
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 19 May 2015
Sec. Behavioral and Evolutionary Ecology
Volume 3 - 2015 | https://doi.org/10.3389/fevo.2015.00050
This article is part of the Research Topic Ballroom Biology: Recent Insights into Honey Bee Waggle Dance Communications View all 10 articles
Honey bee foragers may use both personal and social information when making decisions about when to visit resources. In particular, foragers may stop foraging at resources when their own experience indicates declining resource quality, or when social information, namely the delay to being able to unload nectar to receiver bees, indicates that the colony has little need for the particular resource being collected. Here we test the relative importance of these two factors in a natural setting, where colonies are using many dynamically changing resources. We recorded detailed foraging histories of individually marked bees, and identified when they appeared to abandon any resources (such as flower patches) that they had previously been collecting from consistently. As in previous studies, we recorded duration of trophallaxis events (unloading nectar to receiver bees) as a proxy for resource quality and the delays before returning foragers started trophallaxis as a proxy for social need for the resource. If these proxy measures accurately reflect changes in resource quality and social need, they should predict whether bees continue foraging or not. However, neither factor predicted when individuals stopped foraging on a particular resource, nor did they explain changes in colony-level foraging activity. This may indicate that other, as yet unstudied processes also affect individual decisions to abandon particular resources.
Animals are often faced with the challenge of foraging on resources whose quality and availability change over space and time. In order to maximize foraging success, animals have evolved mechanisms to judge which resources are worth exploiting (Belovsky, 1978; Pyke, 1978; Pleasants, 1989; Van Nest and Moore, 2012). Many animals forage on resources to which they may make multiple trips (such as bees, nectar foraging ants, and birds); in these cases, foragers need to choose when to return to the same resource and when to abandon it to search for a new one. This is known as the “exploitation vs. exploration” trade-off (Krebs et al., 1978; McNamara and Houston, 1985). In social animals, both the information available to make this decision, and the consequences of foraging success, may be shared among individuals. Social insects have been particularly well studied in this respect.
Honey bees (Apis mellifera) provide a great model for social foraging due to their ability to rapidly adapt their foraging efforts to changing resource availability, studied particularly in the context of nectar foraging (Seeley, 1986). This is accomplished through the collective actions and decisions of individual foragers, with the benefits and costs of these decisions affecting the colony as a whole. Individual bees integrate several sources of information, including personal and social, when making decisions about foraging (Biesmeijer and Seeley, 2005). Honey bee foragers use information gained in their own experience, such as memory of time and place, sugar concentration and amount of nectar previously collected, to decide whether to continue or resume foraging on particular resources (Wainselboim et al., 2002; Grüter and Farina, 2009a; Van Nest and Moore, 2012; Al Toufailia et al., 2013). They also make use of various sources of social information, such as information about resource location and quality transmitted via the waggle dance (von Frisch, 1967; Grüter and Farina, 2009b), and information about resource quality and type from nectar samples unloaded in the hive (Grüter and Farina, 2009a). Other communication signals and interactions can also affect foraging decisions, such as the tremble dance (food storer activation) and the stop signal (forager inactivation) (Seeley, 1989; Nieh, 1993; Balbuena et al., 2011; Seeley et al., 2012).
But what kind of information do foragers use to decide when to stop visiting a particular resource? The colony's dynamic ability to allocate foragers to the best resources available can only be maintained if foragers frequently re-evaluate their short-term commitment to resources (Seeley et al., 1991; Detrain and Deneubourg, 2008). While foragers may revisit and check on resources over long periods of time (days or weeks), we are particularly interested in how foragers decide on which resources to continue foraging (Beekman, 2005; Al Toufailia et al., 2013). How do foragers make the decision to stop foraging on a particular resource? Two main processes have been identified. First, an individual personally experiencing a decline in the quality of the resource is more likely to abandon it, and to stop foraging entirely or look for other resources (Seeley et al., 1991; Townsend-Mehler et al., 2010). Second, if the colony's need for foragers in general, or the need for the particular forage brought in by that forager (e.g., if other foragers are bringing higher-quality nectar), has decreased, individuals may also abandon the resource they are currently exploiting (Lindauer, 1952; Seeley, 1989). Foragers get this information from interactions with nestmates, particularly receiver bees (Lindauer, 1952; Seeley, 1989; Biesmeijer and de Vries, 2001).
In honey bees, foragers can assess resource quality directly when foraging, using several criteria, including concentration and volume of the nectar itself, but also the flight distance to the resource from the hive and the likelihood of predation at the resource (von Frisch, 1967; Tan et al., 2013). These measures are integrated by bees and affect both when bees share information about this resource by dancing and the bees' decision to continue foraging on it (Seeley, 1994; De Marco and Farina, 2001). Nectar can be highly temporally and spatially variable, affected by abiotic factors (rainfall, sunlight, nutrients) and biotic factors (pollinator visitation and nectar replacement rates) (Real and Rathcke, 1991; Boose, 1997; Edge et al., 2011). Even over the course of a day nectar volume can change quite rapidly, by several microliters in an hour (Raihan and Kawakubo, 2014).
