
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Epidemiol. , 23 February 2023
Sec. Neurological and Mental Health Epidemiology
Volume 3 - 2023 | https://doi.org/10.3389/fepid.2023.1081757
This article is part of the Research Topic Epidemiology of Epilepsy and Seizures View all 5 articles
Patients with epilepsy carry a risk of premature death which is on average two to three times higher than in the general population. The risk of death is not homogenously distributed over all ages, etiologies, and epilepsy syndromes. People with drug resistant seizures carry the highest risk of death compared to those who are seizure free, whose risk is similar as in the general population. Most of the increased risk is directly related to the cause of epilepsy itself. Sudden unexplained death in epilepsy patients (SUDEP) is the most important cause of epilepsy-related deaths especially in the young and middle-aged groups. Population based studies with long-term follow up demonstrated that the first years after diagnosis carry the highest risk of death, while in the later years the mortality decreases. Improved seizure control and being exposed to a specialized comprehensive care centre may help to reduce the risk of death in patients with epilepsy. The mortality of status epilepticus is substantially increased with case fatality rates between 4.6% and 39%, depending on its cause and duration, and the age of the population studied. The epidemiological data on overall and cause specific mortality as well as their determinants and risk factors are critically reviewed and methodological issues pertinent to the studies on mortality of epilepsy and Status epilepticus are discussed.
Epilepsy is a severe neurological condition affecting approximately up to 70 million people worldwide (1–3). With an incidence of 50/100,000 patient years and a prevalence of 700/100,000, epilepsy accounts for more than 0.5% of the total global burden of disease (1). Each year, 2.4 million people are diagnosed with epilepsy, contributing to 20.6 million disability-adjusted life years lost (1).
There is a large variation in the prevalence of epilepsy in different parts of the world, especially between high- and low-income countries (4). The majority of people with epilepsy (PWE) live in low- and middle-income countries in South-East Asia, Latin America, and sub-Saharan Africa, where the rate of new cases is up to two-fold higher than that of high-income countries (1, 5). Throughout each region, lifetime prevalence rates of epilepsy are 6/1,000 population in Asia and western countries, 17.8/1,000 population in Latin America, and 15.0/1,000 population in sub-Saharan Africa (3, 6, 7). There is also a tendency towards a higher prevalence of epilepsy in rural areas than in urban areas (4, 6).
However, as many as three-quarters of people with epilepsy in low-income countries do not have access to the treatment they need, due to low availability and affordability of antiseizure medications (ASM). Additionally, misconceptions, stigma, and discrimination are greater obstacles to the well-being of people with epilepsy than lack of adequate healthcare in these areas (8).
Persons with epilepsy, who become seizure free on ASMs can almost invariably lead a normal life. The consequences of drug resistant epilepsy are the major driver on the huge burden associated with epilepsy. In drug resistant epilepsy, comorbidities are significantly increased and further complicate the management of this serious neurological condition (9). Comorbidity is defined as the presence of one or more additional conditions co-occurring with a primary condition. The term is used to describe the simultaneous presence of two chronic diseases or conditions in a patient (10). The prevalence of comorbidity increases with age, affecting more than 50% of adults with active epilepsy (11). Comorbidities are associated with several negative outcomes, including a poorer prognosis for seizure control and significantly reduced quality of life. Affective disorders, anxiety, psychosis, autism spectrum disorder and various cognitive impairments are more common in PWE than in the general population (12–17). Under-diagnosis and under-treatment of psychiatric comorbidities might result in worse seizure-control and reduced life-expectancy, with depression and anxiety even leading to self-stigmatization, suicidal ideation, and eventually suicide (18–21). Therefore, the International League Against Epilepsy has developed guidelines for treatment of depression and anxiety in epilepsy (22). Furthermore, people with epilepsy are more likely to have chronic somatic conditions such as stomach/intestinal ulcers, stroke, urinary incontinence, bowel disorders, migraine, Alzheimer disease, myocardial infarction, and chronic fatigue than the general population (11, 20), associated with a worse outcome and increased mortality (23).
Of special concern is the increased mortality rate among people with epilepsy compared to that of the general population (24, 25). Mortality is significantly higher in persons with seizures of a structural or known (symptomatic) cause, as compared to unknown (i.e., cryptogenic) or idiopathic (no structural, metabolic cause, but presumed genetic) cause; mortality is higher in children than in adults, and lower in incidence than in prevalence studies (26). The most important immediate causes of mortality include sudden unexpected death in epilepsy (SUDEP), status epilepticus (SE), injuries, and suicide. Standardized mortality ratios range from 1.6 to 3.0 in high-income countries (25) to 19.8 [95% confidence interval (CI) 9.7–45.1] in low- and middle-income countries (24). Male patients, children and adolescents, as well as patients with lack of access to health facilities show a slightly increased standardized mortality ratio (26). SUDEP among the general population of people with epilepsy reaches an incidence of 1.2 per 1,000 person-years (95% CI, 0.9–1.5), with an incidence of 1.3 (95% CI, 0.9–1.8) in adults above 50 years of age and 1.1 (95% CI, 0.5–2.3) in children under the age of 16 years (27). Major risk factors for SUDEP include the persistence of seizures, nocturnal seizures, and generalized tonic-clonic seizures (28).
To investigate mortality in epilepsy, researchers need to select a representative sample of the population with epilepsy. This can be difficult to do on a global scale, as populations with epilepsy differ in many ways (e.g., frequency of risk factors and age composition). While some factors (e.g., age) can be controlled for in the analysis, others (e.g., distribution of risk factors) cannot. There are many potential sources of data that can be used to identify people with epilepsy, including diagnostic registries at hospitals, EEG laboratories, and groups with increased risk for epilepsy (29). If these registries are thought to have identified most people with epilepsy in a study area, the study is population-based and representative of the general epilepsy population. These studies give good estimate on prevalence overall but may suffer from poor phenotyping of the seizure type, epilepsy syndrome or the causes. If hospital registries are used as the sole source of data, phenotyping and precise diagnosis is a great advantage over population-based studies, however, they are prone to a biased sample that overestimates the mortality of all people with epilepsy. Prognostic studies of mortality rates should ideally include all people newly diagnosed with epilepsy. The best way to achieve this is through incidence studies, which identify all new cases over a specified period of time. An incidence cohort will contain a higher proportion of mild epilepsy cases than a prevalence cohort (29).
In this review, we aim to update current knowledge of the methodological aspects of research on mortality in epilepsy, causes of mortality, mortality in status epilepticus, as well as to set our sights on future directions in mortality research.
For this narrative review, data were collected from MEDLINE®/PubMed® and EMBASE® using specified search criteria (papers published in English from 1980 to 2022; International League Against Epilepsy classification papers published pre-1996 were allowed). The search terms were combinations of the following: “epilepsy” and “mortality”. We used the additional filters: “only full text”, “clinical trial”, “meta-analysis”, “randomized controlled trial”, “review”, and “systematic review”. Overall, we screened 1,042 abstracts. If a title or abstract described a high-quality article that was likely to be eligible for inclusion, the full article was obtained and assessed for relevance. Overall, 135 articles investigating the epidemiology of epilepsy, diagnosis, comorbidities and associated mortality, stigmatization, and treatment were included in this narrative review. A limitation of this approach is that the selected studies are not always comparable, with diverse methodologies and endpoints, but the mail purpose of this narrative review was to give the reader an overview on the topic.
Statistically, in its simplest form, mortality can be quantified by considering a subject at a given point in time as either dead or alive. Consequently, this binary variable might be analyzed using standard methods such as calculating proportions. According to the specific characteristics of the chosen statistical approach (e.g., with or without adjustment for demographic and clinical characteristics), the resulting quantities are referred to by well-established epidemiological terms, which will be outlined in the sequel along with the respective interpretations. It should be noted that these concepts may be considered as special cases within the more general framework of “survival analysis” or “time-to-event analysis” [for an overview, see, Klein & Moeschberger, 2003 (30)]. For this type of analyses, a more fine-scale quantification of mortality is used by considering the time between, e.g., diagnosis of the disease and death. Although this information is usually available, survival analysis approaches are somewhat scarce in epidemiological studies on epilepsy and only four studies addressed this specifically (14, 31–33). At least partially, this might be because in epidemiology, subjects are often observed over quite long time periods, which often results in survival patterns exhibiting complex functional forms. While the survival analysis toolbox also contains flexible modelling approaches, the standard quantities based on proportions might still be preferable, due to their straightforward interpretation. However, especially for life expectancy calculations and comparisons, bridging the gap between the two approaches might yield new insights, which is briefly outlined at the end of this section.
In the sequel, it is assumed that mortality is quantified using the binary indicator “death” vs. “alive”. The proportion of deaths divided by the size of the population of interest (e.g., the population within a specific geographic region) is called (crude) mortality rate. If the population of interest is restricted to subjects with a particular disease (e.g., epilepsy), the term case fatality rate is used instead. Frequently, a specific time point / interval is chosen for these calculations (e.g., 1-year mortality). Further subtle yet important aspects regarding the definition of the “population of interest” (e.g., how to quantify the “subjects at risk”) are not discussed in detail here. In the sequel, for ease of presentation, the term “mortality rate” is used, although the methods might be applied to case fatality rates as well.
