- Department of Neurology, University of Colorado School of Medicine, Aurora, CO, United States
Over 50 million people around the world have epilepsy, and yet, epilepsy recognition and access to care are ongoing issues. Nearly 80% of people with epilepsy live in low-and middle-income countries and face the greatest barriers to quality care. However, there are substantial disparities in care within different communities in high-income countries as well. Across the world, under-recognition of seizures continues to be an issue, leading to diagnostic and treatment delays. This stems from issues surrounding stigma, public education, basic access to care, as well as healthcare worker education. In different regions, people may face language barriers, economic barriers, and technological barriers to timely diagnosis and treatment. Even once diagnosed, people with epilepsy often face gaps in optimal seizure control with the use of antiseizure medications. Additionally, nearly one-third of people with epilepsy may be candidates for epilepsy surgery, and many either do not have access to surgical centers or are not referred for surgical evaluation. Even those who do often experience delays in care. The purpose of this review is to highlight barriers to care for people with epilepsy, including issues surrounding seizure recognition, diagnosis of epilepsy, and the initiation and optimization of treatment.
Introduction
Decades of work have gone into identifying and bringing to light treatment gaps in epilepsy. Despite this work, progress to significantly narrow care divides has been slow. Over the years, issues related to education, stigma, and treatment delays have been consistently reported throughout different regions. At the same time, there have been efforts to improve epilepsy care, particularly in low- and middle-income countries where the treatment gap is greatest. However, significant barriers remain. Even people in high-income countries continue to experience barriers to care such as a lack of specialists, underutilization of epilepsy surgery, and variable resource allocation (1).
Gaps in epilepsy care range from lack of access to care and delayed diagnosis, to delayed treatment and lack of treatment optimization (Figure 1). Recently, this was synthesized in a systematic review aimed at standardizing the definition of the treatment gap and broadly including key drivers into two new primary definitions. First, a conceptual definition, which refers to the overall proportion of people with active epilepsy who do not receive appropriate treatment, and second, an operational definition, which refers specifically to the difference between the total number of people with active epilepsy and the number of those whose seizures are being appropriately treated (2). Standardization of the definition is important for improving the quality of reporting, and enabling higher quality meta-analysis in this area of research. Beyond quantifying the treatment gap, identifying barriers to care, and improving the quality of reporting, collaborative efforts in clinical care, research, education, and advocacy are critical for developing sustainable improvements (3). This review discusses well-recognized gaps in epilepsy care from across the spectrum of barriers to treatment optimization, as well as highlighting ongoing efforts in improvement.
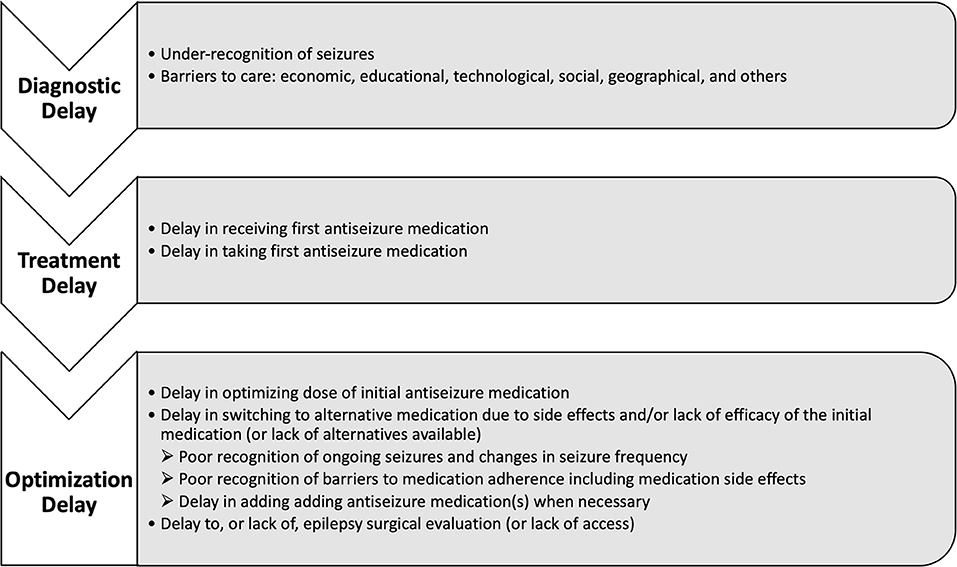
Figure 1. Conceptual framework for the timeline of diagnostic delay in epilepsy and specific barriers at each stage.
Seizure recognition
Difficulties recognizing seizures by patients, families, and healthcare workers is a prominent issue often leading to delays in diagnosis and treatment. In general, the types of seizures least well-recognized are those without motor manifestations. These non-motor seizures can be outwardly subtle and go unassessed for long periods. Over time, recurrent non-motor seizures often culminate in bilateral tonic clonic convulsions, which ultimately prompt medical evaluation and most often lead to a diagnosis of epilepsy.
