- Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Penryn, UK
Camouflage is perhaps the most widespread anti-predator defense in nature, with many different types thought to exist. Of these, resembling the general color and pattern of the background (background matching) is likely to be the most common. Background matching can be achieved by adaptation of individual appearance to different habitats or substrates, behavioral choice, and color change. Although the ability to change coloration for camouflage over a period of hours or days is likely to be widely found among animals, few studies have quantified this against different backgrounds. Here, we test whether juvenile shore crabs (Carcinus maenas) are capable of color change for camouflage by placing them on either black or white (experiment 1) or red and green (experiment 2) backgrounds. We find that crabs are capable of significant changes in brightness, becoming lighter on white backgrounds and darker on black backgrounds. Using models of predator (avian) vision, we show that these differences are large enough in many individuals to lead to perceptible changes in appearance. Furthermore, comparisons of crabs with the backgrounds show that changes are likely to lead to significant improvements in camouflage and potentially reduced detection probabilities. Crabs underwent some changes on the red and green backgrounds, but visual modeling indicated that these changes were very small and unlikely to be detectable. Our experiment shows that crabs are able to adjust their camouflage by changes in brightness over a period of hours, and that this could influence detection probability by predators.
Introduction
Camouflage is perhaps the most widespread and common means to avoid predation in nature, and can be achieved through a variety of mechanisms (Wallace, 1889; Poulton, 1890; Thayer, 1909; Cott, 1940; Stevens and Merilaita, 2009, 2011). It has long been a textbook example of evolution, from the famous example of the peppered moth, Biston betularia (Kettlewell, 1955a; Cook et al., 2012), through to more recent studies of the molecular basis of adaptation to different backgrounds in mice and reptiles (Nachman et al., 2003; Rosenblum, 2006; Rosenblum et al., 2010; Nunes et al., 2011). Despite a partial hiatus in camouflage research for several decades after Cott's (1940) influential book, the subject in the last decade has been resurgent, with a substantial body of work investigating camouflage by biologists in several fields of study, computer scientists, visual psychologists, and art historians (see Stevens and Merilaita, 2009, 2011). Much work has sought to understand the mechanisms by which the various types of camouflage work, including disruptive coloration (e.g., Cuthill et al., 2005; Merilaita and Lind, 2005; Schaefer and Stobbe, 2006; Stevens and Cuthill, 2006; Stevens et al., 2006; Webster et al., 2013; Espinosa and Cuthill, 2014), countershading (e.g., Rowland et al., 2008; Tankus and Yeshurun, 2009), and masquerade (Skelhorn et al., 2010, 2011).
The basis of most types of camouflage is likely to be background matching, whereby the animal resembles the color, pattern, and brightness of the general environment or background (reviewed by Merilaita and Stevens, 2011). Surprisingly, experimental work on background matching has lagged behind that of other camouflage types, although it is clear that the degree of resemblance to the background is a fundamental factor in the likelihood of detection (e.g., Pietrewicz and Kamil, 1977; Stuart-Fox et al., 2003; Vignieri et al., 2010). For background matching, a close fidelity to the substrate or local environment can be achieved by a number of mechanisms. First, populations can change over time so that individuals evolve adaptive matches to their local environment (e.g., Nachman et al., 2003; Rosenblum et al., 2010; Nunes et al., 2011). Second, behavioral mechanisms can allow individuals to choose backgrounds against which they are better hidden (Kettlewell, 1955b; Sargent, 1966; Lovell et al., 2013), or to readjust their position or orientation to blend into the background more effectively on a small scale (Kang et al., 2012). Third, animals can evolve the ability to change color, allowing them to tune their camouflage to the specific environment or background where they are found (Stuart-Fox and Moussalli, 2009).
Color change for camouflage is widely observed in the natural world, although color change also has a range of other functions, including for thermoregulation and communication (reviewed by Stuart-Fox and Moussalli, 2009; Umbers et al., 2014). It has been most extensively studied in cuttlefish, which are capable of rapid changes in color patterns against a wide variety of substrate types, driven by visually inspecting aspects of the background, including pattern contrast and edges (Hanlon, 2007; Kelman et al., 2007; Barbosa et al., 2008; Hanlon et al., 2009; Zylinski et al., 2009). Chameleons are also well known for their powers of color change, and although this may have primarily been driven for communication with conspecifics (Stuart-Fox and Moussalli, 2008), it is also important in concealment (Stuart-Fox et al., 2008).
Most work on color change for camouflage has been undertaken on species that are capable of very rapid (a few seconds) changes in appearance, such as cephalopods, chameleons, and flatfish (Ramachandran et al., 1996; Kelman et al., 2006). However, such rapid color change is seemingly not widespread in other animal groups. In contrast, the ability to change color more slowly, over periods of hours, days, and even weeks and months is likely to be more common in nature, yet has received relatively little study. Comparatively slow color change for camouflage has been shown to occur in, for example, prawns (Keeble and Gamble, 1900), caterpillars (Grayson and Edmunds, 1989), crabs (Stevens et al., 2013), and fish (Clarke and Schluter, 2011), in addition to seasonal changes in mammals and birds in line with the presence and absence of snow cover in winter and summer, respectively (Mills et al., 2013).
Despite the broad occurrence of slower color change, relatively few studies have quantified this and its effect on camouflage. The majority of work has concentrated on determining what changes to chromatophores and pigment dispersion occur when individuals are placed on different backgrounds, but rarely quantify the change in coloration that this causes (but see Hemmi et al., 2006). Much work has been undertaken on crabs in this regard (see discussion in Hemmi et al., 2006; Stevens et al., 2013), especially fiddler crabs (Uca spp.). This has shown that color change can occur via day-night circadian rhythms (e.g., Atkins, 1926; Abramowitz, 1937; Brown and Sandeen, 1948; Fingerman, 1955; Fingerman and Yamamoto, 1967; Darnell, 2012), and that some species have the ability to change color with regards to the background (Brown and Sandeen, 1948; Rao et al., 1967). Work by Hemmi et al. (2006) has shown that fiddler crabs from populations with higher predation risk have lower levels of conspicuousness of their signaling patches (favoring greater concealment), and that simulating greater predation risk causes crabs to reduce their conspicuousness over a period of days.
Recently, Stevens et al. (2013) investigated color change and camouflage in juvenile horned ghost crabs (Ocypode ceratophthalmus). Using digital image analysis to quantify changes in coloration, they found that individuals changed color according to a circadian 24 h rhythm. This made crabs darker at night but lighter and more yellow during the day, and closer in coloration to the sand on the beaches where they live. In addition, when placed on a black or white background, crabs became darker or lighter, respectively. However, simply putting the crabs into the dark did not influence their coloration. Despite these findings, Stevens et al. (2013) only tested for color change on black and white backgrounds and not against other colors (nor has other work investigating pigment dispersion in crabs). In addition, they limited their analyses to reflectance information, rather than modeling predator vision (because the main predator groups were unclear), and they did not measure color change in ultraviolet (UV) light (which many predators can see). In general, little is known about how predators would perceive changes in crab coloration, and whether crabs can also change their color or brightness on other background colors.
Here, we test the ability of juvenile common shore crabs (Carcinus maenas) to change color when put on backgrounds of different brightness (black or white) or color (green or red), and quantify the change in coloration to both avian visual systems and in terms of reflectance only (to account for non-avian predator groups). We also determine how the level of any color change influences the degree of match to the background. The shore crab (sometimes called the green crab) is a very common intertidal species living in a wide range of habitats in the UK, large parts of Europe, and beyond (Crothers, 1966, 1968). It is also a highly invasive species in many parts of the world (Darling et al., 2008; McGaw et al., 2011). C. maenas, makes an ideal species to study camouflage. It is widespread and found in many habitats (Todd et al., 2006), individuals face many predators and subjectively have camouflaged carapace patterns (Crothers, 1968; Todd et al., 2006), and individuals have been reported to have the ability to change color (Powell, 1962a,b).
To human eyes, shore crabs are very effectively camouflaged and individuals show substantial variation in pattern and color (Powell, 1962a; Crothers, 1968; Hogarth, 1978). Juvenile shore crabs are particularly diverse in appearance, utilizing a wide range of colors and patterns (Figure 1), often of high contrast and with striking markings (Hogarth, 1978). Adults are generally more similar to one another and more drab in coloration, although considerable variation still exists, seemingly linked to habitat types (Todd et al., 2006). Both adult and juvenile crabs have a range of predators, including many bird species, especially gulls and shore birds, plus various species of fish and some cephalopods (Crothers, 1968). Associations between phenotype and environment at different spatial scales have been reported in terms of crab patterns, and this is likely due to camouflage, via both direct selection from differential predation and processes such as phenotypic plasticity (Todd et al., 2006, 2012). Variation in juvenile coloration and patterns likely protects them from predators (Hogarth, 1978), with the strong patterns potentially affording them protection through disruptive coloration (Powell, 1962b), although no experimental work has tested this or the mechanisms driving high individual variation.

Figure 1. Variation in juvenile shore crabs, showing the considerable differences in color and pattern that exists among individuals. All crabs used in this experiment were between 2.8 and 12 mm.
