- 1School of Nursing, University of Louisville, Louisville, KY, United States
- 2Division of Infectious Diseases, Centre of Excellence for Research in Infectious Diseases (CERID), University of Louisville, Louisville, KY, United States
- 3Department of Bioinformatics and Biostatistics, School of Public Health and Information Sciences, University of Louisville, Louisville, KY, United States
- 4Department of Anesthesiology, Duke University, Durham, NC, United States
- 5Kornhauser Health Sciences Library, University of Louisville, Louisville, KY, United States
- 6Division of Transplantation, Department of Surgery, University of Louisville, Louisville, KY, United States
- 7Department of Anesthesiology & Perioperative Medicine, University of Louisville, Louisville, KY, United States
- 8Department of Cardiovascular & Thoracic Surgery, University of Louisville, Louisville, KY, United States
- 9Department of Pharmacology & Toxicology, University of Louisville, Louisville, KY, United States
- 10Center for Integrative Environmental Health Sciences, University of Louisville, Louisville, KY, United States
Background: The effects of SARS-CoV-2 have varied between significant waves of hospitalization.
Research question: Are cardiovascular complications different among the first, delta and omicron waves of hospitalized COVID-19 pneumonia patients?
Study design and methods: This was a multi-centre retrospective study of patients hospitalized with SARS-CoV-2 pneumonia: 632 were hospitalized during the first wave (March–July 2020), 1013 during the delta wave (September 2020–March 2021), and 323 during the omicron wave (January 2022–July 2022). Patients were stratified by wave and occurrence of cardiovascular events.
Results: Among all hospitalized patients with cardiovascular events, patients in the omicron wave were younger (62.4 ± 14 years) than patients in the first wave (67.4 ± 7.8 years) and the delta wave (66.9 ± 12.6 years) and had a higher proportion of non-Hispanic White people than in the first wave (78.6% vs. 61.7%). For COVID-19 patients who suffered from cardiovascular events, the omicron wave patients had significantly higher neutrophil/lymphocyte ratio, white blood cell and platelet counts when compared to the first wave. Omicron wave patients had significantly lower albumin and B-type natriuretic peptide levels (only 5.8% of the first wave and 14.6% of the delta wave) when compared to either the first wave or delta wave patients. In COVID-19 patients who suffered cardiovascular events during hospitalization, mortality rate in the omicron wave (26.8%) was significantly lower than the first wave (48.3%), time to mortality for non-survivors of COVID-19 patients who suffered cardiovascular events was significantly longer in the omicron wave (median 16 days) than in the first wave (median 10 days).
Conclusions: Younger and white patients were affected with cardiovascular complications more often by the omicron variant. Despite higher neutrophil/lymphocyte ratio and WBC counts, the omicron patients with cardiovascular events showed lower heart injuries, lower mortality and longer time to mortality for non-survivors when compared to the first and delta waves.
Introduction
The worldwide COVID-19 pandemic has challenged public health infrastructure due to significant morbidity and mortality and created significant social and economic disturbances across the globe (1). Over 6.6 million deaths have been attributed to COVID-19 worldwide (2). Historically, pandemics caused by viral illness have occurred in waves, described as significant incidence spikes subsequent to the initial outbreak. Most countries experienced at least three waves of the COVID-19 pandemic, the first wave in spring 2020, the second wave in late summer and autumn 2020 and the third wave starting from November 2021 (3, 4). Viral effects differed in demographics as well as severity of illness. The second wave of COVID-19 was attributable to the highly contagious delta variant (5). Starting December 2021, the omicron variant has become the dominant variant in many countries (6). Omicron is a new variant of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) with numerous mutations in the N-terminal domain of the viral spike protein and the receptor binding domain. This facilitated the angiotensin-converting enzyme 2 (ACE2) receptor-based cell entry. Therefore, when compared to the delta variant, the omicron variant is 2–3 times more transmissible, which explained peak number of daily new cases of COVID-19 and the quick spread in most of western countries (7).
The effects of COVID-19 on the health and well-being of patients reach far beyond the pulmonary system. As has been reported in numerous studies, COVID-19 may cause cardiovascular complications, such as myocarditis, endothelial dysfunction, thrombotic events, acute myocardial infarction, cardiac fibrosis, arrhythmias, and dysautonomia (8–11). SARS-CoV-2 affects the cardiovascular system through infection of the myocardium, vascular tissues, and circulating cells via the host's ACE-2 receptors for the viral spike protein (10). The pandemic ongoing and the potential for increased cases in future waves have substantial implications for the cardiovascular health globally (9). A recent meta-analysis of 21 studies, including over 77,000 hospitalized COVID-19 cases, evaluated in-hospital cardiovascular events and their impact on mortality. Over 12% of hospitalized patients had cardiovascular comorbidities or risk factors, and over 14% had a cardiovascular event while hospitalized. Cardiovascular comorbidities or risk factors and the development of in-hospital cardiovascular events were significantly associated with mortality (12, 13). These results demonstrated the importance of characterizing the cardiovascular risk profile of COVID-19 patients to identify higher risk patients for effective clinical management. However, there are scarce data on omicron's cardiac involvement especially its comparisons with the first and delta waves.
The University of Louisville COVID-19 Cardiovascular Research Group of the Center of Excellence for Research in Infectious Diseases (CERID) studied the characteristics and outcomes of COVID-19 patients who suffered cardiovascular events and identified risk factors in white and African American populations during the first wave of COVID-19 in Louisville, KY, USA (14). We also evaluated the electrocardiographic and echocardiographic features and their associations with clinical outcomes in this population during the first wave (15, 16). The objective of the present study is to compare the clinical characteristics and outcomes of patients hospitalized with SARS-CoV-2 community-acquired pneumonia (CAP) who suffered in-hospital cardiovascular events in the first wave (March 2020–July 2020), delta wave (September 2020–March 2021) and omicron wave (January 2022–July 2022).
Methods
Informed consent was not required per the exemption by the Institutional Review Board (IRB).
Study design, subjects, and setting
This investigation is a multi-center retrospective observational cohort study of patients hospitalized with SARS-CoV-2 CAP at eight adult, acute-care hospitals in Louisville, KY, USA. COVID-19 hospitalizations between March 2020 and July 2022 were included in this analysis. Patients with (1) a positive reverse transcription-polymerase chain reaction (RT-PCR) for SARS-CoV-2, (2) symptoms including fever, cough, or shortness of breath, and (3) an infiltrate on chest imaging were defined as having SARS-CoV-2 CAP. Patients were followed until hospital discharge or in-hospital death. For this analysis, a cardiovascular event during hospital was defined as any of the following conditions: development of cardiogenic shock, heart failure, acute myocardial infarction, cardiomyopathy, myocarditis, a new, serious arrhythmia, acute worsening of long-term arrhythmia, cerebrovascular accident, pulmonary embolism, deep vein thrombosis, pulmonary edema, or cardiac arrest occurring after hospital admission confirmed by an attending physician at each participating hospital. A new, serious arrhythmia, acute worsening of long-term arrhythmia was defined as any new or worsening arrhythmia that was not sinus rhythm including ventricular, junctional, and atrial arrythmia. Cardiomyopathy was defined as one of the following diagnoses: dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, arrhythmogenic cardiomyopathy or unclassified cardiomyopathy. Acute myocardial infarction was defined as ECG evidence of ST segment elevation or depression, and/or cardiac troponin values at least one value above the 99th percentile of upper reference limit. This cohort represents real world settings where diagnosis and management decisions are made by practicing physicians. The three waves of COVID-19 were defined by the most dominant variant present at the time of the COVID-19 diagnosis in Louisville, KY, USA: the first wave (March 2020–July 2020), delta wave (September 2020–March 2021) and Omicron Wave (January 2022–July 2022).
