
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Epidemiol., 16 July 2024
Sec. Infectious Disease Epidemiology
Volume 4 - 2024 | https://doi.org/10.3389/fepid.2024.1365090
 M. Abdelmasseh1,2
M. Abdelmasseh1,2 A. Cuaranta1,2
A. Cuaranta1,2 A. Iqbal1,2
A. Iqbal1,2 V. Kadiyala3
V. Kadiyala3 J. Willis3
J. Willis3 A. Gorka3
A. Gorka3 E. Thompson1,2
E. Thompson1,2 R. Finley1,2
R. Finley1,2 B. Payne1,2
B. Payne1,2 J. Sanabria1,2*
J. Sanabria1,2*
Introduction: Although Coronavirus disease 2019 (COVID-19) vaccination is critical to control its spread, vaccine hesitancy varies significantly among the United States population; moreover, some vaccine recipients experienced various adverse effects. We aim to assess the impact of COVID-19 vaccine hesitancy in a university-affiliated community, the factors affecting participants’ decisions, and their adverse effects.
Methods: A pre-vaccination online Institutional Review Board IRB-approved survey was emailed in Nov/Dec 2020, 2 months before the implementation of state-policy protocols for COVID-19 vaccination. A post-vaccination survey was emailed in May/June 2021, two months after protocol execution. A third follow-up survey was sent in Nov/Dec 2021, and a fourth was sent in June/July 2022. The study population included three groups of adult participants: university students, faculty, and staff-(MS), university health system patients-(MP), and Cancer Center patients-(MCP). The study was designed as a longitudinal cohort study. Statistical analyses were performed using SPSS.
Results: With a combined response rate of 26% (40,578/157,292) among the four surveys, 15,361 participants completed the first survey (MS = 4,983, MP = 9,551, and MCP = 827). 2/3 of participants (63.5%) were willing to get vaccinated, with a significant difference in acceptance among groups, MS:56.6%, MP:66.2%, and MCP:71.6% (p < 0.05). Vaccine acceptance rates reached 89% in the second survey after the vaccine's approval, with a lower acceptance rate of MS:84.6% than with MP:90.74% and MCP:92.47% participants (p < 0.05). Safety and effectiveness concerns were the main factors affecting participants’ decisions in all the first three surveys; however, participants reported these concerns decreased between pre-vaccination, post-vaccination, and follow-up surveys with 87%, 56%, and 46%, respectively(p < 0.05). More than two-thirds of the participants (70%) reported having either minor/moderate symptoms (61.6%) or major symptoms (8.6%) after getting some of the vaccine doses (p < 0.05).
Conclusion: The hesitance of COVID-19 vaccination was associated with concerns regarding its safety and efficacy. Vaccine acceptance rose higher than expected after protocol execution, likely due to continuous education, whereas safety and efficacy remain factors hindering vaccine acceptance. Continuous education focusing on safety and efficacy of the vaccine can reduce vaccine hesitancy and raise the rates of vaccination.
The COVID-19 (Coronavirus disease 2019) pandemic has impacted nearly every country, infected around 775 million people, and has surpassed 7 million deaths worldwide (1). The Global and United States (US) economies have been heavily affected by a viral disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-COV2), overwhelming healthcare facilities (2, 3). Despite the Centers for Disease Control and Prevention (CDC) guidelines from medical experts and healthcare advisors, the preventive measures of wearing face masks, washing hands frequently, and keeping social distancing (4) have not rescinded the spread of SARS-COV2 throughout the world. With the emergence of new and more contagious variants such as Delta and Omicron, the spread of infection was reaching unprecedented rates worldwide (5–9). The need to raise the vaccination acceptance rates is always important to control infections and relieve the pandemic's burden.
From the initial reported infection of COVID-19 in early December 2019, it took a full year for the world to develop the first vaccine, which was achieved in December 2020 (10). The United Kingdom was the first country to grant emergency authorization for the public administration of the mRNA-based vaccine, followed by the United States (11, 12). The United States Food and Drug Administration (US FDA) authorized the Pfizer-BioNTech COVID-19 vaccine for children aged 12–15 by May 2021. In addition, the US FDA gave the vaccine full authorization in August 2021 (13). Moreover, in October 2021, the US FDA approved vaccinations for children five years or older (14). More than 14 billion doses of the vaccine were administered worldwide; however, only 70.6% of the world population has received at least one dose of a COVID-19 vaccine. In the US, more than 712 million doses were administered, and nearly 70% of the population were vaccinated with a complete primary series of COVID-19 vaccination (10, 15). Governments and industries have come together to overcome the unprecedented challenges of massive distribution logistics. Nevertheless, health policymakers, scientists, and providers are still facing an uphill battle with strategies to increase acceptance of the COVID-19 vaccine, mainly when some vaccine recipients reported experiencing adverse effects post-vaccination. However, adverse event rates were reported more in their phase 3 trials (16), increasing the challenges that improving vaccination rates may face.
