
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci., 20 April 2023
Sec. Water and Wastewater Management
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1142227
This article is part of the Research TopicWater and Wastewater Treatment and Management in the Textile IndustryView all 5 articles
Textile industry wastewater has become a growing concern in recent years due to it has been characterized by a high load of organic dyes, suspended and dissolved solids, alkaline pH, and low biodegradability. As a result, environmental authorities necessitate textile industries to treat effluents before discharge into the environment. Tertiary filters, particularly membrane filtrations, are the most preferable process to recover good-quality water at the tertiary treatment phase, which feeds from secondary effluents, in wastewater treatment processes. However, fouling is still a challenge due to a higher load of suspended solids, colloids, organic matter, and a high level of bio-colloids (mostly from secondary effluents) in the textile wastewater treatment process. Bio-colloids are any colloidal entities of organic matter including microorganisms and their exudates. Hence, a coagulation/flocculation unit process, as a pretreatment option, is critical both at the primary treatment stage and after secondary (biological) effluents to prevent fouling problems at the tertiary filters. We reviewed identifying major foulants causing tertiary filter damage and the available pretreatment option for the removal of these foulants. We focus on and suggest the coagulation/flocculation process as a good pretreatment alternative to prevent filter fouling as it provides a reliable process to treat high water turbidity that arises from a high load of solids and colloids. Amongst different types of foulants, we focus on and present the colloidal solids and bio-colloidal foulants that could be major causes of fouling. These foulants are less understood and expected to be dominant in the textile industry wastewater, and established pretreatment alternatives are not well developed for the bio-foulants fed from the secondary effluent. Thus, these foulants need to be critically identified in the textile wastewater treatment plants to integrate suitable pretreatment options to prevent fouling potentiality. We proposed a coagulation/flocculation unit process as a pretreatment option to reduce colloidal and bio-colloidal fouling before the tertiary treatment stage, next to the secondary effluent, is critical.
The growth of population and rapid industrialization to fulfill the demands for food, shelter, and cloth are increasing nowadays. Hence, a considerable amount of wastewater is being generated from various industries that pose an adverse impact on human health (Badawi et al., 2022). Textile, pharmaceutical, thinner, and metallurgical industries are among the most common wastewater-generating industries (Baaloudj et al., 2022a; Samsami et al., 2020). Textile industries release a considerable amount of dyes to the environment although it is one of the leading contributors to a country’s economic growth. Its effluents play an important role in polluting natural water bodies and deteriorating the environment (Islam and Mostafa, 2020), which results in serious consequences for aquatic biota (Aragaw et al., 2022). Observable and objectionable amounts of dye and pigment are found in water bodies that are discharged from textile effluents. It is assessed that 0.7–1.6 million tons of dyes are produced yearly and 10%–15% is disposed as wastewater, making it major water pollutants (Bhatia et al., 2017; Varghese et al., 2019; Syafiuddin and Fulazzaky, 2020). Consequently, it is of most extreme significance to eliminate colors from wastewater viably to guarantee the protected release of the treated effluent into streams. Adsorption of several textile dyes is reportedly examined for the removal of these colors (Aragaw and Alene, 2022; Aragaw, 2020a; Aragaw, 2020b; Aragaw and Angerasa, 2019). However, adsorption-based removal of colors only cannot be adequate for the final discharge water quality, which could contain a high load of solid matter resulting in danger to the tertiary filter. Recently, microbial-based biological treatment techniques for different industrial wastewaters have been considered as acceptable alternatives so as to be an environmentally friendly strategy (Aragaw, 2021). However, it has a limitation on the final water quality and difficulties of the treatment plan operation.
Although the pollutants’ composition and concentration in textile and dyeing industries are varied significantly, it depends on the chemicals used in different unit processes, dye colors, alkaline pH, high suspended solids (SS), total dissolved solids (TDS), chemical oxygen demand (COD), biological matters (microorganisms), and different anionic (bromides, chlorides, nitrates, and bicarbonates), and metal ions that are well investigated (Jayapal et al., 2022; Yaseen and Scholz, 2019). As a result, discharging untreated or poorly treated textile industry effluents is harmful to the environment and aquatic life and leads to ecological imbalance and associated human health problems. This could further require a huge cost of rehabilitation and could affect the world’s economy in general.
Solids in wastewater can be present in the form of suspended solids, colloids, and dissolved solids. Comparatively, suspended solids (particles in suspension) are usually sized more than 1,000 nm, while colloids are particles that are not in the suspension range from 1 nm to 1,000 nm (Amerian et al., 2019). Unlike suspended solids, colloid solids do not separate when sitting for about enough time. Suspended solids from wastewater could be removed with simple conventional physical treatment techniques such as sedimentation and filtration, and dissolved solids can be easily removed with chemical and biological degradation (Ahmed et al., 2021), while colloid (depending on size and surface charge nature) solids could not be easily removed by conventional physical treatment and/or chemical and biological degradation methods. This is because colloidal materials are very stable in the liquid phase and cannot settle out and/or pass through the filter due to their small size and, hence, are difficult to remove (Kinyua et al., 2016). Thus, the removal of colloidal solids needs to be seen critically as it is the most difficult aspect of conventional wastewater treatment.
There are three basic treatment stages in the wastewater treatment process, namely, primary, secondary, and tertiary. Though all treatment stages are critical for the final discharged water, the tertiary treatment stage is tremendously important to recover good-quality water in the treatment process (Díaz-Garduño et al., 2017). Thus, in addition to the environmental impact of untreated textile industry wastewater, poorly treated effluents can affect the tertiary treatment efficiency of wastewater treatment plants (WWTPs) in terms of operational and resource costs. Particularly, these solids, organics, and bio-colloids (mostly high from the secondary effluent) in wastewater can impact tertiary filter techniques at the tertiary treatment stage, damaging them due to fouling (Chaipetch et al., 2021). Tertiary filters are the most preferable process to recover good-quality water, which feeds from secondary effluents, in wastewater treatment processes. Particularly, membrane filtration is highly effective because it has a higher impurity rejection potential with low associated costs (Abdel-Fatah, 2018). However, fouling is still a challenge due to a higher load of colloids and bio-colloids in the textile industry wastewater treatment process, thus requiring certain pretreatment options.
Among several treatment techniques, coagulation/flocculation is one of the most effective pretreatment techniques to remove dyes and solid materials for the primary treatment stage (Mcyotto et al., 2021). Ghaly et al. (2014) reported in their review paper that total suspended solids (TSS) in the textile was recorded as from 15 to 8,000 mg/L (Ghaly et al., 2014); hence, the coagulation/flocculation pretreatment process is incredibly important for the textile industry effluents. However, colloids and bio-colloids are a challenge for tertiary filers at the tertiary treatment stage. Different coagulants have been used for textile industry effluent treatment. These are aluminum coagulants including aluminum sulfate, aluminum chloride, and sodium aluminate and iron coagulants including ferric sulfate, ferrous sulfate, ferric chloride, and different polyelectrolytes. Though all these coagulants can be used to treat textile industry effluents, their efficiency highly depends on the pH value. Reports confirm that the high efficiency of coagulants is preferable in acidic media (Dotto et al., 2019). Furthermore, natural coagulants provide a more significant solid removal including COD. Therefore, recently, scholars have been searching for and evaluating natural coagulants, from leftover biomass, in the coagulation–flocculation process of dye-containing textile industry effluents as they are environmentally suitable and cost effective (Prabhakaran et al., 2020); synthetic coagulants have been applied for many years (Islam and Mostafa, 2018).
Scholars have prepared several coagulants for the coagulation/flocculation of various kinds of matter in textile wastewater. A coagulation/flocculation unit process, as a pretreatment option, is critical both at the primary treatment stage and after secondary effluents to prevent fouling problems for the tertiary filters. Thus, the objective of the present review is to explore the application of the coagulation/flocculation process as a good pretreatment alternative to prevent filtration fouling in tertiary filtration steps. Hence, the reported coagulation/flocculation treatment of textile industry wastewater for the removal of colloids and bio-colloids is yet to be summarized and evaluated in one single reference. Also, intervention for the conventional treatment process of the textile industry wastewater is required, adding a coagulation/flocculation unit process after secondary treatment. We reviewed the identification of major foulants causing filter damage and the available pretreatment option for the removal of these foulants, focusing on the coagulation/flocculation process as an important pretreatment alternative to prevent fouling in the tertiary filters. The proposed and recommended treatment plant flow layout is demonstrated. We focus on the colloid and bio-colloid foulants that could be major causes of filter fouling, as they are less understood and expected to be dominant in the textile industry wastewater. Also, there are no well-established and developed pretreatment alternatives for bio-foulants. We also recommend that these foulants need to be critically identified in the textile industry WWTP to integrate suitable pretreatment options, at a minimum cost, to prevent fouling potentiality since the effluent composition and concentration could be different depending on the manufacturing process of the textile industry.
A colloid is a type of mixture together with the solution and suspension (Mikhaylin and Bazinet, 2016). To be categorized under a colloid, the size of the substance in the dispersed phase must be between the size of a molecule and what can be seen with the naked eye (i.e., the substance’s dimensions must be between 1 nm and 1 µm. The substance is considered a solution if the dimensions are smaller than this, and if they are larger, then the substance is a suspension (Lu and Weitz, 2013)).
The three main types of colloids are sol (solid in liquid), gel (liquid in solid), and emulsion (liquid in liquid) (Vincent, 2009). The other common classification is based on the nature of the interaction between the dispersed phase and medium. Lyophilic colloids are solvent loving and lyophobic colloids are solvent hating. Lyophilic colloids include soaps, starch, protein, gelatin, and gum, dissolved in water; they are known as hydrophilic. Lyophobic colloids include metals, metal sulfides, and oxides; they are known as hydrophobic. Because of the difference in their characteristics, they react differently to alterations in their environment (Koohestanian et al., 2008). This could be difficult not only for the tertiary filters but also for the pipeline; they can clog and damage the system.
