
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ethol. , 24 June 2024
Sec. Behavioral Development and Play
Volume 3 - 2024 | https://doi.org/10.3389/fetho.2024.1403082
This article is part of the Research Topic Editors' Showcase: Behavioral Development and Play View all 4 articles
Play is a widespread phenomenon in the animal kingdom with observations from all vertebrate classes. The adaptive value of this behaviour, however, remains unclear. In the past century, numerous theories have been put forward, ranging from releasing surplus energy to training species-specific behaviours. However, none of these theories can fully explain the functions of play. A recent neurocognitive theory suggests that play allows the brain to encounter many different and surprising situations that provide it with opportunities to learn about the environment and form predictions about it. This theory has, however, to our knowledge, previously not been experimentally tested. To start exploring the connection between play and cognition, we compared the cognitive capacities of White Leghorn chicks that were stimulated to play in the first five weeks of their lives with chicks that did not receive any play stimulation. More specifically, we wanted to test the connection between specific types of play and cognitive domains. To achieve this, we designed two play treatments: object players that were provided with a variety of toys during their treatment, and social players that were released into an arena with plenty of space and conspecifics, as this has previously been shown to trigger social play. Subsequently, all three treatments (control, object players, social players) were tested in a cognitive test battery consisting of two experiments targeting the social domain and two targeting the physical domain. We found no improvement of cognitive capacities in either play treatment group compared to control subjects, though the social play treatment appears to have affected some behavioural variables recorded during the cognitive tests. Chicks that had played socially were in general bolder, more explorative and had more access to resources in the tests. This might subsequently allow them to exploit their environment more efficiently, which could in turn affect their welfare as they might be more resilient to stress and have more access to resources. More studies will be needed to assess the long-term effects of play on cognitive capacities and welfare of chickens.
Play is a key behaviour in the ontogeny of humans as well as non-human animals (hereafter “animals”). Both humans and animals play most frequently during their juvenile period, implying an important developmental function of this behaviour (e.g. Burghardt, 2005). The paradox of play is, however, our lack of understanding of its fitness benefits or functions. Since play is often energetically demanding and exposes the performers to obvious risks, it can be assumed that this must be outweighed by definite fitness gains for it to be evolutionary conserved to the extent observed (Bateson et al., 2013). The present lack of understanding becomes even more apparent when regarding the five criteria that are commonly used to define play (Burghardt, 2001). Two of the five criteria refer to the absence of functionality of play behaviours. A behaviour is regarded as play when it is firstly incomplete in function and does not contribute to current survival and secondly when it is done for its own sake, i.e., because it is self-rewarding or pleasurable. The other three criteria state that play behaviours differ from serious behaviours (they are e.g. exaggerated, incomplete, or awkward), appear repeatedly during ontogeny, and are expressed only in the absence of stress.
Despite its apparent lack of immediate functions, play has been observed in mammals (e.g. Byers, 1999; Lewis, 2000; Himmler et al., 2016), reptiles (e.g. Dinets, 2015), fishes (Burghardt, 2005), and many bird species. Some birds are among the most playful species in the world like for example keas (Nestor notabilis) (Keller, 1975) and common ravens (Corvus corax) (Heinrich and Smolker, 1998), but even species not classically regarded as playful have been shown to engage in play, such as greater rheas (Rhea americana) (Zeiträg et al., 2023) and even Red Junglefowl and domestic chickens (Gallus gallus) (Lundén et al., 2022). Recently, play-like behaviour has even been reported in insects (Dona et al., 2022). The seeming omnipresence of play suggests deep evolutionary roots of this behaviour.
To begin solving the puzzle of play, many theories about its functions have been brought forward. An early theory proposed a non-adaptive explanation of play stating that play expends excess energy (Spencer, 1872; Burghardt, 2005). Due to the above-mentioned ubiquity of play, this, however, seems unlikely. Later theories have focused on ultimate functions of play. These theories propose, among other things, that play represents a form of physical exercise (Brownlee, 1954), or serves to evaluate the players’ physical abilities (Thompson, 1998). Further, several theories have suggested training effects of play. Play might help players prepare for unexpected situations (Špinka et al., 2001) and train species-specific adult behaviours (Mallpress and Špinka, 2023). A second popular line of theories focuses on social functions of play. Play has been proposed to support social bonding and cooperation (Cordoni, 2009). It has further been suggested that play practices social skills (Bekoff, 1976; Fagen, 1981), and helps define social roles (Blumstein et al., 2013). Despite some evidence supporting these theories (e.g. Blumstein et al., 2013; Nunes, 2014; Perret, 2021), none of them have to date been able to fully explain the functions of play (Burghardt, 2005). It has even been suggested that one functional explanation might not be able to cover all aspects of play (Mallpress and Špinka, 2023).
A different line of investigation explores the role of play in cognitive development. In human developmental psychology, play is proposed to support the development of theory of mind (Leslie, 1987) and imagination (Singer and Singer, 2009). A novel theory by Andersen et al. (2022), combines cognitive and training theories into a neurocognitive framework to explain the functions of play in the ontogeny of humans and animals. This theory states that play does not serve to practice species-specific behaviours, but rather that the exposure to many different and surprising situations during play represents opportunities for the brain to form and test predictions about the environment. Through play, children and animals encounter objects, situations, and social interactions at a much higher frequency than they would normally do. This in turn allows them to experiment with the outcomes of different actions. These experiences can subsequently be used to form predictions about the world, making the brain better at predicting future situations.
Deducing from this theory, animals engaging in play during their juvenile period should show enhanced cognitive capacities compared to animals that did not have the opportunity to play or were playing less during their ontogeny. Further, if play supports the brain in forming predictions about the environment, there should be a connection between the specific types of play an animal has encountered and the situations in which an animal shows improved cognitive capacities.
These hypotheses have, however, to our knowledge not been previously tested experimentally. To begin exploring the connection between play and cognitive development, we exposed White Leghorn chicks to different play treatments and subsequently tested their cognitive capacities.
Chickens are an ideal species to study the connection between play and cognition, as they have been shown to exhibit all three categories of play, i.e., social, object, and locomotor play, during their ontogeny (Lundén et al., 2022). It is also possible to trigger play in chickens through providing them with adequate space (Liu et al., 2020). Lastly, insights into the function of play and enrichment in chickens might have important implications for their welfare in commercial production systems.
To obtain a better understanding of the connection between specific experiences made during play and cognitive capacities, we designed two different play treatment conditions: one group was provided with a variety of objects to play with, and the other was provided with space and pen mates to trigger social play. A third group was not stimulated to play and served as control group. Subjects subsequently completed a cognitive test battery consisting of two physical and two socio-cognitive tasks. We expected to observe improved socio-cognitive skills in subjects of the social play treatment and enhanced physical cognitive skills in the object play treatment. We further expected play stimulated subjects to outperform control subjects in all cognitive tests.