Since the conception of the world's first vaccine for smallpox in 1798 (17, 18), an evolution in the movement of acceptance vs. hesitancy has grown based on vaccine safety and efficacy and its inherent adverse effects (19, 20). In 2015, a group of experts from the World Health Organization (WHO) defined “vaccine hesitancy” as the delay in acceptance or refusal of vaccination despite vaccination services’ availability (21). In 2019, vaccine hesitancy was ranked as one of the top ten threats to global health (22). Vaccine hesitancy and uncertainty have become significant hurdles for reaching desirable societal immunity against targeted diseases in many countries (23, 24). In cooperation with policymakers and other stakeholders, governments and health societies are required to improve the process of providing the necessary information about the vaccine and enhance the understanding and importance of immunization to reach the immunity of the community. The present study aimed to assess the COVID-19 vaccination acceptance and hesitance rates and the factors impacting people's decisions regarding vaccination, besides evaluating the adverse effects participants might have experienced post-vaccination.
The study was designed as a longitudinal cohort study over two years. A pre-vaccination online survey was developed by (Qualtrics® Services), linked to the participants’ emails, and sent upon Institutional Review Board (IRB) approval. The survey was emailed (Supplementary Appendix 1) in Nov/Dec 2020, 2-months before state-policy protocols implementation for COVID-19 vaccine administration. It intended to determine the participants’ acceptance of vaccination. A second post-vaccination survey was emailed (Supplementary Appendix 2) in May/June 2021, 2-months after protocols were executed. The post-vaccination survey was sent weekly with a one-month enrollment period to determine the actual vaccination rates. A third follow-up survey was emailed (Supplementary Appendix 3) in Nov/Dec 2021, 5-months after the second survey. It aimed to determine the reasons affecting participants’ decisions on whether they changed their minds before and after vaccine approval. A fourth and final survey (Supplementary Appendix 4) was sent in June/July 2022 to define adverse events participants may have experienced.
Individual emails were gathered from a central University and Health System warehouse and divided into three groups: (i) University faculty, students, and staff (University Students and Staff/MS); (ii) University School of Medicine affiliated Health System registered patients (General Patients/MP), and (iii) University School of Medicine affiliated Comprehensive Cancer Center registered cancer patients (Cancer Patients/MCP). An individual survey was sent weekly for three weeks with a one-month enrollment period. The software identified duplicates in each survey, which were deleted so that one individual had only one response to each of the four surveys.
The surveys were anonymous to the study team but had a unique IP address. The number of questions ranged from six questions in the first survey, seven to nine questions in the second, eight to ten in the third survey, and fourteen to twenty-three in the fourth (see Supplementary Appendix 1–S4). Each survey contained demographic questions about gender/sex, ethnicity/race, education level, and age category. Tacit consent was given by moving forward from the introductory survey page.
All variables were categorical and described as frequencies and percentages. For predictor variables, we generally used gender, age category, ethnicity, and education and occasionally included trusted sources of information or vaccine type. Outcome variables varied for each survey (i.e., Survey 1—the outcome is whether the respondent will get vaccinated, Survey 2—the outcome is whether the respondent received at least one dose of a vaccine, Survey 3—the outcome is whether the respondent changed their mind about getting vaccinated, Survey 4—the outcome is whether the respondent experienced side effects/symptoms following any of the vaccine doses they received).
Univariate and Multivariate analyses were performed using ordinal and binomial logistic regressions. Area under the curve (AUC) analyses of receiver operating characteristic (ROC) curves were examined for the logistic regression models to determine prediction accuracy. Results were expressed in terms of probability (p < 0.05) and odds ratio estimates with 95% confidence intervals. For logistic regressions, the p-value measures the statistical significance of the estimated coefficients for the predictor variables and tests the null hypothesis that there is no relationship between that predictor and the outcome variables (i.e., the parameter coefficient is equal to 0). Statistical analyses were performed using SPSS version 28 (SPSS-IBM Inc., Chicago, IL, USA).