A honey bee forager can also gain valuable social information about the quality of her resource relative to others exploited by her colony, and the need for this resource, from her nest mates. Foragers, after gathering liquid food such as flower nectar or honeydew, return to the hive to pass this food to another bee, called a “receiver bee,” who will carry it deeper into the hive, process it, and either deposit it in a honey store or pass it on to nurse bees (Seeley, 1995). The time it takes from entering a hive to securing a receiver bee we call “wait time,” and is thought to reflect colony foraging needs in one of two ways (Seeley and Tovey, 1994). Receiver bees have access to multiple foragers, and may thus experience multiple sources of nectar; in response they may be reluctant to accept a lower-quality or novel resource compared to what they have recently experienced (Seeley, 1989; Seeley and Tovey, 1994; Gil and Farina, 2002; Wainselboim and Farina, 2003; Goyret and Farina, 2005). Thus a forager who experiences a longer wait time may be informed that her resource is of poorer quality relative to what is being brought into the hive by others. Difficulty of finding a receiver may also indicate the general state of hive-level foraging to the forager: increased wait time could be a result of a redistribution of workers away from unloading to more pressing colony tasks, or a result of a sudden increase in foragers bringing nectar that overwhelms the capacity of the existing receiver bees to process that nectar (Lindauer, 1952; Seeley and Tovey, 1994). In both of these cases, it may be adaptive for a forager experiencing long wait times to stop foraging on its particular resource. Indeed, in an empirical test using artificial feeders and removal of receiver bees, lower densities of receiver bees resulted in longer wait times, decreased the probability that a forager would perform waggle dances, and increased the probability that a forager would stop foraging on its current resource (Seeley, 1989).
While independently shown to affect foragers' decisions to abandon resources, personal and social information's relative contributions to forager decisions, as well as their importance under natural foraging conditions with many small, temporally and spatially rapidly varying resources, have not been investigated. Does personal or social information more often determine a bee's decision to quit foraging at a resource, and are the bees' decisions fully explained by these two factors, or are other processes also important? For example, bees might simply stop foraging on any particular resource with a fixed probability, which could help the colony maintain flexibility, since it prevents large numbers of foragers from being “locked in” to foraging on particular resources (Detrain and Deneubourg, 2008; Lanan et al., 2012). Does this occur, and how relevant is it compared to quitting in response to the two known factors?
We thus quantify the influences of decreased trophallaxis duration and increased wait time on the decision to abandon resources under natural foraging conditions. Using detailed foraging histories based on in-hive observations of returning foragers, we test (1) the effect of personal information, in the form of a decline in resource quality, on the decision to stop foraging. To do this we compare the average trophallaxis duration (a proxy for nectar load and thus a potential correlate of resource quality) after the last trip before a forager abandons a resource with its previous average trophallaxis duration over recent trips that are likely to be to the same resource. We also test (2) the effect of social information, in the form of wait time to unload nectar, by measuring this directly in the hive, and comparing wait time on the last trip with that on recent trips.
Each experiment was performed with two colonies of about 2000 domestic Italian honeybees (Apis mellifera ligustica) each, with roughly 500 bees individually marked in each colony. They were housed in a glass sided, two frame observation hive with the exit, a clear plastic tube, connected to the hive near the bottom corner. Foragers were marked at the USDA Carl Hayden Bee Center over a period of 1 week prior to the start of the experiment. Foragers were captured by selectively collecting individuals that had left the hive. Individuals were uniquely marked with a colored/numbered tag and paint. After being sealed into their hives for ~24 h, the colony was transported to a new location and left sealed overnight before the beginning of the experiment the next morning.
The two experiments took place in two locations in southern Arizona. Experiments 1a and 1b were located at Appleton–Whittell Research Ranch, an Audubon Society preserve near Elgin, Arizona and took place on June 20 and 27, 2010. Experiments 2a and 2b were performed at the Santa Rita Experimental Range Headquarters in Florida Canyon on Aug 9 and 16, 2010. (These dates and locations correspond to experiments 3 and 4 in Donaldson-Matasci and Dornhaus, 2012).
Hives were opened at dawn and remained open until dusk. During that time all marked bees were recorded coming in and out of the hive. From video recordings taken, we observed all returning marked bees and recorded all instances of trophallaxis within 5 min of entering the hive. Wait time (amount of time from entering the hive until the beginning of the first trophallaxis, an indication of colony foraging needs) and trophallaxis duration (the sum of all trophallaxis event durations in a single hive visit, a proxy for the profitability of the exploited resource) were determined for each returning bee (Wainselboim and Farina, 2003). Trophallaxis duration has been used as a metric for non-invasively determining resource quality (Seeley and Visscher, 1988). We only analyzed foraging histories from foragers who were performing repeated, consistent, successful foraging trips, which we termed to be “employed” (see below); we did this to maximize the likelihood that foragers were indeed repeatedly visiting the same resource. To see if a relationship existed between the decision to quit foraging and declines in trophallaxis duration and/or increases in wait time, we compared these measures on a forager's last trip to the average measure on previous trips of that forager (during its “employment”).
Foraging histories were constructed using the following operational definitions, based on the framework in Biesmeijer and de Vries (2001). We consider a forager to be “employed” while it consistently keeps foraging at the same resource (e.g., a patches of flowers that a bee would return to repeatedly). We operationally defined this as a forager who performs three or more consecutive successful foraging trips (where trophallaxis is performed in the hive after each trip), with less than 2 min variability in duration, and less than 10 min spent in the hive between trips. This was a consistent pattern that emerged from our foraging data, in other words most bees that performed several consecutive successful trips conformed to this pattern. Through the lens of these foraging histories we are able to determine when an individual stops foraging at a particular resource (see Figure 1). We found 29 individual bees out of the 227 individuals observed (184 of which showed at least one successful trophallaxis event) over the 4 experiment days and the 2 colonies that showed such consistent foraging patterns. This was perhaps due to many foragers only performing a few short bouts of trophallaxis over the entire day.