Recently, data from the Human Epilepsy Project (HEP) has shown substantial delays to diagnosis for people with focal epilepsy. Most striking, the delay to diagnosis was 10-times longer in people who initially experienced only non-motor seizures compared to those with motor seizures at epilepsy onset (4). This adds to evidence from several studies over the past decade that have reported substantial delays in diagnosis for people with epilepsy, primarily those with focal epilepsy, and particularly in those with epilepsy characterized by non-motor seizures at epilepsy onset (5, 6). In addition to poor recognition of seizures, a recent review on the topic clarified three primary drivers for the diagnostic delay: “decision delay” (patient-deferred evaluation), “referral delay” (lack of specialist referral), and “attendance delay” (lack or delay of evaluation when referred) (7). There is also evidence that people with new-onset epilepsy related to high-grade tumors, strokes, and older age tend to have a faster times-to-diagnosis than others, highlighting that delay may also vary depending on etiology and age (8). Areas for potentially improving seizure recognition and narrowing these gaps in care will be discussed in regard to specific barriers in the following sections.
Public education and social stigma
Ongoing social stigma surrounding the diagnosis of epilepsy can lead to delayed diagnosis, preventing people from seeking timely medical evaluation. One way to reduce stigma is to improve the public understanding of seizures and epilepsy through education, which can also lead to improvements in diagnosis and treatment. The development of public health campaigns for many diseases have shown success. Within neurology, this has been particularly successful for stroke treatment, in which public awareness campaigns, mnemonics, and quality improvement have dramatically improved the quality of care (9). In designing public awareness campaigns, it is important to understand baseline public knowledge on the topic through utilization of qualitative methods so that such efforts can be aimed at improving true gaps in awareness and knowledge (10, 11). To date, this represents a relative shortcoming in the literature, as baseline public knowledge has been largely investigated through survey studies in different regions around the world rather than using qualitative methods. Although large surveys can have issues from variability in comprehension of questions to the environmental context in which they are asked, these have nonetheless shed light on important themes. For instance, these have shown that although the stigma surrounding epilepsy varies by country and region, it persists in all regions regardless of resources, education, and income level. Unfortunately, door-to-door surveys are problematic due to the social stigma surrounding epilepsy in many of the locations where they are carried out. Specifically, people with epilepsy who have infrequent seizures, or are in remission, may be less likely to disclose their medical history due to the fear of stigmatization with no immediate benefits from responding to surveys accurately (12).
In Africa, notable surveys on the topic include a one conducted in Rwanda in 2005, in which over a thousand individuals were sampled via random cluster sampling, and found that most respondents believed people with epilepsy should not be allowed to go to school (66%), to work (72%), to use public spaces (69%), or to get married (66%), and believed that epilepsy was untreatable (50%) and transmissible (40%) (13). An even larger door-to-door survey including 4500 people in suburban Senegal the same year found that 51% of respondents believed epilepsy was caused by evil spirits and 35% believed it to be contagious (14). The treatment gap in Tanzania as recently as 2016 was reported to be ~45% and had the highest correlations with a lack of education or knowledge of epilepsy or believing in a supernatural cause for epilepsy (15). Even in high income countries, there are gaps in knowledge among people with epilepsy regarding their diagnosis as well as high rates of perceived stigma (16). Stigma exists to variable degrees in all environments, and takes different forms based on local cultures, medical traditions, economic conditions, and politics (17). It can also be substantial among immigrants, which was recently reported in a study carried out in Sweden, where feelings of social isolation were magnified in people with epilepsy who were facing language barriers and an unfamiliar healthcare system (18). These examples underscore the common themes that have been present for thousands of years, and have led to discrimination against people with epilepsy that persists to this day (19).
Current evidence points to an ongoing poor public understanding of seizures and epilepsy. This can be improved through educational initiatives for people with epilepsy, their families, and their communities, and may be a fundamental for improving stigma. An important finding from efforts to identify and reduce stigma has been that public awareness and epilepsy education is negatively correlated with stigma (20, 21). This was confirmed in a recent study in Pakistan, where efforts to improve public awareness of epilepsy led to a significant reduction in both the epilepsy treatment gap as well as stigma (22). These studies highlight a clear target for improving the quality of life and quality of care for people with epilepsy.
Socioeconomic and technological divides
Socioeconomic disparities, as well as frequently co-occurring technological divides, underlie the most significant gaps in epilepsy care worldwide. In several regions, there are ongoing issues with people not being able to access and afford an antiseizure medication, let alone having more than one medication option (23). Beyond basic access to medication, there are also stark differences in quality of life and quality of care for people depending on demographic variables. For many years there has been an ongoing collaborative effort to steer epilepsy research in the direction of identifying and addressing such disparities in care by leaders in the field (24).
Several studies in the United States and North America have shown different rates of seizure control and epilepsy remission along racial and ethnic divides, including delays to diagnosis and decreased utilization of epilepsy surgery in several minority populations (25–27). There have been several studies from different regions around the world highlighting significant differences in quality of life and quality of care along socioeconomic and racial divides (28–31). A recent investigation has also shown higher rates of sudden unexpected death in epilepsy (SUDEP) in people with high socioeconomic disadvantage, which persisted during the study for those with the highest socioeconomic disadvantage over a time when overall rates of SUDEP were decreasing (32). Many barriers to care that have been consistently identified over the years have clear potential for intervention, such as improving medication affordability and improving access to primary and specialist care. However, these issues frequently necessitate larger political solutions as well as healthcare systems solutions and often face economic and political barriers. Strategies to improve access to care are expanding significantly as communication technology improves and telehealth services expand. However, sustainable solutions will likely still rely on a coordinated effort between individuals and communities along with healthcare institutions, government, and non-governmental organizations.
