- Institute of Avian Research “Vogelwarte Helgoland,” Wilhelmshaven, Germany
Changes in the timing of migratory events have been observed recently in many migratory species, most likely in response to climatic change. In the common tern Sterna hirundo we examined such changes in spring arrival date and body mass based on an individual-based longitudinal data set from a transponder marked colony from 1994 to 2012. Although no long-term trend was observed in either trait, strong inter-annual and age-specific variation in arrival date and mass was evident. We investigated whether environmental factors such as (i) global climate phenomena, i.e., North Atlantic and Southern Oscillation Indices, NAOI and SOI, and/or (ii) local factors, i.e., food abundance in the wintering and breeding area represented by fish stock or marine primary productivity, could explain this variation. We found that 2-year-old birds on their first spring migration advanced arrival in relation to spring NAOI and delayed arrival in relation to sprat Sprattus sprattus abundance. The arrival date of 3-year-olds also advanced in relation to NAOI and delayed in relation to winter SOI. In contrast, adults delayed arrival with NAOI and advanced in relation to SOI. Within age groups, earlier annual arrival coincided with higher mass, indicating that a fast and/or early migration did not come at a cost to body condition. Changes in arrival mass relative to environmental covariates were found only in 2-year-olds on their first spring migration: in these birds arrival mass was positively related to herring Clupea harengus and sprat abundance in the breeding area as well as spring NAOI and negatively related to SOI. In conclusion, traits related to migration of common terns were linked with environmental conditions, but showed no long-term trends over the past two decades. Age-related differences were marked, suggesting that common terns might be subject to differing environmental constraints or respond differently to conditions during their annual cycle depending on their age.
Introduction
Physical condition and timing of arrival in the breeding area are important parameters for migratory species with a relatively short reproductive season. In migratory birds, for example, the probability of reproducing successfully increases with an earlier arrival and a greater body mass (Chastel et al., 1995a,b; Kokko and Johnstone, 1999; Bêty et al., 2004; Verhulst and Nilsson, 2008; Descamps et al., 2011; Sergio et al., 2014), and early arrival and a greater mass at arrival have been associated with a reduced mortality risk and increased lifespan (Zhang et al., 2015b). Arrival date and mass are influenced by, among other factors, environmental conditions during migration and in the wintering area (e.g., Hüppop and Hüppop, 2003; Crick, 2004; Visser and Both, 2005; Parmesan, 2006; Newton, 2008). In many species, phenology has been observed to change in response to environmental conditions before and during the pre-breeding migration (e.g., Forchhammer et al., 1998; Frederiksen et al., 2004; Barbraud and Weimerskirch, 2006; Wanless et al., 2009).
Variation in temperature, wind and rainfall influences food availability and foraging success of migrants, which in turn can affect timing of migration and breeding propensity (Stenseth et al., 2002). Migratory seabirds are especially affected by offshore conditions (Schreiber, 2001). Stormy weather, strong winds and rain complicate foraging in piscivorous birds (Dunn, 1973; Finney et al., 1999; Daunt et al., 2006), while local weather also affects prey availability (Misund et al., 1998, 1997). Adverse weather can exhaust birds, causing migration to be prolonged (Newton, 2006). Conditions during winter can affect the physical state and molt of individuals, the length of migration, their ability to return to the colony site and their ability to breed (Schreiber, 2001; Sandvik et al., 2005). In consequence, environmental conditions can affect traits such as arrival date and mass after migration and cause inter-annual variation in reproductive performance and survival probability. Despite within-individual consistency in experienced breeders, substantial variation in arrival dates of birds was found (Bêty et al., 2004; Arnaud et al., 2013; Sergio et al., 2014), indicating plasticity and capacity of response in phenology toward short- and long-term environmental perturbations. Based on an understanding of inter-annual variation in the timing of arrival and of the birds' condition upon arrival at the breeding sites, these traits can be used as indicators of changing environments at wintering sites or on migration routes and as a tool for monitoring population health.
The common tern Sterna hirundo is a small-sized, long-distance migrant and visual hunter, relying on small pelagic fish caught by plunge-diving in the upper water layers (Becker and Ludwigs, 2004). Because of their low body reserves, common terns are sensitive to food shortage (e.g., Frank and Becker, 1992). Arrival date (Ludwigs and Becker, 2002; Dittmann and Becker, 2003; Ezard et al., 2007; Becker et al., 2008a; Arnaud et al., 2013; Zhang et al., 2015b) and arrival mass (Ezard et al., 2007; Limmer and Becker, 2007) in this species are strongly age-dependent and associated with fitness: earlier arrival and higher mass increase breeding success (Wendeln and Becker, 1999; Ezard et al., 2007) and are associated with reduced mortality and an increased lifespan (Zhang et al., 2015b). The common tern therefore represents a good species to examine the dependence of these migratory traits on environmental conditions in its year-round habitats.
Environmental conditions can be represented by global climate phenomena, such as the North Atlantic Oscillation Index NAOI (Hurrell et al., 2001, 2003) or the Southern Oscillation Index SOI (Trenberth and Caron, 2000; Stenseth et al., 2003). They affect local weather and fish abundance over large geographical areas and might therefore be good integrated indicators of overall conditions faced by migrant birds, with more predictive power than local weather variables (Stenseth et al., 2002; Hurrell and Deser, 2009). Food abundance for common terns breeding on western European coasts, such as the studied colony, can be measured in West African fish stock (wintering area, see Figure 1) and North Atlantic herring Clupea harengus and sprat Sprattus sprattus abundance (breeding area, see Figure 1). The preferred wintering area of European common terns (Becker and Ludwigs, 2004) is located in the northwest African upwelling zone with higher than average marine primary productivity, which directly influences fish stock (McGregor et al., 2007). In a preliminary study, some indications were found that NAOI and SOI might influence return rates and arrival dates, but not arrival mass of birds in the study colony (Favero and Becker, 2006), although a similar connection was not found in survival rates (Szostek and Becker, 2015). Instead, marine primary productivity in the wintering area was found to be positively related to common tern survival and recruitment probability (Szostek and Becker, 2015).
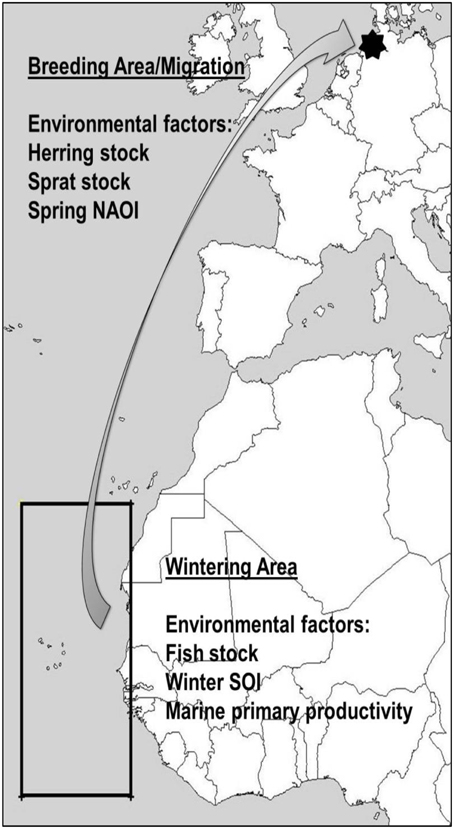
Figure 1. Wintering and breeding area of the Banter See common terns, as well as approximate spring migration route. Environmental covariates are given, along with the geographical locations where they are expected to affect common terns from the study colony.
Measuring time of first arrival at the breeding grounds and body mass upon arrival for individual birds is challenging, especially obtaining large sample sizes and annually repeated measurements. In a long-term integrated population study of common terns on the German North Sea coast, we were able to record annual spring arrival date and body mass with high reliability for a considerable number of known-age individuals over nearly two decades, from 1994 to 2012. Because of the age-related change in these traits (see above), information on the age of individuals is vital to studies investigating changes in arrival date and mass (Ezard et al., 2007).