Colloids are often a source of a relatively high percentage of turbidity, color, biochemical oxygen demand (BOD), and COD of wastewater. It is important to understand the physical and chemical characteristics of colloids before they can enter streams due to the necessity of removing them from wastewater. In colloids, one substance is dispersed in another due to its extremely small size, low particle weight, state of hydration, and surface charge. The foremost behavior of colloids is an excessive charge on the surface, which causes the adsorption of ions from the surrounding solution (Mikhaylin and Bazinet, 2016). Colloids exhibit “Brownian motion,” a bombardment of the particles of the dispersed phase by molecules of the dispersion medium. They are dialyzable so that they can be separated from their crystalloid (low-molecular-weight) counterparts by straining through a semipermeable membrane (Hughes, 2010). The colloids diffuse very slowly compared to soluble ions. In general, colloidal particles exhibit very low osmotic pressure due to their large size relative to the size of soluble ions (Kingsley Ogemdi, 2019). They also have the characteristic of “imbibition” (the taking in of water by gels). Colloidal gels are very often used as ultrafilters, having pores sufficiently small to retain the dispersed phase of a colloidal system but large enough to allow the dispersion medium and its crystalloid solutes to pass through (Kingsley Ogemdi, 2019).
Furthermore, colloidal systems can possess a wide range of plasticity or viscosity. Usually, the viscosity of dilute lyophobic colloids is slightly greater than the viscosity of the pure dispersion medium. However, concentrated lyophobic colloids can have high viscosity. On the other hand, lyophilic systems may reach very high values of viscosity. With these types of colloids, a parabolic, rather than linear, relationship exists between viscosity and the concentration of the dispersed phase (Li et al., 2019). Many colloidal systems, especially lyophilic (gel) systems, exhibit the property of elasticity (Bormashenko et al., 2015). This property permits the gels to resist deformation and, thereby, recover their original shape and size once they have been deformed. If a concentrated beam of light is passed through a colloidal solution in which the dispersed phase has a different refractive index from that of the dispersion medium, its path is milky turbid when viewed perpendicularly. This is known as the Tyndall effect (see Table 1). Generally, being electrically charged to their surroundings is the major property of colloidal suspensions. An electric current passing through a colloidal system causes the positive particles to migrate to the cathode and the negative particles to migrate to the anode (Kingsley Ogemdi, 2019). Thus, these properties of colloids can highly affect the treatment systems, especially clogging the filters and pipelines, which need adequate pretreatment alternatives.
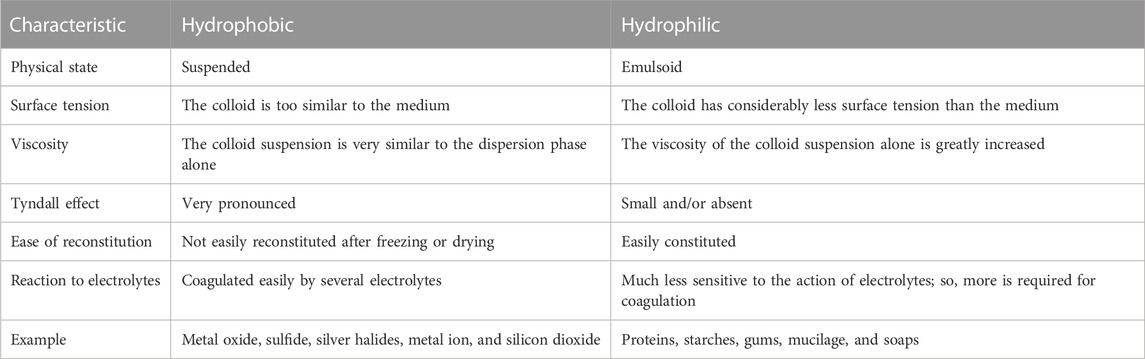
TABLE 1. Type and characteristics of colloidal solids (Kingsley Ogemdi, 2019).
Wastewater contains both colloids and bio-colloids. Colloids can either be organic matter or inorganic matter. Among organic colloids, macromolecules in the water mainly consist of materials such as humic acids, polysaccharides, and proteins, as well as some natural organic matters can be mentioned which lead to organic fouling in the filtration system (Ibrar et al., 2019). Most organic colloids can be enumerated as bio-colloids, but not all organic matter can be taken as bio-colloids. The major inorganic colloids found in wastewater include aluminum silicate minerals, silica, and iron oxides/hydroxides (Jiang et al., 2017). Bio-colloids include microorganisms, some biological debris, polysaccharides, and lipoproteins (Ezugbe and Rathilal, 2020). These particles can cause poor productivity of the filtration processes on a tertiary filter surface, especially a membrane surface, and maybe a salt rejection of the filter (Ibrar et al., 2019). Dye wastewater from the secondary treatment is expected to contain a high load of hydrophobic impurities, which can reduce the flux rate and enhance fouling (Thamaraiselvan and Noel, 2015).
A potential source of hydrophilic colloids in textile industry wastewater includes proteins, starches, soaps, and others that have been used in the different unit operations of fabric and dyeing processes (Azanaw et al., 2022). Most suspended solids smaller than 0.1 mm found in textile industry wastewater can carry negative electrostatic charges. The colloidal particle stability in textile industry wastewater can be determined by the zeta potential (Zp) values. The higher zeta potential indicates the greater repulsion forces between the colloidal particles and assures it is a more stable colloidal suspension, and vice versa for the lower Zp (Dotto et al., 2019). First, small particles should be destabilized and then larger and heavier flocs will be formed in which colloids can be removed by conventional physical treatment. This process comprises mechanisms of clarification such as coagulation/flocculation and sedimentation (Zhang et al., 2018). Coagulation/flocculation is known as one of the most developed and effective processes that can remove most of the colloidal particles in textile wastewater (Zhao et al., 2021; Shewa and Dagnew, 2020). Generally, the coagulation/flocculation mechanisms can be categorized into the following kinds: 1) Simple charge neutralization: The surfaces of colloids are uniformly shielded with negative charges. They can be completely neutralized in a specific dosage of coagulants; at this time, the optimal amounts of coagulants/flocculants will add proportionally with an increase in the initial concentration of the wastewater. 2) Charge patching: Hetero-charges are irregularly distributed and directly generate electrostatic attraction between oil particles. Thus, the zeta potential does not equate to zero at optimal dosage, forming a broad “flocculation window.” 3) Sweeping: The sweeping mechanism is based on inorganic coagulants. However, some flocculants with lower solubility can also form large-sized flocs (Yang et al., 2016). 4) Bridging: In this, the large-molecular-weight flocculants can attach to the fine flocs to aggregate into the large one (Zhao et al., 2021). Destabilization by bridging occurs when a polymer of high molecular weight becomes attached at several adsorption sites to the surface of negatively charged particles along the polymer chain (Hughes, 2010; Zhang et al., 2018). The bridge system may be created by the remaining polymer that remained stretched into the solution and attached to available surface sites of other particulates. The dominant mechanism and treatment efficiency, to a large extent, depend on process conditions (including coagulant/flocculants dosage, pH, and initial concentration and temperature), effluent pollutant concentration load, and the type of coagulants/flocculants.
Chemical coagulation is a complex phenomenon involving various interrelated parameters; hence, it is very critical to define how well a coagulant will function under a given condition. The coagulation/flocculation process is the widely used unit operation, due to its low cost and comparatively easy operation, utilized as a pretreatment option in textile wastewater treatment to reduce fouling in filters (Samsami et al., 2020; Tianzhi et al., 2021; Paixão et al., 2021; Arhin et al., 2016). The coagulation procedure is predominated by charge neutralization, which contributes to the removal of hydrophilic components with small molecular weight (Yu et al., 2003; Saritha et al., 2017). Based on the effectiveness to decolorize textile wastewater, chemical coagulants can be categorized into three parts as described in Figure 1. The application of coagulants such as ferric chloride, alum, and polyelectrolytes can achieve efficient separation of colloid from water in the primary treatment stages. Also, they are used for the pretreatment of secondary effluents before a direct feed to tertiary filters (Baek and Chang, 2009).
The use of alternative coagulants such as pre-hydrolyzed metallic salts is often more effective than the hydrolyzing metallic salts such as ferric chloride, aluminum sulfate (alum), and ferric sulfate which are readily soluble in water (Shewa and Dagnew, 2020; Duan and Gregory, 2003). Pre-hydrolyzed coagulants such as poly-aluminum chloride (PACl), poly-aluminum ferric chloride (PAFCl), polyferrous sulfate (PFS), and polyferric chloride (PFCl) seem to exhibit better color removal even at low temperatures and may also produce a lower volume of sludge (Renault et al., 2009). The effectiveness of PACl products gives more rapid flocculation and strong flocs compared to alum at an equivalent dosage. This can be attributed to the fact that these coagulants are pre-neutralized, have a smaller effect on the pH of the water, and so, reduce the need for pH adjustment.
Commonly used textile dyes are ionic, mostly anionic, and hence, the cationic polymer is preferred as it exhibits better dye removal performance, due to its opposite surface charge attraction (Duan and Gregory, 2003). However, the mechanisms of these products are not well established yet. Furthermore, to conduct and evaluate the phenomenon, it is necessary to consider only the most critical controlling parameters (Verma et al., 2012). Different coagulants affect different degrees of destabilization. The higher the valence of the counter ion, the more its destabilizing effect and the less the dose needed for coagulation. If pH is below the isoelectric point of metal hydroxide while the precipitation of colloids by different coagulants is supported by a suitable polymer, the positively charged polymers will prevail and adsorption of these positive polymers can destabilize negatively charged colloids by charge neutralization (Verma et al., 2012).
Some of the reported chemical coagulation/flocculation technologies used for the textile industry dyes and/or wastewater and their performance have been summarized in Table 1. Alum and ferric chloride and ferric sulfate are the most widely used chemical coagulants with varying dose amounts in textile wastewater treatment. Moreover, natural coagulants have been also considered promising alternatives for the coagulation/flocculation process. Table 1 also summarizes various researchers’ studies on the integrated application of coagulation/flocculation with other different treatment techniques such as membrane filtration, advanced oxidation process, and biological treatment. The combined treatment system resulted in the improved removal efficiency of color, turbidity, total suspended solids, colloids, and COD values.
In addition to the chemical coagulants discussed above, some natural coagulants made from plant extracts also show promising reduction potentials in colloids in the pretreatment or hybrid treatment of textile industry wastewater (Ramos et al., 2021). Natural coagulants and flocculants mainly come from starch, chitosan, cellulose, and other polysaccharide materials which are easily obtained from renewable resources when compared to inorganic salt coagulants and organic synthetic flocculants (Zhao et al., 2021). Natural coagulants could be plant based, animal based, or made from microbes (Shewa and Dagnew, 2020). These natural flocculants also have macromolecular structures and some functional groups, which can neutralize negatively charged particles and reduce the zeta potential to compress the double electric layer. Natural coagulants are less specific to pH adjustment than synthetic chemical coagulants, resulting in the ability to remove colloids in a wide range of solution pH (Verma et al., 2012). In addition, the use of natural coagulants can reduce financial costs, as they are abundant, and they can offer greater safety to public health due to their biodegradable nature (Januário et al., 2021; Saritha et al., 2017). Moreover, they may act as nutrients for microorganisms (Shewa and Dagnew, 2020).
