
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Ethol., 18 November 2024
Sec. Adaptation and Evolution
Volume 3 - 2024 | https://doi.org/10.3389/fetho.2024.1484454
This article is part of the Research TopicSexual Selection and Evolutionary FitnessView all 6 articles
Female preference for longer eyespan has driven exaggerated sexual dimorphism in several species of stalk-eyed flies. Longer eyespan increases a fly’s moment of inertia, and flies experience significant increase in body mass across age as they mature sexually. These costs may impact flight behavior and fitness through maneuverability and predator evasion, and appear ameliorated by co-selection for compensatory traits, as flies with longer eyespans tend to have larger thoraces and wings, allowing them to perform turns similar to flies with shorter eyespans. However, the capacity to compensate for a potentially costly ornament may not be limited to morphological traits which are fixed at the time of eclosion: as flies age, they also accumulate thorax mass and improve their flight performance. The purpose of this study was to investigate the compensatory ability of two populations of stalk-eyed flies (Teleopsis dalmanni and Diasemopsis meigenii) through comparing morphology and flight performance relative to eyespan. ‘Over-compensators’ should exhibit greater morphological and/or performance traits relative to eyespan, whereas ‘under-compensators’ should exhibit relatively less across these metrics. Flight performance was assessed using high-speed videography and variable-density gas-mixtures to determine maximal flight capacity. Young adult flies eclosed as ‘under-compensators’, with less thorax mass, wing velocity and flight capacity relative to their eyespan as compared to older flies. As flies aged and accumulated thorax mass, they became ‘over-compensators’. Thus, compensation for long eye-stalks is not a fixed trait; instead, variation in compensatory ability appears to be associated with the development of thorax muscle and flight performance across age.
Sexual-selection on morphological traits may lead to the exaggeration of ‘ornaments’ over evolutionary time. Although ornaments may reflect the quantity and quality of investment displayed by potential mates, ornaments should be costly to those that bear them (Zahavi, 1975). However, despite the intuition that exaggerated ornaments should impact locomotor performance and behaviors, ornaments have often evolved with little to no decrease in performance (Kotiaho, 2001; Lailvaux and Irschick, 2006; Husak and Swallow, 2011). Husak and Swallow (2011) proposed that, concomitant to female choice of ornaments, direct selection on morphology interacts with the detrimental effects of the ornament to indirectly drive the evolution of performance. Thus, natural selection may limit elaboration and exaggeration of ornaments (Andersson, 1994; Kotiaho, 2001), and selection on the integrated whole organism may result in correlated selection for compensatory traits that reduce the putative negative effects of ornaments (Iwasa et al., 1991; Møller, 1996; Oufiero and Garland, 2007; Irschick et al., 2008; Swallow et al., 2009), which may occur at multiple stages of development and life history (Stearns, 1992; Charnov, 1993; Pitnick et al., 1995; Lailvaux and Husak, 2014).
Stalk-eyed flies (family Diopsidae) are an attractive model-system to explore the interaction between a costly, sexually-selected ornament and morphological traits that offset performance costs. These dipterans possess peculiar head morphology (Supplementary Figure S1), with eyes located laterally on long peduncles (Burkhardt and de la Motte, 1983). Dimorphic species within this family display exaggerated eyespan length in males which, influenced through both female choice and male-male competition, contributes to increased mating success (Burkhardt and de la Motte, 1985; Panhuis and Wilkinson, 1999; Small et al., 2009; Burkhardt and de la Motte, 1987, Burkhardt and de la Motte, 1988; Wilkinson et al., 1998). These exaggerated ornaments in males should impose costs on flight behaviors and maneuvering through increased moment of inertia. However, flies with larger eyespans possess larger thoraces and wings which contribute to their compensatory ability, allowing males to perform turning manuevers similar to females with shorter eyespans (Husak et al., 2011; Swallow et al., 2000; Ribak and Swallow, 2007).
Adult stalk-eyed flies reach sexual maturity after 3-4 weeks of age (Baker et al., 2001; Bath et al., 2015; Bellamy et al., 2013; Bubak et al., 2016; Egge et al., 2011; Pomiankowski et al., 2005; Reguera et al., 2004; Rogers et al., 2005). Growth of the testes and accessory glands increase in length by 200-300% over this period (Baker et al., 2003) and contribute to a significant increase in abdominal mass (Vance et al., 2023). This growth should further impair flight performance across age, particularly because the abdomen does not contribute to aerodynamic force production. Although the dimensions of the thorax and wings are fixed post-eclosion, thorax mass increases with age, improving wing velocity and maximal flight capacity as flies mature sexually (Vance et al., 2023). Thus, compensatory ability may not be determined solely by fixed traits, such as thorax or wing size; rather, compensatory ability may be facilitated by age-related variation in thorax mass. Within a population composed of individuals spanning a broad range of age, there should exist ‘under-compensators’ possessing lesser thorax mass relative to their eyespan, and ‘over-compensators’ possessing greater thorax mass relative to their eyespan.
The purpose of this study is to characterize the relationship between morphological compensation and flight performance of male stalk-eyed flies, Teleopsis dalmanni and Diasemopsis meigenii. Both species are dimorphic, where female preference has driven the exaggeration of eyespan in males. Whereas prior research on compensatory morphology has focused on older, sexually-mature adults (Husak et al., 2011), both T. dalmanni and D. meigenii exhibit significant variation in thorax, abdominal and total body mass, and flight performance, as they age (Vance et al., 2023). Thus, life-time compensatory ability may not be best-characterized by a fixed morphological trait, such as wing size; rather, compensatory ability may instead change as thorax mass, a proxy for the flight musculature, increases with age. We hypothesize that ‘over-compensators’ will demonstrate greater relative flight performance than ‘under-compensators’, and that compensatory ability will increase with age.
Specific details of the methods used to collect and analyze morphological and flight performance data are described in Vance et al. (2023). Adult Teleopsis dalmanni and Diasemopsis meigenii (Supplementary Figure S1A) were collected within one day post-eclosion and housed in 3L chambers, separated by age and species. Male flies (T. dalmanni, n=123; D. meigenii. n=84) selected for assessment of flight performance and allometry possessed intact wings and were free of morphological defect. Flight performance was assessed using variable density, normoxic gas mixtures which consisted of oxygen and nitrogen and/or helium (Dudley, 1995; Vance et al., 2009), and ranged from normodense air (0% Heliox: 21% O2, 79% N2; 1.21kg m–3) to hypodense heliox (100% Heliox: 21% O2, 79% He; 0.41kg m–3) in 0.08kg m–3 increments. An interval-halving method was used to determine the order of hypodense gases presented to the fly, beginning with air. Because the aerodynamic power required to maintain hovering flight is inverse to gas density, maximal flight capacity was determined to be the least-dense gas (LDG, in percent-heliox) where hovering flight was observed.
