
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Epidemiol. , 26 April 2024
Sec. Infectious Disease Epidemiology
Volume 4 - 2024 | https://doi.org/10.3389/fepid.2024.1375975
This article is part of the Research Topic Insights in Infectious Disease Epidemiology: 2023 View all 4 articles
 Mpho L. Sikhosana1,2*
Mpho L. Sikhosana1,2* Richard Welch3
Richard Welch3 Alfred Musekiwa4
Alfred Musekiwa4 Zinhle Makatini1,2
Zinhle Makatini1,2 Joy Ebonwu3
Joy Ebonwu3 Lucille Blumberg3
Lucille Blumberg3 Waasila Jassat3
Waasila Jassat3
Background: Since there are currently no specific SARS-CoV-2 prognostic viral biomarkers for predicting disease severity, there has been interest in using SARS-CoV-2 polymerase chain reaction (PCR) cycle-threshold (Ct) values to predict disease progression.
Objective: This study assessed the association between in-hospital mortality of hospitalized COVID-19 cases and Ct-values of gene targets specific to SARS-CoV-2.
Methods: Clinical data of hospitalized COVID-19 cases from Gauteng Province from April 2020-July 2022 were obtained from a national surveillance system and linked to laboratory data. The study period was divided into pandemic waves: Asp614Gly/wave1 (7 June–22 Aug 2020); beta/wave2 (15 Nov 2020–6 Feb 2021); delta/wave3 (9 May–18 Sept 2021) and omicron/wave4 (21 Nov 2021–22 Jan 2022). Ct-value data of genes specific to SARS-CoV-2 according to testing platforms (Roche-ORF gene; GeneXpert-N2 gene; Abbott-RdRp gene) were categorized as low (Ct < 20), mid (Ct20–30) or high (Ct > 30).
Results: There were 1205 recorded cases: 836(69.4%; wave1), 122(10.1%;wave2) 21(1.7%; wave3) and 11(0.9%;in wave4). The cases' mean age(±SD) was 49 years(±18), and 662(54.9%) were female. There were 296(24.6%) deaths recorded: 241(81.4%;wave1), 27 (9.1%;wave2), 6 (2%;wave3), and 2 (0.7%;wave4) (p < 0.001). Sample distribution by testing platforms was: Roche 1,033 (85.7%), GeneXpert 169 (14%) and Abbott 3 (0.3%). The median (IQR) Ct-values according to testing platform were: Roche 26 (22–30), GeneXpert 38 (36–40) and Abbott 21 (16–24). After adjusting for sex, age and presence of a comorbidity, the odds of COVID-19 associated death were high amongst patients with Ct values 20–30[adjusted Odds Ratio (aOR) 2.25; 95% CI: 1.60–3.18] and highest amongst cases with Ct-values <20 (aOR 3.18; 95% CI: 1.92–5.27), compared to cases with Ct-values >30.
Conclusion: Although odds of COVID19-related death were high amongst cases with Ct-values <30, Ct values were not comparable across different testing platforms, thus precluding the comparison of SARS-CoV-2 Ct-value results.
Since the COVID-19 pandemic emerged, the diagnosis of SARS-CoV-2 has primarily been based on the detection of SARS-CoV-2 RNA gene sequences using reverse transcription polymerase chain reaction (rRT-PCR) testing (1). rRT-PCR is a molecular diagnostic test that amplifies the virus' nucleic acid through the process of thermocycling (2). The number of amplification cycles required for the fluorescence of a gene target to exceed a pre-determined detection threshold level is called the cycle threshold (Ct) value of that particular gene target. The Ct value correlates inversely to the viral load, such that lower Ct values are associated with higher viral loads (2, 3). At the time that this paper was written, there was no Food and Drug Administration (FDA) approved quantitative assay used for reporting absolute SARS-CoV-2 viral copy numbers (4, 5), nor were there any SARS-CoV-2-specific prognostic viral biomarkers available for predicting severe outcomes amongst cases (6). However, evidence from previous studies have shown that higher initial viral loads of SARS-CoV-1 and influenza have been associated with worsening disease course (6), and this could presumably also apply to SARS-CoV-2.
