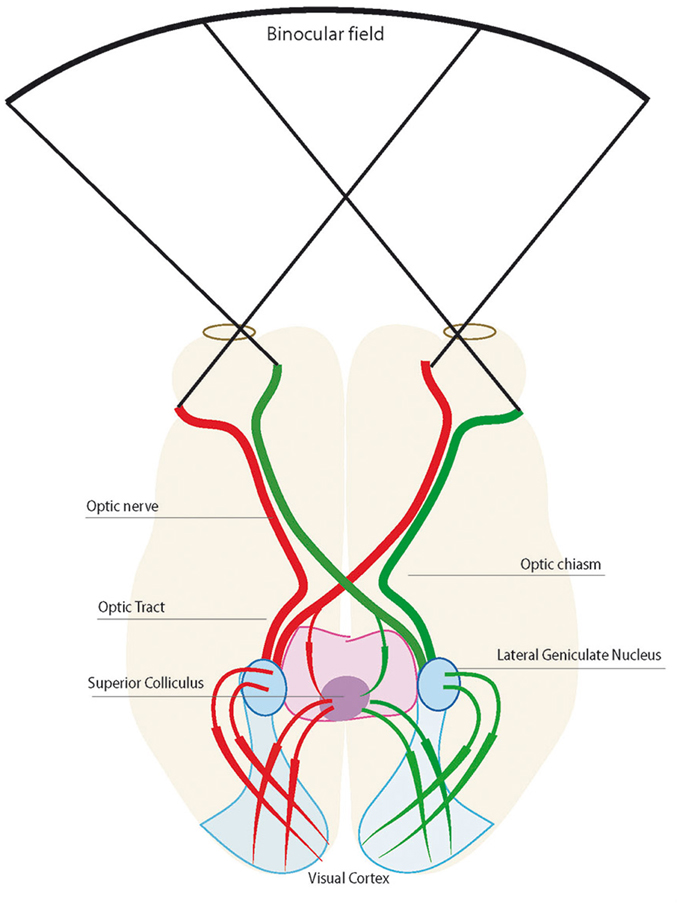
Figure 1. Visual pathway in a primate. The superior colliculus, lateral geniculate nucleus, and visual cortex in each hemisphere receive input from both eyes. Visual information is received by the left halves of the retina (red) of both eyes and transmitted to the left hemisphere. Axons from the right half of each eye (green) terminate in the right hemisphere. Due to the development of frontal eye position and the crossed neuron fibers in the optic chiasm, visual information about the right hand will reach the left hemisphere, which also receives tactile and proprioceptive information. The left hemisphere also steers the right hand, hence, all involved neurons are situated in the same hemisphere. Reprinted from Herrera and Mason (2007) with permission from Elsevier and the authors.
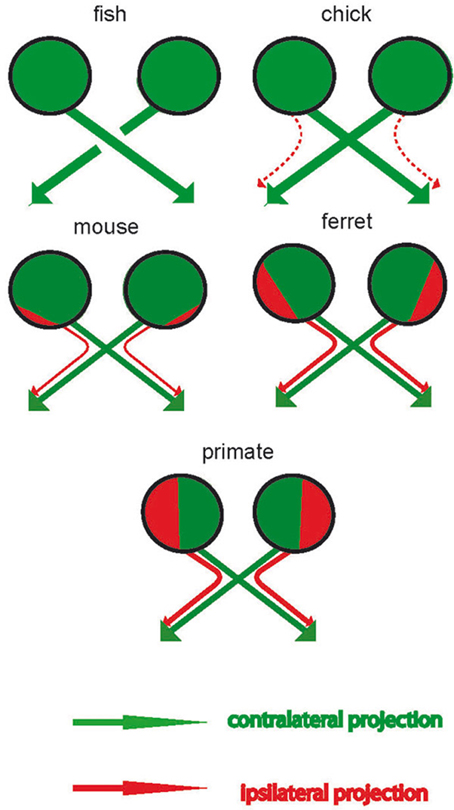
Figure 2. In fish, except Cyclostomes, all retinal ganglial cells project contralaterally and retinal axons do not intermingle at the chiasm. In birds, retinal axons project contralaterally, however, there is a minor transient ipsilateral projection (red dashed lines) that disappears at hatching. Binocular vision is partially developed in mouse and ferret with most retinal axons crossing the midline (green), but they also possess ipsilateral retinal projections (red). Binocular vision is fully developed in mammals with frontally located eyes. Line thickness indicates the proportions of fibers that projects to ipsilateral or contralateral targets. Reprinted from Herrera and Mason (2007) with permission from Elsevier and the authors.
The Eye-forelimb Hypothesis
The EF hypothesis postulates that increased IRP is useful in animals with frontal eyes that regularly use their forelimbs frontally, while reduced IRP is functional in animals using the forelimbs laterally (Larsson, 2011). Limb movement is a multimodal event engaging motoneurons, proprioception, and, possibly, touch, and vision. In essentially all vertebrates each cerebral hemisphere receives little visual, tactile, and proprioceptive information from the ipsilateral forelimb, while multisensory information from the contralateral limb is abundant (Figure 3). Since the involved motor nuclei, somatosensory areas, and vision neurons are situated in the same hemisphere, the neuronal pathways involved are relatively short. However, that would not have been the case without evolutionary modifications of crossed/uncrossed projections in the OC. Reduction in the number of interhemispheric connections reduces brain size (Ringo et al., 1994) and speeds processing (Poffenberger, 1912; Berlucchi et al., 1971). This neural architecture creates hemispheres that are autonomous with respect to motor control and perception of limb movements, and multimodal information about a moving forelimb will converge in the same cerebral hemisphere. The inner ear is also primarily connected to the contralateral hemisphere and hence the hemisphere also receives rapid auditory feedback about the limb that it controls. The OC structure may be functional in the storage and recollection of time-based sequences that is necessary for making predictions, recognizing time-based patterns, and generating behaviors. The neocortex can be viewed as a memory system that builds a model of the world for inference, prediction, and behavior (Hawkins et al., 2009). These authors suggested a hierarchically organized memory system in which time-based sequences of multimodal character are primarily stored locally. Hemispheric autonomy with respect to forelimb coordination is likely to facilitate the storing and recalling of multimodal information in forelimb maneuvers, thereby improving motor learning.
Figure 3. The optic chiasm (OC) in three types of vertebrates. Filled circles represent the superior colliculus (SC). Rectangles represent portions of the left and right visual hemifield. (A) Limbless snakes and caecilians have relatively high proportions of ipsilateral retinal projections, similar to cyclostomes. (B) In animals with laterally placed forelimbs, visual, motor, tactile, and proprioceptive information concerning the forelimb are processed in the contralateral hemisphere. However, that would not have been possible without evolutionary change in the OC, in this case a reduction of IRP compared with cyclostome-like ancestors (C) The cerebral hemispheres of primates receive visual information solely from the contralateral visual hemifield. Again, that would not have been possible without evolutionary change in the OC, in this case increased IRP from the temporal retina. Cats, arboreal marsupials, and fruit bats have similar visual systems. Reprinted from Larsson (2013) with permission from the author/copy-right holder.
