
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci. , 09 June 2023
Sec. Water and Wastewater Management
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1161465
The number of outbreaks of water-borne diseases caused by parasites seems to have increased in recent years. Nevertheless, the occurrence of these pathogens in water generally pays little attention. Waterborne transmission is a major route in the epidemiology of the parasite and therefore poses a serious public health problem. Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon spp. parasites are recognised worldwide as a common cause of diarrhoea. In most cases, it is a dilapidated or poorly maintained standard sanitation and water supply. It is important to perform periodic tests on protozoa, which are often lacking in small laboratories. Since it is necessary to filter large volumes of water for reliable diagnostics and consequently, it is difficult to concentrate them in a large volume of filtrate, it is not easy to detect their presence in the water. Various filtration methods are used to filter these pathogens from water, but cryptosporidial oocysts and microsporidia spores still occur in most of the world’s and Slovak recreational waters. Therefore, it would be appropriate to use the abilities of gill-breathing aquatic animals that filter cryptosporidial oocysts and microsporidia spores from the water by absorbing them with food. Zeolite can also purify water by capturing high concentrations of contaminants, including cryptosporidial oocysts and microsporidial spores.
One of the leading causes of death in children under the age of 5 is acute diarrhoea, especially in developing countries (Mohammad et al., 2021). The reason may also be the widespread occurrence of zoonotic parasites Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon spp. in the environment, including waters, which are transported by the faecal-oral route (Omolabi et al., 2021). Although infections caused by these three parasites are poorly reported, their prevalence is relatively high. Dong et al. (2020) examined the incidence of Cryptosporidium in the general population, patients, school-aged children and the healthy population, with the highest estimated prevalence of Cryptosporidium infection in Mexico (69.6%, 95% CI 66.3–72.8), Nigeria (34.0%, 95% CI 12.4–60.0), Bangladesh (42.5%, 95% CI 36.1–49.0), Republic of Korea (8.3%, 95% CI 4,4–13,2) Although the worldwide prevalence of Encephalitozoon spp. and Enterocytozoon spp. is around 10% (Kucerova-Pospisilova et al., 1998; Halanova et al., 2013; Pan et al., 2015; Zang et al., 2021), in China is up to 41% – 44% incidence of Enterocytozoon bieneusi (Cao et al., 2020). Whereas infection can also be transmitted to humans by water and the environment (Guy et al., 2021), not only by direct contact; cryptosporidiosis, encephalitozoonosis and enterocytozoonosis are among the most common water-borne diseases. Oocysts and spores are transported by rivers, reducing their viability and concentration. Clinical surveillance and monitoring of aquatic pathogens are essential tools to detect and prevent further spread and minimise the outbreak’s extent. However, clinical trials are usually limited to sufficiently ill individuals to seek treatment, leading to underestimating the prevalence of the disease (Yulfi et al., 2021) and providing a delayed outbreak indicator in the community. Analysis of large volumes of water is also needed to screen for pathogens from source and treated drinking water, as amounts from one infectious virion to 500 kL of water are considered sufficient concentrations for viral pathogens to detect them (Zahedi et al., 2021). Microbial contaminants, including bacteria, viruses and unicellular parasites, are the most important components to be controlled in reclaimed water due to potential effects on human health due to short-term exposure and ingestion. Most effects occur shortly after exposure, although the chronic effects of infections are also known. The highest number of conditions reported after contact with drinking, recreational and environmental water containing Cryptosporidium was in the USA in 2014, where up to 24 people lost their lives due to this parasite (Teel et al., 2022). One death after infection with the parasite Encephalitozoon spp. has been reported by tap water (Collier et al., 2021). Deaths against Enterocytozoon spp. are not yet known. Nevertheless, an epidemic has occurred in connection with this parasite, in which 200 people have become infected due to poor treatment of drinking water and its contamination (Cotte et al., 1999). The remaining parasites were also identified in rivers, lakes, drinking and wastewater. Cervero-Aragó et al. (2021) detected from surface waters in Austria Cryptosporidium spp. in 60% of samples. According to Ruan et al. (2021) overall prevalence rate of Encephalitozoon spp. in water was 58.5%. Still, subgroup analysis showed that the prevalence rate in wastewater treatment plants was much higher than in other waters (up to 74.1%). Also, the overall detection rate of Enterocytozoon bieneusi in water was high, up to 64.5% (Ruan et al., 2021). Overview transmission of Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon bieneusi through water is essential for disease risk assessment and for designing effective preventive measures.
In Slovakia, diseases caused by the parasites Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon bieneusi are among the Diseases reported with positive laboratory results. Cryptosporidiosis was first reported in seven HIV/AIDS patients (Čatár and Sobota, 1987).
In 2012, one case was reported in Slovakia, while in 2013, up to 12 cases of cryptosporidiosis were reported (RÚVZ, 2012; ÚVZ, 2013). They were recorded in two siblings in 2013, of whom only a seven-year-old boy showed clinical signs of the disease. Using DNA typing, C. hominis species was identified in these children (Ondriska et al., 2013).
In 2014, one case of an infection caused by Cryptosporidium spp. ÚVZ (2015).
At present, other infections of children from Roma settlements or children’s homes in eastern Slovakia are also known, which were caused mainly by C. hominis and C. muris (Hasajová et al., 2014).
Human cryptosporidiosis was also caused by C. parvum in an immunocompetent patient in Slovakia, with the probable transmission of infectious oocysts occurring by direct contact with infected calves from Zemplínska Teplica and subsequent poor hand hygiene (Mravcová et al., 2020).
In 2003, Čisláková et al., 2003 microsporidia infections in 14% of immunocompromised patients; in 2013, Halánová et al. identified E. bieneusi genotype A and E. cuniculi genotype I in 30.5% of children living in Roma settlements and 2019 Halánová et al. detected E. intestinalis in up to 33% of immunocompromised patients and 5.7% of immunocompetent people.
Although methodologies for detecting pathogens are well described and mastered today, sampling and concentration are still, to some extent, a problem. Costs and logistics are more demanding when larger samples are needed. A promising concentration method appears to be the hollow fibre dead-end ultrafiltration method DEUF (Kahler and Hill, 2020), which serves for the concentration of pathogens in water samples with different physico-chemical properties. It is designed to be performed in the field with minimal equipment and setup. In the DEUF process, water flows into the ultrafilter through the hollow fibre membranes and out of the pores of the ultrafilter, while the microbes are trapped in the hollow fibres. Ultrafilters can filter 10–50 L of turbid surface water or hundreds of litres of ready-made drinking water. The volume of filtered water thus depends on the water quality characteristics and the predicted concentrations of the target microorganisms. Several methods of filtering parasites from water samples are used worldwide, but the most common are filtering techniques using filters, which are summarized in Table 1. After filtration, the ultrafilter is processed in the laboratory. Alternative water filtration techniques are also used, but they must meet the required technical parameters and performance characteristics.