The gasses were mixed using an electronic flow controller (Sable Systems MFC-4) and valves (Tylan FC-2910), which were plumbed into an 8L cylindrical acrylic chamber. During any given gas mixture condition, flies were flown until sustained hovering flight was observed and recorded, or hovering flight was attempted but failed. Hovering flight kinematics were determined from the wing motions in the horizontal plane, recorded by a high-speed (5930 fps) digital video camera (RedLake IDT MotionPro N3-S4). The camera was oriented above the flight chamber and focused such that the depth of field was in focus in the middle 1/3 of the chamber, sufficiently away from the bottom and sides of the chamber to avoid aerodynamic ‘ground’ and ‘edge’ effects (Supplementary Figure S1B). The digital video sequences were analyzed using DLTdv8 (Hedrick, 2008). Flight kinematics were calculated according to Vance and Roberts (2014): wingbeat frequency and wing stroke amplitude (Supplementary Figure S1C) were determined from the average of 10 successive wingstrokes, and average wing velocity was calculated from these kinematics and individual wing-length data. Following assessment of flight performance, flies were euthanized, weighed, dissected and photographed. Custom software (MatLab, The Mathworks) was used to determine eyespan (the distance from the lateral edges of the right and left eyes), thorax and wing morphometrics.
Multiple regression was used to determine the effects of eyespan and body mass on thorax mass; the effects of eyespan and thorax mass on wing velocity; and, the effects of eyespan and wing velocity on maximal flight capacity. Multiple regression was also used to determine the effects of eyespan and age on body mass, thorax mass, wing velocity and maximal flight capacity; because we predict logarithmic growth across age due to the constrains of the exoskeleton, the natural log of age was used for this analysis. Analysis of compensatory traits was performed using methods modified from Husak et al. (2011). Linear regression was used to analyze the relationships of morphology and flight performance across eyespan for each species. Relative values for body mass, thorax mass, wing velocity and flight capacity were calculated from the residual values of each observation relative to the linear regression across eyespan. Because of the mechanistic relationships between these variables, relative thorax mass was evaluated across relative body mass; relative wing velocity was evaluated across relative thorax mass; and, relative flight capacity was evaluated across relative wing velocity. Logarithmic regression was used to evaluate the ontogeny of relative thorax mass, wing velocity and flight capacity across age. Finally, individuals were grouped as ‘over-compensators’ (positive residuals) or ‘under-compensators’ (negative residuals) based on the regression of thorax mass across eyespan. Analysis of variance (ANOVA) was then used to compare the observed values for morphology (eyespan, thorax and wing size), thorax mass and flight performance between the ‘over-compensating’ and ‘under-compensating’ groups.
Evaluating variation in these populations naïve to the age of individuals, flies with longer eyespans were generally heavier (linear regression: T. dalmanni, Td, R2 = 0.19; D. meigenii, Dm R2 = 0.18) and had greater thorax mass (Td, R2 = 0.12; Dm, R2 = 0.20) than flies with shorter eyespans (Figures 1A, B). Wing velocity had no relationship to eyespan in T. dalmanni (Figure 1C; R2<0.01) or D. meigenii (R2 = 0.02), and maximal flight capacity had no relationship to eyespan (Figure 1D; R2 = 0.01 for both species). Multiple regression for the effects of eyespan and body mass on thorax mass revealed no effect of eyespan (P>0.05) and a significant effect of body mass (P<0.001) for both species (Supplementary Table S1; multiple regression: Td, R2 = 0.89; Dm, R2 = 0.93). There was a significant effect of eyespan (P<0.001) and thorax mass (P<0.001) on wing velocity for both species (Td, R2 = 0.80; Dm, R2 = 0.70). And, there was no effect of eyespan (P>0.05) and a significant effect of wing velocity (P<0.001) on flight capacity for both species (Td, R2 = 0.45; Dm, R2 = 0.46). These relationships tested via multiple regression persisted when analyzing the eyespan-relative values (Figure 2): strong, positive relationships were observed between relative body mass and relative thorax mass (Figure 2A; linear regression: Td, R2 = 0.88; Dm, R2 = 0.92), between relative thorax mass and relative wing velocity (Figure 2B; Td, R2 = 0.80; Dm, R2 = 0.70), and between relative wing velocity and relative flight capacity (Figure 2C; Td, R2 = 0.44; Dm, R2 = 0.45).
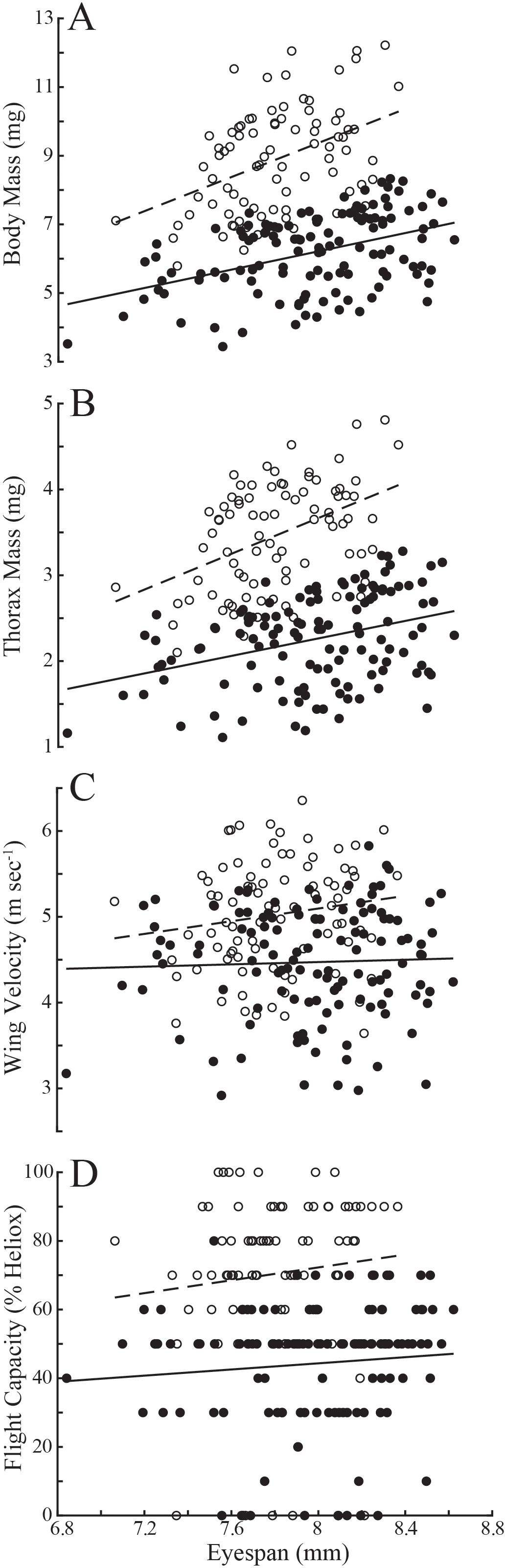
Figure 1. Variation in morphology and flight performance of T. dalmanni (filled circles) and D. meigenii (open circles) across eyespan. (A) Body mass: T. dalmanni (linear regression, solid line), Mb = -4.45 + 1.33eyespan, R2 = 0.19; D. meigenii (dashed line), Mb = -10.48 + 2.48eyespan, R2 = 0.18. (B) Thorax mass: T. dalmanni (solid line), Mt = -1.81 + 0.51eyespan, R2 = 0.12; D. meigenii (dashed line), Mt = -4.66 + 1.04eyespan, R2 = 0.20. (C) Wing velocity T. dalmanni (solid line), v = 3.93 + 0.07eyespan, R2 = 0.001; D. meigenii (dashed line), v = 2.14 + 0.37eyespan, R2 = 0.02. (D) Flight capacity: T. dalmanni (solid line), LDG = 8.51 + 4.48eyespan, R2 = 0.01; D. meigenii (dashed line), LDG = -2.60 + 9.36eyespan, R2 = 0.01. Maximal flight capacity, LDG, is defined as the least dense gas allowing for flight, where 0% Heliox is air (21% O2, 79% N2, 0% He; density: 1.21 kg m-3) and 100% is pure Heliox (21% O2, 0% N2, 79% He; density: 0.41 kg m-3).
