- 1Agricultural Innovation and Technology Transfer Center (AITTC), Mohammed VI Polytechnic University (UM6P), Benguerir, Morocco
- 2College of Sustainable Agriculture and Environmental Sciences, Mohammed VI Polytechnic University, Benguerir, Morocco
- 3Department of Soil Science, Lincoln University, Lincoln, New Zealand
The direct application of phosphate rock (PR) has been found suitable for acidic soils. Still, efforts are needed to improve its reactivity to match grassland P demand. This research aimed to investigate changes in the dissolution of two Moroccan sedimentary PRs (Ben Guerir and Khouribga) in response to four rates of phosphogypsum (PG)—a by-product of the phosphate fertilizer industry. We conducted a 60-day incubation study using two acid soils from New Zealand. The soils were treated with PRs at 100 mgP kg−1 of soil either alone or combined with PG, which was applied at 0, 1, 3 and 9 t ha−1 (approximately the equivalent of 0, 0.9, 2.7, and 8.1 g of PG kg−1 of soil, respectively). The dissolution rates were determined from the differences in residual calcium (Ca) extracted with 1 M HCl. Soil pH, Olsen P, exchangeable aluminium (Al) and Ca and Ca saturation were analyzed at the end of the experiment. Phosphate rocks and PG’s physicochemical properties were characterized. Phosphogypsum addition increased Olsen P by 34% and 59% at 9 t ha−1 compared to 0 t ha−1 in Molesworth and Lindis Peaks soils, respectively. However, PG did not affect the dissolution of PRs in the different of soil types. Khouribga PR was more reactive than Ben Guerir PR, especially in the Molesworth soil where soil pH and base saturation were lower and P retention was higher compared to Lindis Peaks soil. Particle size distribution was the key factor that contributed to the observed greater reactivity of the Khouribga PR. Both PRs showed dissolution rates >50%, suggesting their suitability for direct application on acid soils. Being an important source of sulphur and some P, PG if combined with PR, can promote and complement PR’s direct use as fertilizer on acid soils. Moreover, the development of new fertilizer products by combining these two materials should be encouraged.
1 Introduction
Phosphorus (P) is an essential element for all life forms. It is the second most important macro-nutrient after nitrogen that often limits plant productivity in agricultural and natural ecosystems globally (Elser and Haygarth, 2020; Hou et al., 2020). Low P availability is considered one of the main problems in acid soils which represent over 50% of potentially arable lands in the world (Von Uexküll and Mutert, 1995), especially Ultisols and Oxisols (Reed and Wood, 2016). In acid soils, P availability is mainly limited by adsorption reactions due to low pH, high concentrations of iron (Fe) and aluminium (Al) oxides and hydroxides, and sorption to organic matter and clay minerals (Gessa et al., 2005; Asomaning, 2020). Phosphorus use efficiency (PUE) in acid soil is 10%–15% only because soluble forms of P fertilizer used are easily precipitated as insoluble forms with poor recovery; this leads to the repeated and excessive application of P fertilizer to land (Thomas Sims and Pierzynski, 2005; Cordell et al., 2011).
Phosphate rock (PR) is the basic raw material for manufacturing soluble P fertilizers. Its direct application as fertilizer has been known for a long time ago. Phosphate rock is relatively slow to release soluble P, yet its low price is very attractive as a P fertilizer in comparison to the commercial P fertilizers for which the manufacturing industrial processes are energy-intensive. Moreover, the acceptability of PR for organic farming makes it an obvious choice for common use (Edwards et al., 2010). However, there is still a need to better manage PR dissolution and subsequent availability of P. The direct use of PR is generally limited to a range of situations where the combination of soil properties and cropping systems offer optimal conditions that allow dissolution rates to match short-term plant P demand. Several management options have been proposed to increase PR dissolution such as 1) partial acidulation (Ahmad et al., 2019), 2) biologically mediated solubilization (Magallon-Servin et al., 2020) and 3) incorporation with various additives such as elemental sulphur (César et al., 2020), some industrial wastes (Ahmad et al., 2012) and agro-industrial wastes (Vassilev et al., 2006). However, increasing the number of industrial wastes used as additives in circular manner due to their low price, is an important research topic as there is a need for promising chemical and biotechnological routes that provide a cost-effective solubilization of PR.
Phosphogypsum (PG) is a by-product of the phosphoric fertilizer industry, originating from the wet process of phosphoric acid produced according to the generic reaction:
2 Material and methods
2.1 Soil characteristics
A 60-day incubation experiment was carried out in a laboratory (Lincoln University, NZ) in 2019, using two acid soils. Soils (0–15 cm) were collected from two sites in New Zealand. The “Molesworth” soil was collected from Molesworth station (42° 06′ 17″S, 173° 07′ 33″E), in the Marlborough region, while the “Lindis Peaks” soil was sampled from Lindis Peaks station (44° 46′ 26″S, 169° 27′ 21″E), in the Central Otago region. Plant material and stones were removed, and then the soil was thoroughly mixed, air-dried, and sieved (2 mm). The “Molesworth” is classified as brown soil according to New Zealand (NZ) soil classification (Hewitt, 2010), while “Lindis Peaks” is classified as Pallic. Both soils are classified as Inceptisols in the USDA classification (Schoeneberger et al., 2012). The soil’s physical and chemical characteristics are given in Table 1. The main differences between the two soils were: Base saturation, P retention, exchangeable Al and initial pH (see details in Table 1). These properties were selected because of their role in modulating the solubility of PR in the soil.