Thus, visual feedback from limbs to the limb-steering hemisphere is likely to be highly adaptive. Crossing, or non-crossing, in the OC determines which hemisphere receives visual feedback in forelimb maneuvers, and elaborate mechanisms to regulate IRP proportions exist in the form of multiple axon guidance genes (Herrera and Mason, 2007).
Retinal ganglion cells departure from the retina and propagate in a certain direction by distinguishing guidance cues that are expressed in optic pathway structures, counting the optic disc, optic stalk, OC, and optic tract (Petros et al., 2008). Such guidance cues comprise cell surface ligands e.g., semaphorins in the optic stalk and ephrin B2 in the OC, and a variety of soluble elements for example netrin-1 in the optic disc and Slit1 in areas neighboring the OC (Erskine and Herrera, 2007). In most animals few or no RGC are linked to targets in the ipsilateral side of the brain, while the majority of RGC axons, after crossing the midline at the OC, will be linked to targets in the contralateral hemisphere, located at the ventral-medial hypothalamic (vHT) area (Kim et al., 2014). Subsets of vHT cells express molecules that determine the direction, which RGC axons take at the OC (Kim et al., 2014). For example it has been shown that vHT radial glial cells express ephrinB2, which binds to ephrinB1 receptors expressed in ventral-temporal RGC axons and drive back axons toward the ipsilateral optic tract (Nakagawa et al., 2000; Williams et al., 2003). Other factors e.g., a neuronal cell adhesion molecule expressed in the vHT have been suggested to support the growth of RGC axons across the vHT midline (Williams et al., 2006; Erskine et al., 2011; Kuwajima et al., 2012). Thus, molecular cues guide retinal axons to approximate regions along the rostrocaudal axis of the target. Activity-dependent mechanisms exist that refine this crude map (Hiramoto and Cline, 2014). Hiramoto and Cline showed that retinal mapping in Xenopus laevis might be influenced by the animal's motion direction. The axonal guidance system that determines the OC architecture is an expanding area of research, however many vital mechanisms remains to be discovered (Kim et al., 2014). How axon guidance genes influence, alternatively might be influenced by evolutionary change of species' motor behavior and ecology have been little explored.
The EF hypothesis includes that the selective value of fast and accurate visual steering of limbs, particularly the forelimbs, may result in evolutionary change that turn axonal guidance genes on or off. Thus, the idea presented here is that the OC is an evolutionarily active structure, key to preserving short neural pathways and hemispheric autonomy when eyes or limbs undergo crucial modification to adapt to new ecological niches.
Motor Behavior and Association with Optic Chiasm Structure in Vertebrates
Non-mammals
Fish
Fish represent a diverse group of organisms that includes all gill-bearing aquatic craniate animals that lack limbs with digits: the extant hagfish, lampreys, and cartilaginous and bony fish along with many extinct related groups (Nelson, 2006).
Fish with pectoral fins use them almost exclusively in a lateral direction. Movements and sensory information about the pectoral fin are processed in the contralateral hemisphere. The EF hypothesis predicts that visual information about a pectoral fin will be directed to the contralateral hemisphere, and contralateral retinal projections should predominate. The anatomy and distribution of the RGC in fishes has been reviewed by Ward et al. (1995). Most fish species lack, or have few, IRP (Figure 2). A noteworthy exception is the Cyclostomes (Ward et al., 1995), eel-like animals without paired appendages (Nelson, 2006) (Figure 3A). Paired appendages are likely to have developed in the gnathostome lineage after it separated from cyclostomes (Shimeld and Donoghue, 2012). All living gnathostomes, except snakes, and eels and other lineages with secondary loss, possess paired pelvic, and pectoral appendages that form fins in cartilaginous and bony fish and limbs in tetrapods.
Since Cyclostomes, in general, exhibit IRP, Ward et al. (1995) suggested that the ipsilateral contingent of fibers may be a phylogenetically primitive characteristic. This may be reflected in the Russian sturgeon Acipenser güldenstädti, which has extensive IRP (Reperant et al., 1982). The sturgeon is phylogenetically primitive compared with Teleostans, which largely lack IRP (Ward et al., 1995). However, the idea seems contradictory since phylogenetically advanced animals such as terrestrial predatory mammals and primates possess abundant IRP. Instead, Larsson (2011) proposed that IRP may aid in coordination of bilateral movements in limbless species. For example, when a cyclostome or snake is curled, a given side of the body may be visible by both eyes, while one side may be invisible (Figure 4). In this situation one eye will transmit visual information about the frontal part of body, while the other transmits information about the distal body part. Without IRP, one hemisphere may receive visual feedback about the caudal part of the body, while the contralateral hemisphere will receive information about the rostral parts of the body. In this situation IRP may provide direct visual feedback from the caudal and rostral body to both hemispheres, which may have had adaptive value and possibly resulting in preserved/regained IRP in limbless species.
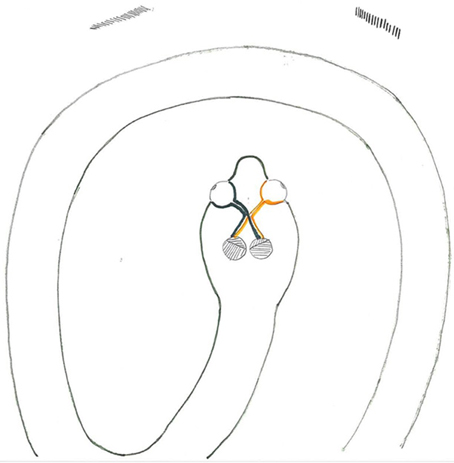
Figure 4. When an eel or snake is curled, a given side of the body may be viewed by both eyes, while the other side is invisible. Here one eye transmits visual information about the rostral part of the body, while the other eye transmits information about the caudal parts. Without IRP, the left hemisphere would only receive visual feedback about the caudal part of the body, while the right hemisphere would only receive information about the more rostral body parts. In this situation IRP will provide visual feedback from caudal and rostral body-parts to both hemispheres.
Larsson (2011) proposed that the evolution of paired appendages, particularly limbs that can be moved autonomously under visual control, increased the adaptive value of contralateral retinal projections (Figure 3B). In accordance with this concept, the pectoral fins of sturgeon are relatively immobile, and there is normally no visible alteration in pectoral fin position during tail-beats. In contrast, many teleosts, which largely lack IRP, use oscillatory movements of pectoral fins to produce propulsive forces (Wilga and Lauder, 1999).
Amphibians
Amphibians comprise the Anura (frogs and toads), the Caudata (salamanders), and the Gymnophiona (caecilians), which is a group of burrowing, limbless species (Noble, 1931). The pattern in amphibians is variable. An interesting example is Xenopus laevis, which has only crossed projections until metamorphosis, at which time it develops forelimbs with claws, binocularity, and IRP (Gaze, 1970; Fritzsch, 1990; Jeffery and Erskine, 2005). In hunting, the forelimbs of Xenopus are held in a flexed position in front of the head and are extended to capture prey and move it to the mouth (Carreño and Nishikawa, 2010). Like in mice (see below) IRP in Xenopus are confined to the temporal retina (Jeffery and Erskine, 2005), which is notable since this area will receive most or all information about the contralateral forelimb.
