- Department of Epidemiology, School of Public Health, Institute of Health, Bule Hora University, Bule Hora, Ethiopia
Background: Vaccines are an effective and ultimate solution that can decrease the burden of coronavirus disease 2019 worldwide. However, poor knowledge and unwillingness to accept this vaccine are key barriers to manage the COVID-19 pandemic in different countries including Ethiopia. Control of the pandemic will depend on the acceptance of coronavirus disease vaccine. However, there is a paucity of evidence on coronavirus disease vaccine acceptance in the study area. The current study was aimed to assess willingness to accept the COVID-19 vaccine and associated factors among adult clients attending Bule Hora University Teaching Hospital, West Guji Zone, southern Ethiopia.
Methods: An institution-based cross-sectional study was conducted among 385 study participants selected by a systematic random sampling technique. Data was collected through observation and structured questionnaires from April 10 to May 30, 2022. The collected data was cleaned and entered into EpiData 3.1 software before being exported to SPSS 25 statistical software for analysis. Bi-variable and multi-variable binary logistic regression model was used to identify the predictors of COVID-19 vaccine acceptance. The strength of association was measured using AOR with 95% confidence interval and significance was declared at p- value < 0.05.
Result: Magnitude of willingness to accept coronavirus disease-19 vaccine was 67.5% (95%Cl: 63–72). Good knowledge [AOR = 2.07, (1.17–3.64)], history of chronic disease [AOR = 2.59, (1.4–4.78)], being a government employee [AOR = 2.35 (1.1–5)], having a favorable attitude [AOR = 14.15 (5.25–37.46)], and good adherence [AOR = 1.74 (1.02–2.97)] were factors that significantly associated with willingness to accept the coronavirus disease 2019 vaccine.
Conclusion: Magnitude of willingness to accept the COVID-19 vaccine was considerable and needs to be improved. Knowledge, attitude, chronic illness, adherence, and being a government employee were factors that associated with willingness to accept the vaccine. Community awareness, advocacy, social mobilization and health education should be given at different levels.
Introduction
The COVID-19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (1). Vaccines are life-saving inventions that have been responsible for the suppression and control of many infectious diseases in many parts of the world (2). In addition to providing direct immunity and preventing disease among vaccinated individuals, they have been shown to protect unvaccinated individuals through herd immunity, if a greater proportion of the population is immune (2). As the number of cases of coronavirus disease (COVID-19) is increasing worldwide, promising COVID-19 vaccine candidates are being produced, like the AstraZeneca, Johnson and Johnson, Sinopharm, and Pfizer vaccines, to fight the coronavirus disease (COVID-19) pandemic; researchers from all over the world have made remarkable efforts to create vaccines against the disease (3–5).
After the incidence of COVID- 19 pandemic, the World Health Organization (WHO) and health care institutions are working on prevention, diagnosis, and treatment including the development of a COVID-19 vaccine, manufactured one year after WHO confirmed COVID-19 to be a global public health emergence. Due to outstanding determination in vaccine research, the COVID-19 vaccines were developed within a very short period of time compared to general vaccine history (6). The AstraZeneca vaccine was developed at the Serum Institute of India (SII) and was provided to Ethiopia on 6 March 2021 with the aim of reducing recent COVID-19 infections (6). The COVID-19 Vaccines Global Access (CovAx) facility allocated 7,620,000 doses of the COVID-19 vaccine for Ethiopia, of which about 2,184,000 doses were already received (7). According to recent global delivery plan, 5.4 million doses of the COVID-19 vaccine were expected to reach Ethiopia by May 2021. The Ministry of Health planned to have 20% of the population in Ethiopia to be vaccinated by the end of 2021 (8).
The main source of vaccine hesitance may be due to considerable amount of misinformation regarding the COVID-19 vaccine that was flowing on social media (9). Globally, willingness to accept the COVID-19 vaccine was reported to be 71.7% in the United Kingdom (10), and ranged from 31% to 74% in Hungary, Japan and Israel (11). The willingness to take COVID-19 vaccine was found to be 40% in China (12). In Africa around 63% of all participants surveyed were eager to accept the COVID-19 vaccine (13). Systematic review and meta-analysis in Ethiopia revealed that the overall magnitude of COVID-19 vaccine acceptance was 56.02% (14).
Therefore, vaccine uptake can be influenced by various risk factors, including the perception that the vaccine may cause adverse effects, attitudes towards vaccination, knowledge of vaccines, misconceptions, fear of unforeseen side effects, social influence, having trust in the health care professional, and having increased information about the COVID-19 vaccine (15). Being female, being an older age, marital status, residence, occupation, not having a health-related job, religion, and educational status were statistically significantly associated with willingness to receive the COVID-19 vaccine (16).
However, poor knowledge and unwillingness to receive the vaccination is a potential barrier to handle the COVID-19 pandemic in the long term and may cause a heavy burden of morbidity, mortality and economic crisis around globe. Since vaccinations are central to the control of COVID-19, its success relies on having safe and effective vaccination and also high levels of vaccine uptake by the public over time (17, 18).
Globally, over 1.3 million doses of the COVID-19 vaccine have been ordered with 4.1% of the individuals being fully vaccinated as of 10 May 2021 (19). In Africa, with 49 countries now rolling out COVID-19 vaccines as of May 2021, more than 30 countries have less than 1% coverage with a continental average of 2.5% (20). Ethiopia received about 2.2 million doses of AstraZeneca COVID-19 vaccines in March 202, and sources disclose that close to 1.9 million people in Ethiopia have already been vaccinated for the first dose of AstraZeneca (21).