Frequently, the probability of dying is influenced by several demographic and clinical characteristics. For example, age and sex are considered as important predictors of death. Consequently, especially the comparison of crude rates between different epidemiological studies might be questionable, due to potential differences in the demographic and clinical characteristics of the underlying respective populations. Therefore, a standardization of the rates, which comprises the following steps, may solve this problem (34):
(1) The first step in all standardization approaches is to calculate mortality rates for specific subgroups that are formed according to those demographic and clinical variables which are considered as important predictors of death. For example, groups according to age decades (i.e., interval 1: 1–10, interval 2: 11–20, interval 3: 21–30, etc.) might be formed, and subsequently, the interval-specific mortality rates mi = di / ni for interval number i are used as age-group-specific estimates.
(2) Now, a straightforward idea would be to apply direct standardization, which is based on the following idea: Assume that the probability of dying is 30 percent. How many deaths are expected in a population of size 1000, then? Intuitively, one would expect 300 deaths (i.e., 1,000 times 0.3). Indeed, this is also sensible from a mathematical viewpoint, and applying this idea to the age-specific rates from step (1) yields the age-group-specific (expected) number of deaths Wi = Ni × mi, where Ni denotes the number of subjects in age group i in the so-called reference population. The reference population might be one of the available well-established standard populations (https://seer.cancer.gov/stdpopulations/, accessed 2022-09-11), or more generally any population that is appropriate with respect to the epidemiological research question.
(3) Finally, the sum of the quantities Wi is calculated and divided by the total size of the reference population (i.e., the sum of the Ni's), in order to obtain a proportion again. So, eventually, the age-adjusted mortality rate is defined as
Thereby, Ni denotes the number of subjects in age group i in the reference population, and N denotes their sum. The quantities mi are the age-group-specific mortality rates in age group i in the study cohort, and k denotes the number of age groups.
By contrast, an indirect standardization approach would replace the calculations outlined above by the following procedure, which tackles the problem in the opposite way (i.e., using the mortality data from the reference population as the “ground truth” for subsequently calculating the expected mortality in the study cohort):
(1) The age-group-specific mortality rates in the reference population are calculated at first.
(2) Then, these rates are multiplied with the numbers of subjects within the specific age groups in the study cohort, in order to obtain an age-group-specific expected number of deaths in the study cohort.
(3) The expected numbers of death from step (2) are summed up, in order to obtain the total number of deaths in the study cohort that would be expected if the mortality rates from the reference population were also true for the study cohort. Finally, the total number of actually observed deaths in the study cohort is often divided by that aforementioned total expected number of deaths, in order to obtain the standardized mortality ratio (SMR).
Complementing the—adjusted or unadjusted—rates, the estimation of life expectancy might be an additional yet less frequently used means of quantifying mortality. The life expectancy at a certain age (also called mean residual life at a given time point) is informally defined as the expected number of years a subject has left to live. It is obvious from this (informal) definition that a higher level of detail regarding the data—instead of only considering “alive” vs. “death” at a prespecified single time point—is required here, which naturally gives rise to adopting methods from survival analysis, as briefly outlined at the beginning of this section. Indeed, several proposals for life expectancy estimation and comparison have been published (e.g., (32)], and methodological research on the corresponding challenges has been quite vivid recently [e.g. (35, 36)].
Looking at mortality data we have to differentiate between the different cohorts. Especially the geographic location of the study influences the numbers, with significantly higher rates in low- and middle-income countries (LMIC) compared to high income countries (HIC) (24, 25). Further, the setting of the cohort is of significance, whether it is population-based or hospital-based, as selection-biases might be introduced. The strength of population-based cohorts is the representative sample ideally including all patients with known or diagnosed epilepsy in a geographic region. The weakness is often an uncertain diagnosis or no verification of the diagnosis at al. For instance, in a landmark study in the UK 1,091 patients were recruited in a prospective population-based cohort, but later epilepsy was confirmed in only 564 (37). Other weaknesses are the usually shorter follow up in population-based cohorts, and finally the regional differences in a given country cannot be addressed, when comparing to a national standard reference population (21, 38). The major strength of hospital-based cohorts is the diagnostic accuracy, the detailed identification of etiology (21, 38). Among other health care restrictions many people in LMIC do not receive appropriate ASMs and epilepsy surgery is hugely underutilized. The so-called treatment gap is defined as “the number of people with active epilepsy not on treatment (diagnostic and therapeutic) or on inadequate treatment, expressed as a percentage of the total number with active epilepsy (39, 40).” The treatment gap was estimated in a systematic review covering Africa, Asia, and Latin America to be as high as 56% of PWE, who do not receive adequate epilepsy treatment (5). In LMICs the availability of basic public health services as well as access to specialized health care for epilepsy varies considerably between LMICs as well as within LMICs between urban areas and rural parts of the respective country. (https://apps.who.int/iris/bitstream/handle/10665/170250/9789240694439_eng.pdf;jsessionid=2248AD37F7F5B801D04BC57A04B5F280?sequence=1, accessed on 2023-01-29) This has significant impact of mortality in LMIC, but mortality data on population-based samples reporting SMRs and life expectancy calculations from LMICs still sparse (7). In LMIC it is almost impossible to ascertain the precise number of deaths because incident studies are difficult, death certificates are notoriously unreliable (not quite different in HICs), autopsy rate is very low, and the high migration rate make large population based prospective studies almost impossible (41). Methods to assess excess mortality on LMICs are often door-to door surveys or selected cohorts.
Standardized mortality rates (SMR) in population-based studies of HICs lie consistently between two and three, with only few outliers, whereas most of the studies reporting SMRs in LMICs vary between three and five. (Table 1). Numbers in hospital-based studies and other selected cohorts are similar, although with more variance (Table 2).
a. Population based cohorts
b. Hospital based cohorts
c. Risk factors for Mortality in Epilepsies
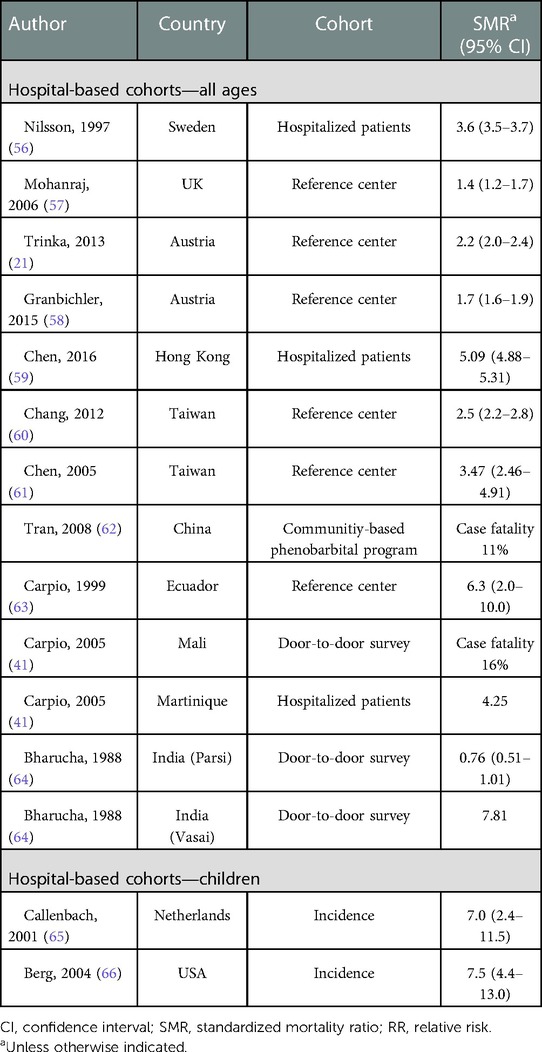
Table 2. Overall mortality in epilepsy patients—selected hospital-based cohorts and other special cohorts.
Etiology of the epilepsy is one of the most important of those factors. Those that suffer from neurological disorders as an underlying cause of the epilepsy, especially when the central nervous system is involved or a learning disorder is present, mortality rates are higher. This is especially true when a brain tumor is present with an up to 50-fold increase in mortality (37, 42, 44, 48, 54, 55, 65–71). Including these patient groups in calculations of mortality in epilepsy is questionable, as the underlying condition determines the chances for premature death rather than the epilepsy itself. Therefore, many studies exclude brain tumors in their estimates. But even then, SMR values remain elevated in all studies, with 2.2–4.3 (21, 37, 42, 44, 45, 47–49).
For cryptogenic and idiopathic epilepsies only marginally increased or normal SMRs have been found, at 0.9–2.1 (37, 42, 44, 45, 47–49).
Even though mortality is increased in all age-groups, a certain age-correlation can be shown, and younger patients show higher values than older age groups (37, 43, 44, 53, 55, 56, 65, 66, 72). A Swedish cohort reported an SMR of 9.5 and 10.7 for age-groups 15 to 39 and 40 to 59, respectively, while it was only non-significantly elevated at 1.3 in those over 80 (56). Similarly, a study from the United Kingdom reported and SMR of 7.6 in those aged 0 to 49, and 2.6 in those over 80 years of age (37). The same was true in an American cohort with 8.5 for ages 0 to 24, and 1.4 for those above 74 (44).
The influence of sex, however, is more controversially discussed. While mortality is elevated for both sexes, some studies have shown higher values for men than women, while others could not confirm this (21, 43, 45, 46, 56). Only one study from Island of an incidence cohort with unprovoked seizures could show that there was a significant difference in SMR between women [0.8 (CI, 95% 0.4–1.5)] and men [2.3 (CI, 95% 1.6–3.1)] (73). Also an older study from the US states an SMR of 1.6 in women, and 2.1 in men, however, confidence intervals were not provided and we therefore do not know if this difference is significant (44).