Human subjects protection
The study was approved by the Institutional Review Board (IRB) at the University of Louisville Human Subjects Research Protection Program Office (IRB number 20.0257) and by the research offices at each participating hospital. Informed consent was not required per the exemption by the IRB.
Study coordinating centre
CERID, located in the University of Louisville Division of Infectious Diseases, implemented all operations of the study. Members of CERID developed the case report form and the study database, collected data from hospital electronic medical records (EMRs), entered data into database software, and performed quality control of collected data by initiating and resolving queries (17). Data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at the University of Louisville Division of Infectious Diseases.
Data collection
Data collected from EMRs included COVID-19 test results; current medications; demographics, medical and social history; physical examination; signs and symptoms of illness; and laboratory, radiologic, and microbiologic findings. Disease management and therapies were noted as well as clinical course, in-hospital complications, and outcomes.
Demographic variables and comorbidities
Demographic data—including age, sex, height, and weight to calculate body mass index (BMI) and race or ethnicity of each patient—were captured to fully evaluate the population sample. Patients were grouped into the following categories: Hispanic; non-Hispanic black; non-Hispanic white; non-Hispanic others. Cardiovascular comorbidities collected included a history of heart failure, congestive heart failure, peripheral vascular disease, cerebrovascular disease, coronary artery disease, essential arterial hypertension, hyperlipidemia, prior myocardial infarction, prior PTCA/CABG, atrial fibrillation, prior deep vein thrombosis and prior pulmonary embolism. Current medication use related to cardiovascular history included aspirin, beta-blockers, angiotensin converting enzyme (ACE) inhibitors, anticoagulants, antiplatelets, statins, spironolactone/eplerenone, calcium channel blockers and angiotensin receptor blockers (ARBs).
Clinical and laboratory variables
Clinical and laboratory data were collected within 48 h of admission or during intensive care unit admission and included partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio, oxygen saturation/fraction of inspired oxygen (SpO2/FiO2) ratio, BMI, and arterial blood gases (ABGs) when available. Other laboratory data collected included hemoglobin; hematocrit; platelets; white blood cell count (WBC); neutrophil count/percentage; lymphocyte count/percentage; neutrophil/lymphocyte ratio (NLR); serum potassium, glucose, blood urea nitrogen (18), creatinine, albumin, and bilirubin; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels; AST/ALT ratio, international normalized ratio (INR) measurement; procalcitonin, D-dimer level; B-type natriuretic peptide (BNP) and N-terminal pro B-type natriuretic peptide (NT-proBNP); interleukin-6 (IL-6); and C-reactive protein (CRP).
Disease management and in-hospital therapies collected included the use steroids, hydroxychloroquine, azithromycin, remdesivir, monoclonal antibodies, heparin, warfarin, ACE inhibitors, angiotensin receptor blockers (ARBs), statin, metformin, plasma therapy, nasal cannula (non-high flow and high-flow), extracorporeal membrane oxygen (ECMO), prone position, neuromuscular blockade/artificial paralysis, inhaled pulmonary vasodilators, inotropes, insulin, non-invasive mechanical Ventilation (NIMV), invasive mechanical ventilation (IMV), vasopressors, antithrombotic prophylaxis and systemic steroids.
The main outcome variables were in-hospital mortality, length of stay (survivors), and days to mortality (non-survivors). Length of stay was defined as the time between admission and discharge from the hospital in days. Days to mortality was defined as the time between admission and in-hospital mortality in days.
Statistical analyses
Descriptive statistics was used to compare demographics, comorbidities, laboratory tests, disease management and therapies and clinical outcomes across the three different COVID-19 waves and development of in-hospital cardiovascular events. Continuous variables were summarized as means and standard deviations (SD) for each group, and categorical variables were summarized as frequencies and percentages for each group. For continuous variables, post-hoc t-tests or Mann-Whitney were performed to examine differences between groups; and one-way analysis of variance (ANOVA) or Kruskal-Wallis were performed to examine differences among groups. For categorical variables, Chi-squared tests were used if the number of observations in each cell was >5; otherwise, Fisher's exact tests were used.
Pairwise and three groups comparisons were carried out for the following group combinations: (1) first wave (March 2020–June 2020) vs. delta wave (September 2020–March 2021) vs. omicron wave (January 2022–July 2022) among patients who suffered cardiovascular events; (2) first wave (March 2020–June 2020) vs. delta wave (September 2020–March 2021); (3) Delta wave (September 2020–March 2021) vs. omicron wave (January 2022–July 2022) and (4) first wave (March 2020–June 2020) vs. omicron wave (January 2022–July 2022).
Kaplan–Meier estimators with log-rank tests or weighted Kaplan-Meier tests were conducted to compare survival times among patients with cardiovascular events for three waves (19). Statistical analyses were conducted using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria, 2020). P-values <0.05 were considered statistically significant.
Results
During the first wave, 120 (18.99%) patients developed in-hospital cardiovascular events and 512 patients who did not; during the delta wave, 180 (17.77%) patients who developed in-hospital cardiovascular events and 833 patients who did not; and lastly, during the omicron wave, 56 (17.33%) patients developed in-hospital cardiovascular events and 267 patients who did not.
Patient demographics stratified by COVID-19 waves and occurrence of cardiovascular event(s) are summarized in Table 1A. Among all hospitalized patients with cardiovascular events, patients in the omicron wave were younger (62.4 ± 14 years) than patients in the first wave (67.4 ± 7.8 years) and the delta wave (66.9 ± 12.6 years) and had a higher proportion of non-Hispanic White people than in the first wave (78.6% vs. 61.7%).
