- 1A Key Laboratory of Groundwater Resources and Environment, Ministry of Education, School of New Energy and Environment, Jilin University, Changchun, China
- 2Department of Bionics Science and Engineering, Jilin University, Changchun, China
- 3Key Laboratory of Automobile Materials, Department of Materials Science and Engineering, Jilin University, Changchun, China
- 4Department of Pathogenic Biology, College of Basic Medical Sciences, Jilin University, Changchun, China
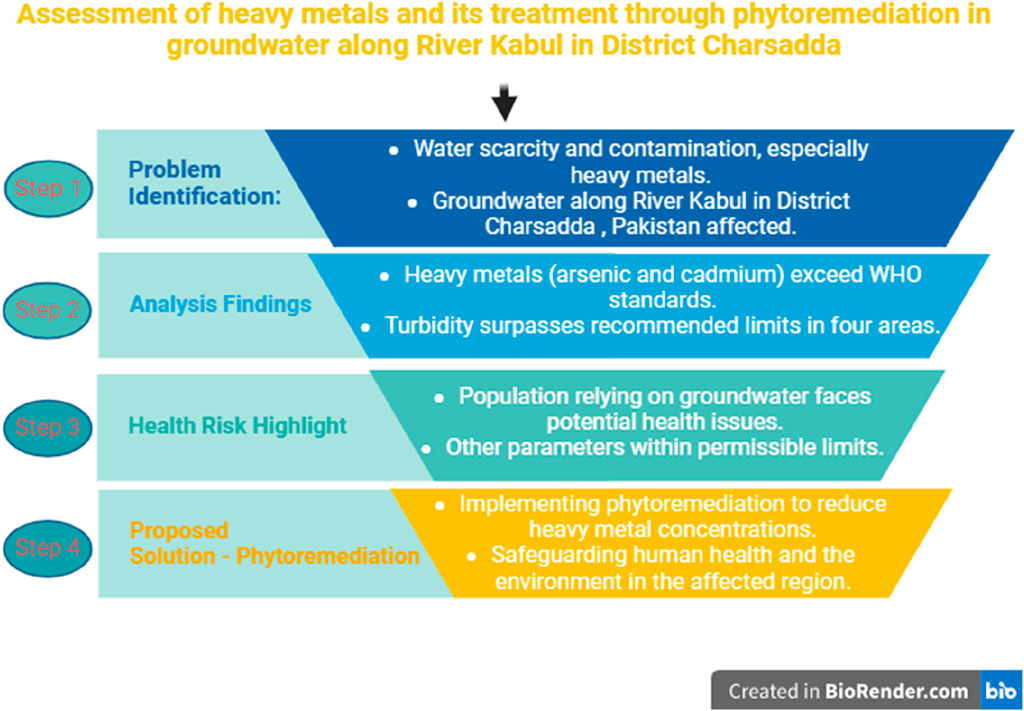
Life cannot exist without water. Water scarcity is caused by massive groundwater decline. Water contamination is the most common problem spreading worldwide quicker than ever, along with anthropogenic water scarcity. Since tainted water can harm health, water contamination is also contributing to water scarcity. Heavy metals in drinking water have plagued most Asian, African, and European nations for decades. Studies reveal that heavy metals have caused damage in Pakistan, India, and China. This study detects heavy metals in groundwater and treats them with phytoremediation along the river Kabul in district Charsadda. It also measures pH, EC, TDS, turbidity, fluoride, phosphates, nitrites, and nitrates. All indicators meet the WHO and national environmental quality criteria for drinking water, except for turbidity, which exceeds the limit of ≤5 NTU in four examined regions, reaching a maximum of 9.99 NTU. Chemical parameters were within the standard limits, except for high concentrations of arsenic (As) and cadmium (Cd) in samples from S2 (15.20 μg/L) and S1 (20.50 μg/L) compared to WHO’s 10 and 5 μg/L standards. However, the limit is within EPA Pakistan’s 50 and 100 μg/L standards for drinking water, which Pakistan still follows. Heavy metals can harm health, even at low levels. Since the majority of the study area’s population relies on groundwater for drinking and other needs, heavy metal pollution of the groundwater can cause many ailments. Thus, phytoremediation is increasingly vital to reduce these heavy metals to WHO limits to protect human health and the environment.
1 Introduction
Nowadays, human activities and their basic needs are growing rapidly due to the increasing population in all countries; as a result, our water resources have been contaminated with a greater quantity of harmful pollutants, especially heavy metals from different sectors (Ullah et al., 2021). Certain adverse effects of urbanization, such as depletion of resources, increasing carbon emissions, and water pollution, are reported regionally and globally; these effects disturb human health and the environment (Ahuti, 2015) Industrialization also has effects on the social and economic changes of people and needs innovations for improvement (Mgbemene et al., 2016). Due to industrial processes, large quantities of pollutants are released into local water bodies, causing problems for human health. Industrialization not only produces HMs but also produces different types of waste that affect the environment. Because of the industrial revolution, large amounts of chemical substances and harmful gases are discharged into the environment.