To capture oocysts of Cryptosporidium spp. and spores Encephalitozoon spp. and Enterocytozoon bieneusi have proven other alternative water filtration techniques (Table 2), but must meet the required technical parameters and performance characteristics. Isolation of Encephalitozoon spp. and Enterocytozoon spp. from raw wastewater and treated wastewater is practised using a combined system (Yamashiro et al., 2017). Primary wastewater samples with a volume of at least 100 L are subjected to centrifugal and concentration techniques (Cantusio et al., 2006), including centrifugation and washing. Subsequently, the wastewater samples are subjected to a membrane filtration technique (Franco et al., 2001).
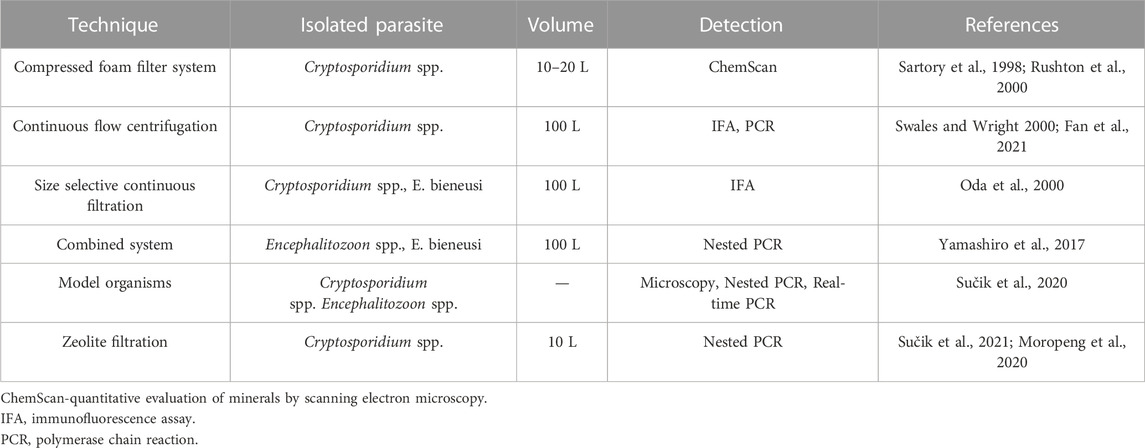
TABLE 2. Alternative techniques used to concentrate Cryptosporidium oocysts, Encephalitozoon spp. and Enterocytozoon bieneusi from the environment.
For the detection of Cryptosporidium spp. the sample concentrate can also be subjected to microscopy using staining. The most used technique for staining oocysts is the Ziehl-Neelsen technique. The two-step Ziehl-Neelsen staining process stains all tissue cells pink with a basic fuchsin solution in the first step. In a second step, the tissue is incubated in an acidic alcohol solution, which decolorizes all cells except the acid-fast cells, which retain their color and appear red. The mechanisms by which this color is produced are not well understood, but it is thought that the interaction of basic fuchsin with components of the bacterial cell wall creates a new molecule that is responsible for the color (Van Deun et al., 2008). Fayer, Morgan, Upton (2000) also used the modified Ziehl-Neelsen technique (Casemore et al., 1985) to detect cryptosporidia. Oocysts of Cryptosporidium spp. appeared pinkish-red, almost spherical, and measured 4–6 μm. Saha et al. (2019) saw cryptosporidial oocysts as light red to dark red spherical bodies, containing granules or bubbles and measuring 8–10 μm. The samples are always microscopically observed at least twice to avoid errors. Results are compared to the Centers for Disease Control and Prevention image gallery. Staining according to Kinyon, staining according to Miláček and Vítovec, dimethyl sulfoxide-carbol fuchsin staining and safranin-methylene blue are used to stain oocysts (Magi et al., 2006).
Light microscopic examination of stained clinical smears, especially stool samples, is an inexpensive method for the detection of microsporidia spores, although it does not allow their identification to the species level. The most commonly used staining technique is the Chromotrope 2R method or its modifications (Feng et Li, 2017).
Transmission electron microscopy (TEM) has long been the gold standard for the identification of microsporidia based on polar filament observation in organisms and is still important for observing and describing the ultrastructural features of developing and mature organisms, but it is too expensive, time-consuming, and unsuitable for routine diagnosis (Weber, Deplazes, Schwartz, 2000; Feng et Li, 2017). Weber’s chromotropic staining was also used by Galván et al. for the examination of microsporidia. (2013). Using optical brighteners, spores are visualized under a fluorescence microscope due to the binding of the optical brighteners to the chitin in the spore wall. Depending on the reagent used as well as the wavelength, the microsporidian spore walls will fluoresce. When using Uvitex 2B, Rylux D (Ostacolor, Prague, Czech Republic) and a wavelength of 405-490 (light during observation, 510 nm), the spores appear as green-white or turquoise oval formations (Valenčáková et Sučik, 2020).
Luka et al., in 2019, in their studies, they presented a label-free interdigitated capacitive biosensor for the detection of oocysts of Cryptosporidium spp. in water samples. Specific anti-Cryptosporidium monoclonal antibodies (IgG3) were covalently immobilized on interdigitated gold electrodes as capture probes, and bovine serum albumin was used to prevent nonspecific adsorption. Antibody immobilization was confirmed by measuring the change in contact angle. Detection was achieved by measuring the relative change in capacitance/dielectric properties due to the formation of the Cryptosporidium-antibody complex. The biosensor was tested for different concentrations of Cryptosporidium spp.
The results show that the developed biosensor can accurately distinguish different captured cell numbers and densities on the biosensor surface. The number of Cryprosporidia oocysts captured on the electrode surface was confirmed using a fluorescein isothiocyanate (FITC) immunofluorescence assay. The response of the developed biosensor depends mainly on the concentration of Cryptosporidium spp. under optimized conditions. The biosensor demonstrated a linear detection range between 15 and 153 cells/mm2 with a detection limit of 40 cells/mm2. The developed label-free capacitive biosensor has great potential for the detection of cryptosporidia in environmental water samples. Furthermore, under optimized conditions, this label-free biosensor can be extended to detect other biomarkers for biomedical and environmental analyses.
Serological tests are more sensitive and specific compared to microscopy. A direct immunofluorescence test (IFA or DFA) was also used for the detection of cryptosporidia by Pignata et al. (2019), using fluorescently labeled Cryptosporidium monoclonal antibody (Cellabs, Sydney, Australia).
As an alternative to examining the presence of Encephalitozoon spp. and Enterocytozoon bieneusi in the host is the use of commercially produced monoclonal antibodies, diagnosed in people with AIDS (Thellier et al., 2005) through stool. However, at certain stages of infection and in cases of chronic infections, excretion of spores through feces is not the rule. Therefore, in the case of a small number of samples (up to 10 samples), it is necessary to supplement the diagnosis with the IFAT serological method or, in the case of a larger number of samples, ELISA for the detection of antibodies against clinically significant types of microsporidia.
The Elisa Reagent test kit (Jining Industry Co., Ltd., Shanghai, China) was used to detect E. cuniculi antibody by Wang et al., 2022. The presence of E. intestinalis and E. bieneusi was proved by immunofluorescence test using species-specific monoclonal antibodies Halánová et al., 2019 in immunocompromised patients in Slovakia.