The limbless caecilian Ichthyophis kohtaoensis, which has weak sight and eyes that are positioned very laterally (Himstedt and Manteuffel, 1985) displays well-developed IRP (Himstedt and Manteuffel, 1985) (Figure 3A). Their IRP can by no means be correlated with binocularity since their small eyes are positioned very laterally far behind the snout (Himstedt and Manteuffel, 1985).
Plethodontid salamanders possess extensive IRP (Wiggers, 1999; Dicke and Roth, 2009). Since they have laterally situated forelimbs that seems to contradict the EF hypothesis. However, for example tropical climbing salamanders, Bolitoglossinae, which represent 50% of existing salamanders, have developed a feeding apparatus involving tongue protraction (Roth and Schmidt, 1993). Their projectile tongue is combined with a more frontal eye position relative to other salamanders (Roth and Schmidt, 1993). The substantial number of IRPs in Bolitoglossinae is most likely the basis for rapid computation of object distance (Wiggers et al., 1995). The combination of IRP and bifocal vision may serve to coordinate actions of body appendages such as a protractile tongue (Martin, 2009; Larsson, 2011).
Reptiles
Extant turtles comprise 240 species (Bellairs, 1970) distributed among 12 families (Gaffney, 1988). Among turtles, the leatherback Trionyx cartilaginous, the only species lacking IRP, has eyes notably more frontally placed and a wider binocular field than does Chinemys, in which IRP are relatively more extensive (Hergueta et al., 1992). Thus, in contrast to mammals, turtles lack a positive association between the proportion of IRP and the degree of frontal vision, the so-called Newton-Muller-Gudden Law (Magnin et al., 1989). The reason is unclear (Hergueta et al., 1992). Trionyx cartilaginous has more frontally placed eyes, and its neck is elongated compared to other turtles (Zug, 2015). Due to this anatomy, it is likely that T. cartilaginous has inferior capacity to visually supervise the contralateral paw compared with other turtle species. A testable prediction of the EF hypothesis is that turtles with IRP have some ability to supervise the contralateral forelimb, which would support that visual control of the forelimbs, not binocular vision per se, stimulates the evolution of IRP.
The RGC projections show contralateral dominance in most lizards and crocodilians and are completely contralateral in chameleons and uromastix lizards (Burns and Goodman, 1967; Repérant, 1975; Bennis et al., 1994; Tarpley et al., 1994; Ward et al., 1995; Derobert et al., 1999; Bruce, 2009). These species exhibit little frontal use of the forelimbs.
Why chameleon species with protractile tongue, in contrast to Bolitoglossinae, have no IRP is an ambiguity shared by the stereopsis and EF hypothesis. Although chameleon species fixate the prey with both eyes before using the protractile tongue, they primarily use accommodation cues, and not stereopsis to estimate the distance (Ott et al., 1998).
Snakes, which lost appendages during evolution, have more abundant IRP (Figure 3A) than lizards, their closest relatives with limbs (Repérant et al., 1992), possibly another indication that IRP aid limbless animals in coordination of bilateral movements.
Birds
In bird embryos, IRPs develop but are greatly reduced just before hatching (Thanos and Bonhoeffer, 1984), and mature birds have no, or extremely few, IRP (Figure 2) (Remy and Gunturkun, 1991; Ward et al., 1995). Since birds are descendants of tetrapod reptiles, it was proposed by Larsson (2011) that bird ontogeny might reflect their phylogenic development: bird predecessors possibly possessed more extensive IRP. Archaeopteryx had forelimbs 120–140% of hindlimb length (Gauthier, 1986), which may have been compatible with forelimbs operating ahead of the animal.
Martin (2009) reported that the maximum binocular field across most avian taxa ranges from 15° to 30°. The assumption that binocularity is required to produce stereopsis has been questioned (Davies and Green, 1994; McFadden, 1994). Although the frontal visual field overlap in woodcock is only around 5%, and thus unlikely to permit stereopsis, they are capable of rapid flight in complex environments suggesting that large binocular fields are not obligatory to take advantage of optic flow information during locomotion (Martin, 2009). Search for disparity sensitive cells in the Wulst of pigeon has failed (Martin, 2009).
Owls have a higher degree of IRP than do most other birds, and their maximum binocular field widths are typically around 50–60° (Pettigrew, 1986a). Owls exhibit double decussation. Despite complete crossing of the optic nerve, information from each part of the retina converge in the brain due to a second crossing, functionally similar to having a large proportion of IRP (Pettigrew, 1986a). Larsson (2011) proposed that the decussation and binocular vision in owls may improve coordination of the lower limbs. Numerous species of owls have been observed foraging on foot (Johnsgard, 1988; McMillian, 1998). Martin (2009) suggested that the primary function of binocular vision in birds is the control of bill and foot position, and that binocular vision aids in timing the opening of the bill or feet, for example when pecking, attacking, gripping prey, or feeding young. New Caledonian crows Corvus moneduloides exhibit the largest known degree of binocular overlap among birds (60°), reinforcing the idea that manipulative behaviors are associated with binocularity (Troscianko et al., 2012).
It has been suggested that nocturnal behavior leads to enrichment of visual structures such as cones, which are associated with binocular vision (Heesy, 2008; Vega-Zuniga et al., 2013). However, several nocturnal predatory birds lack binocular enhancement (Vega-Zuniga et al., 2013). Vega-Zuniga et al. (2013) proposed that birds that lack binocular enhancement such as the nighthawk, a nocturnal predatory bird of the Chordeilinae, represent a challenge to the nocturnal hypothesis as well as to the hypothesis that links binocularity with predatory behavior.
Cockatoos have been widely studied with respect to the relationship between retinal organization and behavioral ecology, since these species display a remarkable variation in niche partition and habitat (Coimbra et al., 2014). Black cockatoos Calyptorhynchus spp. and corellas Cacatua spp. exhibit strongly lateralized visual behaviors (Coimbra et al., 2014), and when foraging primarily use the left eye for fixation and the left foot to collect seed pods, fruit, and tubers (Magat and Brown, 2009). Cockatoos that forage on the ground possess a retinal specialization resembling the dorsotemporal extension (anakatabatic area) found in artiodactyls, and, as in artiodactyls, this structure correlates with the distance between the head and the ground (Coimbra et al., 2014). Retinal ganglion cell densities are significantly higher in the left perifoveal regions in strongly left-footed cockatoo species such as black cockatoos and corellas (Coimbra et al., 2014), further supporting the idea that visual steering of the feet influenced the evolution of retinal specialization. Cockatiels, which do not use the feet in foraging (Magat and Brown, 2009) may have reduced need for visual guidance of the feet, possibly explaining differences in their retinal specializations (Coimbra et al., 2014). The New Zealand parrot kakapo Strigops habroptilus is nocturnal and flightless with short legs and large feet (Powlesland et al., 2006). The Kakapo's eyes are significantly more convergent than seen in other parrots, suggesting an increased binocular overlap possibly related to its nocturnal activity (Corfield et al., 2011). An alternative rationale may be control of bill and foot position (Martin, 2009). The kakapo is a climber, reaching the canopy of 20–30 m tall trees via vines, lianes, and understory shrubs, and often moves from tree to tree through the canopy (Powlesland et al., 2006). When foraging, the kakapo typically grasps a leaf with one foot and strips the nourishing parts of the plant with the beak, until only a ball of indigestible fibers remains (Atkinson and Merton, 2006). The kakapo may be another example of association between binocular vision and precise visual control of body appendages.
