- 1Department of Chemistry and Biochemistry, University of Mississippi, Oxford, MS, United States
- 2School of the Environment, Trent University, Peterborough, ON, Canada
- 3Water Quality and Ecology Research Unit, U.S. Department of Agriculture, Agricultural Research Service, Oxford, MS, United States
Plastics are extensively used in agriculture, but their weathering and degradation generates microplastics (MPs) that can be carried by runoff into water bodies where they can accumulate and impact wildlife. Due to its physicochemical properties, biochar has shown promise in mitigating contaminants in agricultural runoff. However, few studies have examined its effectiveness at removing MPs. In this study, we assessed MP pollution (>30 μm) in runoff from a farm in the Mississippi Delta and examined the effectiveness of biochar (pinewood and sugarcane) to remove MPs from aqueous solutions. Using micro-Fourier Transform Infrared spectroscopy (µ-FTIR), we observed an average of 237 MPs/L (range 27–609) in the runoff, with most particles identified as polyethylene, polyamide, polyvinyl chloride, polyurethane, acrylonitrile butadiene styrene, and polyarylamide. Biochar columns effectively removed MPs from runoff samples with reductions ranging from 86.6% to 92.6%. MPs of different sizes, shapes, and types were stained with Nile red dye (to facilitate observation by fluorescence) and quantified their downward progress with multiple column volumes of water and wet/dry cycles. Smaller MPs penetrated the columns further, but ≥90% of MPs were retained in the ∼20 cm columns regardless of their shape, size, and type. We attribute these results to physical entrapment, hydrophobic behaviors, and electrostatic interactions. Overall, this proof-of-concept work suggests biochar may serve as a cost-effective approach to remove MPs from runoff, and that subsequent field studies are warranted.
1 Introduction
Microplastics (MPs) consist of a suite of synthetic polymers <5 mm in size with varying chemical makeup, shapes, densities, additives, and other characteristics (Thompson et al., 2004; Arthur et al., 2009; Zhang et al., 2018; Padervand et al., 2020). These environmental pollutants, which stems in part from the degradation of plastic litter by natural physical, chemical, and biological processes, can have deleterious effects on certain organisms (Galgani et al., 2013; Cauwenberghe et al., 2015; Mukherjee et al., 2022). MPs pose a critical challenge as they are widespread, persistent, and can bioaccumulate in wildlife, and, potentially, in humans (Mukherjee et al., 2022).
Agricultural production is a prominent source of MPs, as plastics are extensively used in farming for soil moisture retention, weed control, temperature regulation, and irrigation piping (Horton et al., 2017; Jansen et al., 2019; Isari et al., 2021). Notably, plastic mulching has been widely employed for over half a century, resulting in the accumulation of MPs in soils worldwide (Li et al., 2020). The MPs themselves can alter soil biophysical properties, including bulk density, water-holding capacity, and soil microbial interactions with water-stable aggregates (Tang, 2020). Studies have demonstrated the uptake and accumulation of MPs in crop tissue cultures, suggesting the potential transfer of MPs to humans through the food chain (Li et al., 2019). MPs may serve as sources and/or sinks for pesticides in agricultural fields via adsorption of compounds (Wang et al., 2020a). For example, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, and heavy metals are known to adhere to MPs due to various surface interactions, including electrostatic attraction and surface complexation with functional groups like carboxyl groups (Sharma et al., 2024). Furthermore, the practice of biosolids fertilization often introduces MPs, particularly fibers, into soils, leading to increased MP incidence in farmland runoff (Gao et al., 2022; Schell et al., 2022). Additionally, evidence suggests that areas where agricultural plastics such as greenhouse covers, plastic film, and silage film are commonly used tend to have higher levels of MP pollution (Piehl et al., 2018; Jin et al., 2022). These tiny, nearly invisible plastics can also infiltrate soil potentially affecting groundwater systems and human drinking water sources (Re, 2019; Sharma and Sharma, 2024). Runoff from agricultural fields, whether due to rainfall or irrigation, plays a crucial role in the migration of MPs from terrestrial to aquatic systems (Watts et al., 2014; Wang et al., 2020b; Ajith et al., 2021; Mukherjee et al., 2022). Thus, cost-effective solutions are needed to effectively remove MPs from agricultural runoff.
Biochar is obtained by carbonizing biomass in the absence of oxygen (Xiang et al., 2020; Jagadeesh and Sundaram, 2022). Biochar production involves a variety of thermochemical processes, including gasification and pyrolysis (Ambaye et al., 2020). Biochar typically possesses high porosity, large surface area-to-volume ratio, and active surface functional groups. Thus, it serves as an effective adsorbent for pollutant removal from aquatic systems (Lee et al., 2013; Abhishek et al., 2022; Jagadeesh and Sundaram, 2022; Sinha et al., 2022). Applying biochar to soil is an emergent practice and potentially cost-effective method to replenish nutrients, increase agricultural yields, remove contaminants, and sequester carbon (Kuppusamy et al., 2016; McCalla et al., 2022; Dong et al., 2023). Biochar has also been used to remove pollutants, including heavy metals, persistent organic compounds, and, most recently, MPs, from aquatic systems (Wang et al., 2020b; Siipola et al., 2020; Wang et al., 2021a; Magid et al., 2021; Dong et al., 2023; Sharma and Sharma, 2024). Biochar also shows potential in removing MPs in wastewater systems (Wang et al., 2020b; Hsieh et al., 2022).
Removal of MPs by biochar is based on adsorption to-and physical entrapment in-the biochar matrix by various mechanisms, including electrostatic attraction and surface complexation with functional groups like carboxyl groups (Beckingham and Ghosh, 2017; Ye et al., 2020; Wang et al., 2021a; Li et al., 2021; Kumar et al., 2023). Others have highlighted the significance of biochar pyrolyzing temperature, electrostatic interactions, hydrophobicity, specific surface area, pore-filling, and hydrogen bonding in the adsorption process (Magid et al., 2021). Due to the hydrophobic nature of many MPs, they are attracted to the adsorptive sites of biochar in the aqueous media (Beckingham and Ghosh, 2017; Wang et al., 2021a; Kumar et al., 2023). Although considerable knowledge has been gained regarding the adsorption of various chemicals on biochar (Gwenzi et al., 2017; Dai et al., 2019), research on the potential use of biochar in removing MPs is still in the early stages of development (Wang et al., 2020b). Others have recently shown that geomedia (e.g., a mixture of sand and compost materials) in bioretention systems can effectively remove MPs from stormwater runoff (Wolfand et al., 2023).
In this study, we collected and characterized MP pollution in runoff from a farm in the Mississippi Delta, an alluvial region in northwestern Mississippi, using micro-Fourier Transform Infrared (µ-FTIR) spectroscopy. We then passed runoff samples through two types of biochar in glass columns in the laboratory to quantify MP removal efficiency. Additionally, we examined the downward penetration of MPs in the biochar columns based on their size, shape, and type using fluorescent-labeled MPs. This study was not intended to provide a detailed analysis of the MP-biochar sorption mechanisms nor to capture all the effects of environmental conditions on those processes. Instead, the goal was to assess the extent to which MPs are retained in the biochar columns, where high retention rates and minimal penetration suggest that larger field studies are warranted. To our knowledge, this is the first study focused on characterizing MPs in agricultural runoff in the Mississippi Delta. By shedding light on this relatively unexplored aspect of MP pollution, we hope to lay the groundwork for larger-scale field studies aimed at developing effective management strategies for mitigating MP pollution in agricultural settings.
