
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 27 July 2023
Sec. Toxicology, Pollution and the Environment
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1216868
This article is part of the Research Topic Environmental Micropollutants and Stressors: Environmental Impacts, Ecotoxicology, Risk Assessment, and Remediation View all 8 articles
 Cai Zhen-Zhen1
Cai Zhen-Zhen1 Zeng Jian1
Zeng Jian1 Yang Miao-Feng2
Yang Miao-Feng2 Lin Yong-Qing2
Lin Yong-Qing2 Zheng Hui-Dong2
Zheng Hui-Dong2 Luo Dong-Lian2
Luo Dong-Lian2 Jiang Shuang-Cheng2
Jiang Shuang-Cheng2 Guo Tuan-Yu1*
Guo Tuan-Yu1* Zheng Sheng-Hua2*
Zheng Sheng-Hua2*Cage farming is an important means of aquacultural production, while its potential environmental pollution needs to be further investigated. In this study, Dongshan Bay was taken as an example to investigate whether long-term cage farming in a semi-closed bay would cause environmental pollution via vertical distribution assessment. The four sediment cores (YB1, YB2, B1, and B2) were collected from two cage farming areas. Total nitrogen (TN), total organic carbon (TOC), and total phosphorus (TP) were measured. The results showed a negative correlation between TN and TOC and the sediment depth at four sampling sites, and TP was also negatively correlated with the depth at three sites. The average TN (1,405.8, 1,413.8, 1,115.7, and 936.1 mgkg−1) and TP (1,206, 1,141.6, 1,064.6, and 932.8 mgkg−1) values of the four sites were markedly higher than the safety level, with the indexes STN, STP, FF, and ON of the four sites indicating moderate to severe pollution of nitrogen and phosphorus. Particularly, the YB1 and YB2 sites in the area with lower current speed have more severe pollution. The C/N ratio uncovered that the organic matter (OM) might mainly derived from the phytoplankton and nonfibrous plants as a result of excessive fish feed and feces. Collectively, the results indicated that long-term (more than 10 years) cage farming activity in a semi-closed bay such as Dongshan Bay had a negative impact on the environmental quality. Despite limitations in sample size and the absence of stable isotopic analyses, this study enhances our understanding of environmental changes and endogenous pollution risks in shallow marine aquaculture areas. Moreover, it suggests practical approaches such as implementing alternative farming and fallowing periods, should be conducted to mitigate the pollution.
Coastal cultivation plays a crucial role in offering sustainable food production, as a means of addressing the global food shortage (Duarte et al., 2009). In particular, coastal cultivation of seafood such as fish, shrimp, and oysters can provide a range of important nutrients, including protein, omega-3 fatty acids, and vitamins and minerals (Lauritzen, 2021). A study by FAO (FAO, 2020) estimates that nearly half of the world’s fish consumption is derived from aquaculture, with coastal aquaculture being a significant contributor. Among the various coastal cultivations, cage farming that allows for the production of large quantities of seafood in a relatively small area is most important due to low cost, simple operation, and high productivity (Holmer and Kristensen, 1992).
However, intensive cage farming significantly impacts the coastal aquatic environment by generating large quantities of organic waste, including residual feed, fish meal, and fecal pellets (Pillay, 2004). The release and accumulation of these wastes disrupt the marine nutrient cycle, leading to nitrogen and phosphorus eutrophication, phytoplankton proliferation, and algae blooms. (Islam, 2005; Degefu et al., 2011). Moreover, the degradation of organic waste depletes oxygen levels in the water (Brown et al., 1987; Silvert and Sowles, 1996; Kaggwa et al., 2011). While cage farming on open coasts may have minimal effects on sediment and water pollution, long-term and intensive farming in semi-closed bays and enclosed water bodies can potentially cause eutrophication, oxygen depletion, changes in plankton communities, ocean acidification, and other forms of pollution (Zheng et al., 2013; Gao et al., 2021). These environmental impacts can harm farmed fish and reduce economic profitability. The current understanding of the relationship between cage farming and pollution is insufficient for effective management.
Sediment core refers to the vital transformation for biological and chemical pollutants, providing records of the inflow of artificial pollutants and environmental changes over time (Zhang et al., 2008; He et al., 2019). The waste products of aquaculture farming can influence sediment biogeochemistry via sediment metabolism stimulation, oxygen consumption increase, and redox depression, which may lead to nutrient enrichment and plankton community changes (Holby and Hall, 1994; Holmer et al., 2003; Heilskov et al., 2006). Carbon, nitrogen, and phosphorus are vital nutrients in aquatic environments, playing a crucial role in determining the ecological status of ecosystems (Elser et al., 2007; Conley et al., 2009). Measuring their concentrations in marine sediment aids in assessing the ecological condition of water bodies and identifying organic matter resources. (Dai et al., 2007; Goñi et al., 2014). The concentration of total organic carbon (TOC) of marine sediments was measured to investigate the impact of fish farming on Güllük Bay in Turkey (Kucuksezgin et al., 2021). The horizontal distribution profiles of TOC, total nitrogen (TN), and total phosphorus (TP) in the marine sediment of Shido Bay in Japan were investigated to uncover the impact of farming on the environment (Tada et al., 2023). In addition, the TP value was used to evaluate the effect of organic pollution in the seabed of the offshore fish farm in the coast of Manta (Sanz-Lazaro et al., 2021). Hence, sediment core analysis is an effective approach to assessing how fast and how severe the pollution is caused by aquaculture activities.