Mammals
In mammals, the number of IRP to the thalamic nucleus dLGN is positively correlated with the degree of binocular overlap (Pettigrew, 1986a; Barton, 2004). Mammals with laterally positioned eyes exhibit a low level of IRP (Herrera and Mason, 2007) (Table 1B). In rodents, the uncrossed component is about 2–3% (Petros et al., 2008). In mice the IRP are located in a crescent-shaped area bordering the inferior temporal retina (Dräger and Olsen, 1980). As a result of the snout anatomy in rodents (Hughes, 1977), the contralateral paw might be viewed only in the superior quadrant of the visual field, corresponding to the inferior temporal retina. This supports the EF hypothesis, since IRP from the inferior temporal retina of mice is the only potential way to transmit direct visual feedback to the limb-steering hemisphere. Also primates, cats, dogs, and other investigated mammals display IRP exclusively in the temporal retina (Jeffery and Erskine, 2005), which is the retinal part that is likely to receive most information about the contralateral hand or paw.
Vega-Zuniga et al. (2013) compared visual traits of Octodon degus to those of Octodon lunatus, rodents of the South American Octodontidae. The nocturnal O. lunatus visual field shows a 100° frontal binocular overlap, while that of the diurnal O. degus has 50° overlap. The IRP to the dLGN and SC in O. lunatus were found to be almost 10 percent (personal message from Dr Jorge Mpodozis) or five times the number in O. degus. The authors suggested that nocturnal habits may be associated with an enhancement of binocular vision in mammals. Both Octodon species show the ability to climb bushes and small trees; however, since O. lunatus is more commonly found in thickets and O. degus primarily occupies grasslands and flat habitat, climbing is likely to be more frequent in the former. Thus, improved climbing abilities in O. lunatus due to accurate eye-forelimb coordination might be an alternative to the nocturnality hypothesis. A comparative study of the visual systems and the association with climbing abilities in Octodon species would be of interest.
The position and magnitude of retinal specializations correlate with foraging behavior in many species (Hughes, 1977). In browsing and grazing vertebrates living on the ground, the temporal portion of the horizontal streak, a dense distribution of cones, usually forms a dorsotemporal extension (anakatabatic area) that augments spatial resolution in the frontal and inferior visual fields. In artiodactyls this is associated with the distance between the head and the ground (Hughes, 1977; Coimbra et al., 2014) and is likely to enhance visual feedback of the moving hoofs. The peripheral distribution of the herbivore artiodactyl visual streaks suggests significant effects of eye height and the structure of the environment (Schiviz et al., 2008).
Microchiroptera have no (Pettigrew, 1986b) or very few IRP (Scalia et al., 2015). Fruit bats, Megachiroptera, which commonly use claws on the wing to climb trees and manipulate fruit (Zhang et al., 2010), possess a primate-like visual system with a large proportion of IRP and an extensively developed primary visual area (Pettigrew, 1986b; Rosa et al., 1993).
Marsupials
The pattern of retinal input to the dorsal lateral geniculate nucleus (dLGN) in marsupials is variable (Royce et al., 1976; Harman et al., 1990) (Table 1). Plentiful overlap of visual projections in the dLGN is largely analogous to high proportions of IRP. Two diprodonts, the feather tailed glider Acrobates pygmaeus and the honey possum Tarsipes rostratus (Harman et al., 1990) exhibit extensive overlap in the dLGN. Both are fast and agile tree climbers (Branson et al., 1993). Also Marmosa mitis, a South American opossum exhibit extensive overlap in the dLGN (Royce et al., 1976). This animal is arboreal and dexterous; usually lunges forward to pin its prey to the ground with its forefeet before biting it (Macrini, 2004). The polypropodont family Dasuridae, which display extensive overlap in the dLGN, mainly includes carnivorous species, of which many are capable of climbing. Notably, ground dwelling herbivorous wombats known to dig tunnel systems (Evans, 2008) have the least overlap in the dLGN (Triggs, 1996).
Degree of overlap in other marsupials seems to fall between these extremes (Sanderson et al., 1987). For example, the tammar wallaby Macropus eugenii, a small kangaroo belonging to an advanced order of diprotodonts that includes wallabies, kangaroos, wombats, opossums, and the koala (Wimborne et al., 1999), has a binocular field of 50°, and the proportion of IRP is 10% (Wimborne et al., 1999). Although predominately grazers, visual control of forelimbs is required when tammars are browsing (Lentle, 1998).
Primates
Superior visual control of the hand is characteristic of primates (Hughes, 1977). The primate visual system is highly suited to supervision of tasks within arm's length (Hadjidimitrakis et al., 2011; Larsson, 2013). Gaze-centered coordinates are frequently used and are vital to visual directing of the hand (Pouget et al., 2002; Buneo and Andersen, 2006). Ocular dominance usually switches from one eye to another during reaching maneuvers; e.g., the left eye is likely to become dominant when the left hand reaches an object in the left visual field (Khan and Crawford, 2001). Spatial discrimination (Richter et al., 2007) and manual reaction times are superior in the lower visual field (Danckert and Goodale, 2001) where reaching movements in primates typically begin. Vergent eye movements occur most frequently in the space corresponding to the arm's length (Viguier et al., 2001). The representation of 3D shape has been studied in two interconnected areas in the primate brain, recognized to be critical for object grasping: area AIP and area F5a in the ventral premotor cortex; in both areas, 3D-shape selective neurons were co-localized with neurons displaying motor-related activity during object grasping in darkness, indicating a merging of visual and motor information on the same clusters of neurons (Theys et al., 2015).
Predatory Mammals
Many predatory mammals have frontally located eyes and a significant degree of IRP (Jeffery and Erskine, 2005). Primates (predators as well as frugivorous species) display around 45% IRP (Jeffery and Erskine, 2005). Domestic cats display 30% (Herrera and Mason, 2007), dogs 20–22% (Hogan et al., 1999; Lee et al., 1999), and the ferret, a predator, adapted for epigean as well as tunnel locomotion (Horner and Biknevicius, 2010), has approximately 8% IRP (Morgan et al., 1987). However, the predatory bottlenose dolphin Tursiops truncatus displays only crossed projections (Tarpley et al., 1994), and the giant panda Ailuropoda melanoleuca has frontal eyes in spite of a herbivorous diet (Seidensticker and Lumpkin, 2007). The giant panda is a frequent climber. Data about IRP in the giant panda is lacking. Many species of felidae are also tree-climbers and use visually guided paw movements in prey capture (Hughes, 1977). Notably, the sectioning of all crossing fibers at the OC midline was not found to substantially affect disparity detection (stereopsis) in felidae (Lepore et al., 1992).