Although the impact of SARS-CoV-2 rRT-PCR test cycle threshold (Ct) values on adverse outcomes following COVID-19 infection has been investigated, there is currently no consensus regarding the clinical utility of SARS-CoV-2 Ct values. Our study therefore aimed to determine whether there is an association between Ct values of gene targets specific to SARS-CoV-2 and in-hospital COVID-19 related mortality.
This was a sub-study of a parent protocol titled “DATCOV: A surveillance programme for hospitalised and Care Home individuals with COVID-19 in South Africa 2020”. We conducted an anonymised record linkage of SARS-CoV-2 laboratory data from the National Health Laboratory Service's (NHLS) data repository, and clinical data from the South African national hospital surveillance system, DATCOV. DATCOV is an active surveillance system that was established in March 2020 for recording COVID-19 hospitalizations from both public and private healthcare sectors in South Africa (7). Data from both data sources were collected between 01 April 2020–31 Aug 2022 from Gauteng Province (GP), and the only data included in the analyses were for cases whose outcome status was recorded as either “COVID-related death” or “discharged alive”. CDW data were accessed on 02/11/2022 while DATCOV data were accessed on 20/03/2023. The study period was also divided into four pandemic waves as follows: Asp614Gly/wave1 (7 June - 22 Aug 2020); beta/wave2 (15 Nov 2020–6 Feb 2021); delta/wave3 (9 May–18 Sept 2021) and omicron/wave4 (21 Nov 2021–22 Jan 2022).
Clinical data of hospitalized laboratory-confirmed COVID-19 cases from GP with a positive SARS-CoV-2 real-time RT-PCR test or a positive SARS-CoV-2 antigen test were obtained from DATCOV (7). Infection based on a positive SARS-CoV-2 antigen test was subsequently confirmed by molecular testing during hospitalization. A hospital admission was defined according to the definition given by the DATCOV surveillance system, that is, a confirmed hospital stay of ≥1 day regardless of the reason for admission or age (7). All hospitalized SARS-CoV-2 cases from GP reported in the DATCOV surveillance system during the study period, irrespective of age, gender or reason for hospitalization was included in the study. Data recorded in DATCOV included demographic (age, race, sex), exposure, presence of a comorbidity (including diabetes, hypertension, chronic kidney disease, chronic cardiac disease, chronic pulmonary disease or asthma, cancer, tuberculosis or HIV) (8), disease complications, treatment and outcome information (7).
Commercial rRT-PCR assays that were used for SARS-CoV-2 testing during the study period, as well as their respective SARS-CoV-2 specific gene targets were: Roche (ORF gene), GeneXpert (N2 gene) and Abbott (RdRp gene). A laboratory-confirmed SARS-CoV-2 infection was defined as a positive result as per the NHLS virology laboratories' standard operating procedure for SARS-CoV-2 rRT-PCR testing. Only SARS-CoV-2 laboratory results from the NHLS CDW linked to a hospital admission were included in the study. In the case of patients with multiple positive tests during the entire study period, only the Ct value result of the first test conducted immediately before or at hospital admission was included, with all other results belonging to that particular case being removed from the data that would be used in the analyses. SARS-CoV-2 Quality Assurance and SARS-CoV-2 community screening results were excluded. Ct-values were also stratified into three groups: low (Ct < 20); medium (20 ≤ Ct ≤ 30) and high (Ct > 30). These cut-off values were chosen based on cell cultures transfection and viral antigen expression data previously described (6).
Categorical data were expressed as frequencies and percentages, while numerical data were reported as means with their 95% confidence intervals, and results were considered as statistically significant if p was ≤0.05.
Following univariable logistic regression, multivariable logistic regression analysis was used to assess the association between Ct-values and COVID-19-related in-hospital mortality by adjusting for the following predictor variables that have been established as COVID-19 risk factors in the literature: age, sex and the presence of a comorbidity. Results with p values ≤0.05 were considered as statistically significant, and Stata statistical software version 15 was used for data analysis (StataCorp® College Station, Texas, USA).