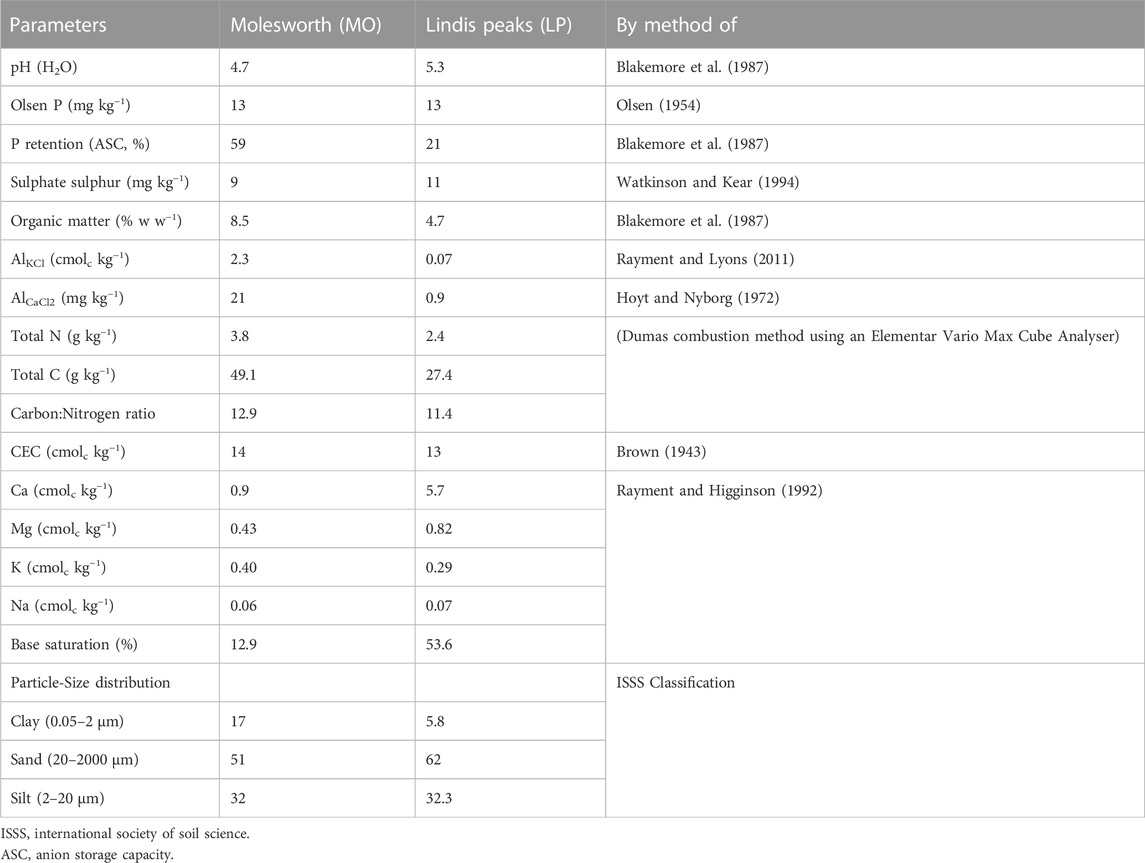
TABLE 1. Soil chemical properties and particle-size distribution before the establishment of the experiments.
2.2 Experimental design and treatments
The sieved soils were subjected to PG and PR treatments either combinedly or separately. This experiment was a 4 × 2 × 2 factorial design with four rates of PG, two PRs and two soils. Four replicates were used for each treatment level. In PG treatment, four rates of PG: 0, 1, 3 and 9 t ha−1 (approximately equivalent to an amount of 0, 0.9, 2.7, and 8.1 g of PG kg−1 of soil, respectively) were applied. The equivalent per hectare of PG rates added to the soils was estimated using a bulk density of 1.1 g cm−3 and a soil depth of 10 cm. In the PR treatments, two Moroccan rock sources (Ben Guerir: BG and Khouribga: Kh) were used. Each PR was applied at 100 mg of P kg−1 of soil (approximately equivalent to an amount of 0.71 and 0.95 g of BG and Kh per kg of soil, respectively, depending on the total P and moisture contents of the PRs). This rate was selected to achieve an optimum Olsen P of 25–30 mg kg−1 for NZ sedimentary soils (Roberts et al., 1994). The chemical compositions of PG and PRs used in this study are presented in Tables 2 and Table 3, respectively. The two sedimentary PRs were characterized and described by Drief (2021); the length of the a-axis of the apatite crystal lattice was determined using the X-ray diffraction method (McClellan and Lehr, 1969), while the particle size distribution was determined by placing each PR on the top of a nest of eleven sieves with a lid and a receiver and subjected to 5 min shaking.
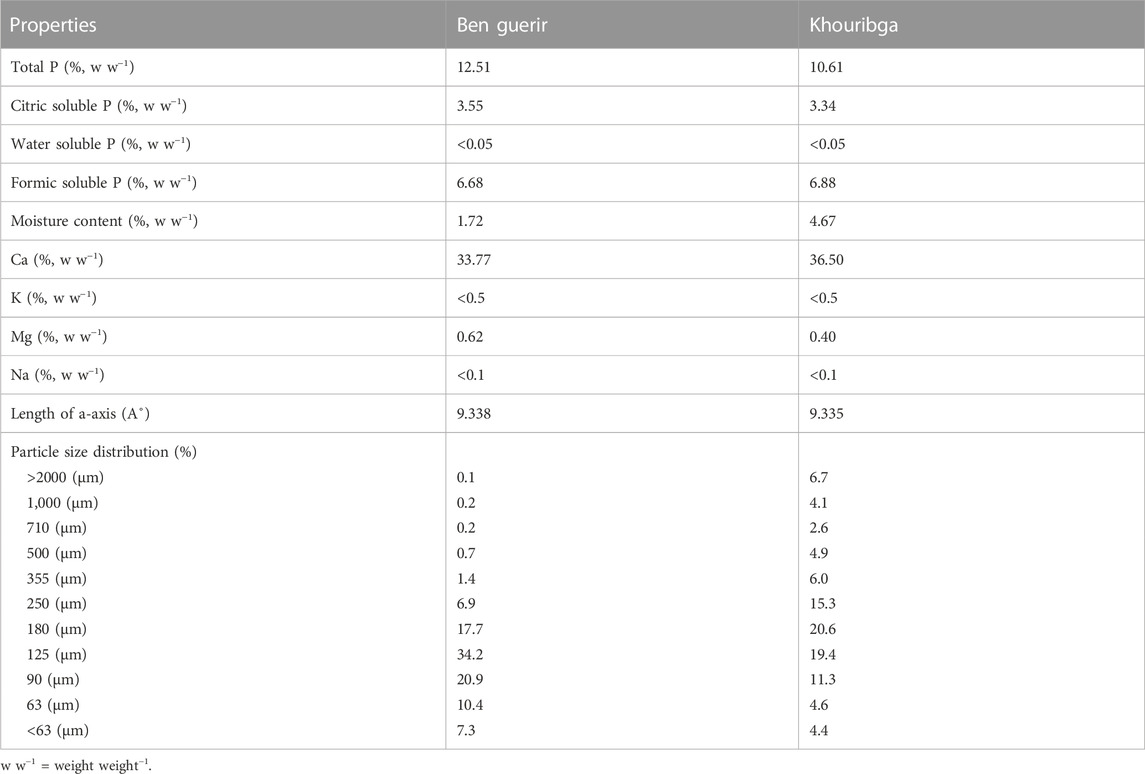
TABLE 3. Chemical composition and particle size distribution of the two Moroccan phosphate rocks used in the experiments.