The more time passes after a first epilepsy diagnosis, the lower the likelihood of premature death. The highest mortality is within the first two years after diagnosis (9, 21, 58). Weather SMR remains elevated at all years later is unclear, but several studies have shown an increased SMR even 20 years following initial diagnosis (21, 42, 44, 47). Epilepsy etiology then seems to be the most important influential factor, with structural etiology showing much higher values in the first years following diagnosis that then decrease significantly until they reach values comparable to those of epilepsies without structural changes (21, 31, 47).
Even though data is limited on this factor, it appears that tonic-clonic seizure (GTCS), primary or secondary, have the most significant influence on mortality. There are only three studies that investigated this: a Swedish cohort that found an SMR of 3.9 for GTCS, and 2.1 for other focal onset seizures (43), a more recent study from Estonia, where GTCS had an SMR of 2.7 compared to 1.5 for focal onset seizures (49), and an American study that reports an SMR of only 1.3 for GTCS, while focal onset seizure showed an SMR of 1.8. However, in the latter study no detailed information on seizure type is available (45).
There are only very few studies on life expectancy in epilepsy (14, 31, 33, 35). In 1974, a subgroup analysis of a population-based study in Warsaw reported a life expectancy of 12.5 years following the diagnosis of epilepsy, which was on average 20 years shorter than that of the general population in Poland at that time (33). Data from the UK National General Practice Study of Epilepsy were used to calculate the life expectancy using the parametric Weibull model (31), a well-recognized statistical technique for exploring the relationship between the survival of a patient, and several explanatory variables. From 564 persons with epilepsy, the life expectancy was reduced by up to 2 years in those with idiopathic and cryptogenic epilepsy, and up to 10 years in those with symptomatic epilepsy, compared to the general population of UK. There was also a decrease in years of life lost with increasing time from epilepsy diagnosis. Unfortunately, the numbers were too small for further subgroup analysis; moreover, the assumptions of the statistical model were rather restrictive insofar that they did not allow for increased life expectancies of the patients. Apart from that, the variability of the life expectancy estimates (e.g., confidence intervals) was not provided, thus rendering the interpretation in comparison with other studies difficult. In addition, the life expectancy was compared with the general UK population neglecting thereby geographic differences of baseline mortality (31). A more recent study analysed the life expectancy of newly diagnosed persons with epilepsy in a large cohort with well-defined adult epilepsy, by comparing life expectancy with that of the general population living in the same geographic area in Tyrol, Austria (35). The authors applied a Weibull regression model using gender, age at diagnosis, epilepsy etiology, and year of diagnosis as covariates at time of epilepsy diagnosis, and 5, 10, 15, and 20 years after diagnosis. Yet, no a priori restrictions were set regarding the mortality rates and life expectancies of the patients. This work confirmed a reduced life expectancy in symptomatic epilepsy until the 1990s, mainly during the first years following diagnosis up to 7.4 years, but on the other hand most subgroups did not show changes in life expectancy compared to the control population. Unexpectedly during the 2000s, life expectancy was even prolonged for those with cryptogenic epilepsy independent of the time since diagnosis (35). These findings cannot be explained easily but are most likely to improved epilepsy care and early identification of drug resistant epilepsy in a comprehensive care centre (74, 75).
Proportional mortality was reported in several hospital- and population-based studies (21, 44, 56, 58, 76–80). Neoplasms account for 5%–26%, and pneumonias for 5%–25% of deaths (77, 78). The largest percentage of deaths is caused by pneumonias in cohorts of institutionalized patients (12%–25%) (77, 78, 80), while the population-based studies and the large Swedish study reported clearly smaller percentages (up to 5.5%) yielding SMRs between 4.0 and 7.2 (44, 56, 76). Deaths from cerebrovascular disease are different, in that the population-based studies showed clearly higher percentages, namely 12%–17% (44, 76), than did the studies of institutionalized patients with only 5%–6% of deaths due to cerebrovascular diseases (77, 80). One exception is the Tyrolean mortality study that, similar to the population-based studies, shows a high percentage of cerebrovascular deaths, namely 15% (21, 79). Accidents are given as the cause of death in 1%–16% of mortalities (21, 33, 44, 76–79, 81). Here, too, the studies conducted in institutionalized patients show clearly higher figures than do the population-based studies (1%–6%) (44, 76). The findings for suicide are particularly divergent, namely up to 21% of deaths reported by some studies (33, 58, 77, 78, 81, 82), and less than 1% of deaths reported by the large population-based studies in Rochester and the United Kingdom (44, 76). Since proportional mortality does not permit any conclusions to be drawn on elevated mortality in an actual as compared to a standard population, the SMR must be calculated for the particular cause of death. In summary, these studies show a consistently elevated SMR for pneumonia (SMR 3.5–10.3), tumors (SMR 1.5–4.8) and cerebrovascular disease (SMR 1.8–5.3) (21, 44, 56, 58, 76–80). Elevated SMRs were also reported for accidents and other external causes of death (SMR 2.4–5.6) (21, 44, 56, 70, 79, 80). The results with regard to suicide are contradictory, as already mentioned: while the population-based studies showed no elevated SMR (44, 76), the other studies showed a clearly elevated SMR ranging from 3.5 to 5.4 (56, 80). A review with special emphasis on the medical risks of epilepsy including physical injuries, mortality and traffic accidents has been published (83).
Wannamaker, who analyzed the literature from 1910 to 1974, reported that 42.7% of deaths are directly related to epilepsy (84). Causes of death directly related to epilepsy are status epilepticus, accidents occurring during an epileptic seizure, bolus death from aspiration and sudden unexpected death in epilepsy patients (SUDEP) (85). Studies conducted in institutionalized patients in Finland and the United Kingdom found occurrences directly related to epilepsy to be the most common cause of death, namely 19%–31% (77, 78, 80). In children with epilepsy, causes of death directly related to epilepsy, such as SUDEP, drowning, injuries, or status epilepticus, are also very common, namely 22%–45% (86, 87). By contrast, the two population-based studies from Rochester (USA) and the United Kingdom show clearly smaller percentages, namely 3%–4% [(44, 76), p. 198]. Differences in case ascertainment, classification criteria and the length of the observation period might play a role here. Identification PWE through population databases or insurance data may lack diagnostic accuracy of epilepsy and also of causes of death (21, 25, 37); and compared to hospital-based cohorts epilepsy may no longer be the primary diagnosis any more, especially when competing diagnoses, such as stroke or dementia appear in the records. In addition, the rate of autopsy may influence the causes, attributed to the death of a PWE. Standardized reporting on death certificates is highly recommended for future research (88).
Status epilepticus (SE) is associated with a significant mortality and accounts for ∼10% of epilepsy-related deaths (89). Two recent reviews summarize all population-based studies on status epilepticus and the respective case fatalities in adults (Table 3) (38, 90).
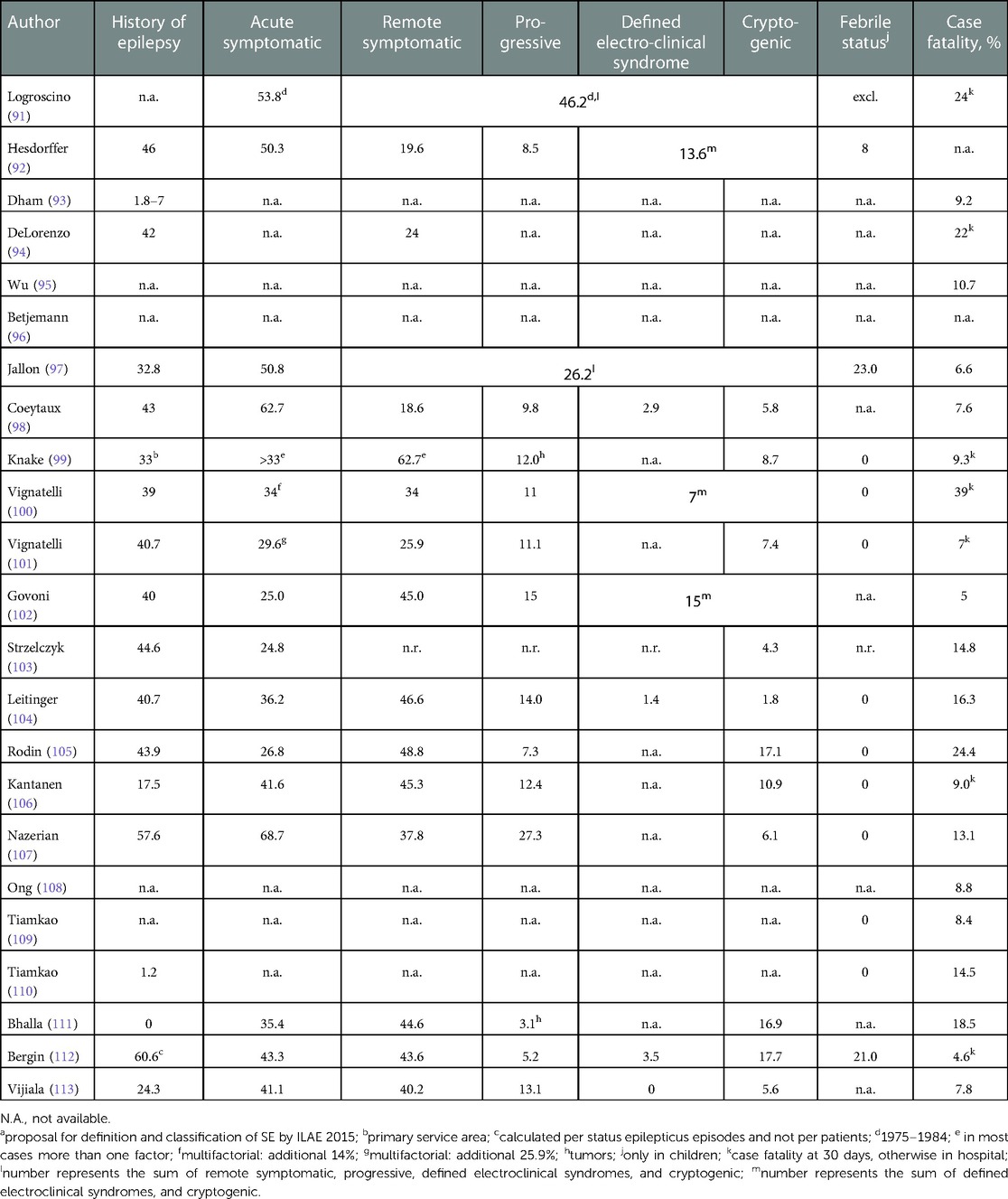
Table 3. Population based studies of adults with status epilepticus (38). (Reproduced with permission from Leitinger and Trinka et al., Epilepsy & Behavior, Elsevier).