In Oromia, currently around 42.2% of health workers accepted the COVID-19 vaccine (22). The suppression of the ongoing community spread of COVID-19 disease is only possible with adequate vaccine coverage to develop herd immunity within the community and through mass media, non-governmental agencies like WHO, and the government continuously working to build vaccine literacy among the public to accept the vaccine when is available and appropriate (23). Regardless of these efforts to reduce the burden of COVID-19 via vaccination and other measures, unwillingness to take the COVID-19 vaccine increased worldwide and hindered the effort to control its spread (24).
However, knowledge, attitude towards the COVID-19 vaccine, adherence level to mitigation measures, and presence of chronic disease were not well known in the southern part of Oromia and the study area (3, 25). Therefore, the current study aimed to assess willingness to accept th COVID-19 vaccine and its predictors.
Materials and methods
Study design and setting
A cross-sectional study was conducted at Bule Hora University Teaching Hospital in West Guji Zone, Oromia, south Ethiopia from April 10 to May 30, 2022. It is 467 km from Addis Ababa. The hospital served around 1,568,547 people and employed 408 staff (117 administrative and 233 clinical staff). In the year 2021, about 3,528 patients will be served in the outpatient service at Bule Hora Teaching Hospital.
Inpatient services at Bule Hora University Teaching Hospital include obstetric, gynaecologic, and neonatal intensive care units, as well as medical and surgical wards. Outpatient services include ANC, postnatal care, ART clinic, PMTCT, family planning, ophthalmology care, psychiatry, dental care, cervical cancer screening, under-5 OPD, emergency OPD, and adult OPD's. Additional services include laboratory and pharmaceutical services, as well as US and x-ray services.
Population and sampling
All clients aged ≥18 years attending Bule Hora University Teaching Hospital were the source population while all randomly selected clients aged ≥18 years and attending Bule Hora University Teaching Hospital during data collection time were the study population. Those clients who had been vaccinated were excluded.
The sample size for the first objective was calculated by using a single population proportion formula considering 59.4% (26) proportion of willingness to receive the COVID-19 vaccine from previous study, with assumptions of confidence level at 95%, a margin of error (d) 5% and adding 10% for non-response as follows:
The sample size for the second specific objective was determined by considering factors that were significantly associated with the outcome variable, two-sided confidence level of 95%, the margin of error of 5%, power of 80% and the ratio of exposed to unexposed 1:1 using EPI-Info software. Considering 10% for nonresponse the final sample size for the second objective was determined. Hence, the largest sample size was taken from first objective, 385.
Six outpatient departments were chosen from the total departments in the hospital using a simple random sampling technique by lottery method. A systematic random sampling procedure was used to choose clients from these six outpatient departments; the first client was selected using a simple random sampling technique, and the others were selected at 9 regular intervals until the required sample size was reached.
Data collection procedure and instruments
The data were collected through interviews and observations using a pre-tested structured questionnaire, which was adapted from published papers (16, 25–27). It consists of four sections including socio-demographic characteristics, knowledge about the COVID-19 vaccine, attitude towards the COVID-19 vaccine, adherence toward COVID-19 mitigation measures, and willingness to receive the COVID-19 vaccine. Prior to data collection, two days of training was given for data collectors and supervisors on the study objectives, subject eligibility criteria and data collection methods. The data was collected by 6 BSc nurses and supervised by 2 BSc nurses.
Operational definitions
Willingness: a state of being prepared or readiness to receive the COVID-19 vaccine. COVID-19 vaccine acceptance was measured using a “Yes” and “No” question: the participant was asked “Are you willing to be vaccinated against COVID-19?” (26).
Knowledge: Eight items were used to assess the knowledge level of the client about the COVID-19 vaccine. Those who correctly answered the question were coded as ″1″, while incorrect answers were given ″0″ values. Participants who scored 70% and above were considered as having good knowledge while those who scored less than 70% were considered as having poor knowledge towards the COVID-19 vaccine (26).
Adherence towards COVID-19 mitigation measures: a composite variable generated from hand washing, using a facemask, keeping physical distance, not travelling to a crowded place, staying at home, and not travelling to any place out of the city in the last 14 days. Hence, an individual was considered as having good adherence towards COVID-19 mitigation measures if they were able to answer “yes” to the median and above of the aforementioned composite variables (28).
Attitude: a settled way of thinking or point-of-view about the COVID-19 vaccine of patients attending the hospital was assessed by assigning one point for each correct answer. The attitude level indicated by the Likert scale: clients who strongly agree, 5 points; agree, 4 points; neutral, 3 points; disagree, 2 points; and strongly disagree, 1 point for positive question and vice versa for negative one. The respondent attitude ranged from 1 to 25 and a cutoff point greater than equal ≥44% (11–25) was considered as a favorable attitude while less <40% were taken as unfavorable attitude toward the COVID-19 vaccine (26).
Data quality control
To ensure data quality, a pre-test was conducted among 5% of the sample size in Yabello General Hospital to ensure the validation of the tool. Amendments were made based on the feedback of the pre-test before the commencement of the final data collection. Two days training was given for the data collectors and supervisors on the aim of the study, clarity of the measuring tool, and ethical considerations. The quality of the data was monitored frequently in the field through close supervision of data collectors.