All experimental protocols were approved by the Linköping Regional Committee for Ethical Approval of Animal Research, license no. 10492–2023. The experiments were conducted in accordance with the ARRIVE guidelines and relevant regulations.
Eggs of White Leghorn chickens (n=65) of the commercial egg-laying hybrid Lohmann LSL-LITE were obtained from a commercial hatchery in Sweden. All eggs were incubated at 38.5°C, 65% relative humidity and rotation once per hour, and subsequently hatched in darkness at the facilities of Linköping University.
On the same day of hatching, chicks were distributed into six home cages ((L) 68,5 cm x (W) 51 cm x (H) 44 cm) in mixed-sex groups of ten to eleven individuals each. Commercial hybrid egg-layers can be feather-sexed at hatch (Lohmann, 2018). The cages were equipped with wood chips, heat roofs, perches, and food and water ad libitum. The cages were subsequently randomly assigned to one of three play conditions: control, object play, or social play (treatment described below), resulting in two cages representing one play condition, respectively. Half of the subjects in every cage were marked with leg rings of the same colour so that each cage contained two groups (marked and unmarked), resulting in four groups of five to six subjects for each play condition. Of the initially 65 subjects, two died during the treatment phase due to causes unrelated to the experiment, resulting in 63 subjects entering the test phase.
From the second week of play treatment, all subjects of each treatment condition were habituated to mealworms three times per week. The first three times, the mealworms were simply strewn into the home cages. After that, mealworms were placed in the same feeding cups that were later used during the test phase and placed in the home cages for ten minutes for the remaining three weeks of play treatment.
To reduce crowding, at the end of the treatment period (after five weeks), subjects were re-distributed from six into nine home cages through taking three individuals from each cage and creating a new group resulting in seven individuals per cage without mixing the treatment groups. To reduce aggression in the new cages, only females were placed in these new groups. We did not observe any aggressive encounters between the newly associated females and did not see differences in their behaviour in the tests compared to the subjects that did not change home cages. All subjects (n=63) were included in the test phase, independent of the reorganisation.
The chicks received three different play treatments to compare chicks that were not stimulated to play during their ontogeny (control) with chicks that predominantly played with objects (object players) and chicks that predominantly played socially with conspecifics (social players).
Starting at five days of age, chicks received their respective play treatment three times per week for five weeks. We decided on this interval of play treatments so that the subjects would always have one day in between handling to keep stress to a minimum. We chose five weeks as the treatment period, as play has previously been shown to peak around this time in chickens and subsequently decline (Lundén et al., 2022). Each session lasted for 30 minutes as a previous study (Lundén et al., 2022) showed that play behaviours usually die down after this time. At the beginning of each treatment session, the subjects were gently caught out of their home cages. Both groups of chicks receiving play stimulation were distributed according to their treatment groups into treatment arenas (120 cm x 120 cm) containing wood chips. They were always placed in the arenas in the same predetermined groups of five to six individuals for the entirety of the treatment period.
For object players (n= 21), these arenas were equipped with ten objects, both lying on the ground as well as hanging from a perch (for a picture of the objects, see Supplementary Materials). The arenas for social players (n=20) did not contain any objects but simply provided space, as this has previously been observed to trigger social play (Liu et al., 2020). Control chicks (n=22) were caught and handled in the same manner as the two play treatment conditions but were placed in cardboard boxes ((L) 48 x (W) 30 x (H) 32 cm) containing wood chips. This controlled for the same amount of handling, but the limited space prevented the occurrence of play behaviours.
Play sessions took place between 8 am and 2 pm. All four groups of one play condition (control, social play, object play) received their treatment simultaneously and the order of play conditions was pseudorandomised between days. All sessions were filmed with video cameras and subsequently all instances of play behaviours (for an ethogram of play behaviours in chicks, see Supplementary Materials) were recorded for the first five minutes and for five minutes in the middle of the session. This was done to record play behaviours occurring in the different treatment groups. A previous study showed that the majority of play behaviours in chickens occur within the first five minutes of a play stimulation (Lundén et al., 2022). Thus, through analysing the first five minutes of our sessions, we obtained a good representation of the occurring play behaviours. We added the second five minutes in the middle of the session to ensure that play behaviours did not significantly change later in the session. For a full analysis of the ontogeny and frequency of play behaviours in White Leghorn chicks, see Lundén et al. (2022). Play behaviours were coded using the program Solomon Coder (Version: beta 19.08.02, Péter, 2017). The same program was subsequently used for all behavioural coding.
The test period started the day after the last play treatment. All tests were performed in the same arenas where the play treatments took place (120 cm x 120 cm), but all objects were removed, and fresh wood chips were distributed on the ground.
To test the subjects’ social dominance, at the age of 38 days, two birds from different play conditions were placed together in the test arena. While dominance in itself is not a cognitive skill, socio-cognitive abilities, such as perception, attention, learning, memory, and inhibitory control, are central to forming and maintaining social relationships including dominance hierarchies (Wascher et al., 2018). We only paired birds of the same sex, as males would not compete with females over food. This resulted in three groupings: social players and object players (n=20; 7 pairs of females, 3 pairs of males), social players and controls (n=18; 3 pairs of females, 6 pairs of males), and object players and controls (n=20; 6 pairs of females, 4 pairs of males). Every subject was only tested once (in one combination) to avoid learning effects.
Social and object players were already familiar with the arenas from their play treatments. To give control birds also time to habituate to this environment, the arena was initially divided into two equally sized compartments using a metal grid. One bird was placed on each side so that they could see each other. To be able to differentiate between subjects, birds were marked with coloured whiteboard markers on their backs.
After ten minutes of habituation, the mesh divider was taken out and a feeding cup containing mealworms was placed in the middle (the same type as the birds had previously been habituated to in the home cages). The cup was fitted with a cardboard lid with a cutout of 2 cm diameter that only allowed one bird to feed at a time. The birds were allowed to roam freely in the arena and feed from the cup for the next ten minutes while the session was video recorded. The frequency and duration of feeding from the cup, the identity of the subject that was feeding first, as well as the frequency of aggressive behaviours (for an ethogram see Supplementary Materials) was subsequently coded from the videos using Solomon Coder.
To test whether play exposure improved motor self-inhibition, chicks were tested in a detour task starting at 40 days old. This is a well-established paradigm that has previously been introduced in birds (for a review see Kabadayi et al., 2018) and specifically in chickens (Ryding et al., 2021). We followed the experimental setup used by Ryding et al. (2021) including two pretest stages.