Potato starch and Moringa oleifera Lam. (MO) can be mentioned due to the presence of water-soluble cationic proteins with a molecular weight ranging from 6 to 16 kDa (kDa), which are capable of destabilizing and precipitating the colloidal particles (Beluci et al., 2019; Samsami et al., 2020). Moringa oleifera could achieve a reduction in turbidity up to 99.9% under optimal conditions (Madjene et al., 2023). Strychnos potatorum (nirmali seeds) and Eirchorrnia crassipes (water hyacinth) are also examined as natural coagulants used for wastewater treatment (Prabhakaran et al., 2020). Sometimes, the natural coagulants are not directly applied; instead, they serve as a flocculating agent because they have a macromolecular structure with a variety of functional groups. Moreover, they can destabilize the charged stable particles mainly through the process of adsorption and neutralization with the functional groups by interparticle bridging (Verma et al., 2012).
Another alternative in the coagulation/flocculation process is the use of biological flocculants (biopolymers/bio-flocculants) derived from bacteria and fungi, which could be environmentally friendly. Chitosan is one of the most promising natural coagulants/flocculants which can potentially substitute inorganic salts and synthetic polyelectrolytes for the removal of particulate and dissolved substances from the wastewater (Shewa and Dagnew, 2020). It is an amino polysaccharide produced by the deacetylation of chitin, the second most abundant biopolymer in the world after cellulose. Its wide application is due to its biodegradability, non-toxic nature, and unique physicochemical properties because of the presence of primary amino groups and high nitrogen content. Moreover, chitosan possesses several intrinsic characteristics such as a high cationic charge density and long polymer chains that make it an effective coagulant/flocculant for wastewater treatment purposes (Teh et al., 2016; Renault et al., 2009).
It is crucial to identify the optimum operating parameters during the addition of coagulants and flocculants. Various coagulants can operate under various optimal conditions. Together with lowering costs and sludge volume, excellent performance requires a thorough understanding of the interaction between the pollutant and coagulant (Alazaiza et al., 2022). The factors that influence the coagulation/flocculation process include temperature, initial pH of wastewater, effluent quality, dosage, (Saritha et al., 2017), coagulant type (Koohestanian et al., 2008), ionic strength (Yang et al., 2016), and mixing conditions (speed and time). Coagulant/flocculant dosage and initial pH are among the most important factors in the unit processes (Zheng et al., 2011). Principally, inadequate dosage or overdosing would result in poor efficiency in flocculation. Therefore, it is significant to determine the optimal dosage to minimize the dosing cost and sludge formation and also to obtain the optimal performance in treatment (Saritha et al., 2017).
Koohestanian et al. (2008) reported that the best removal of organic matter, viruses, colloids, bacteria, and color, and a decrease in turbidity is achieved in optimized parameters (Koohestanian et al., 2008). Because the coagulation/flocculation process intends to remove colloids, which are responsible for turbidity, most of the studies examined the turbidity removal efficiency as the main test parameter, in addition to color removal. For example, turbidity reduction of 85%–98% was achieved from water using the optimum coagulant dosage 10 mg/L for alum and 8 mg/L for ferric chloride (Table 2). Although different coagulants may have different efficiencies for different test parameters, scholars reported that ferric chloride achieved better results than alum for the coagulation treatment system to reduce the concentration of colloidal particles in the effluent. As shown in Table 2, the optimum pH of alum to exhibit higher color removal efficiency is near neutral except for pretreated actual textile wastewater by the coagulation/flocculation process (GilPavas et al., 2017). Moreover, when Fe (III) salts are used as coagulants, pH is a significant influential factor in the destabilization and has a direct implication for the high colloidal concentration in the final discharge. Ultimately, tertiary filters are damaged with fouling. Although the natural pH of ferric chloride solution is acidic, effective color removal can be achieved when the pH is maintained near neutral, but it again depends on the type of dyes used that needs to be removed (Verma et al., 2012). This assures that the coagulation/flocculation treatment of actual textile wastewater is difficult than using a single textile dye because it contains mixtures of various types of dyes and other chemicals. This makes it difficult for pH adjustment, resulting in the treatment process being ineffective. In the case of applications of natural coagulants, the impact of pH is not significant; this makes them a promising alternative in the coagulation/flocculation process (Ihaddaden et al., 2022; Saritha et al., 2017). However, they still have other limitations as mentioned in the previous section.
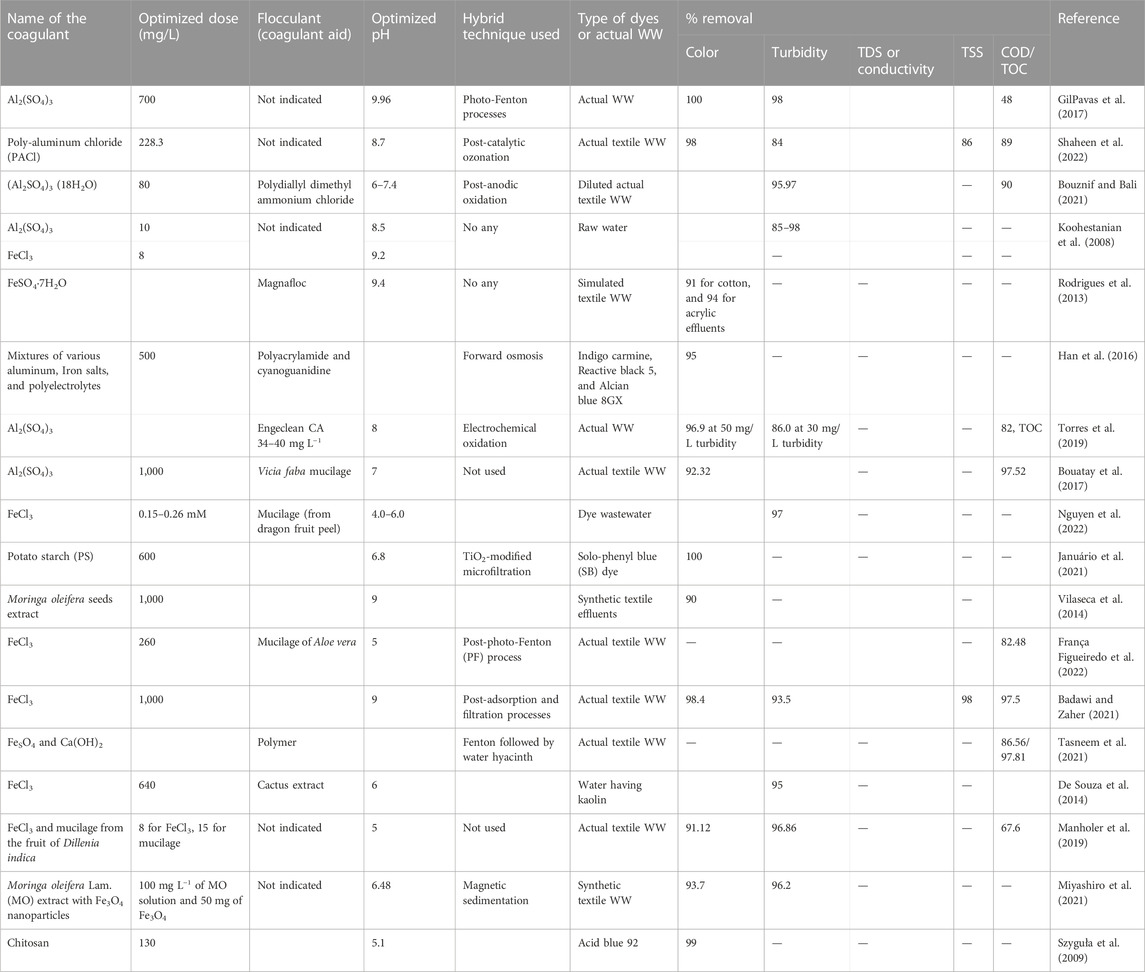
TABLE 2. Summary of the effectiveness of different coagulants studied for colloids and color removal in textile wastewater.
The other important factor affecting the coagulation/flocculation system is the mixing system applied in the coagulation and flocculation stage (Alazaiza et al., 2022). Since textile dyes, with various physical and chemical properties, have been used, different levels of mixing systems are required. Rapid mixing is used to spread out the coagulant throughout the wastewater; on the other hand, slow mixing is a key part of the flocculation stage to get the most favorable performance. Sufficient time must be provided to allow the formation of flocs to permit their efficient removal in the sedimentation process. The time of macro floc formation is one of the operating parameters that are given great consideration in any wastewater treatment plant that involves the coagulation/flocculation process (Saritha et al., 2017).
Temperature also affects the coagulation–flocculation processes; as the wastewater temperature drops, almost all chemical reactions slow down. Cold wastewater can also make dispersing the coagulants evenly in the wastewater more difficult. As a result, coagulation becomes less efficient and needs the utilization of higher coagulant doses to compensate. The performance of inorganic coagulants (alum) is less efficient at a lower temperature because the hydrolysis and precipitation kinetics are low as compared to poly-aluminum chloride (readily hydrolyzed coagulants). Low temperature impedes the aggregation rate of flocs and hinders perikinetic collision (Teh et al., 2016).
One of the major issues related to the coagulation/flocculation process is the toxicity and health hazard possessed by inorganic coagulants and polymeric coagulants (Teh et al., 2016). Although the inorganic salt coagulants and organic synthetic flocculants are effective in the treatment of wastewater, they may cause additional pollution and pose many threats to human health due to the release of residual metal ions or harmful polymer monomers (Zhao et al., 2021; Prabhakaran et al., 2020). Inorganic coagulants need pH adjustment before and after treatment, producing large amounts of sludge and adding undesirable inorganic chemicals like aluminum, iron, sulfate, and chloride to the environment (Dalvand et al., 2016).