2 Materials and methods
2.1 Collection of agricultural runoff and determination of MPs by µ-FTIR
Triplicate samples of agricultural runoff were collected at two sites from a farm near Beasley Lake in the Mississippi Delta (Figure 1). The farm, like others in the region, typically rotates crops, primarily soybean, corn, and cotton, depending on economics and other factors. The samples were collected after a rain event on 12 May 2023 produced significant runoff. The nearest NOAA weather station (<10 km away) recorded 4.2 cm of rainfall on the day. Samples were collected directly into one quart (0.95 L) glass jars and transported to the University of Mississippi in a cooler on ice and stored in a refrigerator at ∼4°C until analysis.

Figure 1. Map showing the location of Beasley Lake in the Mississippi Delta (upper left), runoff sampling locations (BC and NSD) with decimal GPS coordinates (center), and photos of the runoff in ditches (right) and samples in glass Mason jars (lower left).
To quantify and characterize MPs in the runoff, we used the “one pot” method, where sample preparation steps occur in the same jar that the sample was collected in to minimize contamination and losses (Scircle et al., 2020), combined with µ-FTIR analyses for MP identification. Briefly, to isolate MPs, samples underwent digestion with Fenton’s reagent (to remove natural organic matter without damaging the plastics), followed by density separation using ZnCl2 (1.6 g/cm3) (to remove inorganic particles). The MPs were collected off the top layer of solution with a glass pipette before being filtered onto a 10 mm × 10 mm silicon filter (0.2 µm pores). Silicon filters were used as they are transparent in the mid-IR range. Spectra in this region correspond to the vibrational fingerprint of molecules in the sample and thus are commonly used for identifying chemical composition.
Particles on the filter were analyzed by µ-FTIR using a Bruker LUMOS II equipped with a liquid nitrogen-cooled MCT detector (Bruker Corp., Billeraca, MA, United States). An optical image with 8 × magnification was taken of the silicon filter to select particles for spectral analysis. Transmission spectra were then collected in point mode. Both sample and background scans were collected between 4000 and 650 cm-1 with 4 cm-1 resolution and 16 co-added scans. The background was collected from an area of the silicon filter without particles. Data was processed using OPUS 8.7.4 software. Particle chemical compositions were identified by comparing their spectrum to a series of polymer spectral libraries using the program’s Cluster ID function. These libraries include Bruker’s polymer libraries as well as FLOPP and FLOPP-e, free databases of FTIR spectra of virgin polymers and polymers sourced from the environment (De Frond et al., 2021). Only particles with a Hit Quality Index (HQI) of >200 were assigned to a polymer type. Plastic polymers that did not fall into one of these categories were classified as “other plastics,” while particles identified as other materials were classified as non-plastic and excluded from further analysis.
Because we used a Monel screen with ∼30 µm openings in the sample preparation process, we do not include any particles smaller than 30 µm in our results. To avoid plastic contamination during sample preparation, cotton laboratory coats dyed bright orange were worn, nitrile gloves were used, and all work was performed in a clean room. Glassware was thoroughly rinsed with milliQ water and heated at 450°C for 4 h before use, and samples were covered or sealed with aluminum foil until analysis.
2.2 Column study: Biochar and MPs
Sugarcane and pinewood biochar were pyrolyzed at temperatures of 500°C and 900°C, respectively. Bulk density of the sugarcane and pinewood biochars (when gently packed into the columns described below) were 0.2 g/cm3 and 0.4 g/cm3, respectively. A portion of the biochar was gently dry-sieved to assess particle size distribution. Particles of sugarcane biochar (formed at 500°C) and pinewood biochar (formed at 900°C) were also examined by field-emission scanning electron microscopy (SEM) (JSM-7200FLV, JEOL Ltd., Japan). Biochar particle size distributions and SEM images are presented in Section 3.1.
Characteristics of MPs used in the column study are provided in Table 1. The MPs include pristine polyethylene beads (Aldrich Chemical Co., St. Louis, MO) along with MPs generated from weathered plastic debris found on Sardis Lake Dam located in north-central Mississippi. The latter MPs were produced through cryogenic grinding using a cryo mill (Spex CertiPrep, Metuchen, NJ). Together the material represents three different shapes and seven different types of MPs commonly found in the environment.
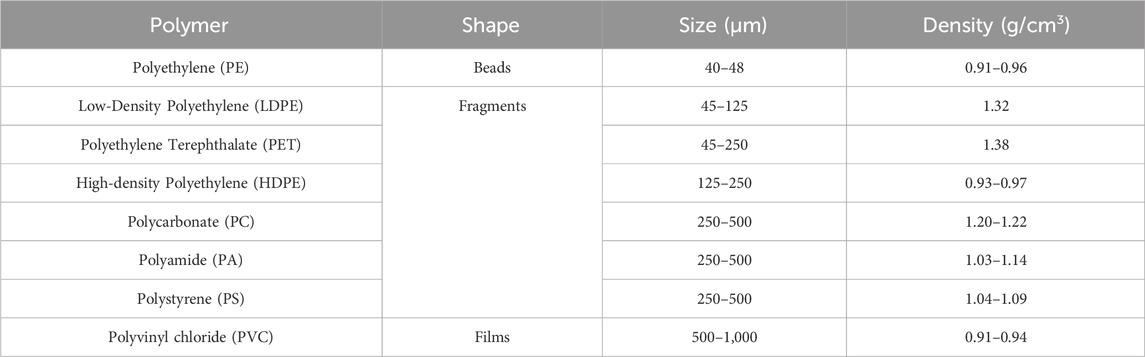
Table 1. Characteristics of Nile-red stained microplastics used in this study. All microplastics (MPs) stemmed from environmentally weathered plastic, except the PE beads, which were purchased (see text for details). MPs in the size ranges reported were obtained after cryomilling of naturally weathered plastics.
To facilitate observation and recovery of MPs, we stained them with Nile red dye which makes them fluoresce brightly under certain wavelengths of light (e.g., 420–510 nm) (Gao et al., 2022). Briefly, 10 mL of Nile red solution (10 μg/mL in methanol) were added to glass vials containing the different types of MPs. The mixture was diluted to 40 mL with Milli-Q water and vials were heated to 70°C for 3 h. After staining, MPs were recovered by filtration, washed with DI water, and dried.
2.3 Column study: Design and approach
The column study was conducted in four phases (Table 2). In phase I, we used 25-mm diameter glass burettes adding ∼50 g of biochar (filling ∼30 cm of the column) and topping it with ∼50 mg of Nile red stained MPs (Figure 2, left side). We then passed three column volumes of filtered (0.45 μm) water (∼1.2 L) through the columns. Although we observed little penetration (<3 cm) of MPs into either the pinewood or sugarcane columns (see Section 2.5 for fluorescence method), the glass burette only allowed for detection of MPs at the exterior (just inside the glass) and there was no way to remove the biochar from the column without disturbing the distribution of MPs.