In pretest stage 1, the chicks were familiarised with being alone in the arena and with transparent materials. To achieve this, we placed chicks individually in the test arena containing a three-sided box made of plexiglass ((L)13 × (W)13 cm x (H) 20 cm). Inside the box, we placed several dried mealworms. Chicks were supposed to move around the transparent walls and enter the box from the open side to retrieve the mealworms. Subjects were considered habituated with transparent materials when they managed to reach the mealworms without pecking the transparent walls three times in a row. Session lengths for this pretest stage varied, with a minimum of ten minutes per day and a maximum of twenty minutes when the potential for passing the stage appeared close. Chicks that did not pass the stage within five days were excluded from the experiment. Six object players, 17 social players, and 17 controls passed the first pretest stage.
In the second pretest stage, subjects were habituated to an opaque cylinder (5 cm in diameter and 8 cm long) containing a mealworm. In this stage, they learned to detour the cylinder to reach the mealworm from the openings on either side. At the beginning of each trial, we placed the cylinder on the opposite side of the arena from where the subject was, with the long side facing the chick. Subjects passed this stage when they managed to retrieve the mealworm five times in a row. They received one session per day on a maximum of three consecutive days. Session lengths varied again, based on subjects’ motivation. Four object players, 17 social players, and 15 controls passed the second pretest stage and thus participated in the test (for possible reasons for the unequal sample sizes, see Discussion).
The test was conducted in the same way as the second pretest stage, but with a transparent cylinder (5 cm in diameter and 8 cm long). Chicks received 15 trials. A trial counted as passed once the mealworm was retrieved from inside the cylinder without pecking on the transparent cylinder, i.e., when they inhibited themselves from pecking on the cylinder but detoured to retrieve the mealworm through the openings on the sides. They received up to three sessions on consecutive days to complete the test. 4 object players, 15 social players, and 14 controls finished the test. The test was video recorded, and subsequently coded using Solomon Coder. We counted whether a trial was passed and how long it took the chick to retrieve the mealworm. In failed trials (trials in which the cylinder was pecked), we further recorded the total amount of pecks. Pecks represent the inability to inhibit the immediate response to peck on the cylinder.
To test chicks’ spatial memory abilities, they completed a memory test at 48 days old following Parois et al. (2017) protocol. In this test, a chick was released into the arena containing eight evenly spaced-out cups containing one mealworm each. Each wall of the arena was marked with a distinct geometrical shape (cross, rectangle, triangle, square) to help the subjects navigate the arena.
Chicks were given one session of a maximum of 10 minutes or until they had eaten all mealworms. Sessions were video recorded and subsequently, we documented the number of visited cups, number of revisited cups, the time in between visits and the total time to visit all cups using Solomon Coder. Errors (revisits) were divided into immediate errors (revisit with no or one other cup in between) and distant errors (revisits after having visited two or more cups in between). Immediate errors occurring after more than 100 seconds from the last visit were also counted as distant errors. Immediate errors are regarded as an attention failure, while distant errors represent actual memory failure. We further calculated the ratio of immediate and distant errors compared to the total number of errors to obtain a better understanding of the distribution of errors and their causes (attention or memory failure). A high ratio of immediate errors suggests reduced attention or a high level of distraction. A high ratio of distant errors, on the other hand, points towards the inability to remember the cups that have already been visited and thus reduced spatial memory capacities.
To test attention and salience of social cues to chicks, we trained two male demonstrators: one that only fed out of a blue bowl and one that only fed out of a yellow bowl. We chose male demonstrators, as males naturally perform “food calling”, i.e., when they find food, they produce a characteristic call that informs hens about the presence of food (Evans & Evans, 1999). The demonstrators were trained through presenting them with both bowls, but only the bowl of the correct colour contained mealworms. We repeated this until demonstrators first approached the bowl of the desired colour in five consecutive trials while both bowls contained mealworms.
The test took place on two consecutive days when subjects were 50 and 51 days old. The arena was divided into two equally sized compartments for the subject and demonstrator, respectively, using a mesh divider that allowed visual contact. Both the subject and demonstrator were allowed to habituate for 5 minutes. After that, the demonstrator fed six consecutive times out of the bowl it was trained on. The side of the correct bowl was thereby pseudorandomised through dice-rolling, but each side could occur maximally two times in a row. After that, the demonstrator was removed from its compartment to avoid it from interfering with the experiment. The subject was subsequently presented with the two bowls, each containing one mealworm, for 12 consecutive trials. The side of the correct colour was again pseudorandomised. The trials were video recorded and subsequently coded using Solomon Coder. For every trial, we recorded the demonstrated colour, the side of the demonstrated colour, and whether the subject chose the correct (demonstrated) colour. A preference for the cup of the demonstrated colour can be interpreted as increased attention to and reliance on social cues rather than reliance on other foraging strategies, such as colour and side.
Each test of the cognitive test battery was analysed separately. To analyse the datasets, we built generalised linear mixed models (GLMM) in RStudio (version 2023.12.1) (RStudio Team, 2020). The models included play treatment, sex, their interaction, and in Experiment 4 also the side and colour of the correct choice as fixed factors. We included cage number (of their initial cage) as random factor. We chose the distribution depending on each dataset. The response variable was chosen separately for each experiment. For more information on each model, see Supplementary Material.
The full models were stepwise reduced using the Akaike information criterion (AIC). AICs of models were compared using the drop1 function. Factors were excluded from the model, when the AIC of a model was more than 2 points lower after removing the factor. The effects of the remaining factors were calculated using likelihood ratio tests (for values of each factor, see Supplementary Material). All deviations from mean values are reported as SEM (standard error from mean).
We observed all three categories of play (social, object, and locomotor play; for a definition of play behaviours, see Supplementary Material) in all three treatment conditions (social play, object play, and control) during the play stimulation sessions. However, we found significant differences in the occurrences of play categories between treatment conditions. We identified a significant effect of treatment (likelihood-ratio test, χ2 = 126.67, df=2, p<0.001) and age (likelihood-ratio test, χ2 = 120.71, df=14, p<0.001) on the number of observed instances of social play. Object and social players played significantly more often socially than control subjects, and social players played significantly more often socially compared to object players. Object players nevertheless played socially, but at a reduced frequency compared to social players (see Figure 1A).
