- 1Department of Biology, College of Staten Island/City University of New York, New York, NY, USA
- 2The Graduate Center, City University of New York, New York, NY, USA
Population level impacts upon seabirds from changing climate are increasingly evident, and include effects on phenology, migration, dispersal, annual survivorship, and reproduction. Most population data on seabirds derive from nesting colonies; documented climate impacts on winter ecology are scarce. We studied interannual variability in winter abundance of six species of alcids (Charadriiformes, Alcidae) from a 58-year time series of data collected in Massachusetts 1954–2011. We used counts of birds taken during fall and winter from coastal vantage points. Counts were made by amateur birders, but coverage was consistent in timing and location. We found significant association between winter abundance of all six species of alcids and climate, indexed by North Atlantic Oscillation (NAO), at two temporal scales: (1) significant linear trends at the 58-year scale of the time series; and (2) shorter term fluctuations corresponding to the 5–8 year periodicity of NAO. Thus, variation in winter abundance of all six species of alcids was significantly related to the combined short-term and longer-term components of variation in NAO. Two low-Arctic species (Atlantic Puffin and Black Guillemot) peaked during NAO positive years, while two high Arctic species (Dovekie and Thick-billed Murre) peaked during NAO negative years. For Common Murres and Razorbills, southward shifts in winter distribution have been accompanied by southward expansion of breeding range, and increase within the core of the range. The proximate mechanism governing these changes is unclear, but, as for most other species of seabirds whose distributions have changed with climate, seems likely to be through their prey.
Introduction
Fluctuating oceanic climate impacts population size (Montevecchi and Myers, 1997; Thompson and Ollason, 2001), survivorship (Sandvik et al., 2005), fecundity (Guinet et al., 1998; Durant et al., 2003, 2005; Wanless et al., 2007), phenology (Aebischer et al., 1990; Gjerdrum et al., 2003; Frederiksen et al., 2004), and both summer and winter distribution (Veit et al., 1997; Manne, 2013) of seabirds. Impacts of climate shifts on seabirds occur at multiple temporal scales, that can be loosely grouped into “short-term” (<1 year: Hunt et al., 1992; Veit et al., 1996), “medium term” (5–10 years) corresponding to fluctuations indexed by El Niño-Southern Oscillation (ENSO) and North Atlantic Oscillation (NAO), and long-term trends (>10 years) that may also reflect longer term fluctuations that our 50 year time series is less likely to resolve (Veit and Montevecchi, 2006). Since seabirds in general have long lifespans and produce relatively few young per year, they are adapted to tolerate short-term environmental disturbances (Veit and Montevecchi, 2006) and populations have recovered from such short-term catastrophes as El Niño events (Schreiber, 2002). Population responses to persistent, long-term changes are less well-documented but climate change on the scale of decades has caused substantial changes in the range and distribution of seabirds (Montevecchi and Myers, 1997; Veit and Montevecchi, 2006; Gaston and Woo, 2008; Nisbet et al., 2013).
Unambiguous, if complex, changes have occurred in the physical and biological characteristics of the North Atlantic Ocean during this century (Stenseth and Mysterud, 2002; Hurrell and Dickson, 2004; Stenseth et al., 2004), and these changes have had substantial biological impacts, even though ascribing such impacts unambiguously to climate is daunting (Hemery et al., 2008; Overholtz and Link, 2009). Difficult to disentangle from climate-induced impacts on seabirds are those resulting from changes in fish stocks due to commercial fisheries. For example, the spectacular collapse of northwestern Atlantic Cod stocks in the early 1990s, and the decline of the herring fishery in the late 1960s and early 1970s were almost certainly primarily the result of overfishing (Fogarty and Murawski, 1998). The collapse of these stocks has had broad-reaching impacts on other components of the ecosystem, including seabirds (Fogarty and Murawski, 1998; Montevecchi and Stenhouse, 2002). Other changes are not so easily attributed to any one particular cause. For example, the enormous increase of sand lance (Ammodytes spp.) during the 1970s in the Northwest Atlantic (Sherman et al., 1981) may have resulted from the disappearance of herring and other predators due to overfishing, but other factors may have been important as well. Many seabirds benefitted from the sand lance increase (Veit and Petersen, 1993; Nisbet et al., 2013), but whether any of these changes has a link to climate forcing is unknown, and it is therefore difficult to disentangle whether impacts on seabirds derive from climate, fisheries, a combination of the two, or some other factor.
Much of the previous work on this subject has focused on the northeast Atlantic, where changing climate is having a measurable effect upon seabirds, and it is thus highly likely that such effects have occurred in the Northwest Atlantic as well. There are, however, interesting differences in the direction of climate-related effects between the two sides of the Atlantic. For example abundance of the widespread copepod Calanus finmarchicus is negatively correlated with NAO in the eastern, but positively correlated with NAO in the western Atlantic (Conversi et al., 2001; Drinkwater et al., 2003), as is also SST and population changes in guillemots (Irons et al., 2008). There is no question that climate-related changes to the pelagic system are occurring, but how these may have impacted seabird abundance off the North American east coast is unknown.