Dongshan Bay located in the southern Fujian Province of China (Chen et al., 2014), is a typical semi-enclosed bay in the northern South China Sea (Figure 1). The bay with a mouth about 5 km wide is of about 20 km long and 15 km wide, covering a sea area about 250 km2. As a key fishery and shell production area in China, Dongshan Bay features a subtropical maritime monsoon climate with a mean annual temperature of 21.0°C (Wu et al., 2017). This climate fosters the growth and reproduction of aquatic animals. In special, a dyke (Bachimen Dyke) has been built in 1961 to cut off the water exchange of Dongshan Bay to the other bay (Zhaoan Bay), making this area an excellent place for aquaculture farming due to the wide, deep, and slow water flow. Since then, the bay becomes one important base for aquaculture products such as large-scale cage farming (Xu et al., 2017; Pan et al., 2021a; Gao et al., 2021). The total aquaculture area and cage culture area in Dongshan Bay attain about 73.8 and 1.72 km2, respectively. The main farming species were prawns, fish, and shellfish (Pan et al., 2021a). The rapid development of the aquaculture industry in Dongshan Bay has led to negative impacts, especially in semi-enclosed bays where water circulation is weaker. Notably, in Bachimen Dyke, the crowding of net cages in these areas results in reduced dissolved oxygen levels, leading to the mass mortality of fish and significant economic losses. In this context, sediment core samples were collected in the cage farming area of Dongshan Bay for investigating the vertical distribution of TOC, TN and TP. In addition, the single indexes such as TN, TP, and the carbon-to-nitrogen ratio (C/N ratio) of organic matter were measured to evaluate the pollution. Based on these analyses, several approaches such as opening Bachimen dyke and alternating farming and fallowing period are suggested to mitigate the pollution. This study offers valuable insights into the environmental effects of cage farming and enhances our understanding of environmental changes and endogenous pollution risks in shallow marine aquaculture areas. Furthermore, it provides a foundation for the scientific deployment and rational layout of cage farming practices that minimize environmental impacts in Dongshan Bay.
Sample sites in Dongshan Bay are shown in Figure 1. YB1 (23.77370°N, 117.4146°E) and YB2 (23.77279°N, 117.4150°E) were set in Bachimen Dyke with a 2–3 m water depth. B1 (117.51737°N, 23.749916°E), and B2 (117.517236°N, 23.749806°E) were set in a more open areas of Dongshan Bay with a 6–7 m water depth. All the four sampling sites are with above 10 years of fish cage farming. As abovementioned, the Bachimen Dyke is a single current channel with a reducing water circulation. The flow velocity of YB1 and YB2 sampling sites are in the range of 0.3–0.5 m/s which are lower than B1 and B2 sites about 1.0–1.1 m/s.
Sediment core samples were collected within 100 cm depth in October 2013 at the sites of YB1, YB2, B1, and B2 in Bachimen Dyke of Dongshan Bay (Figure 1) with a self-gravity bottom sampler (IS2401-B20, WILDCO, United States). The sediment core samples were layered by 0–5, 5–10, 10–20, 20–40, 40–60, 60–80, and 80–100 cm, and stored in a cool and dark place and immediately transported to the laboratory. The sediments were dried and ground to 200 mesh with agate grinder and pestle, and stored in a desiccator at room temperature for later chemical analysis.
The sediment samples were acidified with 1 mol/L HCl to remove carbonates and subsequently rinsed with distilled water several times to neutralize the sample and freeze dried. The pretreatment procedure for total organic carbon (TOC) and total nitrogen (TN) followed Chinese GB/T 12,763.8-2007. The dried sample were set in air at least 24 h until the weight of sample is fixed. TOC and TN were measured with the dry combustion method using an elemental analyzer (Vario Microcube, Elementar, Germany). Total phosphorus (TP) was determined by ultraviolet-visible spectrometry (GENESYS 50, Thermo Scientific, United States) after sequential digestion as described (Kang et al., 2022). The concentrations of organic carbon and nitrogen were determined by calculating the organic matter content using an empirical conversion coefficient. It is important to note that variations in soil textures may introduce bias to the values.
The single evaluation index and the integrated pollution index methods were utilized to assess the TN and TP pollution levels of the sediment cores. The pollution index can be calculated using the following Eqs 1, 2.
where Si is a single evaluation index or standard index; Ci is the measured value of evaluation factor i (mg kg−1); Cs is the standard value of evaluation factor i (mg kg−1). FF is the comprehensive pollution index; F is the average value of all the pollution indices (the average of STN and STP); Fmax is the maximum single pollutant index (the maximum of STN and STP). Among them, the Cs values of TN and TP are 550 mg kg−1 and 600 mg kg−1, respectively, referring to the TN and TP contents that could cause the lowest level of ecological risk effects in sediments issued by the Ministry of Environment and Energy of Ontario, Canada (Persaud et al., 1993). The evaluation criteria for sediment nitrogen and phosphorus pollution are detailed in Table 1.
The organic pollution index (OI) method was introduced to evaluate the organic pollution status of sediments (Qiu et al., 2023), using the following Eqs 3, 4.
where ON is the organic nitrogen content (%) in the sediment, and TN is the total nitrogen content (%) in the sediment; OI is the organic pollution index, and TOC is the organic carbon content (%) in the sediment. The risk classifications of the sediments based on the OI and ON are listed in Table 1.
The raw data was processed using Microsoft Excel 2019 (Microsoft, Redmond, Washington, United States). Graphics were plotted using OriginPro 2019b (OriginLab, Northampton, MA, United States).
