- 1Shaanxi Key Laboratory of Ecological Restoration in Shaanbei Mining Area, Yulin University, Yulin, China
- 2Institute of Ecological Conservation and Restoration, Chinese Academy of Forestry, Beijing, China
During the restoration of degraded vegetation, the mutual matching of underground root systems is crucial for the formation and effective function of the future plant community. In this study, the Amoeba graphic method was integrated to comprehensively establish a root framework index (RFI), based on the three dimensions of root morphology, as well as quantitative and spatial connection characteristics, to quantify the root system architecture. The root development characteristics of alfalfa (T-type), switchgrass (F-type), and mixed planting with alfalfa and switchgrass (T+F-type) were monitored at the test positions. The RFI parameters comprise the acting coefficient of the root framework, root density, root framework degree, and soil bulk density. The RFI values of T-, F-, and T + F-type were 0.38, 0.86, and 1.68, respectively, and were found to provide a well representation of the root structure characteristics. The findings obtained in this study provide scientific support for the ecological construction and evaluation of degraded vegetation.
1 Introduction
Plant roots and soil form a root–soil complex system through network connections, root–soil binding, and biochemical processes, which are highly important for the effective restoration of degraded vegetation and ecological sustainability (Yen, 1987; Li et al., 2017). During the construction of vegetation under unfavorable habitats affected by stress, such as drought, the root system can adapt and maintain the stability of the plant community through bending and winding, decreasing the branching capacity, increasing the connection length, and adjusting the turnover time, thereby allowing for adaptation to a wide range of ecological conditions (Doussan et al., 2003; De Baets et al., 2008; Huang et al., 2019). Therefore, mutual matching of the underground root system during the restoration of degraded vegetation will be key to the formation and effective functioning of plant communities with a well structure in the future (Wang et al., 2019a; Wang et al., 2019b).
For a single vegetation type, the root architecture, i.e., the interconnection and spatial distribution of roots at different levels in the same plant root system, is an important indicator of the root system structure (Guo et al., 2019). Previous studies on root architecture characteristics have focused on quantitative indicators, such as root density (Rd), root length density, effective Rd, and root specific surface area density, as well as other spatial indicators, including the root topology index, connection length, and branching rate (Li et al., 2016a; 2016b; Yang et al., 2018). The characteristics of the horizontal distribution of the uniform root system and the root system bending characteristics have been integrated to describe root system morphology and distribution (Nicoll et al., 2006; Wang et al., 2020). In an innovative approach for quantifying root systems, the Amoeba graphical method has been used in some studies to quantify the overall root system configuration by using three indicators: root diameter, root depth, and root length (Zhang et al., 2016). However, in reconstruction of the vegetation modes of two or more plant species, the root systems of different plants may form a rigid framework with the soil through branching connections, and thus the root architecture concept may not be suitable for representing these structural features and functions. Therefore, it is extremely important to comprehensively characterize root structural traits and develop a quantifiable root framework index (RFI) by effectively integrating root morphological features, quantitative characteristics, and spatial connections (Li et al., 2020a; Li et al., 2020b).
Based on three principles, including scientific credibility, effectiveness, and practicality, in this study, we selected key indicators for describing the root structure according to the dimensions of plant root morphology, quantitative features, and characteristic spatial connections. We used the Amoeba graphic method to establish an RFI for typical plant reconstruction models in loess hilly areas, thereby providing scientific support for the ecological construction and evaluation of degraded vegetation.
2 Materials and methods
2.1 Study area
The study area is located at the Ansai Water and Soil Conservation Comprehensive Experimental Station of the Chinese Academy of Sciences, China (109°19′ 23″E, 36°51′30″N). The annual average rainfall in this area is 505.3 mm, but the inter-annual variation is high, and the distribution is uneven throughout the year. More than 60% of the precipitation is concentrated between July and September. The soil in this area is loessal and silt–loamy, with a clay, silt, and sand content of 9.3%, 57.4%, and 33.3%, respectively. The soil organic matter content is 3.65 g kg−1, and the pH is 8.56.
2.2 Experimental design
The study area had long been used for growing potatoes, millet, and other crops before 2001. The plot was abandoned between 2001 and 2009. In May 2009, the area was reclaimed as agricultural land for growing potatoes, millet, and other crops in three consecutive years (2009–2012). The trial was conducted in mid-May 2012. The experimental treatments involved growing the following plants and combinations: (1) alfalfa (Medicago sativa) with a typical taproot (T) system (251 plants m−2), (2) switchgrass (Panicum virgatum) with a typical fibrous (F) root system (157 plants m−2), and (3) mixed sowing of alfalfa and switchgrass (T + F). Each treatment plot had an area of 27 m2 (9 m × 3 m), with a slope of 20° to the northeast. The topsoil was artificially loosened, and large soil particles were broken into soil particles <1 cm in diameter. No treatments were fertilized before sowing. Because of the small size of the seeds, random sowing per unit area (m2) was conducted after sowing with dry soil of the same quality, to avoid an influence of sowing direction (Li et al., 2015).