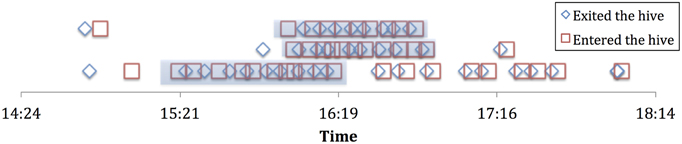
Figure 1. Sample employment histories for 3 employed foragers from experiment 2a. Highlighted portion is the “employment” phase.
To measure the influence of average trophallaxis duration and wait time on colony-level foraging activity, we divided each experiment into 15-min time bins. For each bin we recorded the number of marked foragers who left the hive (employed or unemployed), average length of all trophallaxis events, and the average wait time. Due to the likely presence of autocorrelation in these data series, simply testing for correlations among these factors could lead to erroneous results. Instead, we use a cross-correlation test, which measures the correlations between the two time series as a function of time lag (Venables and Ripley, 2002). If wait time were a major factor affecting foraging activity, we would see a negative correlation (as wait time increases, number of foragers leaving decreases) with a positive time lag (the decrease in the number of foragers would occur after the increase in wait time). If, on the other hand, colony-level foraging activity affected wait time (e.g., because with fewer foragers, bees can unload faster), we would see a positive correlation with a negative lag (decreases in the number of foragers would precede decreases in wait time). Similarly, if changes in trophallaxis duration affected foraging activity, we would see a positive correlation (as resources decline in quality, fewer bees leave the hive) with a positive time lag (a decline in the resource precedes a decline in foragers). We considered only time lags within biologically relevant time scale (less than an hour). To account for multiple testing, i.e., consideration of multiple time lags, we applied a Bonferroni correction (significance level α = 0.05/11, where 11 is the number of potential time lags considered in each experiment). All analyses were performed using the R statistical package (R Core Team, 2013).
Contrary to expectations, we did not find a statically significant effect of either decreased trophallaxis duration or of increasing wait times on individual bees' decision to stop foraging. That is, foragers did not experience a longer-than-average wait time just before quitting any more often than expected by chance (Binomial test p = 0.326, n = 29, see Table 1). Their trophallaxis durations were also not shorter than average any more often than expected by chance (Binomial test p = 0.845, n = 29, see Table 1). Looking at it in a different way, the trophallaxis duration experienced by foragers on their last trip before quitting was not significantly shorter than that experienced on previous trips (Wilcoxon signed-rank test p = 0.56, W = 370.5, n = 29). Neither was wait time on a forager's last trip significantly longer than on previous trips (Wilcoxon signed-rank test p = 0.98, W = 336, n = 29) (Figure 2). These analyses were performed on “employed” foragers, which showed trophallaxis durations on average seven times longer than non-employed categorized foragers (T-test p = 0.0498, n = 4, colony averages for employed and non-employed successful foragers).
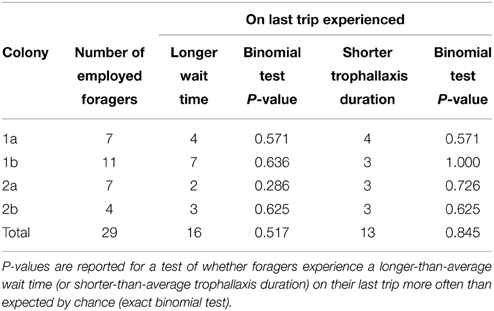
Table 1. The number of “employed” foragers in each experiment, and whether they experienced a longer wait time/shorter trophallaxis duration on the last trip of their employed period compared to the average for previous trips.

Figure 2. Difference between the last and average trophallaxis duration/wait time during a forager's employment period.
The level of colony foraging activity varied considerably throughout the day, as did trophallaxis and wait times (Figure 3). Experiments 1a and 1b (in June) showed strong foraging peaks in the morning, while experiments 2a and 2b (in August) showed more consistent activity across the day, with more foraging in the afternoon.