Previous work by Powell (1962a) tested how the chromatophores of immature adult crabs (of unreported sizes) respond to changes in light and background. He reported that C. maenas has three broad types of chromatophore with red, white, and black pigment, and quantified the degree of concentration of each pigment for crabs on a white and black background. Powell found that chromatophores can either directly respond to light levels (a primary response), or to the nature (e.g., brightness) of the background (a secondary response). On a black background, black pigment disperses and white pigment becomes concentrated, with the opposite occurring on a white background. This change occurs over a period of 30–90 min and the level of response depends on light intensity (i.e., it is mediated by a primary response), although Powell (1962a) found that the secondary response overrides that of the primary one. C. maenas has also been shown to have a 24 h rhythm, becoming darker during the day due to dispersion of dark pigments (Powell, 1962b). The work of Powell (1962a,b) provides a valuable basis for our experiment here, but is limited because crabs were only tested on white and black backgrounds and the change in coloration was not quantified. As far as we are aware, experiments have not tested background responses to different colored substrates (as opposed to black and white), although it is possible that crabs could change color, for instance to become more red, given the pigments they possess.
Methods
Data Collection and Experimental Set Up
Crabs were collected from the upper intertidal zone of Gyllyngvase beach, Falmouth, Cornwall, UK (50°08′33.4690″N, −005°04′07.9716″W) on the 20 and 21 May 2013 for experiment 1 (black vs. white), and 17 and 18 July 2013 for experiment 2 (red and green). We focused on and collected juvenile crabs smaller than 12 mm carapace width because larger crabs are less likely to change color due to increased carapace/cuticle thickness and deposition of calcium carbonate in individuals beyond approximately 14–30 mm width (Powell, 1962b; Crothers, 1968). All crabs were transported back to the laboratory in gray buckets to minimize color change prior to the experiments starting. In the laboratory, crabs were placed in one of two test arenas comprising a shallow tray with the base covered in an intermediate gray background that could be replaced with another color. The tray was filled to 1 cm depth with cool seawater (14 ± 2°C). Crabs were separated using white FloPlast square downpipe cut to size (internal measurements: 61 × 61 × 40 mm) in order to prevent conspecific interactions interfering with any color change, and so that crabs could remain easily identifiable.
Crabs were given 1 h on the intermediate gray background to acclimatize before they were placed into the same tray with either a white or a black (experiment 1) or red or green (experiment 2) background. In each experiment, half of the crabs were placed on each background treatment at any one time. This ensured that at any point in the experiments equal numbers of crabs were placed on each background type to control for potential effects of temperature change or circadian rhythms on color change. After 2 h crabs were gently dried and photographed out of water. They were then returned to the intermediate gray background for 1 h (another acclimatization period; Stevens et al., 2013) before being placed onto the alternative background. After 2 h crabs were photographed again and the experiment ended (at which point crabs were released at the collection site). Each experiment was completed under a UV and human visible Arc Lamp (70W 1.0A power source; EYE Color Arc Lamp with Ventronic, Venture Lighting Europe Ltd. Hertfordshire, UK), and experimental arenas were placed to ensure even lighting occurred over the trays using a black and silver photographic umbrella (Neewer Technology Ltd., Guangdong, China) into which the light was shone.
Color and Brightness Quantification
Photographs were taken using a Nikon D7000 digital camera, which had undergone a quartz conversion to enable UV sensitivity (Advanced Camera Services, Norfolk, UK) and fitted with a Nikon 105 mm Nikkor lens. Images were taken in RAW format with manual white balance and a fixed aperture setting, and converted to uncompressed TIFF files during calibration (see below). A UV/infrared (IR) blocking filter was used for the human visible photos, transmitting wavelengths of 400–700 nm (Baader UV/IR Cut Filter). A UV pass and IR blocking filter was used for the UV photographs (Baader U filter; transmitting between 300 and 400 nm). This resulted in four image layers: long-wavelength (LW), medium-wavelength (MW), short-wavelength (SW), and UV. Each image included a Spectralon gray reflectance standard (Labsphere, Congleton, UK) reflecting light equally at 40% between 300 and 750 nm, and a ruler. Crabs were photographed as quickly as possible in order to prevent stress leading to color change (Detto et al., 2008). Following photography, each image was linearized with regards to light intensity based on camera responses to a set of eight Spectralon gray standards with reflectance values ranging from 2 to 99% (in custom programs written in Image J), because many cameras show non-linear responses in image value to changes in light levels that need to be corrected before accurate data can be obtained (see Westland and Ripamonti, 2004; Stevens et al., 2007a; Garcia et al., 2013). We also equalized the image values (Stevens et al., 2007a; Akkaynak et al., 2014) by removing the effects of varying light conditions with regards to the gray standard, and scaled each image channel (LW, MW, SW, and UV) to reflectance (where an image value of 255 on an 8-bit scale equals 100% reflectance). This gave us four images corresponding to the reflectance of each crab across the visible and UV spectrum, and provides the basis for color analysis independent of any specific visual system or predator group (Stevens, 2011), allowing us to analyze color change in terms of the physical properties of the crabs.
In addition to reflectance information, we also wished to analyze the color change of crabs in terms of the vision of one of their most common predator groups: birds. Broadly, birds can be categorized into one of two main groups based on the sensitivity of their most shortwave sensitive cone type. Species with their sensitivity primarily based in the UV range belong to the UV group, whereas birds with their sensitivity shifted into the violet part of the spectrum (but still capable of detecting UV light, albeit to a lesser degree) belong to a violet-sensitive or VS group (Ödeen and Håstad, 2003; Hart and Hunt, 2007). At least some shore birds and gulls, being major predators of crabs (Crothers, 1968) are likely to fall into the violet group (Ödeen and Håstad, 2003; Hart and Hunt, 2007), we modeled this type of avian system. When modeling a VS bird system, previous work, as here, has generally used the visual sensitivity of the peafowl (Pavo cristatus) as a model species (data from Hart, 2002). To obtain images corresponding to avian vision, we first aligned the LW, MW, SW, and UV layers from the photographs of a given specimen and created an image stack. Following this, we transformed the images to predicted avian cone catch values of the long-wavelength-sensitive (LWS), medium-wavelength-sensitive (MWS), short-wavelength-sensitive (SWS), violet-sensitive (VS), and double cone (D) types by using a polynomial mapping technique to convert from camera to bird color space (see Westland and Ripamonti, 2004; Stevens et al., 2007a; Pike, 2011) using a custom written program in Image J, using a D65 standard irradiance spectrum. The use of digital cameras to study coloration and model animal vision is increasingly widespread and has been shown to be a highly accurate approach to quantify visual signals compared with more conventional approaches to model cone catches using reflectance spectrometry, with a very close correspondence between the two approaches in estimating cone catch values (see Stevens and Cuthill, 2006; Pike, 2011). We have calculated the spectral sensitivity curves of the cameras we use (combined with filters and the lens) using several approaches. These include a method outlined in detail by Pike (2011) using a quadratic programming procedure, and a new method developed in our laboratory (Troscianko and Stevens, unpublished) using dispersing prisms between the lens elements and camera sensor, combined with calibration of wavelength locations on the sensor by using a light source with peaks at known locations. Both methods show close correspondence with one another, and with other approaches using interference filters to calculate camera sensitivity (Pike, 2011). The sensitivity range and peaks of the camera set up in our current study is as follows for each sensor (accounting for lens and filter transmission): UV: 360–400 nm (peak 382 nm), SW: 400–550 nm (peak 462 nm), MW: 420–620 nm (peak 540 nm), LW: 560–700 nm (peak 626 nm). Although this shows low sensitivity below 350 nm (due to the lens properties) this is not a problem because birds with a VS system show reduced sensitivity to shorter wavelengths of UV light, with peak cone responses around 425 nm (Hart, 2002). Indeed, compared to spectrophotometer-based cone-catch estimates (here for peafowl vision) of different squares on a color-chart, our method yields R2 values for each channel from 0.96 to 0.98 (including UV); i.e., extremely close in predicted cone catch values to those obtained with a spectrometer. Note that spectrometers will have other sources of error associated with cone catch modeling not present for cameras, such as issues associated with imprecise angles and distances of the probe to the object/standard. Hence, the camera method is probably even more accurate than the values given above.
For both the four reflectance and five cone catch images, measurements of the entire carapace of each crab were taken, avoiding any areas of specular reflectance where light simply “bounces” back off the carapace surface. For reflectance, we calculated four metrics. First, overall reflectance (brightness) was calculated as (LW+MW+SW+UV)/4. This is a measure of the achromatic intensity across the visible spectrum of the crabs, with higher values meaning individuals are brighter. Note that actual mechanisms of lightness perception in animals are unlikely to be undertaken in the same way. However, mechanisms of luminance perception vary among species and taxa (Osorio and Vorobyev, 2005), and crabs likely face a range of predators of different species (e.g., cephalopods, various fish, and even other crabs) (Crothers, 1968). Our reflectance-based approach is intended to characterize the physical change in appearance of crabs across the spectrum, independent of any assumptions about luminance mechanisms. For color, we calculated two variable types. First, saturation (the amount of color compared to white light) was calculated from the position of each carapace color from the center of a tetrahedral color space (Endler and Mielke, 2005; Stevens, 2011). Here, the four color channel values for each individual are first standardized to a proportion of their total (to remove absolute variation in brightness), and then converted to X, Y, and Z coordinates in the color space. Saturation can be modeled as the shortest (Euclidian) distance of the color from the center of the tetrahedron (Stoddard and Prum, 2008), with greater values potentially leading to higher perceived saturation. See Kelber et al. (2003), Endler and Mielke (2005), and Stevens et al. (2009) for equations and information on color spaces. Finally, we calculated two measures of hue following a previous approach used by Spottiswoode and Stevens (2011); see also (Stevens, 2011) based on putative opponent-style color channels. Opponent color channels are an essential part of color vision. However, which opponent channels exist in birds is not properly known (although there is evidence for some of them Osorio et al., 1999, and multiple opponent channels are known in turtles, which have similar vision Ammermüller et al., 1995; Twig and Perlman, 2004), meaning that exact opponent channels cannot be modeled to gain measures of hue. Our aim here, as in earlier work (Spottiswoode and Stevens, 2011), was to utilize a measure of hue that is inspired by opponent color channels, even if we do not know which precise channels exist. This is constructed as a ratio, as is standard in vision science to model opponent mechanisms (e.g., red/green, or [red−green]/[red+green] for red-green human opponent channels; e.g., Lovell et al., 2005). Komdeur et al. (2005), working with starlings with iridescent purple plumage colors, calculated hue in the form of a nominal opponent channel as: (LW+SW)/(MW+UV). An alternative approach, which we have taken here, is to use a principal component analysis (PCA) to determine the main axis of color variation that exists, and to use this to determine a logical color channel(s) (Komdeur et al., 2005). Following Spottiswoode and Stevens (2011) we performed PCA on a covariance matrix of the standardized data from the four reflectance channels, and used the resultant principal components (PCs) to determine color channels.
