- Department of Neuroscience, University of Lethbridge, Lethbridge, AB, Canada
Play fighting has been one of the most intensely studied forms of play and so has provided some of our deepest insights into the understanding of play in general. As the label implies, this behavior resembles serious fighting, in that the animals compete for an advantage over one another, but unlike true aggression, for play fighting to remain playful, it also incorporates a degree of cooperation and reciprocity – restrained competition seems to be its hallmark. Despite these common features, it should be noted that both the advantage competed over and the mechanisms by which restraint is achieved varies across species. Such variation mitigates simple generalities. For example, how empirical support for a proposed adaptive function in one species not being replicated in another, is to be interpreted. What has emerged over the past few decades is that play fighting is diverse, varying across several dimensions, some superficial, some fundamental, making choosing species to compare a challenge. In this paper, we explore various design features that constitute play fighting and the ways these can be modified across different species and lineages of species. Given that a major pillar of ethology is that description precedes explanation, having a good grasp of the behavioral diversity of play fighting is an essential starting point for detailed analyses of the mechanisms and functions of play. We show that commonalities across species likely involve different mechanisms than do species idiosyncrasies, and that different styles of play fighting likely afford different adaptive opportunities.
Introduction
By tradition, play in non-human animals has been divided into three categories: (a) play with others (“social play”), (b) play with objects (“object play”), and (c) solitary play involving the animal moving its body in peculiar ways (e.g., rapid runs, jumps, body twists and rotations) (“locomotor-rotational play”) (Fagen, 1981; Burghardt, 1998; Burghardt, 2005). Sometimes, components of two or more of the three can be combined (e.g., Biben, 1982; Pellis, 1991; Donaldson et al., 2002; Shimada, 2012; Burghardt et al., 2016; Manitzas Hill et al., 2023). Even in their pure forms, the content of each category can be diverse, for example, social play can involve behavior typically associated with sex, maternal activities, predation, or conspecific aggression (Pellis, 1988; Pellis and Pellis, 2009). The form of social play that appears to simulate conspecific aggression, often referred to as play fighting, is one of the most often reported and most intensely studied forms of play (Pellis and Pellis, 1998a; Pellis and Pellis, 2017a), and has provided some of our deepest insights into the mechanisms and functions of play (for extensive reviews, see Pellis et al., 2010a; Pellis et al, 2014; Vanderschuren and Trezza, 2014; Siviy, 2016; Vanderschuren et al., 2016; Palagi, 2018; Sharpe, 2019; VanRyzin et al., 2020; Achterberg and Vanderschuren, 2023; Cooper et al., 2023; Nunes and Montemayor, 2023; Palagi, 2023; Pellis et al., 2023a). To gain a feel for the phenomenon, consider Mbundi and Ntondo, two captive, three-year-old male lowland gorillas (Gorilla gorilla gorilla) charging, grappling, and wrestling each other (Pellis and Pellis, 2016a). Such a scene has been reported for many mammals, several birds, and even some invertebrates (e.g., Fagen, 1981; Pellis, 1981; Pozis-Francois et al., 2004; Burghardt, 2005; Dapporto et al., 2006; Pruitt et al., 2012). What distinguishes such playful wrestling from serious fighting, including that exhibited by Mbundi and Notondo, is that no resource is gained or lost, injuries are not incurred, the wrestling often leads to further affiliation, and they reverse roles (Smith, 1997). Moreover, the playful version of fighting is often accompanied by gestures that function as “play signals” marking the interactions as playful (Bekoff, 1975; Fagen, 1981; Smith, 1997). In the case of the gorillas, facial gestures are used during play to facilitate further contact (Waller and Cherry, 2012; Palagi et al., 2019).
As the name implies, play fighting resembles serious fighting, although given its differences with serious fighting, some authors prefer to call this behavior rough-and-tumble play as it emphasizes the cooperative, pleasurable side of the behavior (e.g., Palagi, Pellegrini, 2002; Scott and Panksepp, 2003; Burghardt et al., 2016; Smith and StGeorge, 2022), especially when presenting the phenomenon to audiences that may be hostile to any hint of aggression or violence (Pellis et al., 2022a). Nonetheless, a central feature of play fighting is competition, which is what makes it resemble serious fighting, but competition for what? A breakthrough was provided by Owen Aldis (1975), who collected film of play fighting in a range of mammals, including human children. What he found was that animals do not just aimlessly grapple and push one another when play fighting, but rather, most of that grappling and pushing occurs as the partners compete to gain an advantage over one another. For many of the species he had in his sample, that advantage involved biting the competitor, and not just anywhere, but on the same body locations that are bitten during serious fighting. In species in which serious fighting does not involve biting, such as many ungulates, the play fighting also mimics what happens in serious fighting, with the same body locations being head butted or kicked. And in humans, the contestants compete to push and hold each other on the ground, one of the advantages sought during serious fighting (Aldis, 1975).
By noting the similarity of the targets over which the animals struggle for contact between serious and playful fighting, Aldis made it clear that play fighting is a competitive activity. This insight has had a significant impact on the subsequent decades of research on play fighting. First, the image of play fighting as a simulation of serious fighting reinforced the view that the function of play is to practice adult-typical behavior (Groos, 1898), in this case, the behavior patterns used in combat (Smith, 1982). This view continues to guide some current research (e.g., Barrett et al., 2021; Cordoni et al., 2021; Cordoni et al, 2022) and certainly holds sway in the lay media (see most natural history documentaries that discuss play). As will be discussed later, this “practice” view of play fighting is either wrong or incomplete at best (Pellis and Pellis, 1998b; Pellis and Pellis, 2017a). Nonetheless, by identifying that play fighting does not involve aimless grappling and wrestling, but competition for a specific advantage, such as contacting particular body targets, Aldis’ insight provided a framework that could be used to gain deeper understanding of the of the actions performed, and so revolutionized how we think about play.
The design features of play fighting
Competition
What animals compete over, and how this may influence what is or is not classified as play fighting is illustrated by the study of this behavior in rats (Rattus norvegicus). Juvenile rats engage in competitive interactions that resemble fighting. Some early observers referred to some of these interactions as play fighting (e.g., Bolles and Woods, 1964; Poole and Fish, 1975), whereas others considered such behavior immature aggression (e.g., Silverman, 1978; Taylor, 1980). The problem is that the rats use similar behavior patterns in both presumptive types of fighting, including mutual uprights, supine postures, lateral displays and fleeing and chasing, so what distinguishes one of them as play? Using Smith (1997) criteria gets us partially there, but the counterargument would be “of course, no resource is gained, or injury incurred, as the behavior is being performed by immature animals in which the aggression system is not fully formed.” There are many examples of incomplete use of behavior patterns in immature animals that do not achieve a utilitarian end-point and yet would not be considered as play, such as early stages of dust bathing in chickens (Vestergaard et al., 1990), face grooming in infant mice (Golani and Fentress, 1985), and righting in perinatal mammals (Pellis et al., 1991; Pellis et al, 1992b). Although, labeling the interactions of immature rats as play became increasingly popular (e.g., Panksepp and Beatty, 1980; Meaney and Stewart, 1981; Panksepp, 1981; Pellis and Pellis, 1983; but see Hurst et al., 1996), a clear objective way of distinguishing playful from aggressive interactions was yet to emerge. Aldis’ insight that the competition present in play fighting is geared to gaining a species-typical advantage provides a solution.
During serious fighting, rats compete to deliver bites to the lower dorsum and flanks and to the front of the face (Blanchard et al., 1977; Pellis and Pellis, 1987), but during play fighting, rats compete to contact the partner’s nape of the neck, which is nuzzled with the tip of the snout if contacted (Pellis and Pellis, 1987). Indeed, if the snout or the nape is anesthetized, play is suppressed (Siviy and Panksepp, 1987). Moreover, if the receiving rat fails to defend its nape during play fighting, the attacker makes contact, briefly nuzzles it and leaps away (Figure 1), further supporting the view that contacting the nape is the advantage sought during play fighting in rats. Thus, while there is a rough similarity between the tactics used to defend the targets in both types of fighting, the difference in targets leads to them being different in detail. For example, while lateral threat occurs as an offensive tactic during serious fighting, it is used as a defensive tactic during play fighting (Pellis and Pellis, 1987; Pellis and Pellis, 2015). But why the nape?
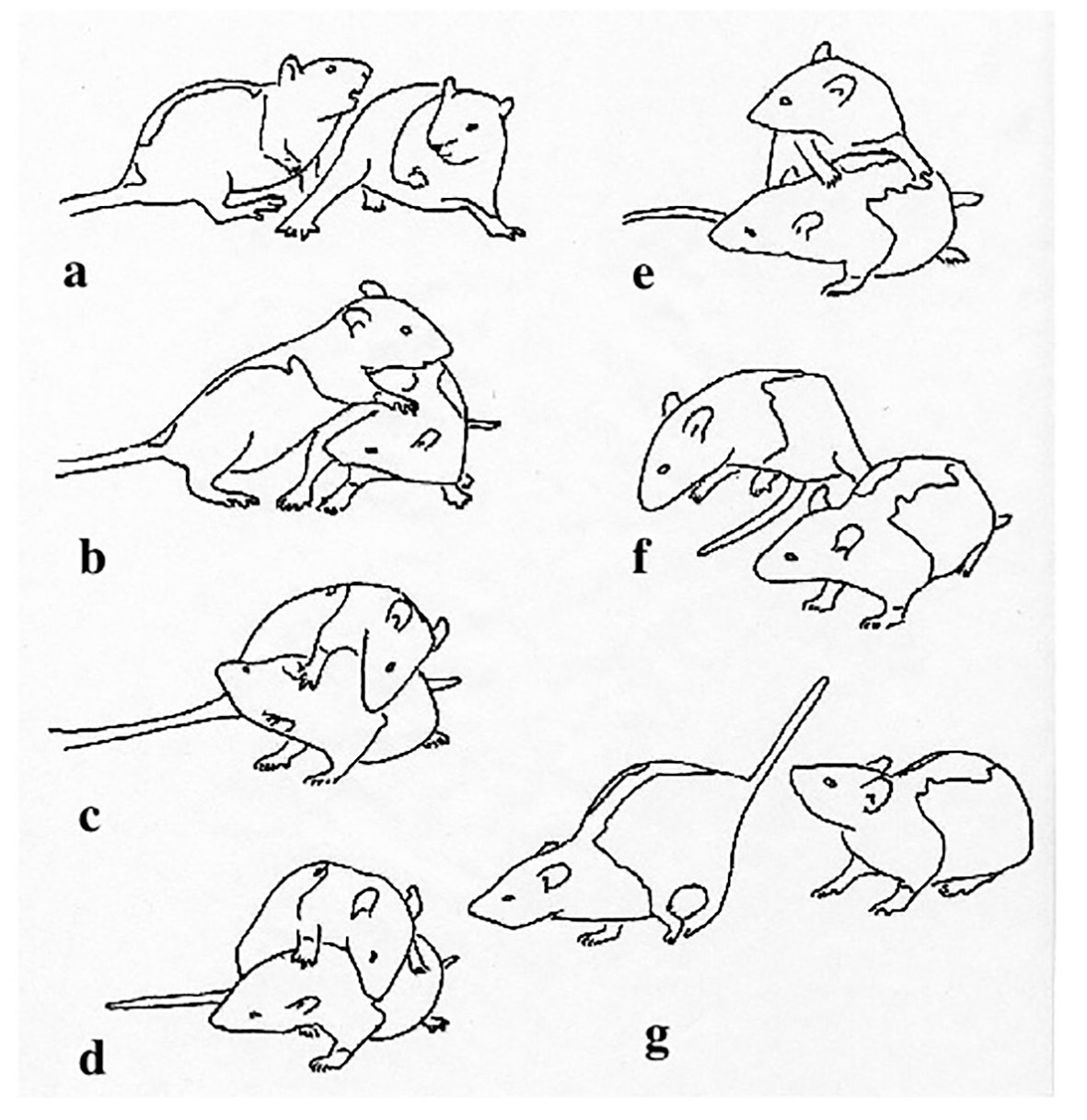
Figure 1 Interaction between two juvenile rats, about 35 days old, showing the animal on the left pouncing on its partner (A, B), contacting the neck and shoulders with its snout (C, D), then, in the absence of an active defense by the recipient, turning (E) and leaping away (F, G). Reprinted from Pellis (1988), with permission from Wiley.
Detailed comparisons of play fighting with serious fighting and sexual encounters of adults revealed that nuzzling the nape occurs during the precopulatory phase of sexual encounters whereby the male contacts the nape (Pellis and Iwaniuk, 2004) and then uses that as a pivot point to mount the female (Whishaw and Kolb, 1985). Reinforcing the sexual connection is the finding that many of the whole-body hops and darts that are inserted into play fighting sequences (Pellis and Pellis, 1983) resemble the solicitation behavior of sexually receptive females during sexual encounters (Thor and Flannelly, 1978; Thor and Holloway, 1983). Comparisons of the targets contacted in a range of murid rodents (members of the same family as rats) revealed that all species attack the same body targets during serious fighting as are attacked by rats (Pellis, 1997), but in each species in which play fighting occurs, the target corresponds to that which is contacted during courtship (Pellis, 1993). These targets can differ across species. For example, deer mice (Peromyscus maniculatus), like rats, compete to nuzzle the nape (Pellis et al., 1989), whereas Djungarian hamsters (Phodopus campbelli) compete to lick the mouth (Pellis and Pellis, 1989). Casting the net more widely across rodents from different familial groupings reveals that some species engage in competition for the same body targets as are bitten during serious fighting, whereas others compete for access to body targets typical of courtship. The latter can include mounting, the terminal phase of courtship. Finally, some species compete for both sexual and aggressive targets (Figure 2). Indeed, during play fighting, the grasshopper mouse (Onychomys leucogaster), which is an obligate carnivore that can catch and dispatch prey of similar size, either competes to lick and nuzzle the shoulders and neck, as in courtship, or bite the nape, as in predation (Pellis and Pellis, 1992a; Pasztor et al., 2001). That is, play fighting can involve competition to contact targets typical of conspecific aggression, amicable behavior, such as that associated with reproduction, or predation (Pellis, 1988). Even when the same targets are involved, play fighting can differ depending on what type of adult functional behavior is being simulated. For example, many felids, such as lions (Panthera leo), compete to bite the nape during play fighting, a target that could be borrowed from either conspecific aggression or predation, but with the latter distinguished by the racking with the hind paws that accompanies predation (Schaller, 1972; Leyhausen, 1979).
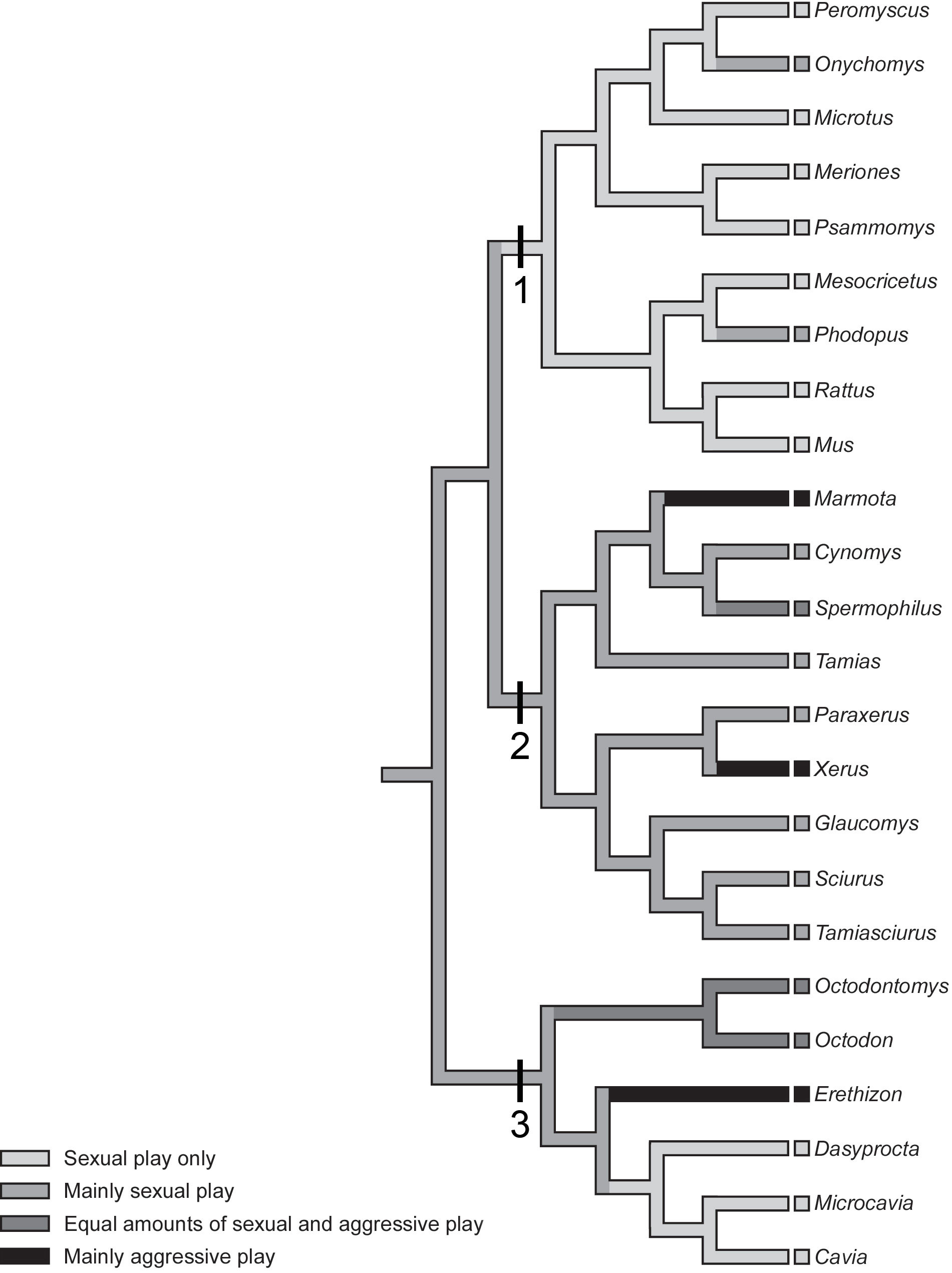
Figure 2 A cladogram, a tree diagram which reveals the pattern of relatedness across a group of species, is shown for rodents, spanning three major divisions of the order in which play has been extensively studied. Note that the cladogram only shows the pattern of relatedness not the actual times of divergence, but to gain a sense of the timescale involved, rodents diverged from their common ancestor with lagomorphs (i.e., rabbits, pikas) about 100 Mya. Clade 1 shows species from the suborder Myomorpha, the mouse-like rodents, including the domestic rat (Rattus), the domestic mouse (Mus), the deer mouse (Peromyscus), the grasshopper mouse (Onychomys), the fat sand rat (Psammomys), the Syrian hamster (Mesocricetus), the Djungarian hamster (Phodopus), the montane vole (Microtus montanus), the prairie vole (M. ochrogaster), and the European vole (M. agrestis). Clade 2 shows species from the suborder Sciuromorpha, the squirrel-like rodents, including the North American ground squirrel (Spermophilus ńee: Utricellus) and the grey tree squirrel (Sciurus). Clade 3, the suborder Hystricomorpha, the guinea pig-like rodents, including the degu (Octodon). Whether the play fighting is mostly aggressive, mostly sexual, or some combination of both, is mapped onto the cladogram. Note that only species that are discussed in the text are named, as the main objective is to give readers a sense of the diversity of types of play fighting. Reprinted from Pellis and Iwaniuk (2004), with permission of the authors.