NAO is a multivariate physical index used to characterize the overall climatological state of the North Atlantic (Hurrell and Dickson, 2004). The NAO is designed to reflect the distribution of sea level atmospheric pressure, but its values correlate strongly with predominant flows of winds and surface currents. The NAO has changed from a pattern of negative values during ~1930–1975 to one of predominantly positive values from 1975 to 2000 (Hurrell and Dickson, 2004; Irons et al., 2008). The more recent period of positive values reflects colder conditions in the Northwest Atlantic; conversely, negative NAO years are associated with warmer waters. Documented and statistically significant shifts in intensity and direction of NAO have occurred in recent years and, as shown below, these shifts have clearly impacted the biology of the North Atlantic (Hurrell and Dickson, 2004).
At a decadal scale, southward shifts in alcid abundance seem to match shifts in NAO (Figure 1). Veit and Guris (2008) found statistical links between Razorbill (Alca torda) and Dovekie (Alle alle) abundance and NAO in New England waters. Razorbills have been increasing steadily since the 1980s, with peak abundance occurring in NAO positive years, while Dovekies have increased dramatically since about 2005, after a general absence since about 1975. Abundance of Dovekies in New England has been more erratic, but also statistically related to NAO, with maximum numbers occurring in NAO-negative years (a pattern strongly supported by maximum abundance in 2010–2011) and thus opposite to the pattern shown by Razorbills. The proximate mechanism for the link between seabirds and NAO remains unclear, but the majority of seabird-climate links has proven to be indirect, and based on changes in seabird prey (Frederiksen et al., 2004; Durant et al., 2005).
Alcid Winter Range
Alcids are notoriously erratic in their dispersal southwards during winter (Nettleship and Birkhead, 1985; Gaston and Jones, 1998) and some species, especially Dovekies, are prone to southward irruptions, very likely in response to abrupt changes in prey availability (Gaston and Jones, 1998). Despite such variability during winter, long-term changes in abundance off the U.S. East Coast are apparent (Veit and Guris, 2008; Nisbet et al., 2013). As recent major shifts in oceanographic climate have also been described for the North Atlantic (Hurrell and Dickson, 2004; Regular et al., 2010), it behooves us to ask whether shifts in winter alcid distribution are related to changes in oceanographic climate. Innovations of this study include an extensive uninterrupted, 58 year time series on seabird abundance from Massachusetts and the fact that it documents the winter, non-breeding distribution of these birds. We therefore hypothesize that fluctuations in oceanic climate impact the variability of winter abundance and distribution of seabirds.
Methods
Massachusetts Time Series
In Massachusetts there are a number of coastal vantage points from which seabirds have been counted annually since the 1930s. The level of effort has been reasonably constant over the period 1954-present; while the number of observers in the field has increased, the number of sites has not, so that the number of hours devoted to counting is unlikely to have changed. Furthermore, both increases and decreases in abundance have occurred during the period we have analyzed, so changes in abundance cannot be simply the consequence of increased effort. Thus, we used this 58 year time series of bird abundance to ask whether changes over that time period in alcid abundance are at least in part related to changes in oceanic climate.
We extracted data on alcids from the publications Records of New England Birds (1954–1968), Bird Observer of Eastern Massachusetts (1972–1986), Bird Observer (1987-present), and North American Birds and its predecessors (1954-present). Gaps for the period 1969–1971 were filled by records maintained at the Massachusetts Audubon Society by Ruth P. Emery (unpublished database). Data in these publications were published as monthly or bimonthly summaries.
The data used in the analyses were estimates of maximum number present in any given winter. We considered that all counts on outer Cape Cod (~50 km long, mostly censused from Provincetown, Figure 2) could possibly involve the same birds, so we conservatively used the maximum number reported during a winter anywhere on outer Cape Cod as the maximum for that year. On the other hand, we assumed that birds counted at Cape Ann or Nantucket were separate from birds counted on outer Cape Cod, and tallied separately, for each winter, maxima from Cape Ann, Cape Cod, and Nantucket. Although the bird abundance data is limited in spatial scope, because of the enormous spatial scope of seabird foraging ranges, samples from a limited area are representative of the ocean basin as a whole (Veit et al., 1997). The species in this analysis collectively cover 35° of latitude, from Northern Greenland to Maine during the nesting season and an even larger area during winter. Thus, this dataset from Massachusetts is not only important for its length; it also samples a substantial portion of the North Atlantic avifauna.
Figure 2. Map of the study area showing the three main census locations: Cape Ann, Provincetown (outer Cape Cod), and Nantucket.
We used NAO index values averaged over December-March for the years in which we extracted data on birds. So birds recorded in November 2000–March 2001 were related to the NAO index for December 2000–March 2001 (Hurrell and Dickson, 2004, http://www.cgd.ucar.edu/cas/jhurrell/indices.html).