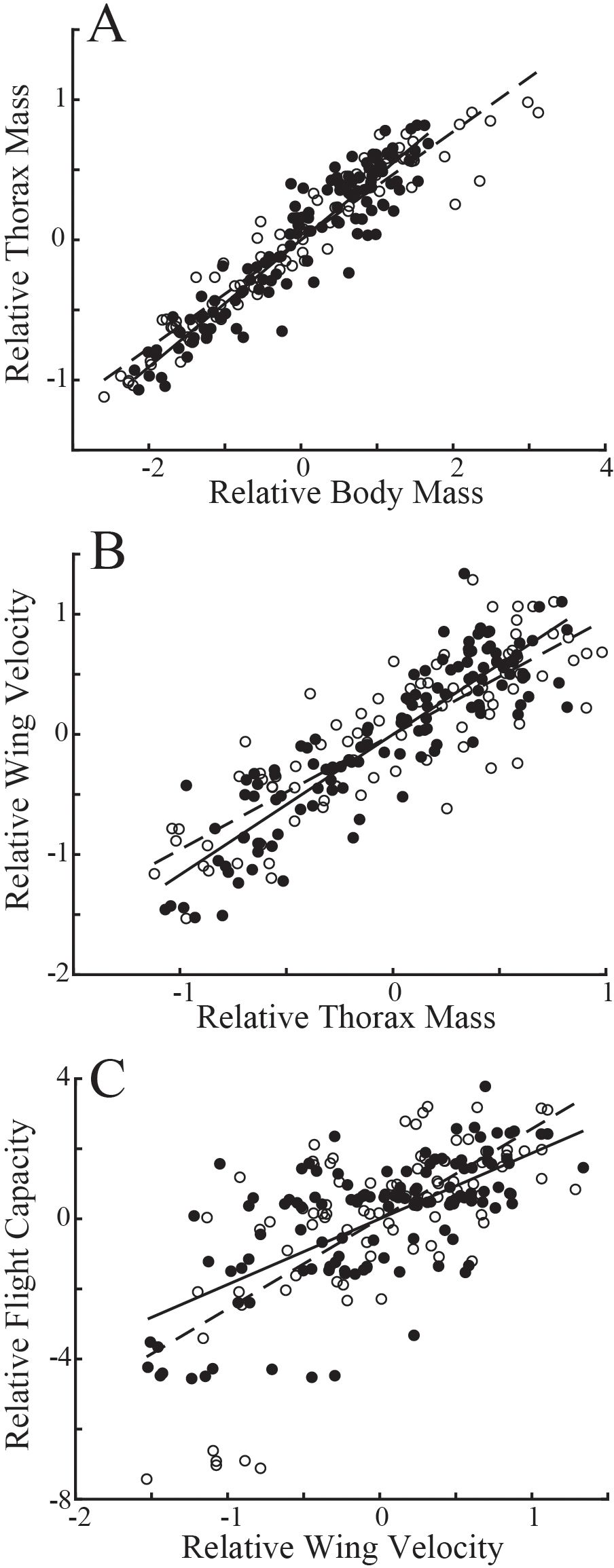
Figure 2. Relationship between compensatory trait residuals in T. dalmanni (filled circles) and D. meigenii (open circles) across eyespan. (A) Relative thorax mass: T. dalmanni (linear regression, solid line), Mt = 0.45 Mb, R2 = 0.88; D. meigenii (dashed line), Mb = 0.39Mt, R2 = 0.91. (B) Relative wing velocity: T. dalmanni (solid line), v = 1.17 Mt, R2 = 0.80; D. meigenii (dashed line), v = 0.96 Mt, R2 = 0.70. (C) Relative Flight capacity: T. dalmanni (solid line), LDG = 1.87v, R2 = 0.44; D. meigenii (dashed line), LDG = 2.57v, R2 = 0.45.
Multiple regression for the effects of eyespan and age were significant for body mass (Table 1; P<0.001 for all comparisons; Td, R2 = 0.77; Dm, R2 = 0.71) and thorax mass (P<0.001 for all comparisons; Td, R2 = 0.76; Dm, R2 = 0.76). Multiple regression for the effects of eyespan on was significant for T. dalmanni (P=0.003) but not for D. meigenii (P=0.536), and was significant for age on wing velocity for both species (P<0.001; Td, R2 = 0.78; Dm, R2 = 0.73); and, the effects of eyespan were not significant (P>0.05) but age was significant on maximal flight capacity for both species (P<0.001; Td, R2 = 0.26; Dm, R2 = 0.52). Again, these relationships evaluated with multiple regression persisted when analyzing the eyespan-relative values across age (Figure 3): Relative thorax mass (Figure 3A; logarithmic regression: Td, R2 = 0.72; Dm, R2 = 0.68), relative wing velocity (Figure 3B; Td, R2 = 0.77; Dm, R2 = 0.71), and relative flight capacity (Figure 3C; Td, R2 = 0.26; Dm, R2 = 0.51) increased logarithmically across age.
