- 1China National Offshore Oil Corporation (CNOOC) Research Institute Ltd., Beijing, China
- 2College of Marine Life Sciences, Ocean University of China, Qingdao, Shandong, China
- 3Marine Carbon Sink Research Center, Shandong Marine Resource and Environment Research Institute, Yantai, Shandong, China
The growing atmospheric mercury (Hg) emissions in China have raised ongoing concerns regarding contamination in marine fish. To better understand the pollution patterns and associated risks, we examined methylmercury (MeHg) content in demersal and pelagic fish from four commonly found families in three geographically distinct bays along the Chinese coast. We identified significant spatial variations in MeHg levels within the same fish family across regions. Specifically, fish collected from the Beibu Gulf in the South China Sea consistently exhibited significantly higher MeHg levels compared to those from the Laizhou Bay in the Northeast and/or Haizhou Bay in the East of China. In contrast, MeHg levels in fish collected from Haizhou Bay consistently remained the lowest. Within each region, we observed significantly higher MeHg concentrations in demersal species compared to pelagic species. This trend was particularly evident in fish species including bartail flathead (Platycephalus indicus), small-scale tongue sole (Cynoglossus microlepis) and greater lizardfish (Saurida tumbil) from the Beibu Gulf (0.50, 0.21, and 0.18 mg/kg dw, respectively), as well as bartail flathead and slender lizardfish (Saurida elongata) from Laizhou Bay (0.09 and 0.12 mg/kg dw, respectively). By comparison, MeHg content in silver pomfret (Pampus argenteus) from all three regions consistently remained relatively lower than in other species. Using target hazardous quotient (THQ) calculations, we estimated potential health risks in local populations associated with the consumption of the studied fish species. Our results showed a lack of apparent health risks to local residents, as all THQ values obtained from the three regions fell within the safe limits (0.02–0.94). However, it remains important to conduct additional assessments and spatiotemporal monitoring that encompass a broader range of species and regions.
1 Introduction
Mercury (Hg) is a ubiquitous and hazardous environmental contaminant, well recognized for its wide-ranging impact on ecosystems and human health (Clarkson, 1997; Boening, 2000). Various forms of Hg originating from human activities or natural geological processes can be introduced into the environment (Hammond, 1971), and undergo the methylation process and transformed into methylmercury (MeHg) (Weber, 1993; Regnell and Watras, 2019). Coastal marine sediments, rich in organic matter and microorganisms that support methylation, play a vital role in shaping methylmercury (MeHg) budgets within coastal regions and the marine food web (Hammerschmidt and Fitzgerald, 2004; Hollweg et al., 2009).
The formation and bioaccumulation of MeHg in the marine environment are influenced by a multitude of factors, such as sediment physicochemical property, organic carbon, bacterial activity, and plankton biomass, resulting in significant spatial heterogeneity (Chen et al., 2008; Zhang Y. et al., 2020). Therefore, assessing Hg levels across geographically distinct regions is essential for obtaining a comprehensive overview of distribution and accumulation patterns, and the better understand of environmental and ecological influences (Clayden et al., 2014; Barbosa et al., 2022; Médieu et al., 2022). However, inter-comparison between existing studies can be problematic due to differences in sample processing method and instrumental analysis (Chen et al., 2008).
National and international legislation efforts have been undertaken to address and mitigate Hg contamination, including the Minamata Convention (Kessler, 2013). However, MeHg remains a significant concern due to ongoing risks such as neurological damage and metabolic disruption associated with the consumption of marine fish in human populations from regions in the United States (Tollefson and Cordle, 1986; Liu et al., 2018), the European Union (Llull et al., 2017), as well as China and other regions (Cao et al., 2020; Basu et al., 2023). Furthermore, increased atmospheric Hg inputs from coal combustion in China (Wu et al., 2016) have been linked to elevated tuna Hg levels compared to other Pacific regions (Médieu et al., 2022).
In this study, we investigated MeHg concentrations in common fish species of the same family or suborder collected from three distinct coast bays in China: the Beibu Gulf (subtropical), Haizhou Bay (temperate), and Laizhou Bay (temperate). We examined regional differences in MeHg levels of the same species or close-related species within the same family, as well as across different species within each region. Given that all three studied bays were situated at urbanized regions, we hypothesized that regional differences in fish MeHg content may exist due primarily to different methylation rate across subtropical and temperate waters. Furthermore, species-specific differences in MeHg content were also expected, particularly between demersal and pelagic species, since coastal sediments are major sites for Hg methylation. We also evaluated the potential factors influencing these variations and assessed human health risks related to the consumption of these fish species for the local populations.