Phosphogypsum and PRs treatments were thoroughly mixed with 100 g of air-dried soil. The treated soils were placed in 200-ml glass jars with screw-top lids left partially open to allow aeration while minimizing water loss through evaporation. The soils were incubated at 25°C in a completely randomized block design. Water was added to the soil during incubation to maintain moisture content at 20%–24% (v v−1). At the end of the incubation period, the lids were removed, and the incubation temperature was raised to 30°C for 5 days to dry the soils. The soils were then sieved (2 mm mesh) for analysis.
2.3 Soil analyses
The extent of PR dissolution in soil was determined from the Ca remaining (ΔCa) in the undissolved PR as described by Bolan and Hedley (1989). The ΔCa values were calculated as the difference between the amounts of Ca extracted from soils amended with PR + PG and that extracted exclusively from PG-treated soil (Eq. (1)), assuming that not all the Ca in PG is soluble. For the measurement of ΔCa, the soils were pre-extracted with 0.5 M BaCl2/TEA solutions at a solid: solution ratio of 1:10 for 1 h to remove the exchangeable Ca. The residual soil was then extracted with 1 M HCl (solid: solution ratio 1: 40 for half an hour). The extracts were then centrifuged at 3,500 rpm for 10 min, and the supernatant was transferred into a separate vial. A subsample of the supernatant was analyzed for total Ca using Inductively Coupled Plasma Optical Emission Spectrophotometry (ICP-OES: Varian 720-ES ICP-OES, Varian, Melbourne, Australia).
The soil pH (1:2.5 soil: water ratio) was measured using deionized water. The plant-available soil P fraction (Olsen P) was estimated using 0.5 M sodium bicarbonate extraction (Olsen, 1954) and was analyzed in a discrete wet chemistry analyzer (Smartchem TM 200, AMS Alliance, Paris, France). In New Zealand, government scientists rigorously examined several labile P soil test methods during the 1970 s and 80 s. After analysis of large data sets from many field experiments, these workers concluded that the Olsen P test was by far the best test of labile soil P under NZ acid pasture soils. This was because it was the best predictor of pasture yield, when compared to the other soil P tests. Since those times, the Olsen P test has been used as the standard method to estimate labile P in New Zealand acid soils, with high success (Mackay et al., 1984; Saunders et al., 1987). Exchangeable Al was extracted using 0.02 M CaCl2 (1:4 soil: extractant ratio) and then analyzed using Inductively Coupled Plasma Optical Emission Spectrophotometry (ICP-OES: Varian 720-ES ICP-OES, Varian, Melbourne, Australia). Calcium saturation (in %) was determined for PG-treated soils only, by extracting the soil cations (Ca2+, K+, Mg2+, Na+, and Al3+) using 1 M ammonium acetate buffered at pH seven followed by analysis with ICP-OES.
2.4 Statistical analysis
Analysis of variance (ANOVA) was carried out to test the differences between means and in cases of significant differences (p < 0.05), subsequent comparison using Tukey’s post-hoc test (p < 0.05) using R statistical software version 4.1.0 (R Development Core Team, 2021) was performed. Soil type, PG rate and PR type were considered fixed factors. Three-way ANOVA was used to identify the main effects of these factors and their interaction effects on PR dissolution. Another three-way ANOVA was carried out to test the significance of the main and interaction effects of soil type, PG rate and treatment (PG alone, PG + BG, and PG + Kh) on soil pH, Olsen P and exchangeable Al. One-way ANOVA was used to identify the differences between the effects of PG rates and PG×PR combinations separately on soil parameters. A two-sample t-test was used to test the differences between the effects of the two soils on PR dissolution and soil parameters. The ANOVA and t-test assumptions, normality (using Shapiro-wilk’s test) and homogeneity of variances (using Levene’s test), were considered during the data analysis. A simple linear regression was used to identify the relationship between Ca saturation and Olsen P and BaCl2-extracted Ca in the soils treated with PG alone.
3 Results
3.1 Dissolution of rock phosphate as affected by phosphogypsum addition and soil type
The dissolution of both Ben Guerir and Khouribga PRs was not affected (p > 0.05) by PG application regardless of soil type (Table 4). However, the dissolution rate between the two PRs was different (p < 0.001) across all soil types and PG rates. Moreover, the dissolution of the investigated PRs was affected by the interaction between PR type and soil type, irrespective of PG rates. This interaction effect is presented in Figure 1 which depicts that the difference in the dissolution between Ben Guerir and Khouribga PRs was significant only for Molesworth soil. Overall average dissolution of Khouribga PR in Molesworth soil was 71% against 56% for Ben Guerir PR, across all PG rates. In contrast, in Lindis Peaks soil, the overall average dissolution remained similar between the two PRs (63% versus 60% for Khouribga and Ben Guerir, respectively).
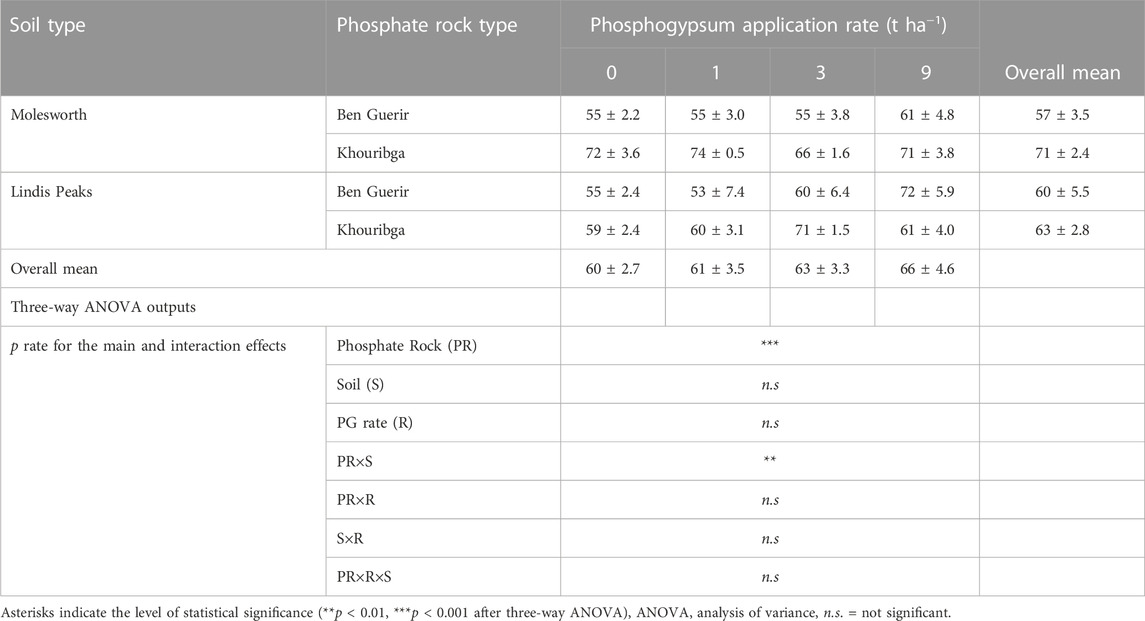
TABLE 4. The dissolution in percentage (%) of two Moroccan phosphate rocks (BG: Ben Guerir and Kh: Khouribga) in two acid grassland soils (Molesworth and Lindis Peaks), as affected by four rates of phosphogypsum (0, 1, 3 and 9 t ha−1) after an incubation of 60 days.