Mortality in SE is associated with drug resistance (104): Refractory (RSE) and Super-Refractory SE (SRSE) are associated with a death rate of up to 39.5% and 37.5%, respectively (103, 106, 114–116). While discharge mortality in nonrefractory SE is 9.6% % (103). In a large case series of new onset refractory SE (NORSE), mortality was 23% (6/26) (117).
In a pediatric population of 100 children, mortality was 10% in SRSE, 1.9% in RSE and 0% in non-refractory SE compared to 3.6% in the total population (118). A retrospective study on 109 patients hospitalized in the neuro-intensice care units for SE revealed intubation, hypotension and a low GCS at presentation as risk factors for an increased mortality (119).
Recurrent SE showed a mortality at 30 days of 2% compared to the initial episode of SE with 22% (120). Within those who relapsed within six months, mortality after the second event at 30 days was 24% compared to the group with relapse after 6 months with 27% (120). In the US, readmission rate at 30 days was 15% after investigation of a nationwide database with 42,232 adults most commonly (45.1%) due to seizures (121). After multivariable analysis, independent risk factors were intracranial hemorrhage (odds 1.56, 95% CI, 1.12–2.18), psychosis, diabetes mellitus, chronic kidney or liver disease, more than three comorbidities, length of stay more than 4 days during index hospitalization, and discharge to a skilled nursing facility (121).
In a 10-year study on 14,487 deaths associated with status epilepticus, cardiac arrest was the cause of death at autopsy in 21.3% (122). Women were at a lower risk of myocardial infarction (odds ratio 0.55, 95% confidence interval 0.51–0.61), patients 45 years or elder had a higher risk of developing myocardial infarction, arrhythmia, heart failure or cardiac arrest compared with people with epilepsy, unspecified convulsions, febrile convulsions, or posttraumatic seizures (122).
In an elderly population in Taiwan the in-hospital mortality of 77 patients with de novo CSE was 38.9% (123). Multivariable analysis revealed the presence of comorbidities (OR 0.23, 95% CI, 0.0059–0.879), low Glasgow Coma Scale (OR 0.045, CI, 0.013–0.160) and de novo SE (OR 0.093, CI, 0.017–0.503) as parameters significantly related to mortality. A recent systematic review about the elderly showed that mortality was reported 71 out of 85 identified studies (124). In another systematic review, mortality within the elderly with SE was highest short-term 22%–38% and long-term 82% which resulted in a standardized mortality ratio, i.e., the relative risk of mortality compared with the general population, was 2.2 (95% CI, 1.6–2.9) in those aged over 65 years (125).
The influence of treatment on the mortality, especially when using anesthetic drugs has been discussed intensively in the recent years. Ferlisi and Shorvon (126) reviewed 159 publications with reported outcome data in 1,061 patients. The long-term outcome was death in 35%, but remarkably higher death rates during treatment were found with phenobarbital/thiopental with 19% compared to 2% with midazolam and 8% with propofol. In a 6-year cohort study on 171 patients with SE, 37% were treated with intravenous anesthetic drugs, the mortality was 18%. It has to be considered, that the side effects of the treatment may significantly contribute to the mortality of RSE and SRSE.
Recently, the impact-of-burden-model was created to provide a framework to implement the various factors (i.e., etiology of SE in form of structural damage and metabolic derangement, burden of status epilepticus, and burden of treatment) a patient with SE is exposed to (Figure 1) (127). In short, this framework shows that the benefit from treatment is the net gain of success of treatment (i.e., reduction of status burden) and burden of treatment. The impact of these burdens depends on the amount of functional reserve or decompensation which are determined by structural pathologies or metabolic derangements and further by comorbidities, age, and several other factors. It becomes apparent that individuals need a treatment adapted to their functional reserve to prevent decompensation and that studies need appropriate outcome parameters. Mortality should only be used in populations with high structural/ metabolic burden, e.g., subarachnoid hemorrhage or cerebral hypoxia due to cardiac arrest. Septicemia or moderate metabolic derangements require functional outcome parameters as readout as people will mostly survive at least on a short time basis.
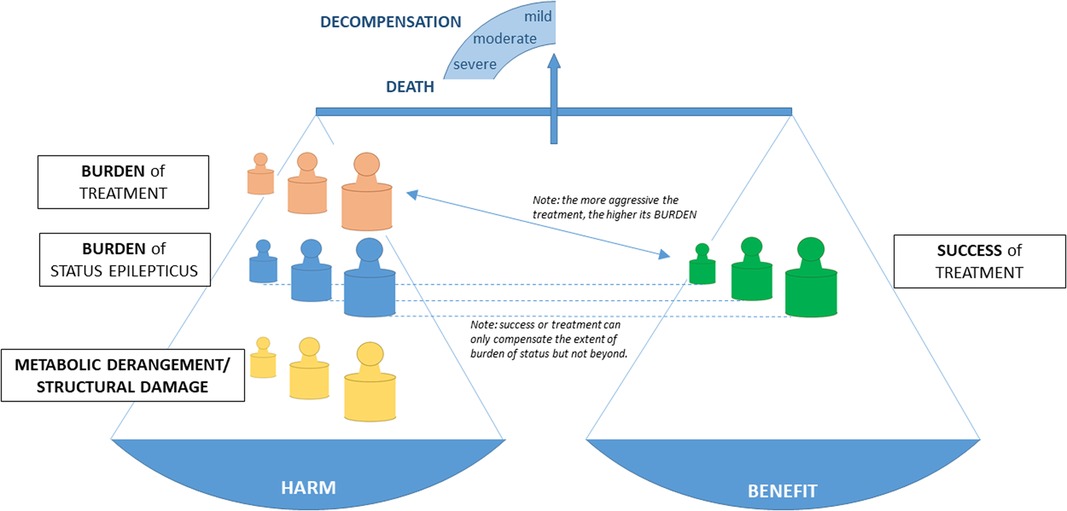
Figure 1. The impact of burden model integrates the structural damage and metabolic derangement, the burden of status, the success of treatment, the burden of treatment, and the impact of these burdens in an individual patient [modified from Trinka and Leitinger, 2022 (127)].
Among epidemiological studies on SE there is a substantial heterogeneity making comparisons between studies difficult. The most important influential factors are: (i) the age profile of the study population and the reference population used for adjustment, (ii) the time to establish the diagnosis of SE, (iii) the inclusion of only first episodes of SE or also of recurrent episodes, (iv) the spectrum of etiologies including a prior history of epilepsy, (v) case ascertainment by ICD-codes or of file-based diagnosis of SE, (vi) the inclusion of both adults and children in one study, (vii) and the timepoint of outcome, i.e., at hospital discharge or at 30 days (38). Overall, there is a lack of recent data on the epidemiology, mortality, and healthcare burden associated with SE using the 2015 ILAE definition of SE. Available data suggest a high burden of illness and mortality, which is associated with, age, etiology, duration, and drug resistance in SE.
Mortality studies on epilepsy have been carried out systematically since the 1950ties. Astonishingly there was the general view until recently, that mortality did not change over time, despite major advancements in treatment including effective ASMs and successful epilepsy surgery. O’Donoghue and Sander used data from the Chalfont Centre for Epilepsy, UK, a residential centre for people with epilepsy, and determined the SMR in the Chalfont population for each 5-year epoch from 1896 to 1965 (128). The authors concluded “that an excess mortality has been associated with chronic epilepsy for 100 years despite major changes in treatment.”. Another more recent systematic review on nine population based studies performed between 1974 and 2006 found SMRs between 1.6 and 5.3 without evidence that the “SMR or the mortality rate of people with epilepsy has changed significantly over time” (129). Several studies demonstrated that the excess mortality is caused by the drug resistant epilepsy patients, and seizure free patients have no increase in SMR (21, 57, 79) (Figure 2).