Discussion
The placement of the eyes, retinal specializations, and the OC architecture seem to be associated with motor behavior in a variety of vertebrates. The necessity of supervising and efficiently processing movements may explain the low proportion of IRP in most fishes, reptiles, and birds. The EF hypothesis accommodates why IRP emanate exclusively from the temporal retina in animals with limbs. Abundant IRPs in primates and most predatory animals is also in accordance with the hypothesis.
There is lack of data on to what extent, and how skillfully, canidae use visually guided paw movements in prey capture, and the relatively high proportion of IRP (20–22%) (Hogan et al., 1999; Lee et al., 1999) in dogs may be at odds with the EF hypothesis. However, wolves are recognized to use their feet and claws for catching/manipulating prey and digging (Moskowitz, 2013).
Visual control of the feet and bill seems to have influenced the evolution of retinal specialization in cockatoos (Coimbra et al., 2014) and possibly in the kakapo and the New Caledonian crow, reinforcing the concept of the primary role of binocular vision in birds as visual control of body appendages (Martin, 2014). The EF hypothesis would predict binocular vision and IRP in species with forelimbs that habitually operate in front of the animal. A predatory lifestyle in terrestrial mammals such as cats or bears typically involves the forelimbs, which are usually frontally positioned and used to hold and manipulate prey, while aquatic predators such as dolphins primarily use the mouth. Thus, the EF hypothesis may explain both why terrestrial mammalian predators possess high proportions of IRP and predatory aquatic animals such as Trionyx and bottlenose dolphins have no IRP. Trionyx and cetaceans are likely to only take advantage of visual feedback to the limb-steering hemisphere, and the EF hypothesis suggests that evolutionary change in position and function of the forelimb resulted in a gradual and complete loss of IRP. That implies that the multiple axon guidance genes, which regulate IRP proportions (Herrera and Mason, 2007) are largely influenced by natural selection. The relatively high proportion of IRP in vertebrates with secondary loss of the limbs demonstrates that such evolutionary change may go in either direction, depending on motor behavior/anatomy in the ecological niche of species.
The primate visual system is highly suited to supervise tasks within the hand's working space (Hadjidimitrakis et al., 2011; Larsson, 2013); indications on that are the switch of ocular dominance during reaching maneuvers (Khan and Crawford, 2001), superior spatial discrimination (Richter et al., 2007) and shorter reaction time in the inferior visual field (Danckert and Goodale, 2001) where the hand commonly operates. Moreover, the fundamentals of stereopsis, vergent eye movements, largely take place within arm's length (Viguier et al., 2001). 3D-shape selective neurons that are co-localized with neurons engaged in grasping (Theys et al., 2015) also supports the EF hypothesis. The EF hypothesis does not contradict the premise that abundant IRP in the OC combined with frontal vision were important in the evolution of stereopsis. However, this review suggests that stereopsis was a byproduct of the evolution of accurate eye limb coordination. The predator hypothesis (Ward et al., 1995), as well as the nocturnal restriction hypothesis (Vega-Zuniga et al., 2013), applies mainly to mammals, whereas the EF hypothesis appears applicable to vertebrates in general. The EF hypothesis might be evaluated through comparative analyses of mammalian and non-mammalian associations among IRP, eye convergence, and visual guidance of the limbs. Such studies are needed to understand the evolution of OC anatomy, retinal specialization, and their association with motor behavior and ecology in vertebrates.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank the three reviewers for many valuable suggestions. Thanks to Carol Mason, Columbia University, USA and Elosie Herrera, Instituto de Neurosciencias, Alicante, Spain, for their support with illustrations. Thanks to the Heart and Lung Clinic, Örebro University Hospital for supporting copy-editing of the manuscript; the Lucidus Consultancy, for editorial advice and copy-editing; and Dr. Jorge Mpodozis for information about octodonts.
References
Allman, J. (1977). “Evolution of the visual system in early primates,” in Progress in psychobiology and Physiological Psychology, eds J. M. Sprague and A. N. Epstein (New York, NY: Academic Press), 1–53.
Atkinson, I. A. E., and Merton, D. V. (2006). Habitat and diet of kakapo (Strigops habroptilus) in the Esperance Valley, Fiordland, New Zealand. Notornis 53, 37–54. Available online at: http://notornis.osnz.org.nz/system/files/Notornis_53_1_37.pdf
Barlow, H. B., Blakemore, C., and Pettigrew, J. D. (1967). Neural mechanism of binocular depth discrimination. J. Physiol. 193, 327–348. doi: 10.1113/jphysiol.1967.sp008360
Barton, R. A. (2004). From the cover: binocularity and brain evolution in primates. Proc. Natl. Acad. Sci. U.S.A. 101, 10113–10115. doi: 10.1073/pnas.0401955101
Bennis, M., Repérant, J., Rio, J. P., and Ward, R. (1994). An experimental re-evaluation of the primary visual system of the European chameleon, Chamaeleo chameleon. Brain Behav. Evol. 43, 173–188. doi: 10.1159/000113633
Berlucchi, G., Heron, W., Hyman, R., Rizzolat, G., and Umiltà, C. (1971). Simple reactions times of ipsilateral and contralateral hand to lateralized visual stimuli. Brain 94, 419–430. doi: 10.1093/brain/94.3.419
Branson, A., Bramwell, M., Kerrod, R., O'toole, C., Parker, S., and Stidworthy, J. (1993). The Illustrated Encyclopedia of Mammals. Oxford: Andromeda Oxford.
Bruce, L. L. (2009). “Evolution of nervous system in reptiles,” in Evolutionary Neuroscience, ed J. H. Kaas (Oxford: Elsevier Science), 125–156.
Buneo, C. A., and Andersen, R. A. (2006). The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44, 2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011
Burns, A. H., and Goodman, D. C. (1967). Retinofugal projections of Caiman sklerops. Exp. Neurol. 18, 105–115. doi: 10.1016/0014-4886(67)90092-1
Carreño, C. A., and Nishikawa, K. C. (2010). Aquatic feeding in pipid frogs: the use of suction for prey capture. J. Exp. Biol. 213, 2001–2008. doi: 10.1242/jeb.043380
Cartmill, M. (1992). New views on primate origins. Evol. Anthropol. 1, 105–111. doi: 10.1002/evan.1360010308
Coimbra, J. P., Collin, S. P., and Hart, N. S. (2014). Topographic specializations in the retinal ganglion cell layer correlate with lateralized visual behavior, ecology, and evolution in cockatoos. J. Comp. Neurol. 522, 3363–3385. doi: 10.1002/cne.23637
Collins, E. T. (1921). Changes in the visual organs correlated with the adoption of arboreal life and with the assumption of the erect posture. Trans. Ophthalm. Soc. 41, 10–90.
Corfield, J. R., Gsell, A. C., Brunton, D., Heesy, C. P., Hall, M. I., Acosta, M. L., et al. (2011). Anatomical specializations for nocturnality in a critically endangered parrot, the kakapo (Strigops habroptilus). PLoS ONE 6:e22945. doi: 10.1371/journal.pone.0022945
Crompton, R. H. (1995). “‘Visual predation,’ habitat structure, and the ancestral primate niche,” in Creatures of the Dark: The Nocturnal Prosimians, eds L. Alterman, G. A. Doyle, and M. K. Izard (New York, NY: Plenum Press), 11–30.