2 Materials and methods
2.1 Study area and sampling
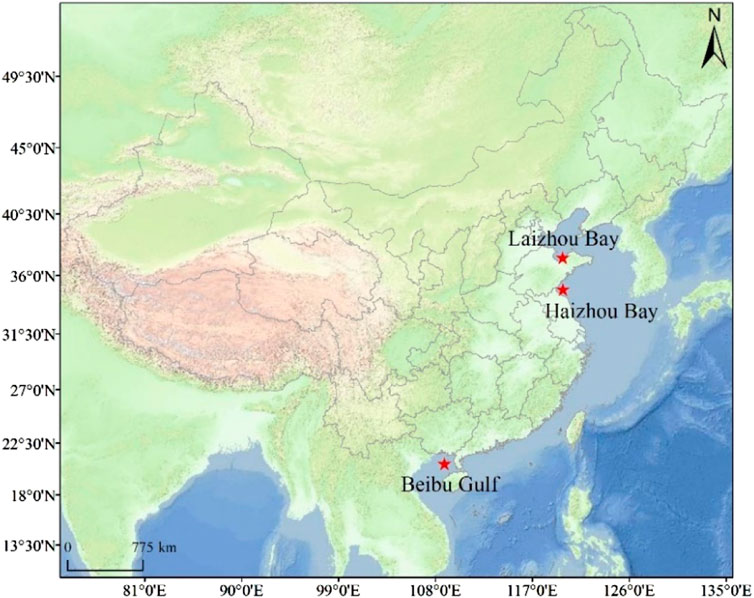
Fish samples were collected during fishery surveys conducted in the offshore area along the Chinese coast to facilitate regional comparisons of MeHg content in common fish species. We selected three specific coastal bays which are geographically distinct regions with vital ecological and economic significance but also face challenges related to pollution due to human activities (Laizhou Bay, Haizhou Bay, and the Beibu Gulf; Figure 1). Fish were collected using trawls in September and October of 2021 following the Animal Research and Ethics Committee of the Ocean University of China guidelines. The sampling period coincides with the rainy season in all three regions due to their location within the East Asian Monsoon system. All samples were immediately placed on ice until returned to the laboratory for further processing. In the laboratory, biological parameters including body length and weight were first measured and recorded, we then used acid-rinsed dissection tools to collect muscle samples from the center of the fish body after removing the skin. Samples were stored in polypropylene centrifuge tubes and kept at −20°C until further analyses. This study focused on identical or closely related species found in the three bays, totalling 96 fish from four families or suborders, i.e., Cynoglossidae, Platycephalidae, Stromateoidei, and Synodontidae. The species, sample size, weight and length are listed in Table 1.
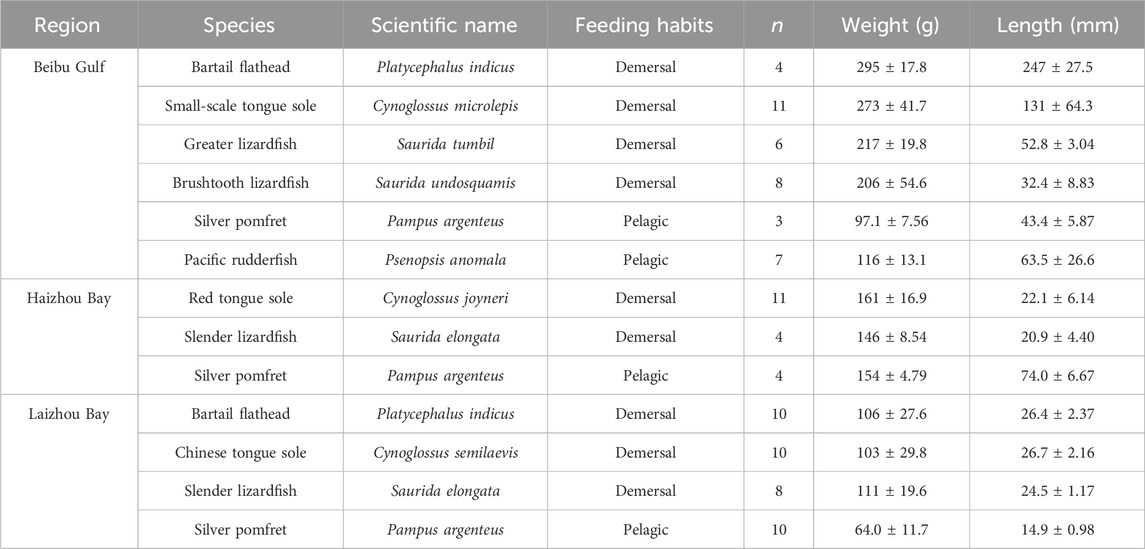
Table 1. Species, sample size (n), weight, and length of sampled fish species from the Beibu Gulf, Haizhou Bay, and Laizhou Bay.
2.2 Methylmercury analysis
Fish muscle samples were freeze-dried and homogenized using a tissue-homogenizer. Approximately 0.10–0.15 g of dried tissue sample were accurately weighed using an analytical balance (Sartorius, Germany), and placed into 50 mL polypropylene centrifuge tubes. Samples were digested using 20 mL of 30% nitric acid solution at 60°C overnight. After digestion, the samples were mixed with 10 mL ultrapure water and centrifugated, and a 200 μL of the supernatant was subsequently collected and transferred into a 50 mL pre-rinsed tube. This fractionation was adjusted to a pH of 6 using potassium hydroxide and sodium acetate solution. Finally, the mixture was added with ultrapure water and 50 μL of a derivatization reagent [NaB(C2H5)4], and left aside for 2 h at room temperature before instrumental analysis. The MeHg content was determined by using gas chromatography-cold vapor atomic fluorescence spectrometry (GC-CVAFS) (Polytech Instrumental Co., Ltd., Beijing, China). The analytical quality control was verified by the analysis of procedure blanks and a reference material, i.e., P43123B (fish powder, Guangzhou Puen Scientific Instrument Co., Ltd.). The average recovery of the reference material (85% ± 10%; n = 13) and the blank content (1.2 ± 1.0 ng; n = 13) were all within the acceptable range. All samples were detected at levels above the detection limit (0.06 pg). All concentrations are expressed in mg/kg dw (dry weight).
2.3 Data analysis
In the present study, all statistical analyses and plotting of the results were performed using R 4.2.0 (R Core Team, 2022). The distribution of MeHg concentrations were checked using Shapiro-Wilk test and Levene’s test, and the values were log-transformed for the analysis of variance (ANOVA; Base R). Tukey’s HSD test (package “stats”) was used for post hoc comparisons in order to assess potential differences in MeHg concentrations of the same fish family among different regions, as well as among different species within the same region. Additionally, the relationship between fish physical parameters (weight and length) and MeHg content across different species and regions were identified using linear mixed effect regression analysis (package “lme4”; Bates et al., 2014) with species and sampling region as random effects. Additionally, regional comparison was performed using weight-adjusted MeHg content, i.e., observed MeHg of each species within a region normalized by the average weight of the same species within that region (Braaten et al., 2017).