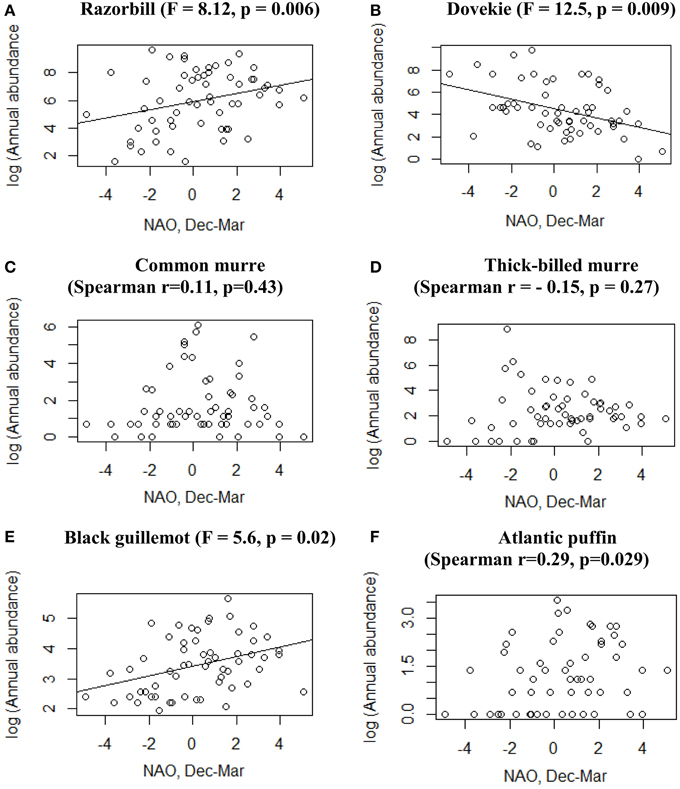
We log transformed all bird abundance data. For four species [Razorbill, Dovekie, Thick-billed Murre (Uria lomvia), and Black Guillemot (Cepphus grylle)] the transformed data did not differ significantly from normal (Kolmogorov–Smirnoff test, p > 0.1), and we used cross-correlation to identify the temporal scale for which correlation between bird abundance and NAO was highest. Maximum correlation was found at a lag of 0 years for all species except Razorbill for which NAO in the previous year (lagged NAO) gave a better fit (Razorbill numbers following NAO). For these four species we used linear regression to relate abundance in Massachusetts to NAO index in the same winter. For the other two species [Atlantic Puffin (Fratercula arctica), and Common Murre (Uria aalge)] the log-transformed data differed significantly (p < 0.05) from the normal distribution so we used Spearman rank correlation coefficients to test for a correlation between abundance and NAO.
To disentangle the possible population temporal autocorrelation from direct effects of NAO on winter abundance, we compared eight regression models for each species: a model with only a year term (assuming a Poisson distribution vs. a negative binomial distribution), models having a single NAO term (Poisson vs. negative binomial), models having both year and NAO (Poisson vs. negative binomial), and models with year, NAO and a lag term for the population size the previous year (Poisson vs. negative binomial). Among those eight models, the model with the lower Akaike Information Criterion (AIC), but only where the lower AIC differed from the next-higher AIC by more than two units (Burnham and Anderson, 2002), was a better fit to the data for that species. If the lower AIC occurred for a model incorporating NAO, then we concluded that even after time series and lagged population effects had been accounted for, NAO acted as a structuring effect for that species' abundance. For ease of visualization, we show graphs of the log-transformed responses (species abundances). All analyses were conducted using R (R Core Development Team, 2012).
Results
Five species (Razorbill, Common Murre, Thick-billed Murre, Black Guillemot, and Atlantic Puffin) increased significantly over the 58-year period (p < 0.05), and Dovekies decreased significantly over the same period (p = 0.019, Figure 3). A significant increasing linear trend is evident for NAO (Figure 3G). Thus, all six species of alcids were significantly related to a 58-year trend in NAO (Figure 3). At shorter timescales, five species were significantly correlated with NAO as revealed by the modeling (Table 1). For four of six species analyzed, the best model was one that included effects of year, NAO and lagged intraspecific abundance (Table 1); a fifth species' best model included only NAO, and the sixth species (Atlantic Puffin) was best modeled by year alone. Thus, the importance of both a long-term (58 year) trend and shorter term (5–8 year) oscillations in NAO significantly impacted winter abundance of five out of six alcid species in New England. For Razorbills, the best model was that which included year, lagged NAO and lagged abundance of Razorbills. In all cases, the chosen model was that assuming the negative binomial distribution; the AIC-values for the negative binomial models were all much lower than those for the Poisson models.
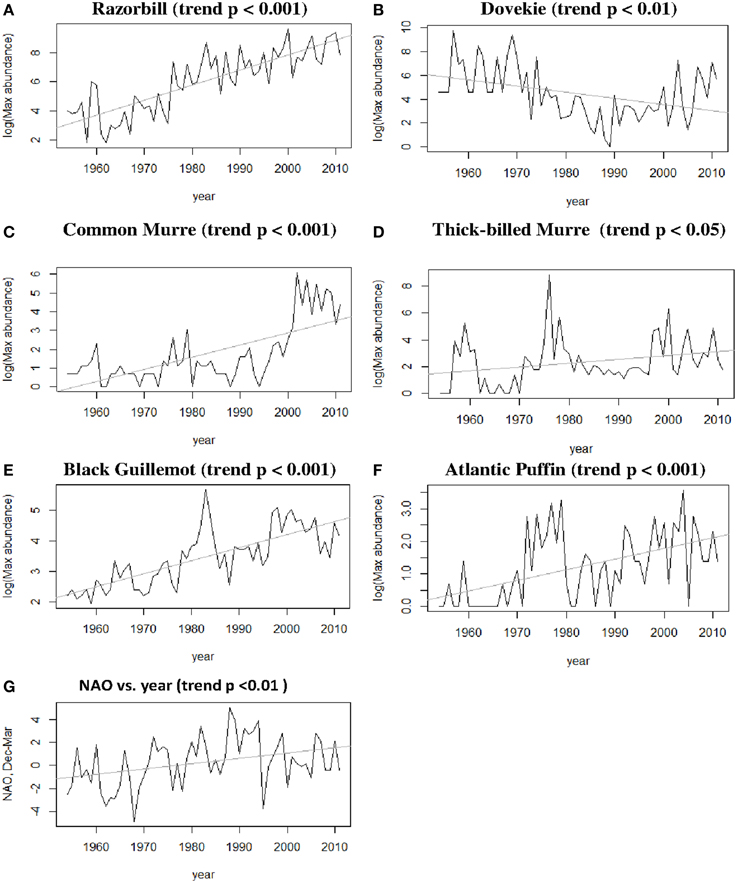
Figure 3. (A–F) Winter abundance (raw time series) of alcids in Massachusetts over the period 1955–2012; (G) NAO over time, showing both trend and periodic fluctuation.