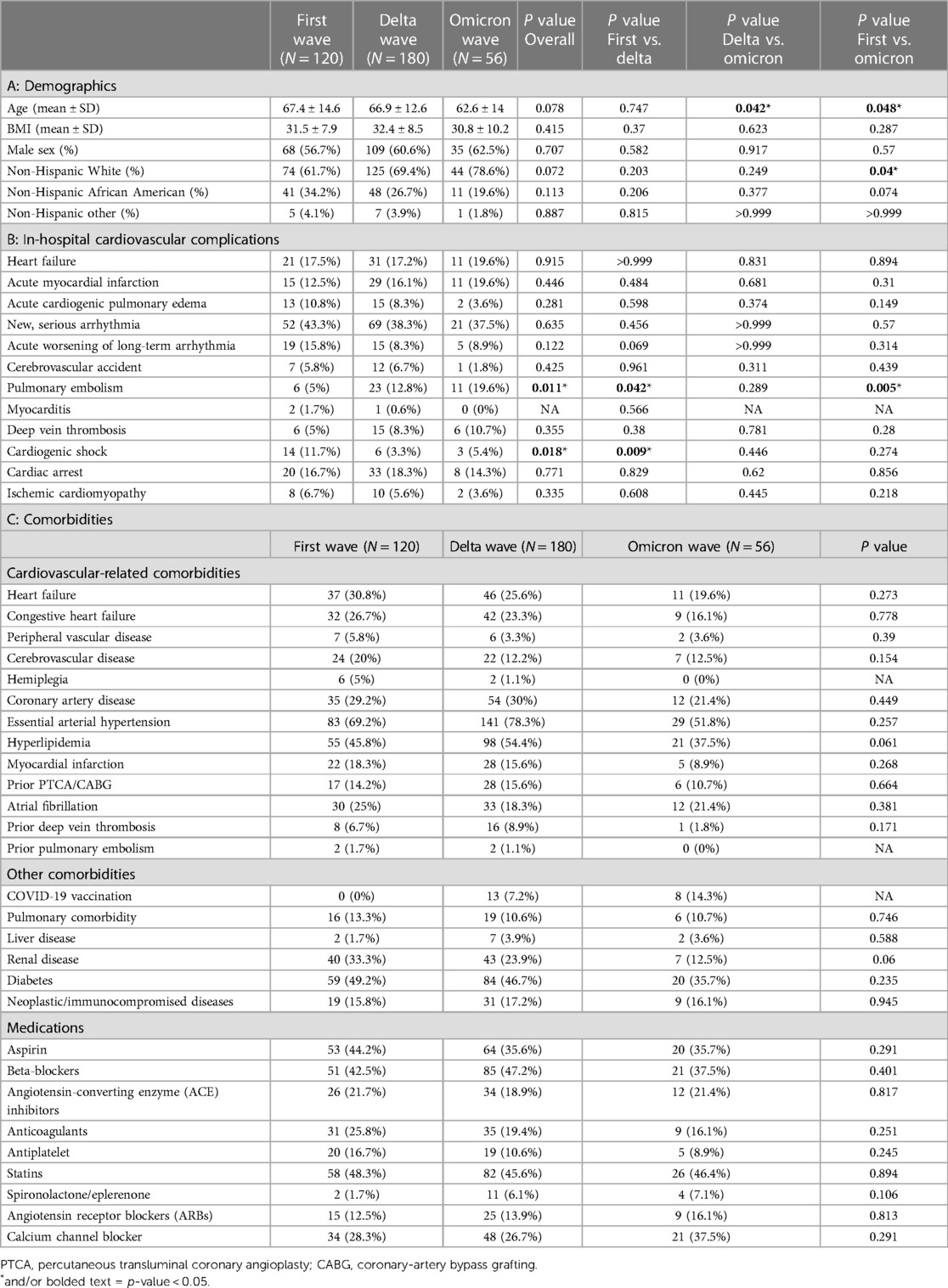
Table 1. Demographics, cardiovascular complications and comorbidities of three waves of COVID-19 patients who developed cardiovascular events during hospitalization. First wave (March 2020–July 2020); Delta wave (September 2020–March 2021); Omicron wave (January 2022–July 2022).
A summary of each cardiovascular complication among patients who developed at least one cardiovascular event during hospitalization is shown in Table 1B. There was a significantly higher proportion of patients who developed cardiogenic shock in the first wave compared to the delta wave (11.7% vs. 3.3%; p = 0.009). While a significantly higher proportion of pulmonary embolism events occurred during the omicron wave compared to the first wave (19.6% vs. 5%; p = 0.05).
Among patients who developed in-hospital cardiovascular events, there was no significant difference across waves for comorbidities. In the delta and omicron waves, COVID-19 vaccination data were also collected. Of note, there were 13 (7.2%) and 8 (14.3%) vaccinated patients who suffered cardiovascular event in the delta wave and omicron wave respectively (Table 1C).
Clinical and laboratory biomarkers such as NLR, WBC counts, platelet counts, neutrophil percentage, lymphocyte percentage, lymphocyte counts, albumin, CRP and BNP values differed significantly among the three waves as shown in Table 2. For COVID-19 patients who suffered from cardiovascular events, the omicron wave patients had significantly higher NLR, WBC and platelet counts when compared to the first wave. Omicron wave patients had significantly lower albumin and BNP levels (only 5.8% of the first wave and 14.6% of the delta wave) when compared to either the first wave or delta wave patients. During hospitalization, the first troponin recorded was significantly different across all three waves (0.2 vs. 1.1 vs. 0.6 ng/ml; p = 0.06). This was mostly driven by the difference between Wave 1 and Wave D (0.2 vs. 1.1 ng/ml; p = 0.008). Peak troponin was also found to be significantly higher in Wave D compared to Wave 1 (2.3 vs. 0.6 ng/ml; p = 0.017).
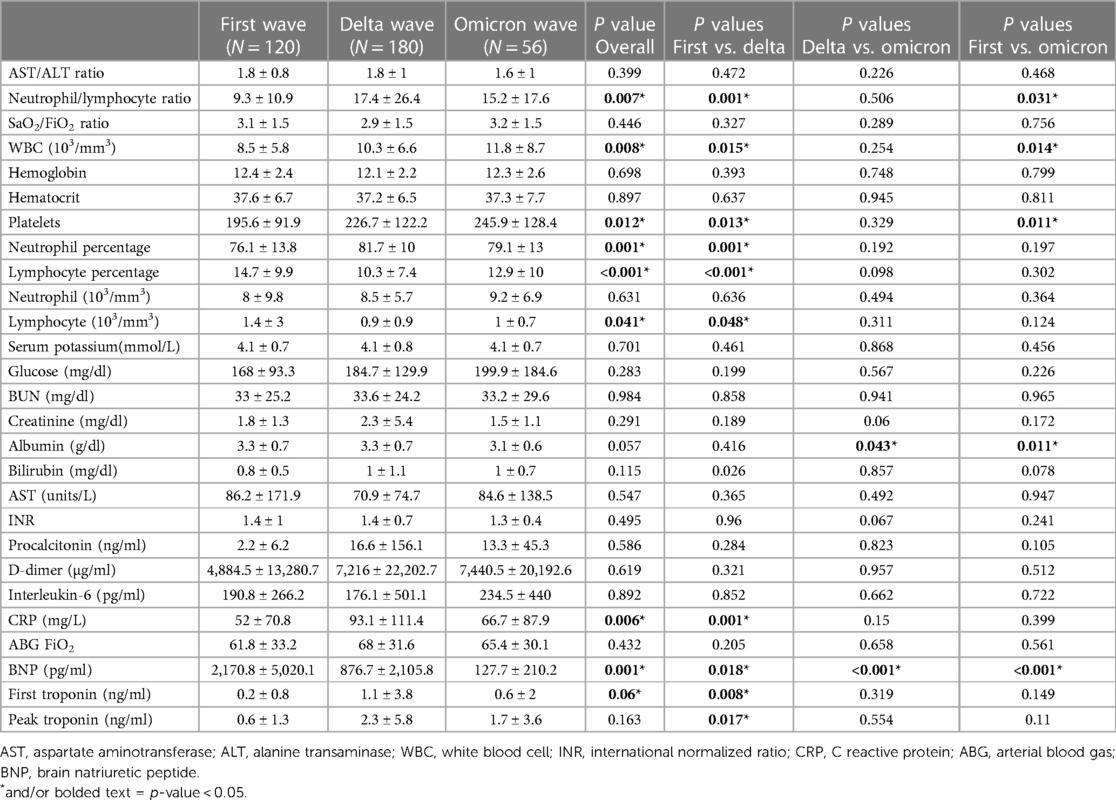
Table 2. Laboratory results of three waves of COVID-19 patients who developed cardiovascular events during hospitalization. First wave (March 2020–July 2020); Delta wave (September 2020–March 2021); Omicron wave (January 2022–July 2022).