Natural polymeric flocculants possess high potential to be an encouraged substitute for other inorganic coagulants and flocculants in recent years. However, these bio-flocculants still have some disadvantages. First, they all have a shorter shelf life because of easy biodegradability. Furthermore, flocs formed by bio-flocculants are inclined to lose stability and strength. In time, high dosages have to be used to achieve equivalent effects, and they are only applied as coagulant aids. Accordingly, the modification of their property and application potential is the subject of research in recent years (Zhao et al., 2021). Polymer flocculants cannot remove the natural organic matter (NOM) components that cause fouling because of the high resistance of concentration polarization, the filter cake layer, and the adsorption layer. Moreover, compared to that produced by sweeping, flocs produced by charge neutralization can generate lower hydraulic resistance. Thus, iron salts and aluminum salts are generally selected as coagulants in the coagulation membrane process (Rasouli et al., 2017). In general, the application of the coagulation–flocculation stage before the tertiary filtration system in textile wastewater treatment systems adds an extra cost of treatment and generation of sludge. However, all the aforementioned limitations might be compensated after the reduction of fouling in the filtration system. It helps to remove bio-foulants generated during biological treatment stages, which are the major causes of membrane damage and fouling; thus, incorporation of the coagulation/flocculation step may help overcome the limited application of filtration systems for textile wastewater treatment.
Moreover, there are different treatment methods; physical methods including sedimentation, floatation, filtration, membrane separation, coagulation/flocculation, ion exchange, advanced oxidation, neutralization, adsorption, electrochemical degradation, and disinfection are employed techniques at different treatment unit processes (Bustos-Terrones et al., 2021). They have been used to remove and recover toxic contaminants from the industrial effluent with varying degrees of success and limitation. Those techniques have advantages and limitations in various wastewater treatment processes as shown in Table 3. Among the limitations, high cost, energy-intensive processes, formation of toxic by-products, and poor removal efficiency can be mentioned, and they also end up with the formation of secondary pollutants (Bhandari et al., 2021), (Vemuri et al., 2021). Ion exchange techniques, adsorption, reverse osmosis, and air stripping, can only extract soluble organic materials which transfer pollutants from one phase to another without eliminating them (Baaloudj et al., 2022b; Jia et al., 2020). The use of membrane technologies for treating dye-containing wastewater is limited due to the short service life of the membrane and the ease to cause pollution (Zhou et al., 2019). Membrane fouling is the other limiting factor in the membrane bioreactor application (Yee et al., 2019). Advanced oxidation processes (AOPs) are a better solution for the rapid removal of non-biodegradable compounds (Kane et al., 2022; Shahzad et al., 2022; Baaloudj et al., 2022b). They comprise all the catalytic and non-catalytic processes that take advantage of the high oxidizing capacity of the hydroxyl radical (OH), and they differ from each other in the way in which this radical is generated (Cuerda-Correa et al., 2020). High cost due to the use of expensive reagents (for example, H2O2) and energy consumption (generation of O3 or UV radiation), sludge production, and toxic by-products are the major shortcomings of AOPs for dye removal (Cuerda-Correa et al., 2020; Moosavi et al., 2020).
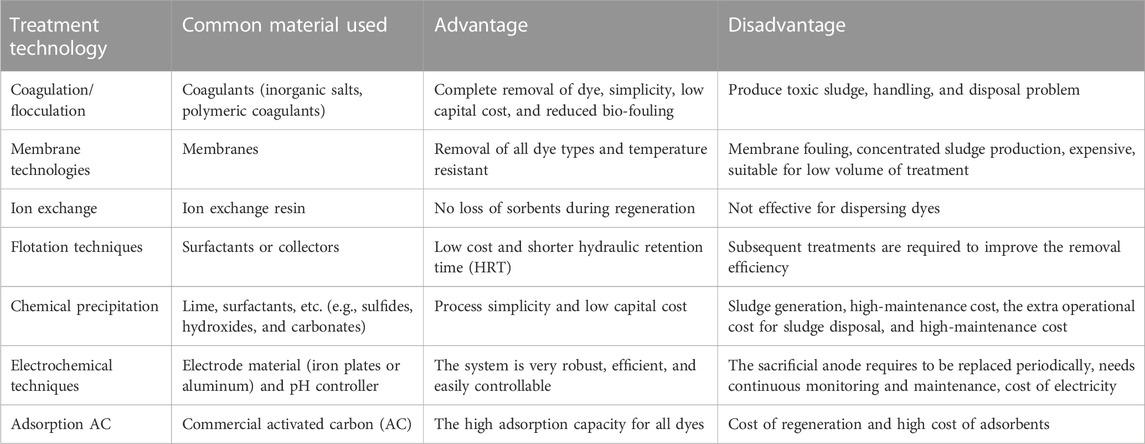
TABLE 3. Merits and demerits of various textile wastewater treatment techniques (Collivignarelli et al., 2019; Aragaw and Bogale, 2021; Yeow et al., 2021).
Secondary effluent treatment (at the tertiary treatment stage) is often employed using a physical process, usually involving filtration technology (membrane, sand, activated carbon filtration, etc.), to remove all solids including colloids, bio-colloids, and nutrients (Qu et al., 2021). Also, tertiary filtration is important to reduce BOD in the final effluent because solids contained BOD responsible organic matters (for instance, algae, planktons, and decayed materials), and phosphorus-associated inorganic matters (Aniyikaiye et al., 2019). Therefore, a tertiary filter is the most preferable process to recover good-quality water from a secondary effluent, fed from a biological treatment tank, as these tertiary treatment techniques have a high rejection of impurities with lower operating costs and higher water quality.
However, colloids and solute macromolecules have been deposited and/or adsorbed onto pores of the filters due to the concentrated contaminants in the secondary effluent, which leads to fouling and damages the filtration system. These contaminant loads in a secondary effluent could contain several constituents including suspended solids, colloids, organic matters, and high-level bio-colloids, resulting in a further elevated fouling potentiality (Marszałek and Żyłła, 2021). Hence, it is critical to identify major foulants in the secondary effluent from the textile wastewater treatment process that causes hindrance in the sustainable application of tertiary treatment and re-designed pretreatment options for the reduction of these foulants. Scholars have been suggesting several pretreatment alternatives, and coagulation/flocculation is one of them (Ćurić and Dolar, 2022). The coagulation/flocculation process, as a pretreatment option, is recommended due to its high efficiency, low cost, and high colloid and bio-colloid removal in the secondary effluent of the textile wastewater treatment process (Dotto et al., 2019).
Out of several fouling types that can happen in tertiary filters, colloidal fouling is a well-established and understood one. However, bio-colloidal fouling (bio-fouling) is less understood and needs consideration during pretreatment alternative development. These foulants are also water quality parameters and can be interrelated with other parameters including electrical conductivity, a measure of total dissolved solids. Most fouling-responsible water quality parameters in textile wastewater including total inorganic colloids, turbidity, colors, and COD can be reduced by different pretreatment alternatives including the coagulation/flocculation process at the primary treatment stage (Ćurić and Dolar, 2022). However, bio-colloids mostly generated from the secondary effluent are not still reduced and are potential foulants for filtration techniques at the tertiary treatment stage. Hence, pretreatment alternatives are required at this stage. Table 4 presents the type of foulants in the secondary effluent that could cause filtration damage, supposed constituents, responsible test parameters, possible pretreatment options, and the corresponding reduced fouling challenges. In the secondary effluent, not only bio-colloids are present but also inorganic solid particles can exist. As can be seen, there are several pretreatment alternatives to treat effluents from the secondary effluent of textile industry wastewater. Coagulation/flocculation as a pretreatment option can be applied for most foulant types constituted in the secondary effluents. Tertiary filtration techniques’ lifetime and good water quality fluxes could primarily be affected by solute build-up, microbial adhesion-derived fouling, attached biofilm formation, and solute adhesion at the filter surface (Khouni et al., 2020). Hence, coagulation/flocculation is effective as a hybrid process for the treatment of wastewater for safe reuse and recovery of water.
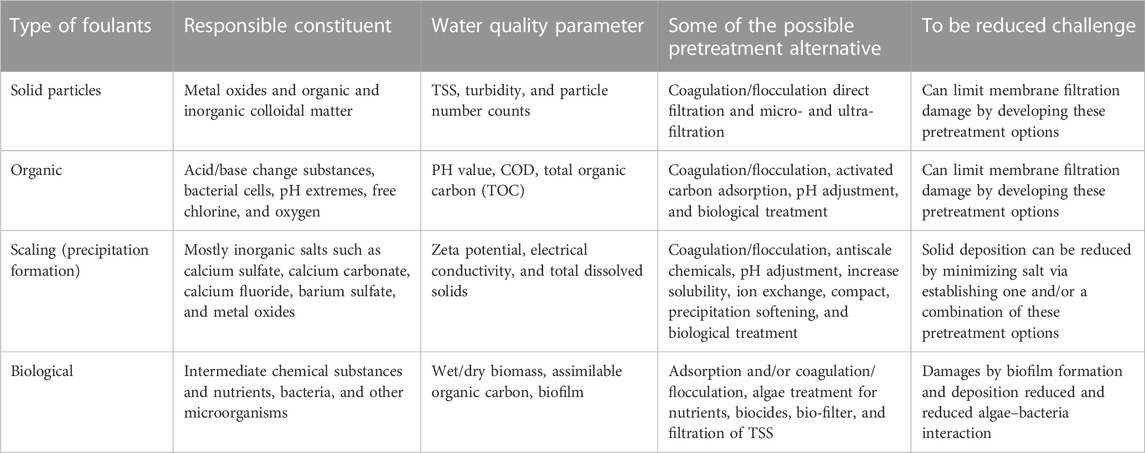
TABLE 4. Major foulants and effluent constituents with their appropriate pretreatment options and evaluation.
Colloidal fouling is a persistent problem in many filtration processes of water and wastewater treatment and is caused by colloidal particles (Ibrar et al., 2019; Nguyen et al., 2019). Colloidal fouling on the filters occurs when colloidal material adheres to its surface or blocks its holes. The cake layer and/or floc accumulated on filters can hinder water from flowing through its pores, resulting in symptoms such as increased pressure difference which results in increased energy consumption. Colloidal fouling is caused by the presence of non-biological organics (e.g., synthetic organic substances) and inorganic particles (e.g., silt or clay) in many feed wastewater, resulting in fouling risk (Warsinger et al., 2015). The major inorganic foulants in textile wastewater can be hydroxides, salts, and bleaching agents, while the organic foulants, especially macromolecules, can be polysaccharides, proteins, and starches.