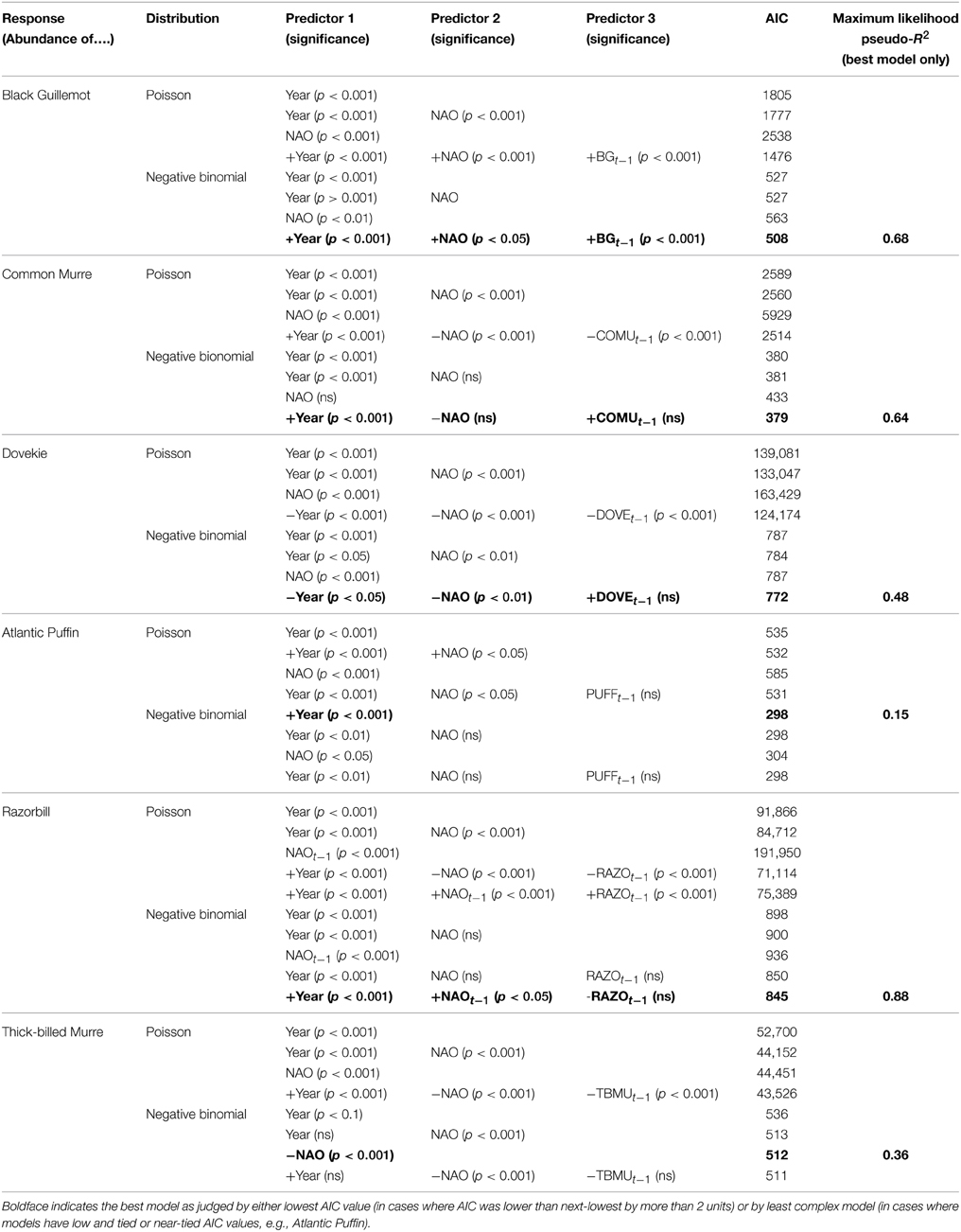
Table 1. Results of model selection for six alcid species wintering population numbers; sign of relationship with predictors given in best model.
Table 1 also shows the maximum likelihood pseudo-R2-values to give an idea of how much variation in alcid abundance is explained by the best models. Atlantic Puffin was the least well-modeled, with a pseudo-R2-value of 0.15, while Razorbill showed the highest pseudo-R2, of 0.88.
