
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 14 February 2025
Sec. Soil Processes
Volume 13 - 2025 | https://doi.org/10.3389/fenvs.2025.1500314
This article is part of the Research TopicAdvances in Soil Pollution Research: Risk Assessment and Ecosystems ManagementView all 8 articles
 Protik Banerjee1
Protik Banerjee1 Harshad V. Kulkarni1,2*
Harshad V. Kulkarni1,2* Allison M. Veach3
Allison M. Veach3 Thiba Nagaraja4
Thiba Nagaraja4 Pousali Pathak1
Pousali Pathak1 Suprem R. Das4,5
Suprem R. Das4,5 Saugata Datta1*
Saugata Datta1*Introduction: The availability and mobility of phosphorus (P) in soils play a crucial role in effectively managing agricultural activities and maintaining healthy soils. Several parameters including soil texture, pH, elemental and mineralogical composition, moisture content, and soil organic matter (SOM) are crucial in controlling the movement of P in soils. This study focuses on assessing geochemical properties of soils from a pristine prairie grassland and an agriculturally dominated land, and their influence on soil P mobility.
Methods: Surface soils were collected from two locations, Konza Prairie Biological Station (KBPS) located in Manhattan (Kansas) which is a native grassland ecosystem, and agricultural land in town of Hays (Kansas).
Results: Results showed that the KPBS soils contained lower water-extractable phosphate (PO43—) concentrations (0.2 ± 0.7 mg/kg) than soils from Hays (1.3 ± 2.4 mg/kg). Bio-available P measured as Bray-P were also lower in KPBS (14.3 ± 7.0 mg/kg) relative to Hays (23.0 ± 23.7 mg/kg). Soils from both the sites contained water-extractable calcium, magnesium and potassium as a primary soluble component likely from carbonate minerals in these calcareous soils. The SOM concentrations measured as loss on ignition (LoI) were greater in KPBS (9.9% ± 1.8%) relative to Hays (5.3% ± 1.7%). Water extractable soil organic carbon (WE-SOC) concentrations were also greater for KPBS (651 ± 274 mg/kg) relative to Hays (288 ± 267 mg/kg). Optical spectroscopic analyses using absorbance and fluorescence properties revealed that the water-extractable SOM in these soils was mainly of terrestrial origin, plant-derived, aromatic, and contained humic-like substances. The intensities of fluorescence peaks A, C, and M, and specific UV absorbance at 254 nm (SUVA 254) of both soils correlate strongly with the Bray-P concentrations, indicating that the source of SOM plays a vital role in controlling soil P mobility.
Conclusion: These findings indicate that natural prairie grassland soils contained lower P concentrations that are primarily insoluble in water and associated with humic and fulvic-like SOM.
Availability of phosphorous (P) in soils is affected by biogeochemical processes. Geochemical processes control the movement and distribution of P in soils in the long term, while biological processes control soil P distribution in the short term by deriving plant available P from soil organic matter (Cross and Schlesinger, 1995). The weathering of primary minerals supplies the plant available pool of phosphate in soils. Dissolution–precipitation (mineral equilibria), adsorption–desorption (interactions between P in solution and solid phases), and mineralization–immobilization are the key processes of the soil P cycle (Filippelli, 2002; Filippelli, 2008; Föllmi, 1996; Haynes, 1982; Kruse et al., 2015; Smil, 2008). These processes influence the amount of P in the soil pore water and solid phases, as well as P available for uptake by plants and microbes and subsequently transfer to surface and groundwaters. Phosphorus is transferred primarily through diffusion from soil aggregates or particles to plant roots (Pierzynski, 2000) with little loss occurring due to erosion and surface runoff. Specific geochemical factors like pH, presence of competing ions and organic matter need to be better understood to have a complete understanding of the P cycle in soils. Depending on parent material, soil type, and soil management practices (e.g., fertilization, tillage) total soil P in topsoil horizons (0–15 cm) may range from 50 to 3,000 mg/kg (Foth and Ellis, 1997; Frossard et al., 2000). The majority of P in mineral soils (50%–75%) is inorganic, and it is mostly coupled with Al and Fe in acidic soils and calcium in alkaline, calcareous soils (Pierzynski, 1991a). Primary minerals (those created by precipitation of P with Al, Ca, and Fe) and secondary minerals (those formed by P adsorbed onto the surface of clay minerals, Fe, and Al oxyhydroxides or carbonates), and P physically occluded inside secondary minerals are all examples of inorganic P. The principal minerals that release P into the soil solution as they weather, vary with the soil type, mainly as a function of time and extent of soil development. Apatites [Ca10(X) (PO4)6, where X is either F, Cl, OH, or CO32-] are the principal mineral sources of P in moderately weathered soils (Pierzynski et al., 2005). Aluminum and iron phosphates, as well as organic forms of P, predominate with higher level of weathering. In grassland ecosystems, a rare decline is observed in the P concentrations where there are no external applications of P fertilizers, hence leading to negative P balance in some cases (McDowell et al., 2020). Organic P is found in two broad pools in mineral soils, one which is susceptible to microbial decomposition and thus biologically available (e.g., inositol phosphates, phospholipids, nucleic acids and their derivatives) and, the other which is stable and highly resistant to microbial activity (the humic acid fraction) (Stevenson, 1982). From 60% to 90% of total P can be present in organic forms in organic soils (>20–30% organic matter [OM] by weight) (Harrison, 1987). In most soils, inositol phosphates make up the majority of organic P, accounting for up to 80% of total organic P (Brookes et al., 1982; Brookes et al., 1984; Turner et al., 2001).
The inorganic and organic forms of soil P are in a constant state of equilibrium with dissolved P in the soil solution, which is mostly present as primary (PO43-) or secondary (HPO42−), (H2PO4−) orthophosphates, varying in concentrations as a function of time pH of the soil. The pH of most agricultural soils is between 4.0 and 9.0. At pH 4.0–5.5 H2PO4−, and HPO42− predominates at pH greater than 8.0. Ion pairs (e.g., CaHPO4, MgHPO4, or CaPO4) can also be found in the soils with pH > 7. However, chemical dissolution of these soluble complexes quickly transforms them to orthophosphate. Although little is known about the soluble organic P species found in soils or their contribution to soil P bioavailability, microbial hydrolysis of dissolved organic P to orthophosphate is thought to be one of the most common processes (Stevenson and Fitch, 1986). It is also dependent on the phosphatase activity in soils which can be controlled by various factors like SOM, pH, nitrogen availability too (Widdig et al., 2019). Soil water systems have a range of P concentrations ranging from 0.01 mg/L (infertile soils) to 7–8 mg/L in fertilized with 0.2 mg/L being the optimal concentration for plant growth (Pierzynski, 1991b; Correll, 1998).
Adsorption and precipitation are key processes involved in P retention in calcareous environments, reducing P availability following fertilizer application. The relative roles of carbonates and oxide clays in P retention in calcareous soils have yielded a variety of outcomes (Afif et al., 1993). Phosphorus accessible to plants is negatively connected to the amount of lime in soil at high application rates, but not to Fe, clay content, or cation exchange capacity (CEC) (Castro and Torrent, 1995). Non-carbonate clays supply most P adsorbing surfaces in many calcareous soils, according to the findings (Ryan et al., 1985; Zhou and Li, 2001), especially at low P concentrations.
Application of organic amendments have been seen to raise the level of bio-availability of P in calcareous soils (Khan et al., 2022). The presence of SOM diminishes the soil’s affinity to adsorb P. This could be owing to organic acids competing for P fixation sites, or the manure complexing exchangeable aluminum fluorides, both of which are potent complexing agents for Al and Fe. Humic and fulvic acids are macromolecular assemblages of diverse organic functional groups found in almost all agricultural soils. It was found that soil humic material increased Olsen P recovery in all soils except those with extremely high sodium (Na) levels (Delgado et al., 2002). According to another study, the presence of metal ions that act as cationic “anchors,” allowing anionic humates and phosphates to associate, which is required for robust interactions between P and humic materials. Humate-metal-P complexes have strong stability constants, with log K values in the range of 4.87–5.92 (Zn- and Mg-anchor, respectively) (Riggle and von Wandruszka, 2005).
Subsurface P retention is influenced by both introduced manure or litter and native organic matter (humic materials). Although humic materials, which are the breakdown products of the biota in the environment, are not a substantial source of P, they do mobilize it in the subsurface. In recent years, the application of extrinsic humates, particularly leonardite humic acid, for soil enhancement has increased. Despite the presence of substantial amounts of organic matter, recent research suggests that the presence of Al and Fe has a considerable impact on P sorption capability (Giesler et al., 2005). The P concentrations has previously been found to decrease organic C sorption to acid mineral soils, implying a ligand exchange mechanism at the surface (Kaiser and Zech, 1996; Delgado et al., 2002). In grasslands of North central America, increased chemical weathering has been observed from a steppe to mesic tallgrass prairies. The surface horizons (A horizon) had decreasing Ca-bound P and increasing amounts of occluded P with increase in precipitation levels. The Ca-bound P was observed to be associated with CaCO3, which showed the importance of carbonate presence controlling soil P levels in in these soils (Ippolito et al., 2010). On the other hand, in agricultural soils, P is often applied in excess through fertilizers, leading to high residual P levels. Particularly in clayey or calcareous soils, due to the immobile nature of P, once it is bound, a major part of the P supplied as fertilizers, remains unavailable for plant uptake (Sattari et al., 2012; Goossens et al., 2022).