FIGURE 1. Comparison of the dissolution rate (%) of the two Moroccan phosphate rocks (BG: Ben Guerir and Kh: Khouribga) within each soil type (Molesworth and Lindis Peaks) separately, across four rates of PG (0, 1, 3 and 9 t ha−1). Asterisks indicate the level of statistical significance (***p < 0.001 after two-samples t-test, n.s = not significant). Circles: individual measurements (n = 16 data points).
3.2 Soil pH, olsen P and exchangeable Al as affected by phosphogypsum and phosphate rock additions
Phosphogypsum addition affected soil pH differently between the two investigated soils. There was an increase in soil pH with a PG rate increase (p < 0.05) in Molesworth soil, whereas a decrease (p < 0.005) in soil pH was observed in Lindis Peaks soil with PG rate increase (Table 5). Soil pH under the combined addition of PR and PG followed the same trend as PG alone and was not influenced by PRs addition (Table 5). Moreover, soil pH was always higher (p < 0.05) in Lindis Peaks soil compared to Molesworth, while exchangeable Al was always higher in Molesworth soil compared to Lindis Peaks soil (Table 5) regardless of the treatment (PG alone, PG + BG, and PG + Kh) and PG rate. In Molesworth soil, the lowest soil exchangeable Al concentrations were recorded at 1 and 3 t ha−1 of PG either applied alone or combined with PRs. Conversely, an increase in exchangeable Al concentration was observed in Lindis Peaks soil with PG rate increase.However, when PG was combined with Khouribga PR, there was a decrease in soil exchangeable Al at 1 and 3 t ha−1 rates compared to the 0 and 9 t ha−1 rates. Moreover, the concentrations of exchangeable Al were always lower under the combined application of PG and PRs compared to PG alone in Lindis Peaks soil.
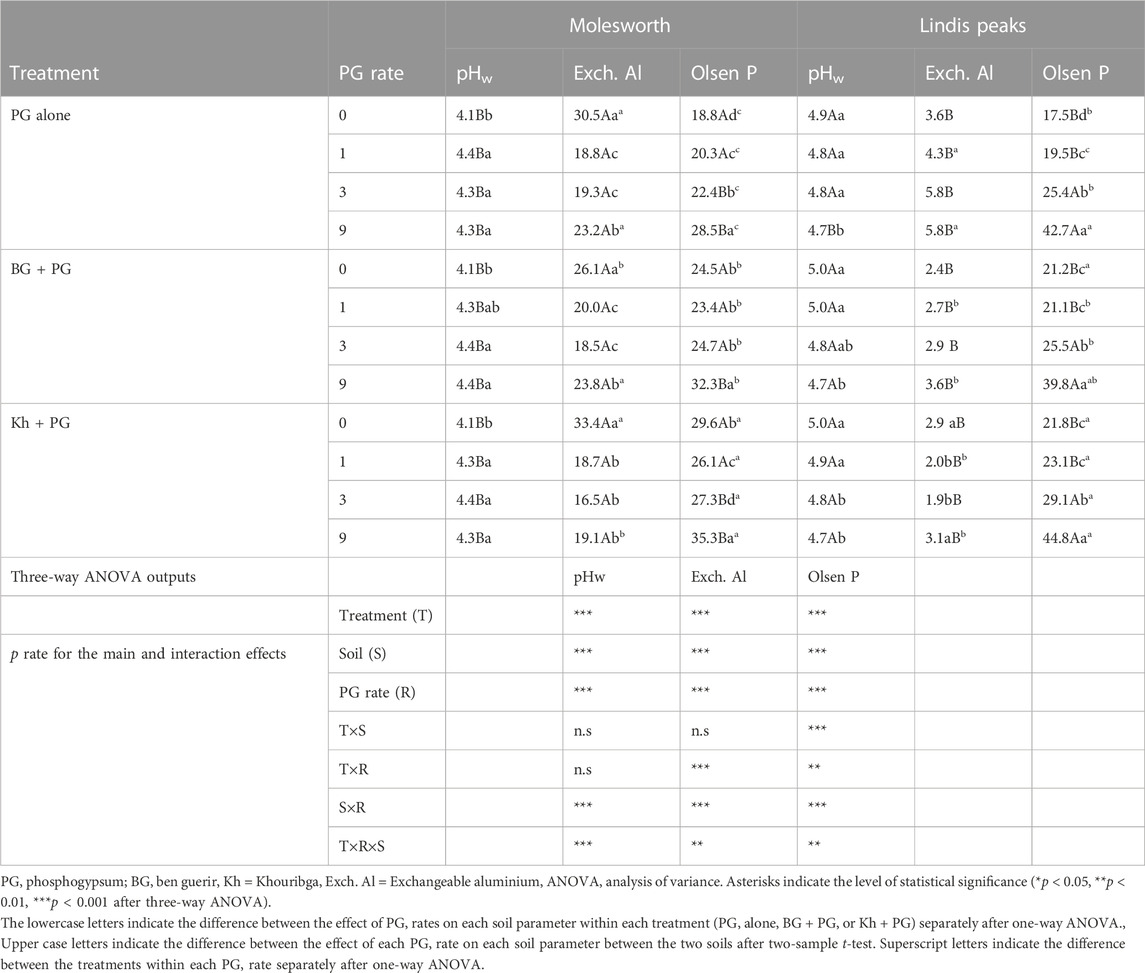
TABLE 5. Effect of a separate and combined application of phosphogypsum and two Moroccan phosphate rocks (Ben Guerir and Khouribga) on soil pH, Olsen P and exchangeable aluminium of two acid grassland soils (Molesworth and Lindis Peaks) after an incubation of 60 days.