Comparative analyses of other taxa have revealed similar patterns. For example, primates can compete to bite body targets like those during conspecific aggression, targets that are groomed during affinitive or sexual encounters, or mounting as in sexual behavior, with species from some branches in the primate tree more likely to do one or the other (Pellis et al, 2023a). Moreover, the play fighting of some species can involve competing for all three (Pellis and Pellis, 2018). The same also appears to be the case for some cetaceans, which can compete for a sexual advantage or an agonistic advantage (Lilley et al., 2020; Ham et al., 2022; Ham et al, 2023b; Ham et al., 2023c). For play involving sexual contacts, it is those cases in which the recipient defends itself that look strikingly like play fighting and so resembles serious fighting. In cases in which the contact is not contested, the play does not resemble fighting and these are typically referred to as sexual play, not play fighting (e.g., Orgeur and Signoret, 1984; Gomendio, 1988). The same distinction applies even for play involving mounting. When mounting by one animal is not contested by its partner, it is not typically referred to as play fighting (Harlow, 1969; Hanby and Brown, 1974). In contrast, when recipients, such as quick-moving ground squirrels (Utricellus spp) actively defend themselves from being mounted, the behavior is more likely to be labeled as play fighting (Nunes et al., 1999; Pasztor et al., 2001) compared to species which do the same thing at a slower pace. For example, when a beluga whale (Delphinapterus leucas) maneuvers to mount the partner playfully or contact it with its penis, and this coupled with the partner rotating to avoid the contact, the action occurs in what seems to human observers, to be in slow motion (Ham et al., 2022). The slower pace of action in the whales compared to the squirrels leads to controversy as to whether such behavior in whales should be labeled as play, much less play fighting.
For rats, play fighting neither simulates serious fighting nor does it represent immature aggression, as the targets are different, but given that the playful targets mimic adult sexual behavior, it could be labeled as immature sex. So, before we can conclude that play fighting is a convergent phenomenon that can involve behavior borrowed from aggression, predation, or sexual encounters, we need to explore how play fighting, whatever its origins, can be distinguished from immature versions of the behavior. Again, Aldis’ insight is crucial to this endeavor.
Cooperation
During serious fighting, an attack is countered by a defense and if the combatants are equally matched in skill, few, if any, attacks reach their target (Geist, 1978), so much so, that the seeming rarity of injury often leads to serious fights being labeled as “ritualized” contests (Archer and Huntingford, 1994). However, the presence of combat-induced injuries has likely been underestimated (e.g., Geist, 1986; Huntingford and Turner, 1987; Ham et al., 2021; Grimes et al., 2022), and detailed analyses of actual combat reveals that when the opponent’s defenses are breached, a combatant will not hesitate to deliver a potentially injurious attack (Geist, 1965, Geist, 1967; Geist, 1971; Pellis et al., 2013). Moreover, if the recipient of an attack successfully wards off the offensive maneuver, it can deliver an offensive attack of its own. That is, in serious fighting, the attacker must overcome the opponent’s defense and avoid a retaliatory attack (Geist, 1978; Blanchard and Blanchard, 1994; Pellis, 1997). Thus, the restraint that is often reported in serious fighting mostly arises from the risk of retaliation. Even though the competition present in play fighting resembles serious fighting – especially, so-called ritualized fighting (Fox, 1969) – it is not the same.
Unlike serious fighting, some of the turn-taking in play fighting results from the cooperative behavior of the partner (Aldis, 1975). So much so, that play fighting was deemed to follow the “50:50 rule” whereby both partners are equally likely to win contests (Altmann, 1962), a view supported by some game theory-based simulations (Dugatkin and Bekoff, 2003). Observations of actual turn-taking during playful encounters have shown that while completely one-sided play relationships are unstable (Suomi, 2005; Wilmer, 1991), the degree of reciprocity, or turn-taking, can deviate from 50:50, depending on the species, sex, and dominance relationships of the participants (e.g., Pellis et al., 1993; Biben, 1998; Bauer and Smuts, 2007; Cordoni and Palagi, 2011; Cordoni et al, 2016; Essler et al., 2016). Thus, some reciprocity, even if uneven, is needed to sustain playfulness (Bekoff, 2014; Palagi et al, 2016b). We are far from a comprehensive comparative evaluation, but based on current evidence, there are at least three ways to incorporate cooperation into play fights to ensure an appropriate degree of reciprocity (Pellis and Pellis, 2017a).
The first option is to launch a playful attack in a manner that leaves the attacker unprotected from a potential counterattack by the partner. This is in marked contrast with serious fighting in which an attack is typically coupled with a defensive maneuver to reduce the risk of retaliation. By not coupling defense with attack during play fighting, it is easier for the defender to launch a successful counterattack. Similarly, unlike serious fighting the defending partner is slower to protect itself, making it easier for its partner to successfully attack. This form of cooperation, in which there is restraint in the execution of combat tactics, has been observed in some rodents and primates, irrespective of differences in whether the targets competed for are borrowed from aggression or amicable behavior (Pellis and Pellis, 1998b). For species not showing such restraint during the execution of combat maneuvers (Thompson, 1998), two further options exist for inserting cooperation.
The second option for promoting cooperation is to avoid taking advantage of a temporary weakness, as illustrated by the play fighting of degus (Octodon degus), a South American rodent. Play fighting in degus involves both sociosexual and aggressive sequences. Sociosexual sequences involve one partner approaching another and then nuzzling and grooming its shoulders, neck, and side of the head. Aggressive sequences involve mutual rearing, boxing, and pushing with the front paws and kicking with the hind legs (Wilson and Kleiman, 1974). The aggressive target is one of the shoulders which is bitten if contacted (Pellis et al, 2010b). Sequences of play fighting involving competition to bite the shoulders look indistinguishable from sequences of serious fighting, with hindleg kicks delivered with such force that the recipient can be hurled to the ground (Figure 3). What distinguishes the two is what happens after a kick is delivered. In serious fighting, the successful kicker immediately runs toward its opponent to deliver a bite, unless the opponent regains balance and faces its attacker before the distance is closed. In contrast, during play fighting, the successful kicker rarely takes advantage of the off-balance partner, but rather, leaves or adopts a relaxed posture, allowing its partner to regain its posture and counterattack (Pellis et al, 2010b). In such cases, reciprocity is not gained by restraining how combat tactics are executed, but by adopting postures that allows the partner to successfully counterattack (Pellis and Pellis, 2017a).
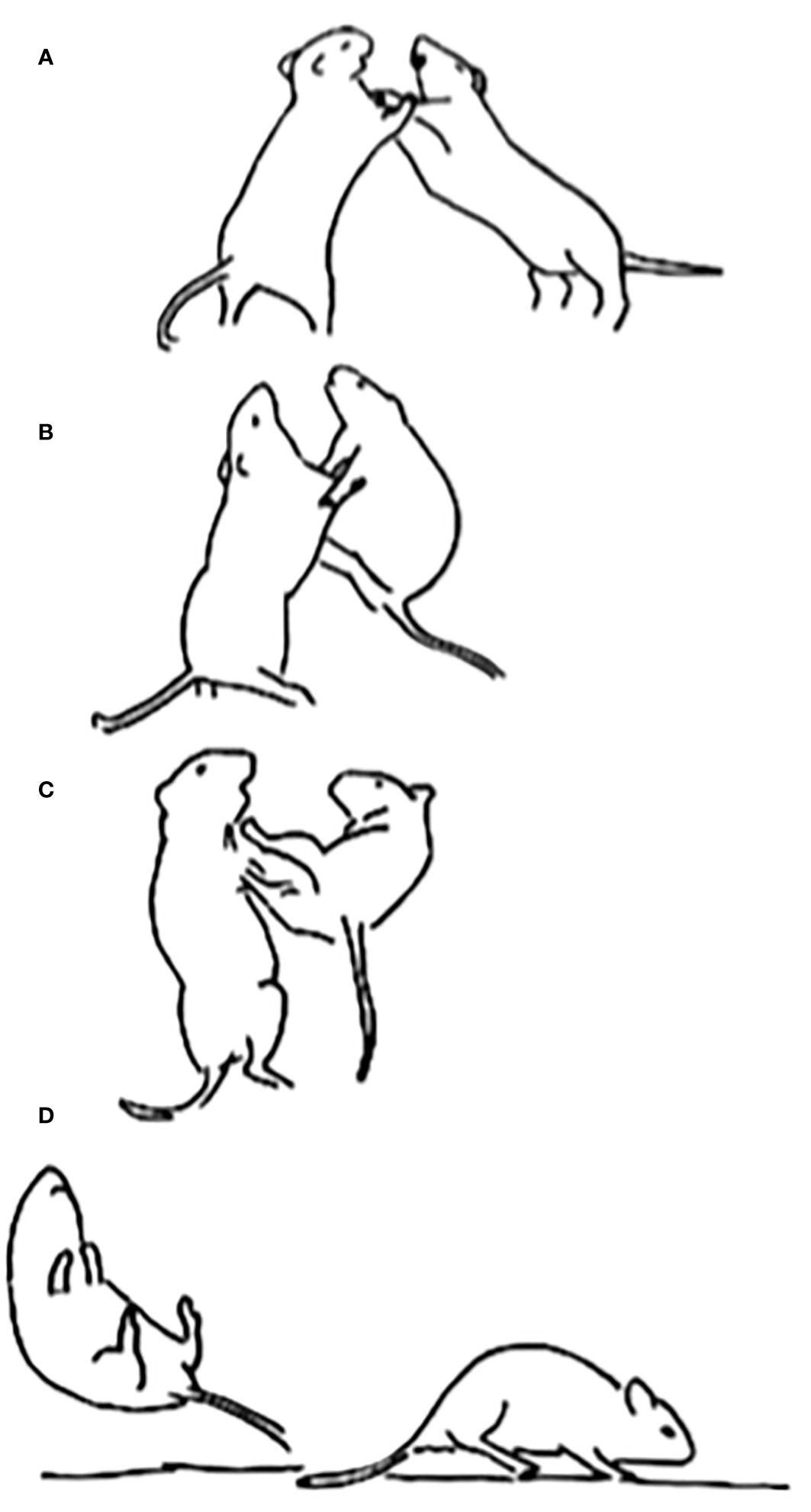
Figure 3 Two adult male degus, introduced as strangers, are shown engaged in combat in a neutral arena. Initially, they rear onto their hind feet and grapple with each other’s forelimbs (A). After maneuvering for several seconds, the degu on the right manages to gain the footing needed to launch a hind leg kick to its opponent’s ventrum (B, C). The kick is then successfully delivered, which sends the opponent flying backwards. The degu that delivered the kick then turns to land on all four of its paws (D). Such kicks are the same when present in play fighting among juveniles. Reprinted from Pellis et al (2010a), with permission from Cambridge University Press.
The third option is to halt the attack only if the partner signals defeat, as illustrated by play fighting in pigs (Suidae). The play fighting in pigs strongly resembles serious fighting, comprising attacking and slashing one another on their faces and shoulders (Fraüdrich, 1974; Barrette, 1986), and is so unrestrained that distinguishing between playful and serious fighting is difficult (Rushen, 1989; Estes, 1993). As the same targets and tactics involved in serious fighting are also used in play fighting, the reduced likelihood of combat injuries most likely arises from the immaturity of their weapon systems, not from the lack of vigor in their actions (e.g., Rushen and Pajor, 1987; Newberry et al, 1988; Silerova et al, 2010). In this way, the play of pigs most closely fits the “playing to win” scenario for play fighting posited by Thompson (1998). The fights of immature Visayan warty pigs (Sus cebifrons) that meet the criteria proposed by Smith (1997) to distinguish playful from serious fighting, were analyzed. As predicted, the combat actions by the piglets did not involve restraint, and if one partner gained the upper hand it pressed the attack, which only ceased if the defender broke free and ran away or if it adopted a submissive posture (Pellis and Pellis, 2016b). Importantly, following withdrawal or submission, in about 30% of cases, the “loser” attacked the “winner” allowing for some reciprocity. A similar pattern was subsequently found in two other species of pigs from two other genera, suggesting that this may be a common way for the extremely rough play of pigs to incorporate a degree of cooperation (Pellis and Pellis, 2017a).
By focusing on the target over which the animals are competing, whether in serious fighting (Geist, 1978; Blanchard and Blanchard, 1994) or in play fighting (Aldis, 1975), the actions performed can be assessed for their functional role as tactics of attack and defense (Pellis and Pellis, 2015; Pellis and Pellis, 2021). In so doing, the lack of coupling of defense with offense during play fighting was found in some animals (Pellis and Pellis, 1998b), and failure to follow up an advantage once gained, albeit in two different ways (Pellis et al, 2010a; Pellis and Pellis, 2016b), was found in other species. An extreme case illustrates the phenomenon and how a functional analysis can discern a deviation of play fighting from what is typical of combat. During both serious and playful fighting, rats rotate to supine placing the targeted body areas out of reach, from which position the defender can use its paws to block the attacker. In turn, the attacker stands over the supine defender, using its forepaws to push and restrain its supine opponent, while attempting to reach over to contact the respective body target (i.e., the lower flanks during serious fighting and the nape during play fighting) (Blanchard et al., 1977; Pellis and Pellis, 1987). But there is a difference beyond the targets involved in using this tactic between the two forms of fighting.
In play fighting, as wrestling so often leads to one partner lying on its back and the other standing on top (Panksepp, 1981; Himmler et al., 2016b), this “pinning” posture is widely used to measure the frequency of play in rats (Pellis et al., 2022b). It is also a common feature of serious fighting and is widely used as a measure of fighting (Adams, 1980; Boice and Adams, 1983). But, in neither playful nor serious fighting is this a static posture. During play the on-bottom rat attempts to make counterattacks against its partner’s nape, and the on-top rat uses its superior position to restrain its partner and make its own further attempts in contacting its partner’s nape (Pellis and Pellis, 1987). Similarly, in serious fighting, the on-top rat maneuvers to gain access to one of its partner’s exposed flanks and the on-bottom rat bites at its partner’s face (Blanchard et al., 1977). In all cases in serious fighting (Blanchard et al., 1977; Pellis and Pellis, 1987), and in most cases during play fighting, the on-top rat holds its partner down with its forepaws while standing firmly anchored on the ground with its hind paws (Figure 4A). However, on occasion during play fighting, the on-top rat stands on its partner’s ventrum with all four paws (Figure 4B). Given that the on bottom rat is squirming, standing on it with all four paws puts the on-top rat in an unstable position (Foroud and Pellis, 2003). Indeed, the chances of a counterattack by the on-bottom rat against an anchored on-top partner being successful is about 30%, but against an unanchored on-top partner, the proportion increases to over 70% (Pellis et al, 2005). By standing on its partner with all four paws, the rat in the on-top, more advantageous position, reduces that advantage, increasing the likelihood of a role reversal and so reciprocity.