Data processing and analysis
The coded data were entered in to EpiData software version 3.1 and it was exported to SPSS version 25 for further analysis. Descriptive statistics were computed to describe sample population characteristics relevant to the variables. Logistic regression was fitted to identify factors associated with willingness to accept the COVID-19 vaccine. The analysis was conducted to select candidate variables for the multivariable model. Those variables that show association with willingness at a p-value less than 0.25 were included in the multivariable logistic regression model. Both crude and adjusted odds ratios with their corresponding 95% confidence interval were used to determine the strength of association. Multicollinearity was checked by using VIF to find correlations between independent variables; no variables with VIF >10 were observed. The model goodness of fit was tested by the Hosmer and Lemeshow statistical test; the model was considered a good fit since it was found to be non-significant for Hosmer and Lemeshow (P = 0.651). Statistical significance was declared at p-value < 0.05.
Results
Socio-demographic characteristics
This study included 385 participants, with a 100% response rate. There were 207 (53.8%) females participants and 215 (55.8%) participants were married. Two hundred fifty (63%) of the participants are between the ages of 18 and 35, with a median age of 30 years. One hundred twenty nine (33.5%) of the respondents had completed secondary education and above. The majority [267 (69.4%)] of participants lived in urban areas; 309 (80.3%) were Oromo by ethnicity; 33.8% worked for the government; and 229 (59.5%) had monthly incomes of at least 4,000 Ethiopian Birr (ETB) (Table 1).
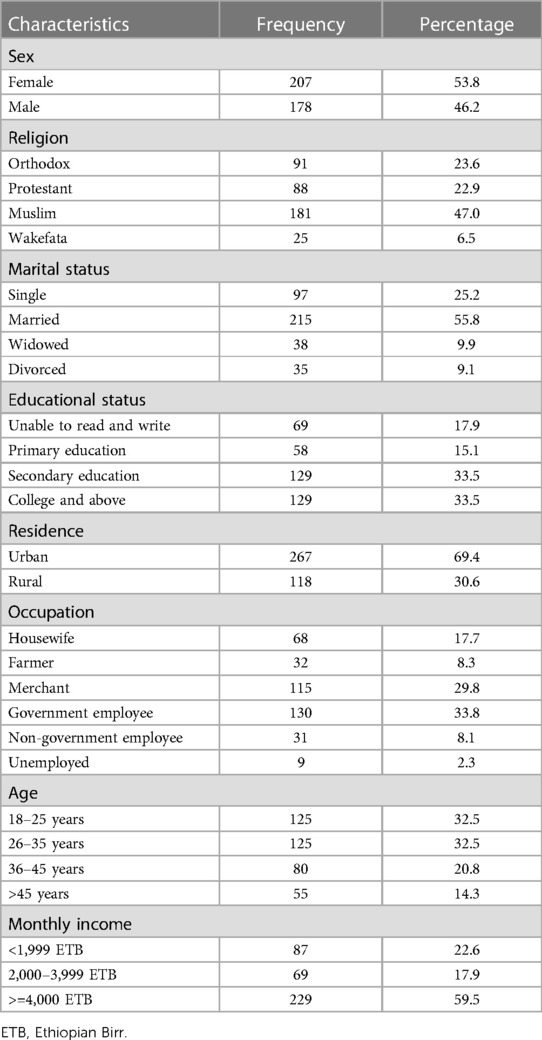
Table 1. Socio-demographic characteristics of the study participants at Bule Hora University teaching hospital, 2022.
Knowledge towards COVID-19 vaccine
Of the 385 study participants, 86 (22.3%) were still of the view that COVID-19 does not exist in Ethiopia. 295 (76.6%) of them were unaware that the vaccine must be administered twice during a 28-day period. In total, 179 (46.5%) of the participants were well-informed about the COVID-19 vaccination (Table 2).
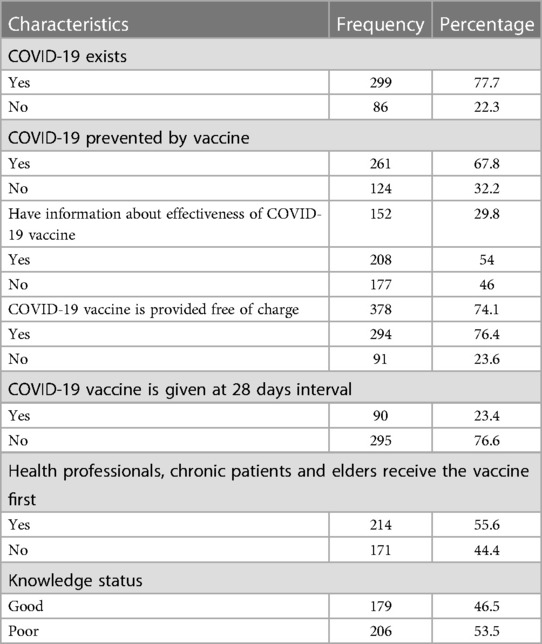
Table 2. Participant knowledge about the COVID-19 vaccine at Bule Hora University teaching hospital, 2022.