2.3 Statistical analysis
Analyses of the acting coefficient of the root framework, root density (Rd), root framework degree (S), and root framework index (RFI) were performed in Microsoft Office 2019. The graphs were generated in SigmaPlot 18.0. The significance of differences was tested with the least significant difference method (p < 0.05).
3 Results
3.1 Construction of the RFI
The root framework should comprehensively characterize the root structure characteristics in terms of the morphological, quantitative, and spatial characteristics of the root system. The four assumptions of the root framework are: (1) the existence of two or more morphological root systems, (2) a certain root biomass, (3) a fine root spatial structure, and (4) specific soil structure properties. In this study, we selected four indicators, including the coefficient of root framework, root density, root framework degree, and soil bulk density—based on our field research and practice, as well as the principles of scientific credibility, effectiveness, and practicability.
1) Acting coefficient of the root framework. Various forms of plant roots differ in terms of their branch connections, root–soil binding, and biochemical effects in the soil. In this study, the acting coefficient of the RFI, i.e., the slope of the linear relationship between the root system mass density and the planting time, was used to characterize the root morphology.
2) Root density. This indicator was to describe the quantitative characteristics of the RFI. The root biomass was obtained by repeatedly washing the roots in a sieve before oven drying at 80 °C to a constant weight. Rd is calculated as follows:
where Rd is the root density (kg m-3), M is the mass of the dried root system (kg), and V is the sample volume (m3).
3) Root framework degree. Based on a previous study (Zhang et al., 2016) and the principles of scientific credibility, effectiveness, and practicability, we selected three indexes—root diameter (X), root length (Y), and root depth (Z)—to establish a three-dimensional coordinate system. The volume formed in Amoeba by connecting the three points was set as the root framework degree and was used to characterize the spatial connection characteristics of the RFI (Figure 1). The root framework degree was calculated with the Amoeba graphical method; therefore, the indicator had mathematical meaning but no physical meaning and thus was regarded as dimensionless. The root framework degree is calculated as follows:
where S is the root framework degree, a is the average root diameter (cm), b is the total root length (cm), and h is the maximum root depth (cm).
4) Soil bulk density. According to the principles of scientific credibility, representativeness, and accessibility, the soil bulk density was selected to indicate the soil structural properties by using following Equation:
where ρ is the soil bulk density (g cm−3), m is the mass of dried soil (g), and v is the volume of the soil sampler (100 cm3).
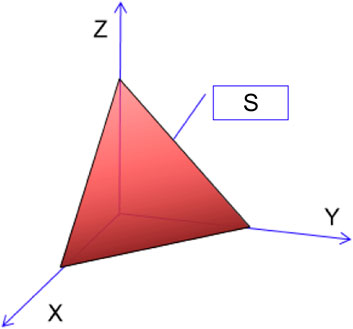
FIGURE 1. Schematic diagram of Amoeba. S, root framework degree; X, root diameter; Y, root length; Z, root depth.
Finally, the root framework index (RFI,%) can be calculated from the following equation:
3.2 Application of RFI
3.2.1 Acting coefficient of the root framework
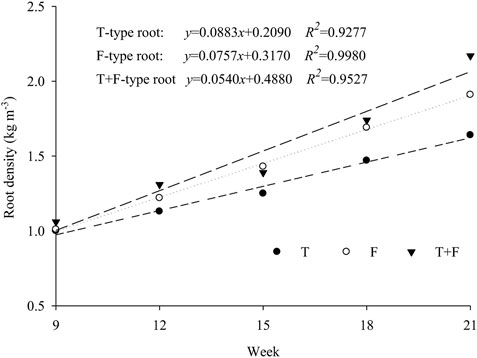
Figure 2 shows that the Rd values for T-type, F-type, and T + F type planting increased in a linear function with the planting age, and the coefficients of determination for the linear regression models all exceeded 0.94. The slope indicators suggested that the acting coefficients for T-, F-, and T + F-type roots were 0.162, 0.227, and 0.265, respectively. These results showed that the root biomass growth rate in mixed T + F plants was greater than that in T- or F-type plants. Moreover, the T + F-type plant combination formed a stable root framework more readily than the T- or F-type treatment.