Cumming, B. G., and DeAngelis, G. C. (2001). The physiology of stereopsis. Annu. Rev. Neurosci. 24, 203–238. doi: 10.1146/annurev.neuro.24.1.203
Danckert, J., and Goodale, M. A. (2001). Superior performance for visually guided pointing in the lower visual field. Exp. Brain Res. 137, 303–308. doi: 10.1007/s002210000653
Davies, M. N. O., and Green, P. R. (1994). “Multiple sources of depth information: an ecological approach,” in Perception and Motor Control in Birds: An Ecological Approach, eds M. N. O. Davies and P. R. Green (Berlin: Springer), 339–356.
Derobert, Y., Médina, M., Rio, J. P., Ward, R., Repérant, J., Marchand, M. J., et al. (1999). Retinal projections in two crocodilian species, Caiman crocodilus and Crocodylus niloticus. Anat. Embryol. (Berl.) 200, 175–191. doi: 10.1007/s004290050271
Dicke, U., and Roth, G. (2009). “The evolution of the amphibian nervous system,” in Evolutionary Neuroscience. Vol. 2, eds J. H. Kaas and T. H. Bullock (Oxford: Elsevier Science), 61–124.
Dräger, U. C., and Olsen, J. F. (1980). Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J. Comp. Neurol. 191, 383–412. doi: 10.1002/cne.901910306
Erskine, L., and Herrera, E. (2007). The retinal ganglion cell axon's journey: insights into molecular mechanisms of axon guidance. Dev. Biol. 308, 1–14. doi: 10.1016/j.ydbio.2007.05.013
Erskine, L., Reijntjes, S., Pratt, T., Denti, L., Schwarz, Q., Vieira, J. M., et al. (2011). VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron 70, 951–965. doi: 10.1016/j.neuron.2011.02.052
Evans, M. C. (2008). Home range, burrow-use and activity patterns in common wombats (Vombatus ursinus). Wildlife Res. 35, 455–462. doi: 10.1071/WR07067
Fritzsch, B. (1990). The evolution of metamorphosis in amphibians. J. Neurobiol. 21, 1011–1021. doi: 10.1002/neu.480210707
Gaffney, E. S. A. P. A. M. (1988). “A phylogeny of turtles,” in The Phylogeny and Classification of the Tetrapods, Vol. 1. Amphibians, Reptiles, Birds. Systematics Association Special, Vol. 35A. ed M. J. Benton (Oxford: Clarendon Press), 157–219.
Gauthier, J. (1986). “Saurischian monophyly and the origin of birds,” in The Origin of Birds and the Evolution of Flight, ed K. Padian (San Francisco, CA: California Academy of Sciences), 1–55.
Hadjidimitrakis, K., Breveglieri, R., Placenti, G., Bosco, A., Sabatini, S. P., and Fattori, P. (2011). Fix your eyes in the space you could reach: neurons in the macaque medial parietal cortex prefer gaze positions in peripersonal space. PLoS ONE 6:e23335. doi: 10.1371/journal.pone.0023335
Harman, A. M., Coleman, L. A., and Beazley, L. D. (1990). Retinofugal projections in a marsupial, Tarsipes rostratus (honey possum). Brain Behav. Evol. 36, 30–38. doi: 10.1159/000115295
Hawkins, J., George, D., and Niemasik, J. (2009). Sequence memory for prediction, inference and behaviour. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364, 1203–1209. doi: 10.1098/rstb.2008.0322
Heesy, C. P. (2008). Ecomorphology of orbit orientation and the adaptive significance of binocular vision in primates and other mammals. Brain Behav. Evol. 71, 54–67. doi: 10.1159/000108621
Heesy, C. P. (2009). Seeing in stereo: the ecology and evolution of primate binocular vision and stereopsis. Evol. Anthropol. 18, 21–35. doi: 10.1002/evan.20195
Hergueta, S., Ward, R., Lemire, M., Rio, J. P., Reperant, J., and Weidner, C. (1992). Overlapping visual fields and ipsilateral retinal projections in turtles. Brain Res. Bull. 29, 427–433. doi: 10.1016/0361-9230(92)90079-D
Herrera, E., and Mason, C. A. (2007). “The evolution of crossed and uncrossed retinal pathways in mammals,” in Evolution of Nervous Systems: Mammals, eds L. Krubitzer and J. Kaas (Oxford: Elsevier), 307–317.
Himstedt, W., and Manteuffel, G. (1985). Retinal projections in the caecilian Ichthyophis kohtaoensis (Amphibia, Gymnophiona). Cell Tissue Res. 239, 689–692. doi: 10.1007/BF00219250
Hiramoto, M., and Cline, H. T. (2014). Optic flow instructs retinotopic map formation through a spatial to temporal to spatial transformation of visual information. Proc. Natl. Acad. Sci. U.S.A. 111, E5105–E5113. doi: 10.1073/pnas.1416953111
Hogan, D., Garraghty, P. E., and Williams, R. W. (1999). Asymmetric connections, duplicate layers, and a vertically inverted map in the primary visual system. J. Neurosci. 19:Rc38.
Horner, A. M., and Biknevicius, A. R. (2010). A comparison of epigean and subterranean locomotion in the domestic ferret (Mustela putorius furo: Mustelidae: Carnivora). Zoology 113, 189–197. doi: 10.1016/j.zool.2009.11.001
Hughes, A. (1977). “The topography of vision in mammals of contrasting life styles,” in Handbook of Sensory Physiology: The Visual System in Vertebrates, ed F. Crescitelli (Berlin, Heidelberg: Springer), 613–756.
Jameson, N. M., Hou, Z. C., Sterner, K. N., Weckle, A., Goodman, M., Steiper, M. E., et al. (2011). Genomic data reject the hypothesis of a prosimian primate clade. J. Hum. Evol. 61, 295–305. doi: 10.1016/j.jhevol.2011.04.004
Jeffery, G., and Erskine, L. (2005). Variations in the architecture and development of the vertebrate optic chiasm. Prog. Retin. Eye Res. 24, 721–753. doi: 10.1016/j.preteyeres.2005.04.005
Johnsgard, P. A. (1988). North American Owls: Biology and Natural History. Washington, DC: Smithsonian Institution Press.
Khan, A. Z., and Crawford, J. D. (2001). Ocular dominance reverses as a function of horizontal gaze angle. Vision Res. 41, 1743–1748. doi: 10.1016/S0042-6989(01)00079-7
Kim, N., Min, K. W., Kang, K. H., Lee, E. J., Kim, H. T., Moon, K., et al. (2014). Regulation of retinal axon growth by secreted Vax1 homeodomain protein. Elife 3:e02671. doi: 10.7554/eLife.02671
Kirk, E. C., Lemelin, P., Hamrick, M. W., Boyer, D. M., and Bloch, J. I. (2008). Intrinsic hand proportions of euarchontans and other mammals: implications for the locomotor behavior of plesiadapiforms. J. Hum. Evol. 55, 278–299. doi: 10.1016/j.jhevol.2008.02.008
Kuwajima, T., Yoshida, Y., Takegahara, N., Petros, T. J., Kumanogoh, A., Jessell, T. M., et al. (2012). Optic chiasm presentation of Semaphorin6D in the context of Plexin-A1 and Nr-CAM promotes retinal axon midline crossing. Neuron 74, 676–690. doi: 10.1016/j.neuron.2012.03.025
Larsson, M. (2011). Binocular vision and ipsilateral retinal projections in relation to eye and forelimb coordination. Brain Behav. Evol. 77, 219–230. doi: 10.1159/000329257
Larsson, M. (2013). The optic chiasm: a turning point in the evolution of eye/hand coordination. Front. Zool. 10:41. doi: 10.1186/1742-9994-10-41
Lee, I., Kim, J., and Lee, C. (1999). Anatomical characteristics and three-dimensional model of the dog dorsal lateral geniculate body. Anat. Rec. 256, 29–39.