Attitude towards the COVID-19 vaccine
Of the respondents, 190 (49.4%) disagree that COVID-19 is a minor disease that does not necessitate vaccination. Yet, 122 (31.7%) of the participants strongly believe that taking additional precautionary measures is far superior to receiving the COVID-19 vaccine. On the other hand, 60 (15.6%) of participants strongly agree that the negative effects of the COVID-19 vaccine outweigh the vaccination benefit, and 43 (11.2%) of respondents strongly agree that being infected with COVID-19 disease is preferable to receiving the vaccine. Overall, 272 (70.6%) of respondents were had a positive attitude towards the COVID-19 vaccine (Table 3).

Table 3. Attitude towards willingness to accept the COVID-19 vaccine among adult clients attending Bule Hora University teaching hospital, 2022.
Adherence to mitigation measures of COVID-19
Of 385 participants, 62.6% did not stay at home when they experienced flu-like symptoms. The majority of participants, 289 (75.1%), covered their mouth during sneezing or coughing. When they arrive for service, approximately 68.6% of respondents wear a face mask. Overall, 215 (55.8%) of the clients adhered to the COVID-19 mitigation measures (Table 4).
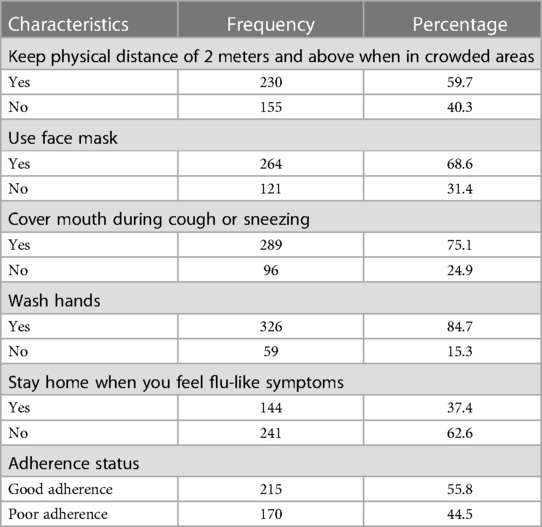
Table 4. Adherence to mitigation measures of COVID-19 toward willingness to accept the COVID-19 vaccine among adult clients attending outpatient services at Bule Hora University teaching hospital, 2022.
Willingness to accept COVID-19 vaccine
According to the findings of this study, nearly 260 (67.5%) (95% Cl: 63–72) of the participants were willing to accept the COVID-19 vaccine if it was provided for free (Figure 1).
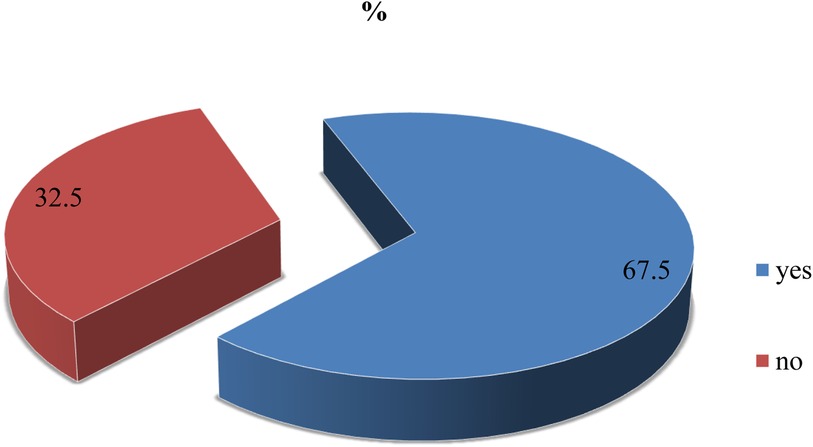
Figure 1. Willingness to accept the COVID-19 vaccine among adult clients attending outpatient services at Bule Hora University teaching hospital, 2022.
Factors associated with willingness to accept COVID-19 vaccine
Bivariable analysis was performed using odds ratio at 95% confidence interval and variables with P- value of < 0.25 in bivariable analysis were considered as candidates for multivariable logistics regression. The result of bivariable logistic analysis shows that residence, age, occupation, chronic illness, knowledge, attitude and adherence were factors associated with willingness to accept the COVID-19 vaccine. In multivariable analysis the occupation, chronic illness, knowledge, attitude, and level of adherence to mitigation measures of the respondents were associated with willingness to accept the COVID-19 vaccine. Government employee were 2 fold more likely willing to accept the COVID-19 vaccine compared to (AOR = 2.35; 95% Cl: 1.1–5.0) their counterparts.
Clients with a history of chronic diseases were 2.59 times more likely to be willing to accept the COVID-19 vaccine (AOR = 2.59:95% Cl: 1.4–4.78) compared to those without a history of chronic illness.
Those clients with good knowledge were 2 times more likely to be willing to accept the COVID-19 vaccine (AOR = 2.07; 95% Cl: 1.17–3.64) than those with poor knowledge. Clients with favorable attitudes towards the COVID-19 vaccine were 14 times more likely to accept the vaccine (AOR = 14.15; 95% Cl: 5.25–37.46) compared to clients with unfavorable attitudes. Those clients with good adherence were 1.74 times more likely to accept the COVID-19 vaccine with (AOR = 1.74; 95%; Cl; 1.02–2.97) when compared to those with poor adherence (Table 5).