We further assessed the human health risks associated with consumption of the studied fish species using the Target Hazard Quotient (THQ) (EPA, 1989) based on the equation below:
where EF is exposure frequency (365 days/year); ED is total exposure duration (4 and 72 years for children and adults, respectively) (Yu et al., 2020); IR is ingestion rate of marine fish in China’s coastal populations (18 and 49 g/day for children and adults, respectively) (Wang et al., 2020); C is MeHg concentration in edible portion of the fish (mg/kg on a wet weight basis employing a conversion factor of 0.8; Li et al., 2023); RfD is oral reference dose for MeHg (1 × 10−4 mg/kg/day); BW is average body weight of an adult (25 and 65 kg for children and adults, respectively); and AT is average exposure time for non-carcinogens (EF × ED). THQ < 1.0 indicates no health risks, while THQ > 1.0 suggests potential risks from fish consumption.
3 Results and discussion
3.1 Regional differences in fish MeHg content
We observed notable variations in MeHg content among the examined fish species in various regions, with fish from the Beibu Gulf consistently exhibiting relatively high levels, while those from Haizhou Bay were consistently low (Figure 2). Specifically, significantly higher concentrations of MeHg were found in the demersal species, the small-scale tongue sole from the Beibu Gulf (0.21 mg/g) compared to the other two tongue sole species from Haizhou Bay and Laizhou Bay (both 0.04 mg/kg; P < 0.001). Similarly, significantly higher MeHg content was found between two Stromateoidei species, both pelagic (silver pomfret and Pacific rudderfish, 0.07 and 0.03 mg/kg, respectively) and two Synodontidae species, both demersal (greater lizardfish and brushtooth lizardfish, 0.18 and 0.14 mg/kg, respectively) from the Beibu Gulf compared to those from Haizhou Bay (silver pomfret, and slender lizardfish, 0.01 and 0.03 mg/kg, respectively; all P ≤ 0.034). Concentrations of MeHg in silver pomfret (0.04 mg/kg) and slender lizardfish (0.11 mg/kg) from Laizhou Bay were also significantly higher than Haizhou Bay (P < 0.001 and P = 0.046, respectively). Additionally, in bartail flathead, MeHg was significantly higher in those collected from the Beibu Gulf than Laizhou Bay (0.50 and 0.09 mg/kg, respectively; P < 0.001).
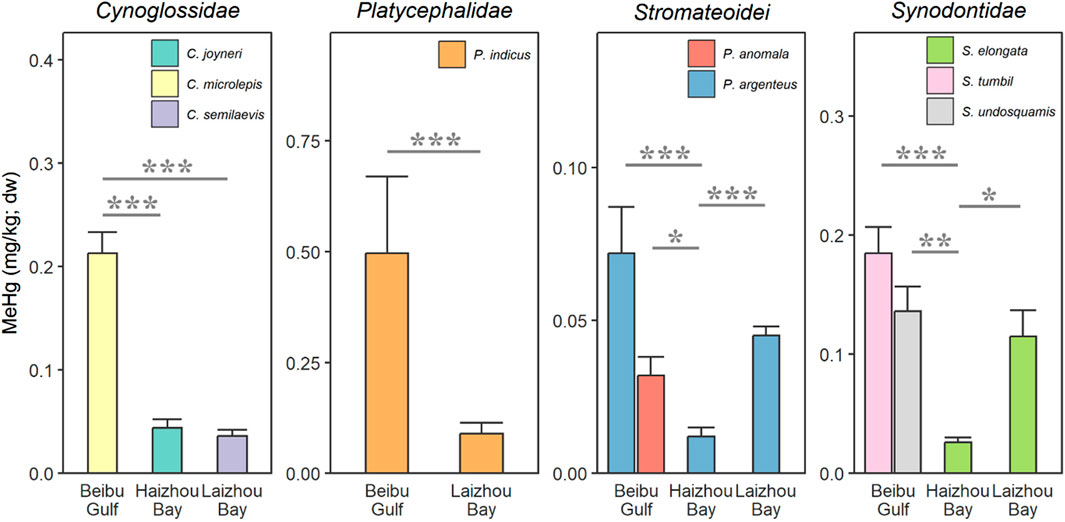
Figure 2. Spatial differences in fish muscle methylmercury (MeHg) content across different families collected from three coastal bays (Beibu Gulf, Laizhou Bay, and Haizhou Bay) in China. The two species from the suborder Stromateoidei are pelagic, while the remaining species are all demersal. Regional comparison for the Platycephalidae family was only conducted between Beibu Gulf and Laizhou Bay, as no available samples were obtained for the Haizhou Bay. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between sampling regions.
In general, the observed concentrations are consistent with those reported in fish collected along the coast of China. For instance, concentrations of MeHg in muscle of bartail flathead and slender lizardfish collected in Laizhou Bay were 0.07 and 0.10 mg/kg dw, respectively, in line with the present findings, whereas those reported for silver pomfret (0.10 mg/kg) was relatively higher compared to the present study (Cao et al., 2020). Such differences may be due to variations in trophic levels within the local food web in conjunction with different contamination profiles between the sediment and water bodies, and warrant in-depth investigation. Nevertheless, muscle MeHg content in silver pomfrets collected from multiple coastal cities of southeastern China was 0.05 mg/kg dw (Zhang et al., 2020). Pacific rudderfish from the Beibu Gulf also exhibited comparable muscle MeHg levels in those previously reported (0.04 ± 0.01 mg/kg dw) (Zhu et al., 2013) and the present study (0.03 ± 0.01 mg/kg dw). Additionally, the current MeHg levels in sole and flathead from the Beibu Gulf appear to be similar to MeHg levels found in muscle of flatfish and common sole (Solea solea), which were approximately 0.39 and 0.44 (mg/kg dw) from the Baltic Sea and France’s Atlantic Coast, respectively (Polak-Juszczak, 2017; Mille et al., 2021). However, the highest levels detected in the species of the present study were considerably lower than those reported for muscle content in common sole from the Western Mediterranean Sea, which were approximately 4.75 (mg/kg dw) (Llull et al., 2017). In fact, it has been shown that the Mediterranean waters exhibit a notable capacity for methylation and serve as a source for the nearby North Atlantic Ocean (Cossa et al., 2022).