Over the past 20 years, PCR-based molecular methods for amplification of gene targets have been developed and increasingly applied to improve sensitivity and species specificity in the diagnosis of both cryptosporidiosis and microsporidiosis (Feng et Li, 2017; Dashti et al., 2022). Compared to traditional methods based on microscopy, these molecular methods can offer potential advantages such as increased sensitivity, higher specificity, faster time to result and easier interpretation.
Researchers in Pakistan (Abbas et al., 2022) amplified the 18S ribosomal RNA gene for the detection of cryptosporidia in water, soil and food using newly designed genus-specific primers through a Multiplex PCR reaction that uses several types of primers to amplify multiple fragments in a single DNA sample.
Standard PCR was used to detect cryptosporidia in a biofilm in the Philippines (Masangkay et al., 2022), which is a fast and simple method used to amplify DNA sequences in vitro, which is based on the principle of enzymatic replication of nucleic acids. To quantify the number of cryptosporidia in biofilms, Koh et al. (2013) used a quantitative polymerase chain reaction (qPCR) technique in which DNA molecules are labeled with a fluorescent dye that is used to monitor and quantify PCR products in real time. Real-time PCR was also used by Elwin et al. (2022) for genotyping Cryptosporidium spp. while monitoring the pool water on the water slide.
E. bieneusi (genotypes similar to C and D), E. intestinalis and E. cuniculi (genotypes I and III) were detected by classical PCR in the analyzed water samples using different pairs of diagnostic primers (Galván et al., 2013).
Cantusio et al. (2006) confirmed the presence of E. bieneusi (AM1, AM3, AM27) in raw wastewater using Nested PCR, which consists of two steps: after the initial 25-35 PCR cycles, another PCR is performed using new primers “nested” into the original primers, which reduces the risk of unwanted products. E. intestinalis and E. bieneusi species were also identified by microsporidial SSU-rDNA amplification in waste, surface and groundwater samples (Fan et al., 2021).
Climate change and population growth are the causes of deteriorating river water quality. Predictions of climate change point to a higher frequency and intensity of extreme precipitation in many areas, while increased discharges of (treated) wastewater can affect faecal pollution, microbiological surface water quality and, ultimately, drinking water safety (Demeter et al., 2021).
Progress in the disposal and treatment of municipal wastewater is a basic prerequisite for sustainable development and environmental protection. At the same time, the existence of water management infrastructure also supports further social and economic growth in the regions (Cao et al., 2021). Slovakia has something to catch up with in terms of public sewers. In January 2018, 68% of the population reached the connection to the public sewerage system, but only less than 40% of the municipalities have a public sewerage system or only a partially built one. Building public sewers is a very important step in protecting groundwater quality. In particular, improper wastewater treatment or leaking sumps pose a significant risk to groundwater (MoEPSR, 2019).
Problems of protozoan parasites (Cryptosporidium) in the aquatic environment are comprehensive. Given that Slovakia is an industrial and agricultural country, we can expect that these microorganisms can contaminate water resources. A possible source of contamination can also be contaminated water with faeces used for irrigation. Therefore, the investigated areas (Figure 1) are interesting for studying these pathogens.
The presence of Cryptosporidium spp. in the waters of Slovakia is generally little studied, microsporidia Encephalitozoon spp. and Enterocytozoon spp. attention has not yet been paid to this area.
The pioneer in this issue was Velická, which in the period 2001–2003, using Micro-Wynd filters, isolated C. parvum from water reservoirs in Slovakia (Klenovec, Hriňová, Nová Bystrica, Málinec, Turček, Starina and Bukovec), while the sample volume was 10 L. After methyl violet staining, according to Miláček and Vítovec (1985) and microscopic determination, 41.4% of the samples were positive for C. parvum, and repeated determinations confirmed these findings. However, cryptosporidia have also been identified in several samples of already treated waters (Velická, 2007). Another researcher was Hatalová et al., who isolated Cryptosporidium spp. from the waters of eastern Slovakia using Artemia metanauplii, with a sample volume of 5 L. The observed parasite was found by Nested PCR in two of the five localities, namely, C. parvum, genotype IIaA16G1R1 in the Nad jazerom urban settlement lake in Košice and C. hominis genotype IeA11G3T3 in the Geča Lakes in the Košice Region (Hatalová et al., 2019). Kalinová et al. (2019), in their study, examined water samples from the Nitra region, used membrane microfilters made of a mixture of cellulose acetate and cellulose nitrate to capture cryptosporidia, and the sample volume was also 5 L. DNA sequencing resulted in four positives out of ten water bodies. The Jelenec and Vráble ponds were positive for C. hominis, genotype IeA11G3T3, Čierne Kľačany for C. parvum, genotype IIaA15G1R1 and C. parvum, genotype IIaA16G1R1 was found in the Slepčany reservoir.
To prove the presence of individual species of Cryptosporidium spp. microscopic methods were used, using the staining method of Miláček, Vitovec (1985), and molecular methods, namely, Nested PCR (Xiao et al., 1999).
At our workplace, we have analyzed the presence of Cryptosporidium spp. and Encephalitozoon spp., in aquatic animals: Rivulogammarus fossarum, Lymnaea stagnalis, Unio tumidus, Dytiscus marginalis, Lymnaea stagnalis. from 5 water reservoirs (Bukovec, Kechnec, Čaňa1, Geča, Čaňa2). Ziel Neelson staining with Kinyoun modification (Ma and Soave, 1983) was used to detect cryptosporidia (Figure 1). Microsporidia were stained by fluorescence (Vávra et al., 1993). Subsequently, we observed the gut of the animal samples or gills in animals with gills using a fluorescence FL-800 microscope (Figure 2). DNA analysis of Cryptosporidium spp. was performed by Nested PCR, using primers Xiao F1/R1 and Xiao F2/R2 (Xiao, 2010). Real-time PCR was used to diagnose Encephalitozoon spp. with primers 530F and 580R (Danišová et al., 2021). After receiving the sequences and comparing them with the homologous sequences from the GenBank using the BLAST program, the presence of Cryptosporidium parvum species in the Čaňa1 and Encephalitozoon cuniculi reservoirs was confirmed in the Čaňa1, Geča, and Čaňa2 reservoirs (Table 3).
Zeolite, which together with quaternary ammonium chloride (QAC) has antimicrobial properties as a filter medium, can also be used for the early inactivation of parasites (Abbaszadegan et al., 2006). Due to the high pore density, it has an effective surface and can thus capture high concentrations of contaminants, which can also be applied to treat drinking water.
We compared the ability of oocyst catchability and properties when handling two types of zeolite, with smaller and larger particles. We came to the conclusion that easier handling was with coarser grained zeolite, it also achieved better results of oocyst filtration, even in a shorter time (Table 4).