Colloidal fouling can be controlled by applying suitable upstream coagulation/flocculation and filtration processes (Jiang et al., 2017), (Ahmed et al., 2020). Similarly, colloidal fouling in the textile industry wastewater can be reduced using the coagulation/flocculation unit process. As shown in Figure 2, commonly installed textile industry wastewater treatment plants contain the coagulation/flocculation process at the primary treatment stages. It is expected that most suspended and dissolved solids and turbidity can be reduced at this stage. If this unit process removal efficiency of these solids is ineffective, a high load of colloidal solids can feed to the secondary treatment and can affect their efficiency as well as make a problem for the tertiary filtration device (Yang et al., 2020). These solids are taken as model colloidal foulants, and the results suggested that salt accumulates on the fouling layer formed by the colloidal particles and increases the cake-enhanced osmotic pressure, leading to a reduction in net osmotic driving force and permeate flux. Physical cleaning with high cross-flow velocity was able to restore the flux, which shows that colloidal fouling is reversible in forward osmosis (FO); however, when particles aggregate under conditions of high salt concentration, due to reverse salt diffusion and high feed solution pH, flux was not recovered (Thamaraiselvan and Noel, 2015). The concept of the critical flux is common in membrane fouling, “below the critical flux, fouling occurs insignificant, whereas, above the critical flux, fouling becomes more severe.” As a result, the effective coagulation/flocculation process is critical at the primary treatment stage for reduction of colloid load. This is directly important to the secondary treatments and its treated effluents that need to be fed to the tertiary stage.
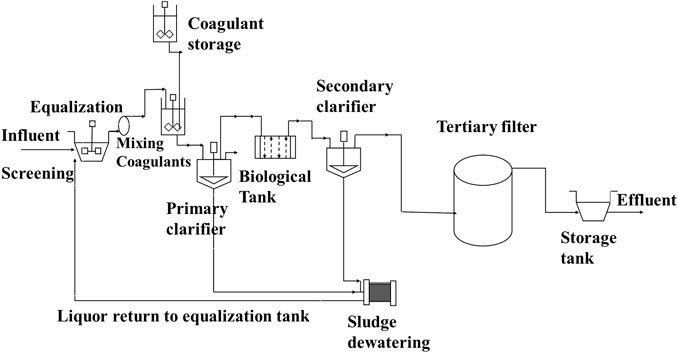
FIGURE 2. Commonly installed textile industry wastewater treatment plants adopted with modification from the work of Bidu et al. (2021).
Furthermore, though effective pretreatment alternatives are critical, more hydrophilic-modified membrane technology is required to minimize fouling due to the presence of hydrophobic impurities in dye-containing wastewater (Samsami et al., 2020). Hydrophilic dynamic membrane technologies using in situ addition of hydrophilic polymers like polyethylene glycol and polyvinyl alcohol can be efficiently used for preventing irreversible fouling by dye molecules (Thamaraiselvan and Noel, 2015). Membrane modification techniques, advanced membrane materials, optimal operational conditions, and adequate pretreatment are commonly used to reduce colloidal fouling and its associated problems (Januário et al., 2021; Cai et al., 2021). The removal of colloidal particles less than 1 nm may not be sufficient to avoid fouling in many cases. The high concentration of the rejected ions in the membrane surfaces could promote the aggregation of dissolved matter into colloidal-sized particles (Al-Amoudi and Lovitt, 2007). In most cases, colloids cause reversible fouling and can be easily removed by hydraulic cleaning measures such as backwash and air scrubbing. However, if the particles and colloid size are smaller than the membrane pore size, then irreversible fouling might occur as these colloidal particles can enter and be trapped within the membrane structure matrix and could not easily be cleaned by hydraulic cleaning. This type of fouling dominates in the case of reverse osmosis applied in good-quality water reclamation when there is an integration of inappropriate pretreatment options (Kim et al., 2020). Therefore, this is important and can be applied to textile wastewater treatment. The pretreatment options for mitigating this fouling can be physical cleaning for the minor fouling and both physical and chemical cleaning for major fouling (Warsinger et al., 2015).
In addition to simple physical cleaning techniques (e.g., hydraulic flushing and osmotic backwashing), coagulation/flocculation and adsorption are widely used pretreatment options as colloidal fouling control mechanisms (Gong et al., 2015). In these pretreatment alternatives, control membrane fouling arose both from inorganic colloid- and organic colloid-containing wastewater. Results showed that membrane filterability is improved by the addition of alum and ferric sulfate due to the effective destabilization of colloids. This can be confirmed by measuring particle size distribution. Some other strategies for controlling membrane fouling are also assessment of parameters such as the silt density index (SDI), Modified Fouling Index (MFI), and turbidity, which could help evaluate colloidal fouling potentiality and the application of media and membrane filtration. The SDI of a feed stream can be measured to predict the relative risk of particulate/colloidal fouling (Jiang et al., 2017). Lime softening or strong acid cation exchange resin for silica removal and application of antifoulants are also strategies that could be applied. Indeed, it can be concluded that colloidal fouling can be minimized both by using effective pretreatment options and by using more hydrophilic-modified membrane technologies during dye-containing textile wastewater.
Bio-fouling for filtration technologies at the tertiary treatment stage is caused by the biofilm formation of bioactive matters including microbes, plankton, plants small animals, and their derivatives. This can result in complex interactions of the filter surface, hydraulic parameters, and bio-foulants. In addition to bio-foulants, soluble solids can be present in secondary effluents. This can be entrapped to coagulated flocs and removes any colloidal particle responsible for fouling (Baek and Chang, 2009). For the inorganic/scaling, colloids, and organic fouling in wastewater reclamation, there are well-established pretreatment (physical or chemical) techniques (Ibrar et al., 2019). However, bio-fouling caused by bio-colloids has been one of the most clinging and not well-understood forms of fouling. Mostly, the sufficient bioactive matter is contained in the secondary effluent to be fed to tertiary treatment. However, most non-bioactive colloidal particles can be removed during preliminary and primary treatments. Reports suggest that bio-foulants, inorganic colloids, and organic matter are in the first, second, and third positions in damaging filtration technologies, especially membrane techniques, respectively (Escobar and Van Der Bruggen, 2015). Bio-foulants are in the first place, compared with non-living colloidal particles, due to the bioactive matter’s ability to reproduce and form a biofilm in favorable conditions (Pichardo-Romero et al., 2020). A high probability of microbial adhesion on the surface could appear due to the large membrane surface areas in the system, which results in establishing themselves over the period and supporting themselves with their extra polymeric substance (Khouni et al., 2020). Indeed, the biofilm can adsorb and concentrates soluble substances near their cell wells; therefore, these soluble substances are locally immobilized and become semisolid-state biofilms. Therefore, due to secondary effluents having a high load of microbes, a dynamic bio-fouling process can be caused by their colonization and growth, resulting in the formation of biofilms; at the time, it can be considered as a membrane bioreactor (Jegatheesan et al., 2016). This is because attached growth bacteria can start to multiply and produce extracellular polymeric substances (EPS), which consist of hetero-polysaccharides and have a high negative surface charge, to form a hydrated gel, which results in biofilm formation on the membrane surface in a multi-step process (Limoli et al., 2015). Therefore, a highly effective pretreatment process is required to reduce the problem in membrane filtration. This needs intervention in the conventional treatment process. We proposed and recommended a flow process for textile industry wastewater treatment plants (Figure 3).
In addition to effective pretreatment being required to reduce filter fouling, filtration device characteristics to interact with bioactive matters need to be optimized. The adhesion of microbes on the surface depends on the membrane materials and their affinity toward microbes. For example, polyether urea has the lowest microbial affinity as compared with polyamide, polysulfone, and polyethersulfone; hence, membrane manufacturers should keep those low-affinity materials for wastewater treatment (Warsinger et al., 2018). Not only active cells but also dead cells could have an adhesion behavior in response to the filter surface. Furthermore, though microbial adhesion depends on their population load, precautions should be taken in minimizing it to below a threshold level; lowering the concentration of a nutrient may have a significant role in preventing bio-fouling, resulting from slowing the adhesion rate (Kucera, 2019).
Furthermore, Al-Abri et al. (2019) recommend chlorination to eliminate/reduce microbial growth, but in the case of higher microbial affinity of the filters such as polyamide, dechlorination is required to protect the device’s lifetime (Al-Abri et al., 2019). However, the use of UV radiation is a promising alternative to chlorination to disinfect the feed. Early removal of microbial food sources such as BOD, TOC, and ammonia reduces the severity of organic foulants. In conclusion, bio-fouling in filters is one of the main factors in the tertiary treatment stage and loss of solid rejection. It is one of the most difficult and least understood fouling types with no established pretreatment techniques; however, the coagulation/flocculation process is advisable for the textile industry wastewater as it contains high solids and organics including synthetic dyes.
Pretreatment alternatives for the removal of foulants are highly significant issues to be dealt with in good-quality water recovery and sustainable application of filtration technologies at the tertiary treatment stage. This issue needs to be considered to reduce filter (devices) life as well as the impact of poorly treated wastewater on the environment. Some short-term strategies such as filter cleaning and employing easy-way pretreatment alternatives (from already established WWTPs) and long-term strategies such as changing/redesigning the WWTP unit process should be applied to minimize/prevent fouling. Among different pretreatment options, coagulation/flocculation is still a cost-comparative alternative for the removal of foulants in wastewater and has been widely practiced since several years. Comparatively, coagulation/flocculation may be considered the better technique for textile industry wastewater amongst other industrial wastewater because of their superior color and colloid removal capacity at the alkaline pH range of the wastewater. Additionally, due to the high load of bioactive matter in the secondary effluent, the coagulation/flocculation process may also be considered a promising pretreatment option for the tertiary treatment stages to prevent fouling. However, the applicability of the coagulation/flocculation process as a pretreatment option for bio-fouling removal in textile wastewater is not established yet. It is well developed and established only in the primary treatment stages. Thus, redesigning wastewater treatment plants by including these pretreatment techniques and evaluating their water recovery efficiency of tertiary treatments of textile wastewater are required.
The following issues need to be looked into account and addressed in the future:
✓ A significant amount of microfibers, which are a kind of colloidal solids, from synthetic textiles are a high constituent in textile industry wastewater, resulting from fabric processing (Ramasamy et al., 2022). However, very limited and/or no studies have been carried out on the characterization and reduction techniques, by coagulation/flocculation pretreatment alternatives, of micro/nano-colloids including microfiber, to be a cause for fouling, in the textile wastewater treatment process.
✓ In addition to it has been required after secondary effluents as a pretreatment option, an effective coagulation/flocculation unit process at the primary treatment stage is critical to reduce colloid load. Thinking this unit process is required both at the primary and after the secondary treatment stage to prevent fouling problems for the tertiary filtration.