3.2.2 Root framework degree
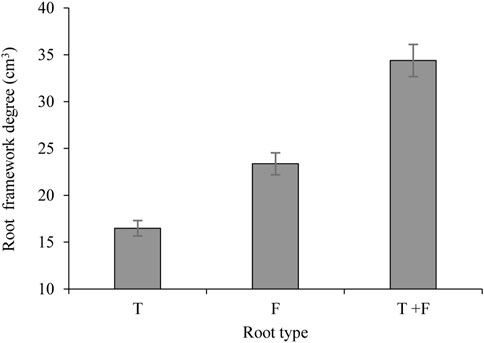
The root framework degree refers to the three-dimensional structure of plant roots in the soil, which reflects the spatial framework of the roots. Figure 3 shows that the root framework degree for the T-, F-, and T + F-type roots was 16.48, 23.32, and 34.40, respectively. The root framework degree for F-type roots was 6.84 higher than that for T-type roots, representing an increase of 41.5%. The root framework value for T + F-type roots was 17.92 and 11.08 greater than that for T- and F-type roots, respectively, representing increases of 108.7% and 50.6%, respectively.
3.2.3 RFI
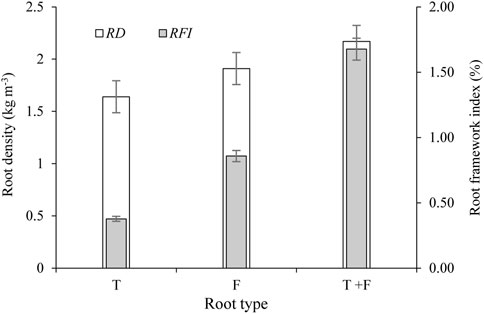
RFI is a parameter used to comprehensively quantify plant root structure in three dimensions according to the root morphology, root coefficient, and root spatial connection characteristics. As shown in Figure 4, the RFI values for T-, F-, and T + F-type plants were 0.387, 0.851, and 1.676, respectively. The RFI value for F-type plants was 0.464 higher than that for the T-type root system. The RFI value for T + F type plants was 1.289 and 0.825 higher than that for T- and F-type roots, respectively, representing increases of 3.3-fold and 0.97-fold. These differences were similar to the changes in Rd. However, the root system spatial connection characteristics were probably incorporated into the RFI, thereby increasing the differences between the treatments. Therefore, the RFI values obtained for T-, F-, and T + F- type plants effectively reflected the characteristics of the root system structures.
4 Discussion
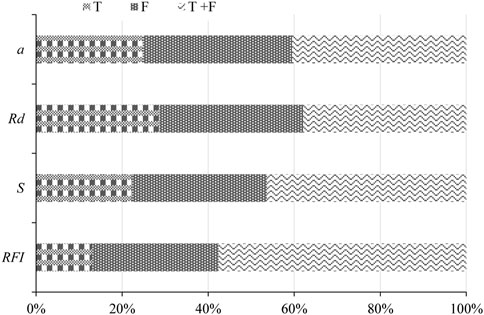
The root density, root specific surface area density, fractal dimension, connection length, and topological structure, as well as other parameters, have been widely used for describing root systems. Some studies have used Rd to evaluate the restoration effect of degraded grassland and have determined an effective density, denoted the optimal root content (Tang et al., 2016; Bo et al., 2019).
Figure 5 shows that the optimal root content may change if the root spatial connection characteristics are considered. The growth characteristics of the root systems in different plant species can vary in terms of the framework level and stability (Niu et al., 2019). RFI was applied to the root–soil complex system formed by two or more plant roots in the soil through branch connections, root–soil binding, and biochemical interactions (Fan et al., 2010). The RFI constructed in this study comprehensively quantifies and characterizes the root structure characteristics based on the dimensions of root morphology, quantitative features, and space; moreover, it objectively reflects the effects of the mutual matching of different root morphologies, and it accurately represents the anchoring effect of deep roots and the reinforcement effect of shallow roots. Moreover, because obtaining the required indicators is simple, the RFI should support studies on the structure of plant roots and the evaluation of plant community ecological construction (Yang et al., 2011).