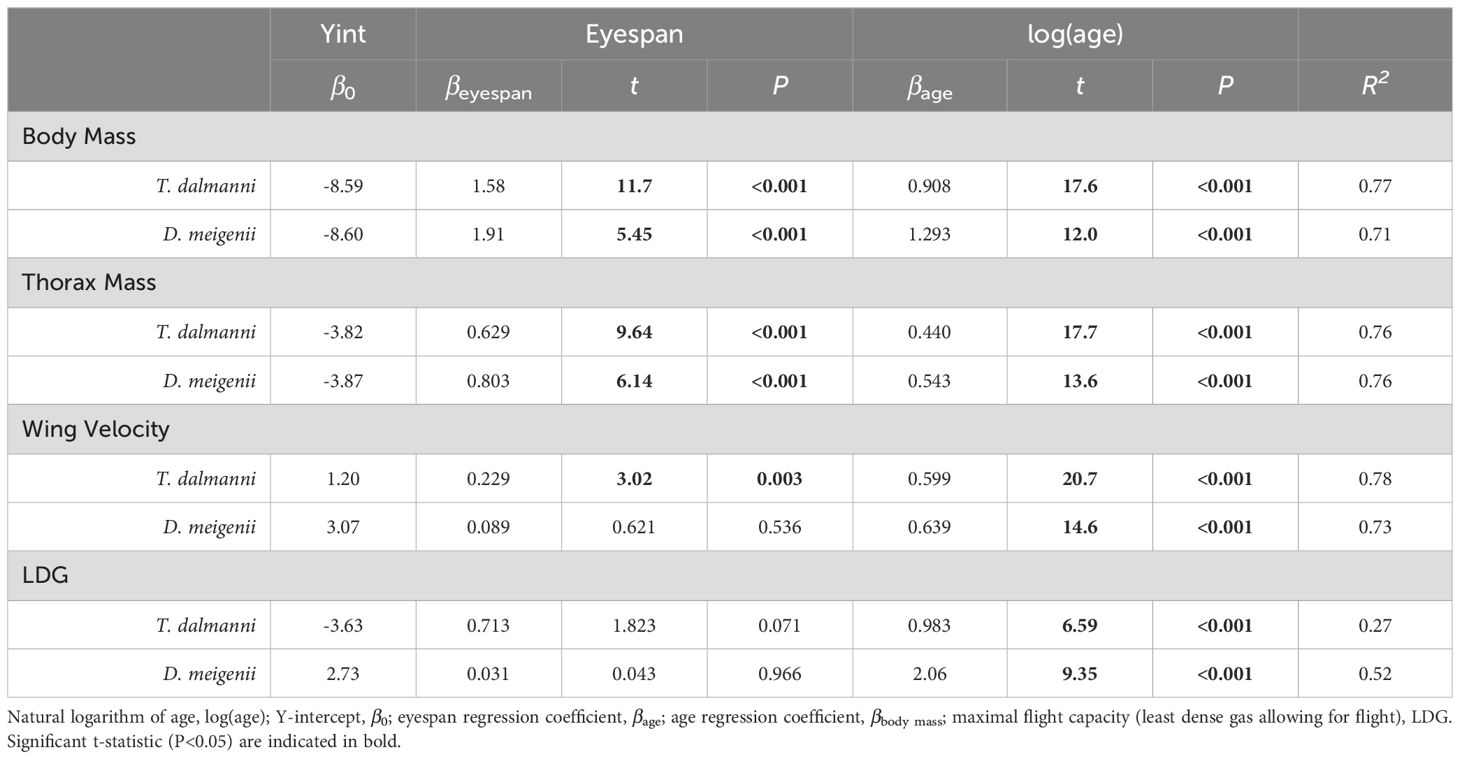
Table 1. Multiple regression for the effects of eyespan and age on body mass, thorax mass and maximal flight performance.
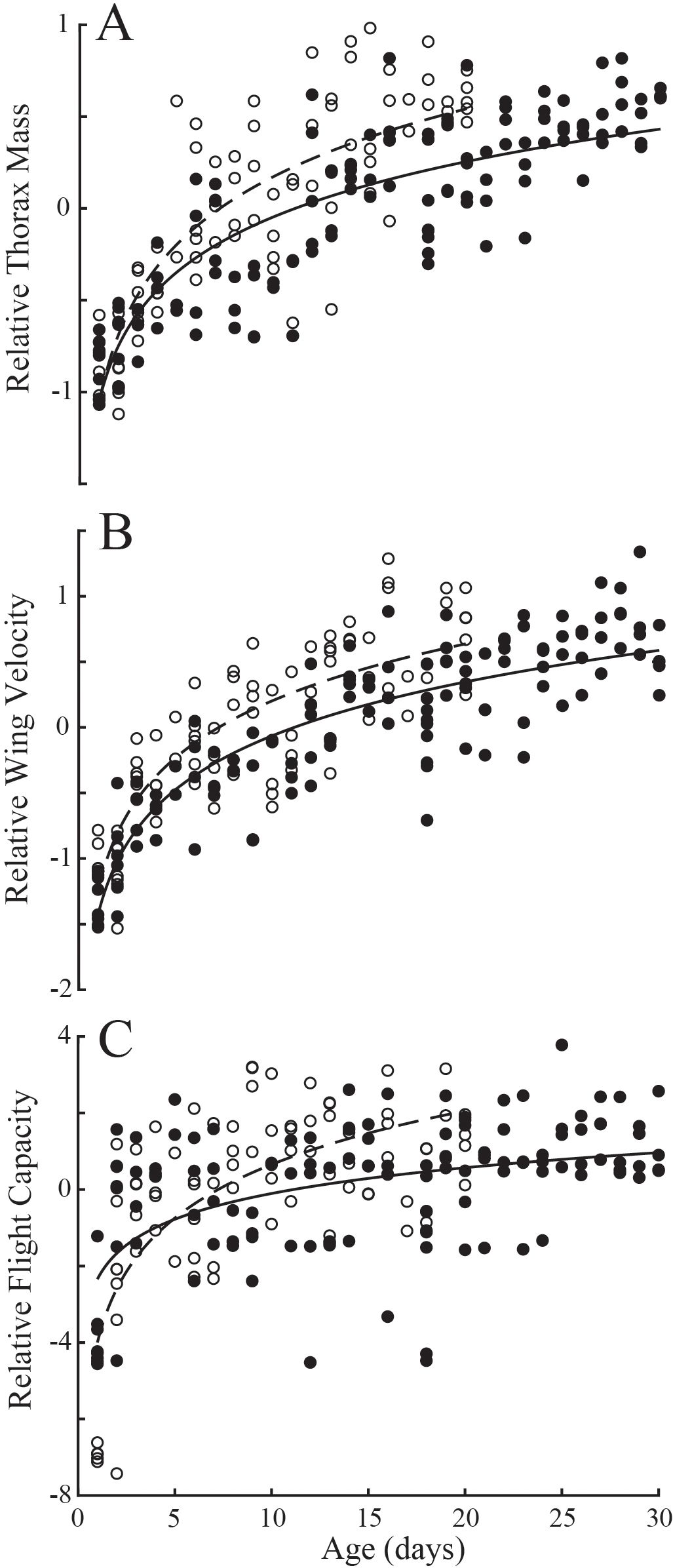
Figure 3. Variation in compensatory trait residuals across age in T. dalmanni (filled circles) and D. meigenii (open circles). (A) Relative thorax mass: T. dalmanni (logarithmic regression, solid line), Mt = -1.05 + 0.44log(age), R2 = 0.72; D. meigenii (dashed line), Mt = -1.06 + 0.53log(age), R2 = 0.68. (B) Relative wing velocity: T. dalmanni (solid line), v = -1.43 + 0.59log(age), R2 = 0.77; D. meigenii (dashed line), v = -1.24 + 0.63log(age), R2 = 0.71. (C) Relative flight capacity: T. dalmanni (solid line), LDG = -2.34 + 0.97log(age), R2 = 0.26; D. meigenii (dashed line), LDG = -4.00 + 2.02log(age), R2 = 0.51.