In this study we focussed on inter-annual variation in arrival date and body mass and disentangled the variation caused by age, sex and environmental factors such as food supply and global climate indices. The aim was to find which of the many environmental constraints faced by the terns year-round exerted most influence and which part of the common tern life-cycle was most limiting. We regarded potential differences between age groups with respect to their sensitivity to the environment and possible carry-over effects between the wintering and breeding seasons. Specifically we wanted to address the following questions: (1) Is there inter-annual variation in spring arrival date and body mass in relation to age and sex? (2) Is there a time trend in arrival date and body mass between 1994 and 2012, against the background of global climate change? (3) Do environmental covariates affect arrival date and body mass and do effects differ between age groups? (4) Are environmental conditions most influential during winter or during migration?
Methods
Study Species
Common terns are small, long-lived, piscivorous seabirds (Becker and Ludwigs, 2004). As common terns are highly visual plunge-divers, their foraging abilities can be severely compromised by stormy or rainy conditions (Dunn, 1973; Becker et al., 1985; Becker and Specht, 1991; Frank and Becker, 1992). Juvenile common terns remain in the wintering area for their first summer, returning to the breeding grounds as 2-year-olds (Becker and Ludwigs, 2004). Most individuals spend a “prospecting” season at the colony as non-breeders before recruitment (Dittmann and Becker, 2003; Dittmann et al., 2007). Arrival mass increases with age until it reaches a plateau at around 6 years (Limmer and Becker, 2007). Arrival date at the colony also advances with age and experience (Dittmann and Becker, 2003; Becker et al., 2008a; Zhang et al., 2015a). First-time arrival date has been linked with age at first reproduction and can be regarded as a proxy for individual quality (Becker et al., 2008a; Zhang et al., 2015b).
Study Site
The Banter See common tern colony is located on the German North Sea coast (53°30′40″N, 08°06′20″E). Since 1984 all chicks at this site have been ringed and since 1992, all fledglings and 101 adult terns from this colony have additionally been injected with small passive transponders (TROVAN ID 100, 11 × 2 mm) that identify individuals remotely and automatically by antenna. In a long-term study, we collected individual life-histories of all native terns at this colony through remote sensing. Antennas were distributed on prominent platforms around the colony site and placed around each nest for 1–2 days to identify breeders. In addition, some platforms were equipped with electronic scales (Sartorius, TE6100; accuracy ± 1 g) which weighed birds automatically, while also recording their identity by antenna. In 1994 the system included 11 antennas and 7 scales and the devices were alternated between resting platforms throughout the colony. The number of registration devices was continuously increased as the colony grew until there were 44 permanent registration platforms (since 2002) of which 22 were also equipped with scales. The system recorded individual presence and mass throughout the breeding season with minimal disturbance to the colony (Becker and Wendeln, 1997; Becker et al., 2008b). For this study we used data between 1994 and 2012. Permissions were granted by the regional authorities “Bezirksregierung Weser-Ems, Stadt Wilhelmshaven” and “Nds. Landesamt für Verbraucherschutz und Lebensmittelsicherheit Oldenburg.”
Arrival Date and Arrival Mass
Common terns are generally registered immediately upon arrival, as they like to sit on the elevated platforms. Arrival dates were defined as the first automatic registration of a bird at the colony (cf. Ludwigs and Becker, 2002; Dittmann and Becker, 2003; Becker et al., 2008a). Dates were disregarded, if the first registration was less than 10 days before the recorded laying date for breeders (9.2% of cases per year), thereby excluding birds first recorded on the nest. Arrival body mass was calculated as the mean of masses measured independently during the first 3 days after arrival at the colony. Measurements that were falsified by wind or rain influencing the reading of the scale were disregarded (for details see: Wendeln and Becker, 1996; Limmer and Becker, 2007). In total the data set comprised 7576 arrival dates and 3664 arrival masses from 1607 individuals. The arrival mass data for each individual included on average 5.05 ± 7.97 (range 1–110) independent measurements.
Environmental Covariates
We focussed on six environmental covariates, concerning food abundance and global weather patterns both in the wintering and breeding area (for details see Table 1). We chose global climate indices (NAOI and SOI) rather than local weather variables for two reasons: firstly we did not want to include too many covariates (Frederiksen et al., 2014) and a compound parameter makes that possible; secondly, exact enough data on where the terns spend their winter months and by which route they migrate are currently not available. The data that are available from geolocation (Becker et al., unpublished) show that adult birds spread widely across the West African coast and do not always use the same migration route. This makes global climate indices more likely to show a broad effect than local weather data. NAOI is likely to affect individuals on migration, although its effects are strongest in the winter and therefore might not be relevant to very late migrating individuals, such as young birds. SOI has global influence, but is most likely to affect birds in the wintering area and we therefore restricted this variable to the winter months.
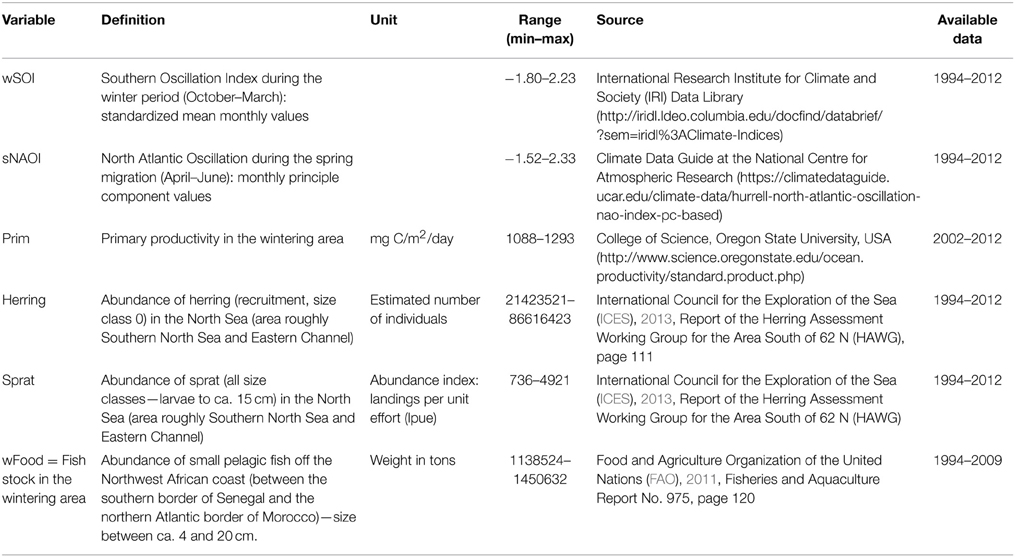
Table 1. Covariates used in statistical analyses: abbreviation, definition, source and available timeframe.
Food abundance in the breeding area and on the final part of migration in the English Channel and North Sea was represented by the most common prey species herring and sprat. These estimates were based on fishery data corrected for fishing effort (ICES, 2013). Fish stock in the wintering habitat was represented by small pelagic fish of various species in consumable size for common terns, including the the most common prey of common terns, sardines Sardina pilchardus; Sardinella sp. and anchovies Engraulis encrasicolus (cf. Dunn and Mead, 1982; Becker and Ludwigs, 2004), but also horse mackerel Trachurus trachurus, t. trecae, Caranx rhonchus and chub mackerel Scomber japonicus. In this case, fishery data were not corrected for fishing effort (FAO, 2011) and therefore this variable must be used with caution. As another indicator of food abundance in the wintering area we included marine primary productivity. Although the relationship between primary productivity and the presence of top-predators is not always straightforward (Grémillet et al., 2008) we used very wide ranging temporal and spatial constraints. So even if a spatial or temporal mismatch were to occur, the average values used in this study are an indication of a year of high or low food abundance.