In this study, we investigate the relationship between the different geochemical properties of the soils, such as the water and chemically extractable fractions for soil P, major ions and organic matter from soils, collected from Kansas. We hypothesize that, in addition to external fertilization in agriculturally-dominated soils, the P mobility would be influenced by presence of competing ions and soil organic matter in pristine prairie grassland soil.
Surface soils were collected from two regions in Kansas (United States). Konza Prairie Biological Station (KPBS; 39.1069, −96.6091) is located (Figure 1) in northeastern Kansas that is mainly characterized by beds of Quaternary sediments, unconsolidated sand, gravel, silt, and clay. North-eastern Kansas consists of Pleistocene glacial deposits with thick accumulations of marine shale, limestone, and chalk, and non-marine deposits of sandstone and shale. The surface geology of KPBS has alternating layers of chert (flint) found in the limestone (Macpherson et al., 2019). The KPBS has been maintained since 1971 and uses a variety of grazing treatments (bison, cattle, non-grazing) and fire regimes (fire return intervals of 1, 2, 4, 10, and 20 years). With an average annual precipitation and air temperature of 835 mm and 13°C, respectively, KPBS is categorized as a mesic grassland (Hayden, 1998; Nippert and Knapp, 2007). The bedrock stratigraphy of Konza is made of limestone and mudstone units (Macpherson, 1996; Smith, 1991). Illite, chlorite, and mixed layer clays of chlorite-illite and chlorite-vermiculite, make up the majority of the minerals in mudstones. The main non-clay mineral found is quartz, with trace to large amounts of calcite. Dolomite and calcite are both present in small amounts in limestone units (Macpherson et al., 2008). The texture of soil found in KPBS mainly consisted of Loam and Silt loam (Web Soil Survey, 2024; Bidwell and McBee, 1973).
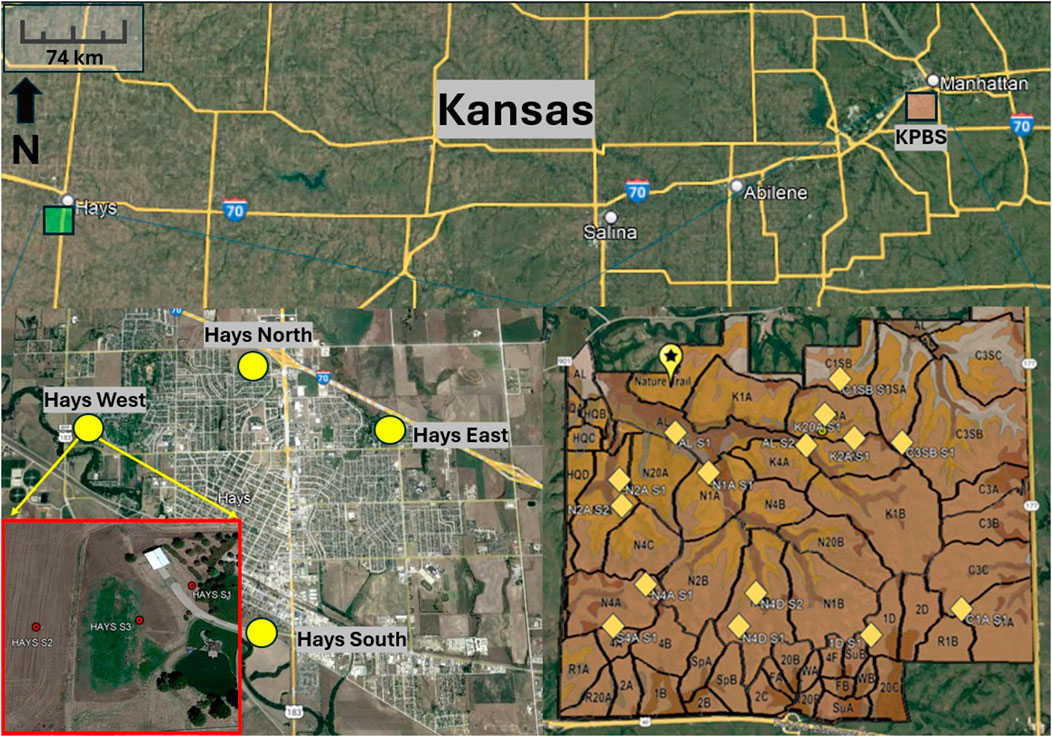
Figure 1. Sampling locations at KPBS Biological Station (KPBS; n = 15) and Hays (n = 12). The samples from KPBS were collected from watersheds that are demarcated by borders in the KPBS area. The samples from Hays were collected each from three distinct land use patterns (agricultural land, pastureland and rangeland).
Soil samples were also collected in Hays (Kansas, United States). The geological formations include the Carlile shale and the Niobrara formation, both of which are from the Cretaceous age. Additionally, there are terrace deposits from the Pleistocene period and recent alluvium. Hays has a sub-humid to semi-arid environment with an average yearly temperature of 11.6°C and average annual precipitation of 596.9 mm. The area of Hays is characterized by mixed grassland type vegetation. In Hays, agricultural practices primarily focus on the cultivation of wheat varieties developed at the Agricultural Research Center-Hays, Kansas State University, and released by the Kansas Agricultural Experiment Station. Specifically, hard white winter wheat cultivars like “Joe” (Zhang et al., 2016), “KS Big Bow” (Zhang et al., 2024), and “Oakley CL” Zhang et al. (2015) have been registered and promoted for cultivation in the region. These wheat varieties are crucial components of the agricultural landscape in Hays, reflecting the emphasis on wheat production in the area. The soil texture found in Hays broadly consists of silty clay loam and clayey loam soils (Web Soil Survey, 2024; Bidwell and McBee, 1973).
The parameters as in soil texture, were broadly derived from the information in the Web Soil Survey website and the “Soils of Kansas” Map by KAES Department of Bidwell and McBee (1973), from which we could identify that the soil texture in the two locations KPBS and Hays were of distinct types viz- clay loam (silty clay loam and clayey loam) and loam (loam and silt loam) type respectively.
A total of 27 soil samples were collected from KPBS (n = 15) and Hays (n = 12) by clearing the surface debris and collecting soil cores at depths up to 15 cm (Figure 1) during August 2022. Surface soil samples were collected as grab samples by using a handheld auger and the loose soil was collected in airtight zip-lock bags. Approximately 700 g to 1 kg of soil was collected from each of the sampling locations. KPBS soils were collected from the following watersheds: AL, 1D, N1A, N2A, N4A, N4D, K1B, K2A, K20A, C1A, C1SB, C3SB, S4B. Hays samples were collected from 4 directional corners of town (North, South, East and West) with 3 individual samples collected at each corner, from three distinct land uses- agricultural, pastureland and rangeland. All soil samples were stored in airtight zip-lock bags, followed by storage in 4°C until laboratory analysis.
Soil pH was measured in 1:1 w/v slurry by mixing 4 g of wet soil in 4 mL of deionized water following Blair (2023), using a HACH PocketPro+ pH meter. Soil samples were dried for 24 h at 60°C, homogenized using a mortar and pestle and sieved using a 0.75 mm mesh. The water leaching experiment was performed by reacting 4 g of crushed soil with 40 mL (1:10 w/v) of ultrapure water (18.3 MΩ cm, pH = 7.11, specific conductance = 1.04 μS/cm) for 24 h. Specific conductance was measured to estimate the amounts of dissolved solids in the supernatant after the extraction experiment was completed. The supernatant filtered using 0.45 µm nylon syringe filter was used to measure cations (Na+, NH4+, K+, Mg2+ and Ca2+) and anions (F−, Cl−, NO2−, NO3−, SO42− and PO43−) using a Dionex Integrion/Aquion High Pressure Ion Chromatography system. Anions were eluted with potassium hydroxide using Dionex IonPac AS18 (4 × 50 mm) separator column, and cations were eluted isocratic with 20 mM methanosulfonic acid using Dionex IonPac C separator column. Ions were measured at The University of Texas at San Antonio (UTSA), department of Earth and Planetary Sciences, Institute for Water Research, Sustainability and Policy (UTSA-IWRSP).
To estimate the amount of assimilable soil P, Bray Phosphorus method (Kovar and Pierzynski, 2009) where soil was extracted using extractant in 1:10 w/v ratio. The extractant solution consists of ammonium fluoride (NH4F) and hydrochloric acid (HCl), and the concentration of extracted P is measured by colorimetry using HACH TNT845 total and reactive phosphorus kits at 610 nm. The Bray-P measurements enable to quantify bioavailable P associated with organic and inorganic fractions in the soil samples.