The sediment cores at YB1, YB2, B1, and B2 presented the declining trend of TN concentrations with the increase in sediment depth (Figure 2A). The vertical distribution of TN content at YB1 and YB2 were variable, where the peak appeared at 20 cm and 40 cm with the concentration of 1739.2 and 1,615.8 mg kg-1, respectively. There was a sharp increase in TN concentration with the sediment depth down to about 60 cm at B1 site with the highest concentration of 1,301.6 mg kg-1. For the B2 station, the TN concentration attained the peak (1,166.9 mg kg-1) at the 5 cm depth and then gradually decreased with the increased depth. The sediment cores at YB2, B1, and B2 showed a declining trend of TP concentrations with sediment depth, while the sediment cores at YB1 presented an increase with sediment depth (Figure 2B). The concentration of TP at YB1 gradually increased with slight fluctuation and arrived at the highest value (1,510.2 mg kg-1) at the 100 cm depth. The TP concentration at B1 peaked around 1923.2 mg kg-1 at the top 5 cm depth, then markedly decreased at the 10 cm depth, and finally increased at the 60 cm depth. In terms of the B2 site, the TP concentration variation was relatively stable throughout the whole core, and the peak TP occurred at 40 cm. However, no apparent change in the TP concentration was noticed in the sediment core of YB2. The sediment cores at YB1, YB2, B1, and B2 presented the declining trend of TOC concentrations with the increasing sediment depth (Figure 2C). YB1 had the highest concentrations of TOC among the four sites with an average concentration of 1.08%. There was a sharp increase in TOC concentration with sediment depth down to about 40 cm at YB1 and a slight increase with sediment depth down to about 60 cm at YB2 (Figure 2C). The B1 and B2 TOC concentrations gradually decreased along with the increase of the sediment depth. The YB1 and YB2 had the highest TOC concentration at 40–60 cm depth, while B1 and B2 showed the highest TOC value at the 10 cm depth. The lack of stable isotopic analyses and limited sample size hindered the clarity of the trends in TN, TP, and TOC contents. Hence, there is a possibility of statistical bias in the content values. While, the TN, TP, and TOC concentrations appeared an overall declining trend with the increased depth in most sediment cores of Dongshan Bay, and most of the peak values presented at upper-middle layers. These vertical profiles of TN, TP, and TOC in the cage farming area of Dongshan Bay are similar to the vertical distribution measured in Monastir Bay, Tunisia, Nao’ao Island, Alian Bay, Jiaozhou Bay, and Daya Bay, China, where mariculture activities were intensive (Huang et al., 2010; Liu et al., 2010; Damak et al., 2020; Pan et al., 2021b; Gu et al., 2021).
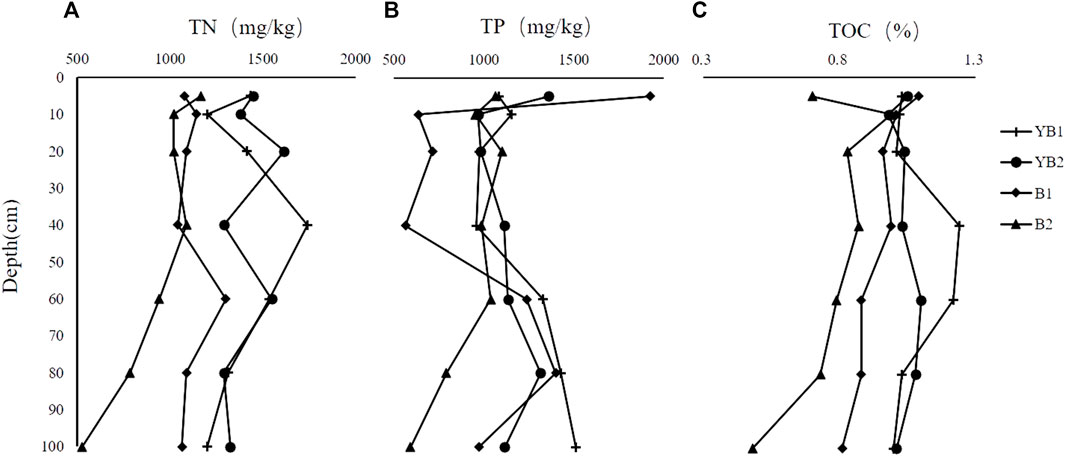
FIGURE 2. Variations of total nitrogen (TN), phosphorus (TP), and organic carbon (TOC) contents in sediment cores of Dongshan Bay, (A) TN, (B) TP, and (C) TOC.
Intensive cage farming introduces a significant amount of organic waste into the aquatic environment, much of which settles on the seafloor, resulting in high concentrations of TN, TP, and TOC in the sediment surface. The decreasing trend of these organic concentrations with depth indicates reduced mineralization and diagenesis. Microorganisms responsible for organic matter decomposition are predominantly found in the surface sediment layer and decrease with depth due to limited dissolved oxygen availability. This anoxic environment greatly weakens the microbial decomposition of organic matter. As a result, most of the vertical distribution profiles of TN, TP, and TOC among the four sites exhibit declining concentrations with increasing depth (Krom and Berner, 1981; Ji et al., 2020). The inverse trend observed in TP concentration at the YB1 site could be attributed to different hydrodynamics affecting the distribution of sinking materials or variations in feeding rates. For instance, densely clustered farming cages can impede bottom water flow, leading to hypoxia or even anoxia conditions. In such cases, active phosphorus cannot be fully oxidized and degraded, resulting in the accumulation of inert phosphorus and an increase in phosphorus content in the bottom sediment (He et al., 2015).
The average and range of the TN and TP concentration at the four sites were shown in Table 2. The overall TN concentration of sediment cores at YB1, YB2, B1, and B2 was in the range of 527.4–1739.2 mg kg-1, with the average value of 1,405.8, 1,413.8, 1,115.7, and 936.1 mgkg−1, respectively (Table 2). The overall TP concentration of sediment cores at YB1, YB2, B1, and B2 was in the range of 592.8–1925.2 mg kg-1, with the average value of 1,206, 1,141.6, 1,064.6, 932.8 mgkg−1, respectively (Table 2). The overall TOC concentration of sediment cores at YB1, YB2, B1, and B2 was in the range of 0.48–1.24%, with the average TOC concentration of 1.08%, 1.04%, 0.95%, and 0.77%, respectively (Table 2). The results indicate that the average TN, TP, and TOC contents at the YB1 and YB2 sites were higher compared to the B1 and B2 sites, which experienced more intense flow velocity. This suggests that cage farming in areas with limited water exchange is more prone to organic matter accumulation. Comparing with other areas, it has been reported that the TN, TP, and TOC concentration of sediments in coastal areas without mariculture activities in Daya Bay, China, was in the range of 650.2–1,327.2 (904.5 ± 81.2) mg·kg-1, 343.2–471.9 (406.3 ± 38.7) mg·kg-1 and 0.69–0.95 (0.82 ± 0.10) %, respectively (Huang et al., 2010). Another observation showed that the TN and TOC concentration in uncultured areas of the Yellow Sea, China were in the range of 0.03–0.08% and 0.34–0.77% (Pan et al., 2021b). The measured average and range of TN, TP, and TOC concentration in the cage farming area of Dongshan Bay were higher than those in the uncultured coastal areas. The higher concentration of organic matter at the upper-middle layers of sediment cores in these areas could be ascribed to the excessive nutrients (nitrogen, phosphorus, carbon) input from mariculture activity. This may be a result of the high density of aquaculture facilities (e.g., cages) and large amounts of organisms (fishes) that can evidently reduce the hydrodynamics of cage farming areas in Dongshan Bay, leading to a higher deposition ratio and higher organic matter concentration.