The relatively higher MeHg concentrations in fish from the Beibu Gulf observed here compared to the other coastal bays may be associated with potentially stronger atmospheric Hg emission owing to biomass burning activities in the Indochina Peninsula (Sheu et al., 2013). Long-range atmospheric transport through the Asian Northeastern Monsoons in autumn may also contribute to elevated atmospheric Hg levels and its subsequent deposition in the northern South China Sea (Liu et al., 2016; Yuan et al., 2023). Furthermore, high precipitation rates, enhanced primary production and microbial activity could also have facilitated the speciation and bioaccumulation of MeHg (Kim et al., 2017; Zhang et al., 2020) in the subtropical Beibu Gulf compared to the other two temperate bays. Indeed, a systematic evaluation of Hg in Chinese coastal sediments has also demonstrated more elevated levels of Hg in sediments from the south coast than those from the east coast of China (Meng et al., 2019). In conjunction, MeHg production could also increase with decreasing latitude, which was primarily influenced by the elevated annual temperature (Dai et al., 2021).
It has been commonly found that MeHg contents correlate with fish size (Andersen and Depledge, 1997; Baeyens et al., 2003; Kehrig et al., 2008). The present study observed a significantly positive association between fish body weight and MeHg concentration, fish collected from the Beibu Gulf also appeared to be relatively heavier in comparison with those from the other regions (Figure 3). Nevertheless, except for bartail flathead (weight-adjusted MeHg: 0.27 and 0.14 mg/kg for Beibu Gulf and Laizhou Bay, respectively; P = 0.08), all regional differences in MeHg content detected here remained significant after adjusting body weight (P range: 0.017 to < 0.001). These results further highlight significant regional disparities and emphasize the need for continued monitoring and assessment to investigate the sources and pathways of MeHg. Moreover, future studies should incorporate fish species of the same sizes and a larger sample size to further assess regional differences fully controlling the size-effect. In addition, within each species, there was a significant positive correlation between body weight and MeHg concentrations for bartail flathead (collected in Laizhou Bay and Beibu Gulf), and a significant negative correlation for silver pomfret (collected in all three bays), whereas no association was found for slender lizardfish (collected in Haizhou Bay and Laizhou Bay; Supplementary Figure S1). These findings suggest that the weight-MeHg concentration relationship as well as the fish size effect may be species- and region-specific, and warrants in-depth revaluation. Additionally, further investigations into the occurrence and distribution of MeHg in the sediment environment of these coastal regions are desired to better assess the baseline contamination pattern of these respective environment and provide further insight into the transfer mechanisms of Hg along the food chain.
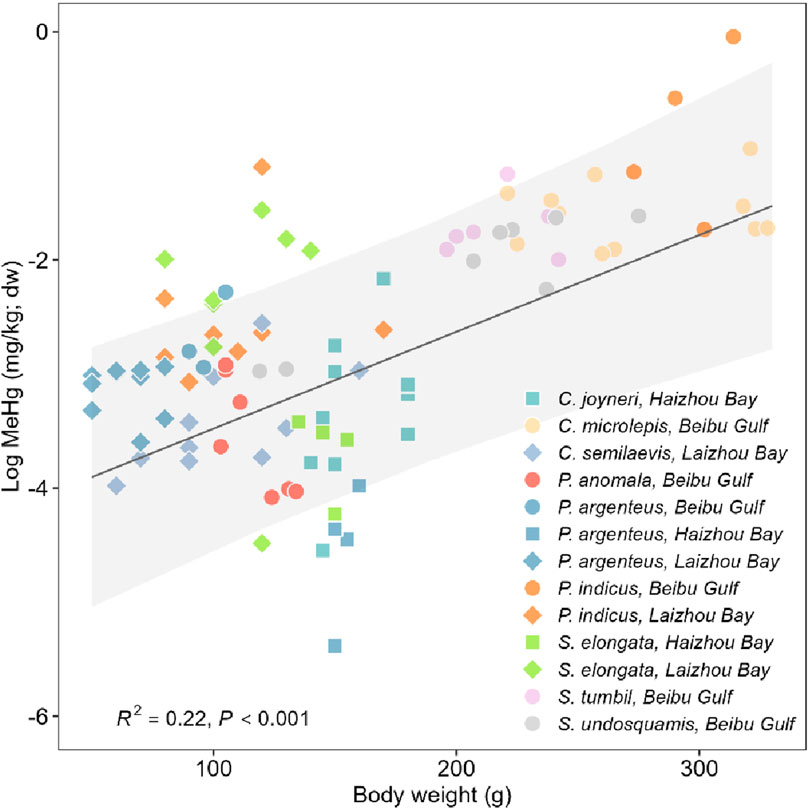
Figure 3. Regression between body weight and methylmercury (MeHg) concentrations in common fish species collected from three coastal bays (Beibu Gulf, Laizhou Bay, and Haizhou Bay) in China. The regression equation for fixed effect weight is: Log (MeHg) = 0.007*Weight − 4.026.
3.2 Species-specific variation in MeHg content
In the present study, we found significant differences across species within each population. Overall, the MeHg content in demersal fish including sole, flathead, and lizardfish showed relatively high concentrations. By comparison, pelagic fish including silver pomfret and Pacific rudderfish had relatively low MeHg concentrations (Figure 4). In the Beibu Gulf, MeHg levels in Pacific rudderfish were significantly lower compared to bartail flathead, small-scale tongue sole and brushtooth lizardfish (P < 0.001, P < 0.001, and P = 0.029, respectively). In Haizhou Bay, MeHg concentrations in silver pomfret was significantly lower than red tongue sole (P < 0.001). In Laizhou Bay, MeHg content in bartail flathead and slender lizard fish was significantly higher than Chinese tongue sole and/or silver pomfret (all P ≤ 0.049).