Statistics
All arrival date and mass variables per age group (Table 2), as well as all covariates were normally distributed (Kolmogorov-Smirnov test: p > 0.05, N = 11–19 years). We tested the environmental covariates for inter-correlation using Pearson's correlation, based on which we eliminated the variable NAOI during autumn migration, as it correlated with winter SOI. None of the other environmental covariates were correlated (p > 0.05, R2 < 0.353).
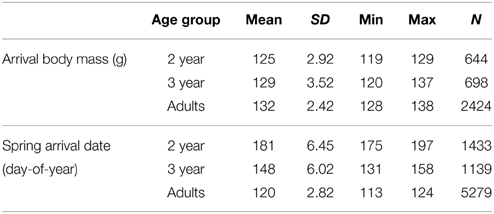
Table 2. Arrival date and body mass, including mean, standard deviation, range per age group (2-year-olds, 3-year-olds and adults) and sample size.
To test for a relationship between arrival date and mass, we performed an additive cross-classified random effects model with normally distributed errors and a Markov chain Monte Carlo estimation algorithm with 100,000 iterations (Browne et al., 2007). Arrival mass (N = 3663 cases from 1138 individuals) was the dependent variable, with age group (ages 2 years, 3 years and adults of 4 years and older; age-group 2 years was the reference group) and sex as fixed factors, as well as arrival date as covariate. Cross-classified random effects were year and bird-ID, which accounted for inter-annual and within-individual variation. These random effects remained in the model irrespective of their significance.
In order to test for year-, age- and sex-effects in arrival date and mass, we used additive cross-classified random effects models in which arrival date (N = 7576 cases from 1607 individuals) and arrival mass (N = 3663 cases from 1138 individuals) were dependent variables with age group and sex as fixed factors as well as year as covariate to test for a trend in time. As above, the cross-classified random effects year and bird-ID accounted for inter-annual and within-individual variation, respectively, and remained in the model irrespective of their significance.
We calculated the effects of environmental covariates, as well as age group and sex on arrival date/arrival mass using additive cross-classified random effect models, as described above. Since birds of different age migrate at different times and might therefore be influenced by different environmental influences, we also included an interaction of each environmental covariate with age group. Full models were simplified by backward stepwise removal of non-significant covariates and interactions. Significance (p < 0.05, two-tailed) was assessed using the Wald statistic, which approximates a χ2 distribution. Statistics were done using MLwiN 2.26 (Rasbah et al., 2012). Please note that a negative relationship between arrival date and an environmental covariate indicates an advanced arrival, while a positive relationship indicates a delayed arrival. Any relationships between arrival date, arrival mass and environmental factors found in this study are not necessarily causal and since we considered only a subsection of possible environmental covariates, there might be other relevant factors not included here. However, reliable data on potentially relevant environmental covariates are scarce and not always readily available.
In fact, not all covariates regarded here were available for the full study period, 1994–2012. The entire set of covariates was available between 2002 and 2009. In order to make best use of the extensive longitudinal data-set available, we ran the analyses for four subsets of data: firstly including those covariates that were available for the entire time period (1994–2012: herring, sprat, NAOI, SOI—Tables 3, 4), then three reduced time periods (2002–2009: all covariates; 1994–2009: herring, sprat, NAOI, SOI, fish stock in the wintering area; 2002–2012: herring, sprat, NAOI, SOI, primary productivity—Supplementary Tables 1–6). Out of the covariates, only fish stock in the wintering area showed a trend over time (R = 0.914, p > 0.001, N = 16 years), possibly due to continuously increasing fishing effort.
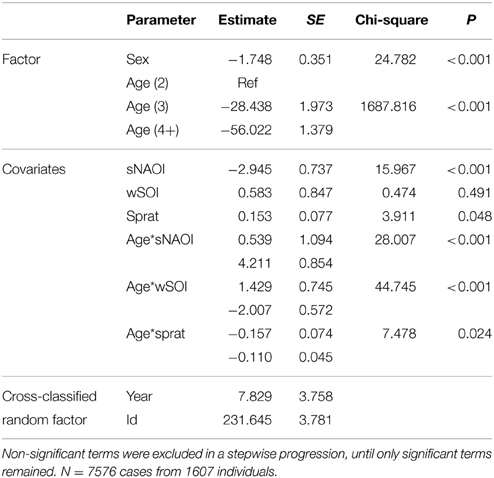
Table 3. Minimal adequate additive cross-classified random effect model describing significant effects of age, sex and environmental covariates on arrival date of common terns (1994–2012).
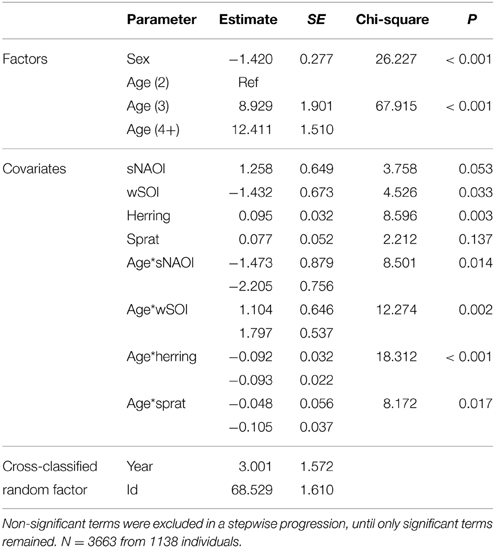
Table 4. Minimal adequate additive cross-classified random effect model describing significant effects of age, sex and environmental covariates on arrival mass of common terns between 1994 and 2012.
Results
Arrival Date
There was no apparent long-term trend for arrival date in any age group (2 year: R2 = 0.008, 3 year: R2 = 0.009, adults: R2 = 0.003; p > 0.05; Figure 2). Mean arrival date (Table 2) per year was not correlated between age groups (adults vs. 3-year-olds: Pearson's R = 0.155, p = 0.526, N = 19 years; adults vs. 2-year-olds: R = −0.111, p = 0.652, N = 19; 2-year-olds vs. 3-year-olds: R = −0.063, p = 0.797, N = 19). Also, incidents of very early or very late arrival were not coordinated between age groups (Supplementary Table 7).
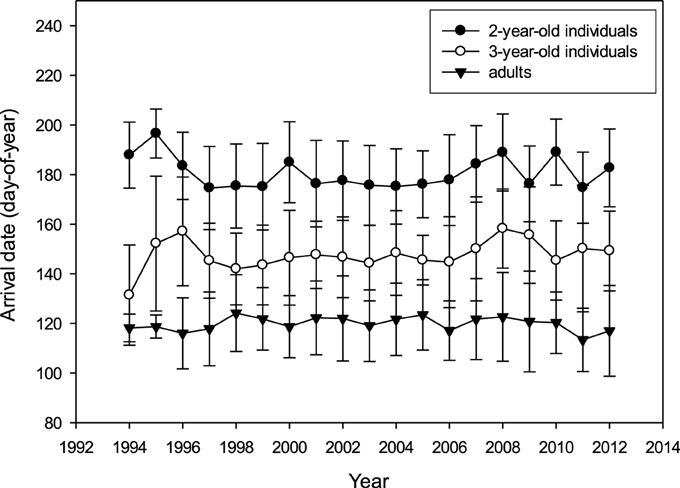
Figure 2. Arrival date (day-of-year: mean ± SD; raw data) at the colony for 2-year-olds, 3-year-olds and adults between 1994 and 2012 (N2y = 1433, N3y = 1139, Nad = 5279).
Accounting for the cross-classified random effects of year and bird-ID (year: estimate = 6.648; SE = 2.877; ID: estimate = 234.452; SE = 3.808), when testing for year-, age- and sex-effects on arrival date there was a significant difference between age groups (2 year: reference; 3 year: estimate = −31.581; SE = 0.658; adults: estimate = −60.201; SE = 0.495; χ 2 = 15782.214; p < 0.001) and between sexes (females compared to males: estimate = −1.740; SE = 0.353; χ 2 = 24.289; p < 0.001), but the model confirmed the absence of a time trend (covariate year: estimate = 0.106; SE = 0.106; χ 2 = 1.000; p = 0.317).