For the avian visual data, our achromatic measure corresponding to perceived lightness was simply the double cone values, since the double cones are widely thought to underlie achromatic vision in birds (Osorio and Vorobyev, 2005). For color, our calculations of hue and saturation were identical, except that we used the data corresponding to the predicted photon catch values of the four single cones. For the above methods, Hue 1 and 2 yield values based on a ratio of color channel or cone catch values (see above), luminance (being cone catches) is by convention normally between 0 and 1, and saturation is between 0 and 0.75. Brightness is reflectance (specified either between 0 and 1, or 0 and 100%).
In addition, we sought to determine whether changes in luminance and color of the crabs were likely to be perceptible. That is, we determined whether the same crabs were distinguishable in color or luminance by comparing the appearance of each individual when on each background. Note that in reality the model is most appropriate to comparing objects side by side and what really matters in camouflage is detection against the background, and we address this issue below. However, we use the above analysis to confirm whether the changes in color and luminance are potentially perceptible or not. To do so, we used a widely implemented log form of the tetrachromatic version of the Vorobyev-Osorio color discrimination model, which assumes that receptor noise limits visual discrimination (Vorobyev and Osorio, 1998; Vorobyev et al., 1998). For color, we used a version utilizing the single cones, and for luminance utilizing the double cones (Siddiqi et al., 2004). We used a Weber fraction value of 0.05 for the most abundant cone type (Eaton, 2005; Stevens, 2011), and relative proportions of cone types in the retina of the peafowl (LW = 0.95, MW = 1.00, SW = 0.86, UV = 0.45; Hart, 2002; Håstad et al., 2005). The Weber value for birds is a best estimate, but although deviations from the chosen value may affect the absolute JND values, it would not affect the relative differences between stimuli. Choosing a Weber value of 0.02 (another widely used value) would lead to higher JND values and hence more likely to suggest differences between stimuli. Hence, a value of 0.05 is more conservative. The output of the model is in just noticeable differences (JNDs), whereby a JND of less than 1.00 means that two stimuli are indiscriminable, values between 1 and 3 that they are only likely to be discriminable under good viewing conditions, and values above this that the stimuli should be distinguishable with increasing ease (Siddiqi et al., 2004).
While the above analysis enables us to determine whether differences between crabs are sufficiently large to lead to perceived differences in appearance, they do not tell us directly if this change leads to an improvement in camouflage; i.e., whether the crab is closer to the background as a result of any color change. Therefore, we compared the appearance of each crab against the color and brightness/luminance of both background types, predicting that crabs should be closer in appearance to the background they were found on compared to when they had been on another background. For the visual system data we modeled JNDs, whereas for the reflectance data we used absolute differences in brightness between crab and background for brightness matching, and Euclidian distances between crab and background in color space for the degree of color similarity.
Experiment 1
In experiment 1, we placed crabs on either a white or a black background after being on the intermediate gray. Although black and white backgrounds may at first be seen as relatively artificial in the context of where shore crabs live, the habitat where individuals were collected does have a range of relatively dark and light colored rocks and stones. Backgrounds were made of waterproof paper (HP LaserJet Tough Paper; Hewlett Packard, Palo Alto, USA), printed on a Hewlett Packard Color LaserJet 2605dn printer at 300 dpi. To ensure that the intermediate gray starting point was midway between white and black we printed (on the same paper type) a range of gray squares made in Photoshop Elements 5.0 (Adobe Systems Inc., San Jose, USA) of different intensities from black through to white. We then measured their reflectance using an Ocean Optics (Dunedin, FL, USA) USB2000+ spectrometer, held at 45° to normal, with illumination by a PX-2 pulsed xenon lamp, and calculated the average reflectance of each square across 400–750 nm (neither the paper or the printer ink reflects UV light), followed by plotting image value against reflectance. We calculated the midpoint gray between white and black on a ratio scale (as Stevens et al., 2007b). The general design of this experiment is as specified above. We used 37 crabs, with carapace widths ranging between 4.67 and 11.89 mm. Results were analyzed with general linear models (GLMs) on the different color and brightness/luminance variables, including effects of background (black or white), the order that crabs were placed on each background, and individual as a random factor. We log transformed the data when normality was not found. JND results were analyzed with Wilcoxon-Mann Whitney one sample tests, testing whether the JND values were higher than discrimination thresholds of 1.00 and 3.00 between the crabs on each background. These tests were directional (rather than testing if values simply differ from zero) because JND values are always greater than zero and we needed to determine if JND values exceeded the critical thresholds given above.
As specified above, we also compared crabs to the color and luminance/brightness of each background type. Here, crabs should be a better match to the black background when they had been placed on a black background compared to when they had been placed on a white background. Conversely, when placed on a white background crabs should be closer to the white than when they had been placed on a black background. Results were analyzed with Wilcoxon-Mann Whitney matched pairs tests comparing the level of match between each individual to the specified background after being placed on each background type.
Experiment 2
Experiment 2 followed the same procedure, except that we used red and green colored backgrounds. Broadly, background colors were based on the red encrusting algae and green algae/seaweed, which are both common at the study site where the crabs were collected, although we did not try to replicate these colors exactly. Both colors were controlled to have the same overall brightness (in terms of reflectance) by photographing different red and green colors printed on the same paper (as above) and measuring their reflectance values in LW, MW, and SW images, followed by calculating the average reflectance value across these so that this was the same for both red and green. By doing so, changes in appearance of crabs may be due to the different colors of the background rather than different brightness (but see Discussion). We used 35 crabs in this experiment, with carapace widths ranging between 2.85 and 11.41 mm. Data for this experiment was consistently non-normal and could not be transformed and so we analyzed the results with Wilcoxon-Mann Whitney Matched Pairs Tests, and the JND data as for experiment 1. As above, we compared crabs to the color and luminance/brightness of each background type. We predicted that if crabs change color on the backgrounds then they should be a better match to the red background when placed on a red background than when the same crabs had been placed on a green background. Conversely, when placed on a green background crabs should be closer to the green than when they had been placed on a red background. Results were analyzed with Wilcoxon-Mann Whitney matched pairs tests comparing the level of match between each individual to the specified background having been placed on each background type.
Results
Experiment 1: Black vs. White
Avian visual modeling
For the calculations of hue (i.e., color type), we found that two PCs explained 97.9% of the variance (PC1: 64.0% and PC2: 33.9%). PC1 was equivalent to the following color channel: hue 1 = (LW+MW)/(SW+UV), which is essentially a ratio of longer to shorter wavelengths. PC2 was equivalent to hue 2 = (LW+UV)/(MW+SW), essentially the ratio of long-wavelength and UV light compared to middle parts of the spectrum.
For luminance, the data were log transformed and there was a significant effect of background [F(1, 73) = 172.36; P < 0.001], and individual [F(36, 73) = 43.67; P < 0.001], but not order [F(1, 73) = 2.16; P = 0.151]; Figure 2. On average, crabs on white backgrounds had higher photon catch luminance values by 0.06, although there was considerable inter-individual variation (Figure 3), with some individuals changing less (minimum difference between white and black = 0.009) and others considerably more (maximum difference between white and black = 0.223). Therefore, individuals are capable of changes in brightness in accordance with the background, with some changes being small, and others large. Individuals that were comparatively lighter on the white background were also generally lighter on the black background (Figure 4).
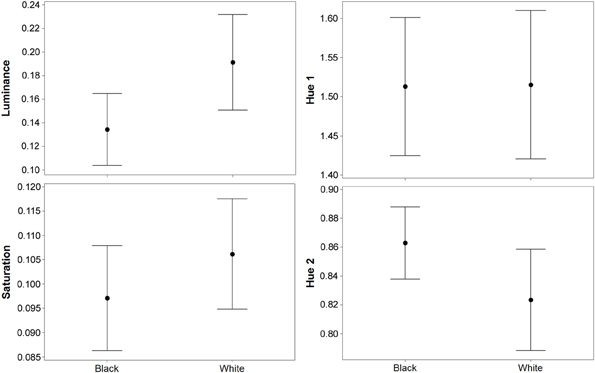
Figure 2. Results (means plus standard error) from experiment 1 (black and white backgrounds) when analyzed with the avian visual model. Differences in luminance (perceived lightness), saturation, and hue 2 were significant, but there was no difference in hue 1. Hue 1 and 2 yield values based on a ratio of color channel or cone catch values (see main text), luminance (being cone catches) is between 0 and 1, and saturation is between 0 and 0.75.