Healthcare worker education
The most widely reported factor contributing to diagnostic delay is under recognition of common seizure symptoms. Specifically, seizures with outwardly subtle symptoms, such as non-motor seizures, are under-recognized (33). Not only are patients more likely to seek medical evaluation after experiencing convulsions, but healthcare workers are more likely to recognize and treat them. A large retrospective cohort study in the United States showed that approximately one-third of patients with newly diagnosed epilepsy remain untreated up to 3 years following an initial diagnosis (34). This study also highlighted the fact that variability of seizure symptoms at epilepsy onset can present a lengthy differential on initial evaluation, which inevitably leads to treatment delays (35). Even when patients present to emergency departments for evaluation of convulsive seizures, a history of preceding seizures (often non-motor) is present in up to half and go largely unrecognized or untreated (36–40). This is a large group of patients who are seen by healthcare providers at a time when they meet diagnostic criteria, yet remain undiagnosed. Additionally, healthcare providers often also fail to adequately identify important family history during initial evaluations. This is an important factor not only for diagnosis, but can have implications for prognosis and counseling, such as in the case of familial mesial temporal lobe epilepsy, which often is not recognized due to not obtaining an adequate family history (41). It is possible that improving seizure education for healthcare workers may improve the quality of medical evaluations when patients present with seizure symptoms and may help to narrow this gap in care.
A study in Nepal found that it is possible to train non-neurologists to accurately diagnose epilepsy in resource-limited settings (42). This is a reasonable goal for making improvements, particularly in low- and middle-income countries where there can be a significant knowledge gap about epilepsy among primary healthcare workers (43). However, even non-neurologist healthcare workers are limited in many low-income countries, and so mindfully distributing primary medical care resources is important for making sustainable improvements (44). A growing opportunity to improve education and awareness among healthcare workers in all settings is technology – web seminars, pre-recorded lectures, and other online learning can supplement training and may be a way of improving epilepsy education particularly outside of large academic centers where people have access to technology (45).
Gaps in treatment
In response to recognition of the pervasive treatment gap in epilepsy, the International League Against Epilepsy (ILAE), International Bureau for Epilepsy (IBE), and World Health Organization (WHO) started the Global Campaign against Epilepsy in 1997. Shortly thereafter, the National Institute of Neurological Disorders and Stroke (NINDS) in the United States in collaboration with the American Epilepsy Society (AES) established benchmarks for epilepsy research in 2001 in order to guide future progress (46). In 2006, results from the collaborative ILAE/IBE/WHO Global Campaign Against Epilepsy, in which data were collected from 160 countries, confirmed a significant gap in epilepsy treatment in the majority of countries with substantial regional variability resulting from economic disparities (47). Subsequently, there were additional reports increasing epilepsy awareness, including a 2015 resolution by the World Health Assembly (WHA) urging member states to implement a coordinated action against epilepsy and its consequences (48). Then in 2020, the 73rd WHA unanimously approved a resolution to develop and implement a 10-year global action plan on epilepsy, and after being discussed by the Executive Board was recommended to be adopted by the 75th WHA in May 2022 (49, 50).
In examining gaps in treatment, a common issue with systematic reviews on the topic is the tendency to exclude non-English publications and exclude many studies on populations in low-income countries, which can lead to sampling bias (51). There is also variation in the definition used for treatment gap, and substantial variability in quality and reporting, making quality meta-analysis challenging. In much of the literature, the epilepsy treatment gap has referred to the proportion of people with untreated epilepsy relative to the prevalence of active disease (52). This is most similar to a recently proposed conceptual definition, though the newer definitions take into account many factors that are important to this issue including gaps in affordability of care and medications, diagnostic gaps, therapeutic gaps, and other issues related to quality of care (2). This effort to standardize the definition is important for ongoing efforts to improve the quality and transparency of reporting, and the consistency between studies. Taking current studies into consideration, gaps in epilepsy care range from poor utilization of antiseizure medications, to poor optimization of antiseizure medications, and poor utilization of epilepsy surgery.
Gaps in antiseizure medication use
A consistent finding across regions is that the most vulnerable people experience the highest treatment gap. Worldwide disparities in care are most striking between areas of high and low socioeconomic status, with high-income and urban areas having the lowest treatment gaps and low-income and rural areas having the highest treatment gaps (2, 53, 54). Since the primary determinant of the treatment gap worldwide appears to be directly related to economic and healthcare-related resources, it is largely a reflection of basic access to medication and healthcare services (55).