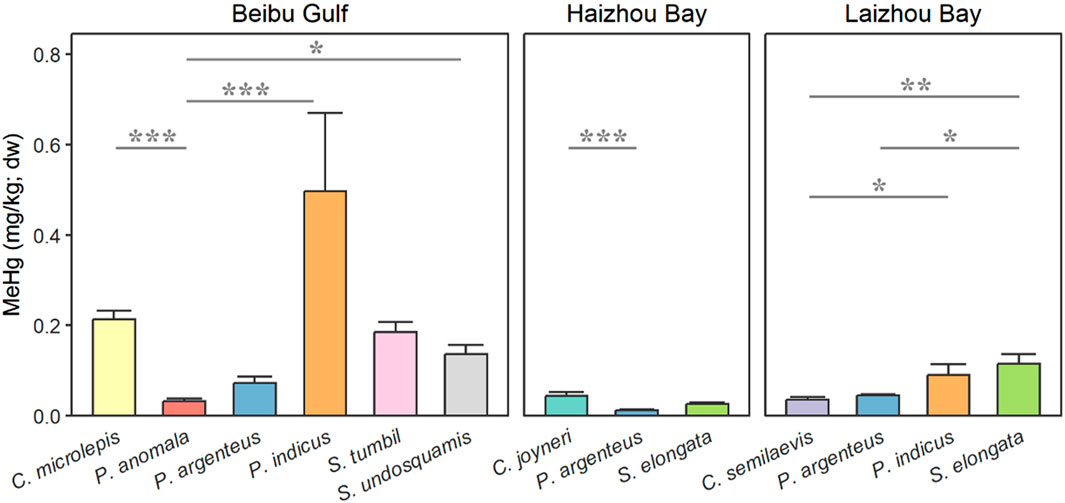
Figure 4. Species differences in the muscle methylmercury (MeHg) content of common fish collected from three coastal bays (Beibu Gulf, Laizhou Bay, and Haizhou Bay) in China. Both P. argenteus and P. anomala are pelagic, while the remaining species are all demersal. Asterisks indicate significant differences (*P < 0.05, **P < 0.01, ***P < 0.001) between species of the same region.
Coastal and estuarine sediments are recognized for their ability to produce MeHg at elevated levels, primarily because of the specific biogeochemical conditions present, such as the abundance of organic matter and sulfate (Chen et al., 2008). Subsequently, MeHg enters the food web through uptake by benthic invertebrate macrofauna, and eventually becomes incorporated into fish tissues from the ingestion of contaminated prey (Mason and Lawrence, 1999; Hammerschmidt and Fitzgerald, 2006). Likewise, various previous study have found considerable species-dependent variability showing demersal species had higher MeHg than pelagic ones (Storelli et al., 2003; Anual et al., 2018; Romero-Romero et al., 2022).
3.3 Human health risks of fish MeHg contamination across different regions
Considering that the fish species examined in this study also serve as important commercial resources for human consumption, it becomes imperative to further assess the potential health risks posed to humans regarding their MeHg content, particularly among the demersal species. In this study, the THQ calculations for various species in different regions indicated no apparent health risks associated with consuming the studied species for either children or adults in the Beibu Gulf, Haizhou Bay, and Laizhou Bay populations (Table 2). Nevertheless, it’s worth noting that the THQ values for both children and adults were notably elevated in the case of bartail flathead from the Beibu Gulf (close to 1.0). This aligns with the significantly higher MeHg concentrations observed in this fish from the Beibu Gulf compared to other species and other regions. Therefore, further monitoring and assessment are warranted.
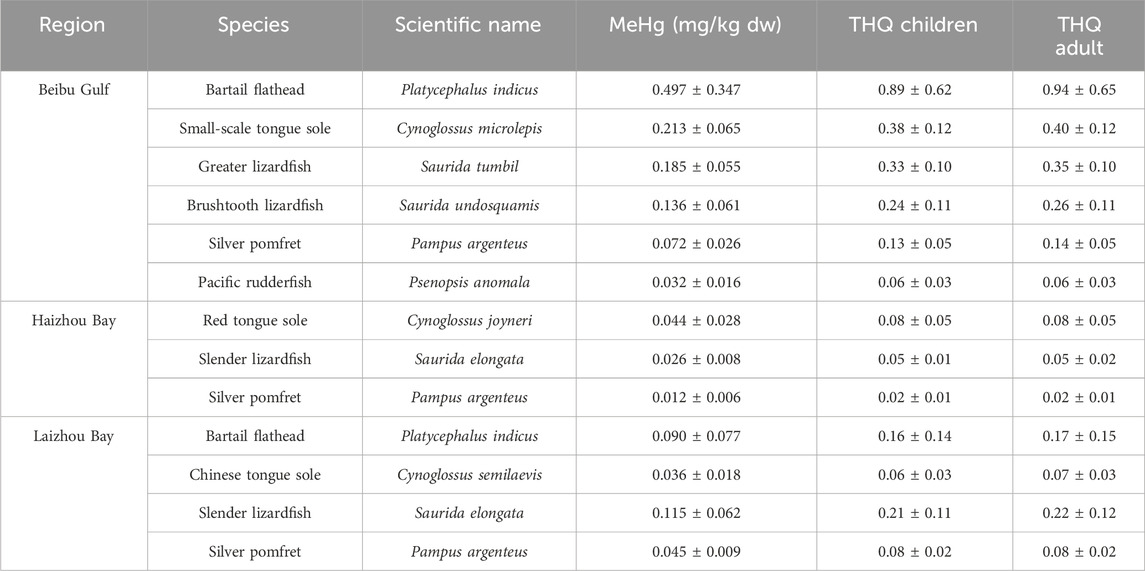
Table 2. Muscle MeHg content and target hazard quotient (THQ) in different fish species from the Beibu Gulf, Haizhou Bay, and Laizhou Bay.