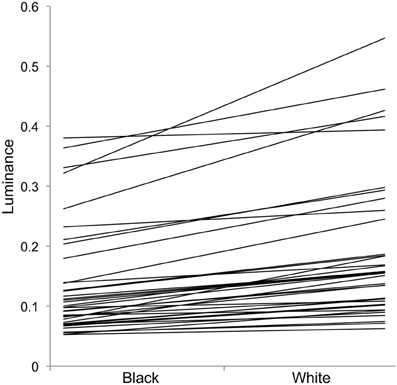
Figure 3. Individual changes in luminance in experiment 1 with crabs on black and white backgrounds. Each individual line represents one individual, showing that individuals vary greatly in overall luminance, and the level of change between the two backgrounds (slope of the lines).
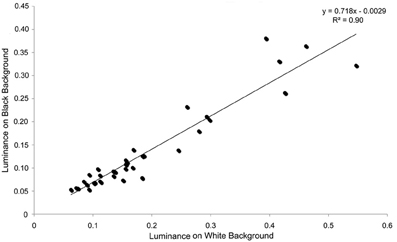
Figure 4. Individual luminance values on black and white backgrounds in experiment 1. There is a very close relationship between the luminance values on individuals against each background, showing that individuals that are relatively bright on one background also tend to be relatively bright on the other background.
For saturation, there was a significant effect of background [F(1, 73) = 7.61; P = 0.009], and individual [F(36, 73) = 10.03; P < 0.001], but not order [F(1, 73) = 0.15; P = 0.697]; Figure 2. On average, crabs on white backgrounds had higher saturation values by 0.009 (maximum difference = 0.059, minimum difference = −0.031). That is, crabs had marginally richer colors on the white background.
For hue 1, the data was log transformed and showed a significant effect of individual [F(36, 73) = 9.08; P < 0.001], but not background [F(1, 73) < 0.01; P = 0.971], or order [F(1, 73) = 0.02; P = 0.903]. However, hue 2 (data also log transformed) showed a significant effect of background [F(1, 73) = 21.11; P < 0.001], and individual [F(36, 73) = 10.24; P < 0.001], but not order [F(1, 73) = 1.86; P = 0.181]; Figure 2. On average, crabs on white backgrounds had lower hue 2 values by 0.031 (minimum difference = −0.048, maximum difference = −0.140), meaning that they were slightly more blue-green in color.
Analysis of discrimination JND values showed that although some differences in color may be perceptible to avian predators (average JND = 2.409, maximum = 6.026, minimum = 0.304) the majority of changes in crab coloration were unlikely to be detectable (just 11 of 37 crabs had JND differences between white and black of more than 3.00). When testing for whether JND values were greater than 1.00, values for color were significantly higher [W(37) = 655.0, P < 0.001]. However, color JND values were not significantly greater than 3.00 [W(37) = 189.0, P = 0.993], suggesting that color changes were generally imperceptible (Figure 5A). In contrast, for luminance the change between white and black backgrounds is likely to be perceptible in the majority of cases (average JND = 7.339, maximum = 17.075, minimum = 0.682), with 33 of 37 crabs having JND differences between white and black of more than 3.00; Figure 5A. When testing for whether JND values were greater than a threshold of 1.00, values for luminance were significantly higher [W(37) = 702.0, P < 0.001]. In addition, luminance JND values were also significantly greater than 3.00 [W(37) = 679.0, P < 0.001], suggesting that luminance changes would be perceptible.
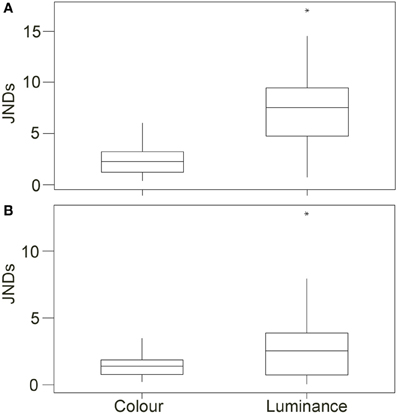
Figure 5. Top (A) Shows the discrimination values (just noticeable differences; JNDs) for color and luminance of the crabs when comparing values for crabs on the white and black backgrounds in experiment 1 [main boxes show medians plus inter-quartile range (IQR), whiskers are lowest and highest values that are within 1.5*IQR from the upper and lower quartiles, asterisks represent outliers]. (B) Shows the same results when comparing crabs in experiment 2 on red and green backgrounds.
We then compared the level of similarity between crabs and the background when individuals had been placed on each background type. Crabs placed on a black background were more similar in luminance JNDs to the black background than crabs were when they had been on the white background [W(37) = 34.0, P < 0.001; Figure 6A], but there was no significant difference in matching for color JNDs [W(37) = 347.0, P = 0.952]. This difference in luminance match was on average just over 6 JNDs, meaning that it is sizable enough to potentially equate to a substantial improvement in camouflage. Likewise, when placed on a white background, crabs were more similar in luminance JNDs to the white background than were crabs after being placed on the black background [W(37) = 703.0, P < 0.001]. Differences were on average just over 7 JNDs. There was also a significant improvement in matching for color JNDs [W(37) = 89.0, P < 0.001], although whether this is large enough to equate to a camouflage benefit is unclear as the average JND difference was only just over 1.00. Note that although the absolute JND values of crabs vs. backgrounds are sometimes high (especially against the white background), this is not our primary concern here because we are not using natural background colors against which the crabs would normally be seen, and so we do not expect very close camouflage as this will also be driven by more long-term color and brightness properties of the carapace to the environment.
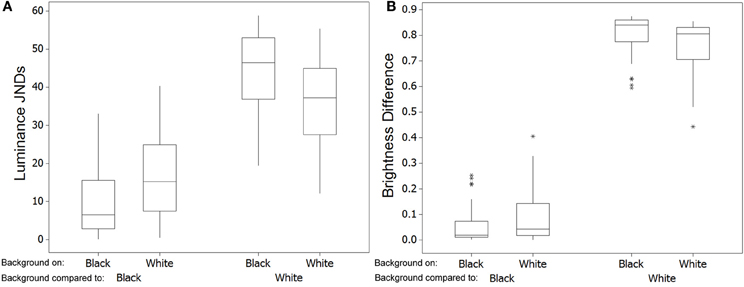
Figure 6. Left (A) Shows the discrimination values (just noticeable differences; JNDs) for luminance when comparing the level of match of crabs with each background [main boxes show medians plus inter-quartile range (IQR), whiskers are lowest and highest values that are within 1.5*IQR from the upper and lower quartiles, asterisks represent outliers, which sometimes overlap]. When crabs had been on a black background they are closer in luminance to the black background than are crabs after being on a white background. Conversely, crabs were more similar in luminance to the white background when they had been on white rather than the black background. In general, being often quite dark, crabs are closer to the black than to the white background. (B) Shows the same comparisons and results except with data being absolute difference in brightness (total reflectance on a scale of 0–1) to the background.
Reflectance
Two PCs explained 98.0% of the variance (PC1: 85.5% and PC2: 12.5%). From the PCA we calculated two color channels: PC1 was equivalent to the following color channel: hue 1 = (LW+MW)/(SW+UV), and PC2 was equivalent to hue 2 (LW+UV)/(MW+SW). These are directly comparable to the color channels for the visual modeling data.
For brightness (log transformed), there were significant effects of background [F(1, 73) = 171.16; P < 0.001], and individual [F(36, 73) = 45.43; P < 0.001], but not order [F(1, 73) = 0.84; P = 0.365]; Figures 7, 8. On average, crabs on white backgrounds had brightness values increased by 4.63% reflectance, although there was considerable inter-individual variation, with some individuals changing less (minimum difference between white and black = 0.59%) and others considerably more (maximum difference between white and black = 18.82%). Therefore, individuals do change in brightness in accordance with the background properties.
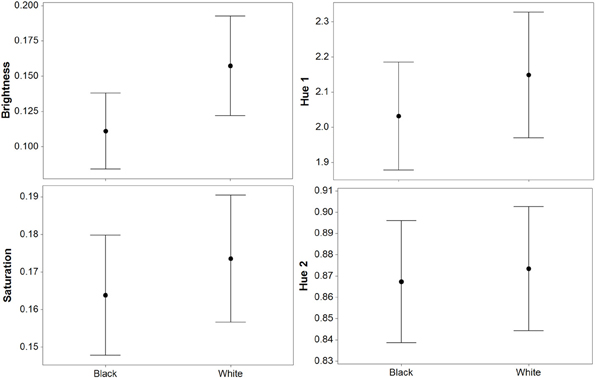
Figure 7. Results (means plus standard error) from experiment 1 (black and white backgrounds) when analyzed with reflectance data. Differences in luminance (perceived lightness) and saturation were significant, but there was no difference in hue 1 or hue 2. Hue 1 and 2 yield values based on a ratio of color channel or cone catch values (see main text), saturation is between 0 and 0.75, and brightness is reflectance between 0 and 1.

Figure 8. Carapace patterns from two crabs tested in experiment 1 showing their brightness when on a black background (left) and on a white background (right). The brightness of all images has been increased by 50% for illustrative purposes. Top crab has a carapace width of 7.1 mm, bottom crab has a width of 6.9 mm.