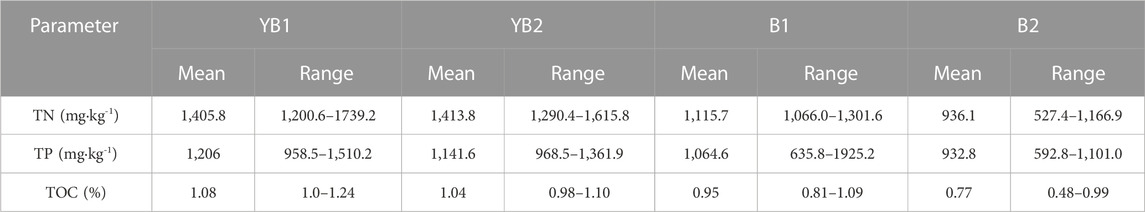
TABLE 2. The total nitrogen (TN), phosphorus (TP) and organic carbon (TOC) contents in core sediments.
According to the sediment nutrient quality standard of Ontario, Canada (Persaud et al., 1993), the average TN (1,405.8, 1,413.8, 1,115.7, and 936.1 mgkg−1) and TP (1,206, 1,141.6, 1,064.6 and 932.8 mgkg−1) value of YB1, YB2, B1, and B2 sites mentioned in Table 2 were far higher than the safety level (TN ≤ 550 mgkg−1, TP ≤ 600 mgkg−1). The average TN single index (STN) of YB1, YB2, B1, and B2 were 2.55, 2.57, 2.03, and 1.70, respectively (Table 3). Almost all the sediment cores were heavily polluted, except for B2 which was moderately polluted with TN. Among all layers of four sediment cores, 60.71% were heavily polluted and 39.28% were moderately polluted with TN. The average TP single index (STP) of YB1, YB2, B1, and B2 was 2.01, 1.90, 1.99, and 1.55, respectively (Table 3), which all overreached the threshold value of severe pollution of TP. Among all the layered sediment samples, 85.72% were heavily polluted and 14.28% were moderately polluted with TP. The average comprehensive pollution index (FF) of nitrogen and phosphorus were 2.72, 2.61, 2.45, and 1.87 for YB1, YB2, B1, and B2, respectively (Table 3). All the sediment cores were polluted, and YB1, YB2, and B1 were severely polluted. The mean ON index in YB1 and YB2 was 0.13%, which was more than the threshold of severe pollution. The mean ON index in the B1 and B2 sites was 0.1% and 0.09%, respectively, indicating a moderate pollution. The range of the average OI index in the four sites was 0.07–0.15, suggesting a slight organic pollution. Taken together, the sediment cores of four sites in caging farming area were all with heavy nitrogen and phosphorus pollution. The pollution risk is more severe in YB1 and YB2 sites which located at the cage farming area of Bachimen Dyke. As in Table 4, compared with the nitrogen and phosphorus pollution of sediment in cage farming areas along the coast, the TN and TP pollution of Dongshan Bay was relatively serious. The range of STN of Dongshan Bay was higher than that of Sansha Bay in China (Wei et al., 2016) and Tanabe Bay in Japan (Uede, 2007), but lower than that of Daya Bay (Huang et al., 2010) and Tangdao Bay (Jiang et al., 2007) in China and Shido Bay in Japan. The range of STP of Dongshan Bay was higher than that of Sansha Bay and Tanabe Bay, while lower than that of Daya Bay and Shido Bay.
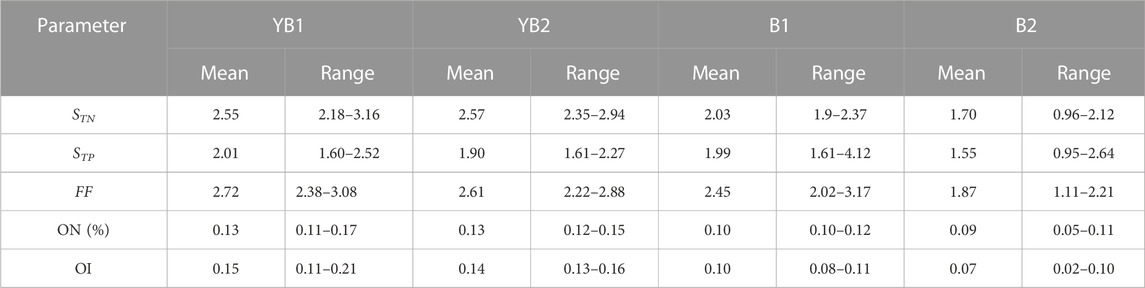
TABLE 3. Risk assessment of nitrogen and phosphorus pollution index in sediment cores of Dongshan Bay.