Arrival Mass
There was no apparent long-term trend for mean arrival mass in any age group (R2 < 0.001; p > 0.05; Figure 3). Mean arrival mass per year was not correlated between age groups (adults vs. 3-year-olds: Pearson's R = 0.201, p = 0.409, N = 19 years; adults vs. 2-year-olds: R = −0.123, p = 0.626, N = 19; 2-year-olds vs. 3-year-olds: R = 0.128, p = 0.611, N = 19). Very high or very low arrival mass did not occur in the same years for different age groups (Supplementary Table 7).
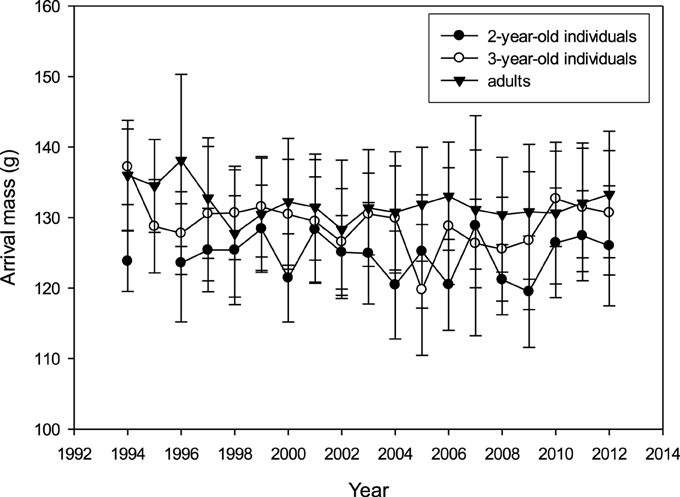
Figure 3. Mass on arrival (mean over first 3 days of colony attendance ± SD; raw data) for 2-year-olds, 3-year-olds and adults between 1994 and 2012 (N2y = 644, N3y = 698, Nad = 2424).
Accounting for the cross-classified random effects of year and bird-ID (year: estimate = 2.136; SE = 1.047; ID: estimate = 69.077; SE = 1.618), when testing for year-, age- and sex-effects on arrival mass there was a significant difference between age groups (2 year: reference; 3 year: estimate = 4.277; SE = 0.502; adults: estimate = 6.416; SE = 0.406; χ 2 = 255.848; p < 0.001) and between sexes (females compared to males: estimate = −1.387; SE = 0.277; χ 2 = 25.056; p < 0.001), but there was no time trend (covariate year: estimate = −0.087; SE = 0.071; χ 2 = 1.508; p = 0.219).
Relationship between Arrival Date and Mass
We found a significant negative correlation between arrival date and mass on arrival (covariate arrival date: estimate = −0.107; SE = 0.006; χ 2 = 360.947; p < 0.001). When accounting for arrival date, the significant but small age group differences in arrival mass between the sexes remained (estimate = −1.666; SE = 0.274; χ 2 = 37.064; p < 0.001), while age effects disappeared (2 year: ref; 3 year: estimate = 0.831; SE = 0.604; adult: estimate = 0.262; SE = 0.734; χ 2 = 3.138; p = 0.208). Cross-classified random factors were year and bird ID (year: estimate = 1.809; SE = 0.915; ID: estimate = 67.321; SE = 1.582). Incidents of very early (or late) arrival date were in some cases coordinated with very high (or low) arrival mass between age groups (Supplementary Table 7).
Environmental Covariates: Effects on Arrival Date
A model with arrival date as dependent variable, age group and sex as factors, year and bird ID as additive cross-classified random factors as well as all environmental factors as covariates (time period 2002–2009) revealed that there were significant differences between sexes, as well as significant interactions of age group with winter SOI and sprat. These interactions showed a positive relationship between arrival date and SOI in 2- and 3-year-olds (i.e., they delayed arrival with higher SOI), while there was no relationship in older birds. In relation to sprat, 2-year-olds advanced arrival with higher sprat abundance, while 3-year-olds and adults delayed arrival. Females consistently arrived between 1 and 2 days earlier than males.
The equivalent model over the period 1994–2012 (including herring, sprat, sNAOI, wSOI; Table 3) revealed similar interactions of age groups with winter SOI and sprat, but also an additional interaction of age with spring NAOI (Table 3, Figure 4A). Here, interactions indicated a negative relationship between arrival date and spring NAOI in 2- and 3-year-olds (they arrived 11 and 9 days earlier over the entire measured range of spring NAOI), while in adults arrival was delayed by 5 days in relation to NAOI. In response to winter SOI, 2- and 3-year-olds delayed arrival (by 2 and 8 days, respectively), as in the short model, while adults now exhibited advanced arrival date with SOI (by 6 days). The relationship with sprat was now positive in 2-year-olds (corresponding to a 6 day delay), while adults still showed a slight positive relationship (2 day delay) and 3-year-olds showed no response. Females consistently arrived between 1 and 2 days earlier than males.
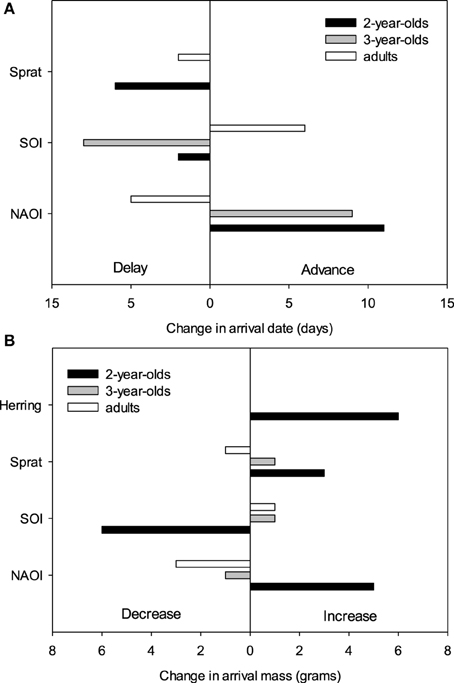
Figure 4. Predicted effects of environmental factors on the change in (A) arrival date (in days, Table 3) and (B) arrival mass (in grams, Table 4) in three age groups (2-year-olds, 3-year-olds and adults) over the observed range of the respective covariates between 1994 and 2012.
Environmental Covariates: Effects on Arrival Mass
Analysis of arrival mass in the same framework (time period 2002–2009) again revealed significant age and sex effects. Out of the environmental covariates, only winter SOI was negatively correlated with arrival mass and in interaction with age group (Supplementary Table 4). The interaction indicated an increase in arrival mass with higher SOI in 2- and 3-year-olds, while adults showed no relationship. Males consistently arrived 2–3 g heavier than females.
The equivalent model over the period 1994–2012 also included the age-SOI interaction, but additionally showed significant age-interactions with spring NAOI, herring and sprat (Table 4, Figure 4B). In the interaction with SOI the response in arrival mass was contrary to the model comprising the short time-span: in 2-year-olds there was a negative relationship (corresponding to a mass decrease of 6 g over the entire measured range of winter SOI), while in 3-year-olds and adults there was a very small increase (1 g). A positive relationship was found between NAOI and arrival mass in 2-year-olds (corresponding to a 5 g increase in arrival mass), while there was a weak negative relationship in 3-year-olds (1 g decrease) and adults (3 g decrease). The interaction with herring was strong in 2-year-olds, who showed an increase in arrival mass with higher herring abundance by 6 g over the entire observed range of herring abundance; 3-year-olds and adults exhibited no response. Sprat showed a weaker positive relationship with arrival mass in 2-year-olds (corresponding to a 3 g increase), with 3-year-olds and adults responding very little (a 1 g in- and decrease, respectively). Between 1994 and 2012, males consistently arrived 1–2 g heavier than females.