Treatment options across three waves of patients who suffered cardiovascular events during hospitalization were compared in Table 3. The proportions of patients were given the following treatments: steroids, remdesivir, monoclonal antibodies, nasal cannula (non-high flow) and non-invasive mechanical ventilation were significantly different across three waves. Specifically, during the omicron wave more patients were treated with monoclonal antibodies compared to the delta and first wave. The use of steroids, remdesivir, and nasal cannula (non-high flow) increased in both the delta and omicron wave compared to the first wave. Non-invasive mechanical ventilation increased from the first wave to the delta wave and lastly, the use of extracorporeal membrane oxygen (ECMO) increased in the omicron wave compared to the delta wave.
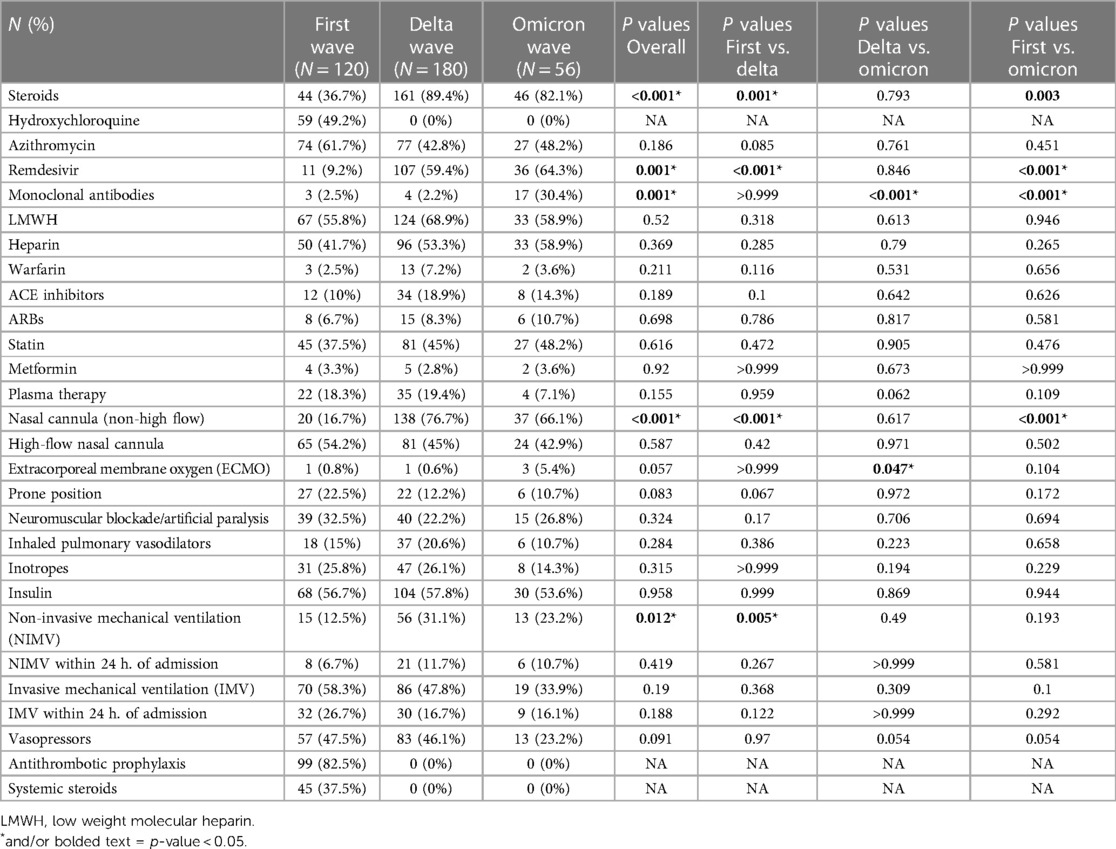
Table 3. Treatment options in three waves of COVID-19 patients who developed cardiovascular events during hospitalization. First wave (March 2020–July 2020); delta wave (September 2020–March 2021); omicron wave (January 2022–July 2022).
Clinical outcomes stratified by waves and cardiovascular events are shown in Table 4A,B. Among COVID-19 patients who developed a cardiovascular event during hospitalization, mortality rate was significantly lower during the omicron wave compared to the first wave (26.8% vs. 48.3%; p = 0.011). In COVID-19 patients who did not develop a cardiovascular event during hospitalization, mortality rate significantly lower during the omicron wave compared to the delta wave (5.6% vs. 13.1%; p = 0.001).
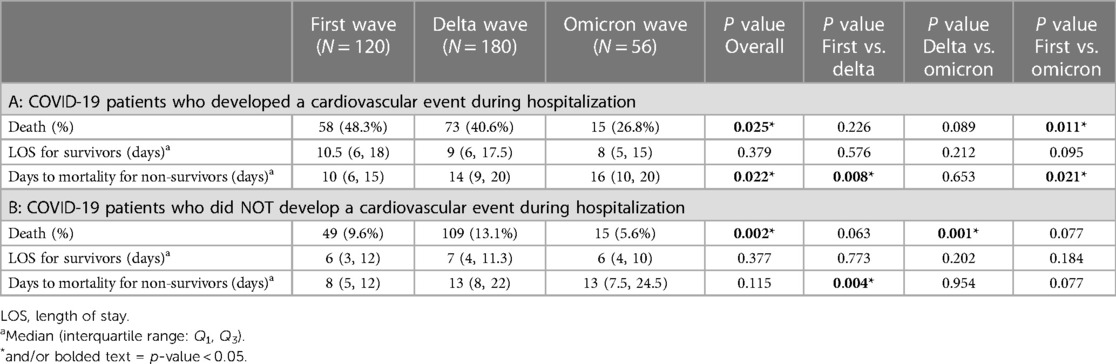
Table 4. Clinical outcomes including death, hospital stay for survivors and days to mortality for non-survivors in three waves of COVID-19 patients who developed cardiovascular events during hospitalization. First wave (March 2020–July 2020); Delta wave (September 2020–March 2021); Omicron wave (January 2022–July 2022).
There was no significant difference in the hospital length of stay for survivors of both COVID-19 patients who did and who did not develop a cardiovascular event during hospitalization across three waves (Table 4B). However, time to mortality for non-survivors of COVID-19 patients who developed an in-hospital cardiovascular event was significantly longer in the omicron wave compared to both the first wave (median 16 vs. 10 days; p = 0.021) and the delta wave (median 10 vs. 14 days; p = 0.008) (Table 4A). In addition, among patients who did not develop a cardiovascular event during hospitalization also had a significantly longer time to mortality for non-survivors during the delta wave compared to the first wave (median 8 vs. 13 days, p = 0.004) (Table 4B).
Kaplan–Meier curves and log-rank tests revealed that there was no significant difference in survival among three waves for COVID-19 patients who suffered cardiovascular events (Figure 1).
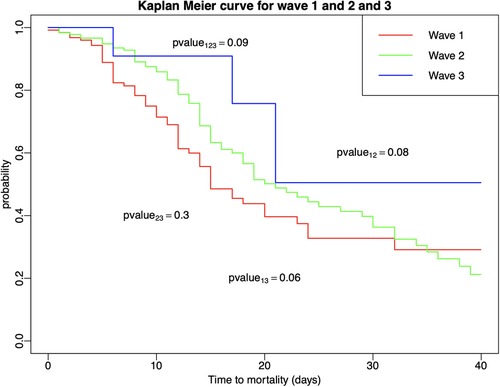
Figure 1. Kaplan–Meier estimators with log-rank tests were conducted to compare survival times among patients who developed in-hospital cardiovascular events across and within the three COVID-19 waves.