Consistently, multiple prior studies have shown that Hg levels in aquatic products from the Beibu Gulf and various other regions along the Chinese coast are within safe limits, posing no apparent health risks (Gu et al., 2018; Zhao et al., 2018; Liu et al., 2019; Qin et al., 2021; Li et al., 2023). On the other hand, certain trace metals, notably arsenic (As), have been found to have elevated THQ values and potential health risks associated with the consumption of specific aquatic products from the Beibu Gulf (Wang et al., 2018; Yang et al., 2021). In the Laizhou Bay, in addition to Hg in predatory fish species (Cao et al., 2020), studies have indicated potential health risks associated with both As and cadmium (Cd) contamination in marine aquatic products (Jiao et al., 2021; Liu et al., 2022). Moreover, in the adjacent waters of the Beibu Gulf within Guangdong province, research has shown that As and Hg pose significantly higher health risk compared to other trace metals (Wang et al., 2023). Consequently, while the observed MeHg levels in fishes from the studied coastal areas are generally safe, continued monitoring of this and other contaminants in a suite of abiotic and biotic compartments is still necessary. Additionally, further studies are needed to understand the mechanisms underlying the varying methylation rates of inorganic Hg across coastal regions, as well as the uptake and accumulation in fish residing in different habitats.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because only some fish related data was used.
Author contributions
TL: Formal Analysis, Investigation, Resources, Writing–original draft. MA: Formal Analysis, Writing–review and editing. JC: Formal Analysis, Writing–review and editing. YL: Formal Analysis, Writing–review and editing. LC: Formal Analysis, Writing–review and editing. JL: Formal Analysis, Writing–review and editing. MZ: Conceptualization, Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The present study was made possible by funding from the Fundamental Research Funds for the Central Universities (202341007 and 202312013).
Conflict of interest
Authors TL and MA were employed by China National Offshore Oil Corporation (CNOOC) Research Institute Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1376882/full#supplementary-material
References
Andersen, J. L., and Depledge, M. H. (1997). A survey of total mercury and methylmercury in edible fish and invertebrates from Azorean waters. Mar. Environ. Res. 44, 331–350. doi:10.1016/s0141-1136(97)00011-1
Anual, Z. F., Maher, W., Krikowa, F., Hakim, L., Ahmad, N. I., and Foster, S. (2018). Mercury and risk assessment from consumption of crustaceans, cephalopods and fish from West Peninsular Malaysia. Microchem. J. 140, 214–221. doi:10.1016/j.microc.2018.04.024
Baeyens, W., Leermakers, M., Papina, T., Saprykin, A., Brion, N., Noyen, J., et al. (2003). Bioconcentration and biomagnification of mercury and methylmercury in North Sea and Scheldt estuary fish. Arch. Environ. Contam. Toxicol. 45, 498–508. doi:10.1007/s00244-003-2136-4
Barbosa, R. V., Point, D., Médieu, A., Allain, V., Gillikin, D. P., Couturier, L. I. E., et al. (2022). Mercury concentrations in tuna blood and muscle mirror seawater methylmercury in the Western and Central Pacific Ocean. Mar. Poll. Bull. 180, 113801. doi:10.1016/j.marpolbul.2022.113801
Basu, N., Bastiansz, A., Dórea, J. G., Fujimura, M., Horvat, M., Shroff, E., et al. (2023). Our evolved understanding of the human health risks of mercury. Ambio 52, 877–896. doi:10.1007/s13280-023-01831-6
Bates, D., Mächler, M., Bolker, B., and Walker, S., 2014. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823.
Boening, D. W. (2000). Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40, 1335–1351. doi:10.1016/s0045-6535(99)00283-0
Braaten, H. F. V., Åkerblom, S., de Wit, H., Skotte, G., Rask, M., Vuorenmaa, J., et al. (2017). Spatial and temporal trends of mercury in freshwater fish in Fennoscandia (1965-2015). NIVA-rapport.
Cao, L., Liu, J., Dou, S., and Huang, W. (2020). Biomagnification of methylmercury in a marine food web in Laizhou Bay (North China) and associated potential risks to public health. Mar. Poll. Bull. 150, 110762. doi:10.1016/j.marpolbul.2019.110762
Chen, C., Amirbahman, A., Fisher, N., Harding, G., Lamborg, C., Nacci, D., et al. (2008). Methylmercury in marine ecosystems: spatial patterns and processes of production, bioaccumulation, and biomagnification. EcoHealth 5, 399–408. doi:10.1007/s10393-008-0201-1
Clarkson, T. W. (1997). The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 34, 369–403. doi:10.3109/10408369708998098
Clayden, M. G., Kidd, K. A., Chételat, J., Hall, B. D., and Garcia, E. (2014). Environmental, geographic and trophic influences on methylmercury concentrations in macroinvertebrates from lakes and wetlands across Canada. Ecotoxicology 23, 273–284. doi:10.1007/s10646-013-1171-9
Cossa, D., Knoery, J., Bănaru, D., Harmelin-Vivien, M., Sonke, J. E., Hedgecock, I. M., et al. (2022). Mediterranean mercury assessment 2022: an updated budget, health consequences, and research perspectives. Environ. Sci. Technol. 56, 3840–3862. doi:10.1021/acs.est.1c03044
Dai, S.-S., Yang, Z., Tong, Y., Chen, L., Liu, S.-Y., Pan, R., et al. (2021). Global distribution and environmental drivers of methylmercury production in sediments. J. Hazard. Mater. 407, 124700. doi:10.1016/j.jhazmat.2020.124700
Epa, U. S. (1989). Assessing human health risks from chemically contaminated fish and shellfish: a Guidance Manual. Washington, D.C: U.S. Environmental Protection Agency.