To quantify the soil organic matter (SOM), the soil samples were first dried at 105°C for 24 h and the differences in weights were calculated for moisture content. Further, the dried soil samples were combusted at 550°C for 4 h and the difference in weights was measured. Loss on ignition (LoI) was calculated as the normalized difference between dry soil mass and combusted soil mass. The WE-SOC concentrations in the soil-water extracts, measured as non-purgable organic carbon (NPOC) concentrations were measured by thermic oxidation using Shimadzu Total Organic Carbon (TOC)/Total Nitrogen (TN) Analyzer. Characterization of Dissolved Organic Matter (DOM) was done by measuring UV–Vis’s absorbance (240 nm–450 nm) and fluorescence (300 nm–600 nm) using Horiba Aqualog Benchtop fluorometer at UTSA-IWRSP. The detailed methods for spectroscopic characterizing DOM are described elsewhere (Kulkarni et al., 2017). Briefly, Absorbance was measured for excitation wavelengths of 240 nm–450 nm with an increment of 3 nm. The fluorescence was simultaneously measured for emission wavelengths of 300 nm–600 nm (instrument default increment of 3.28 nm). Inner filter effect corrections and Rayleigh scatter masking was applied to the raw fluorescence data, and corrected data was normalized to the Raman area of 18.3 MΩ cm Milli-Q ultrapure water (Bahram et al., 2006). Data correction and processing was done using dr-EEM 0.6.6 (Murphy et al., 2013). Absorbance at 254 nm (Abs254) was used to calculate specific ultraviolet absorbance (SUVA), a measure of aromaticity of the dissolved organic matter (Weishaar et al., 2003). Further, spectral slopes of log-transformed absorbance coefficients between 275 nm and 295 nm (S275-295) and between 350 nm and 400 nm (S350-400), and their ratio (Spectral Slope Ratio, SR) were calculated as a measure of molecular weight distribution of DOM (Vähätalo and Wetzel, 2004; Moran et al., 2000; Helms et al., 2008). From the fluorescence data, Fluorescence Index (FI) was calculated as a measure of terrestrially- vs. microbially-derived DOM (McKnight et al., 2001; Cory and McKnight, 2005). The freshness index (FrI), a measure of recent (β) vs. older DOM (α), was calculated as described in prior studies (Parlanti et al., 2000; Wilson and Xenopoulos, 2008; Fellman et al., 2010a). To estimate the extent of humification, humification index (HIX) is calculated (Ohno, 2002; Zsolnay, 2003). Intensities of humic-like peaks (Peaks A, M and C) and protein-like peaks (Peaks B and T) were also calculated from the data as previously described (Coble et al., 1990; Coble, 1996).
A Wilcoxon rank sum test was performed for all response variables soil moisture%, LOI%, pH, EC, WE-SOC, Bray-P, phosphate, fluoride, chloride, nitrate, nitrite, sulphate, sodium, ammonium, magnesium, calcium, peak A, peak B, peak C, peak M, SR, FI, FrI, HIX, BIX, SUVA254 as most variables were not normally distributed. This test was used to determine if variables differed between the KPBS and Hays sites. Next, Spearman’s correlation coefficients were determined for all variables within one site (KPBS versus Hays) to inform specific linear regression analyses to perform. Based on the correlations, linear regression models were performed Bray-P concentrations (response variable) with phosphate, specific conductance, and the DOM spectral signatures Peak A, C, M, and Abs254. Because of significant correlations found, linear regressions were also run with calcium, specific conductance, K+ and TOC. Separate linear regressions were run for site therefore KPBS and Hays have their own linear regression results for these variables. Wilcoxon rank sum tests were performed in the base R package and Spearman correlations with associated p-values were calculated using the function rcorr in the Hmisc package in R (version 4.3.3). The threshold significance level of P < 0.05 was used.
Principal Components Analysis (PCA) is a useful statistical representation that reduces the dimensionality of large datasets and transforms the sets of variables into the principal components (PC) which provides an inter-relationship between multiple variables (Jackson, 1991). Here, the PCA was performed (Figure 6) on the geochemical and organic constituent dataset of Hays and KPBS soil samples including the inorganic and organic parameters (n = 22 variables), using OriginPro 2024b software. The PCA was constructed from a correlation matrix (Supplementary Table S3) with z-score standardization to give a value between −1 and +1 (mean = 0 and SD = 1) for each variable. Two PCs were selected based on the highest eigenvalues. In PCA, ‘loadings’ provide the participation of variables in the PCs, and ‘scores’ are defined as the individual transformed observations. Supporting Data (Supplementary Tables S3, S4) provides the correlation matrix table of the variables included in the PCA.
Soil pH differed between KPBS and Hays (W = 151, p = 0.003) with KPBS having lower but slightly alkaline pH (7.09 ± 0.58) and Hays at 7.98 ± 0.57 (Figure 2A). Specific conductance differed between KPBS (80 ± 33 μS/cm) and Hays (106 ± 34 μS/cm; Figure 2B) with greater conductance in Hays (W = 159, p < 0.001). The moisture content of KPBS soils were on average 15% ± 4% whereas Hays was on average 6% ± 4% (Figure 2C) and were statistically different (W = 12, p < 0.001). Soil OM expressed as LoI% also differed between KPBS and Hays (W = 9, p < 0.001): KPBS had an average of 10% ± 2% whereas in Hays it was found to be 5% ± 2% (Figure 2C). Water extractable soil organic carbon (WE-SOC) also differed between KPBS and Hays (W = 19, p < 0.001): KPBS had greater WE-SOC with an average of 670 ± 259 mg/kg and Hays with average 300 ± 266 mg/kg (Figure 2D). Both water-extractable PO43- ion concentrations and Bray-P concentrations in KPBS soils were found to be (median PO₄³⁻: bdl mg/kg, 10%–90% range: bdl to 0.1 mg/kg; median Bray-P: 15.3 mg/kg, 10%–90% range: 3.9–17.1 mg/kg) and in Hays (median PO₄³⁻: bdl mg/kg, 10%–90% range: bdl to 3.1 mg/kg; median Bray-P: 17.1 mg/kg, 10%–90% range: 8.5–48.5 mg/kg) (Figure 3). Neither did the numbers seem to vary between the sites statistically (W > 107, p > 0.24). Water chemistry was dominated by the presence of calcium, magnesium and potassium cations and SO42−, Cl−, NO3− and NO2− anions. The majority of ions (Ca2+, K+, NH4+, Na+, SO42−, NO2−, NO3−, Cl−, F−) did not differ between sites (p > 0.10). However, Mg2+ weakly differed between KPBS (10 ± 9 mg/kg) and Hays (19.4 ± 10.5; W = 53, p = 0.07) (Figure 4; Supplementary Table S1). Results for Soil pH, Moisture, LoI, SC, WE-SOC, Bray-P, PO43-- are all mentioned in Table 1.
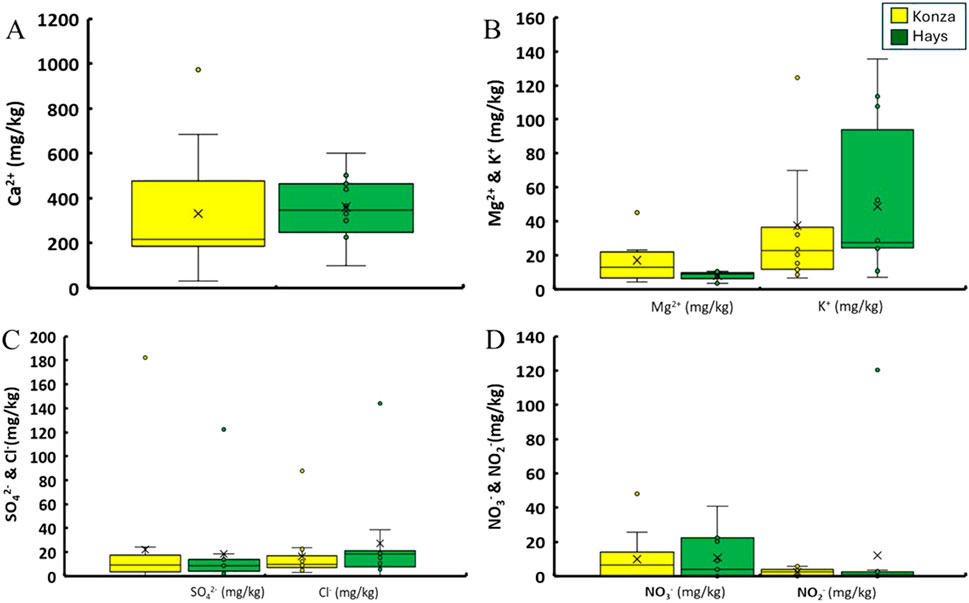
Figure 2. Comparison between the physicochemical parameters observed for soil samples collected from KPBS and Hays. (A) compares the pH of the two sites. (B) compares the specific conductance of the two sites. (C) compares the moisture content and LOI %. (D) the Water extractable soil organic carbon (WE-SOC) concentrations from the two sites.
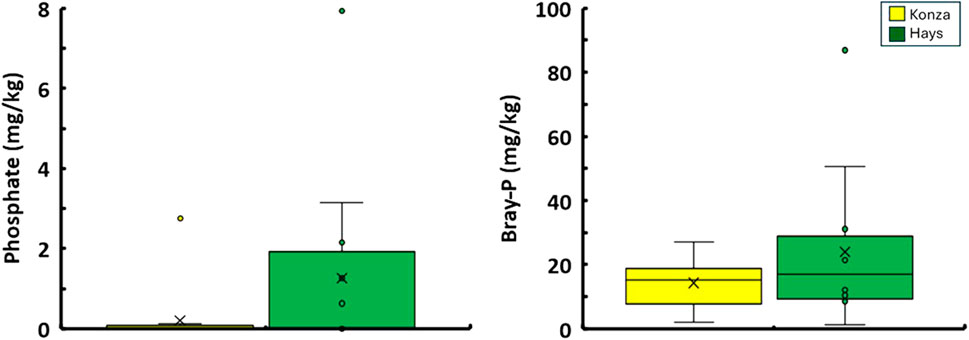
Figure 3. Comparison between the observed forms of P for soil samples collected from KPBS and Hays. (A) compares the concentration of water leachable phosphate ions of the two sites. (B) compares the concentrations of labile forms of soil P (denoted as Bray-P).