Table 5 furthermore demonstrated the most selected treatments as well as the corresponding mortality rates for each treatment option across the three COVID-19 waves of patients who developed cardiovascular events during hospitalization. The mortality rate of patients who received antithrombotic prophylaxis, azithromycin, and invasive mechanical ventilation (IMV) in the first wave are 46 (46.5%), 42 (56.8%) and 51 (72.9%) respectively. Steroids and nasal cannula (non-high flow) were highly used in both the delta and omicron waves. During the delta wave, 70 (43.5%) patients died who received steroids while during the omicron wave, 13 (28.3%) patients died received steroids. Forty-nine (35.5%) patients who received nasal cannula (non-high flow) died during the delta wave, however, 7 (18.9%) patients died in omicron wave. During the delta wave, 49 (39.5%) patients who received LMWH died, while, 10 (27.8%) patients who received Remdesivir died during the omicron wave. There were significant differences in mortality rates for the Top 3 treatment options among the three waves as shown in Figure 2.
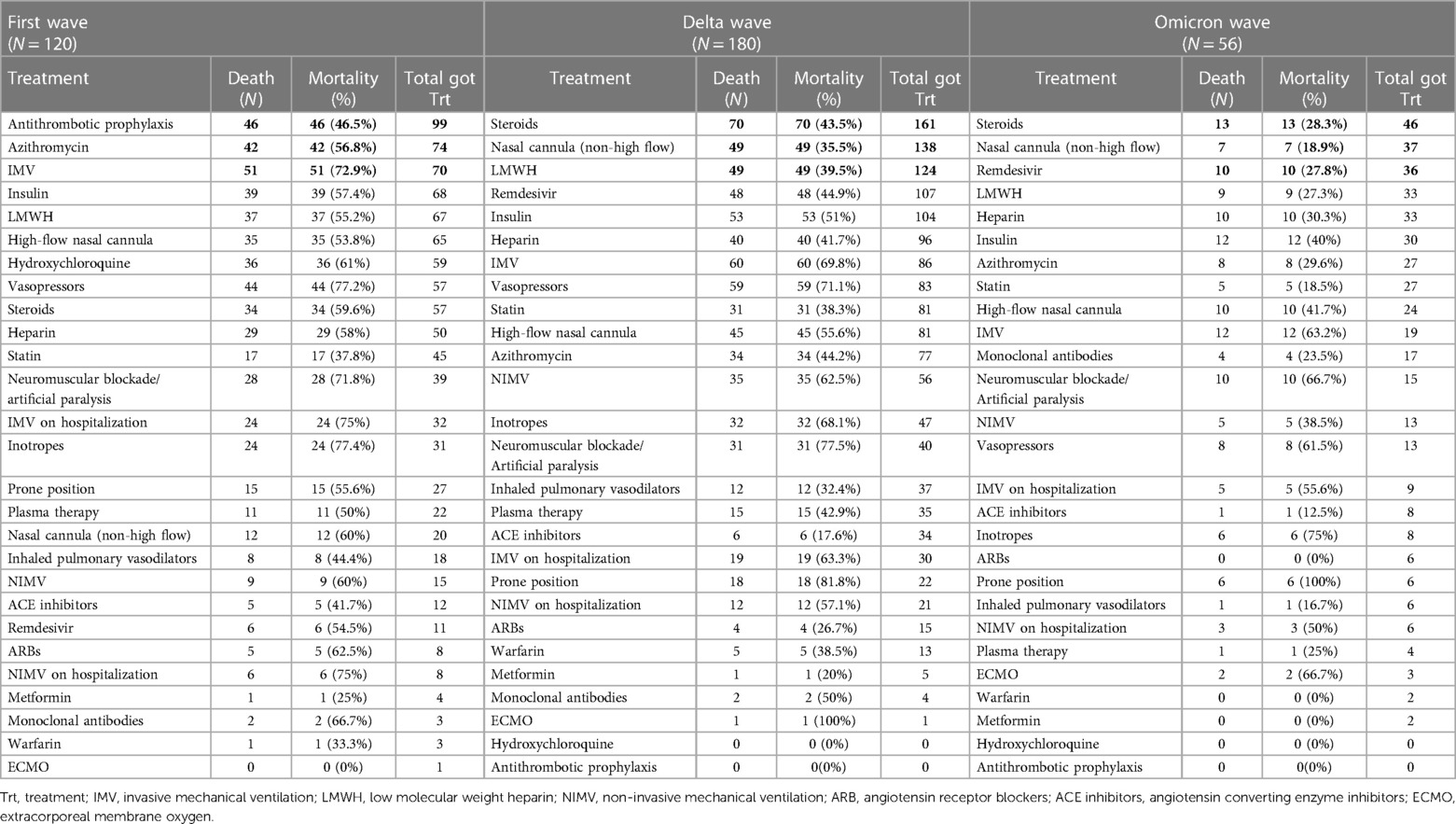
Table 5. Mortality rates for each treatment option in three waves of COVID-19 patients who developed cardiovascular events during hospitalization. First wave (March 2020–July 2020); delta wave (September 2020–March 2021); omicron wave (January 2022–July 2022).
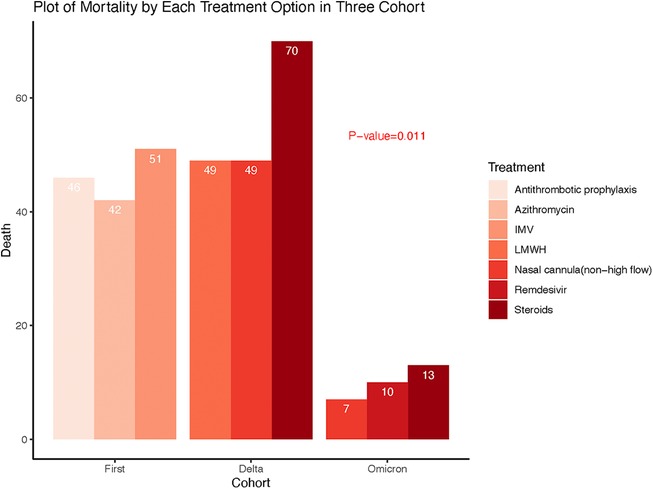
Figure 2. Comparison of mortality rates associated with the top3 treatments between and across three waves of COVID-19.
Discussion
The clinical characteristics, clinical markers, and outcomes of hospitalized COVID-19 patients during the first wave of COVID-19 have been previously reported by the University of Louisville COVID-Cardiovascular Research Group (14). The current study further analysed the cardiovascular complications of the delta and omicron waves in hospitalized COVID-19 pneumonia patients. We found younger and white patients were affected with cardiovascular complications more often by the omicron variant. Despite a few elevated inflammatory markers, omicron patients with cardiovascular events showed significantly lower heart failure marker levels when compared to the first and delta waves. For clinical outcomes, the omicron wave had significantly lower mortality rate and longer time to mortality for non-survivors when compared to the first wave in COVID-19 patients with cardiovascular complications.
Cardiac injury can occur in ≥20% of the hospitalized COVID-19 patients and is associated with a 3% increase in cardiac arrest, a 19% increase in incidence of heart failure, and mortality rates that range from 8% to 69%. Risk factors for cardiac complications include age >70 years, BMI ≥30 kg/m2, male sex, diabetes, pre-existing cardiovascular disease, and moderate to severe pneumonia at hospital presentation. Extensive myocardial inflammation is characterized by diffuse electrocardiographic (ECG) ST elevations and depressed PR intervals with global biventricular dysfunction on echocardiogram whereas regional wall motion abnormalities and localized ECG ST-segment changes are characteristics of focal ischemic damage from macro- or microvascular thrombosis. Apical Left ventricular (LV) wall dyskinesis and mid LV wall akinesis suggests SARS-CoV-2-induced stress-induced cardiomyopathy.