Water pollution is a serious problem that will disturb humans. Various approaches have been used to control this problem, but it is still a concern for humans. Humans and the environment are both affected by these pollutants worldwide. Some heavy metals (HMs) are useful in smaller quantities for humans, such as Ni, Cu, Fe, and As, but they become poisonous in larger quantities (Valko et al., 2016). HMs with a large density are reported to be more toxic, even in lesser amounts (Iram et al., 2013). HMs greatly affect the function of the lungs, kidneys, and brain and also reduce the energy level in humans. Certain HMs are carcinogenic in light of their frequent exposure (Jaishankar et al., 2014). Therefore, it is essential to offer a suitable method for HM uptake from water systems (Tóth et al., 2016).
Several methods, such as ion exchange, coagulation, adsorption, oxidation or reduction, and nanotechnology, have been used to treat HMs in aqueous solutions (Rajasulochana and Preethy, 2016). All these methods have their own advantages and disadvantages, but almost all of them are expensive and time-consuming. A new approach must be planned to safeguard human health and the environment from the consequences of water pollution, and bioremediation is reported to be one of the most promising and efficient techniques for heavy metal removal from water bodies (Raghunandan et al., 2018). This method has developed as a relatively cost-effective procedure and shows efficiency in the removal of heavy metals from water and soil, converting them into non-toxic compounds (Singh and Gupta, 2016). Hydrophytes use heavy metals and other pollutants as food nutrients in the bioremediation method. Bioremediation can be carried out at two sites, such as in situ and ex situ (Kumar et al., 2016). Many species of hydrophytes have been used to treat HMs from industrial wastewater (Laib and Leghouchi, 2012). Hydrophytes are frequently available and can be easily cultivated in aquatic ecosystems. A previous study proved that hydrophyte species remove HMs, thus enhancing the quality of water (Shamshad et al., 2015).
Environmental contaminants include heavy metals and metalloids (Ghani et al., 2022a). HMs can also be considered agricultural soil pollutants due to their potential to adversely affect crop health and productivity when present in high concentrations in the soil (Ghani et al., 2023). HMs exhibit resistance to degradation, and in the absence of plant uptake or leaching, they have the potential to accumulate in the soil and endure for extended durations (Bhatti et al., 2022). The metals commonly identified as contaminants in agricultural soils and known to induce toxic effects in plants at high concentrations include cadmium (Cd), lead (Pb), chromium (Cr), arsenic (As), mercury (Hg), nickel (Ni), copper (Cu), and zinc (Zn) (Nawab et al., 2018; Ghani et al., 2022b). Cd, Pb, As, Hg, and Cr are known to exhibit high toxicity and pose significant risks to plant health at various degrees of contamination (Kumar et al., 2018a).
The aquatic plant water hyacinth is a perennial species that is indigenous to tropical regions of South America. It has already spread to all the continents, except Antarctica. The plants undergo rapid biomass growth and develop dense mats as they reproduce through stolons, which are vegetative runners. Water hyacinth can fully obstruct lakes and wetlands, thereby surpassing indigenous aquatic species, diminishing oxygen levels for fish, and establishing an optimal environment for mosquitoes that transmit diseases. Extensive water hyacinth infestations can impede river transportation and fishing, cause harm to bridges, and obstruct dams. Lake Victoria in Africa and the waterways of Papua New Guinea serve as notable illustrations of regions characterized by substantial populations of water hyacinth, which imposes constraints on transportation and fishing activities, thereby leading to a heightened prevalence of diseases (Kumar et al., 2018b).
In the current research work, two species of hydrophytes, Typha latifolia (cattail) and Echornia crassipes (water hyacinth), were applied because of their abundant availability and easy cultivation everywhere to find their treatment capability for As and Cd at various HM amounts to find a viable and eco-friendly technique to treat HM polluted water, i.e., industrial wastewater.
2 Materials and methods
2.1 Study area
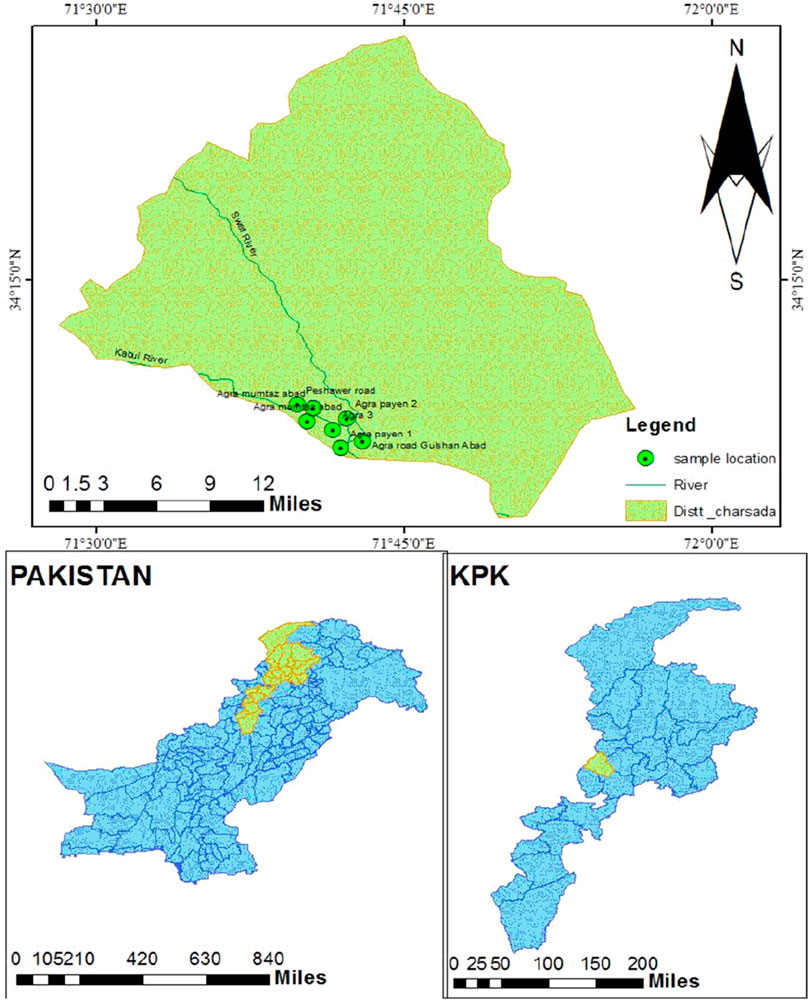
The study area is located in district Charsadda in Khyber Pakhtunkhwa at latitude 34.13338° N and longitude 71.6925° E as given in Figure 1. Charsadda has a total area of approximately 996 km2 and is located at latitude 34.1682° N and longitude 71.7504° E. Charsadda is located almost 30 km away from Peshawar, which is the capital of the province. The total population, according to the 2017 census conducted by the Government of Pakistan, is about 114,565. Three main rivers flow in Charsadda: Kabul, Jindi, and Swat. The study area is known as Sardaryab, located near the bank of river Kabul. The Kabul River emerges from Afghanistan and meets the Indus River at Attock in Pakistan.