There was a significant increase in Olsen P with PG rate increase either applied alone or combined with PRs. However, the highest Olsen P values were recorded under the combined application of PRs and PG (p < 0.001). Within each PG rate, Olsen P-value under the combinations: BG + PG and Kh + PG were always higher (p < 0.05) compared to PG alone in both Molesworth and Lindis Peaks soils (Table 5). Moreover, Kh + PG always gave higher Olsen p values compared to BG + PG in both soils. Excluding the PG effect on Olsen P, Khouribga PR alone contributed to increasing the average Olsen P in Molesworth soil by 48% compared to Ben Guerir PR (7.1 mg kg−1 increase versus 3.7 mg kg−1), and by 81% in Lindis Peaks soil (3.4 mg kg−1 versus 0.6 mg kg−1, respectively), across all PG rates (Figure 2).
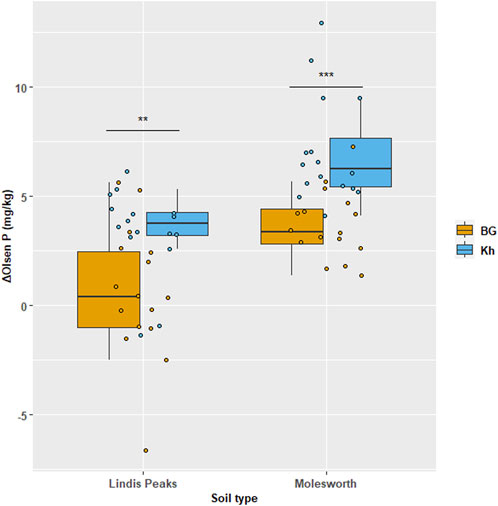
FIGURE 2. The additional effect of two Moroccan rock phosphates (BG: Ben Guerir and Kh: Khouribga) on Olsen P in two acid grassland soils (Molesworth and Lindis Peaks). Individual points (n = 16) represent the of the differences between the Olsen p-value of rock phosphate combined with PG and that of PG alone (ΔOlsen P = Olsen P (BG + PG or Kh + PG) - Olsen P (PG alone. Asterisks indicate the level of statistical significance (**p < 0.01, ***p < 0.001 after two-samples t-test) between BG and Kh within each soil separately.
Calcium saturation of Molesworth soil increased from a min of 56% to a max. of 82% when the PG application rate increased from 0 to 9 t ha−1 in the absence of PR addition (Figure 3B). Likewise, the Ca saturation of Lindis Peaks increased from 9% to 58% (Figure 3B). Moreover, A strong and positive polynomial relationship was found between soil Ca saturation and Olsen P (R2 = 0.93 and 0.99 for Molesworth and Lindis Peaks soils, respectively, Figure 3A) as affected by PG alone. However, the change magnitude of Olsen P with Ca saturation increase was higher for Lindis Peaks soil compared to Molesworth soil, passing from a min. of 17 to max. of 45 mg kg−1 in Lindis Peaks soil against only 18–30 mg kg−1 in Molesworth soil. Also, a strong and positive linear relationship (R2 = 0.95 and 0.96 for Molesworth and Lindis Peaks soils, respectively) was found between BaCl2-extracted Ca and Ca saturation for both soils as affected by PG alone (Figure 3B). However, the slope in the regression equation of Molesworth soil is 2.6 times greater than that of Lindis Peaks (Figure 3B).
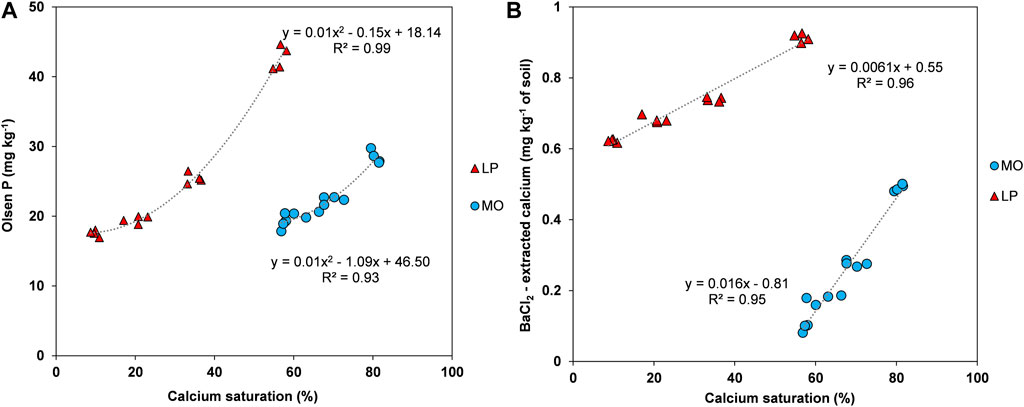
FIGURE 3. Relationships between soil calcium saturation (%) and (A) Olsen P and (B) soil-extracted calcium using 1 M BaCl2/TEA, as affected by four rates of PG (0, 1, 3 and 9 t ha−1) in the absence of phosphate rock addition. MO = Molesworth, LP = Lindis Peaks. Circles and triangles: individual measurements (n = 16).
4 Discussion
In our study, the dissolution of PRs remained unchanged after the PG application, regardless of the application rate. This suggests that either both the acidifying and repressive effects of PG acted equally on PRs solubilization processes in the investigated soils, or that PG treatments had no effect at all. A decline in soil pH with PG was observed in Lindis Peaks soil only, whereas, in Molesworth soil, the pH increased by 0.1–0.3 units under PG application. Non-etheless, the pH values of Molesworth soils with or without PG were very low (pH < 4.5) compared to Lindis peaks. This indicates that the inherent soil acidity of Molesworth was likely the key driver of the dissolution of PRs, and that the resulting increase in soil pH after PG application seemed not to affect the PR dissolution to any great extent. This is evidenced by the fact that + 50% of PRs were dissolved in only a 2-month period irrespective of PG rates. This indicates the suitability of these two Moroccan PRs for direct application on acid soils with sufficient moisture content.
The resulting increase in soil pH in PG-treated Molesworth soils could be ascribed to the ligand exchange reactions in which SO42− replaces OH− releasing it to the soil solution (Hue et al., 1985; Bouray et al., 2020). In contrast, the resulting decrease in Lindis Peaks soil pH can be explained by the large supply of Ca2+ which displaced H+ and Al3+ (which liberates H+ after hydrolysis) into the soil solution (Alva et al., 1988; Alva et al., 1990). Thus, we hypothesize that the PG effect on soil pH likely depends on the balance between Ca2+ and SO42− reactions which in their turn are modulated by soil properties. However, in closed incubation systems where soil volume is limited, promoting high ionic strength conditions, the interpretation of pH (H2O) must be done carefully. Despite the acidifying effects of PG in Lindis peaks soil, PRs dissolution was not affected. This could be due to the repression effect of P and Ca supplied by PG, considering the hypothetical PR dissolution reaction, resulting in the release of H2PO4− and Ca2+ (Chien and Menon, 1995). This is supported by the resulting increase in Olsen P and soil exchangeable Ca proportionally with PG rate increase. Moreover, the linear increase in soil Ca saturation with PG, in both soils, could also have contributed to limiting any eventual positive effect of PG on PRs dissolution. This is because the occupation of soil exchange sites by PG-sourced Ca would not provide a sink for Ca ions released from PR, slowing down its dissolution (Robinson et al., 1992).