Reviews of the treatment gap in different regions have highlighted this point powerfully. In Africa, a recent review of the epilepsy treatment gap among sub-Saharan countries showed a collective treatment gap from 23 studies of nearly 70% (56). A door-to-door questionnaire study conducted in Egypt on 33,818 people found a treatment gap of 83.8% (57). In some African countries the treatment gap has been reported to be particularly high, such as in Madagascar, where an estimated 92% of people with epilepsy remain untreated (58). On other continents, the treatment gap is likewise particularly significant in rural and low-income communities. A door-to-door survey conducted on 55,000 people in China found that 41% of people with epilepsy had never received appropriate treatment and 63% with active epilepsy had not been taking antiseizure medication in the week prior to the survey (59). A separate cross-sectional analysis of 54,976 people in Eastern China found a treatment gap of 58.5%, which was independently associated with having a high seizure frequency and not having health insurance (60). Similar findings have been seen in other studies across Asia from Vietnam and the Philippines to South Kazakhstan, where the treatment gap ranges from 25% to nearly 85%, and has been attributed to socioeconomic barriers, poor public education, limited access to care, limited access to medications, suboptimal use of medications, and unaffordability of medications (61–63). One study from South America found that only about 50% of people with epilepsy São Paulo, Brazil, were taking an antiseizure medication (64).
These reports all paint a bleak picture of the treatment gap, but an even more unfortunate reality is that despite recognition of the problem, there have been few substantial improvements over time. A large systematic review for the WHO published in 2010 reported a gap of 75% in low-income and 50% in most middle- to upper-income countries, but most striking was the finding that over a 20-year period form 1987 to 2007 there was no improvement in the treatment gap (53). Part of this stems from a lack of seizure recognition, which leads to delayed diagnosis on the front end, as well as underutilization of antiseizure medications in people with known diagnoses. This is compounded by issues discussed earlier related to context and location – stigma, education, and socioeconomic variables including access to care and technology. One area to improve the treatment gap, however, may be to improve seizure recognition.
Previous studies have found that when people are evaluated in emergency settings for convulsions, a diagnosis of epilepsy is often missed due to lack of recognizing preceding seizures (5, 36). Improving assessments for people seeking emergency care for first time seizures may have a meaningful impact on improving the time to diagnosis and treatment. This could either be an improvement within the emergency departments, or creating separate first-seizure clinics, which have been successfully implemented in some centers in Australia and shown to improve time-to-diagnosis and treatment (65). Initial diagnosis and treatment is crucial, but delay in this process is not the only gap in care experienced by people with epilepsy.
Once a diagnosis is made, optimizing treatment to prevent further seizures and minimize medication side effects is also crucial. This is an additional layer of complexity that can vary in magnitude depending on local resources. There is substantial variation in medications prescribed for people with epilepsy based on demographic and socioeconomic factors that are often not in line with current recommendations (66, 67). In other words, there are gaps in treatment optimization and the long-term epilepsy care following treatment initiation. One potential way of improving and standardizing care across health systems has recently been explored among children and youth with epilepsy in Project ECHO (Extension for Community Healthcare Outcomes). This model utilizes a hub-and-spoke knowledge-sharing network to leverage expert knowledge in supporting improved care of specialty conditions being treated by a larger network of primary care providers, and was successful when used for improving the quality of care for children and youth with epilepsy (68). Such solutions will be important for improving quality of care considering the limited numbers of specialty-trained physicians, and are increasingly possible through the expanding use of new technologies.
Gaps in epilepsy surgery utilization
Many patients with drug-resistant focal epilepsy who undergo epilepsy surgery are substantially more likely to be seizure free following surgery than those who remain on antiseizure medications alone (69, 70). However, the under-utilization of surgical treatment options for drug-resistant epilepsy has been well described over the years despite evidence to support its use. Even with growing evidence to support epilepsy surgery, as well as several calls-to-action, there remains a persistent knowledge gap among both physicians and patients, as well as a lack of federal funding for research in this area compared to other medical specialties (71, 72). There is also a gap between clinician knowledge and their actions – i.e., even if they are educated in regard to surgical treatment options, there is nonetheless an under-utilization of epilepsy surgery that represents a significant treatment gap (73). Furthermore, epilepsy surgery has been shown to be underutilized in a high-income universal health system, suggesting that access to surgical centers is not the only barrier to utilization (74).
Not only is surgery underutilized in high-income countries, but there are disparities among those who receive it based on age, race, and health insurance (75). In low-income countries the issue is more pronounced since there are limited centers capable of performing epilepsy surgeries. Children face substantial barriers to attaining epilepsy surgery even when it is indicated, often the result of poor understanding of surgery on the part of their families and healthcare providers, as well as due to system disparities in care (76). In all settings, there is a combination of patient-related factors, physician-related factors, and health system factors, which factor into underutilization of epilepsy surgery (77). One method for improving this gap in care may be to expand involvement of epilepsy specialists early in the care of people with epilepsy through the creation of educational networks such as how Project ECHO was utilized for improving the care for children and youth with epilepsy as discussed above. By making inroads into primary care practices and general neurology practices, it may be possible to increase the number of referrals to epilepsy surgical centers and increase acceptance of this as a treatment option among patients and non-specialists.