River Kabul flows along Sardaryab, which is also a major picnic spot. Sardaryab is also the local name of the River Kabul flowing in the district of Charsadda. Almost the whole population of this area is dependent on groundwater for drinking purposes. A major difference in drinking water quality was observed after the area was hit by a massive flood in 2010.
2.2 Sampling collection
Seven groundwater samples were collected from different locations, including Agra Road Gulshanabad, Agra Payan, Agra, Agra Mumtazabad, and Peshawar Road (Sardaryab). Two samples were a composite of 500 mL each, made by mixing two samples for each composite sample of 250 mL. The samples were collected in polythene bottles that were carried to the National Center of Excellence in Geology, University of Peshawar, for the analysis of As and Cd and other parameters, including pH, EC, TDS, turbidity, fluoride, nitrate, nitrite, and phosphate. The geo-coordinates of the selected areas are latitude 34.13338° N and longitude 71.6925° E.
2.3 Sampling, identification, and cultivation of hydrophytes
The selected species of hydrophytes were collected from a local pond in Charsadda, Pakistan. These species were washed with tap water to remove the soil particles and then cleaned with distilled water. Later, the hydrophyte species were examined using a microscope and confirmed by a botanist from the Department of Botany, University of Peshawar, Pakistan. The species were then cultivated in deionized water for almost 4 weeks at room temperature under natural light and used for the experimental work.
2.4 Synthesis of solutions
HM stock solutions were prepared utilizing Cd (NO3)2 and As (NO3)2 of analytical grade in deionized water (Milli-Q grade). Before their use, the growing medium for the hydrophyte species was sterilized for approximately 25–30 min by autoclave at 120 °C; however, the pH ranged from 6.8 to 7.0, with 1 M of NaOH (Singh et al., 2012).
2.5 Experimental setup
All experiments were carried out in open containers (tubs) containing 1 L (L) of wastewater collected from the study area. These open containers were washed with HNO3 (10%) to avoid HM contamination and then washed with distilled water. Individual plants of Typha latifolia (cattail) and Echornia crassipes (water hyacinth) were added to all the containers. The complete experiment was carried out for 1 month under natural light at a room temperature of 22°C ± 1°C.
The removal efficiency (RE) of the hydrophytes was determined using the following formula:
where Ci represents the initial concentration of the contaminant and Cf represents the final concentration after the treatment process (Bilal et al., 2020).
2.6 Analytical methods
After 30 days, the hydrophyte species were collected from each container and then washed several times with 5 mM of ethylenediaminetetraacetic acid (EDTA) and then deionized water to remove HMs.
The samples were dried at 60°C for 2 h in an oven. HMs were extracted from the dead biomass of the hydrophyte species following the advanced method of Rybak et al. (2012). In brief, 3 g of powdered hydrophytes was taken in a beaker containing 20 mL solution of HNO3 (70%) and H2O2 (30%). Whatman filter paper (42) was used for filtration of the prepared solution into a flask of 100 mL, and then the final solution was synthesized using distilled water. The hydrophyte samples were analyzed using an atomic absorption spectrometer AAS (PerkinElmer, United States) with a 100 mL volume at 195.2 nm (wavelength), while argon (Ar) was used as a carrier (NCEG, University of Peshawar, Pakistan).
2.7 Statistical analysis
In this current study, the data were analyzed using statistical analysis. All graphs were created using OriginPro using the individual medium of the hydrophytes to present the mean values, while the map was created using ArcGIS software (Noman et al., 2020).
3 Results and Discussion
3.1 Physiochemical parameters
Different physiochemical parameters were examined, such as PH, electrical conductivity, total dissolved solids, turbidity, arsenic, nitrates, nitrites, fluoride, and phosphates. To obtain the results, the samples were analyzed at NCEG, University of Peshawar, for As, Cd, and other parameters, with the main purpose of the analysis being to check the As and Cd concentration in drinking water and whether the levels could pose health threats.