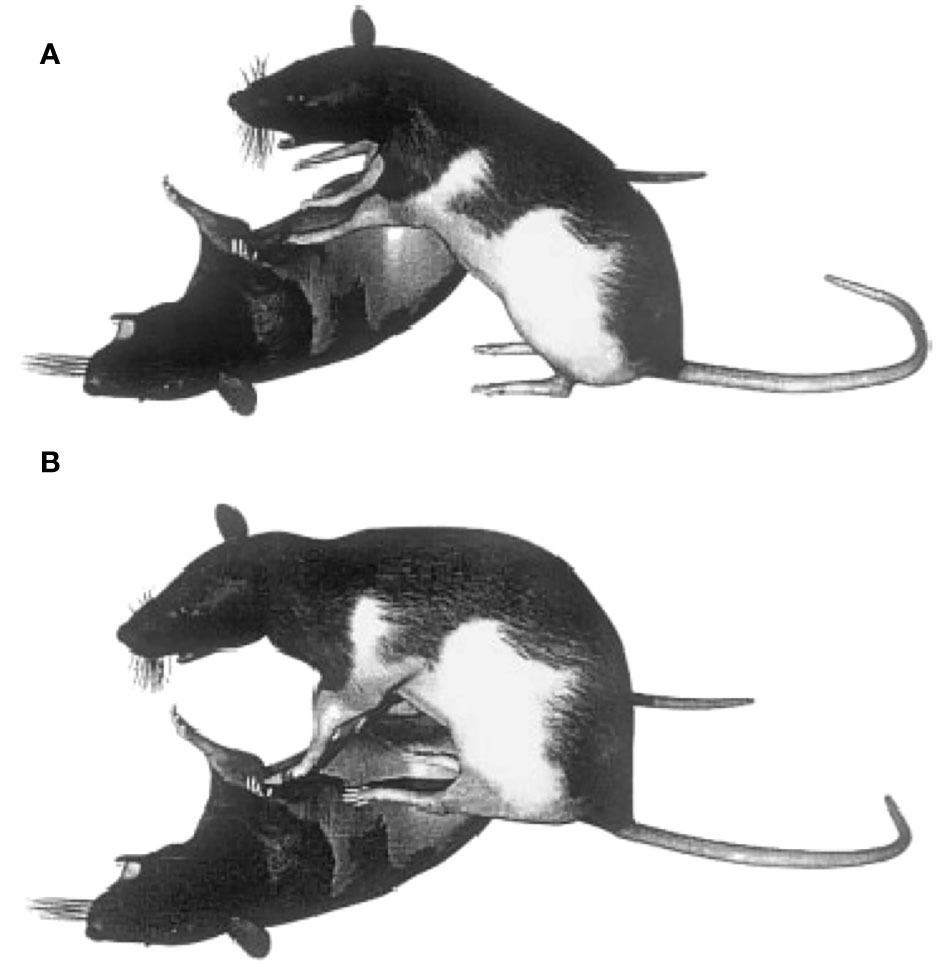
Figure 4 During play fighting rats, often adopt a pinning posture, in which one partner lies on the ground and the other stands on top. This posture typically involves the on-top partner standing on the ground with its hind paws (A) and sometimes standing on the on-bottom partner with all four of its paws (B). Reprinted from Foroud and Pellis (2003) with permission from Wiley.
During adult sexual encounters, if not fully receptive or in the process of selecting the preferred male suitor, female rats evade males by running away (McClintock, 1984; Whishaw and Whishaw, 1996). The female only turns to face the male if cornered and will rarely turn to supine when they do (Pellis and Iwaniuk, 2004). Moreover, once defensively facing the male, counterattacks to the nape do not occur. Thus, while play fighting simulates sexual encounters by competing to contact the nape, it is very different as turning to face the attacker is the most common defense, with rotating to supine as the most likely outcome (Himmler et al., 2016a), and counterattacks to the partner’s nape frequent (Himmler et al., 2016b). A comparative example further highlights that while play fighting can simulate sexual encounters, it shows the fundamental difference to serious fighting. Like rats, adult male prairie voles (Microtus ochrogaster) and montane voles (M. montanus) maneuver to contact and nuzzle the female’s nape during sexual encounters (Pierce et al., 1991), and during play fighting, both species of voles compete to nuzzle each other’s napes. As with rats, during play fighting voles are most likely to defend themselves by turning to face their attacker, rather than evade (Pellis et al., 1989). However, when they face the attacker, like their adult female counterparts, the prairie vole defender is more likely to turn to supine and the montane vole defender is more likely to remain standing on its hind feet. That is, the species differences between the voles more closely mimics the species difference in adult sexual encounters (Pellis and Pellis, 1998a), suggesting a closer motivational link to adult sexual behavior, so can be more plausibly viewed as immature sex. But even so, during play fighting, both species of voles incorporate counterattacks many of which lead to role reversals (Pellis et al., 1989), something not seen in adult sexual encounters (Pierce et al., 1991).
Whether play fighting involves simulation of amicable behavior, such as sexual encounters as in rats and voles, or aggressive behavior, as in pigs and gorillas, they incorporate cooperation in a way to ensure turn-taking and so achieve a degree of reciprocity (Pellis and Pellis, 2017a). That is, one way or another, cooperation is combined with competition for play fighting to remain playful (Palagi, Cordoni et al., 2016). The reciprocation present in play fighting thus makes it different to the behavior it simulates – irrespective of the advantage competed over – quite simply, play fighting in rats cannot be explained away as either immature aggression or immature sex. The competition/cooperation framework initiated by Aldis (1975), especially when applied comparatively, has implications for our understanding of its underlying mechanisms and its adaptive functions. Before exploring these, however, two important issues of potential confusion and misunderstanding need to be discussed.
The first issue concerns sex differences in play fighting, which historically has been thought of as a male-typical behavior. Many of the first detailed studies of play fighting were of species in which males engaged in more of this behavior than females. Researchers sought to identify the mechanisms producing this male-biased sex difference (e.g., Meaney, 1988) and it was a factor used in supporting certain adaptive hypotheses, such as play fighting practicing male combat skills (Smith, 1982). As more species from more lineages of mammals have been studied, however, it has become increasingly apparent that while play fighting may be more frequent in the males of many species, in a sizable minority there is no difference, and in some species, the pattern is reversed, with females playing more than males (Marley et al, 2022). Few studies on non-mammalian vertebrates have provided detailed comparisons between the sexes, but a study of kea (Nestor notabilis), a parrot from New Zealand, suggests that, at least at some ages, play fighting is equally frequent between the sexes, and some forms of this behavior is more frequent in females (Diamond and Bond, 1999). Given the current state of our comparative knowledge of the subject, when studying a new species, it would be a mistake to assume that play fighting is likely to be male-typical. Moreover, while the content of play fighting may differ between the sexes, at least at the same ages, generally, males and females engage in the same species-typical pattern of play (Pellis and Pellis, 2017a), and this is true whether the competition involves body targets typical of aggression, predation, or amicable behavior, including mounting (Pellis and Pellis, 2009). Indeed, where sex differences are found, it may not be in the direction that would seem most intuitive. For example, Richardson’s ground squirrels (U. richardsoni), compete to bite the shoulder, as in aggression, or to mount one another, as in sexual behavior, with about 80% of the interactions involving competition for mounting in both sexes. Whereas there is a sex difference for interactions involving biting, with males engaging in significantly more, there is no sex difference in competing for mounting, even though in adult sexual contexts, mounting is a male-typical behavior (Pasztor et al., 2001). That is, whatever behavior is being simulated during play fighting, including self-evidently male or female typical behavior, it is performed by both sexes. Therefore, as the present paper focuses on some of the general features of play fighting that pertain to both sexes, unless specifically relevant, sex differences will not be considered.
The second issue concerns the use of the term function. The discovery that the grasping, pulling, pushing and wrestling typical of play fighting occurs in the context of the animals competing to gain or avoid a species-typical advantage (Aldis, 1975) means that many of the actions performed during the interactions can be interpreted as tactics of attack and defense (for similar analyses of serious fighting see Geist, 1978; Blanchard and Blanchard, 1994; Pellis, 1997). Consequently, the act of rolling over onto its back by a rat when another rat lunges playfully toward its nape can be described by its function as a defensive tactic that prevents nape contact (Pellis and Pellis, 1987). This use of the word “function” is akin to how it is used in evolutionary morphology, in which “functions are the actions or uses of structures” (Lauder, 1986, p. 11). This is different to the typical use of the word function in most of the ethological literature in which what is inferred are the fitness benefits arising from the behavior (Tinbergen, 1963; Burghardt, 2005). As the immediate function of an action, may or may not have an evolutionary function (Michel and Moore, 1995, pp. 92–96), we use the word function to describe actions during play for their roles as tactics associated with achieving species-typical advantages (Pellis and Pellis, 2015). To distinguish between immediate functions and fitness enhancing functions, the latter are explicitly referred to as adaptive functions.
Implications of the common and idiosyncratic design features of play fighting
During play fighting, macaques (Macaca spp.), like many other cercopithecine monkeys (e.g., Pellis and Pellis, 1997a; Kraus et al., 2019), attack and defend the side of the face, neck, upper arms and shoulders, which are bitten if contacted as in serious fighting (Reinhart et al., 2010). Consequently, these monkeys engage in play fighting that simulates serious fighting. Intriguingly, two researchers who watched the play fighting of young rhesus macaques (M. mulatta) in the same population drew fundamentally different conclusions about why they played. One claimed that the monkeys were rehearsing skills they would need as adults to be effective in combat (Symons, 1978), and the other claimed that such play provided the participants with the opportunity to learn the skills necessary for social cohesion in their complex society (Levy, 1979). We will deal with the problems of inferring adaptive functions about play (Sharpe, 2019) below, but for now, what these divergent views highlight is that when watching the same animals engaged in the same play fighting, one observer focused on its competitive aspects whereas the other on its cooperative aspects, leading them to very different conclusions about the value of what they saw. We want to make our position clear: play fighting is a competitive form of interaction that is balanced by cooperation, so it is not strictly competitive, as is serious fighting. The mixing of competition and cooperation also makes it a different form of interaction to any of the adult functional behavior which it may simulate, and so is not an immature version of those behaviors. Two pieces of evidence strongly support this conclusion.
First, in some species, serious fighting developmentally predates, or co-occurs with, the onset of play fighting (Bekoff, 1977). For example, serious fighting in spotted hyenas (Crocuta crocuta), emerges at the same age as play fighting, and is sufficiently mature to cause serious bodily damage (Frank et al., 1991; Drea et al., 1996). Similarly, at its onset, early after fledging, play fighting in keas is so rigorous that it is difficult to distinguish from serious aggression, only becoming clearly more playful later in the juvenile phase (Diamond and Bond, 1999). Second, many species continue to engage in play fighting as adults, including with other adults (Pellis and Iwaniuk, 2000), and the same pattern of combining competition and cooperation is present (e.g., Pellis, 2002a; Cordoni, 2009; Palagi, 2011; Norscia et al., 2024). For adults, the relative proportion of cooperation to competition may be modified to accommodate more strongly established dominance relationships, but again for the play to remain playful and not escalate to aggression, cooperation still needs to be incorporated (Pellis and Pellis, 1992b; Pellis et al., 1993; Smith et al., 1999). Thus, whether simulating sexual behavior, grooming, predation or conspecific aggression, play fighting is not merely an immature version of those behaviors. Rather, play fighting is a distinct form of behavior with its own life history. But have different forms of play fighting involving different, adult functional behaviors, converged onto a common pattern, in which the idiosyncrasies of the specific behavioral systems are irrelevant, or do the origins of play build on different mechanisms and afford different functions?
The mechanisms underlying play fighting
Most of what we know about the neurobiology and endocrinology of play has come from studying the play fighting of laboratory rats (Achterberg and Vanderschuren, 2023; McCarthy, 2023). Beginning with crude lists of which brain areas and which neurotransmitters do and do not affect play (Panksepp et al., 1984; Thor and Holloway, 1984a; Vanderschuren et al., 1997), we now have a broad outline of the neural circuitry involved. Brain stem circuits provide the behavior patterns, mid-brain and lower forebrain circuits provide motivational and motor regulation, and the cortex provides emotional and cognitive regulation (Siviy, 2016; Vanderschuren et al., 2016). Moreover, new insights about the contribution of previously unsuspected neural structures are being steadily revealed (Gloveli et al., 2023; Zhao and Riters, 2023). However, while some have argued for the existence of a distinct play brain circuit (Panksepp, 1998; Siviy and Panksepp, 2011), the nodes and connections identified so far are common to those important for social behavior in general, especially affinitive social behavior (Chen and Hong, 2018; Luo, 2018; Achterberg and Vanderschuren, 2023). Even so, comparing these brain areas between the sexes and across rat strains indicate that some of those neural circuits influence specific aspects of play (Siviy, 2020; VanRyzin et al., 2020), suggesting that, with advances in mapping dynamic brain circuits showing patterns of activation and communication (Bermudez-Contreras et al., 2018), a distinctive “play circuit” may eventually be characterized in terms of how these widely used areas function together when producing play relative to other affinitive social behavior.
The same lack of comparative data leaves our knowledge of the endocrinological mechanisms and how they influence the development of play fighting at a primitive level. Again, most of what we know is derived from what has been gleaned from studying play fighting in rats (McCarthy, 2023), a species that exhibits a sex difference, with males playing more than females, at least in some rearing conditions (Himmler et al, 2016a; Thor and Holloway, 1984b). The general finding that as a male-typical trait, play fighting is masculinized by exposure to androgens emanating from the gonads and/or adrenal glands early in perinatal development (Meaney, 1988), may account for variations between and within species in which females may be more playful than males (Pederson et al., 1990; Berenbaum and Hines, 1992). Nonetheless, the story may be more complex. In Syrian hamsters (Mesocricetus auratus), steroidal stress hormones have been found to be involved in some age-related changes in play behavior (Taravosh-Lahn and Delville, 2004; Wommack and Delville, 2007b, Wommack and Delville, 2007a). In rats, while some age-related changes in play fighting may involve the masculinizing effects of androgens, some of the changes in females involve the active role of ovarian hormones (Pellis, 2002b). For the progress made to date in mapping the neural circuits involved in play and some of the endocrinological influences on the development of those circuits, we direct readers to some recent reviews (e.g., VanRyzin et al., 2020; Achterberg and Vanderschuren, 2023; McCarthy, 2023). While the comparative data are not yet available to be certain as to what may constitute the general mechanisms at the neurobiological level, they are sufficient to shed new light on two unresolved issues related to the motivational mechanisms that have been thought to regulate play behavior.
Is play a unitary phenomenon?
A major unresolved question about play fighting is whether the mechanisms involved are linked to its different origins or if the same underlying mechanisms are involved irrespective of its origins. To understand this issue, we need to broaden the scope to a commonly held view about play. A supposedly cardinal feature of play is that it mixes behavior patterns from many different adult functional contexts (Meyer-Holzapfel, 1956; Heymer, 1977). This view gains support from reports of play incorporating aspects of the three types of animal play generally recognized: locomotor-rotational play being intermixed with social play, or social play intermixed with object play (see Introduction). Such a view sees play as constituting one common motivational system which can recruit behavior from multiple sources, making play an avenue for producing novel combinations of behavior and so enhancing creativity (Bateson, 2014, Bateson, 2015).
A problem with this view is that different lineages of animals have given rise to species in which play involves only one of the three types to the exclusion of the others. For example, at least two species of octopus from one genus (Octopus spp.) have been reported to engage in object play (Mather and Anderson, 1999; Kuba et al., 2006) as has a species of bumblebee (Bombus terrestris audax) (Galpayage Dona et al., 2022), whereas two other invertebrates, a species of wasp (Polistes dominulus) (Dapporto et al., 2006) and a species of spider (Anelosimus studiosus) (Pruitt et al., 2012) have been reported to engage in social play. Given that some of these authors are experienced observers of play and have logged countless hours of observation of their subject species, it is significant, in our view, that they did not note anything that could be construed as one or other types of play in these species. Extending the comparison to vertebrates, especially the more playful taxa, such as birds and mammals, does not change the picture: some lineages have evolved one or two types of play to the exclusion of the others (Burghardt, 2005; Kaplan, 2020). Thus, it is the exception, not the rule, that all three types of play are present in the same species (Burghardt, 2005), and even then, it is rare that all three show a high degree of complexity (Pellis and Iwaniuk, 2004; Pellis and Pellis, 2017b). Therefore, contrary to the view that mixing types of play is common (Norscia and Palagi, 2016), by default, it must be rare because few lineages have evolved species having two or more types of play. The evolutionary heterogeneity of the emergence of different types of play leads to an alternative hypothesis about the motivational underpinnings of play. Different types of play have been independently evolved, with cases of mixing having arisen secondarily in species from lineages with the cognitive wherewithal to integrate these systems together (Pellis et al, 2019b). This alternative hypothesis fits what is known about play fighting quite well.
Again, Aldis (1975) insight, that play fighting involves competition for particular kinds of contact on specific body targets has proven to be valuable in comparing not only play fighting across species but also within species that engage in more than one type of play fighting. Among rodents, ground squirrels compete during play fighting either to bite the shoulders (aggressive play) or mount the partner (sexual play) (Nunes et al., 1999; Pasztor et al., 2001), Djungarian hamsters compete to lick the mouth (sexual play) or bite the rump (aggressive play) (Pellis and Pellis, 1989), and grasshopper mice compete to nuzzle the sides of the neck (sexual play) or bite the nape of the neck (predatory play) (Pellis et al., 2000). Among some primates, there are similar variations, with play fighting involving competition for biting (aggressive play), mounting or grooming, with the latter derived from sexual encounters as is typical of courtship or from general non-sexual affinitive behavior depending on the species (so sexual or amicable play) (Doyle, 1974; Epps, 1974; Vick and Conley, 1976; Hoffman and Foerg, 1983; Goonan, 1993; Winn, 1994; Pellis and Pellis, 2018). If mixing were the norm, then it would be expected that, during play fighting, species that compete for multiple targets should readily switch between them within the same bout of play. Opposite to this expectation, detailed analyses of the play in some of these species found that, if the competition started with one type of play, it continued and finished with competing for that target, before starting to compete for the target typical of the other type of play fighting (Pellis and Pellis, 1989; Pellis et al., 2000; Pasztor et al., 2001; Pellis and Pellis, 2018). That is, within a single bout of play fighting, there is no mixing, suggesting that each type of play fighting is independent.