Conclusions
Despite continual advances in treatment options for epilepsy, there remain significant barriers to care across the world (Table 1). Disparities exist among communities in all countries, with the greatest gaps in care in low- and middle-income countries. Although recognition of epilepsy has been increasing over time, there are still significant barriers to timely diagnosis and treatment due to under-recognition of seizures. These include issues related to stigma, access to care such as economic, technological, and language barriers, as well as under-recognition of seizures and epilepsy among both the public and healthcare workers. Even once diagnosed, people with epilepsy often face gaps in the optimization of seizure control with the use of medications and surgical evaluation when needed.
Author contributions
JP was solely responsible for concept, design, and drafting of the manuscript.
Acknowledgments
JP thanks the University of Colorado for making this work possible.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zelano J, Klecki J, Christensen J, Tomson T, Malmgren K, Kruja J, et al. The provision of epilepsy care across Europe 2017: A 17-year follow-up survey. Epilepsia open. (2019) 4:144–52. doi: 10.1002/epi4.12306
2. Kwon CS, Wagner RG, Carpio A, Jetté N, Newton CR, Thurman DJ. The worldwide epilepsy treatment gap: a systematic review and recommendations for revised definitions – a report from the ILAE Epidemiology Commission. Epilepsia. (2022) 63:551–64. doi: 10.1111/epi.17112
3. Spiciarich MC, von Gaudecker JR, Jurasek L, Clarke DF, Burneo J, Vidaurre J. Global health and epilepsy: update and future directions. Curr Neurol Neurosci Rep. (2019) 19:30. doi: 10.1007/s11910-019-0947-6
4. Pellinen J, Erica T, Yang A, Price D, Friedman D, Holmes M, et al. Focal non-motor vs motor seizures: the impact on diagnostic delay in focal epilepsy. Epilepsia. (2020) 61:2643–52. doi: 10.1111/epi.16707
5. Firkin AL, Marco DJT, Saya S, Newton MR, O'Brien TJ, Berkovic SF, et al. Mind the gap: Multiple events and lengthy delays before presentation with a ‘first seizure'. Epilepsia. (2015) 56:1534–41. doi: 10.1111/epi.13127
6. Parviainen L, Kälviäinen R, Jutila L. Impact of diagnostic delay on seizure outcome in newly diagnosed focal epilepsy. Epilepsia Open. (2020) 5:605–10. doi: 10.1002/epi4.12443
7. Alessi N, Perucca P, McIntosh AM. Missed, mistaken, stalled: identifying components of delay to diagnosis in epilepsy. Epilepsia. (2021) 62:1494–504. doi: 10.1111/epi.16929
8. Yang M, Tan KM, Carney P, Kwan P, O'Brien TJ, Berkovic SF, et al. Diagnostic delay in focal epilepsy: association with brain pathology and age. Seizure. (2022) 96:121–7. doi: 10.1016/j.seizure.2022.02.004
9. Bray JE, Mosley I, Bailey M, Barger B, Bladin C. Stroke public awareness campaigns have increased ambulance dispatches for stroke in Melbourne, Australia. Stoke. (2011). 42:2154–7.
10. Steriade C. Closing the diagnostic gap in epilepsy: recognizing more than just motor seizures. Epilepsy Curr. (2021) 61:153575972199480. doi: 10.1177/1535759721994807
11. Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet. (2010) 376:1261–71. doi: 10.1016/S0140-6736(10)60809-4
12. Beghi E, Hesdorffer D. Prevalence of epilepsy–an unknown quantity. Epilepsia. (2014) 55:963–7. doi: 10.1111/epi.12579
13. Sebera F, Munyandamutsa N, Teuwen DE, Ndiaye IP, Diop AG, Tofighy A, et al. Addressing the treatment gap and societal impact of epilepsy in Rwanda–Results of a survey conducted in 2005 and subsequent actions. Epilepsy Behav. (2015) 46:126–32. doi: 10.1016/j.yebeh.2015.03.028
14. Ndoye NF, Sow AD, Diop AG, Sessouma B, Séne-Diouf F, Boissy L, et al. Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal an ILAE/IBE/WHO study. Seizure. (2005) 14:106–11. doi: 10.1016/j.seizure.2004.11.003
15. Hunter E, Rogathi J, Chigudu S, Jusabani A, Jackson M, Whittaker RG, et al. The epilepsy treatment gap in rural Tanzania: A community-based study in adults. Seizure. (2016) 36:49–56. doi: 10.1016/j.seizure.2016.02.008
16. Doughty J, Baker GA, Jacoby A, Lavaud V. Cross-cultural differences in levels of knowledge about epilepsy. Epilepsia. (2003) 44:115–23. doi: 10.1046/j.1528-1157.2003.34402.x
17. Thomas S V, Nair A. Confronting the stigma of epilepsy. Ann Indian Acad Neurol. (2011) 14:158. doi: 10.4103/0972-2327.85873
18. Andersson K, Strang S, Zelano J, Chaplin J, Malmgren K, Ozanne A. Multiple stigma among first-generation immigrants with epilepsy in Sweden. Epilepsy Behav. (2021) 115:107638. doi: 10.1016/j.yebeh.2020.107638
19. Pahl K, De Boer HM. Epilepsy and Rights, Atlas Epilepsy Care World Geneva WHO. World Health Organization (2005). p. 72–73.