3.1.1 pH
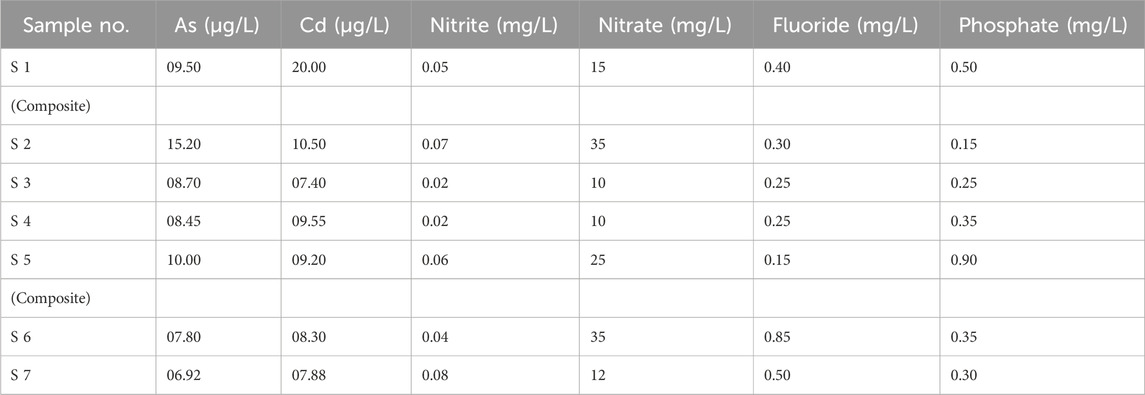
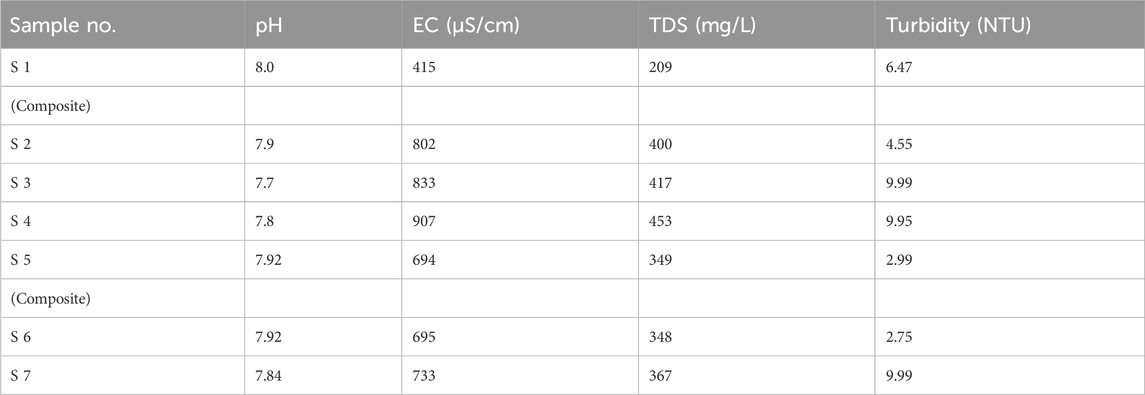
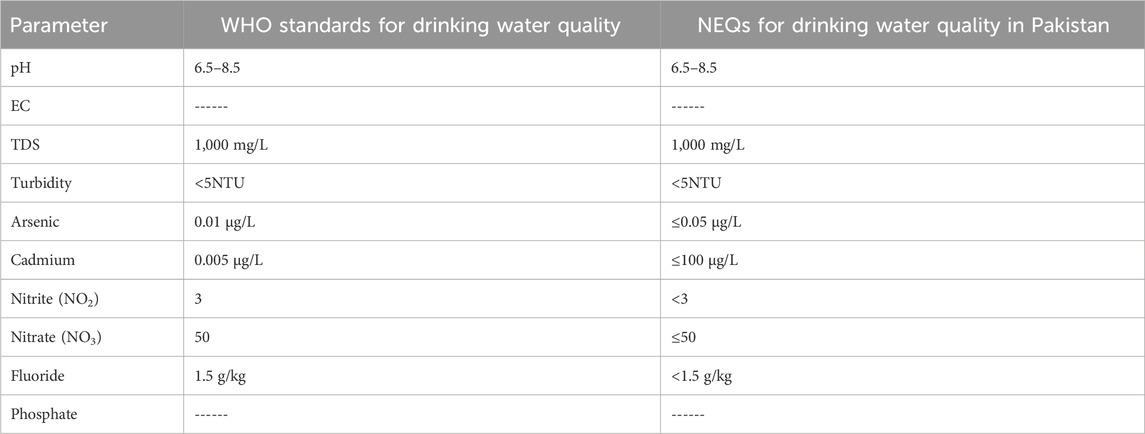
Table 1 represents the pH values of all seven samples collected from different sampling points. According to Table 1, all the pH values are within the acceptable limits of NEQs and WHO (6.5–8.5), and all the values vary from each other.
3.1.2 Electrical conductivity
The electrical conductivity (EC) values of all seven samples are given in Table 1 according to their sampling points. There is no specific limit set for electrical conductivity, but as given in Figure 2 the EC is not very high and is recorded in µS/cm.