This systematic review aimed to compare the methods of concentration Cryptosporidium spp. oocysts and spores Encephalitozoon spp. and Enterocytozoon spp., to point out the possibility of using model organisms as filters and describing these pathogens’ presence in waters in Slovakia. The occurrence of pathogens Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon spp. is a relatively common phenomenon in drinking water sources and represent a significant problem due to the difficulty of detecting their presence. Both oocysts and spores are resistant to the many drinking water disinfectants used, so it is almost impossible to prevent their presence in the water. Due to the high prevalence of Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon spp. in water, emphasis should be placed on its more frequent diagnosis. The mentioned genotypes, found in Slovak water samples, were also found in the other countries, specifically in lakes freely accessible to people for swimming, located in the cottage area, where the sewerage system has not been built yet. Specifically, the IeA11G3T3 genotype is anthroponotic in nature; it was the most common subtype contributing to morbidity in Israel (Grossman et al., 2019), and was found in HIV-positive patients in Thailand (Sannella et al., 2019), Zambia (Mulunda et al., 2020) or Mexico (Urrea-Quezada et al., 2018). Genotype IIaA16G1R1 has been identified in cancer patients not only in Slovakia (Hatalová et al., 2018) but also in Slovenia, Estonia, Romania, Canada, Mexico and Tasmania (Soba et Logar, 2008; Koehler et al., 2014; Lassen et al., 2014; Valenzuela et al., 2014; Iqbal et al., 2015; Vieira et al., 2015). There are also studies confirming this genotype in rivers in Romania (Imre et al., 2017) or in donkeys and horses in Algeria (Laatamna et al., 2015).
As the zoonotic species C. parvum, C. hominis, and E. cuniculi have been identified in several bathing water samples, it can be assessed that little attention is paid to this issue given the risk these parasites pose to human and animal health.
For prevention, it would be appropriate to use gill-breathing aquatic animals to filtrate parasites, which absorb cryptosporidia and microsporidia spores of Encephalitozoon spp. and Enterocytozoon spp. as the results show, gill-breathing aquatic animals are suitable for detecting opportunistic pathogens in water. These species have been studied, among others, by researchers in the Czech Republic. The model organisms that effectively absorb oocysts have been used Margaritifera spp., Rotifera spp., Anostraca spp., Bivalvia spp. and Gastropoda spp. (Križanová, 2007; Rousková, 2008; Kociánová, 2009). This method for filtering opportunistic pathogens from water practised on aquatic organisms appears simple, ecological, economical and time-consuming.
The data suggest that zeolite has removed observed microorganisms from water so that it could be helpful for water and wastewater treatment of small volumes, as evidenced by multiple other world studies (Salazar et al., 2004; Abbaszadegan et al., 2006; Moropeng et al., 2020).
Due to its high pore density, it has an effective surface and thus can capture high concentrations of contaminants, which can also be applied to drinking water treatment. However, the disadvantage is the time-consuming method, so it cannot be used in practice to filter large volumes (100 L and more). Laboratory and field test data in the United States since the mid-1970s suggest that zeolite filter beds have 1.7 to 1.9 times the solids loading capacity/ft3 and excellent filtration performance compared to multimedia, even in tests. gravity filtration (Hansen, 1997; Fuger, 2003).
Based on more than 100 laboratory and field tests (2/3 using pressure vessels and 1/3 using gravity beds) since the mid-1990s, which represent commercial, residential and industrial water filtration projects, it has been concluded that in terms of solids loading capacity, high purity zeolite outperformed multimedia, sand/anthracite and sand because it more efficiently removed fine particles in the 0.5 µ to 10 µ range that escape from the conventional medium (Johnson and Desborough, 2010).
On the contrary, the filtration method consisting of filtration apparatus and filters remains less time-consuming, which is more suitable for filtration of larger volumes and is, therefore, the most used in practice. Several filtration devices and filters made of different materials have been tested, and the multifunctional use of this method is also an advantage.
However, what remains a problem is the neglect of reporting the occurrence of these parasites in the waters and the ignorance of the professional public about the reporting of these diseases. The United Kingdom could be an inspiration in this direction, where the Drinking Water Inspectorate (DWI) requires water companies to carry out risk assessments at all their water supply points and to determine the level of risk posed by Cryptosporidium spp. represents the quality of the final treated water. UK regulations also require companies to design and operate appropriate treatment and disinfection on an ongoing basis. Proven non-compliance with this regulation is a criminal offence in this country (Zahedi et al., 2015). Australia has also issued a warning against these zoonoses occurring in the Northern Territory (NT). People are warned before Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon bieneusi by the Australian Veterinary Association, alerting to the possibility of indirect infection through contaminated water (AVA, 2021). So far, the only standardized method for detecting pathogens Cryptosporodium spp., Encephalitozoon sp. and Enterocytozoon bieneusi is United States Environmental Protection Agency (USEPA) Method 1623.1. An EnviroChek HV filter cartridge with a porosity of 1 µm is used to filter parasites from water samples, filtering 10–50 L of raw water. Immunomagnetic separation is used for the concentration of biological material and differential interference microscopy and fluorescence microscopy for detection, using fluorescein isothiocyanate and 4′,6-diamidino-2-phenylindole for cell staining (Fradette et Charette, 2022). The United States has also issued an EPA document on these parasites (USEPA, 1994) and its supplement (USEPA, 2001b), which contains sufficient information to conclude that Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon bieneusi can cause health problems. They occur in public water mains at levels that may pose a risk to human health.
Cryptosporidium spp., Encephalitozoon spp. and Enterocytozoon spp. are highly prevalent water parasites. These pathogens were detected in both wastewater and treated wastewater, and identical genotypes were detected from different treatment plants, which pose a high risk of infection to humans and animals. To reduce the number of infections caused by this parasite, a set of guidelines on wastewater use would be needed, as water is one of the most common sources of infection. Greater emphasis should also be placed on detecting and disinfection domestic water. Although filtering methods for this purpose are already known, the general public neglects their importance. This is not the case in Slovakia, despite the fact that Cryptosporidium spp. and Encephalitozoon spp. occurred in almost all of the water bodies studied, which are normally used for recreation. Model organisms eating these parasites as well as zeolite with its antimicrobial properties seem to be suitable options for preventing the occurrence of infection.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This research was funded by the grant project of the Ministry of Education, Science, Research and Sports of the Slovak Republic KEGA 004UVLF-4/2023.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, Z., Khan, M. K., Abbas, R. Z., Sindhu, Z. U. D., Sajid, M. S., Munir, A., et al. (2022). Molecular epidemiology of cryptosporidium parvum and giardia lamblia in different water bodies, soil, and vegetables in Pakistan. Health secur. 20 (4), 308–320. doi:10.1089/hs.2021.0118
Abbaszadegan, M., Monteiro, P., Ouwens, R. N., Ryu, H., and Alum, A. (2006). Removal and inactivation of Cryptosporidium and microbial indicators by a quaternary ammonium chloride (QAC)-treated zeolite in pilot filters. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 41 (6), 1201–1210. doi:10.1080/10934520600623091
AVA (2021). Last modified September, 2020. Available at: https://www.ava.com.au/ (Accessed April, 2022).