Omicron accounted for >99% of COVID-19 cases and has become the predominant strain in the United States in December 2021 and. The omicron SARS-CoV2 variant is highly transmissible which might mean an increase in cases, leading to more hospitalizations, cardiovascular complications, and deaths. However, the cardiovascular effects of omicron variant are less well understood and are yet to be addressed. Cardiac complications are thought to be more frequent with the alpha and delta variants than the omicron variant. A retrospective study from Italy showed omicron patients had lower incidence of pulmonary embolism than the delta wave (20). Echocardiographic analysis of 122 COVID-19 infection during the omicron surge in New York City, USA demonstrated that right ventricular (RV) abnormality remained prevalent (34%) in hospitalized omicron patients. RV abnormality was strongly and independently associated with in-hospital mortality (21). Another small study from New York, USA showed significant myocardial injury was associated with high morbidity and mortality (22). Two cases of myocarditis in acutely infected omicron patients were reported from Israel suggested the omicron variant may cause myocarditis, and malignant arrhythmia with hemodynamic instability (23). Interestingly, a recent study revealed that young, otherwise healthy adults who had infections during the omicron wave did not exhibit impairments of cardiovascular health (24).
The current study found that the incidence of cardiovascular events was similar among the first, delta and omicron waves in the Louisville, KY, USA cohort, which is representative of the USA demographics. However, omicron affected younger and white patients more often in terms of cardiovascular complications, which was different from the first and delta waves. South Africa reported patients hospitalized in omicron wave were younger, with less comorbidities and had less need for oxygen therapy, mechanical ventilation and ICU admission when compared to the first and delta waves (25). United Kingdom study of 63,002 omicron patients found that the prevalence of symptoms differs from those of the delta variant, with reduced probability of hospital admission and less involvement of the lower respiratory tract (26). Cardiac complications were not reported in these two studies. A study of omicron COVID-19 patients with myocardial injury from the USA found the omicron wave had similar clinical characteristics as prior waves (27). The current study's findings indicate the omicron variant might affect the cardiovascular system similarly as previous SARS-CoV-2 strains despite less severe respiratory system involvement.
In this study cohort, BNP levels were much lower in the omicron patients with cardiovascular events than those of the first and delta waves. This could indicate omicron variants cause less severe myocardial stretch than prior variants. Troponin levels were significantly higher in the delta wave compared to the first and omicron wave, which may reflect severe myocardial damage or injury particularly in the setting of acute coronary syndromes like myocardial infarction among delta variants. This is consistent with recent findings that troponin elevation was more common in Delta compared to Alpha, and cumulative evidence of cardiac injury (echocardiographic abnormality and/or troponin elevation) was more common in Delta compared with Alpha or Omicron (28). In addition, mortality rate in omicron patients with cardiovascular events was significantly lower than the first wave and the time to mortality for non-survivors was significantly longer in the omicron and delta waves than in the first wave. Another study found omicron patients with myocardial injuries suffered in-hospital mortality at 23.3%, which was significantly lower in than the first (59.3%) and the delta waves (28.1%) (27). A study from South Africa demonstrated that the median length of stay decreased and mortality rate also reduced among omicron patients when compared to prior waves (25).
Previous studies showed higher inflammatory markers such as procalcitonin, D-dimer, interleukin-6 and CRP resulting in a “cytokine storm” or hyperinflammatory state, which is associated with COVID-19 disease severity (29). Hence, differences in inflammatory and other hematological biomarkers may indicate a difference in COVID-19 severity across infection waves. A single-centre study in Spain found that the majority of blood biomarkers were similar across the first and delta waves with regard to disease severity, but significant differences were found in IL-6 and D-dimer (30). Another large study from Turkey compared CRP levels of hospitalized COVID-19 patients with severe or critical disease in the first and second waves, finding significantly higher CRP levels in the second wave (31). Literature shows that cardiac inflammation and microvascular procoagulant changes were lower in the delta wave than the first wave (32). In omicron myocardial injury cases, WBC, CRP, LDH and ferritin were found to be significantly lower than the first and delta waves (27). The current study found that omicron COVID-19 patients with cardiovascular events had higher neutrophil/lymphocyte ratio and WBC counts than the first wave and there was no difference between delta wave and omicron waves in terms of inflammatory markers. There could be multiple reasons for the similarity of inflammatory markers among the three waves in this study. First, steroids are currently the standard of care and could contribute to increase in WBC and reduce the increase of inflammatory markers. Second, the differences may be due to the variations in immuno-pathogenesis among different SARS-COV-2 variants. Third, vaccination might have different efficacy/actions again different variants (26).
Hospital length of stay for survivors was similar across the three COVID-19 waves for patients with and without cardiovascular events. However, days to mortality for non-survivors with cardiovascular events were significantly longer in the delta and omicron waves when compared to the first wave. It can be hypothesized that vaccine administration, earlier administration of evolving therapeutic interventions, and increased knowledge regarding disease progression led to prognostic improvement in the outcomes of the delta and omicron wave cohorts.
Treatments for both COVID-19 and cardiovascular complications could significantly affect the clinical outcomes in different waves of COVID-19 (33–36). During the omicron wave, more patients were treated with monoclonal antibodies compared to the delta and first wave. The use of steroids, remdesivir, and nasal cannula (non-high flow) increased in both the delta and omicron wave compared to the first wave. Non-invasive mechanical ventilation increased from the first wave to the delta wave and lastly, the use of extracorporeal membrane oxygen (ECMO) increased in the omicron wave compared to the delta wave. We then explored the top 3 treatments for COVID-19 patients who suffered cardiovascular events and demonstrated there were distinct differences among the three waves. During the alpha wave, the top 3 treatments were antithrombotic prophylaxis, azithromycin and invasive mechanical ventilations; the top 3 treatments in delta wave were steroids, nasal cannula oxygen and low molecular weight heparin; the top 3 treatments in omicron were steroids, nasal cannula oxygen, and remdesivir. These differences reflected the progressive learning and changes in practice for managing this deadly pandemic by physicians, which could serve as future strategies in newer variants or viruses (37–41).
Long-term sequelae of SARS-CoV-2 infection occur in 18% to 53% of patients, including chest pain, dyspnea, palpitations/tachycardia, and postural orthostatic tachycardia. SARS-CoV-2 hypercoagulability, myocardial inflammation and injury, and microvascular thrombosis, will have significant impact on quality of life, long-term patient functional status, and mortality and require longitudinal follow-up studies and extensive research. How the omicron variants affect the long-term sequelae of COVID-19 is largely unknown and should be extensively studied because the omicron variant was responsible for the largest number of COVID-19 associated hospitalizations (11).
Limitations
There are a few limitations to this study. The results of this retrospective study may not be generalizable to non-hospitalized patients with COVID-19, as their characteristics may differ significantly from hospitalized patients, thereby reducing external validity. Since this was a descriptive study with no regression analysis, no causal inferences can be made concerning risk factors for worse clinical outcomes in our cohort. Information bias may be present given that genomic sequencing was not used to confirm variant data for delta and omicron.