Out of the 157,292 surveys distributed to all participants, the response rate exhibited considerable variations across the four surveys. The first survey achieved a response rate of 10% (n = 15,361), the second survey 7.3% (n = 11,539), and both the third and fourth surveys 4.3% (n = 6,902 and n = 6,776, respectively). Notably, significant differences in response rates among participant groups were observed for each survey (Supplementary Table S1). While the response rate was higher in the MS group (27.4% and 10.5% for the first and fourth surveys, respectively), the MP group showed lower response rates (7.5% and 2.7%). As expected, participants’ demographics differed among groups and by survey (Table 1). The MS group was younger when compared to the MP and the MCP groups (p < 0.05). The education degree was lower in the MS group compared to the MP and MCP groups (p < 0.05). In contrast, the sex distribution at a ratio of 3♀:2♂ among groups was similar for the four surveys. Most of the responders were white, with some diversity in the MS group (p < 0.05).
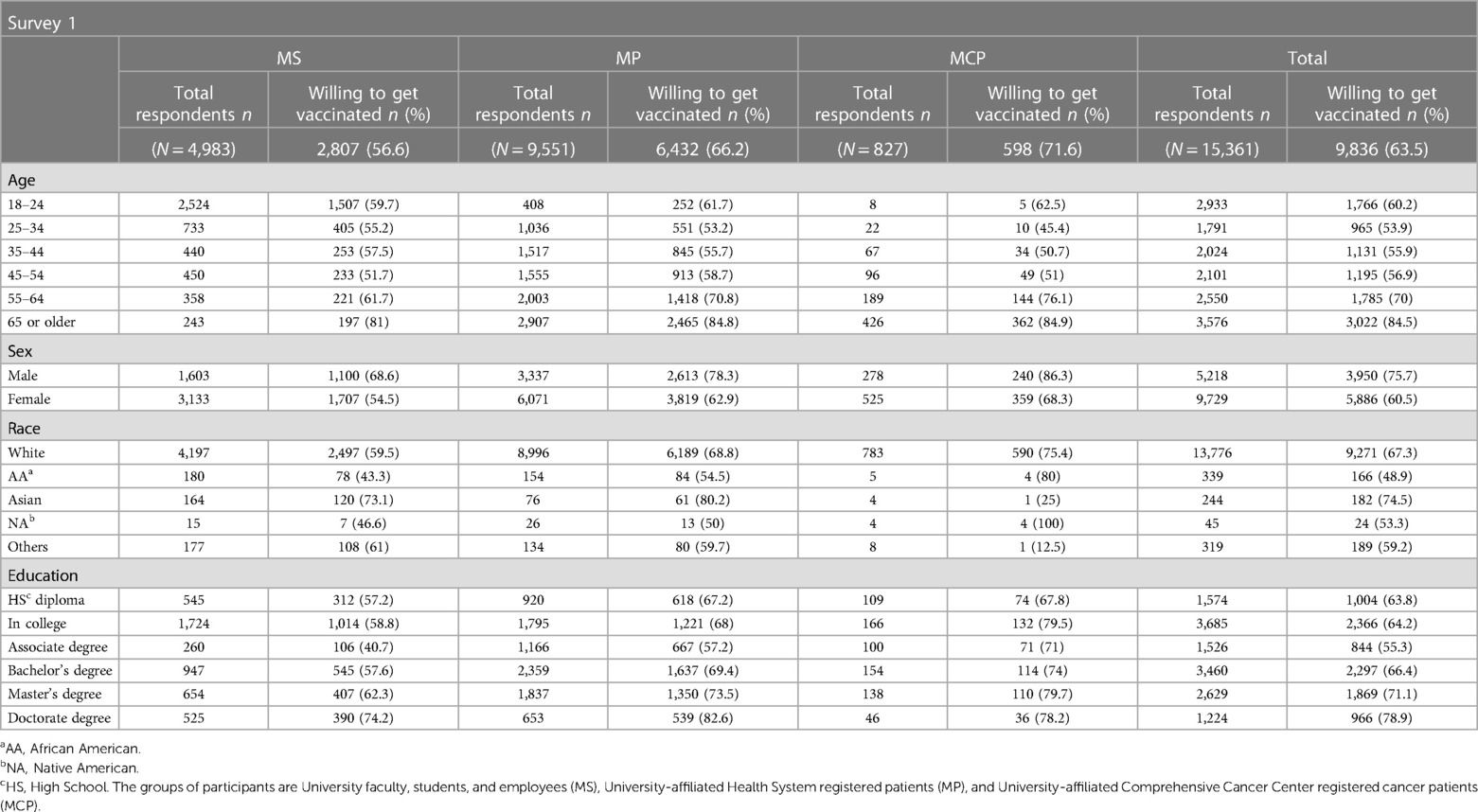
Table 1 Participants’ demographic distribution from survey 1(Pre-vaccination), Survey 2 (post-vaccination), Survey 3 (follow-up), and Survey 4 (Side effects).