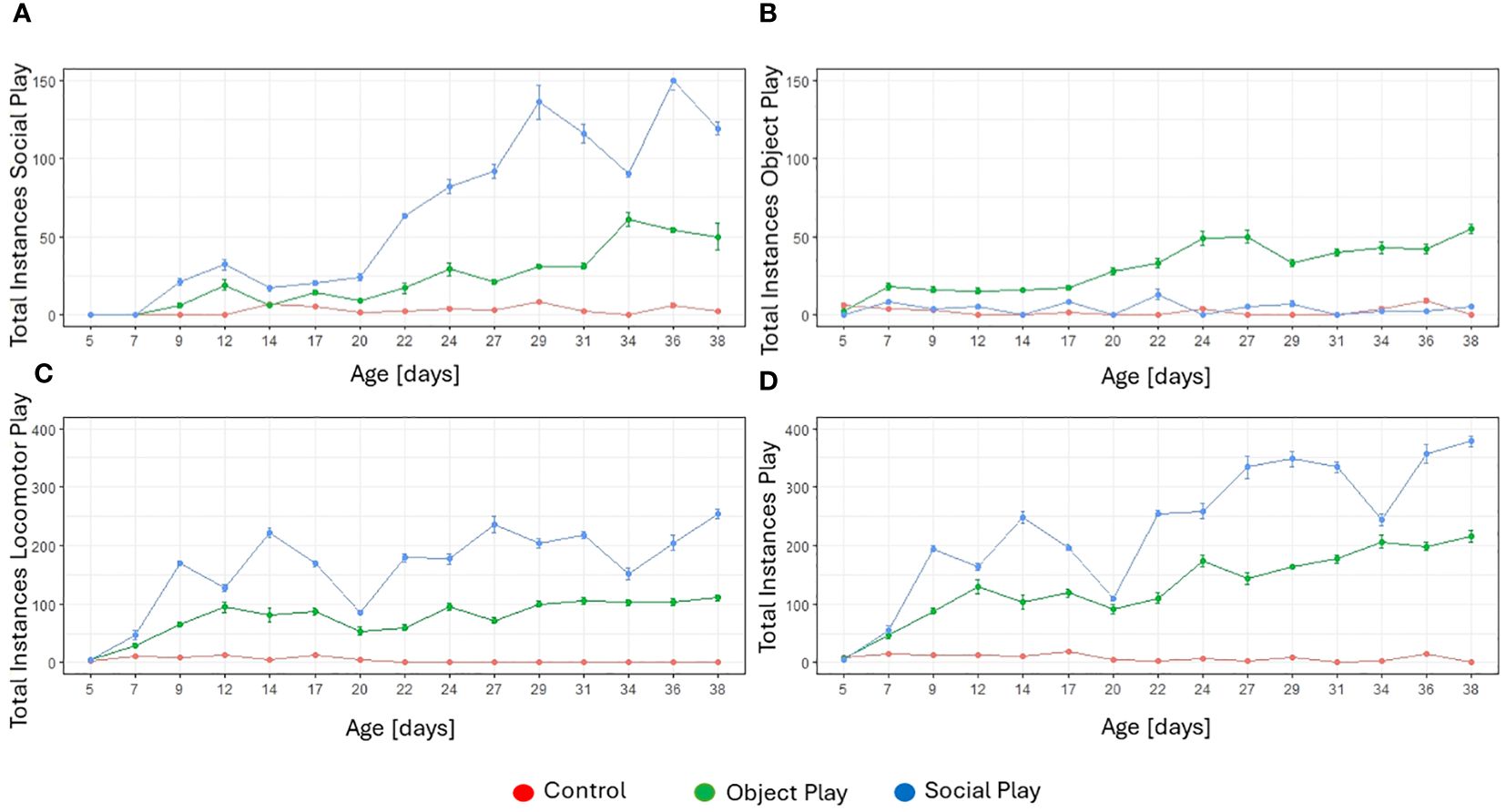
Figure 1 Total instances of play for every treatment group by play category over the five-week treatment period. (A) Total instances of social play. (B) Total instances of object play. (C) Total instances of locomotor play. (D) Total instances of play (all three categories combined).
There was also a significant effect of treatment (likelihood-ratio test, χ2 = 93.23, df=2, p<0.001) and age (likelihood-ratio test, χ2 = 53.43, df=14, p<0.001) on the occurrence of object play. Object players played significantly more often with objects compared to the other two groups. No significant difference was identified between the amount of object play in social players and controls (see Figure 1B). This is perhaps not surprising, as only object players were provided with a range of objects to play with while social players and control birds could only play with objects they found in the arenas, such as wood chips or fallen out feathers.
With respect to locomotor play, we identified a significant effect of treatment (likelihood-ratio test, χ2 = 126.40, df=2, p<0.001) and age (likelihood-ratio test, χ2 = 59.59, df=14, p<0.001). All three treatment groups differed significantly from each other with controls showing least locomotor play, followed by object players, and social players exhibiting most instances of locomotor play (see Figure 1C).
When comparing all instances of play, regardless of the category, a significant effect of treatment (likelihood-ratio test, χ2 = 125.81, df=2, p=<0.001) and age (likelihood-ratio test, χ2 = 116.42, df=14, p<0.001) was identified. Controls played least, followed by object players, and social players overall played most (see Figure 1D). The majority of play instances consisted of locomotor play.
In a competitive setting, play treatment had a significant effect on who was feeding first from the bowl (likelihood-ratio test, χ2 = 7.09, df=2, p=0.029). Social players fed first in 58%, object players in 26%, and control subjects in 16% of the trials (see Figure 2). Thus, social players fed significantly more often first compared to control subjects, while there was no significant difference between control and object players.

Figure 2 Probability of feeding first in a competitive setting of control, object play, and social play treatments. N.S. = not significant, *P<0.05.
Play treatment moreover had a significant effect on the duration of feeding (likelihood-ratio test, χ2 = 14.97, df=2, p<0.001). Social players were feeding on average for 90.46 ± 29.68 seconds, object players for 11.24 ± 5.44 seconds, and control subjects for 7.44 ± 5.32 seconds. Consequently, social players were feeding significantly longer than object players and control subjects, but no significant difference was detected between object players and control subjects.
Only four object players completed the pretest stages and the motor self-inhibition test. Because of that, we grouped both object and social players into one play treatment group for analyses. Play treatment had a significant effect on the number of failed trials, i.e., trials in which subjects pecked on the transparent tube before retrieving the mealworm (likelihood-ratio test, χ2 = 4.47, df=1, p=0.035). Chicks that had received a play treatment failed on average 7 out of 15 trials, while control subjects on average only failed 5 out of 15 trials (see Figure 3).