This study's ethical approval was obtained from the Faculty of Health Sciences Research Ethics Committee of the University of the Witwatersrand (Ethics reference number M2010108), while institutional clearance was obtained from the NHLS Academic Affairs, Research and Quality Assurance. Consent from patients whose data were used in the analysis was waived by the ethics committee because only anonymised data were obtained from the two data sources.
A total of 1205 cases met our study criteria: 836 (69.4%) during wave1; 122 (10.1%) during wave2; 21 (1.7%) during wave3; 11 (0.9%) during wave4. The mean age (±SD) of the cases was 49 years (±18), while 662 (54.9%) of the patients were female. Regarding the cases' clinical outcome, 909 patients were discharged alive, while 296 (24.6%) died, with the distribution of deaths according to pandemic wave being: 241 (81.4%) during wave1; 27 (9.1%) during wave2; 6 (2%) during wave3; 2 (0.7%) during wave4 (p < 0.001). The cases' median (IQR) length of stay was 6 days (2–11). The majority of samples were tested using the Roche testing platform [1,033 (85.7%)], while 169 (14%) samples were tested using the GeneXpert assay and 3 (0.3%) using the Abbott assay. The median (IQR) Ct values according to testing platform were: Roche 26 (22–30); GeneXpert 38 (36–40); Abbott 21 (16–24).
Table 1 shows demographic characteristics of the cases. Some COVID-19 cases were recorded in the CDW database as SARS-CoV-2 positive based on positive Ct-values of non-specific targets according to the NHLS' diagnostic algorithm. In such cases the Ct value of the specific targets would have been recorded as “NULL”, and such cases were removed from the data for the rest of the analysis, the results of which are shown in Table 1. Individuals <20 years had the highest mean Ct-value compared to older cases (p = 0.02), while cases that died had a lower mean Ct-value compared to cases who were discharged alive (p < 0.001).
Table 2 shows results from univariable and multivariable logistic regression analyses.
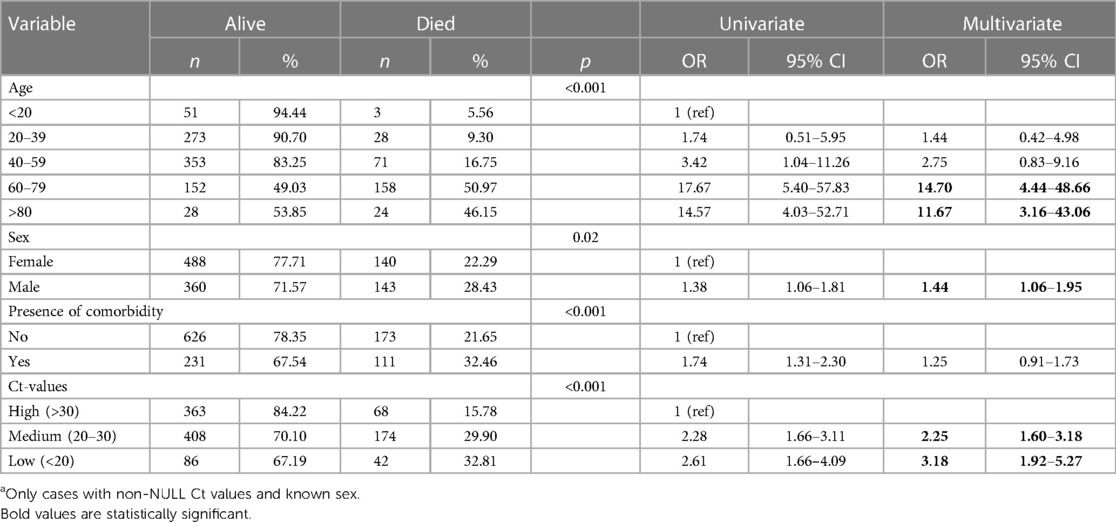
Table 2. Univariate and multivariate analyses of factors associated with in-hospital mortality amongst hospitalized COVID-19 cases, gauteng province, 01 April 2020–31 Aug 2022a.