Discussion
Our analyses revealed strong variation in both arrival date and body mass upon arrival in a small and long-lived piscivorous bird species, the common tern. Surprisingly and in contrast to findings in other species (see refs below), however, there was no long-term trend in either trait. Instead, arrival date was linked with various environmental covariates, with the strength and direction of relationships depending on age. Carry-over effects from the wintering area seemed particularly relevant to arrival date: SOI significantly predicted arrival in all age groups. Conditions during migration and in the breeding habitat, as represented by NAOI and sprat abundance, also appeared influential. The fact that there was no correlated annual fluctuation between age groups and often contrary responses to the same environmental covariates indicates that different age groups experience differing environmental constraints during their annual cycle, which might have a compensatory effect against environmental stochasticity and thereby help maintain population stability. Arrival body mass at the colony was affected by environmental covariates only in the youngest age group on their first spring migration. These 2-year-olds' arrival mass showed a correlation with winter SOI and spring NAOI and was also related to sprat and herring abundance, indicating effects during the latter part of migration.
Arrival Date and Body Mass
Arrival date advances strongly with age (Dittmann and Becker, 2003; Becker et al., 2008a; Zhang et al., 2015a) and experience (Ludwigs and Becker, 2002), as does arrival mass, to a certain extent (Limmer and Becker, 2007). On average, 2-year-olds arrive between 40 and 60 days after adult birds (Becker et al., 2008a; Arnaud et al., 2013). They either need far more time for their first spring migration than adults and 3-year-olds, or they depart from the wintering area later, or both (Sergio et al., 2014). Since 2-year-old individuals are mostly prospectors and very rarely breed they might not be under great pressure to arrive early in the season (Ludwigs and Becker, 2002; Szostek and Becker, 2012). Similarly, 3-year-old terns arrive on average between 20 and 40 days later than adults. Although they gained some experience from their first migration they still either depart later or need more time to migrate than experienced adults, or both (cf. Sergio et al., 2014). Contrary to some earlier studies (Dittmann and Becker, 2003; Becker et al., 2008a) we found that females arrived marginally earlier than males (1–2 days). Males arrived marginally heavier (1–3 g) than females, which is consistent with findings that males are generally slightly larger than females (e.g., Nisbet et al., 2007). Although differences were small, they appeared consistently and were significant in all models.
With regard to inter-annual variation and long-term trends, results for arrival date and body mass were very coherent. Although a distinct age-distribution was apparent in arrival date and to a lesser extent in arrival mass, and variation was strong both within and between years, there was no long-term trend in either variable (Figures 2, 3, also cf. Ezard et al., 2007). In other bird species in the northern hemisphere, however, an advancement in arrival or laying date due to climate change was apparent (e.g., Hüppop and Hüppop, 2003; Crick, 2004; Visser and Both, 2005; Parmesan, 2006), including an advancement of breeding date in relation to spring temperature and NAOI in the Arctic tern Sterna paradisaea (Møller et al., 2006). In British terns (Arctic, common, and sandwich Sterna sandvicensis) an advancement in arrival and laying date was found (Wanless et al., 2009), while in the common terns of the study colony, laying date was unexpectedly found to become delayed over time (Ezard et al., 2007). It is possible that arrival and laying date are influenced by different environmental factors or that cues affecting departure from the wintering area are not linked with those in the breeding area (Both and Visser, 2001; Végvári et al., 2010). This is consistent with the lack of correlations we found between environmental covariates in the wintering and breeding area. Prey fish become increasingly available in the breeding area around early April when water temperatures start to increase, triggering the migration of juvenile herring and sprat into the coastal waters of the Wadden Sea (Dänhardt and Becker, 2014). If an increase in temperatures resulted in earlier herring and sprat availability, it would be an advantage for adult terns to advance their arrival and thereby extend their breeding season.
Arrival date and mass were not correlated between age groups, so there were no “bad” or “good” years over the entire age spectrum, only for the distinct age groups. This indicates that different intrinsic or extrinsic factors might be affecting each age group or that the same factor might change over time within the year and thus might affect the age groups differently as they migrate at different times. Stronger temporal variation in arrival date of 2- and 3-year-olds indicates stronger susceptibility to environmental factors in these younger age groups (Table 2; Favero and Becker, 2006; also for survival: Szostek and Becker, 2012). These age-related differences can be attributed to experience to some extent, but since arrival date is in part a heritable trait and early arrival improves reproductive success, this variation might also represent a target for selection and the lower variability in adults could be due to canalization (Arnaud et al., 2013). Furthermore, variation in plasticity of traits such as arrival date could also be heritable and selected for in interaction with environmental influences (Brommer et al., 2008).
Arrival date and mass were negatively correlated. Generally, the later arriving individuals tend to be the younger ones that find it harder to maintain their body mass in stressful circumstances, such as migration (cf. Dittmann and Becker, 2003; Ezard et al., 2007; Limmer and Becker, 2007). However, arrival mass still decreased with later arrival date when corrected for age group, individual and annual variation. This indicates that an early arrival date is not achieved at a cost to body condition, but is rather an indicator for individual quality (cf. Becker et al., 2008a; Zhang et al., 2015a). In this context, age was not a significant predictor of arrival mass any longer, as this general rule seems to be true for all age groups: Earlier birds are “better” individuals that can maintain a higher body mass.
Environmental Covariates: Effects on Arrival Date
There were strong age-related differences in the relationships between environmental covariates and arrival date. Generally, 2-year-olds and adults often reacted in opposite ways to the same environmental covariate, whereas 3-year-olds responded alternately with their older or younger counterparts or showed no response (Figure 4A). Considering the different time periods that the age groups migrate in and the differing pressures they are under, this is not unexpected.
In the breeding area, sprat, but not herring, was associated with a delay of 2-year-old arrival date by 6 days over the entire measured range of sprat abundance. Sprat in the North Sea (= breeding area) was available to terns on the latter part of their migratory journey, so high abundance and availability of their main prey items might cause them to spend more time foraging during and after migration before visiting the breeding colony, resulting in a later arrival. As 2-year-olds might not be under strong pressure to breed, such a behavioral strategy might give them the chance to improve their body condition after their first spring migration. Furthermore, an effect of NAOI on the advancement of arrival date by 11 days over the total observed range of NAOI was also apparent in 2-year-olds (see Figure 4A, Table 3). During low NAOI years strong wind systems can be suppressed (Hurrell et al., 2003), which may help inexperienced birds migrate faster. Additionally, the NAOI influences food abundance (Attrill and Power, 2002), although the negative relationship indicates an earlier arrival under “worse” feeding conditions. This is consistent with the finding that 2-year-old common terns delay arrival in response to higher sprat abundance. In combination, these findings suggest that young birds engage in a trade-off: when feeding conditions are favorable, they accept a later arrival in exchange for an improved body condition, while they might speed up their migration when food availability is poor. Spring arrival date has been linked to the NAOI in several passerine and non-passerine migrants (e.g., Hüppop and Hüppop, 2003; Žalakevičius et al., 2009), as has timing of breeding in black-legged kittiwake (Rissa tridactyla) and common guillemot (Uria aalge: Frederiksen et al., 2004). The relationship with SOI was very slight in 2-year-olds: it resulted in a 2 day delay with increasing SOI.
The relationship of 3-year-old birds' arrival date with environmental covariates was similar to that of 2-year-olds in direction, but the relative impact was mostly different. Three-year-olds showed an advancement of arrival with rising NAOI of 9 days, which was similar to their younger conspecifics and might well be due to similar underlying mechanisms. Their response to winter SOI, however, was much stronger, resulting in an 8 day delay over the total measured range of this global climate index. The strong delay of arrival date with winter SOI indicates that environmental conditions in the wintering area were an important driver of a fast and/or early migration in terms of weather conditions and possibly food abundance. This would have a lasting carry-over effect on the breeding career of a 3-year-old pre-breeder, as an early arrival improves the chances for a first-time breeding attempt (Ludwigs and Becker, 2002; Becker et al., 2008a). Contrary to younger birds, there was no relationship with sprat abundance, which could be explained by the older birds' improved foraging efficiency, or by the different time of arrival, when other food sources might be more relevant.