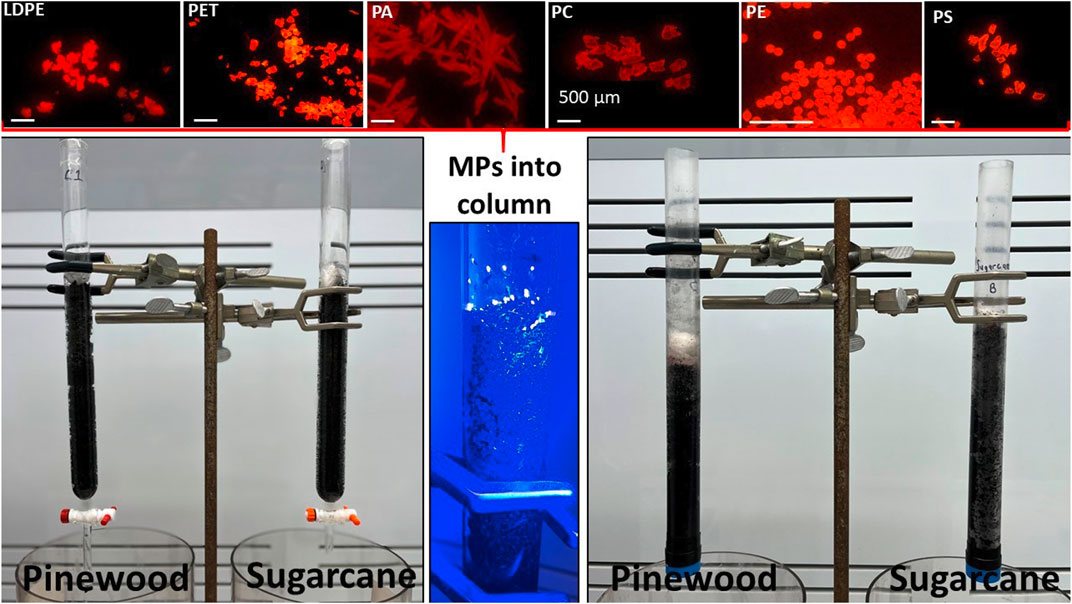
Figure 2. Nile red stained MPs fluorescing in top row from left to right: Low-Density Polyethylene (LDPE), Polyethylene Terephthalate (PET), Polyamide (PA), Polycarbonate (PC), Polyethylene (PE), and Polystyrene (PS). The MPs were introduced to the top of the biochar columns made from either glass burettes (left) or polypropylene tubes (right). The biochar height in the packed columns is ∼20 cm. While fluorescing MPs were observed near the exterior of the glass columns (center), the plastic tubes allowed removal and the sectioning of intact columns of biochar to better assess MP distribution.
In phase II, we used 22-mm diameter polypropylene tubes as columns (Figure 2, right side) because the biochar could be pushed out intact and subsequently divided into 1-cm sections using a metal spatula to carefully inspect each individual section for MPs. To prepare the plastic columns, we sealed one end of the tube with a plastic end cap and punctured it to give a 1–2 mm diameter hole. Glass wool was placed at the bottom of the tubes and 30 g of sugarcane and pinewood biochar were added. Columns (∼30 cm in height) were gently tapped a few times to settle the biochar resulting in a ∼20 cm packed biochar height in column. To prevent MPs from floating on the water added to the column, a glass-wool plug was added to cover the biochar after the MPs were introduced. Specifically, we added 50 mg of each type of Nile red-stained MPs to the top of each column (pinewood and sugarcane). We then passed 400 mL (∼1 column volume) of filtered water through the column vertically (gravity-fed) at 1–2 mL/min (gravity fed). Columns were allowed to dry for ∼24 h and the process was repeated nine times (10 wet-dry cycles). Biochar was then carefully pushed out of the tube with a wooden stick matching the inside diameter of the tube and divided into 1-cm fractions. Each fraction was spread-out on weighing paper and allowed to air-dry before a visual inspection by fluorescence and stereomicroscopy. Representative samples of pinewood and sugarcane were also analyzed by SEM.
In phase III, we repeated the phase II experiment, but this time added exactly 30 MPs of a single polymer to four separate pinewood biochar columns, one column each for PE beads, PA fragments, PC fragments, and LDPE films (Table 1). Using an exact number of MPs in individual columns allowed us to quantify individual MP distributions and assess recoveries.
In phase IV, we passed 1.9 L of agricultural runoff from both BC and NSD sites through pinewood biochar columns (0.95 L through each column, four columns in total). Pinewood biochar was selected for this phase due to its discernible presence of pores and surface features when compared to sugarcane biochar, as evidenced by SEM images (Section 3.2). This choice was made based on the superior structural characteristics of pinewood biochar, which are conducive to enhanced adsorption properties and efficacy in pollutant removal applications. We then analyzed the column effluent using μ-FTIR and compared the results to the sample collected from the same location but analyzed directly (without passing through biochar). This approach tested whether the biochar column retained MPs in agriculture runoff itself (rather than laboratory spiked MPs). The collection of runoff and the μ-FTIR method was detailed earlier.
2.4 Observation of MPs in column fractions by fluorescence and microscopy
Columns spiked with Nile-red stained MPs were examined using a Crime-lite® 82S (Foster + Freeman United States Inc., Ashburn, VA) operated in the 420–510 nm range. Laser safety glasses were used to block light from entering the Crime-lite operator’s eyes. For the recovery experiments, intensely fluorescing particles were counted in each 1-cm column fraction after the biochar was spread on weighing paper and allowed to air-dry. In addition, samples were visually inspected and further examined with a Stemi 508 Stereomicroscope equipped with an Axiocam 105 color digital camera (Carl Zeiss, Jena, Germany).
3 Results and discussion
3.1 Occurrence and characteristics of MPs in the agricultural runoff
Abundances of MPs in runoff from the farm (n = 2) ranged from 27–609 particles/L, with an average of 237 particles/L. These concentrations fall into the wide range reported for agricultural runoff in the literature (Cao et al., 2021). Most MPs were fragments (95.7%), followed by films (2.7%), and fibers (1.6%). While these MP morphologies are commonly encountered in agricultural samples, their relative distribution can vary depending on their sources (Zhang et al., 2020; Choi et al., 2021; Schell et al., 2022). Potential sources include the degradation of larger plastics (e.g., sheeting), application of sewage sludge abundant in fibers, and wind transport and atmospheric deposition from sources off site (Cai et al., 2017; Allen et al., 2019; Leonard et al., 2024). We found polyethylene (PE) as the dominant type of MP measured at the BC sample site, followed by polyarylamide (PARA), PA (nylon), and PVC (poly vinyl chloride) at 21%, 20%, 19%, and 11% respectively (Figure 3A). These plastics are used in agriculture for variety of purposes, including mulching and irrigation polypipe. At our study site, we observed remnants of PE plastic sheeting. PE is also the most used plastic worldwide and accounts for about a third of the total plastic market (Geyer et al., 2017). Other polymer types were also measured, albeit in low concentrations. At the NSD site, acrylonitrile butadiene styrene (ABS, 32%) was the dominant type of MP, followed by PU (21%), PE (18%) and PS (10%), respectively (Figure 3B). The different distribution of MP types in runoff collected at two different sites in the same agricultural field shows the potential for heterogeneity of MPs in field samples. However, for the purposes of this study, the presence and abundance of MPs in the runoff, regardless of their type, indicate suitability for both the column study and for future fieldwork evaluating methods for mitigating MP pollution from agriculture.