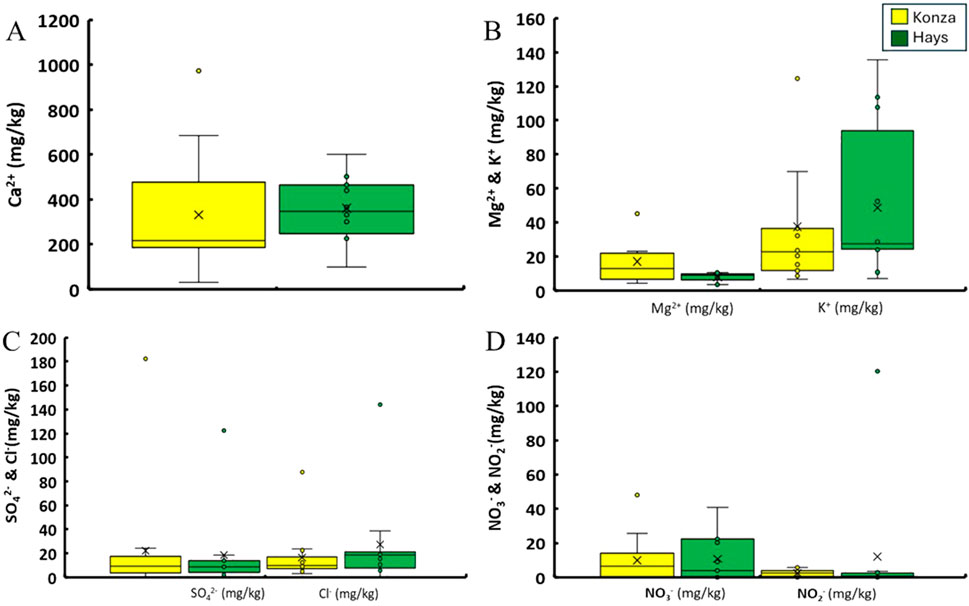
Figure 4. Comparison between the concentration of water leachable ions for soil samples collected from KPBS and Hays. (A) compares the concentration of water leachable calcium ions of the two sites. (B) compares the concentrations of magnesium and potassium ions. (C) shows the concentrations of water leachable sulfate and chloride ions whereas (D) shows the concentrations of nitrate and nitrite ions.
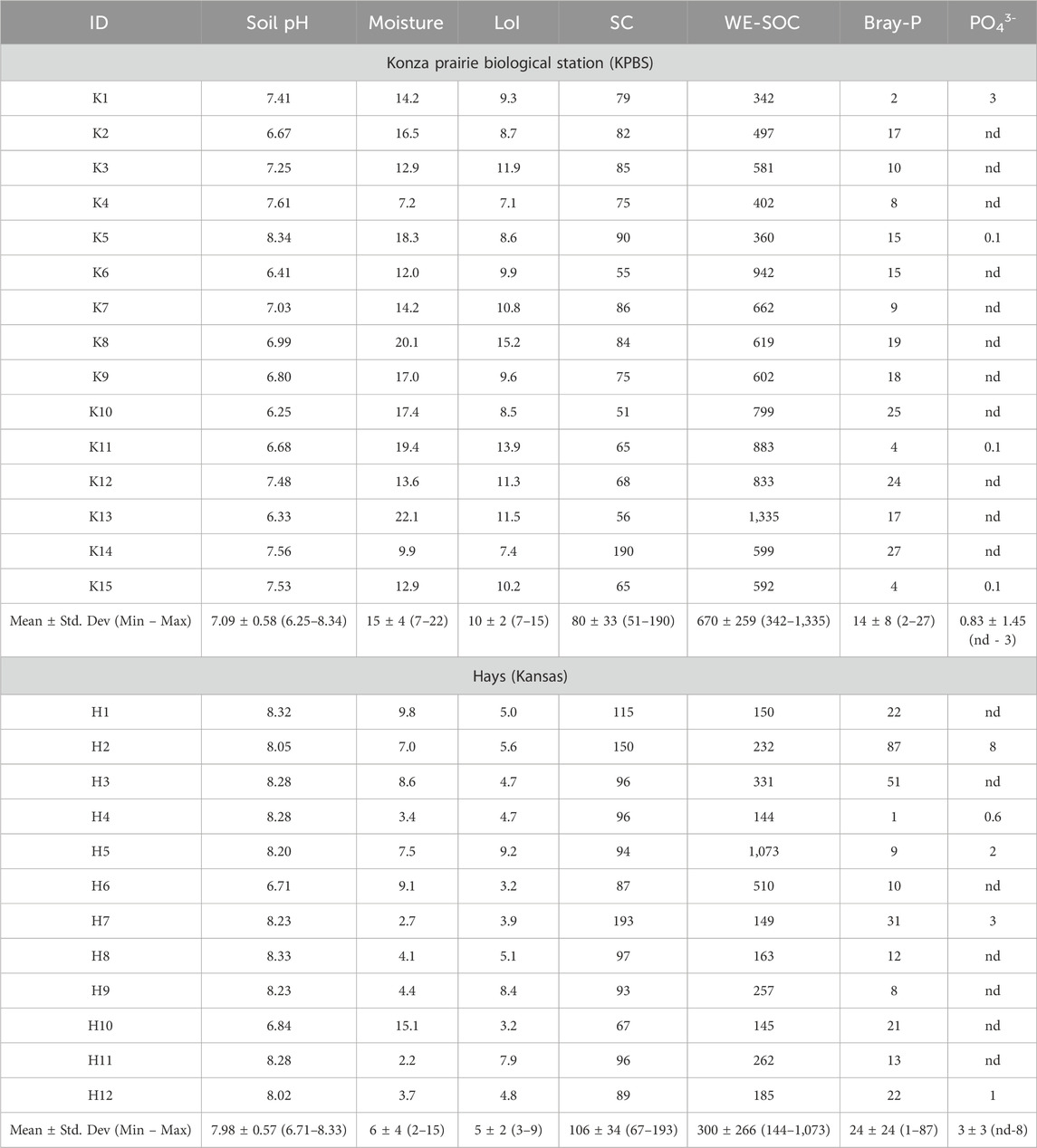
Table 1. Physical and chemical properties of the soil samples collected from KPBS (n = 15) and Hays (n = 12) at 15 cm below the surface. Moisture and loss on ignition (LoI) is reported as %, specific conductance (SC) as µS/cm, and water extractable soil organic carbon (WE-SOC) measured as dissolved organic carbon, Bray-P are expressed as mg/kg.
Soil organic carbon concentration differed between sites (W = 19; p < 0.001) with KPBS having an average 652 ± 275 mg/kg and Hays, on average, 289 ± 27 mg/kg. The specific ultraviolet absorbance (SUVA254) (Supplementary Table S2) also differed between KPBS (1.3 ± 0.9 L/mg.m) and Hays (2.8 ± 1.8 L/mg.m) with greater absorbance in this spectrum seen for Hays versus KPBS (W = 1,321, p = 0.05). At both locations, the soil samples primarily contained humic-like organic matter as characterized by up to 92% ± 3% (KPBS) and 95% ± 2% (Hays) humic-like fluorescence, represented as humification index (HIX) respectively. Fluorescence index (FI) ranged between 1.3 and 1.5 among all samples from both locations indicating terrestrially-derived soil organic matter. Freshness index varied between 0.4 and 0.7 in all samples from both sites indicating a mixture of recently-produced as well as older soil organic matter. The Peak A intensity for the KPBS soils was 7.4 ± 4.5 units and for Hays it was 7.0 ± 3.2 units. The Peak C intensity of KPBS soils was 4.3 ± 2.4 units and for Hays soils, the Peak C intensity was found to be 3.8 ± 1.8 units. The higher intensity of peaks A and C in these soils indicates that the SOM from both these regions have a high molecular weight humic nature that are found generally from terrestrial sources however, it shows that the peak A intensity of KPBS samples was higher than the samples from Hays. The Peak M intensities for soils from KPBS were 4.4 ± 2.6 units, as compared to Hays soils which had Peak M intensity of 3.6 ± 1.6 units. This indicates that The Peak T intensities were very contrasting in the sites, it was found to be 1.4 ± 1.2 units for the samples from KPBS and for Hays it was 0.8 ± 0.4 units, this indicated that the soils form KPBS had more influence of protein tryptophan like OM, indicating higher biological activity in the soils from KPBS as compared to the samples from Hays (Figure 5).

Figure 5. Characteristics of water leachable organic matter of the two sites have been shown in this figure. The excitation emission matrix (EEM plots) obtained from absorbance fluorescence spectroscopy, which depicts the spectroscopic properties of SOM found in KPBS soils and Hays.
The PCA analysis (Figure 6) revealed that in PC1 (23.28%) moisture, Na+, Mg2+, SO42-, TOC, TN, and SOM or LOI had high positive factors loading, and Abs254 had comparatively lower positive factors loading. Soil Ca2+, specific conductance, pH had high negative factors loading and NO3−, NH4+ had comparatively lower negative factors loading. In PC2 (19.45%), K+, Cl−, HIX (Humification Index) had high positive factor loadings, and NO2−, PO43-, Bray-P had comparatively lower positive factors loading, in contrast, FI (Fluorescence Index), BIX (Biological Index), FrI (Freshness Index) had high negative factor loadings. The Hays soil samples’ geochemical data and DOM characteristics were grouped along the negative PC1 axis, dominated by Ca2+, Bray-P, PO43-, HIX, FI, pH, specific conductance, NO3−, and NH4+. In contrast, the majority of the KPBS soil samples dataset was grouped along the positive and to some extent negative PC1 axes with comparative enrichment in LOI, TOC, TN, Ca2+, Abs254 and moisture (Figure 6).