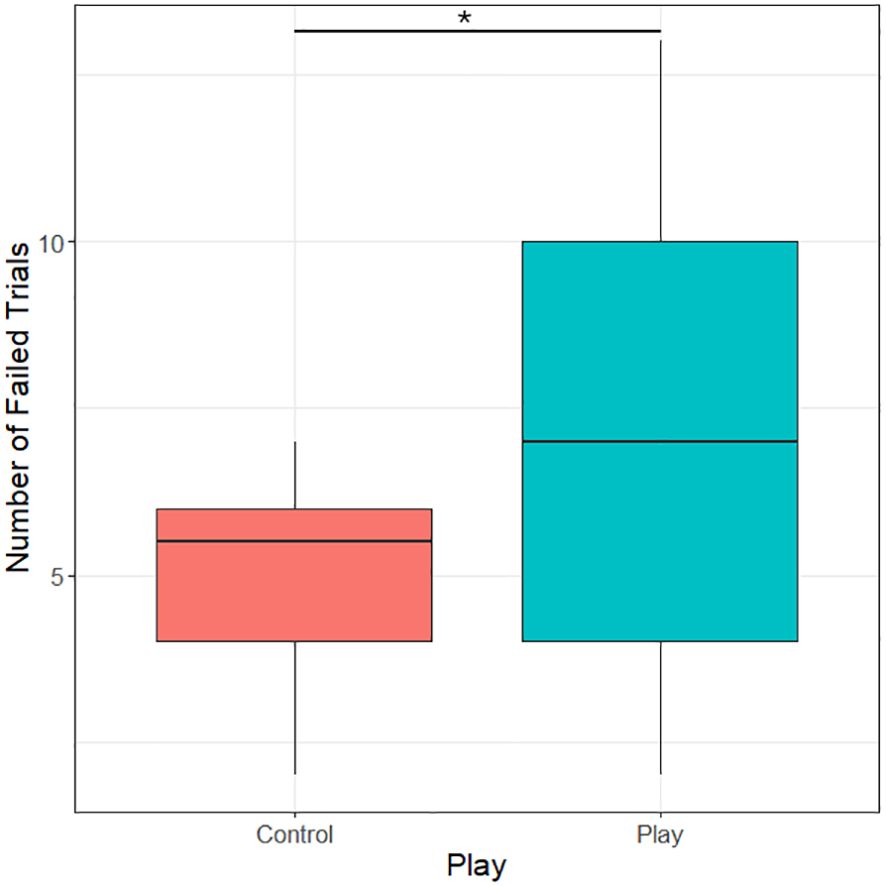
Figure 3 Number of failed trials (trials in which the transparent cylinder was pecked) in the motor self-inhibition test in control subjects and subjects receiving a play treatment. *P<0.05.
We found no significant effect of play treatment on the ratios of global, immediate, and distant errors. In fact, the factor treatment was even excluded from each of these models based on selecting the best-fitting model. Play did thus not improve spatial memory capacities in chicks.
We did, however, find a significant effect of play treatment on the duration to complete the spatial memory test (likelihood ratio test, χ2 = 7.17, df=2, p=0.028). Control chicks took on average 442.30 ± 43.47 seconds, object players 440.80 ± 38.61 seconds, and social players 301.23 ± 44.64 seconds to complete the task (see Figure 4). Thus, social players completed the task significantly quicker than the other two groups. No significant difference between object players and control subjects could be detected.
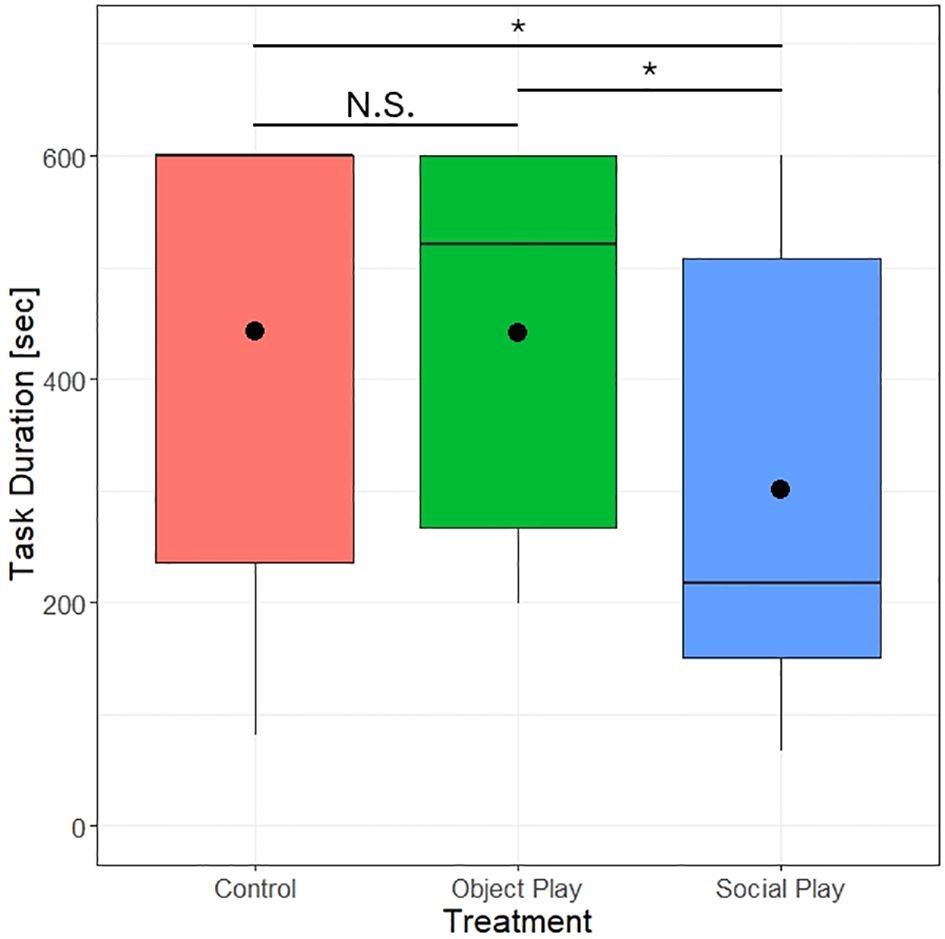
Figure 4 Duration to finish the spatial memory test by play treatments. Points indicate the mean duration to finish the task. N.S. = not significant, *P<0.05.
Play treatment further had a significant effect on the number of visited cups (likelihood ratio test, χ2 = 6.03, df=2, p=0.049). Social players visited on average 7.65 ± 0.17 cups, object players visited 6.59 ± 0.33 cups, and control subjects visited 6.59 ± 0.40 cups. Thus, we again found a significant difference between social players and the other two groups, but no significant difference between object players and control chicks.