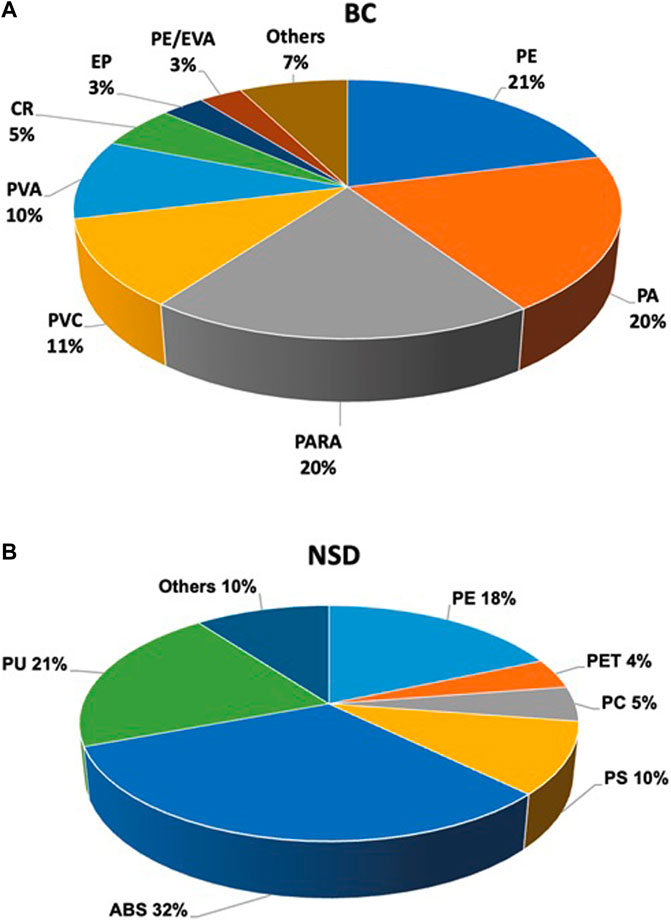
Figure 3. Distribution (%) of microplastics by type in BC (A) and NSD (B) sample sites (n = 2 per site) in agricultural runoff at a farm in the Mississippi Delta. Acronyms not previously defined: CR, crumb rubber; EVA, ethylene vinyl acetate; PVA, polyvinyl alcohol; EP, ethylene propylene rubber; PU, polyurethane; PS, polystyrene.
3.2 Biochar characteristics
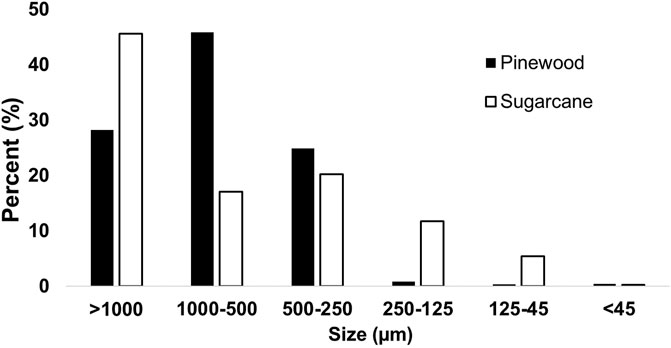
Approximately 70% of the pinewood biochar was between 250 and 1,000 μm, with most of the remaining biochar mass being >1 mm (Figure 4). In contrast, only ∼35% of the sugarcane biochar fell between 250 and 1,000 µm with a greater percentage in both the fines and larger particle fractions.
Both biochars exhibit a rough surface with pores ∼10 µm or less, with pinewood biochar showing more surface features (Figure 5). This is likely due the pinewood’s 400°C higher pyrolysis temperature given that increasing pyrolysis temperature increases biochar surface area and surface features that, in turn, provide more trapping capabilities for particles like MPs (Hsieh et al., 2022).
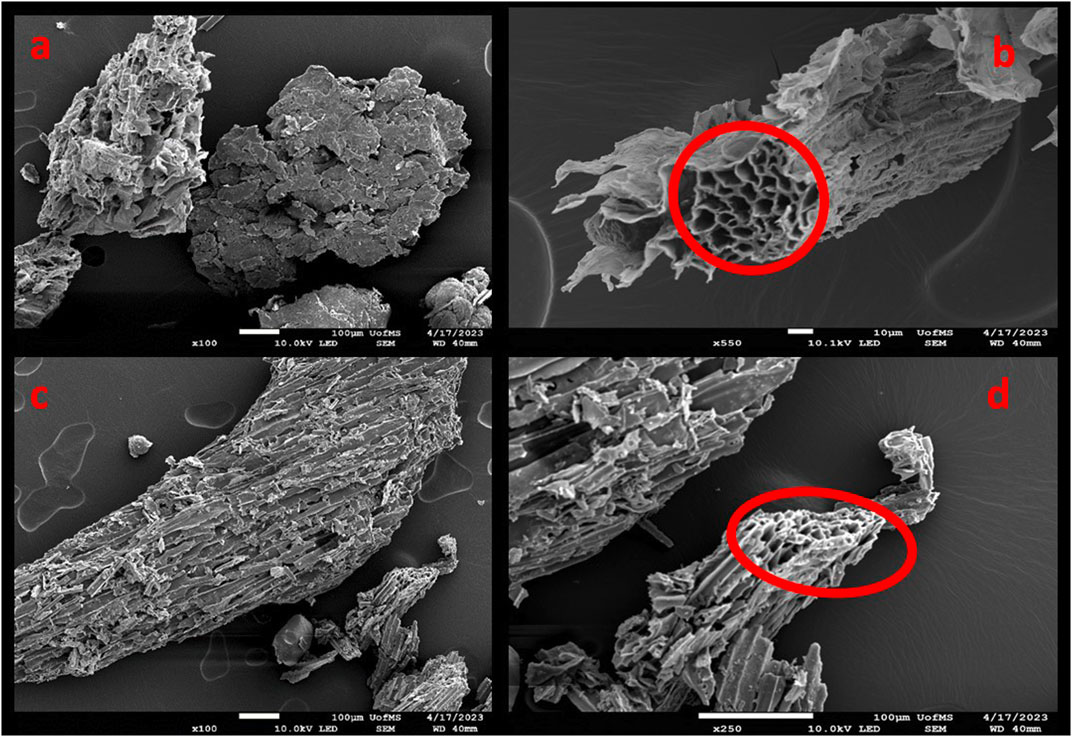
Figure 5. SEM images of sugarcane biochar (A,B) and pinewood biochar (C,D) showing rough surface features and pores (circled in red). Images at 100 × magnification (A,C), 250 × magnification (D), and 550 × magnification (B).
3.3 Effectiveness of biochar columns at trapping MPs
In phase II, we compared the depth of MP penetration into sugarcane and pinewood biochar columns by examining column fractions using microscopy (Figure 6). MPs penetrated deeper into the sugarcane biochar with particles detected in the seventh fraction (up to 7 cm deep), whereas MPs were unable to progress beyond the third fraction (up to 3 cm deep) in the pinewood biochar. This disparity may be attributed to the different biochar particle size distributions and how that affects column packing. Alternatively, these disparities may be due to different pyrolysis temperatures used to produce biochars and how higher temperatures increase surface area and surface features in biochar, or both. The pinewood biochar, generated at 900°C, had more surface features that promote effective trapping, entanglement, or adsorption of MPs in aqueous media compared to the sugarcane biochar formed at 500°C. In a batch sorption experiment, Magid et al. (2021) observed significant adsorption of PS nanoplastics by corncob biomass pyrolyzed at high temperatures. They noted that oxidized corncob biochar displayed notably higher adsorption, exceeding 90%. This was attributed to the presence of hydroxyl groups, which facilitated PS adsorption compared to biochar derived solely from corncob. Furthermore, the adsorption capacity of oxidized corncob biochar for PS increased with pyrolysis temperature. This phenomenon was attributed to various factors, including electrostatic interactions, hydrophobicity, specific surface area, pore-filling, and hydrogen bonding (Magid et al., 2021; Kumar et al., 2023). Similarly, Ganie et al. (2021) reported that biochar prepared at a pyrolysis temperature of 750°C exhibited increased surface area and pore abundance, resulting in drastically higher removal of nanoplastics (>99%) compared to biochar pyrolyzed at 550°C and 350°C, which showed comparatively lower nanoplastics sorption. Research has documented that the pyrolysis temperature used for biochar production significantly influences the aggregation of biochar colloids in aqueous environments (Yang et al., 2019).