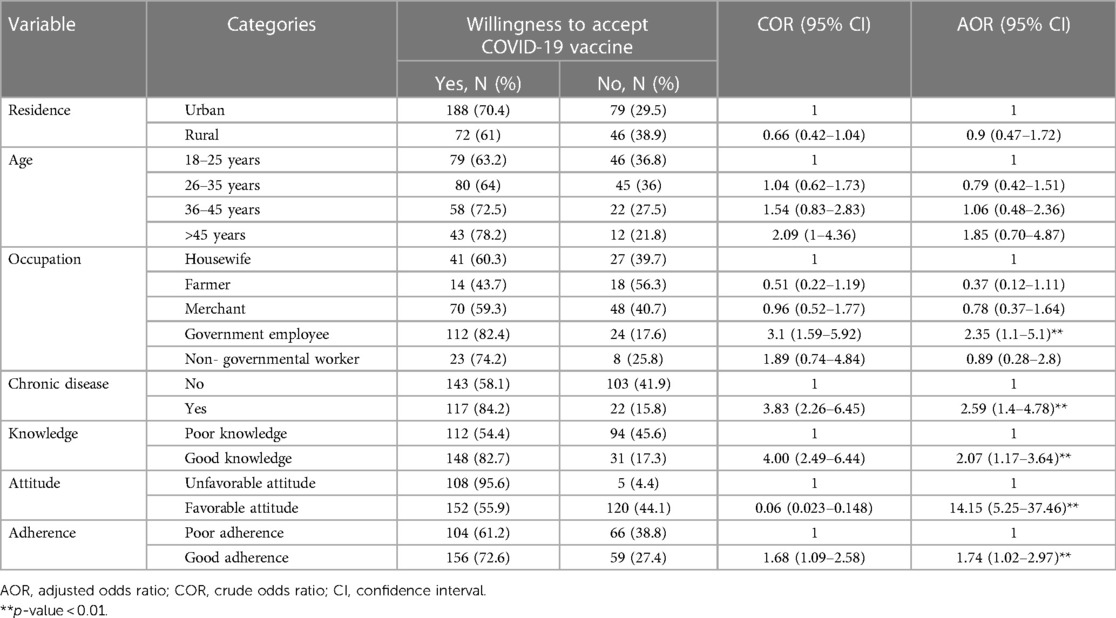
Table 5. Bivariable and multivariable analysis to identify factors associated with willingness to accept the COVID-19 vaccine among adult client attending outpatient services at Bule Hora University teaching hospital, 2022.
Discussion
The COVID-19 vaccine is the best strategy for preventing the spread of COVID-19 worldwide, and willingness to receive the COVID-19 vaccine has a significant impact on COVID-19 mitigation. The magnitude of willingness to accept the COVID-19 vaccine among adult clients who attended Bule Hora University Teaching Hospital was 67.5% (95% Cl: 63–72). Government employees, clients with chronic illness, and clients with good knowledge, favorable attitudes, and good adherence were significantly associated with willingness to accept the COVID-19 vaccine.
This is consistent with other cross-sectional studies done in Korea (61.8%) (29), in Poland (60.3%) (30), in Saudi Arabia (64.7%) (31), 34 African countries (63%) (13), in East Africa (60.2%) (32), in Libya (60.6%) (33), in Northeast Ethiopia (64%) (34) and (69.3%) (35), in Northwest Ethiopia (62.04%) (36), in South Ethiopia and in Gurage (62.9%) (3) and (61%) (25), respectively.
The current study result is lower than vaccine willingness studies conducted in China (90.6%) (37), Austria (89.8%) (38), the United Kingdom (71%) (10) and Israel (74%) (11), respectively. The difference might be due to community awareness level, burden of diseases, and variation in the availability of vaccine types, and socio-demographic characteristics. Moreover, the variation could be explained by differences in awareness on the severity of COVID-19 and access to health care services. However, our result is higher than studies in Tunisia (35%) (39) and in Ethiopia (36.02%) (40), respectively. The difference might be difference in a sample size used; the Tunisian study is smaller than the current study and the Ethiopian study used larger sample size and considered the impact of patients hearing rumors that the COVID-19 vaccine might have negative side effects or might cause the virus itself (40).
In this study, government employees were 2.35 times more likely to be willing to accept the COVID-19 vaccine. This is consistent with studies conducted in Gondar city in North West Ethiopia (41) and an E-survey conducted in Ethiopia (42). This could be due to the fact that individuals employed by the government might have more access to information and knowledge compared to other populations. Clients with a history of chronic disease were 2.59 times more likely to accept the COVID-19 vaccine compared to those with no history of chronic disease. This finding is in line with studies conducted in Wolaita Sodo town (27), in Gurage Zone (3), and a systemic review and meta-analysis in Ethiopia (43). The possible reasons for this could be health education, burden of disease and that first priority was given for individuals with chronic disease to be vaccinated first.
In this study, clients with good knowledge were 2 times more likely to be willing to accept the COVID-19 vaccine compared to those with poor knowledge. This is consistent with a systematic review and meta-analysis in East Africa (32), in Ethiopia (43), North East Ethiopia (26), in southern Ethiopia among adult populations (3) and southern Ethiopia among lactating mothers (25). The possible explanation could be that those who had awareness of the vaccine might know the benefits of being vaccinated, such as halting the transmission of new COVID-19 infections and preventing the possibility of further morbidity and mortality caused by infections.
Clients with favorable attitudes towards the COVID-19 vaccine were 14 fold more likely to accept the vaccine compared to client with unfavorable attitudes. This is consistent with systemic reviews and meta-analysis in East Africa (32), Ethiopia (44), Northeast Ethiopia (45), and also Northwest Ethiopia (46). The possible explanation might be that having a positive attitude towards vaccination and its potential for prevention of further complications associated with COVID-19 might encourage people to show a willingness to receive the available COVID-19 vaccine.