The participant proportion willing to get vaccinated upon the vaccine's approval was 63.5%, ranging from 56.6% in the MS group to 66.2% and 71.6% in the MP and MCP groups, respectively (MS vs. MP and MCP, p < 0.05). Vaccine acceptance rates for each group were higher, reaching 88.6% (85%, 90.3%%, and 92.5% for MS, MP, and MCP groups, respectively) after the vaccine's approval by the FDA. The follow-up survey (survey 3) indicated that 17.2% of the participants accepted vaccination after initially refusing it. Participants reported health workers, family, and friends as the main trusted sources influencing their decisions (Supplementary Figure S1). Safety and efficacy concerns were the main factors affecting participants’ decisions in all three surveys: pre-vaccination (survey 1, 87%), post-vaccination (survey 2, 56%), and follow-up (survey 3, 46%, Figure 1, p < 0.05).
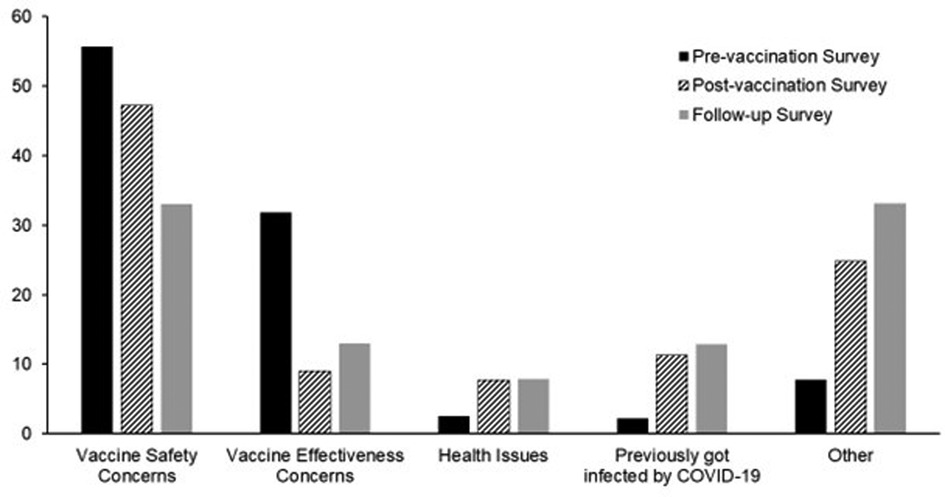
Figure 1 Factors affecting COVID-19 vaccination hesitance in survey 1 (Pre-vaccination), survey 2 (post-vaccination), and survey 3 (follow-up). Safety and efficacy concerns were the main factors affecting participants’ decisions in all three surveys: pre-vaccination (87%), post-vaccination (56%), and follow-up surveys (46%, p < 0.05).
Most participants (70%) reported an adverse event from vaccination, and one out of 10 sought medical evaluation. Minor/moderate symptoms (61.6%) consisted of tiredness/fatigue and pain at the site of injection, observed more frequently after the second dose (36.1% and 28.8% for the tiredness/fatigue and pain at the site of injection, respectively). Major symptoms (8.6%) included anxiety and high-grade fever, which were the most reported after vaccination, mainly also after the second dose (3.9% and 3.7% for anxiety and high-grade fever, respectively, Table 2).