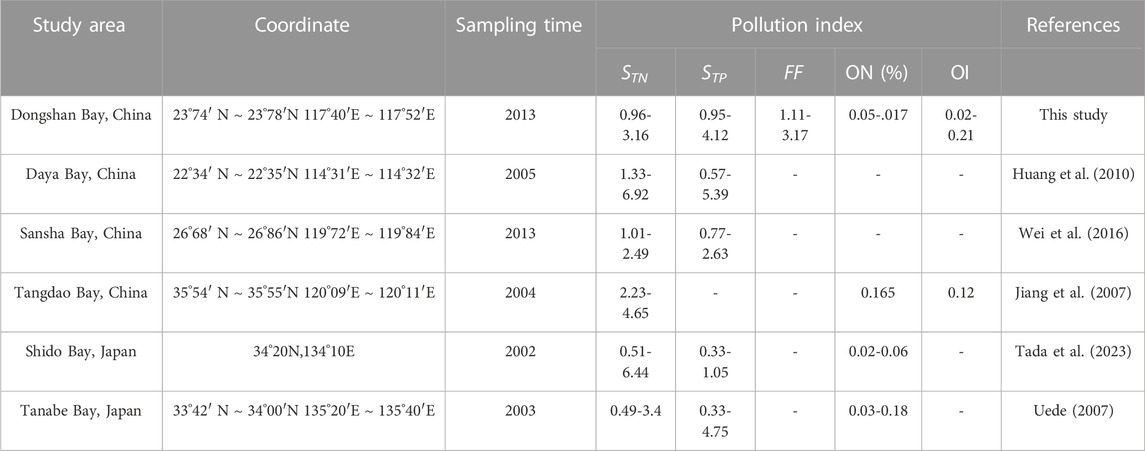
TABLE 4. Comparison of nitrogen and phosphorus pollution indexes of sediments in different cage farming areas.
Excessive discharge of organic matter (carbon, nitrogen, and phosphorus) often occurs as a result of prolonged or intensive aquaculture activities, primarily due to feed and fecal pellet deposition. In coastal areas with slow current speeds, organic waste sinks rapidly, leading to the immediate sedimentation of nutrients in the vicinity of net cages and causing eutrophication. Eutrophication, driven by nitrogen and phosphorus, can trigger excessive plankton growth and result in oxygen-depleted environments, adversely affecting aquatic animal health. Over time, the impact extends beyond the immediate vicinity of the cage farming area, affecting plankton communities, biodiversity, and secondary production on a regional scale. Intensive fish farming contributes to this through the anoxia of the aquatic environment and nutrient enrichment. (Holmer, 2010). For example, salmon farming areas in Norway and Chile have experienced reduced biodiversity due to elevated phosphorus content, while sea bream farming in the Mediterranean region has seen decreased species richness and abundance due to increased organic matter and phosphorus levels (Maldonado et al., 2005; Kutti et al., 2007a; Kutti et al., 2007b; Holmer, 2010; Soto et al., 2019). Occasional occurrences of hundreds of dead fish and red tides in the cage farming area of Bachimen Dyke serve as visible indicators of pollution surpassing acceptable levels (Committee China Bays Annuals Committee, 1994). This observation is further supported by the significant presence of heavy organic matter pollution in our study results. Therefore, it is imperative to mitigate the organic pollution risk and manage the cage farming in a sustainable way. One practical approach to mitigate organic pollution is opening Bachimen Dyke and enhance water exchange, as this facilitates the dispersion and dilution of pollutants. Also, the sustainable production of cage farming relies on the scientific deployment and rational layout of farming operations. In Dongshan Bay. Dongshan Bay is surrounded by high terrain, with good seclusion and wide-open area for cage farming. Consequently, implementing alternative farming and fallowing periods in the farming area can help restore the aquatic environment and benthic fauna. Additionally, cultivating macroalgae such as undaria, gulfweed, and gracilaria in the farming area can serve as a means to absorb excess nutrients and maintain balanced levels of dissolved oxygen. However, to effectively manage a fish farm in an environmentally sustainable manner, it is crucial to consider specific information and physiological conditions related to Dongshan Bay. This includes factors such as hydrodynamics, sediment metabolism, plankton community structure, sediment microorganisms, and temporal dynamics of organic matter. Therefore, obtaining additional information, such as conducting temporal monitoring of organic matter sediment dynamics, would enhance our understanding of the environmental impact of cage farming and enable the construction of a comprehensive and effective management model.
The organic matter (OM) in the sediment mainly comes from the endogenous OM produced by algae and other plants in the water and terrestrial plant debris brought by the erosion of the external watershed. The OM from different sources shows different C/N ratios. According to Mayers and Ishiwatari (Mayers and Ishiwatari. 1993), a C/N value greater than 20 means the pollution comes from fibrous plant detritus, 20–160 from higher terrestrial plants, 4–12 from nonfibrous plants, 0–7 from zooplankton, 6–14 from phytoplankton, and 4–10 from algae. Basically, the high C/N value indicates that the proportion of terrestrial OM is large, while the small value represents the OM of the water body itself. As shown in Figure 3, there was no significant difference in the C/N ratio of sediment cores with varied depths at YB1 and YB2 (Figure 3), while obvious variation of the C/N ratio at different depths of B1 and B2 sediment cores could be observed. The C/N ratio of four sediment cores in Dongshan Bay varied from 7.00 to 11.81, with mean values of 9.01(YB1), 8.81 (YB2), 9.70 (B1), and 9.77 (B2), respectively, indicating the OM in sediment cores mostly comes from phytoplankton and nonfibrous plants in water. Some researchers indicated that the C/N ratio of fish feces was 5.85, and the C/N ratio of fish feed ranged from 4.36 to 7.22 based on the analysis of the sediment of lake cage farming (Wang et al., 2021; Yuan et al., 2023). Considering all sediment cores were collected at Dongshan Bay with above 10 years of fish cage farming, it is plausible that some portion of OM in four sediment cores was from the fish feed and feces. Moreover, with the invasion of this organic waste, phytoplankton in the water body would largely breed due to the nitrogen and phosphorus enrichment, contributing to the most income of OM. Therefore, it was reasoned that the OM mostly came from the phytoplankton and nonfibrous plants in water which could be explained by the excessive fish feed and feces generated by cage farming.