Based on classification via relative thorax size (residuals calculated from linear regressions between eyespan and thorax mass), the eyespans of ‘over-compensators’ was not different than ‘under-compensators’ (Supplementary Figure S2; ANOVA: P>0.05 for both species). Likewise, there was no difference in thorax width or wing area between ‘over-’ and ‘under-compensators’ (P>0.05 for all comparisons). However, wing length of ‘over-compensators’ was 1.6% longer in T. dalmanni (F1,122 = 4.49; P=0.036) and 1.8% longer in D. meigenii (F1,83 = 5.0; P=0.028) than ‘under-compensators’. T. dalmanni ‘over-compensators’ had 32% heavier bodies (Supplementary Figure S2; F1,122 = 136.9; P>0.001), 48% heaver thoraces (F1,122 = 212.9; P>0.001), 27% greater wing velocity (F1,122 = 219.9; P>0.001), and 57% greater flight capacity (F1,122 = 43.4; P>0.001) than ‘under-compensators’. D. meigenii ‘over-compensators’ had 30% heavier bodies (Supplementary Figure S2; F1,83 = 105.9; P>0.001), 33% heavier thoraces (F1,83 = 140.4; P>0.001), 21% greater wing velocity (F1,83 = 94.7; P>0.001), and 38% greater flight capacity (F1,83 = 21.9; P>0.001) than ‘under-compensators’. ‘Over-compensators’ were older flies as compared to ‘under-compensators’ (Td, F1,122 = 150.0, P<0.001; Dm, F1,83 = 86.3, P<0.001). The logarithmic regression representing the population as a function of age transitioned from ‘under-compensating’ to ‘over-compensating’ at 11.1 days for T. dalmanni and 7.2 days for D. meigenii (Figure 3).
In sexually-dimorphic species of stalk-eyed flies, female preference for long eyespans has led to the exaggeration of this ornament in males (Burkhardt and de la Motte, 1985), which significantly affects moment of inertia and flight dynamics (Hedrick et al., 2009), potentially impacting flight behaviors, maneuverability and predator avoidance. Co-evolution of thorax and wing size (Ribak and Swallow, 2007; Husak et al., 2011) reflects compensatory investment into the ‘flight motor’ to offset locomotor costs imposed by bearing longer eyespans. The development of physical traits and behaviors across flies’ life-history, however, doesn’t cease upon eclosion, nor remain fixed across the adult-stage. Sexual maturation in adult stalk-eyed flies occurs over several weeks, resulting in profound increases in abdominal mass (Vance et al., 2023). Since the abdomen does not contribute to the flight motor, this increase in mass should impair flight-dependent behaviors (Isaacs and Byrne, 1998) and reduce flies’ available aerodynamic reserve capacity (Vance et al., 2009). However, relative thorax mass also increases with age (Figure 3A; Table 1), and maintains a very tight relationship with relative body mass (Figure 2A; Supplementary Table S1), which generally maintains the ratio of thorax mass to body mass (Vance et al., 2023).
Although flies with longer eyespan have larger thoraces and wings, fixed morphological traits −although evidence for compensation (Ribak and Swallow, 2007; Husak et al., 2011)− may not serve as the best index for compensatory ability. Instead, eyespan-relative thorax mass was used to categorize individuals within the populations as either ‘under-compensators’ or ‘over-compensators’, as the growth in thorax mass that accompanies sexual maturation has a significant effect on how an individual compares to the population average. All adult flies 1-5 days old were ‘under-compensators’, whereas all 26- to 30-day old T. dalmanni, and all but one 16- to 20-day old D. meigenii, were ‘over-compensators’ (Figure 3A). Likewise, relative wing velocity was strongly associated with relative thorax mass (Figure 2B), and trends in relative wing velocity between young and old flies (Figure 3B) were similar to those observed in relative thorax mass described above. Thus, young adults eclose as ‘under-compensators’ and as they acquire resources and accumulate muscle tissue, they improve their wingbeat frequency and ability to generate wing velocity (Vance et al., 2023), developing into ‘over-compensators’ as they age.
Relative flight capacity was associated with relative wing velocity (Figure 2C) and increased with age in both species (Figure 3C). This relationship is not as distinct as that observed for relative thorax mass and wing velocity. As flies become heavier with age, aerodynamic power demands increase, yet individual variation in wing size, abdomen and overall body mass may interact with the improvements in the ‘flight motor’ (Vance et al., 2023). Nonetheless, the concurrent development of the thorax generally offsets the mass associated with abdominal growth, resulting in improved relative flight capacity across age (Figure 3C). The compensatory ability of T. dalmanni and D. meigenii transitioned from ‘under-compensating’ to ‘over-compensating’ at approximately 11 and 7 days of age, respectively. ‘Over-compensating’ T. dalmanni and D. meigenii had 57% and 38% greater flight capacity, respectively, than ‘under-compensators’, demonstrating a significant behavioral advantage that accompanies the development of the ‘flight motor’.
Prior studies have focused on sexually-mature flies, presumably to avoid the variation in body mass during sexual maturation in young adults from confounding allometric relationships and behaviors (Egge et al., 2011; Ribak and Swallow, 2007; Husak et al., 2011). Eyespan had a significant effect on body mass and thorax mass (Table 1), suggesting that at any age, the larger body planforms associated with longer eyespans were capable of housing more tissue within the body’s segments. This alone may generally compensate for the locomotor costs of bearing larger eyespans, as eyespan had no effect on flight capacity in any comparison tested here. Longer eyespans do not predispose individuals to be better fliers; however, age-related variation in thorax mass impacts the life-history of every fly. The improvement in relative thorax mass, wing velocity and flight capacity across age in T. dalmanni and D. meigenii suggests that compensatory ability is not a constant trait but, rather, develops alongside sexual maturation (Figure 3). Young, sexually-immature flies, in addition to their reduced fecundity, ‘under-compensate’ for the size of their ornament by having lesser thorax mass relative to eyespan than older flies (Figure 3A). This ‘under-compensation’ results in reduced relative wing velocity and flight capacity as compared to ‘over-compensators’, which may impact flight-dependent behaviors, such as maneuvering and predator evasion, and decrease survivorship and fitness. Despite most young flies ‘under-compensating’ for relative flight capacity, a significant fitness advantage should exist for those flies that demonstrate relative flight capacities near or exceeding the population mean as early as possible in their life-history. On the contrary, older flies that fail to accumulate sufficient thorax mass (or accumulate disproportionately greater abdominal mass or possess relatively small wings) may remain ‘under-compensators’ and exhibit flight performance below the population mean; these flies may be at a fitness disadvantage, even if sexually viable.