Univariate and multivariate analyses were performed to determine the factors associated with vaccine hesitance before and after vaccination (Figure 2). On multivariate analyses, age (>44 years old), race (white), gender, and education (bachelor's degree or higher) were factors that statistically held a significant association with participants’ vaccine acceptance prior to vaccination (p < 0.05). After vaccination, age (> 34 years old) and level of education (bachelor's degree or higher) remained significant (p < 0.01). In contrast, age and trusted source were the only factors significantly associated with the change of mind for vaccine hesitance (p < 0.05). Both univariate and multivariate analyses showed that age, gender, level of education, and the type of the vaccine were statistically significant in predicting the adverse events that the participants experienced after any dose (Supplementary Table S2, p < 0.01).
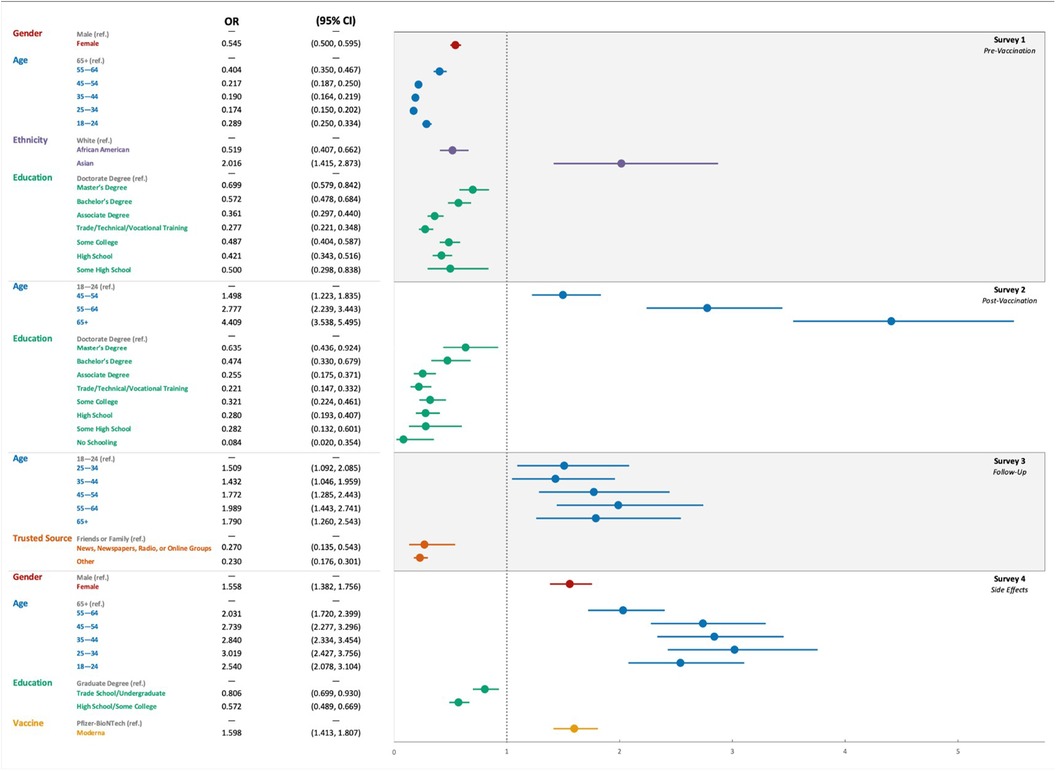
Figure 2 The odds ratio using multivariate analyses for the variables affecting the COVID-19 vaccination hesitance and side effects in the four consecutive surveys: survey 1 (Pre-vaccination), Survey 2 (post-vaccination), Survey 3 (follow-up), and Survey 4 (adverse events).
The need for an effective and safe vaccine to relieve the pandemic's burden of COVID-19 infection has proven to be persistent, especially with frequent virus mutations. The present study focuses on the status of vaccine acceptance among US adults, factors associated with hesitancy and their decisions, and vaccine adverse events. To the best of our knowledge, this study represents one of the largest of its kind to assess COVID-19 vaccination hesitancy. Overall, two-thirds of the study population were willing to get vaccinated, and their main concerns were the safety and efficacy of the vaccine; the percentage increased from 64% to 89% after the vaccine's approval. Moreover, around 17% of the participants eventually changed their minds and accepted the vaccines. Nevertheless, 70% of the participants reported, mainly after the second dose, either minor to moderate symptoms (61.6%) or major symptoms (8.6%) after getting vaccinated.