Conclusion
The present study compared the demographic, clinical, and laboratory characteristics of COVID-19 patients with cardiovascular events in the first, delta and omicron waves of the pandemic. Younger and white patients were affected with in-hospital cardiovascular complications more often by the omicron variant. Despite higher neutrophil/lymphocyte ratio and WBC counts, the omicron patients with cardiovascular events showed lower heart injuries, lower mortality and longer time to mortality for non-survivors when compared to the first and delta waves. Prospective long-term follow-up studies are urgently needed to address long term sequelae from the omicron infections. This information can help to guide triage and treatment of at-risk groups to reduce the risk of poor clinical outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Louisville Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Informed consent was not required per the exemption by the Institutional Review Board (IRB).
Author contributions
LPR: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Supervision. HS: Conceptualization, Data curation, Writing – original draft. TA: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. QX: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. YH: Formal Analysis, Writing – review & editing. VS: Data curation, Methodology, Writing – original draft, Writing – review & editing. FD: Data curation, Methodology, Writing – original draft, Writing – review & editing. TM: Writing – original draft, Writing – review & editing. ECH: Writing – original draft, Writing – review & editing. SF: Writing – original draft, Writing – review & editing, Formal Analysis, Methodology. AG: Writing – original draft, Writing – review & editing. JR: Writing – review & editing, Conceptualization, Writing – original draft. CMJ: Writing – review & editing. RM: Writing – review & editing. RJH: Writing – review & editing. AMW: Writing – review & editing. JJH: Writing – review & editing. FWA: Writing – original draft, Writing – review & editing, Conceptualization. SPC: Writing – original draft, Writing – review & editing. SP: Conceptualization, Writing – original draft, Writing – review & editing. MK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. JH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the National Center for Advancing Translational Sciences [grant number U18TR003787]; the National Institute of Environmental Health Sciences [grant number P30 (P30ES030283)]; National Heart, Lung, and Blood Institute (R01HL158779); National Institute of Allergy and Infectious Diseases (R01AI172873); and Gilead Sciences COMMIT COVID-19 RFP Program [grant number IN-US-983-6063]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Gilead Sciences.
Acknowledgments
Data collection was performed by the Center of Excellence for Research in Infectious Diseases (CERID) group at the University of Louisville Division of Infectious Diseases. Statistical analysis and draft writing were performed by SF and QX under the guidance of MK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soriano V, Ganado-Pinilla P, Sanchez-Santos M, Gomez-Gallego F, Barreiro P, De Mendoza C, et al. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int J Infect Dis. (2021) 105:374–6. doi: 10.1016/j.ijid.2021.02.115
2. Johns Hopkins University and Medicine. Coronavirus Resource Center (2022). Available online at: https://coronavirus.jhu.edu/ (accessed December 24, 2022).
3. Saito S, Asai Y, Matsunaga N, Hayakawa K, Terada M, Ohtsu H, et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. (2021) 82(4):84–123. doi: 10.1016/j.jinf.2020.10.033
4. Iftimie S, Lopez-Azcona AF, Vallverdu I, Hernandez-Flix S, De Febrer G, Parra S, et al. First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in reus, Spain. PLoS One. (2021) 16(3):e0248029. doi: 10.1371/journal.pone.0248029
5. Renardy M, Eisenberg M, Kirschner D. Predicting the second wave of COVID-19 in Washtenaw county, MI. J Theor Biol. (2020) 507:110461. doi: 10.1016/j.jtbi.2020.110461
6. Hachmann NP, Miller J, Collier AY, Ventura JD, Yu J, Rowe M, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. (2022) 387(1):86–8. doi: 10.1056/NEJMc2206576
7. Del Rio C, Omer SB, Malani PN. Winter of omicron-the evolving COVID-19 pandemic. JAMA. (2022) 327(4):319–20. doi: 10.1001/jama.2021.24315
8. Writing Committee Members, Bozkurt B, Das SR, Addison D, Gupta A, Jneid H, et al. 2022 AHA/ACC key data elements and definitions for cardiovascular and noncardiovascular complications of COVID-19: a report of the American college of cardiology/American heart association task force on clinical data standards. J Am Coll Cardiol. (2022) 80(4):388–465. doi: 10.1016/j.jacc.2022.03.355
9. Yan BP, Wong MCS. Cardiovascular complications of COVID-19: a future public health burden requiring intensive attention and research. Hong Kong Med J. (2022) 28(3):199–200. doi: 10.12809/hkmj215131
10. Lo YSA, Jok C, Tse HF. Cardiovascular complications of COVID-19. Hong Kong Med J. (2022) 28(3):249–56. doi: 10.12809/hkmj209217
11. Henning RJ. Cardiovascular complications of COVID-19 severe acute respiratory syndrome. Am J Cardiovasc Dis. (2022) 12(4):170–91.36147783
12. Sabatino J, De Rosa S, Di Salvo G, Indolfi C. Correction: impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS One. (2020) 15(12):e0243471. doi: 10.1371/journal.pone.0243471
13. Sabatino J, De Rosa S, Di Salvo G, Indolfi C. Impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS One. (2020) 15(8):e0237131. doi: 10.1371/journal.pone.0237131
14. Xu Q, Samanapally H, Nathala P, Salunkhe V, Furmanek S, Cahill MN, et al. Outcomes and risk factors for cardiovascular events in hospitalized COVID-19 patients. J Cardiothorac Vasc Anesth. (2021) 35(12):3581–93. doi: 10.1053/j.jvca.2021.03.035
15. Nathala P, Salunkhe V, Samanapally H, Xu Q, Furmanek S, Fahmy OH, et al. Electrocardiographic features and outcome: correlations in 124 hospitalized patients with COVID-19 and cardiovascular events. J Cardiothorac Vasc Anesth. (2022) 36(8 Pt B):2927–34. doi: 10.1053/j.jvca.2022.01.011
16. Furmanek S, Salunkhe V, Pahwa S, Samanapally H, Nathala P, Xu Q, et al. Correlations of before and after event echocardiographic parameters with troponin and BNP in hospitalized COVID-19 patients with cardiovascular events. J Cardiothorac Vasc Anesth. (2022) 36(12):4553–5. doi: 10.1053/j.jvca.2022.08.024
17. Ramirez J, Burden of COVID-19 Scientific Advisory Board. Defining the burden of COVID-19 in the kentuckiana area: incidence, epidemiology & clinical outcomes of patients with COVID-19. Univ Louisville J Respir Infect. (2020) 4(1):a4. doi: 10.18297/jri/vol4/iss1/4
18. Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. (2014) 2014(6):CD003843. doi: 10.1002/14651858.CD003843.pub3