Our results showed that pristine grassland soil samples from KPBS contained lower Bray-P concentrations (∼14 mg/kg) compared to that in agriculturally-dominated sites from Hays (∼24 mg/kg). These results are consistent with the expected effect of external soil fertilization being done at the agriculturally-dominated sites (Robertson and Nash, 2008; Gypser et al., 2021). Blair (2023) provides a compilation of soil chemical properties at KPBS for the past 20 years including Bray-P data. It appears that historical data suggests that the soils in KPBS contain 5 ± 2 mg/kg of Bray-P, which is slightly lower than found in this study which could be attributed to different times and locations of sampling. In both sites, the water extractable PO43- was found to be negligible, suggesting that although a significant concentration of Bray-P was present in the soils, this P was especially soil-bound and did not solubilize with water easily.
Calcium (Ca2+) was the most prominent water extractable ion in both KPBS and Hays soils during the soil-water leaching experiment, which is consistent with the calcareous nature of the soils in Kansas (Padbhushan and Kumar, 2015). Furthermore, the weathering profiles of the soils from both the sites shows a carbonate dissolution signature (Supplementary Figure S1) which shows that these soils are enriched with carbonate minerals and hence they are dominated by Ca2+. A moderate positive correlation (r2 = 0.64) between water extractable specific conductance and Ca2+ concentration in KPBS samples indicates that carbonate dissolution to be the dominant process during the soil-water interactions (Figure 7). However, such correlation was non-existent in Hays samples suggesting that other processes could be involved that our data does not identify. For instance, in highly buffered soils, there are diverging relationships between P sorption, supply potential, and availability, indicating stronger sorption properties in non-calcareous soils compared to calcareous soils (Daly et al., 2015). The presence of different soil parent materials and chemical properties can also influence the strength of the correlation between specific conductance and Ca2+ ions. In soils with contrasting properties, the organic carbon content and chemical composition can significantly impact the relationship between specific conductance and Ca2+ (Yang et al., 2021).
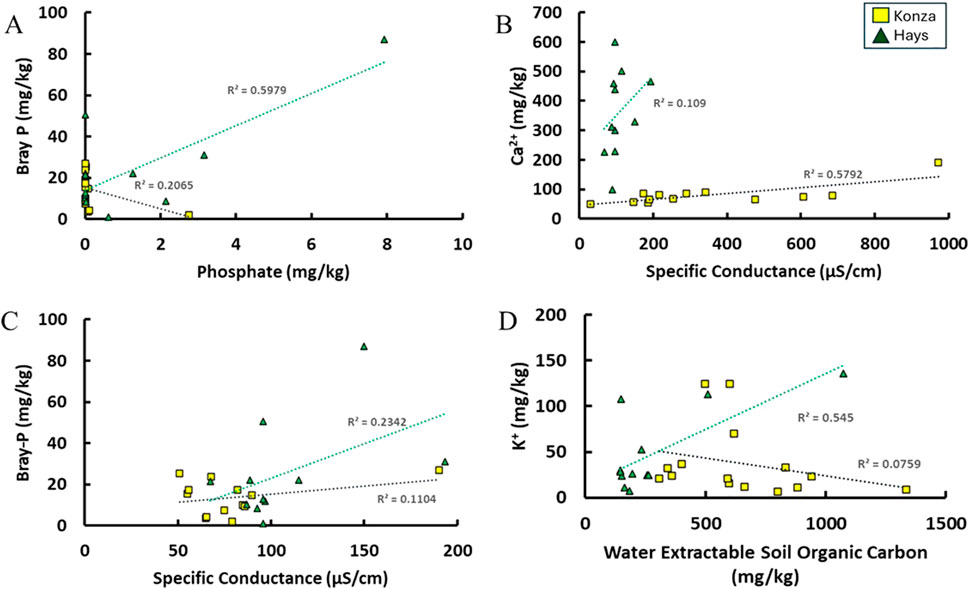
Figure 7. Correlations found in the different parameters which may be controlling P availability in soils from KPBS and Hays. Correlation between Bray-P and water leachable phosphate ions (A), Bray-P and specific conductance (EC) (B), Ca2+ ions and Specific Conductance (C), K+ ions and WE-SOC (D).
Potassium (K+) is one of the macro-nutrients along with P in soil. While our data does not show any correlations between Bray-P and K+ even in fertilized soils from Hays, a moderate positive correlation between K+ and WE-SOC (r2 = 0.52) was observed (Figure 7). Literature (Liao et al., 2020) suggests that calcareous alkaline soils are likely to have lower concentrations of K+ naturally which affects plant growth and nutrient uptake. Prior studies (Robbins and Mayland, 1993; Lund and Doss, 1980; Olatuyi et al., 2009) have suggested that in calcareous soils presence of K+ may enhance P bioavailability through cations exchange as well as through complexation reactions between K+ and WE-SOC, which may explain observed correlations in our dataset.
Soil pH often regulates geochemical processes including phosphorus availability. In our study, soil samples from KPBS showed near-neutral (∼7.09) pH while the Hays soils showed alkaline (∼7.98) pH. High pH in calcareous soils is a common observation that enhances the P mobility through ion exchange (Ige et al., 2005). The higher bray-P concentrations in alkaline Hays soils higher than the near-neutral KPBS soils, could be explained by the following phenomena. Phosphorus dynamics in soil is majorly controlled by the pH while partially by other soil properties. It is thought that P availability will be highest in soils with near neutral pH range of 6.5–7. P binds to Al and Fe in acidic soils while it binds complexes with Ca in alkaline soils. However, in calcareous soils, the P fixation dynamics can become complicated because of high Ca content and carbonate mineral dissolution. In calcareous soils, the equilibrium may favor adsorption-desorption processes (ion exchange: CEC and AEC), thereby overshadowing precipitation and dissolution processes. In calcareous soils, an increase in pH results in a decrease in anion exchange capacity, indicating that phosphate may desorb into the soil solution, thereby enhancing its bioavailability, as represented by the relationship (pH↑, AEC↓, P availability↑). In carbonate soils, the cation exchange capacity increases, and Ca is easily adsorbed by soil particles and does not form complexes with phosphates as described as (pH↑, СEC↑, Ca availability↓, P availability↑). The goal of this study was to understand geochemical interactions between soil P, pH, competing ions and organic matter. Therefore, higher P concentration in Hays soils is not the main result, but rather it’s the interrelationships between geochemical parameters and P, in two soil categories.
Although pH is considered one of the main factors controlling P availability, there are several other factors that come into interplay to demonstrate a combined manner of controlling the P solubility in soils. The equilibrium between the different fractions of soil P, that is the non-labile, Labile, the soluble P fractions also play a key role, which is not always directly controlled simply by pH. Also surface adsorption of P on Al and Fe oxides and the simultaneous precipitation of Al and Fe minerals with P works across a range of pHs. In CaCO3 rich soils, rising pH have also resulted in immobilization of P in forming Ca-P minerals, but when Ca-P minerals reach a state of equilibrium, rising pH does not have any further effect (Penn and Camberato, 2019; Barrow, 2017; von Tucher et al., 2018; Curtin and Syers, 2001). Further, the KPBS soils are also characterized by higher LoI and WE-SOC concentrations than that in Hays soils. Prior studies have reported that presence of SOC containing low molecular weight organic acids may lower the pH in originally alkaline soils limiting the phosphate mobility (Rose et al., 2009; Brindhavani et al., 2022).
The water extractable SOM from both sites KPBS and Hays showed a strong humic-like absorbance fluorescence characteristic (Figure 5). In comparison, the Hays samples showed slightly higher SUVA254 values, a measure of aromaticity of SOM than that in KPBS samples. Further, a moderate positive correlation (r2 = 0.6) was found between Bray-P and absorbance at 254 nm, indicating the association between humic-like SOM and P in soils. Studies have shown that humic substances can enhance the availability and extractability of P in various types of soils, which includes P-fixing soils and acidic soils, where organic inputs have been reported to increase P availability, thereby influencing nutrient uptake by plants (Mehrizi Hejazi et al., 2015; Patidar et al., 2019). Research has indicated that humic substances can compete with P for adsorption sites in soil, leading to higher available P levels in humic OM amended soils (Tunesi et al., 1999). Another study found that the generation of organic acids or humic substances in biologically active compost amendments has been associated with an increase in water-extractable P, highlighting the role of humic substances in influencing P dynamics in soil (Fuentes et al., 2006; Yang et al., 2019). A few studies (Fellman et al., 2010b; Alori et al., 2017) have also reported some of the microbial processes that mobilize P in presence of SOM, which are plausible in our study sites, however additional investigation will be required to confirm those. In our data (Figure 8), fluorescence peaks C and M indicate presence of fulvic-like (or fulvic acid like) organic matter which is an acid-soluble fraction of humic substances. A few studies have indicated that fulvic acids also regulate the nutrients mobility and plant uptake by altering the pH and processes like podsolization (Al-Eezzi and Al-Alawy, 2022; Soares et al., 2023; Cabreira et al., 2024; Heil, 2005). In soils with amorphous Fe and Al oxyhydroxides, humic substances may compete for sorption sites and enhance P mobility (Hawkins et al., 2022), which is also a plausible process in our samples. Therefore, the findings from these prior studies are consistent with our observation in this study where Bray-P concentrations appear to be related to humic-like water extractable SOM.