Prior studies showed the COVID-19 vaccine hesitance as a real impediment to achieving the immunity required to reach herd immunity (25–27). A global survey from 19 countries (n = 13,426) showed a pre-vaccination acceptance rate of 71.5% (28). Nonetheless, the study was skewed from outlier higher rates from two Asian countries (in the range of 85%), which also recorded very high trust in government health recommendations. A study of American US adults (n = 1,056) showed a lower acceptance rate (49%) (29). The hesitance to vaccination varied significantly depending on the other populations surveyed (50%–73%) (30–32).
Concerns with the vaccines pre- and post-approval were similar: safety and efficacy. Although the safety and efficacy profiles were proved to be favorable for most of the vaccines (33–35) and against recent variants (36, 37), adverse events varied in rate and severity among different populations (38–40). Most of the adverse events reported were mild to moderate (the most common were pain at the injection site, fatigue, and headache) (41, 42). Our study concurred with the published literature. Previous studies also showed that healthcare workers were the most trusted source of information regarding COVID-19 vaccines. People tend to trust their healthcare providers rather than the media. Our study proved similar results where nearly half of the participants reported trusting the health workers as their primary source of information.
Although studies have shown a solid scientific base for the needed increase in vaccination rate, substantial efforts are essential by governments and public health officials to enhance vaccine acceptance. Approaching some of the factors that play into the individual decision may pave the way for a paucity of vaccine hesitance. The present study found a significant association between COVID-19 vaccine acceptance and age, gender, race, and education level. While age, gender, and race were variables with no room for modification, general education on public media may dissipate community concerns about the safety and efficacy of the COVID-19 vaccine. Processes and results shown in an easily interpretable and transparent manner may go a long way in trust. A clear and logical approach to actual, theoretical, and fictional implications of an RNA-based vaccine will mitigate differences between those who accept and those who refuse vaccination, avoiding confusion from contradictory facts, especially in the presence of an intense media-originated by anti-vaccination activists (43).
The present study represents the largest in the US to assess COVID-19 vaccination acceptance, with a combined 40,578 responses. Even though there was a low response rate, the study had an adequate sample size; however, race predominance is a limitation to the interpretation and extrapolation of the findings. Moreover, our surveys were sent electronically through email to the participants, which excluded those who didn't have access to emails. In addition, self-reported data are subject to a potential lack of validity and reliability as they may be affected by different biases.
The present study is intended to provide a comprehensive image of the COVID-19 vaccines, assess their acceptance, identify factors that are associated with the individual decisions in the US, and assess their safety outcomes. A low vaccine acceptance rate was associated with a high degree of concern (89%) regarding its efficacy and safety before the vaccines’ approval. Nevertheless, vaccine acceptance rose higher than expected after protocol execution, likely due to continuous education. Safety and efficacy remain factors hindering vaccine acceptance. Most of the reported vaccine adverse events were mild to moderate, with minimal need for medical consulting. Continuous education concerning the importance of vaccination, along with discussing and proving the vaccine's safety and efficacy, can be the main tools to decrease the rates of vaccine hesitancy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Marshall University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Tacit consent was given online by moving forward from the introductory survey page.
MA: Visualization, Validation, Software, Resources, Project administration, Methodology, Formal Analysis, Data curation, Writing – review & editing, Writing – original draft, Investigation, Conceptualization. AC: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Data curation. AI: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Conceptualization. VK: Writing – review & editing, Writing – original draft, Validation, Resources, Methodology, Data curation, Conceptualization. JW: Writing – review & editing, Writing – original draft, Validation, Resources, Methodology, Investigation, Data curation, Conceptualization. AG: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal Analysis, Data curation. ET: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation, Conceptualization. RF: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation, Conceptualization. BP: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation, Conceptualization. JS: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2024.1365090/full#supplementary-material
COVID-19, Coronavirus disease 2019; SARS-COV2, severe acute respiratory syndrome coronavirus 2; CDC, Centers for Disease Control and Prevention; RNA, Ribonucleic acid; mRNA, Messenger Ribonucleic acid; WHO, World Health Organization; IRB, Institutional Review Board; MS, University faculty, students, and staff study group; MP, University School of Medicine affiliated Health System registered patients study group; MCP, University School of Medicine affiliated Comprehensive Cancer Center registered cancer patients study group; FDA, US Food and Drug Administration.
1. Center. JHCR. COVID-19 dashboard (2020) Available online at: https://coronavirus.jhu.edu/map.html (Accessed January 2, 2024).
2. Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. (2020) 78:185–93. doi: 10.1016/j.ijsu.2020.04.018
3. Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. (2020) 8(5):506–17. doi: 10.1016/S2213-2600(20)30161-2
4. Brooks JT, Butler JC, Redfield RR. Universal masking to prevent SARS-CoV-2 transmission—the time is now. JAMA. (2020) 324(7):635–7. doi: 10.1001/jama.2020.13107
6. National Center for I, Respiratory Diseases DoVD. Science Brief: Omicron (B.1.1.529) Variant. Atlanta, GA: CDC COVID-19 Science Briefs. Centers for Disease Control and Prevention (US) Unless a copyright is indicated, information on CDC’s sites, blogs, and applications is in the public domain and may be copied and distributed without permission. (2020).
7. Kandeel M, Mohamed MEM, Abd El-Lateef HM, Venugopala KN, El-Beltagi HS. Omicron variant genome evolution and phylogenetics. J Med Virol. (2021) 94(4):1627–32. doi: 10.1002/jmv.27515
8. Reardon S. How the Delta variant achieves its ultrafast spread. Nature. (2021). doi: 10.1038/d41586-021-01986-w
9. Novelli G, Colona VL, Pandolfi PP. A focus on the spread of the delta variant of SARS-CoV-2 in India. Indian J Med Res. (2021) 153(5&6):537–41. doi: 10.4103/ijmr.ijmr_1353_21
10. World Health Organization (2023). data.who.int, WHO Coronavirus (COVID-19) dashboard > Vaccines [Dashboard]. Available online at: https://data.who.int/dashboards/covid19/vaccines (Accessed January 2, 2024).
11. PFIZER AND BIONTECH CONCLUDE PHASE 3 STUDY OF COVID-19 VACCINE CANDIDATE, MEETING ALL PRIMARY EFFICACY ENDPOINTS [press release]. 11/18/2020. (2020).
12. Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization [press release]. 11/30/2020 (2020).
13. Release FN. FDA Approves First COVID-19 Vaccine. Silver Spring, MD: The U.S. Food & Drug Administration (FDA) (2021).
14. Food and Drug Administration SS, MD. Comirnaty and Pfizer–BioNTech COVID-19 Vaccine. Silver Spring, MD: The U.S. Food & Drug Administration (FDA) (2021) [updated October 202110/4/2022]. Available online at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine
15. Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. Coronavirus Pandemic (COVID-19). Oxford, England: Global Change Data Lab (2020). Published online at: OurWorldInData.org Retrieved from: https://ourworldindata.org/coronavirus
16. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. (2021) 21(7):939–49. doi: 10.1016/S1473-3099(21)00224-3
17. Baxby D. Jenner’s Smallpox Vaccine: The Riddle of Vaccinia Virus and its Origin. Cambridge, England: Cambridge University Press (1981).
18. Baxby D. Smallpox vaccine: ahead of its time. Interdiscip Sci Rev. (2001) 26(2):125–38. doi: 10.1179/isr.2001.26.2.125
19. Karafillakis E, Larson HJ. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. (2017) 35(37):4840–50. doi: 10.1016/j.vaccine.2017.07.061
20. Munoz DC, Llamas LM, Bosch-Capblanch X. Exposing concerns about vaccination in low-and middle-income countries: a systematic review. Int J Public Health. (2015) 60(7):767–80. doi: 10.1007/s00038-015-0715-6
21. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. (2015) 33(34):4161–4. doi: 10.1016/j.vaccine.2015.04.036
22. Akbar WR. Ten Threats to Global Health in 2019. Geneva, Switzerland: World Health Organization (WHO) (2019). Available online at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
23. Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. (2014) 32(19):2150–9. doi: 10.1016/j.vaccine.2014.01.081
24. Lane S, MacDonald NE, Marti M, Dumolard L. Vaccine hesitancy around the globe: analysis of three years of WHO/UNICEF joint reporting form data-2015–2017. Vaccine. (2018) 36(26):3861–7. doi: 10.1016/j.vaccine.2018.03.063
25. Gypsyamber D’Souza DD. Rethinking Herd Immunity and the Covid-19 Response End Game. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health (2021).