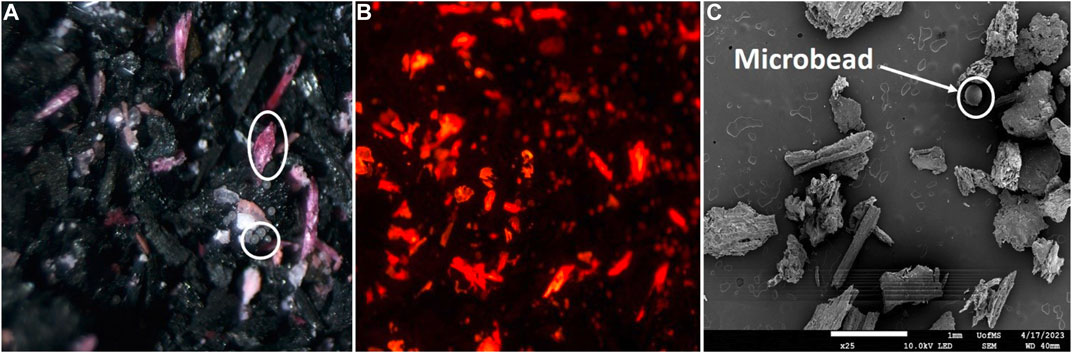
Figure 6. Representative microscopy images of Pinewood biochar column fraction containing microplastics (LDPE, PA, PET, PC, PS, PE, PVC): optical (A), fluorescence (B), and SEM (C). All images are from the same Pinewood biochar column fraction.
The difference between the penetration depth of MPs for the two biochars may also suggest that particles 250–1,000 µm are important in retention as the pinewood biochar had a substantially higher percentage of this “mid-sized” fraction (∼70%) compared to the sugarcane biochar (∼35%). Further, the sugarcane effluent was darker than the pinewood effluent suggesting that the fine biochar particles (present in greater proportion in the sugarcane) were being washed out of the columns, leaving behind a particle distribution skewed to larger sizes. This, in turn, would increase the size of flow paths for MPs to travel down the column, especially for the sugarcane.
Smaller biochar particles generally have a larger specific surface area, providing more active sites for interaction with MPs. This increased surface area may enhance the adsorption capacity of biochar, allowing it to effectively capture MPs from the water. Additionally, smaller biochar particles can penetrate deeper into the aqueous environment, reaching MP sources present throughout the water column. This phenomenon has also been observed in other studies with quartz sand particles (Sharma et al., 2014; Mao et al., 2020). However, the surface morphology of biochar is more complex than that of sand, and the shape and pyrolysis temperature of biochar particles also influence their interaction with MPs (Wang et al., 2020b). Irregularly shaped particles and higher pyrolysis temperatures provide more surface roughness and binding sites compared to spherical or homogeneous particles with low pyrolysis temperatures. In our study, the pinewood biochar is a mixture of both mid-sized and small-sized particles, and its relatively high pyrolysis temperature provides more binding sites for the MPs compared to sugarcane biochar. SEM images show that MPs were trapped or entangled within the pores or rough surface features of the pinewood biochar, which were more prevalent than on the sugarcane biochar. Fine particles (<∼250 μm) with low pyrolysis temperatures should be avoided in future experiments as they would likely be lost from filter socks adapted to house biochar in field experiments.
To better understand the factors affecting the retention of MPs in the biochar columns, we individually examined the penetration depth of several types of MPs (PE beads, PA fragments, PC fragments, and LDPE films), each consisting of 30 MPs. This approach allowed us to assess both MP recoveries and the influence of size and shape on their retention and penetration depth. In each case, we observed 100% recoveries for the spiked MPs.
We observed that the smaller MPs, such as PE beads, exhibited the greatest potential for movement. Additionally, we noted that MP migration depth increased with a greater number of wet-dry cycles. However, most of the particles were found within the upper 7 cm of the columns (Figure 7). This finding aligns with previous research suggesting enhanced vertical migration for smaller-sized MPs, particularly those with spherical shapes, and with increased wet-dry cycles (Kurlanda-Witek et al., 2015; O’Connor et al., 2019). Others have also reported that wet-dry cycles can significantly affect soil behavior and deposited colloids. Consequently, soils can absorb water, leading to swelling and changes in pore structure (Haque et al., 2017; Sharma, 2023). Kumari et al. (2017) observed that wet-dry cycles can influence the transport of contaminants, fertilizers, or pesticides in soil and groundwater. As wet-dry cycles increase, colloids carrying contaminants might be transported back and forth within the soil profile, potentially altering the distribution of contaminants and their availability for migration (Kumar et al., 2022). Similarly, Sharma (2023) showed in their study that wet-dry cycles can cause biochar colloids to undergo repeated cycles of absorption and desorption. As water is absorbed during wet cycles, biochar colloids might be transported deeper into the sediment. During dry cycles, the biochar colloids can become concentrated closer to the sediment surface, leading to redistribution, which can impact nutrient transport, contaminant migration, and soil fertility (Kumar et al., 2022; Sharma, 2023). This highlights the significance of size and shape in determining the penetration depth of MPs in pinewood biochar columns. These findings underscore the importance of considering such factors in the design and implementation of strategies aimed at mitigating MP pollution in agricultural runoff.
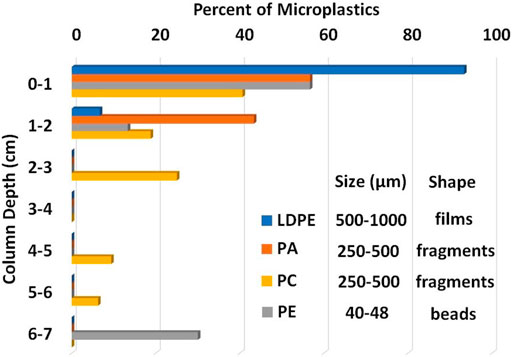
Figure 7. Depth of penetration of different types of MPs in pinewood biochar columns. Note deeper column depths (7–20 cm) are not shown as no particles were found in the biochar in that range.
A few MPs, however, did break through the biochar columns. Three particles of LDPE were found in the effluent, but interestingly their particle sizes were smaller (<500 µm) than those introduced to the column, suggesting possible breakdown during the experiment. In this case, the LDPE particles were films which can be more fragile compared to fragments and beads. For the other columns, there were three fragments of PC, two fragments of PA, and one PE bead in the effluent. It is possible the wet-dry cycles contributed to the enhanced migration by opening pathways. Regardless, the biochar showed ≥90% recovery rate of MPs from the aqueous solutions.