For saturation, there was a significant effect of individual [F(36, 73) = 7.82; P < 0.001], but not background [F(1, 73) = 3.02; P = 0.091], or order [F(1, 73) = 2.75; P = 0.106]. However, when two prominent outliers were removed then the results became significant for background: background [F(1, 71) = 6.35; P = 0.017], individual [F(35, 71) = 10.37; P < 0.001], but not order [F(1, 71) = 1.64; P = 0.208]. On average, crabs on white backgrounds had marginally higher saturation values by 0.009 (maximum difference = 0.077, minimum difference = −0.094; Figure 7).
For hue 1, there was a significant effect of individual [F(36, 73) = 7.15; P < 0.001], but only a borderline non-significant trend for background [F(1, 73) = 4.03; P = 0.052], and no effect of order [F(1, 73) = 1.11; P = 0.299]; Figure 7. For hue 2, there was a significant effect of individual [F(36, 73) = 6.09; P < 0.001] and order [F(1, 73) = 5.40; P = 0.026], but not background [F(1, 73) = 0.41; P = 0.525]. For hue 2 there were two prominent outliers, but when repeating the analysis without them the results were qualitatively the same.
As above, we compared the level of similarity between crabs and the background when individuals had been placed on each background type, here in terms of reflectance information. Crabs placed on a black background were more similar in brightness to the black background than were crabs when placed on the white background [W(37) = 65.0, P < 0.001; Figure 6B], but there was no significant difference in matching for color [W(37) = 273.0, P = 0.239]. This difference in brightness match was on average just over 4% reflectance, although some individuals had much larger differences that would have a much greater effect on concealment. When placed on a white background, crabs were more similar in brightness to the white background than were crabs after being placed on the black background [W(37) = 703.0, P < 0.001]. There was also a significant improvement in matching for color [W(37) = 205.0, P = 0.028].
Experiment 2: Red vs. Green
Avian visual modeling
For the calculations of hue, two PCs explained 95.0% of the variance (PC1: 83.1% and PC2: 11.9%). PC1 was equivalent to following color channel: hue 1 = (LW+MW)/(SW+UV), and PC2 was equivalent to following color channel: hue 2 = (LW+UV)/(MW+SW). These are directly comparable to those for experiment 1.
There was a significant difference between crabs on the red and green backgrounds for luminance [W(35) = 467.0, P = 0.013]; Figure 9. On average, crabs on green backgrounds had slightly higher luminance values by 0.007 (maximum difference = 0.067, minimum difference = −0.065). However, there were no significant differences between red and green backgrounds for saturation [W(35) = 326.0, P = 0.863], hue 1 [W(35) = 310.0, P = 0.941], or hue 2 [W(35) = 231.0, P = 0.171]. Thus, crabs show only minimal changes in color and luminance against these backgrounds.
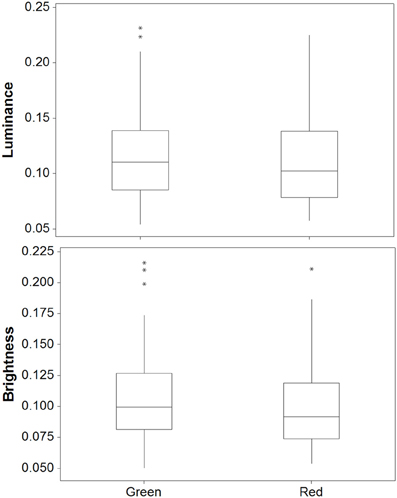
Figure 9. Luminance (top) and brightness (bottom) values for crabs in experiment 2 on red and green backgrounds [main boxes show medians plus inter-quartile range (IQR), whiskers are lowest and highest values that are within 1.5*IQR from the upper and lower quartiles, asterisks represent outliers].
For the discrimination modeling, JND values showed that very few differences in color would have been perceptible to avian predators (average JND = 1.351, maximum = 3.489, minimum = 0.194) with the vast majority of changes in crab coloration unlikely to be detectable (just 1 of 35 crabs had JND differences between green and red of more than 3.00); Figure 5B. When testing for whether JND values were greater than 1.00, values for color were significantly higher [W(35) = 452.0, P = 0.013]. However, average JND values were just 1.351, and values were not significantly greater than 3.00 [W(35) = 2.0, P > 0.950], suggesting that the crabs were not perceptually different in color between the two backgrounds. For luminance, the change between white and black backgrounds was also likely to be perceptible for only some crabs (average JND = 2.906, maximum = 12.839, minimum = 0.010), with just 13 of 35 crabs having JND differences between green and red of more than 3.00. When testing for whether JND values were greater than 1.00, values for luminance were significantly higher [W(35) = 531.0, P < 0.001]. However, luminance JND values were not significantly greater than 3.00 [W(35) = 245.0, P = 0.876].
Crucially, there was no difference in the level of matching for crabs to the red background after being placed on the red or the green background, either for color JNDs [W(35) = 280.0, P = 0.572] or luminance JNDs [W(35) = 375.0, P = 0.326]. This was also true when comparing crabs for the level of matching to the green background after being placed on the green or red background [color: W(35) = 273.0, P = 0.497; luminance: W(35) = 207.0, P = 0.078]. Therefore, the small changes in color and luminance reported above are unlikely to lead to a change in camouflage.
Reflectance
For the calculations of hue, two PCs explained 98.2% of the variance (PC1: 86.6% and PC2: 11.5%). PC1 was equivalent to the following color channel: hue 1 = LW/[(MW+SW+UV)/3], which is equivalent to long-wavelength vs. medium and short-wavelength reflectance, and PC2 was equivalent to following color channel: hue 2 = (LW+UV)/(MW+SW).
There was a significant difference between red and green backgrounds for brightness [W(35) = 458.0, P = 0.020]; Figure 9. Crabs on green backgrounds had higher brightness (reflectance) values, although in practice these differences were very small (average difference = 0.596%, max difference = 6.01%, min difference = −0.521). There were no significant differences between red and green backgrounds for saturation [W(35) = 304.0, P = 0.863], hue 1 [W(35) = 356.0, P = 0.507], or hue 2 [W(35) = 413.0, P = 0.110].
As with the visual modeling, there was no difference in the level of matching for crabs to the red background after being placed on the red or the green background, either for color [W(35) = 329.0, P = 0.825] or brightness [W(35) = 353.0, P = 0.539]. This was also true when comparing crabs for the level of matching to the green background after being placed on the green or red background [color: W(35) = 321.0, P = 0.928; brightness: W(35) = 282.0, P = 0.595].
Discussion
Here, we tested whether juvenile shore crabs changed in appearance when placed on backgrounds of different brightness (black or white) or color (red or green). We found that crabs were capable of changes in luminance (as judged by an avian predator) or brightness (a measure independent of any assumed predator visual system). Crabs became darker on a black background and lighter on a white background. Furthermore, a model of avian discrimination showed that this change in luminance is likely to be substantial enough to be readily detectable to bird predators. Indeed, it is possible when subjectively inspecting the photographs to see changes in some individuals. Perhaps most importantly, we show that the difference in the appearance of crabs is likely to improve their match to the background. When placed on a black background, crabs were a significantly better match to that background in luminance/brightness than when they had been placed on a white background. The converse is also true, whereby crabs that had been on a white background were a significantly better match to the white for luminance/brightness than when they had been on a black background. These differences were on average between 6 and 7 JNDs, implying that the level of change could equate to a genuine reduction in detection probability against the substrate. We also found some changes in color (hue and saturation) on the two background types, although whether such changes were detectable by a receiver is less likely and these rarely seem to equate to improved color matches to the background (see below). In experiment 2, we found some changes in brightness/luminance on the red and green backgrounds, but these were considerably smaller than in experiment 1.
In experiment 1, in addition to the clear differences in brightness/luminance, we found changes in saturation and hue among individuals when on the white and black backgrounds. Initially, this may suggest some adaptive color change response to the backgrounds. However, inspecting this result more carefully highlights the importance of not just considering statistical differences between the two treatments for a given color metric, but also the magnitude of that effect and whether it is detectable. Visual modeling of the color changes suggested that the differences were unlikely to be perceptible to a predatory bird (JNDS were less than 3.00), and the values based on reflectance metrics also showed small differences. We found that when crabs had been on the white background they were a closer match to the white for color than were crabs after being on the black background. However, this is also unlikely to be biologically relevant because the average difference in JNDs was around 1.00, and therefore small. Hence, it is unlikely that such levels of color change would have equated to an actual camouflage advantage for color. More likely, we think it possible that the changes in color were a by-product of the changes in pigment dispersion driven by the adaptive change in brightness. More work is needed to investigate this.
In experiment 2 we also found changes in brightness and luminance values of the crabs. However, these changes were small (on average less than a 1% change in individual reflectance between the backgrounds), with the visual modeling also showing that the differences were often below detection threshold. Again, this highlights the importance of considering the magnitude of color change alongside any statistical differences between groups. Interestingly no changes in color were detected in any of the three color metrics (saturation, hue 1 and hue 2) for either the avian or the reflectance information, and the color vision analysis showed that any changes in color were unlikely to be detected (JNDs were almost all less than 3.00, with an average of just over 1.00). Overall, our findings here show that while shore crabs could be capable of changes in response to background color, they show very little changes for these color stimuli. In line with this, we found no evidence that any changes in color or luminance/brightness equated to an improvement in camouflage. Crabs that had been placed on either a green or red background showed no differences in their level of match to either background type.