After adjusting for sex, age and the presence of a comorbidity, factors found to be associated with COVID-19 related mortality were age groups 60–79 years [adjusted odds ratio (aOR): 14.70, 95% CI: 4.44–48.66] and ≥80 years (aOR: 11.67, 95% CI: 3.16–43.06) compared to cases <20 years; being male (aOR: 1.44, 95% CI: 1.06–1.95); as well as Ct values 20–30 (aOR 2.25; 95% CI: 1.60–3.18) and Ct values <20 (aOR 2.25; 95% CI: 1.60–3.18) compared to Ct values >30.
Although some available data have shown that adverse COVID-19 outcomes are associated with lower Ct values (3, 9–13), there is currently no consensus regarding the impact of SARS-CoV-2 Ct values on disease course, prognosis or infectivity, with some studies showing no association between SARS-CoV-2 Ct values and disease severity or mortality (14–16). Also, the use of single positive Ct values in determining the disease stage or prognosis have been cautioned, with multiple, or specifically, serial Ct values suggested to have better clinical utility instead (17). Equally important is the fact that Ct values of different assays are not interchangeable, owing to the inherent assay-specific differences (14). Our study showed that, compared to cases with high Ct values >30, the odds of COVID19-related mortality increased in cases with Ct values ≤30, particularly in cases with Ct values <20.
There is conflicting data regarding the association between SARS-CoV-2 Ct values and age. While there is data showing that older COVID-19 patients had lower Ct values, and therefore higher viral loads by inference, compared to younger cases (18–20), such findings could not be confirmed in other studies (16, 21). Our study showed that the median Ct-values decreased with age, inferring that older patients had higher viral loads compared to those who were younger (p = 0.02).
Although the risk for severe clinical outcomes and mortality is higher amongst males compared to females following SARS-CoV-2 infection (22), our study findings did not show a significant difference between the mean Ct values amongst men and women (p = 0.55), similar to what has been described in other studies (16, 18, 21, 23). This finding may suggest that, apart from biological sex-based differences between men and women, gender-related determinants of health are likely to contribute to COVID-19 related mortality (22).
Our study showed that there is a relationship between COVID19-related mortality and SARS-CoV-2 PCR gene target Ct values <30, with the relationship being the strongest when Ct values were <20. Although lower Ct values are general understood to infer higher viral loads, an inconclusive relationship between COVID-19 viral loads and disease severity has been described (24). One study demonstrated that the association between disease severity and Ct values depends on COVID-19 cases’ overall burden of comorbidities, with disease severity found to be significantly associated with lower Ct values only in cases with a high comorbidity burden (25). Due to a high burden of comorbidities amongst older patients, this would also explain the likelihood of lower Ct values being described amongst older patients. As mentioned earlier, we found that the median Ct values decreased (inferring higher viral loads) as age increased, and this was also in keeping with the odds of COVID19-related in-hospital mortality being higher amongst cases ≥60 years.
The strength of our study was the use of two large and comprehensive datasets, that provided clinical information as well as the outcome status of all of the cases. The study, however, had a few limitations as well. This study used secondary laboratory data and it was therefore not possible to account for any missing information. Also, any pre-, intra- or post-analytical issues that may have had an impact on the results would have affected the Ct vales, and therefore the study's calculated estimates. Our objective was to determine an association, and not causation. The model that we fitted was also neither predictive nor explanatory, and the purpose of including the established COVID-19 risk factors was to adjust for confounding and not for prediction.
In our study population, we found that lower Ct values were associated with higher odds of COVID19-related death. However, due to inherent assay differences resulting in Ct values not being interchangeable across different testing platforms, the use of Ct values in informing decisions about disease severity and prognosis is cautioned.