3.4 Effectiveness of biochar columns at removing MPs from agricultural runoff
Four samples of agricultural runoff were analyzed for MPs (two from each site, BC and NSD). Duplicate samples (from the same location) were passed through separate columns made of pinewood biochar. The BC samples averaged 416 MPs/L, but after passing duplicate samples through the column, an average of only 31 MPs were recovered, a reduction of 92.6%. Similarly, NSD samples averaged 157 MPs/L, but duplicates run through the column yielded an average of only 21 MPs, a reduction of 86.6%. Columns were effective for all types of MPs in the runoff (Figure 8). These findings suggest biochar used to remove contaminants from agricultural runoff may also be effective at removing MPs. Thus, subsequent field studies on the subject are prudent.
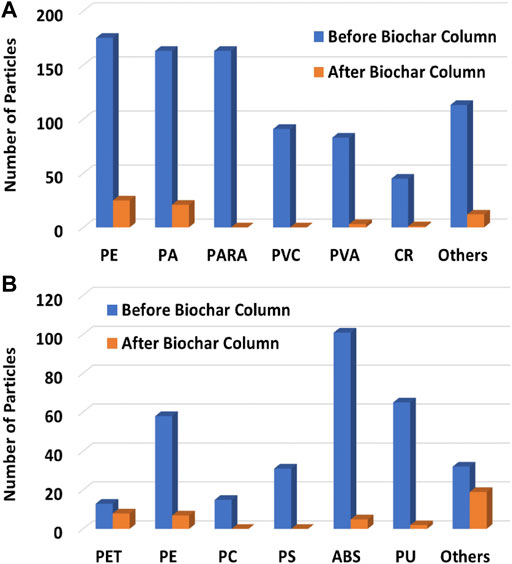
Figure 8. Plot showing the reduction in each type of microplastic after passing agricultural runoff samples through the pinewood biochar column (A) = BC, (B) = NSD.
3.5 Study limitations
While this study provides valuable insights into the potential of biochar as a cost-effective adsorbent for removing MPs from agricultural runoff, there are some limitations that should be acknowledged. First, the laboratory-based setup may not fully replicate real-world conditions, potentially leading to discrepancies between observed and actual MP removal efficiencies. Second, the study focused solely on the characterization and removal of MPs using biochar columns, neglecting other potential factors influencing MP transport and fate in agricultural environments. Also, the sample size and scope of the study were relatively small, limiting the generalizability of the findings to broader agricultural settings. Finally, the study did not explore the long-term effectiveness of biochar in mitigating MP pollution, nor did it consider potential secondary impacts of biochar application on soil health and ecosystem functioning. Addressing these limitations through larger-scale field studies with more diverse experimental conditions will be crucial for obtaining a comprehensive understanding of the efficacy and feasibility of biochar-based interventions for mitigating MP pollution in agricultural runoff from agricultural fields, where plastic is used extensively in agricultural practices, now referred to as plasticulture. Such studies could include, for example, biochar placed in filter socks situated at locations that funnel runoff.
4 Conclusion
This study examined the potential of biochar, an economical carbonaceous material, to capture MPs in agricultural runoff. For the first time, MPs were quantified and characterized in runoff from a farm in the Mississippi Delta. PE was the predominant type of MP, with PA, ABS, PU, PARA, PVC, PS, PVA, PC, PET, CR and PE/EVA, also detected. Most of the MPs were fragments, followed by films and fibers. Passing the runoff through a biochar columns in the laboratory removed between 86% and 92% of the MPs. Using fluorescence-labeled MPs we observed that smaller MPs penetrate more deeply into the columns and that MPs migrated further into sugarcane biochar compared to pinewood biochar, a difference attributed to the biochar’s surface characteristics and particle size distribution. By demonstrating the effectiveness of biochar, a readily available material, in capturing MPs from runoff, this study offers a promising solution for mitigating MP contamination in agricultural environments. The quantification and characterization of MPs in runoff from a farm in the Mississippi Delta provides valuable insights into the prevalence and composition of MP pollution in this agricultural setting. The identification of PE as the predominant type of MP, along with the detection of various other plastic types, underscores the diverse sources contributing to MP pollution in agricultural runoff. Overall, these findings underscore the potential of biochar to be a cost-effective adsorbent for the removal of MPs from agricultural runoff. Future research and implementation efforts should focus on scaling up biochar-based remediation techniques and integrating them into agricultural practices to effectively reduce MP contamination and safeguard environmental and human health.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
BO: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing. JC: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing–original draft, Writing–review and editing. KW: Methodology, Writing–review and editing. EH: Data curation, Writing–review and editing. TG: Investigation, Writing–review and editing. EB: Conceptualization, Data curation, Writing–review and editing. MM: Conceptualization, Data curation, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the National Science Foundation under grant MRI-2116597 and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under award number P20GM103460.
Acknowledgments
The US Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and, where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program (not all prohibited bases apply to all programs). USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this paper is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. We are grateful to the farm owners for allowing us to collect samples on their property. The authors would like to thank I. Lima (USDA-ARS), C. Griggs (USACE-EL) and B. Phillips (UC-Davis) for supplying research material for this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abhishek, K., Srivastava, A., Vimal, V., Gupta, A. K., Bhujbal, S. K., Biswas, J. K., et al. (2022). Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: a state-of-the-art review. Sci. Total Environ. 853, 158562. doi:10.1016/j.scitotenv.2022.158562
Ajith, M., Aswathi, M., Priyadarshini, E., and Rajamani, P. (2021). Recent innovations of nanotechnology in water treatment: a comprehensive review. Bioresour. Technol. 342, 126000. doi:10.1016/j.biortech.2021.126000
Allen, S., Allen, D., Phoenix, V. R., Le Roux, G., Dur´antez Jim´enez, P., Simonneau, A., et al. (2019). Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 12, 339–344. doi:10.1038/s41561-019-0335-5
Ambaye, T. G., Vaccari, M., van Hullebusch, E. D., Amrane, A., and Rtimi, S. (2020). Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 18, 3273–3294. doi:10.1007/s13762-020-03060-w
C. Arthur, J. Baker, and H. Bamford (2009). Proceedings of the international research workshop on the occurrence, effects and fate of micro-plastic marine debris (USA: NOAA Technical Memorandum NOS-OR&R-30).