Gu, Y.-G., Huang, H.-H., Liu, Y., Gong, X.-Y., and Liao, X.-L. (2018). Non-metric multidimensional scaling and human risks of heavy metal concentrations in wild marine organisms from the Maowei Sea, the Beibu Gulf, South China Sea. Environ. Toxicol. Pharmacol. 59, 119–124. doi:10.1016/j.etap.2018.03.002
Hammerschmidt, C. R., and Fitzgerald, W. F. (2004). Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ. Sci. Technol. 38, 1487–1495. doi:10.1021/es034528q
Hammerschmidt, C. R., and Fitzgerald, W. F. (2006). Bioaccumulation and trophic transfer of methylmercury in long island sound. Arch. Environ. Contam. Toxicol. 51, 416–424. doi:10.1007/s00244-005-0265-7
Hammond, A. L. (1971). Mercury in the environment: natural and human factors. Science 171, 788–789. doi:10.1126/science.171.3973.788
Hollweg, T. A., Gilmour, C. C., and Mason, R. P. (2009). Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar. Chem. 114, 86–101. doi:10.1016/j.marchem.2009.04.004
Jiao, Y., Yang, L., Kong, Z., Shao, L., Wang, G., Ren, X., et al. (2021). Evaluation of trace metals and rare earth elements in mantis shrimp Oratosquilla oratoria collected from Shandong Province, China, and its potential risks to human health. Mar. Poll. Bull. 162, 111815. doi:10.1016/j.marpolbul.2020.111815
Kehrig, H. d.A., Howard, B. M., and Malm, O. (2008). Methylmercury in a predatory fish (Cichla spp.) inhabiting the Brazilian Amazon. Environ. Pollut. 154, 68–76. doi:10.1016/j.envpol.2007.12.038
Kessler, R. (2013). The Minamata Convention on Mercury: a first step toward protecting future generations. Natl. Inst. Environ. Health Sci. 121, A304–A309. doi:10.1289/ehp.121-a304
Kim, H., Soerensen, A. L., Hur, J., Heimbürger, L.-E., Hahm, D., Rhee, T. S., et al. (2017). Methylmercury mass budgets and distribution characteristics in the Western Pacific Ocean. Environ. Sci. Technol. 51, 1186–1194. doi:10.1021/acs.est.6b04238
Li, J., Sun, J., Hu, W., Yan, M., and Kang, B. (2023). Exposure status, spatial variation, and health risk assessment of selected heavy metal(loid)s in common commercial fish species of the Beibu Gulf. Mar. Poll. Bull. 195, 115555. doi:10.1016/j.marpolbul.2023.115555
Liu, B., Lv, L., An, M., Wang, T., Li, M., and Yu, Y. (2022). Heavy metals in marine food web from Laizhou Bay, China: levels, trophic magnification, and health risk assessment. Sci. Total Environ. 841, 156818. doi:10.1016/j.scitotenv.2022.156818
Liu, H., Liu, G., Yuan, Z., Ge, M., Wang, S., Liu, Y., et al. (2019). Occurrence, potential health risk of heavy metals in aquatic organisms from Laizhou Bay, China. Mar. Poll. Bull. 140, 388–394. doi:10.1016/j.marpolbul.2019.01.067
Liu, M., Chen, L., Xie, D., Sun, J., He, Q., Cai, L., et al. (2016). Monsoon-driven transport of atmospheric mercury to the South China Sea from the Chinese mainland and Southeast Asia—observation of gaseous elemental mercury at a background station in South China. Environ. Sci. Pollut. R. 23, 21631–21640. doi:10.1007/s11356-016-7432-4
Liu, Y., Buchanan, S., Anderson, H. A., Xiao, Z., Persky, V., and Turyk, M. E. (2018). Association of methylmercury intake from seafood consumption and blood mercury level among the Asian and Non-Asian populations in the United States. Environ. Res. 160, 212–222. doi:10.1016/j.envres.2017.09.031
Llull, R. M., Garí, M., Canals, M., Rey-Maquieira, T., and Grimalt, J. O. (2017). Mercury concentrations in lean fish from the Western Mediterranean Sea: dietary exposure and risk assessment in the population of the Balearic Islands. Environ. Res. 158, 16–23. doi:10.1016/j.envres.2017.05.033
Mason, R. P., and Lawrence, A. L. (1999). Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environ. Toxicol. Chem. 18, 2438–2447. doi:10.1002/etc.5620181109
Médieu, A., Point, D., Itai, T., Angot, H., Buchanan, P. J., Allain, V., et al. (2022). Evidence that Pacific tuna mercury levels are driven by marine methylmercury production and anthropogenic inputs. Proc. Natl. Acad. Sci. 119, e2113032119. doi:10.1073/pnas.2113032119
Meng, M., Sun, R.-y., Liu, H.-w., Yu, B., Yin, Y.-g., Hu, L.-g., et al. (2019). An integrated model for input and migration of mercury in Chinese coastal sediments. Environ. Sci. Technol. 53, 2460–2471. doi:10.1021/acs.est.8b06329
Mille, T., Bisch, A., Caill-Milly, N., Cresson, P., Deborde, J., Gueux, A., et al. (2021). Distribution of mercury species in different tissues and trophic levels of commonly consumed fish species from the south Bay of Biscay (France). Mar. Poll. Bull. 166, 112172. doi:10.1016/j.marpolbul.2021.112172
Polak-Juszczak, L. (2017). Methylmercury in fish from the southern Baltic Sea and coastal lagoons as a function of species, size, and region. Toxicol. Ind. Health. 33, 503–511. doi:10.1177/0748233716685647
Qin, L.-y., Zhang, R.-c., Liang, Y.-d., Wu, L.-c., Zhang, Y.-j., Mu, Z.-l., et al. (2021). Concentrations and health risks of heavy metals in five major marketed marine bivalves from three coastal cities in Guangxi, China. Ecotoxicol. Environ. Saf. 223, 112562. doi:10.1016/j.ecoenv.2021.112562