Bonilla, J. A., Bonilla, T. D., Amir, M., Abdelzaher, A., Scott, T., Lukasik, J., Solo-Gabriele, H., and Palmer, C. (2015). Quantification of Protozoa and viruses from small water volumes. Int. J. Environ. Res. Public Health 12, 7118–7132. doi:10.3390/ijerph120707118
Branco, N., Leal, D. A., and Franco, R. M. (2012). A parasitological survey of natural water springs and inhabitants of a tourist city in southeastern Brazil. Vector Borne Zoonotic 12 (5), 410–417. doi:10.1089/vbz.2011.0679
Cantusio, R., Santos, L. U., and Franco, R. M. B. (2006). Evaluation of activated sludge treatment and the efficiency of the disinfection of Giardia species cysts and Cryptosporidium oocysts by UV at a sludge treatment plant in Campinas, Southeast Brazil. Water Sci. Technol. 54 (3), 89–94. doi:10.2166/wst.2006.453
Cao, S., Xu, M., Jiang, Y., Liu, H., Yuan, Z., Sun, L., Cao, J., and Shen, Y. (2020). Prevalence and genetic characterization of cryptosporidium, giardia and enterocytozoon in chickens from ezhou, hubei, China. Front. Veterinary Sci. 7, 30–225. doi:10.3389/fvets.2020.00030
Cao, Y., Fang, Ch., Deng, J., Yu, F., Ma, D., Chuai, L., Wang, T., Qi, M., and Li, J. (2022). Molecular characterization of Cryptosporidium spp. and Giardia duodenalis in pet dogs in Xinjiang, China. Parasitol. Res. 121 (5), 1429–1435. doi:10.1007/s00436-022-07468-w
Casemore, D. P., Armstrong, M., and Sands, R. L. (1985). Laboratory diagnosis of cryptosporidiosis. J. Clin. Pathology 38 (12), 1337–1341. doi:10.1136/jcp.38.12.1337
Čatár, G., et, , and Sobota, K. (1987). “Cryptosporidiosis – parasitic intestinal infection,” in Reports of scientific conference of the 5th prowazels days (Komárno), 21–23.
Cervero-Aragó, S., Desvars-Larrive, A., Lindner, M., Sommer, R., Häfeli, I., and Walochnik, J. (2021). Surface waters and urban Brown rats as potential sources of human-infective cryptosporidium and giardia in vienna, Austria. Microorganisms 9 (8), 1596. doi:10.3390/microorganisms9081596
Cisláková, L., and Halánová, M. (2003). Mikrosporidiálne infekcie u imunokompromitovaných hospitalizovaných pacientov [Microsporidial infections in immunocompromised hospitalized patients]. Epidemiol Mikrobiol Imunol. 52 (2), 81–83. Slovak.
Collier, S. A., Deng, L., Adam, E. A., Benedict, K. M., Beshearse, E. M., Blackstock, A. J., Bruce, B. B., Derado, G., Edens, C., Fullerton, K. E., Gargano, J. W., Geissler, A. L., Hall, A. J., Havelaar, A. H., Hill, V. R., Hoekstra, R. M., Reddy, S. C., Scallan, E., Stokes, E. K., Yoder, J. S., and Beach, M. J. (2021). Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg. Infect. Dis. 27 (1), 140–149. doi:10.3201/eid2701.190676
Cotte, L., Rabodonirina, M., Chapuis, F., Bailly, F., Bissuel, F., Raynal, C., Gelas, P., Persat, F., Piens, M. A., and Trepo, C. (1999). Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J. Infect. Dis. 180, 2003–2008. doi:10.1086/315112
Danišová, O., and Valenčáková, A. (2021). First detection of blastocystis sp. in pigs in Slovakia and in europe. Parasitol 81, 102235. doi:10.1016/j.parint.2020.102235
Dashti, A., Alonso, H., Escolar-Miñana, C., Köster, P. C., Bailo, B., Carmena, D., et al. (2022). Evaluation of a novel commercial real-time PCR assay for the simultaneous detection of cryptosporidium spp., giardia duodenalis, and entamoeba histolytica. Microbiol. Spectr. 10 (3), e0053122. doi:10.1128/spectrum.00531-22
Demeter, K., Derx, J., Komma, J., Parajka, J., Schijven, J., Sommer, R., Cervero-Aragó, S., Lindner, G., Zoufal-Hruza, C., Linke, R., Savio, D., Ixenmaier, S., Kirschner, A., Kromp, H., Blaschke, A., and Farnleitner, A. (2021). Modelling the interplay of future changes and wastewater management measures on the microbiological river water quality considering safe drinking water production. Sci. Total Environ. 768, 144278. doi:10.1016/j.scitotenv.2020.144278
Dong, S., Yang, Y., Wang, Y., Yang, D., Yang, Y., Shi, Y., Li, C., Li, L., Chen, Y., Jiang, Q., and Zhou, Y. (2020). Prevalence of cryptosporidium infection in the global population: A systematic review and meta-analysis. Acta Parasitol. 65 (4), 882–889. doi:10.2478/s11686-020-00230-1
Elwin, K., Robinson, G., Pérez-Cordón, G., and Chalmers, R. M. (2022). Development and evaluation of a real-time PCR for genotyping of Cryptosporidium spp. from water monitoring slides. Exp. Parasitol. 242, 108366. doi:10.1016/j.exppara.2022.108366
Fan, Y., Wang, X., Yang, R., Zhao, W., Li, N., Guo, Y., Xiao, L., and Feng, Y. (2021). Molecular characterization of the waterborne pathogens Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi, Cyclospora cayetanensis and Eimeria spp. in wastewater and sewage in Guangzhou, China. Parasit. Vectors 14 (1), 66. doi:10.1186/s13071-020-04566-5
Fayer, R., Morgan, U., and Upton, S. J. (2000). Epidemiology of cryptosporidium: Transmission, detection and identification. Int. J. Parasitol. 30 (12), 1305–1322. doi:10.1016/s0020-7519(00)00135-1
Feng, Y., and et Li, N. (2017). “Microsporidia,” in Water and sanitation for the 21st century: Health and microbiological aspects of excreta and wastewater management (global water pathogen project). Editors J. B. Rose, and B. Jiménez-Cisneros
Fradette, M. S., and Charette, S. J. (2022). Working toward improved monitoring of cryptosporidium and giardia (oo)cysts in water samples: Testing alternatives to elution and immunomagnetic separation from USEPA method 1623.1. BMC Res. Notes 15 (1), 254. doi:10.1186/s13104-022-06118-9
Franco, R. M. B., Rocha-Eberhardt, R., and Cantusio, R. (2001). Occurrence of cryptosporidium oocysts and giardia cysts in raw water from the atibaia river, campinas, Brazil. Rev. Inst. Med. Trop. São Paulo 43 (2), 109–111. doi:10.1590/s0036-46652001000200011
Galván, A., Magnet, A., Izquierdo, F., Fenoy, S., Henriques-Gil, N., and del Aguila, C. (2013). Variability in minimal genomes: Analysis of tandem repeats in the microsporidia encephalitozoon intestinalis. Infect. Genet. Evol. 20, 26–33. doi:10.1016/j.meegid.2013.07.024
Grossman, T., Ken-Dror, S., Pavlotzky, E., Vainer, J., Glazer, Y., Sagi, O., Peretz, A., Agmon, V., Marva, E., and Valinsky, L. (2019). Molecular typing of cryptosporidium in Israel. PLoS One 14 (9), 02199777–e220029. doi:10.1371/journal.pone.0219977
Guy, R. A., Yanta, C. A., Muchaal, P. K., Rankin, M. A., Thivierge, K., Lau, R., and Boggild, A. K. (2021). Molecular characterization of Cryptosporidium isolates from humans in Ontario, Canada. Parasit. Vectors 14 (1), 69. doi:10.1186/s13071-020-04546-9
Halánová, M., Valenčáková, A., Malčeková, B., Kváč, M., Sak, B., Kvetonová, D., Bálent, P., and Čisláková, L. (2013). Occurrence of Microsporidia as emerging pathogens in Slovak Roma children and their impact on public health. Ann. Agric. Environ. Med. 20, 695–698.