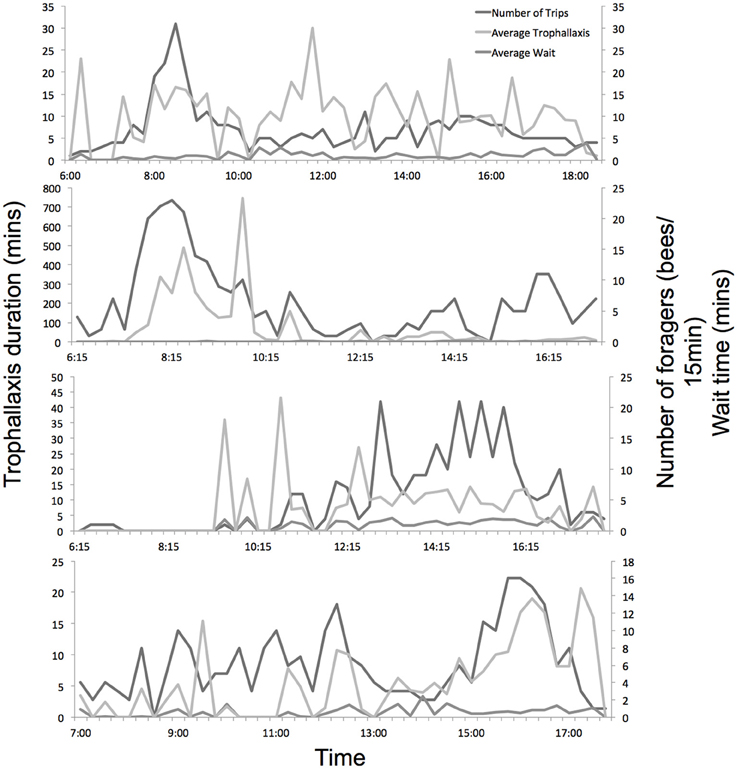
Figure 3. Daily foraging activity, average trophallaxis duration and wait time of all marked foragers across the 4 experiments. Hives were opened and recorded from dawn until dusk.
We found no evidence that changes in trophallaxis duration across all successful foragers affects colony-level foraging activity (Figure 4—Trophallaxis duration). If changes in trophallaxis duration affected foraging activity, we would expect to see a positive correlation (as resources decline in quality, fewer bees leave to forage) with a positive time lag (a decline in the resource is followed by a decline in foraging activity). However, the only significant correlations we observed were positive correlations with negative time lags, suggesting that decreases in foraging activity preceded decreases in trophallaxis duration (experiments 1b and 2b). In the other experiments, no significant correlations were observed.
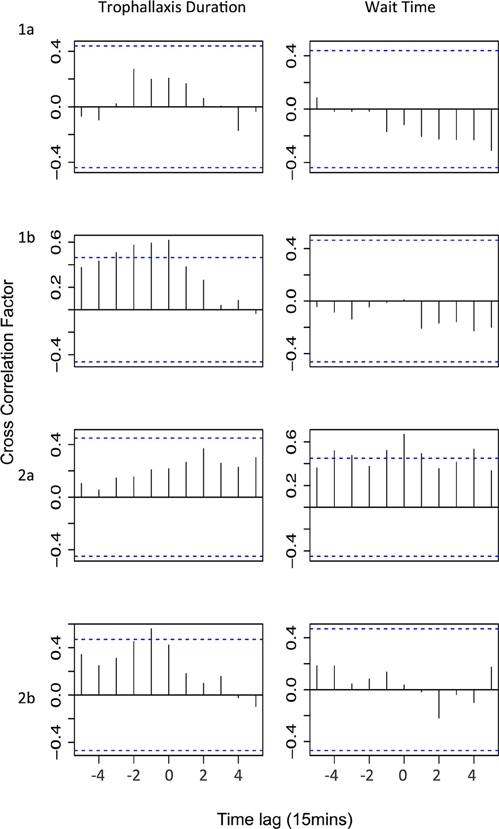
Figure 4. Cross correlation test results showing correlations between either trophallaxis duration or wait time and the number of foragers leaving. Horizontal lines signify critical values corrected for multiple testing (Bonferroni correction: α = 0.0045); a correlation at any time lag above that line is considered statically significant. Time lag is for foragers leaving relative to trophallaxis duration/wait time (i.e., positive time lag indicates that changes in the factor precede changes in foraging activity by the specified time lag).
We also found no evidence that the wait time experienced by foragers influences the colony's foraging effort (Figure 4—Trophallaxis duration). If wait time were a major factor affecting foraging activity, we would expect to see a negative correlation (as wait time increases, number of foragers leaving decreases) with a positive time lag (the decrease in the number of foragers would occur after the increase in wait time). No significant negative correlation between wait time and foraging effort was observed in any experiment. In experiment 2a, significant positive correlations were observed with both positive and negative time lags. A positive correlation with a negative time lag might indicate that high levels of foraging activity tend to increase wait times (because receiver bees are busier), but the occurrence of correlations at positive time lags as well makes it difficult to infer the direction of causation. In the other experiments, there were no significant correlations observed.
Our study aimed to quantify and compare the effects of (1) personal experience of a decline in resource quality and (2) social information about a decrease in the colony's need for a particular resource, in a natural setting. Both of these factors had independently been shown to affect honey bees' short term decisions to stop foraging on artificial food sources (Seeley, 1986; Seeley and Tovey, 1994). We also looked for evidence of these effects at the colony level, by testing whether a honey bee colony's overall foraging activity decreases in response to either factor. In our experiment, neither factor appeared to have a noticeable effect: we saw no relationship between changes in trophallaxis duration (our proxy for resource quality) or wait time to unload (a proxy for colony need) and the decision to quit foraging at either the individual or colony levels.