If different types of play fighting evolved independently and have their own motivational control system, it would be predicted that cases of mixing types of play should be a derived, not an ancestral character state (Pellis et al., 2019a). This prediction was tested by comparing how different types of play fighting are combined among species of primates that have both aggressive and sexual (amicable) play (Pellis et al, 2023b). Whether sequences of both were mixed or not was scored by noting if a counterattack by the recipient was of the same type of play (e.g., amicable → amicable) or different (e.g., amicable → aggressive). Counterattacks that switch the type of play provide evidence for mixing types of play and these could be (1) absent or rare, (2) relatively common or (3) abundant. The distribution of character states was mapped onto a cladogram allowing the most likely ancestral state to be determined. Consistent with the prediction derived from the hypothesis that different types of play are independent, the most likely ancestral state was for little to no mixing to occur, with moderate to abundant mixing being the derived state.
It was only in one genus of New World monkey (Saimiri spp.) that the mixing could be multiple, switching back and forth several times within a bout of play (e.g., amicable → aggressive → amicable → aggressive and so on), indicating that unambiguous mixing of different types of play fighting within a single bout of playing is comparatively rare. A plausible conclusion is that play fighting is heterogeneous, being borrowed independently from different origins (aggression, sexual behavior, grooming, predation) and remains so in most species. If this is true, this would suggest that, if there is a brain circuit for play fighting, there are likely multiple circuits.
As noted earlier, however, even though it may be heterogeneous, play fighting, whatever its origins, has reciprocity in common. Decortication in rats does not change the proportion of play fights that lead to role reversals (Himmler et al., 2016b), suggesting that subcortical mechanisms are sufficient to not only produce play fighting (Pellis et al., 1992a; Panksepp et al., 1994), but also to regulate it to ensure that play fighting remains playful. Cortical mechanisms seem to be important to modulate the play, including bending the degree of reciprocity needed to accommodate partners of different levels of skill and social status (Pellis and Pellis, 2016a). Thus, play fighting likely taps into common mechanisms that make affinitive interactions fair and pleasurable to both partners (Luo, 2018). It is important to note that the neural nodes and circuits revealed to be important for play fighting in rats (Vanderschuren et al., 2016; VanRyzin et al., 2020) involve subcortical systems that are important for all motivational systems (Berridge and Kringelbach, 2013) and engage cortical systems that are important for higher level integration of behavior with changing contexts in general, not just during play, or even social encounters (Mohapatra and Wagner, 2023). Consequently, a full understanding of the neurobiology of play fighting will require teasing apart the circuitry that is unique to each type of play from the subcortical and cortical circuits that are common not only in play but to all (social) behavior.
Competing to touch or touching to compete?
Another unresolved problem concerning the mechanisms that underlie play fighting is that of what exactly animals find pleasurable about this behavior. Play fighting in rats involves competing for nuzzling the nape (Pellis and Pellis, 1987; Siviy and Panksepp, 1987). That the physical act of such touching is essential is illustrated by it being sufficient to satisfying the playful motivation of the players, as shown when the recipient does not defend itself, allowing the attacker unfettered access (Figure 1). Indeed, pharmacological manipulation of a partner so that it moves about but does not respond to playful attacks results in continued nape attacks by the untreated partner (e.g., Thor and Holloway, 1983; Field and Pellis, 1994; Deak and Panksepp, 2006). Perhaps this social touch relies on C-tactile nerve fibers, which are associated with carrying pleasurable interpersonal tactile signals to the brain (Croy et al., 2022; Schirmer et al., 2023). This is a promising facet of play fighting waiting to be explored. Irrespective of the mechanism involved, the importance of touching during play was interpreted by Panksepp (1998) as a core feature of the experience, explaining why the pinning configuration (see Figure 4) is so common during play fights – it enables extensive body contact between the partners, with that contact involving much rubbing and tickling. This inference led to an amazing discovery: rats love to be tickled by experimenters, which the rats reveal by apparently laughing with joy (Panksepp and Burgdorf, 2000). Unfortunately, this finding also led to some false leads. What is true is that rats do find being tickled by human caretakers pleasurable and this has the beneficial effect of making the animals, often the subjects of biomedical research, easier to handle (Cloutier et al., 2012; LaFollette et al., 2017). But there are also two incorrect conclusions that have been derived from it. First, is the conclusion that rats view being tickled by humans as equivalent to playing with a peer (for evidence against this view, see Bombail et al., 2021; Burke et al., 2022; Kisko et al., 2017). Second, is the conclusion that it is the tickling of the partner’s body that makes play with peers a rewarding experience, and that this has been generalized beyond rats (Panksepp and Burgdorf, 2000; Ishiyama Brecht, 2016; Gloveli et al., 2023). It is this second incorrect conclusion that we will focus on here. If bodily contact during play fighting is what is most rewarding for rats, then gaining the pinning configuration should be what animals compete to achieve when playing with peers; however, different strains of rats vary in the likelihood that play fights end in pins (Himmler et al., 2014), and for some, adopting the supine defense position is the tactic of last resort (Himmler et al., 2016b). That is, gaining a bodily position that is supposedly most advantageous for maximizing peer–peer body contact and “tickling” (Panksepp, 1998), is not what the animals stive for during play fighting – gaining and avoiding nape contact is! While contacting the nape still results in tactile sensation, which is rewarding (Siviy and Panksepp, 1987), it is much more limited than the kind offered by the pinning configuration.
Again, Aldis (1975) provides a clue as to how to interpret the touching present in play fighting. He noted that biting during play fighting in the species he examined may not always be directly related to the combat-typical body targets, but rather, the partners may mouth each other indiscriminately. Aldis’ observations suggest that there are two sources of competition during play fighting – one based on contesting access to the combat-typical body target and the other to gain the oral satisfaction of mouthing the partner’s body, with the latter being interpretable within the “tickling” perspective. This distinction resolves several paradoxes about play fighting when considered comparatively and developmentally. When two Asian small-clawed otters (Anonyx cinerea) engage in play fighting, they compete to bite one another on the cheeks. However, if a third partner joins in, it preferentially bites the member of the pair that is on the defensive. During defense, an otter typically lies on its back and the attacker stands over it (much like the pinning described for rats – see Figure 4). The on-top partner uses its forepaws to restrain its supine partner and lunges with its mouth toward an exposed cheek. In turn, the on-bottom otter uses its forepaws and mouth to ward off the attacks. As both interactants are focused on attack and defense of each other’s cheeks, the third otter could attack the on-top otter’s exposed cheeks, but it does not. Rather, it bites at the on-bottom otter’s exposed hind legs, which the bitten otter cannot defend without exposing its cheeks to the on-top attacker. From this position, the third otter mouths the lower portions of the defending otter with little interruption by defensive behavior of the recipient (Pellis, 1984). The same pattern has been documented for Australian magpies (Gymnorhina tibicen). The primary attacker pecks at the side of the head, while the secondary attacker pecks at the feet or tips of the wings of the bird in the defensive position (Pellis, 1981). Similarly, for Tonkean macaques (M. tonkeana), once more than three partners are engaged in a play fight, they stop competing to bite the sides of the face, neck and shoulders and simply mouth whatever body part of the nearest partner is available (Reinhart et al., 2010). That is, play fighting has two distinct forms of biting – one involving competition for specific body targets, with biting those targets being the reward, and more generalized mouthing, where the mouthing itself is what is rewarding and so can be directed at any location on the partner’s body. This distinction can also resolve a developmental puzzle.
For many species as social play first emerges, there is generalized mouthing of the partner’s body which only later becomes focused on the combat-typical body targets (Poole, 1966; Eibl-Eibesfeldt, 1970; Lazar and Beckhorn, 1974; Chalmers, 1984; Pellis and Pellis, 1997a; Pellis and Pellis, 2011). This progression has been interpreted as involving a maturation or learning process that narrows the mouthing to specific locations (Lazar and Beckhorn, 1974). However, if the two types of biting are independent, a different interpretation is possible: they undergo differential maturation, with generalized mouthing developing before target-centered biting. The findings from rats and other species of rodents support the view that targeted and generalized contact involve independent forms of play. First, when a third rat joins a playing pair, it attacks the on-top rat’s exposed nape, it does not nuzzle the unprotected exposed lower body of the defending member of the dyad (Pellis and Pellis, 1998a) Second, from the very first instances of play fighting in the third week after birth, infant rats compete to nuzzle the nape, not nuzzle the partner’s body indiscriminately (Pellis and Pellis, 1997b). Of course, nuzzling is not biting or mouthing. Yet Syrian hamsters which compete to gently nibble the cheeks, target the cheeks from the very first emergence of playful interactions (Pellis and Pellis, 1988). Generalized mouthing or body contact is not part of the play repertoire of rats, or any other murid rodent species so far studied (Pellis and Pellis, 1998a, Pellis and Pellis, 2009). Moreover, whereas the more egalitarian Tonkean macaques show generalized mouthing as well as targeted biting, the more despotic Japanese macaques (M. fuscata) mostly limit their play fighting to targeted biting (Reinhart et al., 2010). This supports Aldis (1975) insight that social play involves two distinct types of touching: generalized touching and targeted contact. Not all species that engage in play fighting have both types, and being independent, generalized and targeted touching have their own developmental trajectories. Moreover, some comparative evidence suggests that touch may not even be essential in targeted play fighting.
Fat sand jirds (Psammomys obesus) compete for access to the sides of the face during play fighting, which involves opposing the tip of the snout with the side of the partner’s face. The opposition can be close (Figure 5A) or more distant (Figure 5B), with actual contact being rare, and gentle when present (Pellis, 1988). Of course, at the distances at which the jirds oppose their faces, the animals are in contact with their vibrissae, which can provide considerable opportunities for social communication (Wolfe et al., 2011; Bobrov et al., 2014), but in this species there is no rubbing or nuzzling and certainly no close bodily contact during play fighting (Pellis, 1988). Among ungulates, play fighting can involve horn and antler clashing, with the contact restricted to these extensions of the body (Geist, 1978; Byers, 1984; Thompson, 1996), and even when teeth are used, the biting and slashing is restricted to the combat-typical body targets (Pellis and Pellis, 2016b). Even among primates in which bodily contact is commonly seen in play fighting (Loizos, 1967; Aldis, 1975), the degree of contact varies markedly from full body-on-body contact to contact at arm’s-length, and even no actual physical contact (Kraus et al., 2019). That is, while touching can be an integral part of play fighting in some species, it can be limited in others. Therefore, while competing for targeted contact may be a universal feature of play fighting, competing for touching itself is not, and when both are present, they likely are independently motivated.
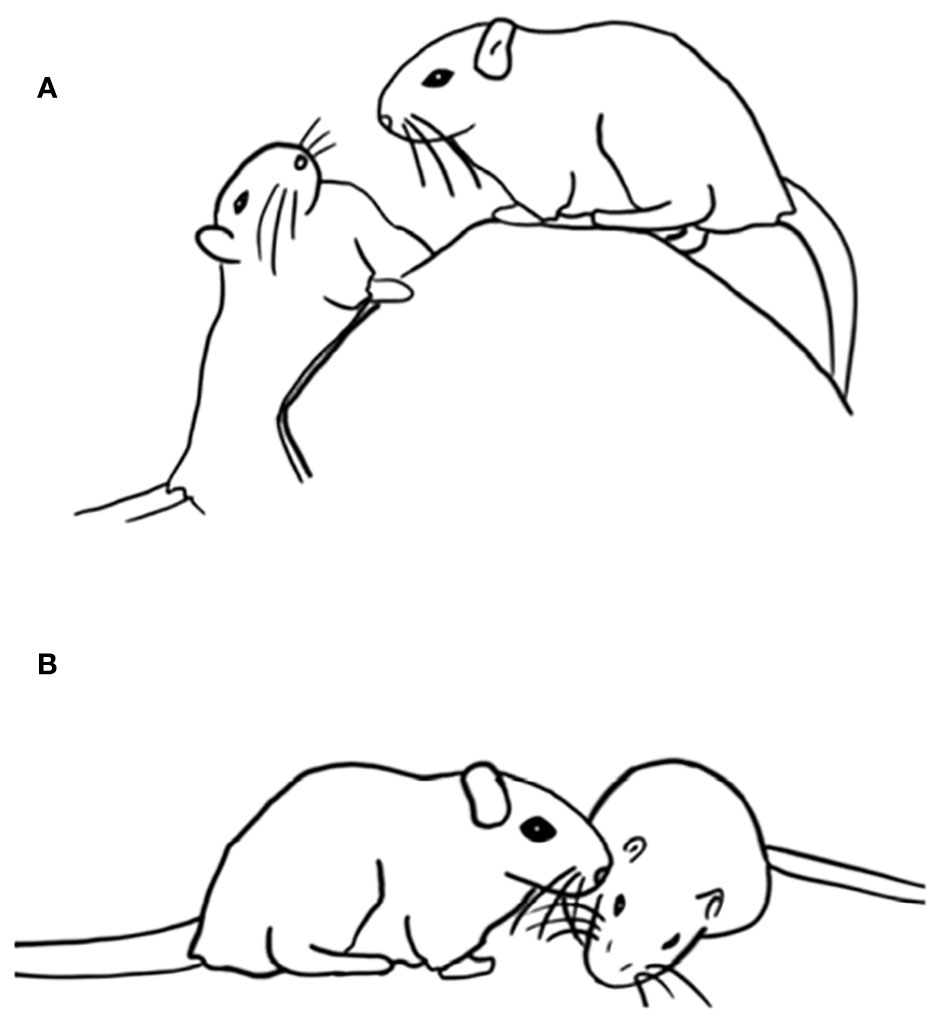
Figure 5 Playful competition in fat sand jirds involves attempting to oppose the snout to the partner’s side of the head. While the opposition may be a head length or more distant (A) or near (B), it rarely involves actual contact. Adapted from Pellis (1988) with permission from Wiley.
Touching for the sake of touching can explain the mutual body-on-body contact seen in some species, such in African elephants (Loxodonta africana), but is such touching sufficient to reinforce the head-to-head competitive play fighting also present in elephants (Lee and Moss, 2014)? In rats, as play fighting involves competing for access to the nape (Pellis and Pellis, 1987), and since anesthetizing the snout and nape suppresses play fighting (Siviy and Panksepp, 1987), touch experienced on these parts of the body must be an integral part of the reward arising from such play. Moreover, recall that rats injected with drugs that allow them to continue to be mobile but no longer playful, still attract playful attacks to their napes from undrugged partners (Thor and Holloway, 1983), supporting the view that contacting the partner’s nape is the goal of play fighting (see Figure 1). However, if undrugged partners are repeatedly exposed to drugged partners, the frequency with which they launch nape attacks decreases, with a completely unresponsive partner being outright aversive (Pellis and McKenna, 1995), suggesting that while contacting the nape is part of what makes play fighting rewarding, it is not enough (Vanderschuren et al., 2016). Some degree of resistance to the contact by the partner seems necessary (Pellis and Pellis, 2009).
Like Visayan warty pigs, warthogs (Phacochoerus africanus) compete with their play partner to strike and slash the sides of the face and shoulders, but in their case, the head is not slender, but broad with lumps and bumps that can catch the opponent’s face (Cumming, 1984). Consequently, warthogs engage in a lot of head-to-head wrestling to gain access to the shoulders, and you would conjecture that if the principal reward of play fighting is to contact the partner on its play target, the opportunity to contact readily available shoulders should be pounced on. Not so. In over 80% of cases when a warthog playfully attacked a partner that was sitting down, it did not directly target an exposed shoulder, but rather, oriented face-to-face and pushed until the partner stood up and engaged in a head-to-head wrestle. The attacker then maneuvered to contact its partner’s shoulders (Pellis and Pellis, 2017a). That is, while contacting the target is rewarding, competing to gain access to the target makes it more rewarding. It seems that part of what makes play rewarding is that it is challenging (Ham et al., 2023c; Rule et al., 2023).
Mouthing or generalized body contact may constitute one form of social play which can explain some phenomena, such as unidirectional play, non-target contact and gentle play (e.g., Lazar and Beckhorn, 1974; Pellis, 1981; Pellis, 1984; Cordoni and Palagi, 2011). But it is a mistake to conclude that touching is what motivates all play fighting. Rather, play fighting involves competing for a target/advantage, which can involve contact or not, and even when contact is involved, it is not a sufficient reward to sustain play fighting (Pellis and Pellis, 2017a). If touch-based play and competitive play are two independent types of social play, they likely involve different, if overlapping, neural control mechanisms, mechanisms that remain to be discerned.