Beckingham, B., and Ghosh, U. (2017). Differential bioavailability of polychlorinated biphenyls associated with environmental particles: microplastic in comparison to wood, coal and biochar. Environ. Pollut. 220, 150–158. doi:10.1016/j.envpol.2016.09.033
Cai, L., Wang, J., Peng, J., Tan, Z., Zhan, Z., Tan, X., et al. (2017). Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: preliminary research and first evidence. Environ. Sci. Pollut. Res. 24, 24928–24935. doi:10.1007/s11356-017-0116-x
Cao, L., Wu, D., Liu, P., Hu, W., Xu, L., Sun, Y., et al. (2021). Occurrence, distribution and affecting factors of microplastics in agricultural soils along the lower reaches of Yangtze River, China. Sci. Total Environ. 794, 148694. doi:10.1016/j.scitotenv.2021.148694
Cauwenberghe, L. V., Claessens, M., Vandegehuchte, M. B., and Janssen, C. R. (2015) Microplastics are taken up by mussels (Mytilus edulis) and lugworms (arenicola marina) living in natural habitats. Environ. Pollut. 199, 10–17. doi:10.1016/j.envpol.2015.01.008
Choi, Y. R., Kim, Y. N., Yoon, J. H., Dickinson, N., and Kim, K. H. (2021). Plastic contamination of forest, urban, and agricultural soils: a case study of Yeoju City in the Republic of Korea. J. Soils Sediments 21, 1962–1973. doi:10.1007/s11368-020-02759-0
Dai, Y., Zhang, N., Xing, C., Cui, Q., and Sun, Q. (2019). The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223, 12–27. doi:10.1016/j.chemosphere.2019.01.161
De Frond, H., Rubinoviz, R., and Rochman, C. M. (2021). Μatr-FTIR spectral libraries of plastic particles (FLOPP and FLOPP-e) for the analysis of microplastics. Anal. Chem. 93 (48), 15878–15885. doi:10.1021/acs.analchem.1c02549
Dong, M., He, L., Jiang, M., Zhu, Y., Wang, J., Gustave, W., et al. (2023). Biochar for the removal of emerging pollutants from aquatic systems: a review. Int. J. Environ. Res. Pub. Health. 20, 1679. doi:10.3390/ijerph20031679
Galgani, F., Hanke, G., Werner, S., and De Vrees, L. (2013). Marine litter within the European marine strategy framework directive. ICES J. Mar. Sci. 70 (6), 1055–1064. doi:10.1093/icesjms/fst122
Ganie, Z. A., Khandelwal, N., Tiwari, E., Singh, N., and Darbha, G. K. (2021). Biochar-facilitated remediation of nanoplastic contaminated water: effect of pyrolysis temperature induced surface modifications. J. Hazard. Mater. 417, 126096. doi:10.1016/j.jhazmat.2021.126096
Gao, Z., Wontor, K., and Cizdziel, J. V. (2022). Labeling microplastics with fluorescent dyes for detection, recovery, and degradation experiments. Molecules 27 (21), 7415. doi:10.3390/molecules27217415
Geyer, R., Jambeck, J. R., and Law, K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3 (7), e1700782. doi:10.1126/sciadv.1700782
Gwenzi, W., Chaukura, N., Noubactep, C., and Mukome, F. N. D. (2017). Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manage. 197, 732–749. doi:10.1016/j.jenvman.2017.03.087
Haque, M. E., Shen, C., Li, T., Chu, H., Wang, H., Li, Z., et al. (2017). Influence of biochar on deposition and release of clay colloids in saturated porous media. J. Environ. Qual. 46 (6), 1480–1488. doi:10.2134/jeq2017.06.0223
Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E., and Svendsen, C. (2017). Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 586, 127–141. doi:10.1016/j.scitotenv.2017.01.190
Hsieh, L., He, L., Zhang, M., Lv, W., Yang, K., and Tong, M. (2022). Addition of biochar as thin preamble layer into sand filtration columns could improve the microplastics removal from water. Water Res. 221, 118783. doi:10.1016/j.watres.2022.118783
Isari, E. A., Papaioannou, D., Kalavrouziotis, I. K., and Karapanagioti, H. K. (2021). Microplastics in agricultural soils: a case study in cultivation of watermelons and canning tomatoes. Water 13 (16), 2168. doi:10.3390/w13162168
Jagadeesh, N., and Sundaram, B. (2022). Adsorption of pollutants from wastewater by biochar: a review. J. Hazard. Mater. Adv. 100226, 100226. doi:10.1016/j.hazadv.2022.100226
Jansen, L., Henskens, M., and Hiemstra, F. (2019) Report on use of plastics in agriculture; schuttelaar and partners edition. The Netherland: Wageningen, 19.
Jin, T., Tang, J., Lyu, H., Wang, L., Gillmore, A. B., and Schaeffer, S. M. (2022). Activities of microplastics (MPs) in agricultural soil: a review of MPs pollution from the perspective of agricultural ecosystems. J. Agric. Food Chem. 70 (14), 4182–4201. doi:10.1021/acs.jafc.1c07849
Kumar, A., Bhattacharya, T., Shaikh, W. A., Mukherjee, S., and Sarkar, B. (2022). A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 4, 22. doi:10.1007/s42773-022-00148-z
Kumar, R., Verma, A., Rakib, M. R. J., Gupta, P. K., Sharma, P., Garg, A., et al. (2023). Adsorptive behavior of micro (nano) plastics through biochar: Co-existence, consequences, and challenges in contaminated ecosystems. Sci. Total Environ. 856, 159097. doi:10.1016/j.scitotenv.2022.159097
Kumari, K. G. I. D., Moldrup, P., Paradelo, M., Elsgaard, L., and de Jonge, L. W. (2017). Effects of biochar on dispersibility of colloids in agricultural soils. J. Environ. Qual. 46 (1), 143–152. doi:10.2134/jeq2016.08.0290
Kuppusamy, S., Thavamani, P., Megharaj, M., Venkateswarlu, K., and Naidu, R. (2016). Agronomic and remedial benefits and risks of applying biochar to soil: current knowledge and future research directions. Environ. Int. 87, 1–12. doi:10.1016/j.envint.2015.10.018
Kurlanda-Witek, H., Ngwenya, B. T., and Butler, I. B. (2015). The influence of biofilms on the mobility of bare and capped zinc oxide nanoparticles in saturated sand and glass beads. J. Contam. Hydrol. 179, 160–170. doi:10.1016/j.jconhyd.2015.06.009
Lee, Y., Eum, P. R. B., Ryu, C., Park, Y. K., Jung, J. H., and Hyun, S. (2013). Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour. Technol. 130, 345–350. doi:10.1016/j.biortech.2012.12.012
Leonard, J., Ravi, S., and Mohanty, S. K. (2024). Preferential emission of microplastics from biosolid-applied agricultural soils: field evidence and theoretical framework. Environ. Sci. Tech. Let. 11, 136–142. doi:10.1021/acs.estlett.3c00850
Li, L., Zhou, Q., Yin, N., Tu, C., and Luo, Y. (2019). Uptake and accumulation of microplastics in an edible plant. Chin. Sci. Bull. 64 (9), 928–934. doi:10.1360/n972018-00845
Li, W., Wufuer, R., Duo, J., Wang, S., Luo, Y., Zhang, D., et al. (2020). Microplastics in agricultural soils: extraction and characterization after different periods of polythene film mulching in an arid region. Sci. Total Environ. 749, 141420. doi:10.1016/j.scitotenv.2020.141420
Li, X., Jiang, X., Song, Y., and Chang, S. X. (2021). Coexistence of polyethylene microplastics and biochar increases ammonium sorption in an aqueous solution. J. Hazard. Mater. 405, 124260. doi:10.1016/j.jhazmat.2020.124260
Magid, A. S. I. A., Islam, M. S., Chen, Y., Weng, L., Li, J., Ma, J., et al. (2021). Enhanced adsorption of polystyrene nanoplastics (PSNPs) onto oxidized corncob biochar with high pyrolysis temperature. Sci. Total Environ. 784, 147115. doi:10.1016/j.scitotenv.2021.147115