3.1.3 Total dissolved solids
The values obtained for total dissolved solids (TDSs) by the analysis of all seven samples from different points in the laboratory are presented in Table 1 .A total of 1,000 mg/L was the standard used by both WHO and Pakistan for drinking water. Hence, the values present in Table 1 are all according to the permissible limit.
3.1.4 Turbidity
According to the WHO and NEQs, the acceptable limit for turbidity in drinking water is < 5NTU. As shown in Table 1, the recorded turbidity values vary. The samples collected from four sampling points have turbidity values that exceed the limit, as represented in Table 1.
3.1.5 Nitrite
The values of nitrites represented in Table 2 show that the level of nitrite in all seven samples is under the permissible limit of 3 mg/L of the WHO and <3 mg/L of Pakistani standards.
3.1.6 Nitrate
The level of nitrate recorded in samples 1, 2, 5, and 6 is under the permissible limit of the WHO and NEQ values of 50 mg/L and ≤50 mg/L, respectively. The values of nitrates in different samples are given in Table 2.
3.1.7 Fluoride
Fluoride values were recorded for samples collected from seven different sampling points. The maximum concentration of fluoride was recorded in sample 6, which was still under the allowable limit of 1.5 mg/L and <1.5 mg/L of the WHO and Pakistan’s standards. Fluoride, if it exceeds the limit, can cause serious health issues.
3.1.8 Phosphate
Different values of phosphates for different sampling points can be seen in Table 2, with the highest recorded value being 0.90 mg/L in sample 5.
3.1.9 Arsenic
Seven samples analyzed in the laboratory for As in groundwater provided different values. The maximum permissible limit for As in groundwater is 10 μg/L, according to the WHO. However, the limit is different in Pakistan for groundwater (50 μg/L). As given in Table 2, samples 2 and 5 have the highest concentration out of all, with the recorded values being 15.20 μg/L and 10.00 μg/L in samples 2 and 5. Both of the values are above the standard limits of the WHO, which means that the population from both sampling points is exposed to arsenic health risks.
3.1.10 Cadmium
The maximum permissible limit for Cd in groundwater is 05 μg/L according to the WHO. However, the limit is different in Pakistan for groundwater (100 μg/L). As given in Table 2, samples 1 and 2 have the highest concentration out of all, with the values observed being 20 and 10.50 μg/L, respectively. Both of the values are above the standard limits of the WHO, thus causing problems for human health in the study area.
3.2 Removal of HMs using Typha latifolia
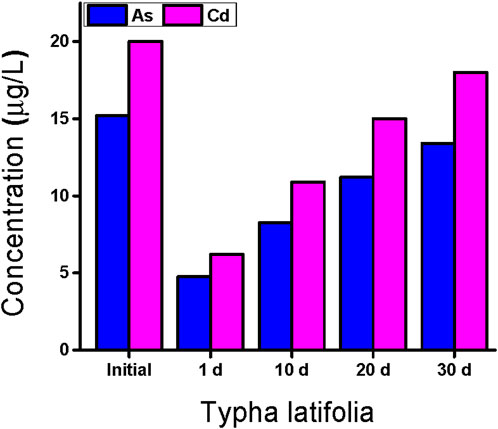
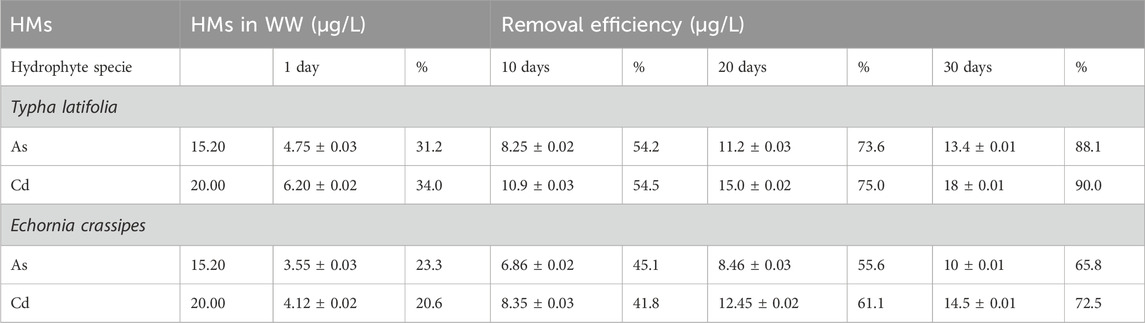
The initial concentrations of Cd and As observed in water samples collected from the study area were 20 and 15.20 μg/L, respectively. The concentration of these HMs was detected to be above the permissible limits set by the WHO as given in Table 3. T. latifolia was cultivated in the water samples for 30 days. After 30 days, the results show that T. latifolia easily grows in wastewater. T. latifolia shows a significant removal of selected HMs such as As and Cd, as reported in Table 4.
The removal efficiency of T. latifolia was reduced after 30 days. The samples were collected at intervals of 10 days. On day 1, T. latifolia showed good removal efficiency for Cd and As, which was 34% and 31.2%, respectively, as reported in Table 4. On day 10, T. latifolia indicated a better removal efficiency against Cd compared to As.