✓ Fouling can be also minimized both by using effective pretreatment options and by using more hydrophilic-modified membrane technologies during dye-containing textile wastewater treatment.
✓ Experimental and simulation modeling will be important to acquire fundamental insights into how the mechanism of these pollutants including bio-foulants affects fouling.
✓ Evaluating them by installing a coagulation/flocculation process as the proposed WWTP flow layout (Figure 3) is required. As a result, in the future, detailed characterization and reduction strategies of colloids and bio-colloids will be an issue in the problem of fouling.
✓ In addition, the effectiveness of certain pretreatment options for the removal of these foulants is to be established.
TA: Conceptualization, Supervising, Writing—First draft, Writing—review and editing, Proofreading. FB: Writing—First draft, Formal analysis, Graphics, and synthesis.
The authors thank the Faculty of Chemical and Food Engineering, Bahir Dar Institute of Technology, for allowing access to the resources.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Fatah, M. A. (2018). Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 9, 3077–3092. doi:10.1016/j.asej.2018.08.001
Ahmed, J., Jamal, Y., and Shujaatullah, M. (2020). Recovery of cooling tower blowdown water through reverse osmosis (Ro): Review of water parameters affecting membrane fouling and pretreatment schemes. Desalin. Water Treat. 189, 9–17. doi:10.5004/dwt.2020.25639
Ahmed, S. F., Mofijur, M., Nuzhat, S., Tasnim, A., Rafa, N., Uddin, A., et al. (2021). Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mat. 416, 125912. doi:10.1016/j.jhazmat.2021.125912
Al-Abri, M., Al-Ghafri, B., Bora, T., Dobretsov, S., Dutta, J., Castelletto, S., et al. (2019). Chlorination disadvantages and alternative routes for biofouling control in reverse osmosis desalination. npj Clean. Water 2, 2. doi:10.1038/s41545-018-0024-8
Al-Amoudi, A., and Lovitt, R. W. (2007). Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Memb. Sci. 303, 4–28. doi:10.1016/j.memsci.2007.06.002
Alazaiza, M. Y. D., Albahnasawi, A., Ali, G. A. M., Bashir, M. J. K., Nassani, D. E., Al Maskari, T., et al. (2022). Application of natural coagulants for pharmaceutical removal from water and wastewater: A review. WaterSwitzerl. 14, 140–216. doi:10.3390/w14020140
Amerian, T., Farnood, R., Sarathy, S., and Santoro, D. (2019). Effects of total suspended solids, particle size, and effluent temperature on the kinetics of peracetic acid decomposition in municipal wastewater. Water Sci. Technol. 12, 2299–2309. doi:10.2166/wst.2020.047
Aniyikaiye, T. E., Oluseyi, T., Odiyo, J. O., and Edokpayi, J. N. (2019). Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 16, 1235. doi:10.3390/ijerph16071235
Aragaw, T. A., and Alene, A. N. (2022). A comparative study of acidic, basic, and reactive dyes adsorption from aqueous solution onto kaolin adsorbent: Effect of operating parameters, isotherms, kinetics, and thermodynamics. Emerg. Contam. 8, 59–74. doi:10.1016/j.emcon.2022.01.002
Aragaw, T. A., and Angerasa, F. T. (2019). Adsorption of basic yellow dye dataset using Ethiopian kaolin as an adsorbent. Data Br. 26, 104504. doi:10.1016/j.dib.2019.104504
Aragaw, T. A., and Bogale, F. M. (2021). Biomass-based adsorbents for removal of dyes from wastewater: A review. Front. Environ. Sci. 9. doi:10.3389/fenvs.2021.764958
Aragaw, T. A., Bogale, F. M., and Gessesse, A. (2022). Adaptive response of thermophiles to redox stress and their role in the process of dye degradation from textile industry wastewater. Front. Physiol. 13, 908370. doi:10.3389/fphys.2022.908370
Aragaw, T. A. (2021). Functions of various bacteria for specific pollutants degradation and their application in wastewater treatment: A review. Int. J. Environ. Sci. Technol. 18, 2063–2076. doi:10.1007/s13762-020-03022-2
Aragaw, T. A. (2020a). Recovery of iron hydroxides from electro-coagulated sludge for adsorption removals of dye wastewater: Adsorption capacity and adsorbent characteristics. Surfaces Interfaces 18, 100439. doi:10.1016/j.surfin.2020.100439
Aragaw, T. A. (2020b). Utilizations of electro-coagulated sludge from wastewater treatment plant data as an adsorbent for direct red 28 dye removal. Data Br. 28, 104848. doi:10.1016/j.dib.2019.104848
Arhin, S. G., Banadda, N., Komakech, A. J., Kabenge, I., and Wanyama, J. (2016). Membrane fouling control in low pressure membranes: A review on pretreatment techniques for fouling abatement. Environ. Eng. Res. 21, 109–120. doi:10.4491/eer.2016.017
Azanaw, A., Birlie, B., Teshome, B., and Jemberie, M. (2022). Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 6, 100230. doi:10.1016/j.cscee.2022.100230
Baaloudj, O., Badawi, A. K., Kenfoud, H., Benrighi, Y., Hassan, R., Nasrallah, N., et al. (2022a). Techno-economic studies for a pilot-scale Bi12TiO20 based photocatalytic system for pharmaceutical wastewater treatment: From laboratory studies to commercial-scale applications. J. Water Process Eng. 48, 102847. doi:10.1016/j.jwpe.2022.102847
Baaloudj, O., Kenfoud, H., Badawi, A. K., Assadi, A. A., El Jery, A., Assadi, A. A., et al. (2022b). Bismuth sillenite crystals as recent photocatalysts for water treatment and energy generation: A critical review. Catalysts 12, 500. doi:10.3390/catal12050500
Badawi, A. K., Ismail, B., Baaloudj, O., and Abdalla, K. Z. (2022). Advanced wastewater treatment process using algal photo-bioreactor associated with dissolved-air flotation system: A pilot-scale demonstration. J. Water Process Eng. 46, 102565. doi:10.1016/j.jwpe.2022.102565
Badawi, A. K., and Zaher, K. (2021). Hybrid treatment system for real textile wastewater remediation based on coagulation/flocculation, adsorption and filtration processes: Performance and economic evaluation. J. Water Process Eng. 40, 101963. doi:10.1016/j.jwpe.2021.101963
Baek, S. O., and Chang, I. S. (2009). Pretreatments to control membrane fouling in membrane filtration of secondary effluents. Desalination 244, 153–163. doi:10.1016/j.desal.2008.04.043
Beluci, N. de C. L., Mateus, G. A. P., Miyashiro, C. S., Homem, N. C., Gomes, R. G., Fagundes-Klen, M. R., et al. (2019). Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TIO 2 -modified membranes to improve the removal of reactive black 5 dye. Sci. Total Environ. 664, 222–229. doi:10.1016/j.scitotenv.2019.01.199
Bhandari, S., Poudel, D. K., Marahatha, R., Dawadi, S., Khadayat, K., Phuyal, S., et al. (2021). Microbial enzymes used in bioremediation. J. Chem. 2021, 1–17. doi:10.1155/2021/8849512
Bhatia, D., Sharma, N. R., Singh, J., and Kanwar, R. S. (2017). Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 47, 1836–1876. doi:10.1080/10643389.2017.1393263
Bidu, J. M., van der Bruggen, B., Rwiza, M. J., and Njau, K. N. (2021). Current status of textile wastewater management practices and effluent characteristics in Tanzania. Water Sci. Technol. 83, 2363–2376. doi:10.2166/wst.2021.133
Bormashenko, E., Whyman, G., and Gendelman, O. (2015). Elastic properties of liquid surfaces coated with colloidal particles. Adv. Condens. Matter Phys. 2015, 1–6. doi:10.1155/2015/206578
Bouatay, F., Eljebsi, N., Dridi-Dhaouadi, S., and Mhenni, F. (2017). Valorization of the Vicia faba mucilage on textile wastewater treatment as a bio-flocculant: Process development and optimization using response surface methodology (RSM). Water Sci. Technol. 75, 629–642. doi:10.2166/wst.2016.480
Bouznif, S., and Bali, M. (2021). Coupling of the coagulation/flocculation and the anodic oxidation processes for the treatment of textile wastewater. Aqua Water Infrastruct. Ecosyst. Soc. 70, 587–599. doi:10.2166/aqua.2021.166
Bustos-Terrones, Y. A., Hermosillo-Nevárez, J. J., Ramírez-Pereda, B., Vaca, M., Rangel-Peraza, J. G., Bustos-Terrones, V., et al. (2021). Removal of BB9 textile dye by biological, physical, chemical, and electrochemical treatments. J. Taiwan Inst. Chem. Eng. 121, 29–37. doi:10.1016/j.jtice.2021.03.041
Cai, Y. H., Galili, N., Gelman, Y., Herzberg, M., and Gilron, J. (2021). Evaluating the impact of pretreatment processes on fouling of reverse osmosis membrane by secondary wastewater. J. Memb. Sci. 623, 119054. doi:10.1016/j.memsci.2021.119054
Chaipetch, W., Jaiyu, A., Jutaporn, P., Heran, M., and Khongnakorn, W. (2021). Fouling behavior in a high-rate anaerobic submerged membrane bioreactor (Anmbr) for palm oil mill effluent (pome) treatment. Membr. (Basel). 11, 649. doi:10.3390/MEMBRANES11090649
Collivignarelli, M. C., Abbà, A., Carnevale Miino, M., and Damiani, S. (2019). Treatments for color removal from wastewater: State of the art. J. Environ. Manage. 236, 727–745. doi:10.1016/j.jenvman.2018.11.094
Cuerda-Correa, E. M., Alexandre-Franco, M. F., and Fernández-González, C. (2020). Advanced oxidation processes for the removal of antibiotics from water. An overview. Overv. Water 12, 102–157. doi:10.3390/w12010102
Ćurić, I., and Dolar, D. (2022). Investigation of pretreatment of textile wastewater for membrane processes and reuse for washing dyeing machines. Membr. (Basel). 12, 449. doi:10.3390/membranes12050449