Residual analyses showed no clear pattern in the residuals for any of the best models. Since the NAO index itself has a positive trend over the timespan we studied (1954–2012, Hurrell and Dickson, 2004), our interpretation is that there are at least two temporal scales over which variation in ocean climate impact winter abundance of alcids off Massachusetts. Put another way, our model shows that variation in winter abundance of alcids off Massachusetts was significantly related to the combined shorter-term and longer-term components of variation in NAO.
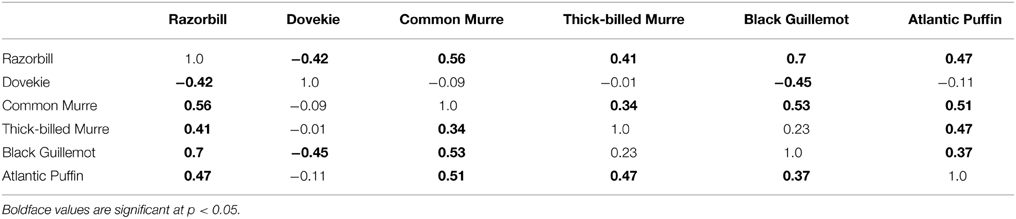
Black Guillemots and Razorbills occurred in high abundance with positive NAO index values, whereas Dovekies and Thick-billed Murres occurred in high abundance with negative NAO. Thus, peak numbers of high-arctic Dovekies and Thick-billed Murres tended to move south to Massachusetts in strongly negative-NAO winters. Common Murre and Atlantic Puffin show small but only borderline significant relationships to NAO on an annual scale. Therefore, response by these birds to NAO varied with the temporal scale at which they were analyzed. The differing response to NAO shown by the different species is reflected in the correlation of abundance among species: The abundances of all species other than Dovekie were positively correlated with one another, and that of Dovekie was negatively correlated with those of the other five species (Table 2).
Discussion
Winter alcid abundance depends significantly on NAO, which in turn shows both a long-term (~50 year) trend and shorter term periodicity (Figure 3). Thus, our analysis supports previous suggestions that climate acts upon seabirds at more than one temporal scale (Schreiber, 2002; Jenouvrier et al., 2005; Veit and Montevecchi, 2006; Irons et al., 2008). Here, we found that four species vary with shorter term NAO fluctuations (Table 1), and all six species vary with the longer-term NAO trend (Figure 3).
The data we have analyzed are on winter abundance. Changes in abundance could reflect either distributional shifts, changes in population size, or a combination of the two. Razorbills, Common Murres, Atlantic Puffins, and perhaps Black Guillemots, are clearly increasing in abundance within their breeding ranges and expanding southward into our study area (Chapdelaine et al., 2001; Bond and Diamond, 2006; Regular et al., 2010). The increase of Atlantic Puffins in our study area has been hastened by reintroduction of birds from Canada to breeding islands in the Gulf of Maine (Kress and Nettleship, 1988). Whatever the proximate cause of increased abundance off Massachusetts in winter, the ultimate cause in most cases seems linked to variation in oceanographic climate.
Shifts in at-sea distribution of seabirds have previously been linked to changing oceanic climate (Veit et al., 1996, 1997; Hyrenbach and Veit, 2003; Péron et al., 2010). The presumption in these studies is that ocean temperature is an index of productivity and advection; consequently, warmer temperatures lead to lowered primary productivity, and thus less secondary productivity and food for seabirds. We postulate that the mechanism underlying the changes in abundance we have described is change in abundance or distribution of seabird prey, which in turn responds to changing oceanic climate, as has been concluded in a number of other recent studies (Frederiksen et al., 2004; Irons et al., 2008; Sydeman et al., 2013) including changes in seabird distribution (Veit et al., 1996, 1997; Gaston and Woo, 2008; Péron et al., 2010).
Razorbills feed on a variety of schooling fishes such as herring and sand lance (Nettleship and Birkhead, 1985). There have been negatively correlated fluctuations in the populations of Atlantic Herring (Clupea harengus) and American Sand Lance (Ammodytes americanus) over the period 1970-present (Sherman et al., 1981; Overholtz and Link, 2007, 2009), one suggestion being these two plankton-feeding fishes replace one another as one gets fished out. A major increase in sand lance in the late 1970s was accompanied by very large numbers of seabirds, including Razorbills, feeding upon them at the time (Veit and Petersen, 1993). Increased abundance of Razorbills, Common Murres, Thick-billed Murres, Black Guillemots, and Atlantic Puffins in Massachusetts occurred during the 1970s (Figure 3) at a time when a dramatic increase in sand lance was documented in the area (Sherman et al., 1981; Veit and Petersen, 1993). Gaston and Woo (2008) show how Razorbills have expanded their breeding range into NW Canada following northward expansion of Capelin (Mallotus vallosus) and sand lance (Ammodytes, spp.).
Both species of Murres and Atlantic Puffins, similarly to Razorbills, eat schooling pelagic fishes including sand lance (Gaston and Hipfner, 2000; Ainley et al., 2002; Lowther et al., 2002; Bond and Diamond, 2006), and, though we lack dietary data from these species in our study area, it seems reasonable that part of their recent increases reflects increasing sandlance abundance. Black Guillemots also eat fish, including sand lance, but they feed in much more inshore waters than any of the other five species and therefore also take a variety of benthic species (Butler and Buckley, 2002). Dovekies are planktivorous (Montevecchi, 2002) and in this study region often focus their foraging over mid shelf fronts where they aggregate over patches of copepods and amphipods (Veit and Guris, 2008).