The removal efficiency for Cd was 54.5%, whereas it was 54.2% for As after 10 days, respectively. On day 20, the removal efficiency of T. latifolia for Cd and As was 75% and 73.6%, respectively, and on day 30, the removal capability of T. latifolia decreased for these HMs. Cd and As were removed by 90% and 88.1% by T. latifolia, respectively, after 30 days (Bilal et al., 2020).
3.3 Removal of HMs using Echornia crassipes
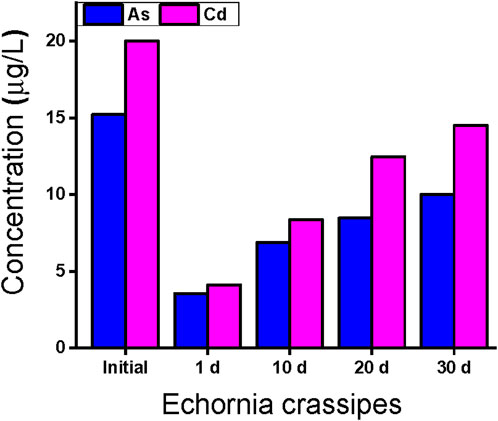
Table 4 reviews the removal efficiency of E. crassipes for the selected HMs in water samples from the study area. The results show that the treatment ability of E. crassipes is less related to T. latifolia, but it is still an efficient species of hydrophyte for the removal of selected HMs. The removal effectiveness of E. crassipes on day 1 was reported to be 23.3% and 20.6% for As and Cd, respectively as reported in Figure 3. These HMs on day 10 were treated significantly by E. crassipes, and 41.8% of Cd and 45.1% of As were removed from wastewater. After day 20, this species showed good efficiency for Cd (61.1%), followed by As (55.6%), and treatment efficiency was reported in the order of Cd >As
On day 30, Cd and As were reduced by 72.5% and 65.8%, respectively, as shown in Table 4. The result shows that E. crassipes is an environmentally friendly and easily cultivated hydrophyte species for the removal of several HMs from water samples collected from the study area.
3.4 Discussion
Nowadays, wastewater from different sources is discharged into local water sources, containing HMs like As, Ni, Pb, Cr, Cd, and Hg. Their toxic effect and long-term persistency in the environment make this an issue of major environmental concern worldwide, particularly in Asian countries like Pakistan, Bangladesh, China, and India. Wastewater containing HMs is discharged into water systems without prior treatment in developing nations like Pakistan, and several methods have been used to remove HMs from these wastewaters (Shamshad et al., 2015). These conventional methods have numerous shortcomings, such as an extensive supply of chemicals, unreliable HM removal, and the generation of toxic waste at the end (Fang et al., 2011). Recently, phytoremediation has emerged as a promising and efficient method to overcome such traditional methods (Tan and Ting, 2012).
Phytoremediation is cost-effective and eco-friendly for the uptake of HMs from wastewater (Taştan et al., 2012). This method has been used to remove HMs from the wastewater of industries on a large scale. The removal of these metals using hydrophytes has been reported as an efficient method for the treatment of HM-polluted water. Hydrophytes have been cultivated in wastewater containing HMs in different concentrations (Karna et al., 2017).
In this study, the result shows that hydrophytes such as T. latifolia and E. crassipes treated Cd and As from the polluted water of the study area. Removal efficiency was different because of the initial concentrations of the selected HMs in the wastewater of the study area. T. latifolia’s efficiency for Cd removal at days 1, 10, 20, and 30 was 34%, 54.5%, 75%, and 90%, respectively, though its initial concentration was detected to be 20 μg/L in the water samples of the study area. As removal efficiency was 31.2%, 54.2%, 73.6%, and 88.1% after days 1, 10, 20, and 30 with a pH range of 6.8–7.0, while its initial concentration was 15.20 μg/L in the water samples. T. latifolia removed the selected HMs to within the acceptable limits of the WHO and PAK-NEQs.
E. crassipes also shows good efficiency for the removal of Cd, which was 20.6%, 41.8%, 61.1%, and 72.5% (see Table 4). The removal efficiency of this species was lower compared to T. latifolia. Both species can grow healthily in polluted water because no yellow part or dead biomass was seen after day 30, and they show good removal efficiency for selected HMs. The current results show that the selected species of hydrophytes are environmentally friendly for the treatment of polluted water. Their simple and healthy growth in wastewater during the experimental period, easy availability, and economical method make this technology acceptable for the treatment of HM-polluted water.
The detected concentration of selected HMs in water samples was above the permissible limits, as shown in Table 2. All these HMs easily cause cancer in living organisms, including humans and animals, and also cause toxic effects in plants (Álvarez and Wendel, 2003) PAK-NEQs of ≤0.05 μg/L and ≤100 μg/L for As and Cd, respectively, in order to maintain safety for human health. The cell walls are the main binding sites for HMs in plant species, especially in hydrophytes (Ma et al., 2016).
The results of this study show that T. latifolia and E. crassipes can remove Cd and As from water samples, although the detected initial concentration was higher in the water samples. Many research articles are present regarding the removal of HMs using both live and dead hydrophyte biomass. Deng et al. (2008) studied Cladophora sp. for the removal of Pb and Cd and found that Cladophora sp. can remove large quantities of these HMs from waterbodies. This study clearly shows the high removal efficiency of selected HMs using algae species. The removal efficiency of these HMs by hydrophytes was reported in the order Typha latifolia > Echornia crassipes. Hydrophytes are cost-effective and efficient compared to the dead biomass of hydrophytes.