The adaptive functions of play fighting
As we encountered earlier, in rhesus monkeys, play fighting was hypothesized to function as practice for serious fighting (Symons, 1978) or to promote social cohesion (Levy, 1979): the revolutionary insights by Aldis (1975) explain these divergent views. If the competition present in play fighting is emphasized, then practice makes sense, but if the cooperation present is emphasized, then social cohesion makes sense. Field-based tests have found evidence in support for both hypotheses in some species (Blumstein et al., 2009; Blumstein et al., 2013b) but not in others (Sharpe, 2005a, Sharpe, 2005b). There may be an objective reason for such discordant findings. Play fighting involves at least a minimum amount of both competition and cooperation being present, but then the relative proportion of each above that minimum can vary across species. The greater the competition relative to the cooperation present, the more closely the behavior patterns used resemble those from adult serious behavior, whereas the greater the cooperation relative to competition present, the less the similarity. That means that, for a species in which play fighting more closely resembles combat, the greater the opportunity for using that play as practice, but the less it resembles combat, the weaker that play is for use as practice, and the more likely it is suitable for use to promote social cohesion or some other prosocial benefit (Pellis and Pellis, 2009; Pellis et al, 2010b). But what evidence is compelling for any given hypothesis for the adaptive function of play?
When is a trait an adaptation?
Field observations have revealed positive correlations between the amount of social play engaged in as juveniles and several developmental benefits in Belding’s ground squirrels (U. beldingi). These include refinement of temperament, promoting less docile responses to threats, increasing cautious behavior, and fostering greater exploration and adaptability of responses in novel situations (Nunes and Montemayor, 2023). However, while a trait may have many benefits, not all benefits need be functions (Hinde, 1975). To qualify as an adaptive function, a trait must have the form that it does because of a history of natural selection for that function, a history that is revealed by design features present that make that trait suitable for the proposed function (Williams, 1966). Vague similarities, like “play fighting resembles serious fighting” (see above), are not sufficient. There needs to be detailed correspondence between the form of the play and the proposed function (Pellis and Pellis, 1998b; Leca, 2023). After all, a particular type of play may be a by-product of some other adaptive or non-adaptive trait, and so require no functional explanation of its own (Leca, 2021a; Leca, 2021b).
An example is shown in the variation in the targets competed over during play fighting across primate species, which can involve biting targets typical of agonistic behavior or targets typical of amicable behavior, with the latter involving either competing to groom one another or mount each other (Pellis and Pellis, 2018). Using the data collected in Pellis et al (2023b), the relative proportion of grooming and mounting was scored for those primate species that exhibited amicable play. Each species was given one of the following character states: (1) more grooming than mounting (≥70%), (2) approximately similar levels of grooming and mounting (>30<70%), or (3) more mounting than grooming (≥70%). The character states were then mapped on a cladogram, revealing that the most likely ancestral state was competing for grooming to be predominant. The lemurs and lorises (i.e., strepsirrhines, the wet-nosed primate species with more ancestral physical and behavioral traits, see Fleagle, 2013) on the left, except for one branch, retain the ancestral pattern whereas the New World monkeys on the right all make the switch to mounting (Figure 6).
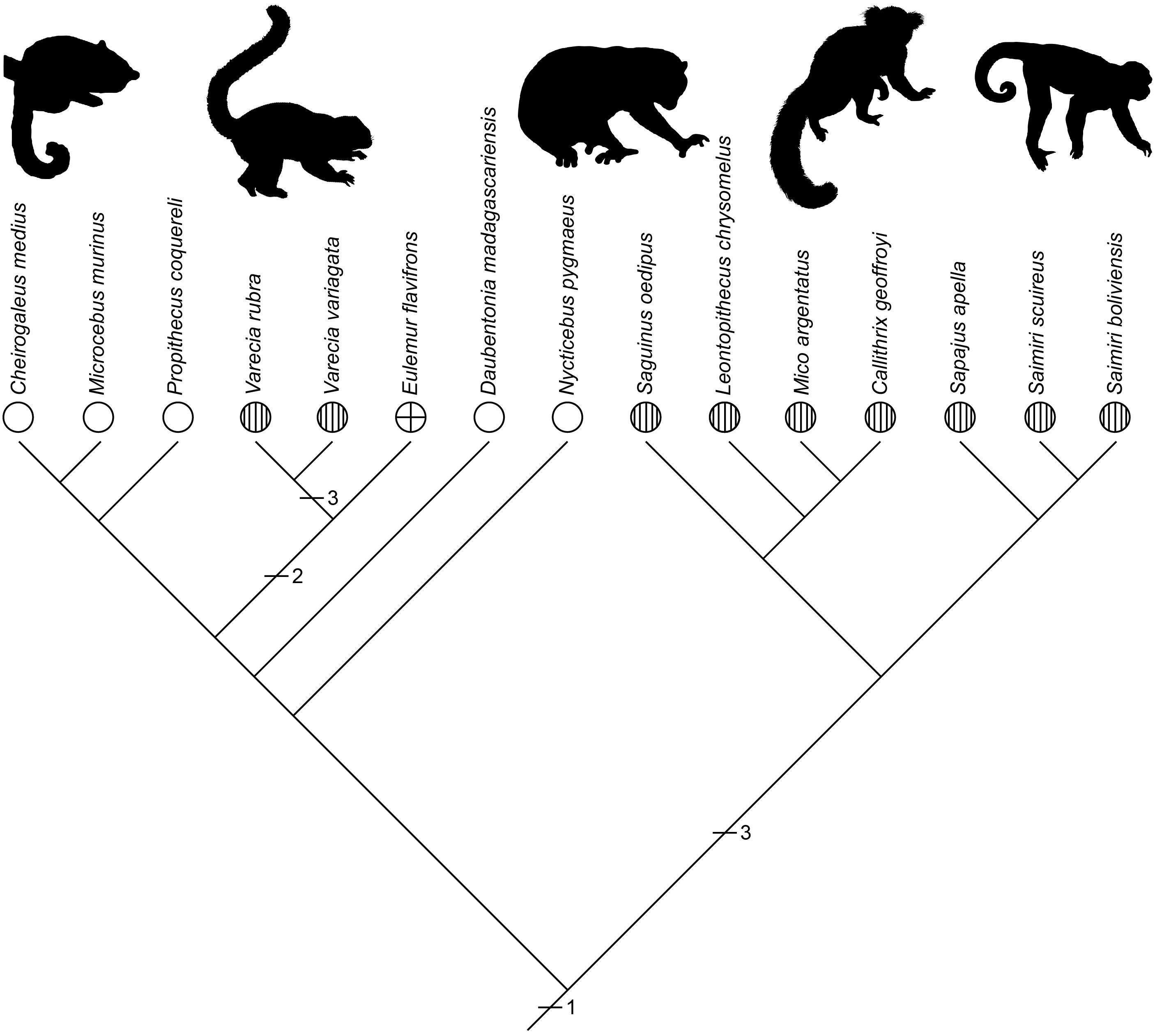
Figure 6 A cladogram representing the pattern of relatedness among primate species that engage in amicable play for which the relative amounts of grooming and mounting could be ascertained, is shown. The species span two major subgroups of primates, the strepsirrhines, a more primitive branch of the clade (see text), which includes the fat tailed dwarf lemur (Cheirogaleus), the gray mouse lemur (Microcebus), Coquerel’s sifaka (Propithecus), two species of ruffed lemur (Varecia), the brown lemur (Eulemur) the aye-aye (Daubentonia) and the pygmy loris (Nycticebus), and the New World monkeys, including the cotton top tamarin (Saguinus), the lion headed tamarin (Leontopithecus), the silver marmoset (Mico), Geoffroy’s marmosets (Callithrix), the brown capuchin (Sapajus) and two species of squirrel monkeys (Saimiri). The character states are: = mostly grooming (1); = equal amounts of both (2); = mostly mounting (3). The horizontal slashes and associated numbers indicate the position and type of evolutionary change. The most parsimonious ancestral state was mostly grooming (i.e., 1).
An adaptationist interpretation could conclude that playful competition over grooming and over mounting have distinct functions, with different lineages benefitting differentially from those functions. But then what about the gradations in-between, is each proportional difference adaptive? There may be a more parsimonious explanation. In strepsirrhines, grooming primarily involves the use of the teeth, whereas in monkeys, grooming is primarily performed with the hands (Bishop, 1962; Jolly, 1966). Indeed, the lower front incisors of strepsirrhines are packed together to form a toothcomb, specially designed for grooming (Szalay and Seligsohn, 1977). In most cases, when competitive grooming arose, one animal grasped and held its partner’s head and began to groom its face orally (Pellis and Pellis, 2018). Even in strepsirrhines in which competing for mounting is as common (e.g., blue-eyed black lemurs – Eulemur) or more common (e.g., ruffed lemurs – Varecia spp.) than competing for grooming, when competition for grooming did occur, it arose from orally grooming the partner’s face. Similarly, while in the monkeys most grooming involved using the hands, the only cases of playful competitive grooming observed was when one animal tried to groom its partner orally. Consequently, a switch in the manner of grooming could have indirectly altered the context that promotes competitive play fighting involving grooming. That is, the switch in emphasizing the type of amicable play is a by-product, not requiring a unique functional explanation (Leca, 2021a). While there are many other socio-ecological factors that need to be considered (see relevant chapters in Mitani et al., 2012) before this conclusion is confirmed, the example shows that differences in the content of play cannot be assumed to be present because of their adaptive value. Even so, this does not mean that natural selection may not be involved in ensuring the use of traits adaptively, however they are initially derived (Leca and Gunst, 2023).
Experimental studies with rats and hamsters have shown that play fighting experiences in the juvenile period facilitate the development of executive functions such as attention, behavioral inhibition, short-term memory, and decision making (Dalley et al., 2004) and do so by altering the neural wiring of the relevant areas of the prefrontal cortex (Pellis et al., 2023a). In turn, improved executive functions result in more effective socio-cognitive skills such as greater impulse control, longer retention of social memories, greater resistance to defeat stress and better inter-animal coordination (e.g., Pellis et al., 1999; Baarendse et al., 2013; Burleson et al., 2016; Schneider et al., 2016; Stark and Pellis, 2020; Marquardt et al., 2023). Critically, observations of rats have revealed that role reversals are among the critical experiences derived from play fighting that leads to improvements in socio-cognitive skills (Pellis et al., 2023a). As noted above, a key feature of play fighting is that it involves cooperation, which in turn, is necessary to produce role reversals (turn-taking), and the studies with rats increasingly point to these beneficial effects arising from partners being relatively balanced in instigating role reversals (Stark et al., 2021; Ham et al., 2024). Not surprisingly, then, in Belding’s ground squirrels, it is social play and not solitary locomotor play that is positively correlated with improved emotional responsivity and social competence (Marks et al., 2017).
As in the case for rats, it would be expected that the forms of social play that involve the most role reversals should be favored in ground squirrels to ensure these beneficial outcomes. However, in the ground squirrels, play fighting can involve either competition to bite the shoulders or competition to mount (Nunes et al., 1999), and a study of a congener, Richardson’s ground squirrels (U. richardsoni), found that counterattacks leading to role reversals were three times more likely during play fights involving biting than those involving mounting (Pasztor et al., 2001). Consequently, it may seem odd that only 20% of all play fighting involves competition over biting in both Belding’s and Richardson’s ground squirrels (Nunes et al., 1999; Pasztor et al., 2001). Perhaps even more perplexing is that in other species of this genus competing for biting constitutes 80% of play fighting (Pellis and Iwaniuk, 2004). Evidence from rats suggests that experience with role reversals can be below the typical level, but if they remain above a critical threshold, those experiences are sufficient to produce the beneficial effects (Pellis et al., 2019b). Thus, it is possible that there can be considerable drift in the relative proportion of types of play fighting across species, if the forms that have the highest rates of role reversals are maintained at above threshold levels. Comparisons across species and lineages so far suggest that play fighting involving competition for courtship, agonism, grooming and predation-based targets tend to involve more role reversals than those involving mounting (Pellis et al., 2000; Pasztor et al., 2001; Himmler et al., 2016a; Pellis and Pellis, 2018; Kraus et al., 2019). However, it may be hubris to conclude that no species have achieved high rates of role reversals when competing for mounting; for example, this possibility has yet to be tested in beluga whales in which mounting play is quite common (Lilley et al., 2020; Ham et al., 2022). Whatever further empirical findings are found, our current state of knowledge is sufficient to disabuse us of expecting that all variations in the content of play fighting require an adaptive explanation (Pellis and Iwaniuk, 2004; Pellis et al, 2023a; see also Figure 6 above).
There are comparative, developmental and neurobiological data amassing that show that there are design features of how, when and with whom rats play as juveniles that support the hypothesis that play fighting is an adaptation evolved to facilitate the development of socio-cognitive skills, not just an incidental by-product (Pellis and Pellis, 1998a; Cooper et al., 2023; Ham and Pellis, 2023; Ham et al., 2023a), and this is extended to other species, including humans (e.g., Nijhoff et al., 2018; Gibb et al., 2021; Paquette and StGeorge, 2023). There are also some other strong candidate functions for play fighting, including its use as an anti-stress mechanism in both juveniles and adults, and as a mechanism by which adults can assess and manage social relationships with other adults (for reviews see Pellis and Pellis, 2009; Pellis and Burghardt, 2017; Kohn, 2019; Palagi, 2023). Finally, an unexplored question is whether, given the positive affective states that are generated by interpersonal touch (von Mohr et al., 2017), more touch-based playful contact is better suited to some of these functions than more competitive-based forms.
Conclusion
The revolution inaugurated by Aldis (1975) produced new descriptive insights into what constitutes play fighting, how it arose and diversified leading species from different lineages converging on to similar patterns (Pellis and Pellis, 2009). In turn, this has led to new insights into both the mechanisms that produce and regulate play and the kinds of functions that it can most plausibly have evolved. Regarding the latter, the major lesson to be learned is that, while there may be many benefits associated with play fighting, discerning which are evolved functions and which are incidental by-products is no simple matter, nor is differentiating between beneficial and irrelevant features of play. Nonetheless, as these issues are becoming clearer, integrating mechanisms with functions is yielding new insights and charting new avenues for the study of play (Himmler et al, 2016b; Leca, 2023; Burghardt et al., 2024).
Author contributions
SP: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft. VP: Conceptualization, Investigation, Writing – review and editing. JH: Conceptualization, Investigation, Visualization, Writing – review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Support for this work was provided by funding by the Natural Sciences and Engineering Council of Canada (NSERC) to SMP (grant number 2018-03706) and JRH (PGS D).
Acknowledgments
We thank the research efforts of many researchers who have advanced our understanding of play. Particularly helpful were the intensive discussions with colleagues at workshops geared to the study of play (National Institute for Mathematical and Biological Synthesis, University of Tennessee, Konrad Lorenz Institute, Austria and the Advanced School of Ethology, Erice, Sicily). We also thank the reviewers and the editor for their helpful comments, and the Harry Frank Guggenheim foundation and the Natural Sciences and Engineering Research Council of Canada that have supported much of the work from our laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achterberg E. J. M., Vanderschuren L. J. M. J. (2023). The neurobiology of social play behavior: past, present and future. Neurosci. Biobehav. Rev. 152, 105319. doi: 10.1016/j.neubiorev.2023.105319
Adams D. B. (1980). Motivational systems of agonistic behavior in muroid rodents: A comparative review and neural model. Aggress. Behav. 6, 295–346. doi: 10.1002/(ISSN)1098-2337
Altmann S. A. (1962). “Social behavior of anthropoid primates: analysis of recent concepts,” in Roots of behavior. Ed. Bliss E. L. (Harper, New York), 277–285.
Archer J., Huntingford F. (1994). “Game theory models and escalation of animal fights,” in Dynamics of aggression. Biological and social processes in dyads and groups. Eds. Potegal M., Knutson J. P. (Lawrence Erlbaum Associates, Hillsdale, NJ), 3–31.
Baarendse P. J. J., Counotte D. S., O’Donnell P., Vanderschuren L. J. M. J. (2013). Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacol 38, 1485–1494. doi: 10.1038/npp.2013.47
Barrett M., Capera M., Morcatty T. Q., Weldon A. V., Hedger K., Maynard K. Q., et al. (2021). Risky business: the function of play in a venomous mammal – the Javan slow loris (Nycticebus javanicus). Toxins 13, 318. doi: 10.3390/toxins13050318
Barrette C. (1986). Fighting behavior of wild Sus scrofa. J. Mammal. 67, 177–179. doi: 10.2307/1381018
Bateson P. (2014). Play, playfulness, creativity and innovation. Anim. Behav. Cogn. 1, 99–112. doi: 10.12966/abc.05.02.2014
Bateson P. (2015). Playfulness and creativity. Curr. Biol. 25, R13–R16. doi: 10.1016/j.cub.2014.09.009
Bauer E. B., Smuts B. B. (2007). Cooperation and competition during dyadic play in domestic dogs, Canis familiaris. Anim. Behav. 73, 489–499. doi: 10.1016/j.anbehav.2006.09.006
Bekoff M. (1975). The communication of play intention: are play signals functional? Semiotica 15, 231–239. doi: 10.1515/semi.1975.15.3.231
Bekoff M. (1977). Mammalian dispersal and the ontogeny of individual behavioral phenotypes. Am. Nat. 111, 715–732. doi: 10.1086/283201
Bekoff M. (2014). “The significance of ethological studies: playing and peeing,” in Domestic dog cognition and behavior. Ed. Horowitz A. (Springer, Berlin, Germany), 59–75.