The resulting interaction between PR source and soil type revealed that the two Moroccan PRs performed differently, but these differences were detectable only in Molesworth soil. This could be attributed to the initial properties of Molesworth soil which were more advantageous for PR dissolution and thus favoring the most reactive PR, which is Khouribga PR in our case. For instance, the lower initial pH of Molesworth soil indicates that there were likely more protons to mediate the dissolution reaction. Moreover, its higher organic matter content (8.5% versus 4.7% in Lindis Peaks) and lower base saturation (12.9% versus 53.6% in Lindis peaks) could have provided an important sink for Ca released during the dissolution (Savini et al., 2006). Additionally, the higher P retention capacity of Molesworth soil (59% versus 21% for Lindis Peaks) might have reduced P entering the soil solution from PR dissolution and consequently enhanced the dissolution rate of PRs. This agrees with previous studies (Smyth and Sanchez, 1982; Yusdar et al., 2007).
The agronomic effectiveness of PR relative to soluble fertilizer (e.g., superphosphate) may be less on soils with high P retention capacity because of soil P fixation, limiting plant available P (Hammond et al., 1986; Babare et al., 1997). Hence, the importance of combining PR with PG as an additional source of P in this type of soil. The higher Al content of Molesworth soil (Whitley et al., 2019; Whitley et al., 2020) might also have been partly involved in the control of P concentration in the soil solution.Hydroxyl-Al species are known to be highly active adsorption surfaces for phosphate (McLean, 1976; Penn and Camberato, 2019). This view is supported by resulting differences in the magnitude of Olsen P increase with calcium saturation increase between the two soils, because the occupation of soil exchangeable site with Ca would necessitate a displacement of Al3+ cation into the soil solution and therefore increases the chances of P complexation and immobilization. Khouribga PR gave higher Olsen P values than Benguerir PR not only in Molesworth but also in Lindis Peaks soil (Figure 2), confirming the superior reactivity of Khouribga PR. This difference in the solubility between these two rocks could be attributed to the particle size distribution; Khouribga PR had a higher percentage of fine particles compared to Ben Guerir PR (Table 3). For instance, the percentage of particle size ≤ 125 µm of Khouribga PR was 72.8% against 39.7% only for Ben Guerir. Therefore, the finer particle size of Khouribga PR likely increased its specific surface area and degree of contact with the soil, and consequently increased its dissolution compared to Benguerir (Kanabo and Gilkes, 1988; Klaic et al., 2017). The unit-cell-a-dimension (Å, Table 3) of the two PRs were similar. This means that the degrees of isomorphic substitution of carbonate (CO32-) for phosphate (PO43-) in the crystalline structure of both PRs were similar and so their chemical stabilities were also similar (Chien et al., 2011).
The resulting decrease of exchangeable Al at 1 and 3 t of PG ha−1 in Molesworth soil confirmed the role that PG may play in mitigating Al toxicity in high Al acid soils, if applied at reasonable rates. This could be attributed to the mechanism of Al displacement on soil exchange sites via Ca2+, followed by Al3+ complexation with SO42− and F− in the soil solution. This view is supported by the finding of a recent study conducted by Bouray et al. (2022). However, higher PG rates should be avoided on acid soils because it may further acidify the soil and thus solubilize Al, as evidenced by the resulting increase in exchangeable Al at 9 t of PG ha−1 compared to 0 and 3 t of PG ha−1 in the present study. The exchangeable Al concentration did not change at 9 t ha−1 compared to 1 and 3 t ha−1 when PG was combined with Khouribga PR in Molesworth soil. Thisconfirms the reactivity of this rock source compared to Ben Guerir PR, because PRs are known to have a liming potential due to the neutralization of H+ protons during the dissolution process (Basak and Biswas, 2016). However, the absence of PRs effects on soil pH in our soils questions the liming potential of Moroccan PRs. Thus, we recommend assessing the liming ability of these PRs and defining their % calcium carbonate equivalent (%CCE) in a separate study, using different application rates instead of one single rate only as was the case in our experiment.
5 Conclusion
Our hypothesis that PG would improve the solubility of the investigated PRs has been rejected. However, the fact that PG did not negatively affect the solubility of the PRs is itself an interesting result, because PG is an important source of S which could complement PR on S and P-deficient soils. This suggests an affordable multi-nutrient fertilizers containing P and S can be developed from the combination of these two relatively cheap materials compared to commercial fertilizers. Khouribga PR dissolution rate was higher than Ben Guerir one, but in Molesworth soil only due to its low pH and base saturation and high P retention capacity. This difference between the two Moroccan PRs has been associated with their particle size distribution because Khouribga PR particles were finer. Both PRs showed dissolution rates >50%, suggesting their suitability for direct application on acid soils. However, in the present study, only two soils were tested without assessing plant effects and responses.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
MB: conceptualization, data collection and analysis, and first draft writing. JM: supervision, funding acquisition, project administration and contributed to manuscript reviewing. MB and JM: investigation and methodology. KM, MG, and RC: supervision, substantially revised and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by Mohammed 6 Polytechnic University (UM6P, Morocco) and Office Chérifien des Phosphates (OCP, Morocco) under a collaborative research program with Lincoln University (contract LU 46500).
Acknowledgments
We thank the AITTC team for their administrative/financial support as well as the insightful discussions. We also thank Lincoln University’s technical staff for laboratory assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, M., Ghoneim, A., Al-Oud, S. S., Alotaibi, K. D., and Nadeem, M. (2019). Acidulated activation of phosphate rock enhances release, lateral transport and uptake of phosphorus and trace metals upon direct-soil application. J. Soil Sci. Plant Nutr. 65 (2), 183–195. doi:10.1080/00380768.2019.1570333
Ahmad, Z., Abd-Elbasit, M. A. M., Inoue, M., Yasuda, H., Honna, T., and Yamamoto, S. (2012). Use of two industrial wastes as soil amendments: Effect on dissolved reactive phosphorus in runoff. Soil Sediment. Contam. 21 (2), 207–226. doi:10.1080/15320383.2012.649376
Alva, A., Sumner, M., and Miller, W. (1990). Reactions of gypsum or phosphogypsum in highly weathered acid subsoils. Soil Sci. Soc. Am. J. 54 (4), 993–998. doi:10.2136/sssaj1990.03615995005400040010x
Alva, A., Sumner, M., and Noble, A. (1988). Alleviation of aluminum toxicity by phosphogypsum. Commun. Soil Sci. Plant Anal. 19 (4), 385–403. doi:10.1080/00103628809367947
Asomaning, S. K. (2020). “Processes and factors affecting phosphorus sorption in soils,” in Sorption in 2020s. Editors G. Kyzas, and N. Lazaridis (IntechOpen), 1–16.