4 Conclusion
The objective of the present study was to assess the efficacy of hydrophytes, specifically Typha latifolia and E. crassipes, in removing Cd and As from wastewater. The efficacy of removing these HMs was influenced by the starting concentration of heavy metals in the water samples. A significant reduction in HMs was observed during the early stages of the experiment while using water solutions. The removal effectiveness of Cd in T. latifolia and E. crassipes was found to be 90% and 72.5%, respectively. Similarly, the removal efficiency of As was found to be 88.1% and 65.8%. It is worth noting that the initial concentration of Cd and As in the water of the study area was 20 and 15.20 μg/L, respectively. It is crucial to understand that these hydrophyte species not only have a long lifespan but also possess the ability to remove heavy metals from water. The high efficiencies exhibited by these species, along with their low input requirements, cost-effectiveness in terms of transportation, and ease of cultivation, render this approach suitable for the large-scale removal of HMs from water.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
SK: conceptualization, data curation, formal analysis, investigation, methodology, project administration, writing–original draft, writing–review and editing. MK: formal analysis, resources, visualization, writing–original draft. SN: software, writing–review and editing. SA: formal analysis, writing–original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Álvarez, I. J. F. W., and Wendel, J. F. (2003). Ribosomal ITS sequences and plant phylogenetic inference. Mol. phylogenetics Evol. 29 (3), 417–434. doi:10.1016/s1055-7903(03)00208-2
Bhatti, Z. I., Ishtiaq, M., Khan, S. A., Nawab, J., Ghani, J., Ullah, Z., et al. (2022). Contamination level, source identification and health risk assessment of potentially toxic elements in drinking water sources of mining and non-mining areas of Khyber Pakhtunkhwa, Pakistan. J. Water Health 20 (9), 1343–1363. doi:10.2166/wh.2022.087
Bilal, Q. W., Noman, M., Shen, Z., Ali, K. S., khan, F., Ali Panhwar, K., et al. (2020). Phytoremediation of contaminated soil Lead and Cadmi-um by Brassica júncea (L.) Czern plant. J. Earth Sci. Environ. Stud. 5 (4), 110–120. doi:10.25177/jeses.5.4.ra.10693
Deng, L., Zhu, X., Su, Y., Su, H., and Wang, X. (2008). Biosorption and desorption of Cd 2+ from wastewater by dehydrated shreds of Cladophora fascicularis. Chin. J. Oceanol. Limnol. 26 (1), 45–49. doi:10.1007/s00343-008-0045-0
Fang, L., Wei, X., Cai, P., Huang, Q., Chen, H., Liang, W., et al. (2011). Role of extracellular polymeric substances in Cu (II) adsorption on Bacillus subtilis and Pseudomonas putida. Bioresour. Technol. 102 (2), 1137–1141. doi:10.1016/j.biortech.2010.09.006
Ghani, J., Nawab, J., Faiq, M. E., Ullah, S., Alam, A., Ahmad, I., et al. (2022a). Multi-geostatistical analyses of the spatial distribution and source apportionment of potentially toxic elements in urban children’s park soils in Pakistan: a risk assessment study. Environ. Pollut. 311, 119961. doi:10.1016/j.envpol.2022.119961
Ghani, J., Nawab, J., Khan, S., Khan, M. A., Ahmad, I., Ali, H. M., et al. (2022b). Organic amendments minimize the migration of potentially toxic elements in soil–plant system in degraded agricultural lands. Biomass Convers. Biorefinery 14, 6547–6565. doi:10.1007/s13399-022-02816-3
Ghani, J., Nawab, J., Ullah, Z., Rafiq, N., Hasan, S. Z., Khan, S., et al. (2023). Multivariate statistical methods and GIS-based evaluation of potable water in urban children’s parks due to potentially toxic elements contamination: a children’s health risk assessment study in a developing country. Sustainability 15 (17), 13177. doi:10.3390/su151713177
Iram, S., Zaman, A., Iqbal, Z., and Shabbir, R. (2013). Heavy metal tolerance of fungus isolated from soil contaminated with sewage and industrial wastewater. Pol. J. Environ. Stud. 22 (3), 691–697. doi:10.7176/JRDM/69-03
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., and Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7 (2), 60–72. doi:10.2478/intox-2014-0009
Karna, R. R., Luxton, T., Bronstein, K. E., Hoponick Redmon, J., and Scheckel, K. G. (2017). State of the science review: potential for beneficial use of waste by-products for in situ remediation of metal-contaminated soil and sediment. Crit. Rev. Environ. Sci. Technol. 47 (2), 65–129. doi:10.1080/10643389.2016.1275417
Kumar, A., Chanderman, A., Makolomakwa, M., Perumal, K., and Singh, S. (2016). Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit. Rev. Environ. Sci. Technol. 46 (6), 556–591. doi:10.1080/10643389.2015.1131562