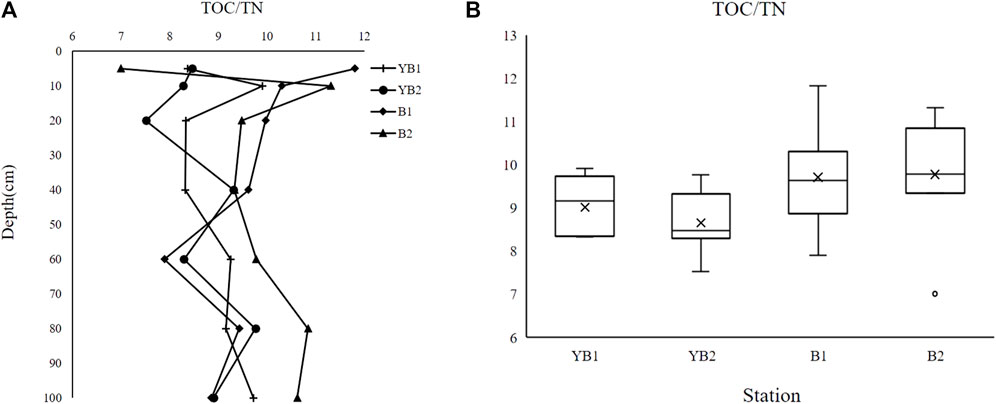
FIGURE 3. TOC/TN ratio in sediment cores of Dongshan Bay. (A), the vertical distribution of TOC/TN ratio in sediment cores at four sites. (B), average TOC/TN ratio in sediment cores at four sites.
The declining trend with sediment depth was observed in TN and TOC concentration at the four sampling sites, i.e., YB1, YB2, B1, and B2 in the semi-closed bay. Likewise, the TP concentration decreased with increased depth at YB2, B1, and B2, while the opposite trend of TP concentration was observed at the YB1 site. The average TN and TP values of YB1, YB2, B1, and B2 were far higher than the safety level. The STN, STP, FF, and ON indexes of the four sites indicated moderate and even severe pollution of nitrogen and phosphorus in Dongshan Bay. Notably, the pollution levels at the YB1 and YB2 sites were found to be more severe compared to the B1 and B2 sites. This suggests that higher water velocities in Dongshan Bay can partially alleviate the impact of organic pollution. The analysis of the C/N ratio revealed that the OM mostly came from the phytoplankton and nonfibrous plants in water which resulted from the excessive fish feed and feces generated by cage farming. These observations suggested cage farming activity for an extended period of time in a semi-closed bay would negatively affect the environment. Hence, we suggest that opening the Bachimen dyke and alternating the farming and fallowing period is needed for mitigating the organic pollution. This study provides foundational knowledge on the impact of cage farming in the Dongshan area, but due to limitations in sample size and insufficient information on temporal dynamics, stable isotope tracing, and other biological and geological factors, the construction of a rigorous scientific model for aquatic environment recovery and precise prediction of pollution risks during farming periods is currently inadequate. Further research is needed to address these gaps.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Sample collection and measurement was done by CZ-Z, ZJ, and YM-F. Formal analysis and plot were made by CZ-Z and LY-Q. GT-Y and ZS-H supervised the study. CZ-Z, ZH-D, LD-L, and JS-C wrote the original draft. All authors contributed to the article and approved the submitted version.
This study was supported by Guiding Project of Fujian Science and Technology Plan (2020Y0059), Fujian Marine Economic Development Special Project (FJHJF-L-2022-21), Fujian Marine Service and Fishery High-quality Development Project (FJHY-YYKJ-2022-1-1) and Fujian Natural Science Foundation (2022J05071).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Brown, J. R., Gowen, R. J., and McLusky, D. S. (1987). The effect of salmon farming on the benthos of a Scottish sea loch. J. Exp. Mar. Biol. Ecol. 109, 39–51. doi:10.1016/0022-0981(87)90184-5
Chen, B., Ji, W., Zhou, K., He, Q., and Fu, T. (2014). Nutrient and eutrophication characteristics of the dongshan bay, South China. Chin. J. Oceanol. Limnol. 32, 886–898. doi:10.1007/s00343-014-3214-3
Committee China Bays Annuals Committee (1994). China bay annuals, China: China Ocean Press, 310–376. (in Chinese).
Conley, D. J., Paerl, H. W., Howarth, R. W., Boesch, D. F., Seitzinger, S. P., Havens, K. E., et al. (2009). Controlling eutrophication: Nitrogen and phosphorus. Science 323, 1014–1015. doi:10.1126/science.1167755
Dai, J., Song, J., Li, X., Yuan, H., Li, N., and Zheng, G. (2007). Environmental changes reflected by sedimentary geochemistry in recent hundred years of Jiaozhou Bay, North China. Environ. Pollut. 145, 656–667. doi:10.1016/j.envpol.2006.10.005
Damak, M., Fourati, R., Elleuch, B., and Kallel, M. (2020). Environmental quality assessment of the fish farms’ impact in the Monastir bay (eastern of Tunisia, central mediterranean): A benthic foraminiferal perspective. Environ. Sci. Pollut. Res. 27, 9059–9074. doi:10.1007/s11356-019-07523-7
Degefu, F., Mengistu, S., and Schagerl, M. (2011). Influence of fish cage farming on water quality and plankton in fish ponds: A case study in the rift valley and north shoa reservoirs, Ethiopia. Aquaculture 316, 129–135. doi:10.1016/j.aquaculture.2011.03.010
Duarte, C. M., Holmer, M., Olsen, Y., Soto, D., Marba, N., Guiu, J., et al. (2009). Will the oceans help feed humanity? BIOSCIENCE 59, 967–976. doi:10.1525/bio.2009.59.11.8
Elser, J. J., Bracken, M. E. S., Cleland, E. E., Gruner, D. S., Harpole, W. S., Hillebrand, H., et al. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142. doi:10.1111/j.1461-0248.2007.01113.x
FAO (Food and Agricultural Organization of the United Nations) (2020). The state of world fisheries and aquaculture 2020. Rome: FAO Fisheries and Aquaculture Department.