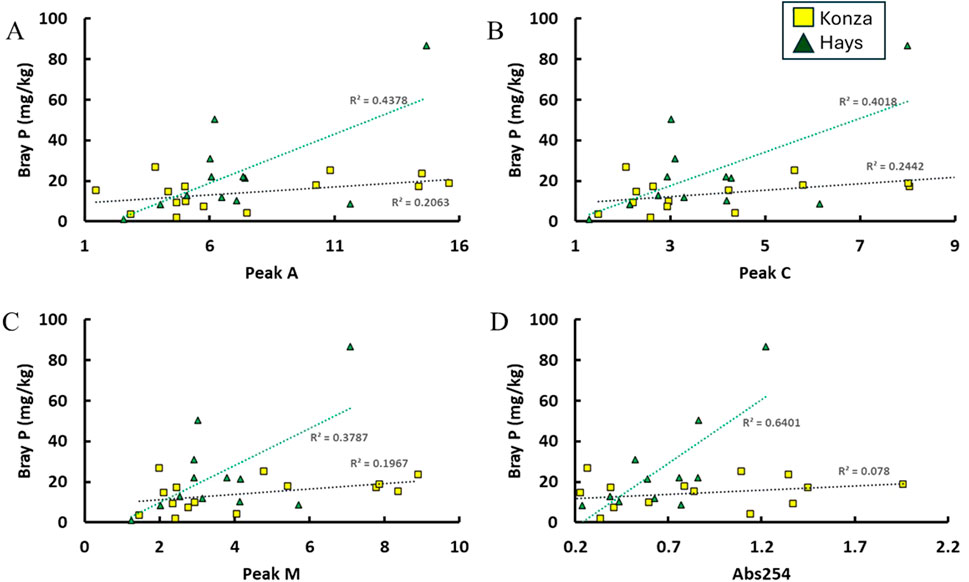
Figure 8. Significant correlations between the spectral signatures of the SOM in both locations and Bray-P values. (A) demonstrates the linear correlations between the Peak A and Bray-P values while the (B) demonstrates correlation between the Peak M and the Bray-P values, (C) shows correlation between Peak M and Bray-P, and (D) shows correlation between the absorbance at 254 nm and the Bray-P values.
In our study, we should also state that the occurrence of SOM (including the quality and quantity) in KPBS creates a better condition for P bio-availability as compared to the conditions in Hays. On the contrary, we find higher levels of bio-available P in Hays, which may indicate the influence of agricultural land use in Hays, via application of higher P-fertilizers. This is in congruence with the recent studies (Meyer et al., 2023; 2021; Degryse and McLaughlin, 2014) which also emphasize land use being one of the contributing factors for determining P availability in soils.
This study provides important insights on P mobility in soils through a comparison between a pristine prairie grassland and agriculturally-influenced regularly fertilized land. The grassland soils were characterized by near-neutral pH, higher moisture content, higher amounts of soil organic matter (SOM), higher water extractable soil organic carbon (WE-SOC), and lower Bray-P concentrations, compared to samples from agriculturally-influenced site. Alkaline soils from the agriculturally influenced site of Hays demonstrates higher influence of ionic activity that may have increased the mobility of P compared to the near-neutral soils of KPBS. So, in this pristine Prairie grassland calcareous soils, the dynamic interaction amongst P retention mechanisms is more likely to be further complicated by the high Ca content due to dissolution of carbonate minerals. Water extractable PO43- concentrations in soils from both sites were negligible highlighting the strong affinity towards solid phase. Among water soluble ions, calcium (Ca2+) was the most dominant cation resulting from the dissolution of carbonate minerals which is common in calcareous soils such as in both sites in this study. Finally, associations between Bray-P and spectral parameters of humic-like water soluble SOM point towards role of humic substances in controlling P mobility through processes like competitive sorption and aqueous complexation. Overall, the findings of this study enhance our understanding of P mobility in natural prairie grassland and in externally fertilized agricultural soils and have broader implications in agricultural and environmental management.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
PB: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. HK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing–original draft, Writing–review and editing. AV: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Writing–review and editing. TN: Investigation, Writing–review and editing. PP: Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing–review and editing. SRD: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing–review and editing. SD: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing–review and editing, Validation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. SD and SRD acknowledge the financial support from the U.S. National Science Foundation (NSF) through the Signals in the Soil (SitS) program, grant number 1935676. SD and SRD also acknowledge A. Chauvet, N. Martsinovich, N. N. Mansouriboroujeni, D. Cameron, J. Lake, J. Nickles - all from University of Sheffield, for useful discussion and UKRI for providing the funding support. SD and SRD also acknowledge A. Mondal and B. Ray at the University of Alabama at Huntsville for their discussions.
The authors acknowledge R. Krishnamoorthy for help in the soil sample collection from Hays.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1500314/full#supplementary-material
Afif, E., Matar, A., and Torrent, J. (1993). Availability of phosphate applied to calcareous soils of west asia and North africa. Soil Sci. Soc. Am. J. 57, 756–760. doi:10.2136/sssaj1993.03615995005700030022x
Al-Eezzi, Y. H., and Al-Alawy, H. H. (2022). Effect of fulvic acid and seaweed on the nutrient uptake and some soil chemical properties. J. Pharm. Negat. Results, 588–593.
Alori, E. T., Glick, B. R., and Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 8, 971. doi:10.3389/fmicb.2017.00971
Bahram, M., Bro, R., Stedmon, C., and Afkhami, A. (2006). Handling of Rayleigh and Raman scatter for PARAFAC modeling of fluorescence data using interpolation. J. Chemom. A J. Chemom. Soc. 20 (3-4), 99–105. doi:10.1002/cem.978
Barrow, N. J. (2017). The effects of pH on phosphate uptake from the soil. Plant soil 410, 401–410. doi:10.1007/s11104-016-3008-9
Bidwell, O. W., and McBee, C. W. (1973). “Kansas agricultural experiment station, USDA,” in Soil conservation service, salina, Kansas, mary clawson “soils of Kansas,” KAES Department of agronomy contribution No. 1359. USDA. Available at: https://esdac.jrc.ec.europa.eu/images/Eudasm/US/us20b.jpg.
Blair, J. M. (2023). NSC01 Chemistry and physical characteristics of soils from Konza LTER watersheds with different fire and grazing treatments. Environmental Data Initiative. doi:10.6073/pasta/73fb69230e21d5a2647a1bd86ebea595
Brindhavani, P. M., Chitdeshwari, T., Selvi, D., Sivakumar, U., and Jeyakumar, P. (2022). Phosphorus releasing potentials of amino acids and low molecular weight organic acids from highly calcareous soils. Int. J. Plant and Soil Sci. 34 (15), 67–78. doi:10.9734/ijpss/2022/v34i1531009
Brookes, P. C., Powlson, D. S., and Jenkinson, D. S. (1982). Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 14 (4), 319–329. doi:10.1016/0038-0717(82)90001-3
Brookes, P. C., Powlson, D. S., and Jenkinson, D. S. (1984). Phosphorus in the soil microbial biomass. Soil Biol. Biochem. 16 (2), 169–175. doi:10.1016/0038-0717(84)90108-1
Cabreira, W. V., de Carvalho Balieiro, F., Pereira, M. G., and dos Santos, R. N. (2024). Corymbia citriodora girdling aiming forest restoration intensifies the production of labile C, N and P in the soil. Commun. Soil Sci. Plant Analysis 55, 2408–2421. doi:10.1080/00103624.2024.2359581
Castro, B., and Torrent, J. (1995). Phosphate availability in calcareous vertisols and inceptisols in relation to fertilizer type and soil properties. Fertil. Res. 40, 109–119. doi:10.1007/bf00750095
Coble, P. G. (1996). Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 51 (4), 325–346. doi:10.1016/0304-4203(95)00062-3
Coble, P. G., Green, S. A., Blough, N. V., and Gagosian, R. B. (1990). Characterization of dissolved organic matter in the Black Sea by fluorescence spectroscopy. Nature 348 (6300), 432–435. doi:10.1038/348432a0
Correll, D. L. (1998). The role of phosphorus in the eutrophication of receiving waters: a review. J. Environ. Qual. 27 (2), 261–266. doi:10.2134/jeq1998.00472425002700020004x
Cory, R. M., and McKnight, D. M. (2005). Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. and Technol. 39 (21), 8142–8149. doi:10.1021/es0506962
Cross, A. F., and Schlesinger, W. H. (1995). A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64 (3-4), 197–214. doi:10.1016/0016-7061(94)00023-4