Thorax size and size wing size in stalk-eyed flies have also evolved to compensate for the inertial and aerodynamic costs associated with having longer eyespan and a larger body (Husak et al., 2011, Husak et al., 2013; Ribak and Swallow, 2007; Ribak et al., 2009). These allometric relationships persist in the T. dalmanni and D. meigenii studied here; flies with longer eyespan had wider thoraces, longer wings, and greater wing area (Vance et al., 2023). Although thorax and wing-size are fixed post-eclosion and presumably do not account for age-related variation in flight performance, selection should favor these traits as they constrain the morphology through which compensatory ability develops across age. Wing size is an important determinant of wing velocity and aerodynamic lift for a given kinematic output, assuming there is sufficient muscle to generate the necessary power. However, there was no difference in wing area despite ‘over-compensators’ having 2% longer wings than ‘under-compensators’. The capacity to accumulate muscle mass across age is limited by the constraints of the thorax exoskeleton, which may ultimately determine the ceiling of maximal aerodynamic performance. Although ‘over-’ and ‘under-compensators’ did not differ in thorax width, it’s unknown whether thorax size influences the rate at which muscle accumulates across age.
Compensatory ability in stalk-eyed flies is critically dependent upon the development of the thorax. The performance of flight muscle in adult insects is influenced by physiological changes that occur after eclosion, including increased pyruvate kinase and citrate synthase activity (Harrison, 1996), and alternative splicing of troponin variants which affect muscle calcium sensitivity (Marden et al., 1999; Schippers et al., 2006). However, few studies in adult insects have characterized developmental changes in thorax mass, especially associated with age-related variation in flight performance. For example, honeybees do not vary thorax mass across caste-specific behaviors (Harrison, 1996), whereas beetles accumulate thorax lipid reserves leading up to dispersal (David et al., 2015). Dragonflies, however, increase thorax mass between tenerel and mature stages (Marden, 1989), which is attributed to muscle growth. Marden (2000) further explains that muscle growth within the fixed exoskeleton of adult insects is possible through the displacement of air sacs. Indeed, microscopic inspection of young flies reveals the presence of voids within the thorax, indicating there is available volume to allow the growth of muscle. We posit that the increased thorax mass in stalk-eyed flies results from growth of muscle, rather than accumulation of energy stores via lipids, as the increased flight performance associated with thorax mass is achieved through increased wingbeat frequency and wing velocity, requiring elevated muscle stiffness and power to drive the indirect actuation of the wings (Vance et al., 2023).
Although our compensation model is inspired by methods employed by Husak et al. (2011), it differs in several ways. Notably, Husak et al. (2011) regressed morphological variables across a proxy for body length (head to wingtip); then, relative compensatory trait (wing size) and relative ornament size (eyespan) were calculated from the residuals of these regressions. Compensation in this framework is assessed by where an individual lies with respect to the regression of relative ornament size across relative compensatory trait; importantly, the regression line represents the average compensatory ability of the population, and does not necessarily reflect an optimum range of compensation that is adaptive. In our model, we regressed non-normalized traits (morphological and flight performance variables) directly across non-normalized ornament size (eyespan; Figure 1). The resulting eyespan-relative traits are the residuals from this regression, as the regression represents the average relationship between trait and ornament across the population; ‘over-’ and ‘under-compensators’ for that trait are positive and negative residual values, respectively. The utility in this modified approach is that relationships between eyespan-relative traits may then be directly compared, particularly between those variables which have mechanistic relationships to one another (Figure 2), or are predicted to vary across age (Figure 3). These trends are largely supported by the multiple regression analyses of these relationships (Table 1 and Supplementary Table S1), and provide further insight into life-time variation in compensatory ability than was revealed by our prior MANCOVA analysis (Vance et al., 2023). However, the multiple regression analyses revealed several results that required further investigation.
First, the multiple regression of eyespan and thorax mass on wing velocity was significant (Supplementary Table S1), however, the partial regression coefficients revealed that wing velocity was inversely related to eyespan. This seems counterintuitive when considering that flies with longer eyespans have greater thorax mass regardless of age (Supplementary Table S1), and a strong relationship exists between thorax mass and wing velocity (Figure 3A). However, aerodynamic forces scale with wing area, and T. dalmanni and D. meigenii with larger eyespans had greater wing area (Vance et al., 2023); less wing velocity should be required to generate a given magnitude of aerodynamic lift. Larger wings are also heavier wings, which may reduce the resonant frequency of the flight motor (Vance and Roberts, 2014). Indeed, the multiple regression for eyespan and thorax mass on wingbeat frequency revealed an inverse relationship between eyespan and frequency (partial regression: P<0.001 for both species). Thus, although larger wings may require less wing velocity, the flight motor may be constrained kinematically, which may compound those challenges faced by younger flies, where lesser-developed thorax muscle may not be as capable of generating power to drive large wings.
Second, the multiple regression for eyespan and age on wing velocity was significant in both species (Table 1), however, there was no relationship between eyespan and wing velocity for D. meigenii (partial regression: P=0.536), and there was a direct relationship between eyespan and wing velocity for T. dalmanii (partial regression: P=0.003). Although the latter result contradicts the inverse relationship revealed by the multiple regression of eyespan and thorax mass on wing velocity discussed above, the effect of the partial regression for eyespan is relatively small compared to the effect for age (Table 1: t=3.02 vs t=20.7). Since flies with longer eyespans may ultimately develop greater thorax muscle than flies with lesser eyespans, it is possible that the mature flight muscle of older, longer-eyespan flies contributed to this positive relationship. But, consider that the linear regression for eyespan and wing velocity is not significant on its own (Figure 1C), and the effect of eyespan on wing velocity does not emerge until one investigates eyespan alongside a separate driving parameter (i.e. thorax mass or age). This instead suggests that the contradicting relationships between eyespan and wing velocity may be influenced by how the driving parameter aligns the data; and, these effects of the driving parameters, revealed by the partial regressions, appear sufficient to maintain these relationships when evaluating the eyespan-relative parameters in compensation space (Figures 2, 3).