Halánová, M., Valenčáková, A., Jarčuška, P., Halán, M., Danišová, O., Babinská, I., et al. (2019). Screening of opportunistic Encephalitozoon intestinalis and Enterocytozoon bieneusi in immunocompromised patients in Slovakia. Cent. Eur. J. Public Health 27 (4), 330–334. doi:10.21101/cejph.a5407
Hansen 1997: Engineering depart-ment, comparison of sand and zeolite filter media: Head loss for gravity beds. Pers. Commun. 19: 68–74. New Mexico State University, La Cruses, NM.
Hasajová, A., Valenčáková, A., Malčeková, B., Danišová, O., Halán, M., Goldová, M., et al. (2014). Significantly higher occurrence of Cryptosporidium infection in Roma children compared with non-Roma children in Slovakia. Eur. J. Clin. Microbiol. Infect. Dis. 33 (8), 1401–1406. doi:10.1007/s10096-014-2082-2
Hatalová, E., Valenčáková, A., Luptáková, L., Špalková, M., Kalinová, J., Halánová, M., Bednárová, V., Gabzdilová, J., Dedinská, K., Ondriska, F., and Boldiš, V. (2019). The first report of animal genotypes of Cryptosporidium parvum in immunosuppressed and immunocompetent humans in Slovakia. Transbound. Emerg. Dis. 66 (1), 243–249. doi:10.1111/tbed.13009
Hatalová, E., Valenčáková, A., Luptáková, L., Špalková, M., Kalinová, J., Halánová, M., et al. (2018). The first report of animal genotypes of Cryptosporidium parvum in immunosuppressed and immunocompetent humans in Slovakia. Transbound. Emerg. Dis. 00, 243–249. doi:10.1111/tbed.13009
Imre, K., Sala, C., Morar, A., Ilie, M. S., Plutzer, J., Imre, M., Dărăbuș, G., et al. (2017). Giardia duodenalis and Cryptosporidium spp. as contaminant protozoa of the main rivers of Western Romania: Genetic characterization and public health potential of the isolates. Environ. Sci. Pollut. Res. 24, 18672–18679. doi:10.1007/s11356-017-9543-y
Iqbal, A., Goldfarb, D. M., Slinger, R., and Dixon, B. R. (2015). Prevalence and molecular characterization of cryptosporidium spp. and giardia duodenalis in diarrhoeic patients in the qikiqtani region, nunavut, Canada. Int. J. Circumpolar Health 74, 27713. doi:10.3402/ijch.v74.27713
Johnson, T. S., and Desborough, G. A. (2010). Zeolite filter media: Setting a new standard for water filtration. Wastewater 11, 255.
Kahler, A. M., and et Hill, V. R. (2020). Detection of cryptosporidium recovered from large-volume water samples using dead-end ultrafiltration. Methods Mol. Biol. 2052, 23–41. doi:10.1007/978-1-4939-9748-0_3
Kalinová, J., Valenčáková, A., Hatalová, E., Danišová, O., Trungelová, M., and Rudolf, H. (2017). Occurrence of Cryptosporidium in the water basins of Nitra region, Slovakia. Acta Trop. 1, 179.
Kociánová, J. 2009: Osud oocýst kryptosporidií v prostředí, při kontaktu s různými skupinami bezobratlých, Jihočeská univerzita v Českých Budějovicích, Přírodovědecká fakulta. 65. Bakalářská práce.
Koehler, A. V., Whipp, M., Hogg, G., Haydon, S. R., Stevens, M., Jex, A. R., and Gasser, R. B. (2014). First genetic analysis of Cryptosporidium from humans from Tasmania, and identification of a new genotype from a traveller to Bali. Electrophoresis 35, 2600–2607. doi:10.1002/elps.201400225
Koh, W., Clode, P. L., Monis, P., and Thompson, R. C. A. (2013). Multiplication of the waterborne pathogen Cryptosporidium parvum in an aquatic biofilm system. Parasit. Vectors 6, 270. doi:10.1186/1756-3305-6-270
Križanová, M. (2007). Interakce mezi mlži (sinanodonta woodiana) a kryptosporidiemi (cryptosporidium parvum), biskupské gymnázium J.N.neumanna jirsíkova 5 370 21, ceské budějovice, středoškolská odborná činnost 2006/2007 obor 08 – ochrana a tvorba životního prostředí, 34.
Kucerova-Pospisilova, Z., and Ditrich, O. (1998). The serological surveillance of several groups of patients using antigens of Encephalitozoon hellem and E. cuniculi antibodies to microsporidia in patients. Folia Parasitol. (Praha) 45, 108–112.
Kumar, T., Abd Majid, M. A., Onichandran, S., Jaturas, N., Andiappan, H., Salibay, C. C., Tabo, H. A., Tabo, N., Dungca, J. Z., Tangpong, J., Phiriyasamith, S., Yuttayong, B., Polseela, R., Do, B. N., Sawangjaroen, N., Tan, T. C., Lim, Y. A., and Nissapatorn, V. (2016). Presence of cryptosporidium parvum and giardia lamblia in water samples from southeast asia: Towards an integrated water detection system. Infect. Dis. Poverty 5, 3. doi:10.1186/s40249-016-0095-z
Laatamna, A. E., Wagnerová, P., Sak, B., Květoňová, D., Xiao, L., Rost, M., McEvoy, J., Saadi, A. R., Aissi, M., and Kváč, M. (2015). Microsporidia and cryptosporidium in horses and donkeys in Algeria: Detection of a novel cryptosporidium hominis subtype family (Ik) in a horse. Vet. Parasitol. 208 (3-4), 135–142. doi:10.1016/j.vetpar.2015.01.007
Lassen, B., Ståhl, M., and Enemark, H. L. (2014). Cryptosporidiosis - an occupational risk and a disregarded disease in Estonia. Acta Veterinaria Scand. 56, 36. doi:10.1186/1751-0147-56-36
Luka, G., Samiei, E., Dehghani, S., Johnson, T., Najjaran, H., and Hoorfar, M. (2019). Label-free capacitive biosensor for detection of cryptosporidium. Sensors (Basel). 19 (2), 258. doi:10.3390/s19020258
Ma, L., Zhang, X., Jian, Y., Li, X., Wang, G., Hu, Y., and Karanis, P. (2019). Detection of Cryptosporidium and Giardia in the slaughterhouse, sewage and river waters of the Qinghai Tibetan plateau area (QTPA), China. Parasitol. Res. 118 (7), 2041–2051. doi:10.1007/s00436-019-06330-w
Ma, P., and Soave, R. (1983). Three-step stool examination for cryptosporidiosis in 10 homosexual men with protracted watery diarrhea. J. Infect. 147, 824–828. doi:10.1093/infdis/147.5.824
Magi, B., Canocchi, V., Tordini, G., Cellesi, C., and Barberi, A. (2006). Cryptosporidium infection: Diagnostic techniques. Parasitol. Res. 98 (2), 150–152. doi:10.1007/s00436-005-0050-6
Masangkay, F. R., Milanez, G. D., Dionisio, J. D., Ormita, L. A. G. L., Alvarez, A. V., and Karanis, P. (2022). Well water sources simultaneous contamination with cryptosporidium and acanthamoeba in east-southeast asia and acanthamoeba spp. in biofilms in the Philippines. Sci. Total Environ. 837, 155752. doi:10.1016/j.scitotenv.2022.155752
Milácek, P., and Vítovec, J. (1985). Differential staining of cryptosporidia by aniline-carbol-methyl violet and tartrazine in smears from feces and scrapings of intestinal mucosa. Folia Parasitol. (Praha). 32 (1), 50.