Dalvand, A., Gholibegloo, E., Ganjali, M. R., Golchinpoor, N., Khazaei, M., Kamani, H., et al. (2016). Comparison of Moringa stenopetala seed extract as a clean coagulant with Alum and Moringa stenopetala-Alum hybrid coagulant to remove direct dye from Textile Wastewater. Environ. Sci. Pollut. Res. 23, 16396–16405. doi:10.1007/s11356-016-6708-z
De Souza, M. T. F., Ambrosio, E., De Almeida, C. A., De Souza Freitas, T. K. F., Santos, L. B., De Cinque Almeida, V., et al. (2014). The use of a natural coagulant (Opuntia ficus-indica) in the removal for organic materials of textile effluents. Environ. Monit. Assess. 186, 5261–5271. doi:10.1007/s10661-014-3775-9
Díaz-Garduño, B., Pintado-Herrera, M. G., Biel-Maeso, M., Rueda-Márquez, J. J., Lara-Martín, P. A., Perales, J. A., et al. (2017). Environmental risk assessment of effluents as a whole emerging contaminant: Efficiency of alternative tertiary treatments for wastewater depuration. Water Res. 119, 136–149. doi:10.1016/j.watres.2017.04.021
Dotto, J., Fagundes-Klen, M. R., Veit, M. T., Palácio, S. M., and Bergamasco, R. (2019). Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 208, 656–665. doi:10.1016/j.jclepro.2018.10.112
Duan, J., and Gregory, J. (2003). Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 102, 475–502. doi:10.1016/S0001-8686(02)00067-2
Escobar, I. C., and Van Der Bruggen, B. (2015). Microfiltration and ultrafiltration membrane science and technology. J. Appl. Polym. Sci. 132, 42042. doi:10.1002/app.42002
Ezugbe, E. O., and Rathilal, S. (2020). Membrane technologies in wastewater treatment: A review. Membr. (Basel) 10, 1–28. doi:10.3390/membranes10050089
França Figueiredo, F., Karoliny Formicoli de Souza Freitas, T., Gonçalves Dias, G., Cesar Lopes Geraldino, H., Paula Jambers Scandelai, A., Junkes Vilvert, A., et al. (2022). Textile-effluent treatment using Aloe vera mucilage as a natural coagulant prior to a photo-Fenton reaction. J. Photochem. Photobiol. A Chem. 429, 113948. doi:10.1016/j.jphotochem.2022.113948
Ghaly, A. E., Ananthashankar, R., Alhattab, M. V. V. R., and Ramakrishnan, V. V. (2014). Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 05, 2157–7048. doi:10.4172/2157-7048.1000182
GilPavas, E., Dobrosz-Gómez, I., and Gómez-García, M. Á. (2017). Coagulation-flocculation sequential with Fenton or Photo-Fenton processes as an alternative for the industrial textile wastewater treatment. J. Environ. Manage. 191, 189–197. doi:10.1016/j.jenvman.2017.01.015
Gong, H., Jin, Z., Wang, X., and Wang, K. (2015). Membrane fouling controlled by coagulation/adsorption during direct sewage membrane filtration (DSMF) for organic matter concentration. J. Environ. Sci. (China) 32, 1–7. doi:10.1016/j.jes.2015.01.002
Han, G., Liang, C., Chung, T., Weber, M., Staudt, C., and Maletzko, C. (2016). Combination of forward osmosis (FO) process with coagulation/fl occulation (CF) for potential treatment of textile wastewater. Water Res. 91, 361–370. doi:10.1016/j.watres.2016.01.031
Hughes, R. (2010). “CO an introduction to colloids; principles, methods and applications,” in Blood cells Editor T. Cosgrove (United States: John Wiley and Sons, Ltd), 1–22.
Ibrar, I., Naji, O., Sharif, A., Malekizadeh, A., Alhawari, A., Alanezi, A. A., et al. (2019). A review of fouling mechanisms, control strategies and real-time fouling monitoring techniques in forward osmosis. WaterSwitzerl. 11, 695. doi:10.3390/w11040695
Ihaddaden, S., Aberkane, D., Boukerroui, A., and Robert, D. (2022). Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J. Water Process Eng. 49, 102952. doi:10.1016/j.jwpe.2022.102952
Islam, M. R., and Mostafa, M. G. (2020). Characterization of textile dyeing effluent and its treatment using polyaluminum chloride. Appl. Water Sci. 10, 119. doi:10.1007/s13201-020-01204-4
Islam, M. R., and Mostafa, M. G. (2018). Removal of a reactive dye from synthetic wastewater using PAC and FeCl3 coagulants. J. Life Earth Sci. 13, 39–44.
Januário, E. F. D., Vidovix, T. B., Bergamasco, R., and Vieira, A. M. S. (2021). Performance of a hybrid coagulation/flocculation process followed by modified microfiltration membranes for the removal of solophenyl blue dye. Chem. Eng. Process. - Process Intensif. 168, 108577. doi:10.1016/j.cep.2021.108577
Jayapal, M., Jagadeesan, H., Krishnasamy, V., Shanmugam, G., Muniyappan, V., Chidambaram, D., et al. (2022). Demonstration of a plant-microbe integrated system for treatment of real-time textile industry wastewater. Environ. Pollut. 302, 119009. doi:10.1016/j.envpol.2022.119009
Jegatheesan, V., Pramanik, B. K., Chen, J., Navaratna, D., Chang, C. Y., and Shu, L. (2016). Treatment of textile wastewater with membrane bioreactor: A critical review. Bioresour. Technol. 204, 202–212. doi:10.1016/j.biortech.2016.01.006
Jia, Y., Ding, L., Ren, P., Zhong, M., Ma, J., and Fan, X. (2020). Performances and mechanism of methyl orange and Congo red adsorbed on the magnetic ion-exchange resin. J. Chem. Eng. Data 65, 725–736. doi:10.1021/acs.jced.9b00951
Jiang, S., Li, Y., and Ladewig, B. P. (2017). A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 595, 567–583. doi:10.1016/j.scitotenv.2017.03.235
Kane, A., Assadi, A. A., Jery, A. El, Badawi, A. K., Kenfoud, H., Baaloudj, O., et al. (2022). Advanced photocatalytic treatment of wastewater using immobilized titanium dioxide as a photocatalyst in a pilot-scale reactor: Process intensification. Mater. (Basel) 15, 4547. doi:10.3390/ma15134547
Khouni, I., Louhichi, G., Ghrabi, A., and Moulin, P. (2020). Efficiency of a coagulation/flocculation–membrane filtration hybrid process for the treatment of vegetable oil refinery wastewater for safe reuse and recovery. Process Saf. Environ. Prot. 135, 323–341. doi:10.1016/j.psep.2020.01.004
Kim, Y., Li, S., and Ghaffour, N. (2020). Evaluation of different cleaning strategies for different types of forward osmosis membrane fouling and scaling. J. Memb. Sci. 596, 117731. doi:10.1016/j.memsci.2019.117731
Kingsley Ogemdi, I. (2019). Properties and uses of colloids: A review. Colloid Surf. Sci. 4, 24. doi:10.11648/j.css.20190402.12
Kinyua, E. M., Mwangi, I. W., Wanjau, R. N., and Ngila, J. C. (2016). Clarification of colloidal and suspended material in water using triethanolamine modified maize tassels. Environ. Sci. Pollut. Res. 23, 5214–5221. doi:10.1007/s11356-015-5766-y
Koohestanian, A., Hosseini, M., and Abbasian, Z. (2008). The separation method for removing of colloidal particles from raw water. Euras. J. Agric. Environ. Sci. 4, 266–273.
Kucera, J. (2019). Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membr. (Basel). 9, 111. doi:10.3390/membranes9090111
Li, X., Li, Y., Xiang, R., Li, S., Li, X., and Zhou, Q. (2019). Effect of dispersion viscosity on microstructure of cordierite foam prepared by thermo-foaming. Ceram. Int. 45, 24487–24492. doi:10.1016/j.ceramint.2019.08.174
Limoli, D. H., Jones, C. J., and Wozniak, D. J. (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 3, 1–30. doi:10.1128/microbiolspec.mb-0011-2014
Lu, P. J., and Weitz, D. A. (2013). Colloidal particles: Crystals, glasses, and gels. Annu. Rev. Condens. Matter Phys. 4, 217–233. doi:10.1146/annurev-conmatphys-030212-184213
Madjene, F., Benhabiles, O., Boutra, A., Benchaib, M., and Bouchakour, I. (2023). Coagulation/flocculation process using Moringa oleifera bio-coagulant for industrial paint wastewater treatment: Optimization by D-optimal experimental design. Int. J. Environ. Sci. Technol. 2023. doi:10.1007/s13762-023-04808-w
Manholer, D. D., De Souza, M. T. F., Ambrosio, E., De Souza Freitas, T. K. F., Geraldino, H. C. L., and Garcia, J. C. (2019). Coagulation/flocculation of textile effluent using a natural coagulant extracted from Dillenia indica. Water Sci. Technol. 80, 979–988. doi:10.2166/wst.2019.342
Marszałek, J., and Żyłła, R. (2021). Recovery of water from textile dyeing using membrane filtration processes. Processes 9, 1833. doi:10.3390/pr9101833
Mcyotto, F., Wei, Q., Macharia, D. K., Huang, M., Shen, C., and Chow, C. W. K. (2021). Effect of dye structure on color removal efficiency by coagulation. Chem. Eng. J. 405, 126674. doi:10.1016/j.cej.2020.126674
Mikhaylin, S., and Bazinet, L. (2016). Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 229, 34–56. doi:10.1016/j.cis.2015.12.006
Miyashiro, C. S., Mateus, G. A. P., dos Santos, T. R. T., Paludo, M. P., Bergamasco, R., and Fagundes-Klen, M. R. (2021). Synthesis and performance evaluation of a magnetic biocoagulant in the removal of reactive black 5 dye in aqueous medium. Mat. Sci. Eng. C 119, 111523. doi:10.1016/j.msec.2020.111523
Moosavi, S., Lai, C. W., Gan, S., Zamiri, G., Akbarzadeh Pivehzhani, O., and Johan, M. R. (2020). Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega 5, 20684–20697. doi:10.1021/acsomega.0c01905
Nguyen, H. H., Tran, L. N., Doan, V. T., Luu, L. M., Nguyen, Q. T., Van Pham, Q., et al. (2022). Coagulation and flocculation of dye wastewater by FeCl3 and mucilage extracted from dragon fruit peel (Hylocereus undatus) in regard of side effects caused by the use of PACl and PAM. Desalin. Water Treat. 250, 181–188. doi:10.5004/dwt.2022.28152