Assessing the costs of bearing ornaments is elusive where compensatory traits and behaviors offset those putative impairments (Oufiero and Garland, 2007; Møller, 1996; Tomkins et al., 2005). Thorax size and wing size appears to have co-evolved with eyespan in stalk-eyed flies (Husak et al., 2011, Husak et al., 2013; Ribak and Swallow, 2007; Ribak et al., 2009); however, ‘over-’ or ‘under-compensating’ for eyespan critically depends on flies’ ability to use this morphology, as determined by the development of thorax muscle and improvement in wing velocity and flight capacity over time. Although selection should favor mechanisms that accelerate an individual’s development towards ‘over-compensation’, there may be a specific range over which ‘over-compensating’ is an optimal strategy, or contexts where ‘over-compensating’ may be maladaptive. Compensatory morphology and behaviors have their own associated costs, including investment into larger body and wing planforms, and the accumulation of resources and energy to facilitate thorax muscle growth, as well as meet the life-time metabolic expenditures associated with elevated flight performance; all of which may compete with concurrent reproductive tissue growth in the abdomen. Future research that investigates variation in compensatory ability, perhaps through constraining or enhancing the time-history of thoracic and abdominal growth, may provide insight into fecundity and functional and costs of bearing large ornaments.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
JV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Funding acquisition, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by a National Science Foundation grant (NSF IOS 1656478) to JV, and a National Science Foundation grant (NSF IOS 1656465) to JS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fetho.2024.1484454/full#supplementary-material
Supplementary Figure S1 | Description of methods, from Vance et al. (2023). (A) The flight performance of male Teleopsis dalmanni (right) and Diasemopsis meigenii (left) were assessed using (B) hypodense, normoxic mixtures of oxygen, nitrogen and helium in an 8-liter cylindrical flight chamber. A high-speed video camera (5930 fps) oriented above the chamber recorded hovering flight. (C) The hovering sequences were digitized, and wing stroke amplitude was calculated from the horizontal angular displacement of the dorsal and ventral wing stroke reversals.
Supplementary Figure S2 | Comparison of ‘over-compensators’ (dark gray) and ‘under-compensators’ (light gray) morphology, flight performance and age (mean ± S.D.). Significant differences (ANOVA, P<0.05) are indicated by asterisks.
Baker R. H., Ashwell R. I. S., Richards T. A., Fowler K., Chapman T., Pomiankowski A. (2001). Effects of multiple mating and male eye span on female reproductive output in the stalk-eyed fly, Cyrtodiopsis dalmanni. Behav. Ecol. 12, 732–739. doi: 10.1093/beheco/12.6.732
Baker R. H., Denniff M., Futerman P., Fowler K., Pokiankowski A., Chapman T. (2003). Acessory gland size influences time to sexual maturity and mating frequency in the stalk-eyed fly, Crytodiopsis dalmanni. Behav. Ecol. 14, 607–611. doi: 10.1093/beheco/arg053
Bath E., Wigby S., Vincent C., Tobias J. A., Seddon N. (2015). Condition, not eyespan, predicts contest outcome in female stalk-eyed flies,Teleopsis dalmanni. Ecol. Evol. 5, 1826–1836. doi: 10.1002/ece3.1467
Bellamy L., Chapman N., Fowler K., Pomiankowski A. (2013). Sexual traits are sensitive to genetic stress and predict extinction risk in the stalk-eyed fly, Diasemopsis meigenii. Evolution 67, 2662–2673. doi: 10.1111/evo.12135
Bubak A. N., Gerkin A. R., Watt M. J., Costabile J. D., Renner K. J., Swallow J. G. (2016). Assessment strategies and fighting patterns in animal contests: a role for serotonin? Curr. Zoology 62, 257–263. doi: 10.1093/cz/zow040
Burkhardt D., de la Motte I. (1983). How stalk-eyed flies eye stalk-eyed flies: Observations and measurements of the eyes of Cyrtodiopsis whitei (Diopsidae, Diptera). J. Comp. Physiol. 151, 407–421. doi: 10.1007/bf00605457
Burkhardt D., de la Motte I. (1985). Selective pressures, variability and sexual dimorphism in stalk-eyed flies (Diopsidae). Die Naturwissenschaften 72, 204–206. doi: 10.1007/BF01195763
Burkhardt D., de la Motte I. (1987). Physiological, behavior, and morphometric data elucidate the evolutive significance of stalked eyes in Diopsidae (Diptera). Entomologia Generalis 12, 221–233. doi: 10.1127/entom.gen/12/1987/221
Burkhardt D., de la Motte I. (1988). Big ‘antlers’ are favored: female choice in stalk-eyed flies (Diptera, Insecta), field collected harems and laboratory experiments. J. Comp. Physiol. A 162, 649–652. doi: 10.1007/BF01342640
Charnov E. L. (1993). Life history invariants: some explorations of symmetry in evolutionary ecology (Oxford: Oxford University Press).
David G., Giffard B., van Halder I., Piou D., Jactel H. (2015). Energy allocation during the maturation of adults in a long-lived insect: implications for dispersal and reproduction. Bulleting Entomological Res. 105, 629–636. doi: 10.1017/S0007485315000553
Dudley R. (1995). Extraordinary flight performance of orchid bees (Apidae: Euglossini) hovering in heliox (80% He/20% O2). J. Exp. Biol. 198, 1065–1070. doi: 10.1242/jeb.198.4.1065
Egge A. R., Brandt Y., Swallow J. G. (2011). Sequential analysis of aggressive interactions in the stalk-eyed fly Teleopsis dalmanni. Behav. Ecol. Sociobiology 65, 369–379. doi: 10.1007/s00265-010-1054-5
Harrison J. M. (1996). Caste-specific changes in honeybee flight capacity. Physiol. Zoology 59, 175–187. doi: 10.1086/physzool.59.2.30156031
Hedrick T. L. (2008). Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspiration Biomimetics 3, 34001. doi: 10.1088/1748-3182/3/3/034001
Hedrick T. L., Cheng B., Deng X. (2009). Wingbeat time and the scaling of passive rotational damping in flapping flight. Science 324, 252–255. doi: 10.1126/science.1168431
Husak J. F., Ribak G., Baker R. H., Rivera G., Wilkinson G. S., Swallow J. G. (2013). Effects of ornamentation and phylogeny on the evolution of wing shape in stalk-eyed flies (Diopsidae). J. Evolutionary Biol. 26, 1281–1293. doi: 10.1111/jeb.12133