Overall, the above lack of color change is surprising because at the location where the crabs were collected the substrates vary greatly in appearance, including reds, browns, yellows, greens, and various shades of grays, corresponding to rocks, algae, seaweed, and sand. Therefore, changing color should be valuable. Although we controlled our red and green backgrounds for overall reflectance, we do not know exactly how the crabs perceive each background type. It is possible that red and green are imperceptible to the crabs, meaning that they judged the backgrounds as the same color. Also, if crabs cannot see longer wavelengths of light then the red background may have appeared darker than the green (although we note that this would predict greater differences in the achromatic response than the minimal changes observed here, which are unlikely to be perceptible or lead to changes in camouflage). What little work has been done on C. maenas vision is inconclusive. Wald (1968) concluded that shore crabs possess two pigment systems with short/medium-wavelength-sensitive and long-wavelength-sensitive receptors (sensitivity curves with peaks near 425 and 565 nm). However, Bruno et al. (1973) found that there is only one single receptor type with maximal sensitivity at 502—506 nm. Martin and Mote (1982) later found a small number of cells with sensitivity peaks around 440 nm, in addition to those with peaks around 508 nm. Other crustaceans do also seem to have color vision (see Kelber et al., 2003). Nonetheless, our primary test was to determine if crabs are capable of changes in color to better match that of the background. Even if there were differences in the luminance perception of the backgrounds this does not detract from the fact that in order to improve camouflage crabs should still be able to change their actual color in line with this, even if they use luminance-based visual mechanisms rather than color vision to do so. Given that the backgrounds were broadly based on natural colors in the environment this may offer an important advantage. It is, for example, well established that cuttlefish show abilities to match different background colors despite being color blind (Marshall and Messenger, 1996). Overall, work with other color types and/or more information about shore crab vision is needed to investigate this further.
As with an earlier study on juvenile ghost crabs (Stevens et al., 2013), the changes in brightness between the black and white backgrounds were highly variable among individuals. Why some individuals are capable of substantial changes in brightness (up to 19% reflectance change between white and black) whereas others change much less (below 1% reflectance change) is unclear, although our modeling suggests that changes in brightness may be perceptible in the majority of crabs in this experiment. This also translated into variation in terms of how well crabs could change to match the different backgrounds. As such, color change in some crabs is likely to lead to substantial improvements in concealment, whereas in others the effect will be smaller. We could find no evidence that this was related to crab size, although presumably above a certain size color change becomes less effective due to thickening of the carapace (Powell, 1962b; Crothers, 1968). Whether or not this variation relates to individual phenotype (e.g., pattern type) is also unclear, but again we could find no evidence that individuals with higher or lower average brightness values underwent greater changes. Given that larger changes in brightness on different backgrounds are likely to lead to significantly improved camouflage, further work is needed to discover why such inter-individual variation exists. One possibility is that changing color is costly in terms of energetics or diet, and only individuals in good condition can do this well. Another possibility is that some crabs may not have developed the necessary visual abilities to judge the background brightness. Secondary responses to the background are thought to be driven by the ratio of light falling directly on the eye compared to the amount of light reflected from the surface (Powell, 1962a; Keeble and Gamble, 1900; Sumner and Keys, 1929). If crabs have not yet developed the necessary morphology or processing to achieve this then it would affect their background response. Finally, it is possible that variation in color change is influenced by other factors, such as interactions with tidal, lunar, and day-night (circadian) color change cycles (Fingerman, 1956; Fingerman et al., 1958).
There are a number of questions that our study has not addressed. First, we are yet to quantify the change in camouflage against more natural backgrounds, and it is possible that color change could occur in this instance. In addition, our experiments were undertaken under artificial lights and it would be worthwhile doing experiments under more natural illumination conditions given that color change is likely to be influenced by light intensity in crabs (Brown and Sandeen, 1948; Powell, 1962a). Furthermore, we did not test the role of temperature on color change. Although the split design of our experiment controlled for the effects of temperature, it is possible that we would have detected color responses at different water temperatures. Previous work with shore crabs by Powell (1962a) found that on black backgrounds there was only an effect of temperature on pigment dispersion at 30°C but not at lower temperatures, whereas on white backgrounds increases in temperature caused an increase in black pigment concentration and white pigment dispersal. Our experiments were also undertaken on small juvenile crabs. Although significant color change is unlikely in large crabs due to loss of carapace transparency, it would be important in future work to determine how color change varies with size. Powell (1962a) also found some evidence of longer term adaptation in crabs to the background over a period of 18 d, and so it will be important to test for color change in crabs within the same molt over longer time periods, as well as across molts, and to assess how this influences camouflage. Finally, although a wealth of studies have shown that improvement in matching to a background decreases the likelihood of detection (as indeed is the essence of how background matching camouflage works), we have not here directly tested how improvement in matching of crabs equates to changes in the probability of detection. Behavioral experiments are required to confirm this effect.
Color change for camouflage is likely to be widespread in nature, and this seems especially true in aquatic and intertidal species. Given the vital importance to most animals of avoiding predators (and for predators to sneak upon their prey), and the fact that most environments are both spatially and temporally heterogeneous, being able to change appearance to tune camouflage to different backgrounds is likely to provide a substantial advantage. Furthermore, because animals must respond to the properties of their backgrounds with their visual systems to achieve a match, understanding the drivers of color change and camouflage can reveal a great deal about how vision works (Zylinski et al., 2009). In recent years, substantial work has sought to understand the types of crypsis that may exist (e.g., disruption, background matching, countershading, distraction, and so on; see above). However, while this has provided numerous insights into the mechanisms underlying camouflage, work has predominantly been undertaken in artificial systems and with artificial prey (e.g., Cuthill et al., 2005; Merilaita and Lind, 2005; Schaefer and Stobbe, 2006; Stevens et al., 2006; Rowland et al., 2008; Webster et al., 2013). There is a pressing need to understand the survival advantage and control of camouflage in real animals. Species that change color over varied timescales and habitats have great potential to address this shortcoming.
Author Contributions
Martin Stevens, Louisa E. Wood, and Alice E. Lown conceived, planned and designed the study. Louisa E. Wood and Alice E. Lown primarily conducted the first experiment and Alice E. Lown the second. Alice E. Lown and Louisa E. Wood calibrated the images and measured them under the guidance of Martin Stevens. Martin Stevens primarily undertook the analysis and visual modeling of the data and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Martin Stevens and Alice E. Lown were supported by a Biotechnology and Biological Sciences Research Council (BBSRC) David Phillips Research Fellowship (BB/G022887/1). We thank Peter Todd for various valuable discussions, and Jolyon Troscianko for help by writing the Image J calibration programmes and assistance with camera calibration. We thank two referees and the Associate Editor for constructive comments on the manuscript and work.
References
Abramowitz, A. A. (1937). The chromatophorotropic hormone of the crustacea: standardization, properties and physiology of the eye-stalk glands. Biol. Bull. 72, 344–365. doi: 10.2307/1537694
Akkaynak, D., Treibitz, T., Xiao, B., Gürkan, U. A., Allen, J. J., Demirci, U., et al. (2014). Use of commercial off-the-shelf digital cameras for scientific data acquisition and scene-specific color calibration. J. Opt. Soc. Am. A 31, 312–321. doi: 10.1364/JOSAA.31.000312
Ammermüller, J., Muller, J. F., and Kolb, H. (1995). The organization of the turtle inner retina. II. analysis of color-coded and directionally selective cells. J. Comp. Neurol. 358, 35–62. doi: 10.1002/cne.903580103
Atkins, D. (1926). On nocturnal colour change in the pea-crab (Pinnotheres veterum). Nature 117, 415–416. doi: 10.1038/117415b0
Barbosa, A., Mäthger, L. M., Buresch, K. C., Kelly, J., Chubb, C., Chiao, C.-C., et al. (2008). Cuttlefish camouflage: the effects of substrate contrast and size in evoking uniform, mottle or disruptive body patterns. Vis. Res. 48, 1242–1253. doi: 10.1016/j.visres.2008.02.011
Brown, F. A., and Sandeen, M. I. (1948). Responses of the chromatophores of the fiddler crab, Uca, to light and temperature. Physiol. Zool. 21, 361–371.
Bruno, M. S., Mote, M. I., and Goldsmith, T. H. (1973). Spectral absorption and sensitivity measurements in single ommatidia of the green crab, Carcinus. Vis. Res. 14, 653–658. doi: 10.1016/0042-6989(74)90060-1
Clarke, J. M., and Schluter, D. (2011). Colour plasticity and background matching in a threespine stickleback species pair. Biol. J. Linn. Soc. 102, 902–914. doi: 10.1111/j.1095-8312.2011.01623.x
Cook, L. M., Grant, B. S., Saccheri, I. J., and Mallet, J. (2012). Selective bird predation on the peppered moth: the last experiment of Michael Majerus. Biol. Lett. 8, 609–612. doi: 10.1098/rsbl.2011.1136
Crothers, J. H. (1968). The biology of the shore crab Carcinus maenas (L.): the life of the adult crab. Field Stud. 2, 579–614.