After excluding all subjects that did not take mealworms in any of the trials (social players: 4, object players: 12, controls: 4), we found no significant effect of play treatment on the probability of choosing the demonstrated colour (likelihood ratio test, χ2 = 0.79, df=2, p=0.66; see Figure 5). We did, however, detect significant effects of demonstrated side with a preference for the right side (likelihood ratio test, χ2 = 10.92, df=1, p<0.001), colour with a preference for blue (likelihood ratio test, χ2 = 7.72, df=1, p=0.0055), and the interaction of play treatment and colour (likelihood ratio test, χ2 = 21.93, df=2, p<0.001). This indicates that side- and colour-biases were masking possible effects of play treatment. None of the play conditions chose the demonstrated colour significantly different from 0.5 (binomial test; social play: p=0.89, object play: p=0.06, control: p=1). No significant effect of play condition was observed on the first choice (likelihood ratio test, χ2 = 1.09, df=2, p=0.58).
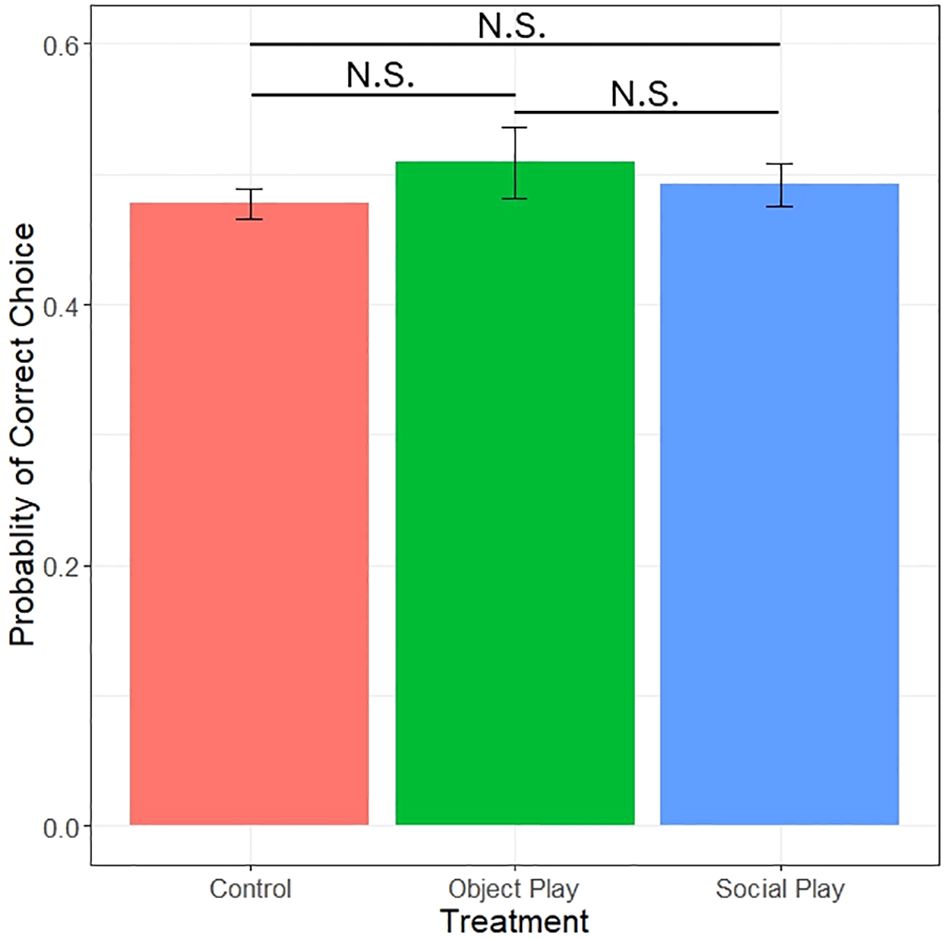
Figure 5 Probability of choosing the demonstrated colour by play treatments. N.S. = not significant.
In the present study, we investigated for the first time the connection between play stimulation and cognitive performance in chickens. In general, we found no evidence for an improvement of cognitive capacities in play stimulated chicks compared to chicks that were not stimulated to play during their ontogeny. However, subjects that had received the social play treatment were behaving more dominantly, were more explorative and quicker in finding food, and were less inhibited when locating food.
The administered play treatments appear to have worked well in the sense that the social play group played most socially, the object play group played most with objects, and the control group barely played at all. Controlling all occurrences of play is, however, difficult. Social players, for example, still played with objects, such as wood chips and feathers that they found in the arena. Similarly, object players played socially as they were moved into the play arenas in groups. In comparison to a previous study (Lundén et al., 2022), chicks in the present study showed more social play, but less instances of object play, though the developmental trend is comparable. The higher frequency of social play might have been caused by larger arenas and play groups in our study. For possible explanations of the low frequency of object play, see below. However, it should be noted that considerable methodological differences between the two studies make direct comparisons difficult. The two studies differ in sampling method, distribution of sexes, and group sizes. For that reason, it is not possible to determine whether the described differences in play frequencies were caused by varying methods, or by actual differences in the play behaviours.
With respect to our initial hypotheses, we found no support for a connection between specific types of play and cognitive domains. It should be noted that as object players also engaged in social play and social players also played with objects, it is difficult to draw conclusions on the connection between specific types of play and the subsequent performance in cognitive tests. We can, however, speculate on the effects of general play frequency on cognitive development, as the social play group overall played most, followed by the object play group and the control group barely played at all.
Moreover, we could not control for play behaviours taking place in the home cages. It should, however, be noted that the home cages provided relatively little space and we did not observe any play behaviours in these cages. This does, however, not mean that chicks did not play in their home cages when no experimenter was present. The differences we observed in the chicks’ behaviour during the test battery, however, indicate that the additional play stimulation did in fact have some effects on their later behaviour.
Due to overcrowding in the home cages, we had to regroup the chicks before the beginning of the test period. This was done through taking three females out of each of the six cages and forming three new additional cages only consisting of females. That means, that these females encountered a new social group as well as a new environment (though the setup and layout of the cages was identical). This might have been a stressful experience and could have, subsequently, altered their performance in the tests. However, overcrowding could have also negatively impacted our experiments. In this study, we did not observe any aggressive encounters between the females in the new cages nor any signs of stress caused by this reorganisation. We allowed the subjects to habituate to their new environment for 20 hours before starting the tests. This interval has previously been shown sufficient for White Leghorn chickens to return to baseline behavioural levels after relocation (Ericsson et al., 2014).