Ministry of the Environment of the Slovak Republic (2021). Ministry of the environment of the Slovak republic. Last modified August. Available at: https://www.minzp.sk/spravy/2019/oktober/po-rokoch-dockali-aj-male-obce-verejne-kanalizacie-vodovody-poputuju-desiatky-milionov-eur.html (Accessed July, 2022).
Mohammad, S. M., Ali, M. S., Abdel-Rahman, S. A., Moustafa, R. A., and Sarhan, M. H. (2021). Genotyping of Cryptosporidium species in children suffering from diarrhea in Sharkyia Governorate, Egypt. J. Infect. Dev. Ctries. 15 (10), 1539–1546. doi:10.3855/jidc.14367
MoPSR (2023). MoPSR yearbook of the Slovak ministry of defense. [online]. [cit. 2023-04-11] Available at: https://www.mod.gov.sk/data/files/4075_rocenka_2019_web-redux.pdf.
Moropeng, R. C. H. (2020). Momba MNB 2020: Mechanism of silver incorporated in biosand zeolite clay granular filters for the removal of Cryptosporidium parvum and Giardia lamblia from surface water at point of use. Desalination Water Treat. 1, 286–295.
Mravcová, K. (2020). Molekulárna diagnostika Cryptosporidium parvum u pacienta zo Slovenska. NewsLab 2, 107–108.
Mulunda, N. R., Hayashida, K., Yamagishi, J., Sianongo, S., Munsaka, G., Sugimoto, C., and Mutengo, M. M. (2020). Molecular characterization of Cryptosporidium spp. from patients with diarrhoea in Lusaka, Zambia. Parasite 27, 53. doi:10.1051/parasite/2020050
Oda, T. (2000). Size selective continuous flow filtration method for detection of Cryptosporidium and Giardia. Water Res. 34 (18), 4477–4481. doi:10.1016/s0043-1354(00)00205-0
Omolabi, K. F., Odeniran, P. O., and Soliman, M. E. (2021). A meta-analysis of Cryptosporidium species in humans from southern Africa (2000-2020). J. Parasit. Dis. 18, 304–316. doi:10.1007/s12639-021-01436-4
Ondriska, F., Vrabcová, I., Brinďáková, S., Kváč, M., Ditrich, O., Boldiš, V., et al. (2013). The first reported cases of human cryptosporidiosis caused by Cryptosporidium hominis in Slovak Republic. Folia Microbiol. 58, 69–73. doi:10.1007/s12223-012-0182-x
Pan, Y., Wang, S., Liu, X., Li, R., Sun, Y., and Gadahi, J. A. (2015). Seroprevalence of encephalitozoon cuniculi in humans and rabbits in China. Iran. J. Parasitol. 10 (2), 290–295.
Pignata, C., Bonetta, S., Bonetta, S., Cacciò, S. M., Sannella, A. R., Gilli, G., et al. (2019). Cryptosporidium oocyst contamination in drinking water: A case study in Italy. Int. J. Environ. Res. Public Health 10 (16), 2055. doi:10.3390/ijerph16112055
Rousková, L. (2008). Role perlooček jako filtrátorů oocyst kryptosporidií ve vodním sloupci, Přírodovědecká fakulta Jihočeské univerzity v Českých Budějovicích. Bakalářská práce 30.
Ruan, Y., Xu, X., He, Q., Li, L., Guo, J., Bao, J., Pan, G., Li, T., and Zhou, Z. (2021). The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasit. Vectors 14 (1), 186. doi:10.1186/s13071-021-04700-x 2021
Rushton, P., Place, B. M., and Lightfoot, N. F. (2000). An evaluation of a laser scanning device for the detection of Cryptosporidium parvum in treated water samples. Lett. Appl. Microbiol. 30 (4), 303–307. doi:10.1046/j.1472-765x.2000.00713.x
RÚVZ Prievidza (2012). Annual report of RÚVZ Prievidza based in Bojnice for the year 2012. [online]. [cit. 2023-04-11] Available at: https://www.ruvzpd.sk/download/VS/Vyrocna_sprava_RUVZPD_2012.pdf.
Saha, R., Saxena, B., Jamir, S. T., and Shekhar, S. (2019). Prevalence of cryptosporidiosis in symptomatic immunocompetent children and comparative evaluation of its diagnosis by Ziehl-Neelsen staining and antigen detection techniques. Trop. Parasitol. 9 (1), 18–22. doi:10.4103/tp.TP_59_18
Salazar, C. (2004). Evaluation of surfactant-modified zeolite for control of Cryptosporidium and Giardia species in drinking water. [online]. [cit. 2023-04-14] Dostupné na internete. Available at: http://www.ees.nmt.edu/outside/alumni/papers/2004t_salazar_cm.pdf.
Sannella, A. R., Suputtamongkol, I., Wongsawat, E., and Cacciò, S. (2019). A retrospective molecular study of Cryptosporidium species and genotypes in HIV-infected patients from Thailand. Parasites Vectors 12, 91. doi:10.1186/s13071-019-3348-4
Sartory, D. P., Parton, A., Parton, A. C., Roberts, J., and Bergmann, K. (2003). Recovery of Cryptosporidium oocysts from small and large volume water samples using a compressed foam filter system. Lett. Appl. Microbiol. 27, 318–322. doi:10.1046/j.1472-765x.1998.00459.x
Soba, B., and Logar, J. (2008). Genetic classification of Cryptosporidium isolates from humans and calves in Slovenia. Parasitology 135, 1263–1270. doi:10.1017/S0031182008004800
Stine, S. W., Song, I., Choi, Ch.I., and Gerba, Ch.P. (2005). Application of microbial risk assessment to the development of standards for enteric pathogens in water used to irrigate fresh produce. J. Food Prot. 68 (5), 913–918. doi:10.4315/0362-028x-68.5.913
Sučik, M., Valenčáková, A., and Galajda, R. (2021). Use of zeolite as a filter to capture opportunistic pathogens in water. Sci. Cannot Wait New Horizons 1, 46.