Cuthill, I. C., Stevens, M., Sheppard, J., Maddocks, T., Párraga, C. A., and Troscianko, T. S. (2005). Disruptive coloration and background pattern matching. Nature 434, 72–74. doi: 10.1038/nature03312
Darling, J. A., Bagley, M. J., Roman, J., Tepolt, C. K., and Geller, J. B. (2008). Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus. Mol. Ecol. 17, 4992–5007. doi: 10.1111/j.1365-294X.2008.03978.x
Darnell, M. Z. (2012). Ecological physiology of the circadian pigmentation rhythm in the fiddler crab Uca panacea. J. Exp. Mar. Biol. Ecol. 426–427, 39–47. doi: 10.1016/j.jembe.2012.05.014
Detto, T., Hemmi, J. M., and Backwell, P. R. Y. (2008). Colouration and colour changes of the fiddler crab, Uca capricornis: a descriptive study. PLoS ONE 3:e1629. doi: 10.1371/journal.pone.0001629
Eaton, M. D. (2005). Human vision fails to distinguish widespread sexual dichromatism among sexually monochromatic birds. Proc. Natl. Acad. Sci. U.S.A. 102, 10942–10946. doi: 10.1073/pnas.0501891102
Endler, J. A., and Mielke, P. W. (2005). Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431. doi: 10.1111/j.1095-8312.2005.00540.x
Espinosa, I., and Cuthill, I. C. (2014). Disruptive colouration and perceptual grouping. PLoS ONE 9:e87153. doi: 10.1371/journal.pone.0087153
Fingerman, M. (1955). Persistent daily and tidal rhythms of color change in Callinectes sapidus. Biol. Bull. 109, 255–264. doi: 10.2307/1538725
Fingerman, M. (1956). Difference in the tidal rhythms of color change of two species of fiddler crab. Biol. Bull. 110, 274–290. doi: 10.2307/1538833
Fingerman, M., Lowe, M. E., and Mobberly, W. C. (1958). Environmental factors involved in setting the phases of tidal rhythm of color change in the fiddler crabs Uca pugilator and Uca minax. Limnol. Oceanogr. 3, 271–282. doi: 10.4319/lo.1958.3.3.0271
Fingerman, M., and Yamamoto, Y. (1967). Daily rhythm of melanophoric pigment migration in eyestalkless fiddler crabs, Uca pugilator (Bosc). Crustaceana 12, 303–319. doi: 10.1163/156854067X00279
Garcia, J. E., Dyer, A. G., Greentree, A. D., Spring, G., and Wilksch, P. A. (2013). Linearisation of RGB camera responses for quantitative image analysis of visible and UV photography: a comparison of two techniques. PLoS ONE 8:e79534. doi: 10.1371/journal.pone.0079534
Grayson, J., and Edmunds, M. (1989). The causes of colour and colour change in caterpillars of the poplar and eyed hawkmoths (Laothoe populi and Smerinthus ocellata). Biol. J. Linn. Soc. 37, 263–279. doi: 10.1111/j.1095-8312.1989.tb01904.x
Hanlon, R. T. (2007). Cephalopod dynamic camouflage. Curr. Biol. 17, 400–404. doi: 10.1016/j.cub.2007.03.034
Hanlon, R. T., Chiao, C.-C., Mäthger, L. M., Barbosa, A., Buresch, K. C., and Chubb, C. (2009). Cephalopod dynamic camouflage: bridging the continuum between background matching and disruptive coloration. Phil. Trans. R. Soc. B 364, 429–437. doi: 10.1098/rstb.2008.0270
Hart, N. S., and Hunt, D. M. (2007). Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, S7–S26. doi: 10.1086/510141
Håstad, O., Victorsson, J., and Ödeen, A. (2005). Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl. Acad. Sci. U.S.A. 102, 6391–6394. doi: 10.1073/pnas.0409228102
Hemmi, J. M., Marshall, J., Pix, W., Vorobyev, M., and Zeil, J. (2006). The variable colours of the fiddler crab Uca vomeris and their relation to background and predation. J. Exp. Biol. 209, 4140–4153. doi: 10.1242/jeb.02483
Hogarth, P. J. (1978). Variation in the carapace pattern of juvenile Carcinus maenas. Mar. Biol. 44, 337–343. doi: 10.1007/BF00390898
Kang, C. K., Moon, J. Y., Lee, S. I., and Jablonski, P. G. (2012). Camouflage through an active choice of a resting spot and body orientation in moths. J. Evol. Biol. 25, 1695–1702. doi: 10.1111/j.1420-9101.2012.02557.x
Keeble, F. W., and Gamble, F. W. (1900). The colour-physiology of Hippolyte varians. Proc. R. Soc. Lond. 65, 461–468. doi: 10.1098/rspl.1899.0059
Kelber, A., Vorobyev, M., and Osorio, D. (2003). Animal colour vision - behavioural tests and physiological concepts. Biol. Rev. 78, 81–118. doi: 10.1017/S1464793102005985
Kelman, E., Baddeley, R., Shohet, A., and Osorio, D. (2007). Perception of visual texture, and the expression of disruptive camouflage by the cuttlefish, Sepia officinalis. Proc. Biol. Sci. 274, 1369–1375. doi: 10.1098/rspb.2007.0240
Kelman, E., Tiptus, P., and Osorio, D. (2006). Juvenile plaice (Pleuronectes platessa) produce camouflage by flexibly combining two separate patterns. J. Exp. Biol. 209, 3288–3292. doi: 10.1242/jeb.02380
Kettlewell, H. B. D. (1955a). Selection experiments on industrial melanism in the Lepidoptera. Heredity 9, 323–342. doi: 10.1038/hdy.1955.36
Kettlewell, H. B. D. (1955b). Recognition of appropriate backgrounds by the pale and black phases of Lepidoptera. Nature 175, 943–944. doi: 10.1038/175943a0
Komdeur, J. M., Oorebeek, M., van Overveld, T., and Cuthill, I. C. (2005). Mutual ornamentation, age, and reproductive performance in the European starling. Behav. Ecol. 16, 805–817. doi: 10.1093/beheco/ari059
Lovell, P. G., Ruxton, G. D., Langridge, K. V., and Spencer, K. A. (2013). Individual quail select egg-laying substrate providing optimal camouflage for their egg phenotype. Curr. Biol. 23, 260–264. doi: 10.1016/j.cub.2012.12.031
Lovell, P. G., Tolhurst, D. J., Paìrraga, C. A., Baddeley, R., Leonards, U., Troscianko, J., et al. (2005). Stability of the color-opponent signals under changes of illuminant in natural scenes. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 22, 2060–2071. doi: 10.1364/JOSAA.22.002060
Marshall, N. J., and Messenger, J. B. (1996). Colour-blind camouflage. Nature 382, 408–409. doi: 10.1038/382408b0
Martin, F. G., and Mote, M. I. (1982). Color receptors in marine crustaceans: a second spectral class of retinular cell in the compound eyes of Callinectes and Carninus. J. Comp. Physiol. A 145, 549–554. doi: 10.1007/BF00612820
McGaw, I. J., Edgell, T. C., and Kaiser, M. J. (2011). Population demographics of native and newly invasive populations of the green crab Carcinus maenas. Mar. Ecol. Prog. Ser. 430, 235–240. doi: 10.3354/meps09037
Merilaita, S., and Lind, J. (2005). Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. Biol. Sci. 272, 665–670. doi: 10.1098/rspb.2004.3000
Merilaita, S., and Stevens, M. (2011). “Chapter 2: crypsis through background matching,” in Animal Camouflage, eds M. Stevens and S. Merilaita (Cambridge: Cambridge University Press), 17–33. doi: 10.1017/CBO9780511852053.002
Mills, S. L., Zimova, M., Oyler, J., Running, S., Abatzoglou, J. T., and Lukacs, P. M. (2013). Camouflage mismatch in seasonal coat color due to decreased snow duration. Proc. Natl. Acad. Sci. U.S.A. 110, 7360–7365. doi: 10.1073/pnas.1222724110
Nachman, M. W., Hoekstra, H. E., and D'Agostino, S. L. (2003). The genetic basis of adaptive melanism in pocket mice. Proc. Natl. Acad. Sci. U.S.A. 100, 5268–5273. doi: 10.1073/pnas.0431157100
Nunes, V. L., Miraldo, A., Beaumont, M. A., Butlin, R. K., and Paulo, O. S. (2011). Association of Mc1r variants with ecologically relevant phenotypes in the European ocellated lizard, Lacerta lepida. J. Evol. Biol. 24, 2289–2298. doi: 10.1111/j.1420-9101.2011.02359.x
Ödeen, A., and Håstad, O. (2003). Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol. Biol. Evol. 20, 855–861. doi: 10.1093/molbev/msg108
Osorio, D., and Vorobyev, M. (2005). Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. Biol. Sci. 272, 1745–1752. doi: 10.1098/rspb.2005.3156
Osorio, D., Vorobyev, M., and Jones, C. D. (1999). Colour vision of domestic chicks. J. Exp. Biol. 202, 2951–2959.