Another possible explanation for changes in population numbers is as a result of changes in fishery operations. Large numbers of alcids, especially Common Murres, are killed as by-catch in gill nets (Montevecchi, 2002). A gill net fishery for Atlantic Cod (Gadus morhua) and other groundfish in and around Newfoundland collapsed in 1992 due to the disappearance of cod, and a corresponding increase in the Common Murre population of Newfoundland has been at least partly attributed to this factor (Regular et al., 2010). In the Massachusetts data, there is a strong increase in Common Murre abundance at about this same time (Figure 3), but there is also a parallel increase in Dovekie abundance that seems unlikely to be related to reduced mortality in gill nets. From the Massachusetts data considered here, the increase in Razorbills seems to be longer term than the increase in Common Murres, and there is little if any apparent acceleration in the early 1990s. Thus, although the closing of the Newfoundland cod fishery almost certainly helped alcid numbers, there are other processes at work to explain changes in numbers of both Dovekies and Razorbills. Finally, chronic oil pollution and hunting, especially of Thick-billed Murres, are likely to impact population growth of alcids (Wiese et al., 2004).
Dovekies are planktivorous (Nettleship and Birkhead, 1985; Gaston and Jones, 1998) while the other five species we studied feed mainly on fish (Gaston and Jones, 1998). This suggests response to climatic oscillations at multiple trophic levels; it should be noted that most alcid diet studies are on diet in the breeding season, and little is known of their winter diets. We know that seabird numbers respond both directly to climate (Schreiber, 2002; Nisbet et al., 2013), and directly to prey base numbers, which may reflect an indirect response to climate via the prey base (Durant et al., 2003; Frederiksen et al., 2004). We have found here a strong response of alcids to climate (as measured by NAO) at multiple timescales, and we can speculate knowledgably that this strong response is comprised of a direct and indirect component. This is the first documentation of alcid response to NAO, and gives greater weight to the importance of managing marine bird populations for their continued persistence in the face of continued climate change.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Marj Rines for providing unpublished recent data on alcids from Massachusetts. Sara Williams provided data on alcid populations in Maine and Sabina Wilhelm provided data from Newfoundland. James Hurrell provided helpful advice on interpretation of the NAO index. Comments from Morten Frederiksen, Tycho Anker-Nilssen, Russell Barry Wynn substantially improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fevo.2015.00038/abstract
References
Aebischer, N. J., Coulson, J. C., and Colebrook, J. M. (1990). Parallel long-term trends across four marine trophic levels and weather. Nature 347, 753–755. doi: 10.1038/347753a0
Ainley, D. G., Nettleship, D. N., Carter, H. R., and Storey, A. E. (2002). “Common murre (Uria aalge),” in The Birds of North America Online, ed A. Poole (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bna.666. Available online at: http://bna.birds.cornell.edu/bna/species/666
Bond, A. L., and Diamond, A. W. (2006). “The common Murre (Uria aalge) on machias seal island, 1999–2005,” in Machias Seal Island: 1999–2005 Progress Report, eds A. L. Bond, M.-P. McNutt, and A. W. Diamond (Fredericton, NB: Atlantic Co-operative Wildlife Ecology Research Network and University of New Brunswick), 1–61.
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer.
Butler, R. G., and Buckley, D. E. (2002). “Black guillemot (Cepphus grylle),” in The Birds of North America Online, ed A. Poole (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bna.675. Available online at: http://bna.birds.cornell.edu/bna/species/675
Chapdelaine, G., Diamond, A. W., Elliott, R. D., and Robertson, G. J. (2001). “Status and population trends of the Razorbill in North America,” in Canadian Wildlife Service Occasional Paper No. 105, (Ottawa, ON), 1–22. Available online at: http://publications.gc.ca/pub?id=392816&sl=0
Conversi, A., Piontkovski, S., and Hameed, S. (2001). Seasonal and interannual dynamics of Calanus finmarchicus in the Gulf of Maine (Northeastern US shelf) with reference to the North Atlantic Oscillation. Deep-Sea Res. II 48, 519–530. doi: 10.1016/S0967-0645(00)00088-6
Drinkwater, K. F., Belgrano, A., Borja, A., Conversi, A., Edwards, M., Greene, C. H., et al. (2003). “The response of marine ecosystems to climate variability associated with the North Atlantic Oscillation,” in The North Atlantic Oscillation: Climatic Significance and Environmental Impact, eds. J. W. Hurrell, Y. Kushnir, G. Ottersen, and M. Visbeck (Washington, DC: American Geophysical Union), 211–234.