A crucial assumption made here is that trophallaxis duration and wait time are valid proxies for resource quality and colony foraging need respectively. These two measures have been tested several times with conflicting results. For trophallaxis duration, Farina and Núñez (1991) and Farina and Wainselboim (2001) found no relationship between resource quality and trophallaxis duration, but Wainselboim and Farina (2003) and Seeley et al. (1991) did. Perhaps these differences are reflections in the variation in methods, particularly in terms of feeders used (capillary tubes vs. multi-well feeders) or where the trophallaxis duration measurements were made (in separate observation chambers or within the hive). In general, no artificial feeder mimics resource delivery of natural resources: flowers deliver tiny and extremely variable nectar amounts, but secrete nectar so slowly that they effectively have no “flow rate” where a bee can wait to fill up, and bees generally visit up to several hundred flowers on each trip (Castellanos et al., 2001). By utilizing trophallaxis duration we are able to make direct comparisons against previous studies (Seeley, 1986) using the same metric, but with natural resources. Thus, while there is perhaps not a consensus on how trophallaxis time relates to resource quality, it is a non-invasive measure previously shown to predict foraging decisions.
Wait time has universally been seen as a source of social information about the need for the particular food brought by a foraging honey bee (Seeley, 1989; Seeley and Tovey, 1994; Gil and Farina, 2002; Wainselboim and Farina, 2003; Goyret and Farina, 2005). What information precisely is contained in this cue, i.e., what social processes affect wait time, has been interpreted somewhat differently in different studies. It may be that the forager mainly receives information about the nutritional status of her colony (Seeley, 1989; Seeley and Tovey, 1994); others conclude that wait time is a reflection on the quality of the foragers resource relative to other resources exploited by the hive (Lindauer, 1961).
While our colony-level analysis included only marked bees (~500, 25% of the colony), they represented a majority of the foragers, thus providing a good measure of colony foraging effort. Nevertheless, for the individual-level analysis we only recorded 29 bees foraging consistently (“employed” according to our operational definition). This sample size is similar to previous studies of this nature (Seeley and Tovey, 1994: 39 foragers; De Marco and Farina, 2001: 17 foragers), however, a larger study, with more foragers recorded as well as including more different days of foraging, would likely have made any effects of both resource quality and colony need for the resource more apparent. We do not conclude from our results that neither factor ever plays a role; after all, the possible effects of both had been demonstrated previously (Seeley, 1986; Seeley and Tovey, 1994). Despite this, however, our results do show that neither factor explains most of the variation in forager decisions.
One reason that we may not have seen an effect of either change in resource quality or wait time on the decision to stop foraging is that the magnitude of both of these effects is small under natural conditions. While several previous manipulative studies have demonstrated these effects, this study is the first that uses natural resources and no manipulation of worker allocation (Seeley, 1986; Seeley and Tovey, 1994; Wainselboim and Farina, 2003; Balbuena et al., 2011). Unlike the artificial feeders used in previous experiments, natural resource quality may change quite dramatically or subtly (Real and Rathcke, 1991; Boose, 1997; Edge et al., 2011). Furthermore, the potentially wide variety of resources being exploited may buffer large changes in the overall quantity and quality of nectar being brought into the hive (Donaldson-Matasci and Dornhaus, 2014). Barring any large-scale simultaneous resource landscape changes, the colony may experience relatively subtle and slow changes in resource intake. Continual adjustments in the ratio of receiver bees to foragers may allow the colony to track those changes without ever experiencing long wait times (Seeley, 1986). Thus colonies under natural conditions may rarely experience the dramatic increase in wait time induced by artificially removing receiver bees from the hive. Wait times could be primarily a byproduct of other colony level functions (such as shifts in worker allocation) rather than a result of resource dynamics. For example the density of bees in the entrance area (often called the dance floor) may be a good indicator to foragers on such shifts, and have been shown to vary throughout the day (Seeley, 1995). Such effects would increase the noise in the wait time cue, and may make its effect on foraging decisions less clear. By comparing these measures over the average time to the last, we hope to capture the greatest amount of change (i.e., the greatest decline in trophallaxis duration). However, it could be with the noise or subtly that natural conditions bring, that foragers use a series of poor indicators to make foraging decisions.
Another possible explanation for the observed lack of effect in our experiment is that both factors are important in nature, but which factor is most influential could change depending on the observed time frame. Our small sample size precluded analyzing the effects separately over different time periods, which might have kept us from finding a significant effect. In the morning, when resources are of higher quality, foraging bees might be willing to wait longer to unload to capitalize on the high quality nectar, in which case these foragers should be relativity insensitive to wait time and highly sensitive to changes in resource quality. Later in the day when there is a higher demand for workers elsewhere in the hive (for example cooling or water collecting) no matter the quality of the resource, the wait time to unload nectar could take precedence in their decision making (Johnson, 2003). At this later time we might then see the sensitivity to wait time increase relative to their response to changing nectar quality. As Figure 3 illustrates, resource quality and unload time were dynamic across the day, which could have been due to the effects of changing resources or additional factors affecting colony organization. However, because we had relatively few employed foragers working consistently across the day, we did not have enough statistical power to test for changes in the importance of each factor over the course of the day. Additionally these factors could impact the decision much differently over a longer time period. While our study looked only at foraging dynamics within a relatively short time frame, previously studies have shown that bees will be more persistent on a previously strong rewarding resource even if it declines in quality (Al Toufailia et al., 2013).