Lentle, R. (1998). Feeding Strategies of the Tammar Wallaby (Macropus Eugenii Desmarest). A thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy, Massey University.
Lepore, F., Samson, A., Paradis, M. C., Ptito, M., and Guillemot, J. P. (1992). Binocular interaction and disparity coding at the 17-18 border: contribution of the corpus callosum. Exp. Brain Res. 90, 129–140. doi: 10.1007/BF00229264
Magat, M., and Brown, C. (2009). Laterality enhances cognition in Australian parrots. Proc. R. Soc. Lond. B. Biol. Sci. 276, 4155–4162. doi: 10.1098/rspb.2009.1397
Magnin, M., Cooper, H. M., and Mick, G. (1989). Retinohypothalamic pathway: a breach in the law of Newton-Muller-Gudden? Brain Res. 488, 390–397. doi: 10.1016/0006-8993(89)90737-3
Martin, G. R. (2009). What is binocular vision for? A birds' eye view. J. Vis. 9, 1–19. doi: 10.1167/9.11.14
Martin, G. R. (2014). The subtlety of simple eyes: the tuning of visual fields to perceptual challenges in birds. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369:20130040. doi: 10.1098/rstb.2013.0040
Martin, R. D. (1979). “Phylogenetic aspects of prosimian behavior,” in The Study of Prosimian Behavior, eds G. A. Doyle and R. D. Martin (New York, NY: Academic Press), 45–77.
McFadden, S. A. (1994). “Binocular depth perception,” in Perception and Motor Control in Birds: An Ecological Approach, eds M. N. O. Davies and P. R. Green (Berlin: Springer), 54–73.
McMillian, M. A. (1998). “Foot-hunting” behavior by a great horned owl. Florida Field Nat. 26, 91–93.
Melin, A. D., Matsushita, Y., Moritz, G. L., Dominy, N. J., and Kawamura, S. (2013). Inferred L/M cone opsin polymorphism of ancestral tarsiers sheds dim light on the origin of anthropoid primates. Proc. Biol. Sci. 280:20130189. doi: 10.1098/rspb.2013.0189
Morgan, J. E., Henderson, Z., and Thompson, I. D. (1987). Retinal decussation patterns in pigmented and albino ferrets. Neuroscience 20, 519–535. doi: 10.1016/0306-4522(87)90108-4
Nakagawa, S., Brennan, C., Johnson, K. G., Shewan, D., Harris, W. A., and Holt, C. E. (2000). Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron 25, 599–610. doi: 10.1016/S0896-6273(00)81063-6
Ott, M., Schaeffel, F., and Kirmse, W. (1998). Binocular vision and accommodation in prey-catching chameleons. J. Comp. Physiol. A 182, 319–330. doi: 10.1007/s003590050182
Petros, T. J., Rebsam, A., and Mason, C. A. (2008). Retinal axon growth at the optic chiasm: to cross or not to cross. Annu. Rev. Neurosci. 31, 295–315. doi: 10.1146/annurev.neuro.31.060407.125609
Pettigrew, J. D. (1978). “Comparison of the retinotopic organization of the visual wulst in nocturnal and diurnal raptors, with a note on the evolution of frontal vision,” in Frontiers of Visual Science, eds S. J. Cool and E. L. Smith (New York, NY: Springer Verlag), 328–335.
Pettigrew, J. D. (1986a). “The evolution of binocular vision,” in Vision Neuroscience, eds K. J. Sanderson, J. D. Pettigrew, and W. R. Levick (Cambridge: Cambridge University Press), 208–222.
Pettigrew, J. D. (1986b). Flying primates? Megabats have the advanced pathway from eye to midbrain. Science 231, 1304–1306. doi: 10.1126/science.3945827
Poffenberger, A. T. (1912). Reaction time to retinal stimulation with specific reference to the time lost in conduction through nerve centres. Arch. Psychol. 23, 1–73.
Poggio, G. F. (1984). “Processing of stereoscopic information in primate visual cortex,” in Dynamic Aspects of Neocortical Function, eds G. M. Edelman, W. E. Gall, and W. M. Cowan (New York, NY: John Wiley), 613–635.
Poggio, G. F., and Poggio, T. (1984). The analysis of stereopsis. Annu. Rev. Neurosci. 7, 379–412. doi: 10.1146/annurev.ne.07.030184.002115
Pouget, A., Ducom, J. C., Torri, J., and Bavelier, D. (2002). Multisensory spatial representations in eye-centered coordinates for reaching. Cognition 83, B1–B11. doi: 10.1016/S0010-0277(01)00163-9
Powlesland, R. G., Merton, D. V., and Cockrem, J. F. (2006). A parrot apart: the natural history of the kakapo (Strigops habroptilus), and the context of its conservation management. Notornis 53, 3–26. Available online at: http://notornis.osnz.org.nz/system/files/Notornis_53_1_37.pdf
Remy, M., and Gunturkun, O. (1991). Retinal afferents to the tectum opticum and the nucleus opticus principalis thalami in the pigeon. J. Comp. Neurol. 305, 57–70. doi: 10.1002/cne.903050107
Repérant, J. (1975). Nouvelles données sur les projections retiniennes chez Caiman sclerops: étude radioautographique. C. R. Acad. Sci. 280, 2881–2884.
Repérant, J., Rio, J. P., Ward, R., Hergueta, S., Miceli, D., and Lemire, M. (1992). “Comparative analysis of the primary visual system of reptiles,” in Biology of the Reptilia. Vol. 4, ed C. G. A. P. Ulinski (Chicago, IL: Chicago University of Chicago Press), 175–240.
Reperant, J., Vesselkin, N. P., Ermakova, T. V., Rustamov, E. K., Rio, J. P., Palatnikov, G. K., et al. (1982). The retinofugal pathways in a primitive actinopterygian, the chondrostean Acipenser güldenstädti. An experimental study using degeneration, radioautographic and HRP methods. Brain Res. 251, 1–23. doi: 10.1016/0006-8993(82)91269-0
Richter, H. O., Wennberg, P., and Raudsepp, J. (2007). The effects of inverting prisms on the horizontal-vertical illusion: a systematic effect of downward gaze. Exp. Brain Res. 183, 9–15. doi: 10.1007/s00221-007-1015-z
Ringo, J. L., Doty, R. W., Demeter, S., and Simard, P. Y. (1994). Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb. Cortex 4, 331–343. doi: 10.1093/cercor/4.4.331
Rosa, M. G., Schmid, L. M., Krubitzer, L. A., and Pettigrew, J. D. (1993). Retinotopic organization of the primary visual cortex of flying foxes (Pteropus poliocephalus and Pteropus scapulatus). J. Comp. Neurol. 335, 55–72. doi: 10.1002/cne.903350105
Roth, G., and Schmidt, A. (1993). The nervous system of Plethodontid salamanders: insight into the interplay between genome, organism, behavior and ecology. Herpetologica 49, 185–194.