During the development of vegetation in unfavorable habitats, such as those affected by drought, roots can adapt and maintain the stability of the plant community by bending and winding, decreasing the branching capacity, increasing the connection length, and adjusting the turnover time, thereby allowing for adaptation to a wide range of ecological conditions. When plants are affected by wind, their underground root systems bend. Most of the root mass is distributed in the downwind direction when plants grow on flat ground, whereas more of the root mass is distributed in the upwind direction when root systems grow on sloping ground. Therefore, future studies using the RFI should comprehensively consider the relationships among soil structure, species attributes, and the external environment, and should weight the parameters in the model to evaluate the stability and sustainability of plant communities on a larger scale (Lifschitz et al., 2022). The RFI constructed from the perspective of mutual matching of underground root systems is helpful for evaluating vegetation restoration effects, and it may support evaluations of regional ecosystem services (Zhu et al., 2019).
5 Conclusion
In this study, based on the root growth characteristics of T-type, F-type, and T + F type plants in a loess hilly area, we established the RFI according to three dimensions: root morphology, functional coefficient, and root spatial connection characteristics. The parameters of the RFI comprise the acting coefficient of the root framework, Rd, root framework degree, and soil bulk density. We determined the RFI values for T-, F-, and T + F-type plants to be 0.38, 0.86, and 1.68, respectively. RFI could effectively reflects the root structure characteristics of vegetation and provides scientific support for ecological construction processes and evaluation of degraded vegetation.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization, QL and DT; methodology, FA and FK; investigation, ZZ and DT; data curation, ZZ; writing—original draft preparation, QL; writing—review and editing, QL and DT; supervision, QL and DT. All authors contributed to the article and approved the submitted version.
Funding
Financial assistance for this study was provided by the projects of the National Natural Science Foundation of China (Grant No. 42207412, 42267071, 41907059), National Key Research and Development Program of China (2022YFF1300802).
Acknowledgments
We also express our gratitude to the reviewers and editors for their constructive comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bo, S. N., Wang, Y. Q., Ma, C., and Li, Y. G. (2019). Quantified effects of fixing soil by root system of Broussonetia papyrifera based on experiment and model. Sci. Soil Water Conserv. 17 (1), 28–34. doi:10.16843/j.sswc.2019.01.004
De Baets, S., Poesen, J., Reubens, B., Wemans, K., Baerdemaeker, J. D., and Muys, B. (2008). Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 305, 207–226. doi:10.1007/s11104-008-9553-0
Doussan, C., Pagès, L., and Pierret, A. (2003). Soil exploration and resource acquisition by plant roots: An architectural and modelling point of view. Agronomie 23 (5-6), 419–431. doi:10.1051/agro:2003027
Fan, C. C., and Chen, Y. W. (2010). The effect of root architecture on the shearing resistance of root permeated soils. Ecol. Eng. 36 (6), 813–826. doi:10.1016/j.ecoleng.2010.03.003
Guo, M. M., Wan, W. L., Shi, Q. H., Chen, T., and Li, J. (2019). An experimental study on the effects of grass root density on gully headcut erosion in the gully region of China's Loess Plateau. Land Degrad. Dev. 30 (17), 2107–2125. doi:10.1002/ldr.3404
Huang, T. L., Tang, L. X., Cheng, L., and Zhang, Q. Y. (2019). Patterns and influencing factors of spatio-temporal variability of soil organic carbon in karst catchment. Sci. Soil Water Conserv. 17 (1), 89–94. doi:10.1504/ijgw.2019.10017599
Li, Q., Cao, Y., Zhang, Z., Tuo, D. F., Bu, Y. J., and Bai, Y. (2016a). Stenotrophomonas maltophilia HW2 enhanced cucumber resistance against cucumber green mottle mosaic virus. Plant Sci. J. 34 (3), 488–495. doi:10.1007/s12374-016-0246-6
Li, Q., Liu, G. B., Yang, J. C., Zhang, Z., and Tuo, D. F. (2020b). Construction and application of a new index for quantifying root erosion resistance: Root framework erosion resistance index. Chin. J. Appl. Ecol. 37 (9), 2955–2962. doi:10.13287/j.1001-9332.202009.010