Babare, A., Gilkes, R., and Sale, P. (1997). The effect of phosphate buffering capacity and other soil properties on North Carolina phosphate rock dissolution, availability of dissolved phosphorus and relative agronomic effectiveness. Aust. J. Exp. Agric. 37 (8), 1037–1049. doi:10.1071/EA96128
Basak, B., and Biswas, D. (2016). Potentiality of Indian rock phosphate as liming material in acid soil. Geoderma 263, 104–109. doi:10.1016/j.geoderma.2015.09.016
Blakemore, L., Searle, P., and Daly, B. (1987). Methods for chemical analysis of soils. Wellington, New Zealand: New Zealand Soil Bureau Scientific. New Zealand Soil Bureau Scientific Report No. 80.
Bolan, N., and Hedley, M. (1989). Dissolution of phosphate rocks in soils. 1. Evaluation of extraction methods for the measurement of phosphate rock dissolution. Fertil. Res. 19 (2), 65–75. doi:10.1007/BF01054677
Bouray, M., Moir, J., Condron, L., and Lehto, N. (2020). Impacts of phosphogypsum, soluble fertilizer and lime amendment of acid soils on the bioavailability of phosphorus and sulphur under lucerne (Medicago sativa). Plants 9 (7), 883. doi:10.3390/plants9070883
Bouray, M., Moir, J. L., Condron, L. M., Lehto, N. J., Bayad, M., Gharous, M. E., et al. (2022). Effect of phosphogypsum application on aluminum speciation in acid pasture soils. J. Soils Sediments. 22, 1959–1975. doi:10.1007/s11368-022-03215-x
Brown, I. C. (1943). A rapid method of determining exchangeable hydrogen and total exchangeable bases of soils. Soil S. C. 56 (5), 353–358. doi:10.1097/00010694-194311000-00004
César, F. R. C. F., Muraoka, T., da Silva, R. C., and Souza-Filho, L. F. (2020). The agronomic benefit of phosphate rock application with elemental sulfur depends on the reactivity and fertilizer placement. J. Plant Nutr. 43 (18), 2773–2784. doi:10.1080/01904167.2020.1793181
Chernysh, Y., Yakhnenko, O., Chubur, V., and Roubík, H. (2021). Phosphogypsum recycling: A review of environmental issues, current trends, and prospects. Appl. Sci. 11 (4), 1575. doi:10.3390/app11041575
Chien, S., and Menon, R. (1995). Factors affecting the agronomic effectiveness of phosphate rock for direct application. Fertil. Res. 41 (3), 227–234. doi:10.1007/BF00748312
Chien, S., Prochnow, L., Tu, S., and Snyder, C. (2011). Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: An update review. Nutr. Cycl. Agroecosyst. 89 (2), 229–255. doi:10.1007/s10705-010-9390-4
Cordell, D., Rosemarin, A., Schröder, J. J., and Smit, A. (2011). Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 84 (6), 747–758. doi:10.1016/j.chemosphere.2011.02.032
Drief, M. (2021). Impact of soil, plant, and fertilizer properties and processes on the dissolution and agronomic effectiveness of Moroccan phosphate rock: A thesis submitted in partial fulfilment of the requirements for the degree of doctor of philosophy at Lincoln university. Christchurch, New Zealand: Lincoln University.
Edwards, A. C., Walker, R. L., Maskell, P., Watson, C. A., Rees, R. M., Stockdale, E. A., et al. (2010). “Improving bioavailability of phosphate rock for organic farming,” in Genetic engineering, biofertilisation, soil quality and organic farming. Editor E. Lichtfouse (Dordrecht: Springer Netherlands), 99–117.
Gessa, C. E., Mimmo, T., Deiana, S., and Marzadori, C. (2005). Effect of aluminium and pH on the mobility of phosphate through a soil-root interface model. Plant soil 272 (1), 301–311. doi:10.1007/s11104-004-5693-z
Hammond, L., Chien, S., and Easterwood, G. (1986). Agronomic effectiveness of Bayovar phosphate rock in soil with induced phosphorus retention. Soil Sci. Soc. Am. J. 50 (6), 1601–1606. doi:10.2136/sssaj1986.03615995005000060044x
Haynes, R., and Williams, P. (1993). Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv. Agron. 49, 119–199. doi:10.1016/S0065-2113(08)60794-4
Hou, E., Luo, Y., Kuang, Y., Chen, C., Lu, X., Jiang, L., et al. (2020). Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat. Commun. 11 (1), 637–639. doi:10.1038/s41467-020-14492-w
Hoyt, P., and Nyborg, M. (1972). Use of dilute calcium chloride for the extraction of plant-available aluminum and manganese from acid soil. Can. J. Soil Sci. 52 (2), 163–167. doi:10.4141/cjss72-020
Hue, N., Adams, F., and Evans, C. (1985). Sulfate retention by an acid BE horizon of an Ultisol. Soil Sci. Soc. Am. J. 49 (5), 1196–1200. doi:10.2136/sssaj1985.03615995004900050025x
Kanabo, I. A. K., and Gilkes, R. J. (1988). The effect of particle size on North Carolina phosphate rock on its dissolution in soil and on levels of bicarbonate-soluble phosphorus. Fertil. Res. 15 (2), 137–145. doi:10.1007/BF01050675
Klaic, R., Plotegher, F., Ribeiro, C., Zangirolami, T. C., and Farinas, C. S. (2017). A novel combined mechanical-biological approach to improve rock phosphate solubilization. Int. J. Min. Process. 161, 50–58. doi:10.1016/j.minpro.2017.02.009
Mackay, A. D., Syers, J. K., Gregg, P. E. H., and Tillman, R. W. (1984). A comparison of 3 soil-testing procedures for estimating the plant availability of phosphorus in soils receiving either superphosphate or phosphate rock. N. Z. J. Agric. Res. 27 (2), 231–245. doi:10.1080/00288233.1984.10430425
Magallon-Servin, P., Antoun, H., Taktek, S., and de-Bashan, L. E. (2020). Designing a multi-species inoculant of phosphate rock-solubilizing bacteria compatible with arbuscular mycorrhizae for plant growth promotion in low-P soil amended with PR. Biol. Fertil. Soils 56 (4), 521–536. doi:10.1007/s00374-020-01452-1
McClellan, G. H., and Lehr, J. R. (1969). Crystal chemical investigation of natural apatites. Am. Min. 54 (9-10), 1374–1391.