In adult birds, relationships between arrival date and environmental covariates were mostly contrary to those in the younger age groups. Generally, we would expect the older and more experienced birds to be less dependent on environmental conditions than their younger and less experienced conspecifics, as they showed far less inter-annual variability in arrival date and body mass, as well as in their vital rates (Ezard et al., 2006; Szostek and Becker, 2012). However, since the sample size was very high for this group it is likely that even correlations with small effect size became significant in these analyses. In any case, the covariates with the strongest effect were related to the global climate indices: Adult birds advanced their arrival date by up to 6 days over the entire measured range of winter SOI in accordance with the tendencies found by Favero and Becker (2006). Similarly, relationships between arrival date and SOI have been reported from the southern hemisphere, although there they appeared in conjunction with a delay in arrival and laying date (Barbraud and Weimerskirch, 2006), or in concurrence with El Niño events (Kalmbach et al., 2001). A tropical roseate tern Sterna dougallii population showed delayed breeding in years of high multivariate ENSO, a climate index related to the SOI, but the effects were also related to local sea surface temperature (Ramos et al., 2002). A significant correlation was also found in adult birds with spring NAOI, which resulted in a delay of up to 5 days, indicating an earlier arrival when weather conditions (Hurrell et al., 2003) and food abundance (Attrill and Power, 2002) are favorable. A very slight delay of 2 days could be attributed to sprat abundance in the breeding area, although such a small delay may not have strong implications for reproductive success or survival. Although arrival date was shown to be delayed in 2-year-old and adult terns in response to prey fish abundance, it is possible that despite a slightly later arrival date, the increase in body condition resulting from increased foraging success might advance laying, as shown in common terns by Wendeln (1997) and Dänhardt and Becker (2014). Diamond and Devlin (2003) found an impact of herring stock in the breeding area on laying dates of puffins Fratercula arctica, yet not on Arctic terns Sterna paradisaea or common terns in the same habitat.
That birds of different age groups migrate at different times of the year and are under varying amounts of pressure to arrive in the breeding area early is not a new idea, but we have shown here that the three age groups are affected differently by environmental constraints in their year-round habitats. While 2-year-olds, as inexperienced hunters (Dunn, 1972 for sandwich terns), are more strongly dependent on food abundance and weather conditions during migration, 3-year-olds show carry-over effects from the wintering habitat and adults seem less strongly affected overall. There is no clear season where environmental conditions appear most limiting to arrival date, this trait seems to be sensitive to conditions during migration as well as being subject to carry-over effects from the wintering area.
Environmental Covariates: Effects on Arrival Mass
The findings concerning arrival mass were mostly consistent with those relating to arrival date. However, differences between age groups were even more strongly pronounced. While 2-year-olds were strongly affected by multiple factors, the older age groups of 3-year-old and adult birds showed no strong response in arrival mass to any environmental covariate (Figure 4B). There was a slight decrease in adult arrival mass in relation to NAOI, but it resulted in only a 3 g change over the entire NAOI range, which birds would be able to recover from quickly during the pre-courtship period (Frank and Becker, 1992; Wendeln and Becker, 1996).
As 2-year-olds are likely to be less proficient foragers (Dunn, 1972) and are also inexperienced as migrants, it is not unexpected that they would show the greatest response in their body condition on arrival. We found a strong increase in response to higher herring abundance in the breeding area of 6 g and a weak increase of 3 g in response to higher sprat abundance. Since clupeoids and especially herring are among the most common and nutritious food sources for common terns in the breeding area (Massias and Becker, 1990; Frank, 1992; Wendeln, 1997), it makes sense that body mass would vary according to the abundance of herring (Wendeln and Becker, 1996). Since herring is available on the last leg of the migratory journey, these results indicate that terns probably feed continuously during migration or that their body condition quickly adapts to the wealth or dearth of food in the breeding area upon arrival (for daily mass changes see Frank and Becker, 1992). In addition, there was an equally strong relationship with the climate indices, which resulted in an increase of 5 g in relation to spring NAOI. This is most likely due to the NAOI affecting prey, other than herring and sprat, causing better feeding conditions during periods of low NAOI (Attrill and Power, 2002). Furthermore, we found a 6 g decrease in arrival mass in response to winter SOI. The most limiting conditions for arrival mass seem to appear during the migration period and the early breeding season, and yet arrival mass is also subject to strong carry-over effects from the wintering area in the youngest age group of common terns.
Concluding Remarks
Our findings underline the importance of age in the study of phenological traits including those related to migration (cf. Ezard et al., 2007). The strong advancement of arrival with age combined with an improvement of body condition reflects the process of canalization in the timing of arrival along with a reduction in variance with age (Arnaud et al., 2013). Yet, even when accounting for age, no long-term trends across the 19 years of the study were evident in either trait. For arrival date in spring this result is in contrast with many other, mainly passerine, bird species, which advanced their arrival over the past decades (refs see above).
Instead, we found effects of environmental factors on arrival date and mass, operating both during the wintering season and spring migration. Most interesting here was our finding that age groups responded very differently to variation in environmental conditions. Still, the relationship between arrival date and environmental factors remains a complicated one. As a long-distance migrant, the common tern is dependent on conditions in two distinct habitats connected by the migration route (Figure 1). Relevant environmental factors are not necessarily synchronous (we found them not to be correlated) and might affect the terns in opposing ways in their wintering area and on migration, or their effects might cancel each other out.
Conflict of Interest Statement
The Reviewer Eric Stienen declares that, despite having collaborated in the past with the author Peter H. Becker, the review process was handled objectively and no conflict of interest exists. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the many field-assistants, technicians, students and volunteers that have contributed to the Banter See project over the years. Thanks to Ad Corten and Andreas Dänhardt for helping us obtain and interpret the relevant fishery data. Two reviewers contributed valuable comments to an earlier version of the manuscript. The project was funded by the German Research Foundation (DFG, Be 916-9).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fevo.2015.00042/abstract
References
Arnaud, C. M., Becker, P. H., Dobson, F. S., and Charmantier, A. (2013). Canalization of phenology in common terns: genetic and phenotypic variations in spring arrival date. Behav. Ecol. 24, 683–690. doi: 10.1093/beheco/ars214
Attrill, M. J., and Power, M. (2002). Climatic influence on a marine fish assemblage. Nature 417, 275–278. doi: 10.1038/417275a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barbraud, C., and Weimerskirch, H. (2006). Antarctic birds breed later in response to climate change. Proc. Natl. Acad. Sci. U.S.A. 103, 6248–6251. doi: 10.1073/pnas.0510397103
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Becker, P. H., Dittmann, T., Ludwigs, J.-D., Limmer, B., Ludwig, S. C., Bauch, C., et al. (2008a). Timing of initial arrival at the breeding site predicts age at first reproduction in a long-lived migratory bird. Proc. Natl. Acad. Sci. U.S.A. 105, 12349–12352. doi: 10.1073/pnas.0804179105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Becker, P. H., Ezard, T. H. G., Ludwigs, J.-D., Sauer-Gürth, H., and Wink, M. (2008b). Population sex ratio shift from fledging to recruitment: consequences for demography in a philopatric seabird. Oikos 117, 60–68. doi: 10.1111/j.2007.0030-1299.16287.x
Becker, P. H., Finck, P., and Anlauf, A. (1985). Rainfall preceding egg-laying—a factor of breeding success in Common Terns (Sterna hirundo). Oecologia 65, 431–436. doi: 10.1007/BF00378919
Becker, P. H., and Specht, R. (1991). Body mass fluctuations and mortality in Common Tern Sterna hirundo chicks dependent on weather and tide in the Wadden Sea. Ardea 79, 45–56.