20. Yeni K, Tulek Z, Simsek OF, Bebek N. Relationships between knowledge, attitudes, stigma, anxiety and depression, and quality of life in epilepsy: a structural equation modeling. Epilepsy Behav. (2018) 85:212–7. doi: 10.1016/j.yebeh.2018.06.019
21. Shi Y, Wang S, Ying J, Zhang M, Liu P, Zhang H, et al. Correlates of perceived stigma for people living with epilepsy: a meta-analysis. Epilepsy Behav. (2017) 70:198–203. doi: 10.1016/j.yebeh.2017.02.022
22. Mogal Z, Aziz H. Epilepsy treatment gap and stigma reduction in Pakistan: a tested public awareness model. Epilepsy Behav. (2020) 102:107979. doi: 10.1016/j.yebeh.2019.106637
23. Pironi V, Ciccone O, Beghi E, Paragua-Zuellig H, Patel AA, Giussani G, et al. Survey on the worldwide availability and affordability of antiseizure medications: Report of the ILAE Task Force on Access to Treatment. Epilepsia. (2022) 63:335–51. doi: 10.1111/epi.17155
24. Theodore WH, Spencer SS, Wiebe S, Langfitt JT, Ali A, Shafer PO, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. (2006) 47:1700–22. doi: 10.1111/j.1528-1167.2006.00633.x
25. Gregerson CHY, Bakian AV, Wilkes J, Knighton AJ, Nkoy F, Sweney M, et al. Disparities in pediatric epilepsy remission are associated with race and ethnicity. J Child Neurol. (2019) 34:928–36. doi: 10.1177/0883073819866623
26. Bensken WP, Navale SM, Andrew AS, Jobst BC, Sajatovic M, Koroukian SM. Delays and disparities in diagnosis for adults with epilepsy: Findings from U.S. Medicaid data. Epilepsy Res. (2020) 166:106406. doi: 10.1016/j.eplepsyres.2020.106406
27. Hamade YJ, Palzer EF, Helgeson ES, Hanson JT, Walczak TS, McGovern RA. Persistent racial and ethnic disparities as a potential source of epilepsy surgery underutilization: Analysis of large national datasets from 2006-2016. Epilepsy Res. (2021) 176:106725. doi: 10.1016/j.eplepsyres.2021.106725
28. Groover O, Morton ML, Janocko NJ, Teagarden DL, Villarreal HK, Drane DL, et al. Mind the gap: health disparities in families living with epilepsy are significant and linked to socioeconomic status. Epileptic Disord. (2020) 22:782–9. doi: 10.1684/epd.2020.1229
29. Jetté N, Quan H, Faris P, Dean S, Li B, Fong A, et al. Health resource use in epilepsy: Significant disparities by age, gender, and aboriginal status. Epilepsia. (2008) 49:586–93. doi: 10.1111/j.1528-1167.2007.01466.x
30. Saadi A, Patenaude B, Mateen FJ. Quality of life in epilepsy-31 inventory (QOLIE-31) scores: a global comparison. Epilepsy Behav. (2016) 65:13–7. doi: 10.1016/j.yebeh.2016.09.032
31. Thurman DJ, Kobau R, Luo YH, Helmers SL, Zack MM. Health-care access among adults with epilepsy: The U.S. National Health Interview Survey, 2010 and 2013, Epilepsy Behav. (2016) 55:184–8. doi: 10.1016/j.yebeh.2015.10.028
32. Cihan E, Hesdorffer DC, Brandsoy M, Li L, Fowler DR, Graham JK, et al. Socioeconomic disparities in SUDEP in the US. Neurology. (2020) 94:e2555–66. doi: 10.1212/WNL.0000000000009463
33. Pellinen J, French J, Knupp KG. Diagnostic delay in epilepsy: the scope of the problem. Curr Neurol Neurosci Rep. (2021) 21:71. doi: 10.1007/s11910-021-01161-8
34. Kalilani L, Faught E, Kim H, Burudpakdee C, Seetasith A, Laranjo S, et al. Assessment and effect of a gap between new-onset epilepsy diagnosis and treatment in the US. Neurology. (2019) 92:e2197–e2208. doi: 10.1212/WNL.0000000000007448
35. Hogan RE. Delay of treatment, after diagnosis, as a contributor to the “Treatment Gap” in epilepsy. Epilepsy Curr. (2019) 19:385–6. doi: 10.1177/1535759719874793
36. Pellinen J, Tafuro E, Baehr A, Barnard S, Holmes M, French J. The impact of clinical seizure characteristics on recognition and treatment of new-onset focal epilepsy in emergency departments. Acad Emerg Med. (2020) 28:412–20. doi: 10.1111/acem.14114
37. Appleton RE. Seizure-related injuries in children with newly diagnosed and untreated epilepsy. Epilepsia. (2002) 43:764–7. doi: 10.1046/j.1528-1157.2002.41101.x
38. Ting YW, Kwong KL. Seizure-related injuries in newly diagnosed childhood epilepsy. Pediatr Neurol. (2010) 42:417–21. doi: 10.1016/j.pediatrneurol.2010.02.010