Pietrewicz, A. T., and Kamil, A. C. (1977). Visual detection of cryptic prey by blue jays (Cyanocitta cristata). Science 195, 580–582. doi: 10.1126/science.195.4278.580
Pike, T. W. (2011). Using digital cameras to investigate animal colouration: estimating sensor sensitivity functions. Behav. Ecol. Sociobiol. 65, 849–858. doi: 10.1007/s00265-010-1097-7
Poulton, E. B. (1890). The Colours of Animals: Their Meaning and Use. Especially Considered in the Case of Insects, 2nd Edn. London: Kegan Paul, Trench Trübner, & Co. Ltd. doi: 10.5962/bhl.title.69899
Powell, B. L. (1962a). The responses of the chromatophores of Carcinus maenas (L. 1758) to light and temperature. Crustaceana 4, 93–102. doi: 10.1163/156854062X00120
Powell, B. L. (1962b). Distribution and rhythmical behaviour of the chromatophores of juvenile Carcinus maenas (L.). J. Anim. Ecol. 31, 251–261. doi: 10.2307/2139
Ramachandran, V. S., Tyler, C. W., Gregory, R. L., Rogers-Ramachandran, D., Duensing, S., Pillsbury, C., et al. (1996). Rapid adaptive camouflage in tropical flounders. Nature 379, 815–818. doi: 10.1038/379815a0
Rao, K. R., Fingerman, M., and Bartell, C. K. (1967). Physiology of the white chromatophores in the fiddler crab, Uca pugilator. Biol. Bull. 133, 606–617. doi: 10.2307/1539922
Rosenblum, E. B. (2006). Convergent evolution and divergent selection: lizards at the White Sands ecotone. Am. Nat. 167, 1–15. doi: 10.1086/498397
Rosenblum, E. B., Römpler, H., Schöneberg, T., and Hoekstra, H. E. (2010). Molecular and functional basis of phenotypic convergence in white lizards at White Sands. Proc. Natl. Acad. Sci. U.S.A. 107, 2113–2117. doi: 10.1073/pnas.0911042107
Rowland, H. M., Cuthill, I. C., Harvey, I. F., Speed, M. P., and Ruxton, G. D. (2008). Can't tell the caterpillars from the trees: countershading enhances survival in a woodland. Proc. Biol. Sci. 275, 2539–2545. doi: 10.1098/rspb.2008.0812
Sargent, T. D. (1966). Background selections of geometrid and noctuid moths. Science 154, 1674–1675. doi: 10.1126/science.154.3757.1674
Schaefer, H. M., and Stobbe, N. (2006). Disruptive coloration provides camouflage independent of background matching. Proc. Biol. Sci. 273, 2427–2432. doi: 10.1098/rspb.2006.3615
Siddiqi, A., Cronin, T. W., Loew, E. R., Vorobyev, M., and Summers, K. (2004). Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. A 207, 2471–2485. doi: 10.1242/jeb.01047
Skelhorn, J., Rowland, H. M., Delf, J., Speed, M. P., and Ruxton, G. D. (2011). Density-dependent predation influences the evolution and behavior of masquerading prey. Proc. Natl. Acad. Sci. U.S.A. 108, 6532–6536. doi: 10.1073/pnas.1014629108
Skelhorn, J., Rowland, H. M., Speed, M. P., and Ruxton, G. D. (2010). Masquerade: camouflage without crypsis. Science 327, 51. doi: 10.1126/science.1181931
Spottiswoode, C. N., and Stevens, M. (2011). How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. Biol. Sci. 278, 3566–3573. doi: 10.1098/rspb.2011.0401
Stevens, M. (2011). Avian vision and egg coloration: concepts and measurements. Avian Biol. Res. 4, 190–206. doi: 10.3184/175815511X13207790177958
Stevens, M., and Cuthill, I. C. (2006). Disruptive coloration, crypsis and edge detection in early visual processing. Proc. Biol. Sci. 273, 2141–2147. doi: 10.1098/rspb.2006.3556
Stevens, M., Cuthill, I. C., Windsor, A. M. M., and Walker, H. J. (2006). Disruptive contrast in animal camouflage. Proc. Biol. Sci. 273, 2433–2438. doi: 10.1098/rspb.2006.3614
Stevens, M., Hopkins, E., Hinde, W., Adcock, A., Connelly, Y., Troscianko, T., et al. (2007b). Field experiments on the effectiveness of eyespots as predator deterrents. Anim. Behav. 74, 1215–1227. doi: 10.1016/j.anbehav.2007.01.031
Stevens, M., and Merilaita, S. (2009). Introduction. Animal camouflage: current issues and new perspectives. Phil. Trans. R. Soc. B 364, 423–427. doi: 10.1098/rstb.2008.0217
Stevens, M., and Merilaita, S. (2011). Animal Camouflage: From Mechanisms and Function. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511852053
Stevens, M., Párraga, C. A., Cuthill, I. C., Partridge, J. C., and Troscianko, T. S. (2007a). Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237. doi: 10.1111/j.1095-8312.2007.00725.x
Stevens, M., Pei Rong, C., and Todd, P. A. (2013). Colour change and camouflage in the horned ghost crab Ocypode ceratophthalmus. Biol. J. Linn. Soc. 109, 257–270. doi: 10.1111/bij.12039
Stevens, M., Stoddard, M. C., and Higham, J. P. (2009). Studying primate color: towards visual system dependent methods. Int. J. Primatol. 30, 893–917. doi: 10.1007/s10764-009-9356-z
Stoddard, M. C., and Prum, R. O. (2008). Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of new world buntings. Am. Nat. 171, 755–776. doi: 10.1086/587526
Stuart-Fox, D., and Moussalli, A. (2008). Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol. 6:e25. doi: 10.1371/journal.pbio.0060025
Stuart-Fox, D., and Moussalli, A. (2009). Camouflage, communication and thermoregulation: lessons from colour changing organisms. Phil. Trans. R. Soc. B 364, 463–470. doi: 10.1098/rstb.2008.0254
Stuart-Fox, D., Moussalli, A., Marshall, N. J., and Owens, I. P. F. (2003). Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim. Behav. 66, 541–550. doi: 10.1006/anbe.2003.2235
Stuart-Fox, D., Moussalli, A., and Whiting, M. J. (2008). Predator-specific camouflage in chameleons. Biol. Lett. 4, 326–329. doi: 10.1098/rsbl.2008.0173
Sumner, F. B., and Keys, A. B. (1929). The effects of differences in the apparent source of illumination upon the shade assumed by a flatfish on a given background. Physiol. Zool. 2, 495–504.
Tankus, A., and Yeshurun, Y. (2009). Computer vision, camouflage breaking and countershading. Phil. Trans. R. Soc. B 364, 529–536. doi: 10.1098/rstb.2008.0211
Thayer, G. H. (1909). Concealing-Coloration in the Animal Kingdom: An Exposition of the Laws of Disguise Through Color and Pattern: Being a Summary of Abbott H. Thayer's Discoveries. New York, NY: Macmillan. doi: 10.5962/bhl.title.57368
Todd, P. A., Briers, R. A., Ladle, R. J., and Middleton, F. (2006). Phenotype-environment matching in the shore crab (Carcinus maenas). Mar. Biol. 148, 1357–1367. doi: 10.1007/s00227-005-0159-2
Todd, P. A., Oh, J., Loke, L. H. L., and Ladle, R. J. (2012). Multi-scale phenotype-substrate matching: evidence from shore crabs (Carcinus maenas L.). Ecol. Complex. 12, 58–62. doi: 10.1016/j.ecocom.2012.09.005
Twig, G., and Perlman, I. (2004). Homogeneity and diversity of color-opponent horizontal cells in the turtle retina: consequences for potential wavelength discrimination. J. Vis. 4, 403–414. doi: 10.1167/4.5.5
Umbers, K. D. L., Fabricant, S. A., Gawryszewski, F. M., Seago, A. E., and Herberstein, M. E. (2014). Reversible colour change in Arthropoda. Biol. Rev. doi: 10.1111/brv.12079. [Epub ahead of print].
Vignieri, S. N., Larson, J. G., and Hoekstra, H. E. (2010). The selective advantage of crypsis in mice. Evolution 64, 2153–2158. doi: 10.1111/j.1558-5646.2010.00976.x
Vorobyev, M., and Osorio, D. (1998). Receptor noise as a determinant of colour thresholds. Proc. Biol. Sci. 265, 351–358. doi: 10.1098/rspb.1998.0302
Vorobyev, M., Osorio, D., Bennett, A. T. D., Marshall, N. J., and Cuthill, I. C. (1998). Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633. doi: 10.1007/s003590050286
Wald, G. (1968). Single and multiple visual systems in arthropods. J. Gen. Physiol. 51, 125–156. doi: 10.1085/jgp.51.2.125
Wallace, A. R. (1889). Darwinism. An Exposition of the Theory of Natural Selection With Some of its Applications. London: Macmillan & Co.
Webster, R. J., Hassall, C., Herdman, C. M., and Sherratt, T. N. (2013). Disruptive camouflage impairs object recognition. Biol. Lett. 9:20130501. doi: 10.1098/rsbl.2013.0501
Westland, S., and Ripamonti, C. (2004). Computational Colour Science using MATLAB. Chichester: John Wiley & Sons Ltd. doi: 10.1002/0470020326
Keywords: camouflage, predation, color change, coloration, anti-predator
Citation: Stevens M, Lown AE and Wood LE (2014) Color change and camouflage in juvenile shore crabs Carcinus maenas. Front. Ecol. Evol. 2:14. doi: 10.3389/fevo.2014.00014
Received: 07 February 2014; Accepted: 11 April 2014;
Published online: 05 May 2014.
Edited by:
Wayne Iwan Lee Davies, University of Western Australia, AustraliaReviewed by:
Thomas Cronin, University of Maryland Baltimore County, USAJan M. Hemmi, The University of Western Australia, Australia
Copyright © 2014 Stevens, Lown and Wood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Stevens, Centre for Ecology and Conservation, University of Exeter, Penryn Campus, Penryn, Cornwall, TR10 9FE, UK e-mail:bWFydGluLnN0ZXZlbnNAZXhldGVyLmFjLnVr
 Martin Stevens
Martin Stevens Alice E. Lown
Alice E. Lown Louisa E. Wood
Louisa E. Wood