In this study, those clients with good adherence to COVID-19 mitigation guidelines were 1.74 times more likely to accept the COVID-19 vaccine when compared to those with poor adherence. This finding is in line with systemic reviews and meta-analysis Ethiopia, in Gonder city residents in northwest Ethiopia (28), in South Ethiopia among lactating mothers (25), and in Northeast Ethiopia (45). This could be because individuals who had good mitigation practices know the burden of COVID-19 infections on the health of the general population.
Strengths
This study focuses on COVID-19 vaccine acceptability and associated factors, which will be useful for decision makers, policy designers, implementers, and managers of health care organizations at all levels to enhance vaccination uptake.
Limitations
Since only Johnson & Johnson and AstraZeneca provided the COVID-19 vaccine at the study site, the age limit has been set at 18 years and above. But, when data collecting was completed, other vaccines, such as Pfizer, were administered to clients aged 12 and up. It would be preferable if clients aged 12 and up were included in the research. Furthermore, due to the nature of the study design, causal inference may not be inferred from this study.
Conclusion
The willingness to receive the COVID-19 vaccination was 67.5% in the study area. Good knowledge, a favorable attitude, a history of chronic illness, being a government employee, and good adherence to mitigation measures were all associated with a higher willingness to accept the COVID-19 vaccine. Health education should be expanded through the Bule Hora University Teaching Hospital's mini media and community education and health promotion regarding the COVID-19 vaccine should be provided. Advocacy and social mobilization should be undertaken to improve community understanding, attitudes towards, and perception of the COVID-19 vaccine.
It is suggested that further community-based qualitative research on COVID-19 vaccination acceptance should be conducted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical clearance was obtained from Bule Hora University research and publication directorate. After the purpose of the study was explained to participants, informed consent with a written signature was obtained. Participants were informed that they could withdraw at any time and/or refrain from responding to questions. Study participants were also informed that all data obtained from them could be kept confidential using code instead of any personal identifiers. Furthermore, the research procedure were conducted in accordance with the principles expressed in the World Medical Association's Declaration of Helsinki.
Author contributions
AH, LA, DJ, AA, and TS conceived the idea and designed the study; led data analysis and interpretation; developed the first draft of the manuscript and made all revisions based on coauthors comments and suggestions. AH, LA, DJ, AA, and TS critically revised the manuscript for important intellectual content; ensured the requirements of submission of the manuscript are met. AH, LA, DJ, AA, and TS contributed towards analysis and data interpretation; revision and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to express our great appreciation to the head of hospitals, participants and data collectors for their selfless provision of continuous support and facilitation during the data collection processes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC. Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci. (2020) 27(1):104. doi: 10.1186/s12929-020-00695-2
2. Alle YF, Oumer KE. Attitude and associated factors of COVID-19 vaccine acceptance among health professionals in debre tabor comprehensive specialized hospital, north central Ethiopia; 2021: cross-sectional study. Virusdisease. (2021) 32((2):272–8. doi: 10.1007/s13337-021-00708-0
3. Abebe H, Shitu S, Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resist. (2021) 14:2015–25. doi: 10.2147/IDR.S312116
5. Janssen Vaccines and PRevention B.V. A study of Ad26. COV2. S for the prevention of SARS-CoV-2-mediated COVID-19 in adult participants. (2020). Available at: clinicaltrials.gov; NCT04505722.
6. European Medicines Agency. COVID-19 vaccines: Development, evaluation, approval and monitoring. Amsterdam: EMA (2020).
7. Ethiopia Cr-o-. Willingness of Ethiopian population to receive COVID-19 vaccine. J Multidiscip Healthc. (2021) 14:1233–43. doi: 10.2147/JMDH.S312637
8. Africa News. Ethiopia launches COVID vaccination in Addis Ababa. Africanews March 14 2021 [Internet]. Available at: https://www.africanews.com/2021/03/14/ethiopia-launches-covid-vaccinations-in-addis-ababa/ (Accessed May 19, 2021).