Durant, J. M., Anker-Nilssen, T., and Stenseth, N.-C. (2003). Trophic interactions under climate fluctuations: the Atlantic Puffin as an example. Proc. R. Soc. Lond. B Biol. Sci. 270, 1461–1466. doi: 10.1098/rspb.2003.2397
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Durant, J. M., Hjermman, D. O., Anker-Nilssen, T., Beaugrand, G., Mysterud, A., Pettorelli, N., et al. (2005). Timing and abundance as key mechanisms affecting trophic interactions in variable environments. Ecol. Lett. 8, 952–958. doi: 10.1111/j.1461-0248.2005.00798.x
Fogarty, M. J., and Murawski, S. A. (1998). Large-scale disturbance and the structure of marine systems: fishery impacts on Georges Bank. Ecol. Appl. 8, S6–S22. doi: 10.1890/1051-0761(1998)8[S6:LDATSO]2.0.CO;2
Frederiksen, M., Wanless, S., Harris, M. P., Rothery, P., and Wilson, L. J. (2004). The role of indistrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J. Appl. Ecol. 41, 1129–1139. doi: 10.1111/j.0021-8901.2004.00966.x
Gaston, A. J., and Hipfner, J. M. (2000). “Thick-billed murre (Uria lomvia),” in The Birds of North America Online, ed A. Poole (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bna.497. Available online at: http://bna.birds.cornell.edu/bna/species/497
Gaston, A. J., and Woo, K. (2008). Razorbills (Alca torda) follow subarctic prey into the Canadian arctic: colonization results from climate change? Auk 125, 939–942. doi: 10.1525/auk.2008.07195
Gjerdrum, C., Vallée, A. M. J., Clair, C. C. S., Bertram, D. F., Ryder, J. L., and Blackburn, G. S. (2003). Tufted Puffin reproduction reveals ocean climate variability. Proc. Natl. Acad. Sci. U.S.A. 100, 9377–9382. doi: 10.1073/pnas.1133383100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Guinet, C., Chastel, O., Koudil, M., Durbec, J., and Jouventin, P. (1998). Effects of warm sea-surface temperature anomalies on the blue petrel at the Kerguelen Islands. Proc. R. Soc. Lond. B Biol. Sci. 265, 1001–1006. doi: 10.1098/rspb.1998.0390
Hemery, G., D'amico, F., Castege, I., Dupont, B., D'elbee, J., Lalanne, Y., et al. (2008). Detecting the impact of oceano-climatic changes on marine ecosystems using a multivariate index: the case of the Bay of Biscay (North Atlantic-European Ocean). Glob. Change Biol. 14, 27–38. doi: 10.1111/j.1365-2486.2007.01471.x
Hunt, G. L. Jr. Priddle, J., Whitehouse, M. J., Veit, R. R., and Heywood, R. B. (1992). Changes in seabird species abundance near South Georgia during a period of rapid change in sea surface temperature. Antarct. Sci. 4, 15–22.
Hurrell, J. W., and Dickson, R. R. (2004). “Climate variability over the North Atlantic,” in Marine Ecosystem and Climate Variation, eds N. C. Stenseth, G. Ottersen, J. W. Hurrell, and A. Belgrano (New York, NY: Oxford University Press), 15–31.
Hyrenbach, K. D., and Veit, R. R. (2003). Ocean warming and seabird communities of the southern California current system (1987–1998): responses at multiple temporal scales. Deep-Sea Res. II 50, 2537–2566. doi: 10.1016/S0967-0645(03)00123-1
Irons, D. B., Anker-Nilssen, T., Gaston, A. J., Byrd, G. V., Falk, K., Gilchrist, G., et al. (2008). Fluctuations in circumpolar seabird populations linked to climate oscillations. Glob. Change Biol. 14, 1455–1463. doi: 10.1111/j.1365-2486.2008.01581.x
Jenouvrier, S., Weimerskirch, W., Barbraud, C., Park, Y.-H., and Cazelles, B. (2005). Evidence of a shift in the cyclicity of Antarctic seabird dynamics linked to climate. Proc. R. Soc. B Biol. Sci. 272, 887–895. doi: 10.1098/rspb.2004.2978
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kress, S. W., and Nettleship, D. N. (1988). Re-establishing the Atlantic Puffin (Fratercula arctica) at a former breeding site in the Gulf of Maine. J. Field Ornithol. 59, 161–170.
Lowther, P. E., Diamond, A. W., Kress, S. W., Robertson, G. J., and Russell, K. (2002). “Atlantic Puffin (Fratercula arctica),” in The Birds of North America Online, ed A. Poole (Ithaca: Cornell Lab of Ornithology). doi: 10.2173/bna.709. Available online at: http://bna.birds.cornell.edu/bna/species/709
Manne, L. L. (2013). “Island species with nowhere to go,” in Wildlife Conservation in a Changing Climate, eds D. Doak, J. Brodie, and E. Post (Chicago, IL: University of Chicago Press), 226–244.
Montevecchi, W. A. (2002). “Interaction between fisheries and seabirds,” in Biology of Marine Birds, eds E. A. Schreiber and J. Burger (Boca Raton, FL: CRC Press), 527–558.
Montevecchi, W. A., and Myers, R. A. (1997). Centurial and decadal oceanographic influences on changes in northern gannet populations and diets in the north-west Atlantic: implications for climate change. ICES J. Mar. Sci. 54, 608–614. doi: 10.1006/jmsc.1997.0265
Montevecchi, W. A., and Stenhouse, I. J. (2002). “Dovekie (Alle alle),” in The Birds of North America Online, ed A. Poole (Ithaca, NY: Cornell Lab of Ornithology). doi: 10.2173/bna.701. Available online at: http://bna.birds.cornell.edu/bna/species/701
Nisbet, I. C. T., Veit, R. R., Auer, S. A., and White, T. P. (2013). Marine Birds of the Eastern US and the Bay of Fundy: Distribution, Numbers, Trends, Threats, and Management. Cambridge, MA: Nuttall Ornithological Monographs.