Husak J. F., Ribak G., Wilkinson G. S., Swallow J. G. (2011). Compensation for exaggerated eye stalks in stalk-eyed flies (Diopsidae). Funct. Ecol. 25, 608–616. doi: 10.1111/j.1365-2435.2010.01827.x
Husak J. F., Swallow J. G. (2011). Compensatory traits and the evolution of male ornaments. Behaviour 148, 1–29. doi: 10.1163/000579510x541265
Irschick D. J., Meyers J. J., Husak J. F., Le Galliard J. F. (2008). How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evolutionary Ecol. Res. 10, 177–196. doi: 10.7275/R58G8HX6
Isaacs B., Byrne D. N (1998). Aerial distribution, flight behavior and eggload: their inter-relationship during dispersal by the sweetpotato whitefly. J. Anim. Ecol. 76, 741–750. doi: 10.1046/j.1365-2656.1998.00236.x
Iwasa Y., Pomiankowski A., Nee S. (1991). The evolution of costly mate preferences. II. The "handicap" principle. Evolution 45, 1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x
Kotiaho J. S. (2001). Costs of sexual traits: A mismatch between theoretical considerations and empirical evidence. Biol. Rev. 76, 365–376. doi: 10.1017/S1464793101005711
Lailvaux S. P., Husak J. F. (2014). The life history of whole-organism performance. Q. Rev. Biol. 89, 285–318. doi: 10.1086/678567
Lailvaux S. P., Irschick D. J. (2006). A functional perspective on sexual selection: insights and future prospects. Anim. Behav. 72, 263–273. doi: 10.1016/j.anbehav.2006.02.003
Marden J. H. (1989). Bodybuilding dragonflies: costs and benefits of maximizing flight muscle. Physiol. Zoology 62, 505–521. doi: 10.1086/physzool.62.2.30156182
Marden J. H. (2000). Variability in the size, composition, and function of insect flight muscle. Annu. Rev. Physiol. 62, 157–178. doi: 10.1146/annurev.physiol.62.1.157
Marden J. H., Fitzhugh G. H., Wolf M. R., Rowan B. (1999). Alternative splicing, muscle calcium sensitivity, and the modulation of dragonfly flight performance. Proc. Natl. Acad. Sciences U.S.A. 96, 15304–15309. doi: 10.1073/pnas.96.26.15304
Møller A. P. (1996). The cost of secondary sexual characters and the evolution of cost-reducing traits. Ibis 138, 112–119. doi: 10.1111/j.1474-919X.1996.tb04317.x
Oufiero C. E., Garland T. Jr (2007). Evaluating performance costs of sexually selected traits. Funct. Ecol. 21, 676–689. doi: 10.1111/j.1365-2435.2007.01259.x
Panhuis T. M., Wilkinson G. S. (1999). Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav. Ecol. Sociobiology 46, 221–227. doi: 10.1007/s002650050613
Pitnick S., Markow T. A., Spicer G. S. (1995). Delayed male maturity is a cost of producing large sperm in Drosophila. Proc. Natl. Acad. Sciences U.S.A. 92, 10614–10618. doi: 10.1073/pnas.92.23.10614
Pomiankowski A., Denniff M., Fowler K., Chapman T. (2005). The costs and benefits of high early mating rates in male stalk-eyed flies, Cyrtodiopsis dalmanni. J. Insect Physiol. 51, 1165–1171. doi: 10.1016/j.jinsphys.2005.06.006
Reguera P., Pomiankowski A., Fowler K., Chapman T. (2004). Low cost of reproduction in female stalk-eyed flies, Cyrtodiopsis dalmanni. J. Insect Physiol. 50, 103–108. doi: 10.1016/j.jinsphys.2003.10.004
Ribak G., Pitts M. L., Wilkinson G. S., Swallow J. G. (2009). Wing shape, wing size, and sexual dimorphism in eye-span in stalk-eyed flies (Diopsidae). Biol. J. Linn. Soc. 98, 860–871. doi: 10.1111/j.1095-8312.2009.01326.x
Ribak G., Swallow J. G. (2007). Free flight maneuvers of stalk-eyed flies: do eye-stalks affect aerial turning behavior? J. Comp. Physiol. A 193, 1065–1079. doi: 10.1007/s00359-007-0259-1
Rogers D. W., Chapman T., Fowler K., Pomiankowski A. (2005). Mating-induced reduction in accessory reproductive organ size in the stalk-eyed fly Cyrtodiopsis dalmanni. BMC. Evolutionary Biol. 5, 37. doi: 10.1186/1471-2148-5-37
Schippers M.-P., Dukas R., Smith R. W., Wang J., Smolen K., McClelland G. B. (2006). Lifetime performance in foraging honeybees: behaviour and physiology. J. Exp. Biol. 209, 3828–3836. doi: 10.1242/jeb.02450
Small J., Cotton S., Fowler K., Pomiankowski A. (2009). Male eyespan and resource ownership affect contest outcome in the stalk-eyed fly, Teleopsis dalmanni. Anim. Behav. 78, 1213–1220. doi: 10.1016/j.anbehav.2009.08.009
Swallow J. G., Hayes J. P., Koteja P., Garland T. Jr (2009). “Selection experiments and experimental evolution of performance and physiology,” in Experimental evolution: concepts, methods, and applications of selection experiments. Eds. Garland T. Jr, Rose M. R. (University of California Press, Berkeley, CA), 301–351.
Swallow J. G., Wilkinson G. S., Marden J. H. (2000). Aerial performance of stalk-eyed flies that differ in eye span. J. Comp. Physiol. B: Biochemical Systemic Environ. Physiol. 170, 481–487. doi: 10.1007/s003600000124
Tomkins J. L., Kotiaho J. S., LeBas N. R. (2005). Phenotypic plasticity in the developmental integration of morphological trade-offs and secondary sexual trait compensation. Proceedings: Biol. Sci. 272, 543–551. Available at: http://www.jstor.org/stable/30047580.
Vance J. T., Pehl K., Acakpo C. J., Swallow J. G. (2023). Compensation for a costly ornament depends on the development of flight performance in stalk-eyed flies. Front. Ethology 2. doi: 10.3389/fetho.2023.1242198
Vance J. T., Roberts S. P. (2014). The effects of artificial wing wear on the flight capacity of the honey bee Apis mellifera. J. Insect Physiol. 65, 27–36. doi: 10.1016/j.jinsphys.2014.04.003
Vance J. T., Williams J. B., Elekonich M. M., Roberts S. P. (2009). The effects of age and behavioral development on honey bee (Apis mellifera) flight performance. J. Exp. Biol. 212, 2604–2611. doi: 10.1242/jeb.028100
Wilkinson G. S., Kahler H., Baker R. H. (1998). Evolution of female mate preferences in stalk-eyed flies. Behav. Ecol. 9, 525–533. doi: 10.1093/beheco/9.5.525
Keywords: sexual selection, ornaments, compensation, flight performance, stalk-eyed flies
Citation: Vance JT and Swallow JG (2024) The development of compensatory ability for a sexually-selected ornament in stalk-eyed flies. Front. Ethol. 3:1484454. doi: 10.3389/fetho.2024.1484454
Received: 21 August 2024; Accepted: 22 October 2024;
Published: 18 November 2024.
Edited by:
Michel Baguette, Muséum National d’Histoire Naturelle, FranceReviewed by:
Sergio Castellano, University of Turin, ItalyCopyright © 2024 Vance and Swallow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jason T. Vance, dmFuY2VqdEBjb2ZjLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.