R Core Team (2022). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Regnell, O., and Watras, C. J. (2019). Microbial mercury methylation in aquatic environments: a critical review of published field and laboratory studies. Environ. Sci. Technol. 53, 4–19. doi:10.1021/acs.est.8b02709
Romero-Romero, S., García-Ordiales, E., Roqueñí, N., and Acuña, J. L. (2022). Increase in mercury and methylmercury levels with depth in a fish assemblage. Chemosphere 292, 133445. doi:10.1016/j.chemosphere.2021.133445
Sheu, G.-R., Lin, N.-H., Lee, C.-T., Wang, J.-L., Chuang, M.-T., Wang, S.-H., et al. (2013). Distribution of atmospheric mercury in northern southeast asia and south China Sea during dongsha experiment. Atmos. Environ. 78, 174–183. doi:10.1016/j.atmosenv.2012.07.002
Storelli, M. M., Giacominelli-Stuffler, R., Storelli, A., D’Addabbo, R., Palermo, C., and Marcotrigiano, G. O. (2003). Survey of total mercury and methylmercury levels in edible fish from the Adriatic Sea. Food Addit. Contam. 20, 1114–1119. doi:10.1080/02652030310001622773
Tollefson, L., and Cordle, F. (1986). Methylmercury in fish: a review of residue levels, fish consumption and regulatory action in the United States. Environ. Health Perspect. 68, 203–208. doi:10.2307/3430265
Wang, Q., Chu, L., Peng, F., Li, J.-Y., Chen, H., and Jin, L. (2020). Contribution of aquatic products consumption to total human exposure to PAHs in Eastern China: the source matters. Environ. Pollut. 266, 115339. doi:10.1016/j.envpol.2020.115339
Wang, W., Lin, C., Wang, L., Jiang, R., Huang, H., Liu, Y., et al. (2023). Contamination, sources and health risks of potentially toxic elements in the coastal multimedia environment of South China. Sci. Total Environ. 862, 160735. doi:10.1016/j.scitotenv.2022.160735
Wang, X.-N., Gu, Y.-G., Wang, Z.-H., Ke, C.-L., and Mo, M.-S. (2018). Biological risk assessment of heavy metals in sediments and health risk assessment in bivalve mollusks from Kaozhouyang Bay, South China. Mar. Poll. Bull. 133, 312–319. doi:10.1016/j.marpolbul.2018.05.059
Weber, J. H. (1993). Review of possible paths for abiotic methylation of mercury (II) in the aquatic environment. Chemosphere 26, 2063–2077. doi:10.1016/0045-6535(93)90032-z
Wu, Q., Wang, S., Li, G., Liang, S., Lin, C.-J., Wang, Y., et al. (2016). Temporal trend and spatial distribution of speciated atmospheric mercury emissions in China during 1978–2014. Environ. Sci. Technol. 50, 13428–13435. doi:10.1021/acs.est.6b04308
Yang, C., Zhang, Z., Liu, Y., Shan, B., Yu, W., Li, H., et al. (2021). Heavy metal pollution and stable isotope ratios (δ13C and δ15N) in marine organisms from the Northern Beibu Gulf, South China Sea. Mar. Poll. Bull. 166, 112230. doi:10.1016/j.marpolbul.2021.112230
Yu, X., Khan, S., Khan, A., Tang, Y., Nunes, L. M., Yan, J., et al. (2020). Methyl mercury concentrations in seafood collected from Zhoushan Islands, Zhejiang, China, and their potential health risk for the fishing community: capsule: Methyl mercury in seafood causes potential health risk. Environ. Int. 137, 105420. doi:10.1016/j.envint.2019.105420
Yuan, C.-S., Chiang, K.-C., Yen, P.-H., Ceng, J.-H., Lee, C.-E., Du, I. C., et al. (2023). Long-range transport of atmospheric speciated mercury from the eastern waters of Taiwan Island to northern South China Sea. Environ. Pollut. 318, 120899. doi:10.1016/j.envpol.2022.120899
Zhang, H., Guo, C., Feng, H., Shen, Y., Wang, Y., Zeng, T., et al. (2020a). Total mercury, methylmercury, and selenium in aquatic products from coastal cities of China: distribution characteristics and risk assessment. Sci. Total Environ. 739, 140034. doi:10.1016/j.scitotenv.2020.140034
Zhang, Y., Soerensen, A. L., Schartup, A. T., and Sunderland, E. M. (2020b). A global model for methylmercury formation and uptake at the base of marine food webs. Glob. Biogeochem. Cycles 34, e2019GB006348. doi:10.1029/2019gb006348
Zhao, B., Wang, X., Jin, H., Feng, H., Shen, G., Cao, Y., et al. (2018). Spatiotemporal variation and potential risks of seven heavy metals in seawater, sediment, and seafood in Xiangshan Bay, China (2011–2016). Chemosphere 212, 1163–1171. doi:10.1016/j.chemosphere.2018.09.020
Keywords: heavy metals, aquatic products, neurotoxicity, potentially toxic elements, marine
Citation: Liu T, An M, Chen J, Liu Y, Chao L, Liu J and Zhang M (2024) Variations in methylmercury contamination levels and associated health risks in different fish species across three coastal bays in China. Front. Environ. Sci. 12:1376882. doi: 10.3389/fenvs.2024.1376882
Received: 26 January 2024; Accepted: 19 July 2024;
Published: 05 August 2024.
Edited by:
Oladele Ogunseitan, University of California, Irvine, United StatesReviewed by:
Inácio Pestana, Fluminense Federal University, BrazilRobert Peter Mason, University of Connecticut, United States
Martin F. Soto-Jimenez, National Autonomous University of Mexico, Mexico
Copyright © 2024 Liu, An, Chen, Liu, Chao, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingliang Zhang, emhhbmdtbDE5ODJAMTI2LmNvbQ==
 Tao Liu1
Tao Liu1 Mingliang Zhang
Mingliang Zhang