Sučik, M., and Valenčáková, A. (2020). Vodné živočíchy ako bioindikátor Cryptosporidium spp. a Encephalitozoon spp. v povrchových a pobrežných vodách. Voda a Klim. zmeny 1, 62–65.
Swales (2000). Evaluation of a continuous flow centrifuge for recovery of Cryptosporidium oocysts from large volume water samples. Water Res. 34, 1962–1966. doi:10.1016/s0043-1354(99)00353-x
Teel, L., Olivieri, A., Danielson, R., Delić, B., Pecson, B., Crook, J., and Pagilla, K. (2022). Protozoa reduction through secondary wastewater treatment in two water reclamation facilities. Sci. Total Environ. 807 (3), 151053. doi:10.1016/j.scitotenv.2021.151053
Thellier, M. (2005). The first united workshop on microsporidia from invertebrate and vertebrate hosts. Folia Parasitol. 52, 1–7. doi:10.14411/fp.2005.001
Urrea-Quezada, A., González-Díaz, M., Villegas-Gómez, I., Durazo, M., Hernández, J., Xiao, L., and Valenzuela, O. (2018). Clinical manifestations of cryptosporidiosis and identification of a new cryptosporidium subtype in patients from sonora, Mexico. Pediatr. Infect. Dis. J. 37 (5), e136–e138. doi:10.1097/INF.0000000000001762
USEPA (1994). Draft drinking water criteria document for cryptosporidium. Prepared by clement international corporation. Washington: Prepared for EPA Office of Water, Office of Science and Technology.
USEPA (2001b). Drinking water criteria document addendum: Cryptosporidium. Washington: United States Environmental Protection Agency, Office of Water.
ÚVZ (2013). Annual report on the activities of public health authorities of the Slovak Republic by individual departments of public health for the year 2013. [online]. [cit. 2023-04-11] Available at: https://www.uvzsr.sk/docs/vs/vyrocna_sprava_SR_2013.pdf.
ÚVZ (2015). Annual report on the activities of public health authorities of the Slovak Republic by individual departments of public health for the year 2015. [online]. [cit. 2023-04-11] Available at: https://www.uvzsr.sk/docs/vs/vyrocna_sprava_SR_2015.pdf.
Valenčáková, A., and Sučik, M. (2020). Alternatives in molecular diagnostics of encephalitozoon and enterocytozoon infections. J. Fungi (Basel) 6 (3), 114. doi:10.3390/jof6030114
Valenzuela, O., González-Díaz, M., Garibay-Escobar, A., Burgara-Estrella, A., Cano, M., Durazo, M., Xiao, L., et al. (2014). Molecular characterization of Cryptosporidium spp. in children from Mexico. PLoS ONE 9, e96128. doi:10.1371/journal.pone.0096128
Van Deun, A., Hossain, M. A., Gumusboga, M., and Rieder, H. L. (2008). Ziehl-neelsen staining: Theory and practice. Int. J. Tuberc. Lung Dis. 12 (1), 108–110.
Vávra, J., Nohýnková, E., Machala, L., and Spála, J. (1993). An extremely rapid method for detection of Microsporidia in biopsy materials from AIDS patients. Folia Parasitol. 40, 273–274.
Velická, Z., Tóthová, L., and Mogoňová, E. (2007). Sledovanie výskytu Cryptosporidium parvum, Giardia Lamblia a Clostridium Perfringens vo vybraných nádržiach a skupinovom vodovode na Slovensku. Acta Environ. Univ. Comen. Bratisl. 15 (1), 90–99.
Vieira, P. M., Mederle, N., Lobo, M. L., Imre, K., Mederle, O., Xiao, L., Matos, O., et al. (2015). Molecular characterisation of Cryptosporidium (Apicomplexa) in children and cattle in Romania. Folia Parasitol. 62, 2015.002. doi:10.14411/fp.2015.002
Wang, Y., Qin, X., Diao, X., Liu, Y., and Liu, J. (2022). Serological survey for antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in pet rabbits in eastern coastal areas of China. J. Vet. Med. Sci. 84 (6), 777–783. doi:10.1292/jvms.21-0660
Weber, R., Deplazes, P., and Schwartz, D. (2000). Diagnosis and clinical aspects of human microsporidiosis. Contributions Microbiol. 6, 166–192. doi:10.1159/000060360
Xiao, L. (2010). Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 124, 80–89. doi:10.1016/j.exppara.2009.03.018
Xiao, L., Morgan, U. M., Limor, J., Escalante, A., Arrowood, M., Shulaw, W., et al. (1999). Genetic diversity within cryptosporidium parvum and related cryptosporidium species. Appl. Environ. Microbiol. 65 (8), 3386–3391. doi:10.1128/AEM.65.8.3386-3391.1999
Yamashiro, S., Fiuza, V. R. D. S., Teixeira, Â. T. L. S., Branco, N., LevyIsabel, C. E., Siqueira de Castro, C. V., and Franco, R. M. B. (2017). Enterocytozoon bieneusi detected by molecular methods in raw sewage and treated effluent from a combined system in Brazil. Mem. Inst. Oswaldo Cruz 112 (6), 403–410. doi:10.1590/0074-02760160435
Yulfi, H., Fakhrur Rozi, M., Andriyani, Y., and Masyithah Darlan, D. (2021). Prevalence of Cryptosporidium spp. and Blastocystis hominis in faecal samples among diarrheic HIV patients in Medan, Indonesia. Med. Glas. (Zenica) 18 (1), 55–61. doi:10.17392/1271-21
Zahedi, A., Monis, P., Deere, D., and Ryan, U. (2021). Wastewater-based epidemiology-surveillance and early detection of waterborne pathogens with a focus on SARS-CoV-2, Cryptosporidium and Giardia. Parasitol. Res. 120 (12), 4167–4188. doi:10.1007/s00436-020-07023-5
Zahedi, A., Paparini, A., Jian, F., Robertson, I., and Ryan, U. (2015). Public health significance of zoonotic Cryptosporidium species in wildlife: Critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 5 (1), 88–109. doi:10.1016/j.ijppaw.2015.12.001
Keywords: Cryptosporidium spp, Encephalitozoon spp, Enterocytozoon spp, filtration, PCR
Citation: Sučik M and Valenčáková A (2023) Methods used for concentrating oocysts of Cryptosporidium spp., spores Encephalitozoon spp. and Enterocytozoon spp. and their occurrence in Slovak water samples. Front. Environ. Sci. 11:1161465. doi: 10.3389/fenvs.2023.1161465
Received: 08 February 2023; Accepted: 19 May 2023;
Published: 09 June 2023.
Edited by:
Mohamed Ashour, National Institute of Oceanography and Fisheries (NIOF), EgyptReviewed by:
Ahmed E. Alprol, National Institute of Oceanography and Fisheries (NIOF), EgyptCopyright © 2023 Sučik and Valenčáková. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monika Sučik, bW9uaWthLnN1Y2lrQHV2bGYuc2s=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.