Overholtz, W. J., and Link, J. S. (2007). Consumption impacts by marine mammals, fish, and seabirds on the Gulf of Maine-Georges Bank Atlantic Herring (Clupea harengus) complex during the years 1977–2002. ICES J. Mar. Sci. 64, 83–96. doi: 10.1093/icesjms/fsl009
Overholtz, W. J., and Link, J. S. (2009). A simulation model to explore the response of the Gulf of Maine food web to large scale environmental and ecological changes. Ecol. Modell. 220, 2491–2502. doi: 10.1016/j.ecolmodel.2009.06.034
R Core Development Team. (2012). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Péron, C., Autier, M., Barbraud, C., Delford, K., Besson, D., and Weimerskirch, H. (2010). Interdecadal changes in at-sea distribution and abundance of subantarctic seabirds along a latitudinal gradient in the southern Indian Ocean. Glob. Change Biol. 16, 1895–1909. doi: 10.1111/j.1365-2486.2010.02169.x
Regular, P. M., Robertson, G. J., Montevecchi, W. A., Shuhood, F., Power, T., Ballam, D., et al. (2010). Relative importance of human activities and climate driving common Murre population trends in the Northwest Atlantic. Polar Biol. 33, 1215–1226. doi: 10.1007/s00300-010-0811-2
Sandvik, H., Erikstad, K. E., Barrett, R., and Yoccoz, N. G. (2005). The effect of climate on adult survival in five species of North Atlantic seabirds. J. Anim. Ecol. 74, 817–831. doi: 10.1111/j.1365-2656.2005.00981.x
Schreiber, E. A. (2002). “Climate and weather effects on seabirds,” in Biology of Marine Birds, eds J. Burger and E. A. Schreiber (Boca Raton, FL: CRC Press), 179–215.
Sherman, K., Jones, C., Sullivan, L., Smith, W., Berrin, P., and Egsymont, E. (1981). Congruent shifts in sand eel abundance in western and eastern North Atlantic ecosystems. Nature 291, 486–489. doi: 10.1038/291486a0
Stenseth, N. C., and Mysterud, A. (2002). Climate, changing phenology, and other life – history traits: nonlinearity and match-mismatch to the environment. Proc. Natl. Acad. Sci. U.S.A. 99, 13379–13381. doi: 10.1073/pnas.212519399
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stenseth, N. C., Ottersen, G., Hurrell, J. W., and Belgrano, A. (eds.). (2004). Marine Ecosystems and Climate Variation—The North Atlantic. A Comparative Perspective. New York, NY: Oxford University Press.
Sydeman, W. J., Santora, J. A., Thompson, S. A., Marinovic, B., and Di Lorenzo, E. (2013). Increasing variance in North Pacific climate relates to unprecedented ecosystem variability off California. Glob. Change Biol. 19, 1662–1675. doi: 10.1111/gcb.12165
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thompson, P. M., and Ollason, J. C. (2001). Lagged effects of ocean climate change on fulmar population dynamics. Nature 413, 417–420. doi: 10.1038/35096558
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Veit, R. R., and Guris, P. A. (2008). Recent increases in alcid abundance in the New York Bight and New England Waters. N. J. Birds 34, 83–87.
Veit, R. R., McGowan, J. A., Ainley, D. G., Wahl, T. R., and Pyle, P. (1997). Apex marine predator declines ninety percent in association with changing oceanic climate. Glob. Change Biol. 3, 23–28. doi: 10.1046/j.1365-2486.1997.d01-130.x
Veit, R. R., and Montevecchi, W. A. (2006). The influences of global climate change on marine birds. Acta Zool. Sin. 52, 165–168.
Veit, R. R., and Petersen, W. R. (1993). Birds of Massachusetts. Lincoln, MA: Massachusetts Audubon Society.
Veit, R. R., Pyle, P., and McGowan, J. A. (1996). Ocean warming and long-term change in pelagic bird abundance within the California current system. Mar. Ecol. Prog. Ser. 139, 11–18. doi: 10.3354/meps139011
Wanless, S., Frederiksen, M., Daunt, F., Scott, B. E., and Harris, M. P. (2007). Black-legged kittiwakes as indicators of environmental change in the North Sea: evidence from long-term studies. Prog. Oceanogr. 72, 30–38. doi: 10.1016/j.pocean.2006.07.007
Keywords: alcid, climate change, NAO, multiscale, Northwest Atlantic, Massachusetts
Citation: Veit RR and Manne LL (2015) Climate and changing winter distribution of alcids in the Northwest Atlantic. Front. Ecol. Evol. 3:38. doi: 10.3389/fevo.2015.00038
Received: 01 December 2014; Accepted: 26 March 2015;
Published: 10 April 2015.
Edited by:
Morten Frederiksen, Aarhus University, DenmarkReviewed by:
Morten Frederiksen, Aarhus University, DenmarkTycho Anker-Nilssen, Norwegian Institute for Nature Research, Norway
Russell Barry Wynn, National Oceanography Centre, UK
Copyright © 2015 Veit and Manne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard R. Veit, Department of Biology, College of Staten Island/City University of New York, 2800 Victory Blvd., Staten Island, New York, 10314 NY, USAcnJ2ZWl0MjNAZ21haWwuY29t
 Richard R. Veit
Richard R. Veit Lisa L. Manne
Lisa L. Manne