19. Pepe MS, Fleming TR. Weighted Kaplan-Meier statistics: a class of distance tests for censored survival data. Biometrics. (1989) 45(2):497–507. doi: 10.2307/2531492
20. Corriero A, Ribezzi M, Mele F, Angrisani C, Romaniello F, Daleno A, et al. COVID-19 variants in critically ill patients: a comparison of the delta and omicron variant profiles. Infect Dis Rep. (2022) 14(3):492–500. doi: 10.3390/idr14030052
21. Salem Omar AM, Alam L, Talebi S, Garcia-Sastre A, Narula J, Argulian E. Right ventricular abnormality in patients hospitalized with COVID-19 infection during omicron variant surge. Am J Cardiol. (2022) 173:158–60. doi: 10.1016/j.amjcard.2022.03.035
22. Alam L, Omar AMS, Talebi S, Narula J, Argulian E. Echocardiographic findings in patients with COVID-19 with myocardial injury during the omicron variant surge. Am J Cardiol. (2022) 172:168–9. doi: 10.1016/j.amjcard.2022.03.008
23. Fishman B, Goitein O, Berkovitch A, Rahav G, Matetzky S. First report of myocarditis in two patients with COVID-19 omicron variant: case report. Eur Heart J Case Rep. (2022) 6(10):ytac407. doi: 10.1093/ehjcr/ytac407
24. Skow RJ, Nandadeva D, Grotle AK, Stephens BY, Wright AN, Fadel PJ. Impact of breakthrough COVID-19 cases during the omicron wave on vascular health and cardiac autonomic function in young adults. Am J Physiol Heart Circ Physiol. (2022) 323(1):H59–64. doi: 10.1152/ajpheart.00189.2022
25. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. (2022) 327(6):583–4. doi: 10.1001/jama.2021.24868
26. Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, Mcneal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. Br Med J. (2022) 376:e069761. doi: 10.1136/bmj-2021-069761
27. Case BC, Shea C, Rappaport H, Cellamare M, Zhang C, Zhu M, et al. The evolving impact of myocardial injury in patients with COVID-19 amid the omicron wave of the pandemic. Am J Cardiol. (2023) 190:54–60. doi: 10.1016/j.amjcard.2022.11.041
28. Khan RS, Ordog T, Hong SD, Schmitz AH, Thattaliyath B, Sharathkumar AA. Evolution of cardiovascular findings in multisystem inflammatory syndrome in children (MIS-C) across COVID-19 variants: common trends and unusual presentations. Pediatr Cardiol. (2024) 45(3):552–9. doi: 10.1007/s00246-023-03397-2
29. Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. (2021) 51(1):e13429. doi: 10.1111/eci.13429
30. Mollinedo-Gajate I, Villar-Alvarez F, Zambrano-Chacon MLA, Nunez-Garcia L, De La Duena-Munoz L, Lopez-Chang C, et al. First and second waves of coronavirus disease 2019 in Madrid, Spain: clinical characteristics and hematological risk factors associated with critical/fatal illness. Crit Care Explor. (2021) 3(2):e0346. doi: 10.1097/CCE.0000000000000346
31. Sargin Altunok E, Satici C, Dinc V, Kamat S, Alkan M, Demirkol MA, et al. Comparison of demographic and clinical characteristics of hospitalized COVID-19 patients with severe/critical illness in the first wave versus the second wave. J Med Virol. (2022) 94(1):291–7. doi: 10.1002/jmv.27319
32. Mughal MS, Kaur IP, Wang C, Alhashemi R, Buemio A, Patton CD, et al. Variation in clinical characteristics, outcomes, and mortality of hospitalized patients with COVID-19 during the second wave of the pandemic: a single-center experience. J Investig Med. (2021) 69(8):1479–82. doi: 10.1136/jim-2021-001876
33. Wang B, Li M, Yu J, Zhang Y. A case report: application of NMBAs significantly improve oxygenation of the COVID-19 patient. J Anesth Transl Med. (2023) 2(4):10–2. doi: 10.58888/2957-3912-2023-04-003
34. Wang T, Lu Y, Liu X, Chen S. Homemade portable high-flow nebulized oxygen therapy device used during the COVID-19 pandemic to improve blood oxygen saturation in critically ill patients: a two-case report. J Anesth Transl Med. (2023) 2(4):1–5. doi: 10.58888/2957-3912-2023-04-001
35. Feng Y, Wei H, Jiang B. Controversies concerning emergency tracheal intubation in patients with COVID-19. J Anesth Transl Med. (2023) 2(1):15–8. doi: 10.58888/2957-3912-20230207-3
36. Chen H, Zhang L, Wang Y, Li A, Zhang Y, Wu Y. Effect of anesthesia intensive care unit during the COVID-19 pandemic. J Anesth Transl Med. (2023) 2(1):19–20. doi: 10.58888/2957-3912-20230312
37. Tang L, Wang C, Chen X, Chen X, Yang J, Liu H, et al. Perioperative infection control recommendations during the SARS-CoV-2 omicron variant pandemic. J Anesth Transl Med. (2023) 2(1):1–5. doi: 10.58888/2957-3912-20230207-1
38. Mei B, Dai Q, Shang Z, Chen X, Yang J, Liu H, et al. Timing of surgery in patients with novel coronavirus infection: basing on current epidemiological characteristics and the impact on physiological functions. J Anesth Transl Med. (2023) 2(1):6–9. doi: 10.58888/2957-3912-20230107
39. Wang T, Xia H, Ma L, Yang X, Wang Y, Huang J, et al. Recommendations on perioperative management and emergency intubation for patients with omicron. J Anesth Transl Med. (2023) 2(1):10–4. doi: 10.58888/2957-3912-20230207-2
40. Lu Z, Ma Q, Xu L, Ji J, Tian Y, Huang J, et al. The implication of preoperative transthoracic echocardiography evaluation in patients with hypertrophic cardiomyopathy undergoing noncardiac surgery by anesthesiologists: two case reports. J Anesth Transl Med. (2023) 2(3):42–5. doi: 10.58888/2957-3912-2023-03-06
Keywords: SARS-CoV-2, COVID-19, omicron, delta, cardiac, vascular, outcome
Citation: Roser LP, Samanapally H, Ali T, Xu Q, Han Y, Salunkhe V, Deepti F, McGuffin T, Huang EC, Furmanek S, Glynn A, Ramirez J, Jones CM, Mariyappa R, Hogue RJ, Williams AM, Huang JJ, Arnold FW, Clifford SP, Pahwa S, Kong M and Huang J (2024) Different clinical characteristics and outcomes of adult hospitalized SARS-CoV-2 pneumonia patients complicated by cardiovascular events during the first, delta and omicron waves of COVID-19. Front. Epidemiol. 4:1342917. doi: 10.3389/fepid.2024.1342917
Received: 22 November 2023; Accepted: 4 March 2024;
Published: 18 April 2024.
Edited by:
Lukas J. Motloch, Paracelsus Medical University, AustriaReviewed by:
Rudin Pistulli, University of Münster, GermanyDiana Gareeva, Bashkir State Medical University, Russia
© 2024 Roser, Samanapally, Ali, Xu, Han, Salunkhe, Deepti, McGuffin, Huang, Furmanek, Glynn, Ramirez, Jones, Mariyappa, Hogue, Williams, Huang, Arnold, Clifford, Pahwa, Kong and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiapeng Huang amlhcGVuZy5odWFuZ0Bsb3Vpc3ZpbGxlLmVkdQ==
 Lynn P. Roser1
Lynn P. Roser1 T’shura Ali
T’shura Ali Qian Xu
Qian Xu Yuchen Han
Yuchen Han Fnu Deepti
Fnu Deepti Alex Glynn
Alex Glynn Julio Ramirez
Julio Ramirez Ryan J. Hogue
Ryan J. Hogue Alexander M. Williams
Alexander M. Williams Justin J. Huang
Justin J. Huang Maiying Kong
Maiying Kong Jiapeng Huang
Jiapeng Huang on behalf of the COVID-19 CV Research Group (COVID-CVRG) of the Centre of Excellence for Research in Infectious Diseases (CERID) Coronavirus Study Group
on behalf of the COVID-19 CV Research Group (COVID-CVRG) of the Centre of Excellence for Research in Infectious Diseases (CERID) Coronavirus Study Group