Li, Q., Liu, G. B., Zhang, Z., Tuo, D. F., Bai, R. R., and Qiao, F. F. (2017). Relative contribution of root physical enlacing and biochemistrical exudates to soil erosion resistance in the Loess soil. Catena 153, 61–65. doi:10.1016/j.catena.2017.01.037
Li, Q., Liu, G. B., Zhang, Z., Tuo, D., and Miao, X. (2016b). Structural stability and erodibility of soil in an age sequence of artificial robinia pseudoacacia on a hilly loess plateau. Pol. J. Environ. Stud. 25, 1595–1601. doi:10.15244/pjoes/62390
Li, Q., Liu, G. B., Zhang, Z., Tuo, D., and Xu, M. (2015). Effect of root architecture on structural stability and erodibility of topsoils during concentrated flow in hilly Loess Plateau. Chin. Geogr. Sci. 25 (6), 757–764. doi:10.1007/S11769-014-0723-0
Li, Q., Yang, J. C., and Zhang, J. Q. (2020a). Progress of research on soil erosion resistance of plant roots and future prospects. J. Agric. Resour. Env. 37 (1), 17–23. doi:10.13254/j.jare.2018.0316
Lifschitz, M., Tommasino, E., Zabala, J. M., Grunberg, K., Ramos, J. C., and Tomás, M. A. (2022). Combined effect of salinity and hypoxia in seedlings of two varieties of Panicum coloratum: Morphology, root system architecture, oxidative damage and antioxidant response. Ann. Appl. Biol. 180 (2), 283–293. doi:10.1111/aab.12733
Nicoll, B. C., Berthier, S., Achim, A., Gouskou, K., Danjon, F., and Beek, L. P. H. V. (2006). The architecture of Picea sitchensis structural root systems on horizontal and sloping terrain. Trees-Struct. Funct. 20 (6), 701–712. doi:10.1007/s00468-006-0085-z
Niu, M., Chen, J. H., Zhou, D. S., Xie, T. Z., Bie, P. F., Zhao, R., et al. (2019). Topological characteristics of the root systems of four native broad-leaved trees in the central Sichuan hilly region. J. Nanjing For. Univ. 43, 3–11. doi:10.3969/j.issn.1000-2006.201811010
Tang, K. M., Zhang, G. H., and Sun, Z. L. (2016). Seasonal variation in soil detachment capacity of grasslands and its influencing factors. Sci. Soil Water Conserv. 14 (6), 18–25. doi:10.16843/j.sswc.2016.06.003
Wang, J. S., Sun, J., Yu, Z., Li, Y., Tian, D. S., Wang, B. X., et al. (2019b). Vegetation type controls root turnover in global grasslands. Glob. Ecol. Biogeogr. 28 (4), 442–455. doi:10.1111/geb.12866
Wang, J., Zhao, W. W., Liu, Y., and Jia, L. Z. (2019a). Effects of plant functional traits on soil conservation. A review. Acta Ecol. Sin. 39 (9), 3355–3364. doi:10.5846/stxb201804020737
Wang, Z. H., Chiarucci, A., Fang, H., and Chen, M. H. (2020). An interspecific variation in rhizosphere effects on soil anti-erodibility. Sci. Rep-uk. 10, 2411–2435. doi:10.1038/s41598-020-58784-z
Yang, F., Liu, L., Wang, W. K., Zhao, G. Z., and Guan, P. (2011). Distribution characteristics comparison of Salix psam-mophila roots under different landforms in Mu Us Desert. Agric. Sci. Technol. 12 (7), 1059–1061. doi:10.16175/j.cnki.1009-4229.2011.07.031
Yang, Z. Y., Zhou, B. Z., Chen, Q. B., Ge, X. G., Wang, X. M., Cao, Y. H., et al. (2018). Effects of drought on root architecture and non-structural carbohydrate of Cunninghamia lanceolata. Acta Ecol. Sin. 38 (18), 6729–6740. doi:10.5846/stxb201803260604
Yen, C. P. (1987). “Tree root patterns and erosion control,” in Proceedings of the international workshop on soil erosion and its countermeasures. Editor S. Jantawat (Bangkok, Thailand: Soil and Water Conservation Society of Thailand)
Zhang, Z. M. (2016). Quantifying root architecture and evaluation: Case study over songnen meadow steppes plants. Changchun, China: Master thesis of northeast normal University.
Keywords: root density, root framework degree, root framework index, root structure, degraded vegetation
Citation: Li Q, Ai F, Kang F, Zhang Z and Tuo D (2023) Construction and application of a new index for root architecture quantification in arid and semi-arid regions. Front. Environ. Sci. 11:1214372. doi: 10.3389/fenvs.2023.1214372
Received: 29 April 2023; Accepted: 20 June 2023;
Published: 07 July 2023.
Edited by:
Gergely Tóth, Institute of Advanced Studies, HungaryReviewed by:
Weiming Yan, Northwest A&F University, ChinaKalman Rajkai, Hungarian Academy of Sciences, Hungary
Copyright © 2023 Li, Ai, Kang, Zhang and Tuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dengfeng Tuo, ZGZ0dW9AY2FmLmFjLmNu
 Qiang Li
Qiang Li Feng Ai1
Feng Ai1 Dengfeng Tuo
Dengfeng Tuo