39. Shorvon SD, Goodridge DMG. Longitudinal cohort studies of the prognosis of epilepsy: contribution of the National General Practice Study of Epilepsy and other studies. Brain. (2013) 136:3497–510. doi: 10.1093/brain/awt223
40. Holper S, Foster E, Chen Z, Kwan P. Emergency presentation of new onset versus recurrent undiagnosed seizures: a retrospective review. Emerg Med Australas. (2020) 32:430–7. doi: 10.1111/1742-6723.13420
41. Perucca P, Crompton DE, Bellows ST, McIntosh AM, Kalincik T, Newton MR, et al. Familial mesial temporal lobe epilepsy and the borderland of déjà vu. Ann Neurol. (2017) 82:166–76. doi: 10.1002/ana.24984
42. Patterson V, Gautam N, Pant P. Training non-neurologists to diagnose epilepsy. Seizure. (2013) 22:306–8. doi: 10.1016/j.seizure.2013.02.001
43. Buddhiraja R, Sharma S, Sharma S, Bansal RK, Setia RK, Bansal N, et al. Epilepsy knowledge, attitudes, and practices among primary healthcare providers in an Indian district. Epilepsy Behav. (2020) 104:106899. doi: 10.1016/j.yebeh.2019.106899
44. Bhalla D, Preux PM. Manpower gap: an important barrier against reduction of the treatment gap of epilepsy. Seizure. (2013) 22:586–7. doi: 10.1016/j.seizure.2013.03.010
45. Weber DJ, Moeller JJ. Epilepsy education: recent advances and future directions. Curr Neurol Neurosci Rep. (2019) 19:35. doi: 10.1007/s11910-019-0946-7
46. Engel J. Progress in epilepsy: reducing the treatment gap and the promise of biomarkers. Curr Opin Neurol. (2008) 21:150–4. doi: 10.1097/WCO.0b013e3282f4edc3
47. Dua T, De Boer HM, Prilipko LL, Saxena S. Epilepsy Care in the World: results of an ILAE/IBE/WHO Global Campaign Against Epilepsy survey. Epilepsia. (2006) 47:1225–31. doi: 10.1111/j.1528-1167.2006.00595.x
48. Covanis A, Guekht A, Li S, Secco M, Shakir R, Perucca E. From global campaign to global commitment: The World Health Assembly's Resolution on epilepsy. Epilepsia. (2015) 56:1651–7. doi: 10.1111/epi.13192
49. Guekht A, Brodie M, Secco M, Li S, Volkers N, Wiebe S. The road to a World Health Organization global action plan on epilepsy and other neurological disorders. Epilepsia. (2021) 62:1057–63. doi: 10.1111/epi.16856
50. Kestel D. Draft Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders 2022–2031. (2022). Available online at: https://www.who.int/news/item/28-04-2022-draft-intersectoral-global-action-plan-on-epilepsy-and-other-neurological-disorders-2022-2031 (accessed 22 May, 2022).
51. Burneo JG. About systematic reviews and the treatment gap in epilepsy. Epilepsia. (2009) 50:1293–4. doi: 10.1111/j.1528-1167.2008.01999.x
52. Kale R. Global Campaign Against Epilepsy:the treatment gap. Epilepsia. (2002) 43 (Suppl 6):31–3. doi: 10.1046/j.1528-1157.43.s.6.13.x
53. Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. (2010) 88:260–6. doi: 10.2471/BLT.09.064147
54. Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. (2008) 49:1491–503. doi: 10.1111/j.1528-1167.2008.01693.x
55. Meyer ACL, Dua T, Boscardin WJ, Escarce JJ, Saxena S, Birbeck GL. Critical determinants of the epilepsy treatment gap: a cross-national analysis in resource-limited settings. Epilepsia. (2012) 53:2178–85. doi: 10.1111/epi.12002
56. Owolabi LF, Owolabi SD, Adamu B, Jibo AM, Alhaji ID. Epilepsy treatment gap in Sub-Saharan Africa: Meta-analysis of community-based studies. Acta Neurol Scand. (2020) 142:3–13. doi: 10.1111/ane.13246
57. Shehata G, El tallawy H, Farghaly W, Rageh T, Sayed M, Abdelwarith A, et al. Spectrum of epilepsy - prevalence, impact, and treatment gap: an epidemiological study from Al-Quseir, Egypt. Neuropsychiatr Dis Treat. (2016) 12:1111. doi: 10.2147/NDT.S87765
58. Ratsimbazafy V, Andrianabelina R, Randrianarisona S, Preux PM, Odermatt P. Treatment gap for people living with epilepsy in Madagascar. Trop Doct. (2011) 41:38–9. doi: 10.1258/td.2010.100254
59. Wang WZ, Wu JZ, Wang DS, Dai XY, Yang B, Wang TP, et al. The prevalence and treatment gap in epilepsy in China: an ILAE/IBE/WHO study. Neurology. (2003) 60:1544–5. doi: 10.1212/01.WNL.0000059867.35547.DE