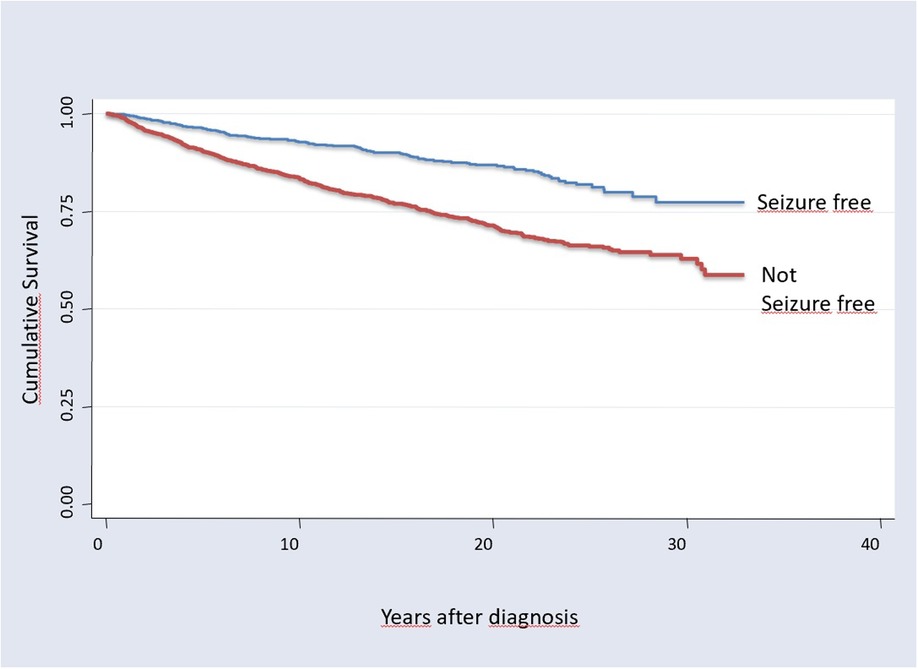
Figure 2. Kaplan maier survival curves of 3,334 patients in tyrol, Austria, with more than 30 years observation; overall 48,595 person years. Seizure free patients have a significant better overall survival (log rank test χ = 80,3, p < 0.0001), [from Trinka, 2005 (79)].
A time trend analysis of the Tyrolean Epilepsy Cohort (21, 58, 74, 79) found a decrease of mortality since 1980: The overall SMR decreased from 3.0 (95% CI, 2.1–4.3) in 1980–1989, to 2.7 (95% CI, 2.0–3.5) in 1990–1999 and to 1.4 (95% CI, 1.0–2.0) in 2000–2007 (74). The reason for this decline is not easy to explain, but the early recognition of drug resistant epilepsy and the introduction of epilepsy surgery in this centre are the most plausible explanations (74). More recently a large study from the Comprehensive Calgary Epilepsy Programme, Alberta, Canada found a clear significant association of levels of specialized epilepsy care and excess mortality in patients with epilepsy (75). Among the 23 653 incident cases the overall standardized mortality rate was 7.2%. It was 9.4%for those receiving nonspecialist care, 5.6%for those seen by a neurologist, and 2.8%for those seen in the Comprehensive Calgary Epilepsy Programme. The hazard ratio (HR) of mortality was significantly lower in those receiving neurologist (HR, 0.85; 95% CI, 0.77–0.93) and Comprehensive Calgary Epilepsy Programme (HR, 0.49; 95% CI, 0.38–0.62) care (75). This study clearly showed that specialized care of epilepsy patients saves lives.
In Status epilepticus, similarly a decrease of deaths from SE was observed: Neligan and Walker analysed SE mortality data from 2001 to 2013, and compared it to annual age group populations for England and Wales: All epilepsy deaths significantly decreased (Spearman's q −0.733, p = 0.004), which is predominantly due to a decrease in SE deaths (Spearman's q −0.917, p < 0.001) (89). Along the same lines, the number of patients admitted to CCU for SE in the UK were rising three-fold from the early 2000 years to the early 2010 years whereas acute hospital mortality was decreasing in 35,595 CCU cases, especially in neurological critical care units from 8.1% to 4.4% in the same period of time (130). In sum, these finding supports the hypothesis that the policy of early and aggressive treatment of SE (Trinka and Leininger, Continuum 2022) may be improving the outcome, and most importantly decrease mortality.
After more than 50 years of modern public health research, it has been shown that the premature death of patients with epilepsies, more specifically drug resistant epilepsies, and SE can be reduced by adequate treatments and comprehensive care. But still according to the WHO Epilepsy Report (https://apps.who.int/gb/ebwha/pdf_files/EB146/B146_12-en.pdf/, accessed 2023-01-29, and https://www.who.int/news/item/28-04-2022-draft-intersectoral-global-action-plan-on-epilepsy-and-other-neurological-disorders-2022-2031, accessed 2023-01-29), epilepsies rank fifth among all neurological causes for disability-adjusted life years (DALYs) (131). Worldwide, an estimated 125,000 people die each year due to epilepsy (131). The risk of premature mortality for people with epilepsy is estimated at now is three times that of the general population (25). In some low-resource settings around the world, this risk may be increased up to seven-fold (24, 132). However, up to 70% of people with epilepsy could become seizure-free following an accurate diagnosis and use of cost-effective and commonly available, ASMs. The research gap in mortality of epilepsy and status epilepticus between HIC and LMICs is huge and more research in LMICs is urgently needed. Moreover, the socioeconomic determinants of mortality are often neglected or understudies. Modifiable risk factors are well known, with access to appropriate treatments as the most important one. Ideally future research on mortality will involve more population-based incident cohorts from LMIC and collect data on comorbidities, and specific causes of death, as well as socioeconomic data. The reference population for such studies should take regional differences of mortality into equation.
The recently approved WHO Intersectorial Global Action plan for Epilepsies and other Neurological Disorders (133) calls for a multi-stakeholder approach driven at the national and local level to reduce the treatment gap, stigma, and aim for 70% of the people with epilepsy to be seizure free. There is hope that these measures will dramatically reduce the premature mortality in the future.
All authors contributed significantly to conception and design of the presented paper, as well as drafting of the paper. ET additionally contributed significantly to revising the paper for intellectual content gave final approval of the submitted version of the manuscript. All authors contributed to the article and approved the submitted version.
GZ gratefully acknowledges the support of the WISS 2025 project “IDA-Lab Salzburg” (20204-WISS/225/197-2019 and 20102-F1901166-KZP)
ET reports personal fees from EVER Pharma, Marinus, Arvelle, Angelini, Argenx, Medtronic, Bial-Portela & Ca, NewBridge, SK, Pharma, GL Pharma, GlaxoSmithKline, Boehringer Ingelheim, LivaNova, Eisai, UCB, Biogen, Sanofi, Jazz Pharma, and Actavis. His institution received grants from Biogen, UCB Pharma, Eisai, Red Bull, Merck, Bayer, the European Union, FWF Österreichischer Fond zur Wissenschaftsforderung, Bundesministerium für Wissenschaft und Forschung, and Jubiläumsfond der Österreichischen Nationalbank. GZ gratefully acknowledges the support of the WISS 2025 project “IDA-Lab Salzburg” (20204-WISS/225/197-2019 and 20102-F1901166-KZP). ML has no conflicts of interest in the past five years. The remaining authors have no conflicts of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Katchanov J, Birbeck GL. Epilepsy care guidelines for low-and middle-income countries: from WHO mental health GAP to national programs. BMC Med. (2012) 10(1):1–5. doi: 10.1186/1741-7015-10-107
3. Yemadje LP, Houinato D, Quet F, Druet-Cabanac M, Preux PM. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. (2011) 52(8):1376–81. doi: 10.1111/j.1528-1167.2011.03099.x
4. Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. (2010) 51(5):883–90. doi: 10.1111/j.1528-1167.2009.02481.x
5. Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. (2008) 49(9):1491–503. doi: 10.1111/j.1528-1167.2008.01693.x
6. Mac TL, Tran DS, Quet F, Odermatt P, Preux PM, Tan CT. Epidemiology, aetiology, and clinical management of epilepsy in Asia: a systematic review. Lancet Neurol. (2007) 6(6):533–43. doi: 10.1016/S1474-4422(07)70127-8
7. Trinka E, Kwan P, Lee B, Dash A. Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia. (2019) 60:7–21. doi: 10.1111/epi.14458
8. World Health Organization, Global Campaign against Epilepsy, Programme for Neurological Diseases, Neuroscience (World Health Organization), International Bureau for Epilepsy, World Health Organization. Department of Mental Health, ... & International League against Epilepsy. (2005). Atlas: epilepsy care in the world. World Health Organization.
9. Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. (2014) 37:59–70. doi: 10.1016/j.yebeh.2014.05.031
10. Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. (1970) 23(7):455–68. doi: 10.1016/0021-9681(70)90054-8
11. Gaitatzis A, Sisodiya SM, Sander JW. The somatic comorbidity of epilepsy: a weighty but often unrecognized burden. Epilepsia. (2012) 53(8):1282–93. doi: 10.1111/j.1528-1167.2012.03528.x
12. Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: can a natural history be developed? Lancet Neurol. (2008) 7(2):151–60. doi: 10.1016/S1474-4422(08)70018-8
13. LaFrance WC Jr, Kanner AM, Hermann B. Psychiatric comorbidities in epilepsy. Int Rev Neurobiol. (2008) 83:347–83. doi: 10.1016/S0074-7742(08)00020-2
14. Dreier JW, Lauersen TM, Tomson T, Plana-Ripoll O, Christensen J. Cause-specific mortality and life years lost in people with epilepsy: a Danish cohort study. Brain. (2022) 146(1):124–34. doi: 10.1093/brain/awac042
15. Rainer LJ, Granbichler CA, Kobulashvili T, Kuchukhidze G, Rauscher C, Renz N, et al. Prevalence of comorbidities, and affective disorders in epilepsy: a latent class analysis approach. Epilepsy Res. (2022) 182:106917. doi: 10.1016/j.eplepsyres.2022.106917
16. Trinka E, Kienpointner G, Unterberger I, Luef G, Bauer G, Doering LB, et al. Psychiatric comorbidity in juvenile myoclonic epilepsy. Epilepsia. (2006) 47(12):2086–91. doi: 10.1111/j.1528-1167.2006.00828.x
17. Rainer LJ, Kronbichler M, Kuchukhidze G, Trinka E, Langthaler PB, Kronbichler L, et al. Emotional word processing in patients with juvenile myoclonic epilepsy. Front Neurol. (2022) 13. doi: 10.3389/fneur.2022.875950
18. Kwon OY, Park SP. Depression and anxiety in people with epilepsy. J Clin Neurol. (2014) 10(3):175–88. doi: 10.3988/jcn.2014.10.3.175
19. Kwon OY, Park SP. Frequency of affective symptoms and their psychosocial impact in Korean people with epilepsy: a survey at two tertiary care hospitals. Epilepsy Behav. (2013) 26(1):51–6. doi: 10.1016/j.yebeh.2012.10.020
20. Trinka E. Epilepsy: comorbidity in the elderly. Acta Neurol Scand. (2003) 108:33–6. doi: 10.1034/j.1600-0404.108.s180.5.x
21. Trinka E, Bauer G, Oberaigner W, Ndayisaba JP, Seppi K, Granbichler CA. Cause-specific mortality among patients with epilepsy: results from a 30-year cohort study. Epilepsia. (2013) 54(3):495–501. doi: 10.1111/epi.12014
22. Kerr MP, Mensah S, Besag F, De Toffol B, Ettinger A, Kanemoto K, et al. International consensus clinical practice statements for the treatment of neuropsychiatric conditions associated with epilepsy. (2011).
23. Janszky I, Hallqvist J, Tomson T, Ahlbom A, Mukamal KJ, Ahnve S. Increased risk and worse prognosis of myocardial infarction in patients with prior hospitalization for epilepsy—the Stockholm Heart Epidemiology Program. Brain. (2009) 132(10):2798–804. doi: 10.1093/brain/awp216
24. Levira F, Thurman DJ, Sander JW, Hauser WA, Hesdorffer DC, Masanja H, et al. Premature mortality of epilepsy in low-and middle-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. (2017) 58(1):6–16. doi: 10.1111/epi.13603
25. Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR, et al. The burden of premature mortality of epilepsy in high-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. (2017) 58(1):17–26. doi: 10.1111/epi.13604
26. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. (2020) 54(2):185–91. doi: 10.1159/000503831
27. Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: a nationwide population-based cohort study. Neurology. (2017) 89(2):170–7. doi: 10.1212/WNL.0000000000004094
28. Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology and the American epilepsy society. Epilepsy Curr. (2017) 17(3):180–7. doi: 10.5698/1535-7511.17.3.180
29. Forsgren L, Hauser WA, Olafsson E, Sander J, Sillanpää M, Tomson T. Mortality of epilepsy in developed countries: a review. Epilepsia. (2005) 46:18–27. doi: 10.1111/j.1528-1167.2005.00403.x
30. Klein JP, Moeschberger ML. Survival analysis: Techniques for censored and truncated data. Vol 1230. New York: Springer (2003).
31. Gaitatzis A, Johnson AL, Chadwick DW, Shorvon SD, Sander JW. Life expectancy in people with newly diagnosed epilepsy. Brain. (2004) 127(11):2427–32. doi: 10.1093/brain/awh267
32. Granbichler CA, Zimmermann G, Oberaigner W, Kuchukhidze G, Ndayisaba JP, Taylor A, et al. Potential years lost and life expectancy in adults with newly diagnosed epilepsy. Epilepsia. (2017) 58(11):1939–45. doi: 10.1111/epi.13902
33. Zielihski JJ. Epilepsy and mortality rate and cause of death. Epilepsia. (1974) 15(2):191–201. doi: 10.1111/j.1528-1157.1974.tb04941.x
35. Jin P, Zeleniuch-Jacquotte A, Liu M. Generalized mean residual life models for case-cohort and nested case-control studies. Lifetime Data Anal. (2020) 26(4):789–819. doi: 10.1007/s10985-020-09499-w
36. Andersen PK. Life years lost among patients with a given disease. Stat Med. (2017) 36(22):3573–82. doi: 10.1002/sim.7357
37. Cockerell OC, Johnson AL, Sander JW, Shorvon SD. Prognosis of epilepsy: a review and further analysis of the first nine years of the British National General Practice Study of Epilepsy, a prospective population-based study. Epilepsia. (1997) 38(1):31–46. doi: 10.1111/j.1528-1157.1997.tb01075.x
38. Leitinger M, Trinka E, Zimmermann G, Granbichler CA, Kobulashvili T, Siebert U. Epidemiology of status epilepticus in adults: apples, pears, and oranges—a critical review. Epilepsy Behav. (2020) 103:106720. doi: 10.1016/j.yebeh.2019.106720
40. Meinardi H, Scott RA, Reis R, On Behalf Of The Ilae Commission on the Developing World JS. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. (2001) 42(1):136–49. doi: 10.1046/j.1528-1157.2001.32800.x
41. Carpio A, Bharucha NE, Jallon P, Beghi E, Campostrini R, Zorzetto S, et al. Mortality of epilepsy in developing countries. Epilepsia. (2005) 46:28–32. doi: 10.1111/j.1528-1167.2005.00404.x
42. Lhatoo SD, Johnson AL, Goodridge DM, MacDonald BK, Sander JW, Shorvon SD. Mortality in epilepsy in the first 11 to 14 years after diagnosis: multivariate analysis of a long-term, prospective, population-based cohort. Ann Neurol. (2001) 49(3):336–44. doi: 10.1002/ana.70
43. Lindsten H, Nyström L, Forsgren L. Mortality risk in an adult cohort with a newly diagnosed unprovoked epileptic seizure: a population-based study. Epilepsia. (2000) 41(11):1469–73. doi: 10.1111/j.1528-1157.2000.tb00124.x
44. Hauser WA, Annegers JF, Elveback LR. Mortality in patients with epilepsy. Epilepsia. (1980) 21(4):399–412. doi: 10.1111/j.1528-1157.1980.tb04088.x
45. Benn EK, Hauser WA, Shih T, Leary L, Bagiella E, Dayan P, et al. Estimating the incidence of first unprovoked seizure and newly diagnosed epilepsy in the low-income urban community of Northern Manhattan, New York City. Epilepsia. (2008) 49(8):1431–9. doi: 10.1111/j.1528-1167.2008.01564.x
46. Morgan CL, Kerr MP. Epilepsy and mortality: a record linkage study in a UK population. Epilepsia. (2002) 43(10):1251–5. doi: 10.1046/j.1528-1157.2002.38701.x
47. Olafsson E, Allen Hauser W, Gudmundsson G. Long-term survival of people with unprovoked seizures: a population-based study. Epilepsia. (1998) 39(1):89–92. doi: 10.1111/j.1528-1157.1998.tb01279.x
48. Neligan A, Bell GS, Johnson AL, Goodridge DM, Shorvon SD, Sander JW. The long-term risk of premature mortality in people with epilepsy. Brain. (2011) 134(2):388–95. doi: 10.1093/brain/awq378
49. Rakitin A, Liik M, Oun A, Haldre S. Mortality risk in adults with newly diagnosed and chronic epilepsy: a population-based study. Eur J Neurol. (2011) 18(3):465–70. doi: 10.1111/j.1468-1331.2010.03195.x
50. Ding D, Wang W, Wu J, Yang H, Li S, Dai X, et al. Premature mortality risk in people with convulsive epilepsy: long follow-up of a cohort in rural China. Epilepsia. (2013) 54(3):512–7. doi: 10.1111/epi.12048
51. Mu J, Liu L, Zhang Q, Si Y, Hu J, Fang J, et al. Causes of death among people with convulsive epilepsy in rural West China: a prospective study. Neurology. (2011) 77(2):132–7. doi: 10.1212/WNL.0b013e318223c784
52. Banerjee TK, Ray BK, Das SK, Hazra A, Ghosal MK, Chaudhuri A, et al. A longitudinal study of epilepsy in Kolkata, India. Epilepsia. (2010) 51(12):2384–91. doi: 10.1111/j.1528-1167.2010.02740.x
53. Sillanpää M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. (2010) 363(26):2522–9. doi: 10.1056/NEJMoa0911610
54. Nickels KC, Grossardt BR, Wirrell EC. Epilepsy-related mortality is low in children: a 30-year population-based study in Olmsted County, MN. Epilepsia. (2012) 53(12):2164–71. doi: 10.1111/j.1528-1167.2012.03661.x
55. Camfield CS, Camfield PR, Veugelers PJ. Death in children with epilepsy: a population-based study. Lancet. (2002) 359(9321):1891–5. doi: 10.1016/S0140-6736(02)08779-2
56. Nilsson L, Tomson T, Farahmand BY, Diwan V, Persson PG. Cause-specific mortality in epilepsy: a cohort study of more than 9,000 patients once hospitalized for epilepsy. Epilepsia. (1997) 38(10):1062–8. doi: 10.1111/j.1528-1157.1997.tb01194.x
57. Mohanraj R, Norrie J, Stephen LJ, Kelly K, Hitiris N, Brodie MJ. Mortality in adults with newly diagnosed and chronic epilepsy: a retrospective comparative study. Lancet Neurol. (2006) 5(6):481–7. doi: 10.1016/S1474-4422(06)70448-3
58. Granbichler CA, Oberaigner W, Kuchukhidze G, Bauer G, Ndayisaba JP, Seppi L, et al. Cause-specific mortality in adult epilepsy patients from Tyrol, Austria: hospital-based study. J Neurol. (2015) 262(1):126–33. doi: 10.1007/s00415-014-7536-z
59. Chen Z, Liew D, Kwan P. Excess mortality and hospitalized morbidity in newly treated epilepsy patients. Neurology. (2016) 87(7):718–25. doi: 10.1212/WNL.0000000000002984
60. Chang YH, Ho WC, Tsai JJ, Li CY, Lu TH. Risk of mortality among patients with epilepsy in southern Taiwan. Seizure. (2012) 21(4):254–9. doi: 10.1016/j.seizure.2012.01.006
61. Chen RC, Chang YC, Chen THH, Wu HM, Liou HH. Mortality in adult patients with epilepsy in Taiwan. Epileptic Disord. (2005) 7(3):213–9. doi: 10.1046/j.1528-1157.2002.38701.x
62. Tran DS, Zen J, Strobel M, Odermatt P, Preux PM, Huc P, et al. The challenge of epilepsy control in deprived settings: low compliance and high fatality rates during a community-based phenobarbital program in rural Laos. Epilepsia. (2008) 49(3):539–40. doi: 10.1111/j.1528-1167.2007.01529_3.x
63. Carpio A, Hauser WA, Lisanti N, Aguirre RI, Roman M, Placencia M, et al. Prognosis of epilepsy in Ecuador. A preliminary report. In: Epilepsia. Vol. 40. Philadelphia, PA, USA: Lippincott Williams & Wilkins (1999). p. 110.
64. Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of epilepsy in the parsi community of bombay. Epilepsia. (1988) 29(2):111–5. doi: 10.1111/j.1528-1157.1988.tb04405.x
65. Callenbach PM, Westendorp RG, Geerts AT, Arts WFM, Peeters EA, van Donselaar CA, et al. Mortality risk in children with epilepsy: the Dutch study of epilepsy in childhood. Pediatrics. (2001) 107(6):1259–63. doi: 10.1542/peds.107.6.1259
66. Berg AT, Shinnar S, Testa FM, Levy SR, Smith SN, Beckerman B. Mortality in childhood-onset epilepsy. Arch Pediatr Adolesc Med. (2004) 158(12):1147–52. doi: 10.1001/archpedi.158.12.1147
67. Forsgren L, Edvinsson SO, Nyström L, Blomquist HK. Influence of epilepsy on mortality in mental retardation: an epidemiologic study. Epilepsia. (1996) 37(10):956–63. doi: 10.1111/j.1528-1157.1996.tb00533.x
68. Loiseau P, Loiseau J, Picot MC. One-year mortality in Bordeaux cohort: the value of syndrome classification. Epilepsia. (2005) 46:11–4. doi: 10.1111/j.1528-1167.2005.00401.x
69. Berg AT, Nickels K, Wirrell EC, Geerts AT, Callenbach PM, Arts WF, et al. Mortality risks in new-onset childhood epilepsy. Pediatrics. (2013) 132(1):124–31. doi: 10.1542/peds.2012-3998
70. Granbichler CA, Nashef L, Selway R, Polkey CE. Mortality and SUDEP in epilepsy patients treated with vagus nerve stimulation. Epilepsia. (2015) 56(2):291–6. doi: 10.1111/epi.12888
71. Keezer MR, Bell GS, Neligan A, Novy J, Sander JW. Cause of death and predictors of mortality in a community-based cohort of people with epilepsy. Neurology. (2016) 86(8):704–12. doi: 10.1212/WNL.0000000000002390
72. Moseley BD, Wirrell EC, Wong-Kisiel LC, Nickels K. Early onset epilepsy is associated with increased mortality: a population-based study. Epilepsy Res. (2013) 105(3):410–4. doi: 10.1016/j.eplepsyres.2013.03.002
73. Rafnsson V, Ólafsson E, Hauser WA, Gudmundsson G. Cause-specific mortality in adults with unprovoked seizures. Neuroepidemiology. (2001) 20(4):232–6. doi: 10.1159/000054795
74. Granbichler CA, Oberaigner W, Kuchukhidze G, Ndayisaba JP, Ndayisaba A, Taylor A, et al. Decrease in mortality of adult epilepsy patients since 1980: lessons learned from a hospital-based cohort. Eur J Neurol. (2017) 24(5):667–72. doi: 10.1111/ene.13267
75. Lowerison MW, Josephson CB, Jetté N, Sajobi TT, Patten S, Williamson T, et al. Association of levels of specialized care with risk of premature mortality in patients with epilepsy. JAMA Neurol. (2019) 76(11):1352–8. doi: 10.1001/jamaneurol.2019.2268
76. Cockerell OC, Hart YM, Sander JW, Goodridge DMG, Shorvon SD, Johnson AL. Mortality from epilepsy: results from a prospective population-based study. Lancet. (1994) 344(8927):918–21. doi: 10.1016/S0140-6736(94)92270-5
77. Iivanainen M, Lehtinen J. Causes of death in institutionalized epileptics. Epilepsia. (1979) 20(5):485–91. doi: 10.1111/j.1528-1157.1979.tb04830.x
78. Klenerman P, Sander JW, Shorvon SD. Mortality in patients with epilepsy: a study of patients in long term residential care. J Neurol Neurosurg Psychiatry. (1993) 56(2):149–52. doi: 10.1136/jnnp.56.2.149
79. Trinka E. Ursachenspezifische Mortalität von Patienten Mit Epilepsien in Tirol. Tyrol, Austria: Private University for Health Sciences, Medical Informatics and Technology Tyrol (2005).
80. White S, Mclean AM, Howland C. Anticonvulsant drugs and cancer a cohort study in patients with severe epilepsy. Lancet. (1979) 314(8140):458–61. doi: 10.1016/S0140-6736(79)91505-8
81. Krohn W. Causes of death among epileptics. Epilepsia. (1963) 4(1-4):315–21. doi: 10.1111/j.1528-1157.1963.tb05228.x
82. Brink Henriksen P. The mortality of epileptics. Epilepsy and insurance Social Studies on epilepsy No 5. (1967). p 5–12.
83. Tomson T, Beghi E, Sundqvist A, Johannessen SI. Medical risks in epilepsy: a review with focus on physical injuries, mortality, traffic accidents and their prevention. Epilepsy Res. (2004) 60(1):1–16. doi: 10.1016/j.eplepsyres.2004.05.004
84. Wannamaker BB. A perspective on death of persons with epilepsy. Epilepsy Sudden Death. (1990) 49:318–24. doi: 10.1016/j.yebeh.2015.04.010
85. Gaitatzis A, Sander JW. The mortality of epilepsy revisited. Epileptic Disord. (2004) 6(1):3–13. doi: 10.1001/jamaneurol.2013.578 15075062
86. Harvey AS, Nolan T, Carlin JB. Community-based study of mortality in children with epilepsy. Epilepsia. (1993) 34(4):597–603. doi: 10.1111/j.1528-1157.1993.tb00434.x
87. Sillanpää M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. (1998) 338(24):1715–22. doi: 10.1056/NEJM199806113382402
88. Middleton OL, Atherton DS, Bundock EA, Donner E, Friedman D, Hesdorffer DC, et al. National association of medical examiners position paper: recommendations for the investigation and certification of deaths in people with epilepsy. Acad Forensic Pathol. (2018) 8(1):119–35. doi: 10.23907/2018.009
89. Neligan A, Walker MC. Falling status epilepticus mortality rates in England and Wales: 2001–2013? Epilepsia. (2016) 57(7):e121–4. doi: 10.1111/epi.13402
90. Lu M, Faure M, Bergamasco A, Spalding W, Benitez A, Moride Y, et al. Epidemiology of status epilepticus in the United States: a systematic review. Epilepsy Behav. (2020) 112:107459. doi: 10.1016/j.yebeh.2020.107459
91. Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Time trends in incidence, mortality, and case-fatality after first episode of status epilepticus. Epilepsia. (2001) 42(8):1031–5. doi: 10.1046/j.1528-1157.2001.0420081031.x
92. Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. (1998) 50(3):735–41. doi: 10.1212/WNL.50.3.735
93. Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care. (2014) 20(3):476–83. doi: 10.1007/s12028-013-9935-x
94. DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. (1996) 46(4):1029–35. doi: 10.1212/WNL.46.4.1029
95. Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology. (2002) 58(7):1070–6. doi: 10.1212/WNL.58.7.1070
96. Betjemann JP, Josephson SA, Lowenstein DH, Burke JF. Trends in status epilepticus—related hospitalizations and mortality: redefined in US practice over time. JAMA Neurol. (2015) 72(6):650–5. doi: 10.1001/jamaneurol.2015.0188
97. Jallon P, Coeytaux A, Galobardes B, Morabia A. Incidence and case-fatality rate of status epilepticus in the Canton of Geneva. Lancet. (1999) 353(9163):1496. doi: 10.1016/S0140-6736(99)00583-8
98. Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland:(EPISTAR). Neurology. (2000) 55(5):693–7. doi: 10.1212/WNL.55.5.693
99. Knake S, Rosenow F, Vescovi M, Oertel WH, Mueller HH, Wirbatz A, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. (2001) 42(6):714–8. doi: 10.1046/j.1528-1157.2001.01101.x
100. Vignatelli L, Tonon C, D’alessandro R. Epilepticus BG for the S of S. Incidence and short-term prognosis of status epilepticus in adults in Bologna, Italy. Epilepsia. (2003) 44(7):964–8. doi: 10.1046/j.1528-1157.2003.63702.x
101. Vignatelli L, Rinaldi R, Galeotti M, De Carolis P, D’Alessandro R. Epidemiology of status epilepticus in a rural area of northern Italy: a 2-year population-based study. Eur J Neurol. (2005) 12(11):897–902. doi: 10.1111/j.1468-1331.2005.01073.x
102. Govoni V, Fallica E, Monetti VC, Guerzoni F, Faggioli R, Casetta I, et al. Incidence of status epilepticus in Southern Europe: a population study in the health district of Ferrara, Italy. Eur Neurol. (2008) 59(3-4):120–6. doi: 10.1159/000111873
103. Strzelczyk A, Ansorge S, Hapfelmeier J, Bonthapally V, Erder MH, Rosenow F. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. (2017) 58(9):1533–41. doi: 10.1111/epi.13837