In addition to variation within a single environment, differences in foraging conditions between environments could have shaped the foraging patterns we saw (Sherman and Visscher, 2002). Whether personal or social information is most important in an individual's decision to stop foraging at a particular resource may change depending on the foraging environments. For example previous work has shown that the benefit a colony receives from communication via the waggle dance depends on the resource environment (Donaldson-Matasci and Dornhaus, 2012). This could be true for the benefits of using a particular type of personal or social information (like waiting times) as well. For example, in environments with short lived, rich resources, using personal information about resource quality may allow a forager to secure a highly profitable resource before it disappears, regardless of possibly out-of-date social information. If resources are long-lived, the colony-level foraging effort should perhaps be more driven by colony need than resource availability. In that case, following wait time to learn about colony needs may ensure that the colony's nectar collection and processing rates are well balanced and efficient. Generally each of these sources of information have been shown to vary in their accuracy, with personal information being more accurate about a single exploited resource, but naïve about the resource landscape (Franks et al., 2003). Social information is thought to operate on a slower timescale than personal, potentially leading inaccuracy about specific resource due to transmission errors and the potential for it to be outdated (Rendell et al., 2010). However, social information allows for comparison among resources without requiring direct comparison by individuals. Thus what may favor the use of either social or personal information may be driven by the need for short term accuracy on about a specific resource (personal) or longer term information across resources (social) in a particular context. Further more different types of social and personal information exist and may be affected by environmental conditions separately. For example the waggle dance may be more suitable for ephemeral resources due to its fast response time, while floral odors shared among foragers may lend to steady resource patches.
In addition to being context dependent, what information a foraging honey bee uses to quit foraging on a particular resource could vary among individuals and among colonies. It has been shown that nectar response thresholds (the concentration of sucrose at which individuals respond) vary among individuals and colonies (Pankiw and Page, 2000). Individual variation in nectar response thresholds could provide a mechanism for the variation we see in the decision to abandon a resource, with high threshold individuals being more likely to abandon a resource when it declines in quality and low threshold individuals being more persistent. Similarly, inter-individual variation in sensitivity to wait time could obscure the colony-level correlation between increased wait time and quitting foraging. Future studies with larger numbers of marked individuals foraging over the course of several days could show whether individuals are consistent across their foraging careers in their sensitivity to declines in resource quality and/or wait time.
We have focused on two sources of information that foraging honey bees might use in making the decision to abandon a resource: personal information about resource quality, and social information about colony needs. However, it is likely that there is a stochastic element to their decision-making as well. Some have argued that individuals living in groups can afford to be less precise: individual variance in decision-making may be compensated by the reliability of the system as a whole (Oster and Wilson, 1978). Furthermore, some randomness in individual behavior can actually be good, in the context of collective behavior, because it may allow the group to respond more flexibly to changing environmental conditions (Deneubourg et al., 1983, 1986; Seeley et al., 1991; Detrain and Deneubourg, 2008; Townsend-Mehler and Dyer, 2011). For example, individuals may sometimes persist in foraging at even rather poor nectar sources (“inspectors”), just in case the resource increases in quality (Biesmeijer and de Vries, 2001; Biesmeijer and Seeley, 2005; Granovskiy et al., 2012). Likewise it could be advantageous for some individuals to abandon even a strong nectar source, in order to keep the colony from overcommitting to any single resource while potentially missing out on even stronger ones. Given the potential for rapid resource dynamics, a colony being “locked into” one or a few resources may miss newly emerging ones (Detrain and Deneubourg, 2008; Lanan et al., 2012).
If there is a strong element of randomness in a forager's decision to abandon a resource, it may be difficult to detect the subtler effects of personal or social information under natural foraging conditions. Our results may reflect a complex interplay of factors influencing honey bee decision making in natural environments, but the potential importance of stochasticity in these systems should not be overlooked.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MCD was funded by the University of Arizona's Center for Insect Science through a NIH Training Grant #1K12GM000708. AD was funded by NSF grants no. IOS-1045239 and DBI-1262292. Honey bee colonies were kindly provided by R. Page at Arizona State University and G. DeGrandi-Hoffman at the USDA Carl Hayden Bee Research Center, and housed at the latter's facility with assistance from M. Chambers and T. Deeby. We thank E. Francis (SASI), L. Kennedy (AWRR), M. Heitlinger (SRER) and R. Smith (UADS) for their cooperation and support at our field sites. We gratefully acknowledge the field work assistance of N. Matasci, G. Barraza, J. Brown, J. Chappell, M. Hughes, J. Icely, N. Narkhede, S. Williams and Y. Zhu.