McLean, E. O. (1976). Chemistry of soil aluminum. Ommun. Soil Sci. Plant Anal. 7 (7), 619–636. doi:10.1080/00103627609366672
Olsen, S. R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington D.C.: US Department of Agriculture.
Outbakat, M. b., Choukr-Allah, R., Bouray, M., Gharous, M. E., and Mejahed, K. E. (2023). “Phosphogypsum: Properties and potential use in agriculture,” in Biosaline agriculture as a climate change adaptation for food security. Editors R. Choukr-Allah, and R. Ragab (Switzerland AG: Springer Nature).
Outbakat, M. B., Choukrallah, R., El Gharous, M., El Omari, K., Soulaimani, A., and Mejahed, K. (2022). Does phosphogypsum application affect salts, nutrients, and trace elements displacement from saline soils? Front. Environ. Sci. 1381. doi:10.3389/fenvs.2022.964698
Penn, C. J., and Camberato, J. J. (2019). A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9 (6), 120. doi:10.3390/agriculture9060120
Rayment, G. E., and Lyons, D. J. (2011). “Exchange acidity (H+ + Al3+) by 1 M potassium chloride,” in Soil chemical methods: Australasia. Editors G. E. Rayment, and D. J. Lyons (Collingwood, Victoria, Australia: CSIRO publishing), 335–338.
Rayment, G., and Higginson, F. R. (1992). Australian laboratory handbook of soil and water chemical methods. Melbourne, Australia: Inkata Press Pty Ltd.
Reed, S. C., and Wood, T. E. (2016). “Soil phosphorus cycling in tropical soils: An ultisol and oxisol perspective,” in Soil phosphorus. Editors R. Lal, and B. A. Stewart (Boca Raton: Taylor & Francis), 247–284.
Roberts, A., Webb, T., Morton, J., O'Connor, M., and Edmeades, D. (1994). “Building a solid foundation-sulphur, phosphorus and potassium requirements for the sedimentary soiIs of North Canterbury,” in Proceedings of the New Zealand Grassland Association, 7–12.
Robinson, J., Syers, J., and Bolan, N. (1992). Importance of proton supply and calcium-sink size in the dissolution of phosphate rock materials of different reactivity in soil. Eur. J. Soil Sci. 43 (3), 447–459. doi:10.1111/j.1365-2389.1992.tb00151.x
Saadaoui, E., Ghazel, N., Ben Romdhane, C., and Massoudi, N. (2017). Phosphogypsum: Potential uses and problems–a review. Int. J. Environ. Stud. 74 (4), 558–567. doi:10.1080/00207233.2017.1330582
Saunders, W., Sherrell, C., and Gravett, I. (1987). Calibration of Olsen bicarbonate phosphorus soil test for pasture on some New Zealand soils. N. Z. J. Agric. Res. 30 (3), 387–394. doi:10.1080/00288233.1987.10421899
Savini, I., Smithson, P., and Karanja, N. (2006). Effects of added biomass, soil pH and calcium on the solubility of Minjingu phosphate rock in a Kenyan Oxisol. Arch. Agron. Soil Sci. 52 (1), 19–36. doi:10.1080/03650340500471922
Schoeneberger, P. J., Wysocki, D. A., and Benham, E. C. (2012). Field book for describing and sampling soils. Lincoln, Nebraska, USA: Natural Resources Conservation Service, USDA, National Soil Survey Center.
Smyth, T., and Sanchez, P. (1982). Phosphate rock dissolution and availability in Cerrado soils as affected by phosphorus sorption capacity. Soil Sci. Soc. Am. J. 46 (2), 339–345. doi:10.2136/sssaj1982.03615995004600020026x
Thomas Sims, J., and Pierzynski, G. M. (2005). “Chemistry of phosphorus in soils,” in Chemical processes in soils. Editors M. A. Tabatabai, and D. L. Sparks (USA: Soil Science Society of America, Inc.), 151–192.
Vassilev, N., Medina, A., Azcon, R., and Vassileva, M. (2006). Microbial solubilization of rock phosphate on media containing agro-industrial wastes and effect of the resulting products on plant growth and P uptake. Plant Soil 287 (1), 77–84. doi:10.1007/s11104-006-9054-y
Von Uexküll, H., and Mutert, E. (1995). Global extent, development and economic impact of acid soils. Plant soil 171 (1), 1–15. doi:10.1007/BF00009558
Watkinson, J., and Kear, M. (1994). High performance ion chromatography measurement of sulfate in 20 m M phosphate extracts of soil. Commun. Soil Sci. Plant Anal. 25 (7-8), 1015–1033. doi:10.1080/00103629409369095
Whitley, A. E., Moir, J. L., Lehto, N. J., and Paramashivam, D. (2020). Extractable aluminium in New Zealand andisols and Inceptisols. Geoderma Reg. 22, e00315. doi:10.1016/j.geodrs.2020.e00315
Whitley, A., Moir, J., and Almond, P. (2019). A meta-analysis of exchangeable aluminium in New Zealand soils using the National Soils Database. Soil Res. 57 (2), 113–123. doi:10.1071/SR18246
Keywords: phosphogypsum, phosphate rock, dissolution, acid soil, phosphorus, incubation study
Citation: Bouray M, Moir J, El Mejahed K, Choukr-Allah R and El Gharous M (2023) Does phosphogypsum addition affect phosphate rock dissolution in acid soils?. Front. Environ. Sci. 11:1130881. doi: 10.3389/fenvs.2023.1130881
Received: 23 December 2022; Accepted: 17 April 2023;
Published: 05 May 2023.
Edited by:
Yuncong Li, University of Florida, United StatesReviewed by:
Warren Dick, The Ohio State University, United StatesJajati Mandal, University of Salford, United Kingdom
Copyright © 2023 Bouray, Moir, El Mejahed, Choukr-Allah and El Gharous. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moussa Bouray, bW91c3NhLmJvdXJheUB1bTZwLm1h
 Moussa Bouray
Moussa Bouray Jim Moir
Jim Moir Khalil El Mejahed
Khalil El Mejahed Redouane Choukr-Allah1,2
Redouane Choukr-Allah1,2 Mohamed El Gharous
Mohamed El Gharous