Berenbaum S. A., Hines M. (1992). Early androgens are related to childhood sex-typed toy preferences. Psychol. Sci. 3, 203–206. doi: 10.1111/j.1467-9280.1992.tb00028.x
Bermudez-Contreras E., Chekhov S., Sun J., Tarnowsky J., McNaughton B. L., Mohajerani M. H. (2018). High-performance, inexpensive setup for simultaneous multisite recording of electrophysiological signals and mesoscale voltage imaging in the mouse cortex. Neurophotonics 5, 025005. doi: 10.1117/1.NPh.5.2.025005
Berridge K., Kringelbach M. L. (2013). Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr. Opin. Neurobiol. 23, 294–303. doi: 10.1016/j.conb.2013.01.017
Biben M. (1982). Object play and social treatment of prey in bush dogs and crab-eating foxes. Behaviour. 79, 201–211. doi: 10.1163/156853982X00256
Biben M. (1998). “Squirrel monkey play fighting: making the case for a cognitive training function for play,” in Animal play: evolutionary, comparative, and ecological perspectives. Eds. Bekoff M., Byers J. A. (Cambridge University Press, Cambridge, UK), 161–162.
Bishop A. (1962). Control of the hand in lower primates. Ann. N.Y. Acad. Sci. 102, 316–337. doi: 10.1111/j.1749-6632.1962.tb13649.x
Blanchard R. J., Blanchard D. C. (1994). “Environmental targets and sensorimotor systems in aggression and defence,” in Ethology and psychopharmacology. Eds. Cooper S. J., Hendrie C. A. (John Wiley and Sons, New York), 133–157.
Blanchard R. J., Blanchard D. C., Takahashi T., Kelley M. J. (1977). Attack and defensive behaviour in the albino rat. Anim. Behav. 25, 622–634. doi: 10.1016/0003-3472(77)90113-0
Blumstein D. T., Chung L. K., Smith J. E. (2013b). Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris). Proc. R. Soc B: Biol. Sci. 280, 20130485. doi: 10.1098/rspb.2013.0485
Blumstein D. T., Wey T. W., Tang K. (2009). A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc. R. Soc B: Biol. Sci. 276, 3007–3012. doi: 10.1098/rspb.2009.0703
Bobrov E., Wolfe J., Rao R. P., Brecht M. (2014). The representation of social facial touch in rat barrel cortex. Curr. Biol. 24, 109–115. doi: 10.1016/j.cub.2013.11.049
Boice R., Adams N. (1983). Degrees of captivity and aggressive behavior in domestic Norway rats. Bull. Psychon. Soc 21, 149–152. doi: 10.3758/BF03329980
Bolles R. C., Woods P. J. (1964). The ontogeny of behavior in the albino rat. Anim. Behav. 12, 427–441. doi: 10.1016/0003-3472(64)90062-4
Bombail V., Brown. S. M., Hammond T. J., Meddle S. L., Nielsen B. L., Tivey E. K. L., et al. (2021). Crying with laughter: adapting the tickling protocol to address individual differences among rats in their response to playful handling. Front. Vet. Sci. 24, 677872. doi: 10.3389/fvets.2021.677872
Burghardt G. M. (1998). “Play,” in Comparative psychology: A handbook. Eds. Greenberg G., Haraway M. (Garland, New York, NY), 757–767.
Burghardt G.M. (2005). “The genesis of play,” in Testing the limits (MIT Press, Cambridge, MA). doi: 10.7551/mitpress/3229.001.0001
Burghardt G. M., Albright J. D., Davis K. M. (2016). Motivation, development and object play: Comparative perspectives with lessons from dogs. Behaviour 153, 767–793. doi: 10.1163/1568539X-00003378
Burghardt G. M., Pellis S. M., Schank J. C., Smaldino P. E., Vanderschuren L. J. M. J., Palagi E. (2024). Animal play and evolution: Seven timely research questions about enigmatic phenomena. Neurosci. Biobehav. Rev. 160, 105617. doi: 10.1016/j.neubiorev.2024.105617
Burke C. J., Pellis S. M., Achterberg, E J. M. (2022). Who’s laughing? Play, tickling and ultrasonic vocalizations in rats. Phil. Trans. R. Soc B 377, 20210184. doi: 10.1098/rstb.2021.0184
Burleson C. A., Pedersen R. W., Seddighi S., DeBusk L. E., Burghardt G. M., Cooper M. A. (2016). Social play in juvenile hamsters alters dendritic morphology in the medial prefrontal cortex and attenuates effects of social stress in adulthood. Behav. Neurosci. 130, 437–447. doi: 10.1037/bne0000148
Byers J. A. (1984). “Play in ungulates,” in Play in animals and humans. Ed. Smith K. (Basil Blackwell, Oxford, UK), 5–41.
Chalmers N. (1984). “Social play in monkeys: theories and data,” in Play in animals and humans. Ed. Smith P. K. (Blackwell, Oxford, UK), 119–141.
Chen P., Hong W. (2018). Neural circuit mechanisms of social behavior. Neuron 98, 16–30. doi: 10.1016/j.neuron.2018.02.026
Cloutier S., Panksepp J., Newberry R. C. (2012). Playful handling by caretakers reduces fear of humans in laboratory rats. App. Anim. Behav. Sci. 140, 161–171. doi: 10.1016/j.applanim.2012.06.001
Cooper M. A., Grizzell J. A., Whitten C. J., Burghardt G. M. (2023). Comparing the ontogeny, neurobiology, and function of social play in hamsters and rats. Neurosc. Rev. 147, 105102. doi: 10.1016/j.neubiorev.2023.105102
Cordoni G. (2009). Social play in captive wolves (Canis lupus): not only an immature affair. Behaviour 146, 1363–1385. doi: 10.1163/156853909X427722
Cordoni G., Collarini E., Gioia M., Norscia I. (2022). Play fighting versus real fighting in piglets: similar patterns, different structure. Behav. Process. 203, 104778. doi: 10.1016/j.beproc.2022.104778
Cordoni G., Gioia M., Demuru E., Norscia I. (2021). The dark side of play: play fighting as a substitute for real fighting in domestic pigs, Sus scrofa. Anim. Behav. 175, 21–31. doi: 10.1016/j.anbehav.2021.02.016
Cordoni G., Nicotra V., Palagi E. (2016). Unveiling the “secret” of play in dogs (Canis lupus familiaris): asymmetry and signals. J. Comp. Psych. 130, 278–287. doi: 10.1037/com0000035
Cordoni G., Palagi E. (2011). Ontogenetic trajectories of chimpanzee social play: similarities with humans. PloS One 6, e27344. doi: 10.1371/journal.pone.0027344
Croy I., Fairhurst M. T., McGlone ,. F. (2022). The role of C-tactile nerve fibers in human social development. Curr. Opin. Behav. Sci. 43, 20–26. doi: 10.1016/j.cobeha.2021.06.010
Cumming D. H. M. (1984). “Wild pigs and boars,” in The encyclopaedia of mammals. Ed. MacDonald D. (Allen and Unwin, London), 500–503.
Dalley J. W., Cardinal R. N., Trevor W., Robbins T. W. (2004). Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neurosci. Biobehav. Rev. 28, 771–784. doi: 10.1016/j.neubiorev.2004.09.006
Dapporto L., Turillazzi S., Palagi E. (2006). Dominance interactions in young adult paper wasp (Polistes dominulus) foundresses: a playlike behavior? J. Comp. Psych. 120, 394–400. doi: 10.1037/0735-7036.120.4.394
Deak T., Panksepp J. (2006). Play behavior in rats pretreated with scopolamine: increased play solicitation by the non-injected partner. Physiol. Behav. 87, 120–125. doi: 10.1016/j.physbeh.2005.09.009
Donaldson T. M., Newberry R. C., Sipinka M., Cloutier S. (2002). Effects of early experience on play behaviour of piglets after weaning. App. Anim. Behav. Sci. 79, 221–231. doi: 10.1016/S0168-1591(02)00138-7
Doyle G. A. (1974). “The behaviour of the lesser bushbaby,” in Prosimian biology. Eds. Martin R. D., Doyle G. A., Walker A. C. (The University of Pittsburgh Press, Pittsburgh, PA), 213–231.
Drea C. M., Hawk J. E., Glickman S. E. (1996). Aggression decreases as play emerges in infant spotted hyenas: preparation for joining the clan. Anim. Behav. 51, 1323–1336. doi: 10.1006/anbe.1996.0136
Dugatkin L. A., Bekoff M. (2003). Play and the evolution of fairness: a game theory model. Behav. Process 60, 209–214. doi: 10.1016/S0376-6357(02)00120-1
Eibl-Eibesfeldt I. (1970). “Ethology,” in The biology of behavior (Holt, Rinehart and Winston, New York, NY).
Epps J. (1974). “Social interactions of Perodicticus potto kept in captivity in Kampala, Uganda,” in Prosimian biology. Eds. Martin R. D., Doyle G. A., Walker A. C. (The University of Pittsburgh Press, Pittsburgh, PA), 233–244.
Essler J. L., Cafazzo S., Marshall-Pescini S., Virányi Z., Kotrschal K., Range F. (2016). Play behavior in wolves: using the ‘50:50’ rule to test for egalitarian play styles. PloS One 11, e0154150. doi: 10.1371/journal.pone.0154150
Estes R. D. (1993). “The safari companion,” in A guide to watching african mammals (Chelsea Green, Post Mills, VT).
Field E. F., Pellis S. M. (1994). Differential effects of amphetamine on the attack and defense components of play fighting in rats. Physiol. Behav. 56, 325–330. doi: 10.1016/0031-9384(94)90202-X
Foroud A., Pellis S. M. (2003). The development of ‘roughness’ in the play fighting of rats: a Laban Movement Analysis perspective. Dev. Psychobiol. 42, 35–43. doi: 10.1002/dev.10088
Fox M. W. (1969). The anatomy of aggression and its ritualization in Canidae: a developmental and comparative study. Behaviour 35, 242–258. doi: 10.1163/156853969X00224
Frank L. G., Glickman S. E., Licht P. (1991). Fatal sibling aggression, precocial development, and androgens in neonatal spotted hyenas. Sci. 252, 702–704. doi: 10.1126/science.2024122
Fraüdrich H. (1974). “A comparison of behaviour in the Suidae,” in The behaviour of ungulates and its relation to management, 24th ed. Eds. Geist V., Walther F. (IUCN, Morges, Switzerland), 133–143.
Galpayage Dona H. S., Solvi C., Kowalewska A., Mäkelä K., MaBouDi H., Chittka L. (2022). Do bumble bees play? Anim. Behav. 194, 239–251. doi: 10.1016/j.anbehav.2022.08.013
Geist V. (1967). On fighting injuries and dermal shields of mountain goals. J. Wildl. Manage. 31, 192–194. doi: 10.2307/3798378
Geist V. (1978). “On weapons, combat and ecology,” in Advances in the study of communication and affect (vol. 4) aggression, dominance and individual spacing. Eds. Krames L., Pliner P., Alloway T. (Plenum Press, New York, NY), 1–30.
Geist V. (1986). New evidence for high frequency of antler wounding in cervids. Can. J. Zool. 64, 380–384. doi: 10.1139/z86-059
Gibb R., Coelho L., Van Rootselaar N. A., Halliwell C., MacKinnon M., Plomp I., et al. (2021). Promoting executive function skills in preschoolers using a play-based program. Front. Psychol. 12, 720225. doi: 10.3389/fpsyg.2021.720225
Gloveli N., Simonnet J., Tang W., Concha-Miranda M., Maier E., Dvorzhak A., et al. (2023). Play and tickling responses map to the lateral columns of the rat periaqueductal gray. Neuron 111, 3041–3052. doi: 10.1016/j.neuron.2023.06.018
Golani I., Fentress J. C. (1985). Early ontogeny of face grooming in mice. Dev. Psychobiol. 18, 529–544. doi: 10.1002/dev.420180609
Gomendio M. (1988). The development of different types of play in gazelles: implications for the nature and functions of play. Anim. Behav. 36, 825–836. doi: 10.1016/S0003-3472(88)80165-9
Goonan P. M. (1993). Behaviour and reproduction of the slender loris (Loris tardigradus) in captivity. Folia Primatol 60, 146–157. doi: 10.1159/000156683
Grimes C., Brent L. J. N., Weiss M. N., Franks D. W., Balcomb K. C., Ellifrit D. K., et al. (2022). The effect of age, sex, and resource abundance on patterns of rake markings in resident killer whales (Orcinus orca). Mar. Mamm. Sci. 38, 941–958. doi: 10.1111/mms.12908
Ham J. R., Lilley M. K., Hill M., H. M. (2021). Conspecific scarring on wild belugas (Delphinapterus leucas) in Cunningham Inlet. Behaviour 158, 663–683. doi: 10.1163/1568539X-bja10086
Ham J. R., Lilley M. K., Hill M., H. M. (2023a). “Non-conceptive sexual behavior in cetaceans: comparison of form and function,” in Sex in cetaceans. Eds. Würsig B., Orbach D. N. (Springer Nature, Cham, Switzerland), 129–151.
Ham J. R., Lilley M. K., Lelekach J., Miller M. R., Robeck T. R., Pellis S. M., et al. (2022). The emergence and early development of socio-sexual behavior in beluga calves (Delphinapterus leucas). Behav. Process 200, 104695. doi: 10.1016/j.beproc.2022.104695
Ham J. R., Lilley M. K., Miller M. R., Leca J.-B., Pellis S. M., Hill M. ,. H. M. (2023b). Self-handicapping in object play: How belugas (Delphinapterus leucas) make play difficult. Int. J. Play 12, 67–80. doi: 10.1080/21594937.2022.2152535
Ham J. R., Lilley M. K., Wincheski R. J., Miranda J., Velarde Dediós A. G., Kolodziej K., et al. (2023c). Playful mouth-to-mouth interactions of belugas (Delphinapterus leucas) in managed care. Zoo Biol. 42, 730–743. doi: 10.1002/zoo.21788
Ham J. R., Pellis S. M. (2023). The Goldilocks principle: balancing novelty and familiarity in choosing play partners in male juvenile rats. Anim. Behav. Cogn. 10, 304–328. doi: 10.26451/abc
Ham J. R., Szabo M., Annor-Bediako J., Stark R. A., Iwaniuk A. N., Pellis S. M. (2024). Quality not quantity: Deficient juvenile play experiences lead to altered medial prefrontal neurons and socio-cognitive skill deficiencies. Dev. Psychobiol. 66, e22456. doi: 10.1002/dev.22456
Hanby J. P., Brown C.E. (1974). The development of sociosexual behaviours in Japanese macaques, Macaca fuscata. Behaviour 49, 152–195. doi: 10.1163/156853974X00444
Harlow H.F. (1969). Age-mate or peer affectional systems. Adv. Study Behav. 2, 333–383. doi: 10.1016/S0065-3454(08)60072-8
Himmler S. M., Himmler B. T., Pellis V. C., Pellis S.M. (2016a). Play, variation in play and the development of socially competent rats. Behaviour 153, 1103–1137. doi: 10.1163/1568539X-00003307
Himmler S. M., Himmler B. T., Stryjek R., Modlińska K., Pisula W., Pellis S. M. (2016b). Pinning in the play fighting of rats: a comparative perspective with some methodological recommendations. Int. J. Comp. Psych. 29, 1–14. doi: 10.46867/ijcp
Himmler S. M., Modlińska K., Stryjek R., Himmler B. T., Pisula W., Pellis S. M. (2014). Domestication and diversification: a comparative analysis of the play fighting of the brown Norway, Sprague-Dawley, and Wistar strains of laboratory rats. J. Comp. Psych. 128, 318–327. doi: 10.1037/a0036104
Hinde R. A. (1975). “The concept of function,” in Function and evolution of behaviour. Eds. Baerends G., Beer C., Manning A. (Clarendon Press, Oxford, UK), 3–15.
Hoffman R., Foerg R. (1983). Development of locomotion and social behavior in infants of fat-tailed dwarf lemur (Cheirogaleus medius). Z. Saugetierd. 48, 129–136.
Huntingford F. A., Turner A. K. (1987). Animal conflict (London, UK: Chapman and Hall). doi: 10.1007/978-94-009-3145-9
Hurst J. L., Barnard C. J., Hare R., Wheeldon E. B., West C. D. (1996). Housing and welfare in laboratory rats: time-budgeting and pathophysiology in single sex groups. Anim. Behav. 52, 335–360. doi: 10.1006/anbe.1996.0179
Ishiyama S., Brecht M. (2016). Neural correlates of ticklishness in the rat somatosensory cortex. Sci 354, 757–760. doi: 10.1126/science.aah5114
Jolly A. (1966). Lemur social behavior and primate intelligence. Sci. 153, 501–506. doi: 10.1126/science.153.3735.501
Kaplan G. (2020). Play behaviour, not tool using, relates to brain mass in a sample of birds. Sci. Rep. 10, 20437. doi: 10.1038/s41598-020-76572-7
Kisko T. M., Wöhr M., Pellis V. C., Pellis S. M. (2017). From play to aggression: high-frequency 50-kHz ultrasonic vocalizations as play and appeasement signals in rats. Curr. Top. Behav. Neurosci. 30, 91–108. doi: 10.1007/7854_2015_432
Kohn G. M. (2019). How social systems persist: learning to build a social network in an uncertain world. Anim. Behav. 154, 1–6. doi: 10.1016/j.anbehav.2019.06.006
Kraus K. L., Pellis V. C., Pellis S. M. (2019). Targets, tactics and cooperation in the play fighting of two genera of Old-World monkeys (Mandrillus and Papio): accounting for similarities and differences. Int. J. Comp. Psych. 32, 1–25. doi: 10.46867/ijcp
Kuba M. J., Byrne R. A., Meisel D. V., Mather J. A. (2006). When do octopuses play? Effects of repeated testing, object type, age, and food deprivation on object play in Octopus vulgaris. J. Comp. Psych. 120, 184–190. doi: 10.1037/0735-7036.120.3.184
LaFollette M. R., O’Haire M. E., Cloutier S., Blankenberger W. B., Gaskill B.N. (2017). Rat tickling: a systematic review of applications outcomes and moderators. PloS One 12, e0175320. doi: 10.1371/journal.pone.0175320
Lauder G. V. (1986). “Homology, analogy, and the evolution of behavior,” in Evolution of animal behavior. Eds. Nitecki M. H., Kitchell J. A. (Oxford University Press, New York), 9–40.