Kumar, V., Singh, J., and Chopra, A. K. (2018a). Assessment of plant growth attributes, bioaccumulation, enrichment, and translocation of heavy metals in water lettuce (Pistia stratiotes L.) grown in sugar mill effluent. Int. J. phytoremediation 20 (5), 507–521. doi:10.1080/15226514.2017.1393391
Kumar, V., Singh, J., and Chopra, A. K. (2018b). Assessment of phytokinetic removal of pollutants of paper mill effluent using water hyacinth (Eichhornia crassipes [Mart.] Solms). Environ. Technol. 39 (21), 2781–2791. doi:10.1080/09593330.2017.1365944
Laib, E., and Leghouchi, E. (2012). Cd, Cr, Cu, Pb, and Zn concentrations in ulva lactuca, codium fragile, jania rubens, and dictyota dichotoma from rabta bay, jijel (Algeria). Environ. Monit. Assess. 184 (3), 1711–1718. doi:10.1007/s10661-011-2072-0
Ma, Y., Oliveira, R. S., Freitas, H., and Zhang, C. (2016). Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front. plant Sci. 7, 918. doi:10.3389/fpls.2016.00918
Mgbemene, C. A., Nnaji, C. C., and Nwozor, C. (2016). Industrialization and its backlash: focus on climate change and its consequences. J. Environ. Sci. Technol. 9 (4), 301–316. doi:10.3923/jest.2016.301.316
Nawab, J., Ghani, J., Khan, S., and Xiaoping, W. (2018). Minimizing the risk to human health due to the ingestion of arsenic and toxic metals in vegetables by the application of biochar, farmyard manure and peat moss. J. Environ. Manag. 214, 172–183. doi:10.1016/j.jenvman.2018.02.093
Noman, M., Jun, W., Ullah, S., Ali, K. S., Faisal Khan, B., Tasleem, R., et al. (2020). Chitral gol national park, 121–129.
Raghunandan, K., Kumar, A., Kumar, S., Permaul, K., and Singh, S. (2018). Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 8 (1), 71.
Rajasulochana, P., and Preethy, V. (2016). Comparison on efficiency of various techniques in treatment of waste and sewage water–A comprehensive review. Resource-Efficient Technol. 2 (4), 175–184. doi:10.18799/24056529/2016/4/63
Rybak, A., Messyasz, B., and Łęska, B. (2012). Freshwater Ulva (Chlorophyta) as a bioaccumulator of selected heavy metals (Cd, Ni and Pb) and alkaline earth metals (Ca and Mg). Chemosphere 89 (9), 1066–1076. doi:10.1016/j.chemosphere.2012.05.071
Shamshad, I., Khan, S., Waqas, M., Ahmad, N., Ur-Rehman, K., and Khan, K. (2015). Removal and bioaccumulation of heavy metals from aqueous solutions using freshwater algae. Water Sci. Technol. 71 (1), 38–44. doi:10.2166/wst.2014.458
Singh, L., Pavankumar, A. R., Lakshmanan, R., and Rajarao, G. K. (2012). Effective removal of Cu2+ ions from aqueous medium using alginate as biosorbent. Ecol. Eng. 38 (1), 119–124. doi:10.1016/j.ecoleng.2011.10.007
Singh, S., and Gupta, V. K. (2016). Ayurvedic approach for management of ankylosing spondylitis: a case report. Int. J. Pharmacol. Biol. Sci. 10 (1), 53–56. doi:10.1016/j.jaim.2015.10.002
Tan, W. S., and Ting, A. S. Y. (2012). Efficacy and reusability of alginate-immobilized live and heat-inactivated Trichoderma asperellum cells for Cu (II) removal from aqueous solution. Bioresour. Technol. 123, 290–295. doi:10.1016/j.biortech.2012.07.082
Taştan, B. E., Duygu, E., and Dönmez, G. (2012). Boron bioremoval by a newly isolated Chlorella sp. and its stimulation by growth stimulators. water Res. 46 (1), 167–175. doi:10.1016/j.watres.2011.10.045
Tóth, G., Hermann, T., Da Silva, M. R., and Montanarella, L. (2016). Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 88, 299–309. doi:10.1016/j.envint.2015.12.017
Ullah, S., Noman, M., Ali, K. S., Siddique, M., Sahak, K., Hashmi, S. K., et al. (2021). Treatment of industrial wastewater (IWW) and reuse through advanced oxidation processes (AOPs): a comprehensive overview. IOSR J. Environ. Sci. 15 (1), 04–14. doi:10.9790/2402-1501010414
Keywords: aquatic plant, heavy metals, groundwater, phytoremediation, wastewater
Citation: Khan S, Kamal M, Noor S and Afzal SM (2024) Assessment of heavy metals and its treatment through phytoremediation in groundwater along River Kabul in district Charsadda. Front. Environ. Sci. 12:1392892. doi: 10.3389/fenvs.2024.1392892
Received: 28 February 2024; Accepted: 27 March 2024;
Published: 03 July 2024.
Edited by:
Tarek O. Said, National Institute of Oceanography and Fisheries (NIOF), EgyptReviewed by:
Gehan El Zokm, National Institute of Oceanography and Fisheries (NIOF), EgyptSyed Hafizur Rahman, Jahangirnagar University, Bangladesh
Copyright © 2024 Khan, Kamal, Noor and Afzal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shehryar Khan, a2hhbnNoZXJ5YXIzMThAZ21haWwuY29t
 Shehryar Khan
Shehryar Khan Masroor Kamal2
Masroor Kamal2