The data analyzed in this study is subject to the following licenses/restrictions: Only anonymized data were provided to the investigators for analysis. Requests to access these datasets should be directed to National Institute of Communicable Diseases;bHVjaWxsZWJAbmljZC5hYy56YQ==.
The studies involving humans were approved by Faculty of Health Sciences Research Ethics Committee of the University of the Witwatersrand (Ethics reference number M2010108) and institutional clearance was obtained from the NHLS Academic Affairs, Research and Quality Assurance. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Consent from patients whose data were used in the analysis was waived by the ethics committee because only anonymised data were obtained from the two data sources.
MS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. RW: Conceptualization, Formal Analysis, Software, Writing – original draft, Writing – review & editing. AM: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. ZM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. JE: Methodology, Writing – original draft, Writing – review & editing. LB: Methodology, Writing – original draft, Writing – review & editing. WJ: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI-P, et al. Potential rapid diagnostics. Vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J Clin Med. (2020) 9:623. doi: 10.3390/jcm9030623
2. Machado BAS, Hodel KVS, Barbosa-Júnior VG, Soares MBP, Badaró R. The main molecular and serological methods for diagnosing COVID-19: an overview based on the literature. Viruses. (2021) 13:40. doi: 10.3390/v13010040
3. Shenoy S. SARS-CoV-2 (COVID-19). viral load and clinical outcomes; lessons learned one year into the pandemic: a systematic review. World J Crit Care Med. (2021) 10(4):132–50. doi: 10.5492/wjccm.v10.i4.132
4. Poon KS, Wen-Sim Tee N. Caveats of reporting cycle threshold values from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) qualitative polymerase chain reaction assays: a molecular diagnostic laboratory perspective. Clin Infect Dis. (2021) 73(9):e2851–2. doi: 10.1093/cid/ciaa1399
5. Food and Drug Administration. Genetic Variants of SARS-CoV-2 May Lead to False Negative Results with Molecular Tests for Detection of SARS-CoV-2 - Letter to Clinical Laboratory Staff and Health Care Providers. Available online at: https://www.fda.gov/medical-devices/letters-health-care-providers/genetic-variants-sars-cov-2-may-lead-false-negative-results-molecular-tests-detection-sars-cov-2 (accessed Novemeber 20, 2021).
6. Trunfio M, Venuti F, Alladio F, Longo BM, Burdino E, Cerutti F, et al. Diagnostic SARS-CoV-2 cycle threshold value predicts disease severity. Survival and six-month sequelae in COVID-19 symptomatic patients. Viruses. (2021) 13(2):281. doi: 10.3390/v13020281
7. Jassat W, Mudara C, Ozougwu L, Tempia S, Blumberg L, Davies MA, et al. Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: a cohort study. Lancet Glob Health. (2021) 9(9):e1216–25. doi: 10.1016/S2214-109X(21)00289-8
8. Jassat W, Abdool Karim SS, Mudara C, Welch R, Ozougwu L, Groome MJ, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Health. (2022) 10(7):e961–9. doi: 10.1016/S2214-109X(22)00114-0
9. Wright J, Achana F, Diwakar L, Semple MG, Carroll WD, Baillie K, et al. Cycle threshold values are inversely associated with poorer outcomes in hospitalized patients with COVID-19: a prospective. Observational cohort study conducted at a UK tertiary hospital. Int J Infect Dis. (2021) 111:333–5. doi: 10.1016/j.ijid.2021.08.022
10. Waudby-West R, Parcell BJ, Palmer CNA, Bell S, Chalmers JD, Siddiqui MK. The association between SARS-CoV-2 RT-PCR cycle threshold and mortality in a community cohort. Eur Respir J. (2021) 58(1):2100360. doi: 10.1183/13993003.00360-2021