Lazar J. W., Beckhorn G. D. (1974). Social play or the development of social behavior in ferrets (Mustela putorius)? Am. Zool. 14, 405–414.
Leca J. B. (2021a). “By-products of adaptations,” in Encyclopedia of evolutionary psychological science. Eds. Shackelford T. K., Weekes-Shackelford V. A. (Springer, Cham, Switzerland), 850–860.
Leca J. B. (2021b). “Evolutionary by-products,” in Encyclopedia of evolutionary psychological science. Eds. Shackelford T. K., Weekes-Shackelford V. A. (Springer, ham, Switzerland), 2638–2648.
Leca J. B. (2023). Towards a three-level neo-Tinbergenian approach to object play: structure, causes and consequences of a behavioral puzzle. Neurosci. Biobehav. Rev. 152, 105290. doi: 10.1016/j.neubiorev.2023.105290
Leca J. B., Gunst N. (2023). The exaptive potential of (object) play behavior. Int. J. Play 12, 40–52. doi: 10.1080/21594937.2022.2152184
Lee P. C., Moss C. J. (2014). African elephant play, competence and social complexity. Anim. Behav. Cognit. 1, 144–156. doi: 10.12966/abc.05.05.2014
Levy J. S. (1979). Play behavior and its decline during development in rhesus monkeys (Macaca mulatta) (Chicago, IL: University of Chicago).
Leyhausen P. (1979). “Cat behavior,” in The predatory and social behavior of domestic and wild cats (Garland, New York, NY).
Lilley M. K., Ham J. R., Hill H. M. (2020). The development of socio-sexual behavior in belugas (Delphinapterus leucas) under human care. Behav. Process. 171, 104025. doi: 10.1016/j.beproc.2019.104025
Loizos C. (1967). “Play in higher primates: a review,” in Primate ethology. Ed. Morris D. (Aldine, Chicago, IL), 176–218.
Luo J. (2018). The neural basis of and a common neural circuitry in different types of prosocial behavior. Front. Psychol. 9, 859. doi: 10.3389/fpsyg.2018.00859
Manitzas Hill H. M., Ortiz N., Kolodziej K., Ham J. R. (2023). Social games that belugas (Delphinapterus leucas) play. Int. J. Play 12, 81–100. doi: 10.1080/21594937.2022.2152536
Marks K. A., Vizconde D. L., Gibson E. S., Roriguez J. R., Nunes S. (2017). Play behavior and responses to novel situations in juvenile ground squirrels. J. Mammal. 98, 1202–1210. doi: 10.1093/jmammal/gyx049
Marley C., Pollard T. M., Barton R. A., Street S. E. (2022). A systematic review of sex differences in rough and tumble play across non-human mammals. Behav. Ecol. Sociobiol. 76, 158. doi: 10.1007/s00265-022-03260-z
Marquardt A. E., VanRyzin J. W., Fuguen R. W., McCarthy M. M. (2023). Social play experience in juvenile rats is indispensable for appropriate socio-sexual behavior in adulthood in males but not females. Front. Behav. Neurosci. 16, 1076765. doi: 10.3389/fnbeh.2022.1076765
Mather J. A., Anderson R. C. (1999). Exploration, play and habituation in octopuses. (Octopus dofleini) J. Comp. Psych. 113, 333–338. doi: 10.1037//0735-7036.113.3.333
McCarthy M. M. (2023). Neural control of sexually dimorphic social behavior; Connecting development to adulthood. Annu. Rev. Neurosci. 46, 321–339. doi: 10.1146/annurev-neuro-121522-110856
McClintock M. K. (1984). Group mating in the domestic rat as a context for sexual selection: consequences for the analysis of sexual behavior and neuroendocrine responses. Adv. Study Behav. 14, 1–50. doi: 10.1016/S0065-3454(08)60298-3
Meaney M. J. (1988). The sexual differentiation of social play. Trends Neurosci. 11, 54–58. doi: 10.1016/0166-2236(88)90164-6
Meaney M. J., Stewart J. (1981). A descriptive study of social development in the rat (Rattus norvegicus). Anim. Behav. 29, 34–45. doi: 10.1016/S0003-3472(81)80149-2
Michel G. F., Moore C. L. (1995). “Developmental psychobiology,” in An interdisciplinary science (MIT Press, Cambridge, MA). doi: 10.7551/mitpress/2392.001.0001
Mitani J. C., Call P. M., Kappeler P. M., Palombit R. A., Silk J. B. (2012). The evolution of primate societies (Chicago, IL: The University of Chicago Press). doi: 10.7208/chicago/9780226531731.001.0001
Mohapatra A. N., Wagner S. (2023). The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder. Front. Psychiat. 14, 1205199. doi: 10.3389/fpsyt.2023.1205199
Newberry R. C., Wood-Gush D. G. M., Hall J. W. (1988). Playful behaviour of piglets. Behav. Process. 17, 205–216. doi: 10.1016/0376-6357(88)90004-6
Nijhoff S. L., Vinkers C. H., van Geelan S. M., Duiff S. N., Achterberg E. J. M., van der Net J., et al. (2018). Healthy play, better coping: the importance of play for the development of children in health and disease. Neurosci. Biobehav. Rev. 95, 421–429. doi: 10.1016/j.neubiorev.2018.09.024
Norscia I., Hecker M., Caselli M., Collarini E., Aldama B. G., Santos S. B., et al. (2024). Social play in African savannah elephants may inform selection against aggression. Curr. Zool. doi: 10.1093/cz/zoae009
Norscia I., Palagi E. (2016). The missing lemur link: an ancestral step in human evolution (Cambridge, UK: Cambridge University Press). doi: 10.1017/CBO9781139060059
Nunes S., Montemayor M. M.P. (2023). Multiple benefits of juvenile play: a ground squirrel perspective Neurosci. Biobehav. Rev. 147, 105099. doi: 10.1016/j.neubiorev.2023.105099
Nunes S., Muecke E.-M., Anthony J. A., Batterbee A. S. (1999). Endocrine and energetic mediation of play behavior in free-living Belding’s ground squirrels. Horm. Behav. 36, 153–165. doi: 10.1006/hbeh.1999.1538
Orgeur P., Signoret J.P. (1984). Sexual play and its functional significance in the domestic sheep (Ovis aries L.). Physiol. Behav. 33, 111–118. doi: 10.1016/0031-9384(84)90021-0
Palagi E. (2011). “Playing at every age: modalities and potential functions in non-human primates,” in Oxford handbook of the development of play. Ed. Pellegrini A. D. (Oxford University Press, Oxford, UK), 70–82.
Palagi E. (2018). Not just for fun! Social play as a springboard for adult social competence in human and non-human primates. Behav. Ecol. Sociobiol. 72, 90. doi: 10.1007/s00265-018-2506-6
Palagi E. (2023). Adult play and the evolution of tolerant and cooperative societies. Neurosci. Biobehav. Rev. 148, 105124. doi: 10.1016/j.neubiorev.2023.105124
Palagi E., Burghardt G. M., Smuts B., Cordoni G., Dall’Olio S., Fouts H. N., et al. (2016a). Rough-and-tumble play as a window on animal communication. Biol. Rev. 91, 311–327. doi: 10.1111/brv.12172
Palagi E., Cordoni G., Demuru E., Bekoff M. (2016b). Fair play and its connection with social tolerance, reciprocity and the ethology of peace. Behaviour 153, 1195–1216. doi: 10.1163/1568539X-00003336
Palagi E., Norscia I., Pressi S., Cordoni G. (2019). Facial mimicry and play: a comparative study in chimpanzees and gorillas. Emot. 19, 665–681. doi: 10.1037/emo0000476
Panksepp J. (1981). The ontogeny of play in rats. Dev. Psychobiol. 14, 327–332. doi: 10.1002/dev.420140405
Panksepp J. (1998). Affective neuroscience (New York, NY: Oxford University Press). doi: 10.1093/oso/9780195096736.001.0001
Panksepp J., Beatty W. W. (1980). Social deprivation and play in rats. Behav. Neural Biol. 30, 197–206. doi: 10.1016/S0163-1047(80)91077-8
Panksepp J., Burgdorf J. (2000). 50-kHz chirping (laughter)? in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav. Brain Res. 115, 25–38. doi: 10.1016/S0166-4328(00)00238-2
Panksepp J., Normansell L., Cox J. F., Siviy S. M. (1994). Effects of neonatal decortication on the social play of juvenile rats. Physiol. Behav. 56, 429–443. doi: 10.1016/0031-9384(94)90285-2
Panksepp J., Siviy S. M., Normansell L. (1984). The psychobiology of play: theoretical and methodological perspectives. Neurosci. Biobehav. Rev. 8, 465–492. doi: 10.1016/0149-7634(84)90005-8
Paquette D., StGeorge J. M. (2023). Proximate and ultimate mechanisms of human father-child rough-and-tumble play. Neurosci. Biobehav. Rev. 149, 105151. doi: 10.1016/j.neubiorev.2023.105151
Pasztor T. J., Smith L. K., MacDonald N. L., Michener G. R., Pellis S.M. (2001). Sexual and aggressive play fighting of sibling Richardson’s ground squirrels. Aggress. Behav. 27, 323–337. doi: 10.1002/ab.1015
Pederson J. M., Glickman S. E., Frank L. G., Beach F.A. (1990). Sex differences in the play behavior of immature spotted hyenas, Crocuta crocuta. Horm. Behav. 24, 403–420. doi: 10.1016/0018-506X(90)90018-S
Pellegrini A. D. (2002). “Rough-and-tumble play from childhood through adolescence: development and possible functions,” in Blackwell handbook of childhood social development, 1st ed. Eds. Smith P. K., Hart C. (Blackwell, Malden, MA), 438–453.
Pellis S. M. (1981). A description of social play by the Australian magpie Gymnorhina tibicen based on Eshkol-Wachman notation. Bird Behav. 3, 61–79. doi: 10.3727/015613881791560685
Pellis S. M. (1984). Two aspects of play-fighting in a captive group of oriental small-clawed otters Amblonyx cinerea. Z. Tierpsychol. 65, 77–83. doi: 10.1111/j.1439-0310.1984.tb00374.x
Pellis S. M. (1988). Agonistic versus amicable targets of attack and defense: consequences for the origin, function and descriptive classification of play-fighting. Aggress. Behav. 14, 85–104. doi: 10.1002/(ISSN)1098-2337
Pellis S. M. (1991). How motivationally distinct is play? A preliminary case study. Anim. Behav. 42, 851–853. doi: 10.1016/S0003-3472(05)80129-0
Pellis S. M. (1993). Sex and the evolution of play fighting: a review and a model based on the behavior of muroid rodents. J. Play Theory Res. 1, 56–77. doi: 10.1002/1098-2337(1993)19:5<385::AID-AB2480190508>3.0.CO;2-%23
Pellis S. M. (1997). Targets and tactics: the analysis of moment-to-moment decision making in animal combat. Aggress. Behav. 23, 107–129. doi: 10.1002/(ISSN)1098-2337
Pellis S. M. (2002a). “Keeping in touch: play fighting and social knowledge,” in The cognitive animal: empirical and theoretical perspectives on animal cognition. Eds. Bekoff M., Allen C., Burghardt G. M. (MIT Press, Cambridge, MA), 421–427.
Pellis S. M. (2002b). Sex-differences in play fighting revisited: Traditional and non-traditional mechanisms for sexual differentiation in rats. Arch. Sex Behav. 31, 11–20.
Pellis S. M., Blundell M. A., Bell H. C., Pellis V. C., Krakauer A. H., Patricelli G. L. (2013). Drawn into the vortex: the facing-past encounter and combat in lekking male greater sage-grouse (Centrocercus urophasianus). Behaviour 150, 1567–1599. doi: 10.1163/1568539X-00003110
Pellis S. M., Burghardt G. M. (2017). “Play and exploration,” in APA handbook of comparative psychology: vol 1. Basic concepts, method, neural substrate, and behavior. Eds. Call J., Burghardt G. M., Pepperberg I., Snowdon C., Zentall T. (American Psychological Association, Washington, DC), 699–722.
Pellis S. M., Field E. F., Whishaw I. Q. (1999). The development of a sex-differentiated defensive motor-pattern in rats: A possible role for juvenile experience. Dev. Psychobiol. 35, 156–164. doi: 10.1002/(ISSN)1098-2302
Pellis S. M., Iwaniuk A. N. (2000). Adult-adult play in primates: comparative analyses of its origin, distribution and evolution. Ethol 106, 1083–1104. doi: 10.1046/j.1439-0310.2000.00627.x
Pellis S. M., Iwaniuk A. N. (2004). Evolving a playful brain: a levels of control approach. Int. J. Comp. Psychol. 17, 90–116. doi: 10.46867/IJCP.2004.17.01.01
Pellis S. M., McKenna M. M. (1995). What do rats find rewarding in play fighting? An analysis using drug-induced non-playful partners. Behav. Brain Res. 68, 65–73. doi: 10.1016/0166-4328(94)00161-8
Pellis S. M., Pasztor T. J., Pellis V. C., Dewsbury D. A. (2000). The organization of play fighting in the grasshopper mouse (Onychomys leucogaster): mixing predatory and sociosexual targets and tactics. Aggress. Behav. 26, 319–334. doi: 10.1002/(ISSN)1098-2337
Pellis S. M., Pellis V. C. (1983). Locomotor-rotational movements in the ontogeny and play of the laboratory rat Rattus norvegicus. Dev. Psychobiol. 16, 269–286. doi: 10.1002/dev.420160403
Pellis S. M., Pellis V. C. (1987). Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggress. Behav. 13, 227–242. doi: 10.1002/(ISSN)1098-2337
Pellis S. M., Pellis V. C. (1988). Play-fighting in the Syrian golden hamster Mesocricetus auratus Waterhouse, and its relationship to serious fighting during post-weaning development. Dev. Psychobiol. 21, 323–337. doi: 10.1002/dev.420210404
Pellis S. M., Pellis V. C. (1989). Targets of attack and defense in the play fighting by the Djungarian hamster Phodopus campbelli: links to fighting and sex. Aggress. Behav. 15, 217–234. doi: 10.1002/(ISSN)1098-2337
Pellis S. M., Pellis V. C. (1992a). An analysis of the targets and tactics of conspecific attack and predatory attack in Northern grasshopper mice Onychomys leucogaster. Aggress. Behav. 18, 301–316. doi: 10.1002/(ISSN)1098-2337
Pellis S. M., Pellis V. C. (1992b). Juvenilized play fighting in subordinate male rats. Aggress. Behav. 18, 449–457. doi: 10.1002/(ISSN)1098-2337
Pellis S. M., Pellis V. C. (1997a). Targets, tactics and the open mouth face during play fighting in three species of primates. Aggress. Behav. 23, 41–57. doi: 10.1002/(ISSN)1098-2337
Pellis S. M., Pellis V. C. (1997b). The pre-juvenile onset of play fighting in rats (Rattus norvegicus). Dev. Psychobiol. 31, 193–205. doi: 10.1002/(ISSN)1098-2302
Pellis S. M., Pellis V. C. (1998a). The play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci. Biobehav. Rev. 23, 87–101. doi: 10.1016/S0149-7634(97)00071-7
Pellis S. M., Pellis V. C. (1998b). “The structure-function interface in the analysis of play fighting,” in Play behavior: comparative, evolutionary, and ecological aspects. Eds. Bekoff M., Byers J. A. (Cambridge University Press, Cambridge, UK), 115–140.
Pellis S. M., Pellis V. C. (2009). “The playful brain,” in Venturing to the limits of neuroscience (Oneworld Press, Oxford, UK).
Pellis S. M., Pellis V. C. (2011). To whom the play signal is directed: a study of headshaking in black-handed spider monkeys (Ateles geoffroyi). J. Comp. Psychol. 125, 1–10. doi: 10.1037/a0020547
Pellis S. M., Pellis V. C. (2015). Are agonistic behavior patterns signals or combat tactics – or does it matter? Targets as organizing principles of fighting. Physiol. Behav. 146, 73–78. doi: 10.1016/j.physbeh.2015.03.038
Pellis S. M., Pellis V. C. (2016a). “Play and cognition: the final frontier,” in Animal cognition: principles, evolution, and development. Ed. Olmstead M. C. (Nova Science, New York, NY), 201–230.
Pellis S. M., Pellis V. C. (2016b). Play fighting in Visayan warty pigs (Sus cebifrons): insights on restraint and reciprocity in the maintenance of play. Behaviour 153, 727–747. doi: 10.1163/1568539X-00003346
Pellis S. M., Pellis V. C. (2017a). What is play fighting and what is it good for? Learn. Behav. 45, 355–366. doi: 10.3758/s13420-017-0264-3
Pellis S. M., Pellis V. C. (2017b). “Play behavior,” in Encyclopedia of animal cognition and behavior. Eds. Vonk J., Shackleford T. (Springer, New York, NY), 1–13.