Both the social and the object play treatment groups engaged in locomotor play. As both treatment conditions were released into arenas of the same size, we expected to observe comparable rates of locomotor play. However, object players showed significantly less locomotor play compared to the social play treatment. Possibly, the setup of the object play arenas might have caused the low frequency of locomotor play in this treatment group. In this setup, three objects were hanging from a diagonal perch. These objects formed a diagonal divider (though very sparse and see-through) in the arena. Our findings indicate that space is the main trigger of play in young chicks as had previously been described in broilers (Liu et al., 2020). The hanging objects might have inhibited play behaviours. This finding might have consequences with how chicken pens should be set up and stresses the importance of providing chickens with open, free space.
The object play treatment had no apparent effects on the cognitive capacities of the chicks. It should, however, be noted that the object play group appeared more fearful and less food motivated during the cognitive tests than the other two treatment groups (social players and controls). The object play group even had to be removed from the analyses of two experiments because too few individuals participated in the tasks (motor self-inhibition and social information transfer tests). It is unclear what caused the behaviour of this group. It could have been caused by a coincidental combination of fearful individuals or few very fearful individuals unsettling the rest of the group. Alternatively, the behaviour could in fact have been caused by the object play treatment itself. The amount of colours, textures, and shapes of novel objects provided in the arenas might potentially have been stressful to the young chicks. This could also explain the low number of observed playful interactions with the objects. A gradual introduction of objects might have reduced this effect. However, as subjects have been introduced to the objects so early in their ontogeny, we would have expected them to habituate to them. In fact, previous studies show that early exposure to more complex environments including objects such as balls, strings, or drawings on the wall reduced fearfulness in chicks (Jones and Waddington, 1992). Furthermore, during the play treatments, the chicks in our study did not appear stressed or fearful in the video recordings and performed different play behaviours, though at a reduced frequency compared to the social play condition. Further studies are needed to shed light on whether this was a coincidence or if something in the play treatment has caused the fearful behaviour of the subjects receiving the object play treatment.
The social play treatment appears to have had an effect on some of the behavioural variables recorded in the cognitive test battery, even though we did not observe an overall improvement of cognitive capacities. It should further be noted that some of the effects are small (number of visited cups in the spatial memory test, number of failed trials in motor self-inhibition test). The enhanced exploratory tendencies of chicks that were stimulated to play socially might allow them to gather more information about the environment as more exploration typically leads to more encounters with different objects and situations. The cognitive benefits suggested by Andersen et al. (2022) might still occur, but possibly at a later stage. Alternatively, instead of play allowing the brain to learn about the environment, it might stimulate explorative behaviour which could in turn help the brain in forming predictions about the environment. Positive effects on cognitive performance might thus only become apparent later in life. The subjects in this study were tested between six and eight weeks old, which could have been too young to see the potential benefits play had on their cognitive capacities. A follow-up study testing the same subjects later in life would shed more light on the cognitive benefits of play stimulation. Nevertheless, more studies will be needed to fully understand the connection between play and cognitive development.
As we found no support for the neurocognitive theory of play brought forward by Andersen et al. (2022), some of the alternative theories might still be more fitting to explain the functions of play. The cognitive test battery in this study was not designed to test for any training effects of play (as suggested by e.g. Mallpress and Špinka, 2023). The only possible training effect we observed was between the social play treatment and the social dominance test, with subjects that had played more socially being dominant over control chicks. This finding is in line with previously described theories about the function of play in forming social hierarchies (e.g. Bekoff, 1976; Fagen, 1981). Blumstein et al. (2013), for example found that directional outcomes of early social play in yellow-bellied marmots (Marmota flaviventris) is correlated to later dominance relationships. In our study, interacting playfully in a competitive way with conspecifics (through e.g. sparring) might have trained the chicks to successfully compete with others over resources. More studies specifically designed to demonstrate training effects of play will be needed to fully understand the involvement of play in training species-specific behaviours.
One factor that might have affected our findings is the fact that we used the same arenas we used in the play treatments for the cognitive tests due to space constraints. This means that subjects of different play treatments might have different associations with this space and hence might act differently. Social players were familiar with the arenas, object players might have expected objects and thus might have had a negative association with being placed in the arenas without the familiar toys, and control subjects had not seen the arenas previously and might thus either have enjoyed having more space or have been stressed by the novel environment. This could explain why social players generally performed best in the tests, as they should be least stressed or distracted by the test environment. To mitigate this effect, we included a habituation phase during the first test (social dominance test) to allow the control subjects to get used to the novel environment. Nevertheless, if familiarity were the driver behind the subjects’ behaviour, we would expect to find a change in the findings as we progressed through the tests. The birds were in the arenas for tests every day for two weeks. Hence, potential initial neophobia in control chicks should not have affected later tests. Similarly, even if object players were initially surprised by the lack of the toys, they should have habituated to their absence during the following days.
Taken together, our findings show that chicks that were engaging in higher frequencies of play during their ontogeny appeared to be more explorative, bolder, and dominant over other individuals. This might in turn allow them to have more access to resources as we, for example, observed that they were feeding significantly longer in a competitive setting. These findings might also have welfare implications for chickens. Chickens that have played during their ontogeny might be better at finding food and other resources and have an advantage in monopolising those, which could in turn lead to physical advantages and more resilience to stress. Future lines of investigation should cover long-term effects of play stimulation on cognitive performance and investigate physical and welfare markers to obtain a better understanding of the effects of play stimulation during the ontogeny on chickens.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Linköping Regional Committee for Ethical Approval of Animal Research, license no. 10492-2023. The study was conducted in accordance with the local legislation and institutional requirements
CZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. PJ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. CZ and this project were funded by the Carl Trygger Foundation under grant number CTS 21:1533
We are thankful to Austeja Rutkauskaite for her help with the husbandry of the research subjects as well as support during data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fetho.2024.1403082/full#supplementary-material
Andersen M. M., Kiverstein J., Miller M., Roepstorff A. (2022). Play in predictive minds: A cognitive theory of play. Psychol. Rev. 130 (2), 462–479. doi: 10.1037/rev0000369.