11. Rao SN, Manissero D, Steele VR, Pareja J. A narrative systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect Dis Ther. (2020) 9:573–86. doi: 10.1007/s40121-020-00324-3
12. Shah VP, Farah WH, Hill JC, Hassett LC, Binnicker MJ, Yao JD, et al. Association between SARS-CoV-2 cycle threshold values and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. Open Forum Infect Dis. (2021) 8(9):ofab453. doi: 10.1093/ofid/ofab453
13. Choudhuri J, Carter J, Nelson R, Skalina K, Osterbur-Badhey M, Johnston A, et al. SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality. PLoS One. (2020) 15(12):e0244777. doi: 10.1371/journal.pone.0244777
14. Binnicker MJ. Can the severe acute respiratory syndrome coronavirus 2 polymerase chain reaction cycle threshold value and time from symptom onset to testing predict infectivity? Clin Infect Dis. (2020) 71(10):2667–8. doi: 10.1093/cid/ciaa735
15. Camargo JF, Lin RY, Komanduri KV. Lack of correlation between the SARS-CoV-2 cycle threshold (ct) value and clinical outcomes in patients with COVID-19. J Med Virol. (2021) 93(10):6059–62. doi: 10.1002/jmv.27171
16. Penney J, Jung A, Koethe B, Doron S. Cycle threshold values and SARS-CoV-2: relationship to demographic characteristics and disease severity. J Med Virol. (2022) 94(8):3978–81. doi: 10.1002/jmv.27752
17. Public Health England. Understanding cycle threshold (Ct) in SARS-CoV-2 RT-PCR (publishing.service.gov.uk). Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926410/Understanding_Cycle_Threshold__Ct__in_SARS-CoV-2_RT-PCR_.pdf (accessed October 12, 2022).
18. Maltezou HC, Raftopoulos V, Vorou R, Papadima K, Mellou K, Spanakis N, et al. Association between upper respiratory tract viral load, comorbidities, disease severity, and outcome of patients with SARS-CoV-2 infection. J Infect Dis. (2021) 223(7):1132–8. doi: 10.1093/infdis/jiaa804
19. Magleby R, Westblade LF, Trzebucki A, Simon MS, Rajan M, Park J, et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis. (2021) 73(11):e4197–205. doi: 10.1093/cid/ciaa851
20. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. (2020) 20(5):565–74. doi: 10.1016/S1473-3099(20)30196-1
21. Salvatore PP, Dawson P, Wadhwa A, Rabold EM, Buono S, Dietrich EA, et al. Epidemiological correlates of polymerase chain reaction cycle threshold values in the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. (2021) 72(11):e761–7. doi: 10.1093/cid/ciaa1469
22. Alwani M, Yassin A, Al-Zoubi RM, Aboumarzouk OM, Nettleship J, Kelly D, et al. Sex-based differences in severity and mortality in COVID-19. Rev Med Virol. (2021) 31(6):e2223. doi: 10.1002/rmv.2223
23. Kleiboeker S, Cowden S, Grantham J, Nutt J, Tyler A, Berg A, et al. SARS-CoV-2 viral load assessment in respiratory samples. J Clin Virol. (2020) 129:104439. doi: 10.1016/j.jcv.2020.104439
24. Dadras O, Afsahi AM, Pashaei Z, Mojdeganlou H, Karimi A, Habibi P, et al. The relationship between COVID-19 viral load and disease severity: a systematic review. lmmunity Inflamm Dis. (2022) 10(3):e580. doi: 10.1002/iid3.580
Keywords: SARS-CoV-2, Ct values, mortality, South Africa, PCR
Citation: Sikhosana ML, Welch R, Musekiwa A, Makatini Z, Ebonwu J, Blumberg L and Jassat W (2024) Association between SARS-CoV-2 gene specific Ct values and COVID-19 associated in-hospital mortality. Front. Epidemiol. 4:1375975. doi: 10.3389/fepid.2024.1375975
Received: 24 January 2024; Accepted: 8 April 2024;
Published: 26 April 2024.
Edited by:
Shailendra Saxena, King George’s Medical University, IndiaReviewed by:
Nicole Rübsamen, University of Münster, Germany© 2024 Sikhosana, Welch, Musekiwa, Makatini, Ebonwu, Blumberg and Jassat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mpho L. Sikhosana bHNpa2hvc2FuYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.