26. Amer SA, Shah J, Abd-Ellatif EE, El Maghawry HA. COVID-19 vaccine uptake among physicians during the second wave of COVID-19 pandemic: attitude, intentions, and determinants: a cross-sectional study. Front Public Health. (2022) 10:823217. doi: 10.3389/fpubh.2022.823217
27. Ali HT, Ashour Y, Rais MA, Barakat M, Rezeq TA, Sharkawy MM, et al. Unravelling COVID-19 vaccination attributes worldwide: an extensive review regarding uptake, hesitancy, and future implication. Ann Med Surg (Lond). (2023) 85(7):3519–30. doi: 10.1097/MS9.0000000000000921
28. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2020) 27:225–8. doi: 10.1038/s41591-020-1124-9
29. Chodosh S. Why only half of Americans say they would get a COVID-19 vaccine. Popular Science. (2020) Available online at: https://www.popsci.com/story/health/covid-19-vaccine-poll/ (Accessed January 2, 2024).
30. Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. (2020) 26:100495. doi: 10.1016/j.eclinm.2020.100495
31. Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. (2020) 38(42):6500–7. doi: 10.1016/j.vaccine.2020.08.043
32. Kreps S, Prasad S, Brownstein JS, Hswen Y, Garibaldi BT, Zhang B, et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Netw Open. (2020) 3(10):e2025594. doi: 10.1001/jamanetworkopen.2020.25594
33. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389
34. Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years of age. N Engl J Med. (2022) 387(6):525–32. doi: 10.1056/NEJMoa2203209
35. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. (2021) 385(12):1078–90. doi: 10.1056/NEJMoa2110475
36. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. (2021) 385(7):585–94. doi: 10.1056/NEJMoa2108891
37. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Amir O, Freedman L, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. (2022) 386(18):1712–20. doi: 10.1056/NEJMoa2201570
38. Hatmal MM, Al-Hatamleh MAI, Olaimat AN, Mohamud R, Fawaz M, Kateeb ET, et al. Reported adverse effects and attitudes among arab populations following COVID-19 vaccination: a large-scale multinational study implementing machine learning tools in predicting post-vaccination adverse effects based on predisposing factors. Vaccines (Basel). (2022) 10(3):366. doi: 10.3390/vaccines10030366
39. Camacho Moll ME, Salinas Martínez AM, Tovar Cisneros B, García Onofre JI, Navarrete Floriano G, Bermúdez de León M. Extension and severity of self-reported Side effects of seven COVID-19 vaccines in Mexican population. Front Public Health. (2022) 10:834744. doi: 10.3389/fpubh.2022.834744
40. Amer SA, Al-Zahrani A, Imam EA, Ishteiwy EM, Djelleb IF, Abdullh LR, et al. Exploring the reported adverse effects of COVID-19 vaccines among vaccinated arab populations: a multi-national survey study. Sci Rep. (2024) 14(1):4785. doi: 10.1038/s41598-024-54886-0
41. Guerra-Estévez D, Palomo-Palomo C, Parrado-González A, Estaire-Gutiérrez J, Reyes-Malia M, Romero-Alonso MM. Self-reported adverse events within the seven days following the spikevax® (moderna) vaccination. Farm Hosp. (2022) 46(5):301–7. doi: 10.7399/fh.13245
42. Sánchez-Saez F, Peiró S, Cuenca L, Vanaclocha H, Limón R, Salas D, et al. Side effects during the week after first dose vaccination with four COVID-19 vaccines. Results of the ProVaVac survey study with 13,837 people in Spain. Vaccine. (2022) 40(41):5942–9. doi: 10.1016/j.vaccine.2022.08.028
Keywords: COVID-19, SARS-COV2, vaccine, survey, hesitancy, pandemic, adverse events
Citation: Abdelmasseh M, Cuaranta A, Iqbal A, Kadiyala V, Willis J, Gorka A, Thompson E, Finley R, Payne B and Sanabria J (2024) COVID-19 vaccination hesitance and adverse effects among US adults: a longitudinal cohort study. Front. Epidemiol. 4: 1365090. doi: 10.3389/fepid.2024.1365090
Received: 3 January 2024; Accepted: 1 July 2024;
Published: 16 July 2024.
Edited by:
Mairaj Ahmed Ansari, Jamia Hamdard University, IndiaReviewed by:
Alberto Firenze, University of Palermo, Italy© 2024 Abdelmasseh, Cuaranta, Iqbal, Kadiyala, Willis, Gorka, Thompson, Finley, Payne and Sanabria. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. Sanabria, c2FuYWJyaWFqQG1hcnNoYWxsLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.