Al Toufailia, H., Grüter, C., and Ratnieks, L. W. (2013). Persistence to unrewarding feeding locations by honeybee foragers (Apis mellifera): the effect of experience, resource profitability, and season. Ethology 119, 1096–1106. doi: 10.1111/eth.12170
Balbuena, M. S., Molinas, J., and Farina, W. M. (2011). Honeybee recruitment to scented food sources: correlations between in-hive social interactions and foraging decisions. Behav. Ecol. Sociobiol. 66, 445–452. doi: 10.1007/s0265-011-1290-3
Beekman, M. (2005). How long will honey bees (Apis mellifera L.) be stimulated by scent to revisit past-profitable forage sites? J. Comp. Physiol. A. 191, 1115–1120. doi: 10.1007/s00359-005-0033-1
Belovsky, G. E. (1978). Diet optimization in a generalist herbivore: the moose. Theor. Populat. Biol. 134, 105–134. doi: 10.1016/0040-5809(78)90007-2
Biesmeijer, J. C., and de Vries, H. (2001). Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav. Ecol. Sociobiol. 49, 89–99. doi: 10.1007/s002650000289
Biesmeijer, J. C., and Seeley, T. D. (2005). The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 59, 133–142. doi: 10.1007/s00265-005-0019-6
Boose, D. L. (1997). Sources of variation in floral nectar production rate in Epilobium canum (Onagraceae): implications for natural selection. Oecologia 110, 493–500. doi: 10.1007/s004420050185
Castellanos, M. C., Wilson, P., and Thomson, J. D. (2001). Dynamic nectar replenishment in flowers of Penstemon (Scrophulariaceae). Am. J. Bot. 89, 111–118. doi: 10.3732/ajb.89.1.111
De Marco, R., and Farina, W. (2001). Changes in food source profitability affect the trophallactic and dance behavior of forager honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 50, 441–449. doi: 10.1007/s002650100382
Deneubourg, J., Aron, S., Goss, S., Pasteels, J., and Duerinck, G. (1986). Random behaviour, amplification processes and number of participants: how they contribute to the foraging properties of ants. Phys. D Nonlin. Phenom. 22, 176–186. doi: 10.1016/0167-2789(86)90239-3
Deneubourg, J. L., Pasteels, J. M., and Verhaeghe, J. C. (1983). Probabilistic behaviour in ants: a strategy of errors? Theor. Biol. 105, 259–271. doi: 10.1016/S0022-5193(83)80007-1
Detrain, C., and Deneubourg, J. (2008). Collective decision-making and foraging patterns in ants and honeybees. Adv. Insect Physiol. 35, 123–173. doi: 10.1016/S0065-2806(08)00002-7
Donaldson-Matasci, M., and Dornhaus, A. (2012). How habitat affects the benefits of communication in collectively foraging honey bees. Behav. Ecol. Sociobiol. 66, 583–592. doi: 10.1007/s00265-011-1306-z
Donaldson-Matasci, M., and Dornhaus, A. (2014). Dance communication affects consistency, but not breadth, of resource use in pollen-foraging honey bees. PLoS ONE 9:e107527. doi: 10.1371/journal.pone.0107527
Edge, A., Nest, B. N., Johnson, J. N., Miller, S. N., Naeger, N., Boyd, S. D., et al. (2011). Diel nectar secretion rhythm in squash (Cucurbita pepo) and its relation with pollinator activity. Apidologie 43, 1–16. doi: 10.1007/s13592-011-0087-8
Farina, W. M., and Núñez, J. A. (1991). Trophallaxis in the honeybee, Apis mellifera (L.) as related to the profitability of food sources. Anim. Behav. 42, 389–394. doi: 10.1016/S0003-3472(05)80037-5
Farina, W. M., and Wainselboim, A. J. (2001). Changes in the thoracic temperature of honeybees while receiving nectar from foragers collecting at different reward rates. J. Exp. Biol. 204, 1653–1658.
Franks, N. R., Dornhaus, A., Fitzsimmons, J., and Stevens, M. (2003). Speed versus accuracy in collective decision making. Proc. Biol. Sci. 270, 2457–2463. doi: 10.1098/rspb.2003.2527
Gil, M., and Farina, W. M. (2002). Foraging reactivation in the honeybee Apis mellifera L.: factors affecting the return to known nectar sources. Naturwissenschaften 89, 322–325. doi: 10.1007/s00114-002-0323-1
Goyret, J., and Farina, W. M. (2005). Non-random nectar unloading inter- actions between foragers and their receivers in the honeybee hive. Naturwissenschaften 92, 440–443. doi: 10.1007/s00114-005-0016-7
Granovskiy, B., Latty, T., Duncan, M., Sumpter, D., and Beekman, M. (2012). How dancing honey bees keep track of changes: the role of inspector bees. Behav. Ecol. 23, 588–596. doi: 10.1093/beheco/ars002
Grüter, C., and Farina, W. M. (2009a). Past experiences affect interaction patterns among foragers and hive-mates in honeybees. Ethology 115, 790–797. doi: 10.1111/j.1439-0310.2009.01670.x
Grüter, C., and Farina, W. M. (2009b). The honeybee waggle dance: can we follow the steps? Trends Ecol. Evol. 24, 242–247. doi: 10.1016/j.tree.2008.12.007
Johnson, B. R. (2003). Organization of work in the honeybee: a compromise between division of labour and behavioural flexibility. Proc. R. Soc. Lond. B Biol. Sci. 270, 147–152. doi: 10.1098/rspb.2002.2207
Krebs, J., Kacelnik, A., and Taylor, P. (1978). Test of optimal sampling by foraging great tit. Nature 275, 27–31. doi: 10.1038/275027a0
Lanan, M. C., Dornhaus, A., Jones, E. I., Waser, A., and Bronstein, J. L. (2012). The trail less traveled: individual decision-making and its effect on group behavior. PLoS ONE 7:e47976. doi: 10.1371/journal.pone.0047976
Lindauer, M. (1952). Ein beitrag zur frage der arbeitsteilung im bienenstaat. Z. Vergl. Physiol. 34, 253–259. doi: 10.1007/BF00298048
McNamara, J. M., and Houston, A. I. (1985). Optimal foraging and learning. J. Theor. Biol. 117, 231–249. doi: 10.1016/S0022-5193(85)80219-8
Nieh, J. C. (1993). The stop signal of honey bees: reconsidering its message. Behav. Ecol. Sociobiol. 33, 51–56. doi: 10.1007/BF00164346
Oster, G. F., and Wilson, E. O. (1978). Caste and Ecology in the Social Insects. Princeton, NJ: Princeton University Press.