Gao, C., Yu, F., Chen, J., Huang, Z., Jiang, Y., Zhuang, Z., et al. (2021). Anthropogenic impact on the organic carbon sources, transport and distribution in a subtropical semi-enclosed bay. Sci. Total Environ. 767, 145047. doi:10.1016/j.scitotenv.2021.145047
Goñi, M. A., Moore, E., Kurtz, A., Portier, E., Alleau, Y., and Merrell, D. (2014). Organic matter compositions and loadings in soils and sediments along the Fly River, Papua New Guinea. Geochimica Cosmochimica Acta 140, 275–296. doi:10.1016/j.gca.2014.05.034
Gu, Y-G., Wang, Y., Ouyang, J., Jordan, R. W., and Jiang, S. (2021). Impacts of coastal aquaculture on sedimentary phosphorus speciation and fate: Evidence from a seaweed cultivation area off Nan'ao Island, South China. Mar. Pollut. Bull. 171, 112719. doi:10.1016/j.marpolbul.2021.112719
He, L., Zhong, H., Liu, G., Dai, Z., Brookes, P. C., and Xu, J. (2019). Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 252, 846–855. doi:10.1016/j.envpol.2019.05.151
He, T., Yang, W., Xie, J., and Yu, H. (2015). Distribution characteristics and environmental significance of carbon, nitrogen and phosphorus in core sediments of Daya Bay. Mar. Environ. Sci. 34, 524–529. (in Chinese). doi:10.13634/j.cnki.mes.2015.04.009
Heilskov, A. C., Alperin, M., and Holmer, M. (2006). Benthic fauna bio-irrigation effects on nutrient regeneration in fish farm sediments. J. Exp. Mar. Biol. Ecol. 339, 204–225. doi:10.1016/j.jembe.2006.08.003
Holby, O., and Hall, P. O. J. (1994). Chemical fluxes and mass balances in a marine fish cage farm. III. Silicon. Aquaculture 120, 305–318. doi:10.1016/0044-8486(94)90087-6
Holmer, M., Duarte, C. M., Heilskov, A., Olesen, B., and Terrados, J. (2003). Biogeochemical conditions in sediments enriched by organic matter from net-pen fish farms in the Bolinao area, Philippines. Mar. Pollut. Bull. 46, 1470–1479. doi:10.1016/S0025-326X(03)00281-9
Holmer, M., and Kristensen, E. (1992). Impact of marine fish cage farming on metabolism and sulfate reduction of underlying sediments. Mar. Ecol. Prog. Ser. 80, 191–201. doi:10.3354/meps080191
Holmer, M. (2010). Environmental issues of fish farming in offshore waters: Perspectives, concerns and research needs. Aquac. Environ. Interact. 1, 57–70. doi:10.3354/aei00007
Huang, X., Guo, F., and Huang, L. (2010). Distribution characteristics and pollution of nitrogen and phosphorus in core sediments of marine culture area in Dapeng Cove. J. Trop. Oceanogr. 29, 91–97. (in Chinese). doi:10.11978/j.issn.1009-5470.2010.01.091
Islam, M. S. (2005). Nitrogen and phosphorus budget in coastal and marine cage aquaculture and impacts of effluent loading on ecosystem: Review and analysis towards model development. Mar. Pollut. Bull. 50, 48–61. doi:10.1016/j.marpolbul.2004.08.008
Ji, S., Chen, Y., Wang, X., Zhang, Z., Wang, D., and Li, Y. (2020). Chemical synthesis of single atomic site catalysts. Chem. Rev. 120, 11900–11955. doi:10.1021/acs.chemrev.9b00818
Jiang, Z., Cui, Y., Chen, B., and Wang, G. (2007). Assessment on sediment quality and pollution degree of cage culture area in Tangdao bay. J. Agro-Environment Sci. 2007, 394–399. (in Chinese).
Kaggwa, M. N., Liti, D. M., and Schagerl, M. (2011). Small tropical reservoirs and fish cage culture: A pilot study conducted in machakos district, Kenya. Aquac. Int. 19, 839–853. doi:10.1007/s10499-010-9403-y
Kang, T-W., Yang, H. J., Han, J. H., Han, Y-U., Kim, M-S., Kim, J., et al. (2022). Identifying pollution sources of sediment in Lake Jangseong, Republic of Korea, through an extensive survey: Internal disturbances of past aquaculture sedimentation. Environ. Pollut. 306, 119403. doi:10.1016/j.envpol.2022.119403
Krom M, D., and Berner R, A. (1981). The diagenesis of phosphorus in a nearshore marine sediment. Geochimica Cosmochimica Acta 45, 207–216. doi:10.1016/0016-7037(81)90164-2
Kucuksezgin, F., Pazi, I., Gonul, L. T., Kocak, F., Eronat, C., Sayin, E., et al. (2021). The impact of fish farming on the water column and marine sediments in three coastal regions from eastern Aegean coast. Environ. Sci. Pollut. Res. 28, 29564–29580. doi:10.1007/s11356-021-12695-2
Kutti, T., Ervik, A., and Hansen, P. K. (2007a). Effects of organic effluents from a salmon farm on a fjord system. I. Vertical export and dispersal processes. Aquaculture 262, 367–381. doi:10.1016/j.aquaculture.2006.10.010
Kutti, T., Hansen, P. K., Ervik, A., Høisæter, T., and Johannessen, P. (2007b). Effects of organic effluents from a salmon farm on a fjord system. II. Temporal and spatial patterns in infauna community composition. Aquaculture 262, 355–366. doi:10.1016/j.aquaculture.2006.10.008
Lauritzen, L. (2021). A spotlight on seafood for global human nutrition. Nature 598, 260–262. doi:10.1038/d41586-021-02436-3
Liu, S. M., Zhu, B. D., Zhang, J., Wu, Y., Liu, G. S., Deng, B., et al. (2010). Environmental change in Jiaozhou Bay recorded by nutrient components in sediments. Mar. Pollut. Bull. 60, 1591–1599. doi:10.1016/j.marpolbul.2010.04.003
Maldonado, M., Carmona, M. C., Echeverría, Y., and Riesgo, A. (2005). The environmental impact of mediterranean cage fish farms at semi-exposed locations: Does it need a re-assessment? Helgol. Mar. Res. 59, 121–135. doi:10.1007/s10152-004-0211-5
Mayers, P. A., and Ishiwatari, R. (1993). Lacustrine organic geochemistry-an overview of indicators of organic matter sources and diagenesis inlake sediments. Org. Geochem 20, 867–900. doi:10.1016/0146-6380(93)90100-p
Pan, Z., Liu, Q., Jiang, R., Li, W., Sun, X., Lin, H., et al. (2021a). Microplastic pollution and ecological risk assessment in an estuarine environment: The Dongshan Bay of China. Chemosphere 262, 127876. doi:10.1016/j.chemosphere.2020.127876
Pan, Z., Tan, Y-M., Gao, Q-F., Dong, S-L., Fang, X-D., and Yan, J-L. (2021b). A 120-year record of burial fluxes and source apportionment of sedimentary organic carbon in Alian Bay, China: Implication for the influence of mariculture activities, and regional environment changes. Aquaculture 535, 736421. doi:10.1016/j.aquaculture.2021.736421
Persaud, D., Jaagumagi, R., and Hayton, A. (1993). Guidelines for the protection and and management of aquatic sediment quality in Ontario. Ottawa: Ontario Ministry of the Environment and Energy, p3.