Bateson P., Bateson P. P. G., Martin P. (2013). Play, playfulness, creativity and innovation (Cambridge: Cambridge University Press). doi: 10.1017/CBO9781139057691
Bekoff M. (1976). “Animal play: Problems and perspectives,” in Perspectives in ethology, vol. 2. (Boston, MA: Springer), 165–188. doi: 10.1007/978-1-4615-7572-6_4
Blumstein D. T., Chung L. K., Smith J. E. (2013). Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris). Proc. R. Soc. B: Biol. Sci. 280, 20130485. doi: 10.1098/rspb.2013.0485
Brownlee A. (1954). Play in domestic cattle in Britain: An analysis of its nature. Br. Veterinary J. 110, 48–68. doi: 10.1016/S0007-1935(17)50529-1
Burghardt G. M. (2001). “Play,” in Developmental psychobiology. Handbook of behavioral neurobiology, vol. 13 . Ed. Blass E. M. (Boston MA: Springer), 317–356. doi: 10.1007/9781-4615–1209-7_9
Burghardt G. M. (2005). The genesis of animal play: Testing the limits (Cambridge, MA: MIT press). doi: 10.7551/mitpress/3229.001.0001
Byers J. A. (1999). The distribution of play behaviour among Australian marsupials. J. Zoology 247, 349–356. doi: 10.1111/j.1469-7998.1999.tb00997.x
Cordoni G. (2009). Social play in captive wolves (Canis lupus): Not only an immature affair. Behaviour 146, 1363–1385. doi: 10.1163/156853909X427722
Dinets V. (2015). Play behavior in crocodilians. Anim. Behav. Cogn. 2, 49–55. doi: 10.12966/abc.02.04.2015
Dona H. S. G., Solvi C., Kowalewska A., Mäkelä K., MaBouDi H., Chittka L. (2022). Do bumble bees play? Anim. Behav. 194, 239–251. doi: 10.1016/j.anbehav.2022.08.013
Ericsson M., Fallahsharoudi A., Bergquist J., Kushnir M. M., Jensen P. (2014). Domestication effects on behavioural and hormonal responses to acute stress in chickens. Physiol. Behav. 133, 161–169. doi: 10.1016/j.physbeh.2014.05.024
Evans C. S., Evans L. (1999). Chicken food calls are functionally referential. Anim. Behav. 58, 307–319. doi: 10.1006/anbe.1999.1143
Heinrich B., Smolker R. (1998). “Play in common ravens (Corvus corax),” in Animal play: Evolutionary, comparative, and ecological perspectives. Eds. Bekoff M., Byers J. A. (Cambridge: Cambridge University Press), 27–44. doi: 10.1017/CBO9780511608575.003
Himmler S. M., Himmler B. T., Pellis V. C., Pellis S. M. (2016). Play, variation in play and the development of socially competent rats. Behaviour 153, 1103–1137. doi: 10.1163/1568539X-00003307
Jones R. B., Waddington D. (1992). Modification of fear in domestic chicks, Gallus gallus domesticus, via regular handling and early environmental enrichment. Anim. Behav. 43, 1021–1033. doi: 10.1016/S0003-3472(06)80015-1
Kabadayi C., Bobrowicz K., Osvath M. (2018). The detour paradigm in animal cognition. Anim. Cogn. 21, 21–35. doi: 10.1007/s10071-017-1152-0
Keller R. (1975). Das Spielverhalten der Keas (Nestor notabilis Gould) des Zürcher Zoos. Z. für Tierpsychologie 38, 393–408. doi: 10.1111/j.1439-0310.1975.tb02012.x
Leslie A. M. (1978). Pretense and representation: The origins of “theory of mind”. Psychol. Rev. 94, 412. doi: 10.1037/0033-295X.94.4.412
Lewis K. P. (2000). A comparative study of primate play behaviour: Implications for the study of cognition. Folia Primatol. 71, 417–421. doi: 10.1159/000052740
Liu Z., Torrey S., Newberry R. C., Widowski T. (2020). Play behaviour reduced by environmental enrichment in fast-growing broiler chickens. Appl. Anim. Behav. Sci. 232, 105098. doi: 10.1016/j.applanim.2020.105098
Lohmann (2018). Management guide hatchery (Lohmann Tierzucht). Available at: www.lohmann-breeders.com.
Lundén G., Oscarsson R., Hedlund L., Gjøen J., Jensen P. (2022). Play ontogeny in young chickens is affected by domestication and early stress. Sci. Rep. 12, 13576. doi: 10.1038/s41598–022-17617-x
Mallpress D. E., Špinka M. (2023). The practicality of practice: A model of the function of play behaviour. Ecol. Evol. 13, e10521. doi: 10.1002/ece3.10521
Nunes S. (2014). Juvenile social play and yearling behavior and reproductive success in female Belding’s ground squirrels. J. Ethol. 32, 145–153. doi: 10.1007/s10164-014-0403-7
Parois S., Calandreau L., Kraimi N., Gabriel I., Leterrier C. (2017). The influence of a probiotic supplementation on memory in quail suggests a role of gut microbiota on cognitive abilities in birds. Behav. Brain Res. 331, 47–53. doi: 10.1016/j.bbr.2017.05.022
Perret M. (2021). Litter sex composition influences competitive performance during first reproduction in male mouse lemurs. Physiol. Behav. 228, 113196. doi: 10.1016/j.physbeh.2020.113196
RStudio Team (2020). RStudio: integrated development for R. RStudio (Boston, MA: RStudio, PBC). Available at: http://www.rstudio.com/.
Ryding S., Garnham L. C., Abbey-Lee R. N., Petkova I., Kreshchenko A., Løvlie H. (2021). Impulsivity is affected by cognitive enrichment and links to brain gene expression in red junglefowl chicks. Anim. Behav. 178, 195–207. doi: 10.1016/j.anbehav.2021.06.007
Singer D. G., Singer J. L. (2009). The house of make-believe: Children’s play and the developing imagination (Cambridge, MA: Harvard University Press). doi: 10.2307/j.ctvk12s32
Špinka M., Newberry R. C., Bekoff M. (2001). Mammalian play: Training for the unexpected. Q. Rev. Biol. 76, 141–168. doi: 10.1086/393866
Thompson K. V. (1998). “Self assessment in juvenile play,” in Animal play: Evolutionary, comparative, and ecological perspectives. Eds. Bekoff M., Byers J. A. (Cambridge: Cambridge University Press), 183–204. doi: 10.1017/CBO9780511608575.003
Wascher C. A., Kulahci I. G., Langley E. J., Shaw R. C. (2018). How does cognition shape social relationships? Philos. Trans. R. Soc. B: Biol. Sci. 373, 20170293. doi: 10.1098/rstb.2017.0293
Keywords: cognitive development, play, chicken, social play, object play
Citation: Zeiträg C and Jensen P (2024) The effects of play stimulation on cognitive capacities of chickens. Front. Ethol. 3:1403082. doi: 10.3389/fetho.2024.1403082
Received: 18 March 2024; Accepted: 10 June 2024;
Published: 24 June 2024.
Edited by:
Christine Drea, Duke University, United StatesReviewed by:
Leonie Jacobs, Virginia Tech, United StatesCopyright © 2024 Zeiträg and Jensen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Zeiträg, Y2xhdWRpYS56ZWl0cmFnQGxpdS5zZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.