Pankiw, T., and Page, R. E. (2000). Response thresholds to sucrose predict foraging division of labor in honeybees. Behav. Ecol. Sociobiol. 47, 265–267. doi: 10.1007/s002650050664
Pleasants, J. M. (1989). Optimal foraging by nectarivores: a test of the marginal-value theorem. Am. Nat. 134, 51–71. doi: 10.1086/284965
Pyke, G. H. (1978). Optimal foraging: movement patterns of bumblebees between inflorescences. Theor. Populat. Biol. 13, 72–98. doi: 10.1016/0040-5809(78)90036-9
R Core Team. (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Availible online at: http://www.r-project.org
Raihan, J., and Kawakubo, N. (2014). Nondestructive and continuous observation of nectar volume using time-interval photography. Plant Species Biol. 29, 212–215. doi: 10.1111/1442-1984.12007
Real, L., and Rathcke, B. (1991). Individual variation in nectar production and its effect on fitness in kalmia latifolia. Ecology 72, 149–155. doi: 10.2307/1938910
Rendell, L., Boyd, R., Cownden, D., Enquist, M., Eriksson, K., Feldman, M. W., et al. (2010). Why copy others? Insight from the social learning strategies tournament. Science 328, 208–213. doi: 10.1126/science.1184719
Seeley, T. D. (1986). Social foraging by honeybees: how colonies allocate foragers among patches of flowers. Behav. Ecol. Sociobiol. 19, 343–354. doi: 10.1007/BF00295707
Seeley, T. D. (1989). Social foraging in honey bees: how nectar foragers assess their colony's nutritional status. Behav. Ecol. Sociobiol. 24, 181–199. doi: 10.1007/BF00292101
Seeley, T. D. (1994). Honey bee foragers as sensory units of their colonies. Behav. Ecol. Sociobiol. 34, 51–62. doi: 10.1007/BF00175458
Seeley, T. D. (1995). Wisdom of the Hive: The Social Phyisology of Honeybee Colonies. Cambridge, MA: Harvard University Press.
Seeley, T. D., Camazine, S., and Sneyd, J. (1991). Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 28, 277–290. doi: 10.1007/BF00175101
Seeley, T. D., and Tovey, C. (1994). Why search time to find a food-storer bee accurately indicates the relative rates of nectar collecting and nectar processing in honey bee colonies. Anim. Behav. 47, 311–316. doi: 10.1006/anbe.1994.1044
Seeley, T. D., and Visscher, P. K. (1988). Assessing the benefit of cooperation in honeybee foraging: search costs, forage quality, and competitive ability. Behav. Ecol. Sociobiol. 22, 229–237. doi: 10.1007/BF00299837
Seeley, T. D., Visscher, P. K., Schlegel, T., Hogan, P. M., Franks, N. R., and Marshall, J. A. R. (2012). Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science 335, 108–111. doi: 10.1126/science.1210361
Sherman, G., and Visscher, K. P. (2002). Honeybee colonies achieve fitness through dancing. Nature 419, 920–922. doi: 10.1038/nature01127
Tan, K., Hu, Z., Chen, W., Wang, Z., Wang, Y., and Nieh, J. C. (2013). Fearful foragers: honey bees tune colony and individual foraging to multi-predator presence and food quality. PLoS ONE 8:e75841. doi: 10.1371/journal.pone.0075841
Townsend-Mehler, J. M., and Dyer, F. C. (2011). An integrated look at decision-making in bees as they abandon a depleted food source. Behav. Ecol. Sociobiol. 66, 275–286. doi: 10.1007/s00265-011-1275-2
Townsend-Mehler, J. M., Dyer, F. C., and Maida, K. (2010). Deciding when to explore and when to persist: a comparison of honeybees and bumblebees in their response to downshifts in reward. Behav. Ecol. Sociobiol. 65, 305–312. doi: 10.1007/s00265-010-1047-4
Van Nest, B. N., and Moore, D. (2012). Energetically optimal foraging strategy is emergent property of time-keeping behavior in honey bees. Behav. Ecol. 23, 649–658. doi: 10.1093/beheco/ars010
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S, 4th Edn. New York, NY: Springer-Verlag.
Wainselboim, A. J., and Farina, W. M. (2003). Trophallaxis in honeybees, Apis mellifera (L.), as related to their past experience at the food source. Anim. Behav. 66, 791–795. doi: 10.1006/anbe.2003.2256
Keywords: social insects, foraging, honey bees, Apis mellifera, decision making, collective behavior
Citation: Rivera MD, Donaldson-Matasci M and Dornhaus A (2015) Quitting time: When do honey bee foragers decide to stop foraging on natural resources? Front. Ecol. Evol. 3:50. doi: 10.3389/fevo.2015.00050
Received: 03 January 2015; Accepted: 04 May 2015;
Published: 19 May 2015.
Edited by:
Roger Schürch, University of Sussex, UKReviewed by:
Shawn M. Wilder, The University of Sydney, AustraliaCopyright © 2015 Rivera, Donaldson-Matasci and Dornhaus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael D. Rivera, School of Integrative Biology, University of Illinois, 505 S. Goodwin Ave., Urbana, IL 61801, USA,bWRyaXZlcjNAaWxsaW5vaXMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.