Qiu, Z., Liu, Q., Zhang, R., Zhan, C., Liu, S., Zhang, J., et al. (2023). Distribution characteristics and pollution assessment of phosphorus forms, TOC, and TN in the sediments of Daye Lake, Central China. J. Soils Sediments 23, 1023–1036. doi:10.1007/s11368-022-03398-3
Sanz-Lazaro, C., Casado-Coy, N., Calderero, E. M., and Villamar, U. A. (2021). The environmental effect on the seabed of an offshore marine fish farm in the tropical Pacific. J. Environ. Manag. 300, 113712. doi:10.1016/j.jenvman.2021.113712
Silvert, W., and Sowles, J. W. (1996). Modelling environmental impacts of marine finfish aquaculture. J. Appl. Ichthyology 12, 75–81. doi:10.1111/j.1439-0426.1996.tb00066.x
Soto, D., León-Muñoz, J., Dresdner, J., Luengo, C., Tapia, F. J., and Garreaud, R. (2019). Salmon farming vulnerability to climate change in southern Chile: Understanding the biophysical, socioeconomic and governance links. Rev. Aquac. 11, 354–374. doi:10.1111/raq.12336
Tada, K., Nakakuni, M., Koomklang, J., Yamaguchi, H., and Ichimi, K. (2023). The impact of fish farming on phosphorus loading of surface sediment in coastal complex aquaculture. Fish. Sci. 89, 375–386. doi:10.1007/s12562-022-01666-2
Uede, T. (2007). Chemical characteristics of seabed sediments and phosphorus forms in fish aquaculture. Nippon. Suisan Gakkaishi 73, 62–68. doi:10.2331/suisan.73.62
Wang, M., Wang, X., He, X., Zhao, Q., and Liu, J. (2021). Sources of sedimentary organic matter from the cage fish farm in Lake Poyang, China. J. Lake Sci. 33, 158–167. (in Chinese). doi:10.18307/2021.0108
Wei, Z., Han, H., Yu, K., Ding, P., Hu, M., Huo, Y., et al. (2016). The content of carbon, nitrogen and phosphorus in the surface sediment at the mariculture area in the enclosed Sansha Bay. Mar. Sci. 40, 77–86. (in Chinese). doi:10.11759/hykx20151105002
Wu, L., He, D., Ji, Z., You, W., Tan, Y., Zhen, X., et al. (2017). Protection efficiency assessment and quality of coastal shelterbelt for Dongshan Island at the coastal section scale. J. For. Res. 28, 577–584. doi:10.1007/s11676-016-0325-z
Xu, Y., Sun, Q., Ye, X., Yin, X., Li, D., Wang, L., et al. (2017). Geochemical analysis of sediments from a semi-enclosed bay (Dongshan Bay, southeast China) to determine the anthropogenic impact and source. Chemosphere 174, 764–773. doi:10.1016/j.chemosphere.2017.01.081
Yuan, X-Y., Meng, C., Liu, H., and Sun, B. (2023). Magnetically driven nanorobots based on peptides nanodots with tunable photoluminescence for rapid scavenging reactive α-dicarbonyl species and effective blocking of advanced glycation end products. Food Chem. 422, 136252. doi:10.1016/j.foodchem.2023.136252
Zhang, C., Wu, L., Luo, Y., Zhang, H., and Christie, P. (2008). Identifying sources of soil inorganic pollutants on a regional scale using a multivariate statistical approach: Role of pollutant migration and soil physicochemical properties. Environ. Pollut. 151, 470–476. doi:10.1016/j.envpol.2007.04.017
Keywords: age farming, sediment cores, vertical distribution, pollution assessment, Dongshan Bay
Citation: Zhen-Zhen C, Jian Z, Miao-Feng Y, Yong-Qing L, Hui-Dong Z, Dong-Lian L, Shuang-Cheng J, Tuan-Yu G and Sheng-Hua Z (2023) Vertical distribution and pollution assessment of TN, TP, and TOC in the sediment cores of cage farming areas in Dongshan Bay of southeast China. Front. Environ. Sci. 11:1216868. doi: 10.3389/fenvs.2023.1216868
Received: 04 May 2023; Accepted: 19 July 2023;
Published: 27 July 2023.
Edited by:
Zhenbei Wang, Beijing Forestry University, ChinaReviewed by:
Moslem Sharifinia, Iranian Fisheries Science Research Institute, IranCopyright © 2023 Zhen-Zhen, Jian, Miao-Feng, Yong-Qing, Hui-Dong, Dong-Lian, Shuang-Cheng, Tuan-Yu and Sheng-Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo Tuan-Yu, Z3R5MjAwOTk5QDE2My5jb20=; Zheng Sheng-Hua, ZGF1cGhpbkAxMzkuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.