Mao, M., Zheng, X., Chen, C., Zhao, K., Yan, C., Sharma, P., et al. (2020). Coupled effect of flow velocity and structural heterogeneity on transport and release of kaolinite colloids in saturated porous media. Environ. Sci. Pollut. Res. 27, 35065–35077. doi:10.1007/s11356-020-09806-w
McCalla, L. B., Phillips, B. M., Anderson, B. S., Voorhees, J. P., Siegler, K., Faulkenberry, K. R., et al. (2022). Effectiveness of a constructed wetland with carbon filtration in reducing pesticides associated with agricultural runoff. Arch. Environ. Contam. Toxicol. 82 (3), 317–329. doi:10.1007/s00244-021-00909-0
Mukherjee, A. G., Wanjari, U. R., Bradu, P., Patil, M., Biswas, A., Murali, R., et al. (2022). Elimination of MPs from the aquatic milieu: a dream to achieve. Chemosphere 135232. doi:10.1016/j.chemosphere.2022.135232
O’Connor, D., Pan, S., Shen, Z., Song, Y., Jin, Y., Wu, W. M., et al. (2019). Microplastics undergo accelerated vertical migration in sand soil due to small size and wet-dry cycles. Environ. Pollut. 249, 527–534. doi:10.1016/j.envpol.2019.03.092
Padervand, M., Lichtfouse, E., Robert, D., and Wang, C. (2020). Removal of microplastics from the environment. A review. Environ. Chem. Lett. 18, 807–828. doi:10.1007/s10311-020-00983-1
Piehl, S., Leibner, A., Löder, M. G., Dris, R., Bogner, C., and Laforsch, C. (2018). Identification and quantification of macro-and microplastics on an agricultural farmland. Sci. Rep. 8 (1), 17950. doi:10.1038/s41598-018-36172-y
Re, V. (2019). Shedding light on the invisible: addressing the potential for groundwater contamination by plastic microfibers. Hydrogeol. J. 27, 2719–2727. doi:10.1007/s10040-019-01998-x
Schell, T., Hurley, R., Buenaventura, N. T., Mauri, P. V., Nizzetto, L., Rico, A., et al. (2022). Fate of MPs in agricultural soils amended with sewage sludge: is surface water runoff a relevant environmental pathway? Environ. Pollut. 293, 118520. doi:10.1016/j.envpol.2021.118520
Scircle, A., Cizdziel, J. V., Missling, K., Li, L., and Vianello, A. (2020). Single-pot method for the collection and preparation of natural water for microplastic analyses: microplastics in the Mississippi river system during and after historic flooding. Environ. Toxicol. Chem. 39 (5), 986–995. doi:10.1002/etc.4698
Sharma, P. (2023). Biochar colloids mobilization by consecutive fluid displacement in unsaturated condition. Groundw. Sustain. Dev. 23, 101030. doi:10.1016/j.gsd.2023.101030
Sharma, P., Bao, D., and Fagerlund, F. (2014). Deposition and mobilization of functionalized multiwall carbon nanotubes in saturated porous media: effect of grain size, flow velocity and solution chemistry. Environ. earth Sci. 72, 3025–3035. doi:10.1007/s12665-014-3208-7
Sharma, P., and Sharma, P. (2024). Micro(nano)plastics: invisible compounds with a visible impact. F1000 Res. 13, 69. doi:10.12688/f1000research.142212.1
Sharma, P., Sharma, P., and Abhishek, K. (2024). Sampling, separation, and characterization methodology for quantification of microplastic from the environment. J. Hazard. Mater. Adv. 100416, 100416. doi:10.1016/j.hazadv.2024.100416
Siipola, V., Pflugmacher, S., Romar, H., Wendling, L., and Koukkari, P. (2020). Low-cost biochar adsorbents for water purification including microplastics removal. Appl. Sci. 10, 788. doi:10.3390/app10030788
Sinha, R., Kumar, R., Sharma, P., Kant, N., Shang, J., and Aminabhavi, T. M. (2022). Removal of hexavalent chromium via biochar-based adsorbents: state-of-the-art, challenges, and future perspectives. J. Environ. Manage. 317, 115356. doi:10.1016/j.jenvman.2022.115356
Tang, K. H. D. (2020). Effects of microplastics on agriculture: a mini-review. Asian J. Environ. Ecol. 13 (1), 1–9. doi:10.9734/ajee/2020/v13i130170
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., et al. (2004). Lost at sea: where is all the plastic? Science 304, 838. doi:10.1126/science.1094559
Wang, J., Sun, C., Huang, Q. X., Chi, Y., and Yan, J. H. (2021a). Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 419, 126486. doi:10.1016/j.jhazmat.2021.126486
Wang, T., Yu, C., Chu, Q., Wang, F., Lan, T., and Wang, J. (2020a). Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 244, 125491. doi:10.1016/j.chemosphere.2019.125491
Wang, Z., Sedighi, M., and Lea-Langton, A. (2020b). Filtration of microplastic spheres by biochar: removal efficiency and immobilisation mechanisms. Water Res. 184, 116165. doi:10.1016/j.watres.2020.116165
Watts, A. J., Lewis, C., Goodhead, R. M., Beckett, S. J., Moger, J., Tyler, C. R., et al. (2014). Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 48, 8823–8830. doi:10.1021/es501090e
Wolfand, J., Poor, C. J., Taylor, B. L., Morrow, E., Radke, A., and Diaz-Gunning, E. (2023). Microplastics: the occurrence in stormwater runoff and the effectiveness of bioretention systems for removal. J. Environ. Eng. 149 (11), 04023078. doi:10.1061/JOEEDU.EEENG-7285
Xiang, W., Zhang, X., Chen, J., Zou, W., He, F., Hu, X., et al. (2020). Biochar technology in wastewater treatment: a critical review. Chemosphere 252, 126539. doi:10.1016/j.chemosphere.2020.126539
Yang, W., Shang, J., Sharma, P., Li, B., Liu, K., and Flury, M. (2019). Colloidal stability and aggregation kinetics of biochar colloids: effects of pyrolysis temperature, cation type, and humic acid concentrations. Sci. Total Environ. 658, 1306–1315. doi:10.1016/j.scitotenv.2018.12.269
Ye, S., Cheng, M., Zeng, G., Tan, X., Wu, H., Liang, J., et al. (2020). Insights into catalytic removal and separation of attached metals from natural-aged microplastics by magnetic biochar activating oxidation process. Water Res. 179, 115876. doi:10.1016/j.watres.2020.115876
Zhang, K., Shi, H., Peng, J., Wang, Y., Xiong, X., Wu, C., et al. (2018). Microplastic pollution in China’s inland water systems: a review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 630, 1641–1653. doi:10.1016/j.scitotenv.2018.02.300
Keywords: microplastics, biochar, agricultural runoff, plasticulture, microplastic removal, column study, FTIR microscopy, fluorescence
Citation: Olubusoye BS, Cizdziel JV, Wontor K, Heinen E, Grandberry T, Bennett ER and Moore MT (2024) Removal of microplastics from agricultural runoff using biochar: a column feasibility study. Front. Environ. Sci. 12:1388606. doi: 10.3389/fenvs.2024.1388606
Received: 20 February 2024; Accepted: 21 May 2024;
Published: 10 June 2024.
Edited by:
Oladele Ogunseitan, University of California, Irvine, United StatesReviewed by:
Prabhakar Sharma, Nagaland University, IndiaJie Wang, China Agricultural University, China
Copyright © 2024 Olubusoye, Cizdziel, Wontor, Heinen, Grandberry, Bennett and Moore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James V. Cizdziel, Y2l6ZHppZWxAb2xlbWlzcy5lZHU=
 Boluwatife S. Olubusoye
Boluwatife S. Olubusoye James V. Cizdziel
James V. Cizdziel Kendall Wontor
Kendall Wontor Edward Heinen1
Edward Heinen1 Tony Grandberry
Tony Grandberry