Curtin, D. I., and Syers, J. K. (2001). Lime-induced changes in indices of soil phosphate availability. Soil Sci. Soc. Am. J. 65 (1), 147–152. doi:10.2136/sssaj2001.651147x
Daly, K., Styles, D., Lalor, S., and Wall, D. (2015). Phosphorus sorption, supply potential and availability in soils with contrasting parent material and soil chemical properties. Eur. J. Soil Sci. 66 (4), 792–801. doi:10.1111/ejss.12260
Degryse, F., and McLaughlin, M. J. (2014). Phosphorus diffusion from fertilizer: visualization, chemical measurements, and modeling. Soil Sci. Soc. Am. J. 78 (3), 832–842. doi:10.2136/sssaj2013.07.0293
Delgado, A., Madrid, A., Kassem, S., Andreu, L., and del Carmen del Campillo, M. (2002). Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 245, 277–286. doi:10.1023/a:1020445710584
Fellman, J. B., Hood, E., and Spencer, R. G. (2010b). Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: a review. Limnol. Oceanogr. 55 (6), 2452–2462. doi:10.4319/lo.2010.55.6.2452
Fellman, J. B., Spencer, R. G., Hernes, P. J., Edwards, R. T., D'Amore, D. V., and Hood, E. (2010a). The impact of glacier runoff on the biodegradability and biochemical composition of terrigenous dissolved organic matter in near-shore marine ecosystems. Mar. Chem. 121 (1-4), 112–122. doi:10.1016/j.marchem.2010.03.009
Filippelli, G. M. (2002). The global phosphorus cycle. Rev. mineralogy Geochem. 48 (1), 391–425. doi:10.2138/rmg.2002.48.10
Filippelli, G. M. (2008). The global phosphorus cycle: past, present, and future. Elements 4 (2), 89–95. doi:10.2113/gselements.4.2.89
Föllmi, K. B. (1996). The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Science Rev. 40 (1-2), 55–124. doi:10.1016/0012-8252(95)00049-6
Frossard, E., Condron, L. M., Oberson, A., Sinaj, S., and Fardeau, J. C. (2000). Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 29 (1), 15–23. doi:10.2134/jeq2000.00472425002900010003x
Fuentes, B., Bolan, N., Naidu, R., and Mora, M. D. L. L. (2006). Phosphorus in organic waste-soil systems. J. Soil Sci. Plant Nutr. 6 (2), 64–83. doi:10.4067/s0718-27912006000200006
Giesler, R., Andersson, T., Lo, ̈vgren L., and Persson, P. (2005). Phosphate sorption in aluminum- and iron-rich humus soils. SoilSci Soc. Am. J. 69, 77–86. doi:10.2136/sssaj2005.0077a
Goossens, E. M. C., Schrijver, A. D., Schelfhout, S., Vanhellemont, M., Verheyen, K., and Mertens, J. (2022). Phosphorus puts a mortgage on restoration of species-rich grasslands on former agricultural land. Restor. Ecol. 30 (4). doi:10.1111/rec.13523
Gypser, S., Schütze, E., and Freese, D. (2021). Single and binary Fe-and Al-hydroxides affect potential phosphorus mobilization and transfer from pools of different availability. Soil Syst. 5 (2), 33. doi:10.3390/soilsystems5020033
Harrison, A. F. (1987). Mineralisation of organic phosphorus in relation to soil factors, determined using isotopic 32P labelling.
Hawkins, J. M. B., Vermeiren, C., Blackwell, M. S. A., Darch, T., Granger, S. J., Dunham, S. J., et al. (2022). The effect of soil organic matter on long-term availability of phosphorus in soil: evaluation in a biological P mining experiment. Geoderma 423, 115965. doi:10.1016/j.geoderma.2022.115965
Hayden, B. P. (1998). Regional climate and the distribution of tallgrass prairie. Grassland dynamics: long-term ecological research in tallgrass prairie. New York: Oxford University Press, 19–34.
Haynes, R. J. (1982). Effects of liming on phosphate availability in acid soils: a critical review. Plant soil 68, 289–308. doi:10.1007/bf02197935
Heil, C. A. (2005). Influence of humic, fulvic and hydrophilic acids on the growth, photosynthesis and respiration of the dinoflagellate Prorocentrum minimum (Pavillard) Schiller. Harmful algae 4 (3), 603–618. doi:10.1016/j.hal.2004.08.010
Helms, J. R., Stubbins, A., Ritchie, J. D., Minor, E. C., Kieber, D. J., and Mopper, K. (2008). Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 53 (3), 955–969. doi:10.4319/lo.2008.53.3.0955
Ige, D., Akinremi, O., Flaten, D., Ajiboye, B., and Kashem, M. (2005). Phosphorus sorption capacity of alkaline manitoba soils and its relationship to soil properties. Can. J. Soil Sci. 85 (3), 417–426. doi:10.4141/s04-064
Ippolito, J. A., Blecker, S. W., Freeman, C. L., McCulley, R. L., Blair, J. M., and Kelly, E. F. (2010). Phosphorus biogeochemistry across a precipitation gradient in grasslands of central North America. J. Arid Environ. 74 (8), 954–961. doi:10.1016/j.jaridenv.2010.01.003
Kaiser, K., and Zech, W. (1996). Defects in estimation of aluminum in humus complexes of podzolic soils by pyrophosphate extraction. Soil Sci. 161 (7), 452–458. doi:10.1097/00010694-199607000-00005
Khan, K. S., Ali, M. M., Naveed, M., Rehmani, M. I. A., Shafique, M., Ali, H. M., et al. (2022). Co-application of organic amendments and inorganic p increase maize growth and soil carbon, phosphorus availability in calcareous soil. Front. Environ. Sci. 10. doi:10.3389/fenvs.2022.949371
Kovar, J. L., and Pierzynski, G. M. (2009). Methods of phosphorus analysis for soils, sediments, residuals, and waters second edition. South. Coop. Ser. Bull. 408.
Kruse, J., Abraham, M., Amelung, W., Baum, C., Bol, R., Kühn, O., et al. (2015). Innovative methods in soil phosphorus research: a review. J. plant Nutr. soil Sci. 178 (1), 43–88. doi:10.1002/jpln.201400327
Kulkarni, H. V., Mladenov, N., Johannesson, K. H., and Datta, S. (2017). Contrasting dissolved organic matter quality in groundwater in Holocene and Pleistocene aquifers and implications for influencing arsenic mobility. Appl. Geochem. 77, 194–205. doi:10.1016/j.apgeochem.2016.06.002
Liao, J., Liang, D., Jiang, Q., Mo, L., Pu, G., and Deng, Z. (2020). Growth performance and element concentrations reveal the calcicole-calcifuge behavior of three adiantum species. BMC Plant Biol. 20 (1), 327. doi:10.1186/s12870-020-02538-6
Lund, Z., and Doss, B. (1980). Residual effects of dairy cattle manure on plant growth and soil properties1. Agron. J. 72 (1), 123–130. doi:10.2134/agronj1980.00021962007200010025x
Macpherson, G. L. (1996). Hydrogeology of thin limestones: the Konza Prairie long-term ecological research site, Northeastern Kansas. J. Hydrology 186 (1-4), 191–228. doi:10.1016/s0022-1694(96)03029-6
Macpherson, G. L., Roberts, J. A., Blair, J. M., Townsend, M. A., Fowle, D. A., and Beisner, K. R. (2008). Increasing shallow groundwater CO2 and limestone weathering, Konza Prairie, USA. Geochimica Cosmochimica Acta 72 (23), 5581–5599. doi:10.1016/j.gca.2008.09.004
Macpherson, G. L., Sullivan, P. L., Stotler, R. L., and Norwood, B. S. (2019). Increasing groundwater CO2 in a mid-continent tallgrass prairie: controlling factors. E3S Web Conf. 98, 06008. doi:10.1051/e3sconf/20199806008
McDowell, R., Dodd, R., Pletnyakov, P., and Noble, A. (2020). The ability to reduce soil legacy phosphorus at a country scale. Front. Environ. Sci. 8, 6. doi:10.3389/fenvs.2020.00006
McKnight, D. M., Boyer, E. W., Westerhoff, P. K., Doran, P. T., Kulbe, T., and Andersen, D. T. (2001). Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 46 (1), 38–48. doi:10.4319/lo.2001.46.1.0038
Mehrizi Hejazi, M., Sarcheshmehpour, M., and Ebrahimi, Z. (2015). The effects of some humic substances and vermicompost on phosphorus transformation rate and forms in a calcareous soil. J. soil Sci. plant Nutr. 15 (1), 249–260.