60. Ding X, Zheng Y, Guo Y, Shen C, Wang S, Chen F, et al. Active epilepsy prevalence, the treatment gap, and treatment gap risk profile in eastern China: a population-based study. Epilepsy Behav. (2018) 78:20–4. doi: 10.1016/j.yebeh.2017.10.020
61. Moalong KMC, Espiritu AI, Fernandez MLL, Jamora RDG. Treatment gaps and challenges in epilepsy care in the Philippines. Epilepsy Behav. (2021) 115:107491. doi: 10.1016/j.yebeh.2020.107491
62. Guekht A, Zharkinbekova N, Shpak A, Hauser WA. Epilepsy and treatment gap in urban and rural areas of the Southern Kazakhstan in adults. Epilepsy Behav. (2017) 67:98–104. doi: 10.1016/j.yebeh.2016.11.028
63. Tuan NA, Tomson T, Allebeck P, Chuc NTK, Cuong LQ. The treatment gap of epilepsy in a rural district of Vietnam: a study from the EPIBAVI project. Epilepsia. (2009) 50:2320–3. doi: 10.1111/j.1528-1167.2009.02298.x
64. Noronha ALA, Marques LH, Borges MA, Cendes F, Guerreiro CAM, Min LL. Assessment of the epilepsy treatment gap in two cities of south-east of Brazil. Arq Neuropsiquiatr. (2004) 62:761–3. doi: 10.1590/S0004-282X2004000500003
65. McIntosh AM, Tan KM, Hakami TM, Newton MR, Carney PW, Yang M, et al. Newly diagnosed seizures assessed at two established first seizure clinics: Clinic characteristics, investigations, and findings over 11 years. Epilepsia Open. (2020) 6:171–80. doi: 10.1002/epi4.12460
66. Hamer HM, Kostev K. Sociodemographic disparities in administration of antiepileptic drugs to adults with epilepsy in Germany: a retrospective, database study of drug prescriptions. CNS Drugs. (2014) 28:753–9. doi: 10.1007/s40263-014-0187-x
67. Pisu M, Richman J, Piper K, Martin R, Funkhouser E, Dai C, et al. Quality of antiepileptic treatment among older medicare beneficiaries with epilepsy: a retrospective claims data analysis. Med Care. (2017) 55:677–83. doi: 10.1097/MLR.0000000000000724
68. Joshi S, Gali K, Radecki L, Shah A, Hueneke S, Calabrese T, et al. Integrating quality improvement into the ECHO model to improve care for children and youth with epilepsy. Epilepsia. (2020) 61:1999–2009. doi: 10.1111/epi.16625
69. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. (2001) 345:311–8. doi: 10.1056/NEJM200108023450501
70. Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. (2012) 307:922–30. doi: 10.1001/jama.2012.220
71. Solli E, Colwell NA, Say I, Houston R, Johal AS, Pak J, et al. Deciphering the surgical treatment gap for drug-resistant epilepsy (DRE): a literature review. Epilepsia. (2020) 61:1352–64. doi: 10.1111/epi.16572
72. Samanta D, Leigh Hoyt M, Scott Perry M. Healthcare professionals' knowledge, attitude, and perception of epilepsy surgery: a systematic review. Epilepsy Behav. (2021) 122:108199. doi: 10.1016/j.yebeh.2021.108199
73. Sirven JI. The silent gap between epilepsy surgery evaluations and clinical practice guidelines. Eur J Neurol. (2010) 17:522–3. doi: 10.1111/j.1468-1331.2009.02892.x
74. Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology. (2016) 86:72–8. doi: 10.1212/WNL.0000000000002249
75. McClelland S, Guo H, Okuyemi KS. Racial disparities in the surgical management of intractable temporal lobe epilepsy in the United States: a population-based analysis. Arch Neurol. (2010) 67:577–83. doi: 10.1001/archneurol.2010.86
76. Beatty CW, Lockrow JP, Gedela S, Gehred A, Ostendorf AP. The missed value of underutilizing pediatric epilepsy surgery: a systematic review. Semin Pediatr Neurol. (2021) 39:100917. doi: 10.1016/j.spen.2021.100917
Keywords: neurology, diagnoisis, underutilization, healthcare delivery, disparities, accessibility, barriers
Citation: Pellinen J (2022) Treatment gaps in epilepsy. Front. Epidemiol. 2:976039. doi: 10.3389/fepid.2022.976039
Received: 22 June 2022; Accepted: 18 July 2022;
Published: 01 August 2022.
Edited by:
Zhibin Chen, Monash University, AustraliaReviewed by:
Martin J. Brodie, International Bureau for Epilepsy, United KingdomPiero Perucca, The University of Melbourne, Australia
Copyright © 2022 Pellinen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacob Pellinen, SmFjb2IuUGVsbGluZW5AY3VhbnNjaHV0ei5lZHU=
†ORCID: Jacob Pellinen orcid.org/0000-0002-0747-7210
 Jacob Pellinen
Jacob Pellinen