Becker, P. H., and Wendeln, H. (1997). A new application for transponders in population ecology of the common tern. Condor 99, 534–538. doi: 10.2307/1369963
Bêty, J., Giroux, J.-F., and Gauthier, G. (2004). Individual variation in timing of migration: causes and reproductive consequences in greater snow geese (Anser caerulescens atlanticus). Behav. Ecol. Sociobiol. 57, 1–8. doi: 10.1007/s00265-004-0840-3
Both, C., and Visser, M. E. (2001). Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298. doi: 10.1038/35077063
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brommer, J. E., Rattiste, K., and Wilson, A. J. (2008). Exploring plasticity in the wild: laying date-temperature reaction norms in the common gull Larus canus. Proc. Biol. Sci. 275, 687–693. doi: 10.1098/rspb.2007.0951
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Browne, W. J., McCleery, R. H., and Sheldon, B. C. (2007). Using cross-classified multivariate mixed response models with application to life history traits in great tits (Parus major). Stat. Model. 7, 1–23. doi: 10.1177/1471082X0700700301
Chastel, O., Weimerskirch, H., and Jouventin, P. (1995a). Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76, 2240–2246. doi: 10.2307/1941698
Chastel, O., Weimerskirch, H., and Jouventin, P. (1995b). Influence of body condition on reproductive decision and reproductive success in the blue petrel. Auk 112, 964–972. doi: 10.2307/4089027
Crick, H. Q. P. (2004). The impact of climate change on birds. Ibis (Lond. 1859). 146, 48–56. doi: 10.1111/j.1474-919X.2004.00327.x
Dänhardt, A., and Becker, P. H. (2014). Saisonale Abundanzmuster pelagischer Schwarmfische und die Brutphänologie von Flussseeschwalben Sterna hirundo. Corax. 22, 71–77.
Daunt, F., Afanasyev, V., Silk, J., and Wanless, S. (2006). Extrinsic and intrinsic determinants of winter foraging and breeding phenology in a temperate seabird. Behav. Ecol. Sociobiol. 59, 381–388. doi: 10.1007/s00265-005-0061-4
Descamps, S., Bêty, J., Love, O. P., and Gilchrist, H. G. (2011). Individual optimization of reproduction in a long-lived migratory bird: a test of the condition-dependent model of laying date and clutch size. Funct. Ecol. 25, 671–681. doi: 10.1111/j.1365-2435.2010.01824.x
Diamond, A. W., and Devlin, C. M. (2003). Seabirds as indicators of change in marine ecosystems: ecological monitoring on Machias Seal Island. Environ. Monit. Assess. 88, 153–175. doi: 10.1023/A:1025560805788
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dittmann, T., and Becker, P. H. (2003). Sex, age, experience and condition as factors affecting arrival date in prospecting common terns, Sterna hirundo. Anim. Behav. 65, 981–986. doi: 10.1006/anbe.2003.2128
Dittmann, T., Ezard, T. H. G., and Becker, P. H. (2007). Prospectors' colony attendance is sex-specific and increases future recruitment chances in a seabird. Behav. Processes 76, 198–205. doi: 10.1016/j.beproc.2007.05.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dunn, E. K. (1972). Effect of age on the fishing ability of Sandwich Terns Sterna sandvicensis. Ibis (Lond. 1859). 114, 360–366.
Dunn, E. K. (1973). Changes in fishing ability of terns associated with windspeed and sea surface conditions. Lett. Nat. 244, 520–521. doi: 10.1038/244520a0
Dunn, E. K., and Mead, C. (1982). Relationship between sardine fisheries and recovery rates of ringed terns in West Africa. Seabird Rep. 6, 98–104.
Ezard, T. H. G., Becker, P. H., and Coulson, T. (2006). The contributions of age and sex to variation in common tern population growth rate. J. Anim. Ecol. 75, 1379–1386. doi: 10.1111/j.1365-2656.2006.01162.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ezard, T. H. G., Becker, P. H., and Coulson, T. (2007). Correlations between age, phenotype, and individual contribution to population growth in common terns. Ecology 88, 2496–2504. doi: 10.1890/06-2020.1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
FAO. (2011). FAO Fisheries and Aquaculture Report No. 975. Report of the FAO Working Group on the Assessment of Small Pelagic Fish off Northwest Africa. Banjul, the Gambia.
Favero, M., and Becker, P. H. (2006). “Effects of the North Atlantic Oscillation and El Nino-Southern Oscillation on return rates, body mass and timing of migration of Common Terns Sterna hirundo breeding in Germany,” in Waterbirds Around the World, eds C. A. Galbraith, G. C. Boere, and D. A. Stroud (Edinburgh: The Stationary Office), 405–409.
Finney, S. K., Wanless, S., and Harris, M. P. (1999). The Effect of weather conditions on the feeding behaviour of a diving bird, the common guillemot uria aalge. J. Avian Biol. 30, 23–30. doi: 10.2307/3677239
Forchhammer, M. C., Post, E., and Stenseth, N. C. (1998). Breeding phenology and climate. Nature 391, 29–30. doi: 10.1038/34070
Frank, D. (1992). The influence of feeding conditions on food provisioning of chicks in common terns Sterna hirundo nesting in the German Wadden Sea. Ardea 80, 45–55.
Frank, D., and Becker, P. H. (1992). Body mass and nest reliefs in common terns Sterna hirundo exposed to different feeding conditions. Ardea 80, 57–69.
Frederiksen, M., Harris, M. P., Daunt, F., Rothery, P., and Wanless, S. (2004). Scale-dependent climate signals drive breeding phenology of three seabird species. Glob. Chang. Biol. 10, 1214–1221. doi: 10.1111/j.1529-8817.2003.00794.x
Frederiksen, M., Lebreton, J.-D., Pradel, R., Choquet, R., and Gimenez, O. (2014). Identifying links between vital rates and environment: a toolbox for the applied ecologist. J. Appl. Ecol. 51, 71–81. doi: 10.1111/1365-2664.12172
Grémillet, D., Lewis, S., Drapeau, L., van Der Lingen, C. D., Huggett, J. A., Coetzee, J. C., et al. (2008). Spatial match-mismatch in the Benguela upwelling zone: should we expect chlorophyll and sea-surface temperature to predict marine predator distributions? J. Appl. Ecol. 45, 610–621. doi: 10.1111/j.1365-2664.2007.01447.x
Hüppop, O., and Hüppop, K. (2003). North Atlantic Oscillation and timing of spring migration in birds. Proc. Biol. Sci. 270, 233–240. doi: 10.1098/rspb.2002.2236
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hurrell, J. W., and Deser, C. (2009). North Atlantic climate variability: the role of the North Atlantic Oscillation. J. Mar. Syst. 79, 231–244. doi: 10.1016/j.jmarsys.2009.11.002
Hurrell, J. W., Kushnir, Y., Ottersen, G., and Visbeck, M. (2003). An overview of the North Atlantic Oscillation. Geophys. Monogr. 134, 1–35. doi: 10.1029/134GM01
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hurrell, J. W., Kushnir, Y., and Visbeck, M. (2001). The North Atlantic Oscillation. Science 291, 603–605. doi: 10.1126/science.1058761
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
ICES. (2013). ICES HAWG Report 2013: Report of the Herring Assessment Working Group for the Area South of 62 N (HAWG) 12-21 March 2013 ICES Headquarters, Copenhagen International Council for the Exploration of the Sea. Copenhagen. ICES CM 2013/ACOM:06.
Kalmbach, E., Ramsay, S. C., Wendeln, H., and Becker, P. H. (2001). A study of neotropic cormorants in central Chile: possible effects of El Nino. Waterbirds 24, 345–351. doi: 10.2307/1522064
Kokko, H., and Johnstone, R. A. (1999). Social queuing in animal societies: a dynamic model of reproductive skew. R. Soc. B 266, 571–578. doi: 10.1098/rspb.1999.0674
Limmer, B., and Becker, P. H. (2007). The relative role of age and experience in determining variation in body mass during the early breeding career of the Common Tern (Sterna hirundo). Behav. Ecol. Sociobiol. 61, 1885–1896. doi: 10.1007/s00265-007-0429-8
Ludwigs, J.-D., and Becker, P. H. (2002). The hurdle of recruitment: influences of arrival date, colony experience and sex in the common tern Sterna hirundo. Ardea 90, 389–399.