Meyer, G., Bell, M. J., Kopittke, P. M., Lombi, E., Doolette, C. L., Brunetti, G., et al. (2023). Mobility and lability of phosphorus from highly concentrated fertiliser bands. Geoderma 429, 116248. doi:10.1016/j.geoderma.2022.116248
Meyer, G., Bell, M. J., Lombi, E., Doolette, C. L., Brunetti, G., Novotny, E. H., et al. (2021). Phosphorus speciation in the fertosphere of highly concentrated fertilizer bands. Geoderma 403, 115208. doi:10.1016/j.geoderma.2021.115208
Moran, M. A., Sheldon Jr, W. M., and Zepp, R. G. (2000). Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol. Oceanogr. 45 (6), 1254–1264. doi:10.4319/lo.2000.45.6.1254
Murphy, K. R., Boehme, J. R., Brown, C., Noble, M., Smith, G., Sparks, D., et al. (2013). Exploring the limits of dissolved organic matter fluorescence for determining seawater sources and ballast water exchange on the US Pacific coast. J. Mar. Syst. 111, 157–166. doi:10.1016/j.jmarsys.2012.10.010
Nezat, C. A., Blum, J. D., Yanai, R. D., and Park, B. B. (2008) “Mineral sources of calcium and phosphorus in soils of the northeastern United States,”Soil Sci. Soc. Am. J. 72, 1786–1794. doi:10.2136/sssaj2007.0344
Nippert, J. B., and Knapp, A. K. (2007). Soil water partitioning contributes to species coexistence in tallgrass prairie. Oikos 116 (6), 1017–1029. doi:10.1111/j.0030-1299.2007.15630.x
Ohno, T. (2002). Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. and Technol. 36 (4), 742–746. doi:10.1021/es0155276
Olatuyi, S. O., Akinremi, O. O., Flaten, D. N., and Crow, G. H. (2009). Accompanying cations and anions affect the diffusive transport of phosphate in a model calcareous soil system. Can. J. soil Sci. 89 (2), 179–188. doi:10.4141/cjss07118
Padbhushan, R., and Kumar, D. (2015). Distribution of boron in different fractions in some alkaline calcareous soils. Commun. Soil Sci. Plant Analysis 46 (8), 939–953. doi:10.1080/00103624.2015.1018521
Parlanti, E., Wörz, K., Geoffroy, L., and Lamotte, M. (2000). Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 31 (12), 1765–1781. doi:10.1016/s0146-6380(00)00124-8
Patidar, J., Sharma, Y., and Tagore, G. S. (2019). Phosphorus fractions in contrasting soil orders in central India. Int. J. Curr. Microbiol. Appl. Sci. 8 (01), 3050–3059. doi:10.20546/ijcmas.2019.801.325
Penn, C. J., and Camberato, J. J. (2019). A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9 (6), 120. doi:10.3390/agriculture9060120
Pierzynski, G. M. (1991a). The chemistry and mineralogy of phosphorus in excessively fertilized soils. Crit. Rev. Environ. Sci. Technol. 21 (3-4), 265–295. doi:10.1080/10643389109388418
Pierzynski, G. M. (1991b). The chemistry and mineralogy of phosphorus in excessively fertilized soils. Crit. Rev. Environ. Sci. Technol. 21 (3-4), 265–295. doi:10.1080/10643389109388418
Pierzynski, G. M. (2000). Methods of phosphorus analysis for soils, sediments, residuals, and waters.
Pierzynski, G. M., McDowell, R. W., and Thomas Sims, J. (2005). Chemistry, cycling, and potential movement of inorganic phosphorus in soils. Phosphorus Agric. Environ. 46, 51–86. doi:10.2134/agronmonogr46.c3
Riggle, J., and von Wandruszka, R. (2005). Binding of inorganic phosphate to dissolved metal humates. Talanta 66 (2), 372–375. doi:10.1016/j.talanta.2004.11.003
Robbins, C., and Mayland, H. (1993). Calcium, magnesium, and potassium uptake by crested wheatgrass grown on calcareous soils. Commun. Soil Sci. Plant Analysis 24 (9-10), 915–926. doi:10.1080/00103629309368848
Robertson, F. A., and Nash, D. M. (2008). Phosphorus and nitrogen in soil, plants, and overland flow from sheep-grazed pastures fertilized with different rates of superphosphate. Agric. Ecosyst. and Environ. 126 (3-4), 195–208. doi:10.1016/j.agee.2008.01.023
Rose, T. J., Rengel, Z., Ma, Q., and Bowden, J. W. (2009). Phosphorus accumulation by field-grown canola crops and the potential for deep phosphorus placement in a Mediterranean-type climate. Crop Pasture Sci. 60 (10), 987–994. doi:10.1071/cp08367
Ryan, J., Curtin, D., and Cheema, M. A. (1985). Significance of iron oxides and calcium carbonate particle size in phosphate sorption by calcareous soils. Soil Sci. Soc. Am. J. 49, 74–76. doi:10.2136/sssaj1985.03615995004900010014x
Sattari, S. Z., Bouwman, A. F., Giller, K. E., and Ittersum, M. v. (2012). Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. 109 (16), 6348–6353. doi:10.1073/pnas.1113675109
Smil, V. (2008). Energy in nature and society: general energetics of complex systems. Reg. Environ. Change 9, 57–58. doi:10.1007/s10113-008-0073-5
Smith, G. N. (1991). Geomorphology and geomorphic history of the Konza prairie research natural area, Riley and Geary Counties. Kansas: Doctoral dissertation, Kansas State University.
Soares, D. D. A., Sekiya, B. M. S., Modesto, V. C., Nakao, A. H., Freitas, L. A., Souza, I. M. D. D., et al. (2023). Accumulated carbon fractions in tropical sandy soils and their effects on fertility and grain yield in an integrated crop–livestock system. Sustainability 15 (18), 13829. doi:10.3390/su151813829
Stevenson, F. J. (1982). Organic forms of soil nitrogen. Nitrogen Agric. soils 22, 67–122. doi:10.2134/agronmonogr22.c3
Stevenson, F. J., and Fitch, A. (1986). Chemistry of complexation of metal ions with soil solution organics. Interact. soil minerals Nat. organics microbes 17, 29–58. doi:10.2136/sssaspecpub17.c2
Tunesi, S., Poggi, V., and Gessa, C. (1999). Phosphate adsorption and precipitation in calcareous soils: the role of calcium ions in solution and carbonate minerals. Nutrient Cycl. Agroecosyst. 53, 219–227. doi:10.1023/a:1009709005147
Turner, M. G., Gardner, R. H., O'neill, R. V., and O'Neill, R. V. (2001). Landscape ecology in theory and practice, 401. New York: Springer.
Vähätalo, A. V., and Wetzel, R. G. (2004). Photochemical and microbial decomposition of chromophoric dissolved organic matter during long (months–years) exposures. Mar. Chem. 89 (1-4), 313–326. doi:10.1016/j.marchem.2004.03.010
von Tucher, S., Hörndl, D., and Schmidhalter, U. (2018). Interaction of soil pH and phosphorus efficacy: long-term effects of P fertilizer and lime applications on wheat, barley, and sugar beet. Ambio 47, 41–49. doi:10.1007/s13280-017-0970-2
Web Soil Survey (2024). Available at: https://websoilsurvey.nrcs.usda.gov/app/WebSoilSurvey.aspx.
Weishaar, J. L., Aiken, G. R., Bergamaschi, B. A., Fram, M. S., Fujii, R., and Mopper, K. (2003). Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. and Technol. 37 (20), 4702–4708. doi:10.1021/es030360x
Widdig, M., Schleuss, P. M., Weig, A. R., Guhr, A., Biederman, L. A., Borer, E. T., et al. (2019). Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front. Environ. Sci. 7, 185. doi:10.3389/fenvs.2019.00185
Wilson, H. F., and Xenopoulos, M. A. (2008). Ecosystem and seasonal control of stream dissolved organic carbon along a gradient of land use. Ecosystems 11, 555–568. doi:10.1007/s10021-008-9142-3
Yang, F., Zhang, S., Song, J., Du, Q., Li, G., Tarakina, N. V., et al. (2019). Synthetic humic acids solubilize otherwise insoluble phosphates to improve soil fertility. Angew. Chem. 131 (52), 18989–18992. doi:10.1002/ange.201911060
Yang, H., Xie, Y., Zhu, T., and Zhou, M. (2021). Reduced organic carbon content during the evolvement of calcareous soils in karst region. Forests 12 (2), 221. doi:10.3390/f12020221
Zhang, G., Fritz, A. K., Li, Y., Bowden, R. L., Bai, G., Chen, M.-S., et al. (2024). Registration of ‘KS Big Bow’hard white winter wheat. J. Plant Registrations 18 (2), 388–392. doi:10.1002/plr2.20354
Zhang, G., Martin, T. J., Fritz, A. K., Miller, R., Chen, M. S., Bowden, R. L., et al. (2015). Registration of ‘oakley CL’wheat. J. Plant Registrations 9 (2), 190–195. doi:10.3198/jpr2014.04.0023crc
Zhang, Y., Zhang, S., Wang, R., Jiang, Y., Zhang, Y., Li, H., et al. (2016). Impacts of fertilization practices on ph and the ph buffering capacity of calcareous soil. Soil Sci. and Plant Nutr. 62 (5-6), 432–439. doi:10.1080/00380768.2016.1226685
Zhou, M., and Li, Y. (2001). Phosphorus-sorption characteristics of calcareous soils and limestone from the Southern Everglades and adjacent farmlands. Soil Sci. Soc. Am. J. 65, 1404–1412. doi:10.2136/sssaj2001.6551404x
Keywords: phosphorus, soil organic matter (SOM), bio-availability, land use, grassland ecosystem, agriculture
Citation: Banerjee P, Kulkarni HV, Veach AM, Nagaraja T, Pathak P, Das SR and Datta S (2025) Geochemical factors influencing the phosphorus mobility in Konza prairie grassland and agriculture-dominated soils in north-eastern Kansas. Front. Environ. Sci. 13:1500314. doi: 10.3389/fenvs.2025.1500314
Received: 23 September 2024; Accepted: 07 January 2025;
Published: 14 February 2025.
Edited by:
Oliver Matthias Wiche, Zittau/Görlitz University of Applied Sciences, GermanyReviewed by:
Precious Okoroafor, City Department of Climate Protection and Adaptation, GermanyCopyright © 2025 Banerjee, Kulkarni, Veach, Nagaraja, Pathak, Das and Datta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harshad V. Kulkarni, aGFyc2hhZEBpaXRtYW5kaS5hYy5pbg==; Saugata Datta, c2F1Z2F0YS5kYXR0YUB1dHNhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.