9. Singh L, Bansal S, Bode L, Budak C, Chi G, Kawintiranon K, et al. A first look at COVID-19 information and misinformation sharing on twitter. arXiv. (2020) 52. doi: 10.48550/arXiv.2003.13907
10. Freeman D, Loe BS, Chadwick A, Vaccari C, Waite F, Rosebrock L, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (oceans) II. Psychol Med. (2020):1–15. doi: 10.1017/s0033291720005188
11. Goodwin R, Ben-Ezra M, Takahashi M, Luu LN, Borsfay K, Kovács M, et al. Psychological factors underpinning vaccine willingness in Israel, Japan and Hungary. Sci Rep. (2022) 12(1):439. doi: 10.1038/s41598-021-03986-2
12. Hong J, Xu XW, Yang J, Zheng J, Dai SM, Zhou J, et al. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in eastern China: a cross-sectional survey. J Integr Med. (2022) 20(1):34–44. doi: 10.1016/j.joim.2021.10.004
13. Anjorin AA, Odetokun IA, Abioye AI, Elnadi H, Umoren MV, Damaris BF, et al. Will Africans take COVID-19 vaccination? PLoS One. (2021) 16(12):e0260575. doi: 10.1371/journal.pone.0260575
14. Mekonnen BD, Mengistu BA. COVID-19 vaccine acceptance and its associated factors in Ethiopia: a systematic review and meta-analysis. Clin Epidemiol Glob Health. (2022) 14:101001. doi: 10.1016/j.cegh.2022.101001
15. Zewude B, Habtegiorgis T, Hizkeal A, Dela T, Siraw G. Perceptions and experiences of COVID-19 vaccine side-effects among healthcare workers in southern Ethiopia: a cross-sectional study. Pragmat Obs Res. (2021) 12:131–45. doi: 10.2147/POR.S344848
16. Belsti Y, Gela YY, Akalu Y, Dagnew B, Getnet M, Abdu Seid M, et al. Willingness of Ethiopian population to receive COVID-19 vaccine. J Multidiscip Healthc. (2021) 14:1233–43. doi: 10.2147/JMDH.S312637
17. Paul E, Steptoe A, Fancourt D. Attitudes towards vaccines and intention to vaccinate against COVID-19: implications for public health communications. Lancet Reg Health Eur. (2021) 1:100012. doi: 10.1016/j.lanepe.2020.100012
18. Williams L, Flowers P, McLeod J, Young D, Rollins L. The catalyst project team. Social patterning and stability of intention to accept a COVID-19 vaccine in Scotland: will those most at risk accept a vaccine? Vaccines (Basel). (2021) 9(1):17. doi: 10.3390/vaccines9010017
19. Bongomin F, Olum R, Andia-Biraro I, Nakwagala FN, Hassan KH, Nassozi DR, et al. COVID-19 vaccine acceptance among high-risk populations in Uganda. Ther Adv Infect Dis. (2021) 8:20499361211024376. doi: 10.1177/20499361211024376
20. Tessema SK, Nkengasong JN. Understanding COVID-19 in Africa. Nat Rev Immunol. (2021) 21(8):469–70. doi: 10.1038/s41577-021-00579-y
21. AllAfrica. Ethiopia receives the first Astra Zeneca COVID-19 vaccine. All Africa March 7, 2021. Available at: https://allafrica.com/stories/202103070285.html
22. Bereda G, Bereda G. Eagerness to acceptance of COVID-19 vaccine among health care workers in Oromia regional state, Ethiopia. An online based cross-sectional study, 2021. Austin J Pulm Respir Med. (2021) 8(3):1077. doi: 10.26420/austinjpulmrespirmed.2021.1077
23. Narapureddy BR, Muzammil K, Alshahrani MY, Alkhathami AG, Alsabaani A, AlShahrani AM, et al. COVID-19 vaccine acceptance: beliefs and barriers associated with vaccination among the residents of KSA. J Multidiscip Healthc. (2021) 14:3243–52. doi: 10.2147/JMDH.S340431
24. Frederiksen LSF, Zhang Y, Foged C, Thakur A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol. (2020) 11:1817. doi: 10.3389/fimmu.2020.01817
25. Mose A. Willingness to receive COVID-19 vaccine and its determinant factors among lactating mothers in Ethiopia: a cross-sectional study. Infect Drug Resist. (2021) 14:4249–59. doi: 10.2147/IDR.S336486
26. Berihun G, Walle Z, Berhanu L, Teshome D. Acceptance of COVID-19 vaccine and determinant factors among patients with chronic disease visiting dessie comprehensive specialized hospital, northeastern Ethiopia. Patient Prefer Adherence. (2021) 15:1795–805. doi: 10.2147/PPA.S324564
27. Zewude B, Habtegiorgis T. Willingness to take COVID-19 vaccine among people most at risk of exposure in southern Ethiopia. Pragmat Obs Res. (2021) 12:37–47. doi: 10.2147/POR.S313991
28. Azene ZN, Merid MW, Muluneh AG, Geberu DM, Kassa GM, Yenit MK, et al. Adherence towards COVID-19 mitigation measures and its associated factors among Gondar city residents: a community-based cross-sectional study in northwest Ethiopia. PLoS One. (2020) 15(12):e0244265. doi: 10.1371/journal.pone.0244265
29. Chun JY, Kim SI, Park EY, Park SY, Koh SJ, Cha Y, et al. Cancer patients’ willingness to take COVID-19 vaccination: a nationwide multicenter survey in Korea. Cancers (Basel). (2021) 13(15):411. doi: 10.3390/cancers13153883
30. Brodziak A, Sigorski D, Osmola M, Wilk M, Gawlik-Urban A, Kiszka J, et al. Attitudes of patients with cancer towards vaccinations-results of online survey with special focus on the vaccination against COVID-19. Vaccines (Basel). (2021) 9(5):411. doi: 10.3390/vaccines9050411
31. Al-Mohaithef M, Padhi BK. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J Multidiscip Healthc. (2020) 13:1657–63. doi: 10.2147/JMDH.S276771