104. Vignatelli L, Rinaldi R, Galeotti M, De Carolis P, D'Alessandro R. Epidemiology of status epilepticus in adults: a population-based study on incidence, causes, and outcomes. Epilepsia. (2019) 60(1):53–62. doi: 10.1111/epi.14607
105. Rodin E, Krogstad MH, Aukland P, Lando M, Møller HS, Gesche J, et al. High long-term mortality after incident status epilepticus in adults: results from a population-based study. Epilepsia. (2019) 60(1):33–41. doi: 10.1111/epi.14602
106. Kantanen AM, Sairanen J, Kälviäinen R. Incidence of the different stages of status epilepticus in Eastern Finland: a population-based study. Epilepsy Behav. (2019) 101:106413. doi: 10.1016/j.yebeh.2019.07.014
107. Nazerian P, Lazzeretti D, Vanni S, Donnarumma E, Magazzini S, Ruggiano G, et al. Incidence, management and short-term prognosis of status epilepticus in the emergency department: a population survey. Eur J Emerg Med. (2019) 26(3):228–30. doi: 10.1097/MEJ.0000000000000568
108. Ong CT, Sheu SM, Tsai CF, Wong YS, Chen SCC. Age-dependent sex difference of the incidence and mortality of status epilepticus: a twelve year nationwide population-based cohort study in Taiwan. PloS one. (2015) 10(3):e0122350. doi: 10.1212/WNL.0000000000002984
109. Tiamkao S, Pranboon S, Thepsuthammarat K, Sawanyawisuth K. Incidences and outcomes of status epilepticus: a 9-year longitudinal national study. Epilepsy Behav. (2015) 49:135–7. doi: 10.1016/j.yebeh.2015.04.040
110. Tiamkao S, Pranbul S, Sawanyawisuth K, Thepsuthammarat K, Group IER. A national database of incidence and treatment outcomes of status epilepticus in Thailand. Int J Neurosci. (2014) 124(6):416–20. doi: 10.3109/00207454.2013.850084
111. Bhalla D, Tchalla AE, Mignard C, Marin B, Mignard D, Jallon P, et al. First-ever population-based study on status epilepticus in French Island of La Reunion (France)–Incidence and fatality. Seizure. (2014) 23(9):769–73. doi: 10.1016/j.seizure.2014.06.009
112. Bergin PS, Brockington A, Jayabal J, Scott S, Litchfield R, Roberts L, et al. Status epilepticus in Auckland, New Zealand: incidence, etiology, and outcomes. Epilepsia. (2019) 60(8):1552–64. doi: 10.1111/epi.16277
113. Vijiala S, Alvarez V. Epidemiology of status epilepticus in a non-urban area in Switzerland. Acta Neurol Scand. (2021) 143(4):413–20. doi: 10.1111/ane.13383
114. Ferlisi M, Hocker S, Grade M, Trinka E, Shorvon S, et al. Preliminary results of the global audit of treatment of refractory status epilepticus. Epilepsy Behav. (2015) 49:318–24. doi: 10.1016/j.yebeh.2015.04.010
115. Hocker SE, Britton JW, Mandrekar JN, Wijdicks EFM, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol. (2013) 70(1):72–7. doi: 10.1001/jamaneurol.2013.578
116. Fang YT, Lee TL, Tu YH, Lin SH, Chien ME, Huang CW, et al. Factors associated with mortality in patients with super-refractory status epilepticus. Sci Rep. (2022) 12(1):1–10. doi: 10.1038/s41598-021-99269-x
117. Matthews E, Alkhachroum A, Massad N, Letchinger R, Doyle K, Claassen J, et al. New-onset super-refractory status epilepticus: a case series of 26 patients. Neurology. (2020) 95(16):e2280–5. doi: 10.1212/WNL.0000000000010787
118. Briassoulis G, Stefanogianni C, Zaganas I, Raissaki M, Briassoulis P, Ilia S. Specific characteristics and current diagnostic and treatment modalities performance of super refractory status epilepticus in children: a comparative study. Eur J Paediatr Neurol. (2022) 37:32–9. doi: 10.1016/j.ejpn.2022.01.004
119. Tatlidil I, Ture HS, Akhan G. Factors affecting mortality of refractory status epilepticus. Acta Neurol Scand. (2020) 141(2):123–31. doi: 10.1111/ane.13173
120. Orlandi N, Gozzi A, Giovannini G, Turchi G, Cioclu MC, Vaudano AE, et al. Recurrent status epilepticus: clinical features and recurrence risk in an adult population. Seizure. (2022) 97:1–7. doi: 10.1016/j.seizure.2022.02.012
121. Dhakar MB, Thurman DJ, Haider HA, Rodriguez AR, Jette N, Faught E. Thirty-day readmission after status epilepticus in the United States: insights from the nationwide readmission database. Epilepsy Res. (2020) 165:106346. doi: 10.1016/j.eplepsyres.2020.106346
122. Cheng CY, Hsu CY, Wang TC, Jeng YC, Yang WH. The risk of cardiac mortality in patients with status epilepticus: a 10-year study using data from the centers for disease control and prevention (CDC). Epilepsy Behav. (2021) 117:107901. doi: 10.1016/j.yebeh.2021.107901
123. Verma A, Kumar A, Sachan D. Comparison of clinical profile and outcome of de novo convulsive status epilepticus with those with a past history of epilepsy in the elderly populace. Acta Neurol Taiwan. (2022) 31(3):101–6. doi: 10.1046/j.1528-1157.2003.63702.x
124. Sadeghi M, Eshraghi M, Akers KG, Hadidchi S, Kakara M, Nasseri M, et al. Outcomes of status epilepticus and their predictors in the elderly—a systematic review. Seizure. (2020) 81:210–21. doi: 10.1016/j.seizure.2020.08.021
125. Chin RF, Neville BGR, Scott RC. A systematic review of the epidemiology of status epilepticus. Eur J Neurol. (2004) 11(12):800–10. doi: 10.1111/j.1468-1331.2004.00943.x
126. Shorvon S, Ferlisi M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. (2012) 135(8):2314–28. doi: 10.1093/brain/aws091
127. Trinka E, Leitinger M. Management of status epilepticus, refractory status epilepticus, and super-refractory status epilepticus. Continuum. (2022) 28(2):559–602. doi: 10.1212/CON.0000000000001103
128. O’Donoghue MF, Sander J. A historical perspective on the mortality associated with chronic epilepsy. Acta Neurol Scand. (1997) 96(3):138–41. doi: 10.1111/j.1600-0404.1997.tb00255.x
129. Neligan A, Bell GS, Shorvon SD, Sander JW. Temporal trends in the mortality of people with epilepsy: a review. Epilepsia. (2010) 51(11):2241–6. doi: 10.1111/j.1528-1167.2010.02711.x
130. Damian MS, Ben-Shlomo Y, Howard R, Harrison DA. Admission patterns and survival from status epilepticus in critical care in the UK: an analysis of the intensive care national audit and research centre case mix programme database. Eur J Neurol. (2020) 27(3):557–64. doi: 10.1111/ene.14106
131. Collaborators G 2016 E. Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18(4):357. doi: 10.1016/S1474-4422(18)30454-X
132. Mesraoua B, Deleu D, Hassan AH, Gayane M, Lubna A, Ali MA, et al. Dramatic outcomes in epilepsy: depression, suicide, injuries, and mortality. Curr Med Res Opin. (2020) 36(9):1473–80. doi: 10.1080/03007995.2020.1776234
133. Intersectoral Global Action Plan on epilepsy and other neurological disorders 2022–2031. (2021). Available from: https://www.who.int/publications/m/item/intersectoral-global-action-plan-on-eepilepsy-and-other-neurological-disorders-2022-2031
Keywords: seizures, death rate, standardized mortality ratio, case fatality, epilepsy
Citation: Trinka E, Rainer LJ, Granbichler CA, Zimmermann G and Leitinger M (2023) Mortality, and life expectancy in Epilepsy and Status epilepticus—current trends and future aspects. Front. Epidemiol. 3:1081757. doi: 10.3389/fepid.2023.1081757
Received: 27 October 2022; Accepted: 31 January 2023;
Published: 23 February 2023.
Edited by:
Ding Ding, Fudan University, ChinaReviewed by:
Jianxiang Liao, Shenzhen Children’s Hospital, China© 2023 Trinka, Rainer, Granbichler, Zimmermann and Leitinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eugen Trinka ZS50cmlua2FAc2Fsay5hdA==
†ORCID Markus Leitinger orcid.org/0000-0001-8552-6762
Specialty Section: This article was submitted to Neurological and Mental Health Epidemiology, a section of the journal Frontiers in Epidemiology
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.