Massias, A., and Becker, P. H. (1990). Nutritive value of food and growth in common tern sterna hirundo chicks. Ornis Scand. 21, 187–194. doi: 10.2307/3676778
McGregor, H. V., Dima, M., Fischer, H. W., and Mulitza, S. (2007). Rapid 20th-century increase in coastal upwelling off northwest Africa. Science 315, 637–639. doi: 10.1126/science.1134839
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Misund, O. A., Melle, W., and Fernö, A. (1997). Migration behaviour of norwegian spring spawning herring when entering the cold front in the Norwegian Sea. Sarsia 82, 107–112.
Misund, O. A., Vilhjálmsson, H. Í., Jákupsstovu, S. H., Røttingen, I., Belikov, S., Asthorsson, O., et al. (1998). Distribution, migration and abundance of norwegian spring spawning herring in relation to the temperature and zooplankton biomass in the Norwegian sea as recorded by coordinated surveys in spring and summer 1996. Sarsia 83, 117–127.
Møller, A. P., Flensted-Jensen, E., and Mardal, W. (2006). Rapidly advancing laying date in a seabird and the changing advantage of early reproduction. J. Anim. Ecol. 75, 657–665. doi: 10.1111/j.1365-2656.2006.01086.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Newton, I. (2006). Can conditions experienced during migration limit the population levels of birds? J. Ornithol. 147, 146–166. doi: 10.1007/s10336-006-0058-4
Nisbet, I. C. T., Bridge, E. S., Szczys, P., and Heidinger, B. J. (2007). Sexual dimorphism, female-female pairs, and test for assortative mating in common terns. Waterbirds 30, 169–179. doi: 10.1675/1524-4695(2007)30[169:SDFPAT]2.0.CO;2
Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100
Ramos, J. A., Maul, A. M., Ayrton, V., Bullock, I., Hunter, J., Bowler, J., et al. (2002). Influence of local and large-scale weather events and timing of breeding on tropical roseate tern reproductive parameters. Mar. Ecol. Prog. Ser. 243, 271–279. doi: 10.3354/meps243271
Rasbah, J., Steele, F., Browne, W., Prosser, B., and Goldstein, H. (2012). A User's Guide to MLwiN, Version 2.26. Bristol: Centre for Multilevel Modelling, University of Bristol.
Sandvik, H., Erikstad, K. E., Barrett, R. T., and Yoccoz, N. G. (2005). The effect of climate on adult survival in five species of North Atlantic seabirds. J. Anim. Ecol. 74, 817–831. doi: 10.1111/j.1365-2656.2005.00981.x
Schreiber, E. A. (2001). “Climate and weather effects on seabirds,” in Biology of Marine Birds, eds E. A. Schreiber and J. Burger (Boca Raton, FL: CRC Press), 179–215.
Sergio, F., Tanferna, A., De Stephanis, R., López Jiménez, L., Blas, J., Tavecchia, G., et al. (2014). Individual improvements and selective mortality shape lifelong migratory performance. Nature 515, 410–413. doi: 10.1038/nature13696
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stenseth, N. C., Mysterud, A., Ottersen, G., Hurrell, J. W., Chan, K.-S., and Lima, M. (2002). Ecological effects of climate fluctuations. Sci. Compass 297, 1292–1296. doi: 10.1126/science.1071281
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stenseth, N. C., Ottersen, G., Hurrell, J. W., Mysterud, A., Lima, M., Chan, K.-S., et al. (2003). Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc. R. Soc. B 270, 2087–2096. doi: 10.1098/rspb.2003.2415
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Szostek, K. L., and Becker, P. H. (2012). Terns in trouble: demographic consequences of low breeding success and recruitment on a common tern population in the German Wadden Sea. J. Ornithol. 153, 313–326. doi: 10.1007/s10336-011-0745-7
Szostek, K. L., and Becker, P. H. (2015). Survival and local recruitment are driven by environmental carry-over effects from the wintering area in a migratory seabird. Oecologia. doi: 10.1007/s00442-015-3298-2. (in press).
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trenberth, K. E., and Caron, J. M. (2000). The southern oscillation revisited: sea level pressures, surface temperatures, and precipitation. J. Clim. 13, 4358–4365.
Végvári, Z., Bókony, V., Barta, Z., and Kovács, G. (2010). Life history predicts advancement of avian spring migration in response to climate change. Glob. Chang. Biol. 16, 1–11. doi: 10.1111/j.1365-2486.2009.01876.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Verhulst, S., and Nilsson, J.-A. (2008). The timing of birds' breeding seasons: a review of experiments that manipulated timing of breeding. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 363, 399–410. doi: 10.1098/rstb.2007.2146
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Visser, M. E., and Both, C. (2005). Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. doi: 10.1098/rspb.2005.3356
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wanless, S., Frederiksen, M., Walton, J., and Harris, M. P. (2009). Long-term changes in breeding phenology at two seabird colonies in the western North Sea. Ibis (Lond. 1859) 151, 274–285. doi: 10.1111/j.1474-919X.2008.00906.x
Wendeln, H. (1997). Body mass of female common terns (Sterna hirundo) during courtship: relationships to male quality, egg mass, diet, laying date and age. Colon. Waterbirds 20, 235–243. doi: 10.2307/1521689
Wendeln, H., and Becker, P. H. (1996). Body mass change in breeding Common Terns Sterna hirundo. Bird Study 43, 85–95. doi: 10.1080/00063659609460998
Wendeln, H., and Becker, P. H. (1999). Effects of parental quality and effort on the reproduction of common terns. J. Anim. Ecol. 68, 205–214. doi: 10.1046/j.1365-2656.1999.00276.x
Žalakevičius, M., Bartkevičienė, G., Ivanauskas, F., and Nedzinskas, V. (2009). The Response of spring arrival dates of non-passerine migrants to climate change: a case study from Eastern Baltic. Acta Zool. Litu. 19, 155–171. doi: 10.2478/v10043-009-0029-0
Zhang, H., Vedder, O., Becker, P. H., and Bouwhuis, S. (2015a). Age-dependent trait variation: the relative contribution of within-individual change, selective appearance and disappearance in a long-lived seabird. J. Anim. Ecol. doi: 10.1111/1365-2656.12321. [Epub ahead of print].
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: age-related changes, arrival date, body mass, environmental impact, long-distance migrant, timing of migration, NAOI, SOI
Citation: Szostek KL, Bouwhuis S and Becker PH (2015) Are arrival date and body mass after spring migration influenced by large-scale environmental factors in a migratory seabird? Front. Ecol. Evol. 3:42. doi: 10.3389/fevo.2015.00042
Received: 31 October 2014; Accepted: 06 April 2015;
Published: 29 April 2015.
Edited by:
Morten Frederiksen, Aarhus University, DenmarkReviewed by:
Morten Frederiksen, Aarhus University, DenmarkBørge Moe, Norwegian Institute for Nature Research, Norway
Eric Stienen, Instituut voor Natuur- en Bosonderzoek, Belgium
Copyright © 2015 Szostek, Bouwhuis and Becker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: K. Lesley Szostek, Institute of Avian Research “Vogelwarte Helgoland,” An der Vogelwarte 21, D-26386 Wilhelmshaven, Germany,bGVzbGV5LnN6b3N0ZWtAaWZ2LXZvZ2Vsd2FydGUuZGU=
 K. Lesley Szostek
K. Lesley Szostek Sandra Bouwhuis
Sandra Bouwhuis Peter H. Becker
Peter H. Becker