32. Alemayehu A, Demissie A, Yusuf M, Gemechu Lencha A, Oljira L. COVID-19 vaccine acceptance and determinant factors among general public in east Africa: a systematic review and meta-analysis. Health Serv Res Manag Epidemiol. (2022) 9:23333928221106269. doi: 10.1177/23333928221106269
33. Elhadi M, Alsoufi A, Alhadi A, Hmeida A, Alshareea E, Dokali M, et al. Knowledge, attitude, and acceptance of healthcare workers and the public regarding the COVID-19 vaccine: a cross-sectional study. BMC Public Health. (2021) 21(1):955. doi: 10.1186/s12889-021-10987-3
34. Adane M, Ademas A, Kloos H. Knowledge, attitudes, and perceptions of COVID-19 vaccine and refusal to receive COVID-19 vaccine among healthcare workers in northeastern Ethiopia. BMC Public Health. (2022) 22(1):128. doi: 10.1186/s12889-021-12362-8
35. Taye BT, Amogne FK, Demisse TL, Zerihun MS, Kitaw TM, Tiguh AE, et al. Coronavirus disease 2019 vaccine acceptance and perceived barriers among university students in northeast Ethiopia: a cross-sectional study. Clin Epidemiol Glob Health. (2021) (12):100848. doi: 10.1016/j.cegh.2021.100848
36. Taye EB, Taye ZW, Muche HA, Tsega NT, Haile TT, Tiguh AE. COVID-19 vaccine acceptance and associated factors among women attending antenatal and postnatal cares in central Gondar zone public hospitals, northwest Ethiopia. Clin Epidemiol Glob Health. (2022) 14:100993. doi: 10.1016/j.cegh.2022.100993
37. Zhou Q, Tian T, Ni J, Zhao X, Li H, Yang Y, et al. COVID-19 vaccination acceptance in China after it becomes available: a cross-sectional study. Vaccines (Basel). (2021) 9(12):3883. doi: 10.3390/vaccines9121398
38. Duong MC, Nguyen HT, Duong BT. Who influences the public intention to get a COVID-19 vaccine and what are the public references and concerns? A population survey in Vietnam. Infect Chemother. (2021) 53(4):753–66. doi: 10.3947/ic.2021.0122
39. Khiari H, Cherif I, M’ghirbi F, Mezlini A, Hsairi M. COVID-19 vaccination acceptance and its associated factors among cancer patients in Tunisia. Asian Pac J Cancer Prev. (2021) 22(11):3499–506. doi: 10.31557/APJCP.2021.22.11.3499
40. Tadele Admasu F. Knowledge and proportion of COVID-19 vaccination and associated factors among cancer patients attending public hospitals of Addis Ababa, Ethiopia, 2021: a multicenter study. Infect Drug Resist. (2021) 14:4865–76. doi: 10.2147/IDR.S340324
41. Shitu K, Wolde M, Handebo S, Kassie A. Acceptance and willingness to pay for COVID-19 vaccine among school teachers in Gondar city, northwest Ethiopia. Trop Med Health. (2021) 49(1):63. doi: 10.1186/s41182-021-00337-9
42. Rikitu Terefa D, Shama AT, Feyisa BR, Ewunetu Desisa A, Geta ET, Chego Cheme M, et al. COVID-19 vaccine uptake and associated factors among health professionals in Ethiopia. Infect Drug Resist. (2021) 14:5531–41. doi: 10.2147/IDR.S344647
43. Belay GM, Alemu TG, Techane MA, Wubneh CA, Assimamaw NT, Tamir TT, et al. COVID-19 vaccine acceptance rate and its predictors in Ethiopia: a systematic review and meta-analysis. Hum Vaccin Immunother. (2022) 18(6):2114699. doi: 10.1080/21645515.2022.2114699
44. Mose A, Wasie A, Shitu S, Haile K, Timerga A, Melis T, et al. Determinants of COVID-19 vaccine acceptance in Ethiopia: a systematic review and meta-analysis. PLoS One. (2022) 17(6):e0269273. doi: 10.1371/journal.pone.0269273
45. Tefera Z, Assefaw M. A mixed-methods study of COVID-19 vaccine acceptance and its determinants among pregnant women in northeast Ethiopia. Patient Prefer Adherence. (2022) 16:2287. doi: 10.2147/PPA.S374217
Keywords: willingness, COVID-19, vaccine, acceptance, Ethiopia
Citation: Huka AE, Alemeyehu L, Jara D, Ayele A and Shifa T (2023) Predictors of willingness to accept COVID-19 vaccine among adults. Front. Epidemiol. 3:1240557. doi: 10.3389/fepid.2023.1240557
Received: 15 June 2023; Accepted: 5 October 2023;
Published: 27 October 2023.
Edited by:
Tobias Kurth, Charité University Medicine Berlin, GermanyReviewed by:
Suhaily Mohd Hairon, Universiti Sains Malaysia Health Campus, MalaysiaLuiz Ricardo Berbert, Federal University of Rio de Janeiro, Brazil
© 2023 Huka, Alemeyehu, Jara, Ayele and Shifa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alo Edin Huka amlibG9zYTFAZ21haWwuY29t
Abbreviations ANC, antenatal care; AOR, adjusted odds ratio; ART, anti-retroviral therapy; CI, confidence interval; COR, crude odds ratio; COVAX, coronaviruses disease vaccine global access; COVID-19, coronaviruses disease 2019; ETB, Ethiopia Birr; HIV, human immunodeficiency virus; IESO, integrated emergency surgery and obstetric; MCH, maternal and child health; MOH, Ministry of Health; OPD, out patient department; PMTCT, prevention of mother to child transmission; SARS, COV-2 severe acute respiratory syndrome Cov-2; SPSS, statistical package for social sciences; WHO, World Health Organization.
 Alo Edin Huka
Alo Edin Huka Lami Alemeyehu
Lami Alemeyehu Angefa Ayele
Angefa Ayele