Royce, G. J., Ward, J. P., and Harting, J. K. (1976). Retinofugal pathways in two marsupials. J. Comp. Neurol. 170, 391–413. doi: 10.1002/cne.901700309
Sanderson, K. J., Nelson, J. E., Crewther, D. P., Crewther, S. G., and Hammond, V. E. (1987). Retinogeniculate patterns in diprotodont marsupials. Brain Behav. Evol. 30, 22–42. doi: 10.1159/000118636
Scalia, F., Rasweiler, J. J., Danias, J. (2015). Retinal projections in the short-tailed fruit bat, carollia perspicillata, as studied using the axonal transport of cholera toxin b subunit: comparison with mouse. J. Comp. Neurol. 523, 1756–1791. doi: 10.1002/cne.23723
Schiviz, A. N., Ruf, T., Kuebber-Heiss, A., Schubert, C., and Ahnelt, P. K. (2008). Retinal cone topography of artiodactyl mammals: influence of body height and habitat. J. Comp. Neurol. 507, 1336–1350. doi: 10.1002/cne.21626
Shimeld, S. M., and Donoghue, P. C. (2012). Evolutionary crossroads in developmental biology: cyclostomes (lamprey and hagfish). Development 139, 2091–2099. doi: 10.1242/dev.074716
Silcox, M., Sargis, E., Block, J., and Boyer, D. (2007). “Primate origins and supraordinal relationships: morphological evidence,” in Primate Evolution and Human Origins, eds W. Henke and I. Tattersall (New York, NY: Springer-Verlag), 831–859.
Tan, Y., and Li, W. H. (1999). Trichromatic vision in prosimians. Nature 402, 36. doi: 10.1038/46947
Tarpley, R. J., Gelderd, J. B., Bauserman, S., and Ridgway, S. H. (1994). Dolphin peripheral visual pathway in chronic unilateral ocular atrophy: complete decussation apparent. J. Morphol. 222, 91–102. doi: 10.1002/jmor.1052220109
Thanos, S., and Bonhoeffer, F. (1984). Development of the transient ipsilateral retinotectal projection in the chick embryo:a numerical fluorescence-microscopic analysis. J. Comp. Neurol. 224, 407–414. doi: 10.1002/cne.902240308
Theys, T., Romero, M. C., van Loon, J., and Janssen, P. (2015). Shape representations in the primate dorsal visual stream. Front. Comput. Neurosci. 9:43. doi: 10.3389/fncom.2015.00043
Tigges, J. (1970). Retinal projections to subcortical optic nuclei in diurnal and nocturnal squirrels. Brain Behav. Evol. 3, 121–134. doi: 10.1159/000125466
Triggs, B. (1996). The Wombat: Common Wombats in Australia. Sydney, NSW: University of New South Wales Press.
Troscianko, J., Von Bayern, A. M., Chappell, J., Rutz, C., and Martin, G. R. (2012). Extreme binocular vision and a straight bill facilitate tool use in New Caledonian crows. Nat. Commun. 3, 1110. doi: 10.1038/ncomms2111
Vega-Zuniga, T., Medina, F. S., Fredes, F., Zuniga, C., Severin, D., Palacios, A. G., et al. (2013). Does nocturnality drive binocular vision? Octodontine rodents as a case study. PLoS ONE 8:e84199. doi: 10.1371/journal.pone.0084199
Viguier, A., Clément, G., and Trotter, Y. (2001). Distance perception within near visual space. Perception 30, 115–124. doi: 10.1068/p3119
Ward, R., Reperant, J., Hergueta, S., Miceli, D., and Lemire, M. (1995). Ipsilateral visual projections in non-eutherian species: random variation in the central nervous system? Brain Res. Rev. 20, 155–170. doi: 10.1016/0165-0173(94)00009-e
Wheatstone, C. (1838). Contributions to the physiology of vision. Part the first. On some remarkable, and Hitherto unobserved, phenomena of binocular vision. Phil. Trans. R. Soc. Lond. 128, 371–394. doi: 10.1098/rstl.1838.0019
Wiggers, W. (1999). Projections of single retinal ganglion cells to the visual centers: an intracellular staining study in a plethodontid salamander. Vis. Neurosci. 16, 435–447. doi: 10.1017/S0952523899163053
Wiggers, W., Roth, G., Eurich, C., and Straub, A. (1995). Binocular depth perception mechanisms in tongue-projecting salamanders. J. Comp. Physiol. A 176, 365–377. doi: 10.1007/BF00219062
Wilga, C. D., and Lauder, G. V. (1999). Locomotion in sturgeon: function of the pectoral fins. J. Exp. Biol. 202, 2413–2432.
Williams, S. E., Grumet, M., Colman, D. R., Henkemeyer, M., Mason, C. A., and Sakurai, T. (2006). A role for Nr-CAM in the patterning of binocular visual pathways. Neuron 50, 535–547. doi: 10.1016/j.neuron.2006.03.037
Williams, S. E., Mann, F., Erskine, L., Sakurai, T., Wei, S., Rossi, D. J., et al. (2003). Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39, 919–935. doi: 10.1016/j.neuron.2003.08.017
Wimborne, B. M., Mark, R. F., and Ibbotson, M. R. (1999). Distribution of retinogeniculate cells in the tammar wallaby in relation to decussation at the optic chiasm. J. Comp. Neurol. 405, 128–140.
Zhang, J. S., Jones, G., Zhang, L. B., Zhu, G. J., and Zhang, S. Y. (2010). Recent surveys of bats (Mammalia: Chiroptera) from China II. Pteropodidae. Acta Chiropterol. 12, 103–116. doi: 10.3161/150811010X504626
Keywords: ipsilateral retinal projections, predator, prey, vision, coordination, evolution, stereopsis
Citation: Larsson ML (2015) Binocular vision, the optic chiasm, and their associations with vertebrate motor behavior. Front. Ecol. Evol. 3:89. doi: 10.3389/fevo.2015.00089
Received: 14 March 2015; Accepted: 16 July 2015;
Published: 29 July 2015.
Edited by:
Angelika Stollewerk, Queen Mary University of London, UKReviewed by:
John Bickle, Mississippi State University, USAAntonio Benítez-Burraco, University of Huelva, Spain
Gad Katzir, University of Haifa, Israel
Copyright © 2015 Larsson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matz L. Larsson, Hjärt- och Lungkliniken, Örebro University Hospital, Örebro, Sweden,bGFyc3Nvbi5tYXR6QGdtYWlsLmNvbQ==;bWF0ei5sYXJzc29uQHJlZ2lvbm9yZWJyb2xhbi5zZQ==
 Matz L. Larsson
Matz L. Larsson