Nguyen, T. T., Kook, S., Lee, C., Field, R. W., and Kim, I. S. (2019). Critical flux-based membrane fouling control of forward osmosis: Behavior, sustainability, and reversibility. J. Memb. Sci. 570–571, 380–393. doi:10.1016/j.memsci.2018.10.062
Paixão, R. M., Reck, I. M., da Silva, L. H. B. R., Baptista, A. T. A., Bergamasco, R., Vieira, M. F., et al. (2021). Discolouration of contaminated water with textile dye through a combined coagulation/flocculation and membrane separation process with different natural coagulants extracted from Moringa oleifera Lam. seeds. Can. J. Chem. Eng. 99, 1976–1983. doi:10.1002/cjce.23932
Pichardo-Romero, D., Garcia-Arce, Z. P., Zavala-Ramírez, A., and Castro-Muñoz, R. (2020). Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes 8, 182. doi:10.3390/pr8020182
Prabhakaran, G., Manikandan, M., and Boopathi, M. (2020). Treatment of textile effluents by using natural coagulants. Mat. Today Proc. 33, 3000–3004. doi:10.1016/j.matpr.2020.03.029
Qu, F., Yang, Z., Li, X., Yu, H., Pan, Z., Fan, G., et al. (2021). Membrane fouling control by UV/persulfate in tertiary wastewater treatment with ultrafiltration: A comparison with UV/hydroperoxide and role of free radicals. Sep. Purif. Technol. 257, 117877. doi:10.1016/j.seppur.2020.117877
Ramasamy, R., Aragaw, T. A., and Balasaraswathi Subramanian, R. (2022). Wastewater treatment plant effluent and microfiber pollution: Focus on industry-specific wastewater. Environ. Sci. Pollut. Res. 29, 51211–51233. doi:10.1007/s11356-022-20930-7
Ramos, M. D. N., Lima, J. P. P., de Aquino, S. F., and Aguiar, A. (2021). A critical analysis of the alternative treatments applied to effluents from Brazilian textile industries. J. Water Process Eng. 43, 102273. doi:10.1016/j.jwpe.2021.102273
Rasouli, Y., Abbasi, M., and Hashemifard, S. A. (2017). Investigation of in-line coagulation-MF hybrid process for oily wastewater treatment by using novel ceramic membranes. J. Clean. Prod. 161, 545–559. doi:10.1016/j.jclepro.2017.05.134
Renault, F., Sancey, B., Badot, P. M., and Crini, G. (2009). Chitosan for coagulation/flocculation processes - an eco-friendly approach. Eur. Polym. J. 45, 1337–1348. doi:10.1016/j.eurpolymj.2008.12.027
Rodrigues, C. S., Madeira, L. M., and Boaventura, R. A. (2013). Treatment of textile dye wastewaters using ferrous sulphate in a chemical coagulation/flocculation process. Environ. Technol. 34, 719–729. doi:10.1080/09593330.2012.715679
Samsami, S., Mohamadi, M., Sarrafzadeh, M. H., Rene, E. R., and Firoozbahr, M. (2020). Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 143, 138–163. doi:10.1016/j.psep.2020.05.034
Saritha, V., Srinivas, N., and Srikanth Vuppala, N. V. (2017). Analysis and optimization of coagulation and flocculation process. Appl. Water Sci. 7, 451–460. doi:10.1007/s13201-014-0262-y
Shaheen, O., Ikhlaq, A., Ullah, U., Yaqub, U., Akram, A., Kalim, I., et al. (2022). Application of poly aluminum chloride and alum as catalyst in catalytic ozonation process after coagulation for the treatment of textile wastewater. J. Environ. Manage. 323, 115977. doi:10.1016/j.jenvman.2022.115977
Shahzad, W., Badawi, A. K., Rehan, Z. A., Muhammad, A., Ali, R., Shah, F., et al. (2022). Enhanced visible light photocatalytic performance of Sr0.3(Ba,Mn)0.7ZrO3 perovskites anchored on graphene oxide. Ceram. Int. 48, 24979–24988. doi:10.1016/j.ceramint.2022.05.151
Shewa, W. A., and Dagnew, M. (2020). Revisiting chemically enhanced primary treatment of wastewater: A review. Sustain 12, 5928. doi:10.3390/SU12155928
Syafiuddin, A., and Fulazzaky, M. A. (2020). Decolorization kinetics and mass transfer mechanisms of Remazol Brilliant Blue R dye mediated by different fungi. Biotechnol. Rep. 29, e00573. doi:10.1016/j.btre.2020.e00573
Szyguła, A., Guibal, E., Palacín, M. A., Ruiz, M., and Sastre, A. M. (2009). Removal of an anionic dye (Acid Blue 92) by coagulation-flocculation using chitosan. J. Environ. Manage. 90, 2979–2986. doi:10.1016/j.jenvman.2009.04.002
Tasneem, A., Sarker, P., Akter, S., Mouna, S. S. P., Rahaman, M. S., Mohinuzzaman, M., et al. (2021). Textile wastewater treatment by combination of chemical and phytoremediation processes. Pollution 7, 43–54. doi:10.22059/poll.2020.304569.835
Teh, C. Y., Budiman, P. M., Shak, K. P. Y., and Wu, T. Y. (2016). Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 55, 4363–4389. doi:10.1021/acs.iecr.5b04703
Thamaraiselvan, C., and Noel, M. (2015). Membrane processes for dye wastewater treatment: Recent progress in fouling control. Crit. Rev. Environ. Sci. Technol. 45, 1007–1040. doi:10.1080/10643389.2014.900242
Tianzhi, W., Weijie, W., Hongying, H., and Khu, S. T. (2021). Effect of coagulation on bio-treatment of textile wastewater: Quantitative evaluation and application. J. Clean. Prod. 312, 127798. doi:10.1016/j.jclepro.2021.127798
Torres, N. H., Souza, B. S., Ferreira, L. F. R., Lima, A. S., Dos Santos, G. N., and Cavalcanti, E. B. (2019). Real textile ef fl uents treatment using coagulation/fl occulation followed by electrochemical oxidation process and ecotoxicological assessment. Chemosphere 236, 124309. doi:10.1016/j.chemosphere.2019.07.040
Varghese, A. G., Paul, S. A., and Latha, M. S. (2019). Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environ. Chem. Lett. 17, 867–877. doi:10.1007/s10311-018-00843-z
Vemuri, B., Xia, L., Chilkoor, G., Jawaharraj, K., Sani, R. K., Amarnath, A., et al. (2021). Anaerobic wastewater treatment and reuse enabled by thermophilic bioprocessing integrated with a bioelectrochemical/ultrafiltration module. Bioresour. Technol. 321, 124406. doi:10.1016/j.biortech.2020.124406
Verma, A. K., Dash, R. R., and Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. 93, 154–168. doi:10.1016/j.jenvman.2011.09.012
Vilaseca, M., López-Grimau, V., and Gutiérrez-Bouzán, C. (2014). Valorization of waste obtained from oil extraction in Moringa oleifera seeds: Coagulation of reactive dyes in textile effluents. Mater. (Basel) 6, 6569–6584. doi:10.3390/ma7096569
Vincent, B. (2009). “Introduction to colloidal Dispersions;Principles, methods and applications,” in Colloid science: Principles, methods and applications Editor T. Cosgrove (United Kingdom: Bristol Colloid Centre), 1–13. doi:10.1002/9781444305395.ch1
Warsinger, D. M., Chakraborty, S., Tow, E. W., Plumlee, M. H., Bellona, C., Loutatidou, S., et al. (2018). A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 81, 209–237. doi:10.1016/j.progpolymsci.2018.01.004
Warsinger, D. M., Swaminathan, J., Guillen-Burrieza, E., Arafat, H. A., and Lienhard, V. J. H. (2015). Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 356, 294–313. doi:10.1016/j.desal.2014.06.031
Yang, R., Li, H., Huang, M., Yang, H., and Li, A. (2016). A review on chitosan-based flocculants and their applications in water treatment. Water Res. 95, 59–89. doi:10.1016/j.watres.2016.02.068
Yang, X., López-Grimau, V., Vilaseca, M., and Crespi, M. (2020). Treatment of textile wastewater by cas, mbr, and mbbr: A comparative study from technical, economic, and environmental perspectives. Water 12, 1306. doi:10.3390/w12051306
Yaseen, D. A., and Scholz, M. (2019). Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 16, 1193–1226. doi:10.1007/s13762-018-2130-z
Yee, T. L., Rathnayake, T., and Visvanathan, C. (2019). Performance evaluation of a thermophilic anaerobic membrane bioreactor for palm oil wastewater treatment. Membr. (Basel) 9, 55. doi:10.3390/membranes9040055
Yeow, P. K., Wong, S. W., and Hadibarata, T. (2021). Removal of azo and anthraquinone dye by plant biomass as adsorbent – A review. Biointerface Res. Appl. Chem. 11, 8218–8232. doi:10.33263/BRIAC111.82188232
Yu, J., Sun, D. D., and Tay, J. H. (2003). Characteristics of coagulation-flocculation of humic acid with effective performance of polymeric flocculant and inorganic coagulant. Water Sci. Technol. 47, 89–95. doi:10.2166/wst.2003.0024
Zhang, J., Zhang, Q., and Maa, J. P. Y. (2018). Numerical simulation of coagulation processes of colloidal particles: A lattice Boltzmann approach. J. Hydraul. Res. 56, 857–870. doi:10.1080/00221686.2017.1422194
Zhao, C., Zhou, J., Yan, Y., Yang, L., Xing, G., Li, H., et al. (2021). Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 765, 142795. doi:10.1016/j.scitotenv.2020.142795
Zheng, H., Zhu, G., Jiang, S., Tshukudu, T., Xiang, X., Zhang, P., et al. (2011). Investigations of coagulation-flocculation process by performance optimization, model prediction and fractal structure of flocs. Desalination 269, 148–156. doi:10.1016/j.desal.2010.10.054
Keywords: bio-colloids, colloids, fouling, textile effluent, pretreatment alternatives
Citation: Aragaw TA and Bogale FM (2023) Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front. Environ. Sci. 11:1142227. doi: 10.3389/fenvs.2023.1142227
Received: 11 January 2023; Accepted: 05 April 2023;
Published: 20 April 2023.
Edited by:
Santiago Septien Stringel, University of KwaZulu-Natal, South AfricaReviewed by:
Ahmed K. Badawi, Ahmad Badawi, EgyptCopyright © 2023 Aragaw and Bogale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadele Assefa Aragaw, dGFhYWFkODJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.