Pellis S. M., Pellis V. C. (2018). I am going to groom you”: multiple forms of play fighting in gray mouse lemurs (Microcebus murinus). J. Comp. Psychol. 132, 6–15. doi: 10.1037/com0000082
Pellis S. M., Pellis V. C. (2021). Understanding animal behaviour: what to measure and why (Cambridge, UK: Cambridge University Press). doi: 10.1017/9781108650151
Pellis S. M., Pellis V. C., Bell H. C. (2010a). The function of play in the development of the social brain. Am. J. Play 2, 278–296.
Pellis S. M., Pellis V. C., Burke C. J., Stark R. A., Ham J. R., Euston D. R., et al. (2022a). Measuring play fighting in rats: a multilayered approach. Curr. Protoc. 2, e337. doi: 10.1002/cpz1.337
Pellis S. M., Pellis V. C., Dewsbury D. A. (1989). Different levels of complexity in the playfighting by muroid rodents appear to result from different levels of intensity of attack and defense. Aggress. Behav. 15, 297–310. doi: 10.1002/ab.2480150405
Pellis S. M., Pellis V. C., Foroud A. (2005). “Play fighting: aggression, affiliation and the development of nuanced social skills,” in Developmental origins of aggression. Eds. Tremblay R., Hartup W. W., Archer J. (Guilford Press, New York, NY), 47–62.
Pellis S. M., Pellis V. C., Ham J. R. (2023a). The evolution of aggressive and amicable play fighting in primates: A phylogenetic perspective. Int. J. Play 12, 101–112. doi: 10.1080/21594937.2022.2152537
Pellis S. M., Pellis V. C., Ham J. R., Achterberg E. M. J. (2022b). The rough-and-tumble play of rats as a natural behaviour suitable for studying the social brain. Front. Behav. Neurosci. 16, 1033999. doi: 10.3389/fnbeh.2022.1033999
Pellis S. M., Pellis V. C., Ham J. R., Stark R. A. (2023b). Play fighting and the development of the social brain: the rat’s tale. Neurosci. Biobehav. Rev. 145, 105037. doi: 10.1016/j.neubiorev.2023.105037
Pellis S. M., Pellis V. C., Himmler B. T. (2014). How play makes for a more adaptable brain: a comparative and neural perspective. Am. J. Play 7, 73–98.
Pellis S. M., Pellis V. C., Himmler B. T., Modlińska K., Stryjek R., Kolb B., et al. (2019a). Domestication and the role of social play on the development of sociocognitive skills in rats. Int. J. Comp. Psychol. 32, 1–12. doi: 10.46867/ijcp
Pellis S. M., Pellis V. C., McKenna M. M. (1993). Some subordinates are more equal than others: play fighting amongst adult subordinate male rats. Aggress. Behav. 19, 385–393.
Pellis S. M., Pellis V. C., Nelson J. E. (1992a). The development of righting reflexes in the pouch young of the marsupial Dasyurus hallucatus. Dev. Psychobiol. 25, 105–125. doi: 10.1002/dev.420250204
Pellis S. M., Pellis V. C., Pelletier A., Leca J.-B. (2019b). Is play a behavior system, and, if so, what kind? Behav. Process. 160, 1–9. doi: 10.1016/j.beproc.2018.12.011
Pellis S. M., Pellis V. C., Reinhart C. J. (2010b). “The evolution of social play,” in Formative experiences: the interaction of caregiving, culture, and developmental psychobiology. Eds. Worthman C., Plotsky P., Schechter D., Cummings C. (Cambridge University Press, Cambridge, UK), 404–431.
Pellis V. C., Pellis S. M., Teitelbaum P. (1991). A descriptive analysis of the post-natal development of contact-righting in rats (Rattus norvegicus). Dev. Psychobiol. 24, 237–263. doi: 10.1002/dev.420240405
Pellis S. M., Pellis V. C., Whishaw I. Q. (1992b). The role of the cortex in play fighting by rats: developmental and evolutionary implications. Brain Behav. Evol. 39, 270–284. doi: 10.1159/000114124
Pierce J. D., Pellis V. C., Dewsbury D. A., Pellis S. M. (1991). Targets and tactics of agonistic and precopulatory behavior in montane and prairie voles: their relationship to juvenile play-fighting. Aggress. Behav. 17, 337–349. doi: 10.1002/(ISSN)1098-2337
Poole T. B., Fish J. (1975). An investigation of playful behaviour in Rattus norvegicus and Mus musculus (Mammalia). J. Zool. 175, 61–71. doi: 10.1111/j.1469-7998.1975.tb01391.x
Pozis-Francois O., Zahavi A., Zahavi A. (2004). Social play in Arabian babblers. Behaviour 141, 425–450. doi: 10.1163/156853904323066720
Pruitt J. N., Burghardt G. M., Riechert S. E. (2012). Non-conceptive sexual behavior in spiders: a form of play associated with body condition, personality type, and male intrasexual selection. Ethol. 118, 33–40. doi: 10.1111/j.1439-0310.2011.01980.x
Reinhart C. J., Pellis V. C., Thierry B., Gauthier C.-A., VanderLaan D. P., Vasey P. L., et al. (2010). Targets and tactics of play fighting: competitive versus cooperative styles of play in Japanese and Tonkean macaques. Int. J. Comp. Psychol. 23, 166–200. doi: 10.46867/IJCP.2010.23.02.05
Rule J. S., Goddu M. K., Chu J., Pinter V., Reagen E. R., Bonawitz E., et al. (2023). Fun isn’t easy: children choose more difficult options when ‘playing for fun’ vs. “trying to win. doi: 10.31234/osf.io/q7wh4
Rushen J. (1989). “Offence and defence and the assessment of fighting ability by domestic pigs,” in Ethoexperimental approaches to the study of behavior. Eds. Blanchard R. J., Brain P. F., Blanchard D. C., Parmigiani S. (Kluwer, Dordrecht, The Netherlands), 375–384.
Rushen J., Pajor E. (1987). Offence and defence in fighting between young pigs (Sus scrofa). Aggress. Behav. 13, 329–346. doi: 10.1002/(ISSN)1098-2337
Silerova J., Sipinka M., Siárová R., Algers B. (2010). Playing and fighting by piglets around weaning on farms, employing individual or group housing of lactating sows. App. Anim. Behav. Sci. 124, 83–89. doi: 10.1016/j.applanim.2010.02.003
Schaller G. B. (1972). “The serengeti lion,” in A study of predator-prey relations (The University of Chicago Press, Chicago, IL).
Schirmer A., Croy I., Ackerley R. (2023). What are C-tactile afferents and how do they relate to “affective touch”? Neurosci. Biobehav. Rev. 151, 105236. doi: 10.1016/j.neubiorev.2023.105236
Schneider P., Bindila L., Schmahl C., Bohus M., Meyer-Lindenberg A., Lutz B., et al. (2016). Adverse social experiences in adolescent rats result in enduring effects on social competence, pain sensitivity and endocannabinoid signaling. Front. Behav. Neurosci. 10, 203. doi: 10.3389/fnbeh.2016.00203
Scott E., Panksepp J. (2003). Rough-and-tumble play in human children. Aggress. Behav. 29, 539–551. doi: 10.1002/ab.10062
Sharpe L. L. (2005a). Play fighting does not affect subsequent fighting success in wild meerkats. Anim. Behav. 69, 1023–1029. doi: 10.1016/j.anbehav.2004.07.013
Sharpe L. L. (2005b). Play does not enhance social cohesion in a cooperative mammal. Anim. Behav. 70, 551–558. doi: 10.1016/j.anbehav.2004.08.025
Sharpe L. L. (2019). “Fun, fur, and future fitness: the evolution of play in mammals,” in The cambridge handbook of play: developmental and disciplinary perspectives. Eds. Smith P. K., Roopnarine J. L. (Cambridge University Press, Cambridge, UK), 49–66.
Shimada M. (2012). “Social object play among juvenile Japanese macaques: comparison between the provisioned Arashiyama–Kyoto troop and the non-provisioned kinkazan troop,” in The monkeys of stormy mountain: 60 years of primatological research on the Japanese macaques of arashiyama. Eds. Leca J.-B., Huffman M. A., Vasey P. L. (Cambridge University Press, Cambridge, UK), 258–283.
Siviy S. M. (2016). A brain motivated to play: insights into the neurobiology of playfulness. Behaviour 153, 819–844. doi: 10.1163/1568539X-00003349
Siviy S. M. (2020). How strain differences could help decipher the neurobiology of mammalian playfulness: what the less playful Fischer344 rat can tell us about play. Int. J. Play 9, 9–24. doi: 10.1080/21594937.2020.1721024
Siviy S. M., Panksepp J. (1987). Sensory modulation of juvenile play in rats. Dev. Psychobiol. 20, 39–55. doi: 10.1002/dev.420200108
Siviy S. M., Panksepp J. (2011). In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci. Biobehav. Rev. 35, 1821–1830. doi: 10.1016/j.neubiorev.2011.03.006
Smith P. K. (1982). Does play matter? Functional and evolutionary play. Behav. Brain Sci. 5, 139–184. doi: 10.1017/S0140525X0001092X
Smith P. K. (1997). “Play fighting and real fighting. Perspectives on their relationship,” in New aspects of human ethology. Eds. Schmitt A., Atzwanger K., Grammar K., Schaüfer K. (Plenum Press, New York, NY), 47–64.
Smith L. K., Fantella S.-L., Pellis S. M. (1999). Playful defensive responses in adult male rats depend upon the status of the unfamiliar opponent. Aggress. Behav. 25, 141–152. doi: 10.1002/(ISSN)1098-2337
Smith P. K., StGeorge J. M. (2022). Play fighting (rough-and-tumble play) in children: developmental and evolutionary perspectives. Int. J. Play. 12, 113–126. doi: 10.1080/21594937.2022.2152185
Stark R., Pellis S. M. (2020). Male Long Evans rats reared with a Fischer 344 peer during the juvenile period show deficits in social competency: A role for play. Int. J. Play 9, 76–91. doi: 10.1080/21594937.2020.1720142
Stark R. A., Ramkumar R., Pellis S. M. (2021). Deficient play-derived experiences in juvenile Long Evans rats reared with a Fischer 344 partner: A deficiency shared by both sexes. Int. J. Comp. Psychol. 34, 1–18. doi: 10.46867/IJCP.2021.34.5592
Suomi S. J. (2005). “Genetic and environmental factors influencing the expression of impulsive aggression and serotonergic functioning in rhesus monkeys,” in Developmental origins of aggression. Eds. Tremblay R. E., Hartup W. W., Archer J. (Guilford Press, New York, NY), 63–82.
Symons D. (1978). “Play and aggression,” in A study of rhesus monkeys (Columbia University Press, New York, NY).
Szalay F. S., Seligsohn D. (1977). Why did the strepsirrhine tooth comb evolve? Folia Primatol 27, 75–82. doi: 10.1159/000155778
Taravosh-Lahn K., Delville Y. (2004). Aggressive behavior in female golden hamsters: Development and the effect of repeated social stress. Horm. Behav. 46, 428–435. doi: 10.1016/j.yhbeh.2004.03.007
Taylor G. T. (1980). Fighting in juvenile rats and the ontogeny of agonistic behavior. J. Comp. Physiol. Psychol. 94, 953–961. doi: 10.1037/h0077816
Thompson K. V. (1996). Play-partner preferences and the function of social play in infant sable antelope, Hippotragus Niger. Anim. Behav. 52, 1143–1155. doi: 10.1006/anbe.1996.0261
Thompson K. V. (1998). “Self-assessment in juvenile play,” in Animal play: evolutionary, comparative, and ecological perspectives. Eds. Bekoff M., Byers J. A. (Cambridge University Press, Cambridge, UK), 183–204.
Thor D. H., Flannelly K. J. (1978). Sex-eliciting behavior of the female rat: discrimination of receptivity by anosmic and intact males. Behav. Biol. 23, 326–340. doi: 10.1016/S0091-6773(78)91351-2
Thor D. H., Holloway J. W.R. (1983). Play solicitation behavior in juvenile male and female rats. Anim. Learn. Behav. 11, 173–178. doi: 10.3758/BF03199645
Thor D. H., Holloway J. W.R. (1984a). Social play in juvenile rats: a decade of methodological and experimental research. Neurosci. Biobehav. Rev. 8, 455–464. doi: 10.1016/0149-7634(84)90004-6
Thor D. H., Holloway J. W.R. (1984b). Developmental analysis of social play behavior in juvenile rats. Bull. Psychon. Soc 22, 587–590. doi: 10.3758/BF03333916
Tinbergen N. (1963). On aims and methods of ethology. Z. Tierpsychol. 20, 410–433. doi: 10.1111/j.1439-0310.1963.tb01161.x
Vanderschuren L. J. M. J., Achterberg E. J. M., Trezza V. (2016). The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 70, 86–105. doi: 10.1016/j.neubiorev.2016.07.025
Vanderschuren L. J. M. J., Niesink R. J. M., van Ree J. M. (1997). The neurobiology of play behavior in rats. Neurosc. Biobehav. Rev. 21, 309–326. doi: 10.1016/S0149-7634(96)00020-6
Vanderschuren L. J. M. J., Trezza V. (2014). What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. Curr. Top. Behav. Neurosci. 16, 189–212. doi: 10.1007/7854_2013_268
VanRyzin J. W., Marquardt A. E., McCarthy M. M. (2020). Developmental origins of sex differences in the neural circuitry of play. Int. J. Play 9, 58–75. doi: 10.1080/21594937.2020.1723370
Vestergaard K., Hogan J. A., Kruijt J. P. (1990). The development of a behavior system: dustbathing in the Burmese red jungle-fowl: I. The influence of the rearing environment on the organization of dustbathing. Behaviour 112, 99–116. doi: 10.1163/156853990X00707
Vick L. G., Conley J. M. (1976). An ethogram for Lemur fulvus. Primates 17, 125–144. doi: 10.1007/BF02382847
von Mohr M., Kirsch L. P., Fotopoulou A. (2017). The soothing function of touch: affective touch reduces feelings of social exclusion. Sci. Rep. 7, 13516. doi: 10.1038/s41598-017-13355-7
Waller B. M., Cherry L. (2012). Facilitating play through communication: significance of teeth exposure in the gorilla play face. Am. J. Primatol. 74, 157–164. doi: 10.1002/ajp.21018
Whishaw I. Q., Burke C. J., Pellis S. M. (2021). Does play shape hand use skill in rats? Exp. Brain Res. 239, 1895–1909. doi: 10.1007/s00221-021-06097-6
Whishaw I. Q., Kolb B. (1985). The mating movements of male decorticate rats: evidence for subcortically generated movements by the male but regulation of approaches by the female. Behav. Brain Res. 17, 171–191. doi: 10.1016/0166-4328(85)90042-7
Whishaw I. Q., Whishaw G. E. (1996). Conspecific aggression influences food carrying: studies on a wild population of rats. Aggress. Behav. 22, 47–66. doi: 10.1002/(ISSN)1098-2337
Williams G. C. (1966). Adaptation and natural selection: A critique of some current evolutionary thought (Princeton, NJ: Princeton University Press).
Wilmer A. H. (1991). Behavioral deficiencies of aggressive 8–9-year-old boys: an observational study. Aggress. Behav. 17, 135–154. doi: 10.1002/1098-2337(1991)17<135::AID-AB2480170303>3.0.CO;2-E
Wilson S. C., Kleiman D. G. (1974). Eliciting play: a comparative study. Am. Zool. 14, 341–370. doi: 10.1093/icb/14.1.341
Winn R. M. (1994). Preliminary study of the sexual behaviour of three aye-ayes (Daubentonia Madagascariensis) in captivity. Folia Primatol. 62, 63–73. doi: 10.1159/000156764
Wolfe J., Mende C., Brecht M. (2011). Social facial touch in rats. Behav. Neurosci. 125, 900–910. doi: 10.1037/a0026165
Wommack J. C., Delville Y. (2007a). Stress, aggression, and puberty: Neuroendocrine correlates of the development of agonistic behavior in golden hamsters. Brain Behav. Evol. 70, 267–273. doi: 10.1159/000105490
Wommack J. C., Delville Y. (2007b). Cortisol controls the pubertal development of agonistic behavior in male golden hamsters via type II corticosteroid receptors. Horm. Behav. 51, 306–312. doi: 10.1016/j.yhbeh.2006.11.007
Keywords: targets, tactics, aggression, sex, affiliation, predation, adaptation, evolutionary by-product
Citation: Pellis SM, Pellis VC and Ham JR (2024) Play fighting revisited: its design features and how they shape our understanding of its mechanisms and functions. Front. Ethol. 3:1362052. doi: 10.3389/fetho.2024.1362052
Received: 27 December 2023; Accepted: 08 April 2024;
Published: 22 April 2024.
Edited by:
Christine Drea, Duke University, United StatesReviewed by:
Michael Potegal, University of Minnesota Twin Cities, United StatesMystera M. Samuelson, University of Nebraska Medical Center, United States
Copyright © 2024 Pellis, Pellis and Ham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sergio M. Pellis, cGVsbGlzQHVsZXRoLmNh
 Sergio M. Pellis
Sergio M. Pellis Vivien C. Pellis
Vivien C. Pellis Jackson R. Ham
Jackson R. Ham